Amino derivatives of androstanes and androstenes as medicaments for cardiovascular disorders
A technology of androstane and alkyl groups, applied in the field of new amino derivatives, can solve problems such as side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
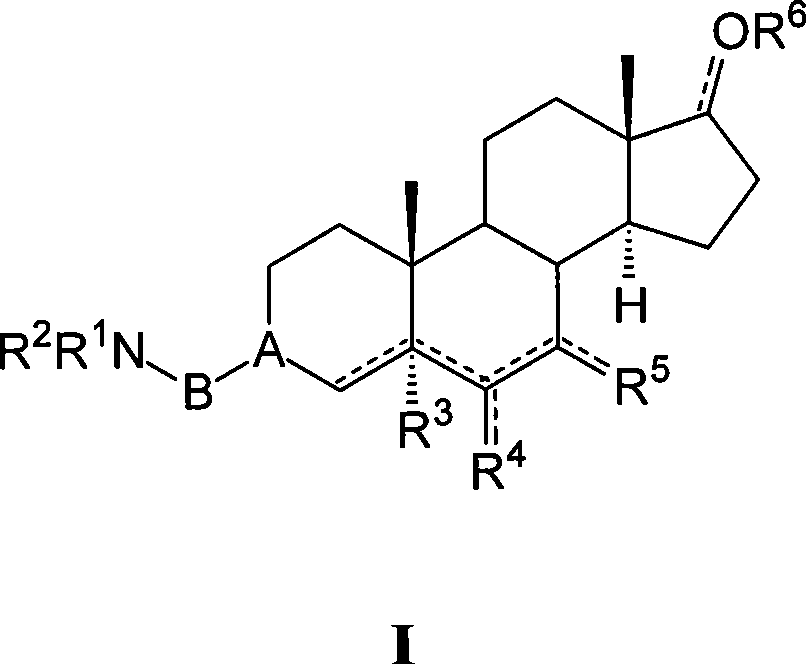
[0362] (E, Z) 3-(2-Aminoethoxyimino)-17-oxoandrostane-6α-yl fumarate nitrate Ester (I-aa)
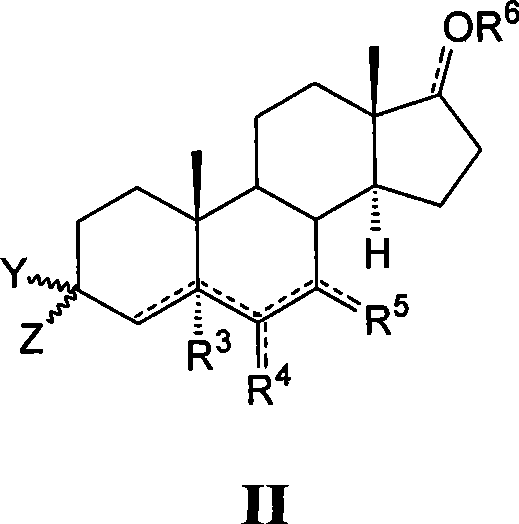
[0363] To a stirred THF solution (30 mL) of 3,17-dioxoandrostane-6α-ylnitrate (II-aa, Preparation 1, 1.14 g) was rapidly added dropwise 2-aminoethoxyamino di Hydrochloride (223mg), Na 2 HPO 4 12H 2 O (2.30 g) in water (11.6 mL). After 1.5 hours, NaCl (1.8 g) was added and the mixture was stirred for 10 minutes. The phases were separated and the aqueous phase was extracted with THF (2x). The combined organic extracts were washed with Na 2 SO 4 Dry, filter and evaporate to dryness. By flash chromatography (SiO 2 , CHCl 3 / MeOH / 26% NH4OH 90 / 10 / 1) to purify the crude product. To this concentrated fraction was added a stoichiometric amount of fumaric acid in MeOH. Add EtOAc / Et 2 After a 1 / 1 mixture of O, the precipitate was filtered to give the title compound I-aa (0.57 g, 33%) as a white solid. 1 H-NMR (300MHz, DMSO-d 6 , ppm from TMS): δ 8.76 (bb, 4H), 6.41 (s, 1H), 4.98 ...
Embodiment 2
[0365] (E, Z) 3-(2-Aminoethoxyimino)-17-oxoandrostane-6β-ylfumarate nitrate Ester (I-ab)
[0366] Starting from 3,17-dioxoandrostane-6β-ylnitrate (II-ab, preparation 2) and 2-aminoethoxyamino dihydrochloride as described in Example 1, at 60% prepared in high yields. 1 H-NMR (300MHz, DMSO-d 6 , ppm from TMS): δ 8.41 (bb, 4H), 6.40 (s, 2H), 5.23 (m, 0.5H), 5.19 (m, 0.5H), 4.03 (m, 2H), 3.05 (m, 1H ), 2.96 (m, 2H), 2.45-0.70 (m, 19H), 1.00 (s, 1.5H), 0.99 (s, 1.5H), 0.80 (s, 3H).
Embodiment 3
[0368] (E, Z) 3-(2-Aminoethoxyimino)-6α-cyanoandrostan-17-one fumarate (I-ac)
[0369] Starting from 6α-cyanoandrostane-3,17-dione (II-ac, preparation 3) and 2-aminoethoxyamino dihydrochloride as described in Example 1, in 65% yield prepared. 1 H-NMR (300MHz, DMSO-d 6 , ppm from TMS): δ 9.07 (bb, 4H), 6.40 (s, 2H), 4.07 (m, 2H), 3.24 (m, 0.5H), 3.06 (m, 0.5H), 2.99 (m, 2H ), 2.77 (m, 1H), 2.45-0.70 (m, 19H), 0.88 (s, 1.5H), 0.87 (s, 1.5H), 0.77 (s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com