Malaria vaccine
A malaria and vaccine technology, applied in the direction of allergic diseases, immunoglobulin, and vector-borne diseases, can solve the problems of unused malaria vaccines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
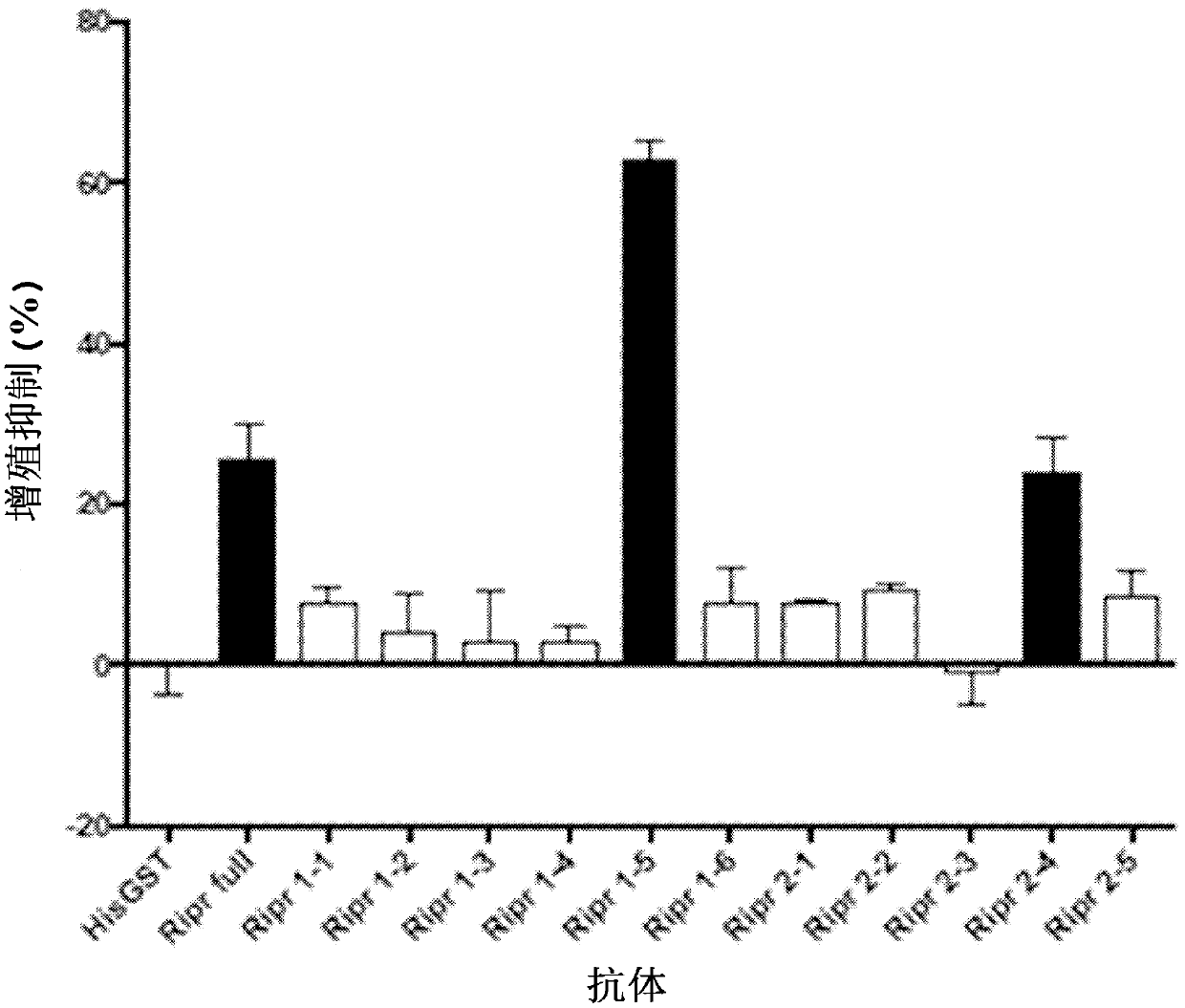
[0126] Use a vector containing the codon-optimized sequence of wheat encoding Ripr linked at the C-terminus to the coding sequence of the His tag, namely pEU-E01-MCS; The vector of the sequence of one of the cloned 11 fragments, pEU-E01-GST-TEV-N2, served as a template for transcription. All peptides were synthesized using templates containing the Met coding sequence at the N-terminus and the 6xHis coding sequence at the C-terminus.
[0127] Encoding SEQ ID NO: 21 ~ 1086 amino acids, SEQ ID NO: 2 amino acids 720 ~ 934 (SEQ ID NO: 4), and SEQ ID NO: 2 amino acids 648 ~ 830 (SEQ ID The wheat codon-optimized sequences of NO: 8) are shown in SEQ ID NO: 5, SEQ ID NO: 6, and SEQ ID NO: 9, respectively. Totally transcribed mRNA was used for protein synthesis using wheat germ cell-free protein synthesis kit WEPRO®7240H (cell free Science). The resulting solution containing the synthetic protein was affinity purified by using Ni Sepharose 6 Fast Flow (GE Healthcare). The antigen sol...
Embodiment 2
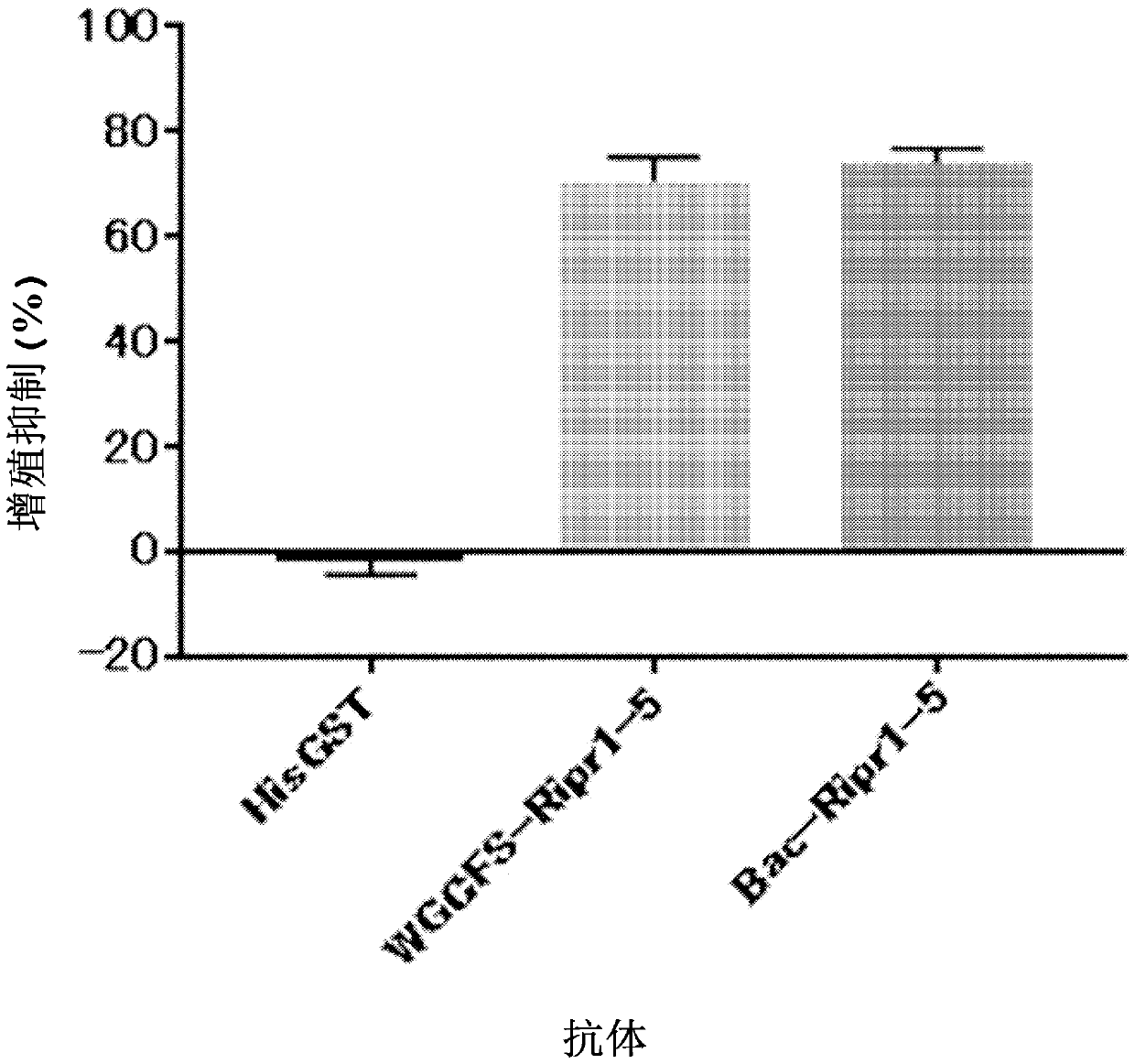
[0148] The baculovirus codon-optimized sequence encoding Ripr1-5 (SEQ ID NO: 10) linked at the N-terminus to the Gp67 secretion signal coding sequence and at the C-terminus to the His-tag coding sequence was subcloned into the pFastBac1 vector. By using the obtained expression vector and DH10Bac competent cells, recombinant bacmids were prepared. The recombinant bacmid was transfected into Sf9 insect cells with Cellfectin II reagent to produce recombinant baculovirus. The recombinant baculovirus was amplified and used to infect Sf9 insect cells to express Ripr1-5. The culture supernatant was collected, and Ripr1-5 was purified by Ni-NTA affinity column and Superdex200 gel filtration column.
[0149] In the same manner as in Example 1, a rabbit polyclonal antibody was obtained by using Ripr1-5 (Bac-Ripr1-5) produced using the baculovirus / insect cell expression system. The inhibitory activity of the rabbit polyclonal antibody against the proliferation of Plasmodium was compare...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com