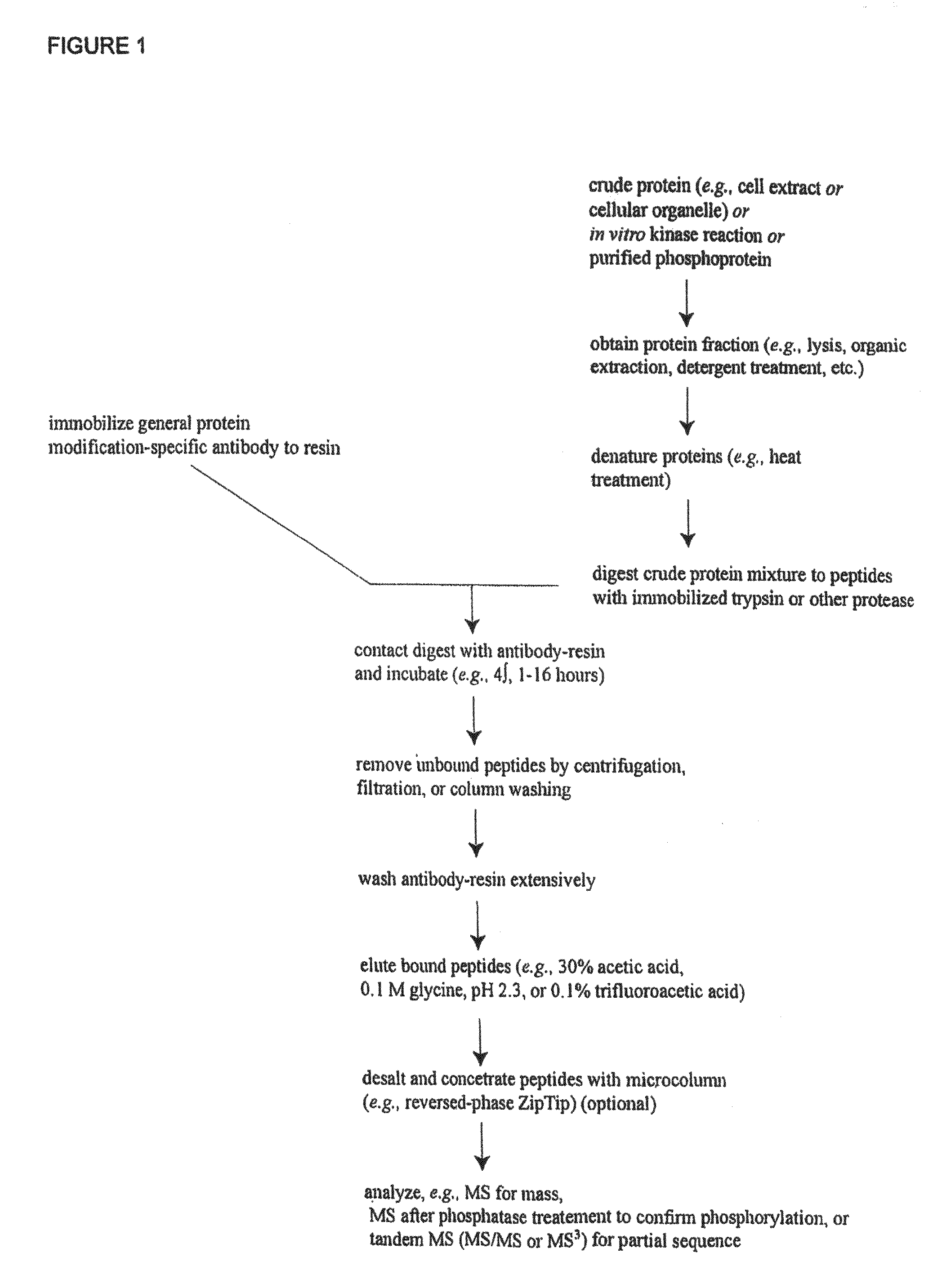
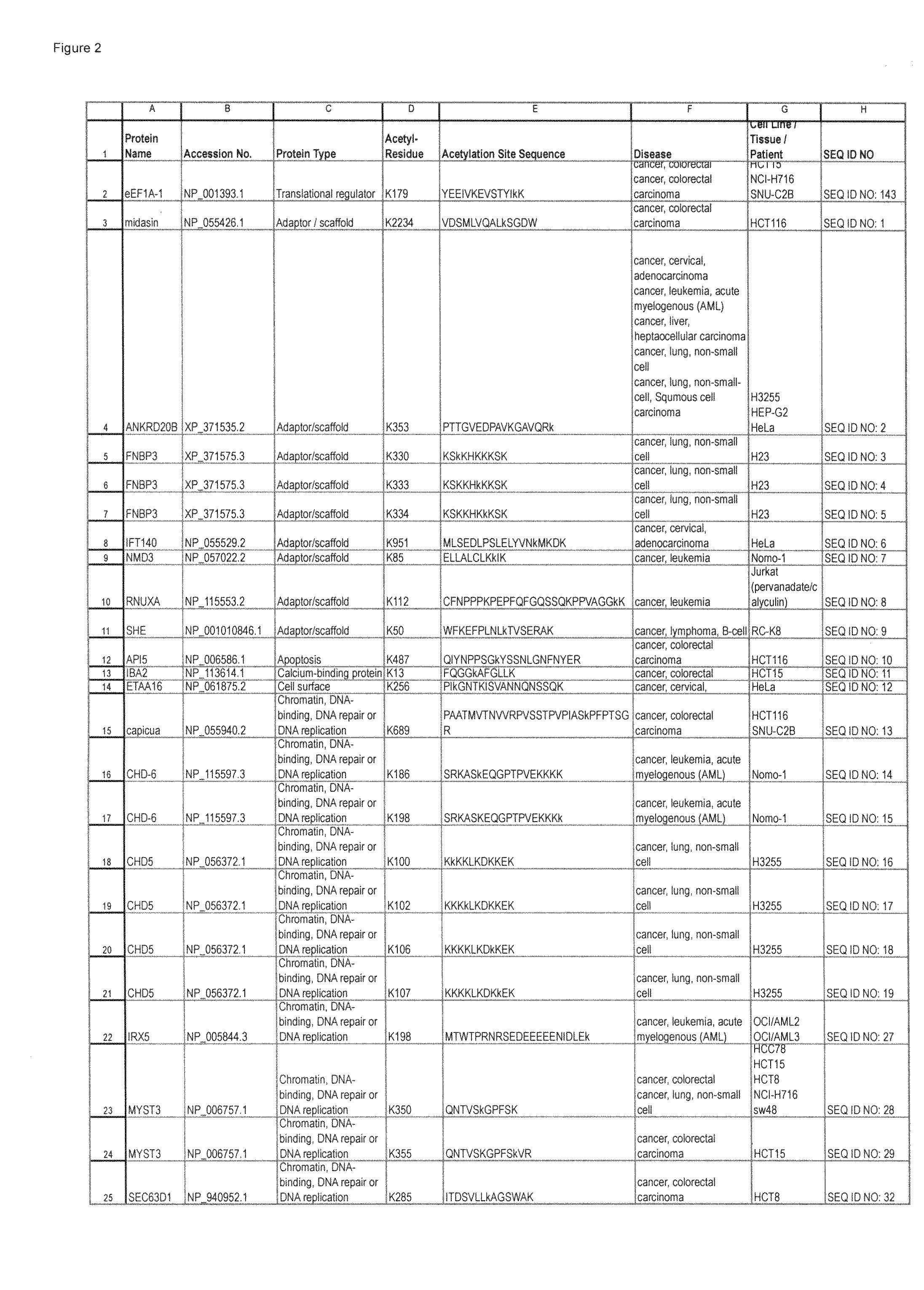
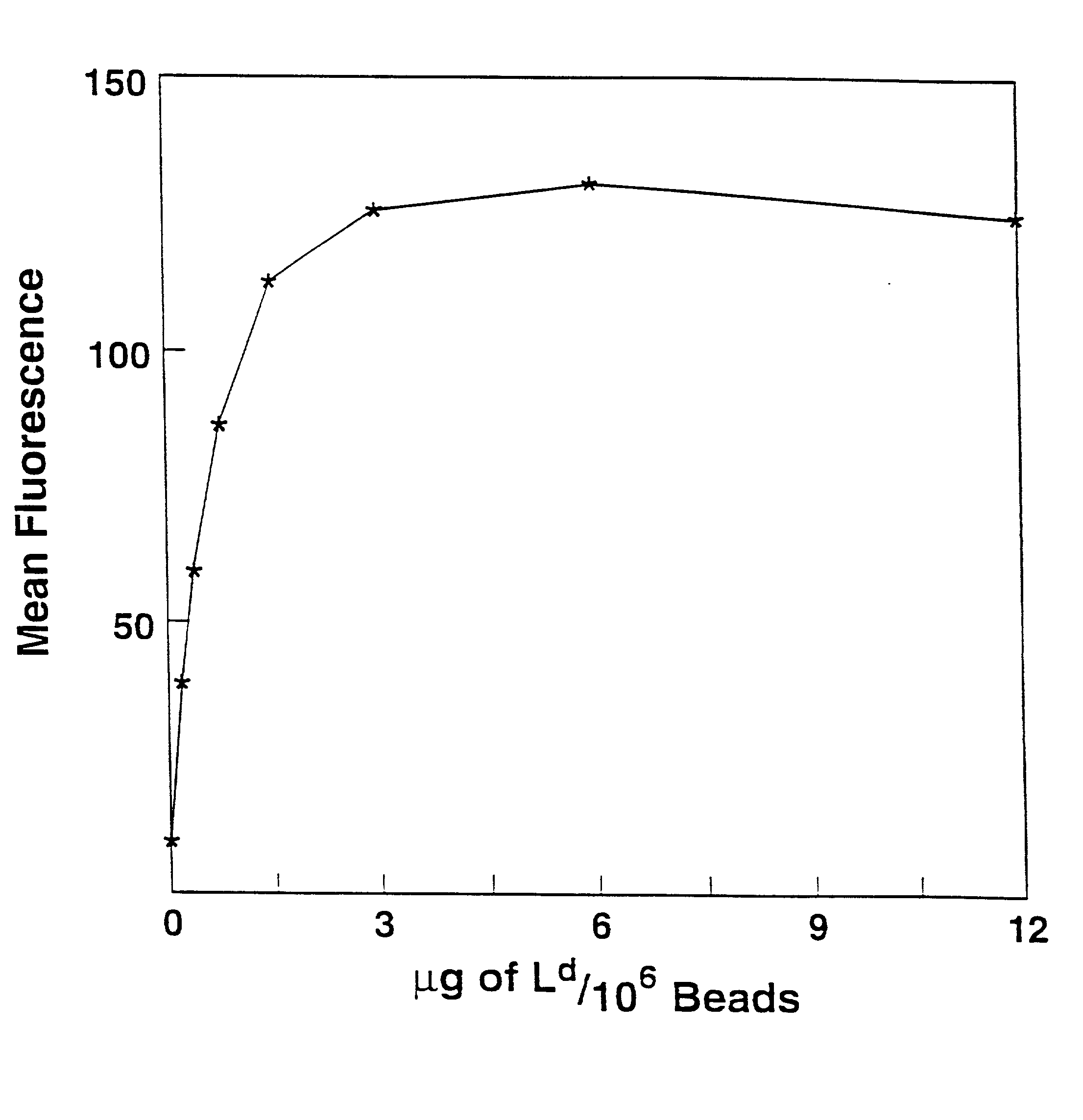
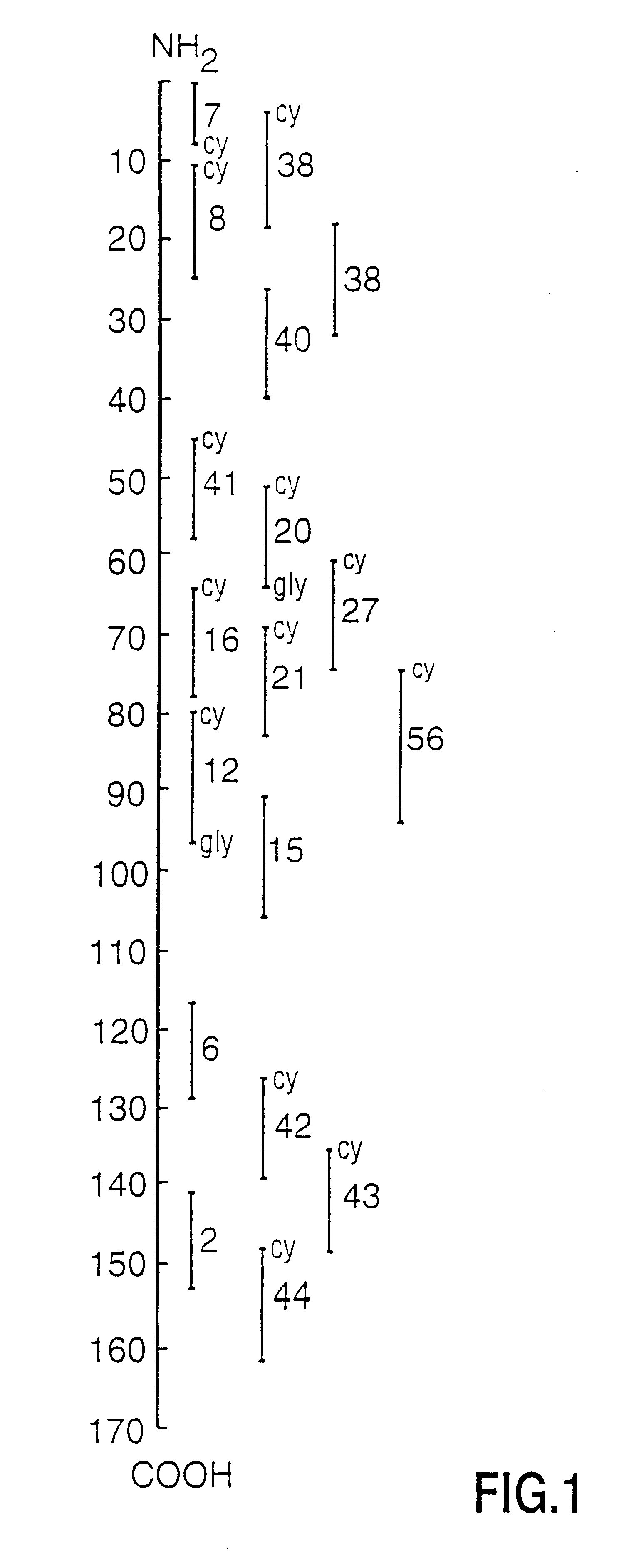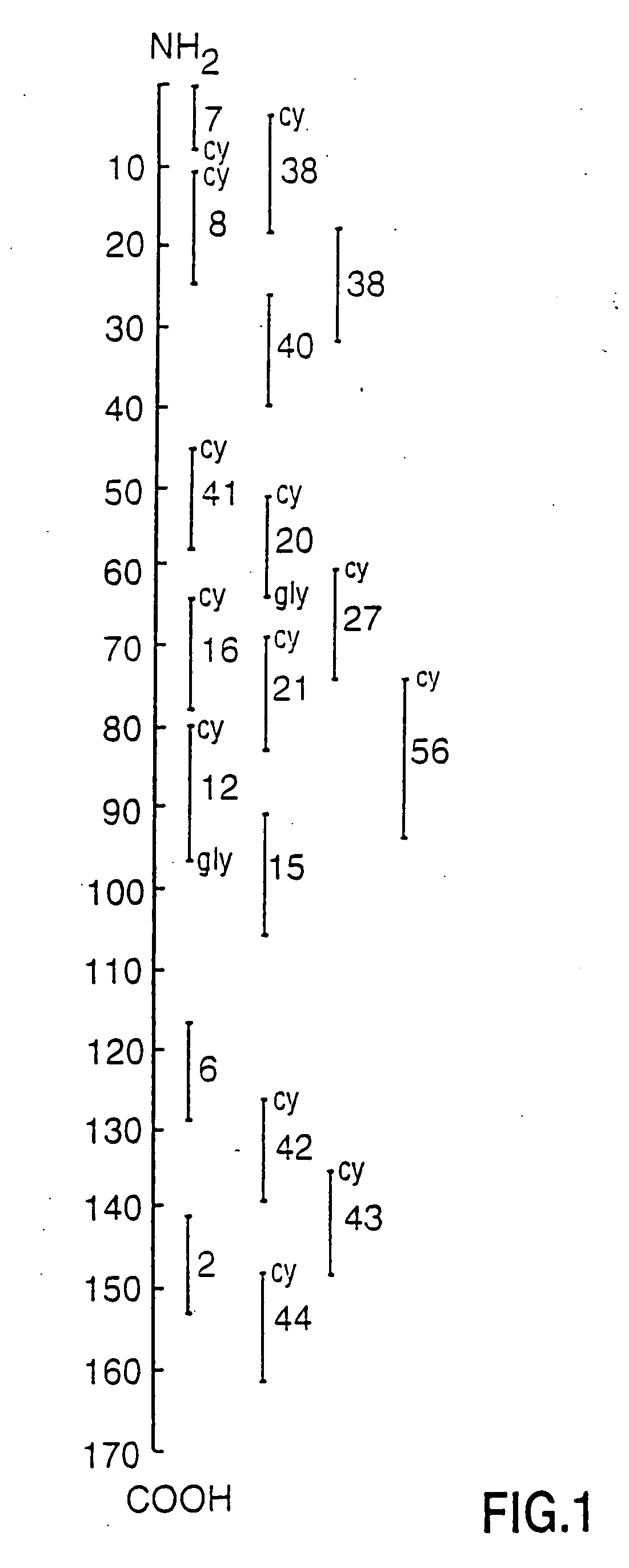Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
86 results about "Peptide specificity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
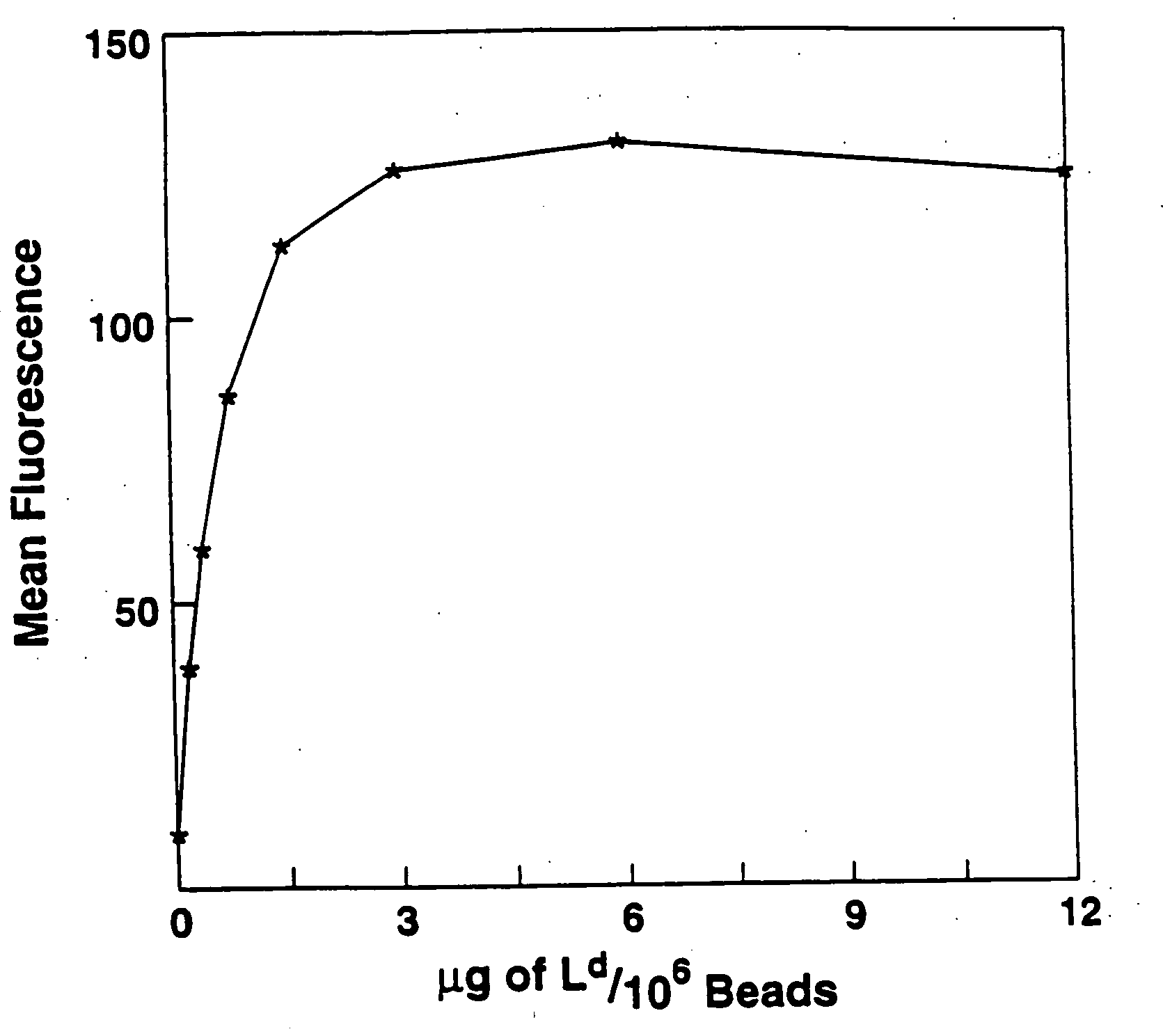
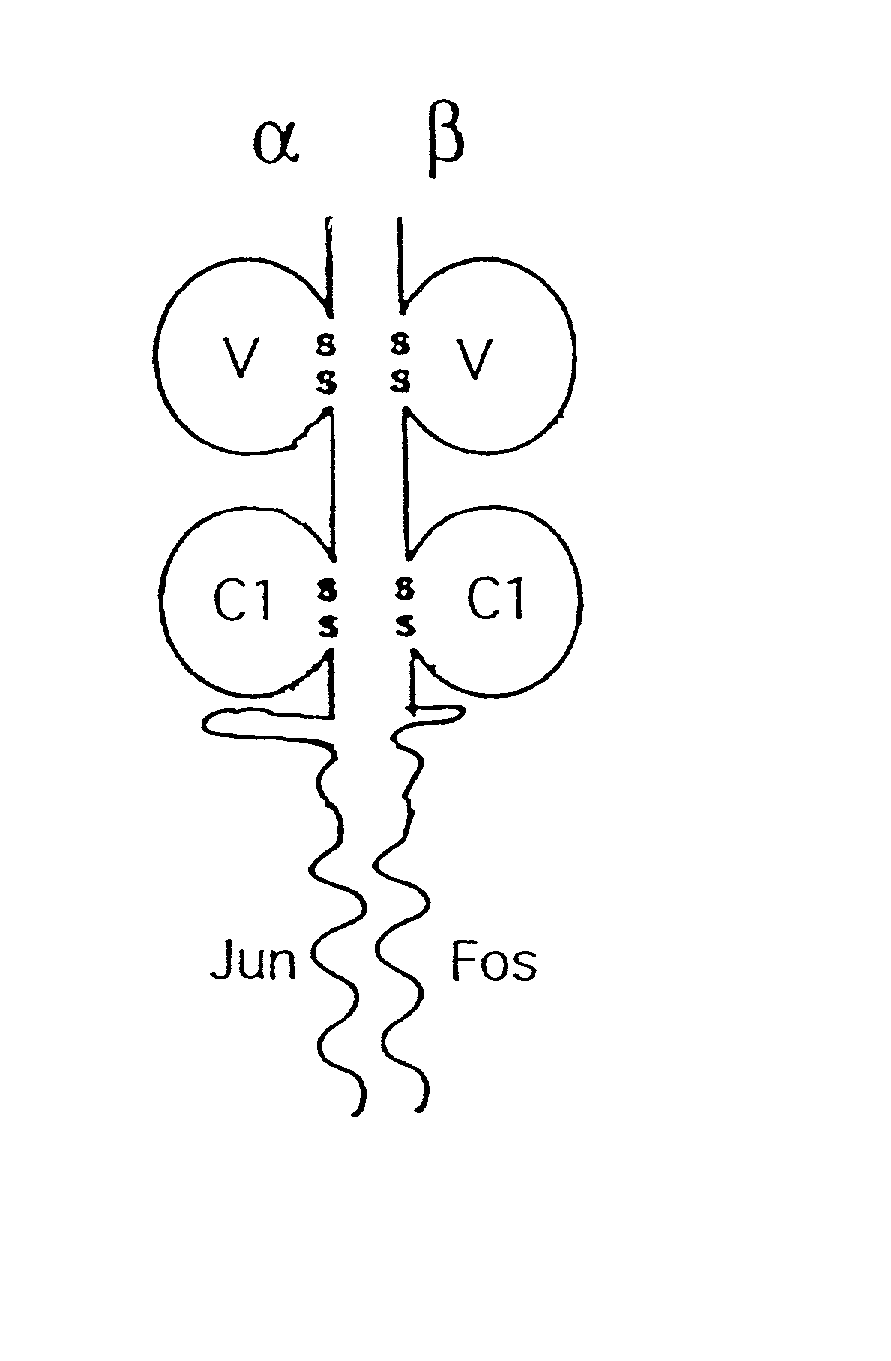
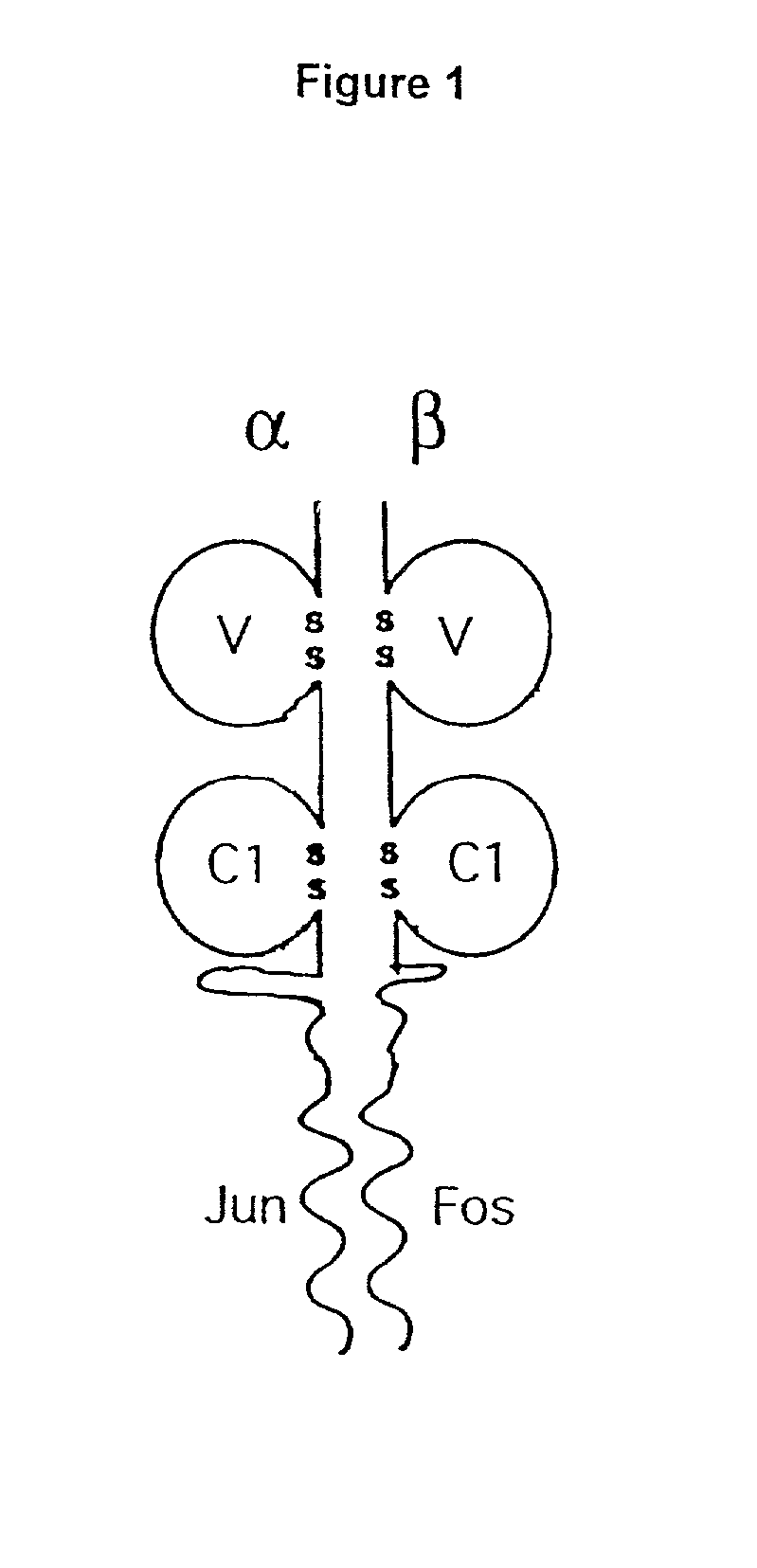
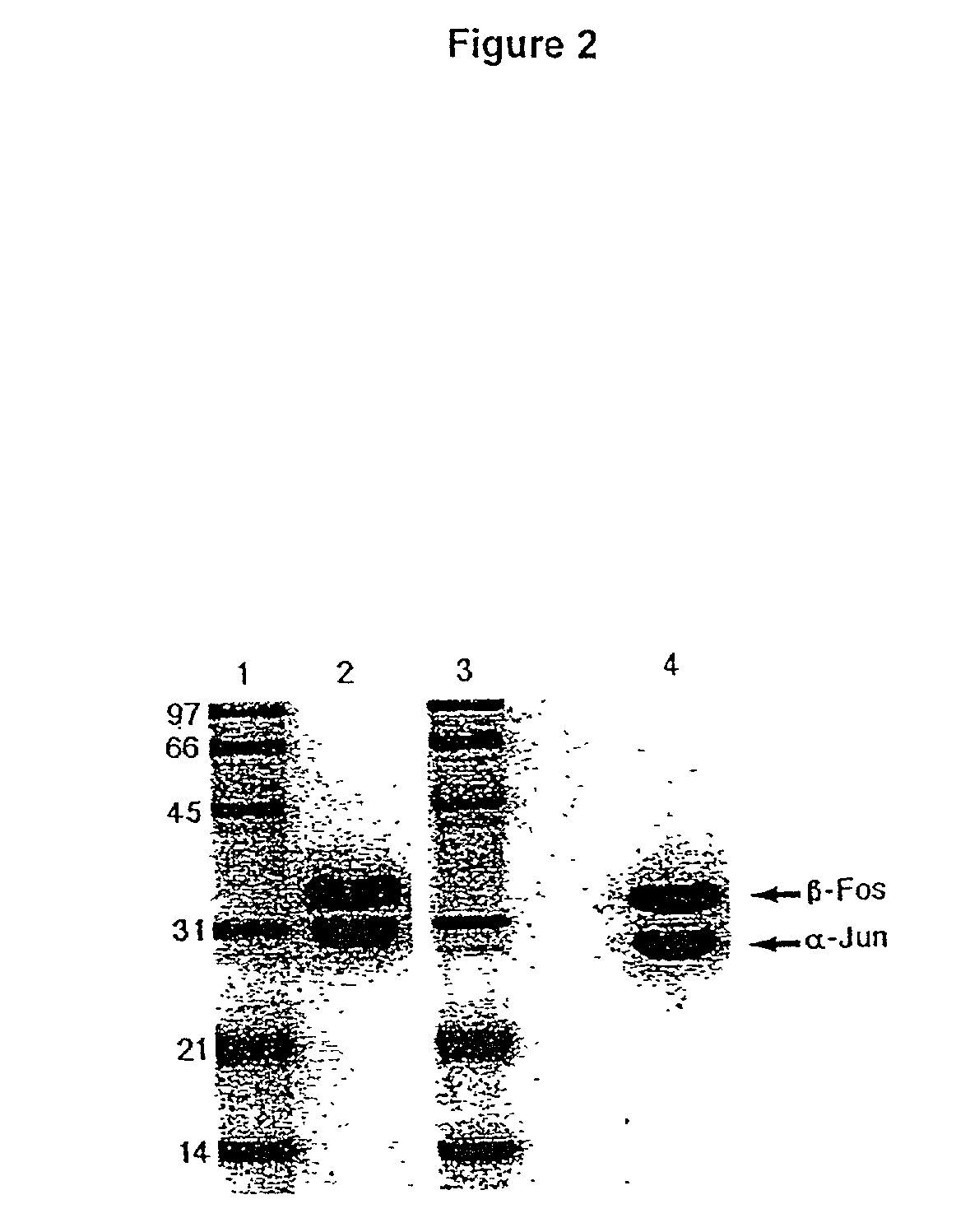
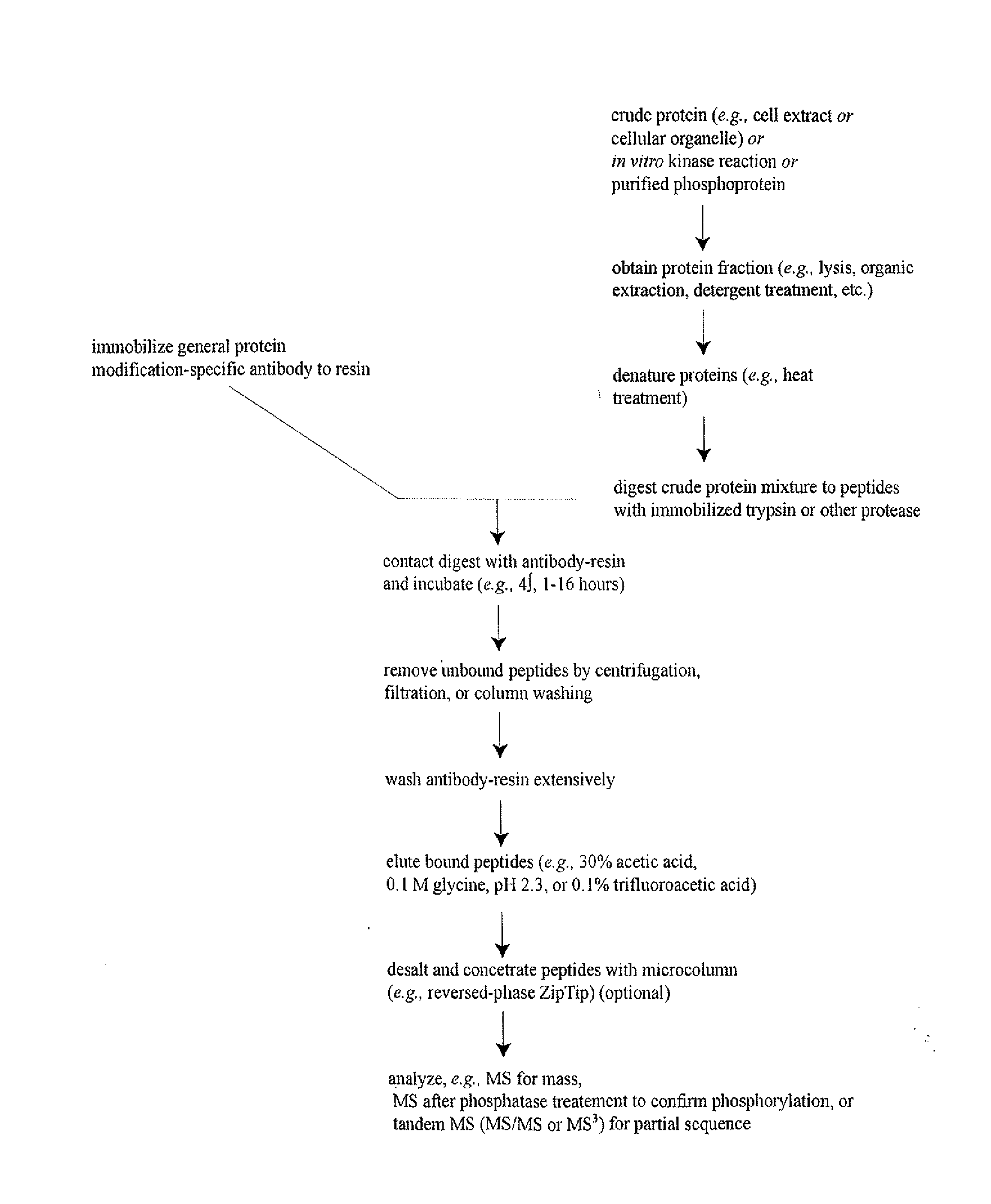
Purification of antigen-specific T cells
InactiveUS20060134125A1Snake antigen ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsMHC class IAbnormal tissue growth
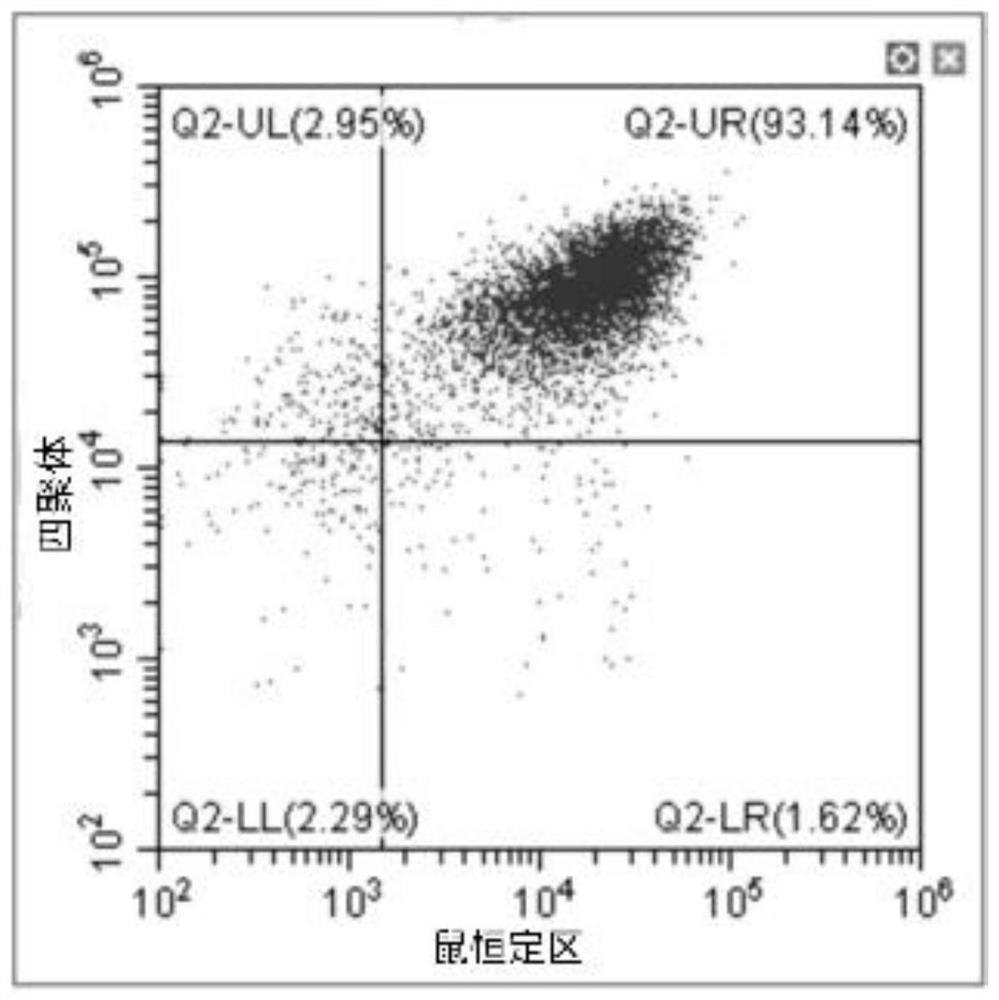
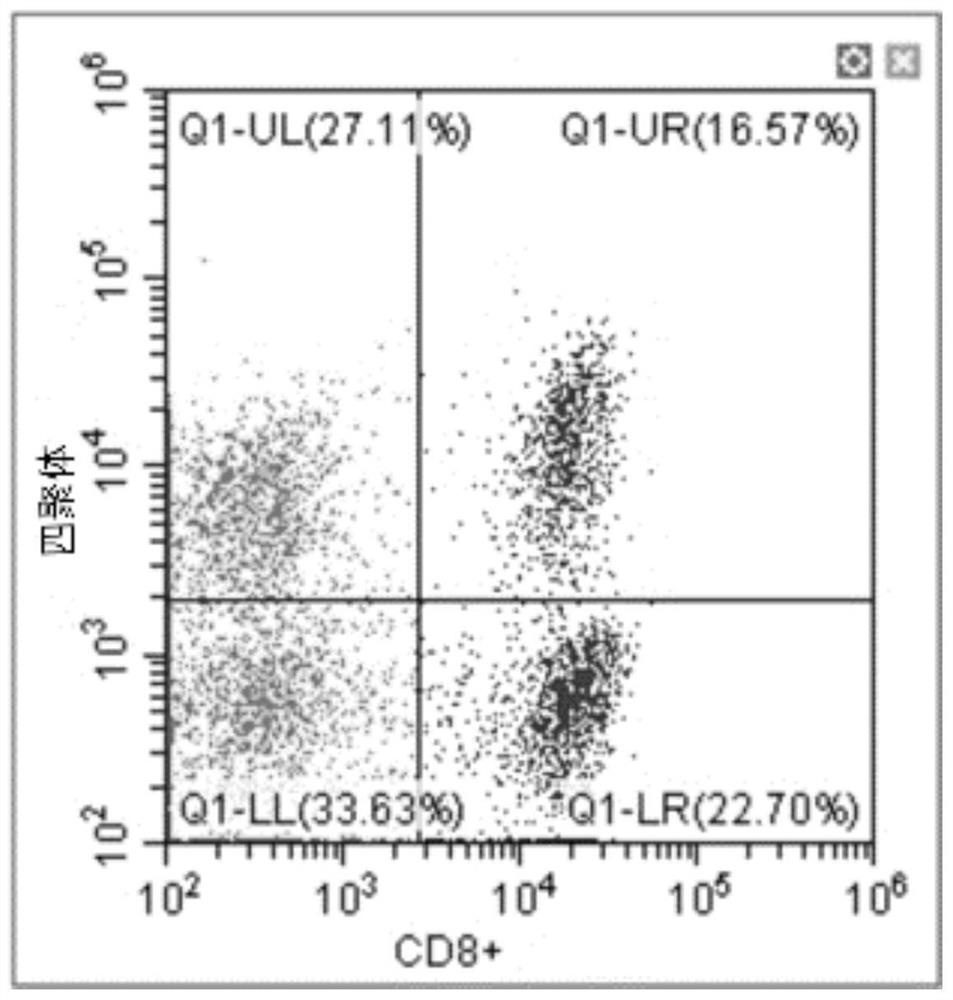
A new method to capture, purify and expand antigen-specific T lymphocytes has been developed using magnetic beads coated with recombinant MHC class I molecules. This method was optimized using homogenous populations of naive T cells purified from mice transgenic for the 2C T cell receptor (TCR). These T cells were captured on beads coated with MHC class I molecules and the relevant antigenic peptides. MHC and peptide specificity was confirmed by the usage of irrelevant MHC peptide combinations. An enrichment of 800 to 1600 fold was measured, using 2C T cells mixed with irrelevant T cells, starting from a 2C T cell frequency of 1 / 3000. The same approach was used to purify antigen-specific CD8+ T cells from total CD8+ T cells from naive mice. The recovered cells could be expanded and specifically kill target cells in vitro; they had a significant effect in vivo as well. We expect this procedure to be suitable to purify and expand in vitro tumor- and virus-specific killer T cells for use in cell therapy.
Owner:ORTHO PHARMA
C-peptide specific assay procedure
InactiveUS6929924B2Highly accurate C-peptideStrong specificityAnimal cellsBiological material analysisC-peptideProinsulin
The present invention relates to a specific C-peptide assay method which can eliminate all interference due to proinsulin and its intermediates, in particular des-31,32-proinsulin and / or des-64,65-proinsulin. It also relates to antibodies for carrying out this assay.
Owner:BIO RAD EURO GMBH
Soluble T cell receptor
InactiveUS20020142389A1High affinityStrong specificityBacteriaPeptide/protein ingredientsHeterologousExtracellular Structure
The present invention relates to a recombinant soluble T cell receptor. The T cell receptor (TCR) is refolded and comprises a recombinant TCR alpha or gamma chain extracellular domain having a first heterologous C-terminal dimerisation peptide; and a recombinant TCR beta or delta chain extracellular domain having a second C-terminal dimerisation peptide which is specifically heterodimerised with the first dimerisation peptide to form a heterodimerisation domain, which may be a coiled coil domain. The invention also provides nucleic acid sequences encoding the recombinant TCR and a method for producing the recombinant TCR. The TCR may be labelled with a detectable label so as to enable the detection of specific MHC-peptide complexes. Alternatively, it can be linked to a therapeutic agent such as a cytotoxic agent or an immunostimulating agent so as to deliver such an agent to the site of a specific MHC-peptide complex.
Owner:JAKOBSEN BENT KARSTEN +4
Purification of antigen-specific T cells
InactiveUS7125964B2Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsMHC class IMagnetic bead
Owner:ORTHO MCNEIL PHARM INC
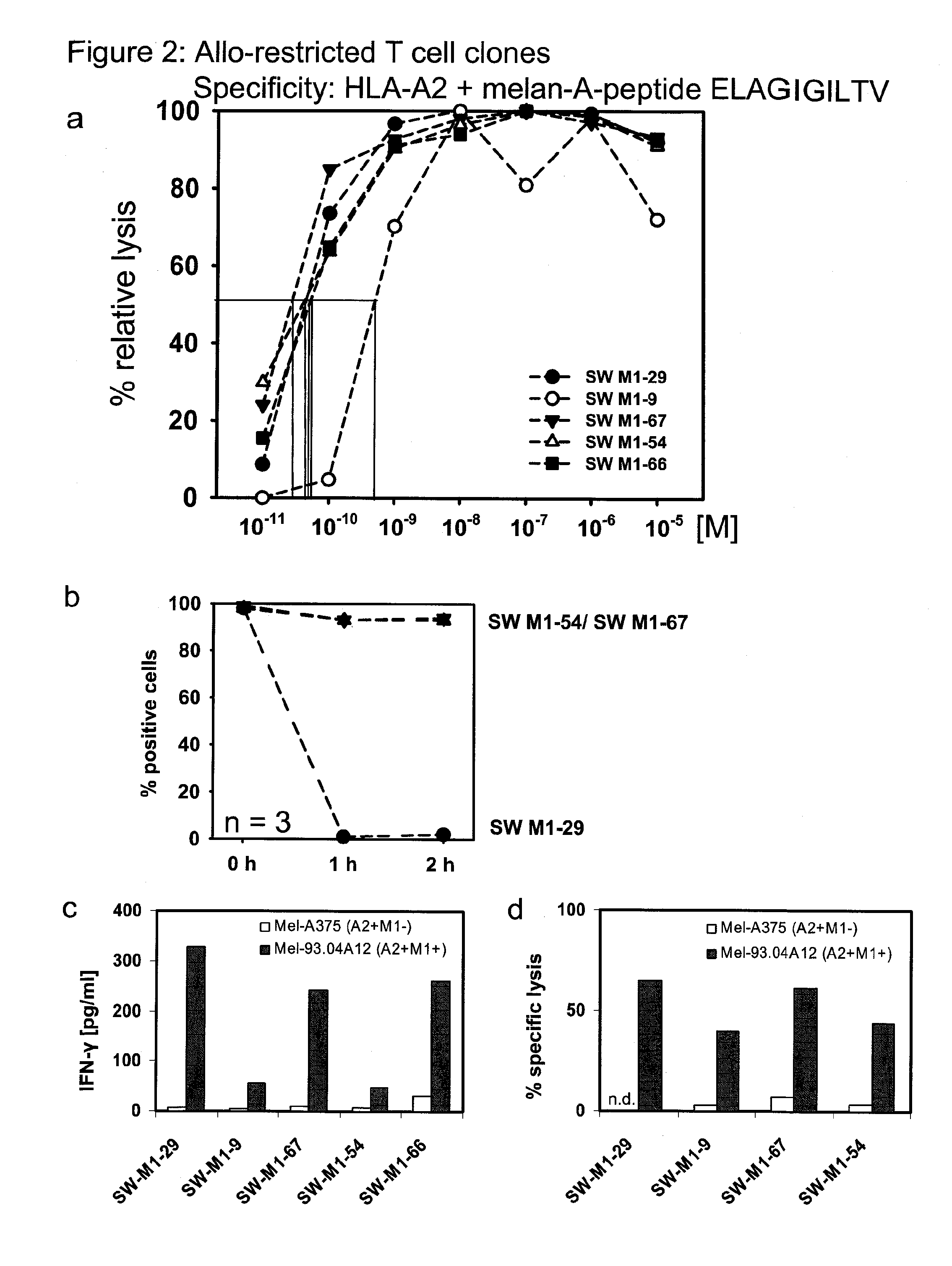
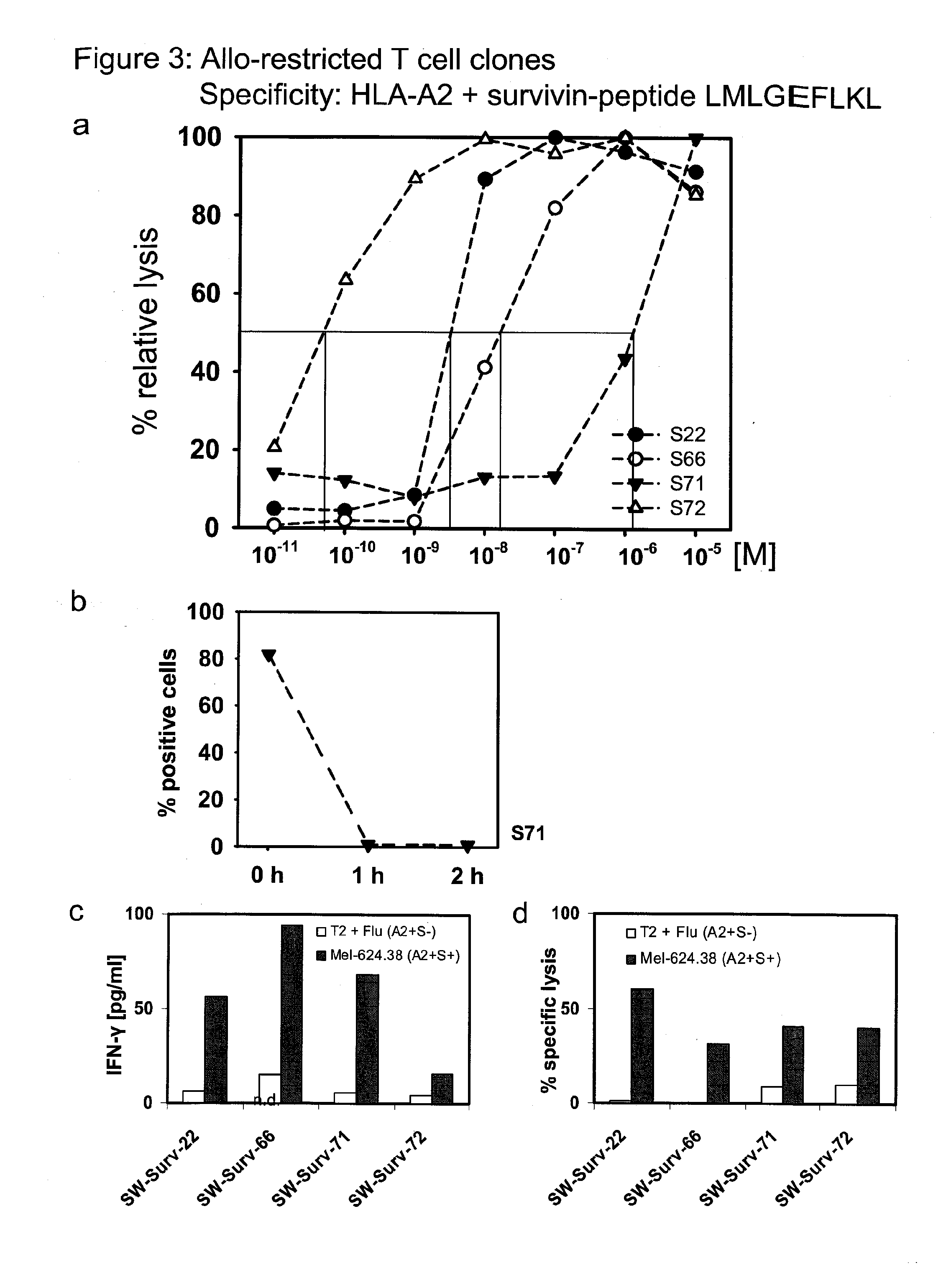
Repertoire of allo-restricted peptide-specific t cell receptor sequences and use thereof
InactiveUS20120128704A1Effectively minimize immune selectionEasy to useOrganic active ingredientsBiocideDiseaseTyrosinase
The present invention is directed to a kit-of-parts or composition containing nucleic acid sequences coding for high-avidity, allo-restricted TCR, wherein the TCR are independently directed against the tyrosinase antigen, the melan-A antigen and the survivin antigen. The invention is further directed to a kit-of-parts or composition containing at least three groups of transgenic lymphocytes transformed with vectors coding for TCR against said antigens. Furthermore, the present invention provides a pharmaceutical composition and its use in the treatment of diseases involving malignant cells expressing said tumor-associated antigens. The invention further relates to a nucleic acid molecule coding for a TCR that recognizes the survivin antigen, a TCR encoded thereby and a T cell expressing said TCR. Further, the invention discloses a vector, a cell and a pharmaceutical composition encoding / containing same and their use in the treatment of diseases involving malignant cells expressing survivin.
Owner:HELMHOLTZ ZENT MUNCHEN DEUTES FORSCHUNGSZENT FUR GESUNDHEIT & UMWELT +1
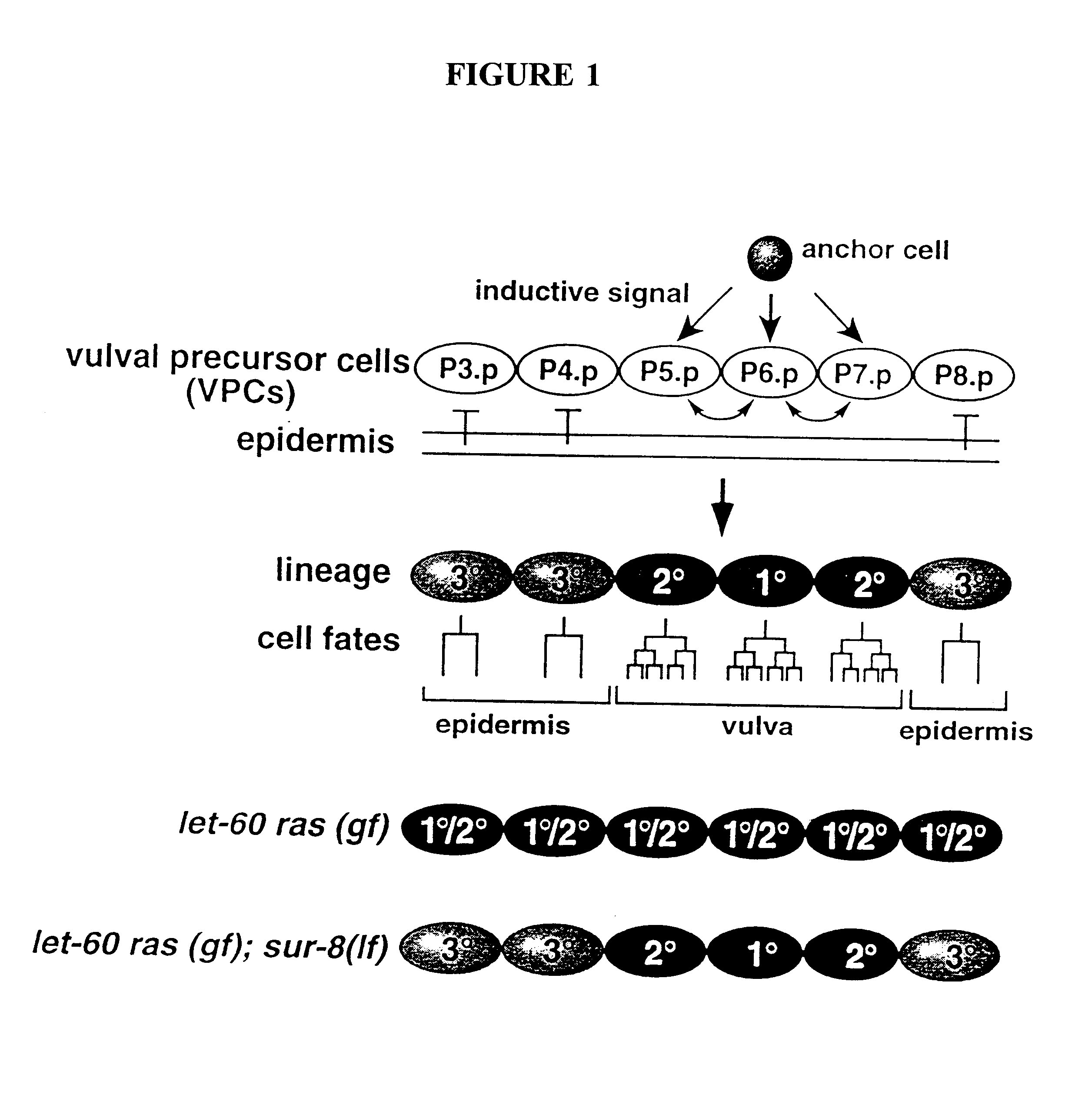
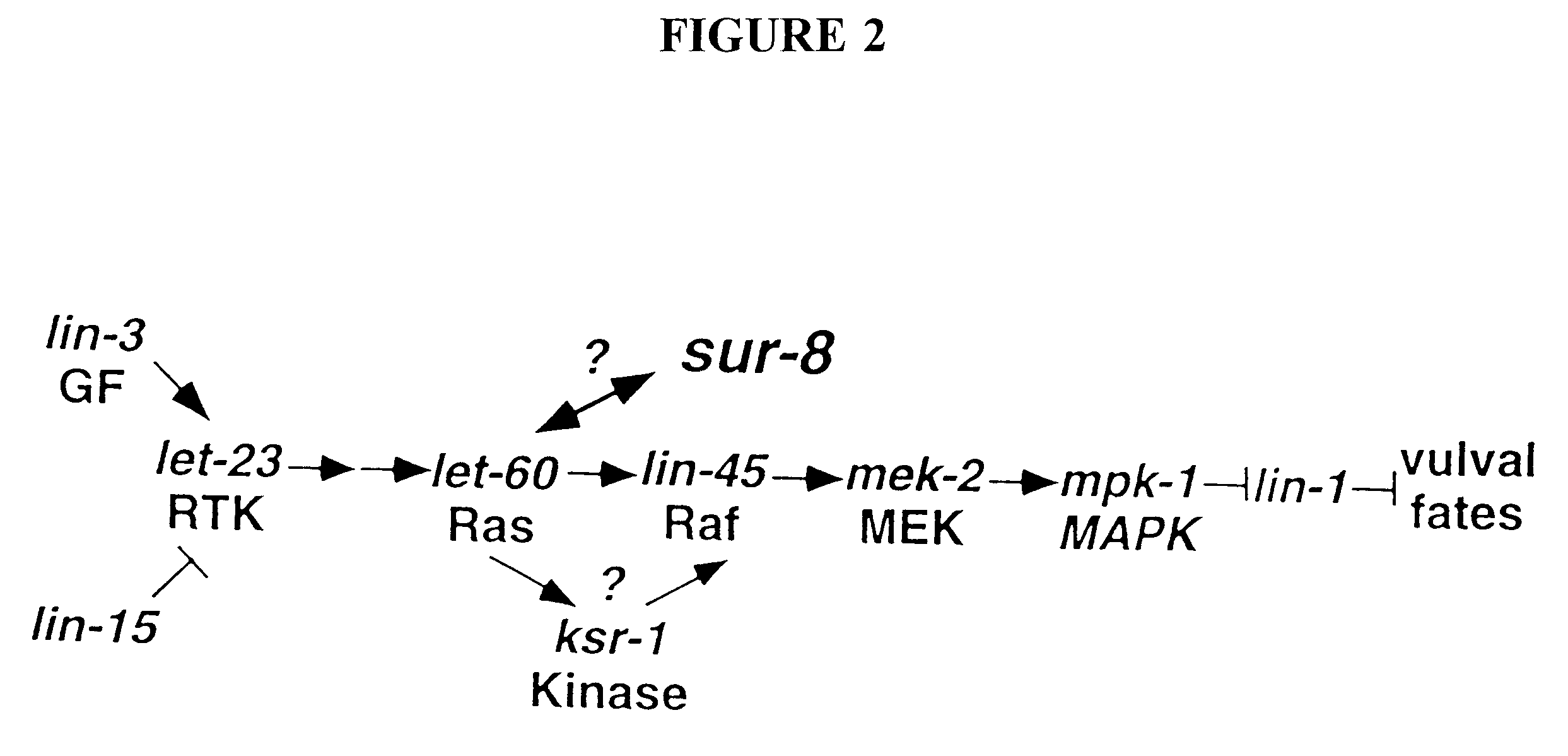
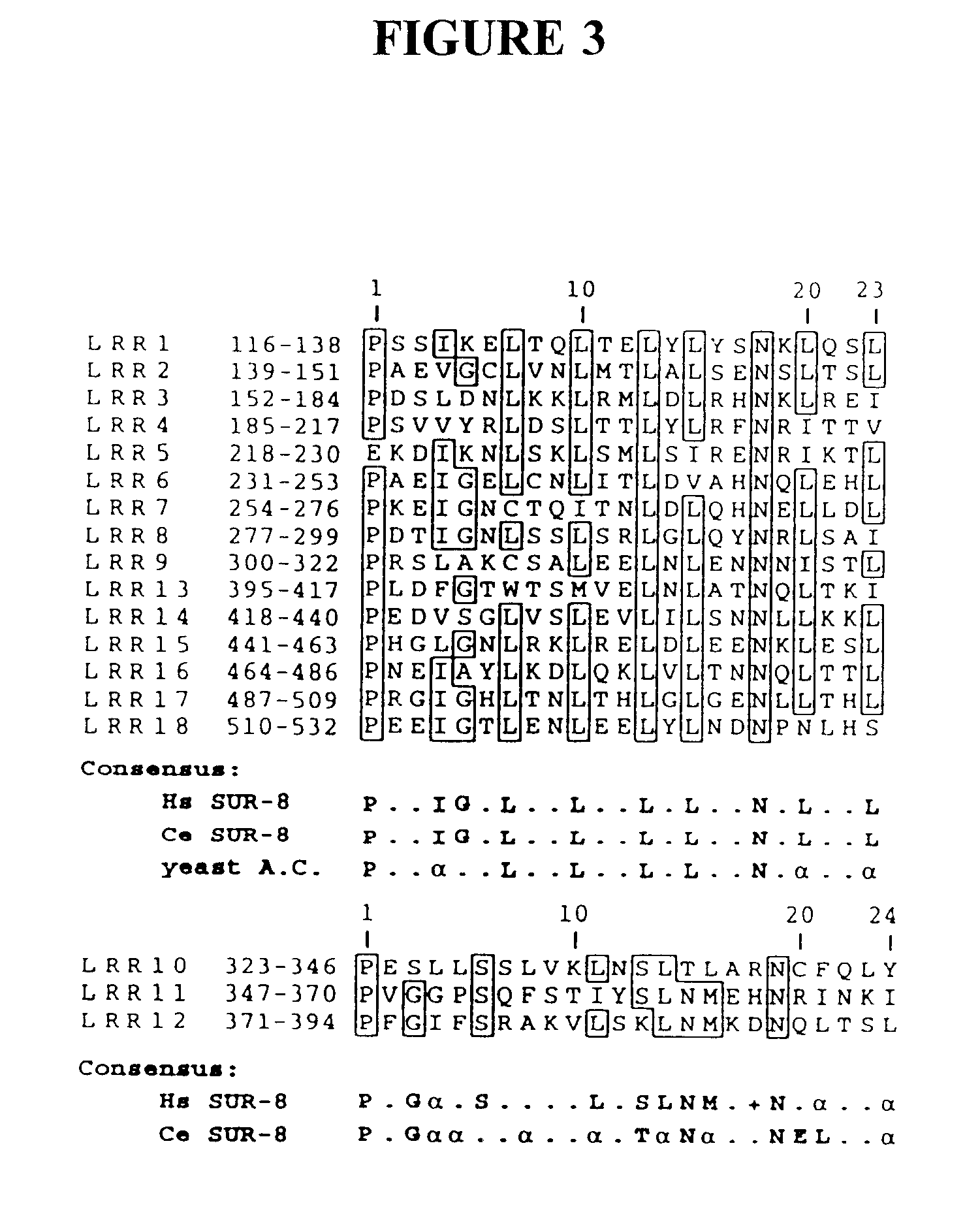
Ras suppressor SUR-8 and related compositions and methods
The present invention relates to Ras suppressors, in particular the Ras suppressor SUR-8. The present invention provides isolated nucleotide sequence encoding SUR-8, isolated SUR-8 peptides, antibodies that specifically bind SUR-8, methods for the detection of SUR-8, methods for producing SUR-8 transgenic animals, non-human animals expressing SUR-8, and methods for screening compounds for the ability to alter SUR-8 associated signal transduction.
Owner:UNIV TECH
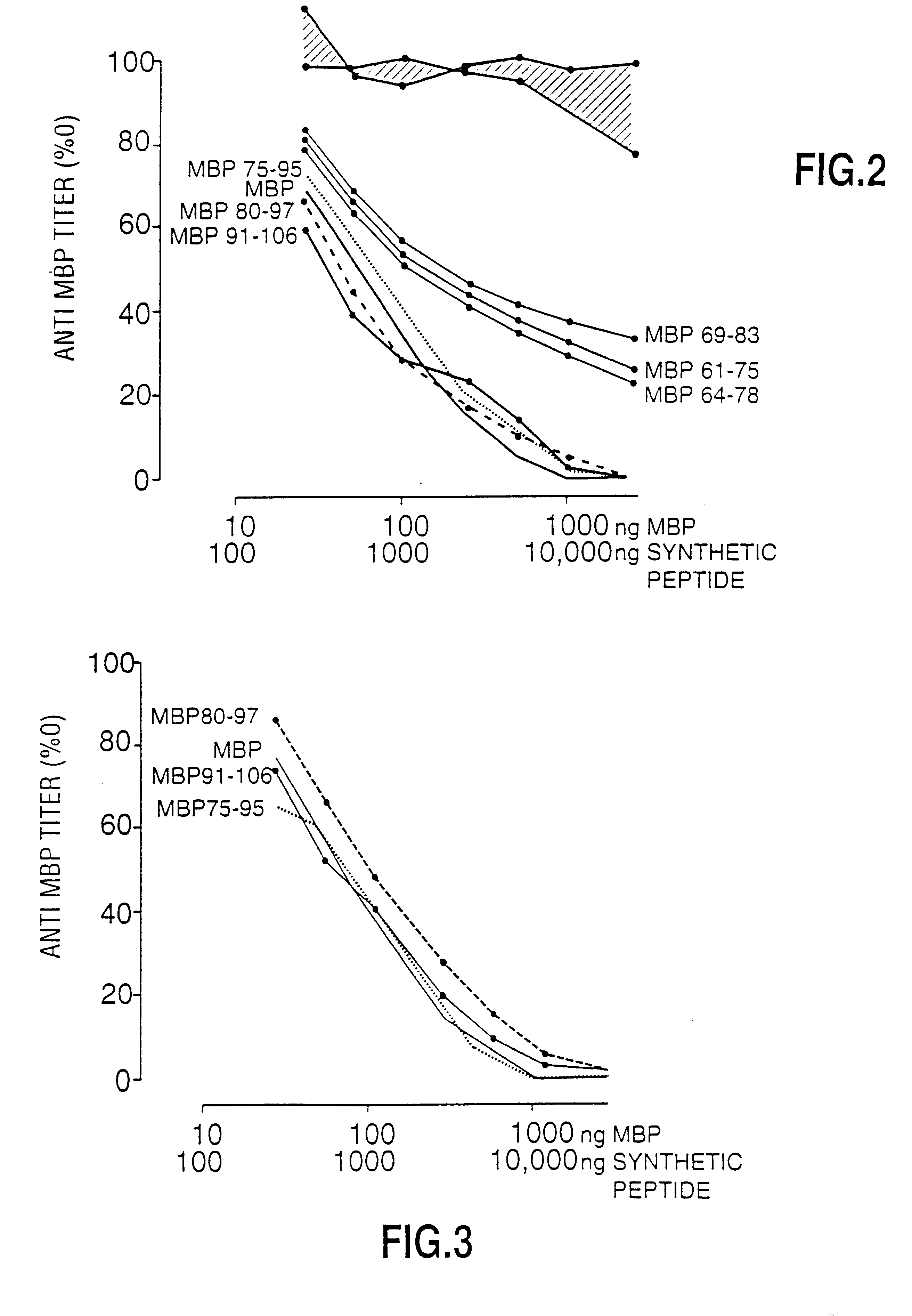
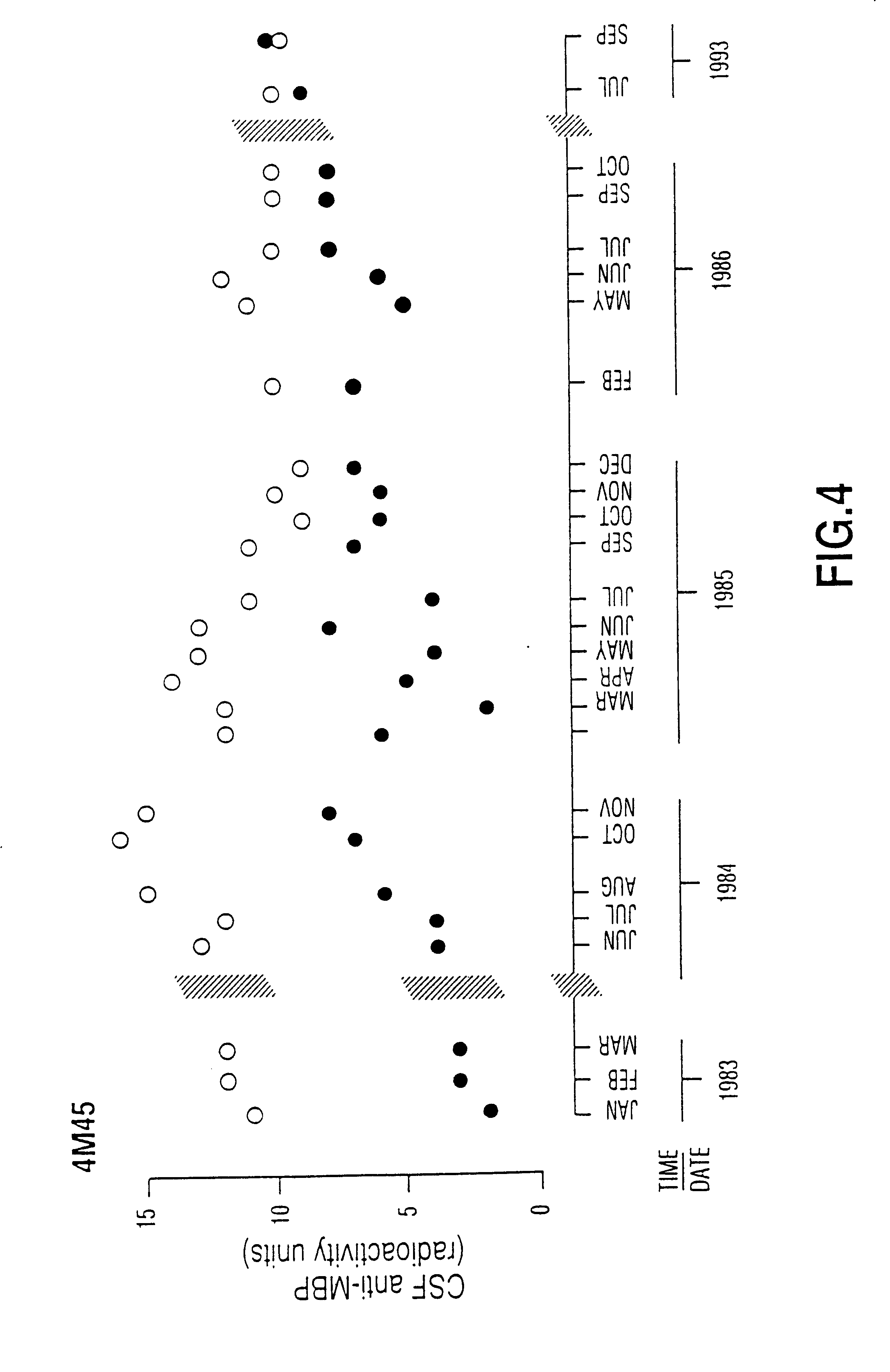
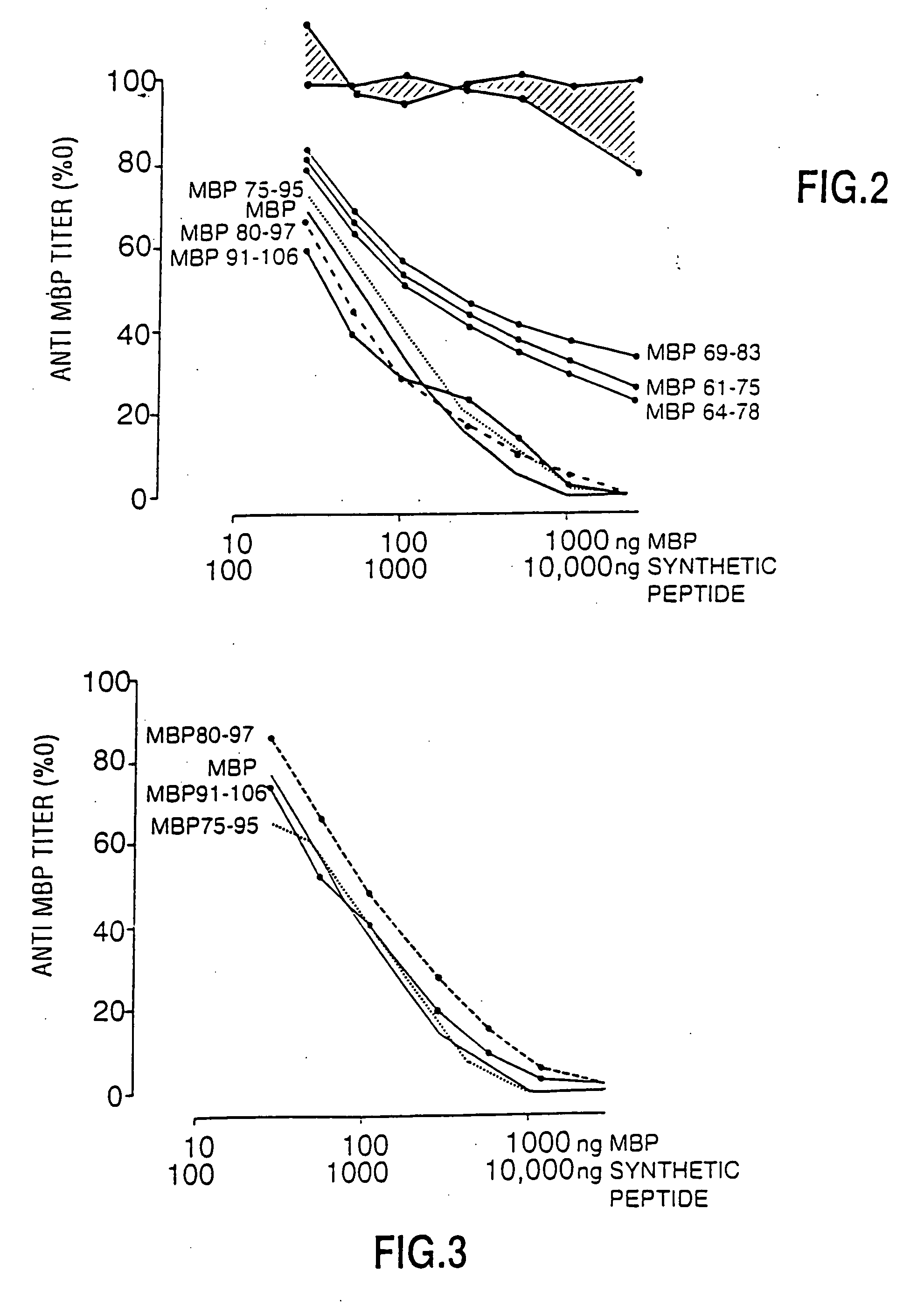
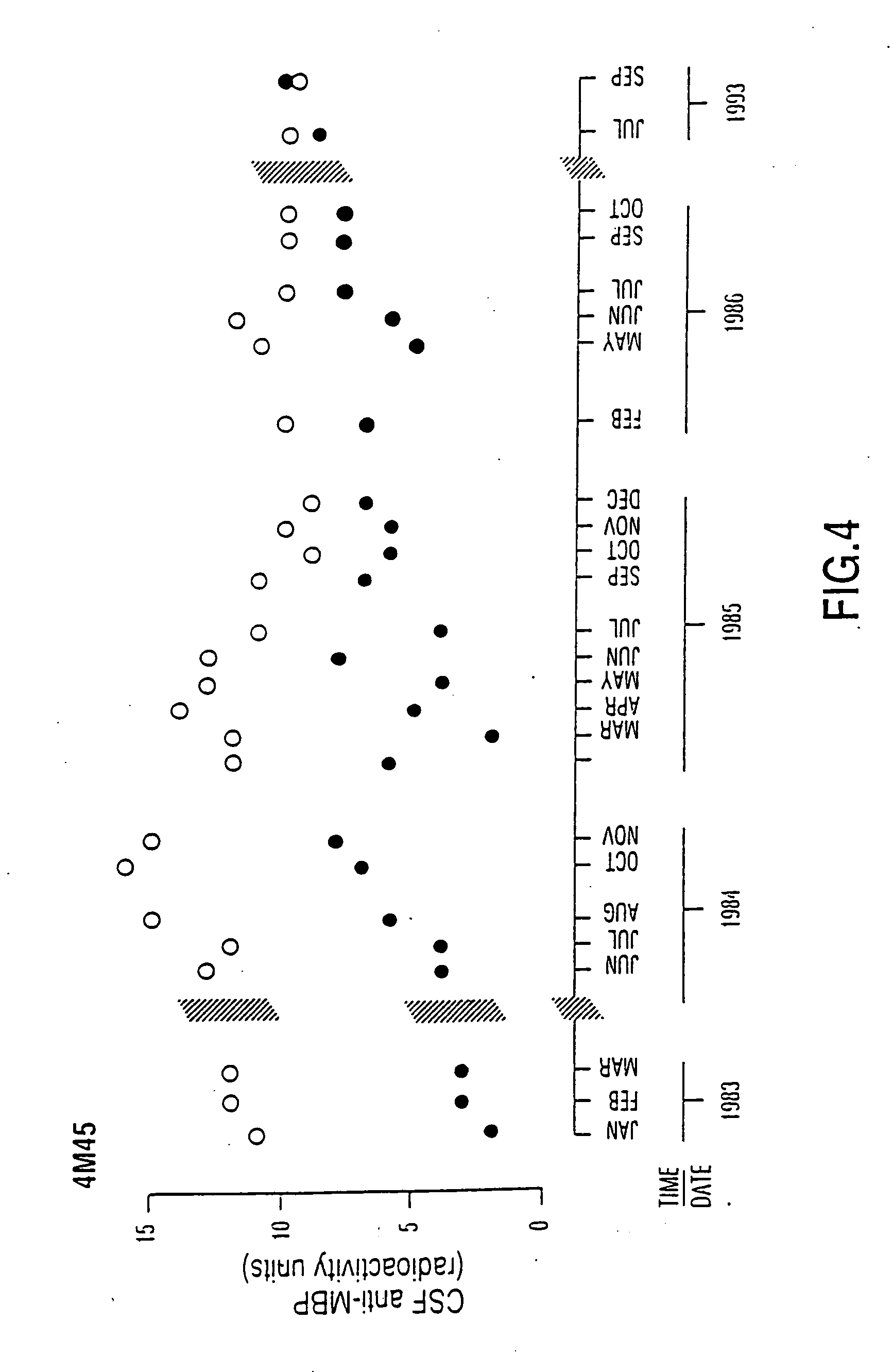
Peptide specificity of anti-myelin basic protein and the administration of myelin basic protein peptides to multiple sclerosis patients
InactiveUS6252040B1Promote tolerancePromote resultsNervous disorderPeptide/protein ingredientsWhole bodyADAMTS Proteins
Human myelin basic protein (h-MBP) has a molecular weight of 18.5 KD and contains 170 amino acid residues. Synthetic peptides ranging in length from about 8 to 25 residues and covering the entire length of the protein have been produced. Antibodies to h-MBP (anti-MBP) were found to be neutralized by the synthetic peptides, in vitro, which span the h-MBP from about amino acid residue 61 to about amino acid residue 106. The peptides, which cover both the amino (about residues 1 to 63) and carboxy (about residues 117 to 162) terminals of h-MBP did not neutralize purified anti-MBP. Intrathecal administratin of peptide MBP(75-95), MBP(86-95), or MBP(82-98) produced complete binding-neutralization of free (F) anti-MBP with no change in bound (B) levels. A control peptide MBP35-58 had no effect on F or B anti-MBP levels. Intravenous administration of MBP(75-95), MBP(86-95), or MBP(82-98) resulted in significant decline of F and B CSF anti-MBP levels. Administration of MBP synthetic peptides to MS patients either intrathecally or intravenously did not have any adverse neurological effects and systemic complications did not occur. The MBP epitope for MS anti-MBP has been localized to an area between amino acid 86 and amino acid 95.
Owner:ALBERTA THE GOVERNORS OF THE UNIV
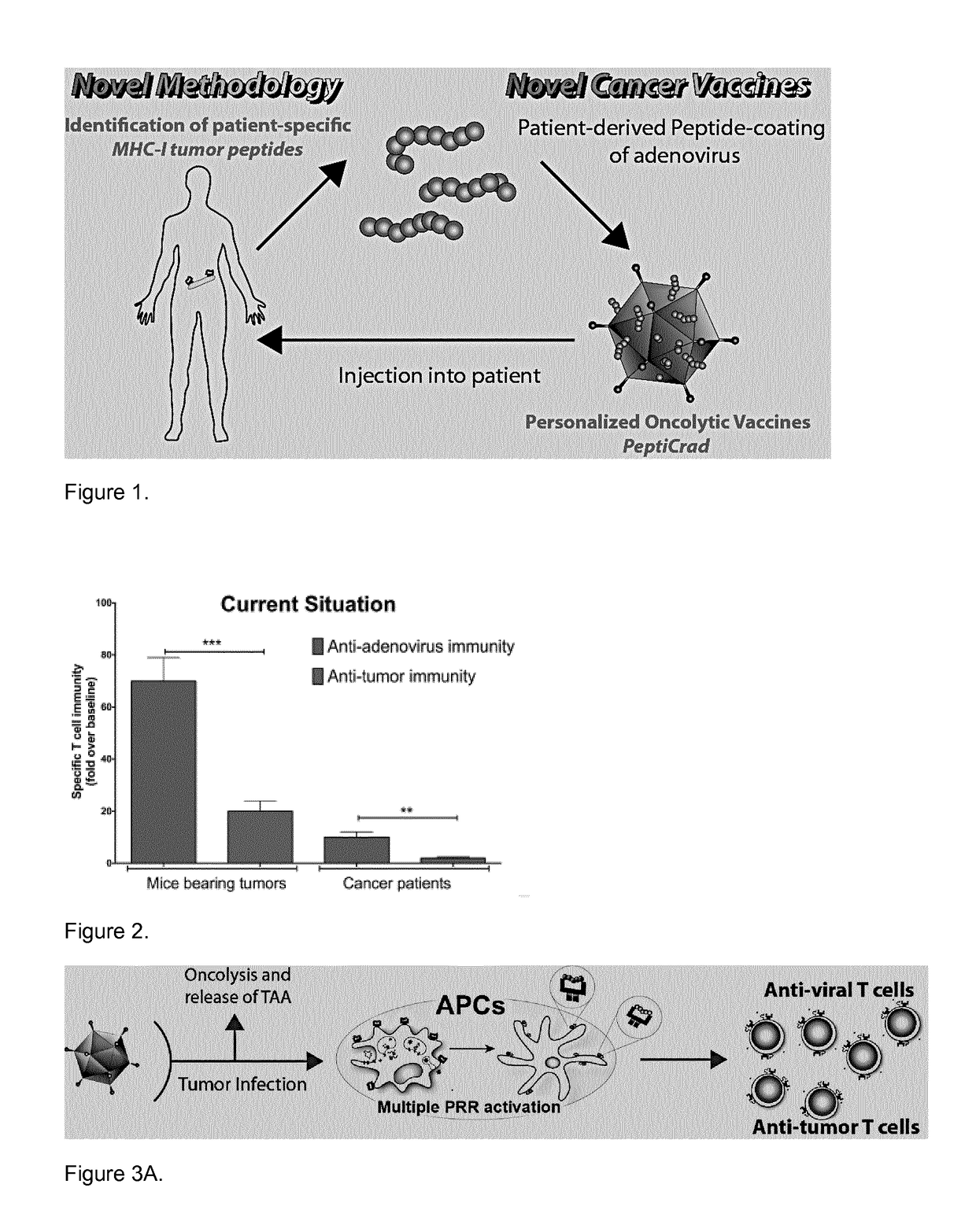
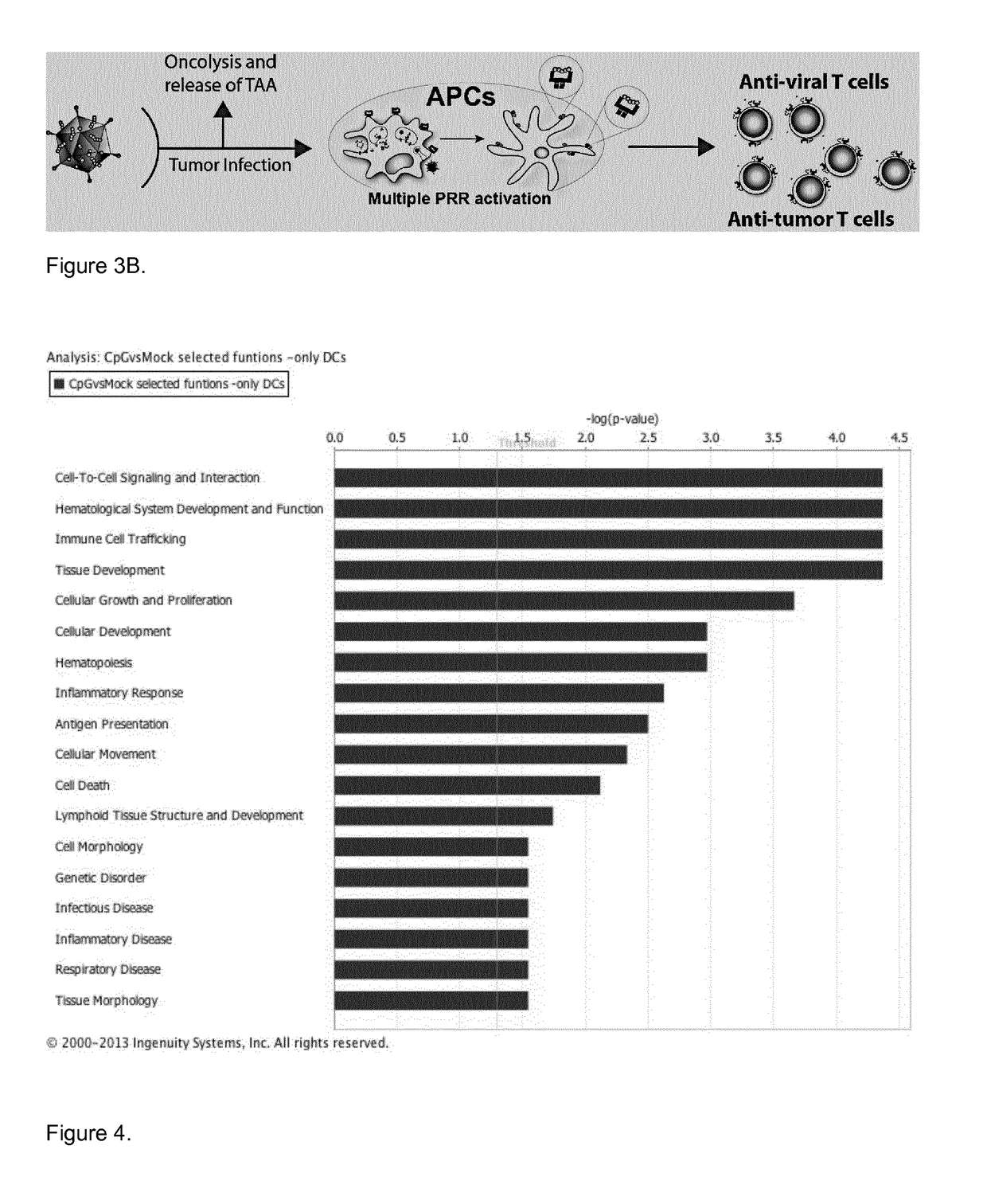
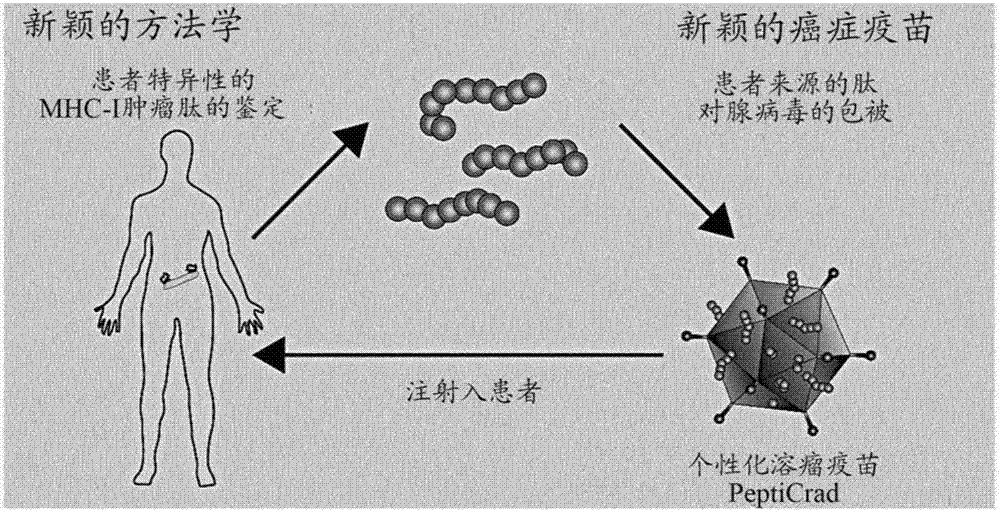
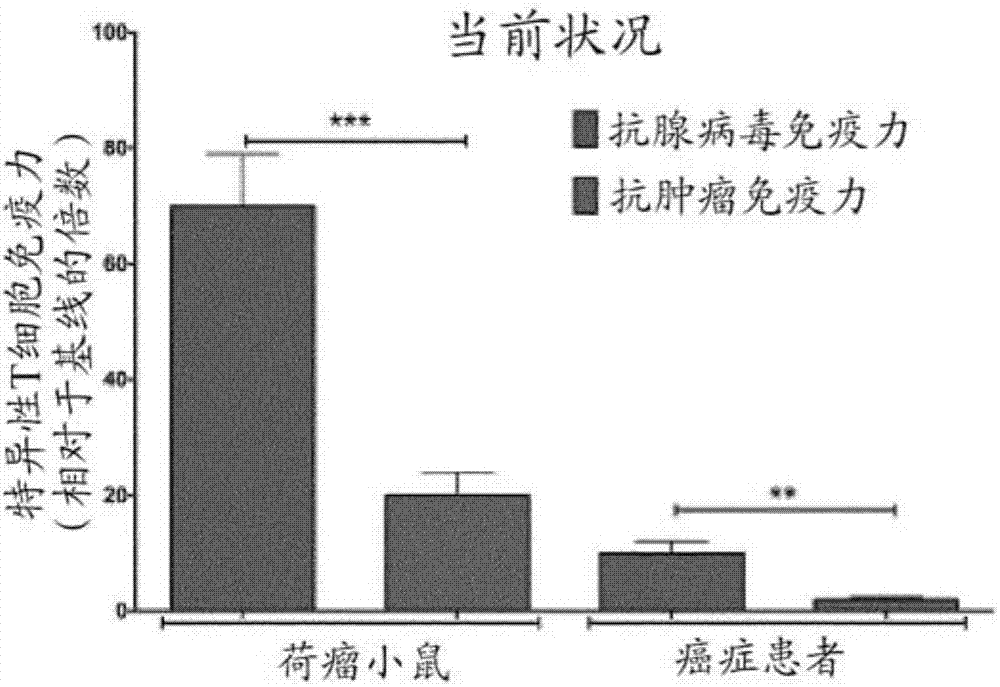
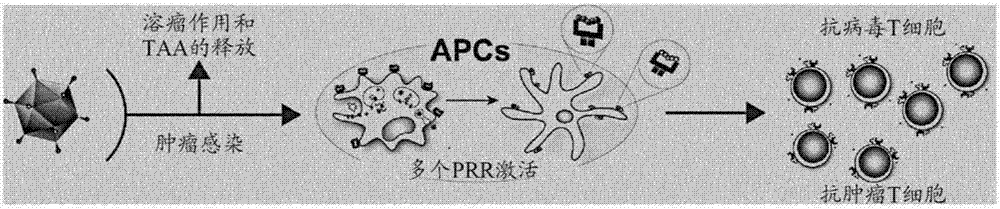
Modified adenoviruses for cancer vaccines development
ActiveUS20170080069A1Quickly and cost-effectivelyConstant and rapid monitoringBiological material analysisAntiviralsViral vectorCapsid
The present invention relates to adenoviral vectors, wherein the viral capsid has been coated with polypeptides, which are capable of stimulating a peptide-specific immune response in a subject and uses thereof. Furthermore, the present invention relates to methods of treating diseases, e.g. cancer, by adenoviral vectors which have been coated by polypeptides causing peptide-specific immune response. Also the present invention relates to a method of coating adenoviral vectors by specific peptides as well as to a method of identifying those peptides suitable for coating the capsid of an adenoviral vector.
Owner:VALO THERAPEUTICS OY
High Surface Area Substrate for Cell Culture
A cell culture microcarrier includes (1) a polystyrene microcarrier base having a remnant of a carboxylic acid group, and (ii) a polypeptide conjugated to the base via the remnant of the carboxylic acid group. The polypeptide may contain a cell adhesive sequence, such as RGD. Cells cultured with such microcarriers exhibit peptide-specific binding to the microcarriers.
Owner:CORNING INC
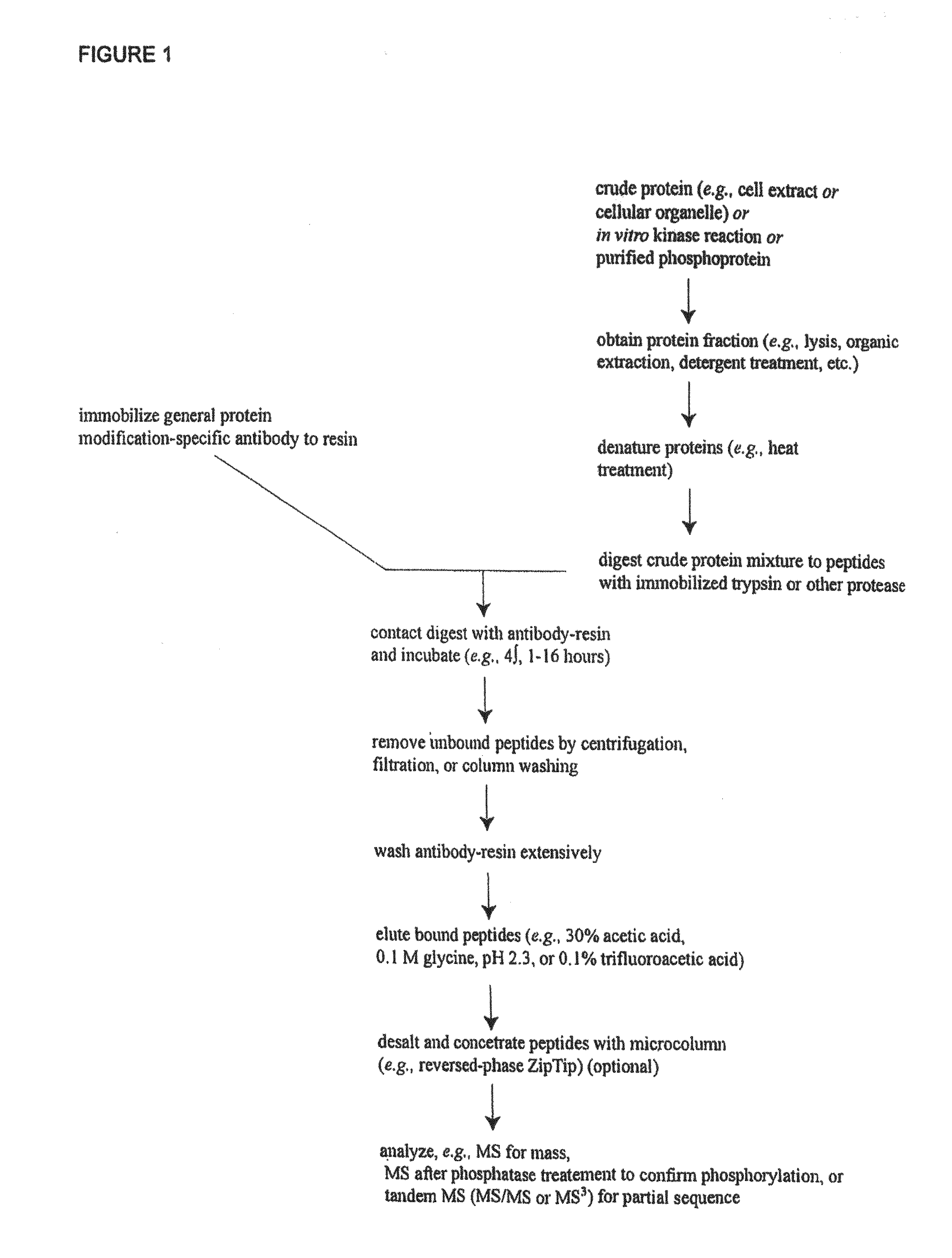
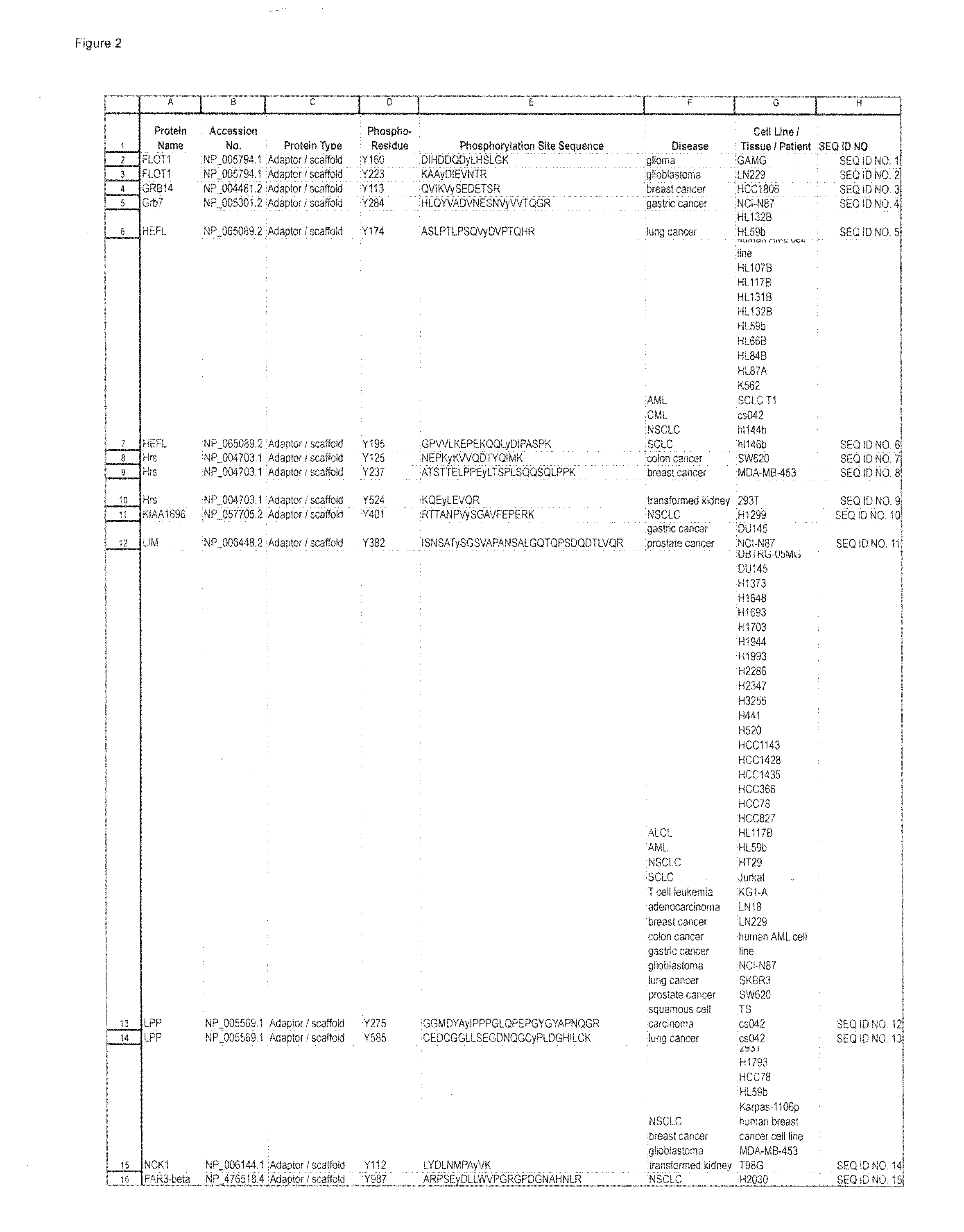
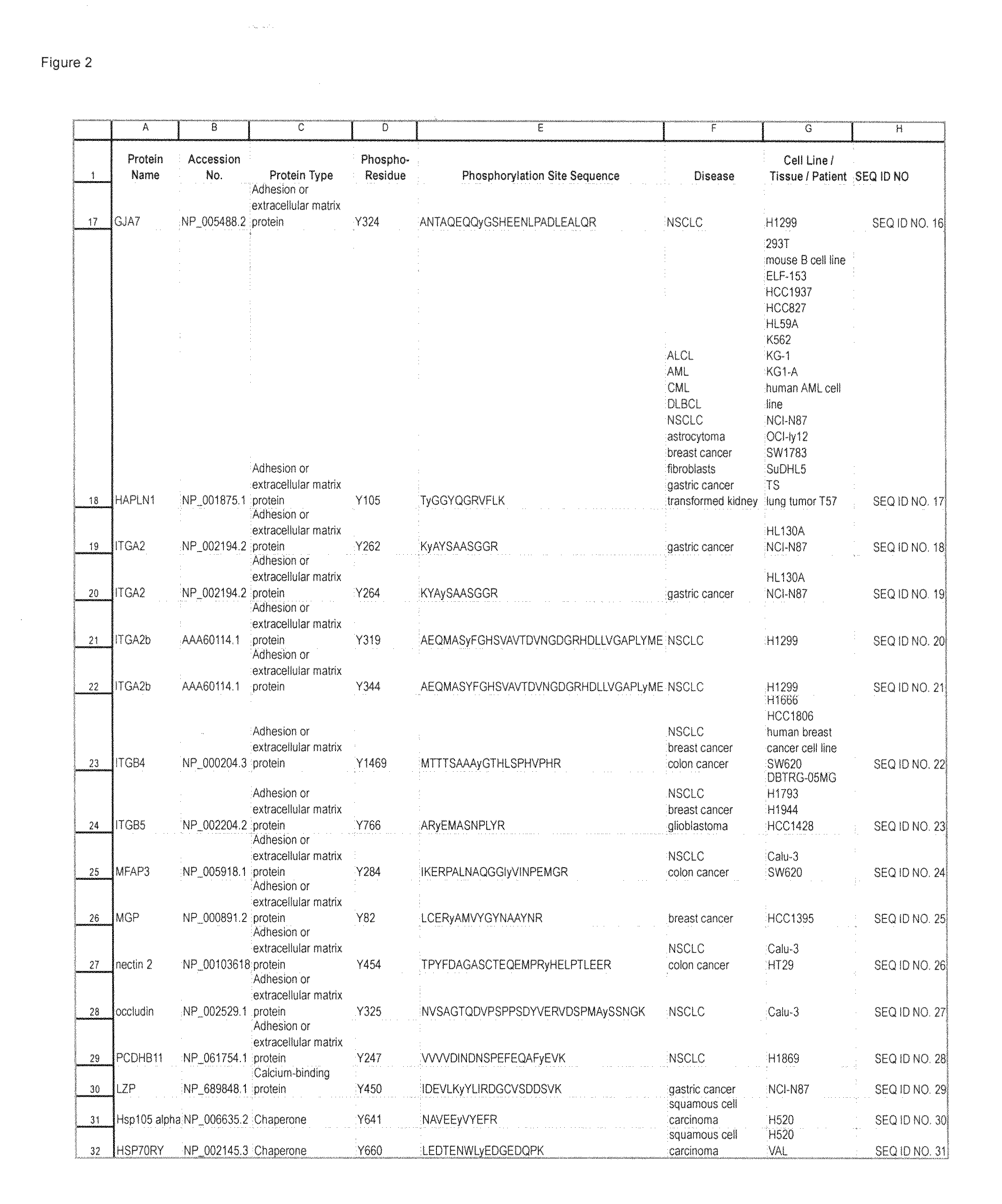
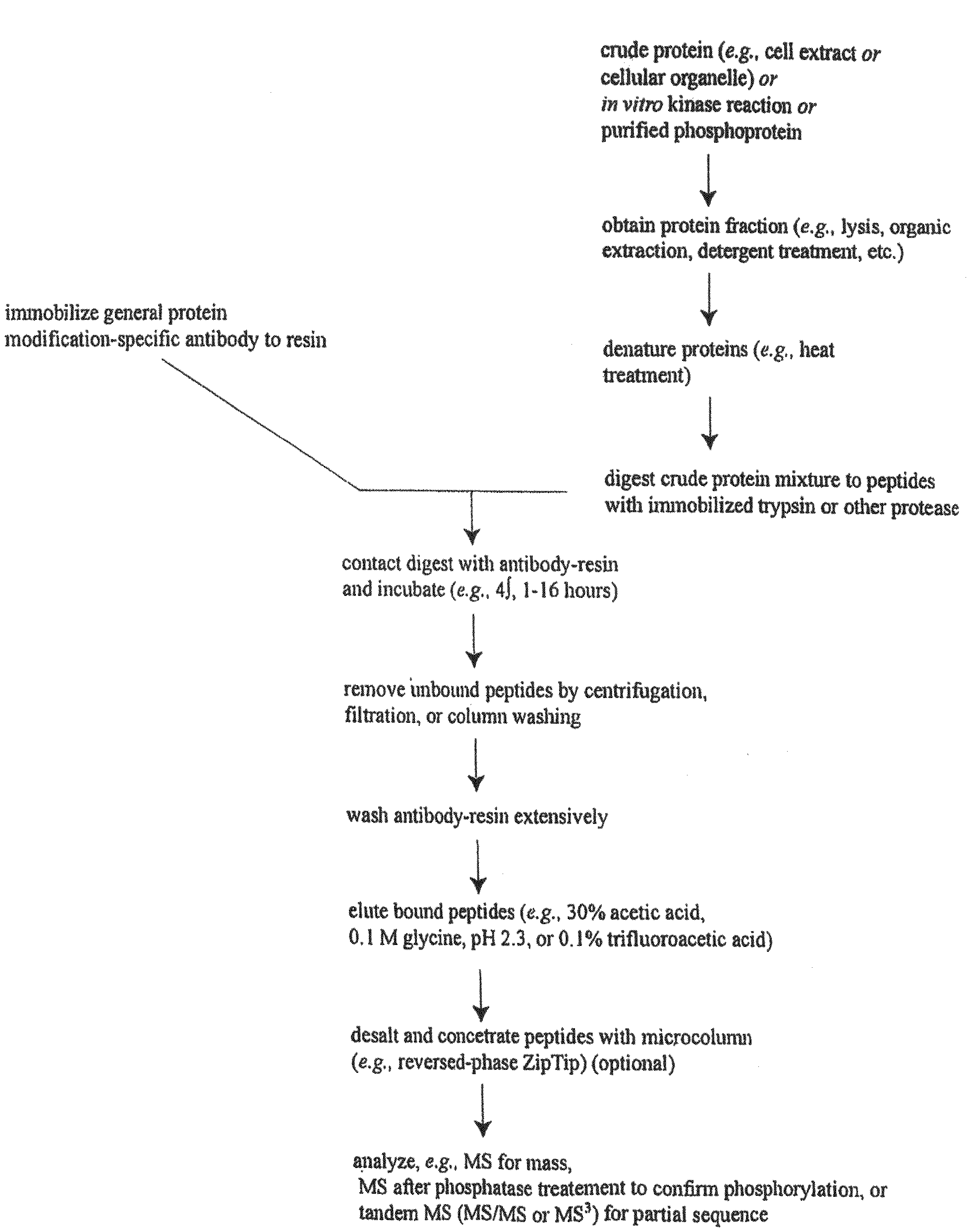
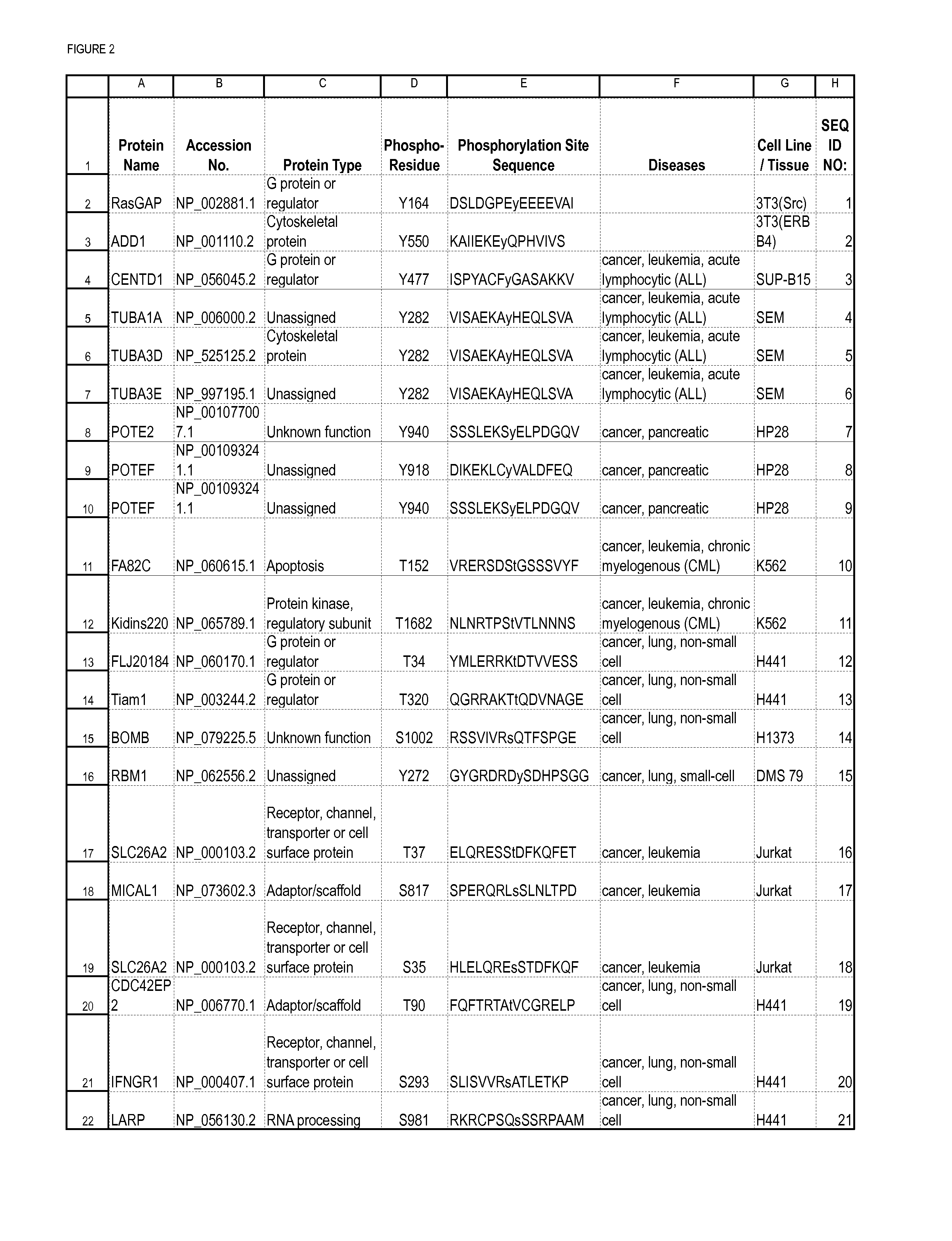
Tyrosine Phosphorylation Sites
InactiveUS20100129928A1Immunoglobulins against cell receptors/antigens/surface-determinantsBiological testingPeptideAntibody
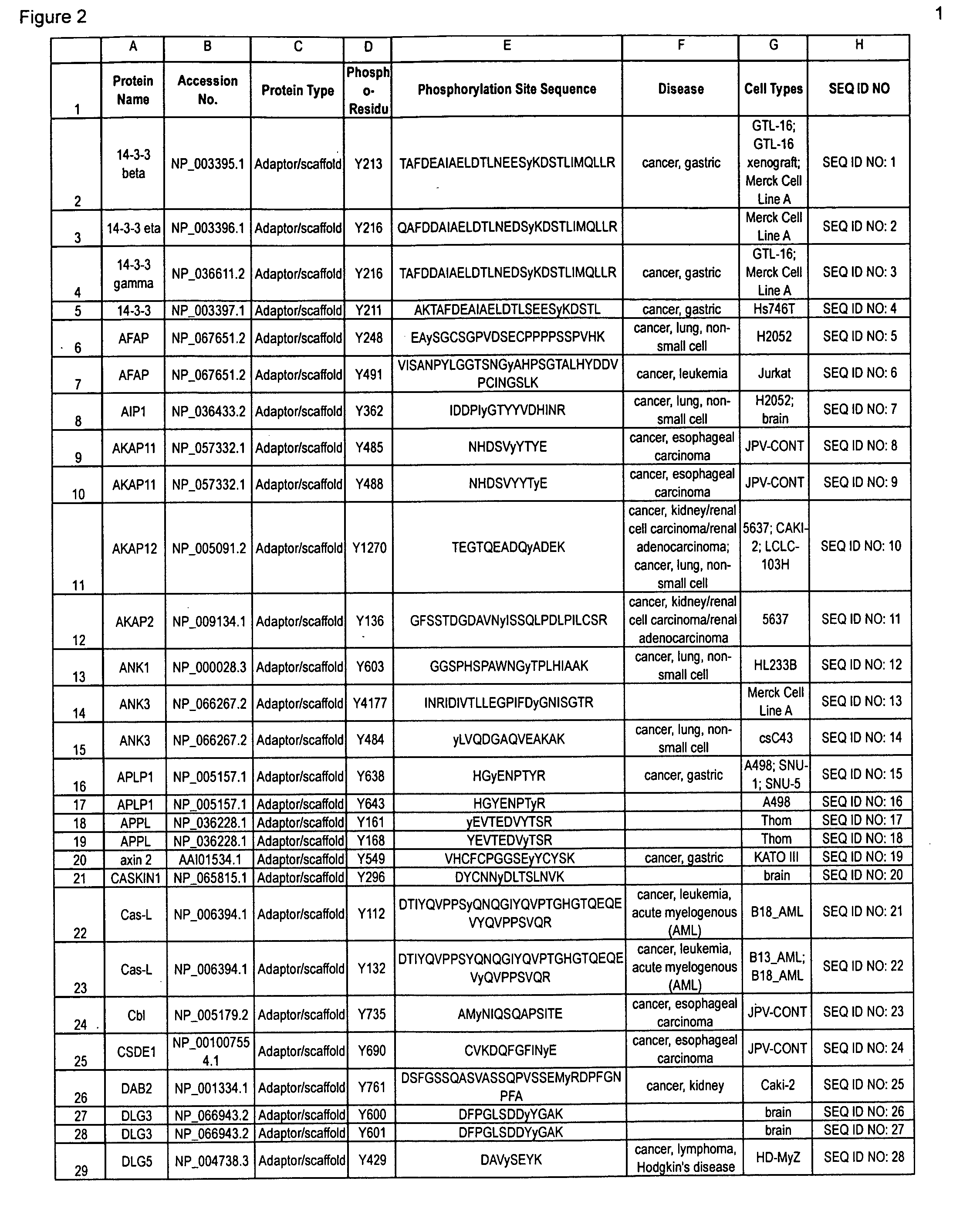
The invention discloses 347 novel phosphorylation sites identified in carcinoma, peptides (including AQUA peptides) comprising a phosphorylation site of the invention, antibodies specifically bind to a novel phosphorylation site of the invention, and diagnostic and therapeutic uses of the above.
Owner:CELL SIGNALING TECHNOLOGY
Tyrosine phosphorylation sites
InactiveUS20090305297A1Microbiological testing/measurementImmunoglobulins against animals/humansPhosphorylation siteLeukemia
The invention discloses 397 novel phosphorylation sites identified in carcinoma and / or leukemia, peptides (including AQUA peptides) comprising a phosphorylation site of the invention, antibodies specifically bind to a novel phosphorylation site of the invention, and diagnostic and therapeutic uses of the above.
Owner:CELL SIGNALING TECHNOLOGY
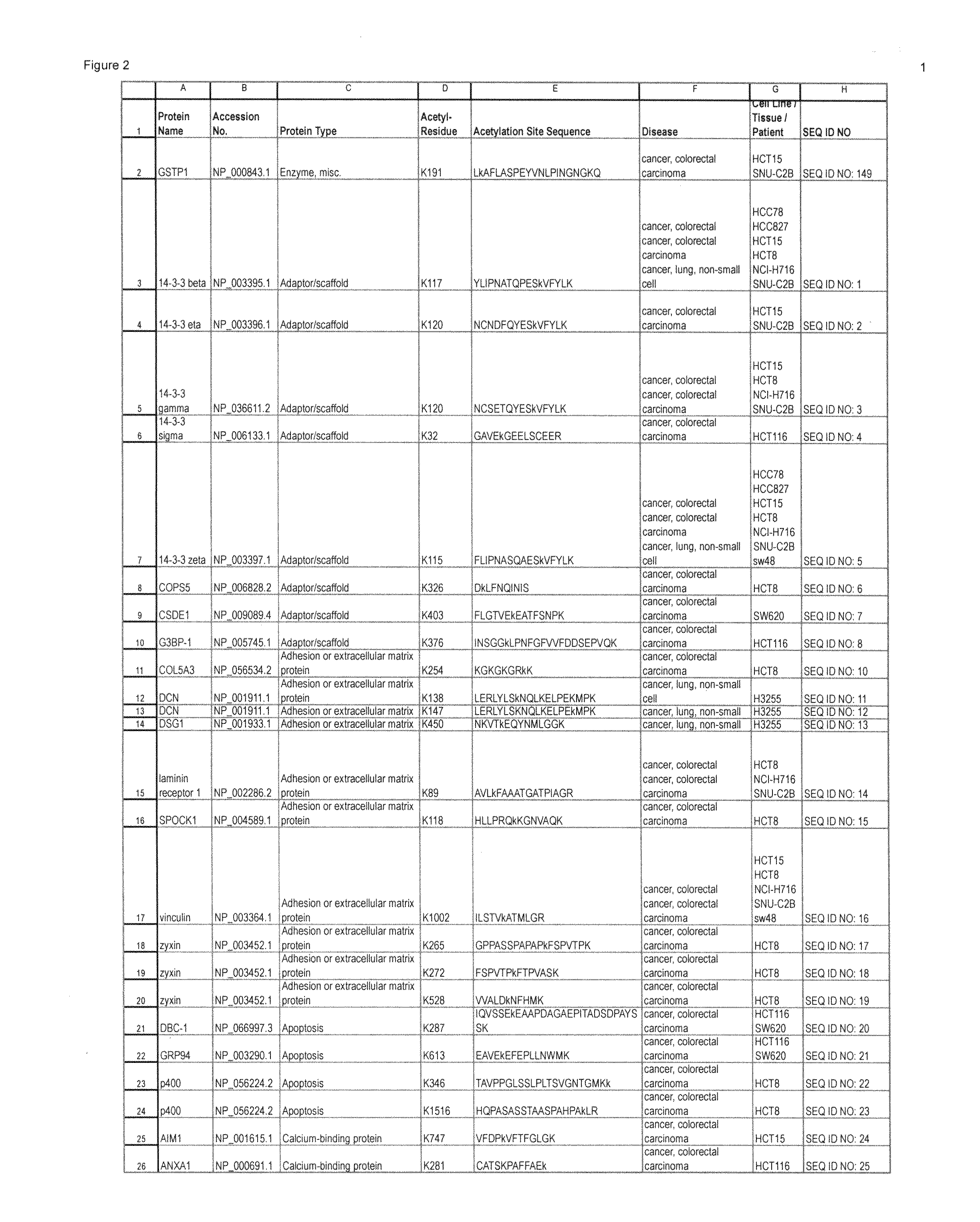
Lysine acetylation sites
InactiveUS20100092992A1Isotope introduction to peptides/proteinsImmunoglobulinsAntiendomysial antibodiesAcetylation
The invention discloses 322 novel acetylation sites identified in various cancers, peptides (including AQUA peptides) comprising a acetylation site of the invention, antibodies specifically bind to a novel acetylation site of the invention, and diagnostic and therapeutic uses of the above.
Owner:HORNBECK PETER +5
Purification of antigen-specific t cells
A new method to capture, purify and expand antigen-specific T lymphocytes has been developed using magnetic beads coated with recombinant MHC class I molecules. This method was optimized using homogenous populations of naive T cells purified from mice transgenic for the 2C T cell receptor (TCR). These T cells were captured on beads coated with MHC class I molecules and the relevant antigenic peptides. MHC and peptide specificity was confirmed by the usage of irrelevant MHC peptide combinations. An enrichment of 800 to 1600 fold was measured, using 2C T cells mixed with irrelevant T cells, starting from a 2C T cell frequency of {fraction (1 / 3000)}. The same approach was used to purify antigen-specific CD8+ T cells from total CD8+ T cells from naive mice. The recovered cells could be expanded and specifically kill target cells in vitro; they had a significant effect in vivo as well. We expect this procedure to be suitable to purify and expand in vitro tumor- and virus-specific killer T cells for use in cell therapy.
Owner:LUXEMBURG ALAIN T +2
Tyrosine phosphorylation sites
InactiveUS20090053831A1Immunoglobulins against cell receptors/antigens/surface-determinantsFermentationPeptideCarcinoma
The invention discloses 405 novel phosphorylation sites identified in carcinoma and / or leukemia, peptides (including AQUA peptides) comprising a phosphorylation site of the invention, antibodies specifically bind to a novel phosphorylation site of the invention, and diagnostic and therapeutic uses of the above.
Owner:CELL SIGNALING TECHNOLOGY
Cytotoxic t-cell epitope peptide and use thereof
InactiveCN107709351AMicrobiological testing/measurementImmunoglobulins against animals/humansImmunotherapeutic agentEBV Infections
The present invention provides a cytotoxic T-cell (cytotoxic T lymphocyte, abbreviated hereinafter as CTL) epitope peptide specific to the Epstein-Barr virus (described hereinafter as EBV), a vaccinefor treating or preventing EBV infection or EBV-positive cancer using this peptide, a passive immunotherapeutic agent for EBV, and a method for assaying CTL specific to EBV. The present invention alsoprovides an HLA-A*24:02-restricted epitope peptide comprising an IYTEVRELV sequence (SEQ ID NO: 43) from a cytoskeleton-associated protein (cytoskeleton-associated protein 4: CKAP4 hereinafter, alsoknown as: CLIMP-63, ERGIC-63, P63). The peptide-specific cytotoxic T-cells (CTL hereinafter) can attack malignant tumor cells that express a high level of CKAP4.
Owner:MEDICAL & BIOLOGICAL LAB CO LTD
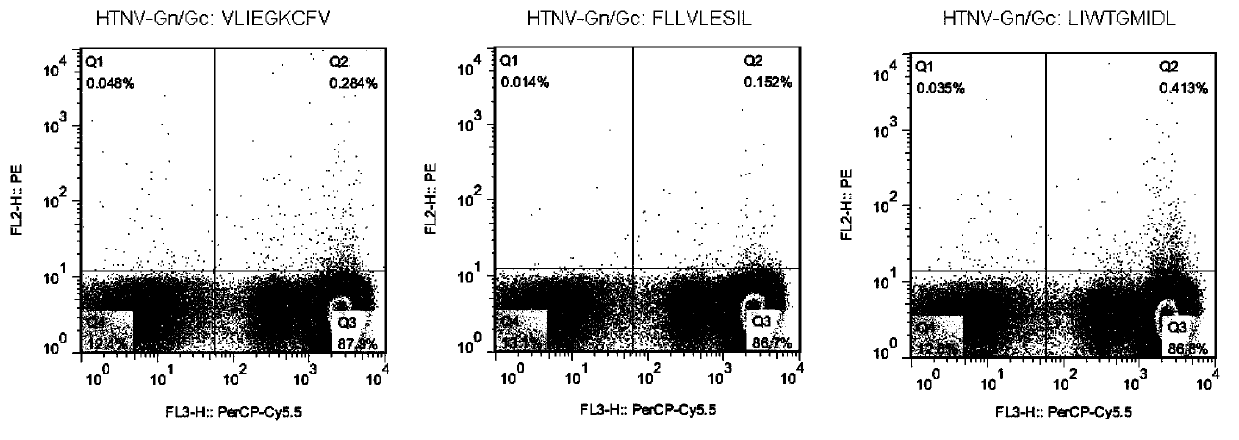
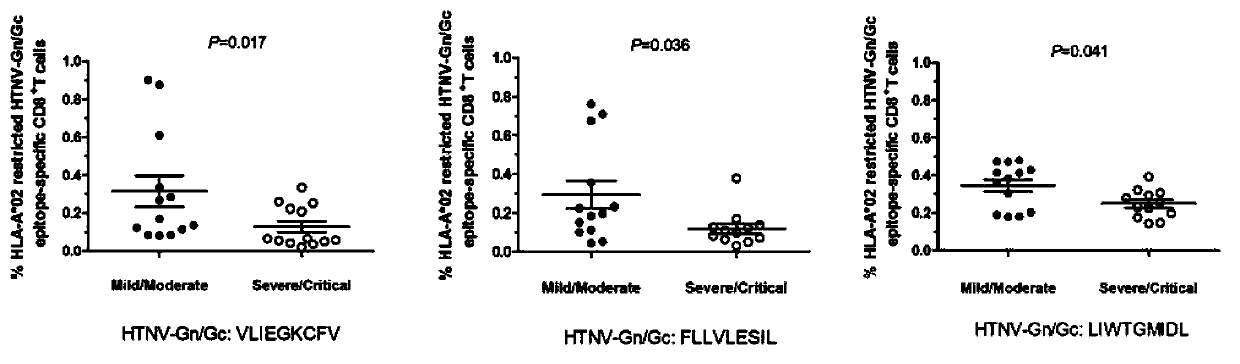
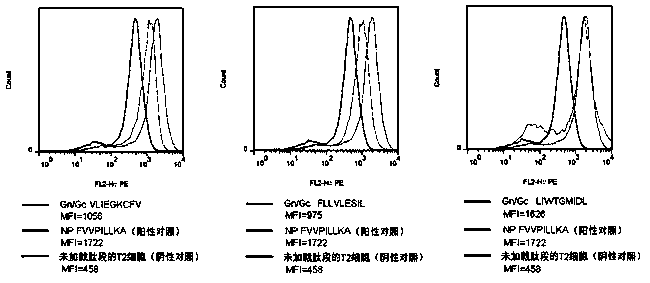
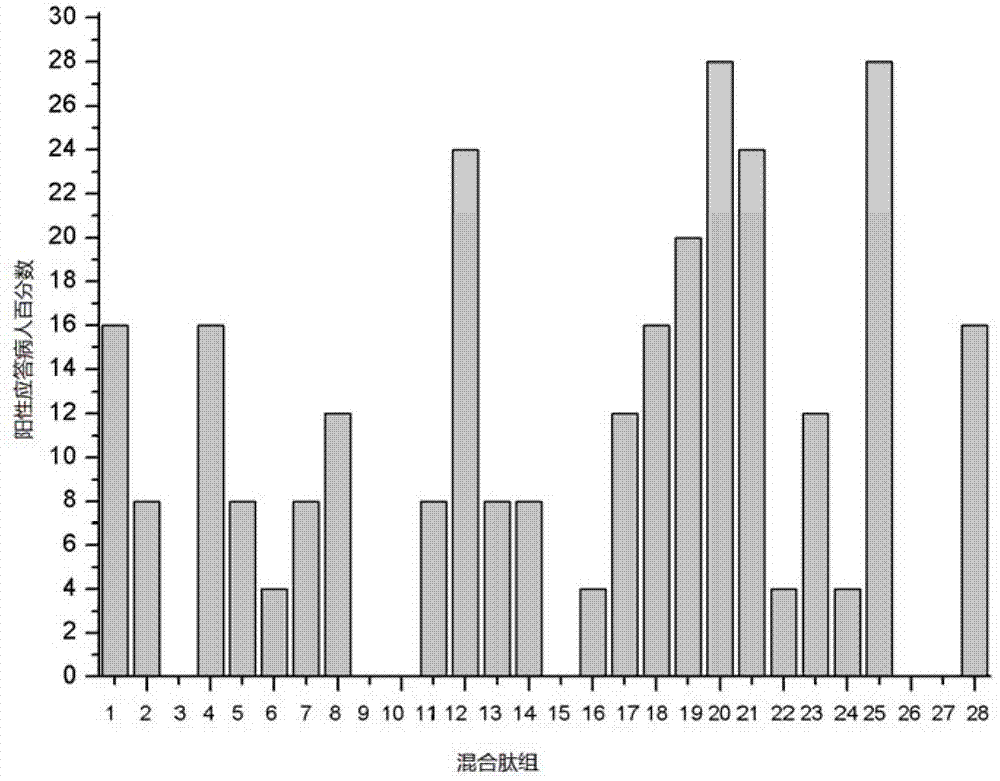
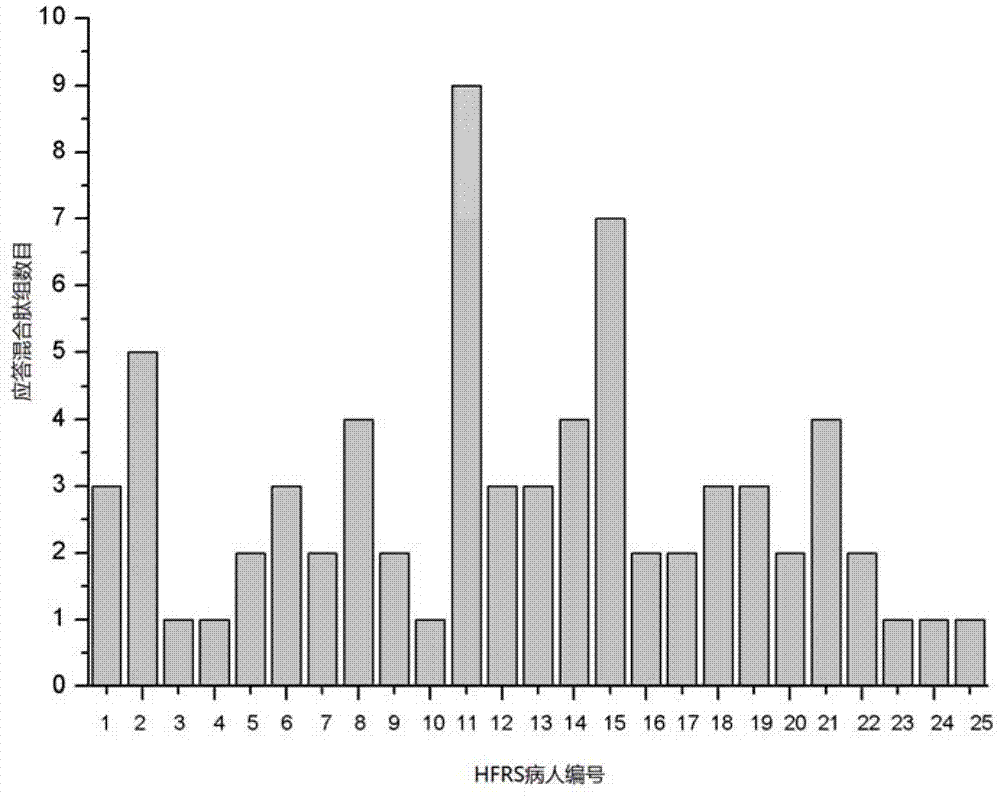
CTL epitope peptide of HTNV-Gn/Gc specificity and polymer and application thereof
ActiveCN103421118AStrong immune responseStrong specificityViral antigen ingredientsAntiviralsDiseaseCtl epitope
The invention discloses CTL epitope peptide of HTNV-Gn / Gc specificity limited by HLA-A*02, as well as application thereof. The nucleotide sequence of the CTL epitope peptide is shown in SEQ ID No: 1-3. The CTL epitope peptide of HTNV-Gn / Gc specificity is epitope polypeptide limited by HLA-A*02 molecule, can induce the CD8+T cell to generate strong cell immune response, excrete IFN-gamma; the frequency of excreting IFN-gamma can be used for indicating the severity degree of an HFRS patient, i.e., the higher the frequency of excreting IFN-gamma is, the less severe the HFRS condition is; therefore, the CTL epitope peptide can be applied to the manufacturing of CTL epitope peptide vaccine, inducing of cytotoxicity T cell capable of generating CTL epitope peptide specificity, or manufacturing of HLA-A*02 / epitope peptide-compound tetramer, and has excellent development and application prospect in the field of immunological therapy of HFRS specificity.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Serine, Threonine, and Tyrosine Phosphorylation Sites
The invention discloses 990 novel phosphorylation sites identified in carcinoma and leukemia, peptides (including AQUA peptides) comprising a phosphorylation site of the invention, antibodies specifically bind to a novel phosphorylation site of the invention, and diagnostic and therapeutic uses of the above.
Owner:CELL SIGNALING TECHNOLOGY
HLA-A*0201 restrictive CTL epitope of neuritin protein, and application of HLA-A*0201 restrictive CTL epitope
The invention pertains to the field of biomedicine, relates to an antigen cytotoxic T cell (CTL) epitope, and particularly relates to a HLA-A*0201 restrictive CTL epitope of a neuritin protein. The amino acid sequence of the HLA-A*0201 restrictive CTL epitope is showed as SEQ ID No.1, SEQ ID No.2 or SEQ ID No.3. The invention also relates to an application of the CTL epitope in preparation of tumor therapeutic peptide vaccines. The CTL epitope can bind to HLA-A*0201 with a high affinity, such that a stable composite is obtained, peptide specific CTL responses can be induced, the peptide specific CTL is stimulated to secrete a high level of IFN-gamma, and specificity killing effect on tumour cells is produced. The CTL epitope can be used to prepare tumor therapeutic peptide vaccine such as glioma therapeutic peptide vaccine, and has potential and good development and application prospects in the field of tumor specific immune therapy.
Owner:中国人民解放军南京军区福州总医院四七六医院
Coated oncolytic adenoviruses for cancer vaccines
The present invention relates to adenoviral vectors, wherein the viral capsid has been coated with polypeptides, which are capable of stimulating a peptide-specific immune response in a subject and uses thereof. Furthermore, the present invention relates to methods of treating diseases, e.g. cancer, by adenoviral vectors which have been coated by polypeptides causing peptide-specific immune response. Also the present invention relates to a method of coating adenoviral vectors by specific peptides as well as to a method of identifying those peptides suitable for coating the capsid of an adenoviral vector.
Owner:瓦洛治疗公司
Peptide specificity of anti-myelin basic protein and the administration of myelin basic protein peptides to multiple sclerosis patients
Human myelin basic protein (h-MBP) has a molecular weight of 18.5 KD and contains 170 amino acid residues. Synthetic peptides ranging in length from about 8 to 25 residues and covering the entire length of the protein have been produced. Antibodies to h-MBP (anti-MBP) were found to be neutralized by the synthetic peptides, in vitro, which span the h-MBP from about amino acid residue 61 to about amino acid residue 106. The peptides, which cover both the amino (about residues 1 to 63) and carboxy (about residues 117 to 162) terminals of h-MBP did not neutralize purified anti-MBP. Intrathecal administratin of peptide MBP(75-95), MBP(86-95), or MBP(82-98) produced complete binding-neutralization of free (F) anti-MBP with no change in bound (B) levels. A control peptide MBP35-58 had no effect on F or B anti-MBP levels. Intravenous administration of MBP(75-95), MBP(86-95), or MBP(82-98) resulted in significant decline of F and B CSF anti-MBP levels. Administration of MBP synthetic peptides to MS patients either intrathecally or intravenously did not have any adverse neurological effects and systemic complications did not occur. The MBP epitope for MS anti-MBP has been localized to an area between amino acid 86 and amino acid 95.
Owner:THE GOVERNORS OF THE UNIV OF ALBERTA
Lysine acetylation sites
The invention discloses 332 novel acetylation sites identified in various cancers, peptides (including AQUA peptides) comprising a acetylation site of the invention, antibodies specifically bind to a novel acetylation site of the invention, and diagnostic and therapeutic uses of the above.
Owner:CELL SIGNALING TECHNOLOGY
Modulation of structured polypeptide specificity
ActiveUS20160046673A1Improve stabilityProteolytic stability is enhancedSenses disorderPeptide/protein ingredientsCrystallographyPeptide ligand
Owner:BICYCLERD LTD
Preparation of antigen-presenting human gamma delta t cells and use in immunotherapy
InactiveUS20090130074A1Improve purification effectEfficient workBiocideBacterial antigen ingredientsDendritic cellInfectious Disorder
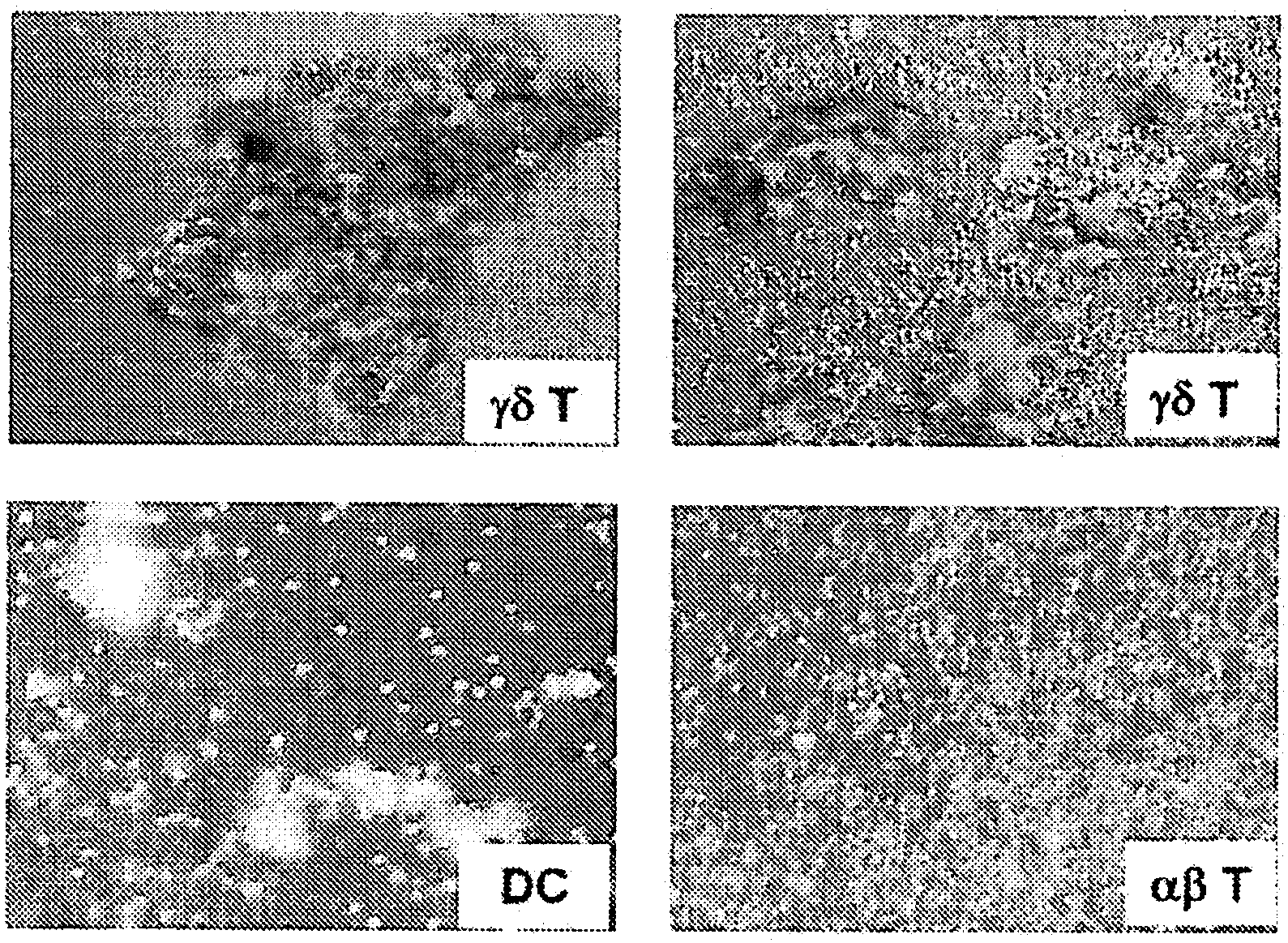
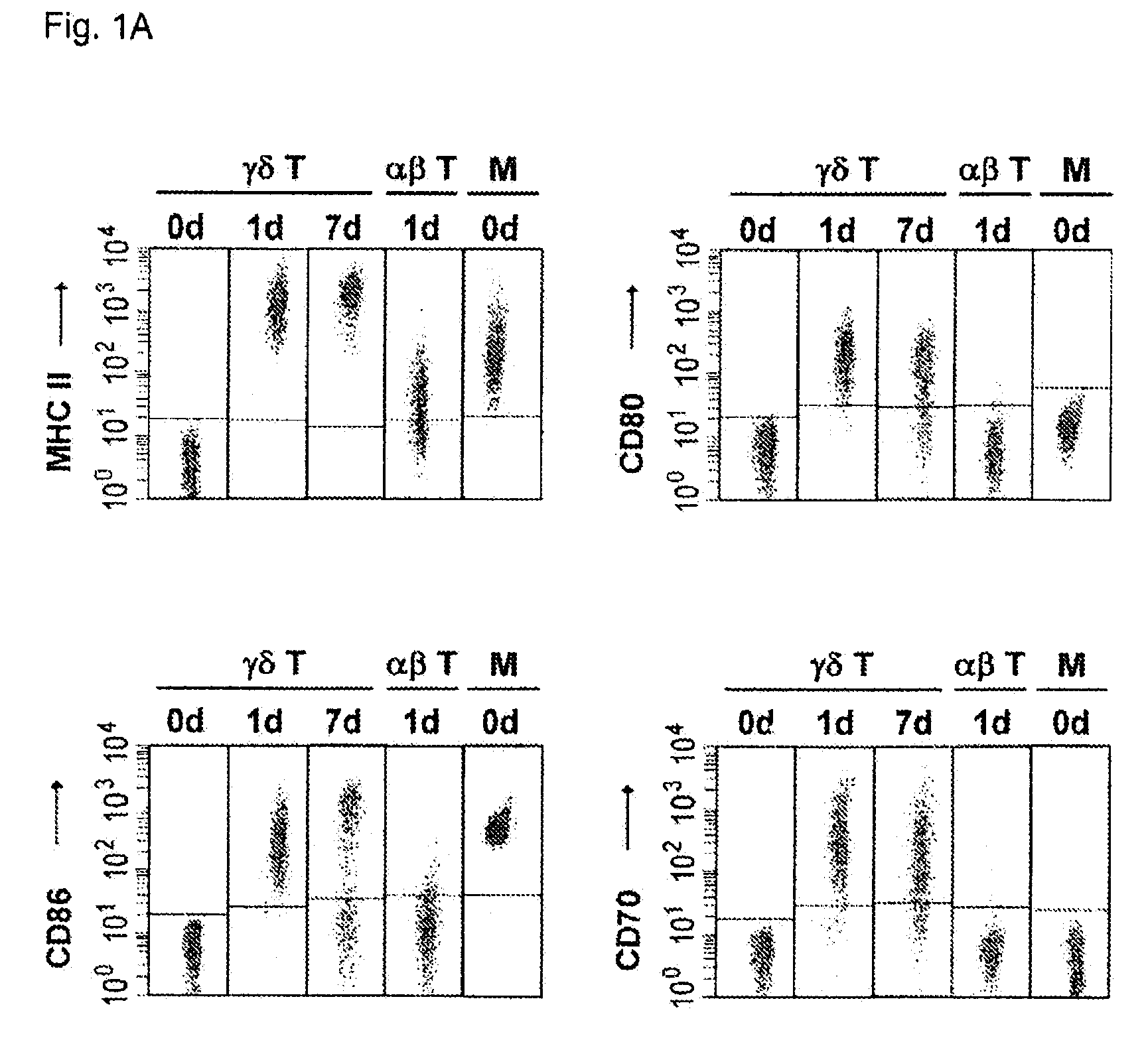
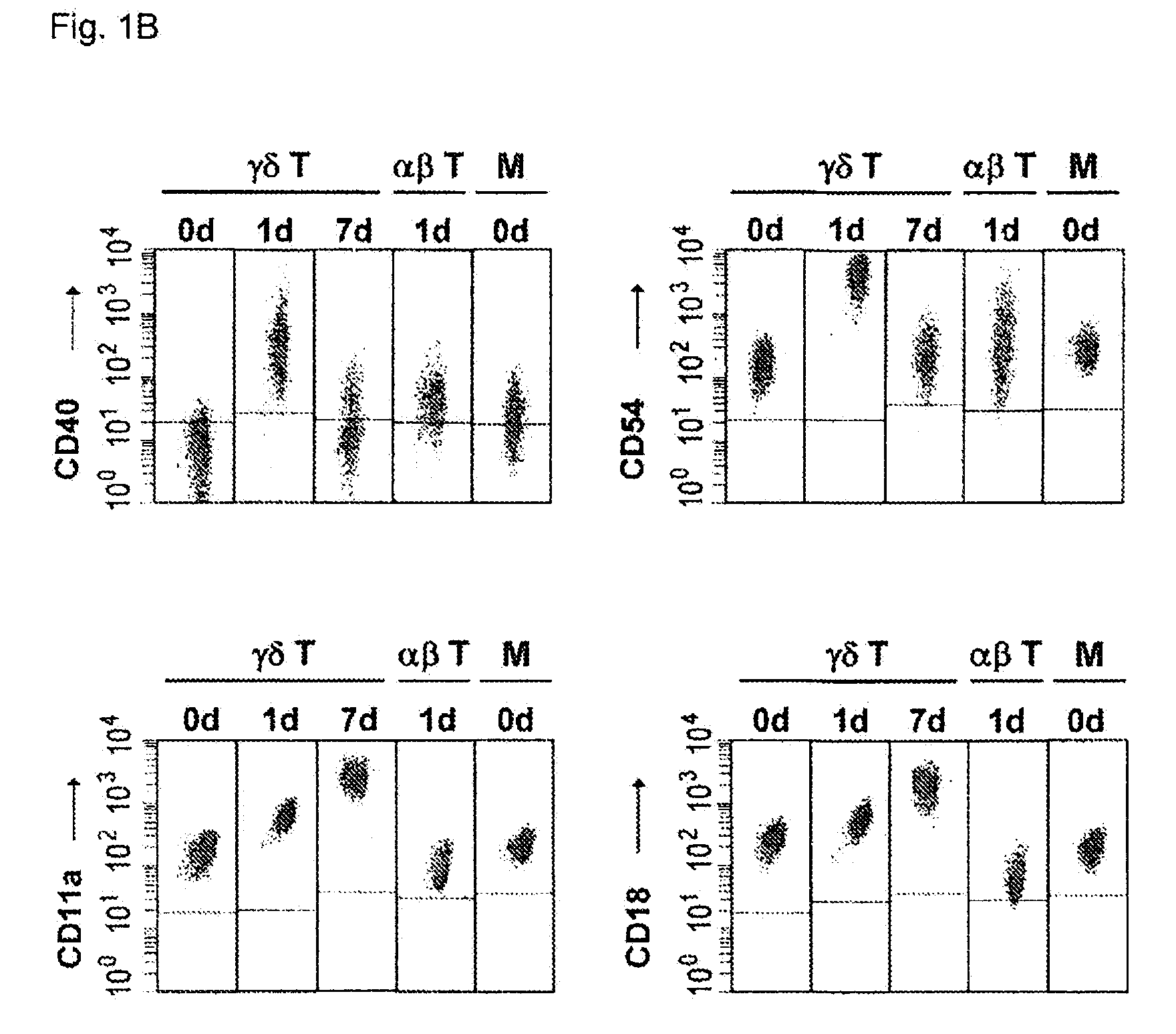
Cytotoxic αβ T cells form an essential component in immunity to infections and tumors, and are also implicated in host defense against these challenges. The present disclosure demonstrates the ability of activated γδ T cells to cross-present exogenous antigens to CD8+αβ T cells, a process previously thought to be mediated best by dendritic cells. In particular, the present disclosure provides a method for cross-presentation of antigen derived from tumor cell or microbial organisms such as viruses, bacteria, yeasts, parasites, and the like, or from cells infected with such organisms, to a CD8+αβ T cell. Still further, the present disclosure provides a method for treatment of a tumor or a chronic or recurrent infectious disease, comprising delivering an antigen-presenting autologous γδ T cell population above into a patient requiring such treatment. Still yet further, a method is described for preparing a peptide-specific effector T cell.
Owner:UNIV COLLEGE CARDIFF CONSULTANTS LTD
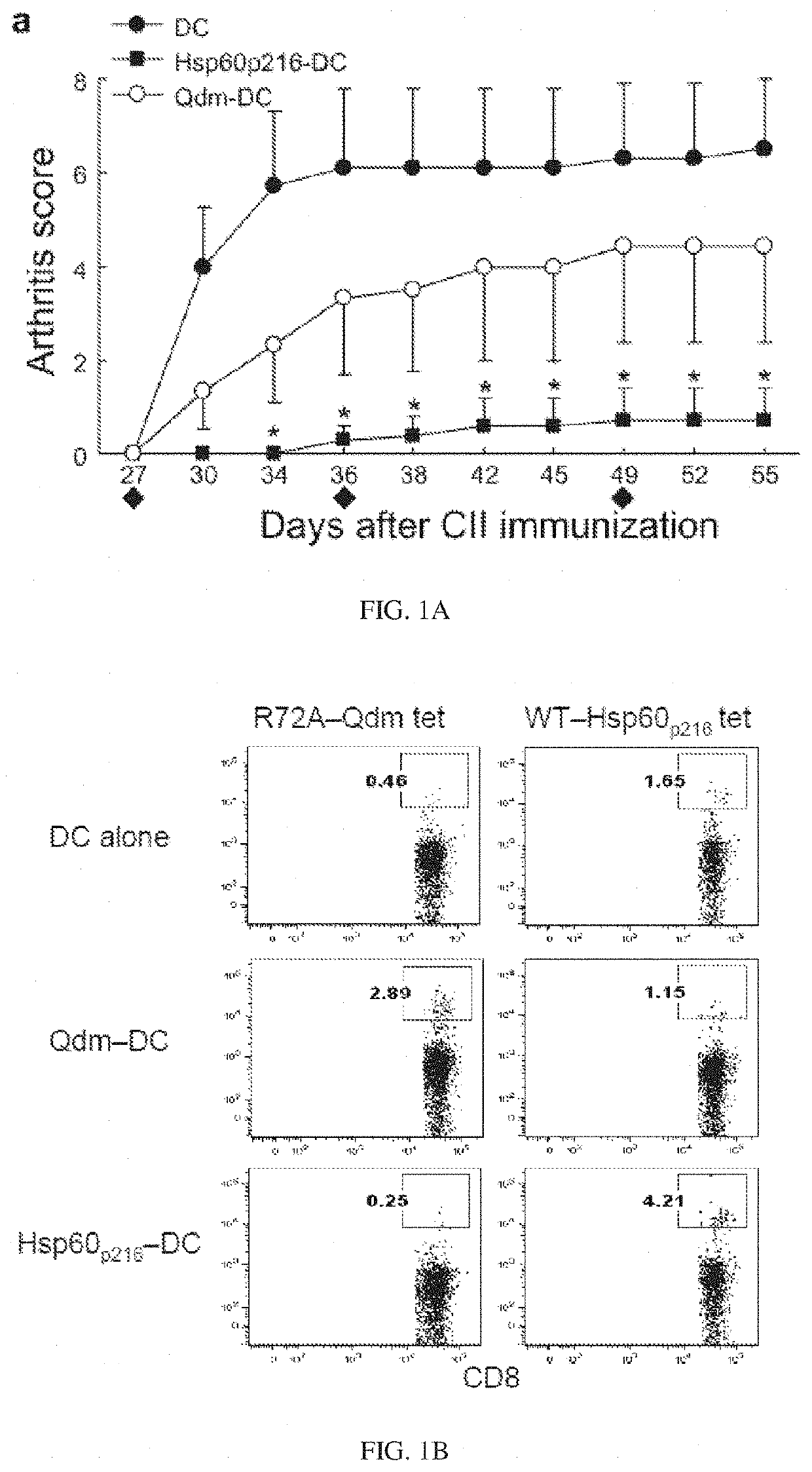
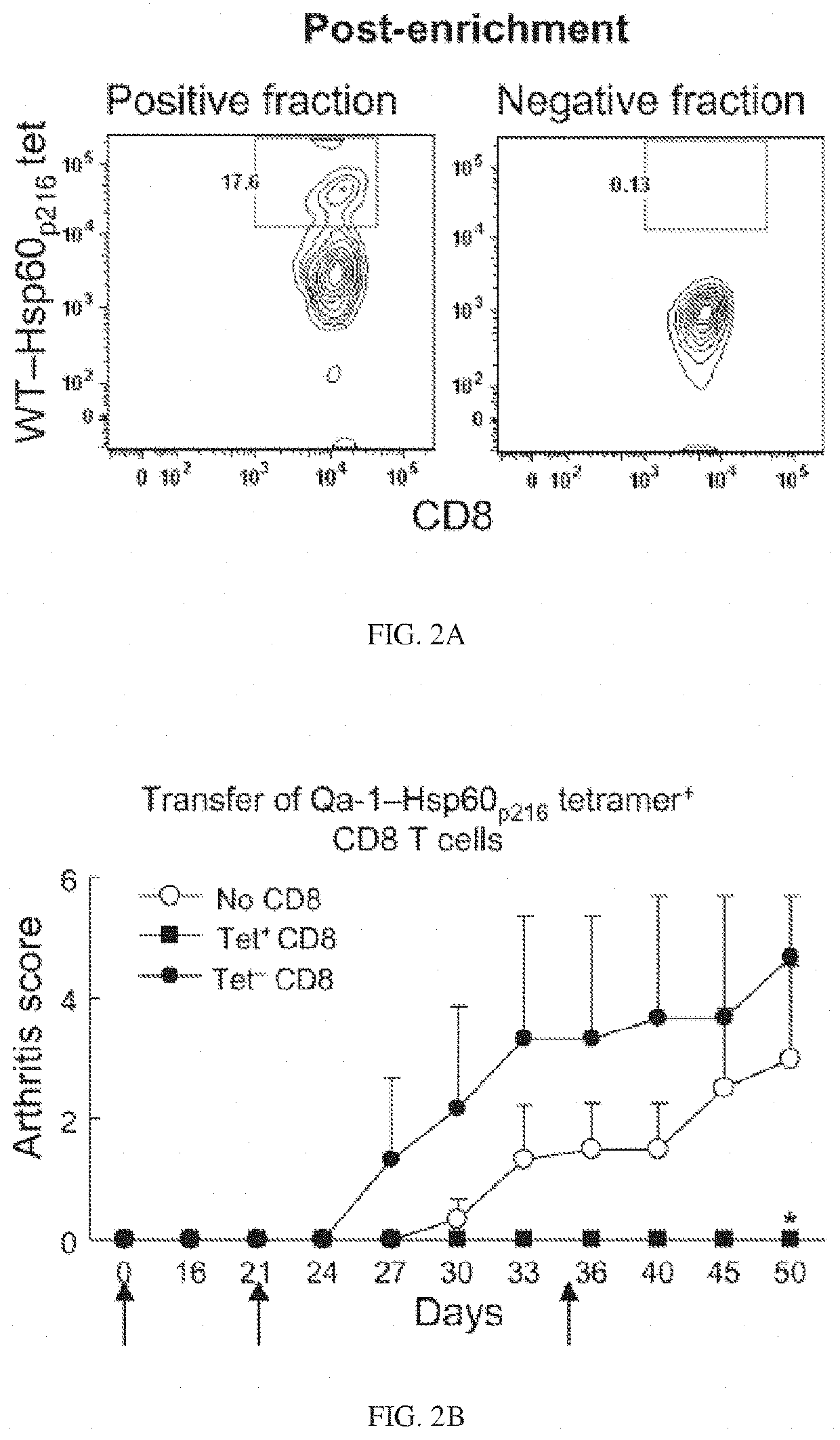
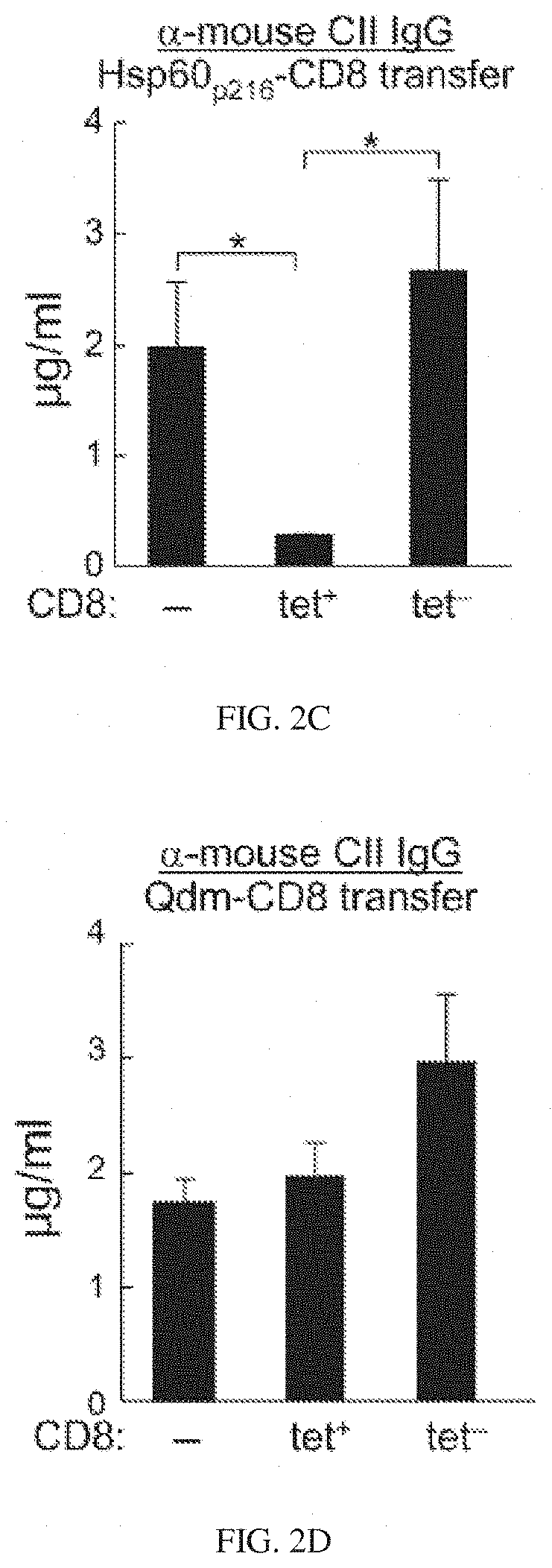
Targeted expansion of Qa-1-peptide-specific regulatory CD8 T cells to ameliorate arthritis
ActiveUS10548957B2Mammal material medical ingredientsDepsipeptidesImmunologic disordersAutoimmune condition
Nanoparticles to treat autoimmune diseases and HIV infection are provided. The nanoparticles comprise a biocompatible polymer and a complex, wherein the complex is a major histocompatibility complex (MHC) class I antigen E (HLA-E) linked to a peptide, and wherein the HLA-E-peptide complex is linked to the surface of the nanoparticle. The present invention also relates to methods for treating autoimmune diseases and HIV infection.
Owner:DANA FARBER CANCER INST INC
Composition for preventing and treating skin disease comprising substance specifically binding to vimentin-derived peptide
PendingCN111148529APrevent and treat skin diseasesCosmetic preparationsPeptide/protein ingredientsAntiendomysial antibodiesCutaneous Disorders
Owner:疫免医学有限公司
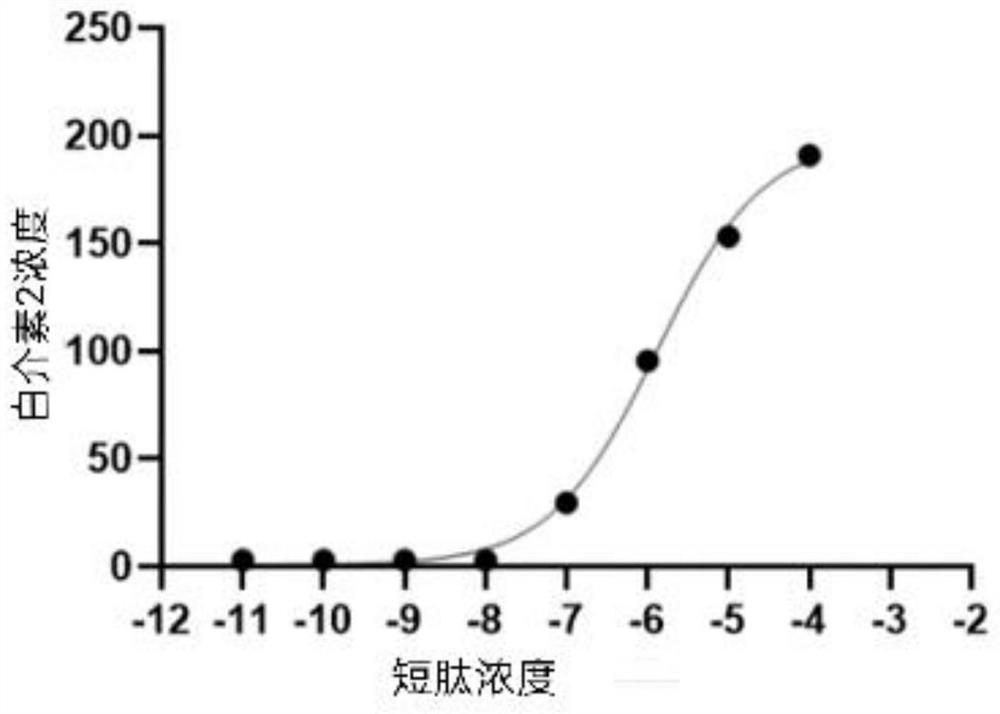
Method for inducing early t memory response with short peptides Anti-tumor vaccine
ActiveUS20180169200A1Large capacityEffective immunityCancer antigen ingredientsAntigen/adjuvant combination ingredientsPeptide TWhite blood cell
The present invention relates to a therapeutic peptide T specific immune therapy for use in the treatment of a cancer of an HLA-A2 (Human Leukocyte Antigen A2) positive patient, said treatment comprises a priming period consisting in two to three administrations of said therapeutic peptide T specific immune therapy, thereby inducing a memory T cell response.
Owner:OSE IMMUNOTHERAPEUTICS
Hantaan virus glucoprotein specific T cell epitope peptide and application thereof
ActiveCN102731617AStrong immune responseViral antigen ingredientsImmunoglobulins against virusesCtl epitopeSpecific immunity
The invention discloses an HTNV-Gn / Gc specific T cell epitope peptide and an application thereof. The amino acid sequence of the HTNV-Gn / Gc specific T cell epitope peptide is one selected from SEQ ID NO. 1 to SEQ ID NO. 75. The HTNV-Gn / Gc specific T cell epitope peptide comprises CD4<+>T cell epitope and CTL epitope, and can respectively induce CD4<+>T cell and CD8<+>T cell to generate intensive cellular immunologic response and to secrete high-level IFN-gamma. The HTNV-Gn / Gc specific T cell epitope peptide can be used in the preparation of T cell epitope peptide vaccines, and can be used in inducing CD4<+>T cell or cytotoxicity T cell generating epitope peptide specificity. Therefore, the HTNV-Gn / Gc specific T cell epitope peptide has a good exploitation and application prospect in the field of HFRS specific immunotherapy.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Monoclonal antibody specifically recognizing modification site after translation of p53 and kit for assaying modification site containing the same
The present invention provides a monoclonal antibody recognizing modification after translation of p53 in a manner specific to a modification site, an antibody microarray comprising the antibody immobilized on a substrate, etc. Disclosed is a monoclonal antibody which reacts specifically with a peptide consisting of an amino acid sequence of at least 6 consecutive amino acids containing a predetermined amino acid residue of the amino acid sequence represented by SEQ ID NO: 1, wherein the amino acid residue is phosphorylated or acetylated, or with a peptide having one to several arbitrary amino acids added to the above peptide, but does not react with the above peptide which is not phosphorylated or acetylated.
Owner:HOKKAIDO UNIVERSITY +1
Biosensing method for detecting activity of histone modification enzyme and screening inhibitor thereof based on non-modified antibody mediate nano-gold assembly
InactiveCN102590509ASimple designSimple and fast operationColor/spectral properties measurementsBiological studiesMonoclonal antibody
The invention discloses a colorimetric biosensing method for analyzing and detecting activity of histone modification enzyme and screening inhibitor thereof based on non-modified antibody mediate nano-gold. The method comprises the following steps of: designing substrate peptide of histone modification enzyme; determining the mixing self-assembly ratio of the substrate peptide of the histone modification enzyme to control peptide; and quantitatively detecting the activity of the histone modification enzyme and screening the corresponding inhibitor by colorimetry based on nano-gold assembly of non-labeled antibody mediate modified histone modification enzyme substrate peptide. The specificity interaction of the histone modification enzyme and the substrate peptide of the histone modification enzyme is detected, and the substrate peptide modified on the nano-gold whch is modified by the histone modification enzyme can be subjected to specific binding with the corresponding monoclonal antibodies to ensure that the nano-gold is assembled, the activity of the histone modification enzyme is detected, and the inhibitor of the histone modification enzyme is screened by ultraviolet quantitative analysis on the nano-gold which modifies the substrate peptide of the histone modification enzyme. The method is high in sensitivity and specificity, and is convenient and easy to operate and can be used for biological research, medical research, medicine screening and the like.
Owner:HUNAN UNIV
T cell receptor for recognizing EBV antigen and application of T cell receptor
ActiveCN112940108AGood killing effectImmunoglobulin superfamilyPeptide/protein ingredientsAntigenComplementarity determining region
The invention discloses a T cell receptor for recognizing an EBV antigen and application of the T cell receptor. The T cell receptor (TCR) comprises an alpha chain containing a variable region and / or a beta chain containing a variable region, wherein the variable region of the alpha chain comprises a complementarity determining region 3 (CDR3) containing an amino acid sequence of AVVNNNDMR (SEQ ID NO: 3); and / or the variable region of the beta chain comprises a complementarity determining region 3 (CDR3) comprising ASSPGRWYEQY (SEQ ID NO: 6). The TCR provided by the invention can be specifically combined with an EBV antigen short peptide, and T cells transducing the TCR can be specifically activated and have a very strong killing effect on target cells.
Owner:HENAN CANCER HOSPITAL +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com