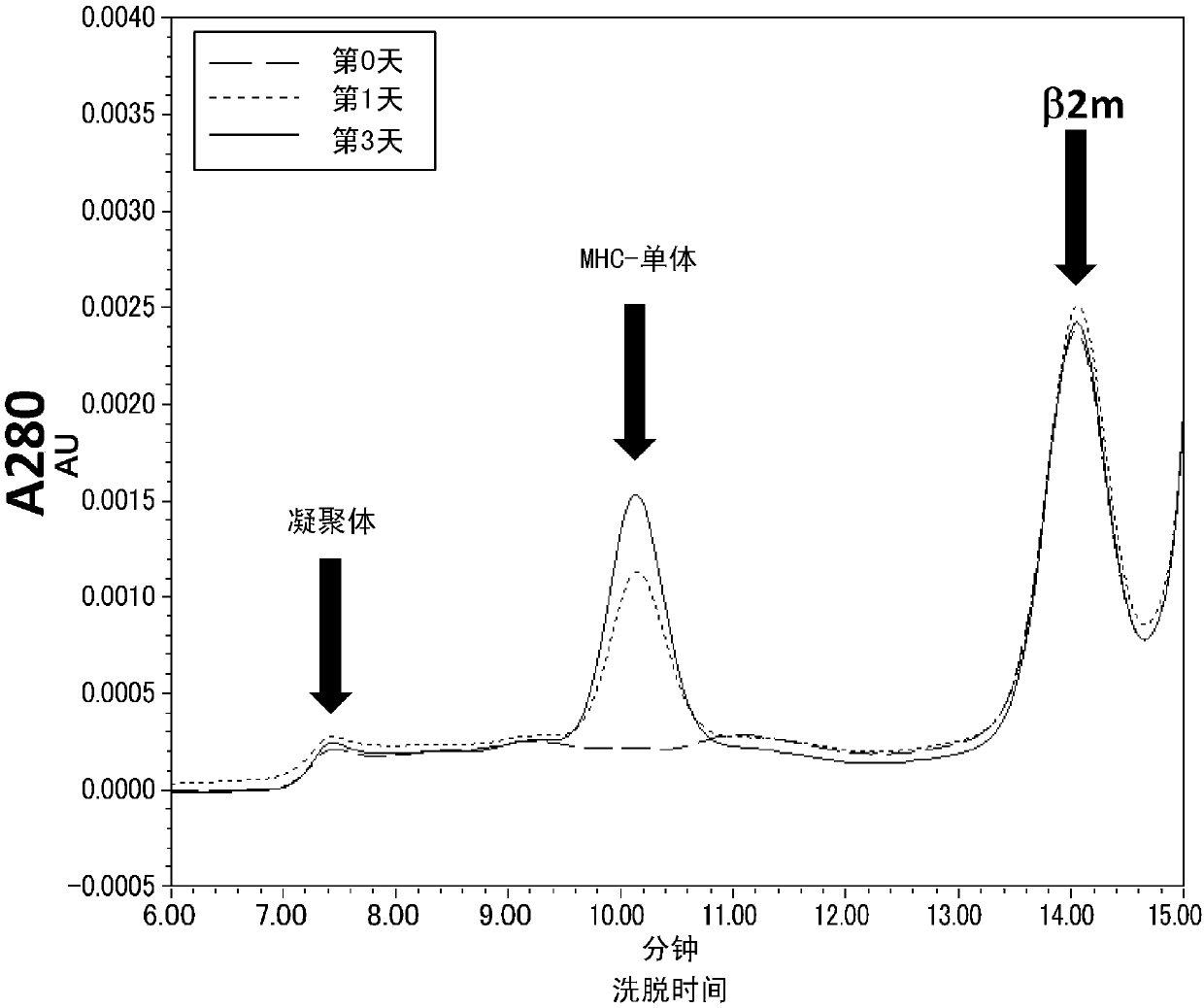
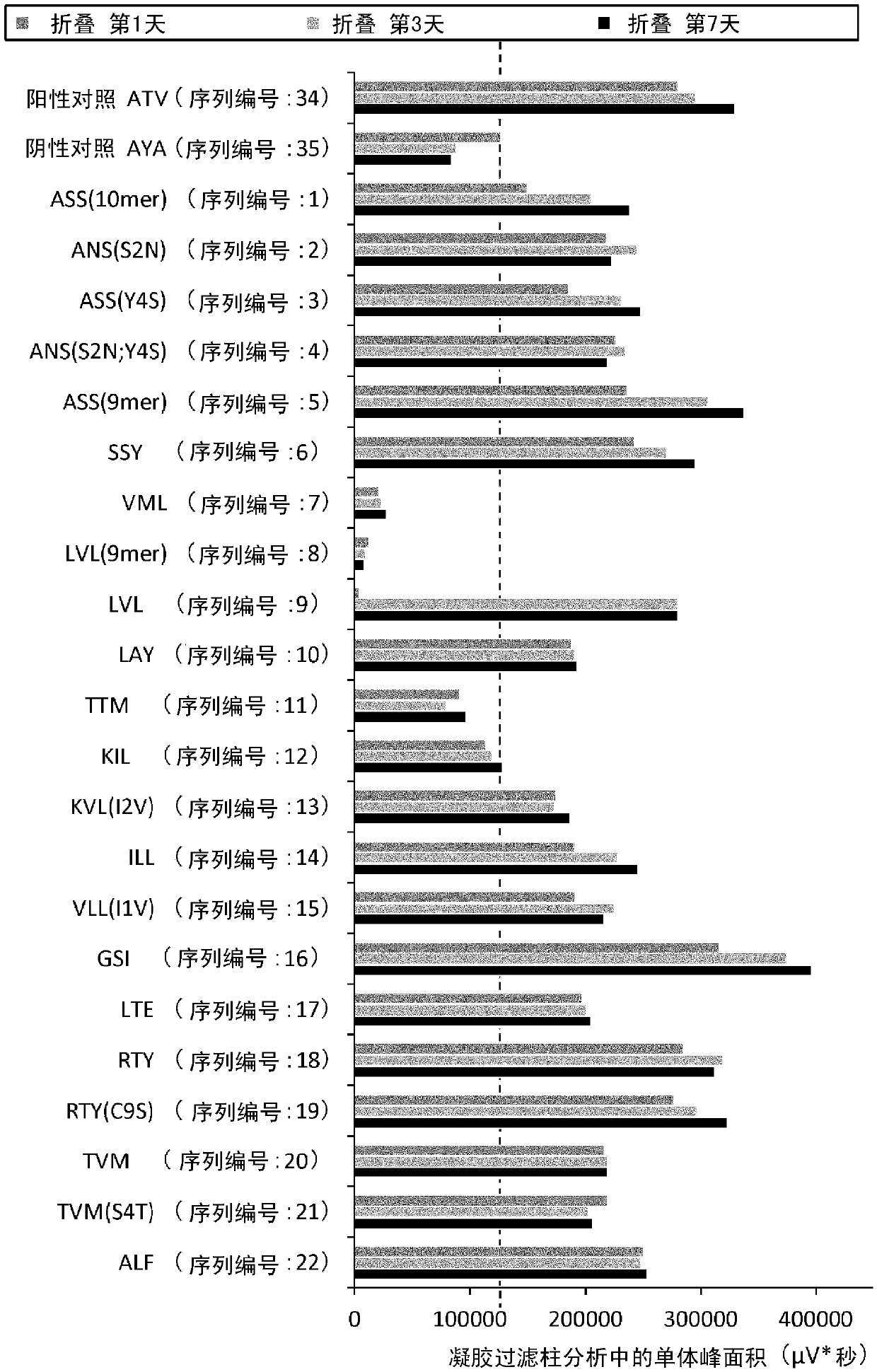
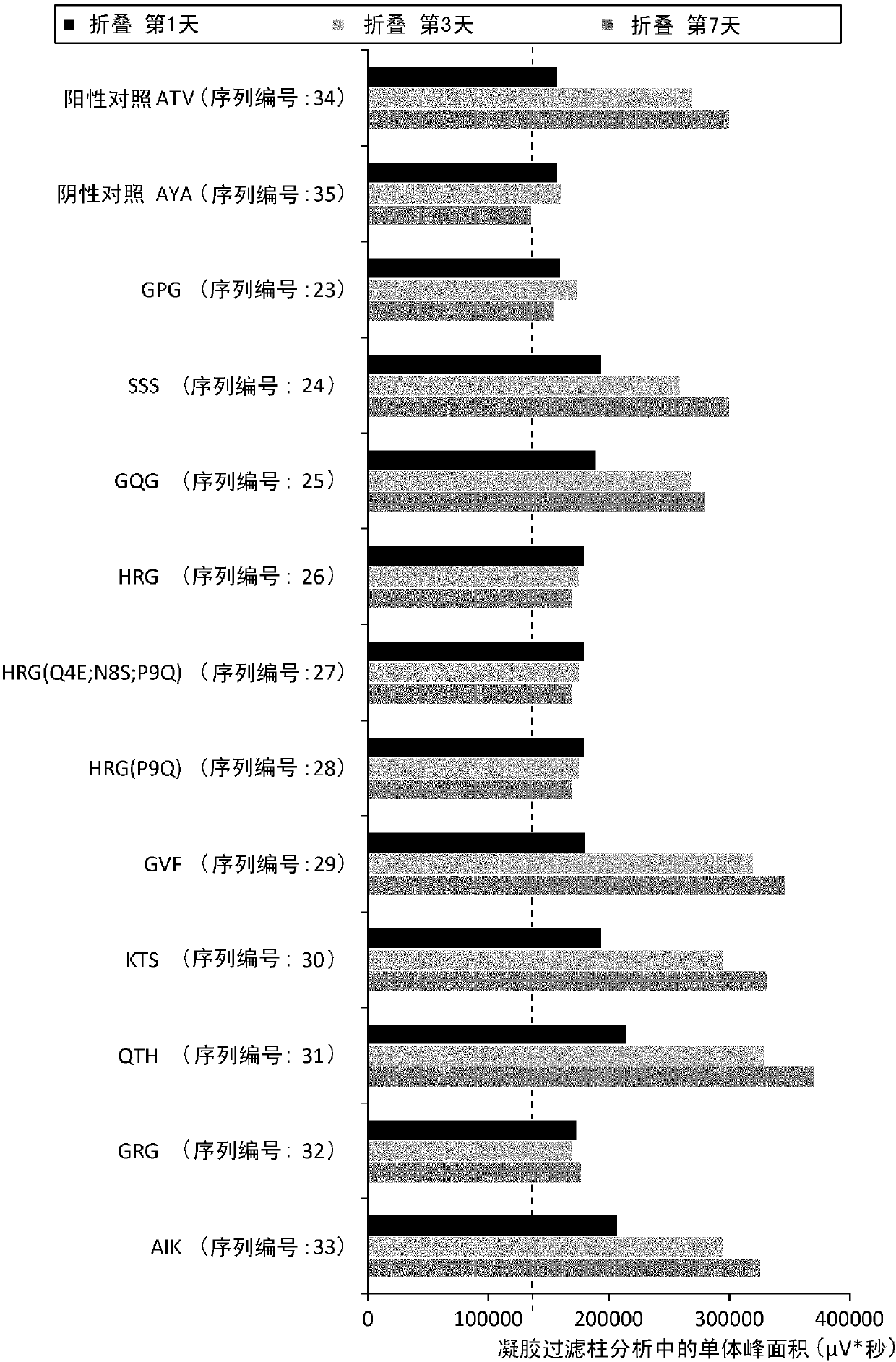
Cytotoxic t-cell epitope peptide and use thereof
A cytotoxic, epitope peptide technology, applied in the field of anti-cancer agents, passive immunotherapy agents, cancer vaccines and anti-cancer agents, CTL peptides, can solve the problem of unknown CKAP4 antigenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0365] [Selection of EBV-specific CTL epitope candidate peptides]
[0366]EBV is classified into type 1 and type 2 according to differences in expressed proteins and the like. Most of the infections and lurking in humans are type 1, and type 2 infections mainly AG876 are rare in Asia (Dambaugh T, Hennessy K, Chamnankit L, Kieff E. U2region of Epstein-Barr virus DNA may encode Epstein-Barr nuclear antigen2. Proc Natl Acad Sci US A. 1984 Dec;81(23):7632-6). However, there are also reports of EBV co-infection cases in the same individual, and there are also reports of ethnic and regional diversity of EBV infection strains (Apolloni A, Sculley TB. Detection of A-type and B-type Epstein-Barr virus in throat washings and lymphocytes. Virology. 1994 Aug 1; 202(2): 978-81, Correa RM, Fellner MD, Alonio LV, Durand K, Teyssie AR, Picconi MA. Epstein-barr virus (EBV) in healthy carriers: Distribution of genotypes and 30 bp deletion in latent membrane protein-1 (LMP-1) oncogene. J Med V...
Embodiment 2
[0515] [Prediction of CKAP4-specific HLA-A*24:02-restricted epitope peptides]
[0516] CKAP4 is a type II membrane protein with a full length of 602 amino acids, and no isoform has been reported. The selection of CKAP4-specific CTL epitope candidate peptides of the present invention was carried out on HLA-A*24:02 which is retained by about 60% of Japanese. Specifically, it was carried out referring to a plurality of software published on the Internet that can search for CTL epitope candidate peptides consisting of 8 to 12 amino acids having a binding motif to HLA-A*24:02. As a result, 10 CTL epitope candidate peptides consisting of 9 to 10 amino acids having a binding motif of HLA-A*24:02 were selected from the amino acid sequence of CKAP4, and the peptides were synthesized. Synthetic CTL epitope candidate peptides are shown below.
[0517] Synthetic HLA-A*24:02-binding CKAP4-specific CTL epitope candidate peptide
[0518] Arg Leu Gly Arg Ala Leu Asn Phe Leu Phe (Serial Num...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com