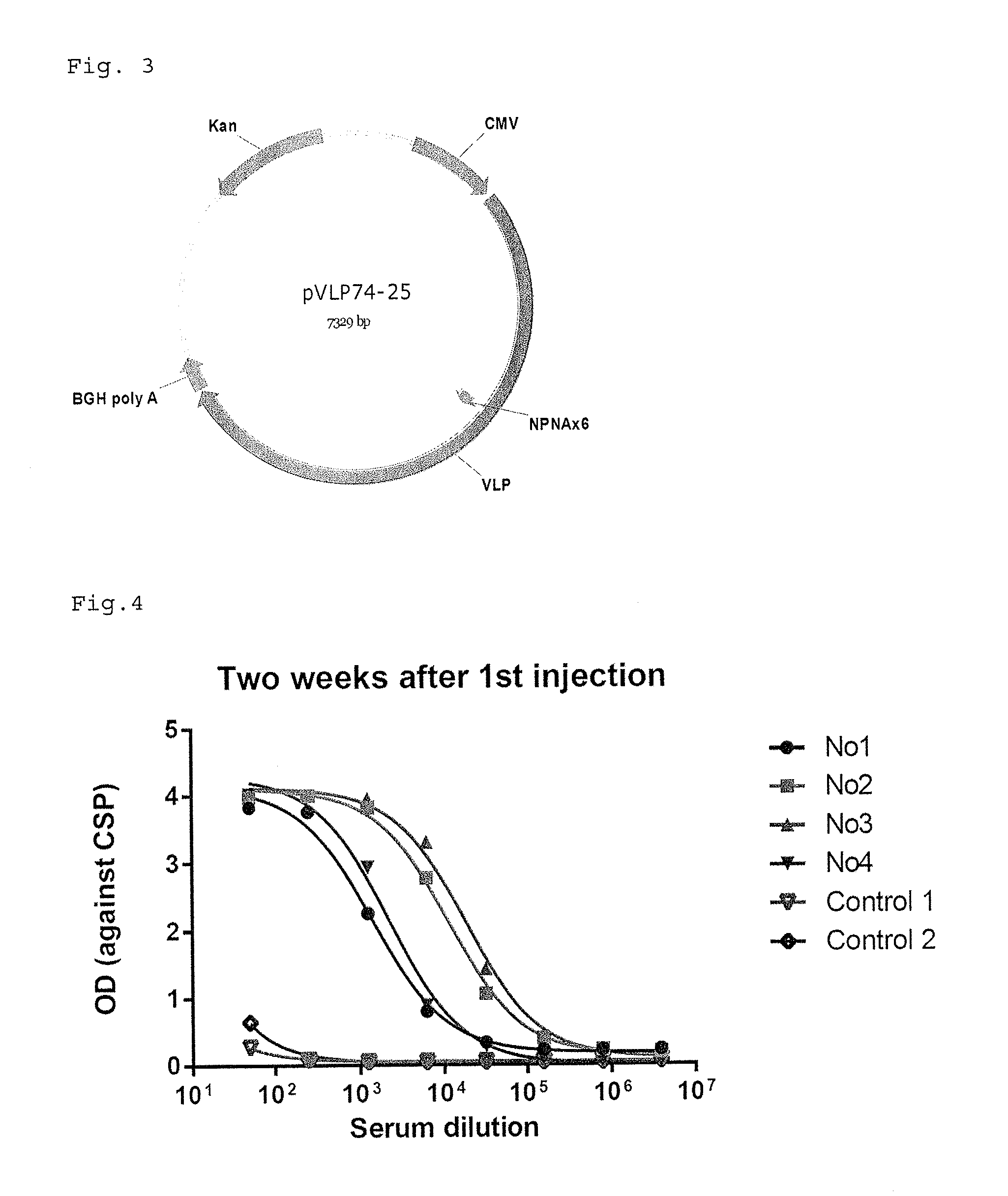
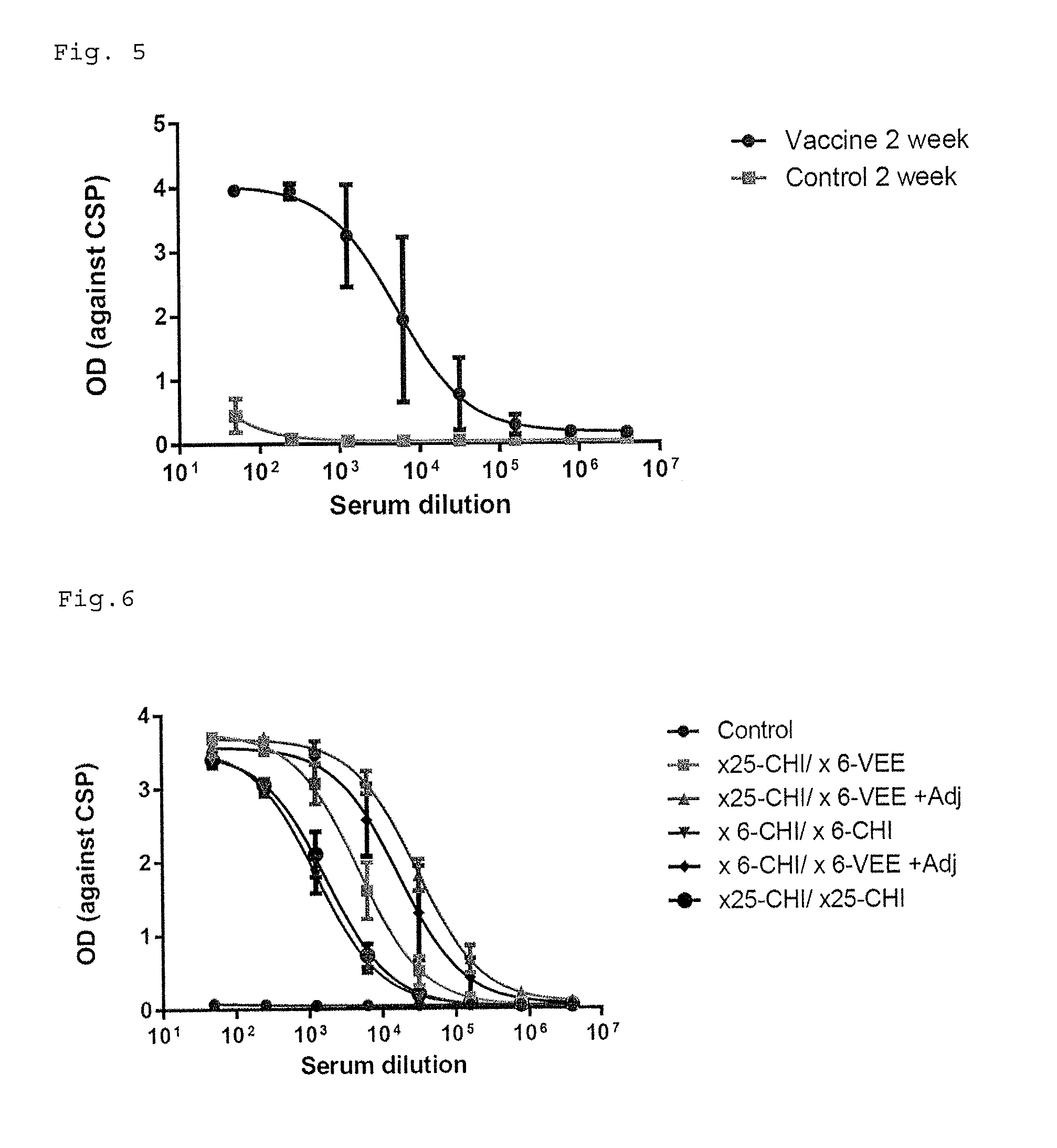
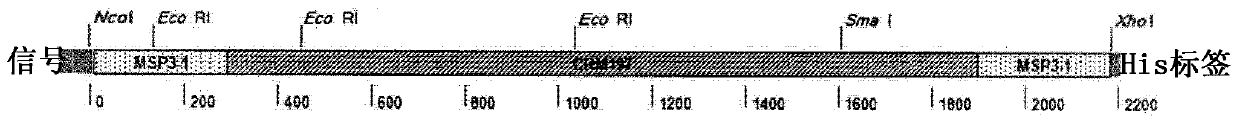
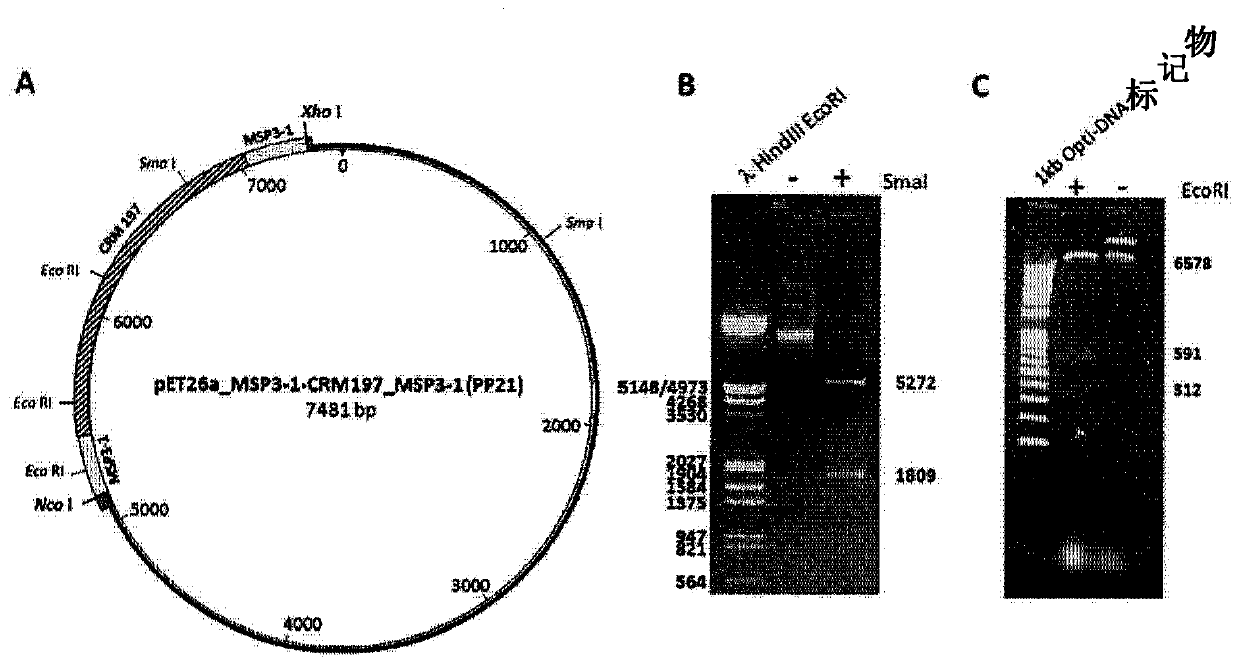
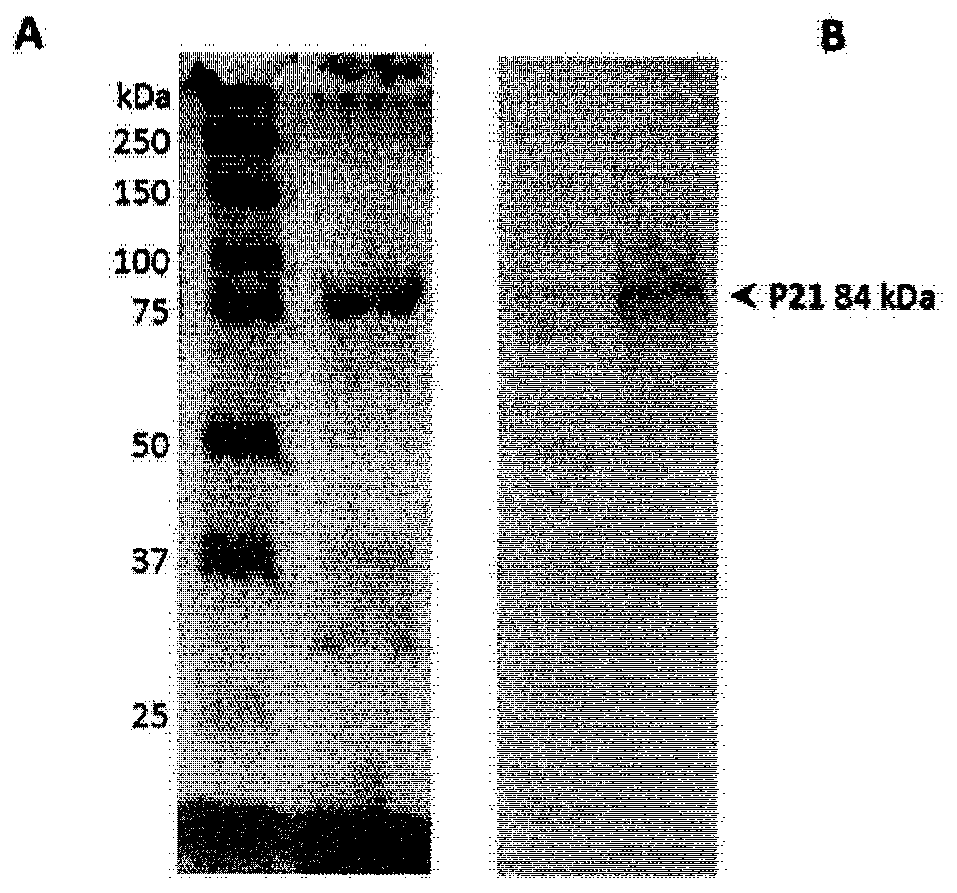
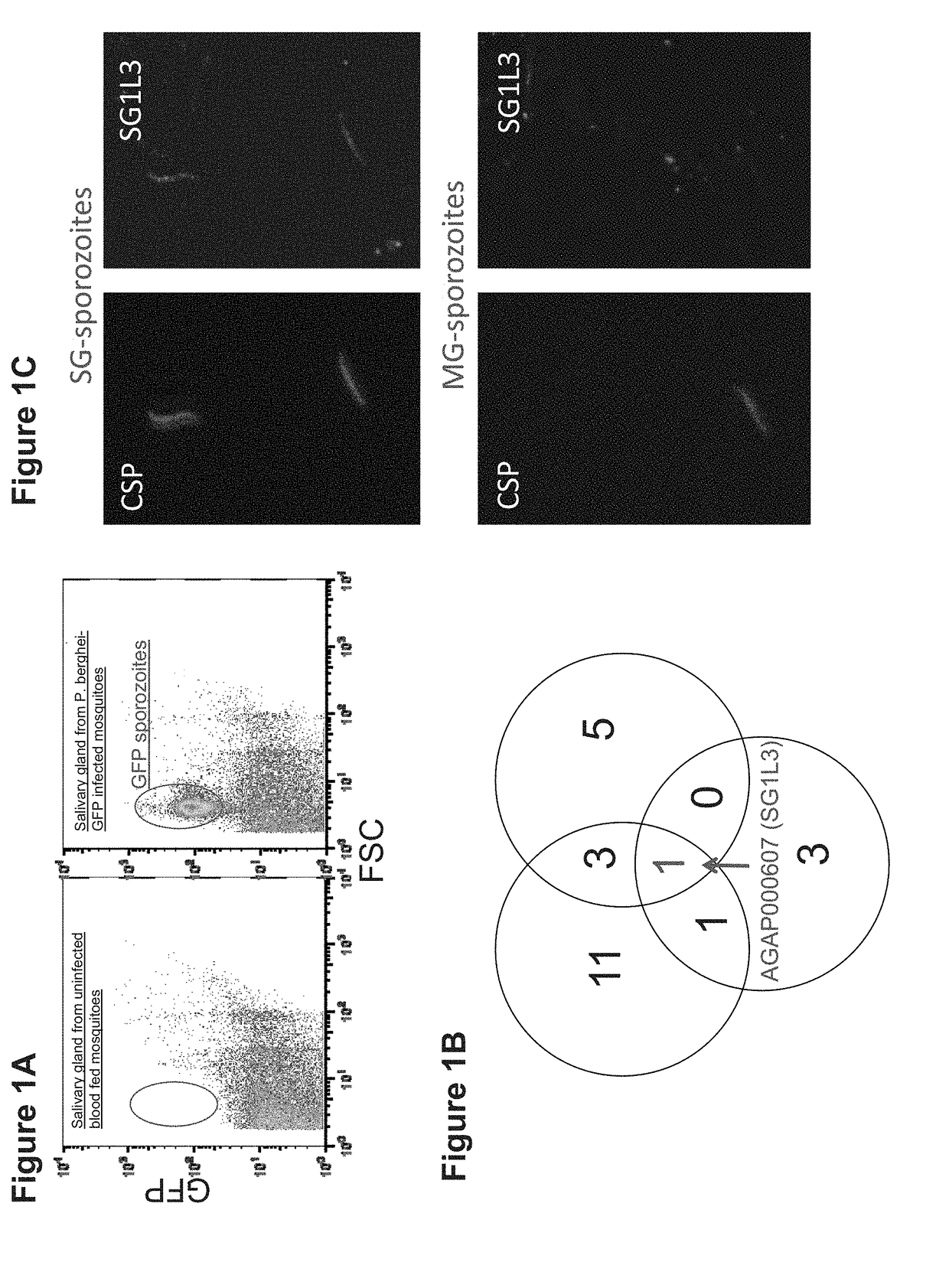
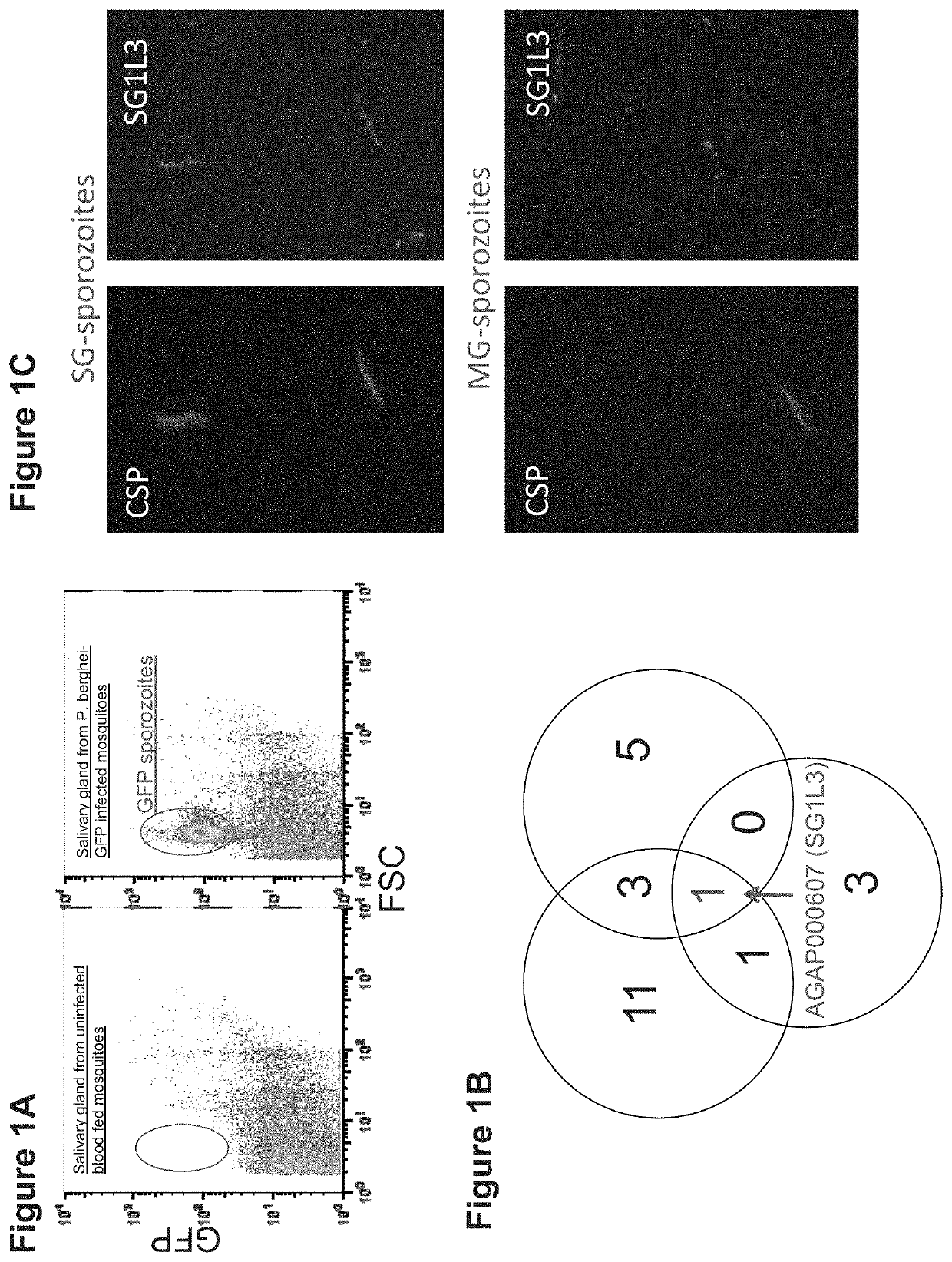
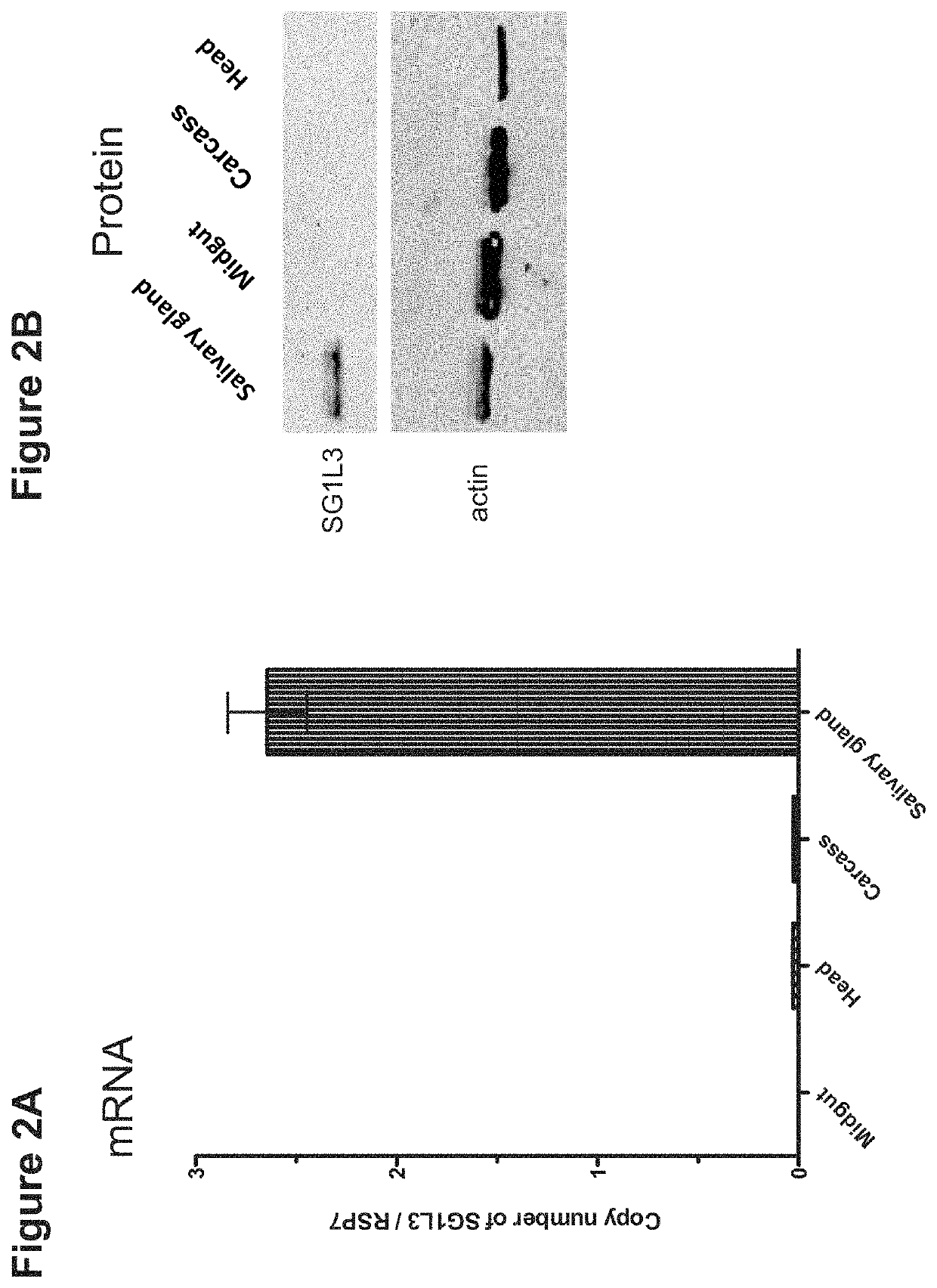
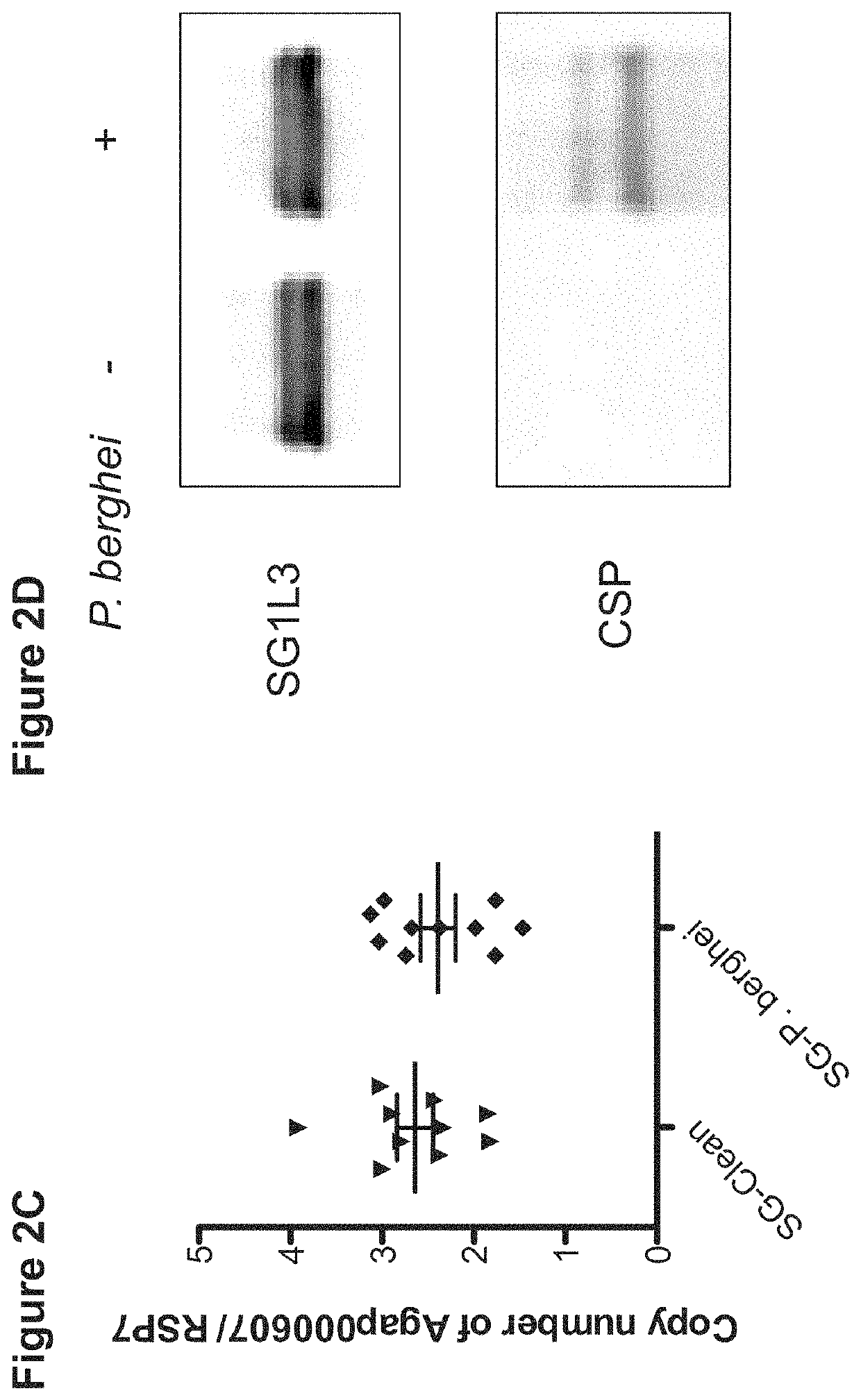
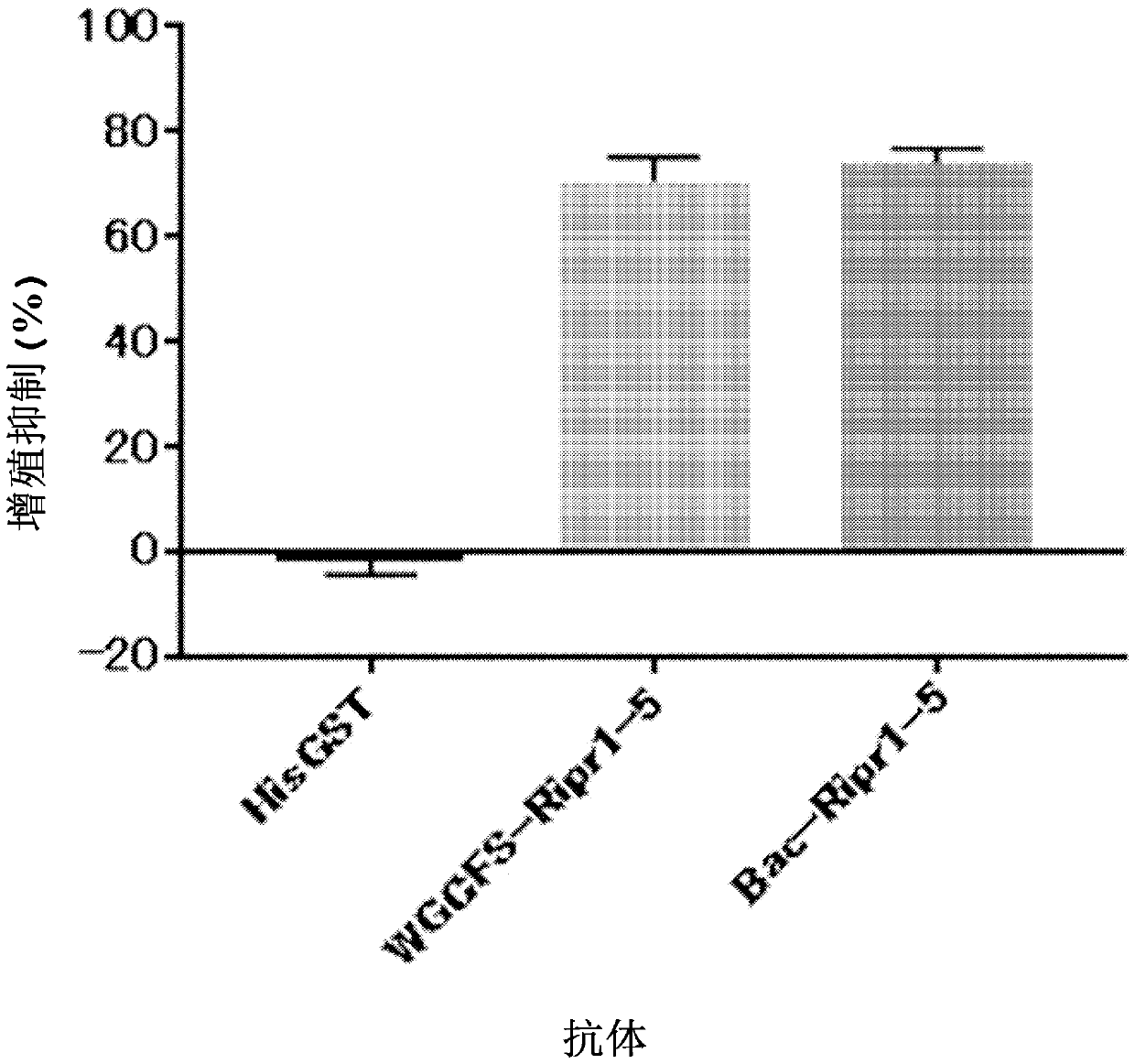
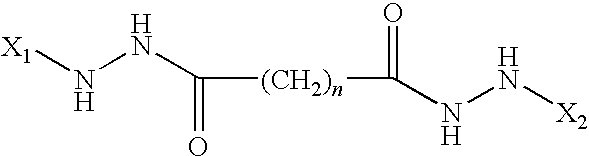Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
35 results about "Malarial Vaccines" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
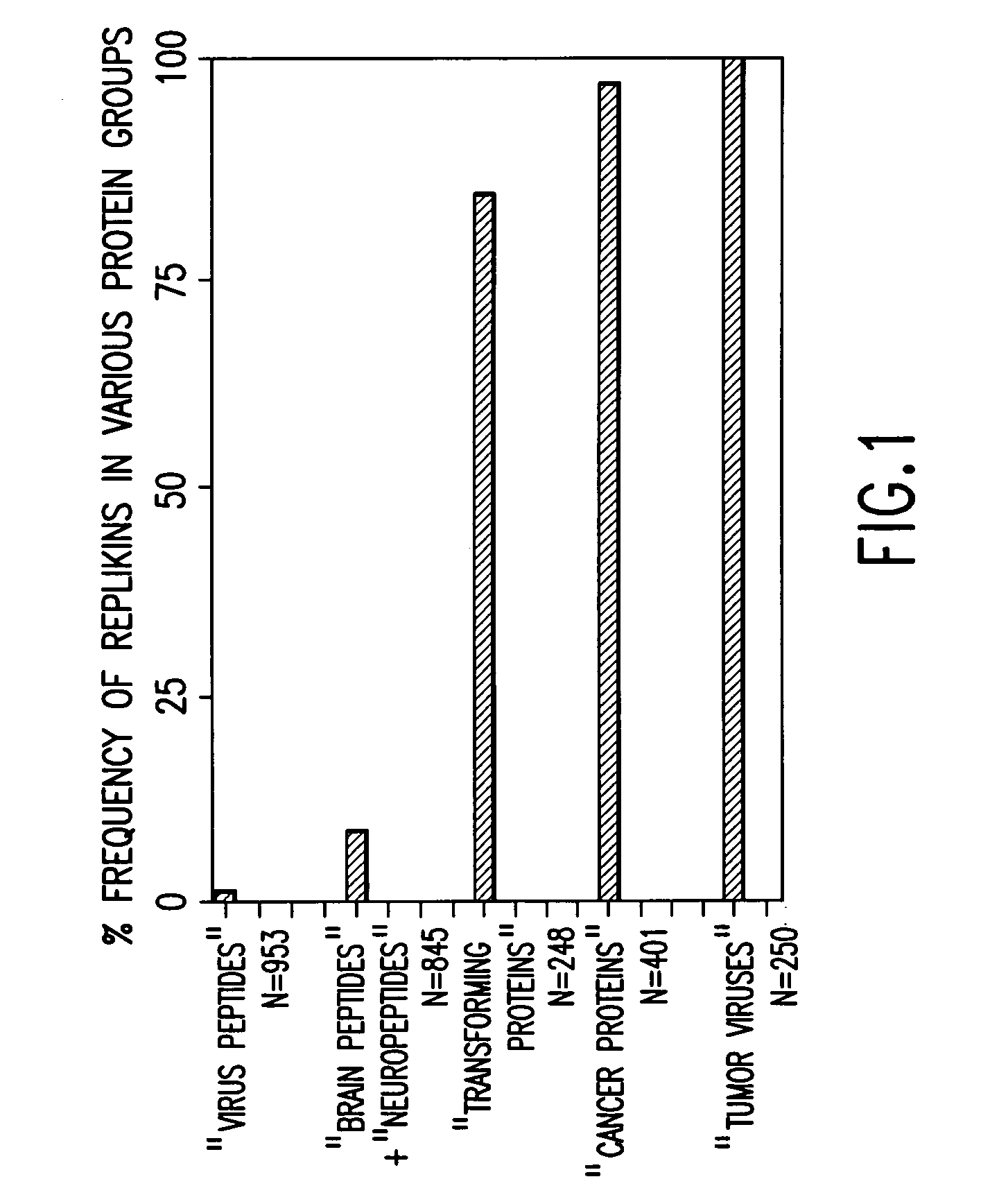
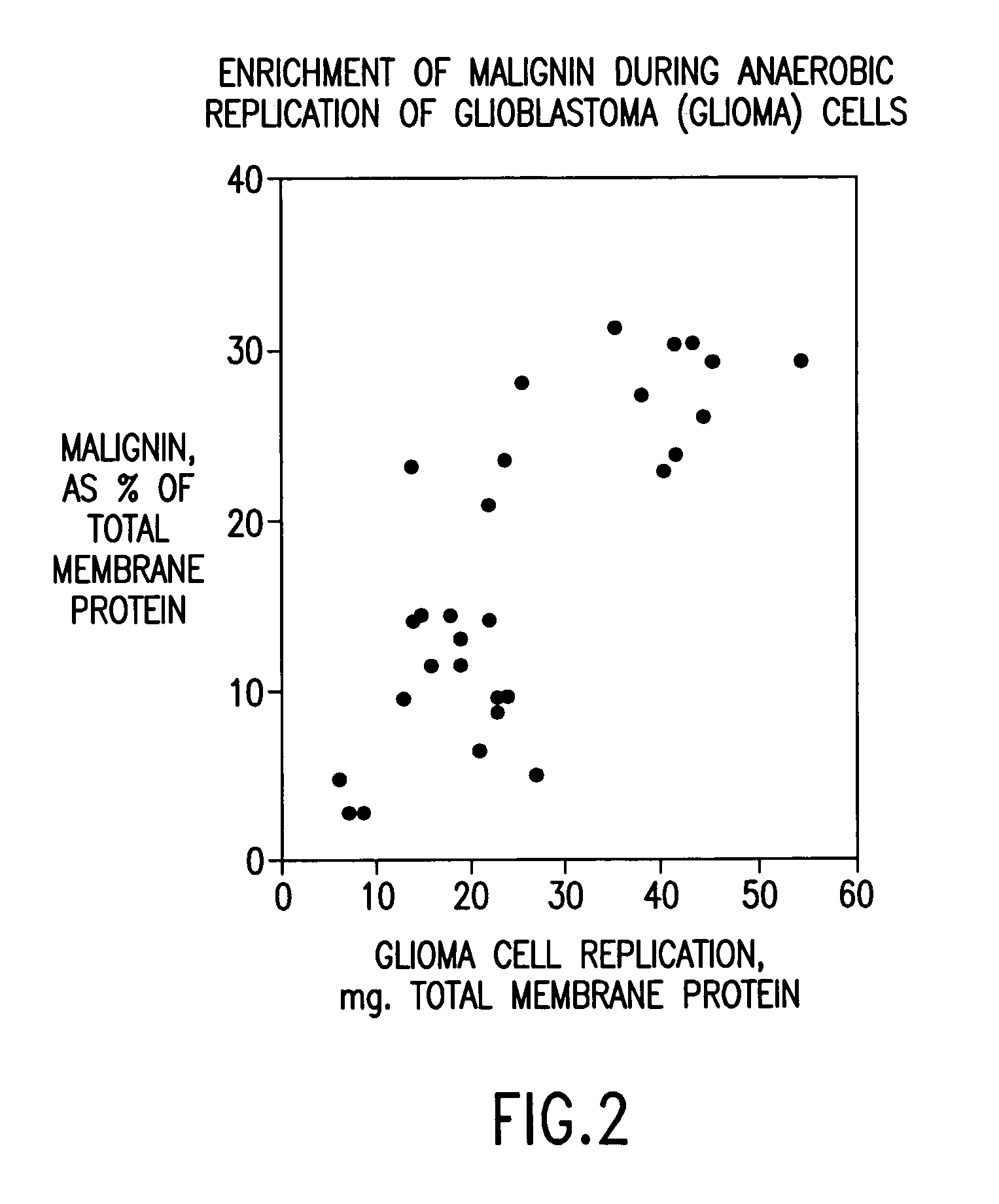
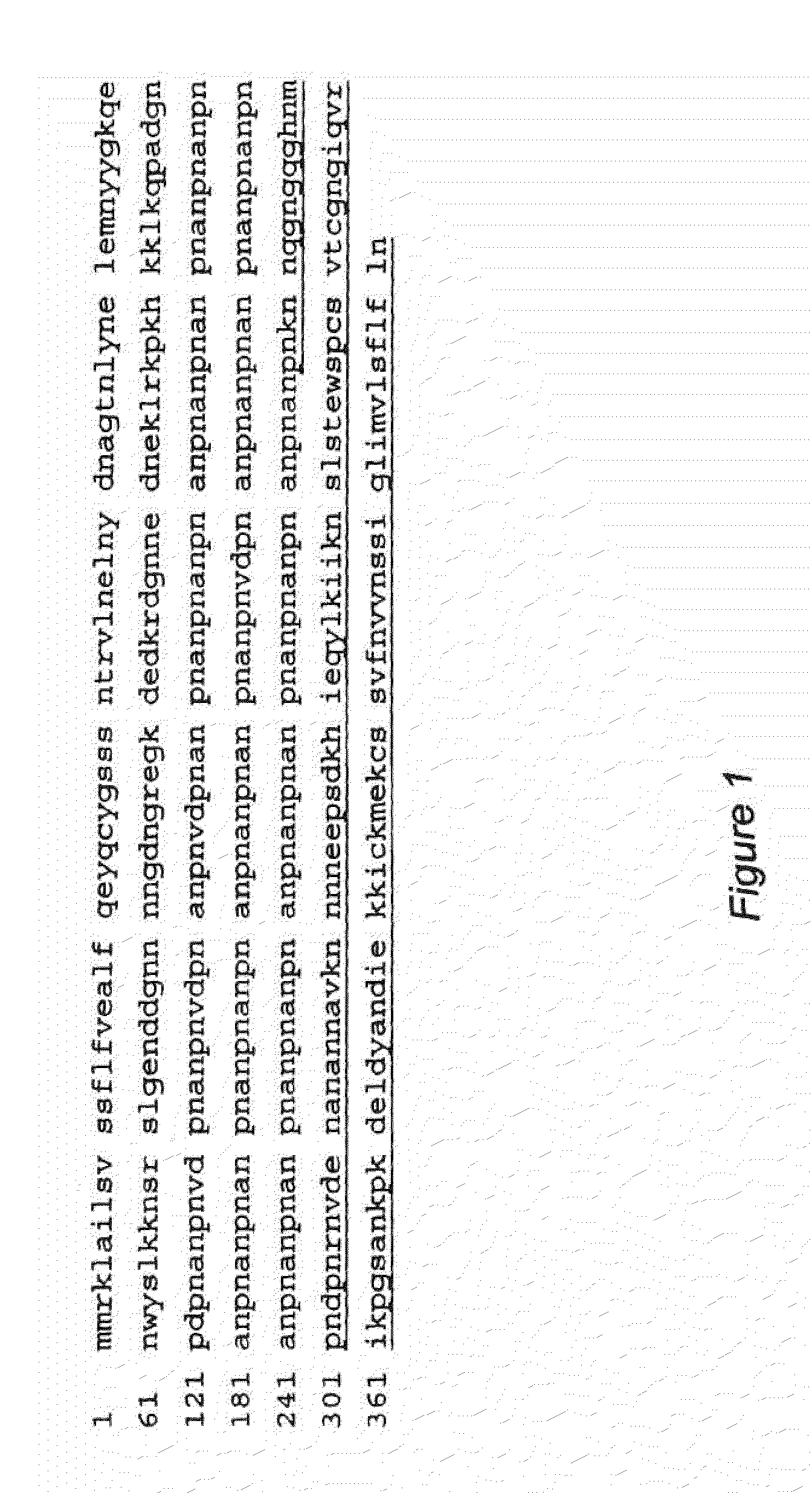
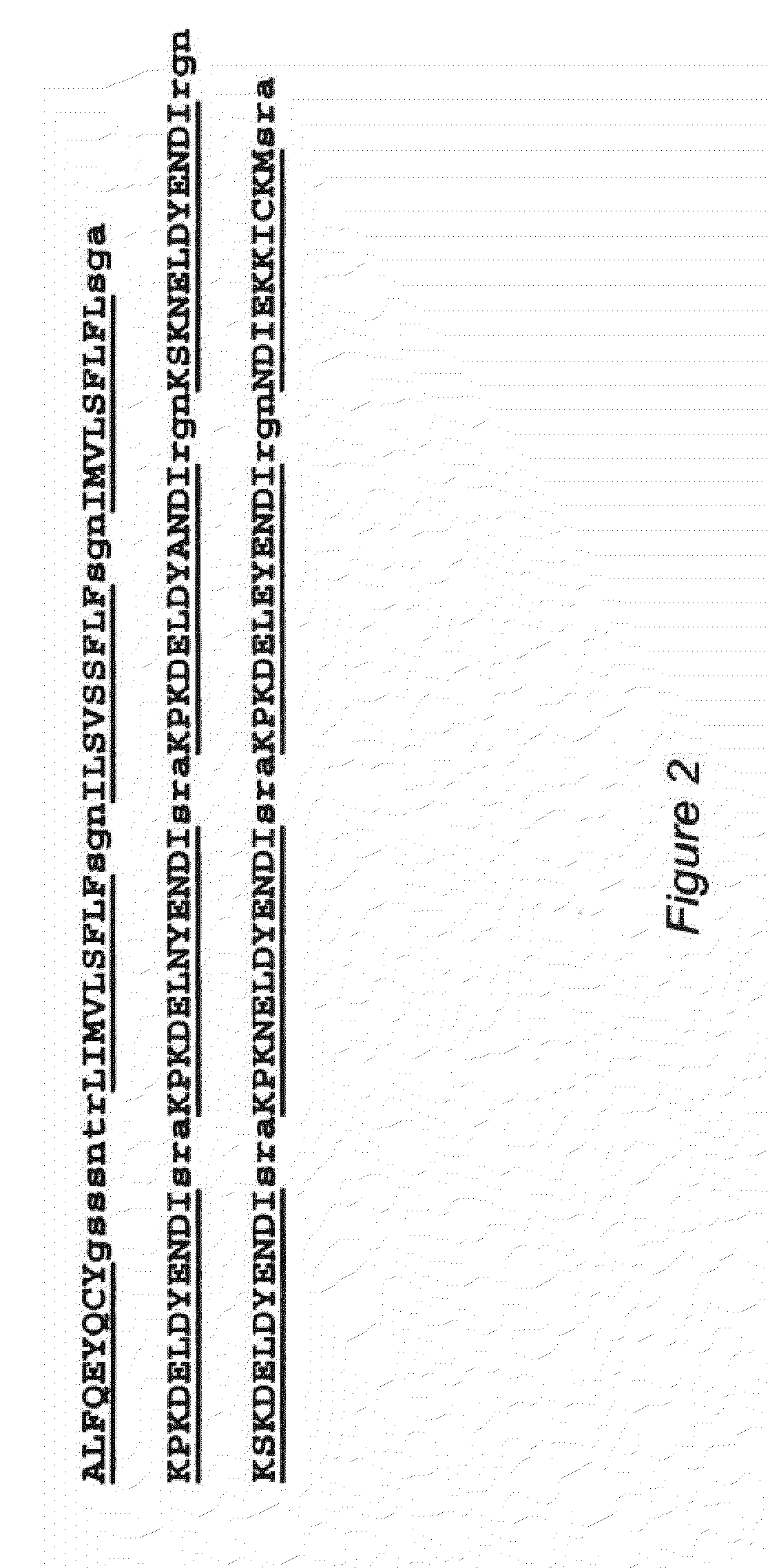
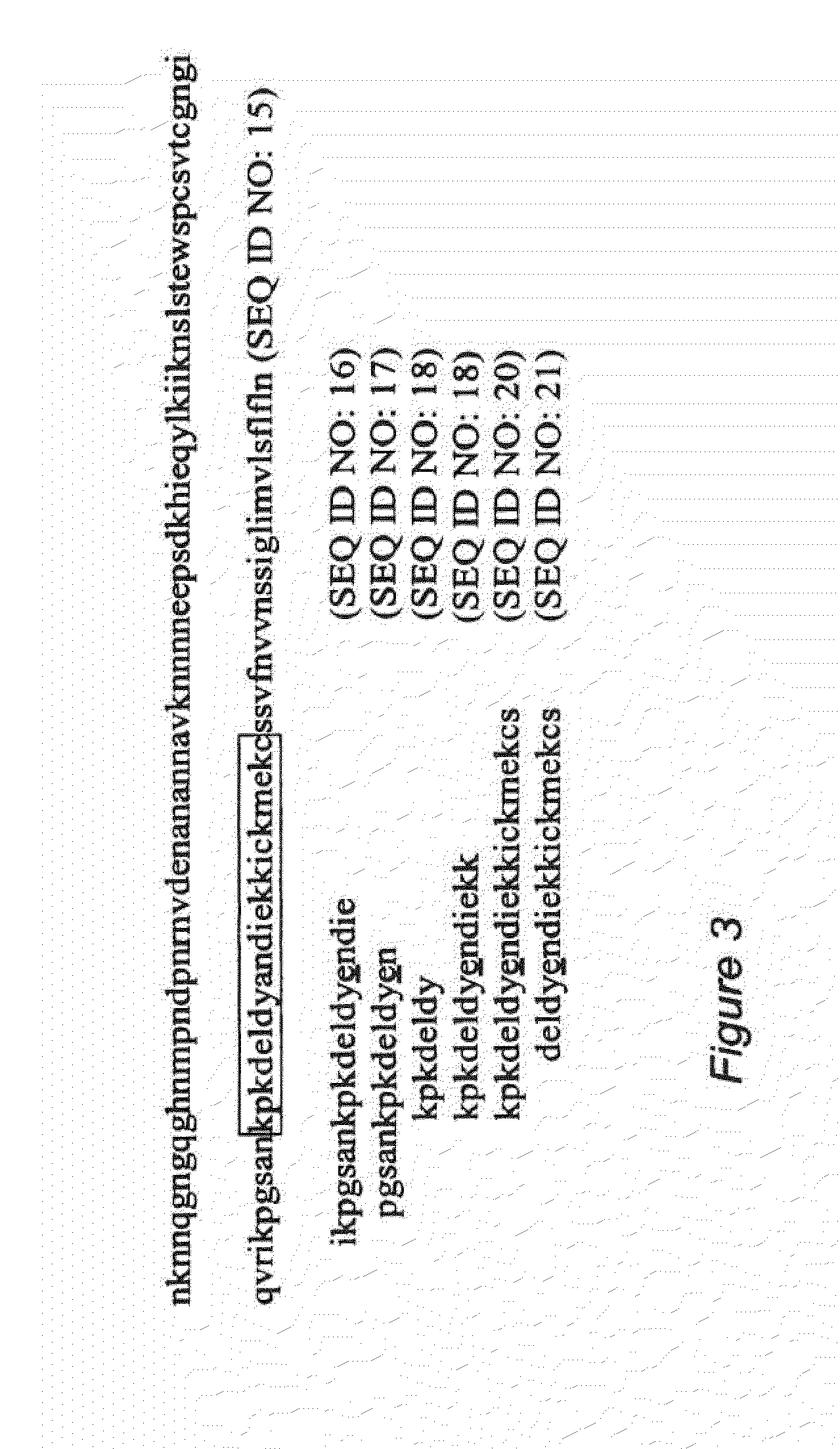
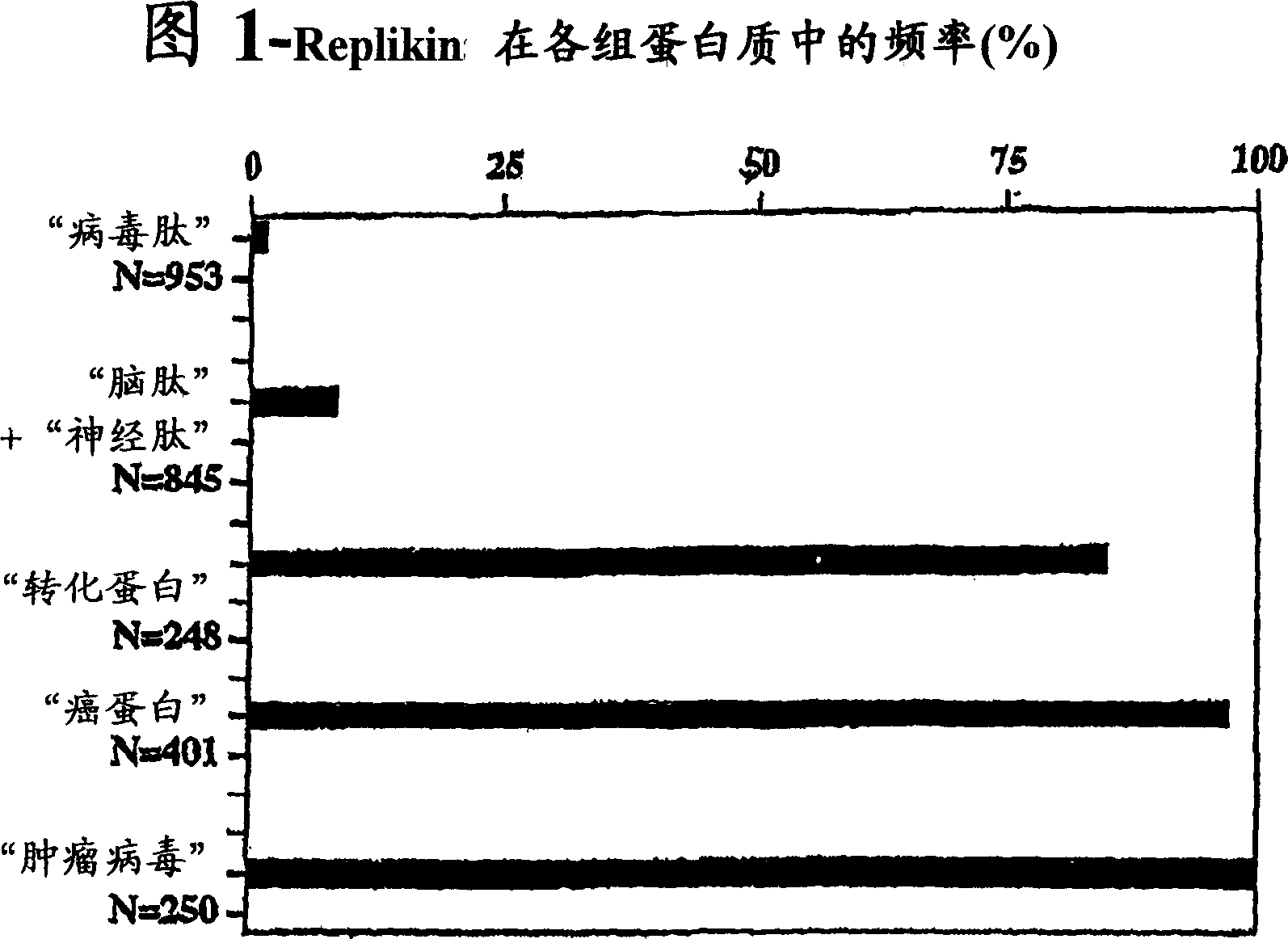
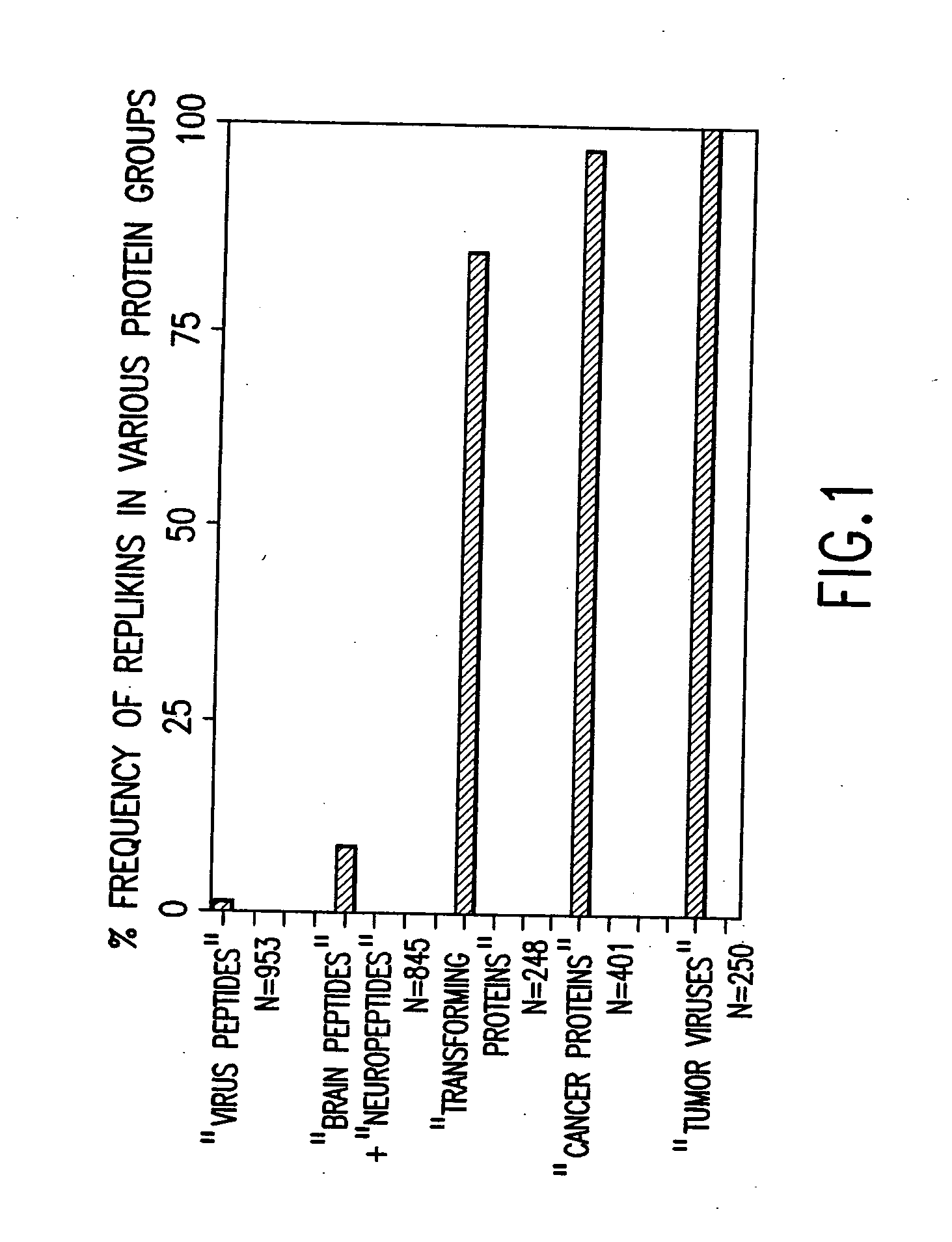
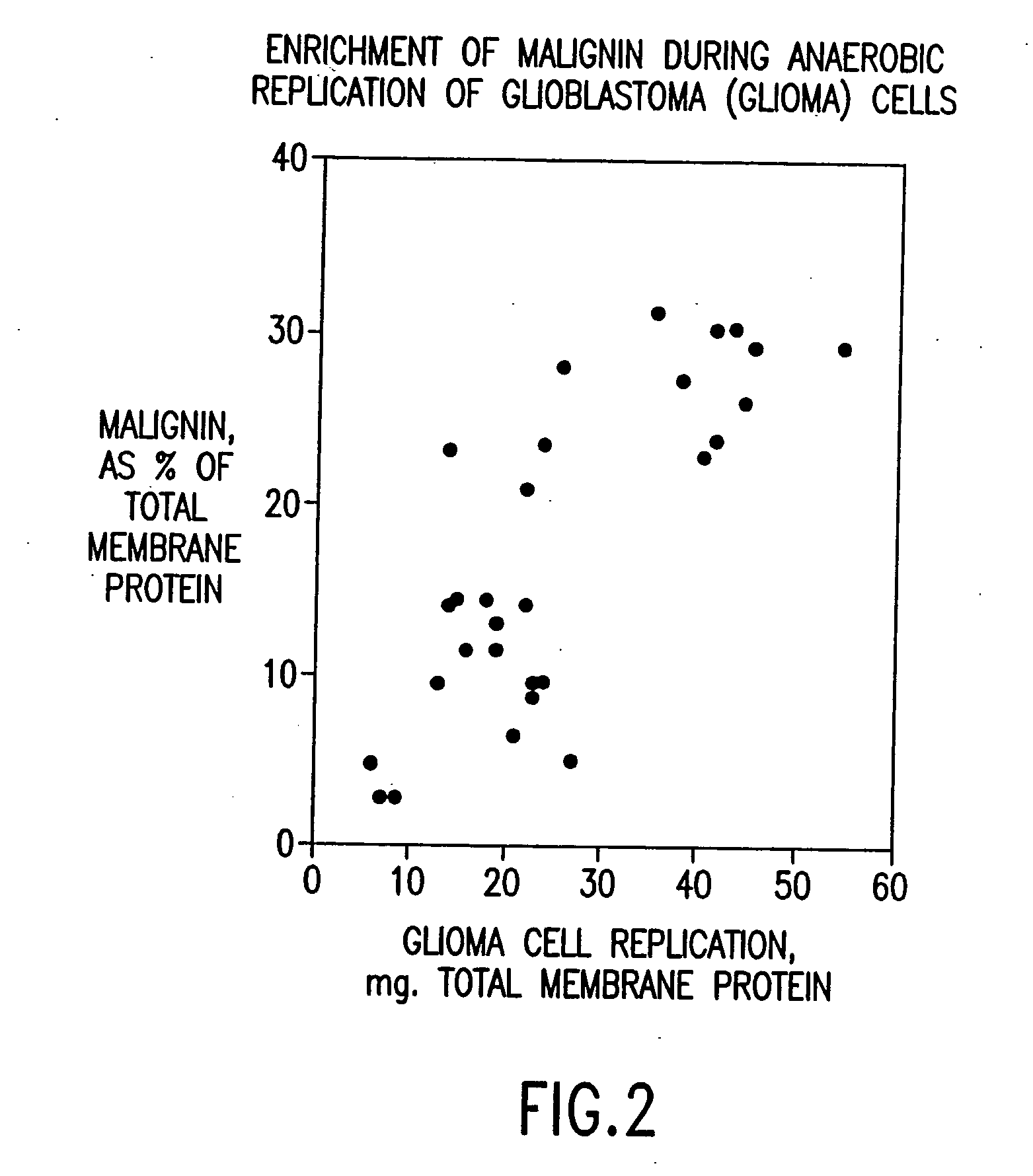
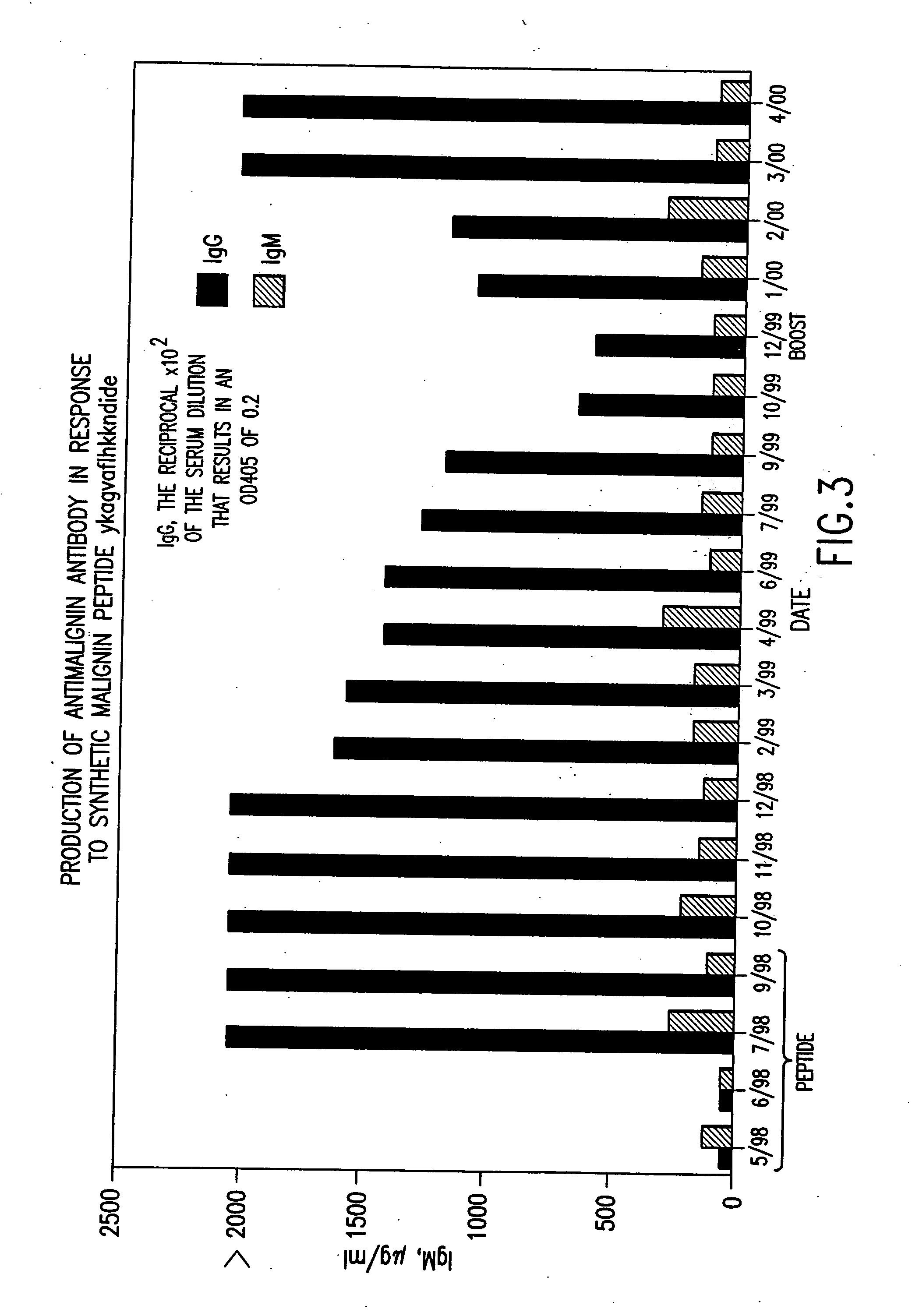
Replikin peptides in rapid replication of glioma cells and in influenza epidemics
Peptides of influenza virus hemagglutinin protein and Plasmodium falciparum malaria antigen, antibodies specific for the peptides, influenza vaccines, malaria vaccines and methods of stimulating the immune response of a subject to produce antibodies to influenza virus or malaria are disclosed. Also disclosed are methods for formulating vaccines for influenza virus.
Owner:BOGOCH SAMUEL +1
Preparation and usage of plasmodium fusion antigen
InactiveUS7101556B2Simple and low-cost methodBacteriaPeptide/protein ingredientsImmunogenicityGenetic engineering
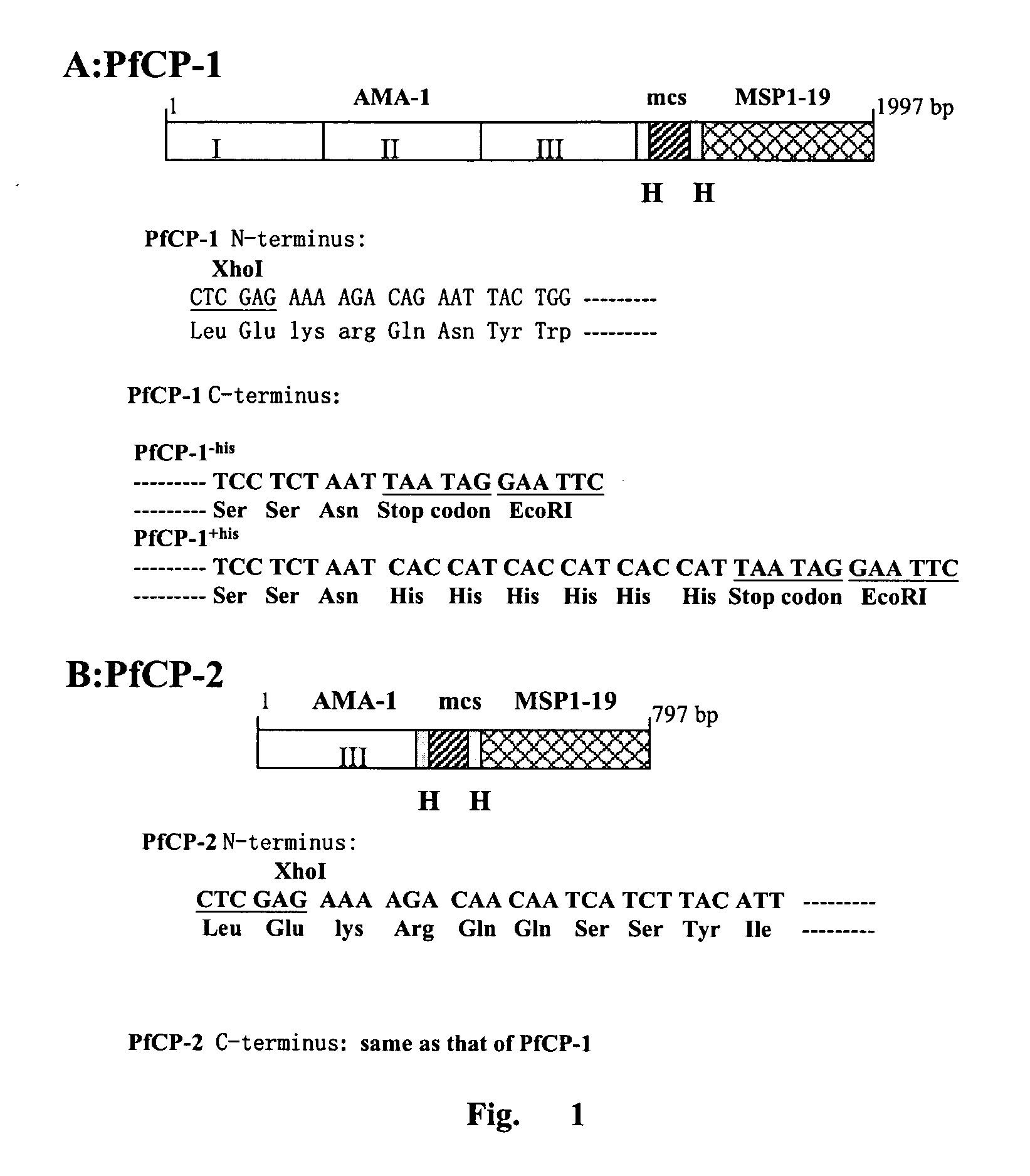
The invention provides a fusion protein comprising the Plasmodium merozoite surface protein-1 (MSP1) and the Plasmodium apical membrane antigen 1 (AMA-1), the encoding DNA sequence, the vector containing the sequence, the host cell containing the vector, and the genetic engineering method for preparing the fusion protein and the usage for producing anti-malarial vaccine. The AMA-1 / MSP1 fusion protein of the present invention has excellent immunogenicity and can cause an effective immune response against Plasmodium in individuals.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Serum antibody assay for determining protection from malaria, and pre-erythrocytic subunit vaccines
Disclosed herein are diagnostic assays for identifying individuals that are protected against Plasmodium falciparum caused malaria. Such assays are particularly useful for determining not only the protective efficacy of Pf whole parasite vaccines for individual subjects, but also within populations of vaccinated subjects. The assays comprise the use of proteomes representing at least 50% of Pf, preferably coupled to a solid phase as a fixed array. The arrays are used to probe the sera of human subjects, particularly subjects of human clinical trials of whole parasite malaria vaccines as well as public health vaccination campaigns. Serum samples with antibody profiles most strongly reactive in multiplex to CSP and MSP5 demonstrate a sensitivity of from 92% to 100% and a specificity of from 84% to 89%.
Owner:UNITED STATES OF AMERICA +2
Process for producing antigenic substance
InactiveUS20060233789A1Wide rangeHinders its propagationBiocideSnake antigen ingredientsEmbryoMalaria
An object of the present invention is to provide a means for producing an antigenic component with the retained native antigenicity using a cell-free protein synthesis. In particular, it is an object to provide a means for producing an antigenic component without depending on codon usage, like expressing an antigenic component from a gene containing a large amount of AT. The present inventors have made a strenuous study to solve the matters described above and successfully completed the present invention by preparing an antigenic component with the retained antigenicity, in particular a malaria antigen useful for manufacturing a malaria vaccine, through a system with the use of a wheat embryo among cell-free protein synthesis means.
Owner:CELLFREE SCI
Blood stage malaria vaccine
An immunogenic composition for use as a blood-stage malaria vaccine, a method of producing the immunogenic composition and a method of treatment of malaria are provided. The immunogenic composition includes isolated or purified merozoites, or red blood cells infected with merozoites, treated with centanamycin or tafuramycin A. The immunogenic composition does not include an adjuvant. A single dose of the immunogenic composition is sufficient to protect an animal against subsequent malaria infection by the same isolate, strain or species of Plasmodium used in the immunogenic composition, or by one or more heterologous isolates, strains or species of Plasmodium.
Owner:GRIFFITH UNIVERSITY +2
Three-component-multistage malaria vaccine
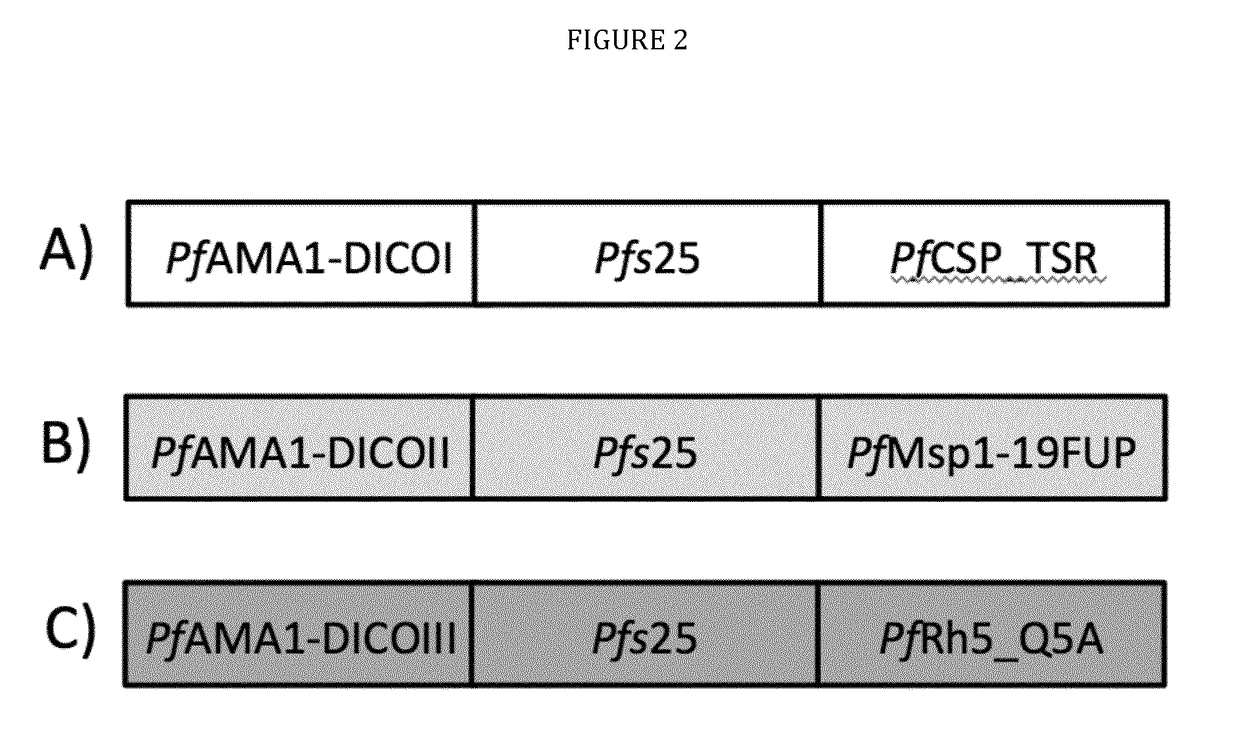
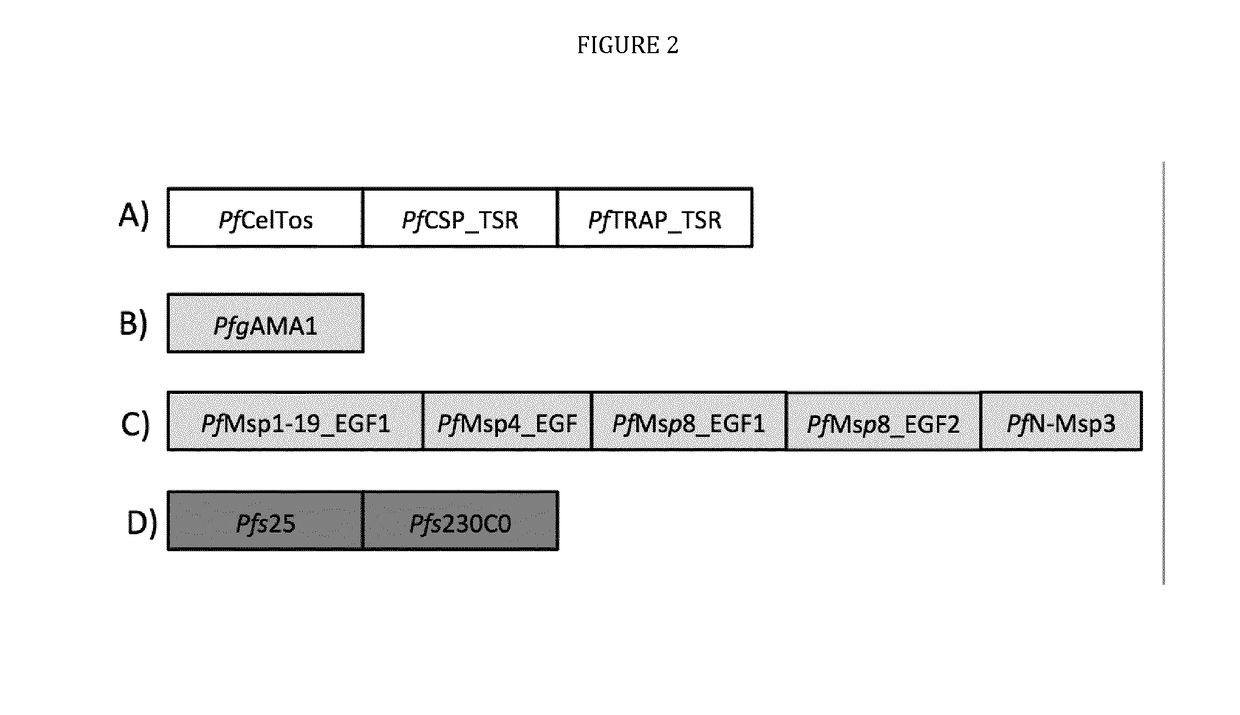
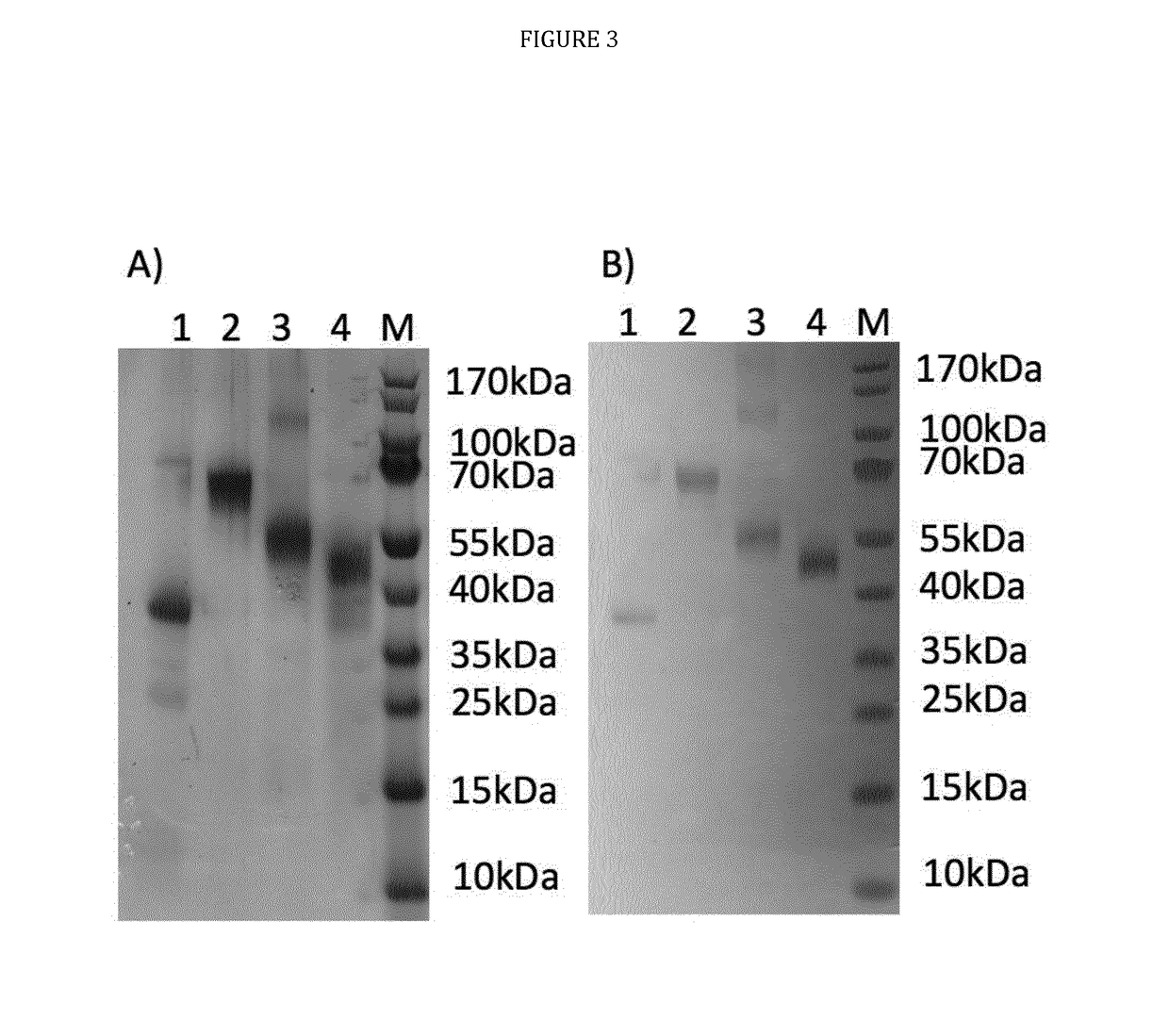
The technology provided herein relates to novel malaria vaccines composed of different recombinant proteins, in particular recombinant fusion proteins comprising several different Plasmodium falciparum antigens from the pre-erythrocytic, the blood, and the sexual parasite main stages. The proteins may be used in a mixture vaccine formulation to elicit protective immune responses in humans. Nucleic acid molecules encoding said recombinant proteins, vectors and host cells containing the nucleic acids and methods for preparation and producing such proteins are also disclosed, as well as antibodies induced or generated by the use of said malaria vaccines and the use of such antibodies or recombinant derivatives for passive immunotherapy.
Owner:FRAUNHOFER GESELLSCHAFT ZUR FOERDERUNG DER ANGEWANDTEN FORSCHUNG EV
Preparation and usage of plasmodium fusion antigen
InactiveUS20040063190A1Simple and low-cost methodBacteriaPeptide/protein ingredientsPlasmodium merozoiteGenetic engineering
The invention provides a fusion protein comprising the Plasmodium merozoite surface protein-1 (MSP1) and the Plasmodium apical membrane antigen 1 (AMA-1), the encoding DNA sequence, the vector containing the sequence, the host cell containing the vector, and the genetic engineering method for preparing the fusion protein and the usage for producing anti-malarial vaccine. The AMA-1 / MSP1 fusion protein of the present invention has excellent immunogenicity and can cause an effective immune response against Plasmodium in individuals.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Vaccines against pregnancy-associated malaria
InactiveUS20160136253A1Improve purification effectImprove stabilityProtozoa antigen ingredientsWhole-cell/virus/DNA/RNA ingredientsImmunogenicityNucleotide
The present invention relates to combinations of polypeptides or of polynucleotides corresponding to a specific region of the N-terminal portion of the VAR2CSA protein of different parasitic families or lines of Plasmodium falciparum, and to their use in the prevention of pregnancy-associated malaria. The invention also relates to immunogenic compositions and to vaccines useful for preventing malaria in pregnant women.
Owner:INSTITUT DE RES & DEV POUR LE DEVPEMENT
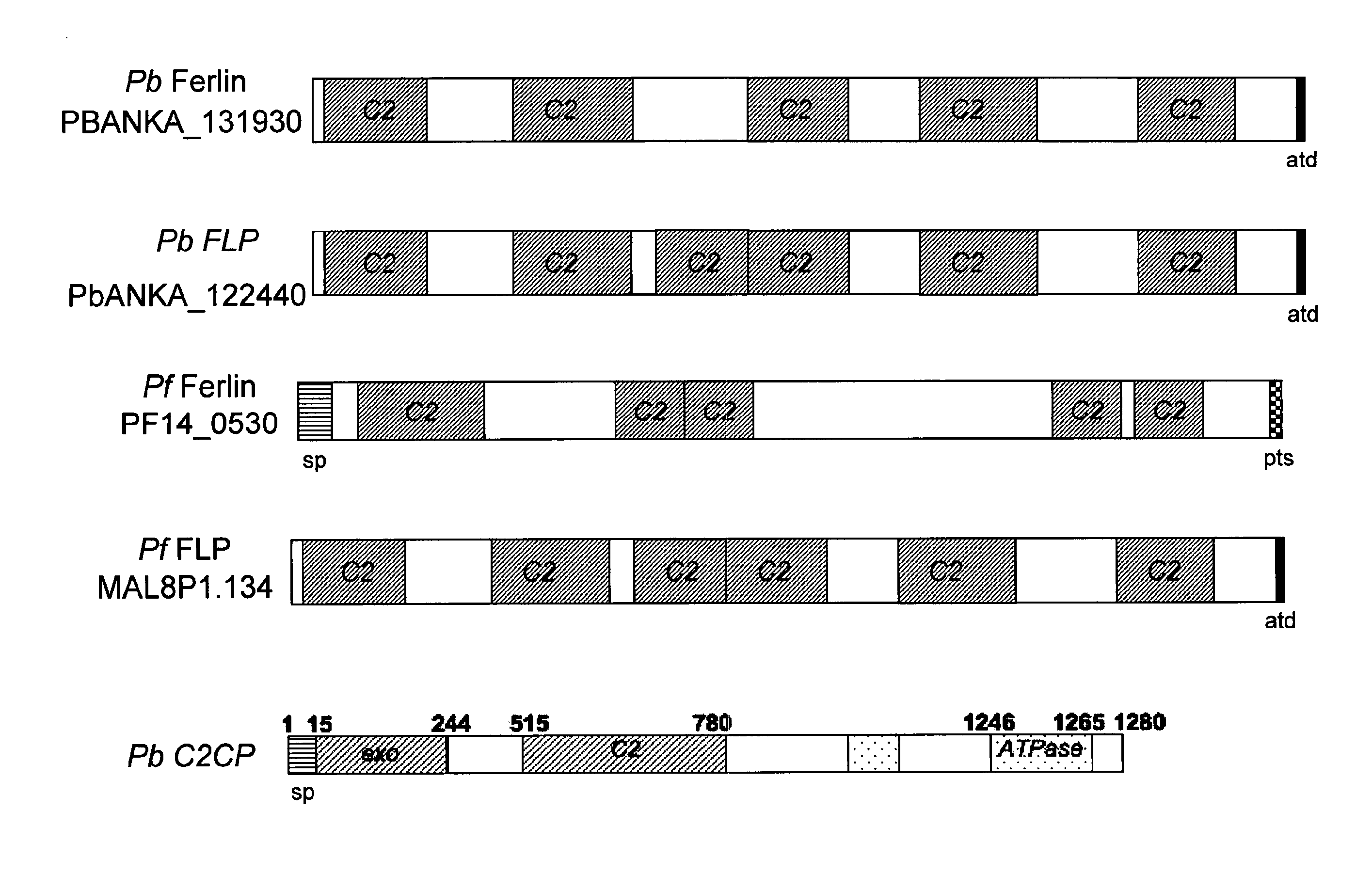
Malaria vaccines based on apicomplexan ferlins, ferlin-like proteins and other C2-domain containing proteins
The present invention relates to peptides comprising at least one antigenic determinant or epitope of an apicomplexan Ferlin, Ferlin-like protein and / or another C2-domain containing protein for use as malaria vaccines. It further relates to compositions comprising said peptides and to the use of such compositions as malaria vaccines.
Owner:MALVA GMBH
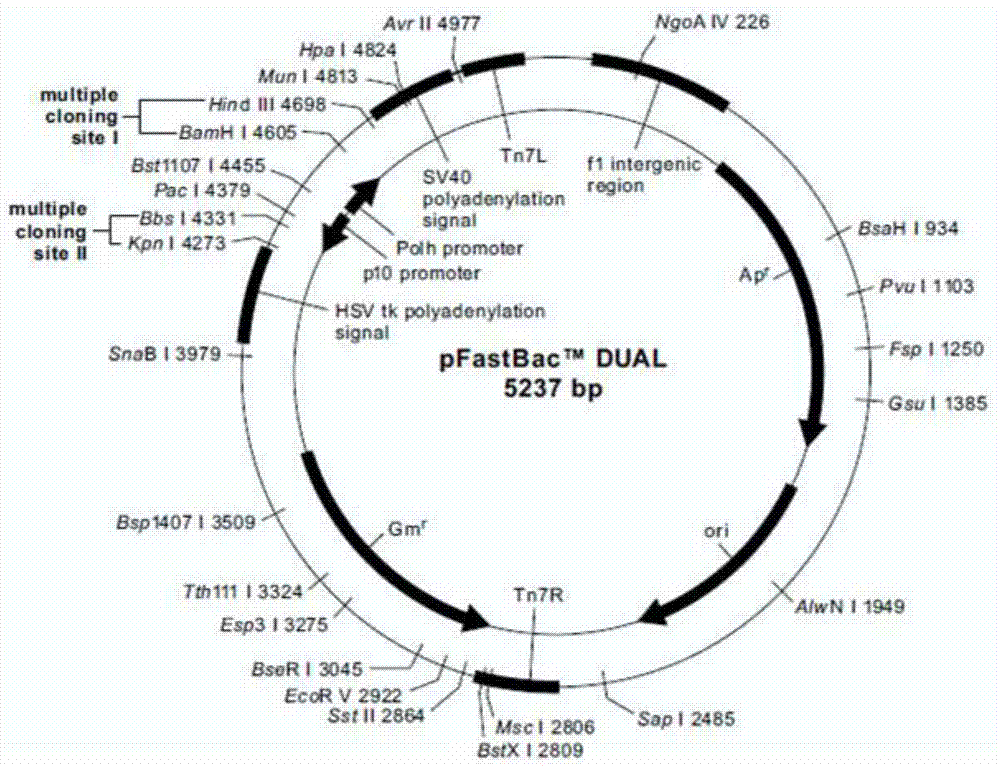
Recombinant vector, recombinant baculovirus prepared with the same and application of virus in preparation of malaria vaccines
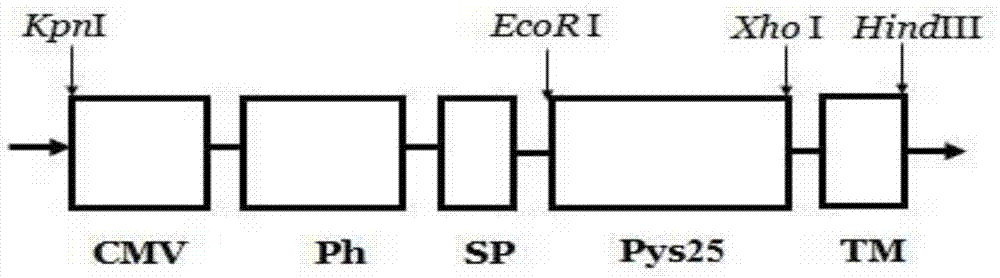
InactiveCN104711289AHigh homologyIncrease productionGenetic material ingredientsAntiparasitic agentsTiterHindIII
The invention provides a recombinant vector, a recombinant baculovirus prepared with the vector and application of the virus in preparation of malaria vaccines. The recombinant vector is constructed by inserting a section of recombinant sequence into the pFastBacDual vector. The recombinant sequence is formed by: according to CMV, Ph, SP and TM sequences, adding EcoR I enzyme site and Xho I enzyme site between SP and TM, adding KpnI enzyme site in front of CMV-F, and adding HindIII enzyme site after TM-R. The recombinant baculovirus is obtained by: inserting Plasmodium yoelii Pys25 antigen gene into the recombinant vector, conducting homologous recombination with the genome of a shuttle vector Bacmid through transposition, then transfecting a bombyx mori cell, and performing packaging in the bombyx mori cell. Through simple separation and purification of the recombinant baculovirus, an antibody with good titer can be prepared, thus providing a reference for study of P25 malaria vaccines.
Owner:特菲(天津)生物医药科技有限公司
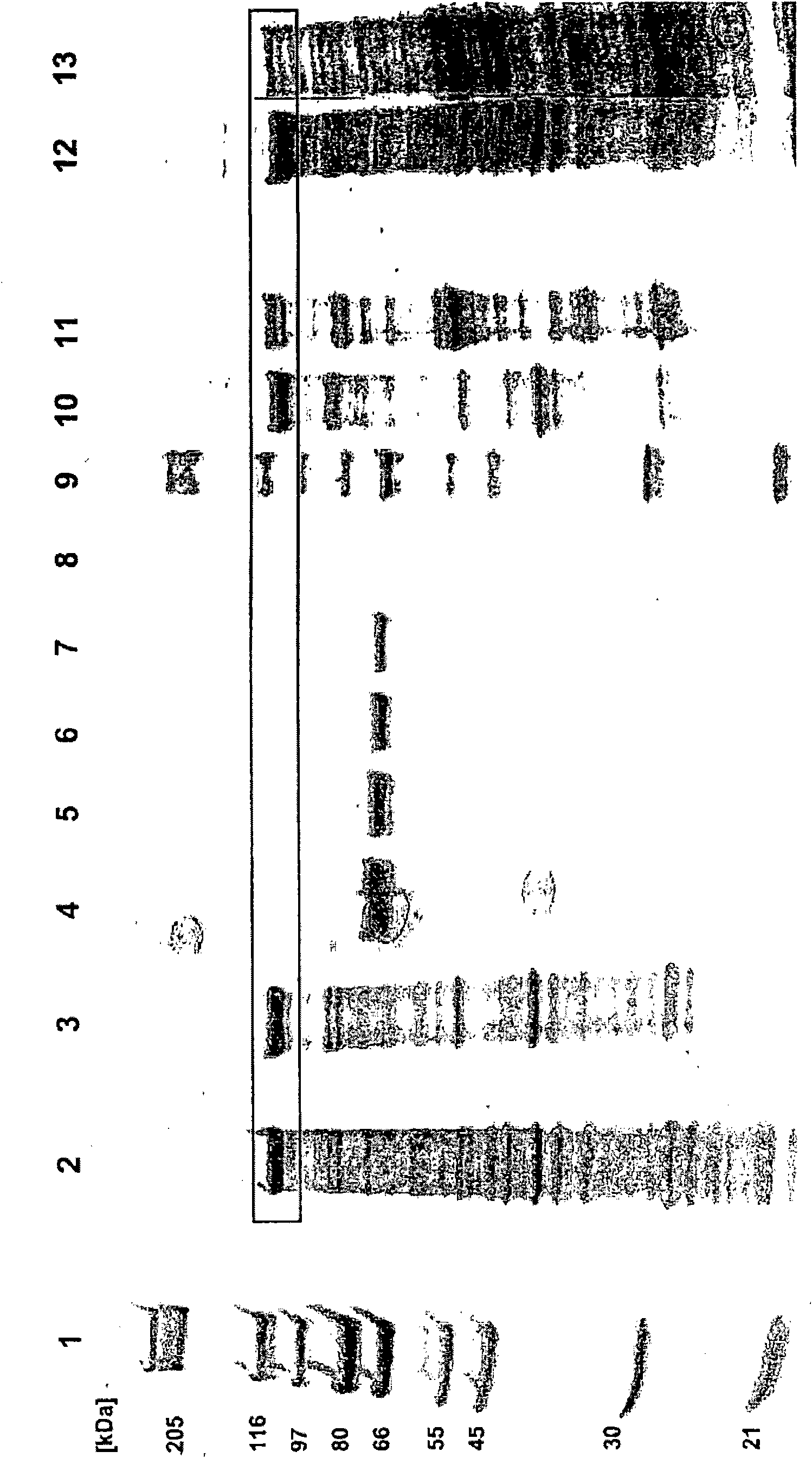
Plasmodium falciparum AMA-1 protein and uses thereof
InactiveUS20060264619A1Eliminate the problemImprove responseSugar derivativesDepsipeptidesMalarial parasitesBiology
In this application is described the expression and purification of a recombinant Plasmodium falciparum (3D7) AMA-1 ectodomain. The method of the present invention produces a highly purified protein which retains folding and disulfide bridging of the native molecule. The recombinant AMA-1 is useful as a diagnostic reagent, for use in antibody production, and as a protein for use alone, or as part of, a vaccine to prevent malaria.
Owner:LANAR DAVID +3
Conjugates of Plasmodium falciparum surface proteins as malaria vaccines
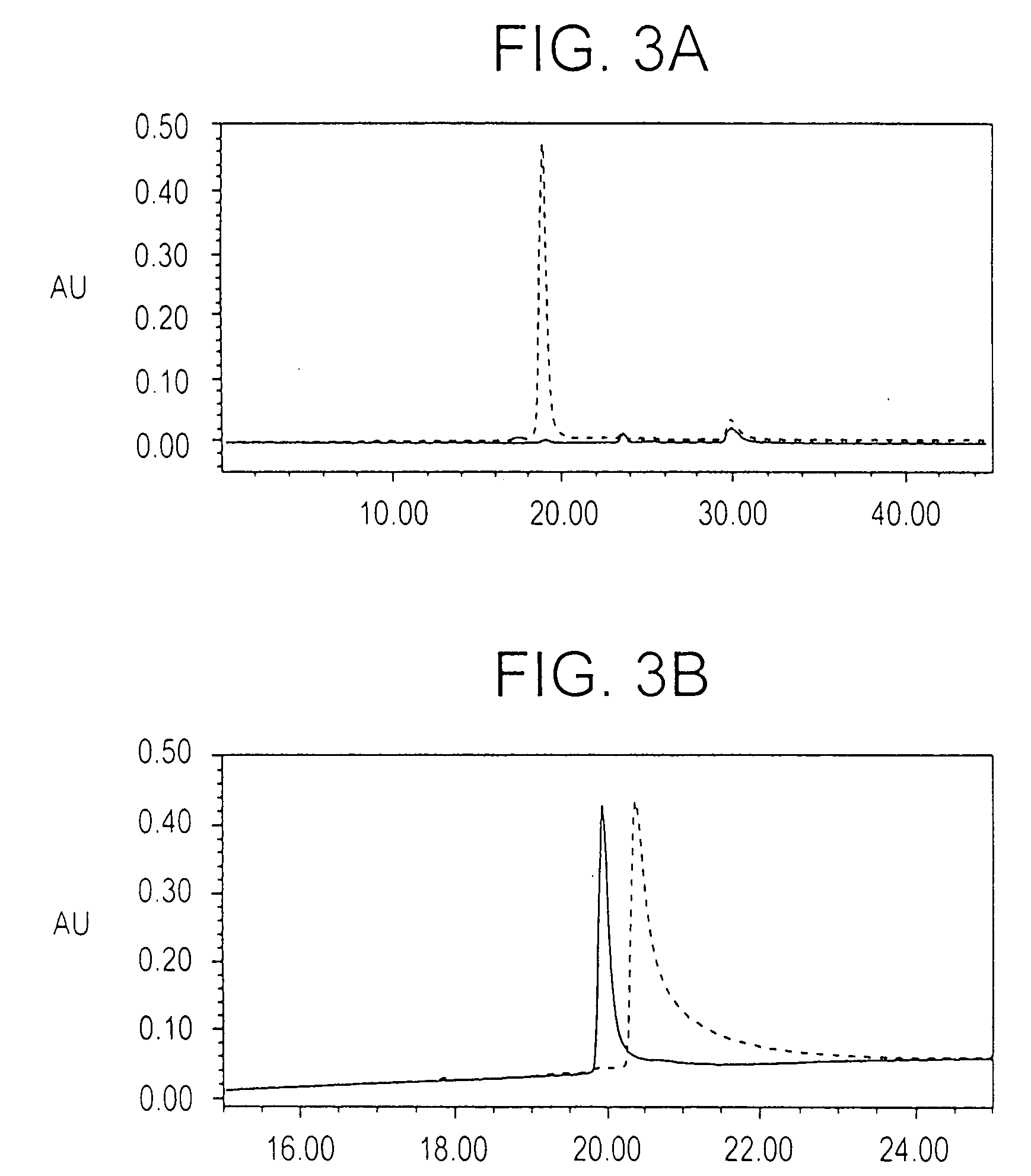
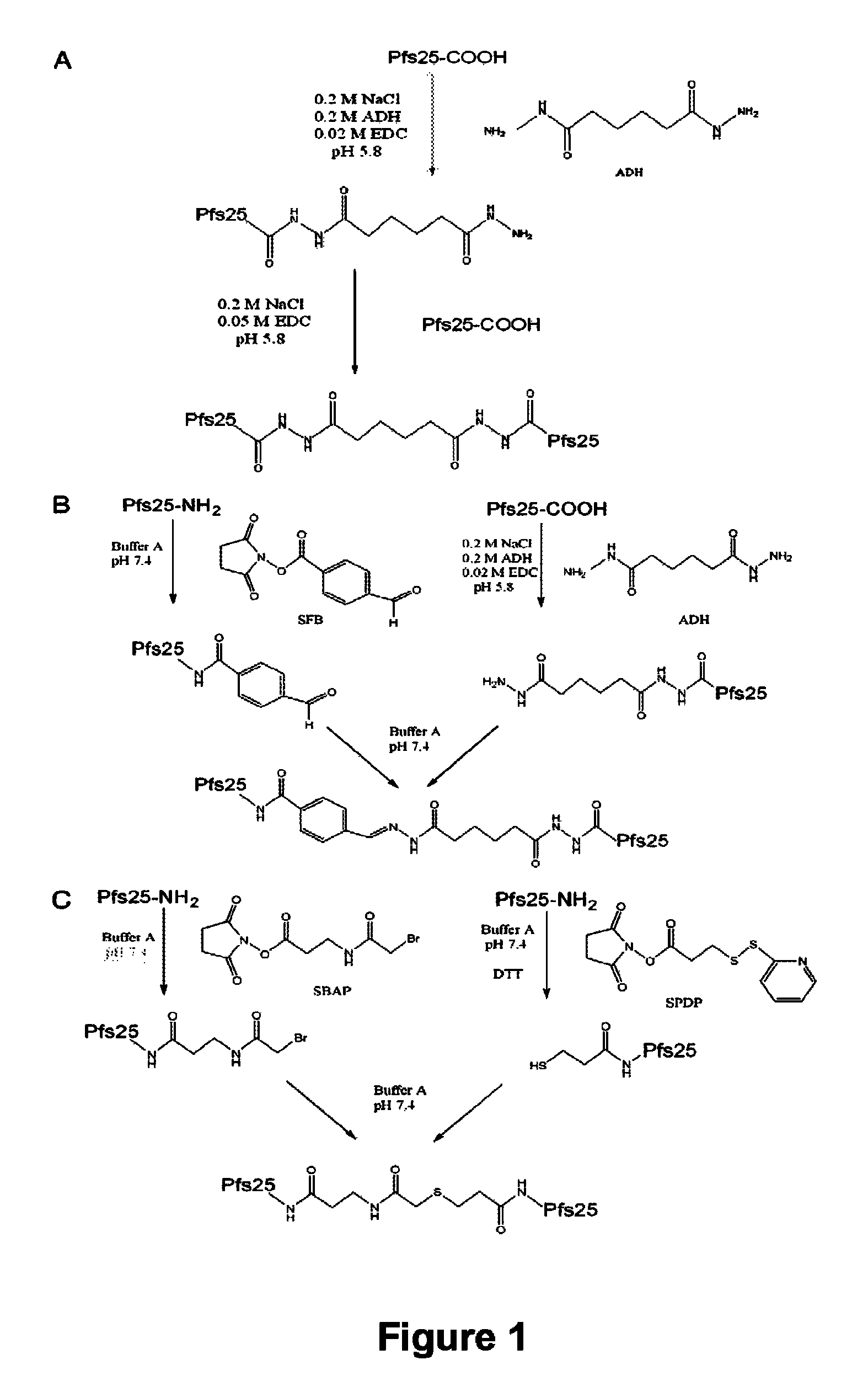
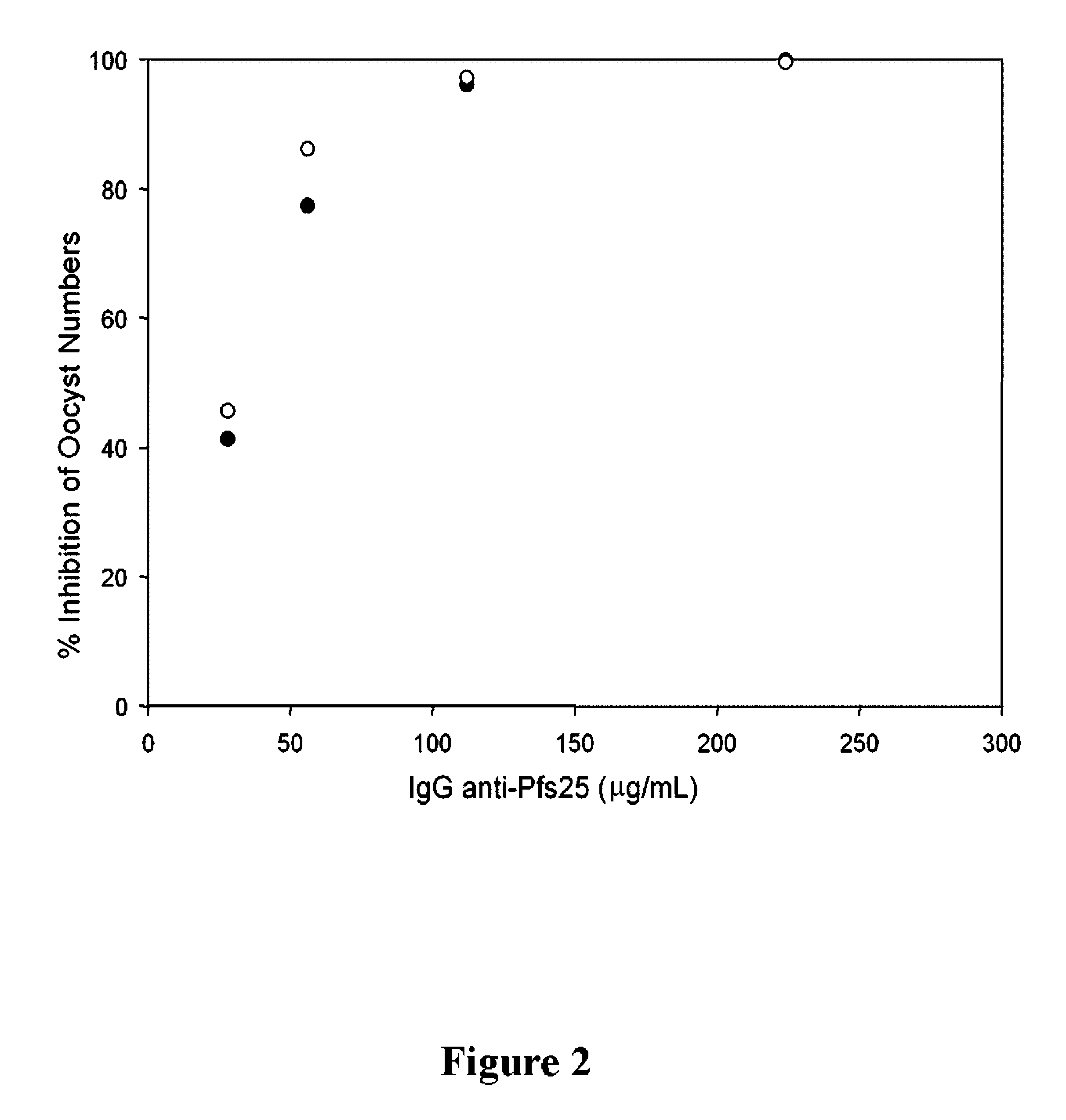
InactiveUS8383133B2Cell receptors/surface-antigens/surface-determinantsAntibody mimetics/scaffoldsMalarial parasiteTGE VACCINE
Conjugates of ookinete surface protein Pfs25 are provided that are efficacious as vaccines against Plasmodium falciparum, the most severe form of malaria. Conjugates of ookinete surface protein Pvs25 for use as a vaccine against Plasmodium vivax are also provided. Methods for preparing the conjugates, which comprise the ookinete surface protein bound onto itself or onto another protein by a linking group, are also provided.
Owner:UNITED STATES OF AMERICA
Malaria vaccine
ActiveUS20060024324A1Improve purification effectSimple filterPeptide/protein ingredientsProtozoaStaphylococcus lactisHybrid protein
A fusion protein, from P. falciparum Glutamate-rich protein(GLURP) genetically coupled to P. falciparum Merozoite surface protein 3 (MSP3) was produced in Lactococcus lactis as a secreted recombinant GLURP-MSP3 hybrid protein and experiments showed that the GLURP-part of the hybrid increased the overall antibody response. Immunizations with the hybrid protein consistently generated a stronger antibody response against the individual GLURP and MSP3 domains than a mixture of the two recombinant molecules injected at one site or the individual recombinant molecules injected simultaneously at two different sites. The difference was most pronounced for the MSP3-specific antibody response suggesting that T cell epitopes located in the GLURP RO-region provide help for B-cell epitopes in the MSP3 region. Moverover, when the animals were injected with a mixture of GLURP and MSP3, individual mice tended to mount a predominant antibody response against either molecule: in some animals GLURP was immunodominant whereas in other animals MSP3 was the dominant immunogen. Additionally, the hybrid was also more antigenic than the individual recombinant proteins since the ELISA-titer of naturally occurring IgG antibodies, in clinically
Owner:STATENS SERUM INST
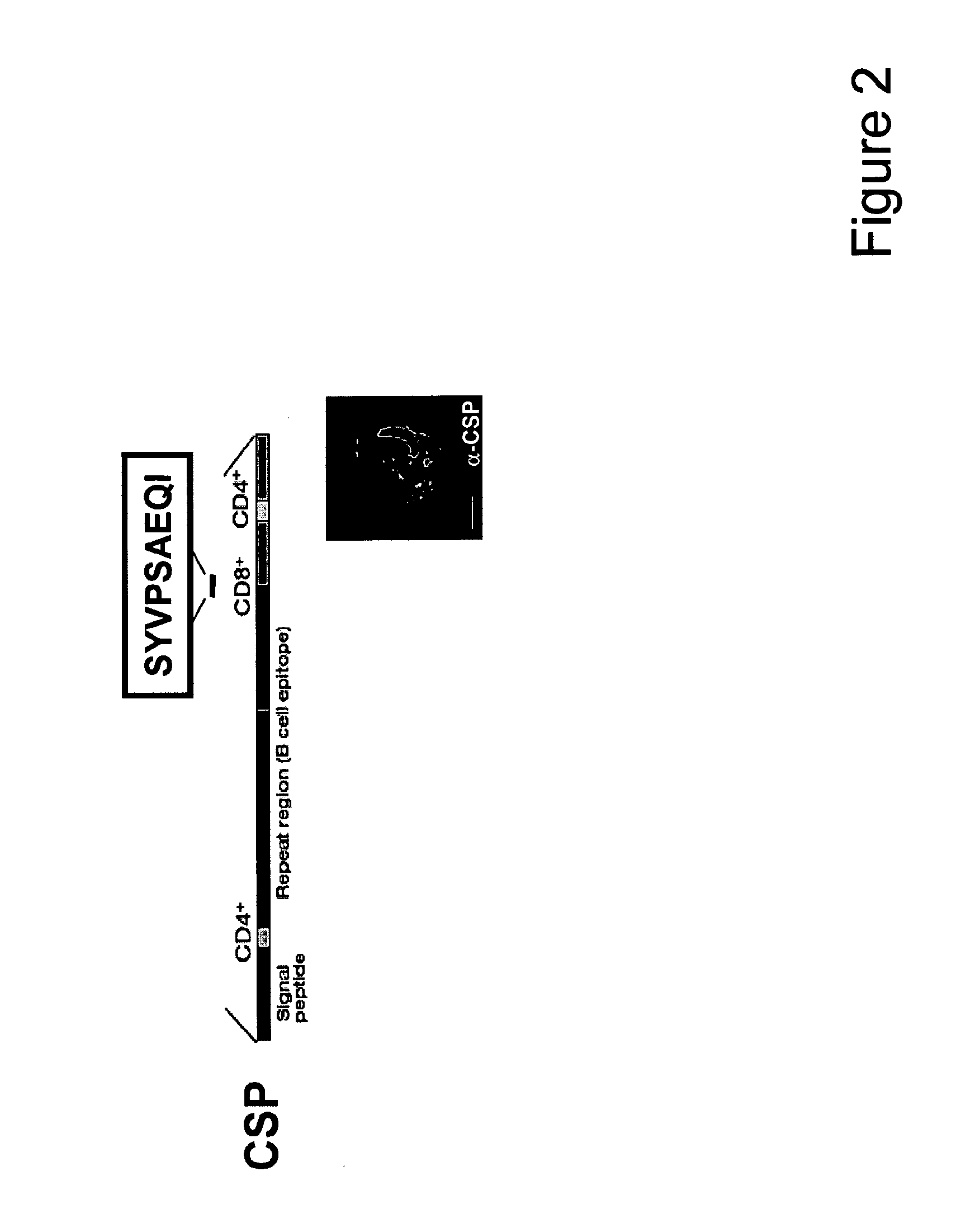
Malaria vaccine compositions and constituents which elicit cell mediated immunity
Malaria vaccines based on polyepitope constructs that elicit cell-mediated immunity against a broad spectrum of malaria parasites and which cover the majority of HLA alleles are provided. Epitopes in the polyepitope constructs are from regions of the Plasmodium falciparum circumsporozoite protein (CSP) known to contain CD4 and CD8 T cell epitopes, and include both epitopes from highly variable and highly conserved regions of CSP.
Owner:INT AIDS VACCINE INITIATIVE INC
Multi-component-multistage malaria vaccines
InactiveUS20170106071A1Antibody mimetics/scaffoldsBiological material analysisMalarial parasitePassive Immunizations
The present disclosure relates to novel malaria vaccines composed of different recombinant proteins, in particular recombinant fusion proteins comprising several different Plasmodium falciparum antigens from the pre-erythrocytic the blood, and the sexual parasite stages. The proteins and / or fusion proteins will be used in a mixture vaccine formulation to elicit protective immune responses in humans. Nucleic acid molecules encoding said recombinant proteins, vectors, host cells containing the nucleic acids and methods for preparation and producing such proteins; Antibodies induced or generated by the use of said malaria vaccines or said nucleic acid molecules encoding said proteins and / or fusion proteins and the use of such antibodies or recombinant derivatives for passive immunotherapy.
Owner:FRAUNHOFER GESELLSCHAFT ZUR FOERDERUNG DER ANGEWANDTEN FORSCHUNG EV
Malaria vaccine
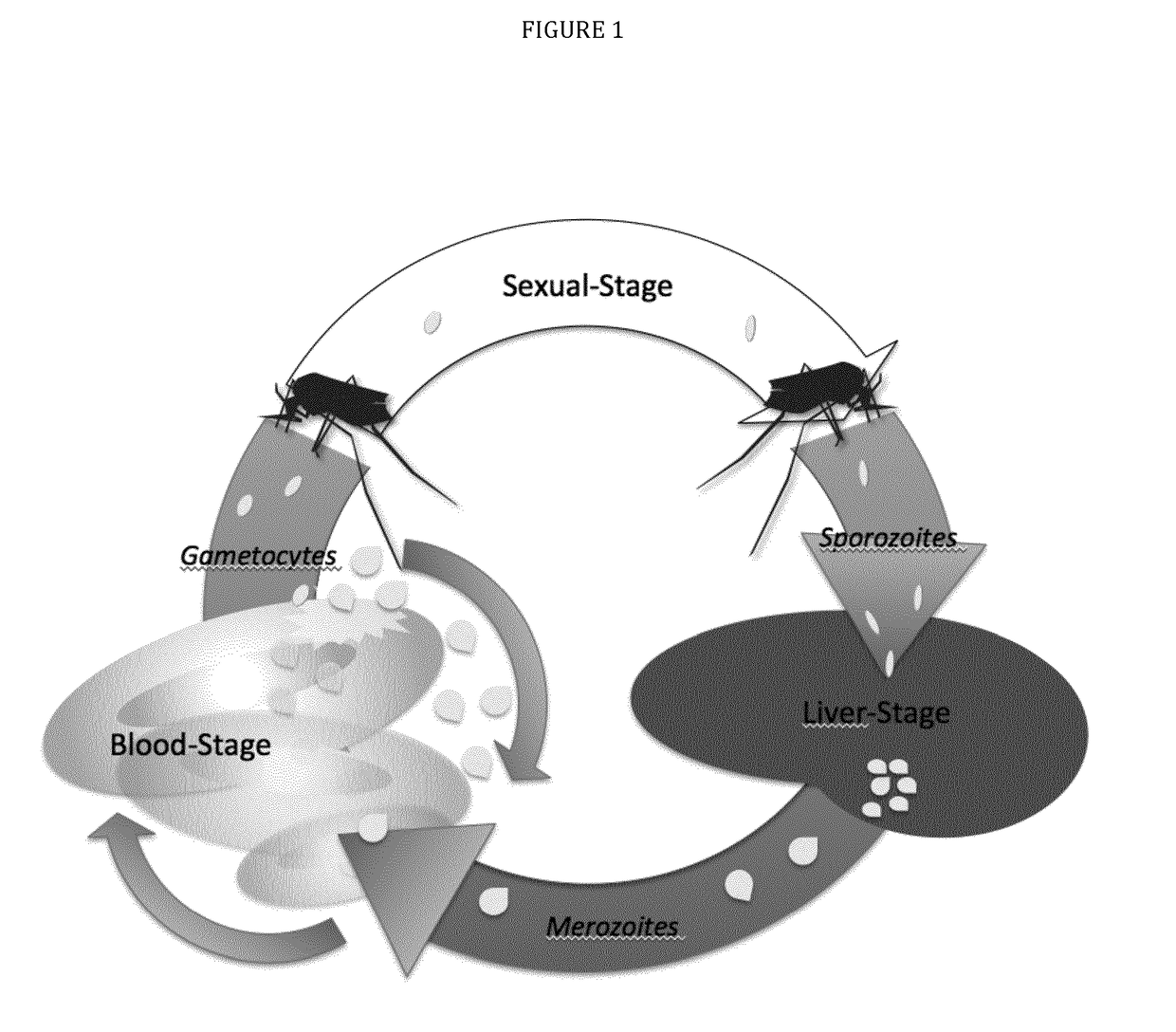
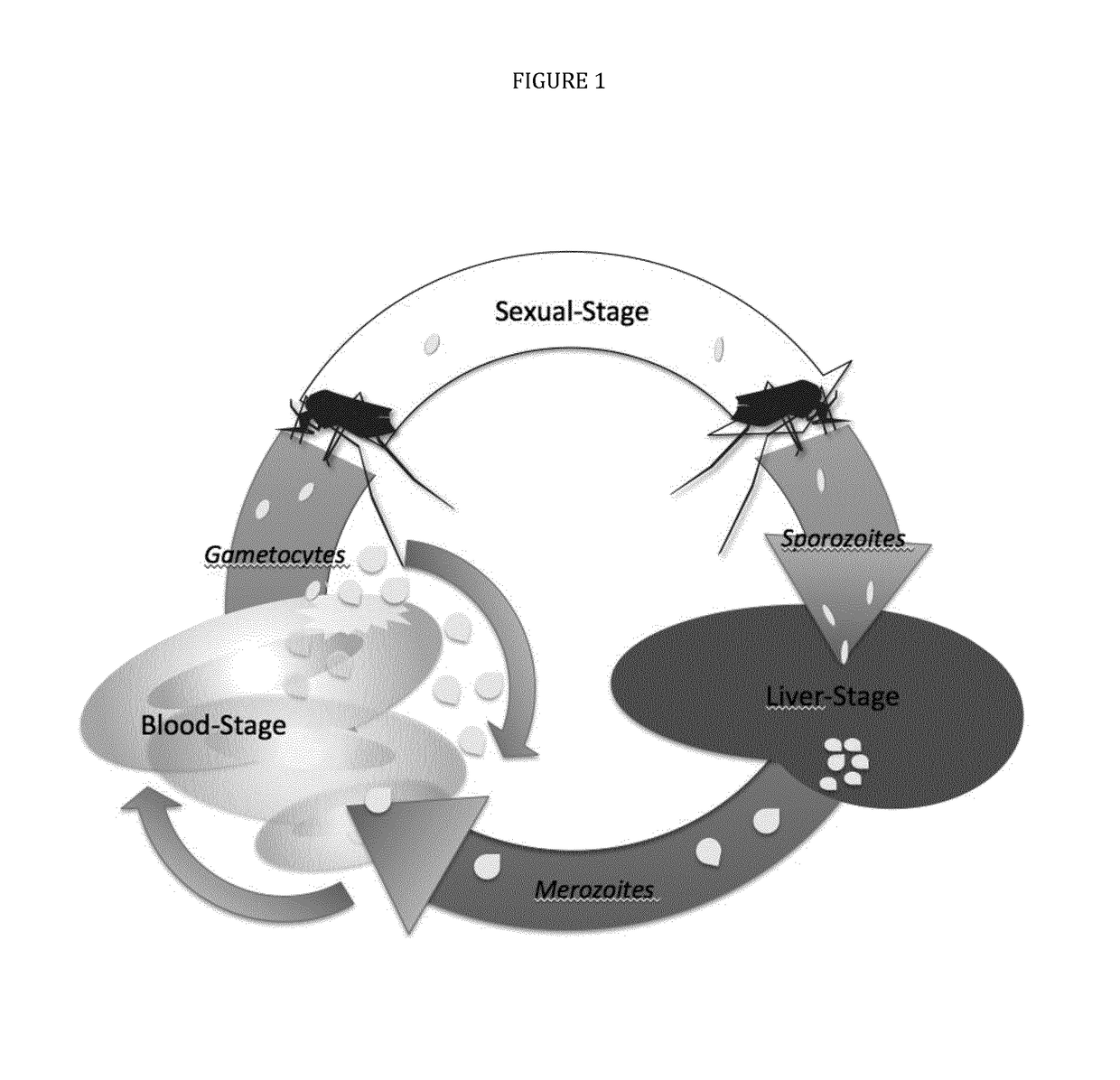
The present invention features immunogenic compositions based on pre-fertilization or post-fertilization antigens expressed in the circulating gametocytes in the peripheral blood of infected persons or on the malaria parastes' stages of develop-ment in the mosquito midgut including extracellular male and female gametes, fertilized zygote and ookinete. The invention also features methods to prevent the transmission of malaria using the immunogenic compositions of the invention.
Owner:US ARMY MEDICAL RES MATERIEL COMMAND USAMRMC +1
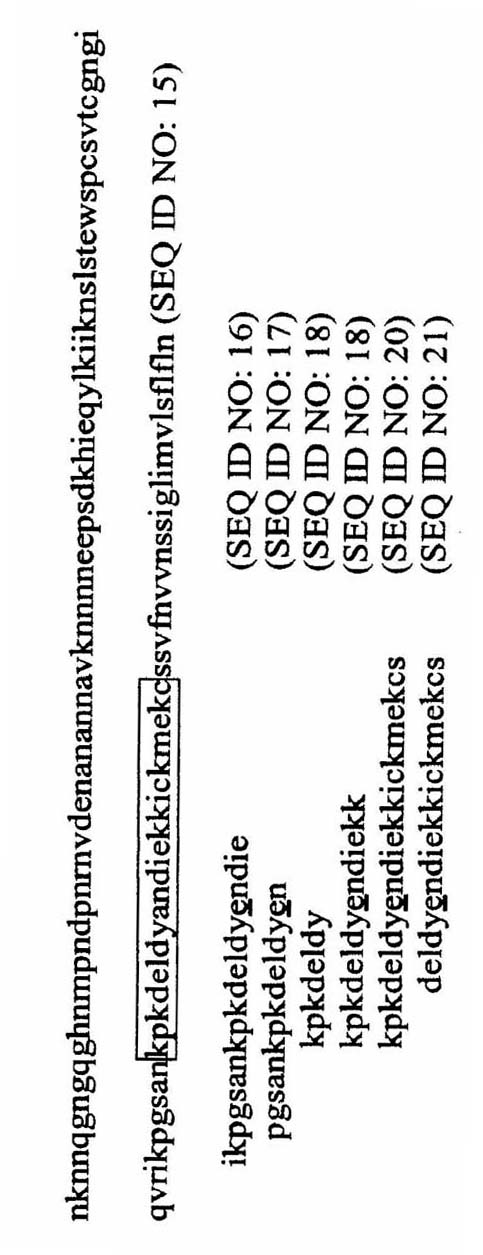
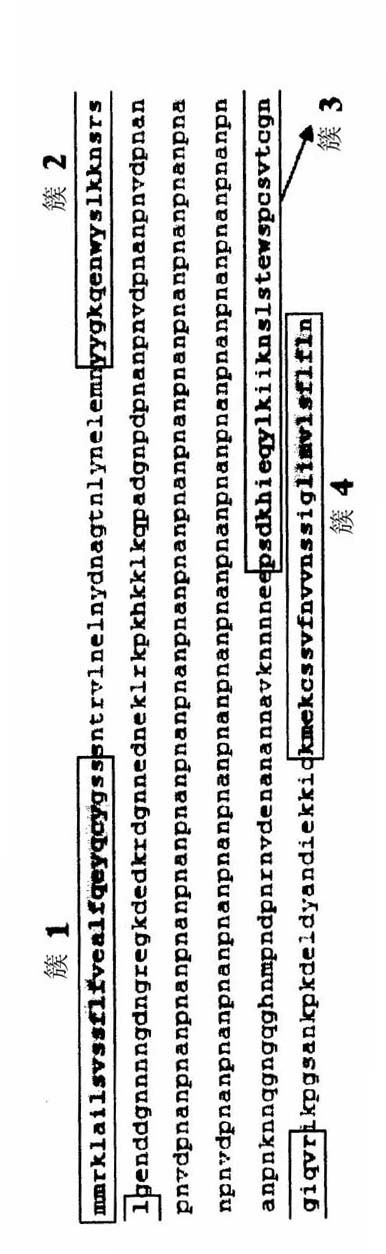
REPLIKIN peptides and uses thereof
The present invention discloses influenza virus hemagglutinin protein and peptide of malaria antigen of Plasmodium falciparum (Plasmodium falciparum), antibody specific to said peptide, influenza vaccine, malaria vaccine and immunity stimulating patients to produce anti-influenza virus or malaria antibody method of response. The invention also discloses methods for formulating vaccines against influenza viruses. The invention discloses an isolated peptide of Bacillus anthracis (Bacillus anthracis) anthrax toxin lethal factor protein pX01-107, an antibody specific to the peptide and an antibody that stimulates patients to produce anti-Bacillus anthracis anthrax toxin lethal factor protein pX01-107 method of the immune response. The present invention also discloses isolated peptides of the smallpox virus surface antigen S precursor protein, antibodies specific to said peptides and methods of stimulating an immune response in a patient to produce antibodies against the smallpox virus surface antigen S precursor protein.
Owner:塞缪尔·博戈奇 +1
Biofusion proteins as Anti-malaria vaccines
ActiveCN109963586AImproving immunogenicityAntibody mimetics/scaffoldsCarrier-bound antigen/hapten ingredientsHeterologousImmunogenicity
The invention relates to fusion proteins which comprise at least one antigenic amino acid sequence fused to a carrier heterologous protein sequence, wherein the antigenic sequence comprises an epitopic sequence of a Plasmodium protein and the carrier heterologous protein sequence is a sequence that is immunogenic in humans. The proteins are useful as anti-malaria vaccines.
Owner:VAC4ALL PTE LTD
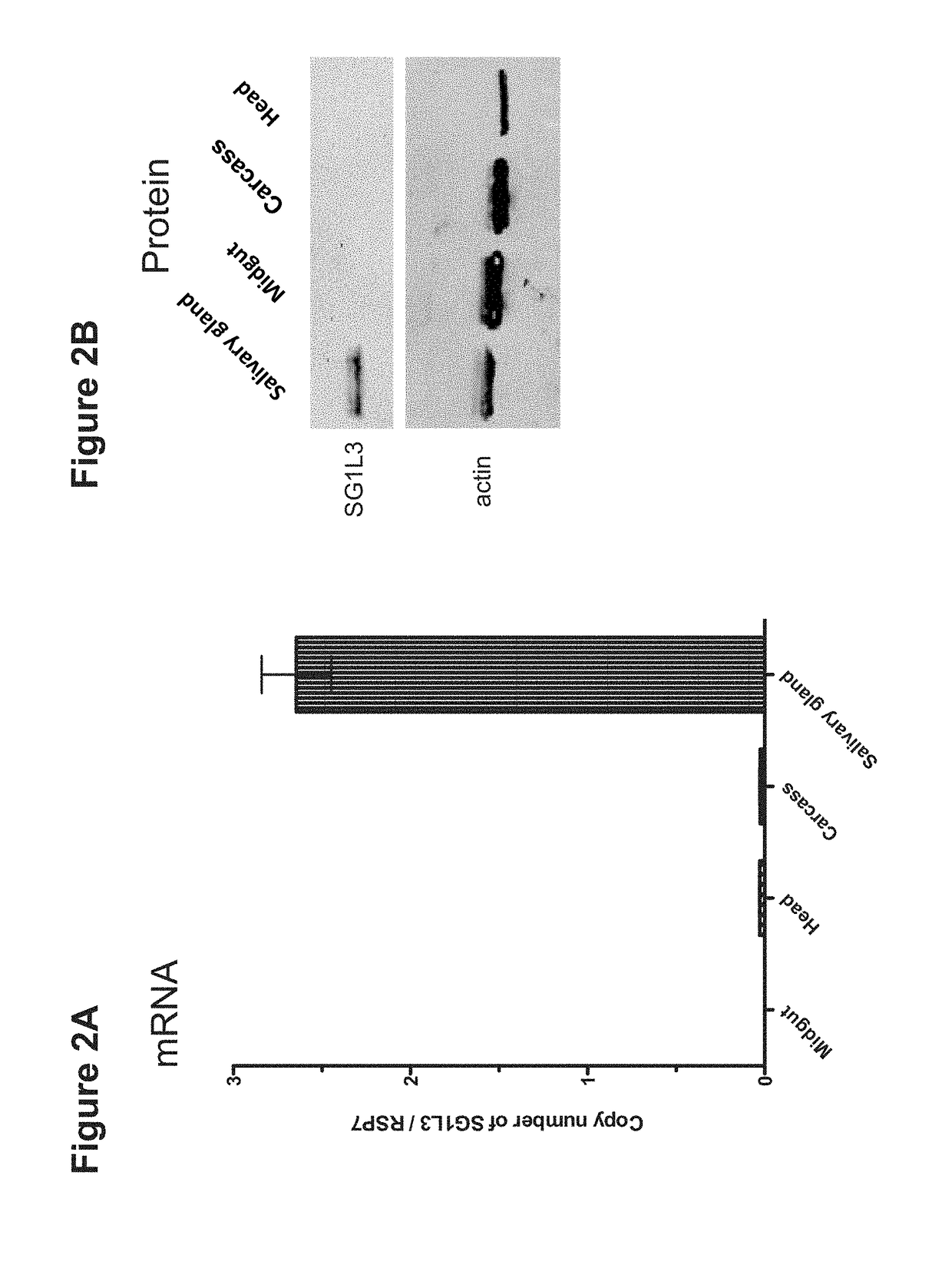
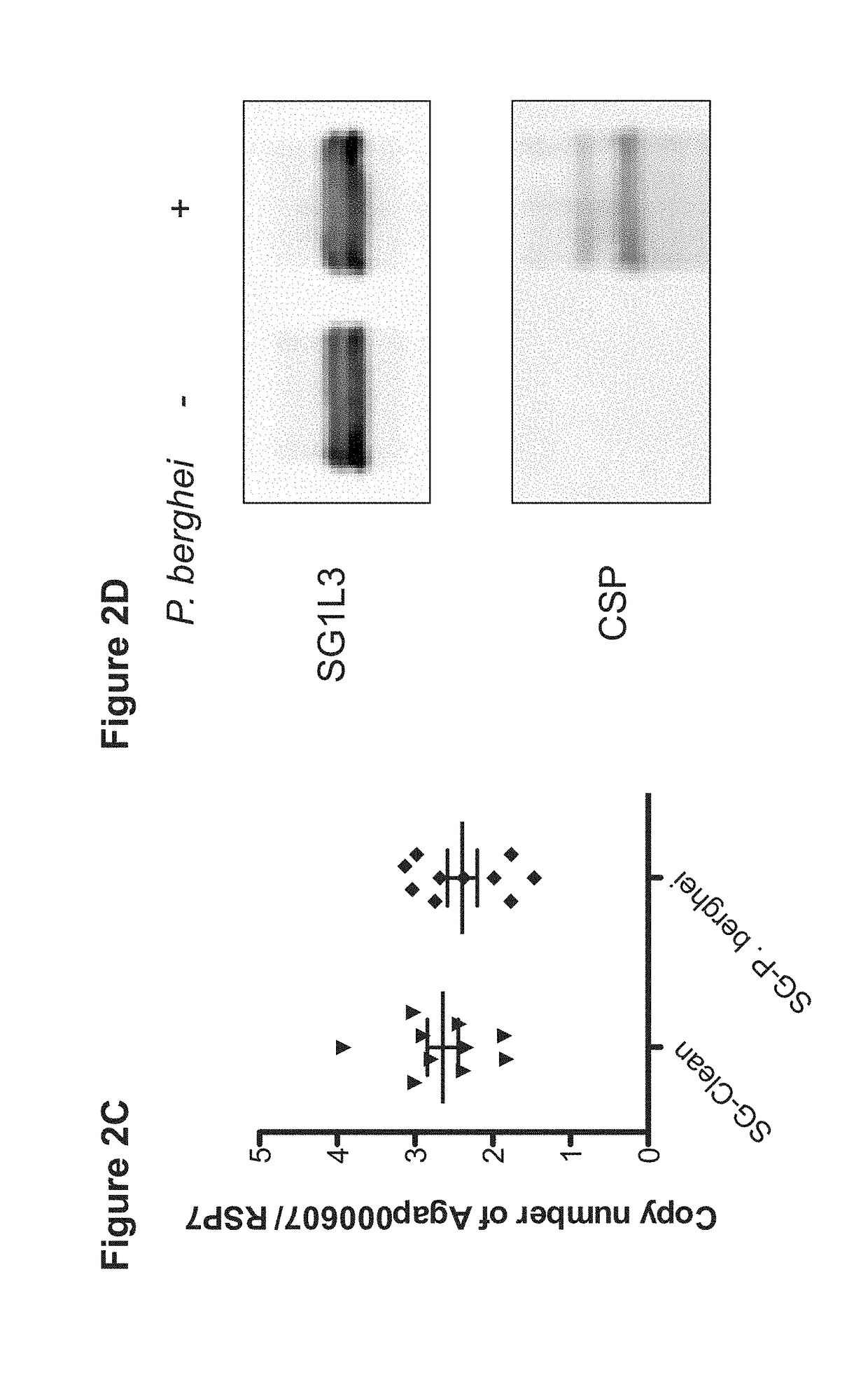
Mosquito Saliva Protein Malaria Vaccine
Owner:YALE UNIV
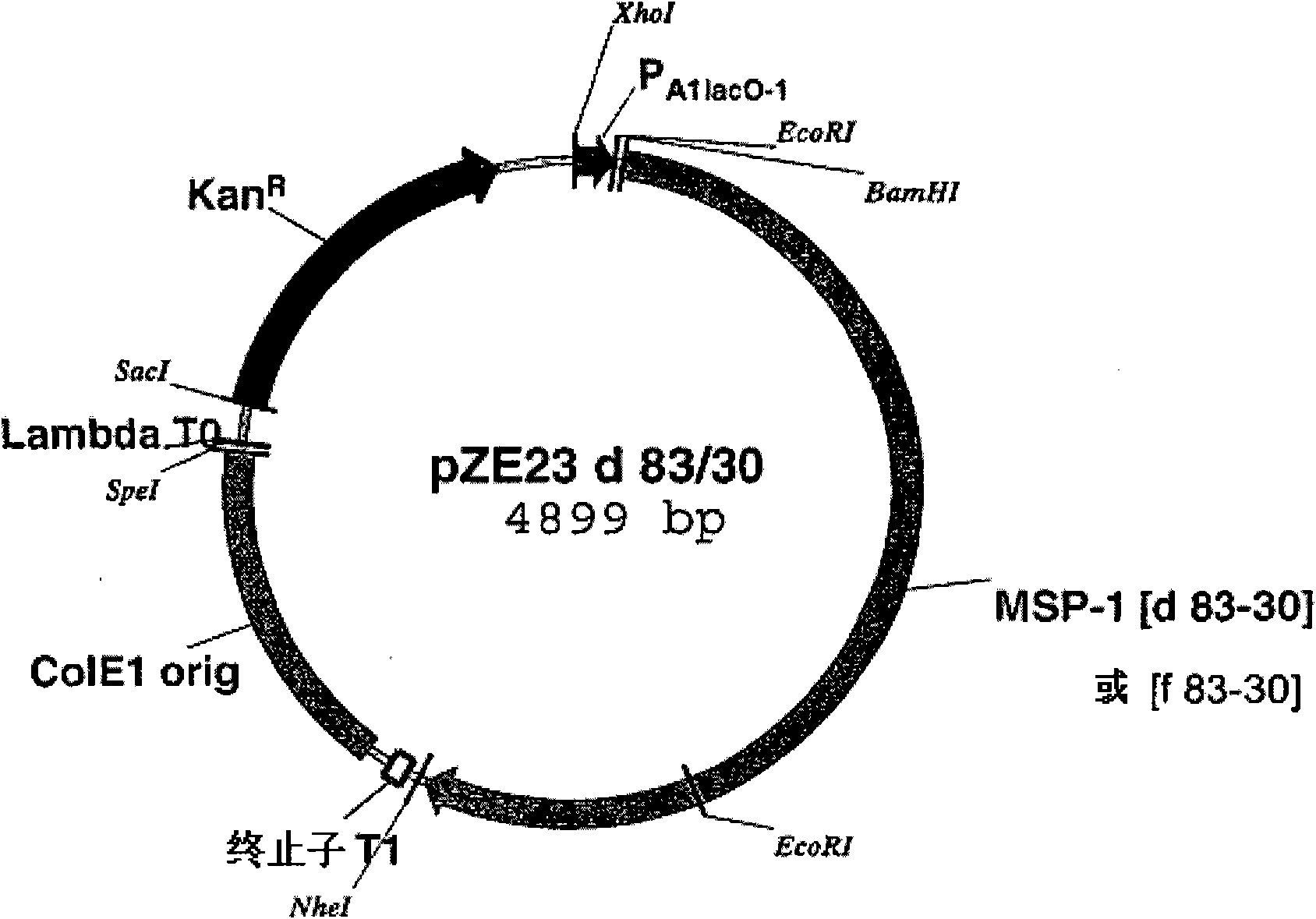
Recombinant malaria vaccine
Owner:赫尔曼·比雅尔
Mosquito saliva protein malaria vaccine
Owner:YALE UNIV
Malaria vaccine
The present invention provides a polypeptide and a malaria vaccine comprising the polypeptide, the polypeptide comprising any of the following amino acid sequences: (a) an amino acid sequence represented by SEQ ID NO. 4 or SEQ ID NO. 8; (b) an amino acid sequence represented by SEQ ID NO. 4 or SEQ ID NO. 8, in which 1-10, preferably 1-5, and more preferably 1-3 amino acids have been substituted, deleted, added or inserted; and (c) an amino acid sequence having at least 95%, preferably at least 97%, and more preferably at least 99% sequence identity with SEQ ID NO. 4 or SEQ ID NO. 8.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD +1
Plasmodium falciparum HLA class I restricted T-cell epitopes
The invention relates immunogenic polypeptides and epitopes from Plasmodium falciparum protein AMA 1. The epitopes contain HLA class I binding motifs and stimulate an anti-malaria CD8+ T-cell response. The polypeptides can be incorporated into immunogenic formulations against malaria. Additionally, the antigens are useful for facilitating evaluation of immunogenicity of candidate malaria vaccines.
Owner:SEDEGAH MARTHA +1
Multivalent Malaria Transmission-Blocking Vaccines
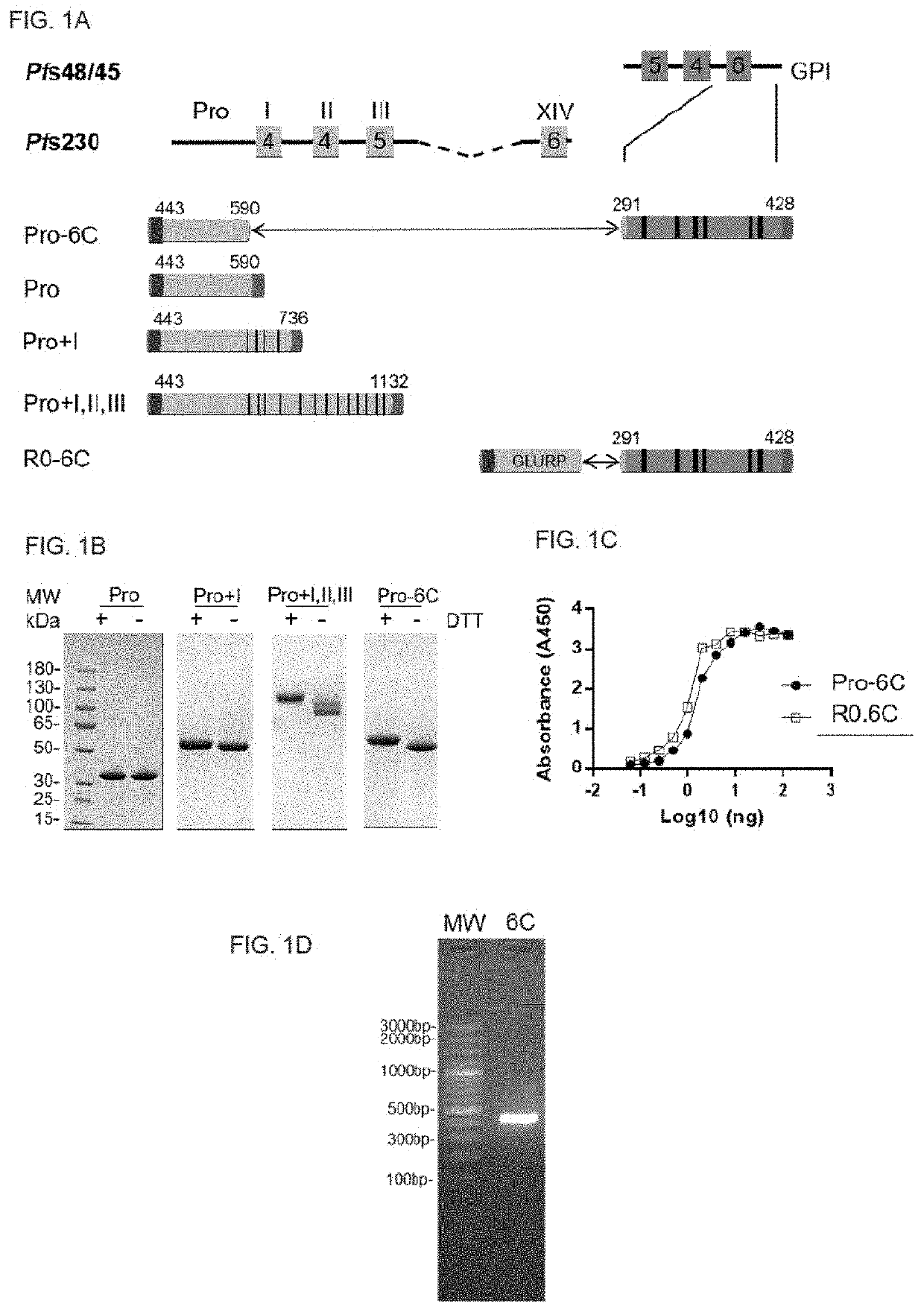
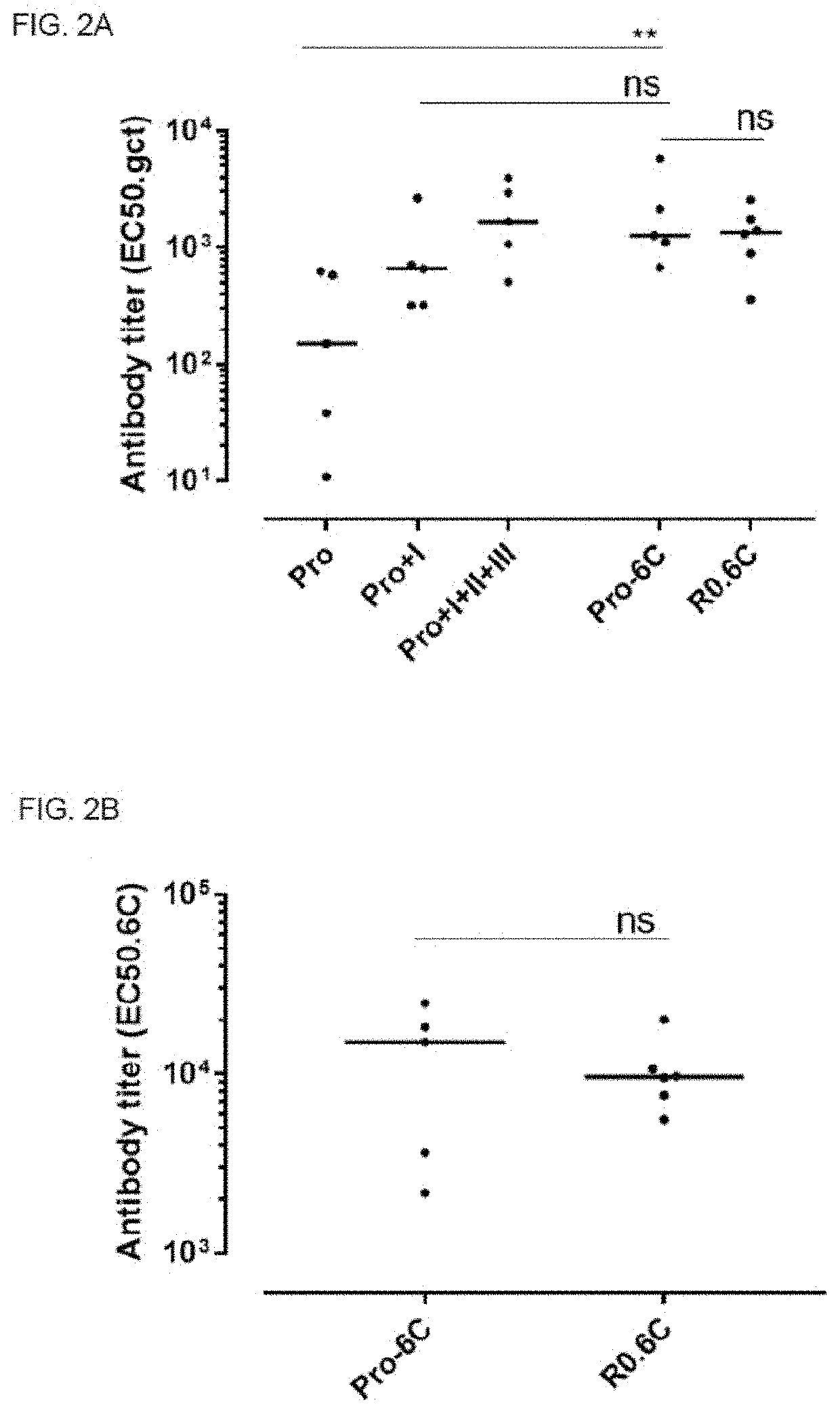
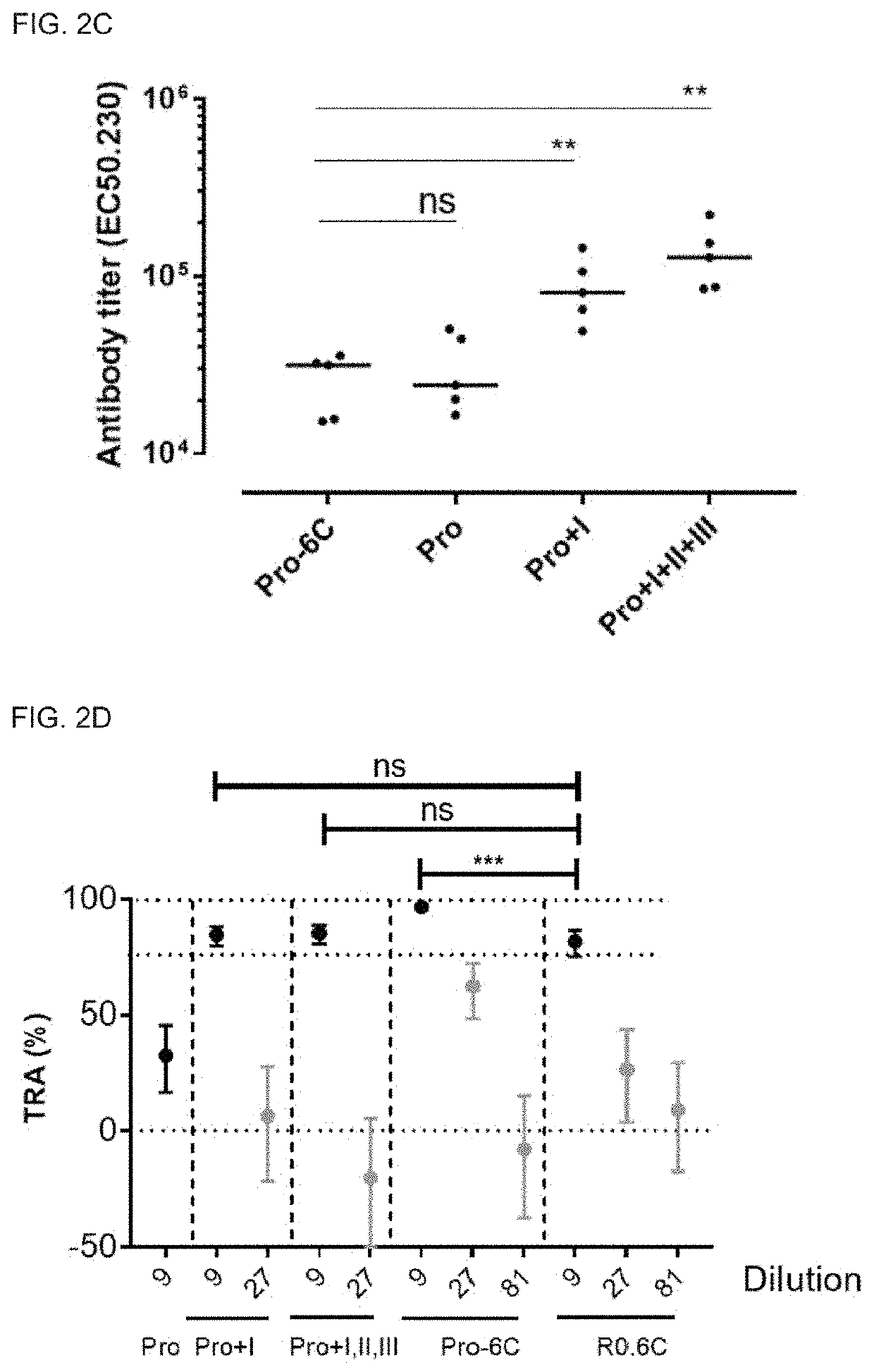
PendingUS20220194996A1Easy to produceAntibody mimetics/scaffoldsPeptide preparation methodsAntigenMalaria
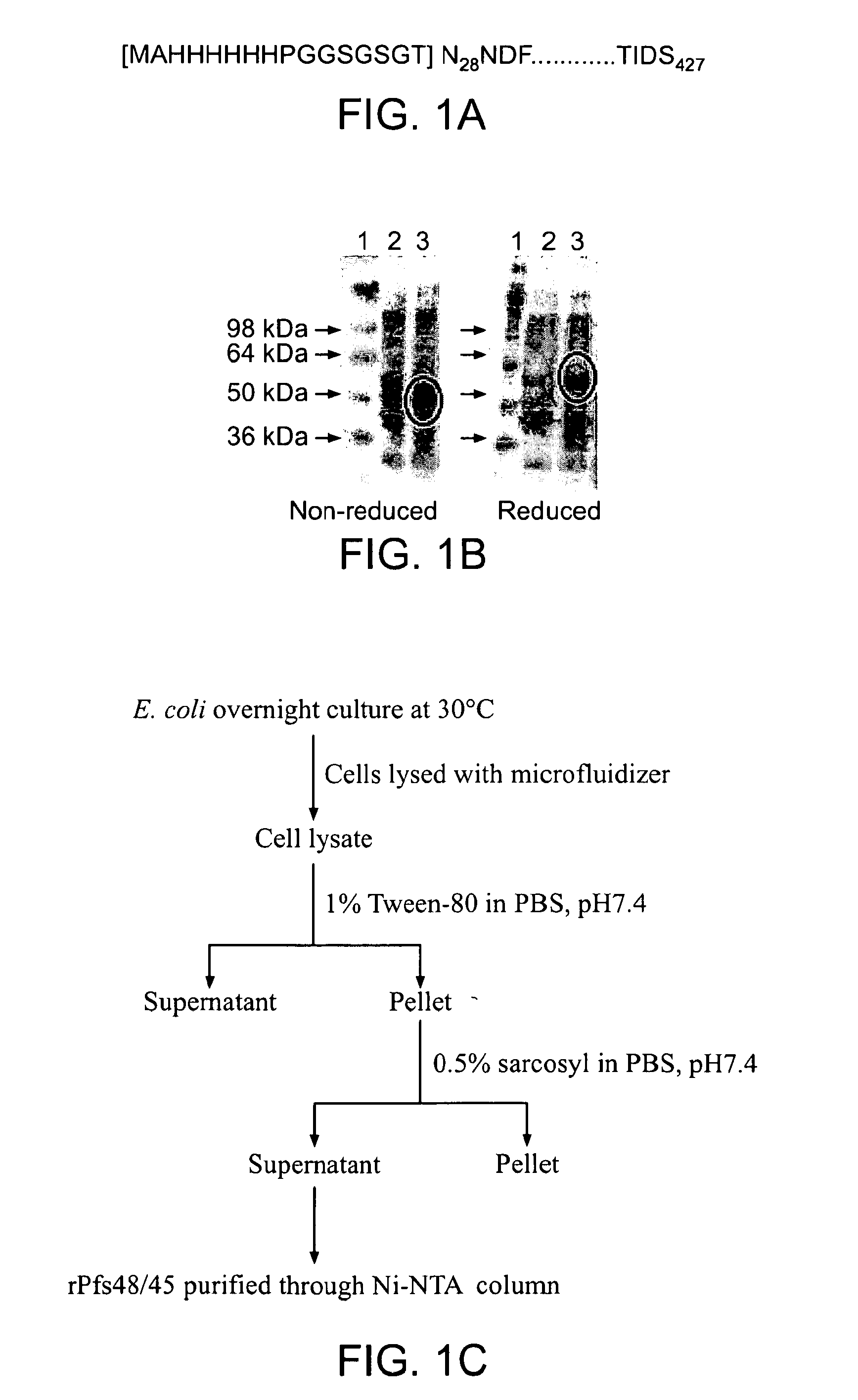
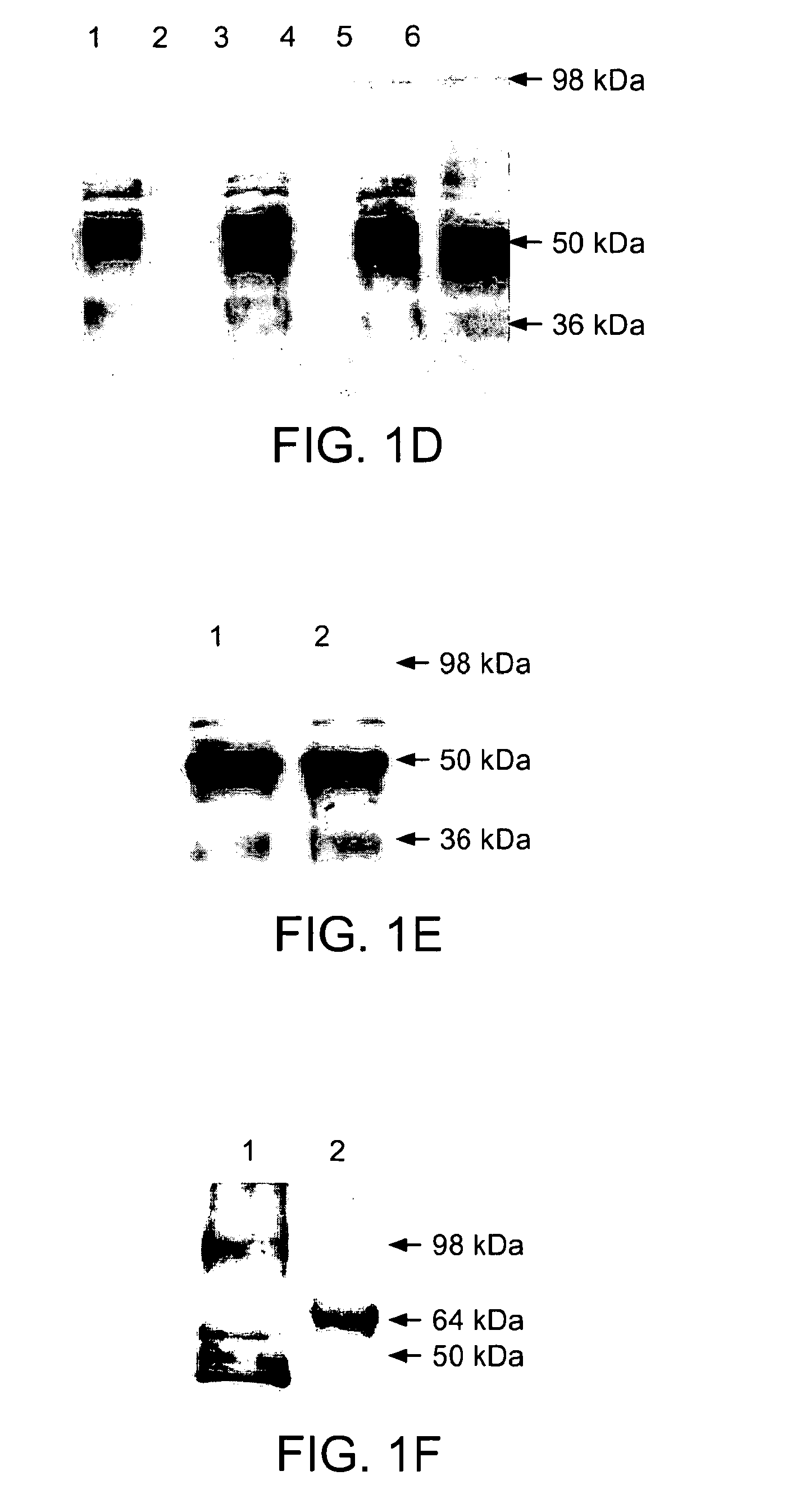
The present invention relates to a method for recombinant production of a fusion protein comprising multiple malaria antigens for inducing immune responses comprising a combination of antibodies. In particular, the fusion proteins of the present invention comprise fragments of both Pfs230 and Pfs48 / 45 to lower the required threshold of functional antibodies and to reduce the risk of escape mutations. Thus, the fusion proteins of the present invention are suitable for use in a multivalent malaria vaccine.
Owner:STATENS SERUM INST
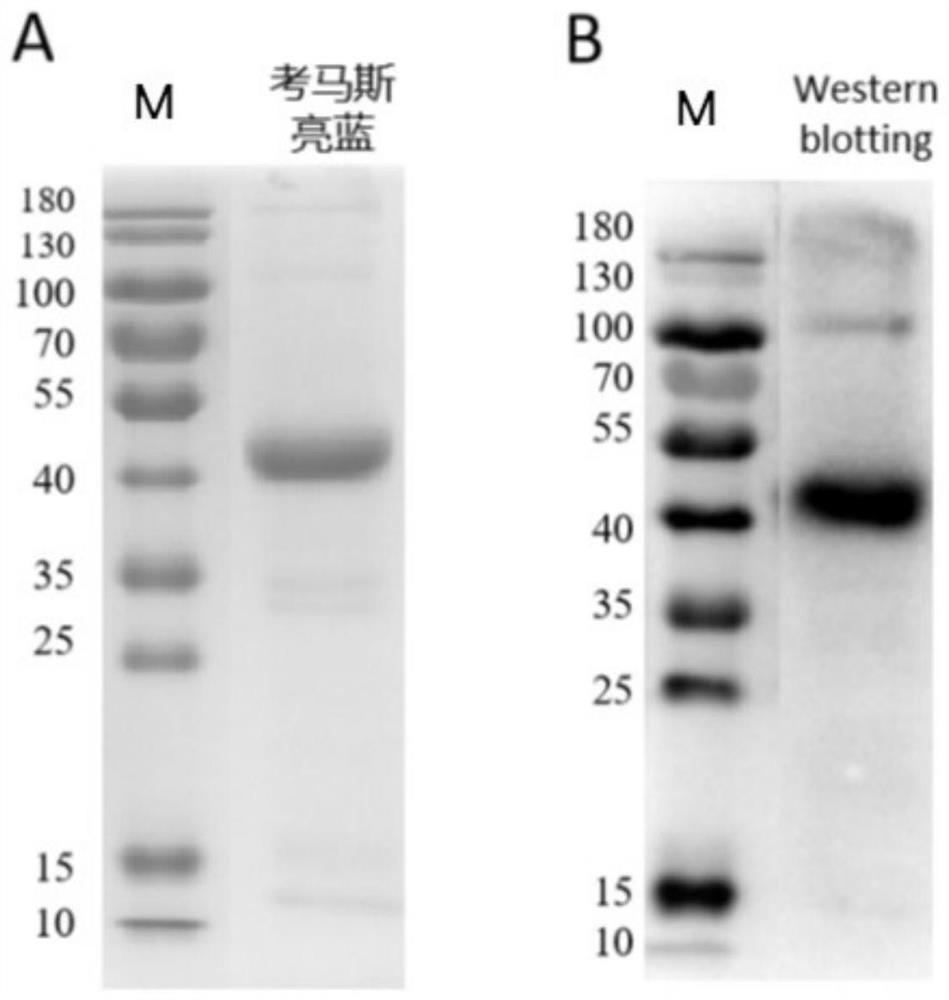
An antibody that inhibits the growth of Plasmodium cynomolgus monkeys in vitro
ActiveCN111978413BEnhanced inhibitory effectBacteriaAntibody mimetics/scaffoldsAntigenPlasmodium vivax merozoite
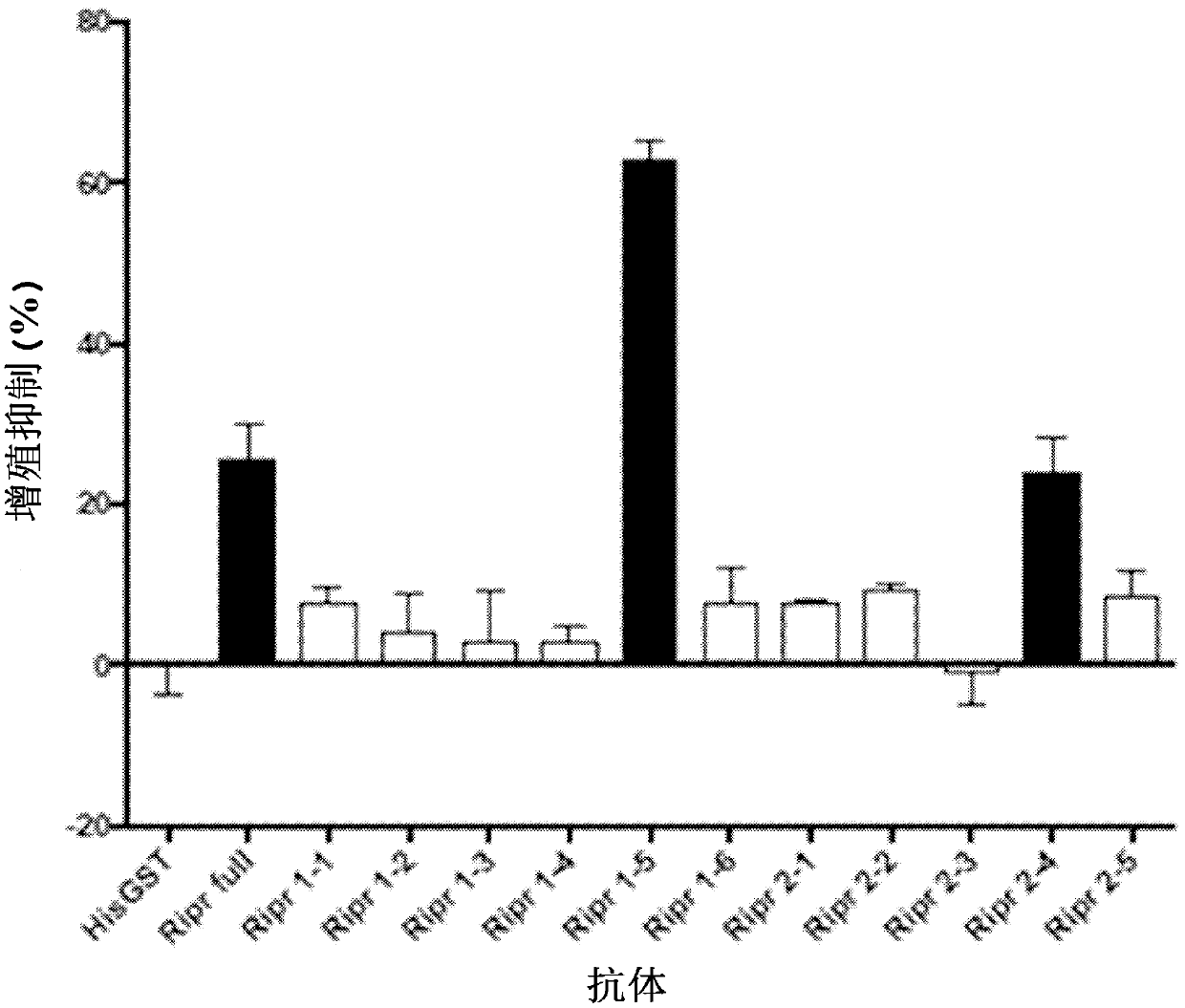
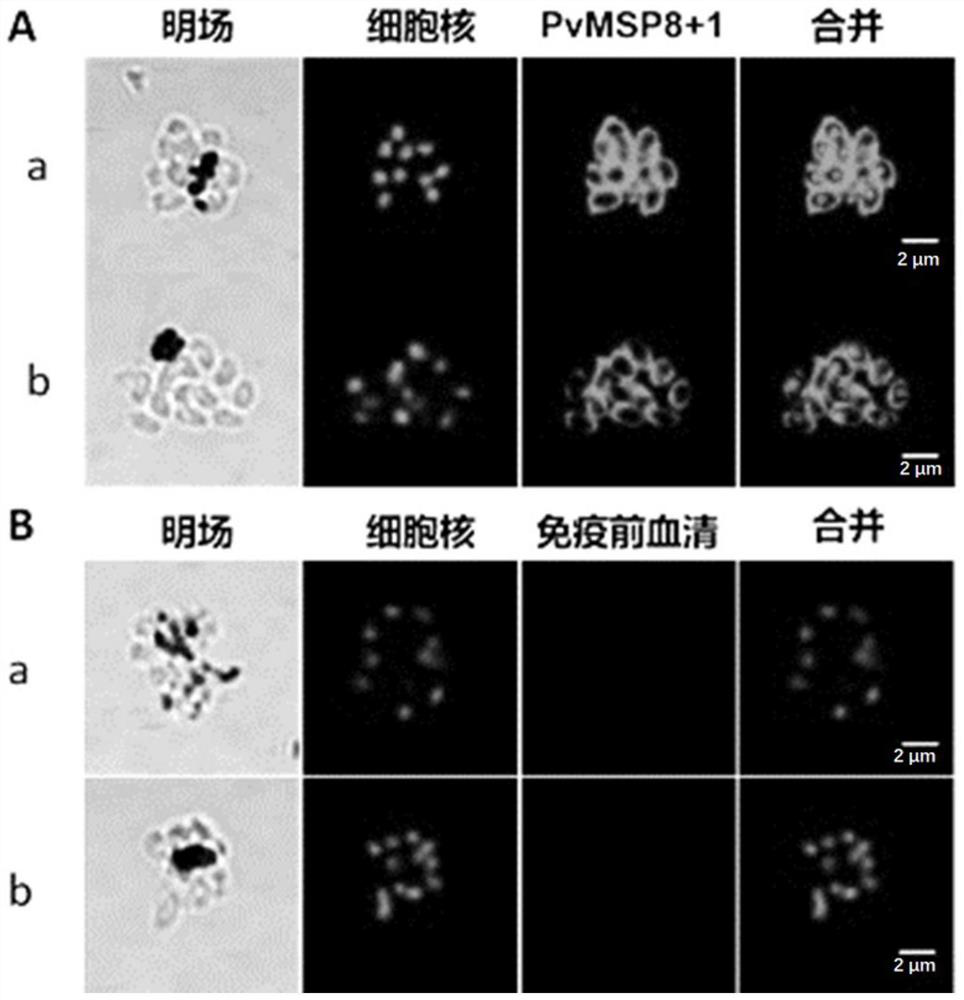
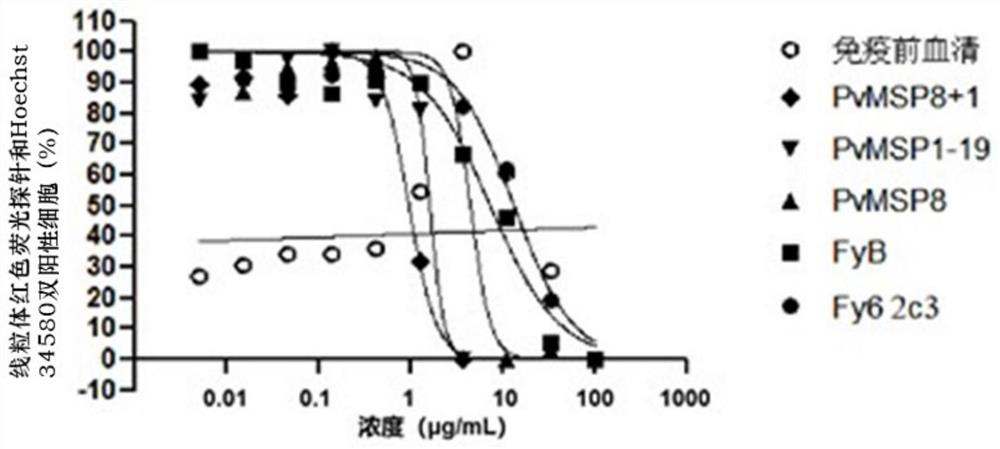
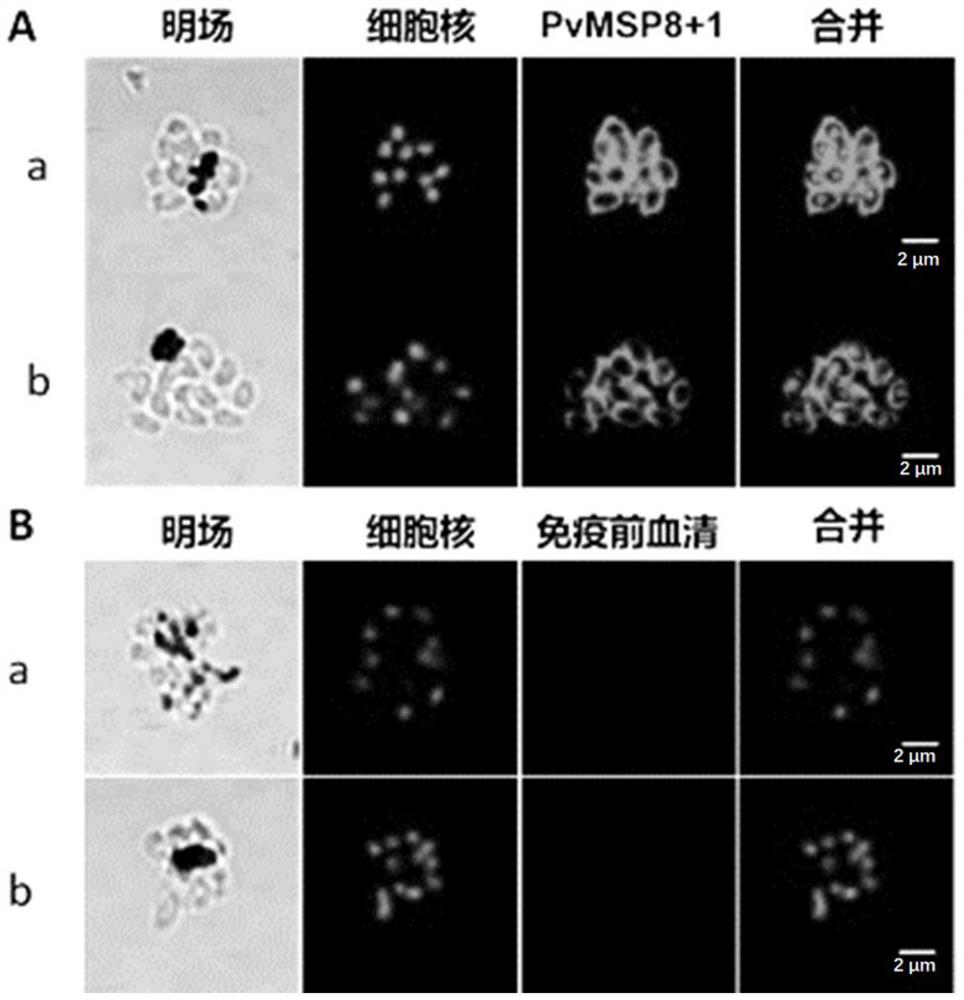
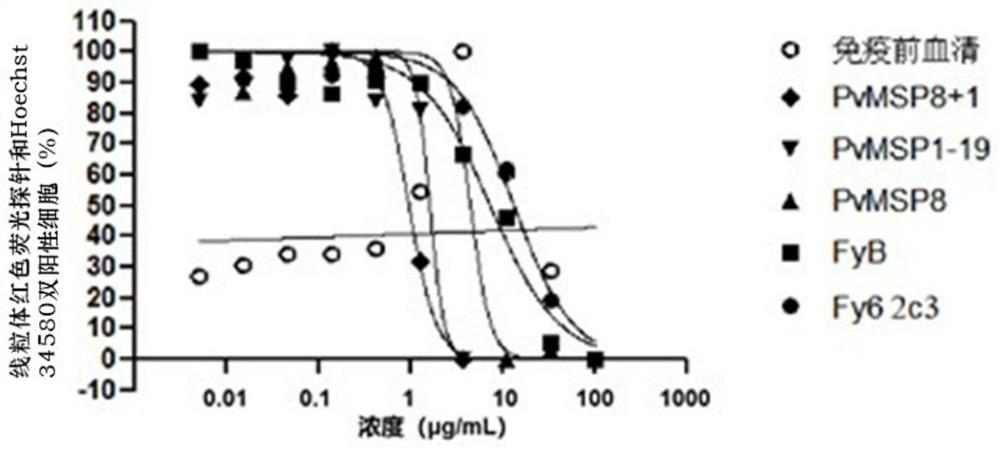
The invention discloses an antibody for inhibiting the growth of Plasmodium cynomolgus monkey in vitro, belonging to the fields of parasitology and immunology. The antibody is prepared from PvMSP8+1 recombinant protein, and the PvMSP8+1 recombinant protein is a fusion protein composed of Plasmodium vivax merozoite surface protein 8 (PvMSP8) and the C-terminal of 1 (PvMSP1‑19). In the in vitro growth inhibition assay, the PvMSP8+1 antibody showed a superior inhibitory effect than the PvMSP8 and PvMSP1‑19 single antigen antibodies. This provides a certain scientific basis for the development of multi-antigen malaria vaccines, and has broad application prospects.
Owner:JIANGNAN UNIV
Replikin peptides in rapid replication of glioma cells and in influenza epidemics
InactiveUS20070053916A1Improve concentrationAntibacterial agentsVirusesInfluenza virus A hemagglutininViral Vaccine
Peptides of influenza virus hemagglutinin protein and Plasmodium falciparum malaria antigen, antibodies specific for the peptides, influenza vaccines, malaria vaccines and methods of stimulating the immune response of a subject to produce antibodies to influenza virus or malaria are disclosed. Also disclosed are methods for formulating vaccines for influenza virus.
Owner:BOGOCH SAMUEL +1
Malaria vaccine compositions and constituents which elicit cell mediated immunity
Malaria vaccines based on polyepitope constructs that elicit cell-mediated immunity against a broad spectrum of malaria parasites and which cover the majority of HLA alleles are provided. Epitopes in the polyepitope constructs are from regions of the Plasmodium falciparum circumsporozoite protein (CSP) known to contain CD4 and CD8 T cell epitopes, and include both epitopes from highly variable and highly conserved regions of CSP.
Owner:AERAS GLOBAL TB VACCINE FOUND
Adenoviral vector-based malaria vaccines
ActiveUS20140335128A1Induce immune responseWhole-cell/virus/DNA/RNA ingredientsDsDNA virusesMalarial parasiteAntigen
The invention provides a method of inducing an immune response against malaria in a mammal. The method comprises intramuscularly administering to a mammal a composition comprising a pharmaceutically acceptable carrier and either or both of (a) a first adenoviral vector comprising a nucleic acid sequence encoding a P. falciparum circumsporozoite protein (CSP) operably linked to a human CMV promoter, and / or (b) a second adenoviral vector comprising a nucleic acid sequence encoding a P. falciparum apical membrane antigen 1 (AMA-1) antigen operably linked to a human CMV promoter.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY
Antibody for inhibiting growth of Plasmodium cynomolgi in vitro
ActiveCN111978413AEnhanced inhibitory effectBacteriaAntibody mimetics/scaffoldsAntigenPlasmodium cynomolgi
The invention discloses an antibody for inhibiting growth of Plasmodium cynomolgi in vitro, and belongs to the field of parasitics and immunology. The antibody is prepared from a PvMSP8 + 1 recombinant protein, and the PvMSP8 + 1 recombinant protein is a fusion protein composed of Plasmodium vivax merozoite surface protein 8 (PvMSP8) and the C terminal (PvMSP1-19) of 1. In an in-vitro growth inhibition test, the PvMSP8 + 1 antibody shows an inhibition effect superior to that of PvMSP8 and PvMSP1-19 monoantigen antibody. A certain scientific basis is provided for people to develop the multi-antigen malaria vaccine, and the antibody has a wide application prospect.
Owner:JIANGNAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com