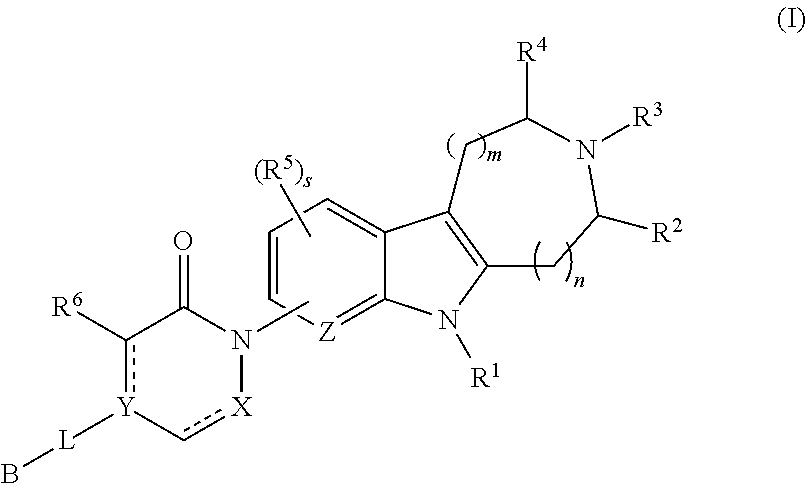
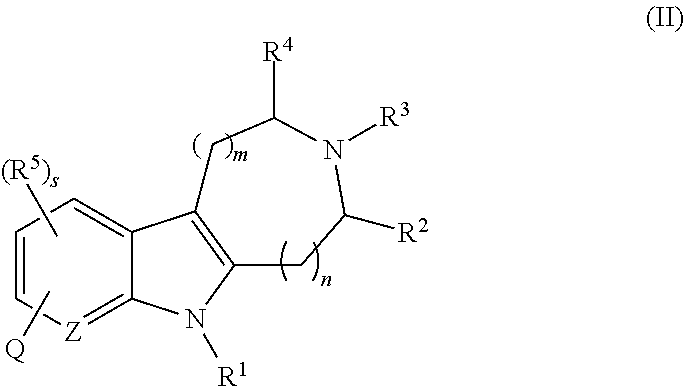
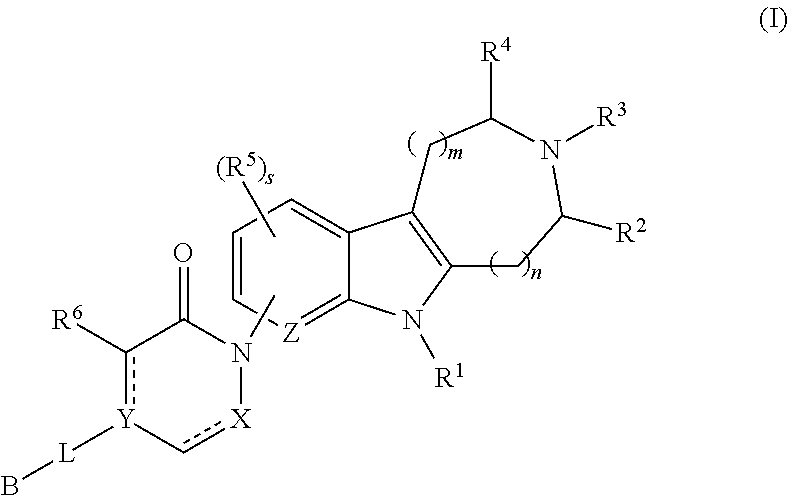
Azinone-substituted azapolycycle mch-1 antagonists, methods of making, and use thereof
a technology of azapolycycline and azapolycycline, which is applied in the field of pyridone-substituted azapolycycline, can solve the problems of economic and social costs, excessive body fat, and excessive weight, and achieves increased body mass index, increased waist circumference, and high affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Analytical Methods and Materials
[0143]Unless otherwise noted, reagents and solvents were used as received from commercial suppliers. Proton nuclear magnetic resonance (NMR) spectra were obtained on Bruker spectrometers at 300, 400 or 500 MHz. Spectra are given in ppm (δ) and coupling constants, J, are reported in Hertz. Tetramethylsilane (TMS) was used as an internal standard. Mass spectra were collected using either a Finnigan LCQ Duo LCMS ion trap electrospray ionization (ESI) or a mass Varian 1200L single quadrapole mass spectrometer (ESI). High performance liquid chromatograph (HPLC) analyses were obtained using a Luna C18(2) column (250×4.6 mm, Phenomenex) or a Gemini C18 column (250×4.6 mm, Phenomenex) with UV detection at 254 nm or 223 nm using a standard solvent gradient program (Method A or Method B).
Method A:
[0144]
TimeFlow(min)(mL / min)% A% B0.01.090.010.0201.010.090.0301.010.090.0A = Water with 0.05% Trifluoroacetic AcidB = Acetonitrile with 0.05% Trifluoroacetic Acid
Metho...
example 2
Preparation of 4-(Benzyloxy)-1-(11-methyl-2,3,5,6,11,11b-hexahydro-1H-indolizino[8,7-b]indol-9-yl)pyridin-2(1H)-one hydrochloride
a) 9-Bromo-11-methyl-2,3,5,6,11,11b-hexahydro-1H-indolizino[8,7-b]indole
[0146]
[0147]To a solution of 2-(6-bromo-1-methyl-1H-indol-3-yl)ethanamine (6.50 g, 25.7 mmol) in CH2Cl2 (50 mL) were added 4-bromobutyryl chloride (4.50 mL, 38.6 mmol) and NEt3 (7.20 mL, 51.4 mmol). After 2 h, the reaction mixture was diluted with CH2Cl2 and filtered through a layer of Celite. The filtrate was concentrated to give a brown oil. The oil was diluted with benzene (120 mL) and treated with POCl3 (12 mL, 0.13 mol). The reaction mixture was heated at reflux for 1 h. After the mixture was concentrated, the residue was purified by flash column chromatography (silica gel, 10% CH3OH in CH2Cl2) to give the crude product (6.1 g) as a brown oil. The oil was diluted with EtOH and cooled to 0° C. NaBH4 (0.60 g, 16 mmol) was added, and the reaction was stirred from 0° C. to room temper...
example 3
Preparation of (−)4-(Benzyloxy)-1-(11-methyl-2,3,5,6,11,11b-hexahydro-1H-indolizino[8,7-b]indol-9-yl)pyridin-2(1H)-one hydrochloride
[0150]
[0151]The (−) enantiomer was obtained by chiral separation of the racemate with a XHIPAΛΠAK AD column (heptane / EtOH, 40 / 60 (0.1% DEA)). The resulting solid (40 mg, 0.094 mmol) was dissolved in CH3OH (1 mL) and treated with 2 N HCl in Et2O (1 mL). The reaction was stirred at room temperature until complete. The solvent was removed under reduced pressure, and the resulting solid was dried under vacuum to give the title compound (40 mg, 92%) as a yellow powder: mp 224-228° C.; 1H NMR (500 MHz, CD3OD) δ 7.68 (d, J=8.0 Hz, 1H), 7.66 (d, J=8.0 Hz, 1H), 7.51-7.37 (m, 6H), 7.10 (dd, J=8.5, 2.0 Hz, 1H), 6.39 (dd, J=7.5, 2.5 Hz, 1H), 6.19 (d, J=2.5 Hz, 1H), 5.23 (s, 2H), 5.13 (t, J=8.5 Hz, 1H), 3.83-3.79 (m, 1H), 3.75 (s, 3H), 3.72-3.69 (m, 1H), 3.65-3.58 (m, 2H), 3.20-3.16 (m, 2H), 2.86-2.83 (m, 1H), 2.39-2.34 (m, 2H), 2.22-2.17 (m, 1H); ESI MS m / z 426 [M+...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com