Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
468 results about "NAFLD - Nonalcoholic Fatty Liver Disease" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
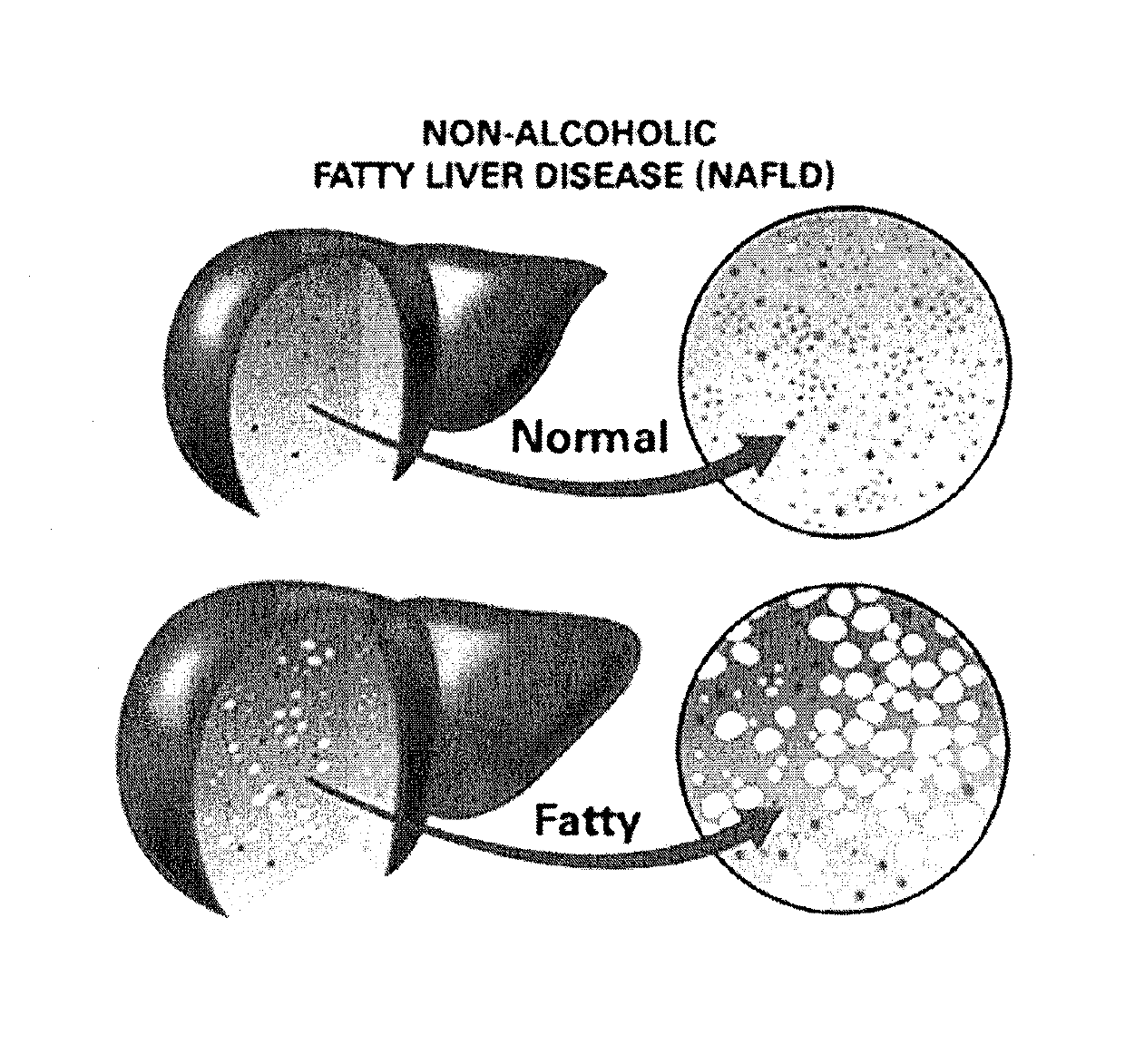
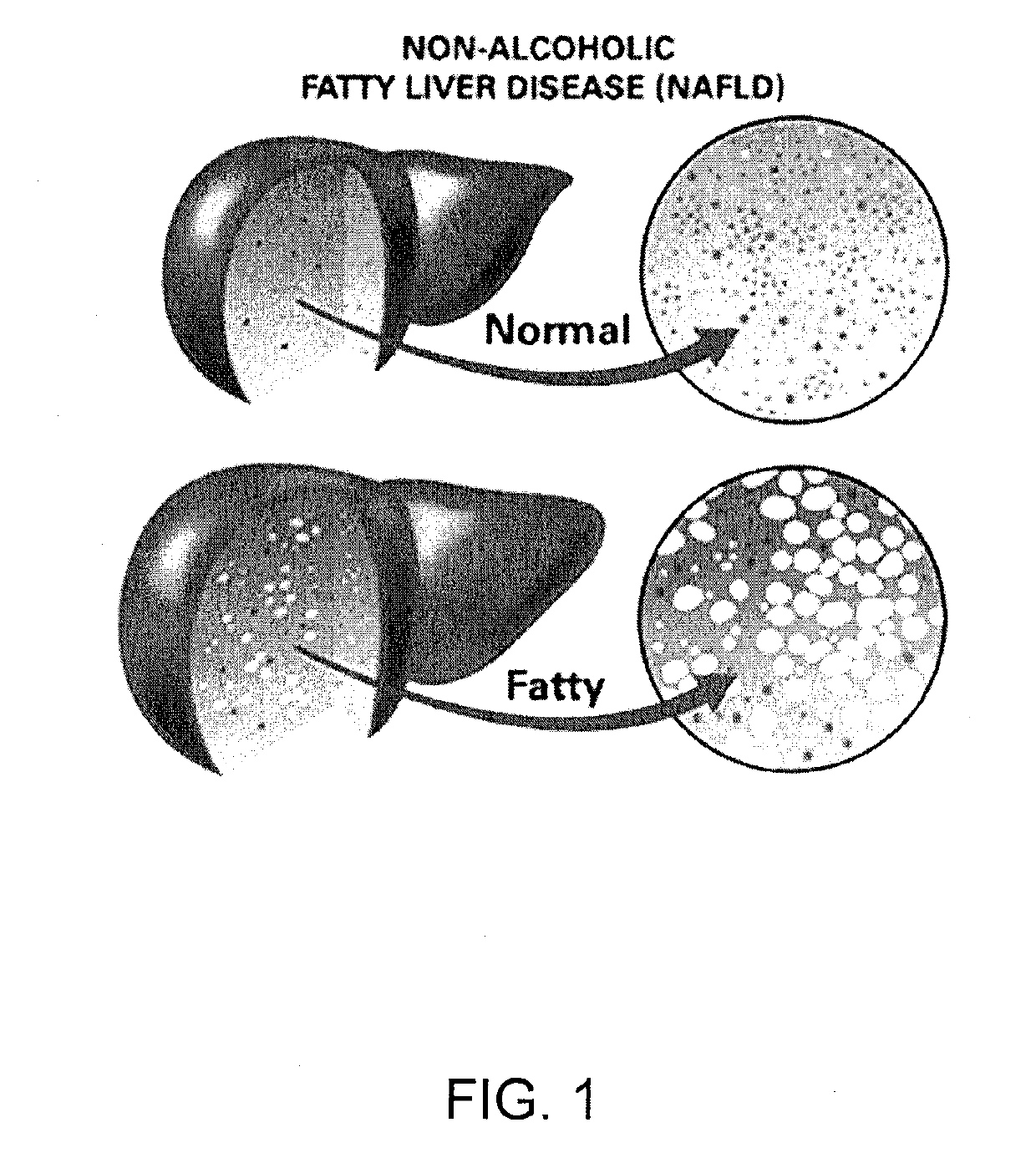
Non-alcoholic fatty liver disease (NAFLD) is one of the types of fatty liver which occurs when fat is deposited (steatosis) in the liver due to causes other than excessive alcohol use. Non-alcoholic steatohepatitis (NASH) is the most extreme form of NAFLD.
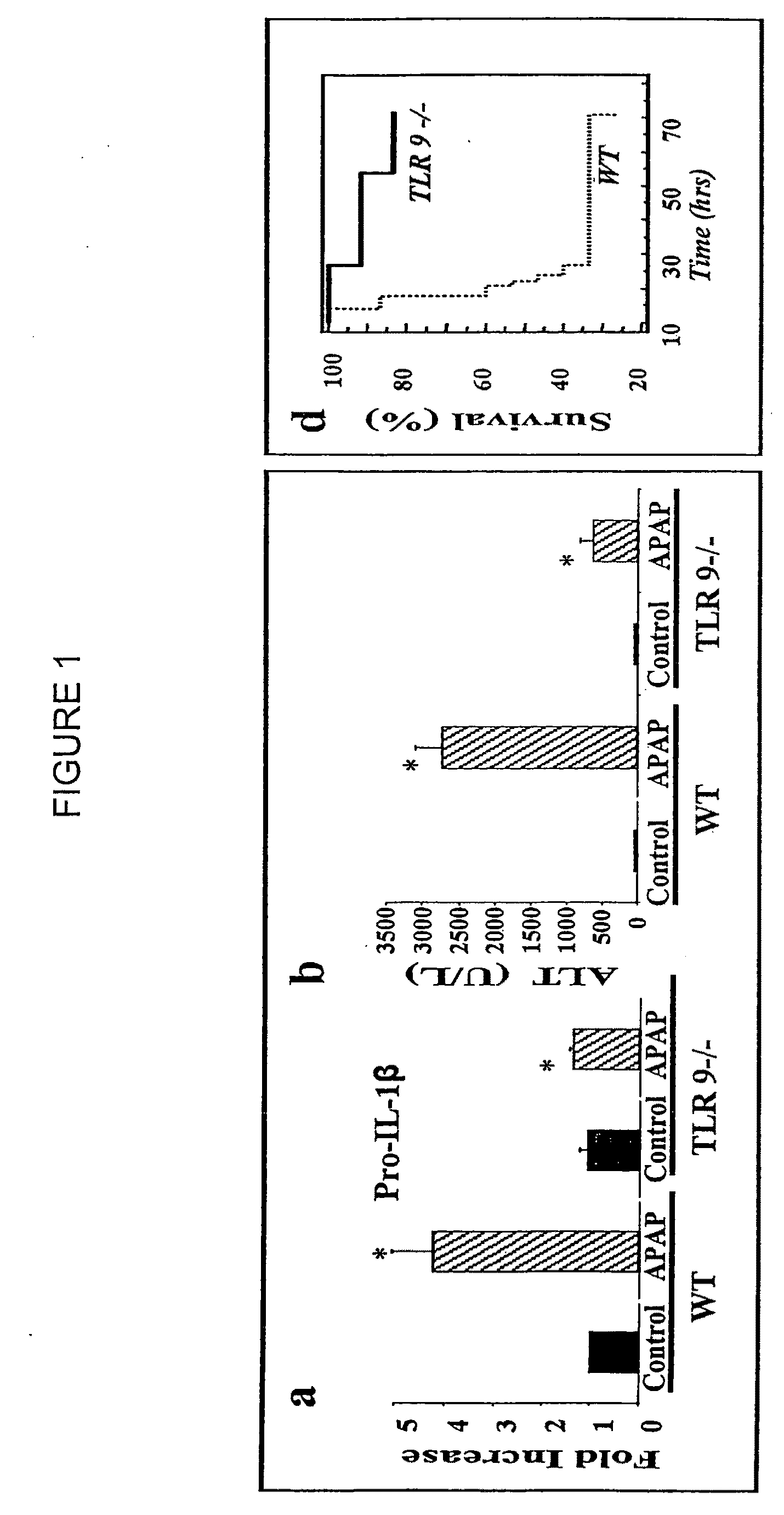
Method of reducing drug-induced adverse side effects in a patient
InactiveUS20060252670A1Good treatment effectReduce adverse side effectsBiocideSenses disorderSide effectPeroxisome Proliferation
The invention relates to the discovery that farnesoid X receptor (FXR) agonists can be used in combination with peroxisome proliferation activated receptor gamma (PPARγ) agonists to reduce drug-induced adverse side effects in patients suffering from conditions such as insulin resistance, Type II diabetes, metabolic syndrome, non-alcoholic fatty liver disease (NAFLD), non-alcoholic steatohepatitis (NASH), and heart disease. Particularly, the present invention encompasses methods for treating patients suffering from drug-induced adverse side effects with selective PPARγ, dual PPARα / γ and pan PPARα / γ / δ agonists in combination with FXR agonists.
Owner:INTERCEPT PHARMA INC
Nuclear sulfated oxysterol, potent regulator of lipid homeostasis, for therapy of hypercholesterolemia, hypertriglycerides, fatty liver diseases, and atherosclerosis
ActiveUS8399441B2Lower Level RequirementsImprove suppression propertiesOrganic active ingredientsMetabolism disorderLipid formationMetabolite
The sulfated oxysterol 5-cholesten-3β, 25-diol 3-sulphate, a nuclear cholesterol metabolite that decreases lipid biosynthesis and increases cholesterol secretion and degradation, is provided as an agent to lower intracellular and serum cholesterol and / or triglycerides, and to prevent or treat lipid accumulation-associated inflammation and conditions associated with such inflammation. Methods which involve the use of this sulfated oxysterol to treat conditions associated with high cholesterol and / or high triglycerides and / or inflammation (e.g. hypercholesterolemia, hypertriglyceridemia, non-alcoholic fatty liver diseases, atherosclerosis, etc.) are also provided.
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS +1
Application of GLP-1R/GCGR double-target agonist polypeptide to treatment of fatty liver diseases, hyperlipidemia and arteriosclerosis
ActiveCN106046145AImprove biological activityExtended half-lifePeptide/protein ingredientsMetabolism disorderFibrosisDrug biological activity
The invention relates to application of a polypeptide compound having double agonist effects on a glucagon-like peptide-1 receptor and a glucagon receptor. The polypeptide compound has the characteristics of high enzymolysis stability, high bioactivity, no adverse reaction, etc., can alleviate abnormal rise in the levels of total cholesterol and triglyceride in blood induced by diabetes and high-meal diet, lowers the level of liver enzyme, improves liver damage and fibrosis stage and is applicable to prevention or treatment of diseases like non-alcoholic fatty liver diseases, hyperlipidemia and arteriosclerosis.
Owner:SHENZHEN TURIER BIOTECH CO LTD
Method and System for Treating Non-Alcoholic Fatty Liver Disease
ActiveUS20170106026A1Inhibit progressEarly detectionUnknown materialsObesity treatmentIntestinal microorganismsInsulin resistance
A method and system for treating non-alcoholic fatty liver disease (NAFLD) involves the modulation of the gut microbial of a person suffering from NAFLD, and in particular the provision of a probiotic therapy configured to reduce liver aminotransferases, total-cholesterol, TNF-α and to improve insulin resistance in NAFLD patients.
Owner:SEED HEALTH INC
Compositions and methods for treating nonalcoholic fatty liver disease-associated disorders
The invention relates to compositions containing cholesterol absorption inhibitors alone or in combination with other therapeutic agents for treating non-alcoholic fatty liver disease-associated disorders by administering a therapeutically effective amount of the compositions to a subject in need thereof.
Owner:IRONWOOD PHARMA
Method and system for reducing the likelihood of developing liver cancer in an individual diagnosed with non-alcoholic fatty liver disease
ActiveUS20190117709A1Inhibit progressEarly detectionOrganic active ingredientsDigestive systemFiberMonoacylglycerol acyltransferase
A method for reducing the likelihood of developing non-alcoholic steatohepatitis (NASH) in an individual diagnosed with non-alcoholic fatty liver disease involves providing in the gut of an individual a population of beneficial bacteria selected from the group consisting of Lactobacillus species, and at least 6 grams per day of fiber to the individual to maintain a therapeutically effective amount of the beneficial bacteria in the gut of the individual. In certain embodiments, monoacylglycerolacyltransferase-3 (MGAT3) synthesis is inhibited to lower triacylglycerol (TAG) production, while in others, expression of diacylglycerolacyltransferase-2 (DGAT-2) is inhibited. The beneficial bacteria are preferably modified to produce increased amounts of butyrate and are also encapsulated in a frangible enclosure. Levels of Roseburia are preferably increased while the levels of Akkermansia spp. in the individual's gut microbiome are reduced.
Owner:SEED HEALTH INC
Pharmaceutical compositions for the treatment/prophylaxis of non-alcoholic fatty liver disease
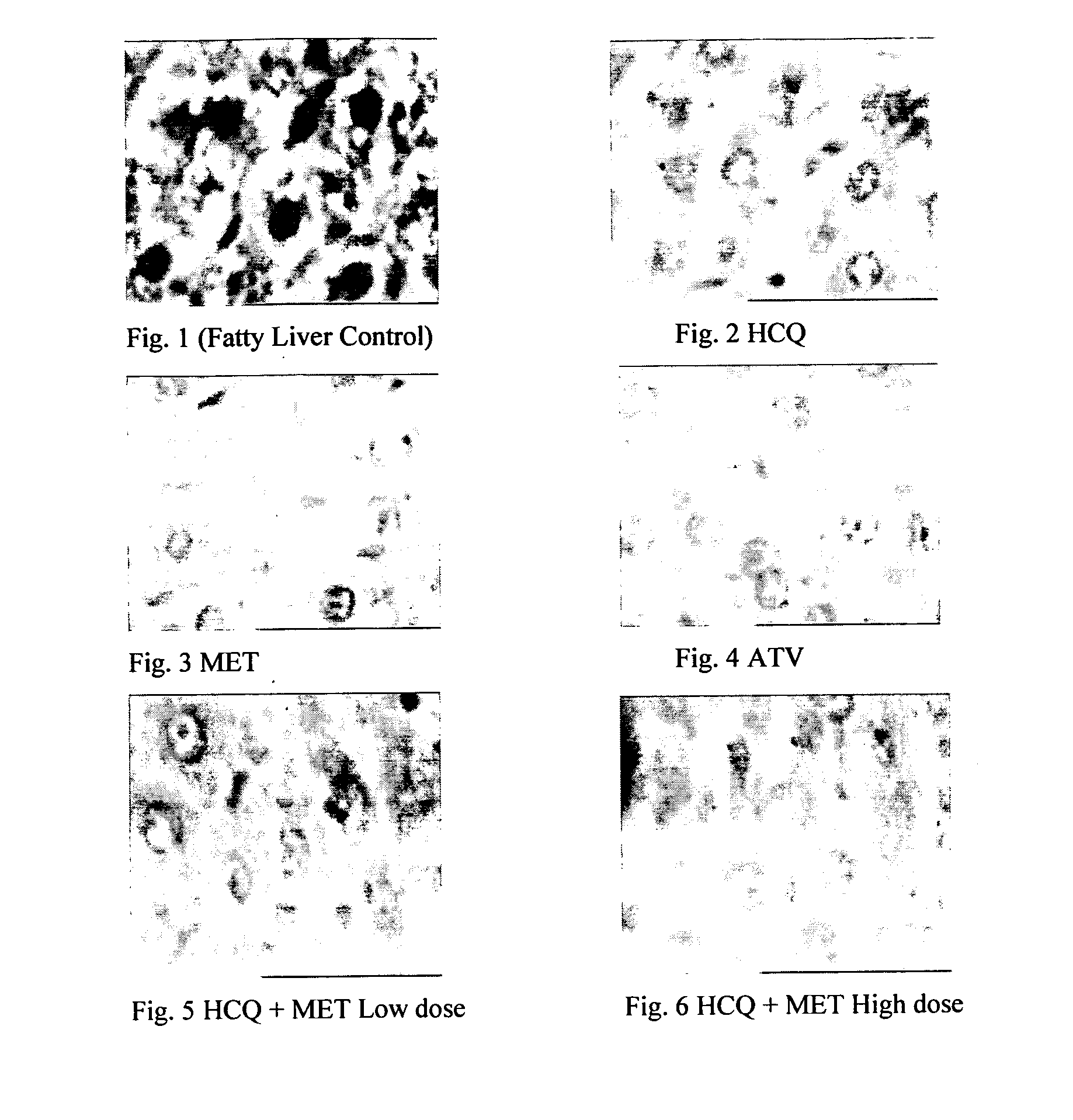
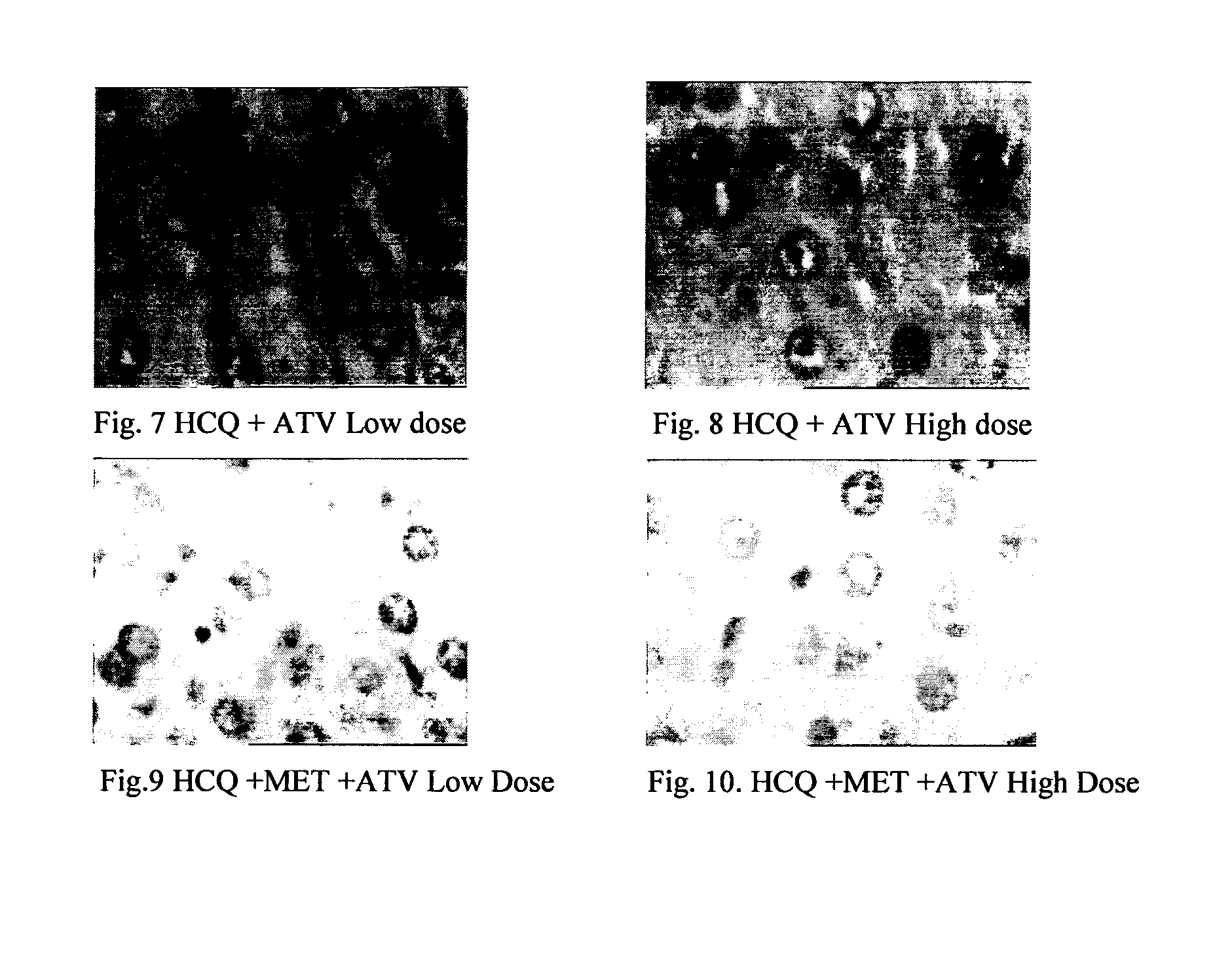
InactiveUS20120202849A1Lowering/preventing accumulation of fatBiocideOrganic chemistryDiseaseHydroxychloroquine
Disclosed herein is a novel synergistic pharmaceutical composition comprising hydroxychloroquine with insulin sensitizing agents and lipid lowering agents such as statins along with pharmaceutical excipients / carriers useful in treating Non-Alcoholic Fatty Liver Disease.
Owner:IPCA LAB LTD
Treatment for non-alcoholic-steatohepatitis
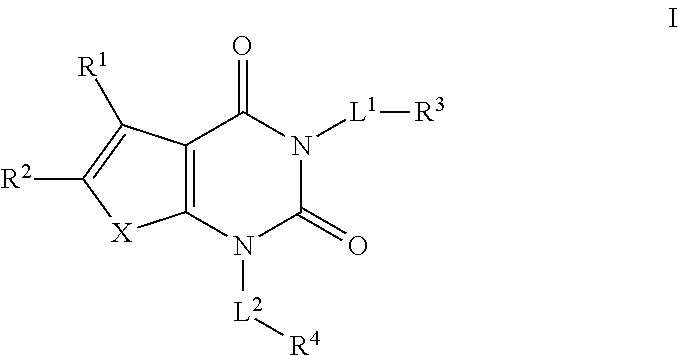
The present invention provides methods of treating a subject with non-alcoholic fatty liver disease (NAFLD), insulin resistance, obesity or hyperlipidemia, comprising administering to the subject an effective amount of a compound according to Formula I:or a physiologically acceptable salt thereof.
Owner:ARES TRADING SA
Acc inhibitor combination therapy for the treatment of non-alcoholic fatty liver disease
The present invention provides methods of treating, stabilizing or lessening the severity or progression of a non-alcoholic fatty liver disease using an ACC inhibitor alone or with one or more additional therapeutic agents.
Owner:GILEAD APOLLO LLC +1
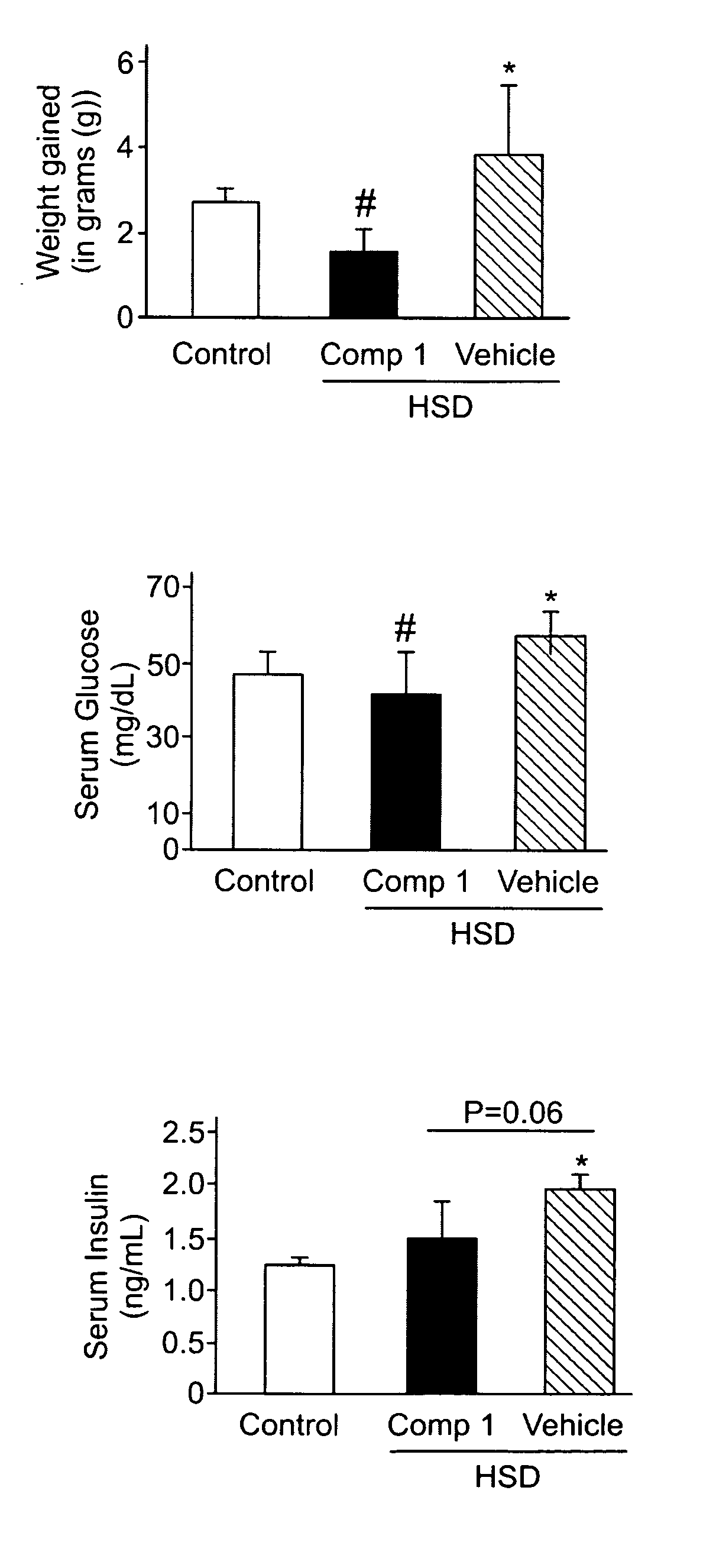
Pharmaceutical composition for the prevention or the treatment of non-alcoholic fatty liver disease and the method for prevention or treatment of non-alcoholic fatty liver disease using the same
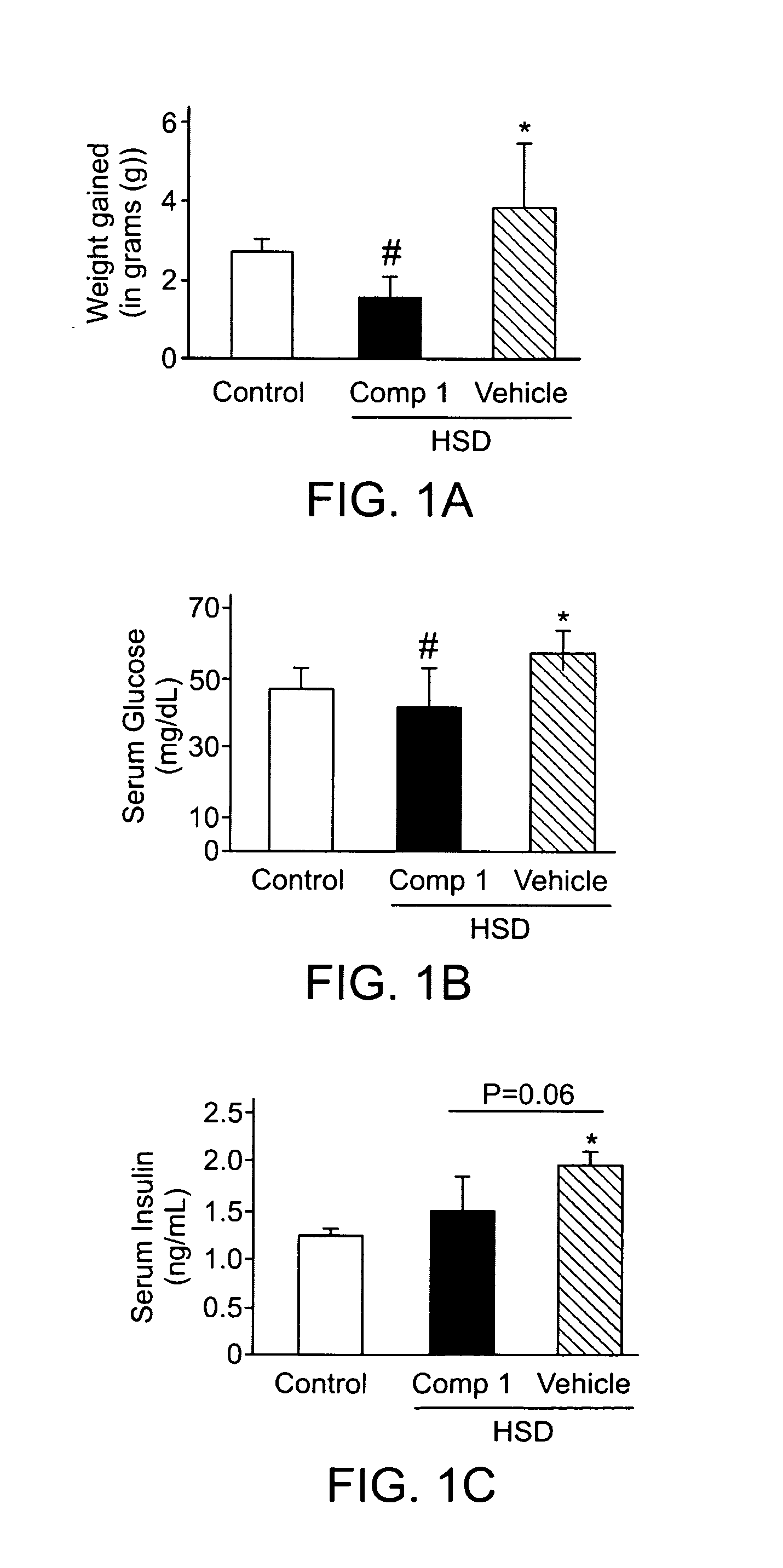
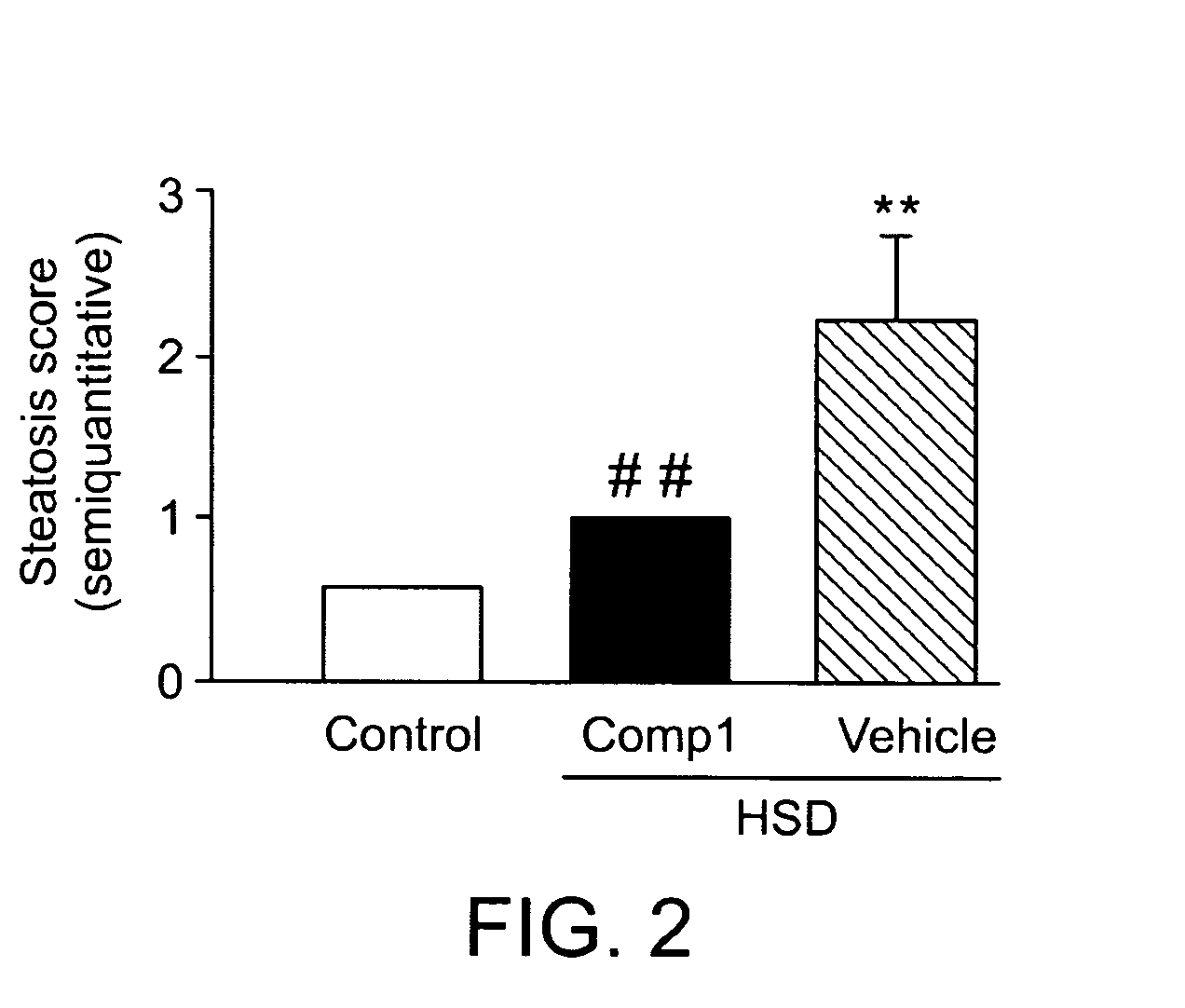
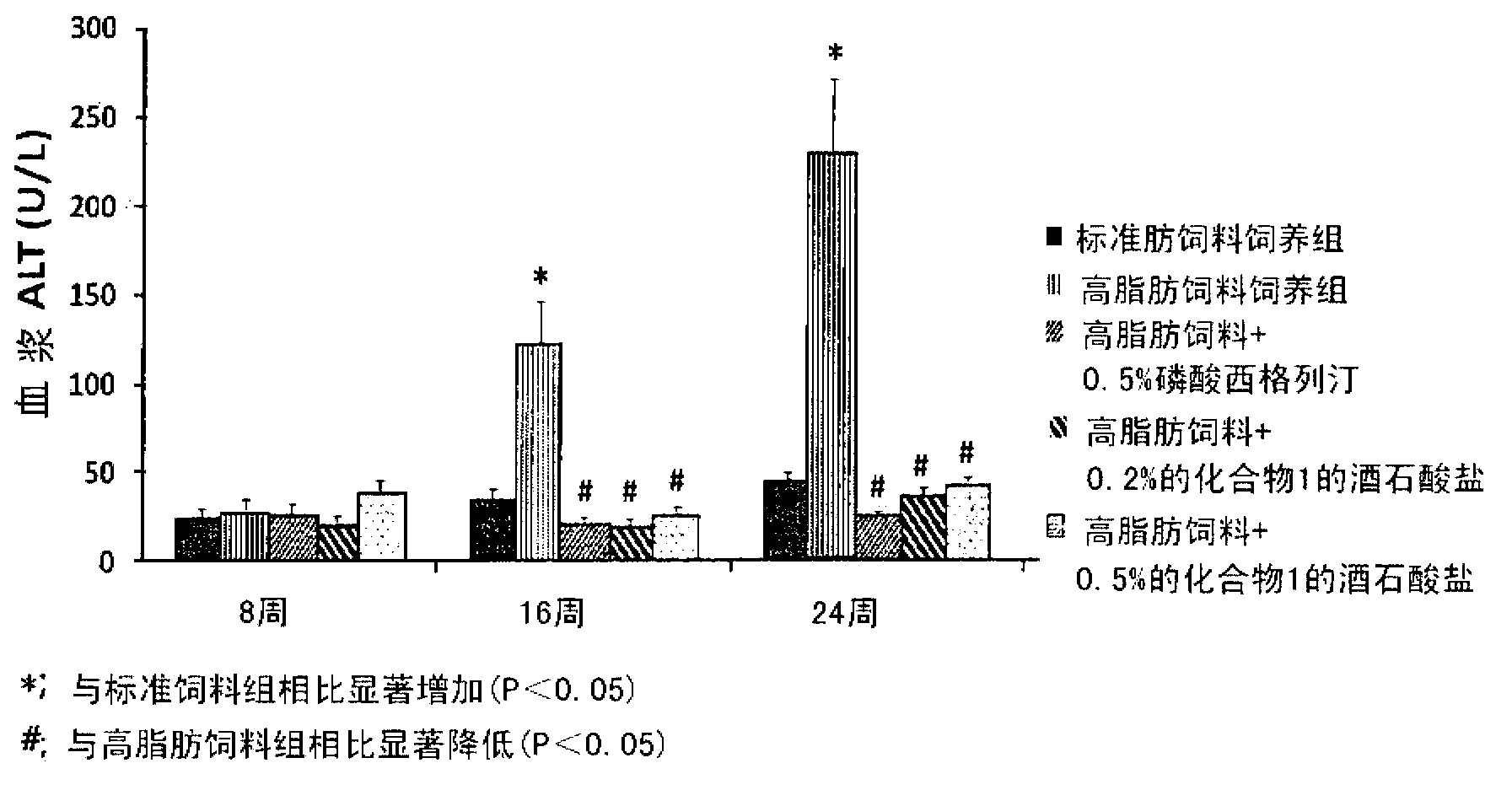
InactiveCN102883721AInhibit progressInhibitory activityOrganic active ingredientsMetabolism disorderSitagliptinBULK ACTIVE INGREDIENT
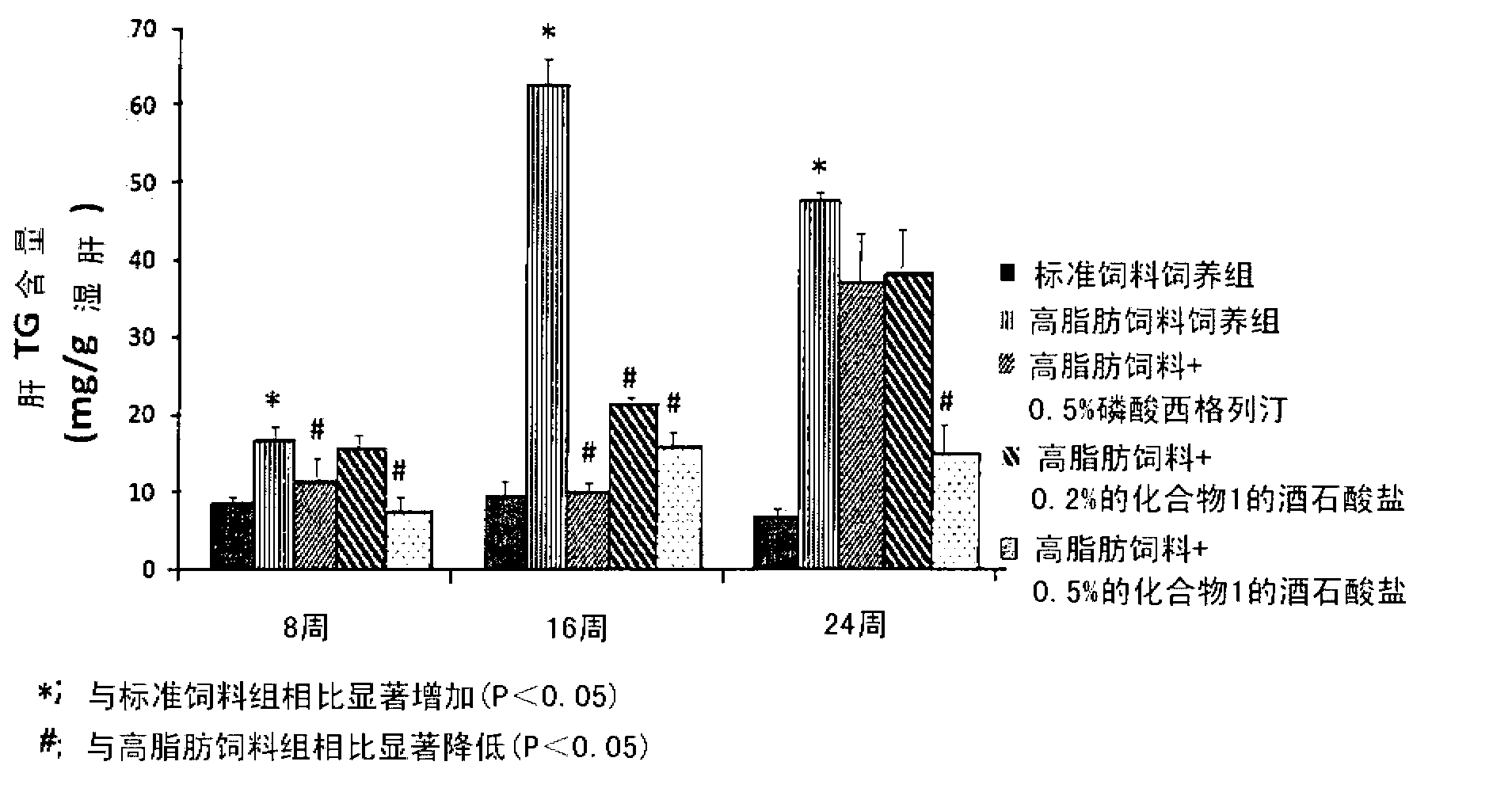
The present invention provides a pharmaceutical composition for the prevention and treatment of a non-alcoholic fatty liver disease (NAFLD), containing an active ingredient selected from the group consisting of Compound 1 represented by formula 1, sitagliptin, vildagliptin, linagliptin or a pharmaceutically acceptable salt thereof. Further, the present invention provides a method for the prevention or treatment of a non-alcoholic fatty liver disease, including administering an effective amount of an active ingredient selected from the group consisting of Compound 1 represented by formula 1, sitagliptin, vildagliptin, linagliptin or a pharmaceutically acceptable salt thereof to a mammal including a human in need thereof.; Further, the present invention provides use of Compound 1 represented by formula 1, sitagliptin, vildagliptin, linagliptin or a pharmaceutically acceptable salt thereof, for manufacturing a pharmaceutical composition for the prevention or treatment of a non-alcoholic fatty liver disease.
Owner:DONG A PHARMA
Diagnostics and methods for treatment of non-alcoholic hepatic steatosis and hepatic steatohepatitis, and prevention of complications thereof
ActiveUS20190255084A1Increased riskPrecise definitionPeptide/protein ingredientsDigestive systemHepatitis c viralLiver steatosis
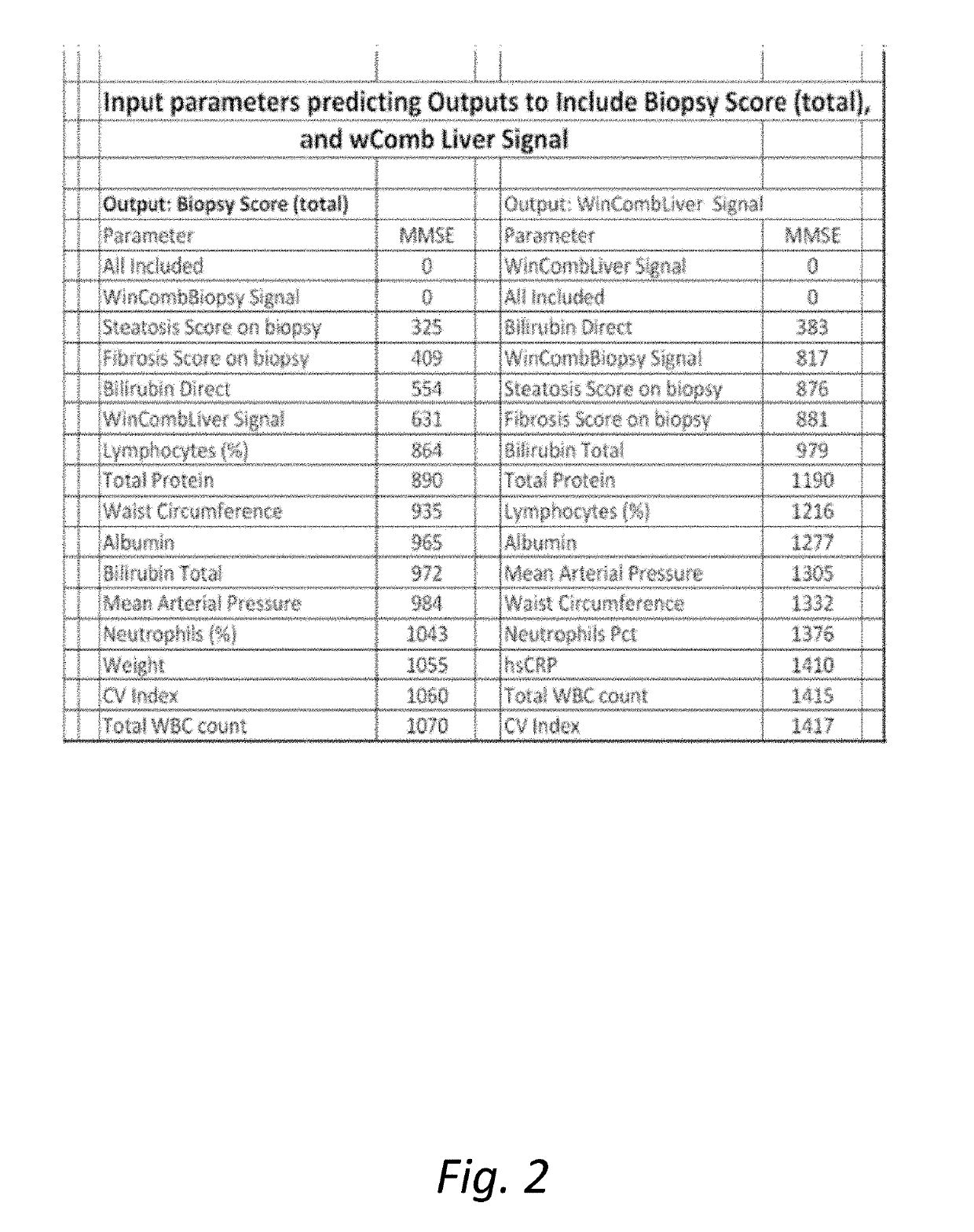
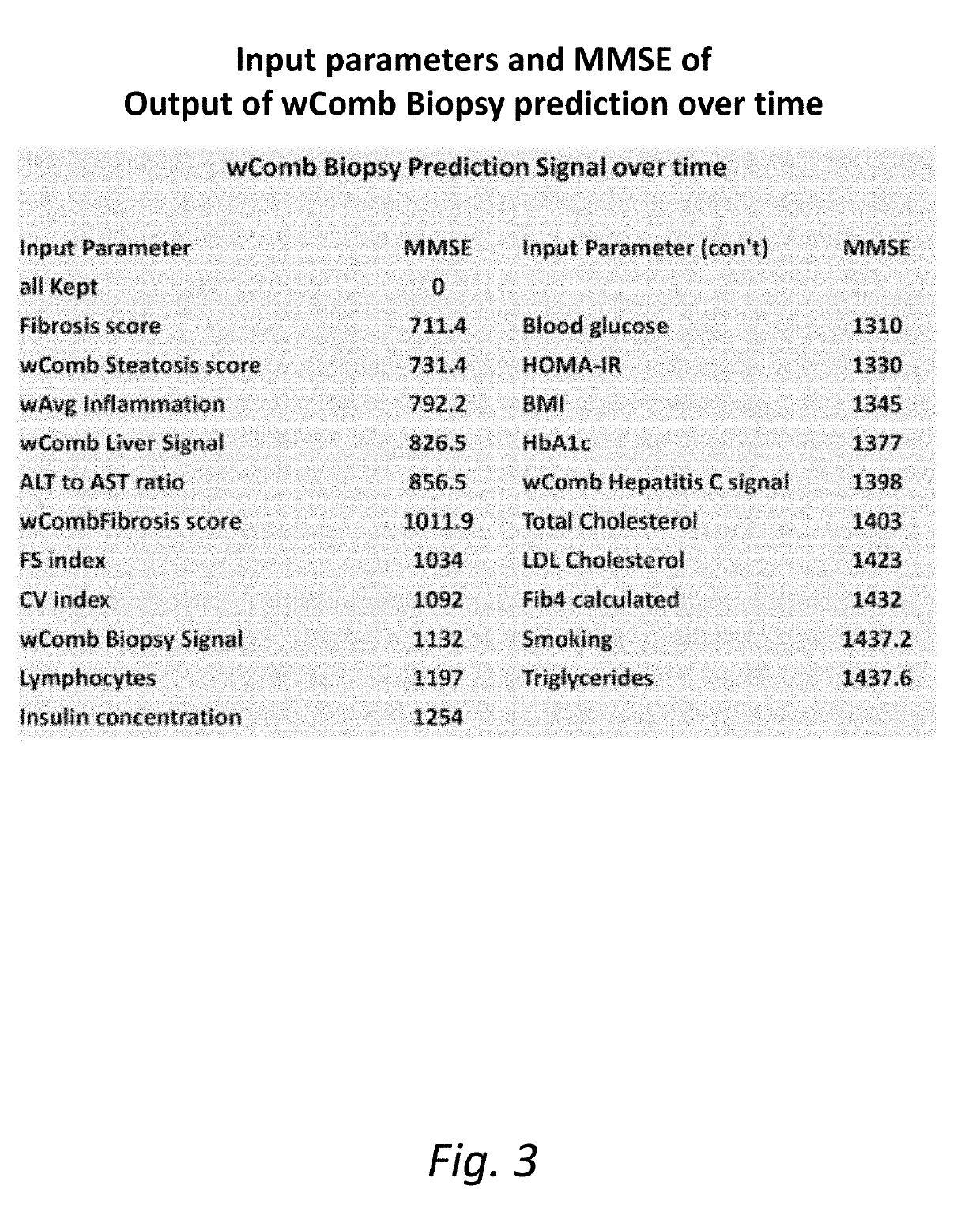
The present invention is directed to a System characterization of NASH that combines Modeling and Biomarkers, enabling pharmaceutical compositions and methods of treatment that relate to the inhibition, resolution and / or prevention of Non Alcoholic Fatty Liver Disease (NAFLD) and Non Alcoholic Steatohepatitis (NASH). Said conditions are Liver related complications among the array of manifestations of metabolic syndromes, including Type 2 diabetes, hyperlipidemia, weight gain, abdominal obesity, insulin resistance, hypertension, atherosclerosis, fatty liver diseases and certain chronic inflammatory states that lead to these manifestations, among others. In additional aspects, the present invention relates to compositions and methods which may be used to treat, inhibit or reduce the likelihood of NASH and NAFLD complications in patients with hepatitis viral infections, including Hepatitis B and Hepatitis C viral infections, as well as the secondary disease states and / or conditions which are often associated with such viral infections, including hepatic steatosis (steatohepatitis), cirrhosis, fatty liver and hepatocellular cancer, metabolic syndrome complications including cardiovascular diseases, neurodegenerative diseases and premature ageing, among other disease states or conditions.
Owner:APHAIA PHARMA AG +1
Methods of treating diabetes and other blood sugar disorders
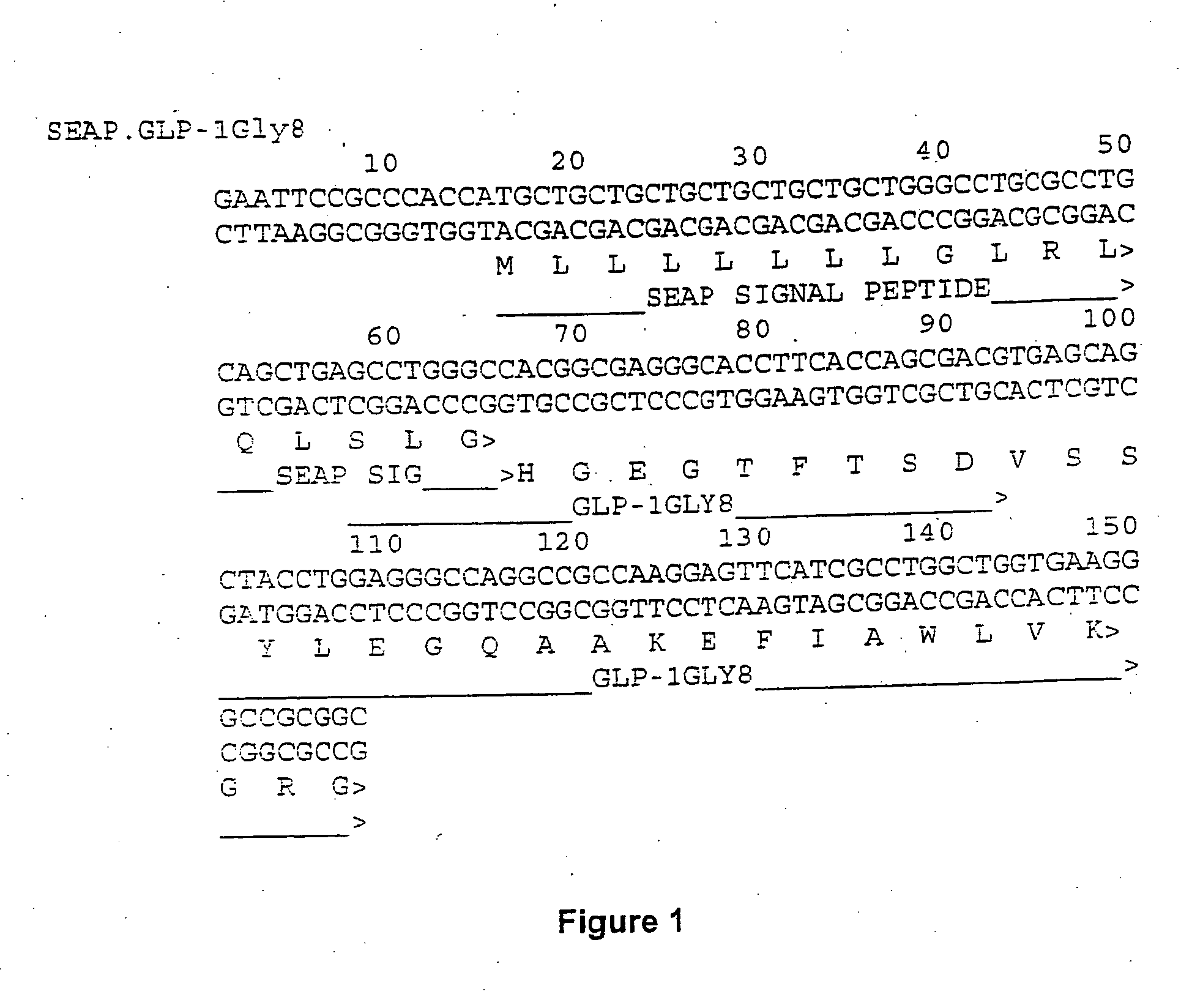
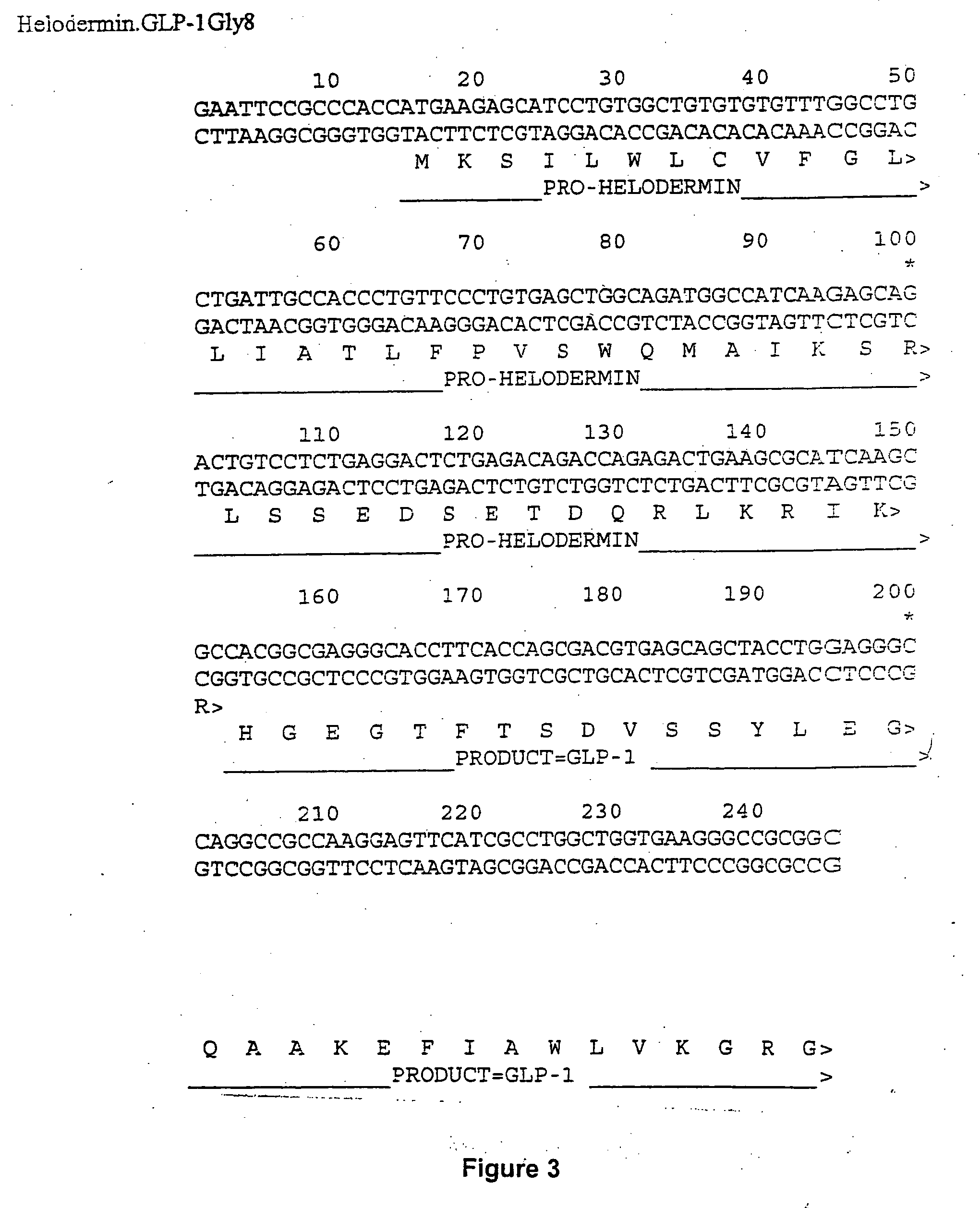
Evidence is emerging that lipid accumulation in the liver and the muscle contributes to insulin resistance in type II diabetes and the metabolic syndrome (1). This has prompted an investigation of the relationship between lipid accumulation in the liver, serum triglyceride levels, and glucose disposal. These studies demonstrate that liver fat positively correlated to fasting triglyceride levels and negatively correlated to glucose diposal (2). Therefore, strategies to prevent lipid accumulation in liver would have therapeutic value for treatment of type II diabetes, metabolic syndrome and non-alcoholic fatty liver disease. The invention described here relates to continuous administration of GLP-1 or its analogs obtained by either gene or cell therapy that results in reduction serum triglycerides and reduction of lipid accumulation in the liver for treatment of type II diabetes, the metabolic syndrome or non-alcoholic fatty liver disease.
Owner:GENZYME CORP
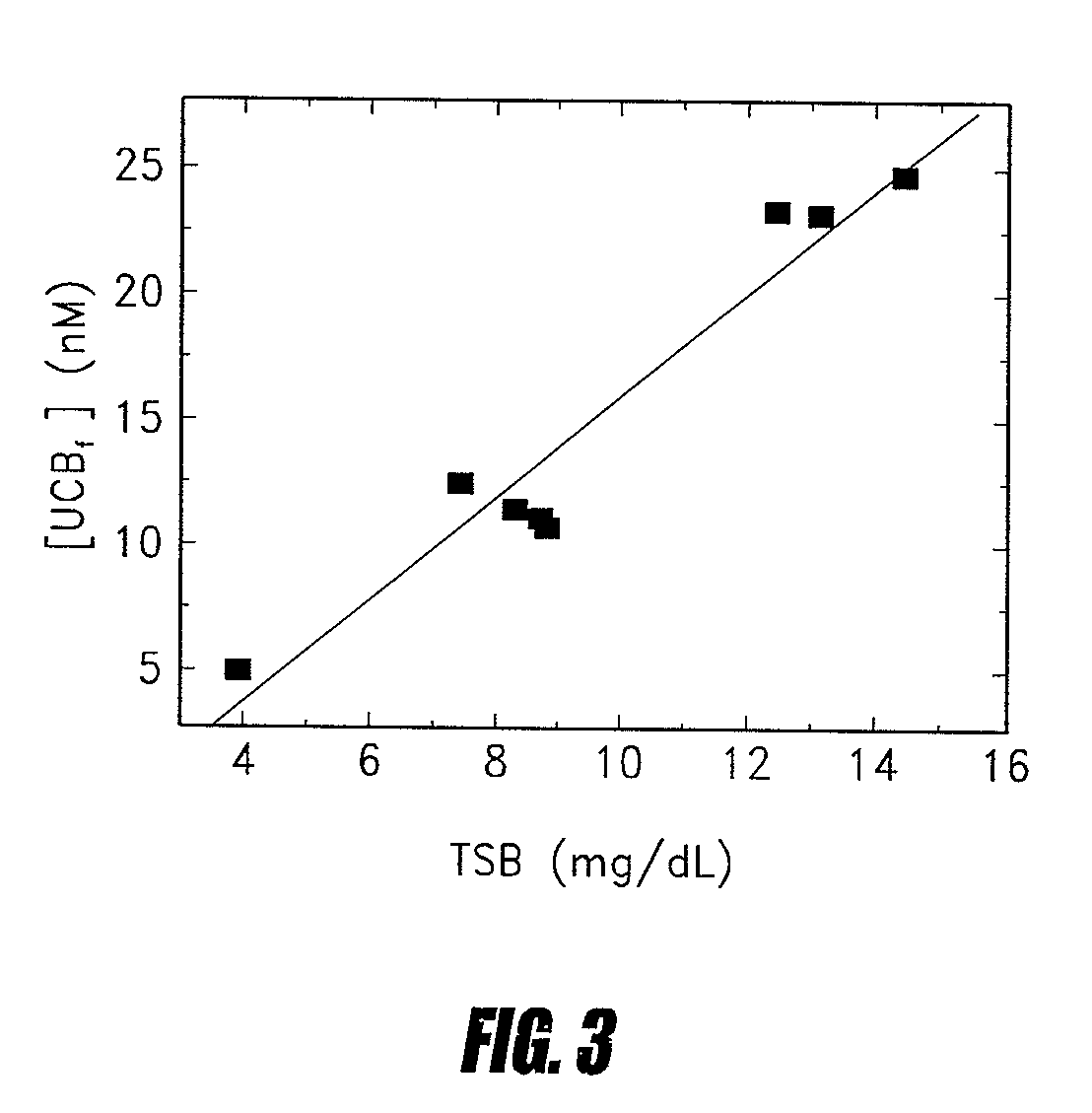
Use of probes for unbound metabolites
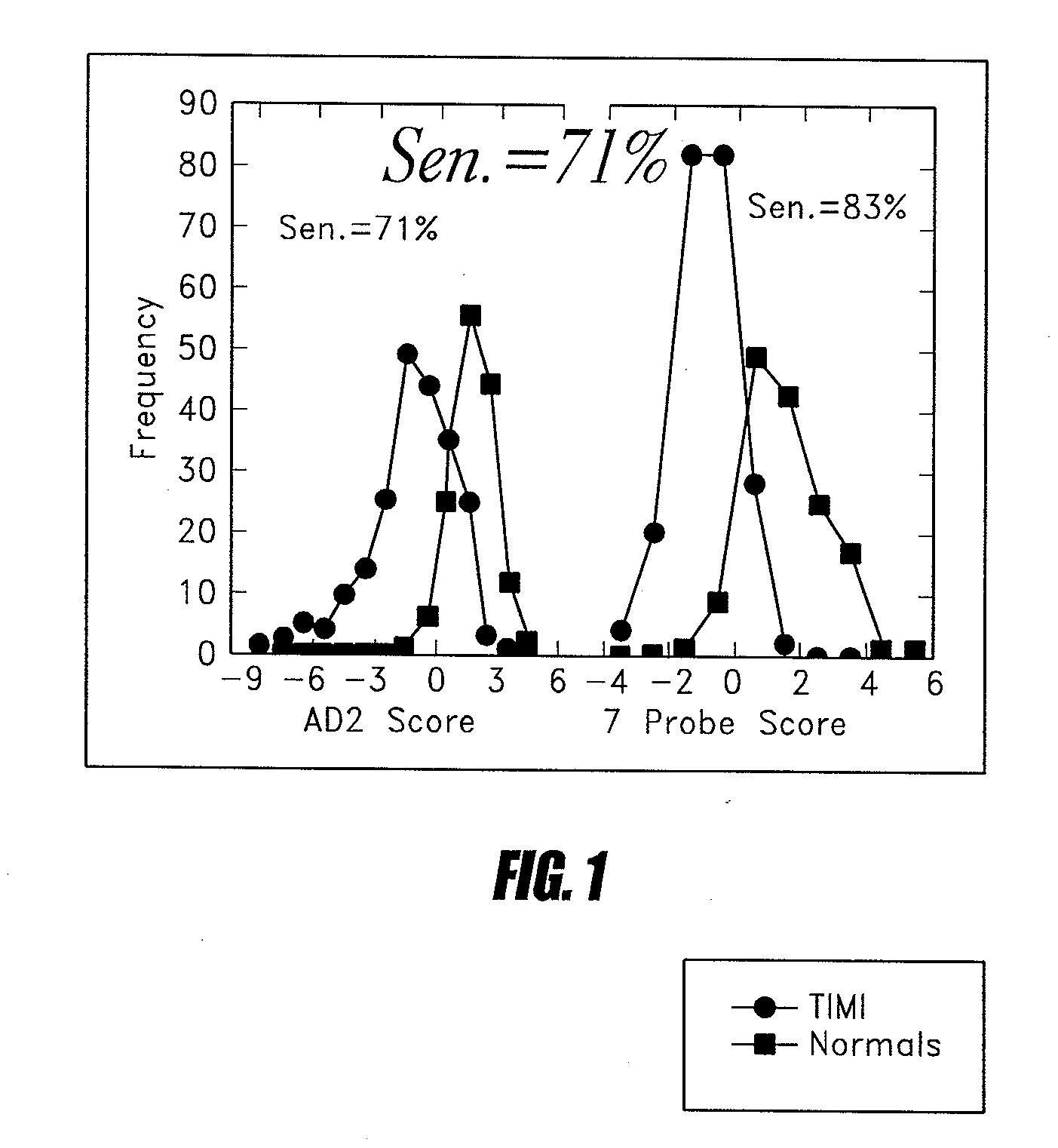
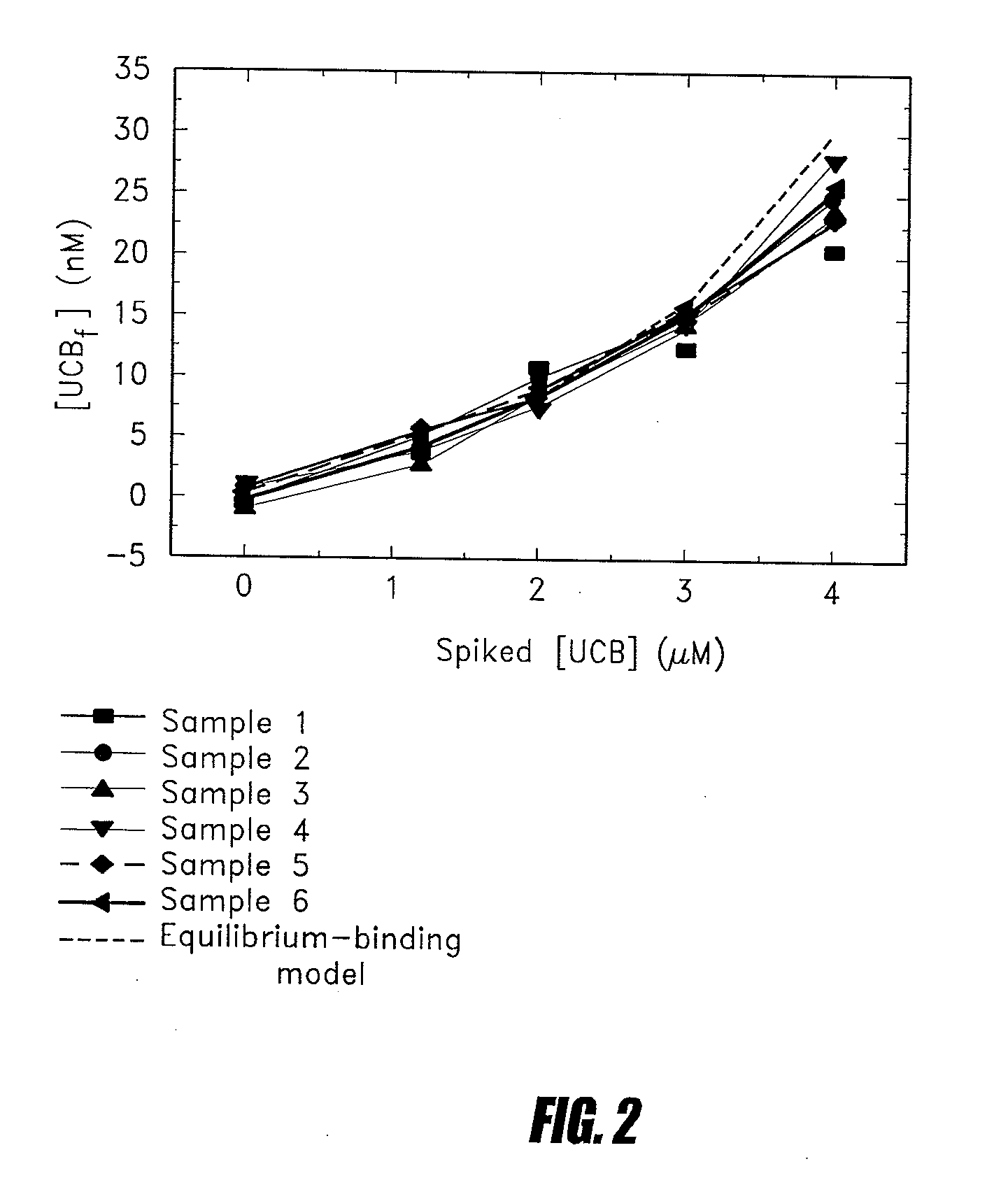
Methods of determining levels of unbound metabolites are disclosed. Probes derived from fatty acid binding protein muteins are described that bind preferentially to a number of unbound metabolites including oleate, stearate, linoleate, palmitate, arachidonate and unconjugated bilirubin. A profile for a patient is determined using one or more of the described probes. The profile is useful in diagnosis of disease, particularly myocardial infarction, non-alcoholic fatty liver disease (NAFLD), diabetes, stroke, sepsis and neonatal jaundice. The responses of multiple probes to a test sample are used to classify the degree of acute coronary syndrome by comparison to multi-probe profiles generated from unstable angina, non ST elevation myocardial infarction, and ST elevation myocardial infarction.
Owner:KLEINFELD ALAN
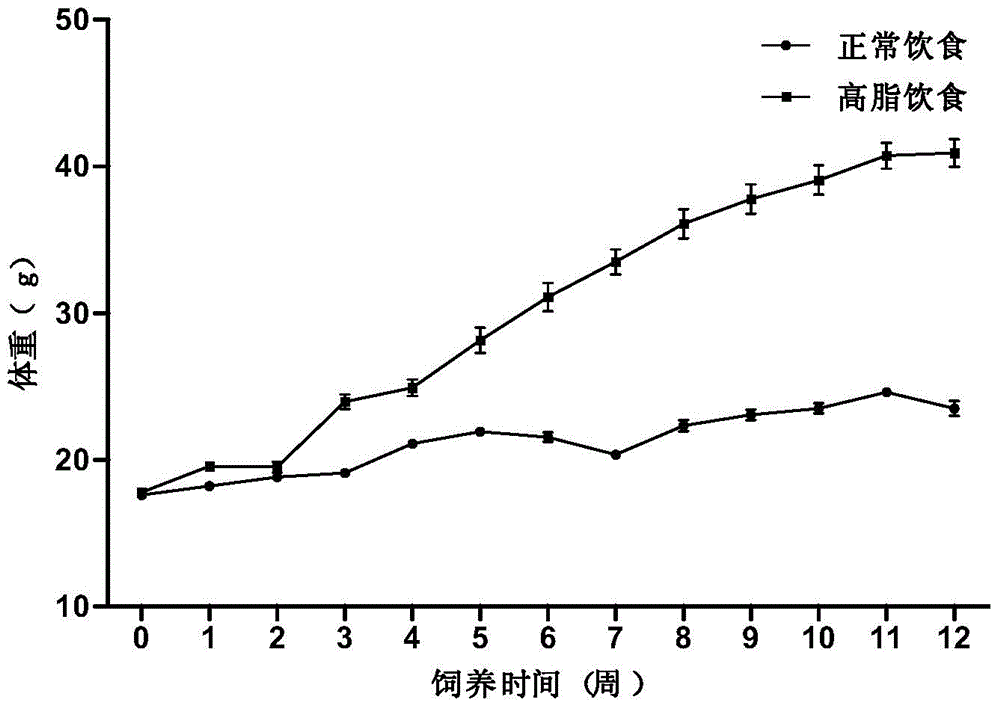
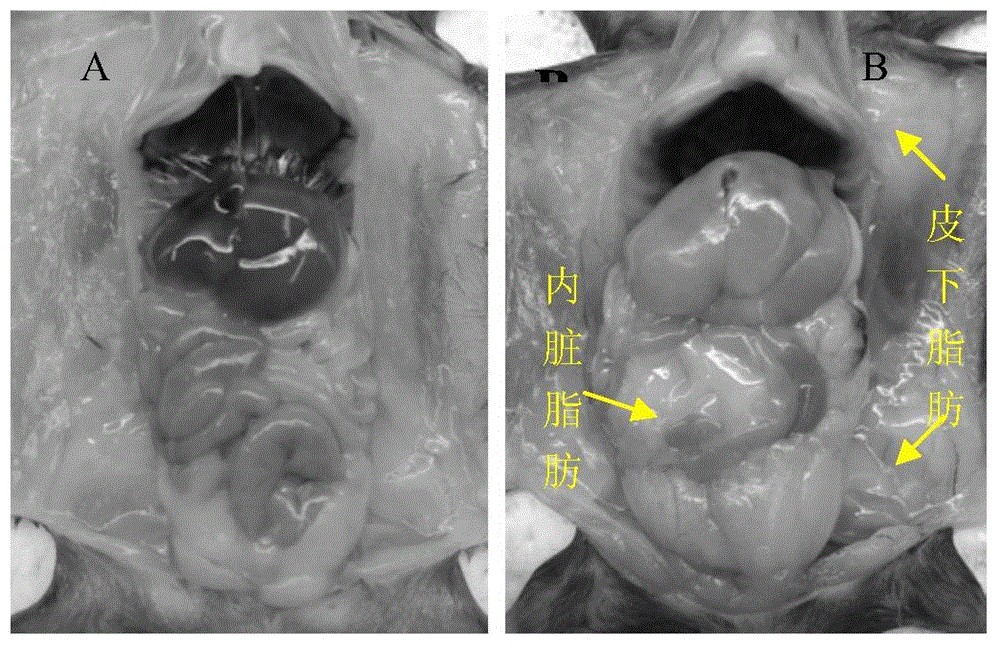
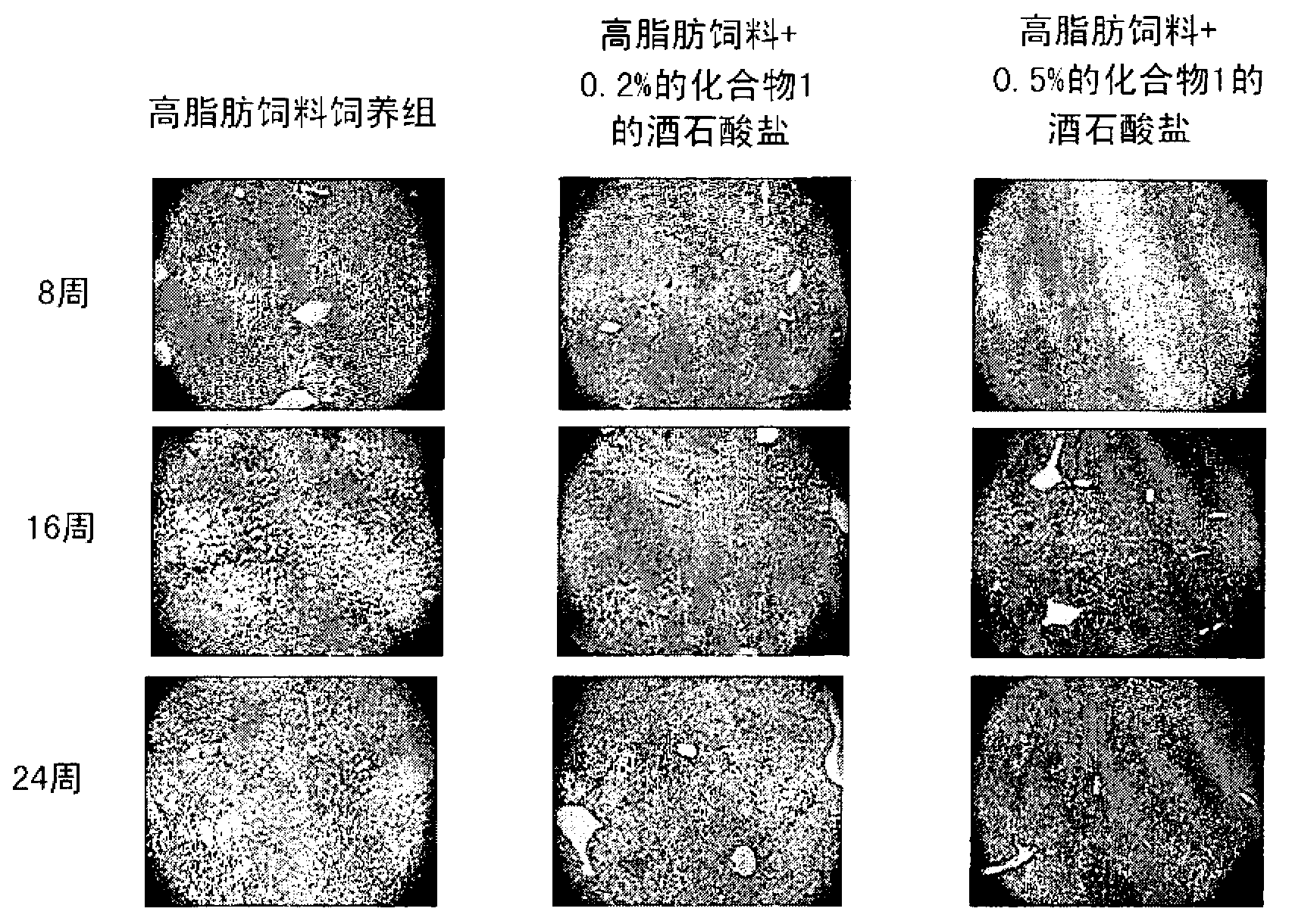
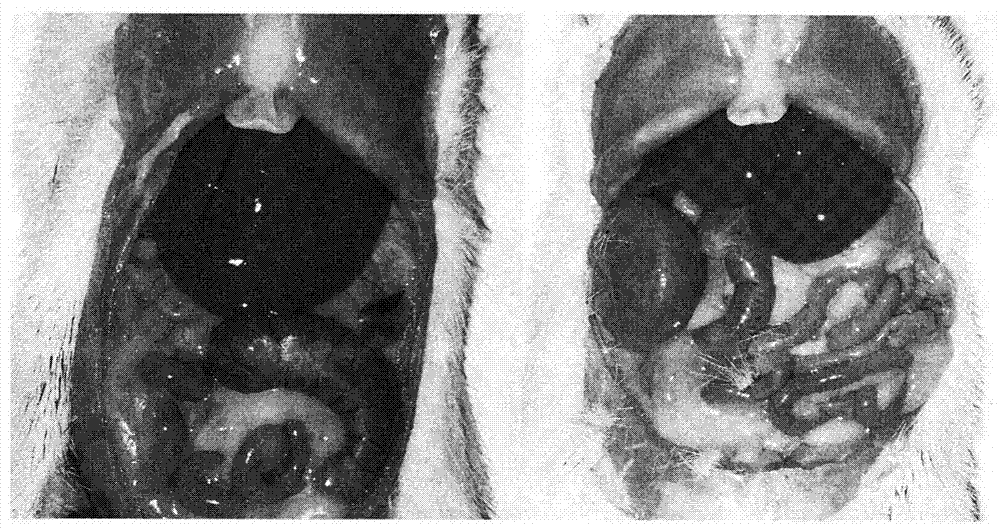
Application of composite high-fat forage to construct non-alcoholic fatty liver disease rat model
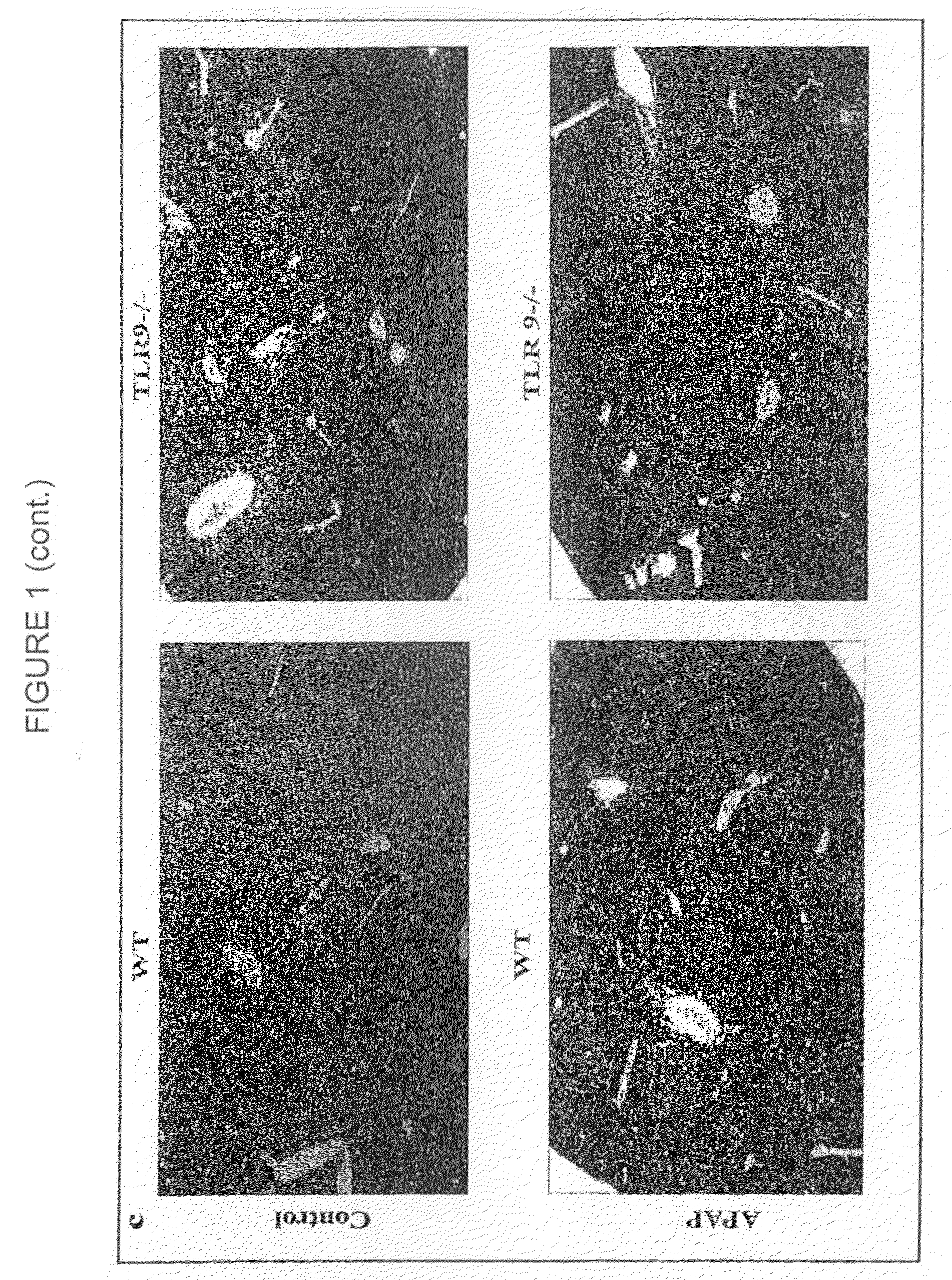
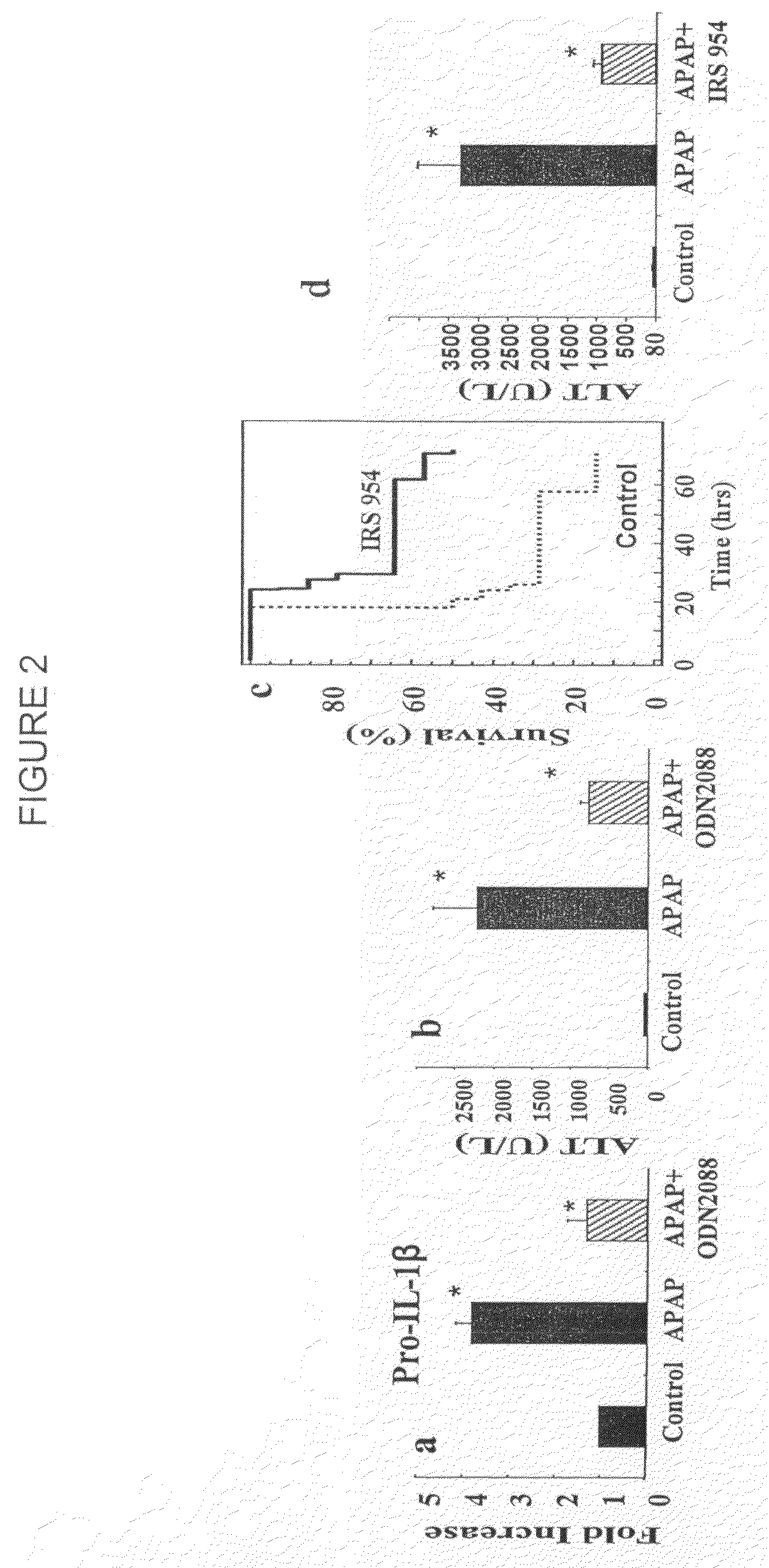
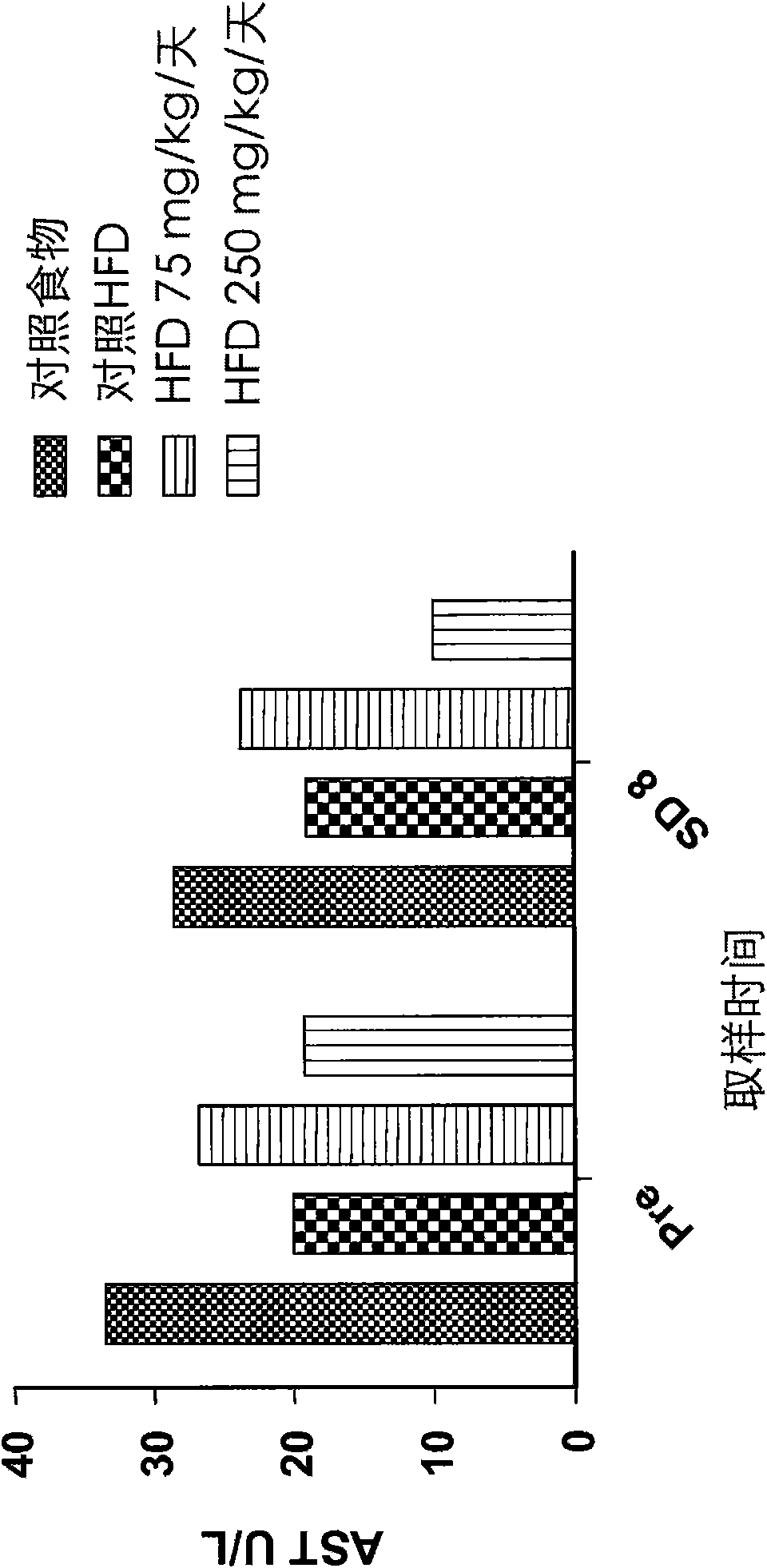
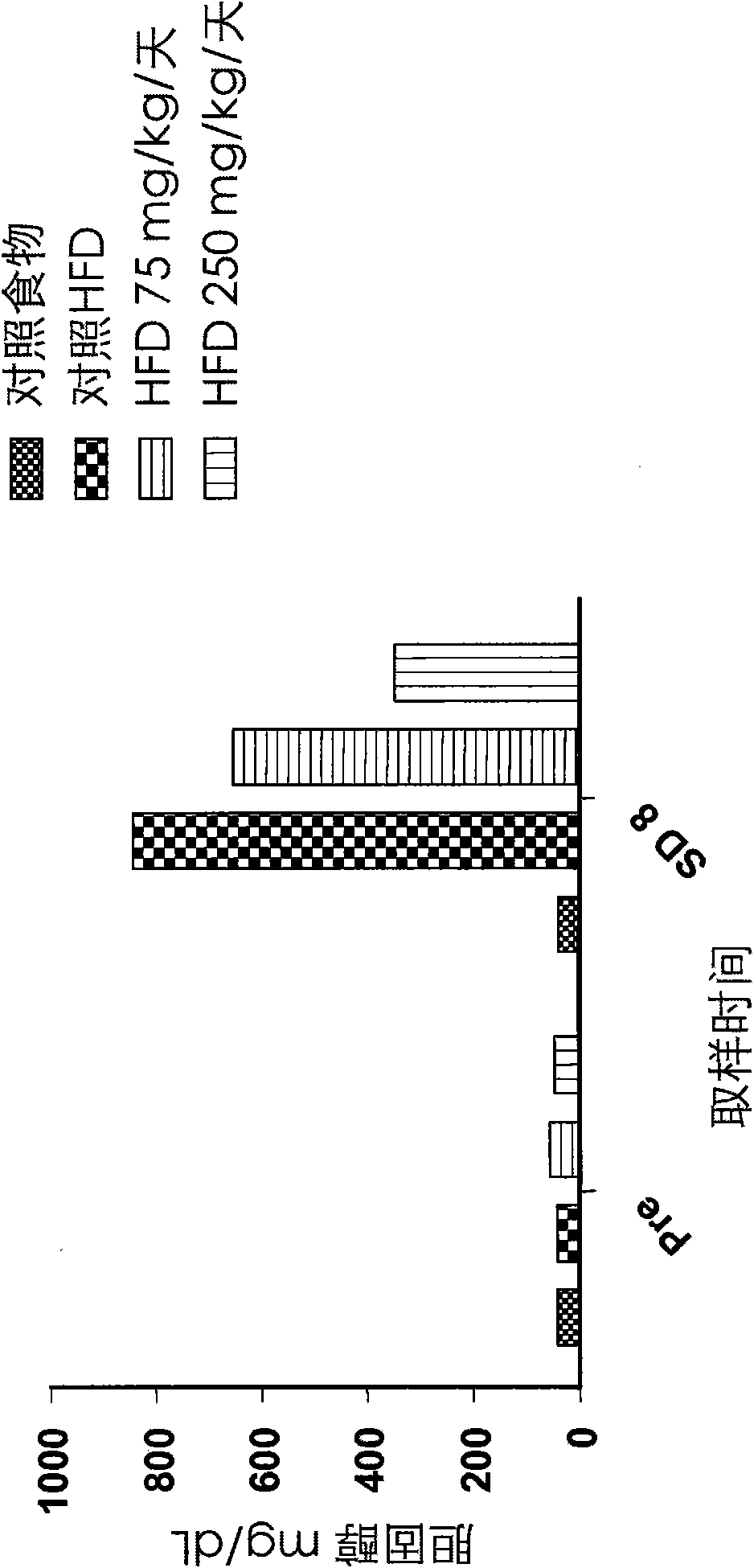
The invention belongs to the field of animal experiment models, and discloses application of a composite high-fat forage to construct a non-alcoholic fatty liver disease rat model. The high-fat forage is composed of the following raw materials in percent by mass: 77.5% of a rat basic forage, 10% of egg, 10% of coconut oil, 2% of cholesterol, 0.5% of bile salt, and 500mg / kg / d of sodium valproate calculated according to the rat weight. After 8 weeks, rats all have typical non-alcoholic fatty live symptoms, and liver has a lot of fat accumulation along with inflammatory cell infiltration. The model establishing time is short, the success rate is high, and the composite high-fat forage is applicable to pathogenesis research induced by high-fat-diet combined medicines with liver-toxicity side effect, screening of related control measures and efficacy evaluation of treatment medicines.
Owner:NANJING GENERAL HOSPITAL NANJING MILLITARY COMMAND P L A +1
Method of treating non-alcoholic fatty liver disease and steatohepatitis
A compound of Formula (I):or a metabolite thereof, or an ester of the compound of Formula (I) or the metabolite thereof, or a pharmaceutically acceptable salt of each thereof, wherein m, n, X1 and X2 are as defined herein, is useful for treating non-alcoholic fatty liver disease (NAFLD) or non-alcoholic steatohepatitis (NASH).
Owner:MEDICINOVA INC
Azabicycloalkane-indole and azabicycloalkane-pyrrolo-pyridine mch-1 antagonists, methods of making, and use thereof
Novel MCH-1 receptor antagonists are disclosed. These compounds are used in the treatment of various disorders, including obesity, anxiety, depression, non-alcoholic fatty liver disease, and psychiatric disorders. Methods of making these compounds are also described in the present invention.
Owner:ALBANY MOLECULAR RESEARCH INC
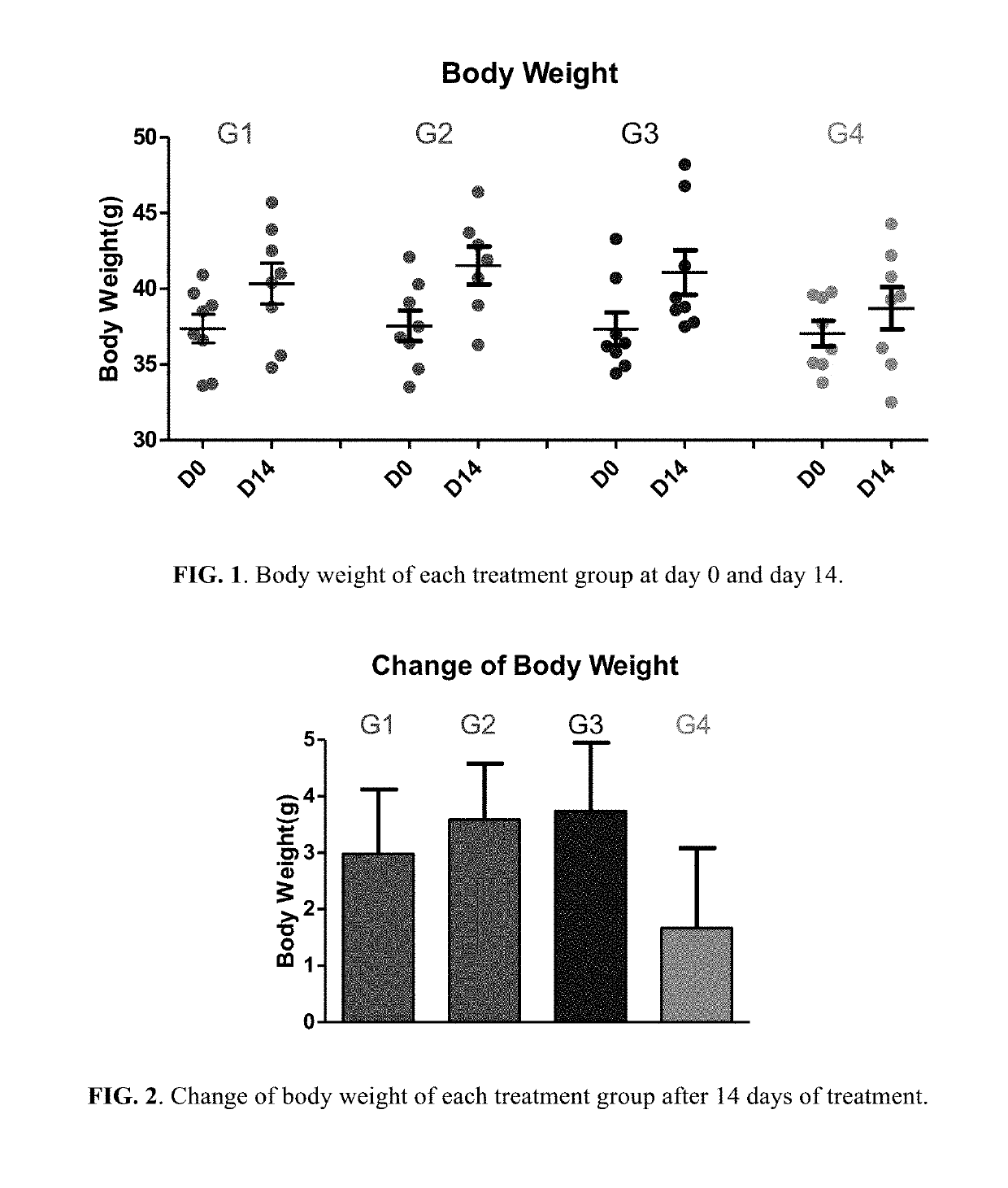
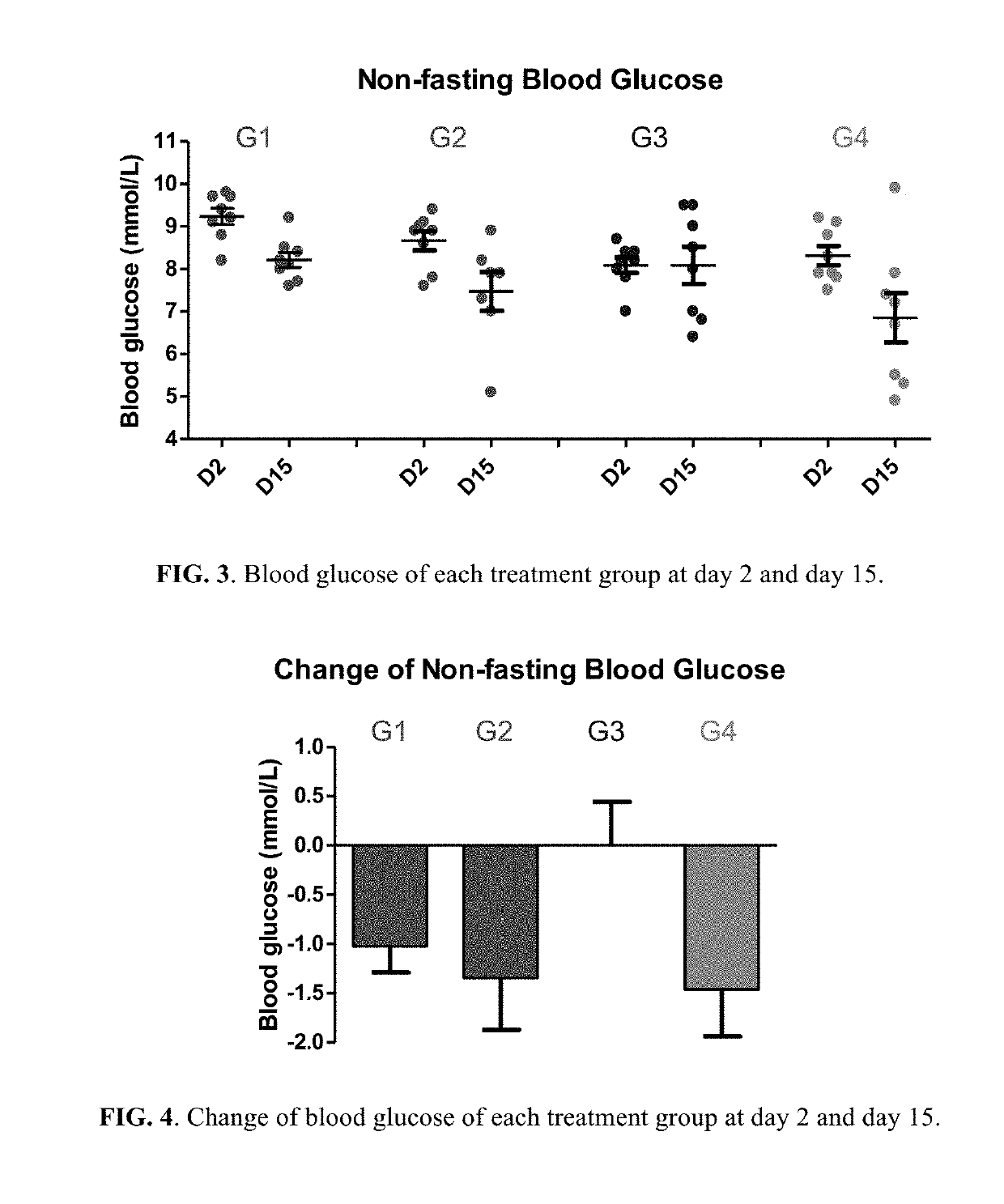
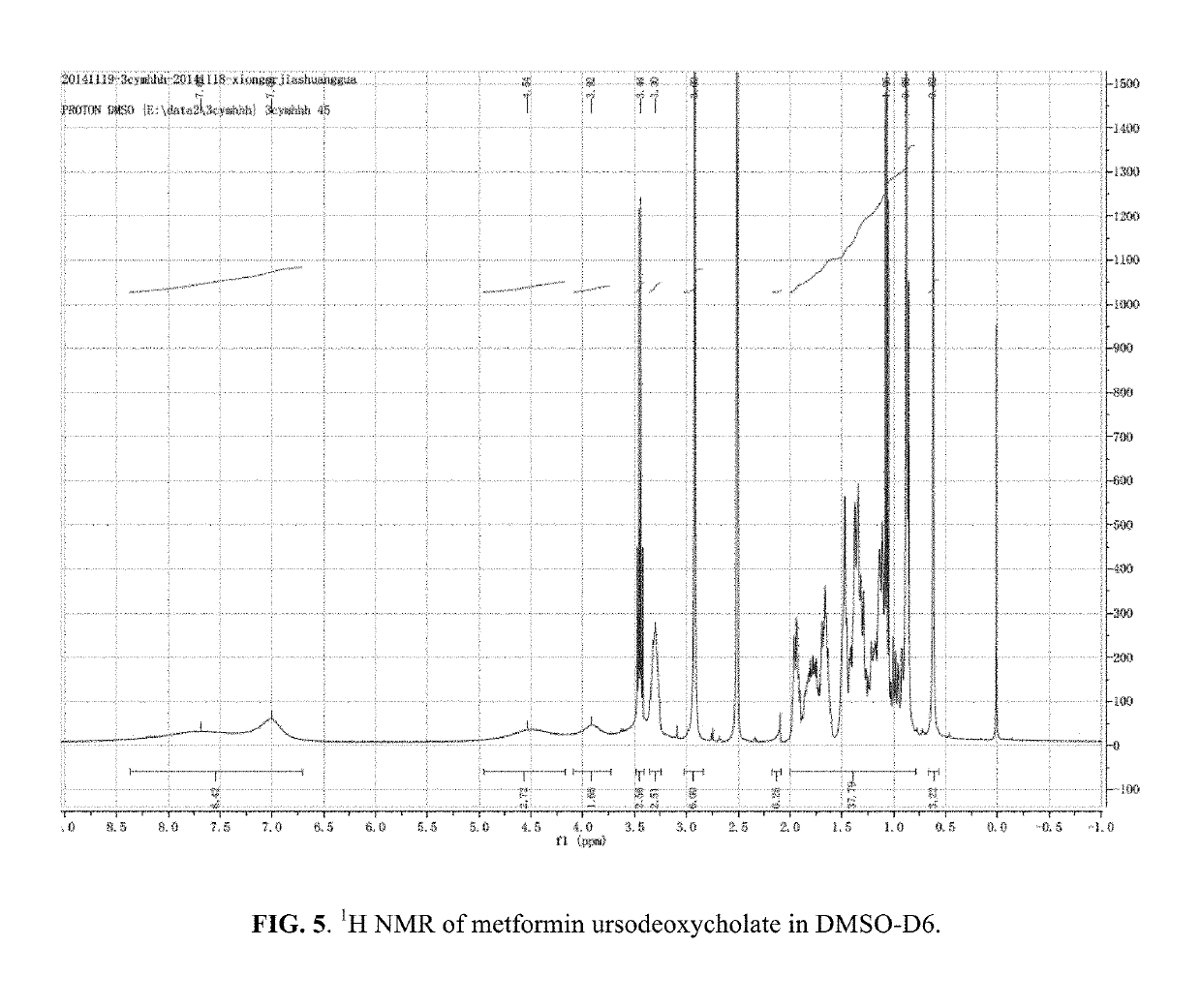
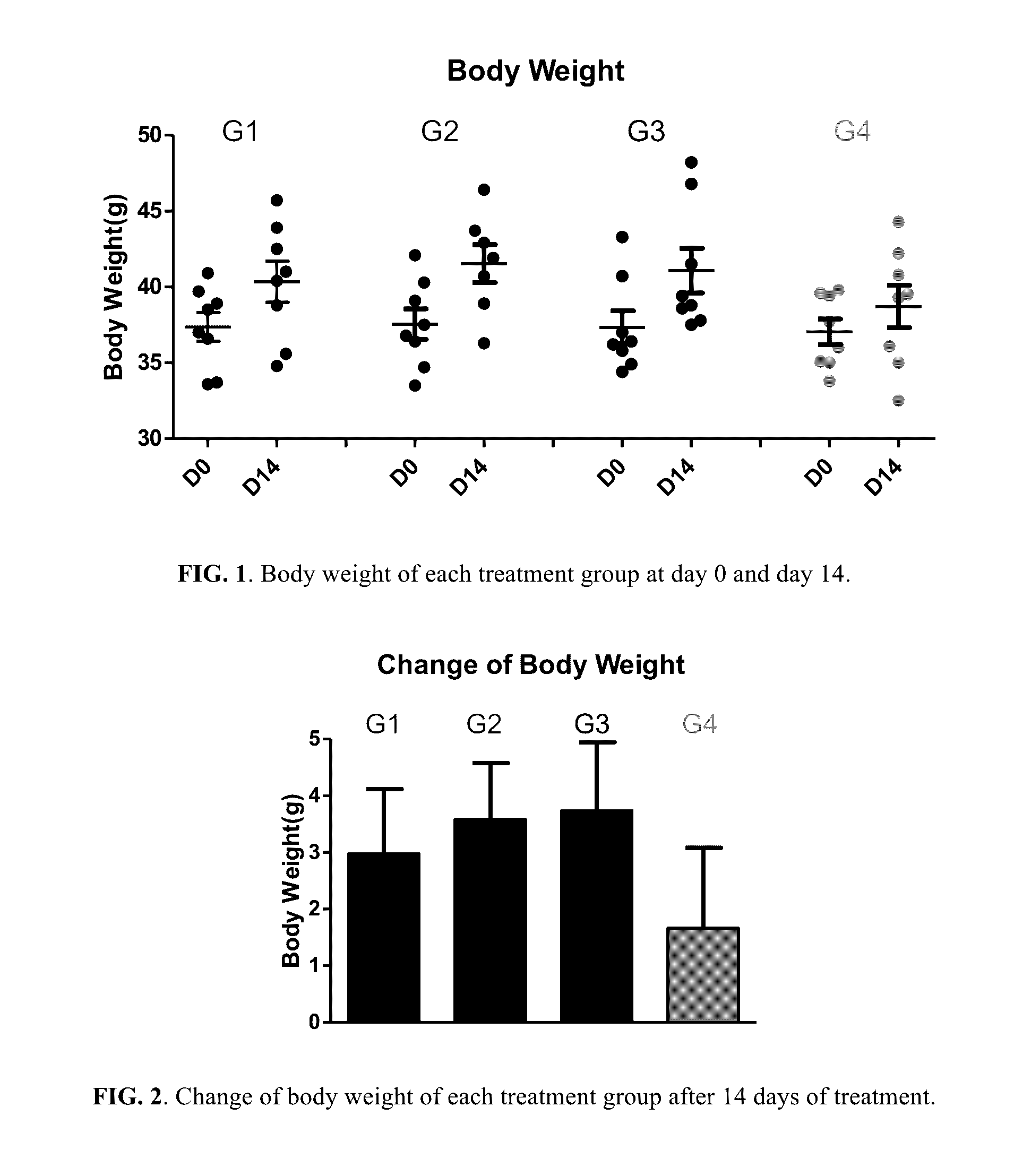
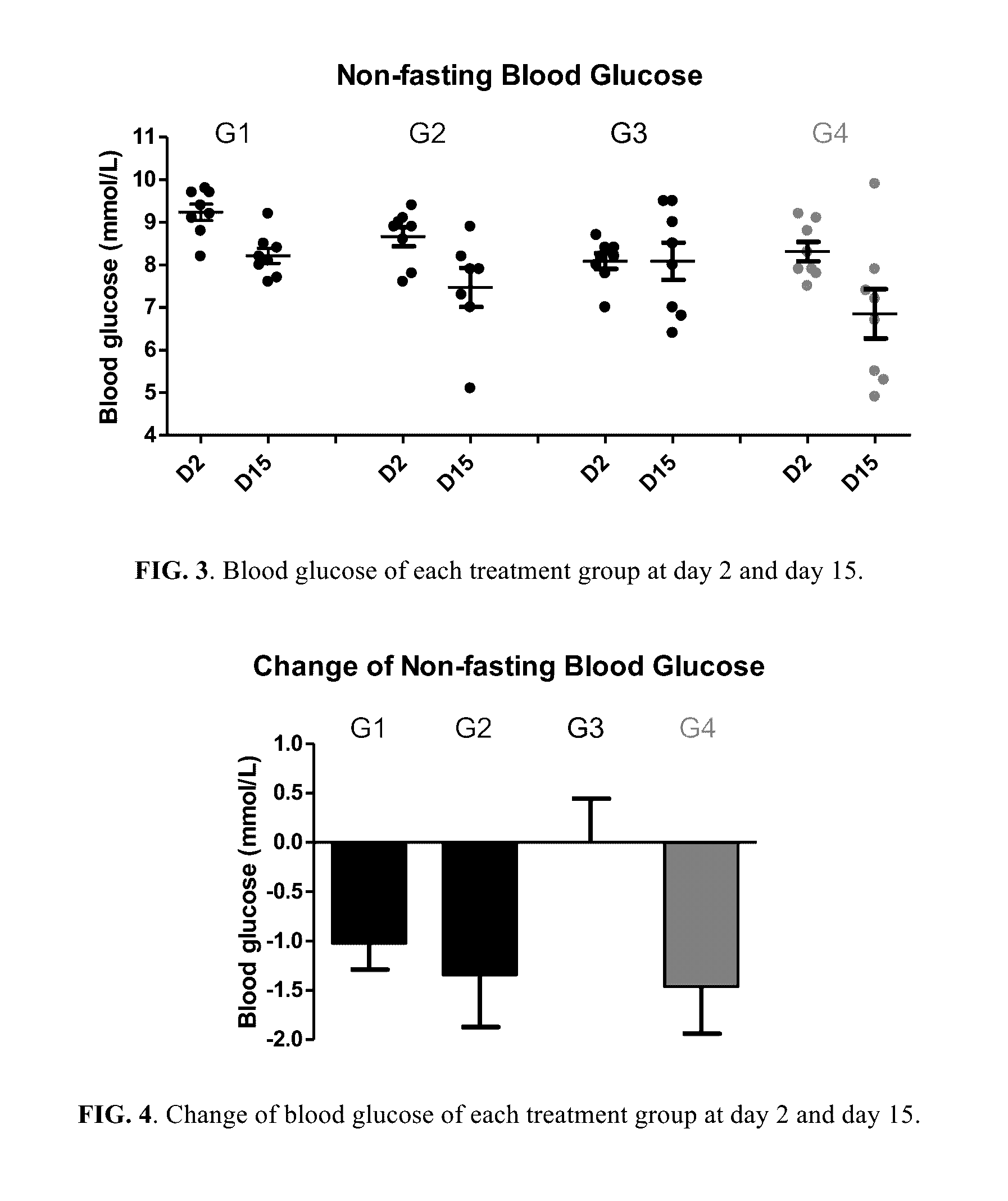
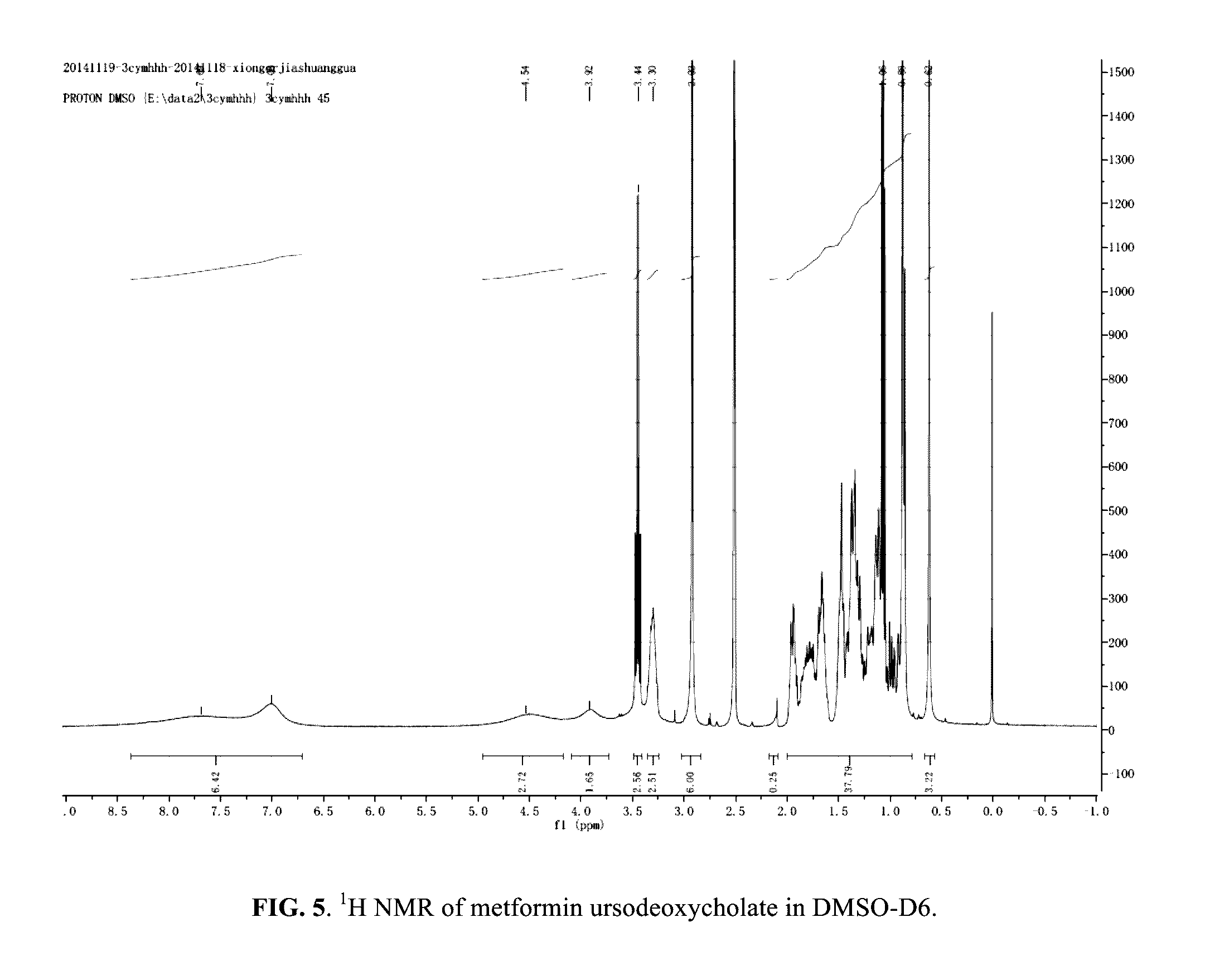
Berberine salts, ursodeoxycholic salts and combinations, methods of preparation and application thereof
Owner:SHENZHEN HIGHTIDE BIOPHARM
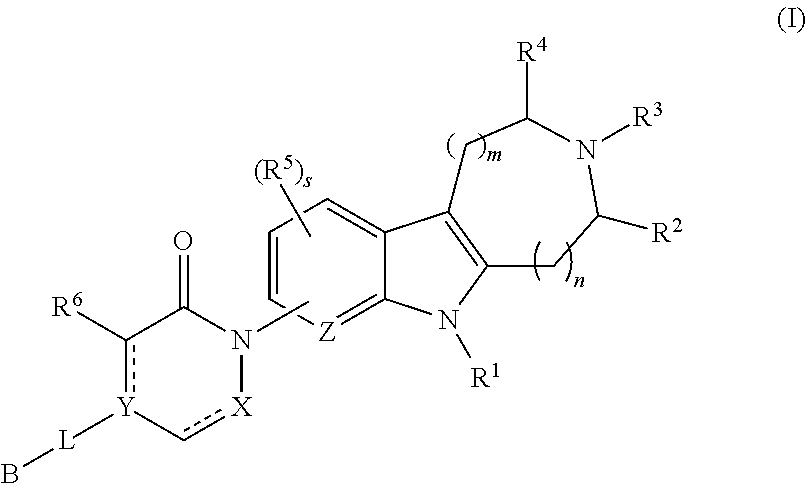
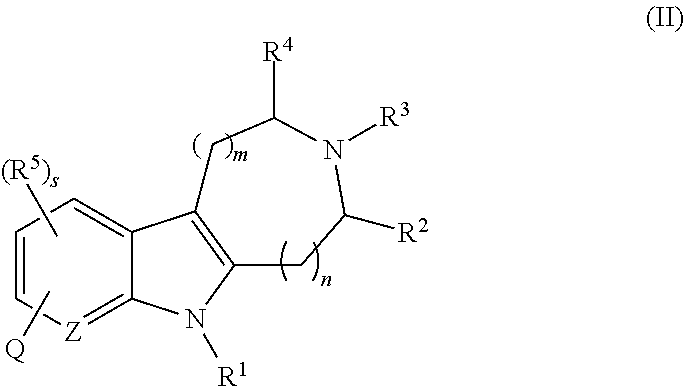
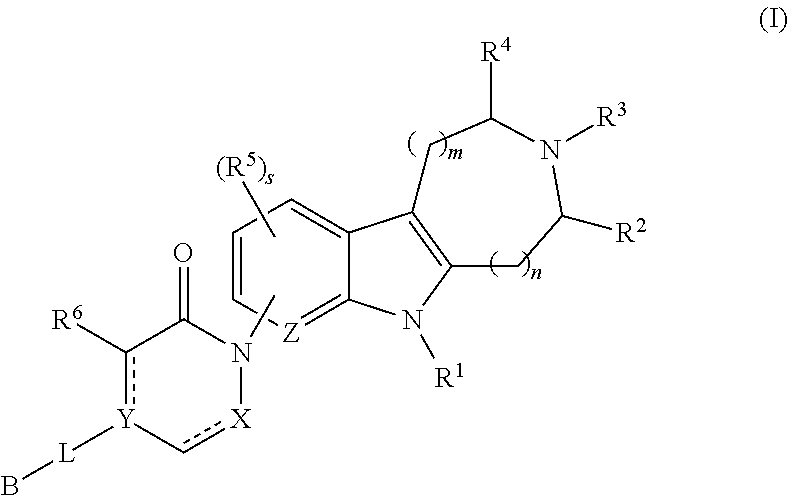
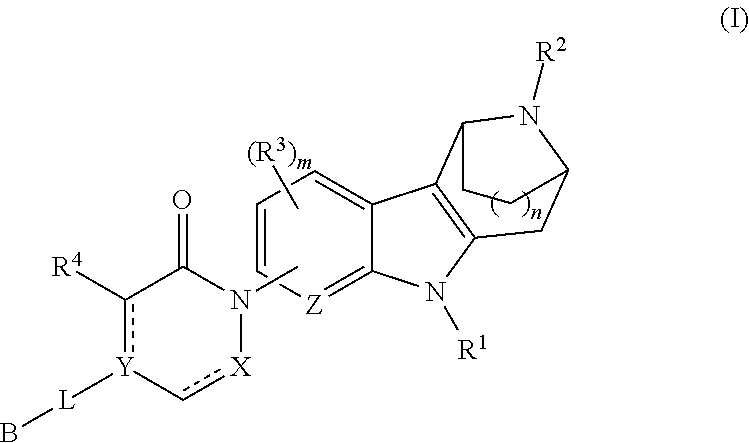
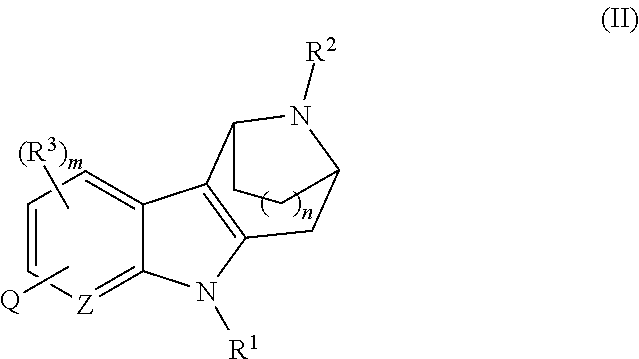
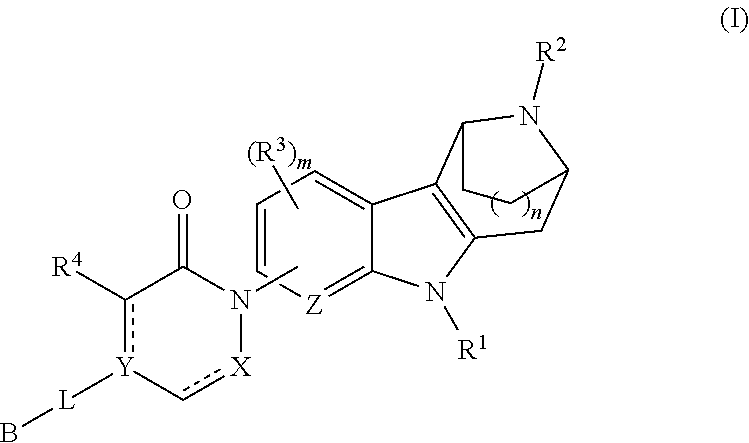
AZINONE-SUBSTITUTED AZEPINO[b]INDOLE AND PYRIDO-PYRROLO-AZEPINE MCH-1 ANTAGONISTS, METHODS OF MAKING, AND USE THEREOF
Novel MCH-1 receptor antagonists are disclosed. These compounds are used in the treatment of various disorders, including obesity, anxiety, depression, non-alcoholic fatty liver disease, and psychiatric disorders. Methods of making these compounds are also described in the present invention.
Owner:HARMONY BIOSCIENCES LLC +1
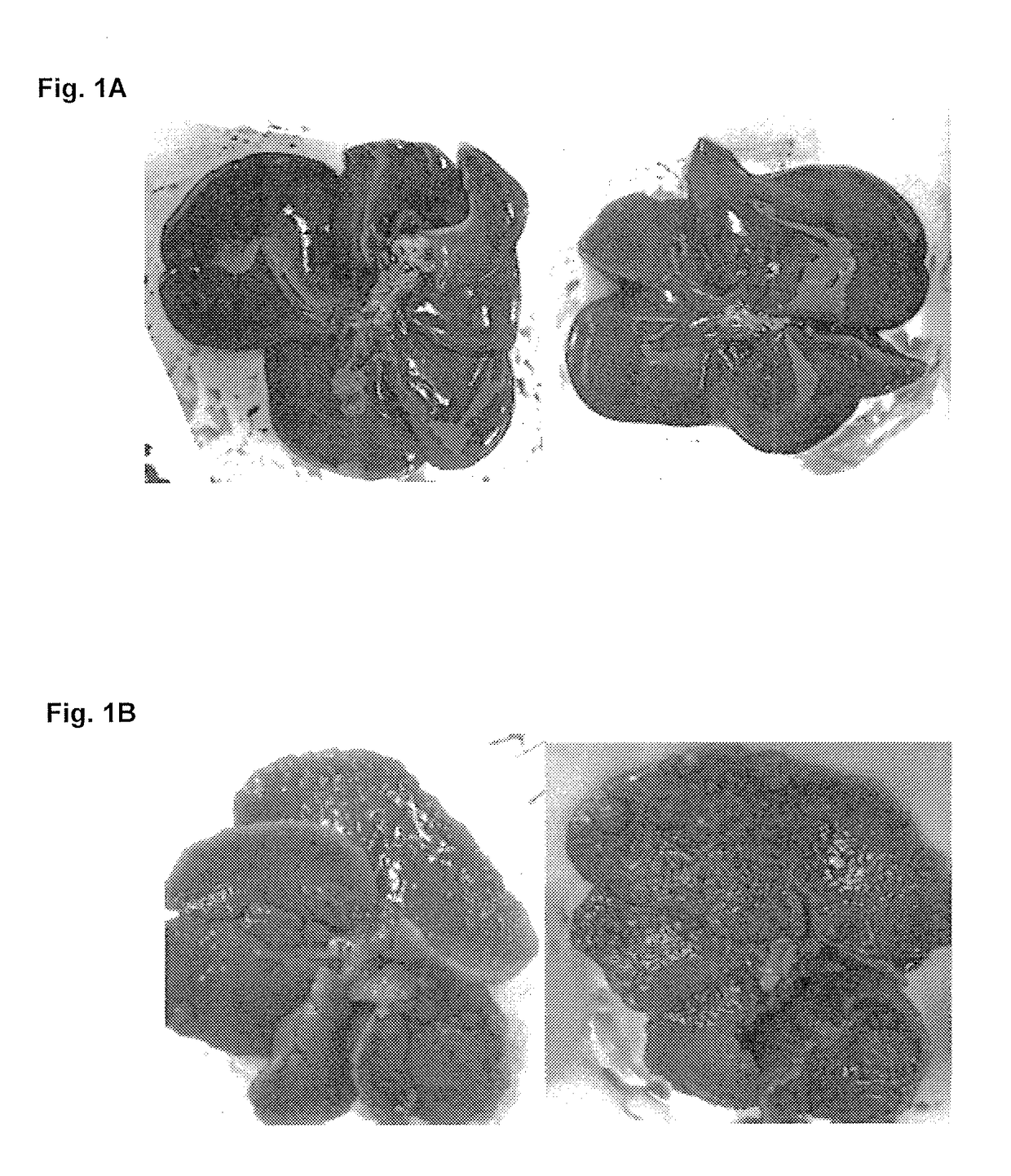
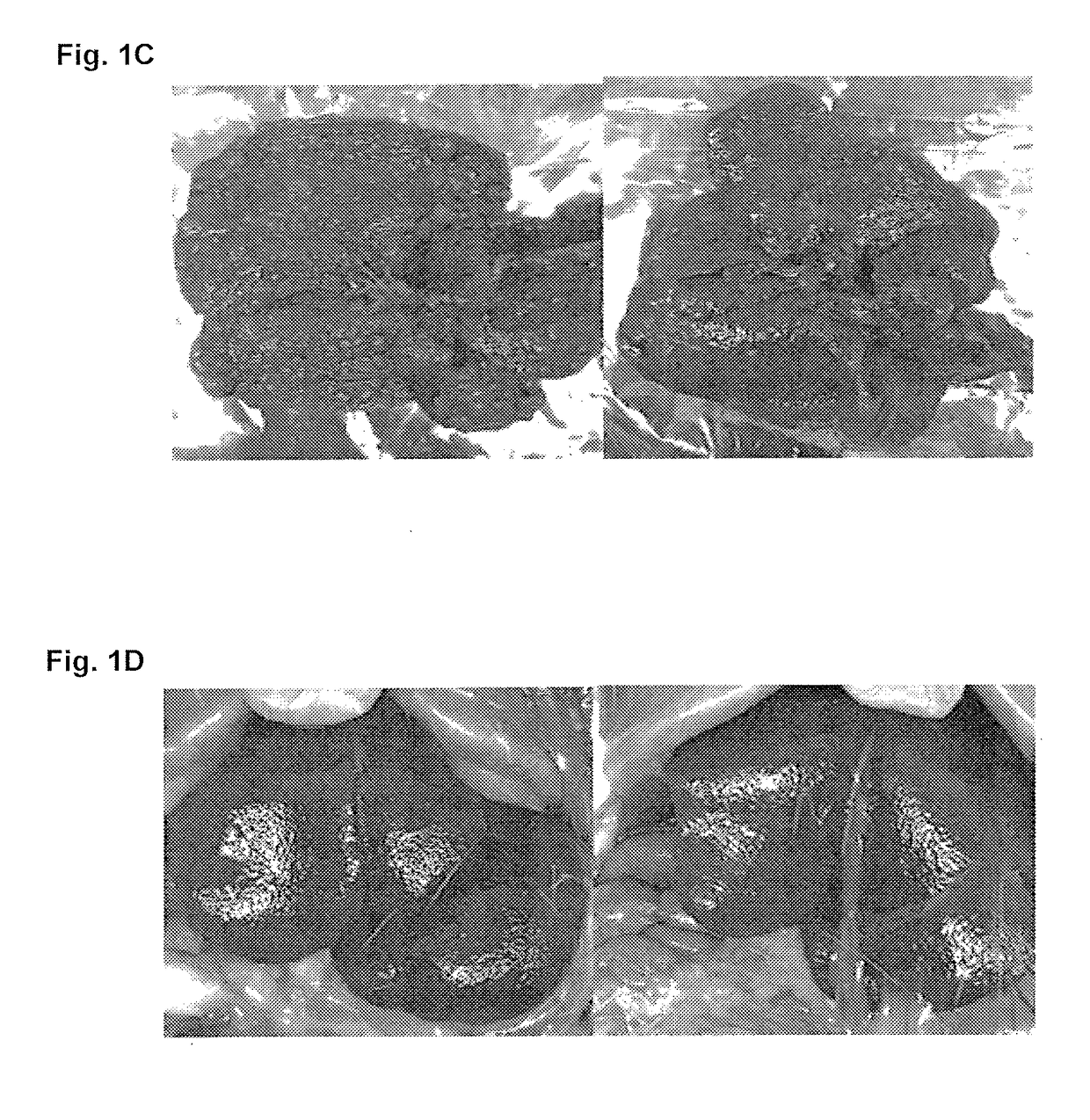
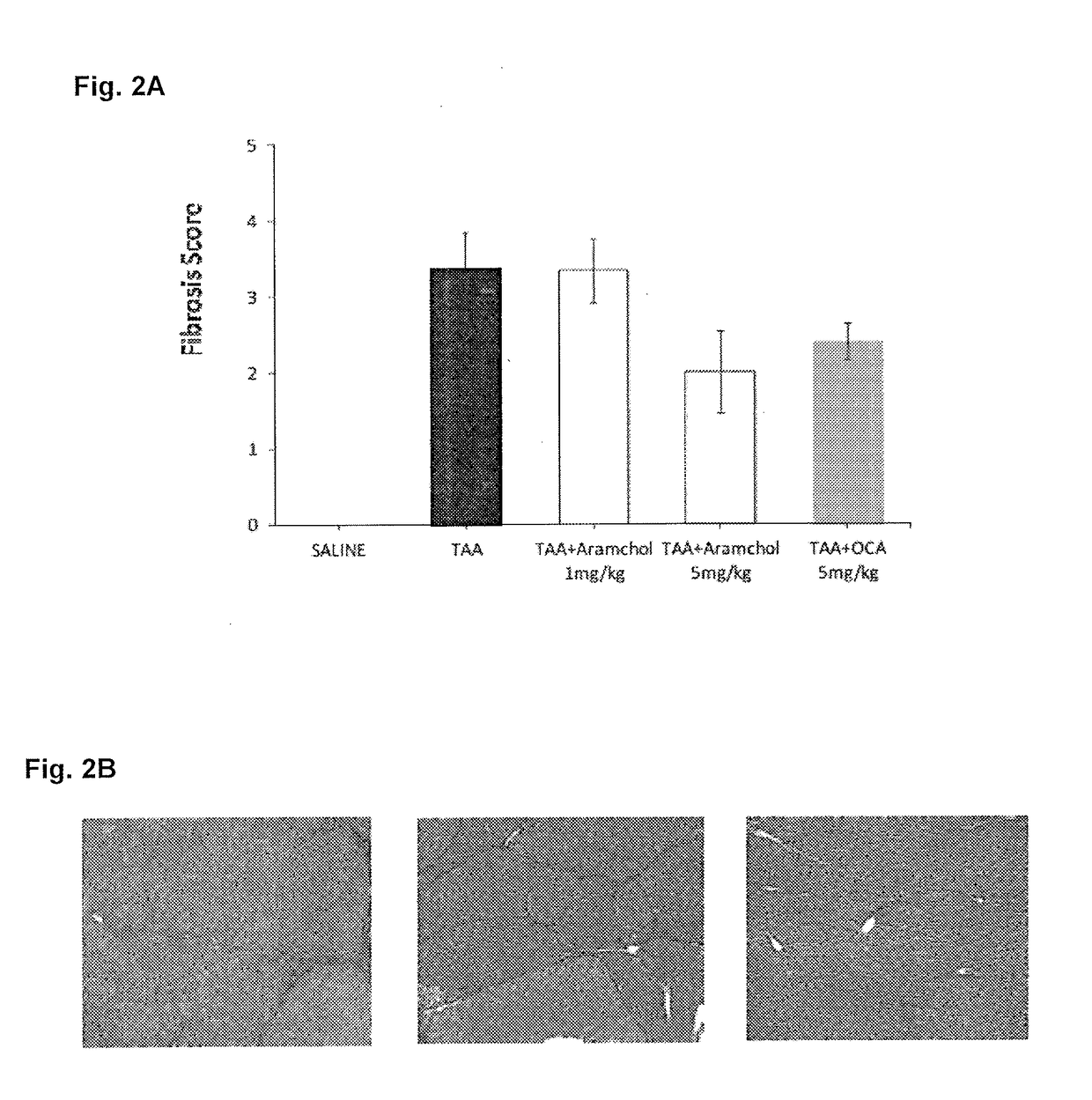
Treatment for hepatic fibrosis
ActiveUS20180125862A1Improve the situationBlood cholesterol concentrationDigestive systemSkeletal disorderLiver fibrosisNon alcoholic
The invention relates to the treatment and reduction of fibrosis, specifically hepatic fibrosis. More specifically, embodiments of the invention provide compositions and methods useful for the treatment and inhibition of hepato-fibrotic conditions associated with Non-Alcoholic Fatty Liver Disease (NAFLD) and Non-Alcoholic Steatohepatitis (NASH), employing the use of 3β-arachidylamido-7α,12α-dihydroxy-5β-cholan-24-oic acid (Aramchol) or a pharmaceutically acceptable salt thereof. In other embodiments, the treatment and inhibition of hepato-fibrotic conditions caused by contact with hepatotoxic chemical substances or by mechanical obstruction is contemplated.
Owner:GALMED RES & DEV LTD
Compositions and methods for reducing hepatotoxicity associated with drug administration and treating non-alcoholic fatty liver disease, non-alcoholic steatohepatitis and associated cirrhosis
InactiveUS20090239831A1Improve efficiencyEnhancing armamentariumBiocideSalicyclic acid active ingredientsActive agentHigh doses
The present invention relates to the discovery that acetylsalicylic acid (ASA or aspirin), salicylic acid (SA) and related salicylate esters and their pharmaceutically acceptable salts, when coadministered in effective amounts with a drug or other bioactive agent which typically (in the absence of the salicylate compound) produces significant hepatotoxicity as a secondary indication, will substantially reduce or even eliminate such hepatotoxicity. Favorable therapeutic intervention results from the use of the present invention having the effect of reducing hepatotoxicity associated with the administration of certain drugs and other bioactive agents and in certain instances of allowing the administration of higher doses of a compound which, without the coadministration, would produce hepatotoxicity which limits or even negates the therapeutic value of the compound. The invention also relates to methods of reducing the likelihood of a patient at risk for non-alcoholic fatty liver diseases (NAFLD), including non-alcoholic steatohepatitis (NASH), or treating NAFLD or NASH including primary NASH, NASH secondary to liver transplantation (NASH post-liver transplantation) or cirrhosis represent alternative aspects of the present invention.
Owner:YALE UNIV
Methods of treating non-alcoholic steatohepatitis (nash) using cysteamine products
The disclosure relates, in general, to treatment of fatty liver disorders comprising administering compositions comprising cysteamine products. The disclosure provides administration of enterically coated cysteamine compositions to treat fatty liver disorders, such as non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH).
Owner:RGT UNIV OF CALIFORNIA
Nuclear sulfated oxysterol, potent regulator of lipid homeostasis, for therapy of hypercholesterolemia, hypertriglycerides, fatty liver diseases, and atherosclerosis
ActiveUS20100273761A1Improve suppression propertiesDecrease lipid biosynthesisOrganic active ingredientsAntipyreticLipid formationMetabolite
The sulfated oxysterol 5-cholesten-3β, 25-diol 3-sulphate, a nuclear cholesterol metabolite that decreases lipid biosynthesis and increases cholesterol secretion and degradation, is provided as an agent to lower intracellular and serum cholesterol and / or triglycerides, and to prevent or treat lipid accumulation-associated inflammation and conditions associated with such inflammation. Methods which involve the use of this sulfated oxysterol to treat conditions associated with high cholesterol and / or high triglycerides and / or inflammation (e.g. hypercholesterolemia, hypertriglyceridemia, non-alcoholic fatty liver diseases, atherosclerosis, etc.) are also provided.
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS +1
Apoptosis signal-regulating kinase 1 inhibitors and methods of use thereof
The present invention discloses compounds of Formula (I), and pharmaceutically acceptable salts and esters thereof:which inhibit the Apoptosis signal-regulating kinase 1 (ASK-1), which associated with autoimmune disorders, neurodegenerative disorders, inflammatory diseases, chronic kidney disease, cardiovascular disease. The present invention further relates to pharmaceutical compositions comprising the aforementioned compounds for administration to a subject suffering from ASK-1 related disease. The invention also relates to methods of treating an ASK-1 related disease in a subject by administering a pharmaceutical composition comprising the compounds of the present invention. The present invention specifically relates to methods of treating ASK-1 associated with hepatic steatosis, including non-alcoholic fatty liver disease (NAFLD) and non-alcohol steatohepatitis disease (NASH).
Owner:ENANTA PHARM INC
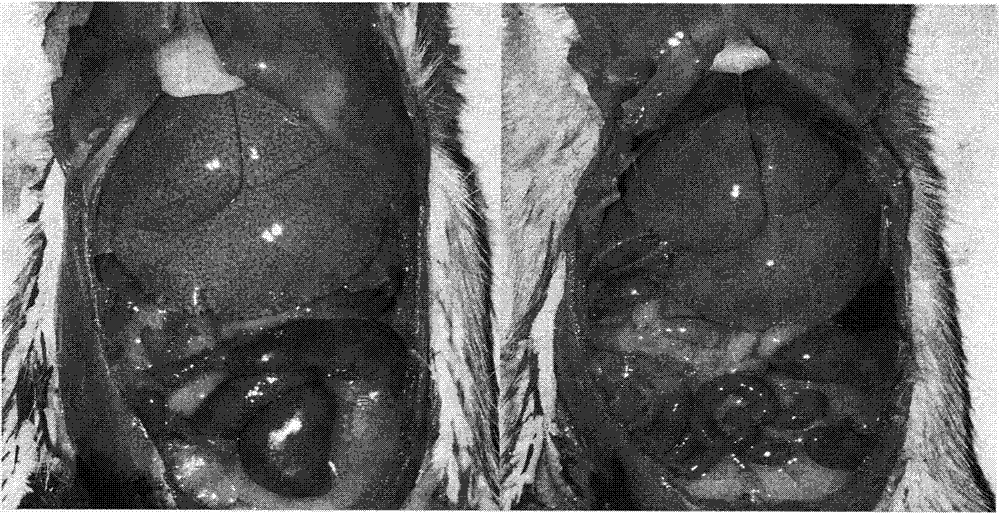
Method for establishing non-alcoholic fatty liver disease combined with viral hepatitis mouse model
InactiveCN104365543AAffect eatingGood repeatabilityViral/bacteriophage medical ingredientsAnimal feeding stuffLiver histologyHigh fat
The invention discloses a method for establishing a non-alcoholic fatty liver disease combined with viral hepatitis mouse model. The method comprises the steps that high-fat feed is used for feeding a C3H / HeN small mouse for 12 weeks, the body weight, the liver weight, transaminase, the glucolipid metabolism index and liver histological change of the small mouse are observed, and a non-alcoholic fatty liver disease small mouse is cultivated. Then 10 PFU of MHV-3 infected mice are selected, and the survival rate, the liver histology and intrahepatic virus replication of the infected mice are observed, so that the non-alcoholic fatty liver disease combined with viral hepatitis mouse model similar to a human disease process is established. The established model provides a forceful tool for virus dynamics, immunological changes and prevention and control measures systematically studying non-alcoholic fatty liver disease combined with viral hepatitis.
Owner:TONGJI HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI TECH
Method and system for reducing the likelihood of developing liver cancer in an individual diagnosed with non-alcoholic fatty liver disease
ActiveUS10512661B2Improvements in intestinal dysbiosisReduce intestinal permeabilityOrganic active ingredientsDigestive systemDiseaseTriacylglycerol VLDL
A method for reducing the likelihood of developing non-alcoholic steatohepatitis (NASH) in an individual diagnosed with non-alcoholic fatty liver disease involves providing in the gut of an individual a population of beneficial bacteria selected from the group consisting of Lactobacillus species, and at least 6 grams per day of fiber to the individual to maintain a therapeutically effective amount of the beneficial bacteria in the gut of the individual. In certain embodiments, monoacylglycerolacyltransferase-3 (MGAT3) synthesis is inhibited to lower triacylglycerol (TAG) production, while in others, expression of diacylglycerolacyltransferase-2 (DGAT-2) is inhibited. The beneficial bacteria are preferably modified to produce increased amounts of butyrate and are also encapsulated in a frangible enclosure. Levels of Roseburia are preferably increased while the levels of Akkermansia spp. in the individual's gut microbiome are reduced.
Owner:SEED HEALTH INC
A novel composition for nonalcoholic fatty liver disease (NAFLD)
Owner:CADILA HEALTHCARE LTD
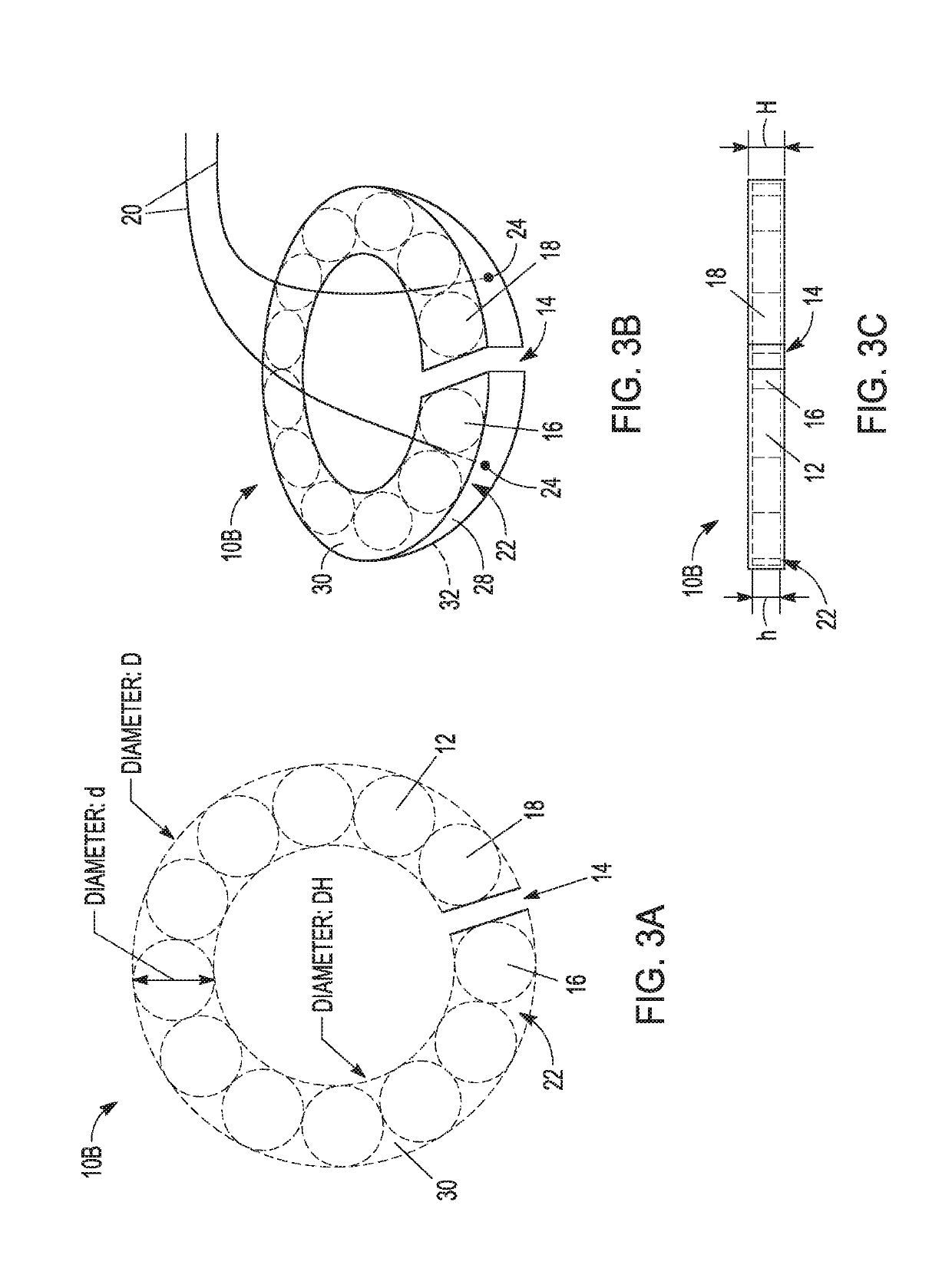
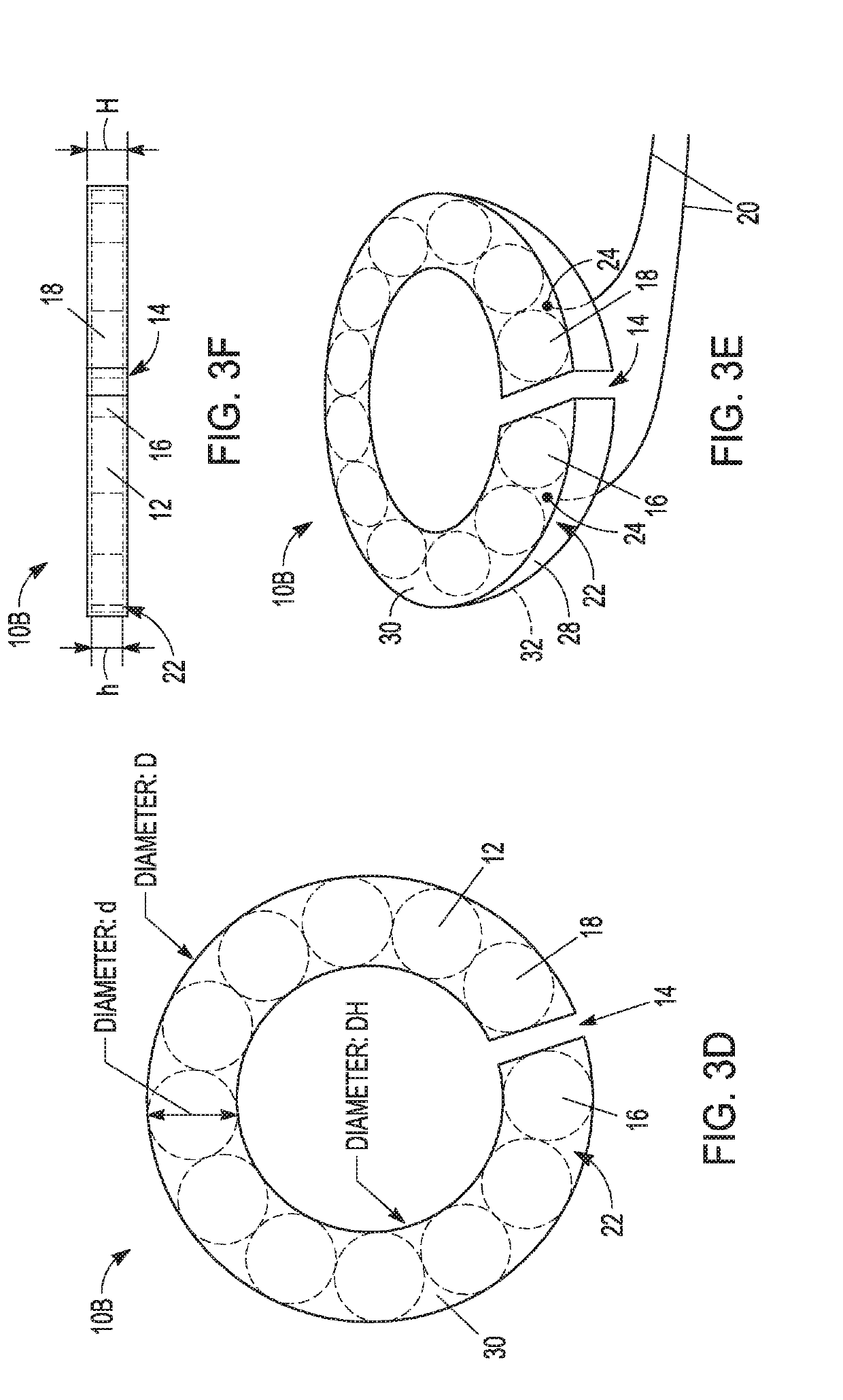
Magnetic anastomosis devices
Embodiments of the present invention provide a magnetic anastomosis device (MAD), a delivery catheter for the MAD, and a method for treating at least one of diabetes, obesity, digestive disease, and non-alcoholic fatty liver disease. In embodiments of the present invention, a pair of the MADs are delivered in body lumen by endoscopic and / or laparoscopic techniques. An anastomosis between two adjacent body lumens is formed by compression of the pair of MADs followed by necrosis and regeneration of the lumen tissue. The bodily fluids are then redirected through the formed anastomosis to relieve disease symptoms.
Owner:NEUROTRONIC
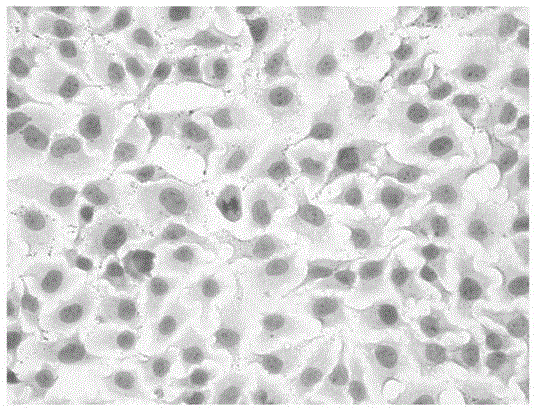
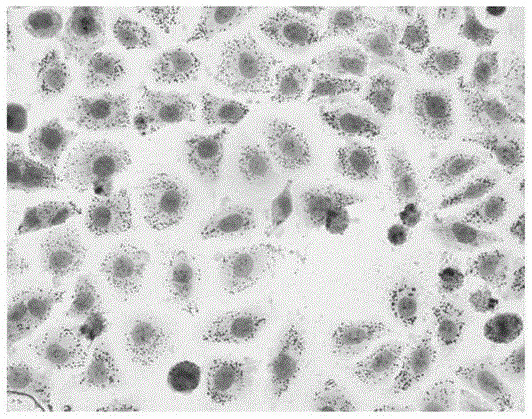
Enzymatically decomposed clam oligopeptide having recovery effect on non-alcoholic fatty liver disease cell model and preparation method of calm oligopeptide
ActiveCN104387451AReduce mortalityGood repeatabilityMicrobiological testing/measurementPeptidesLipid formationStaining
The invention relates to an enzymatically decomposed clam oligopeptide having a recovery effect on a non-alcoholic fatty liver disease (NAFLD) cell model. The clam oligopeptide is characterized by comprising an amino acid sequence of Gln Leu Asn Trp Asp. The invention also relates to a preparation method of the enzymatically decomposed clam oligopeptide having the recovery effect on the NAFLD cell model. Compared with the prior art, the following advantages can be achieved: the NAFLD cell model is established by virtue of induction of palmitic acid and the damaged liver cells in a body are simulated sufficiently, and the result indicates that the TG content of the in-vitro NAFLD cell model established 48 hours after induction by 15 micrograms / milliliter palmitic acid increased remarkably in comparison with that of normal liver cells; oil red O staining shows that the number of intracellular lipid droplets of the model group is increased in contrast with that of the cells of a normal group, and the cell mortality rate is low and the repeatability is good, and therefore, the modeling method is simple, convenient and feasible; meanwhile, the enzymatically decomposed clam oligopeptide having the obvious recovery effect on the NAFLD cell model can be separated out from clams.
Owner:ZHEJIANG OCEAN UNIV
Azinone-substituted azapolycycle mch-1 antagonists, methods of making, and use thereof
Novel MCH-1 receptor antagonists are disclosed. These compounds are used in the treatment of various disorders, including obesity, anxiety, depression, non-alcoholic fatty liver disease, and psychiatric disorders. Methods of making these compounds are also described in the present invention.
Owner:ALBANY MOLECULAR RESEARCH INC
Berberine salts, ursodeoxycholic salts and combinations, methods of preparation and application thereof
Owner:SHENZHEN HIGHTIDE BIOPHARM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com










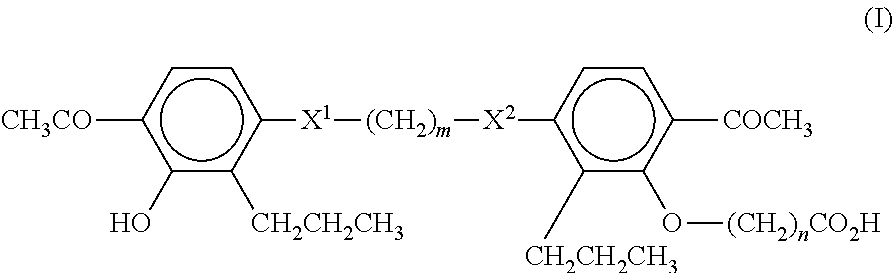
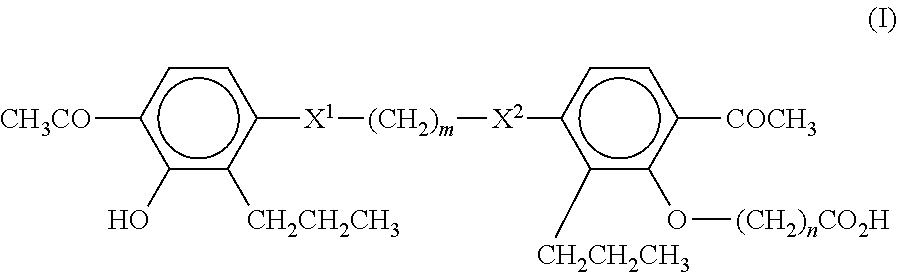

![AZINONE-SUBSTITUTED AZEPINO[b]INDOLE AND PYRIDO-PYRROLO-AZEPINE MCH-1 ANTAGONISTS, METHODS OF MAKING, AND USE THEREOF AZINONE-SUBSTITUTED AZEPINO[b]INDOLE AND PYRIDO-PYRROLO-AZEPINE MCH-1 ANTAGONISTS, METHODS OF MAKING, AND USE THEREOF](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/571c3ea7-937e-44b0-b1a9-b64302e134e3/US20110003793A1-20110106-C00001.png)
![AZINONE-SUBSTITUTED AZEPINO[b]INDOLE AND PYRIDO-PYRROLO-AZEPINE MCH-1 ANTAGONISTS, METHODS OF MAKING, AND USE THEREOF AZINONE-SUBSTITUTED AZEPINO[b]INDOLE AND PYRIDO-PYRROLO-AZEPINE MCH-1 ANTAGONISTS, METHODS OF MAKING, AND USE THEREOF](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/571c3ea7-937e-44b0-b1a9-b64302e134e3/US20110003793A1-20110106-C00002.png)
![AZINONE-SUBSTITUTED AZEPINO[b]INDOLE AND PYRIDO-PYRROLO-AZEPINE MCH-1 ANTAGONISTS, METHODS OF MAKING, AND USE THEREOF AZINONE-SUBSTITUTED AZEPINO[b]INDOLE AND PYRIDO-PYRROLO-AZEPINE MCH-1 ANTAGONISTS, METHODS OF MAKING, AND USE THEREOF](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/571c3ea7-937e-44b0-b1a9-b64302e134e3/US20110003793A1-20110106-C00003.png)