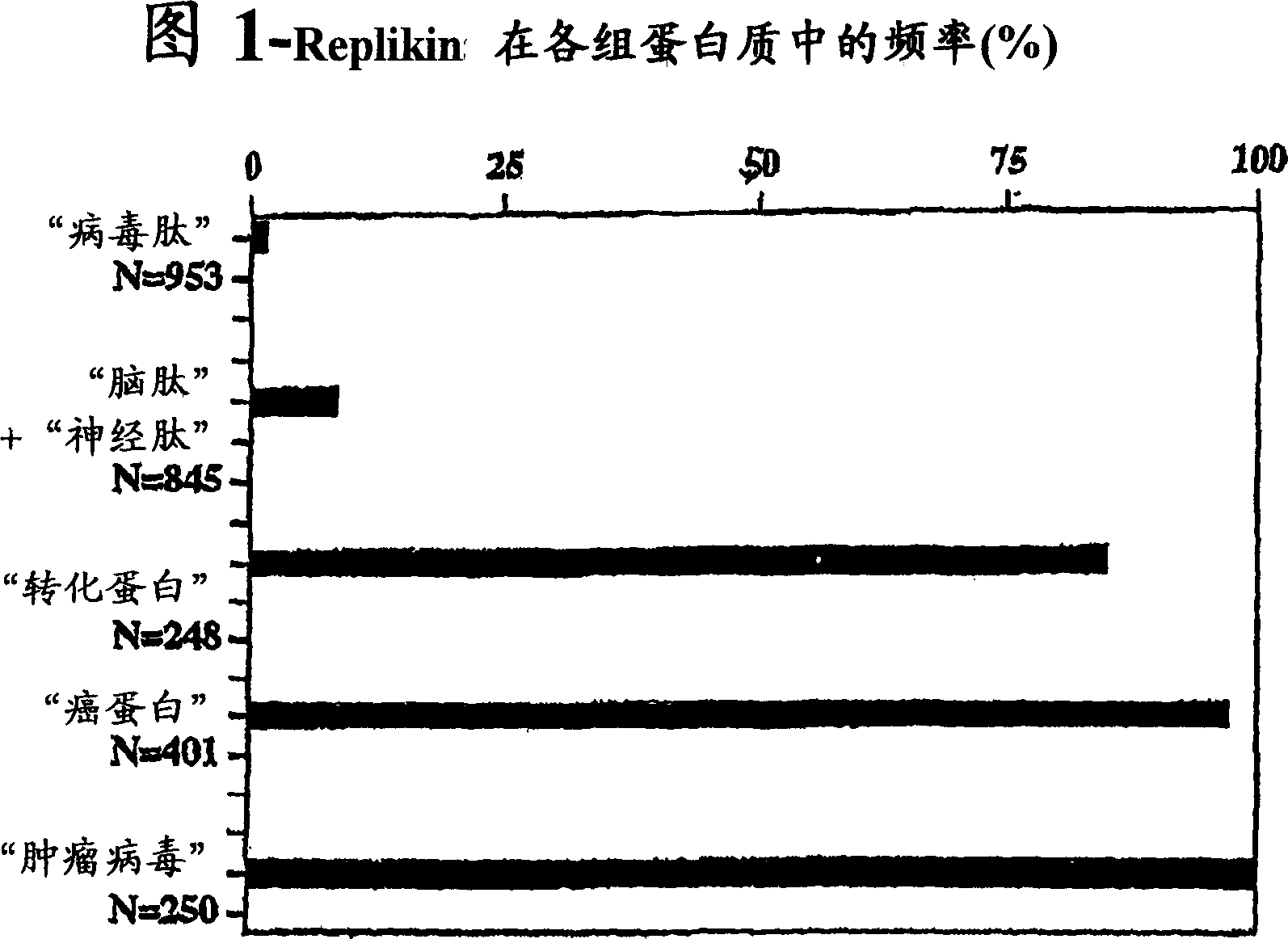
REPLIKIN peptides and uses thereof
A peptide sequence, influenza virus technology, applied in the field of peptides with shared structural features, Replikin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0435] REPLIKIN EXTRACTION, ISOLATION AND IDENTIFICATION METHODS AND USE OF REPLIKIN TO TARGET, MARK OR DESTROY REPLIKIN CONTAINING Organisms
[0436] a) Algae
[0437] The following algae were collected from the Bermuda water station and were either extracted the same day or frozen at -20°C and extracted the next day. The algae were prepared in a cold room (0-5° C.) in a Waring blender in 1 gram aliquots in a neutral buffer such as 100 cc. 0.005 M phosphate buffer pH 7 (“phosphate buffer” ) for 15 minutes, centrifuged at 3000 rpm, the supernatant was concentrated by evaporation, and then dialyzed against cold phosphate buffer to obtain a volume of approximately 15 ml. The volume of this extraction solution is recorded, an aliquot sample is taken for protein analysis, and the remaining sample is fractionated to obtain protein fractions with a pK range between 1-4.
[0438] A preferred method of fractionation is chromatography as follows:
[0439] The extraction solution was...
Embodiment 2
[0453] As an example of the diagnostic use of Replikin: Aglyco 1OB or the 16-mer Replikin can be used as an antigen to capture its corresponding antibody present in serum and quantify the amount of said antibody for diagnostic purposes, as in U.S. 6,242,578 B1 Shown in Figures 2, 3, 4 and 7.
[0454] As an example of the production of drugs linked to Replikin for labeling, nutritional or destruction purposes: the 16-mer Replikin is injected into rabbits to generate specific antibodies against the 16-mer Replikin, as shown in U.S. 6,242,578 B1 Embodiment 6 and Figure 9A and Figure 9B.
[0455] As an example of labeling Replikin with drugs: labeling specific cells containing the Replikin with an antibody against the 16-mer Replikin is shown in Figure 5 and Example 6 of U.S. 6,242,578 B1.
[0456]As an example of destroying Replikin with drugs: inhibit or destroy specific cells containing the Replikin with an antibody against the 16-mer Replikin, as shown in U.S. 6,242,578 B1 F...
Embodiment 3
[0458] The presence and concentration of Replikin in the sequence data of influenza virus hemagglutinin protein or neuraminidase protein isolates was analyzed by visual scanning of the sequence or by using the computer program described herein based on the three-point recognition system. analyze. Influenza virus isolates are obtained and amino acids of the influenza hemagglutinin and / or neuraminidase proteins are obtained by any method known in the art, for example by sequencing the hemagglutinin or neuraminidase genes and deriving protein sequences therefrom sequence. Sequences were scanned for the presence of novel Replikins, the conservation of Replikins over time, and the concentration of Replikins in each isolate. Comparing Replikin sequences and concentrations to amino acid sequences obtained from earlier (eg, about 6 months to about 3 years ago) isolates provides new variants used to predict the most likely cause of influenza in the upcoming flu season strain data and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com