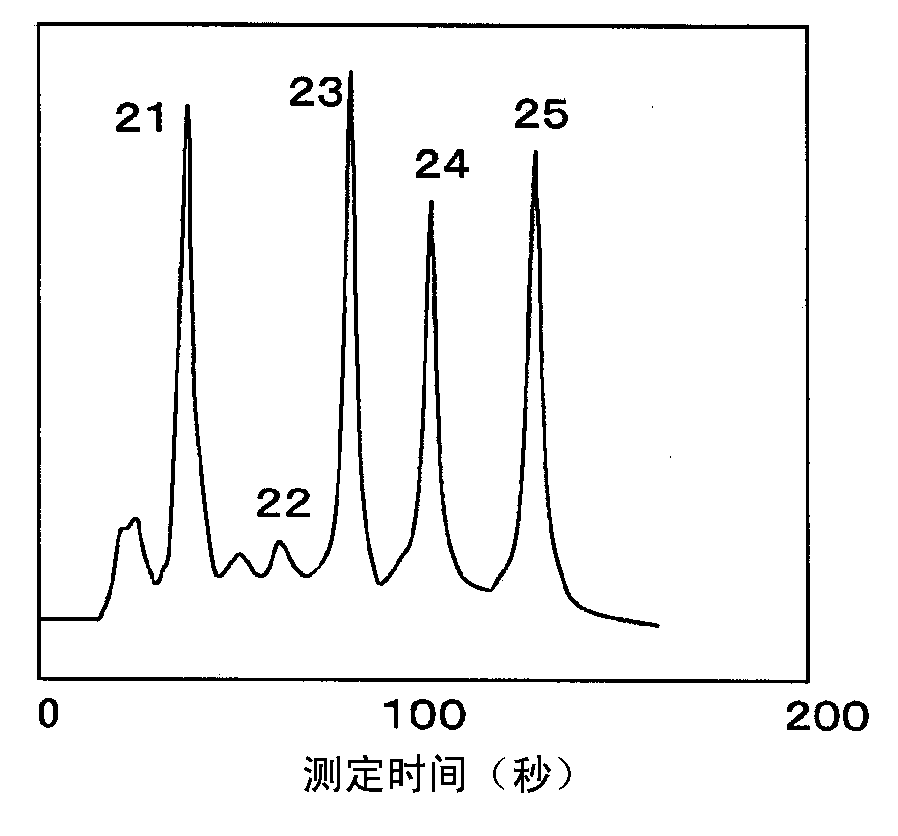
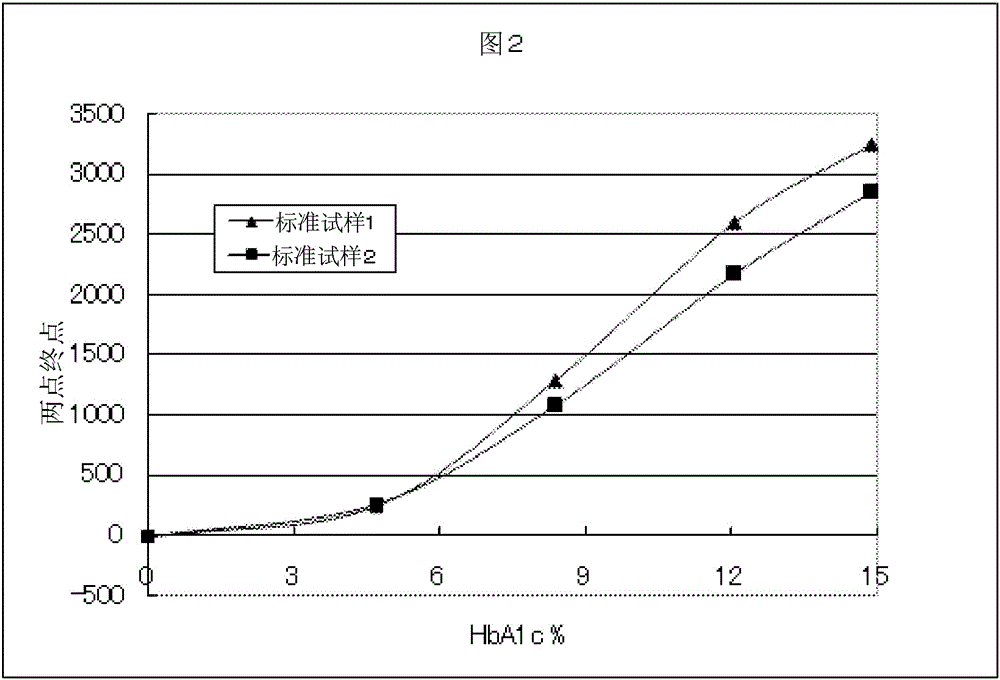
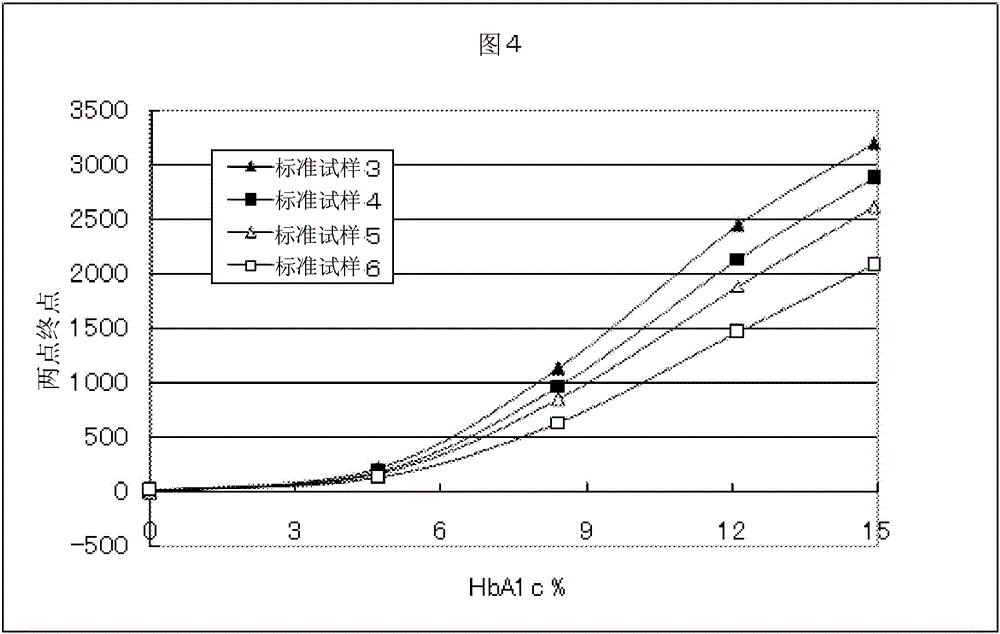
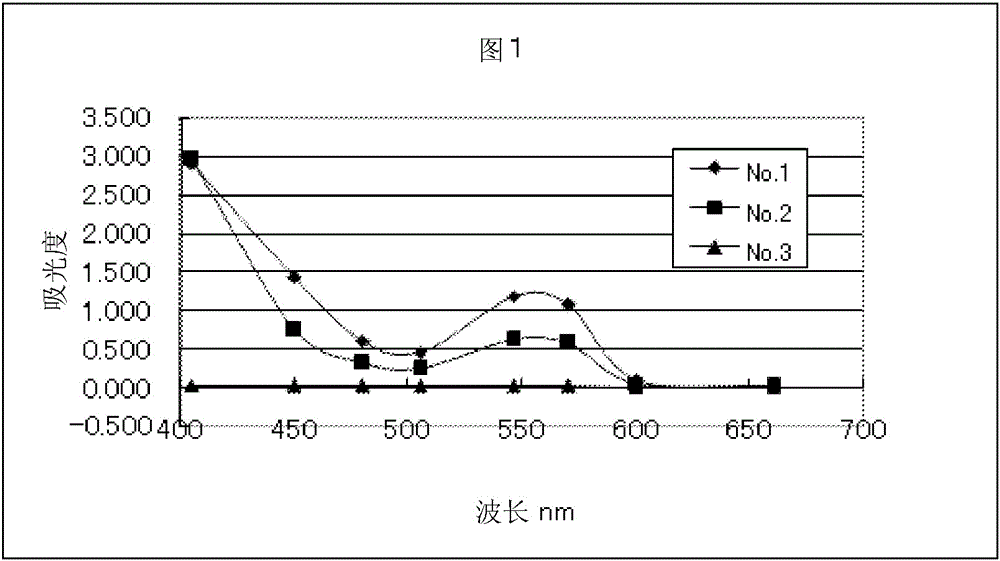
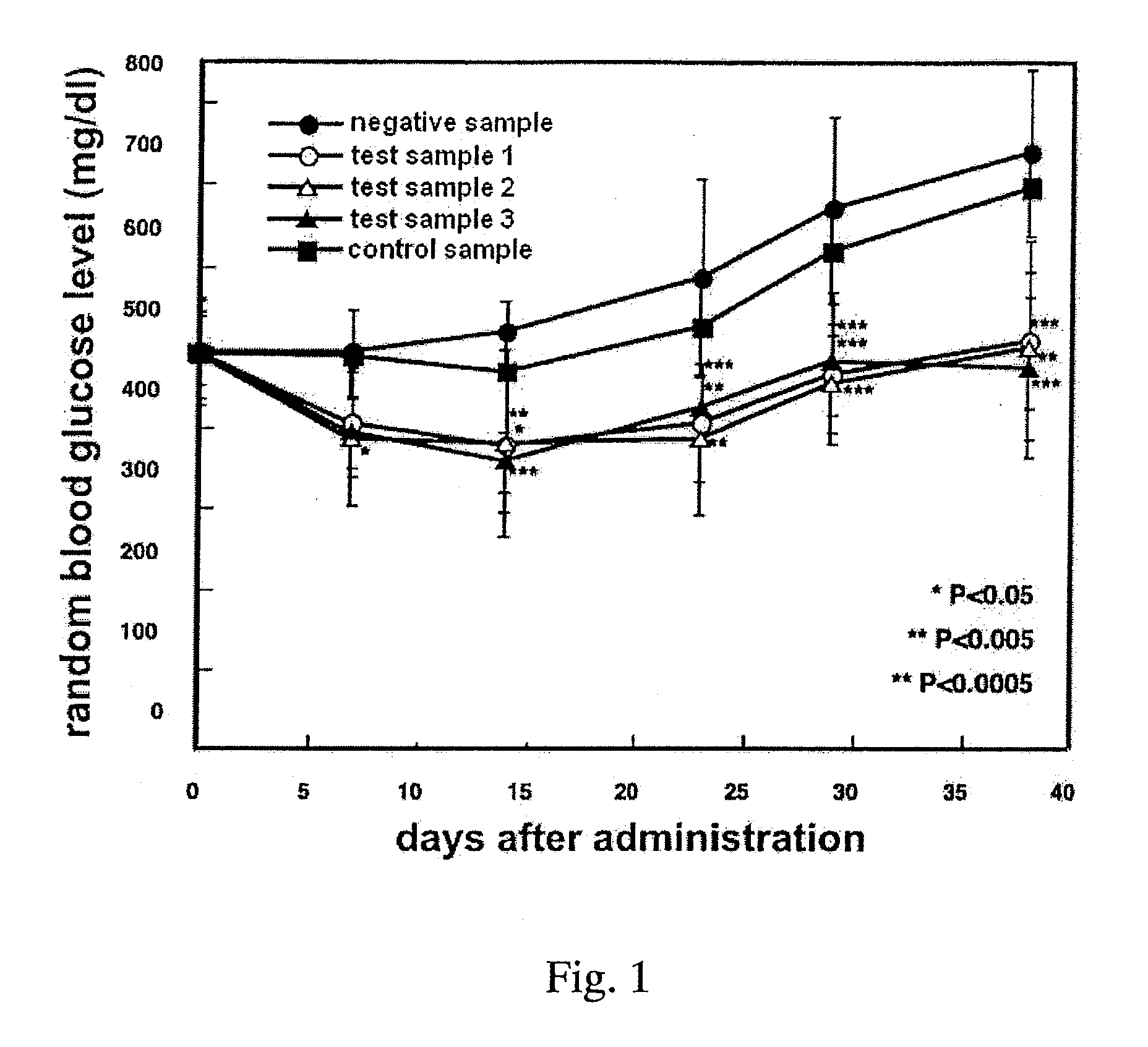
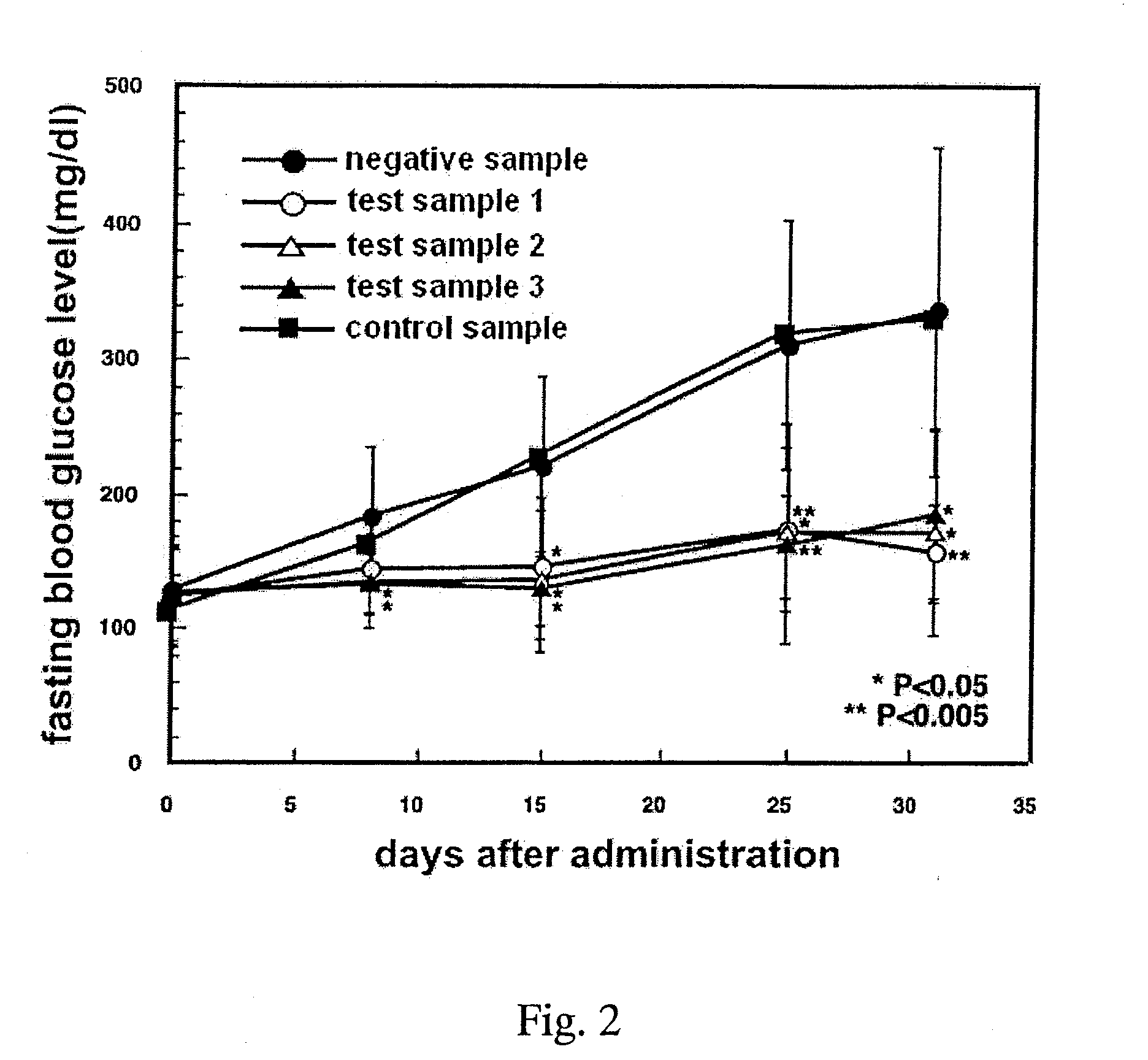
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
46 results about "Haemoglobin A1c" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Hemoglobin A1c is a protein on the surface of red blood cells that sugar molecules stick to, usually for the life of the red blood cell (about three months). The higher the level of glucose in the blood, the higher the level of hemoglobin A1c is detectable on red blood cells.
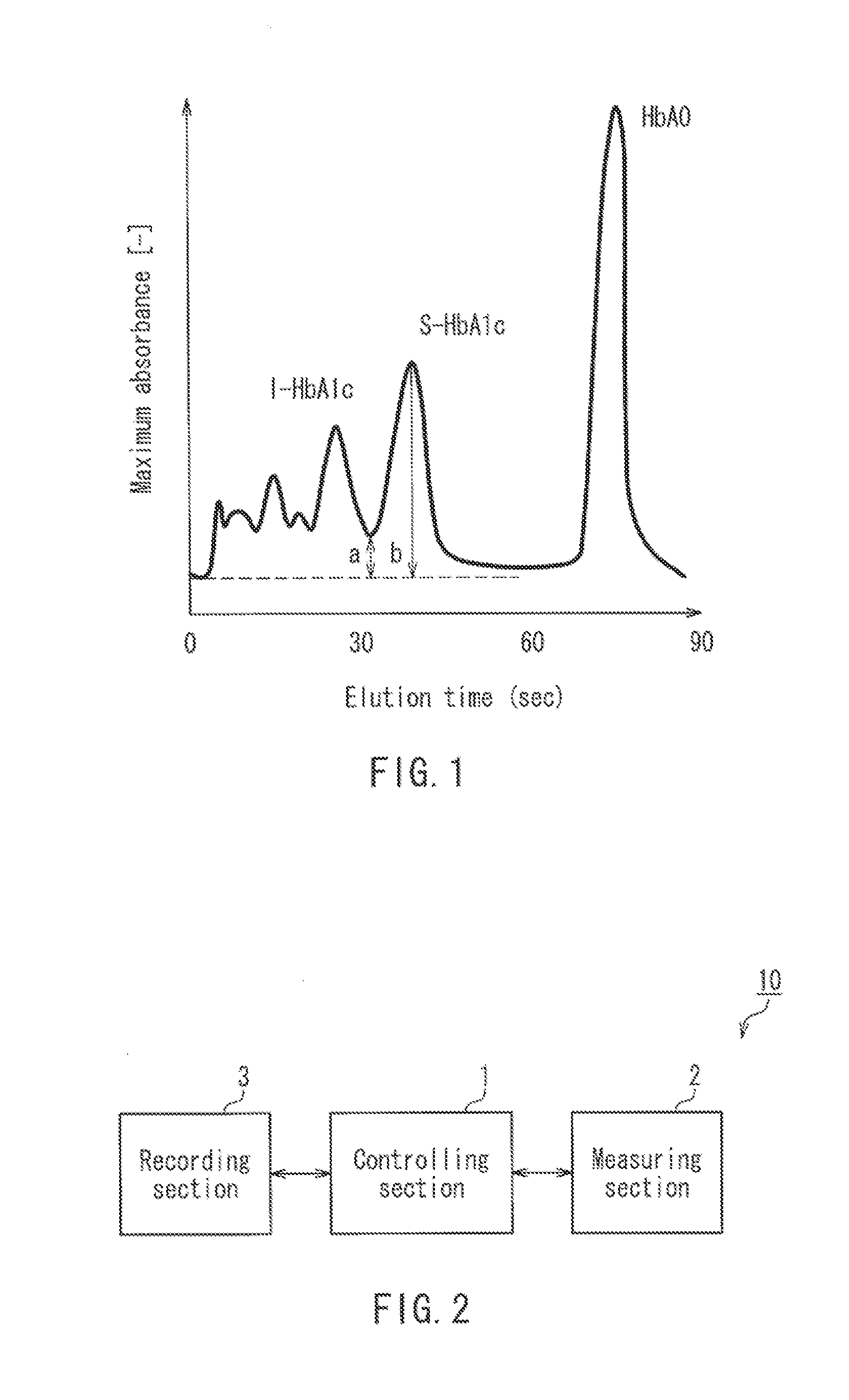
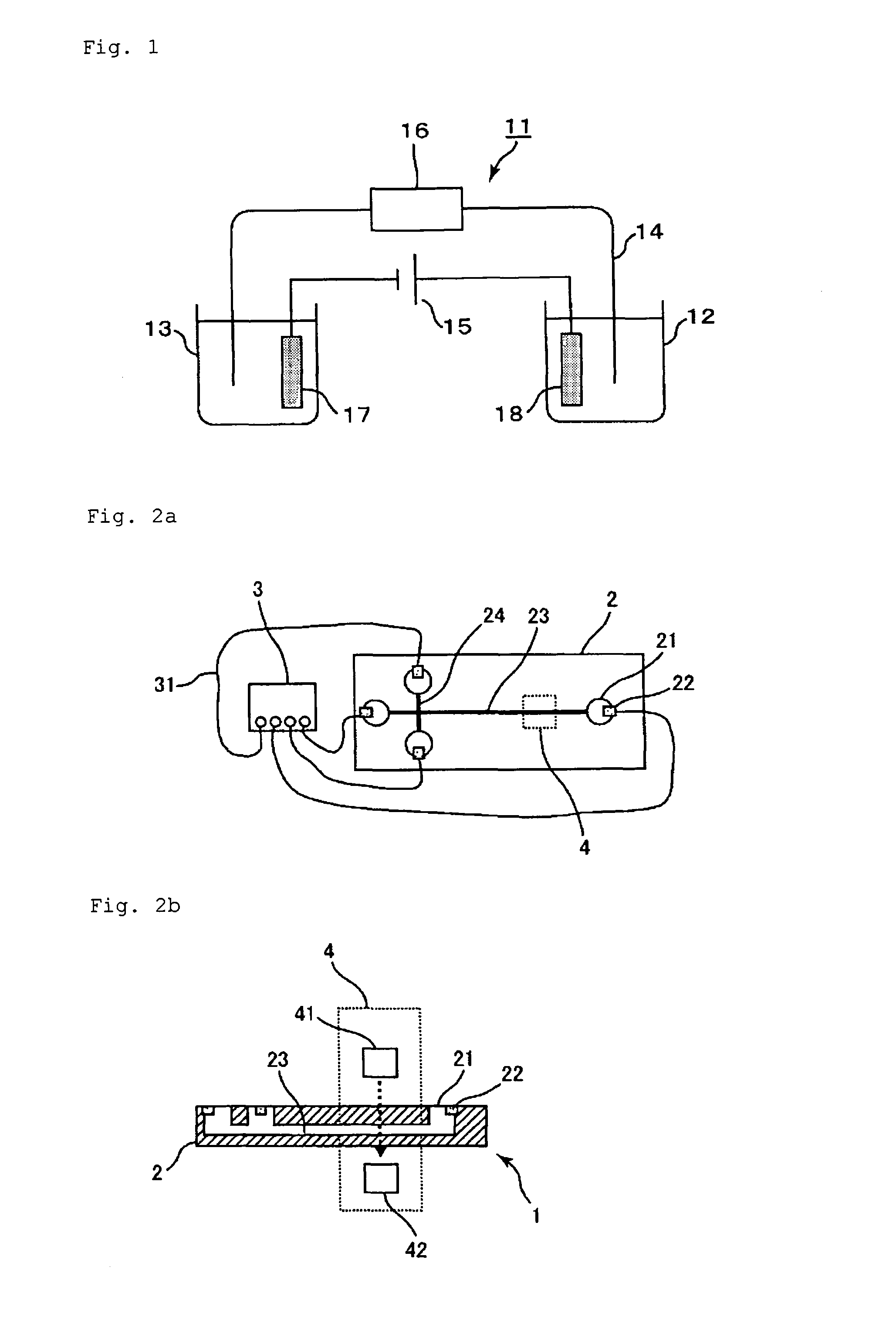
Method for determination of hemoglobins
InactiveUS6428704B1Difficult to obtainReduce generationIon-exchange process apparatusComponent separationOrganic acidHaemoglobin A1c
A method for determining hemoglobins is provided which is suitable for determination of stable hemoglobin A1c. A method for determining hemoglobins by cation exchange liquid chromatography wherein an eluent is used containing a chaotropic ion and further an inorganic acid, an organic acid and / or any salt thereof having a buffer capacity in the 4.0-6.8 pH range.
Owner:SEKISUI CHEM CO LTD
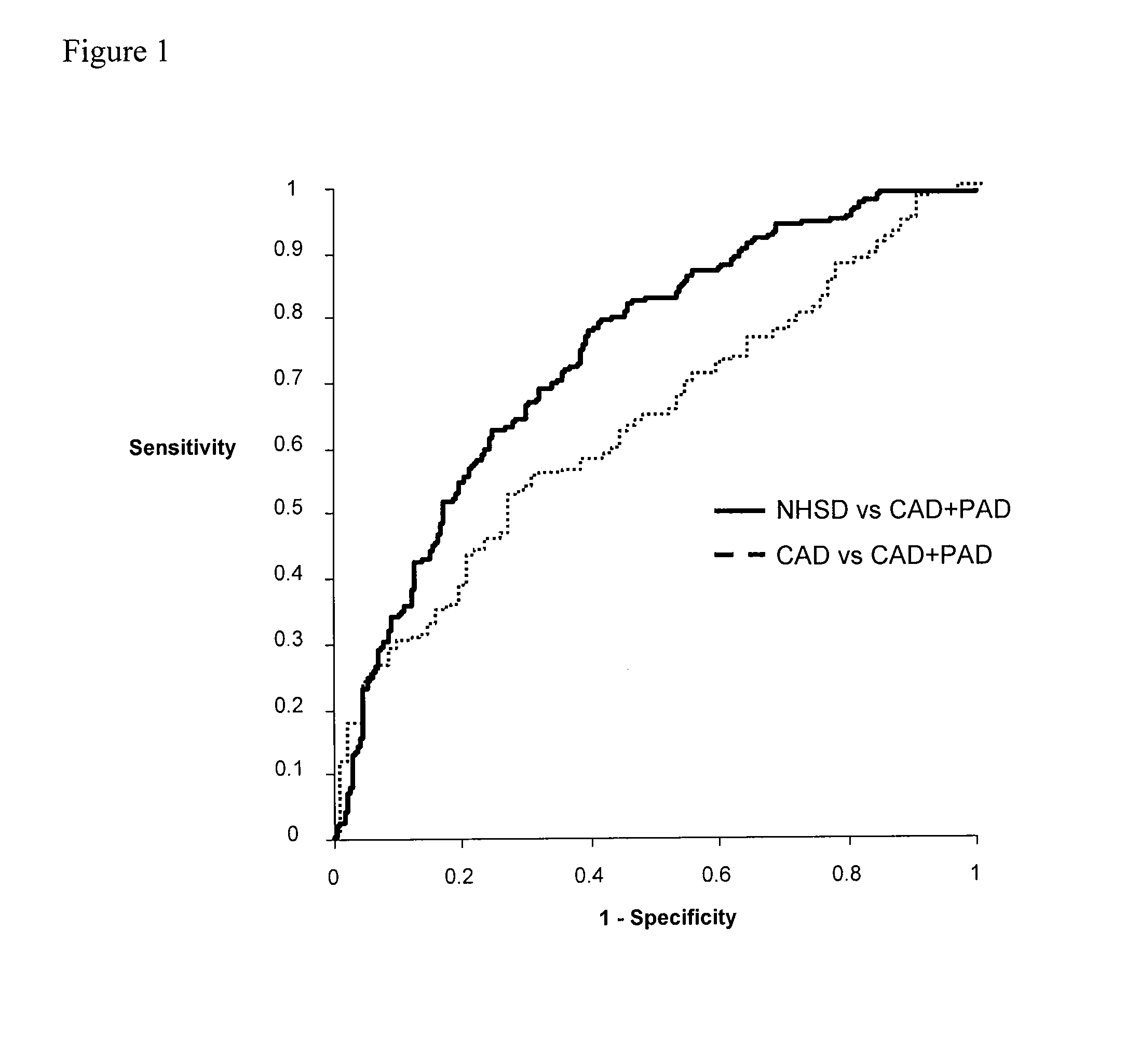
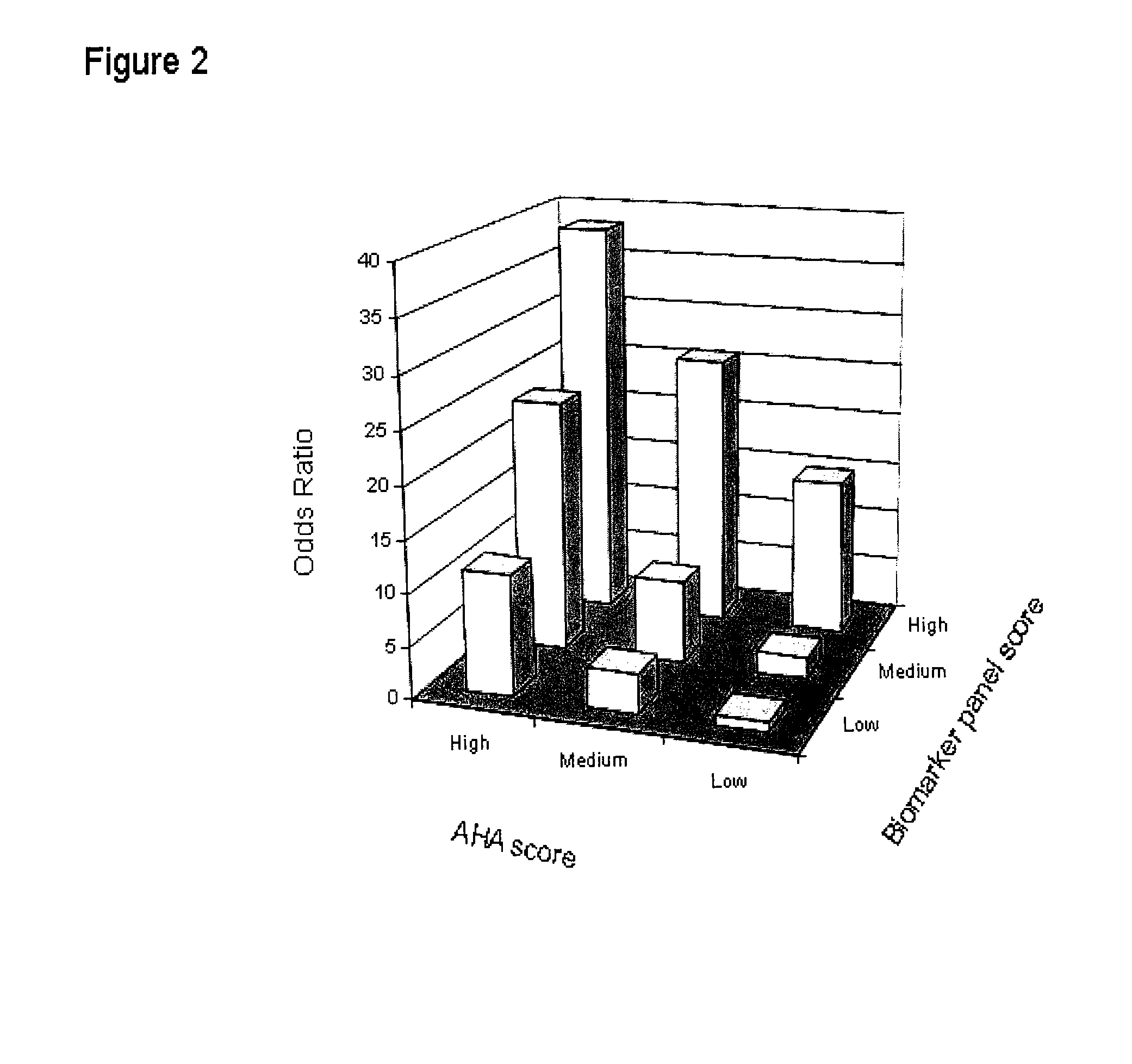
Panel of biomarkers for peripheral arterial disease
ActiveUS20080171396A1Increases clinical index of suspicionGreat scrutinyDisease diagnosisBiological testingHaemoglobin A1cD-Glucose
The invention provides biomarkers including β-2-microglobulin, Cystatin C, hsCRP and glucose as well as methods for using the biomarkers for diagnosing and / or assessing the risk of peripheral artery disease in a subject. In some embodiments, the subject being tested may be suffering from or at risk of other circulatory diseases, including coronary artery disease. Hemoglobin A1c or other proxies for measuring glucose levels may be substituted for or measured in addition to glucose in the context of the present invention.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV +1
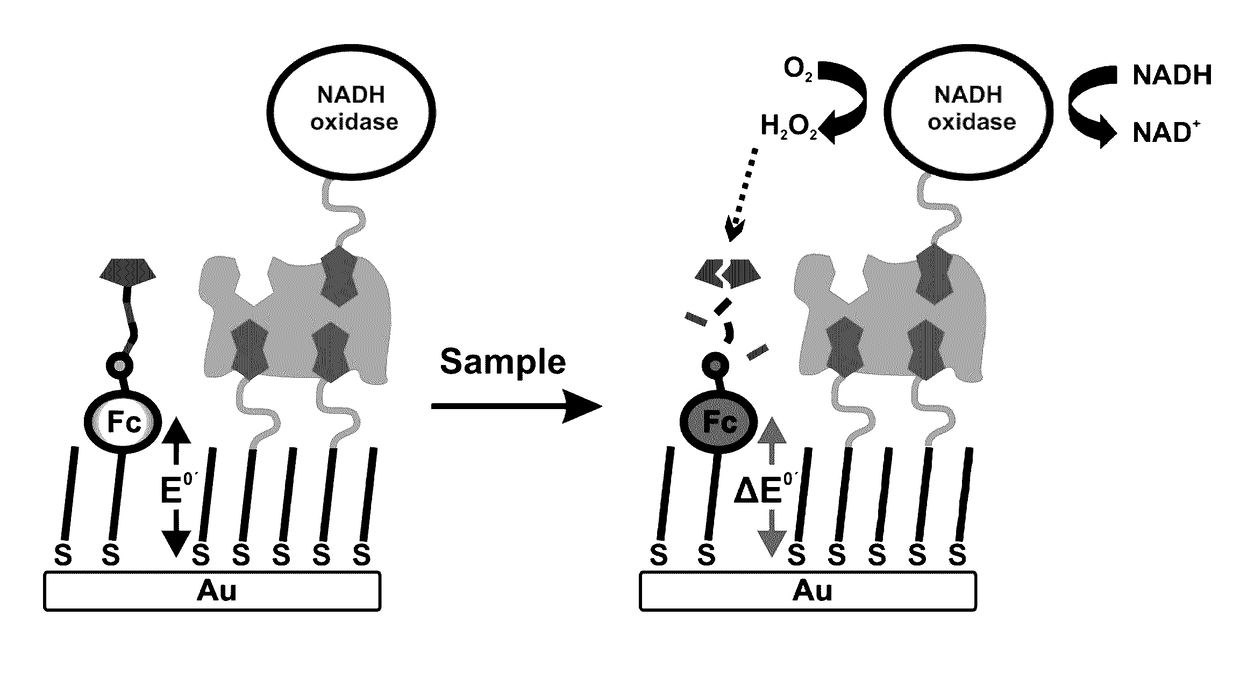
Single, Direct Detection Of Hemoglobin A1C Percentage Using Enzyme Triggered Redox Altering Chemical Elimination (E-Trace) Immunoassay
InactiveUS20130098777A1Accurate measurementImmobilised enzymesBioreactor/fermenter combinationsPhysical chemistryRedox
The disclosure also relates to novel compositions and methods for the single, direct detection of Hemoglobin A1c percentage in a sample, using conversion of functional groups attached to a transitional metal complex, resulting in quantifiable electrochemical signal at two unique potentials, Eo1 and Eo2.
Owner:OHMX CORP
Ratiometric Immunoassay Method and Blood Testing Device
InactiveUS20120142025A1Bioreactor/fermenter combinationsBiological substance pretreatmentsPoint of careAnalyte
The invention is to devices and methods for rapid determination of analytes in liquid samples. The devices and methods incorporate a sample dilution feature and multiple immunosensors for performing a ratiometric immunoassay on a first analyte and a second analyte, for example, hemoglobin and hemoglobin A1c or albumin and glycosylated albumin. The devices are preferably capable of being used in the point-of-care diagnostic field.
Owner:ABBOTT POINT CARE
Hemoglobin A1c Determination Method, Enzyme to be Used therefor, and Production Method Thereof
ActiveUS20080113381A1Reduce actionReduced activityBacteriaHydrolasesProteinase activityScreening method
There is provided a method for specifically determining a glycated β-chain N-terminal of glycated hemoglobin using enzymes without a separation operation, and a determination reagent kit therefor.A protease that cleaves a glycated amino acid and / or a glycated peptide from a glycated β-chain N-terminal without substantially cleaving a glycated amino acid or a glycated peptide from a glycated α-chain N-terminal of glycated hemoglobin or a fragment thereof is screened. The method of specifically determining a glycated β-chain N-terminal of glycated hemoglobin and the determination reagent kit are provided by using the protease obtained by the screening method.According to the present invention, a glycated β-chain N-terminal of glycated hemoglobin can specifically be determined without a separation operation.
Owner:ASAHI KASEI PHARMA
Handheld Dedicated Device for Diabetes Management
InactiveUS20070012324A1Reduce riskEasily and accurately track whatSurgeryDiagnostic recording/measuringCholesterolHand held
Handheld dedicated device for diabetes management. The device has nutritional information for 35,000 food items. Food items from fast food restaurants and various brands of food. The device provides a way of looking up all this information. The device can store information entered by the user of the device on food eaten, glucose levels, medication, insulin and exercise data. It also provides a way to save information from blood tests like Hemoglobin A1c which is an average of blood glucose levels over three months, blood pressure, and cholesterol. The input source for the device is keypad or voice command means.
Owner:NIRKONDAR RATNA +1
Diagnostic assay
InactiveUS6265223B1Improve performanceEase of sample handlingSamplingLaboratory glasswaresBlood collectionHaemoglobin A1c
For blood or other physiological fluid sample collection kits that use filter paper to collect the sample the performance of the kit and associated analytical method can be improved by using a material having properties which are superior to those of standard filter paper or modified filter paper routinely used in standard biological assays. Certain materials currently available for uses other than blood collection, storage, or transport have properties that are advantageous as employed in assays of biological fluids, including the use of specific glass fiber blotting materials for collecting blood samples for hemoglobin or hemoglobin A1c monitoring.
Owner:FLEXSITE RES & DEV
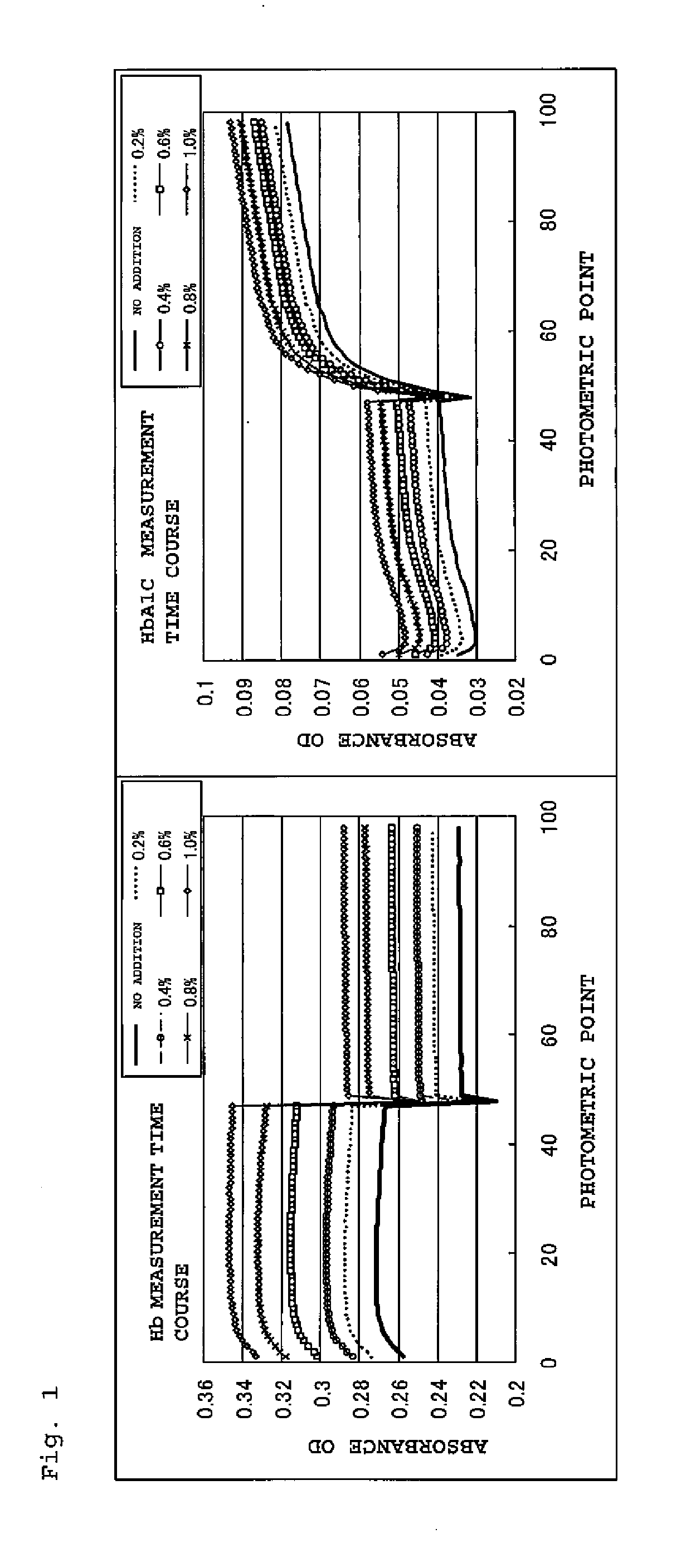
Hemolysis reagent composition for hemoglobin a1c quantitative analysis using enzymatic method
ActiveUS20140370539A1Accelerates hemolysis rateShorten the timeOrganic chemistryMicrobiological testing/measurementHemolysisReaction rate
Disclosed is a hemolysis reagent composition for the quantitative analysis of glycated hemoglobin (HbAlc) via an enzymatic method. The hemolysis reagent compositions of the present invention containing a zwitterionic surfactant and a nitrous compound considerably accelerate hemolysis rate due to the presence of the zwitterionic surfactant, and also induce the N-terminal β-chain of hemoglobin released by hemolysis to be exposed to the outside of the hemoglobin molecule and assists the participation of a greater number of molecules in the reaction, thereby improving reaction sensitivity and reaction rate of the glycated hemoglobin, and the nitrous compound makes the protein structure of the hemoglobin flexibly modified so that the amino acid sequence of the N-terminal β-chain of hemoglobin can be readily cut off, thereby significantly reducing the entire time required for the measurement and improving the accuracy of the measurement.
Owner:I SENS INC
Hemoglobin determination method
ActiveUS20100282607A1Improve accuracyReduced measurement timeElectrolysis componentsVolume/mass flow measurementHemoglobin determinationSulfated polysaccharides
An object of the present invention is to provide a method for measuring hemoglobin using electrophoresis, in particular a method for measuring hemoglobin that enables high accuracy measurement of stable hemoglobin A1c and a method for simultaneously measuring stable hemoglobin A1c and abnormal hemoglobins.The present invention provides a method for measuring hemoglobin using electrophoresis, which comprises: immobilizing an ionic polymer on an inner surface of a migration path; and using a buffer solution containing a sulfated polysaccharide.
Owner:SEKISUI CHEM CO LTD
Method for immunologically assaying hemoglobin a1c in specimen
InactiveUS20150316541A1Easy to handleReduce processing timeBiological testingLatex particleAgglutination
Means that enables an immunoassay of hemoglobin A1c in a whole blood sample without pretreatment of the sample is disclosed. The immunoassay of hemoglobin A1c in a sample according to the present invention comprises bringing latex particles into contact with a non-pretreated whole blood sample in a hypotonic solution, and then bringing hemoglobin A1c adsorbed on the latex particles into contact with an anti-hemoglobin A1c antibody. The immunoassay is preferably carried out by an agglutination method. The hypotonic solution is, for example, a buffer containing a Good's buffer at a concentration of 0.02 to 0.2 mol / L, which Good's buffer has the maximum buffer capacity in the neutral to alkaline region.
Owner:FUJIREBIO CO LTD
Diagnostic assay
InactiveUS6177283B1Improve performanceEase of sample handlingBioreactor/fermenter combinationsBiological substance pretreatmentsBlood collectionCellulose
For blood or other physiological fluid sample collection kits that use filter paper to collect the sample, the performance of the kit and associated analytical method can be improved by using a material having properties which are superior to those of standard filter paper or modified filter paper routinely used in standard biological assays. Certain materials currently available for uses other than blood collection, storage, or transport have properties that are advantageous as employed in assays of biological fluids, including the use of specific cellulose blotting materials for collecting blood samples for hemoglobin or hemoglobin A1c monitoring.
Owner:FLEXSITE RES & DEV
Measuring device and measuring method
InactiveCN102564977AEasy to measureEasy to operateMaterial analysis by observing effect on chemical indicatorColor/spectral properties measurementsMeasurement deviceBlood specimen
Owner:TOYOBO CO LTD
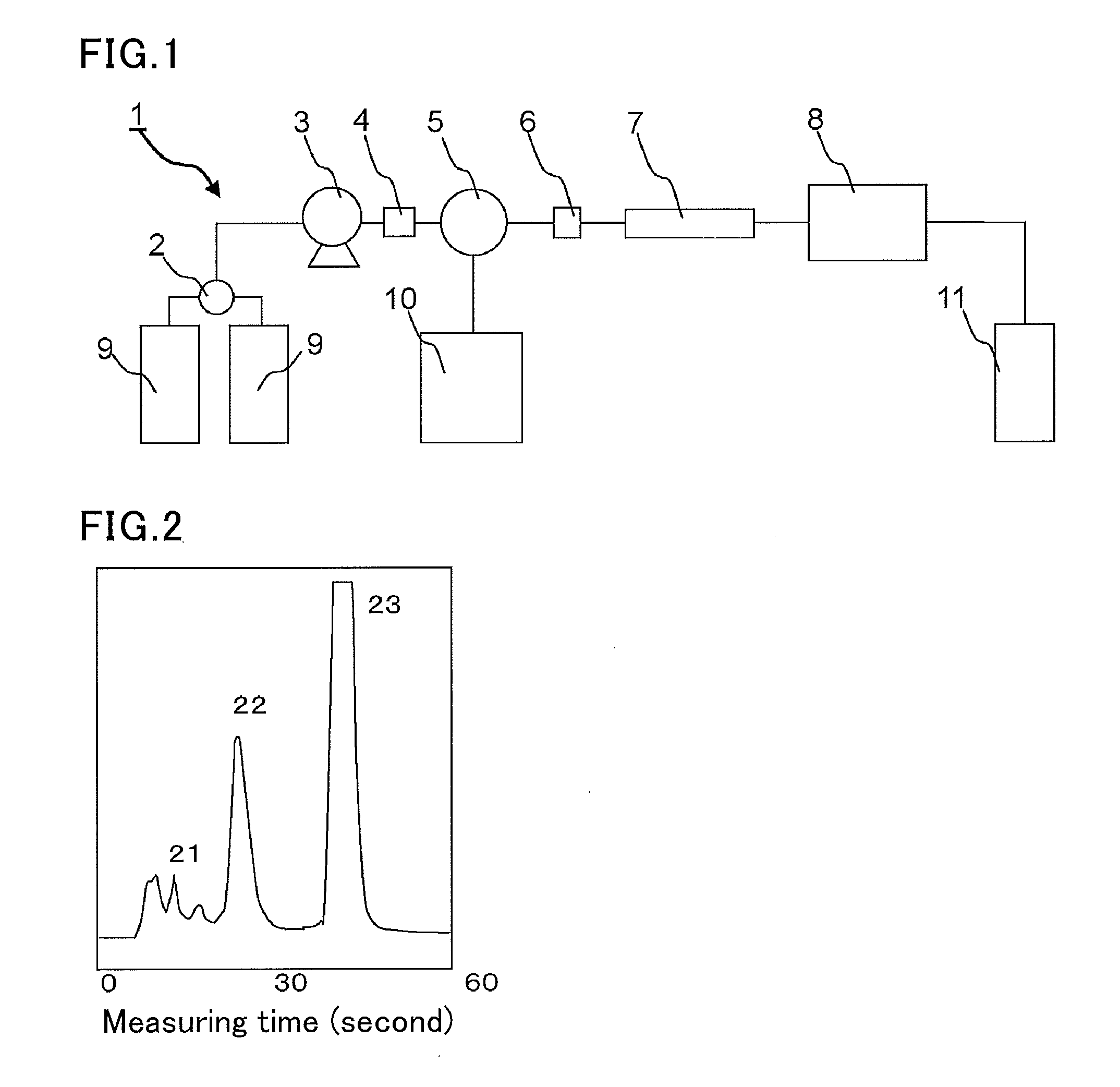
Method for measuring hemoglobins
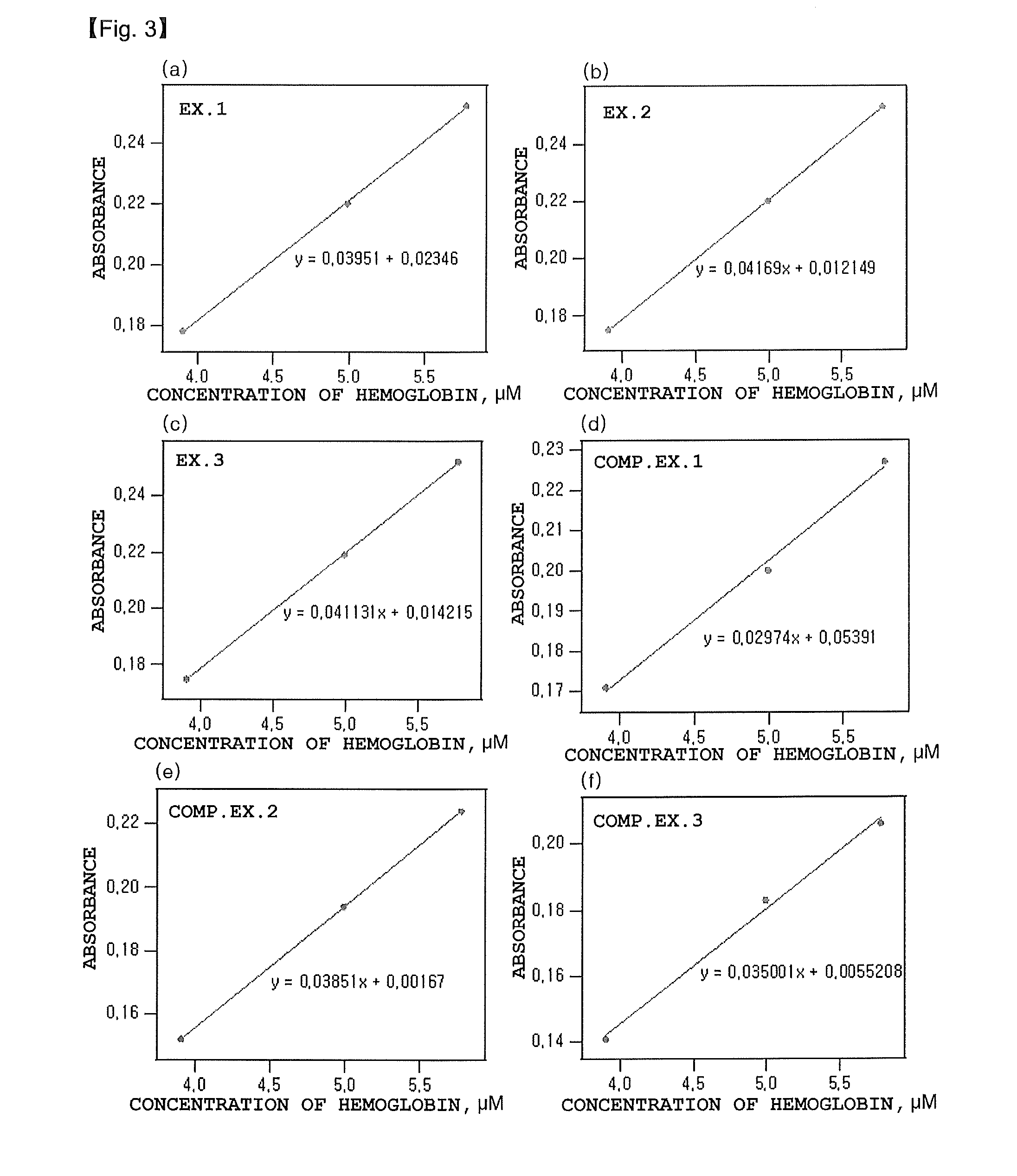
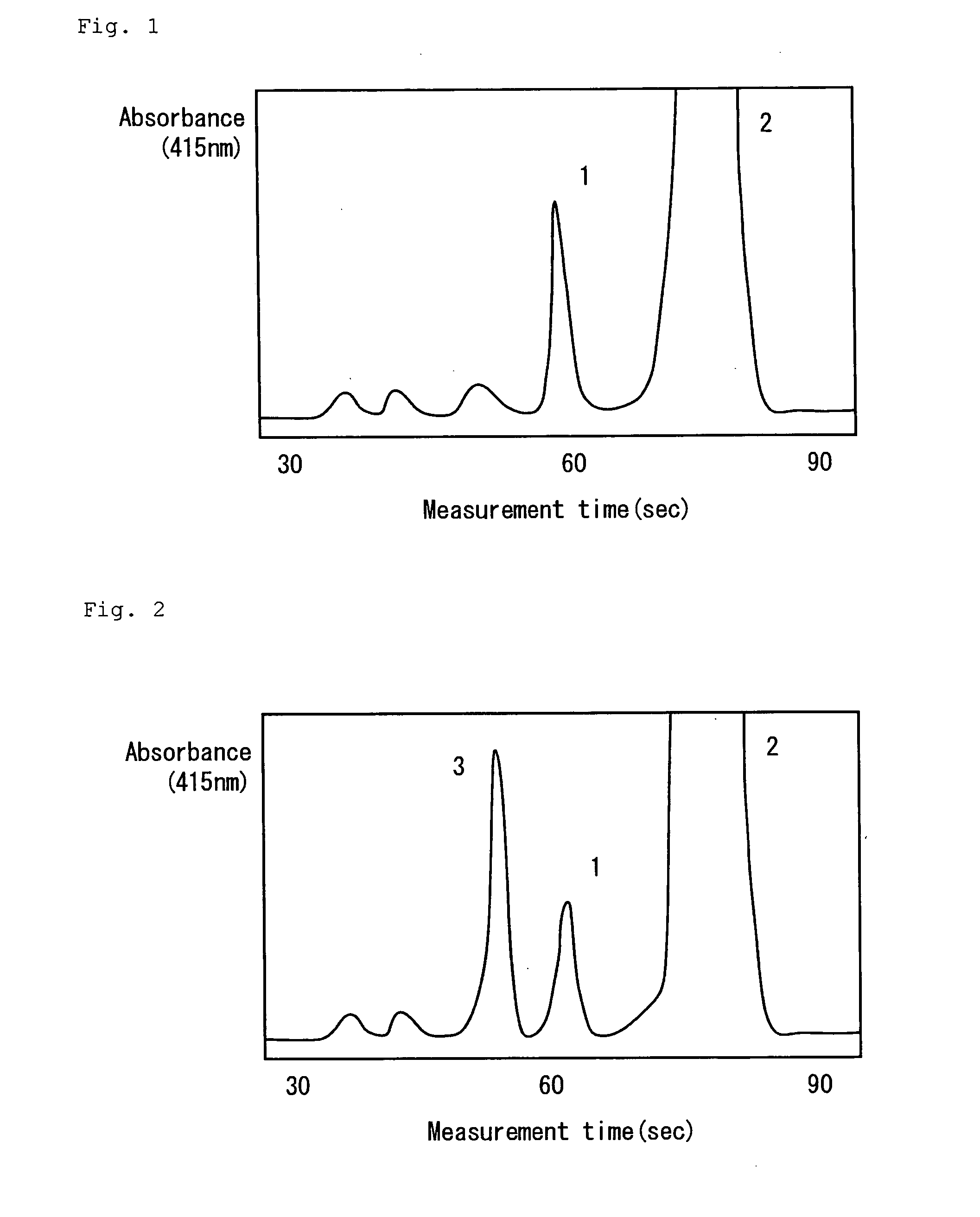
ActiveCN103649745AAddressing Assay ShortcomingsChromatographic cation exchangersComponent separationIon exchangeCrosslinked polymers
The purpose of the present invention is to provide: a method for measuring hemoglobins, which can measure hemoglobins within a short time and with high accuracy; and a method for measuring hemoglobin A1c, a method for measuring hemoglobin A1c and hemoglobin F simultaneously, a method for measuring hemoglobin A1c and hemoglobin A2 simultaneously and a method for measuring hemoglobin A1c and an abnormal hemoglobin simultaneously, each of which utilizes the above-mentioned method for measuring hemoglobins. The present invention is a method for measuring hemoglobins by liquid chromatography using a column which has cation-exchangeable particles filled therein as a column filler and has a pressure value of 9.8 plus103 to 29.4 plus105 Pa inclusive when an eluent to be used for the measurement is fed therethrough at a flow rate of 1.0 mL / minute, wherein the cation-exchangeable particles comprise crosslinked polymer particles and a polymer containing a cation-exchange group and provided on the surfaces of the crosslinked polymer particles.
Owner:SEKISUI MEDICAL CO LTD
Method for immunologically assaying hemoglobin A1c in specimen
InactiveCN105122060AEasy to handleReduce processing timeBiological testingLatex particleAgglutination
Owner:FUJIREBIO CO LTD
HEMOGLOBIN A1c-SPECIFIC APTAMER, HEMOGLOBIN-SPECIFIC APTAMER, AND APPLICATIONS THEREOF
ActiveUS20150111306A1Easy to synthesizeReserve accuracySugar derivativesBiological material analysisDiabetes mellitusAptamer
The present invention provides a Hemoglobin A1c-specific aptamer and a Hemoglobin-specific aptamer. The aptamers were selected in vitro using SELEX and a microfluidic chip system. The aptamers established low free energy, thus were more stable than conventional antibodies. The high specificity of the aptamers to Hemoglobin A1c or Hemoglobin allows them to be effectively used in diagnosis of diabetes and / or anemia.
Owner:NATIONAL TSING HUA UNIVERSITY
HEMOGLOBIN A1c MEASUREMENT METHOD AND MEASUREMENT KIT
PendingCN105683374AImprove reaction efficiencyAvoid unwanted reactionsMaterial analysis by observing effect on chemical indicatorMicrobiological testing/measurementHemoglobin A1c measurementSpecific activity
Provided is an amadoriase characterized in acting on the beta-chains of hemoglobin A1c (HbA1c) to generate hydrogen peroxide. A HbA1c measurement method and measurement reagent kit using same are also provided. A HbA1c measurement method uses an amadoriase characterized in having a specific activity of 0.1 U / mg or more with respect to alphaF6P and in oxidizing the amino terminus of the beta-chains of HbA1c to generate hydrogen peroxide, and a measurement reagent kit comprises said amadoriase. The present invention provides a measurement method and measurement kit that are able to quantify HbA1c rapidly, easily and with good precision.
Owner:KIKKOMAN CORP
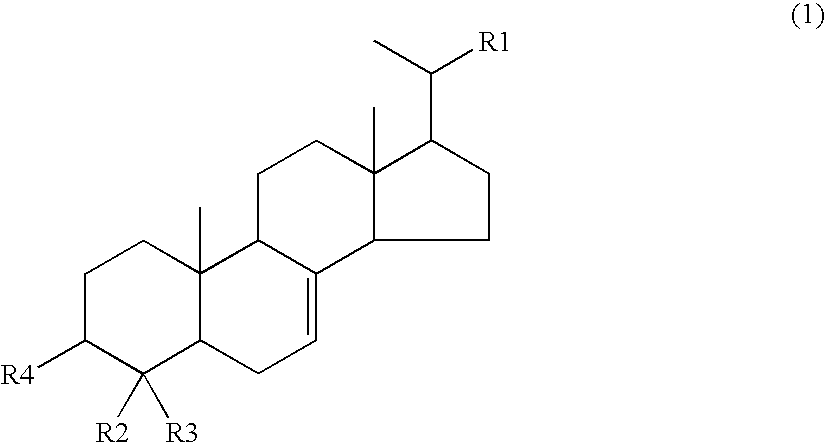
Drug for improving hyperglycemia
ActiveUS20100240632A1Safely ingestedImproving hyperglycemiaOrganic active ingredientsSenses disorderAcute hyperglycaemiaAlcohol
A compound having a hyperglycemia improving effect and a hemoglobin A1c lowering action such as 4-methylcholest-7-en-3-ol, 4-methylergost-7-en-3-ol and 4-methylstigmast-7-en-3-ol is used as an active ingredient of a drug or food or drink for improving hyperglycemia.
Owner:MORINAGA MILK IND CO LTD
Saturation binding ratiometric assay
InactiveUS20190137522A1Eliminate significant source of imprecisionEffective amountBiological testingDetection of post translational modificationsMagnetic beadTotal hemoglobin
Methods, devices, and reagents are described for performing ratiometric assays for hemoglobin A1c. The methods involve a direct ratio determination between Hb A1c and normalized total hemoglobin utilizing a saturating amount of hemoglobin so that Hb A1c binds proportionately to a substrate. In some applications, the assay utilizes LOCI or other proximity label for signal generation and / or labeled magnetic beads. The methods can be configured as homogeneous or heterogeneous assays.
Owner:MANNEH VICTOR +1
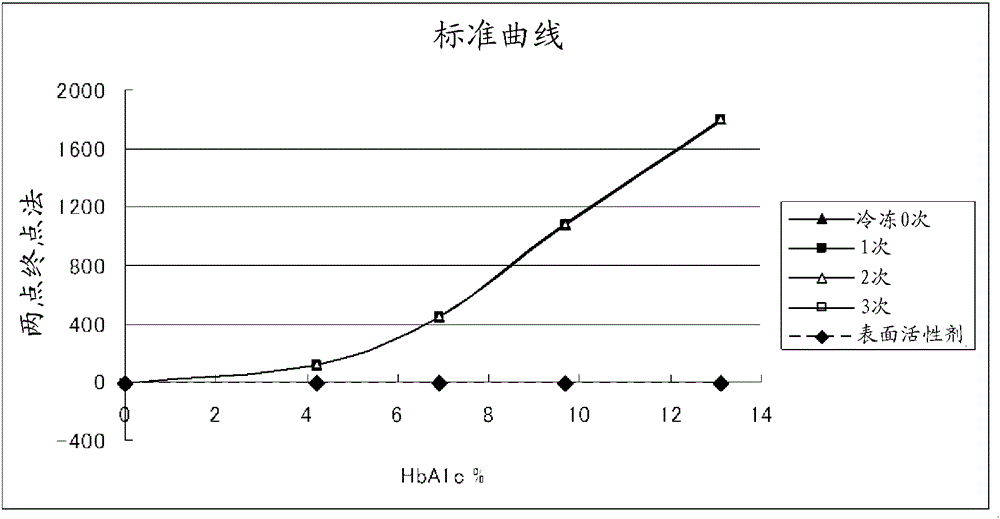
CALIBRATION METHOD IN MEASUREMENT OF HEMOGLOBIN A1c
ActiveUS20120103054A1Easy CalibrationShort timeSludge treatmentComponent separationHaemoglobin A1cEnvironmental geology
A calibration method that enables calibration easily in a short time in a measurement of hemoglobin A1c by use of a separation analysis is provided. In a measurement of a hemoglobin A1c amount by use of a separation analysis, a one-point calibration using a single calibration standard is performed to obtain calibration data to be used for correcting a measured value.
Owner:ARKRAY INC
Panel of biomarkers for peripheral arterial disease
InactiveUS7998743B2Increases clinical index of suspicionGreat scrutinyDisease diagnosisBiological testingCoronary artery diseaseBeta-2-Microglobulin Protein
The invention provides biomarkers including β-2-microglobulin, Cystatin C, hsCRP and glucose as well as methods for using the biomarkers for diagnosing and / or assessing the risk of peripheral artery disease in a subject. In some embodiments, the subject being tested may be suffering from or at risk of other circulatory diseases, including coronary artery disease. Hemoglobin A1c or other proxies for measuring glucose levels may be substituted for or measured in addition to glucose in the context of the present invention.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV +1
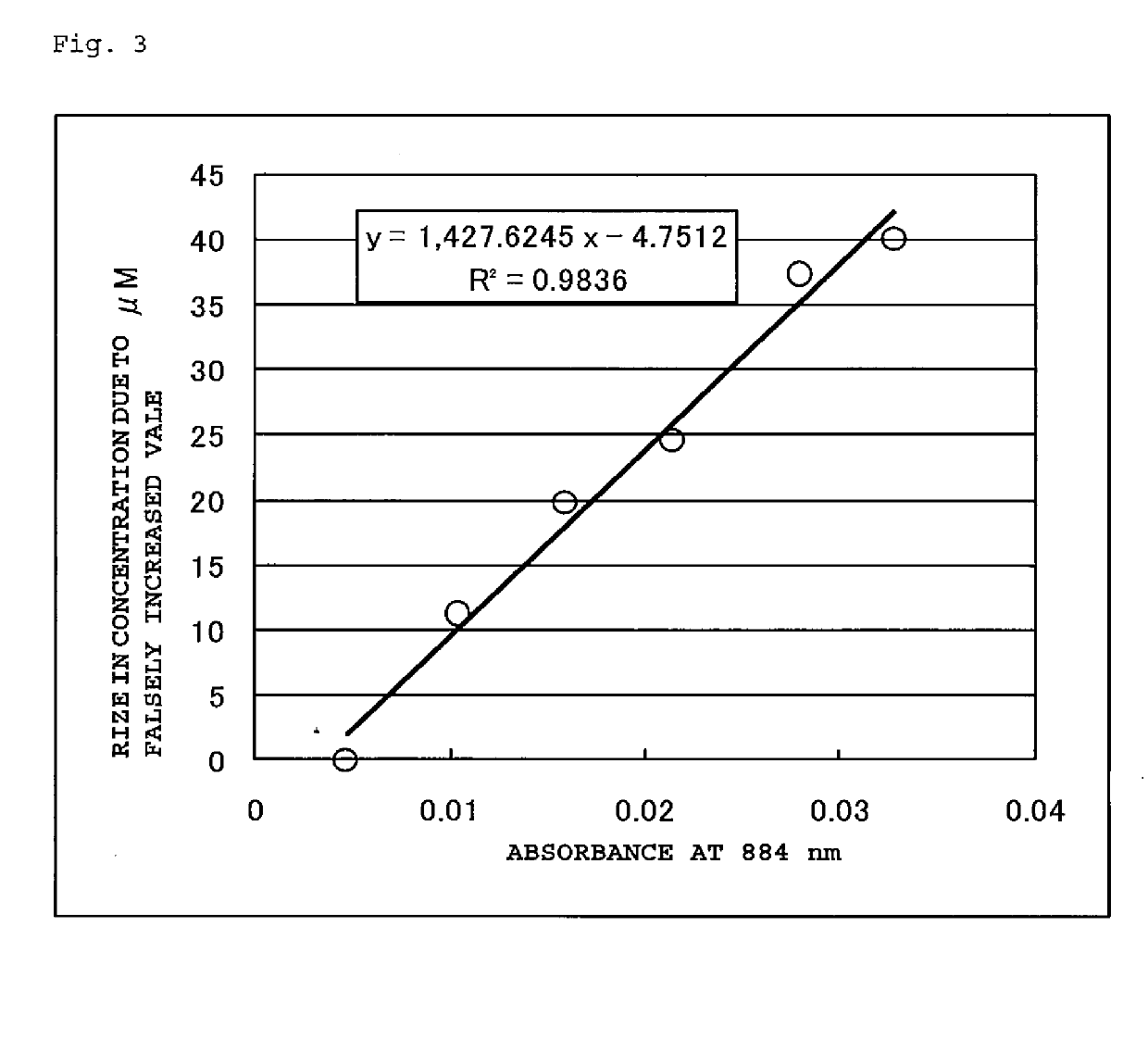
METHOD FOR MEASURING HbA1c
ActiveUS20190169674A1Accurate measurementMicrobiological testing/measurementBiological material analysisLength waveWhole blood sample
Provided is a method of avoiding the influence of a coexisting substance in the measurement of HbA1c % for a whole blood sample by an enzymatic method. Specifically, provided is a method of measuring a ratio of a hemoglobin A1c concentration to a hemoglobin concentration in a sample by an enzymatic method, the method including: a first step of optically measuring the hemoglobin concentration; and a second step of optically measuring the hemoglobin A1c concentration, wherein, when HbA1c % is calculated by dividing the hemoglobin A1c concentration measured in the second step by the hemoglobin concentration measured in the first step, the hemoglobin concentration serving as a denominator, which is measured at a first wavelength, is corrected using a result measured at a second wavelength.
Owner:SEKISUI MEDICAL CO LTD
Hypoglycemic preparation used for human body and its preparation method
InactiveCN107468717AFunction increaseCorrect disorderBacteriaMetabolism disorderDiabetes mellitusIntestinal microorganisms
The invention provides a hypoglycemic preparation for the human body. The hypoglycemic preparation includes at least one kind of probiotics, and the amount of each kind of probiotics is 107-1013 CFU, and the total amount of the probiotics in each hypoglycemic preparation is 8*107-8*1013 CFU. The invention further discloses a preparation method of a hypoglycemic preparation used for the human body. According to the hypoglycemic preparation used for the human body and its preparation method, the gastrointestinal function can be promoted, the body's immune system is regulated so as to correct the intestinal microbial disturbance, and the preparation has the effects of reducing hemoglobin A1c and treating the diabetes.
Owner:陈元秀
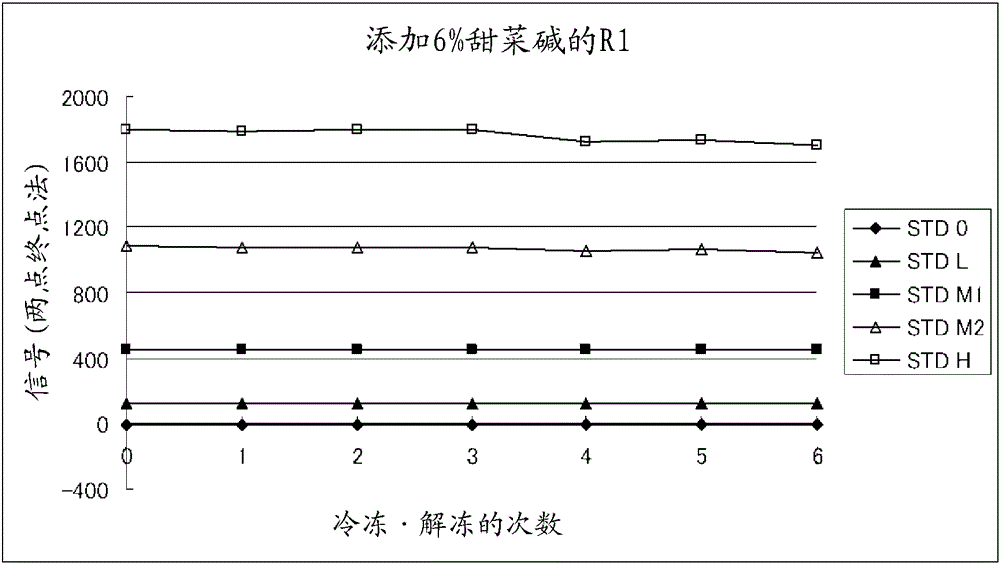
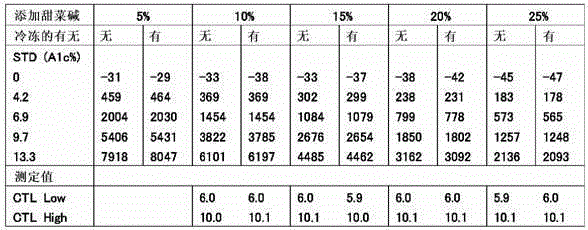
Method for preventing deterioration of unsensitized latex reagent
Provided is a novel means for suitably maintaining the dispersion state of a latex reagent in an immunoassay kit and preventing the occurrence of coagulation and sedimentation due to changes in temperature so that a reagent is protected from deterioration in measurement performance, said means also being applicable to immunoagglutination measurement kits in which a small amount of a latex reagent is dispensed. An immunoassay reagent according to the present invention comprises unsensitized latex particles and trimethyl glycine in a solvent. The concentration of trimethyl glycine in the reagent is preferably 5-10 w / v%. The reagent can be suitably used as an immunoassay reagent for hemoglobin A1c, for example.
Owner:FUJIREBIO CO LTD
METHOD FOR MEASURING STABLE HEMOGLOBIN A1c USING LIQUID CHROMATOGRAPHY, AND METHOD FOR SIMULTANEOUS MEASUREMENT OF STABLE HEMOGLOBIN A1c AND ABNORMAL HEMOGLOBIN USING LIQUID CHROMATOGRAPHY
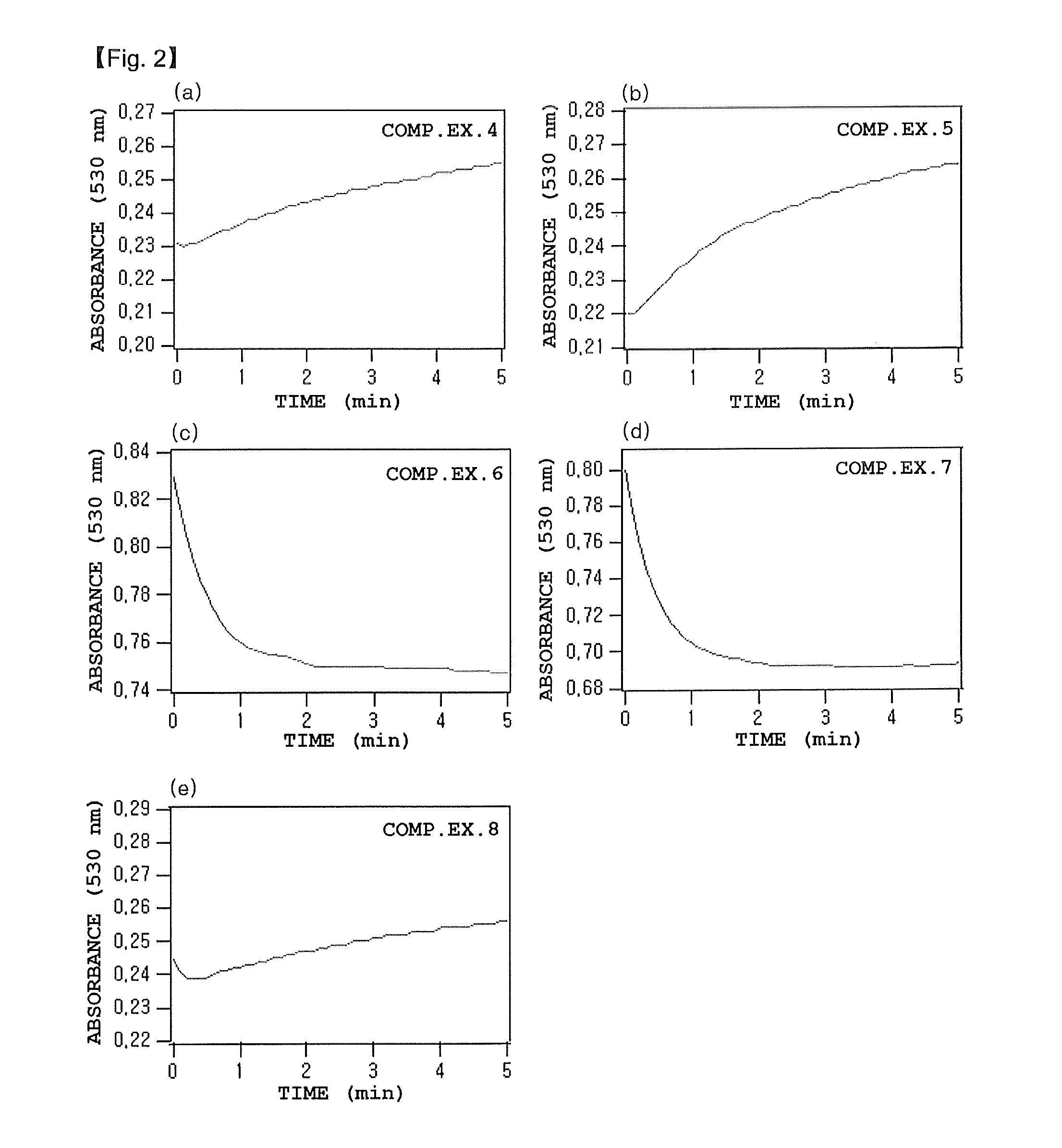
InactiveUS20140238113A1Good reproducibilityShort timeSamplingComponent separationSilicone oilChemistry
An object of the present invention is to provide a method capable of measuring stable hemoglobin A1c using liquid chromatography in a short time with good reproducibility.The present invention is a method for measuring stable hemoglobin A1c using liquid chromatography, installing on a flow path of a liquid chromatograph a filter whose surface is treated with a solution containing 1 to 50% by weight of a silicone oil or a solution containing 1 to 50% by weight of a silicone resin, and setting a pressure value generated in a measurement system of the liquid chromatograph to 9.8×103 Pa or more and 19.6×105 Pa or less.
Owner:SEKISUI MEDICAL CO LTD
Cellular hemoglobin a1c quality controls
Intact erythrocytes with selected and often elevated levels of hemoglobin A1c (HbA1c) for use as quality controls for HbA1c assays and assay instruments are prepared by hypotonic dialysis of erythrocytes from a healthy mammal to permeabilize the erythrocyte membranes, infusion of the permeabilized erythrocytes with hemoglobin A1c, and de-permeabilization of the infused erythrocytes. Quality controls of essentially any level of HbA1c can be prepared in this manner and once prepared will be useful for monitoring the entire assay procedure, including the lysis of the erythrocytes in a typical sample.
Owner:BIO RAD LAB INC
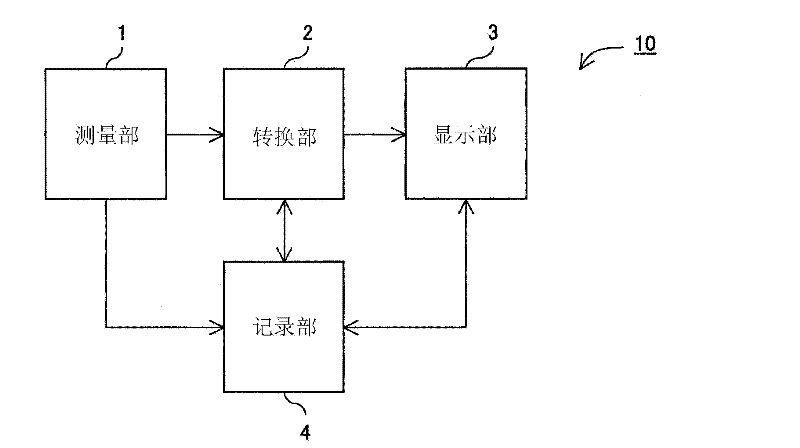
HbA1c measurement result display method, display device, and display program
InactiveCN102539594AInhibition of measurement resultsComponent separationHealth-index calculationDisplay deviceNormal values
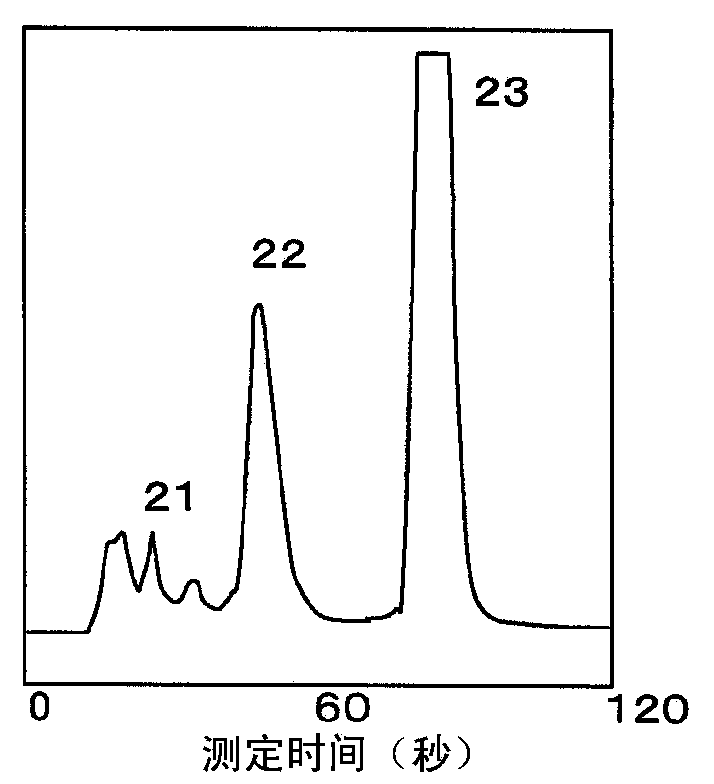
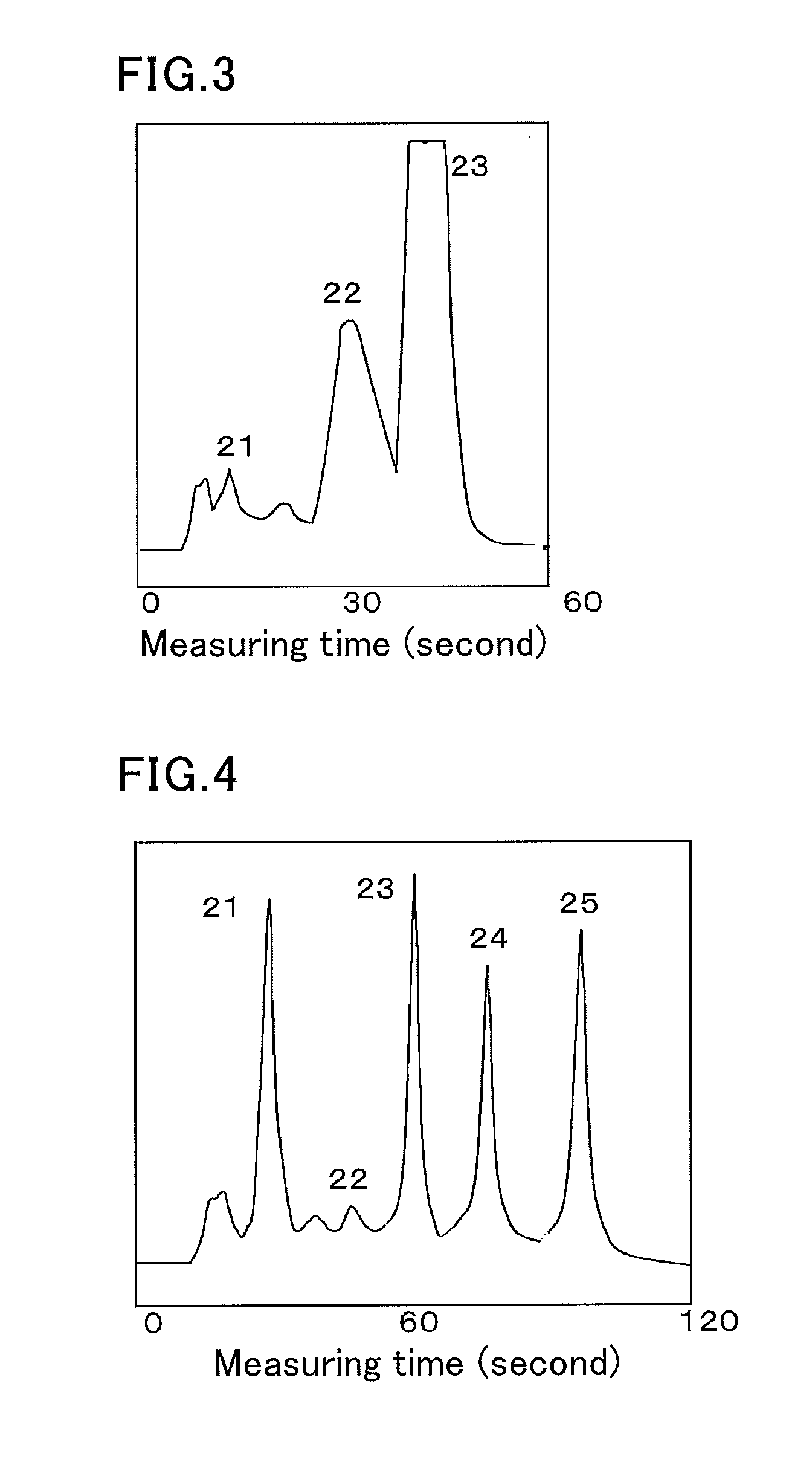
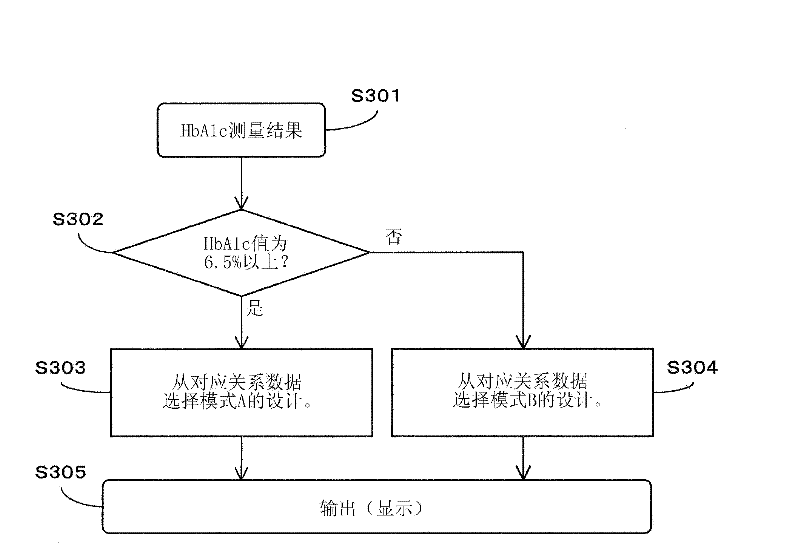
The invention provides an HbA1c measurement result display method, display device, and display program. The method for displaying HbA1c measurement results is provided, which makes it possible to prevent overlooking a sample that exhibits an HbAlc value at a predetermined level different from a normal value, for example, a sample that exhibits a diabetic type. The method is a display method for displaying spectrum data as results of measurement of hemoglobin A1c (HbAlc) in a sample by separation analysis, and the method includes applying one of at least two different types of designs to a peak area for HbAlc in the spectrum data, according to an amount of HbAlc in the sample, or according to the amount of HbAlc and an amount of blood glucose in the sample.
Owner:ARKRAY INC
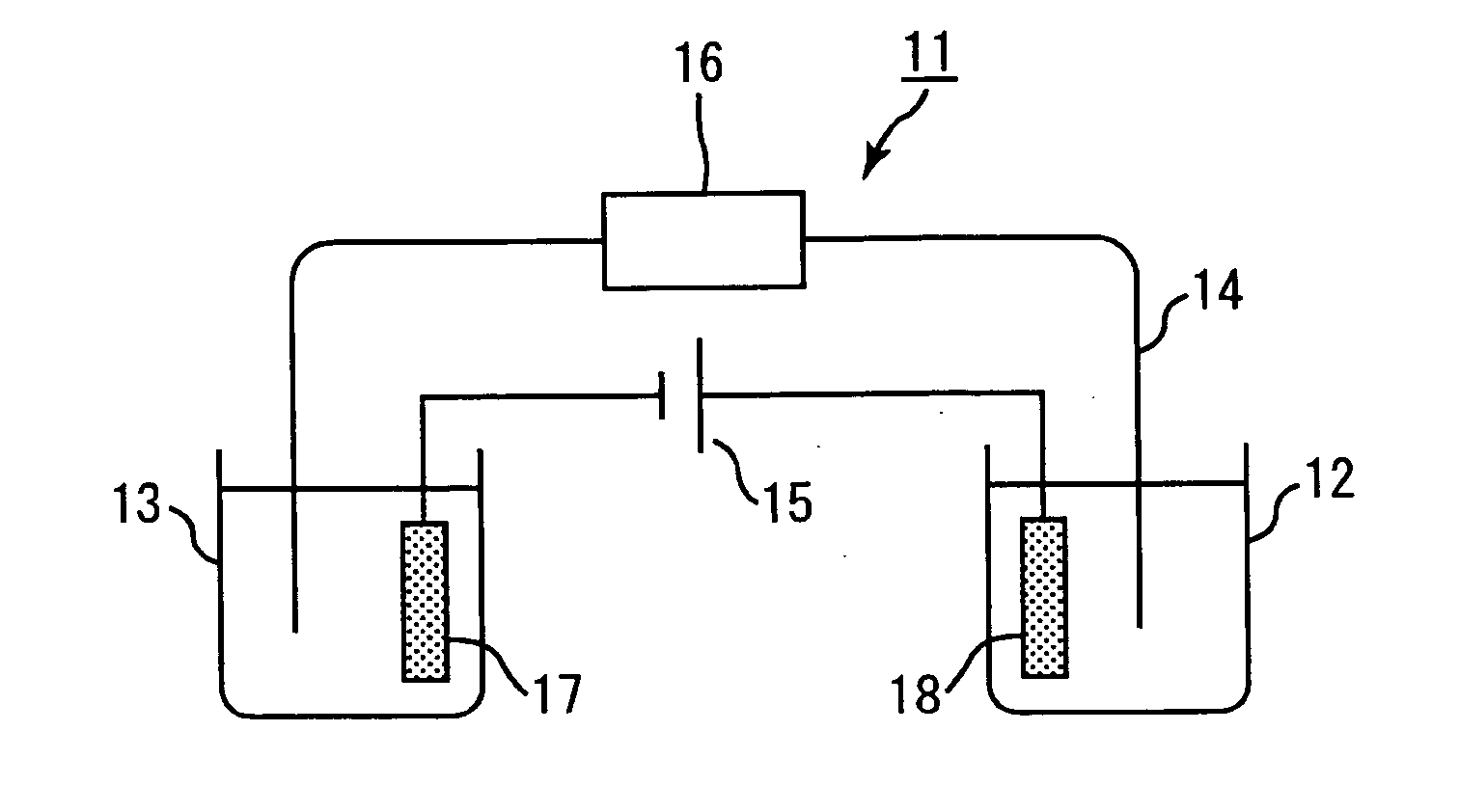
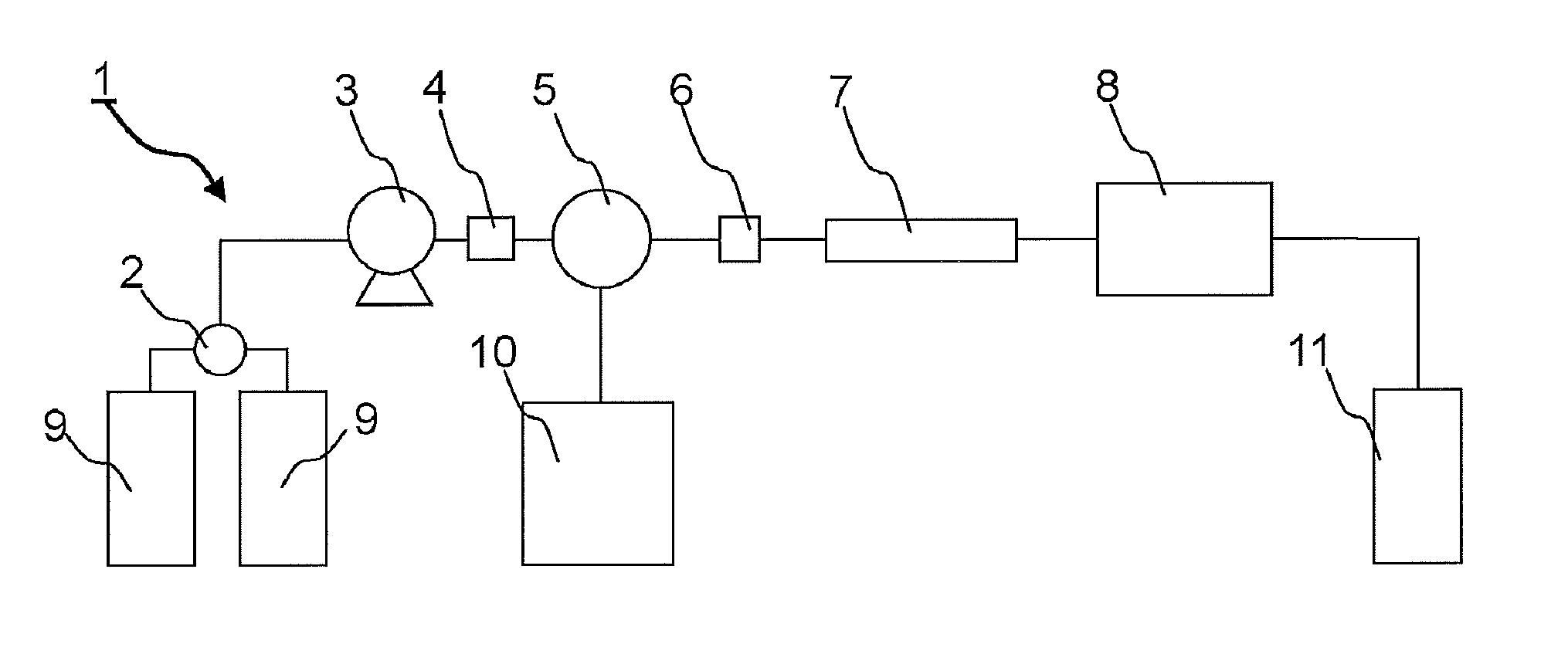
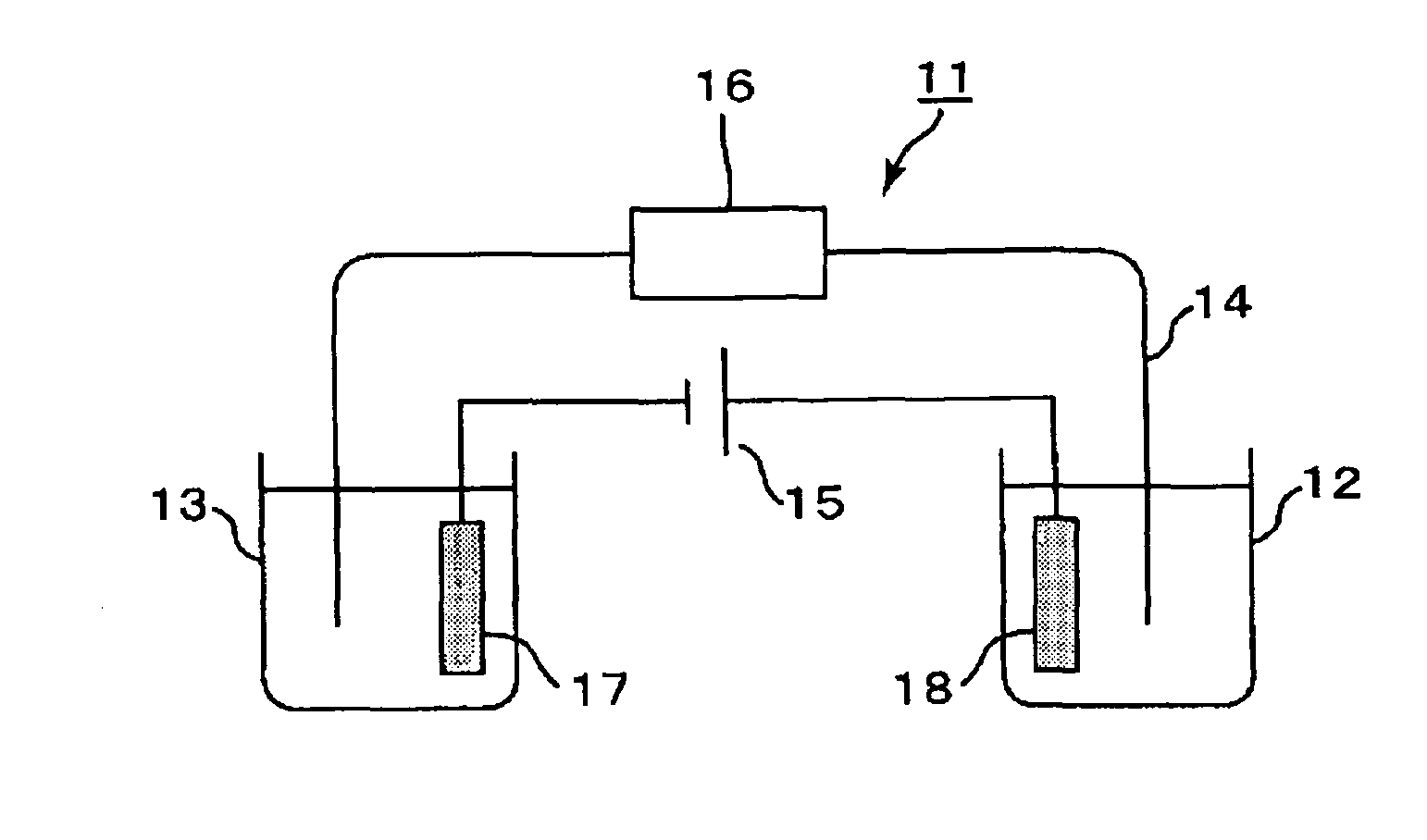
Hemoglobin measurement method and electrophoresis apparatus
InactiveUS9017536B2High precision measurementSludge treatmentVolume/mass flow measurementDiabetes mellitusMedicine
An object of the present invention is to provide a method for measuring hemoglobin that enables short-time, high-accuracy measurement of hemoglobin, in particular stable hemoglobin A1c, which is used as a diagnostic indicator of diabetes mellitus, and an electrophoresis apparatus that is suitably used in this measurement method. The present invention provides a method for measuring hemoglobins using electrophoresis, which includes: using a migration path having an inner surface coated with a cationic substance to be immobilized on the inner surface or a migration path having an inner surface made of a cationic material; and using a buffer solution containing a water-soluble polymer having an anionic group as an electrophoresis buffer solution.
Owner:SEKISUI CHEM CO LTD
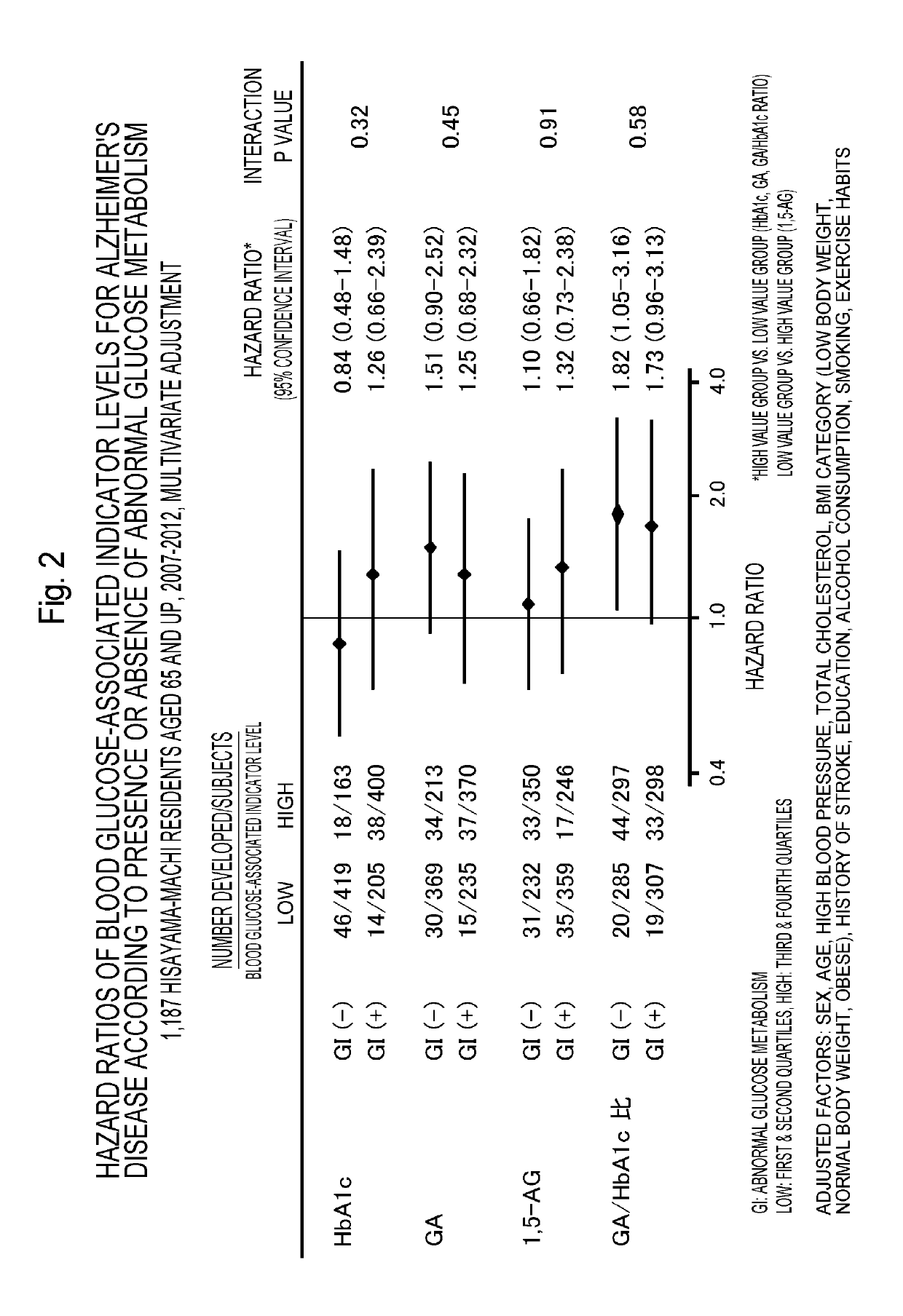
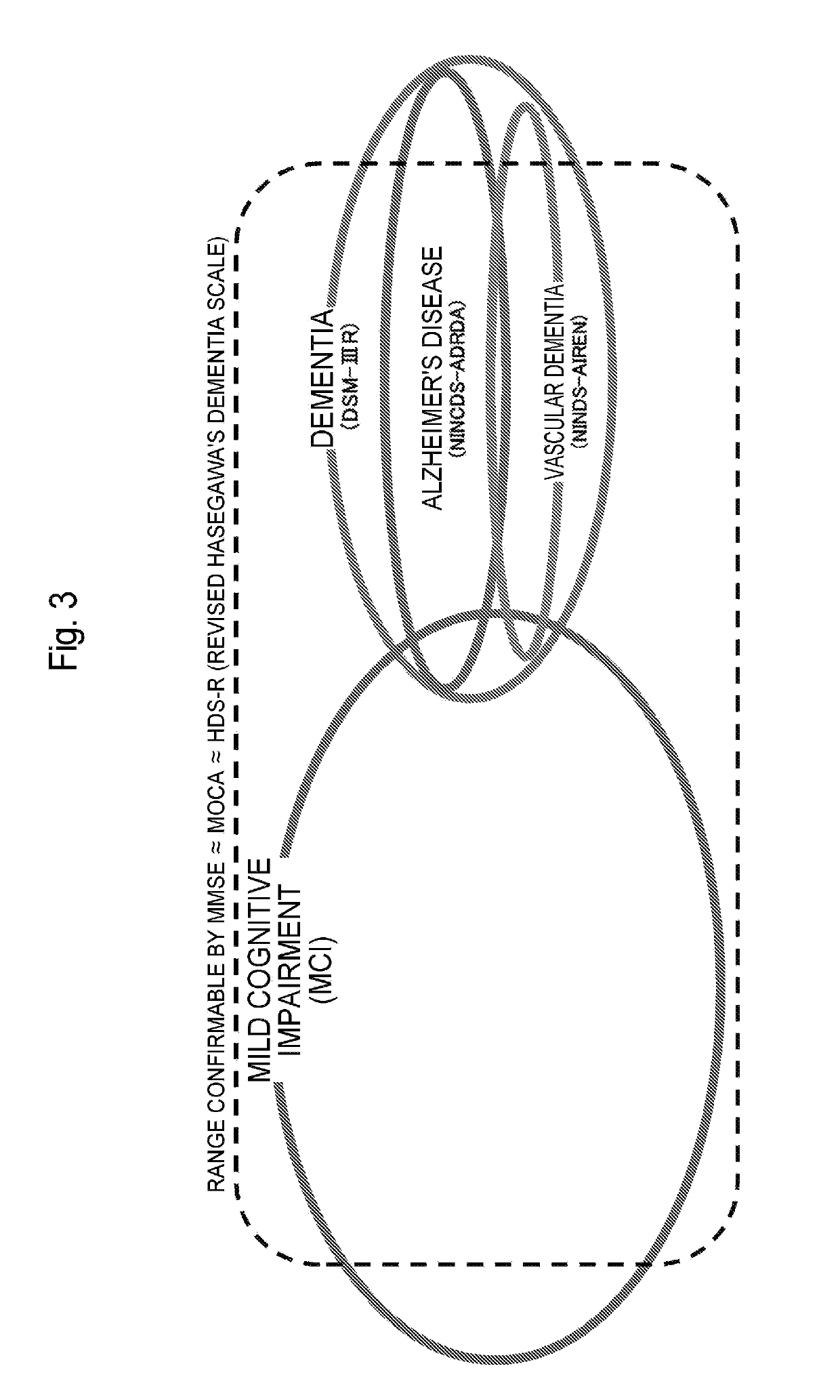
Method for determining alzheimer's disease risk
InactiveUS20190265256A1Determined accurately and easily and rapidly and cheaplyEasily and rapidly and cheaplyCompound screeningApoptosis detectionDisease riskGlycoalbumin
With the present invention, glycoalbumin and hemoglobin A1c are measured in a blood sample, and the incidence of or the existence or non-existence of a development risk of Alzheimer's disease can then be determined based on the calculated glycoalbumin / hemoglobin A1c ratio. Compounds for treating or preventing Alzheimer's disease can also be selected using this glycoalbumin / hemoglobin A1c ratio.
Owner:KYUSHU UNIV +1
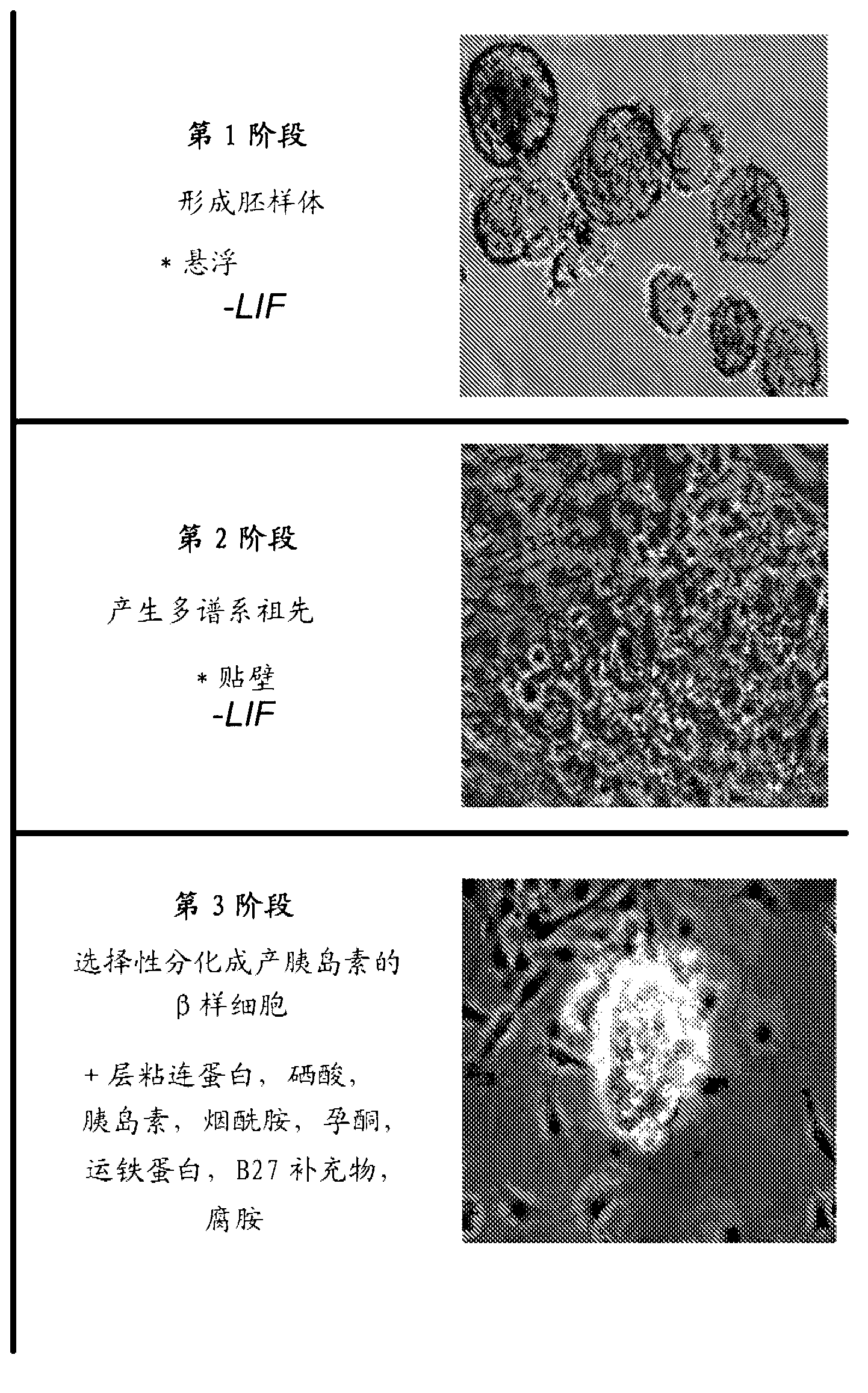
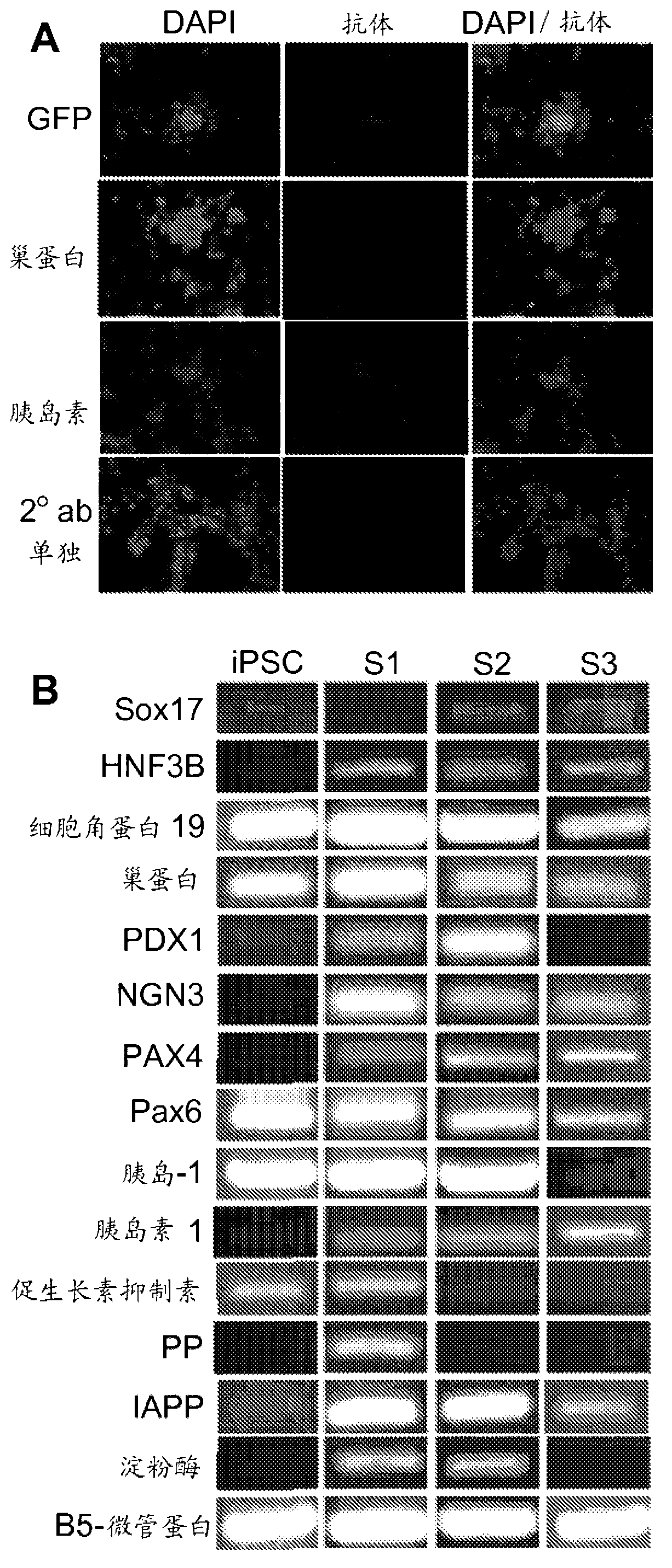
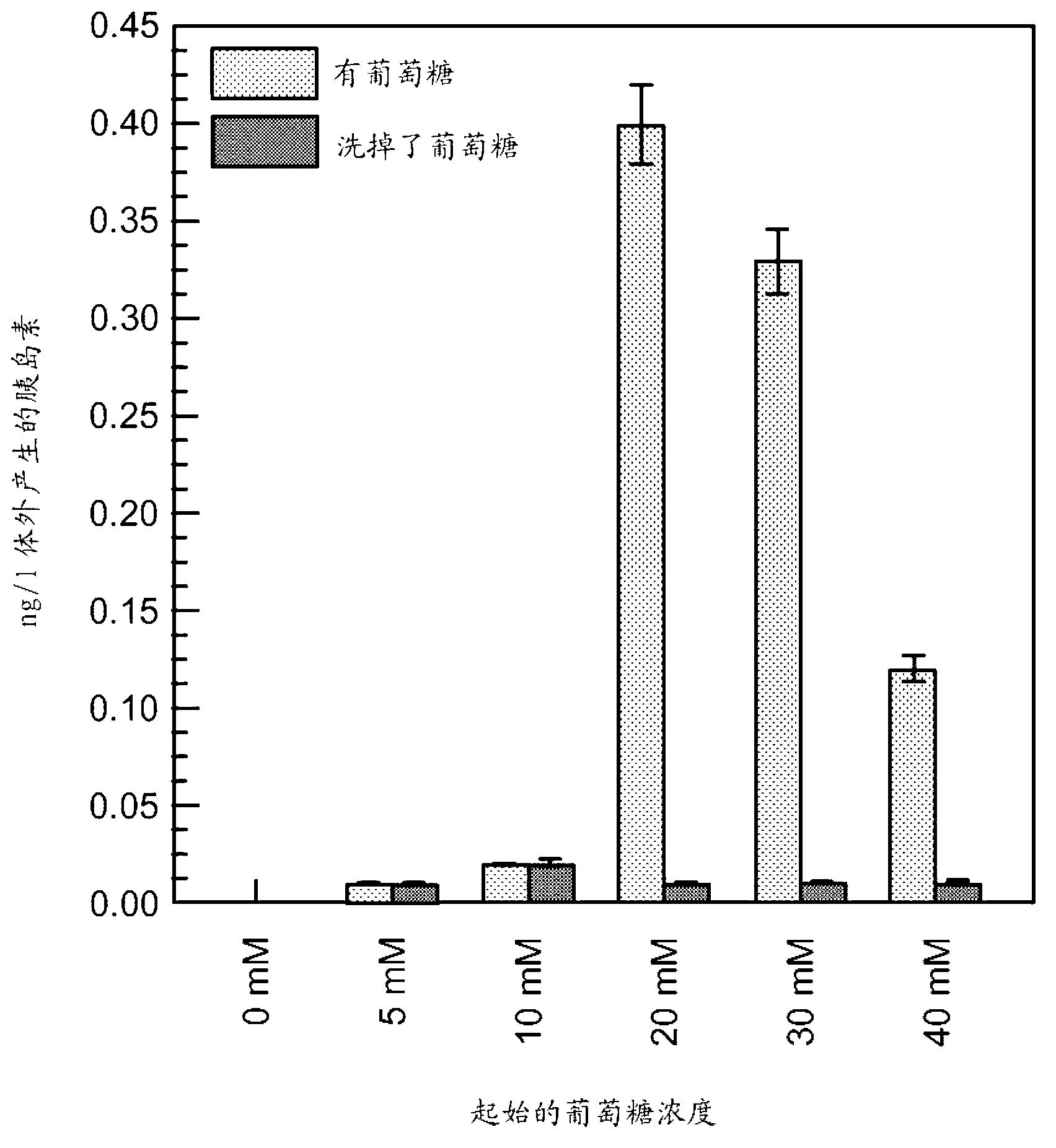
Methods and compositions for treating diabetes with iPS derived pancreatic beta-like cells
Diabetes mellitus is characterized by either the inability to produce insulin (Type 1 diabetes) and or as insensitivity to insulin secreted by the body (Type 2 diabetes). In either case, the body is unable to efficiently move blood glucose across cell membranes to be utilized. This leads to a variety of local and systemic detrimental effects. Current treatments for diabetes focus on exogenous insulin administration and dietary control. Provided herein are treatments of diabetes using a cellular therapy to ameliorate symptoms associated with both reduced insulin secretion and insulin sensitivity. Using induced-pluripotent stem (iPS) cells, beta-like (ss-like) cells similar to the endogenous insulin secreting cells were derived.; These ss-like cells secreted insulin in response to glucose, and corrected a hyperglycemic phenotype in a mouse model of Type 2 diabetes via an iPS cell transplant. Within the Type 2 diabetes mouse model, a long term correction of hyperglycemia was achieved as measured by blood glucose and hemoglobin AIc measurements. Reduction of hyperglycemia was also seen in a chemically-induced mouse model for Type 1 diabetes.
Owner:Y 马 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com