Biofusion proteins as Anti-malaria vaccines
A fusion protein and protein technology, which can be used in drug combinations, fusion polypeptides, antibody mimics/scaffolds, etc., and can solve problems such as high cost, high technical requirements, and difficulty in reproduction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0116] Previous studies have shown that frequent malaria exposure confers immunity in adults, which prevents or alleviates the disease. This immunity can be passively transferred by administration of immunoglobulin (IgG) from malaria-immunized adults.
[0117] A parasite killing mechanism, termed antibody-dependent cell-mediated inhibition (ADCI), was identified. Screening of broad genomic DNA libraries by ADCI led to the identification of merozoite-surface protein 3 (MSP3) and subsequently several other antigens, including PEBS.
[0118] Identification of cytotropic antibodies as a surrogate for protection: ADCI-mediating cytotropic (leukocyte-binding) anti-MSP3 antibodies IgG1 and IgG3 were found to be consistently associated with a reduced risk of malaria in epidemiological studies in many different settings. The antibodies also mediate protection against P. falciparum in infected animals.
[0119] Such studies indicate the properties of an effective immune response and g...
Embodiment 4
[0124] Example 4 provides the results using the LSA5-CRM construct.
[0125] Materials and methods
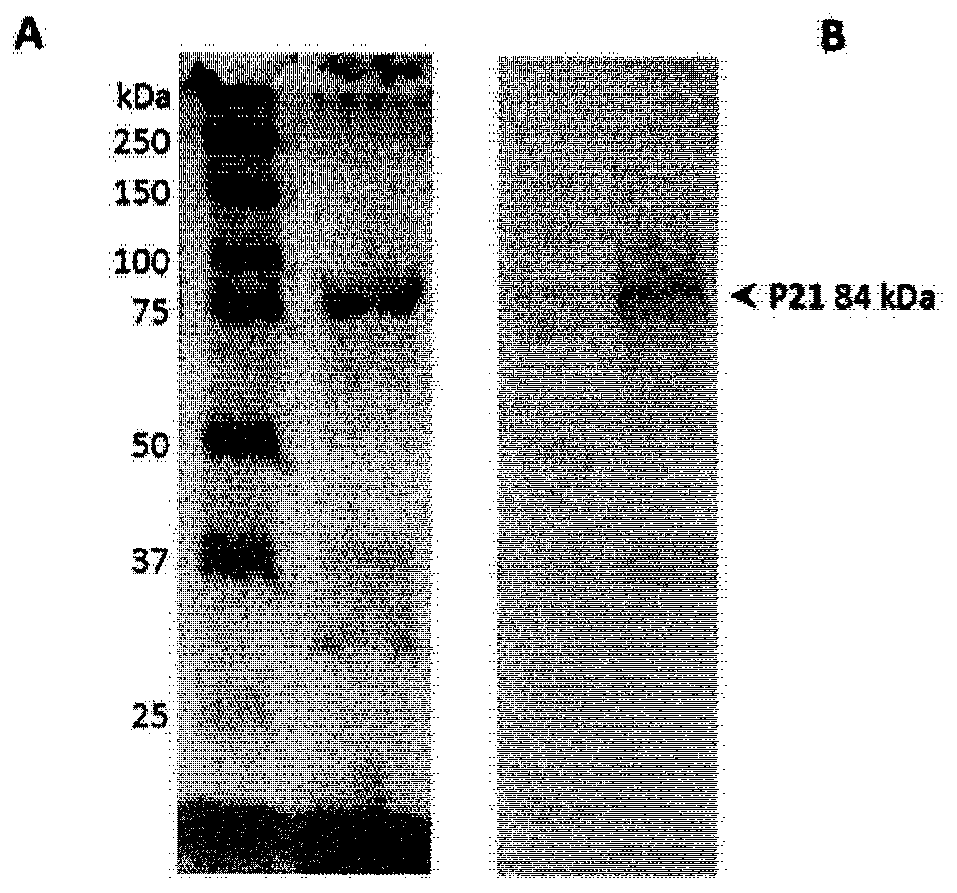
[0126] Generation of expression constructs and production of biofusion proteins in E. coli
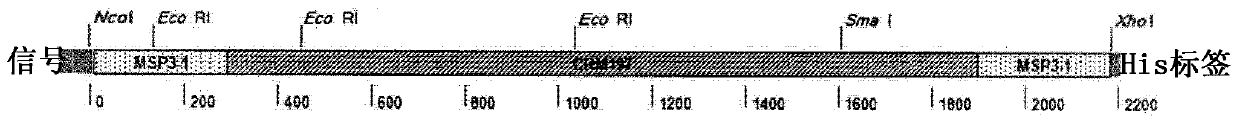
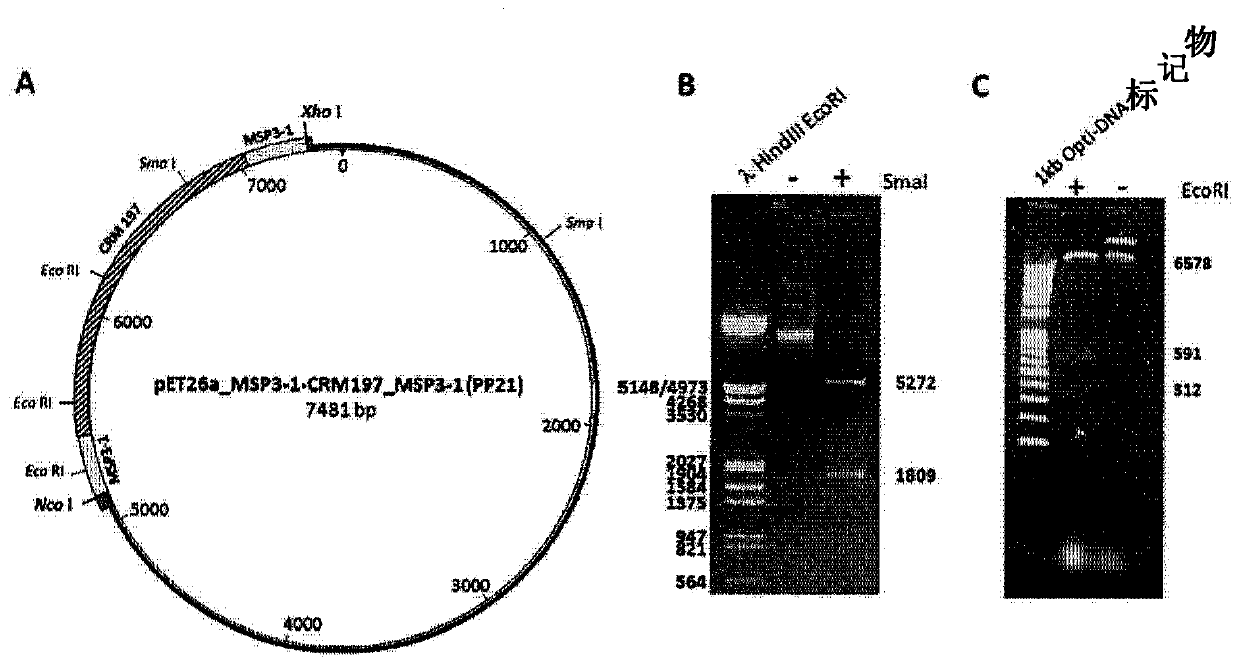
[0127] The gene sequence encoding a chimeric immunogen consisting of detoxified diphtheria toxin CRM197 covalently linked at the N-terminus and C-terminus to the malaria peptide antigen was chemically synthesized (GenScript) ( figure 1 ) and codon-optimized for production in E. coli. Restriction sites for Nco I and Xho I were added at the 5' and 3' ends, respectively, for cloning in the expression plasmid pET-26a (Novagen). The sequence inserted into the shuttle plasmid pUC57 was checked by sequencing and accelerated lyophilized (4 μg).
[0128] The MSP3-1 peptide in PP21 (plasmodB acc. Nbr PF3D7_1035400) corresponds to amino acids (AA) 155 to 249 in MSP3-1.
[0129] Other hybrid immunogens have been designed against CRM197 (Table 3). In PP22, the MSP3-1 peptide was replaced by t...
Embodiment 1
[0209] Example 1: Immunogenicity of PP21, the CRMP97-MS3 fusion construct
[0210] The immune response induced by PP21 was evaluated in the following animal models:
[0211] · BALB-C mice
[0212] · C57BL mice
[0213] ·Squirrel Monkey
[0214] • Human immunogenic mouse model (HIMM) in which cells from human spleen tissue transplanted into immunocompromised NSG mice were immunized in vivo and sera and cells from the immunized animals were used for immune analysis.
[0215] All experiments were performed in a comparative manner using the benchmark antigen MSP3-LSP (a long synthetic peptide covering the region 186-271 of MSP3-1) used in clinical trials and using Montanide ISA 720, since laboratory mice are usually sensitive to hydrogen oxidized Aluminum reacts poorly and the route of injection in HIMM does not allow the use of aluminum hydroxide, and mainly due to the aim of comparing constructs rather than adjuvants, it is known that in humans, aluminum hydroxide adjuvanted ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com