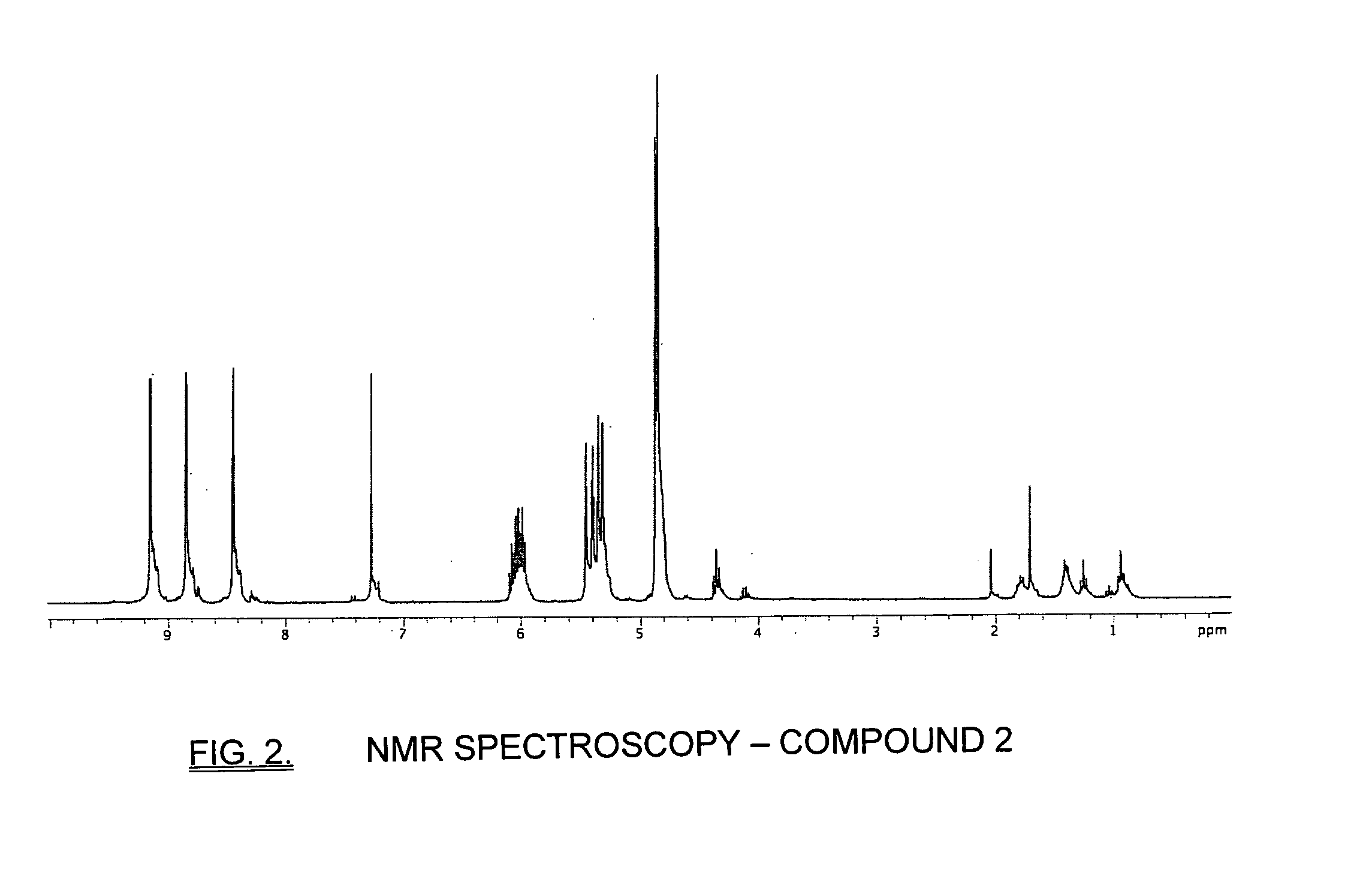
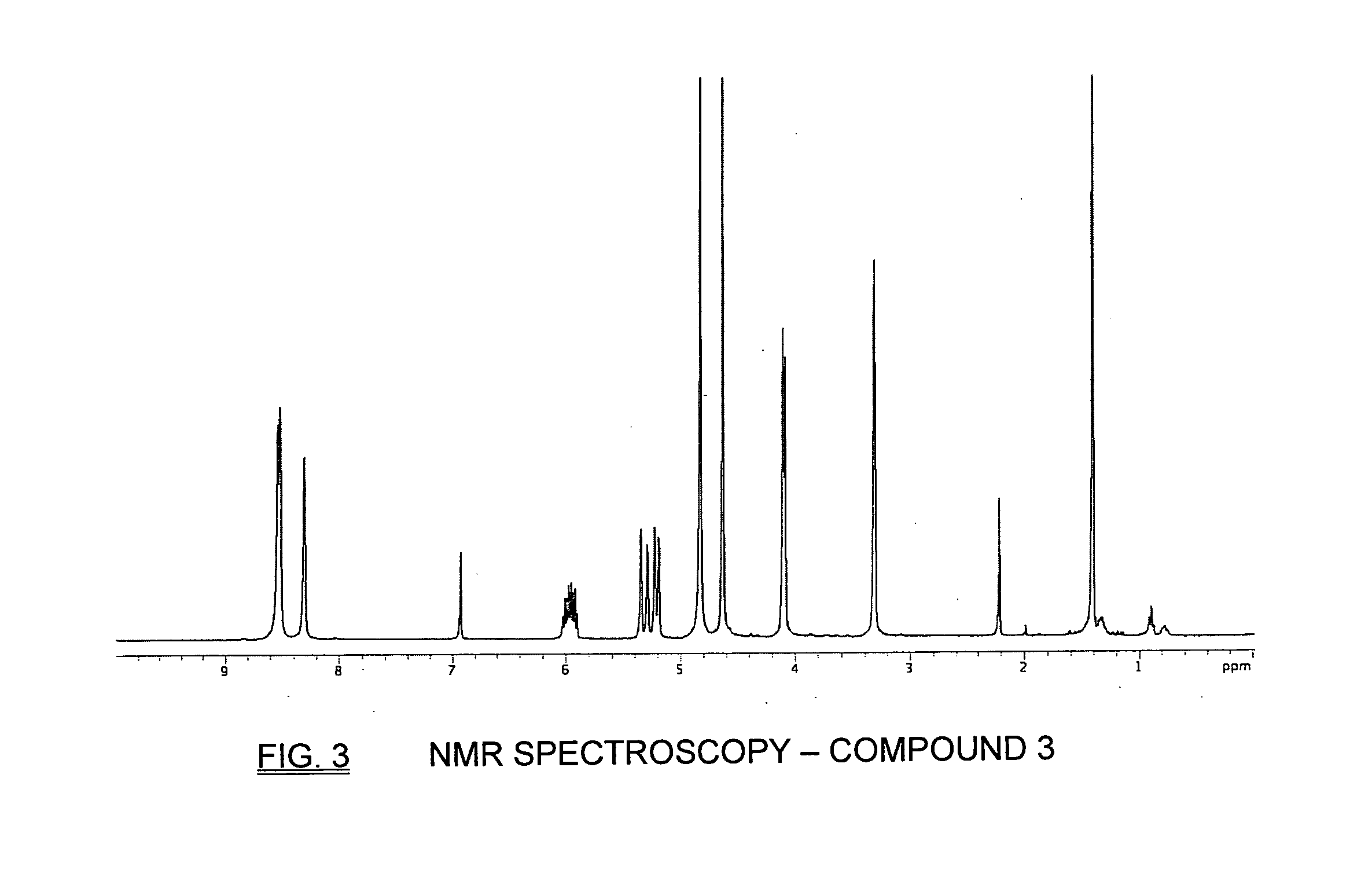
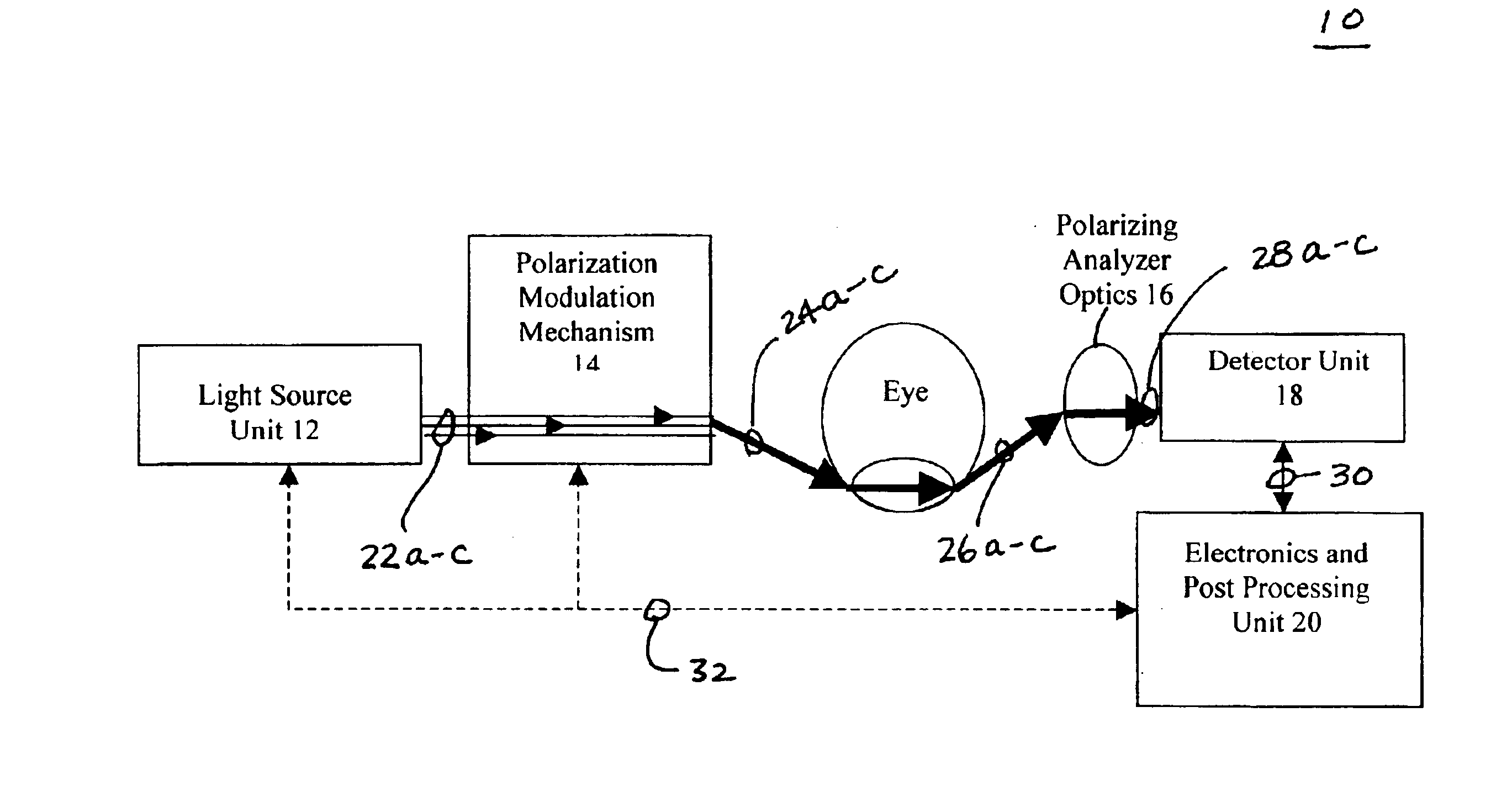
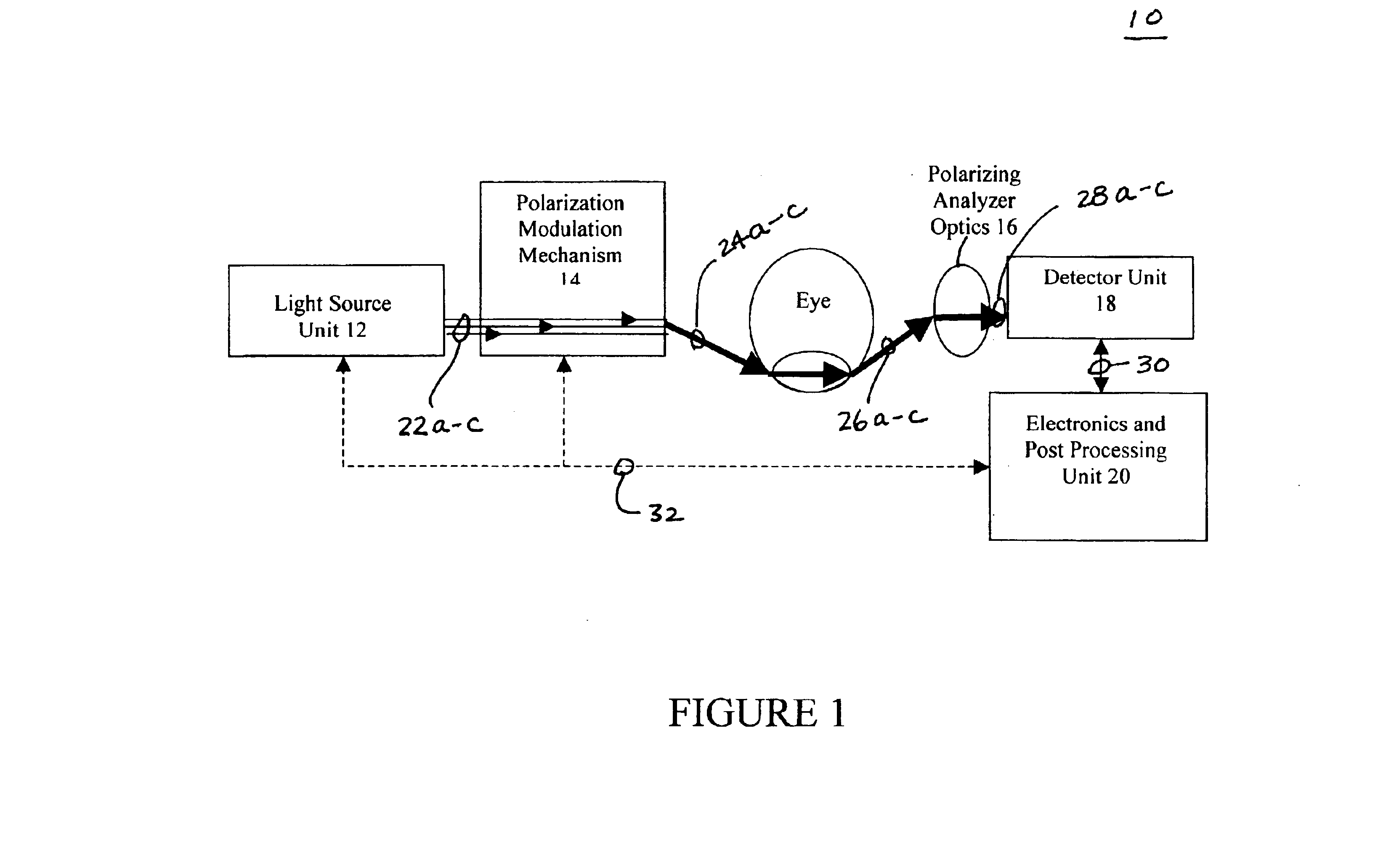
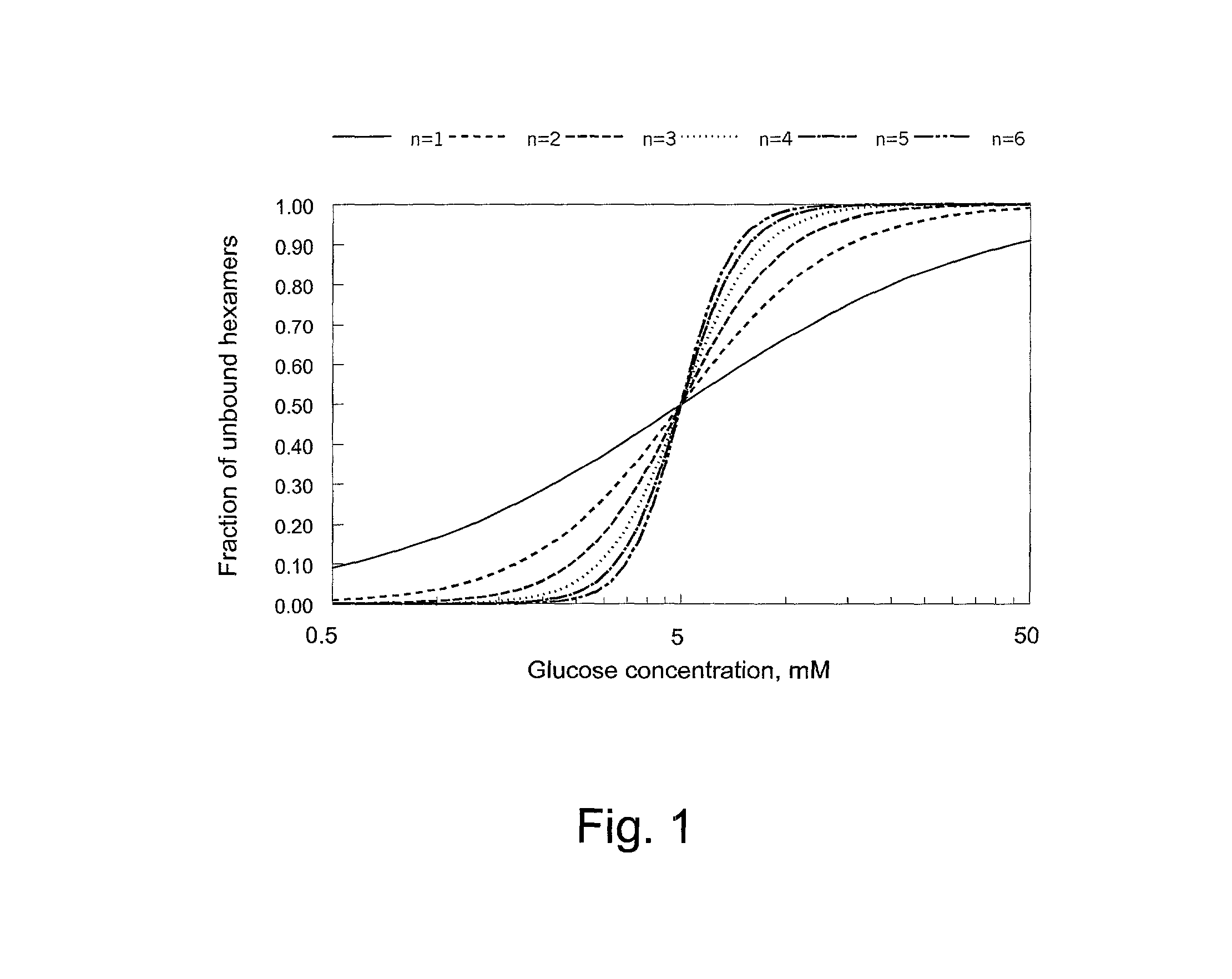
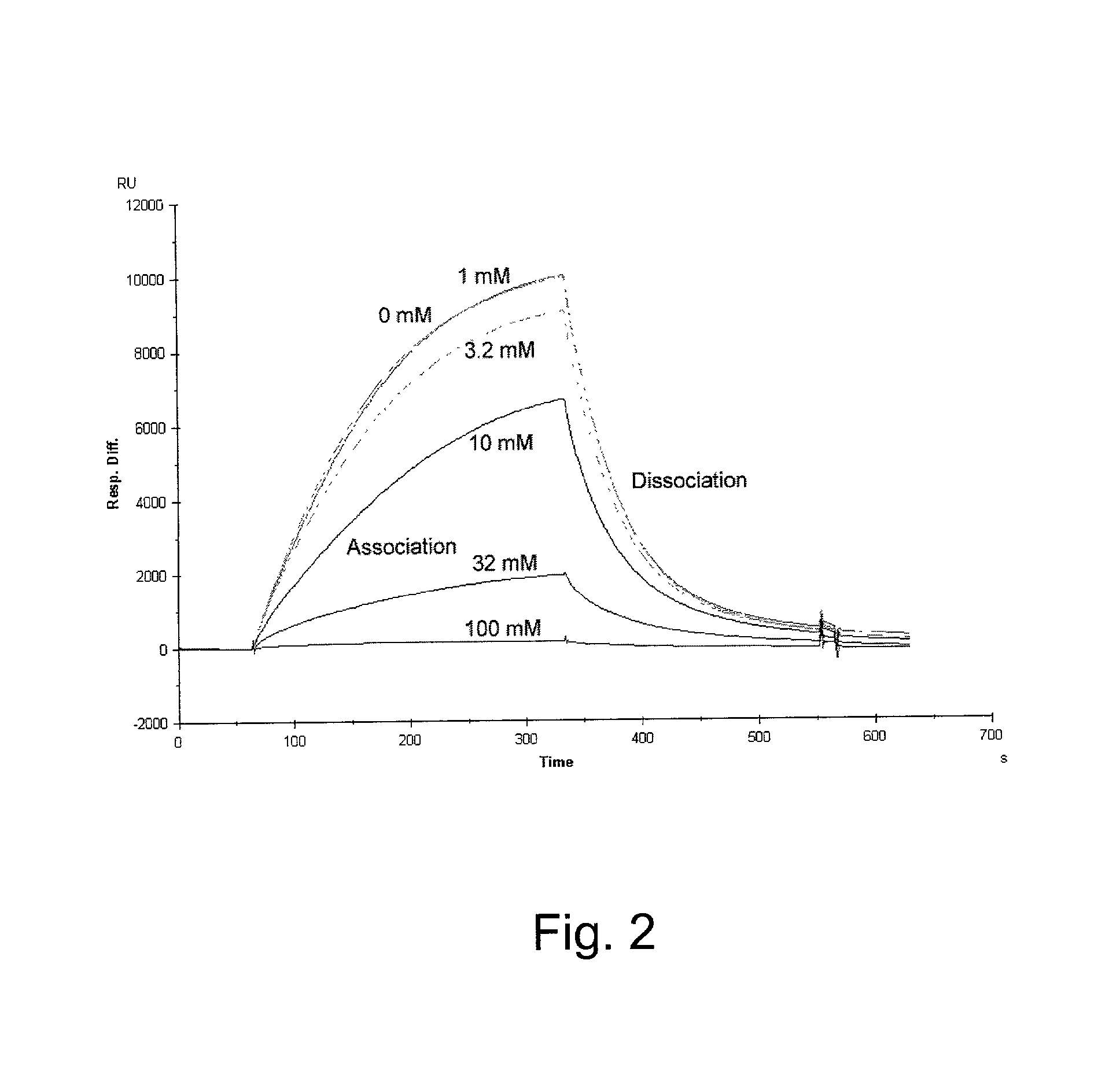
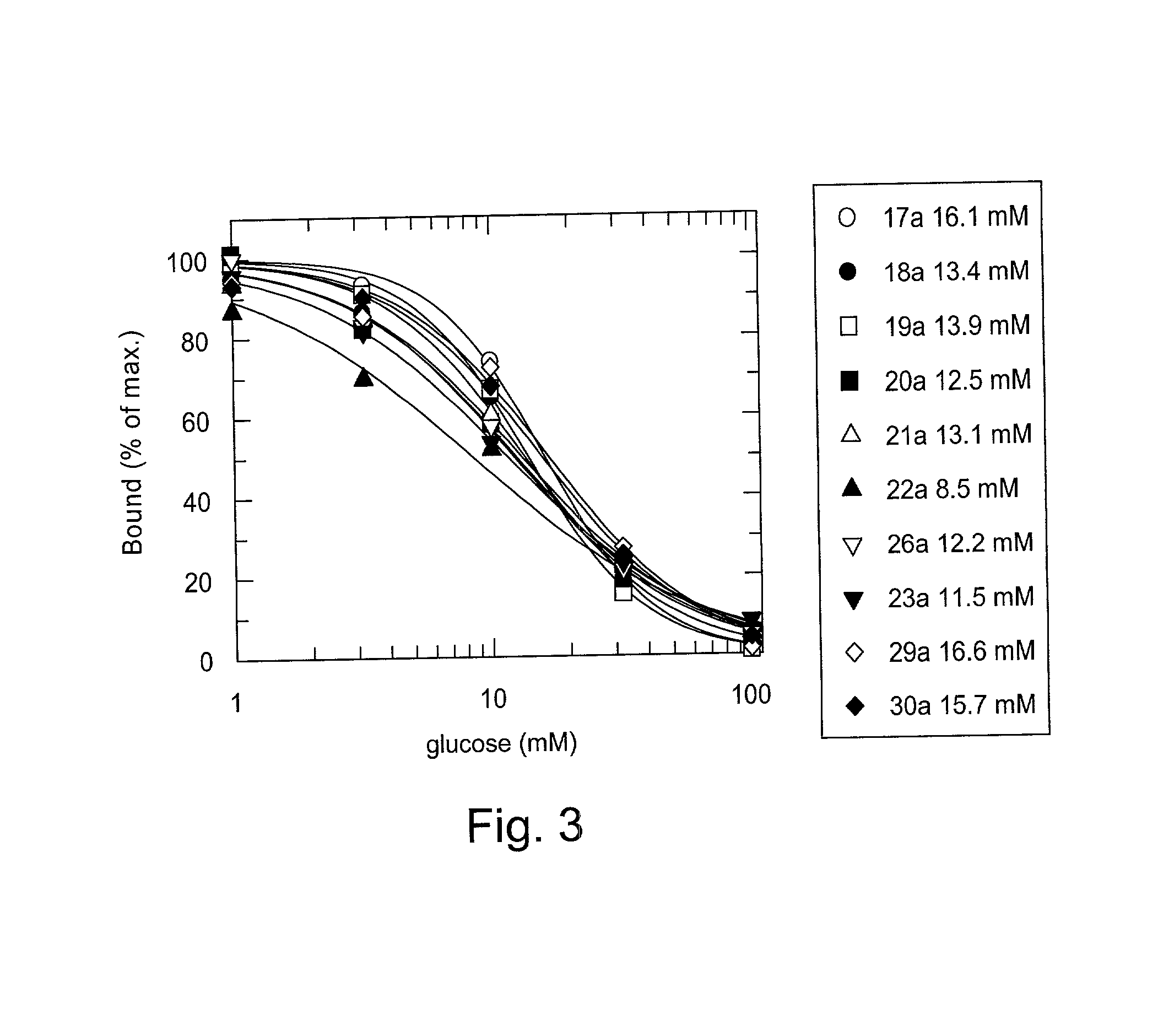
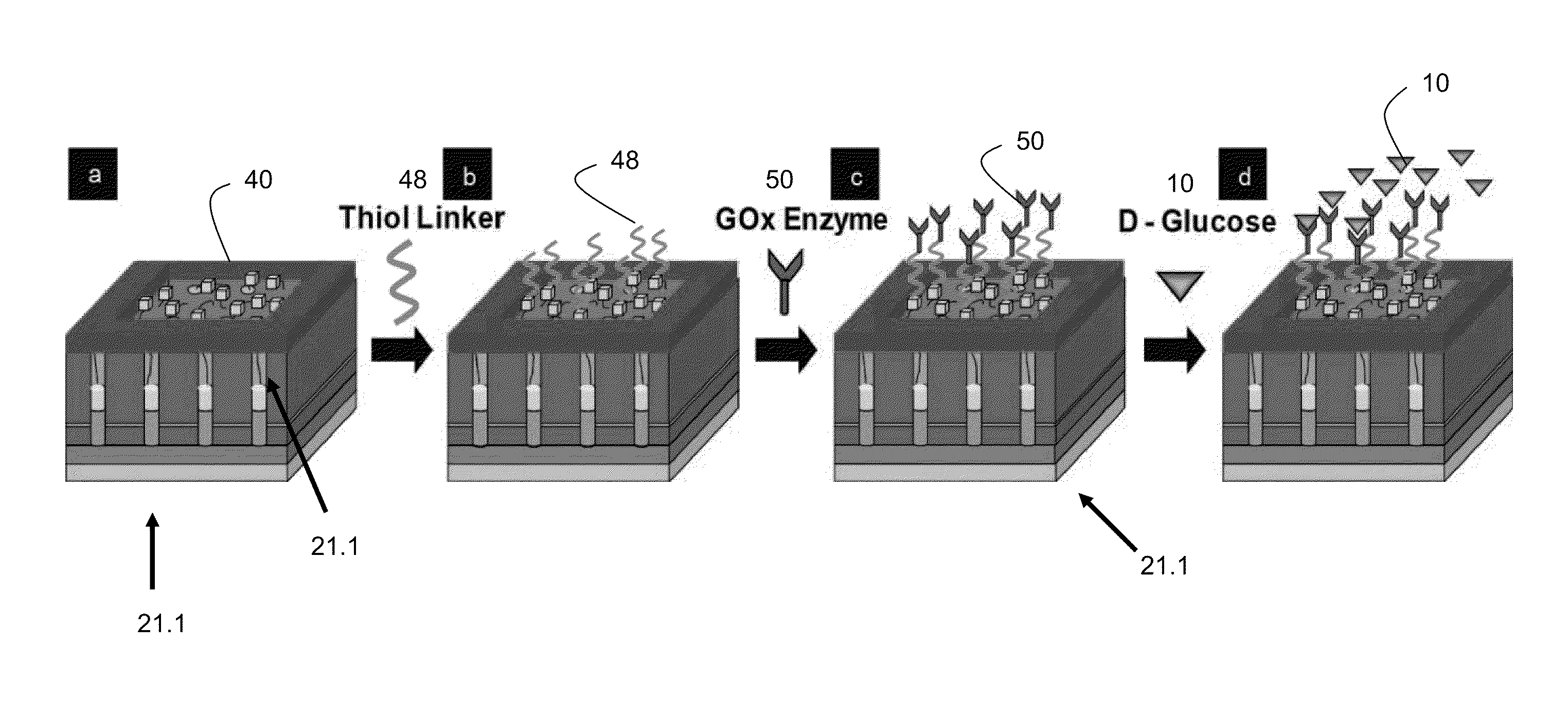
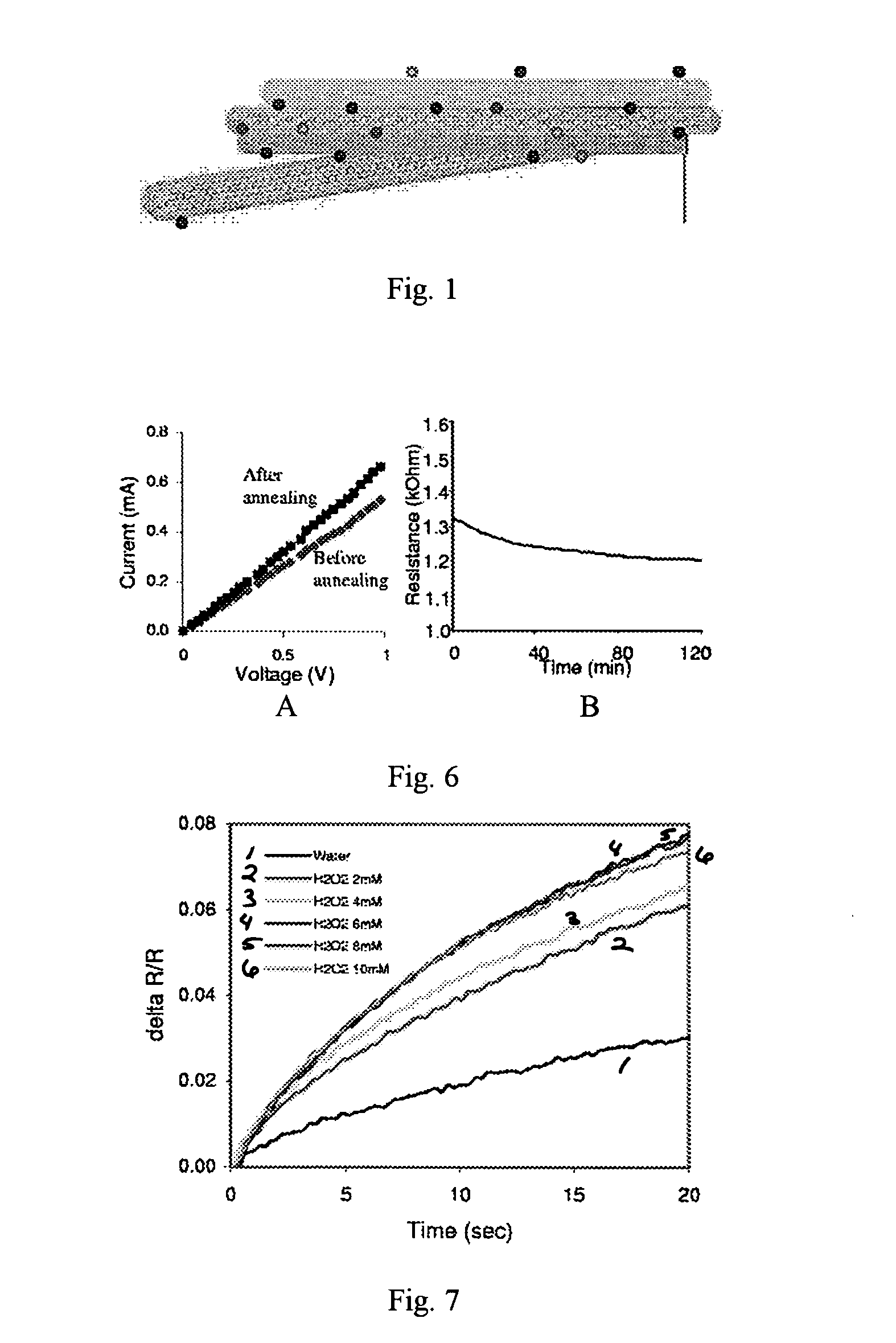
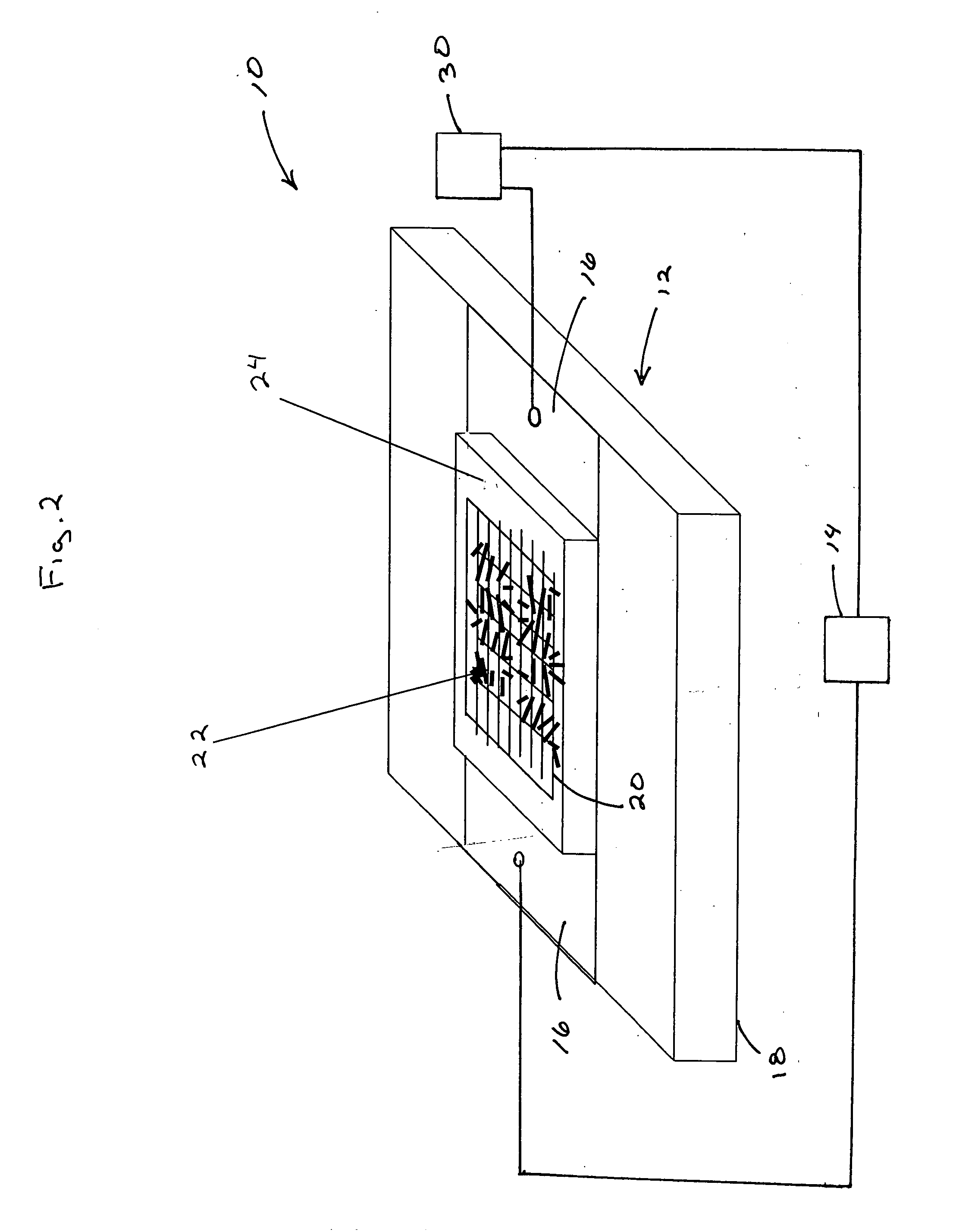
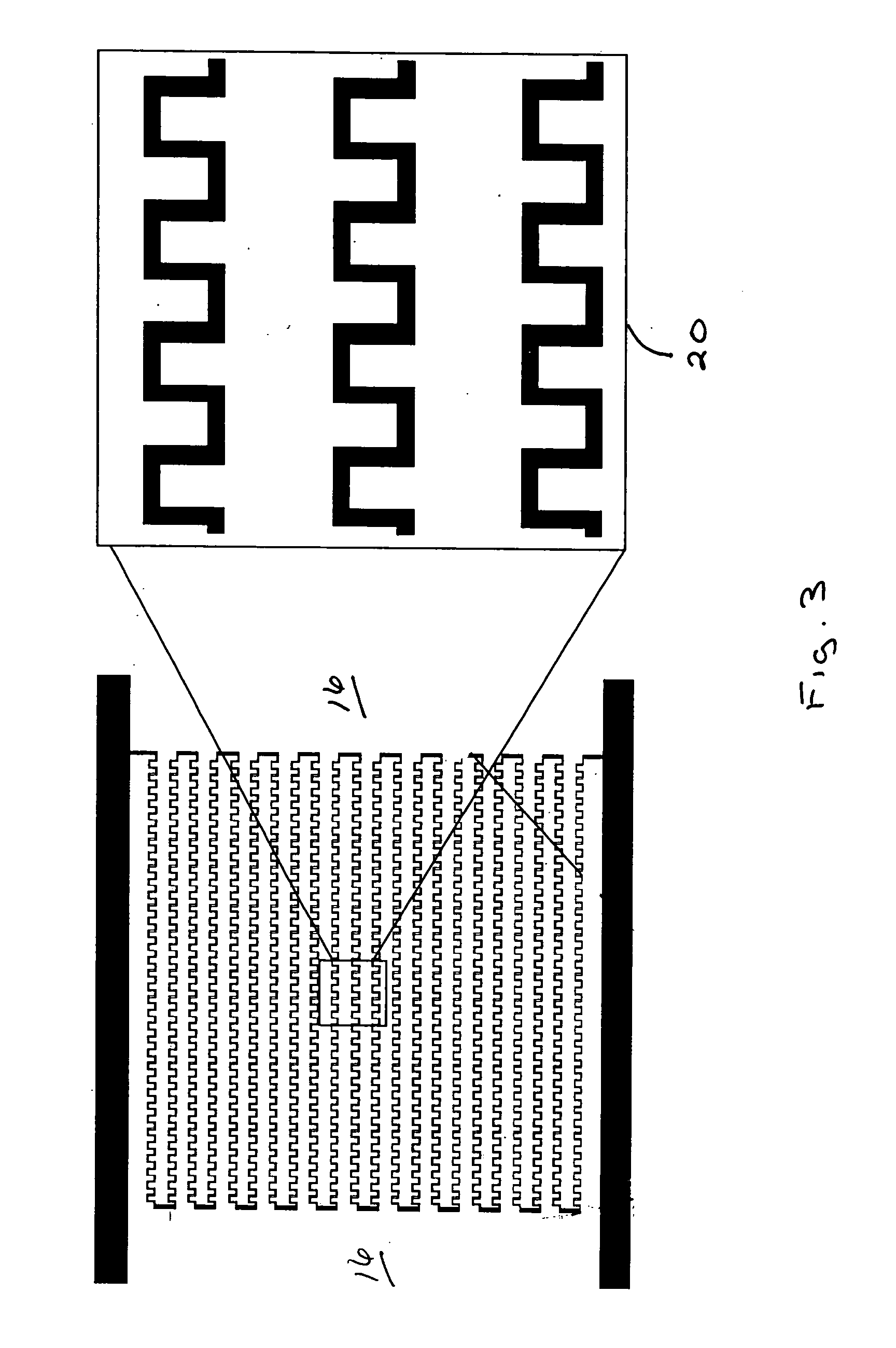
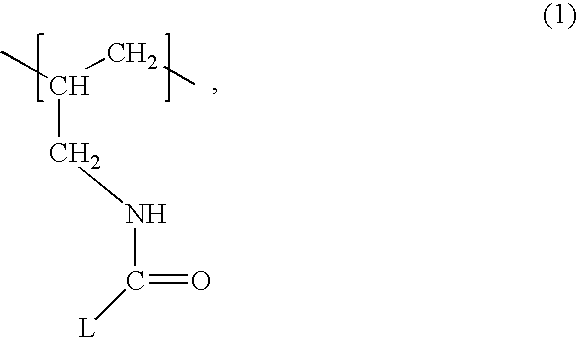Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
131 results about "Glucose sensing" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The appropriate secretion of insulin from pancreatic β-cells is critically important to the maintenance of energy homeostasis. The β-cells must sense and respond suitably to postprandial increases of blood glucose, and perturbation of glucose-sensing in these cells can lead to hypoglycaemia or hyperglycaemias and ultimately diabetes.
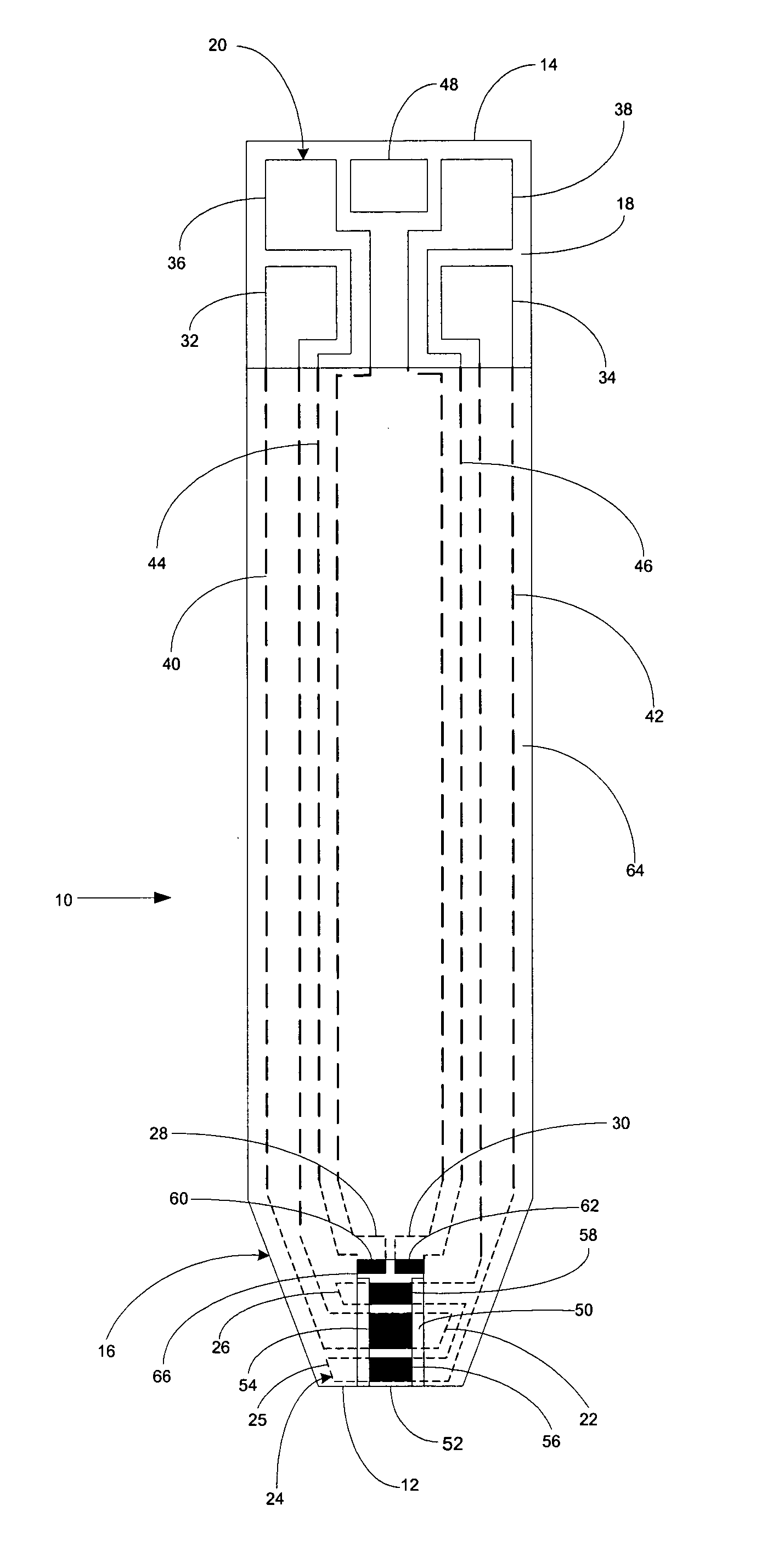
Systems and methods for blood glucose sensing
InactiveUS6946299B2Immobilised enzymesBioreactor/fermenter combinationsElectrical conductorGlucose polymers
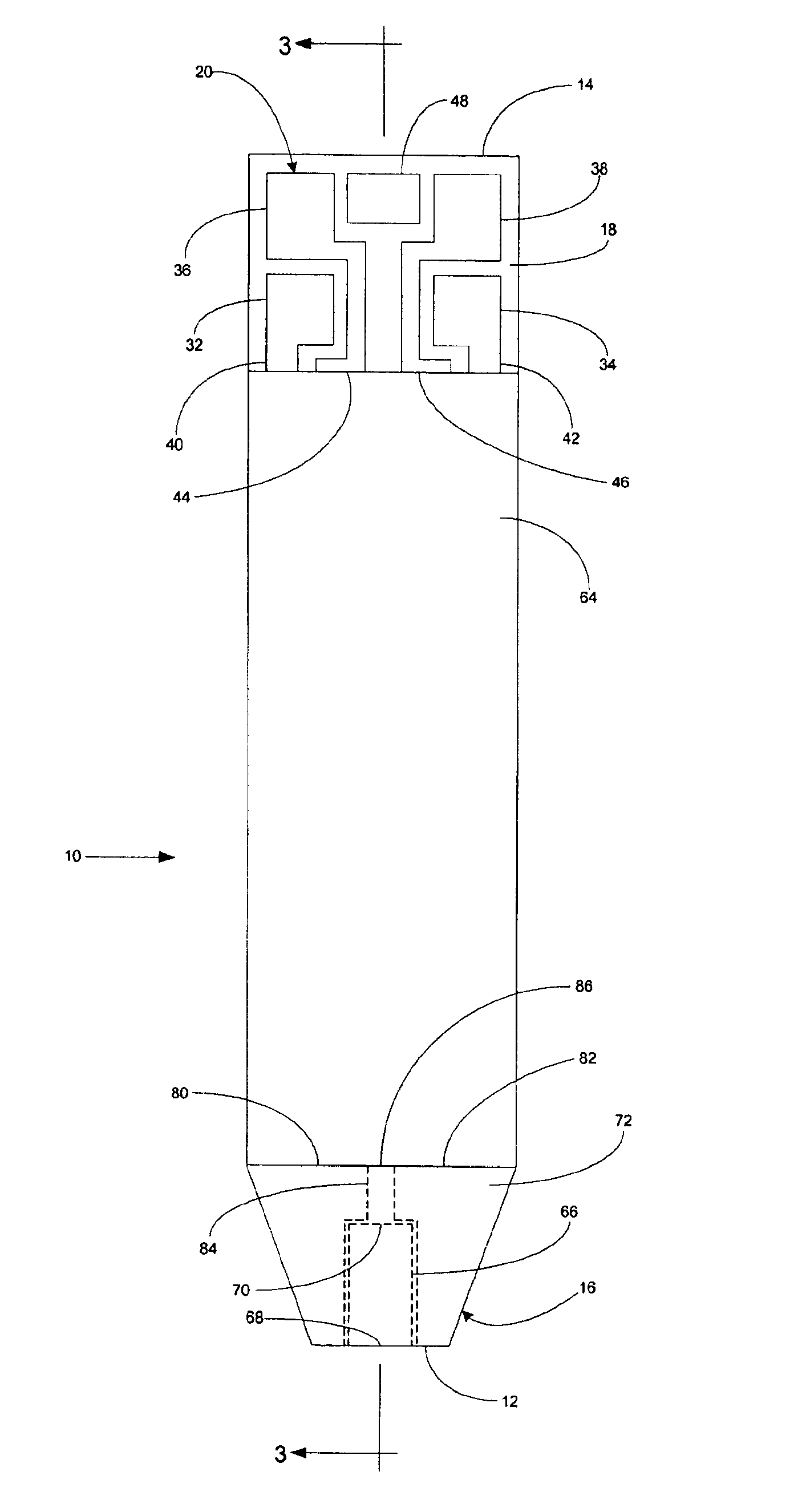
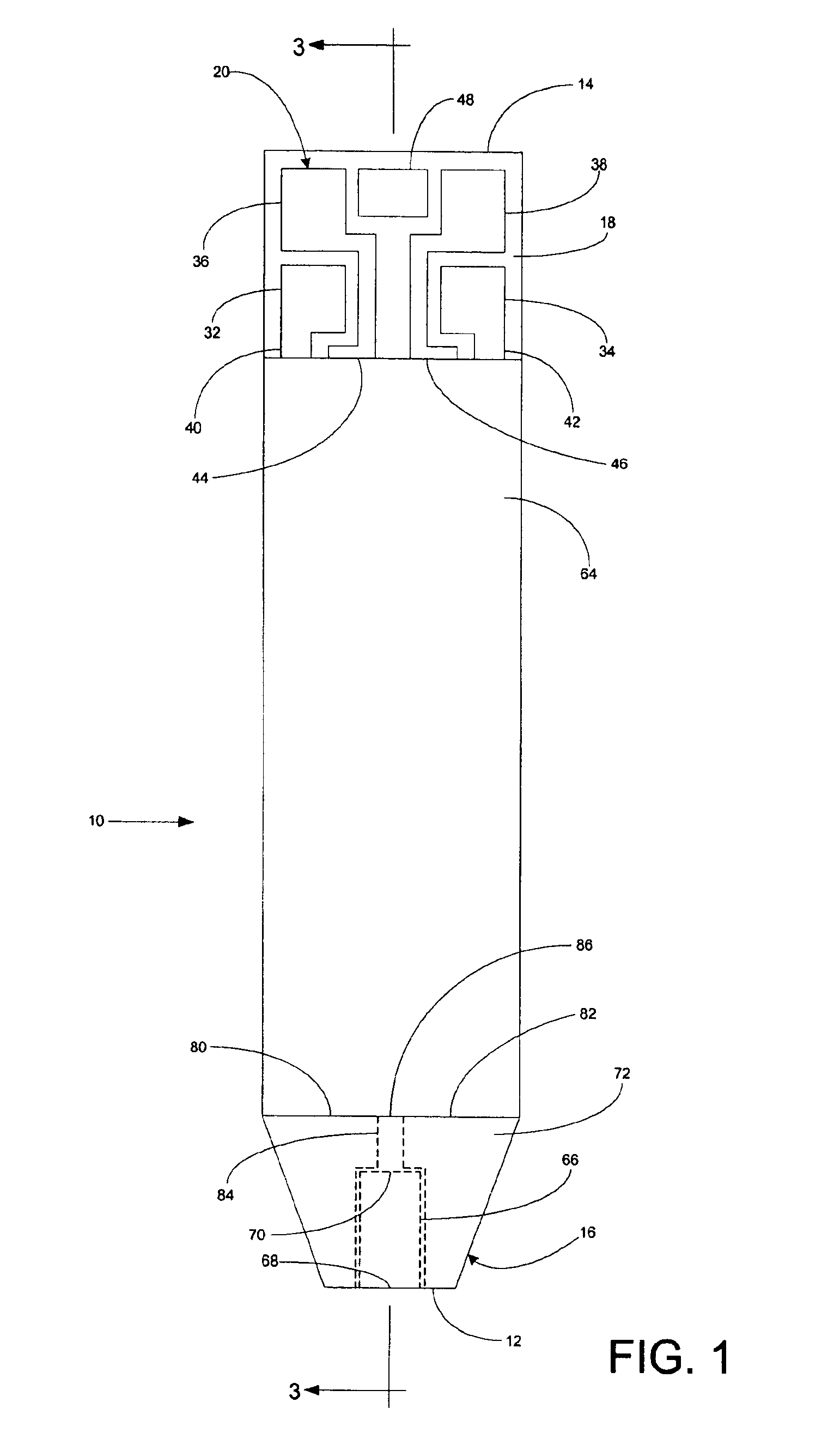
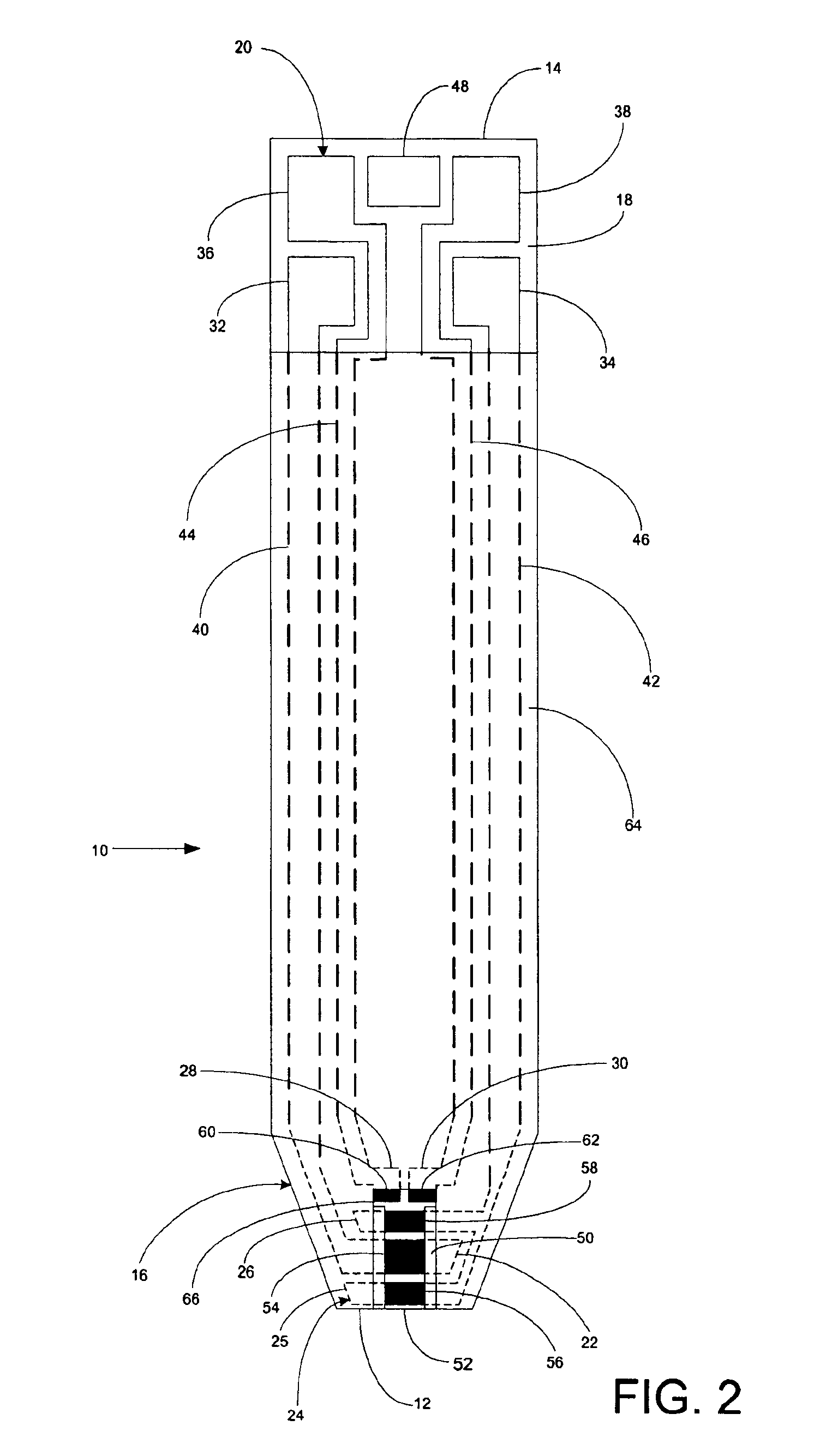
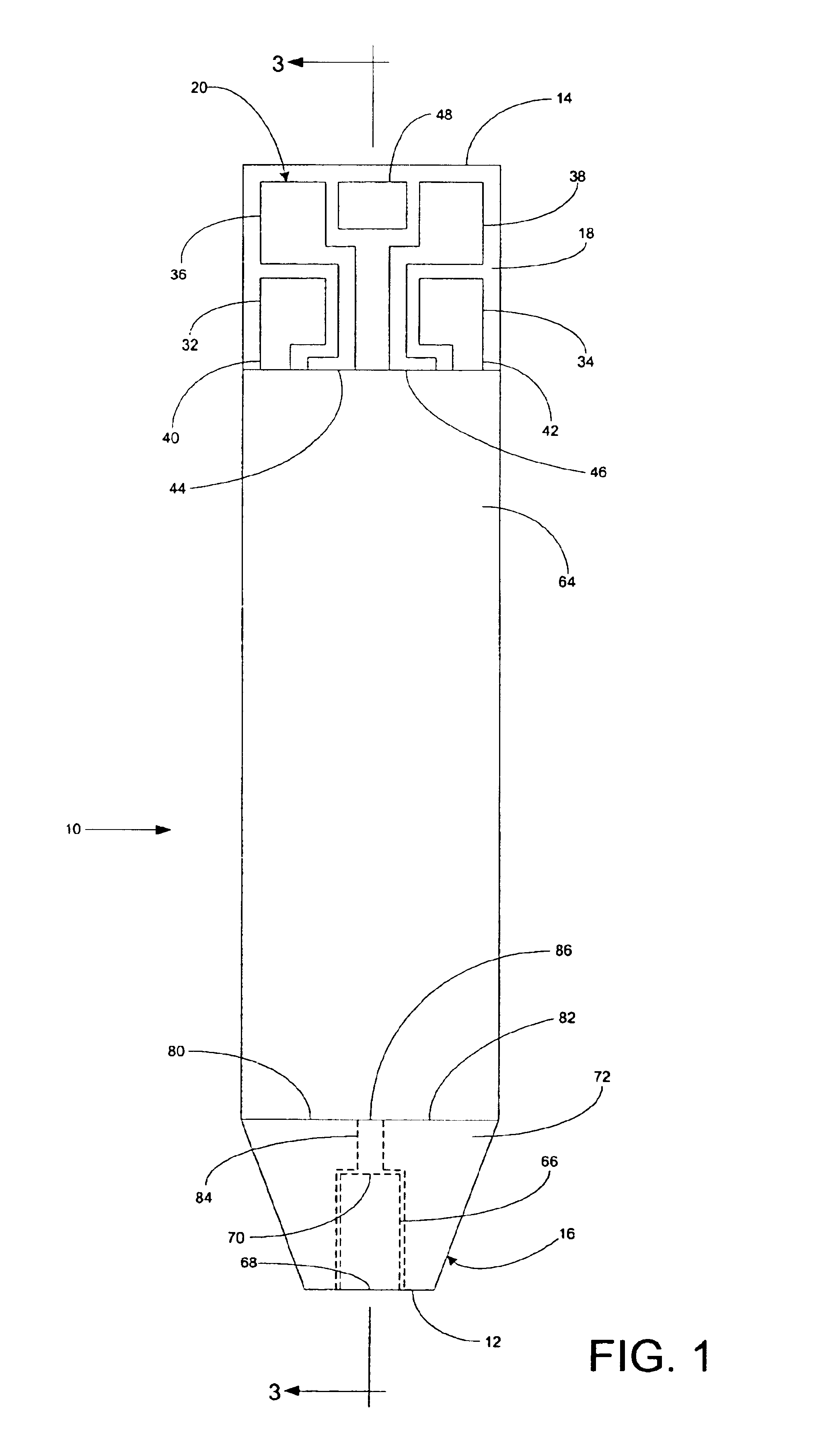
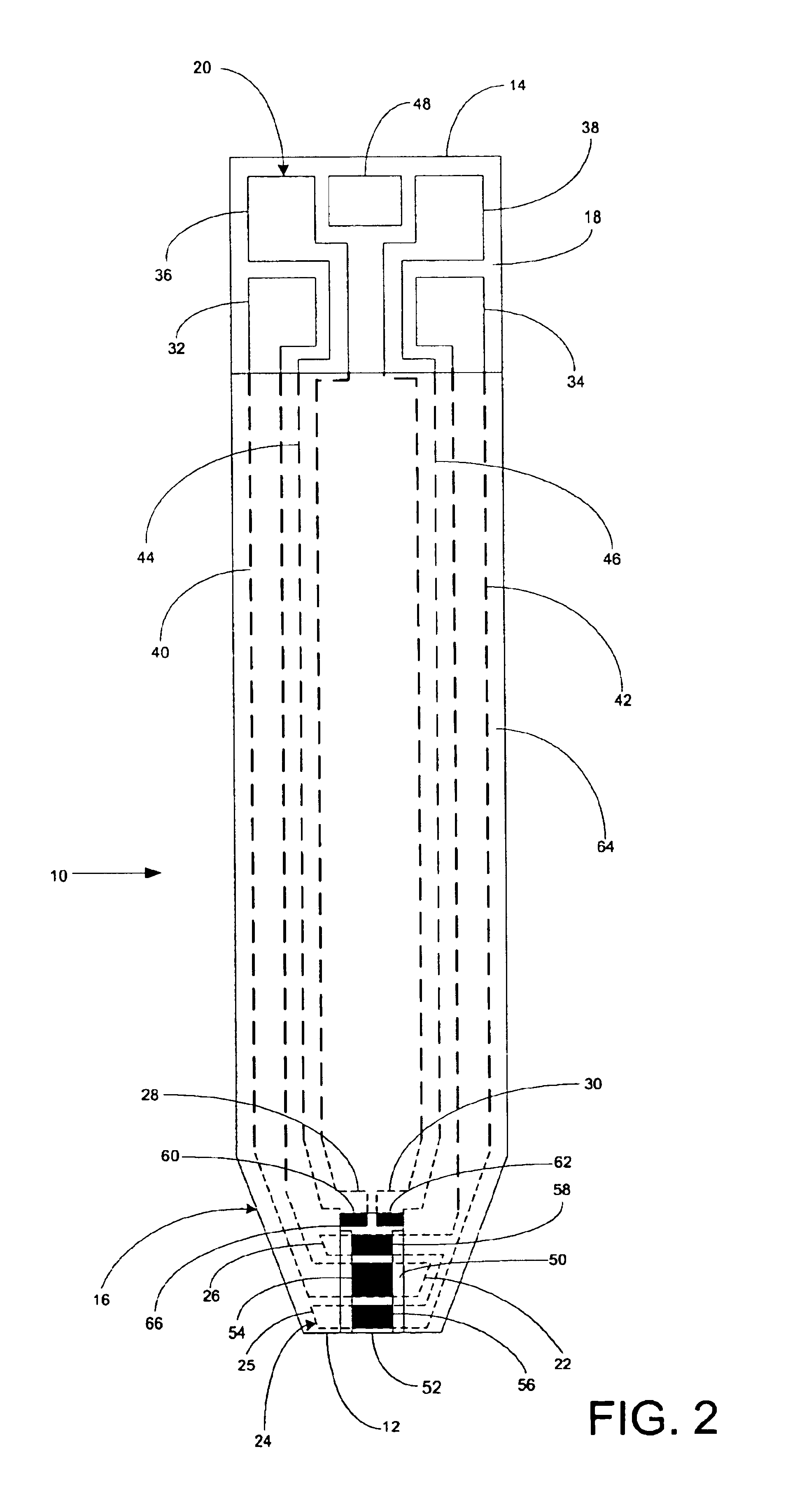
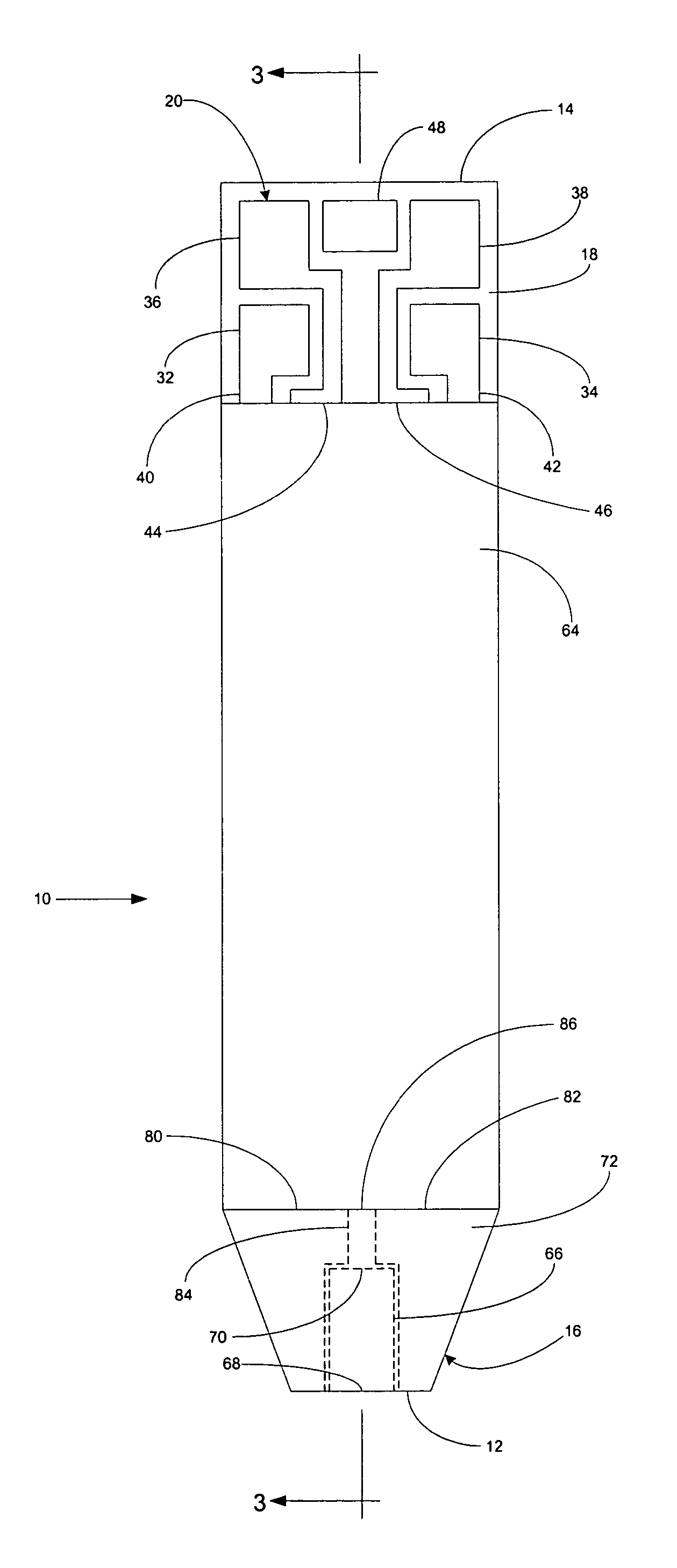
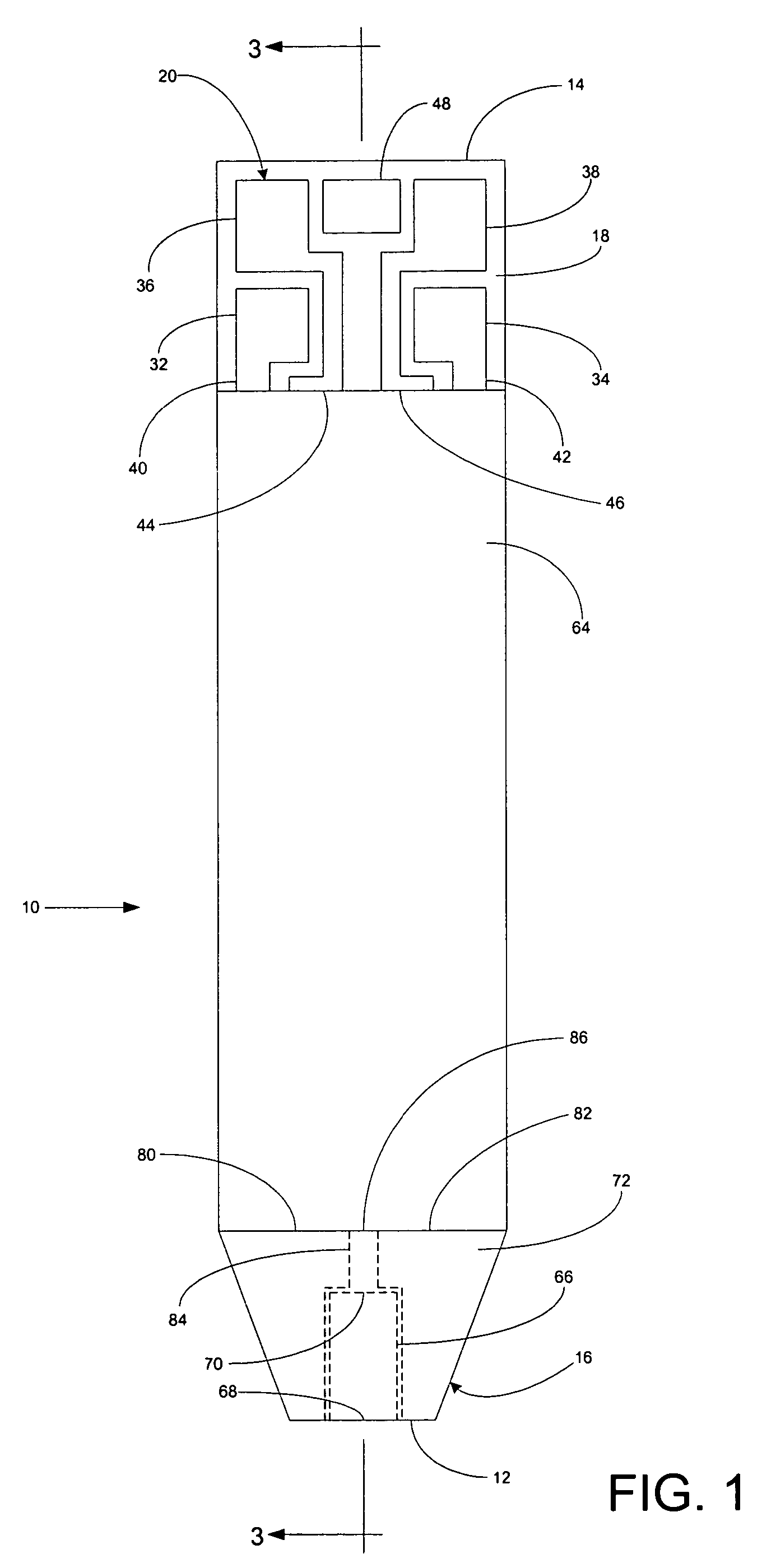
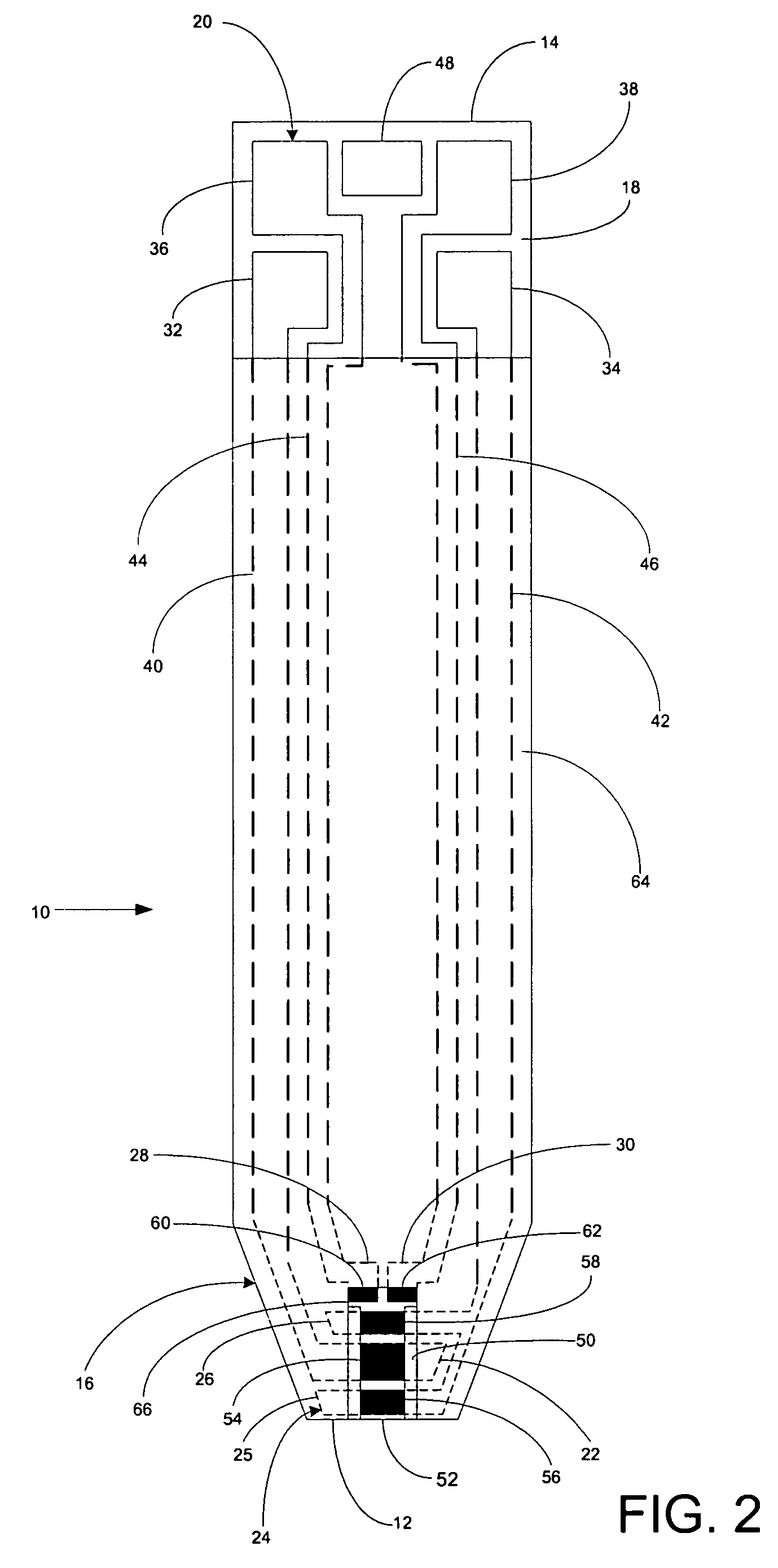
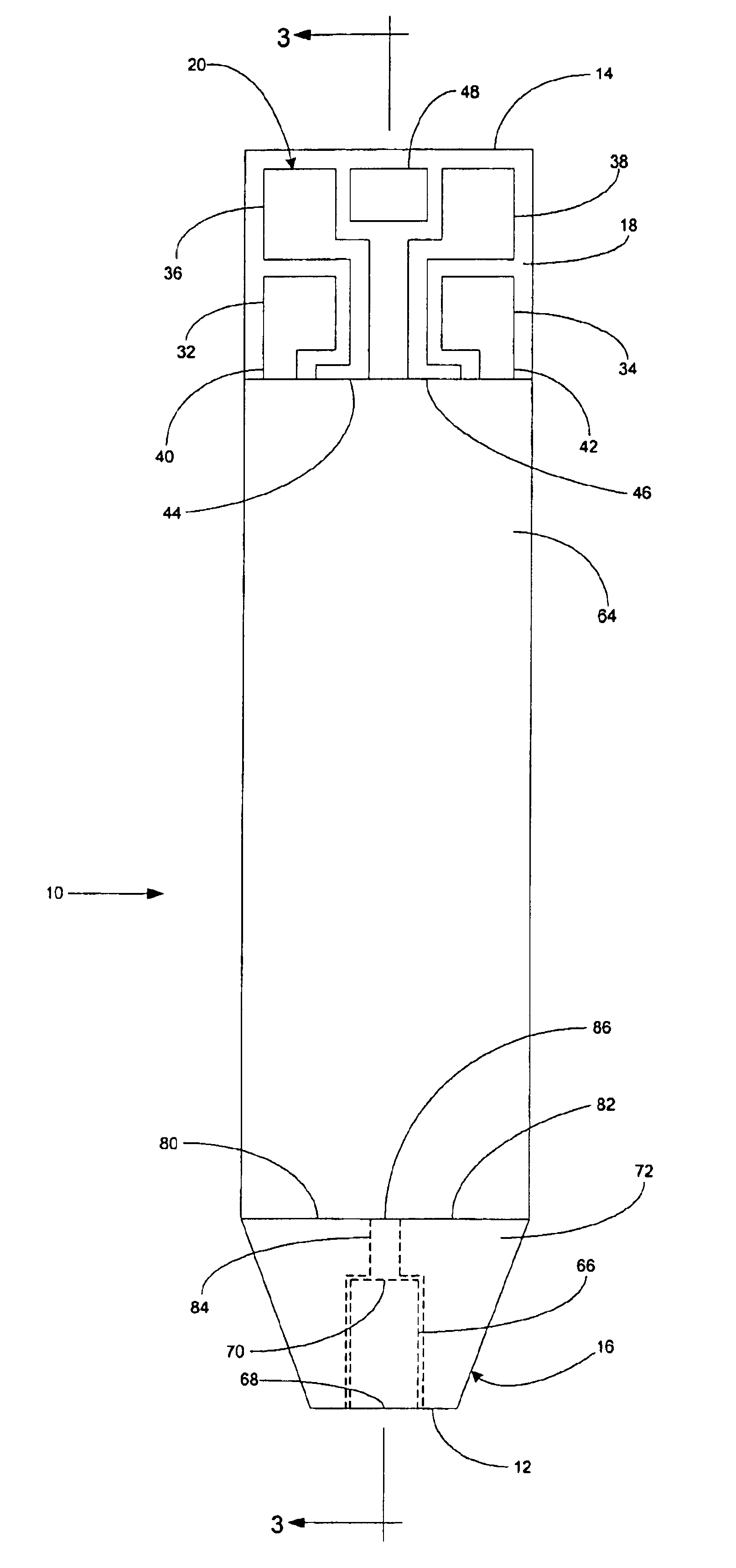
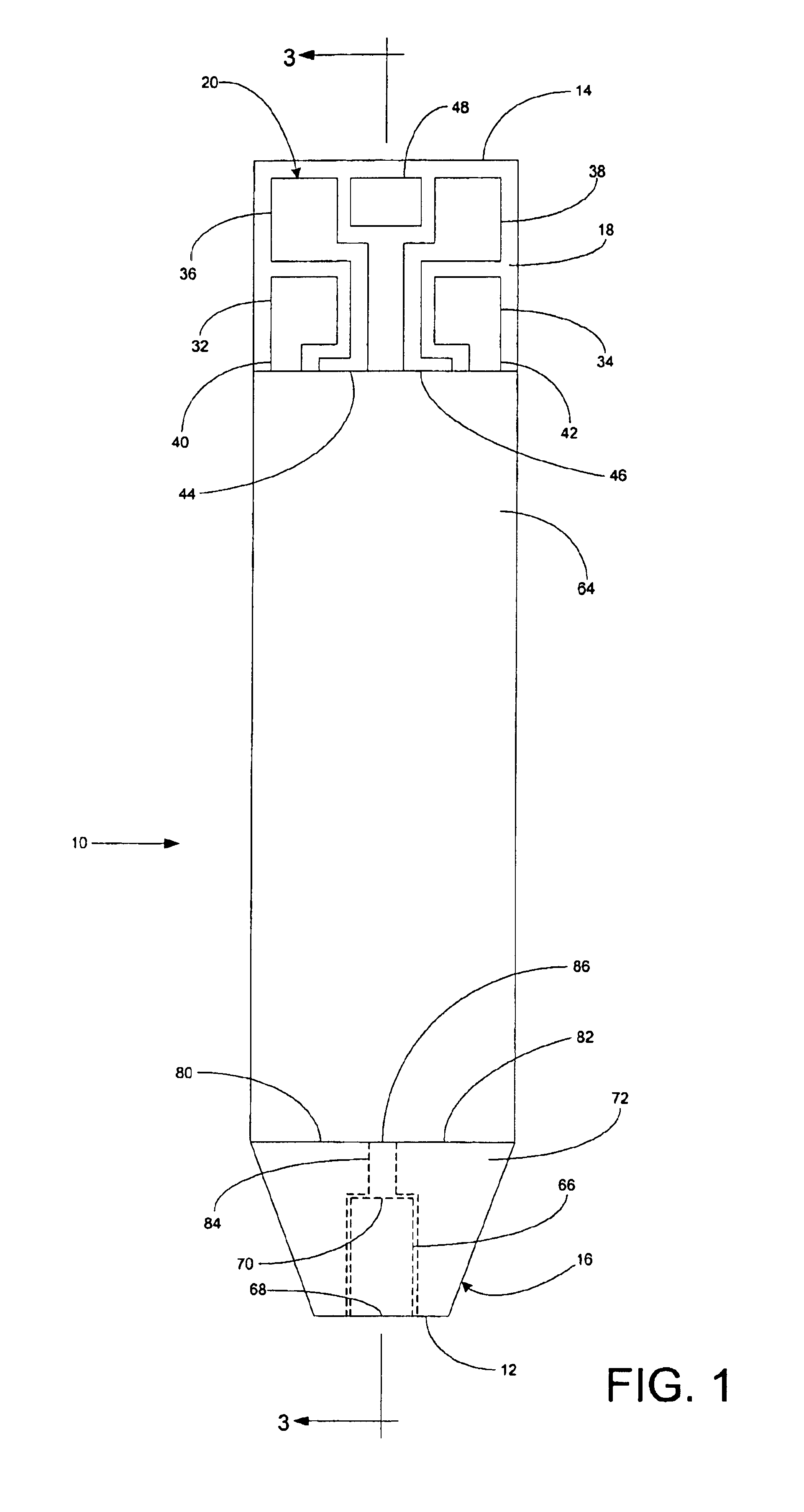
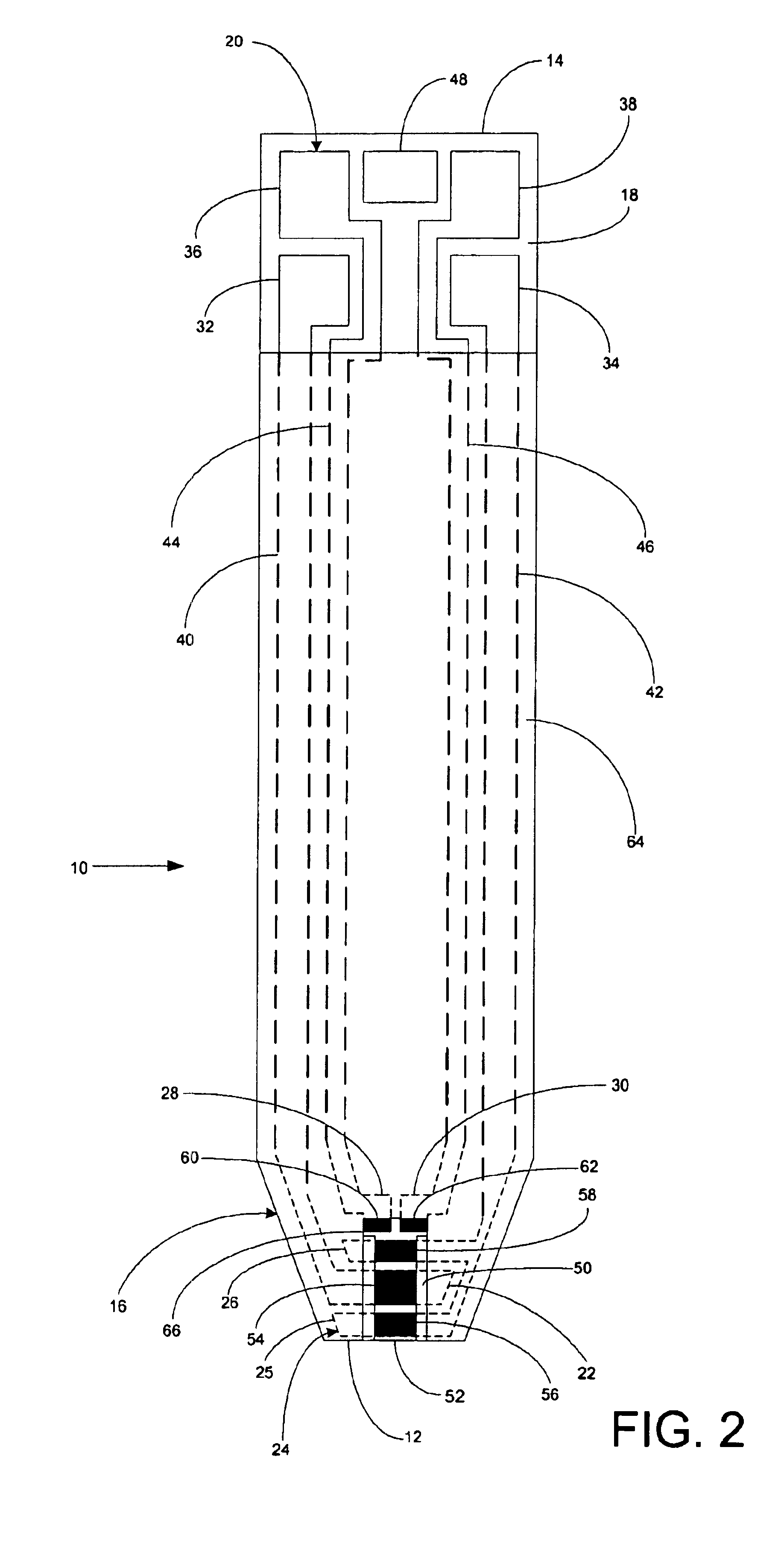
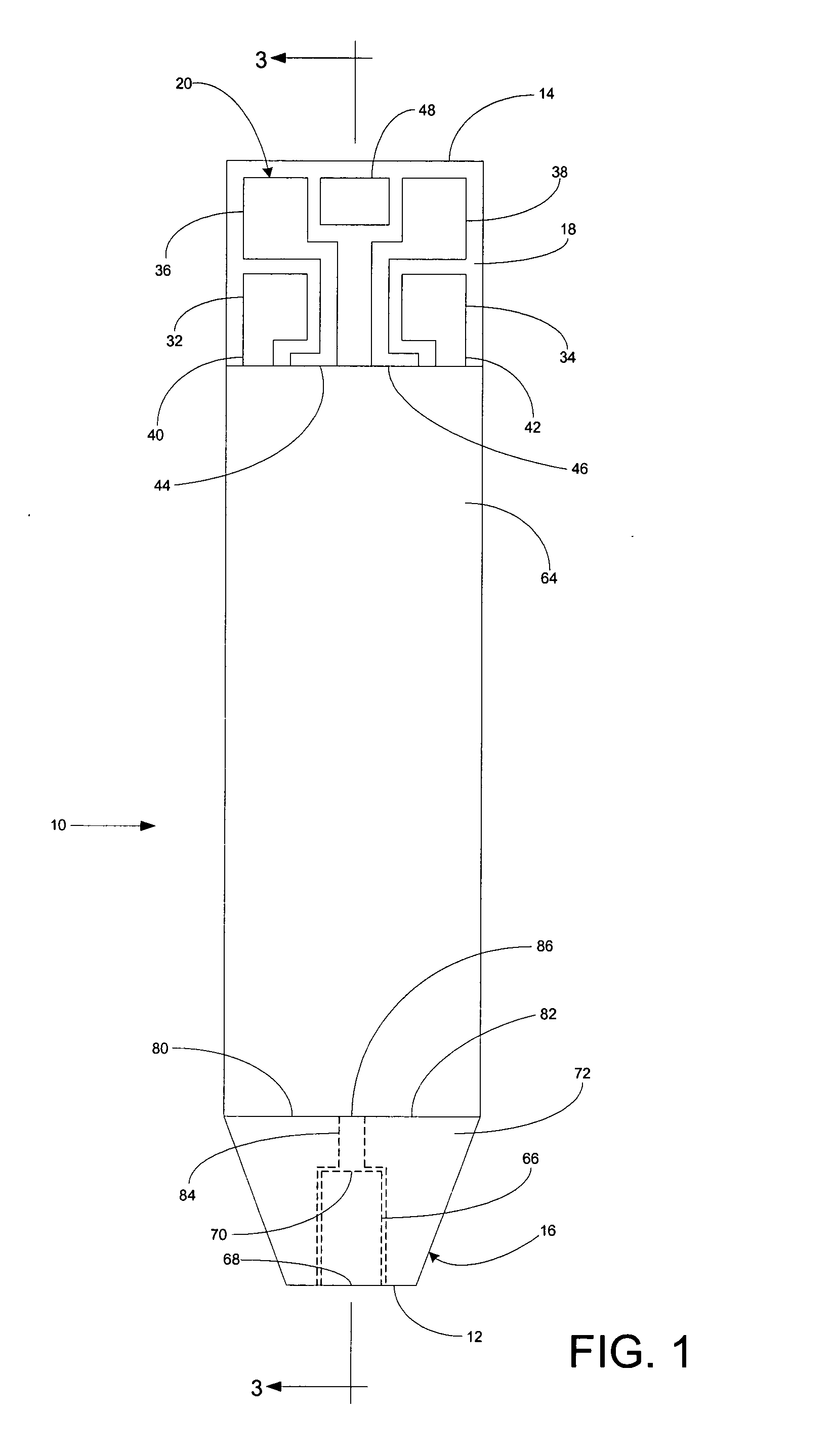
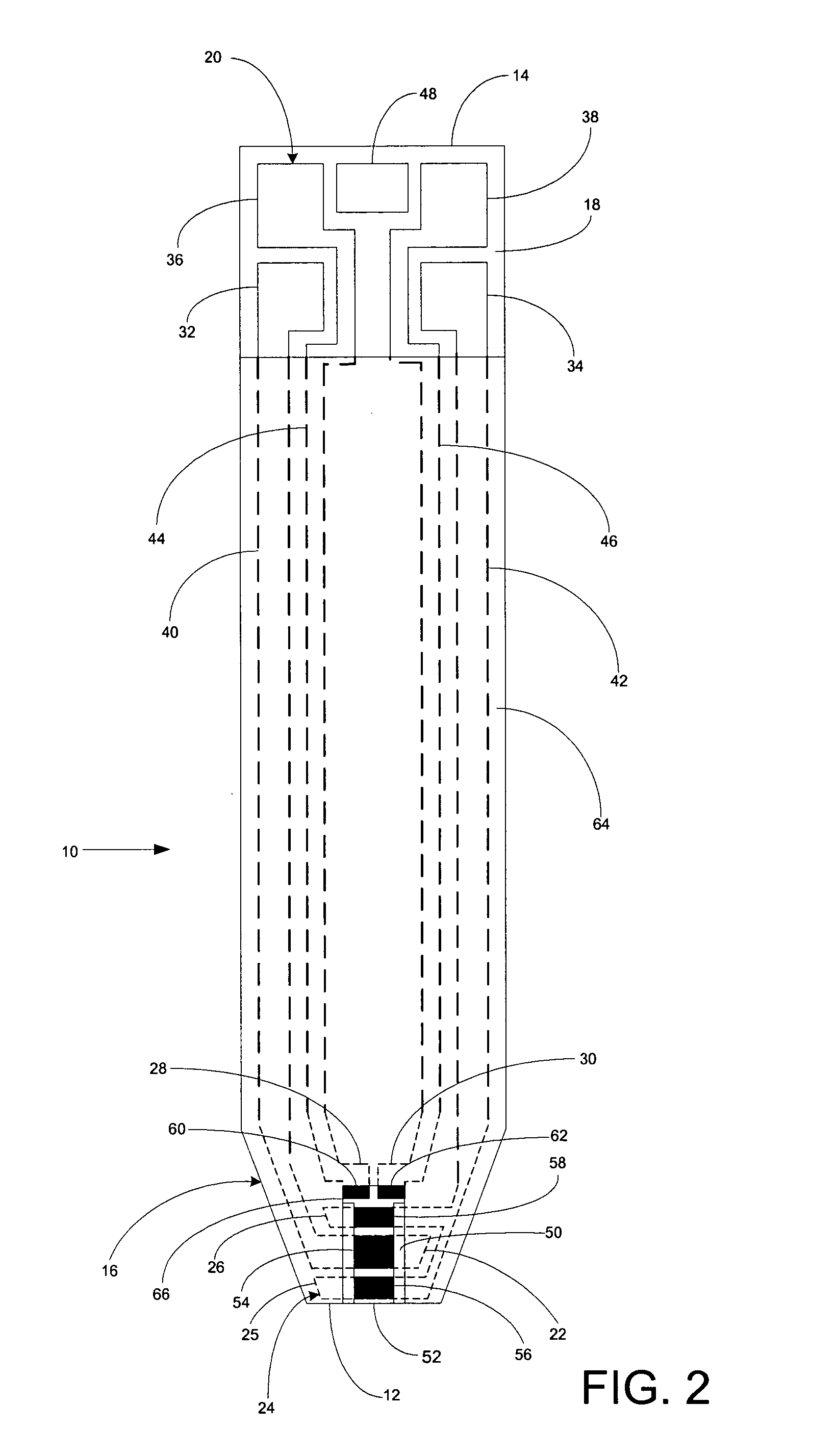
A system for measuring a glucose level in a blood sample includes a test strip and a meter. The test strip includes a sample chamber, a working electrode, a counter electrode, fill-detect electrodes, and an auto-on conductor. A reagent layer is disposed in the sample chamber. The auto-on conductor causes the meter to wake up and perform a test strip sequence when the test strip is inserted in the meter. The meter uses the working and counter electrodes to initially detect the blood sample in the sample chamber and uses the fill-detect electrodes to check that the blood sample has mixed with the reagent layer. The meter applies an assay voltage between the working and counter electrodes and measures the resulting current. The meter calculates the glucose level based on the measured current and calibration data saved in memory from a removable data storage device associated with the test strip.
Owner:TRIVIDIA HEALTH
Systems and methods for blood glucose sensing
InactiveUS6959247B2Immobilised enzymesBioreactor/fermenter combinationsElectrical conductorGlucose polymers
A system for measuring a glucose level in a blood sample includes a test strip and a meter. The test strip includes a sample chamber, a working electrode, a counter electrode, fill-detect electrodes, and an auto-on conductor. A reagent layer is disposed in the sample chamber. The auto-on conductor causes the meter to wake up and perform a test strip sequence when the test strip is inserted in the meter. The meter uses the working and counter electrodes to initially detect the blood sample in the sample chamber and uses the fill-detect electrodes to check that the blood sample has mixed with the reagent layer. The meter applies an assay voltage between the working and counter electrodes and measures the resulting current. The meter calculates the glucose level based on the measured current and calibration data saved in memory from a removable data storage device associated with the test strip.
Owner:TRIVIDIA HEALTH
Systems and methods for blood glucose sensing
InactiveUS7160251B2Immobilised enzymesBioreactor/fermenter combinationsElectrical conductorElectrode pair
A system for measuring a glucose level in a blood sample includes a test strip and a meter. The test strip includes a sample chamber, a working electrode, a counter electrode, fill-detect electrodes, and an auto-on conductor. A reagent layer is disposed in the sample chamber. The auto-on conductor causes the meter to wake up and perform a test strip sequence when the test strip is inserted in the meter. The meter uses the working and counter electrodes to initially detect the blood sample in the sample chamber and uses the fill-detect electrodes to check that the blood sample has mixed with the reagent layer. The meter applies an assay voltage between the working and counter electrodes and measures the resulting current. The meter calculates the glucose level based on the measured current and calibration data saved in memory from a removable data storage device associated with the test strip.
Owner:TRIVIDIA HEALTH
Systems and methods for blood glucose sensing
InactiveUS6964871B2Immobilised enzymesBioreactor/fermenter combinationsElectrical conductorEngineering
A system for measuring a glucose level in a blood sample includes a test strip and a meter. The test strip includes a sample chamber or other testing zone, a working electrode, a counter electrode, fill-detect electrodes, and an auto-on conductor. A reagent layer is disposed in the testing zone. The auto-on conductor causes the meter to wake up and perform a test strip sequence when the test strip is inserted in the meter. The meter uses the working and counter electrodes to initially detect the blood sample in the sample chamber and uses the fill-detect electrodes to check that the blood sample has mixed with the reagent layer. The meter applies an assay voltage between the working and counter electrodes and measures the resulting current. The meter calculates the glucose level based on the measured current and calibration data saved in memory from a removable data storage device associated with the test strip.
Owner:TRIVIDIA HEALTH
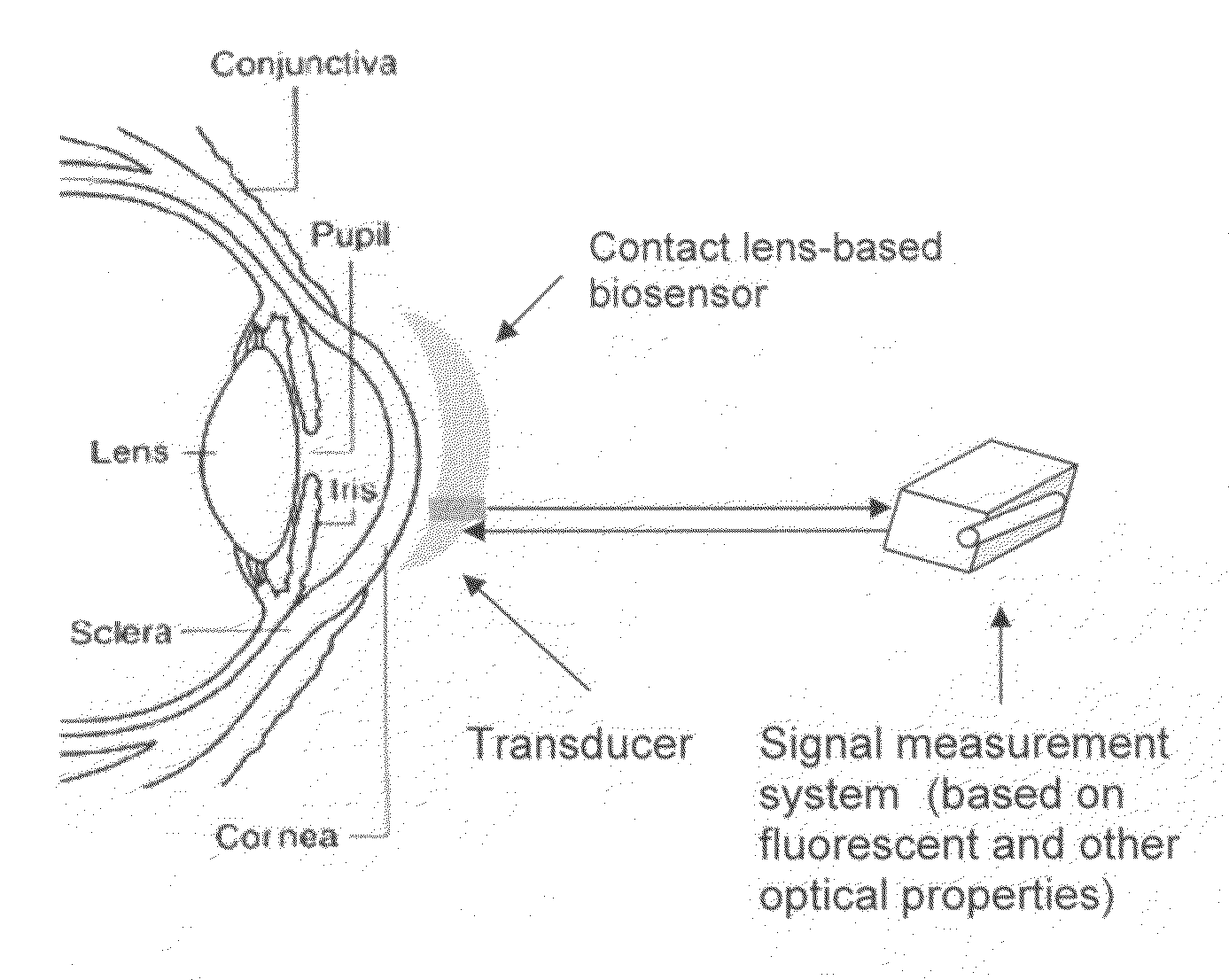
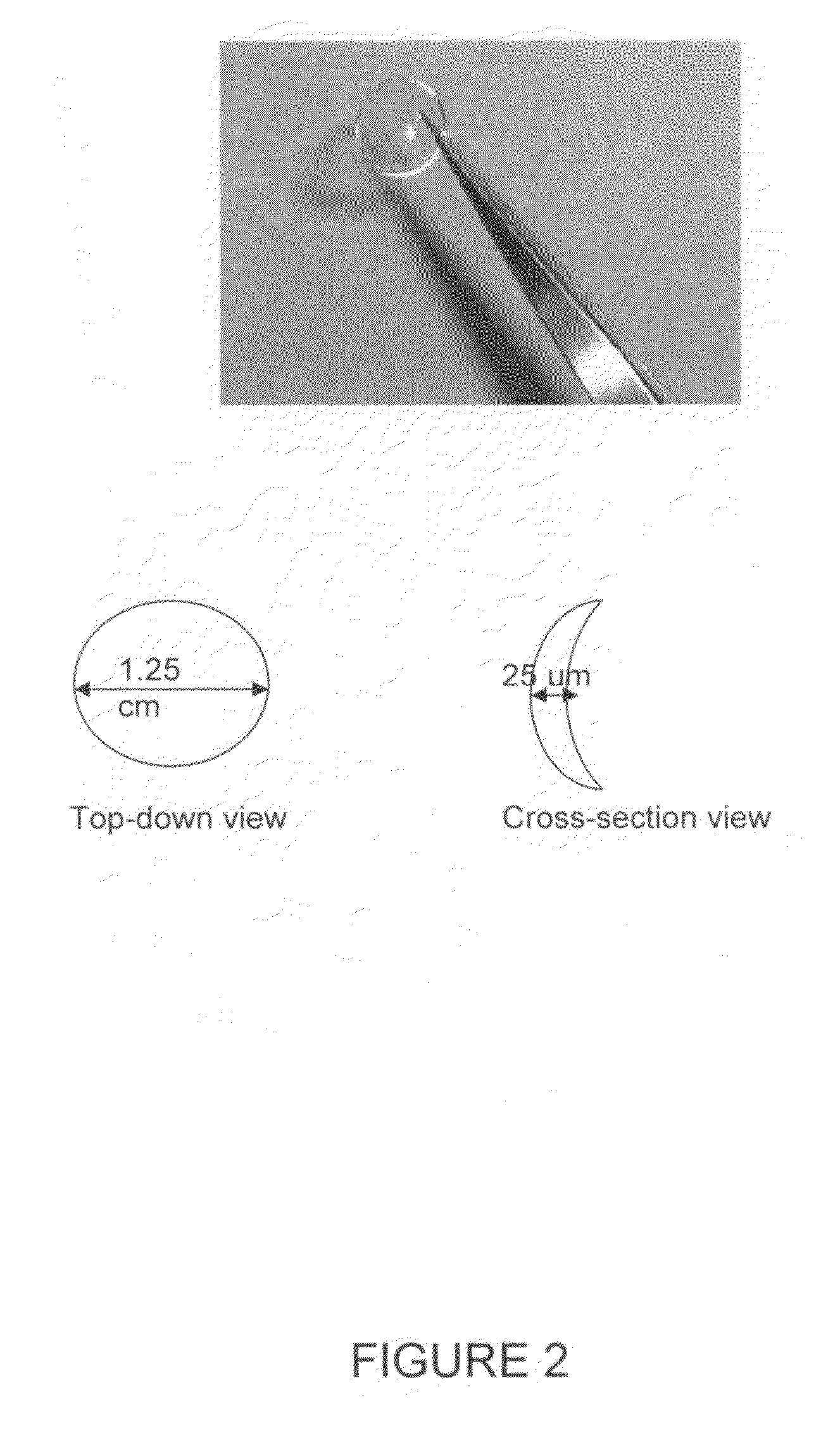
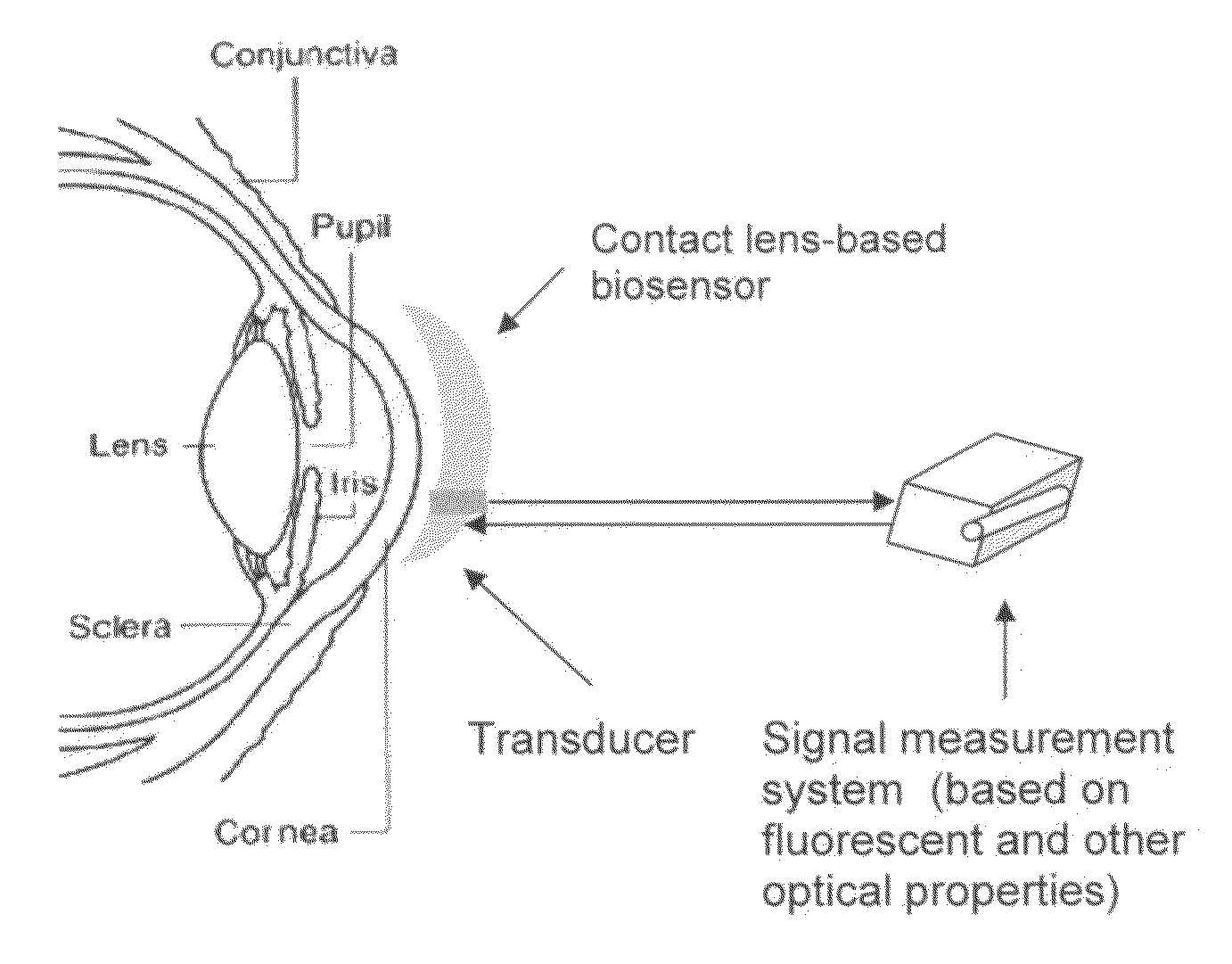
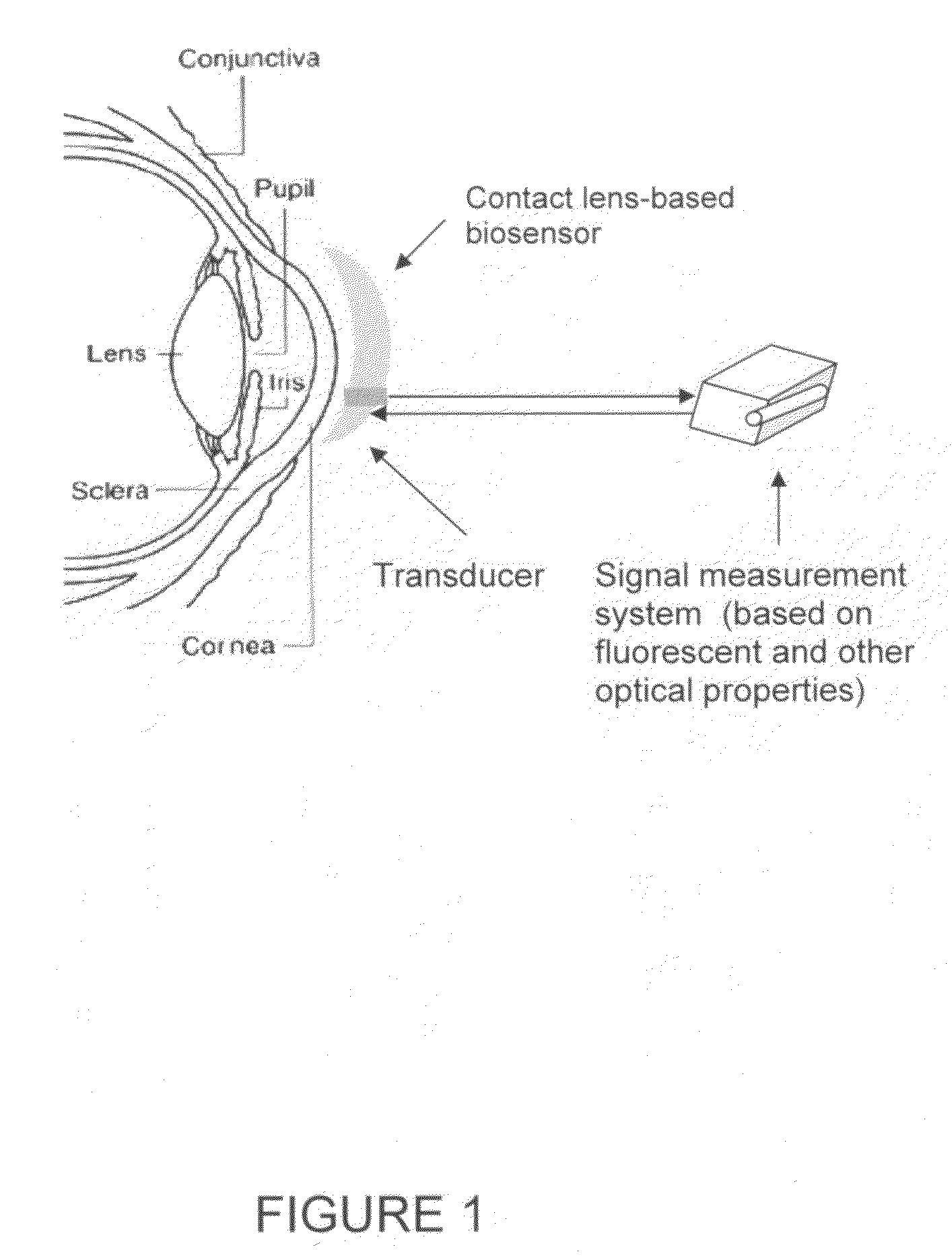
Contact lens integrated with a biosensor for the detection of glucose and other components in tears
ActiveUS20100113901A1Improve behaviorLess discomfortOptical articlesDiagnostic recording/measuringFluorescenceMonitors blood glucose
The present invention provides contact lens with integrated biosensor for the continuous, non-invasive monitoring of physiological glucose by employing biocompatible nanostructure-laden lens materials. These contact lenses can be worn by diabetics who can colorimetrically see changes in their contact lens color or other fluorescence-based properties, giving an indication of tear and blood glucose levels. This invention for the glucose biosensor based on the new disposal contact lens provides a safe, convenient and non-expensive glucose sensing device. The sensing device disclosed herein provides an efficient and noninvasive solution for monitoring blood glucose.
Owner:ZHANG JIN +1
Systems and methods for blood glucose sensing
InactiveUS20050045476A1Immobilised enzymesBioreactor/fermenter combinationsElectrical conductorBlood sugar
A system for measuring a glucose level in a blood sample includes a test strip and a meter. The test strip includes a sample chamber, a working electrode, a counter electrode, fill-detect electrodes, and an auto-on conductor. A reagent layer is disposed in the sample chamber. The auto-on conductor causes the meter to wake up and perform a test strip sequence when the test strip is inserted in the meter. The meter uses the working and counter electrodes to initially detect the blood sample in the sample chamber and uses the fill-detect electrodes to check that the blood sample has mixed with the reagent layer. The meter applies an assay voltage between the working and counter electrodes and measures the resulting current. The meter calculates the glucose level based on the measured current and calibration data saved in memory from a removable data storage device associated with the test strip.
Owner:TRIVIDIA HEALTH
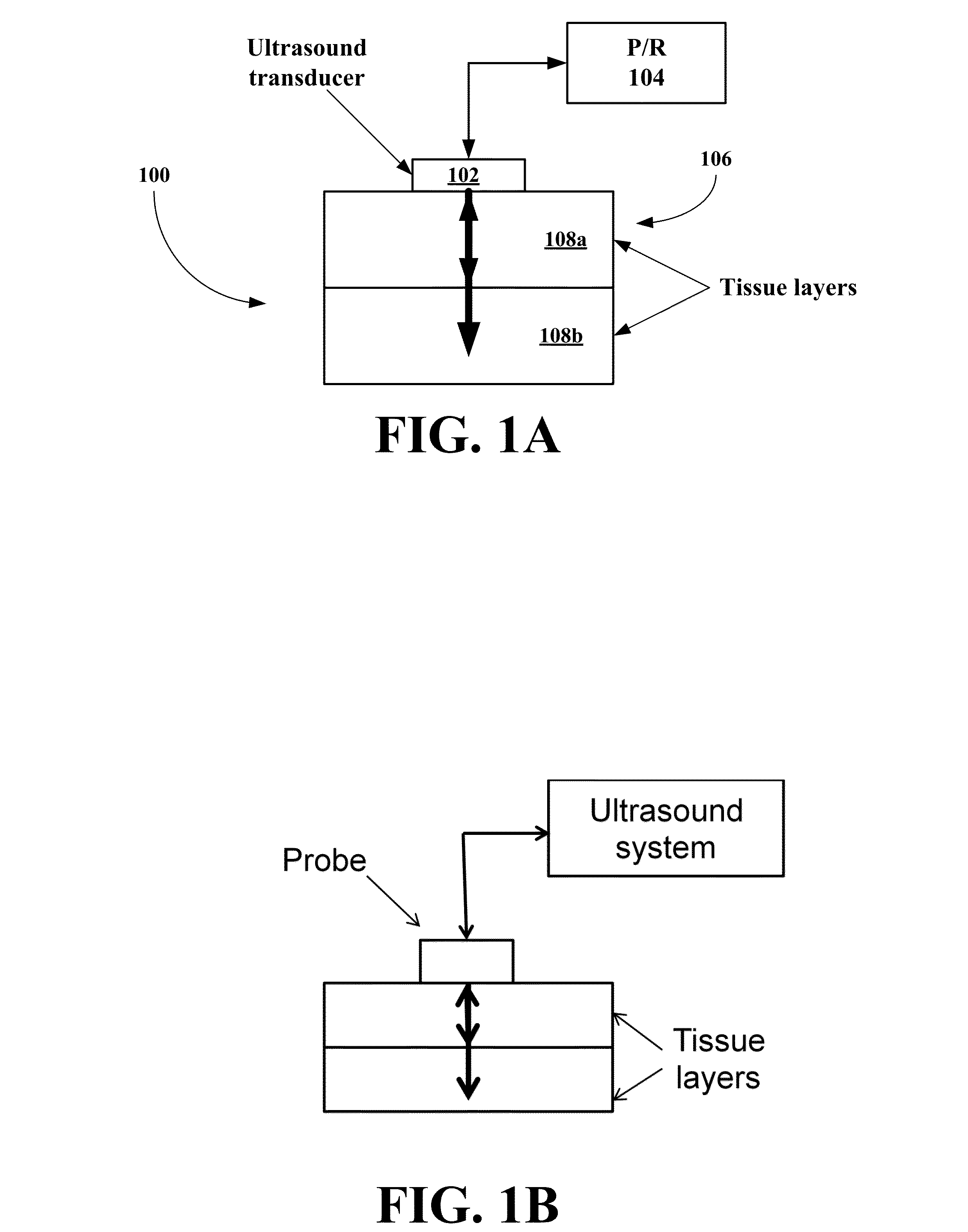
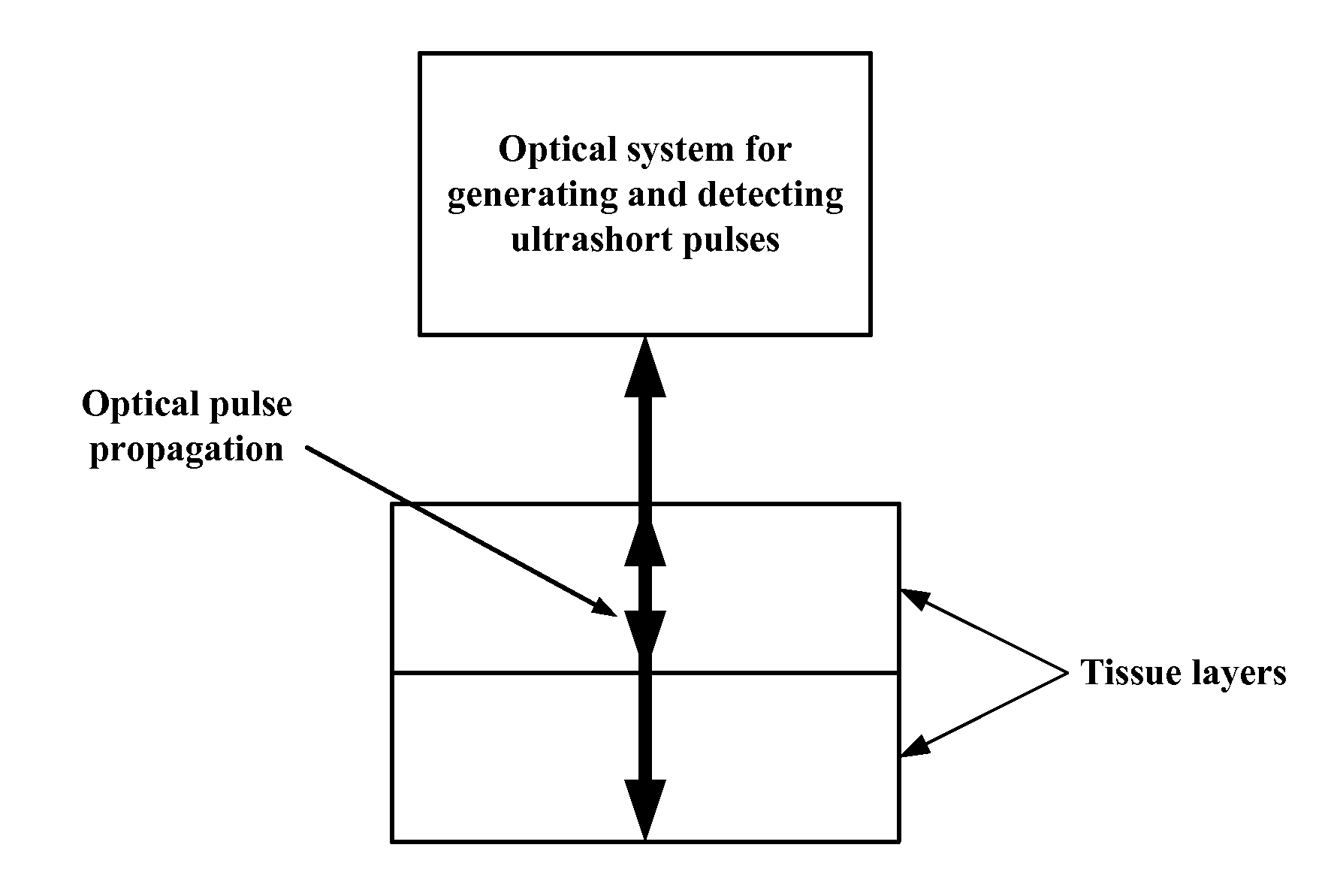
Noninvasive glucose sensing methods and systems
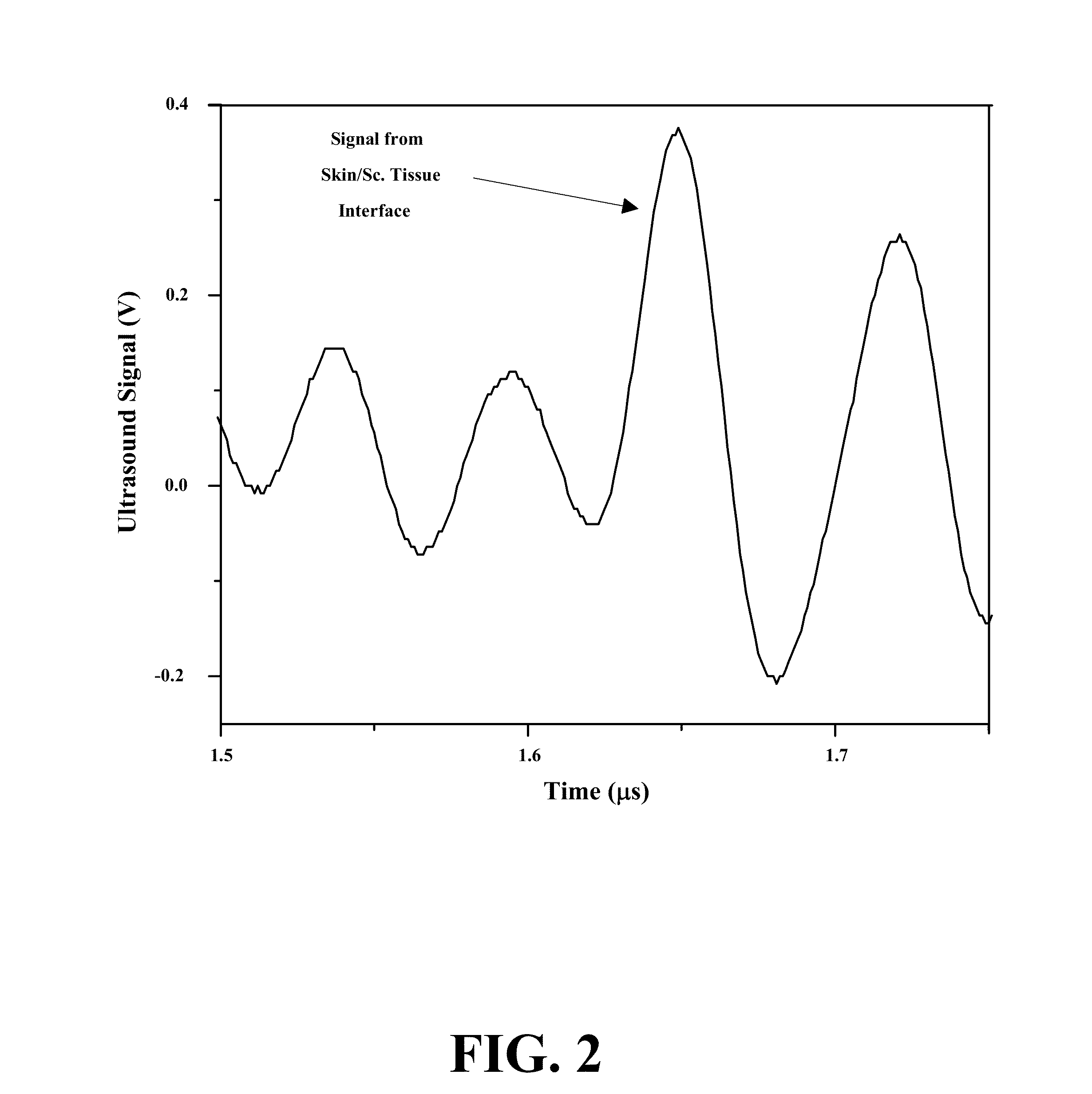
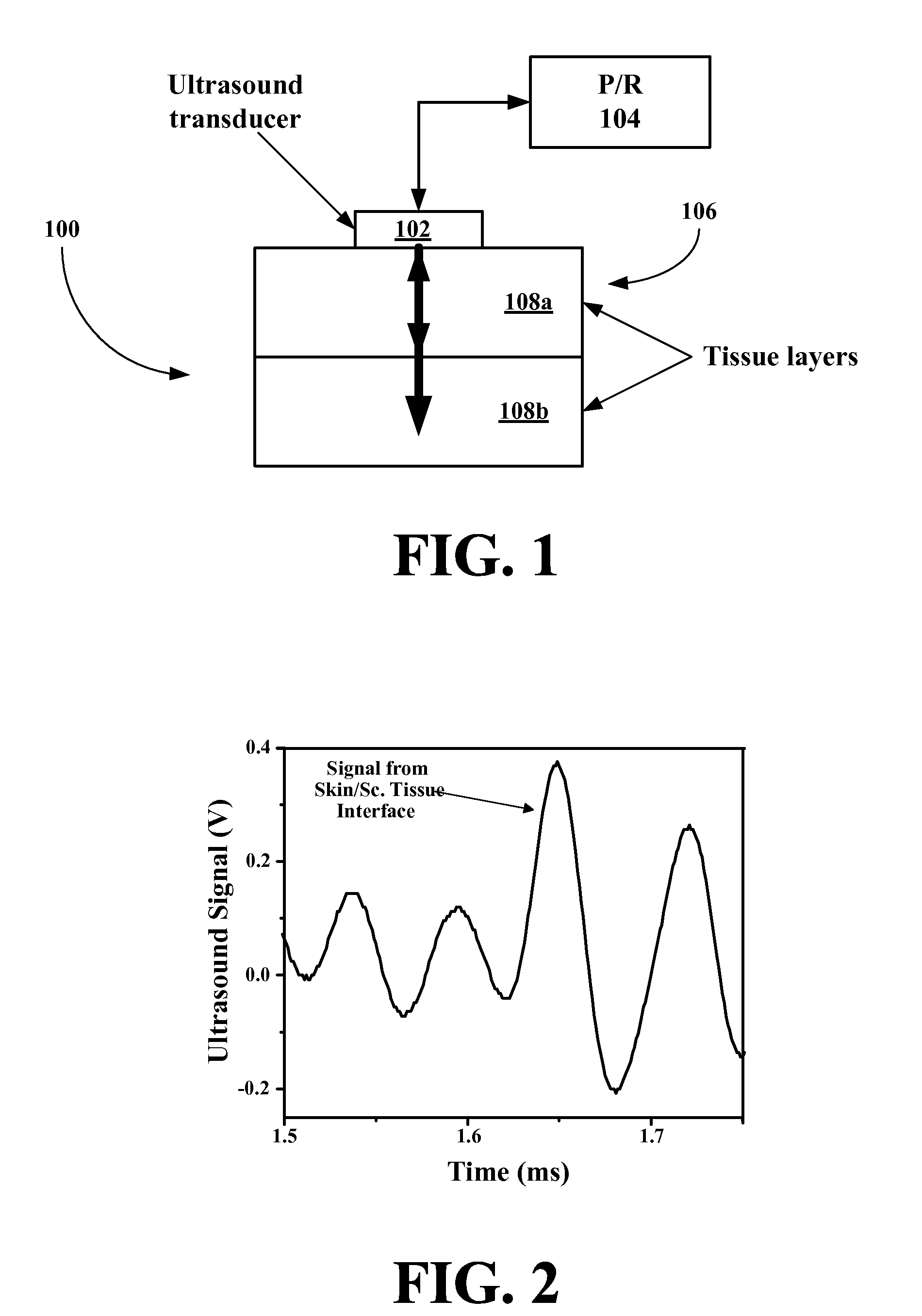
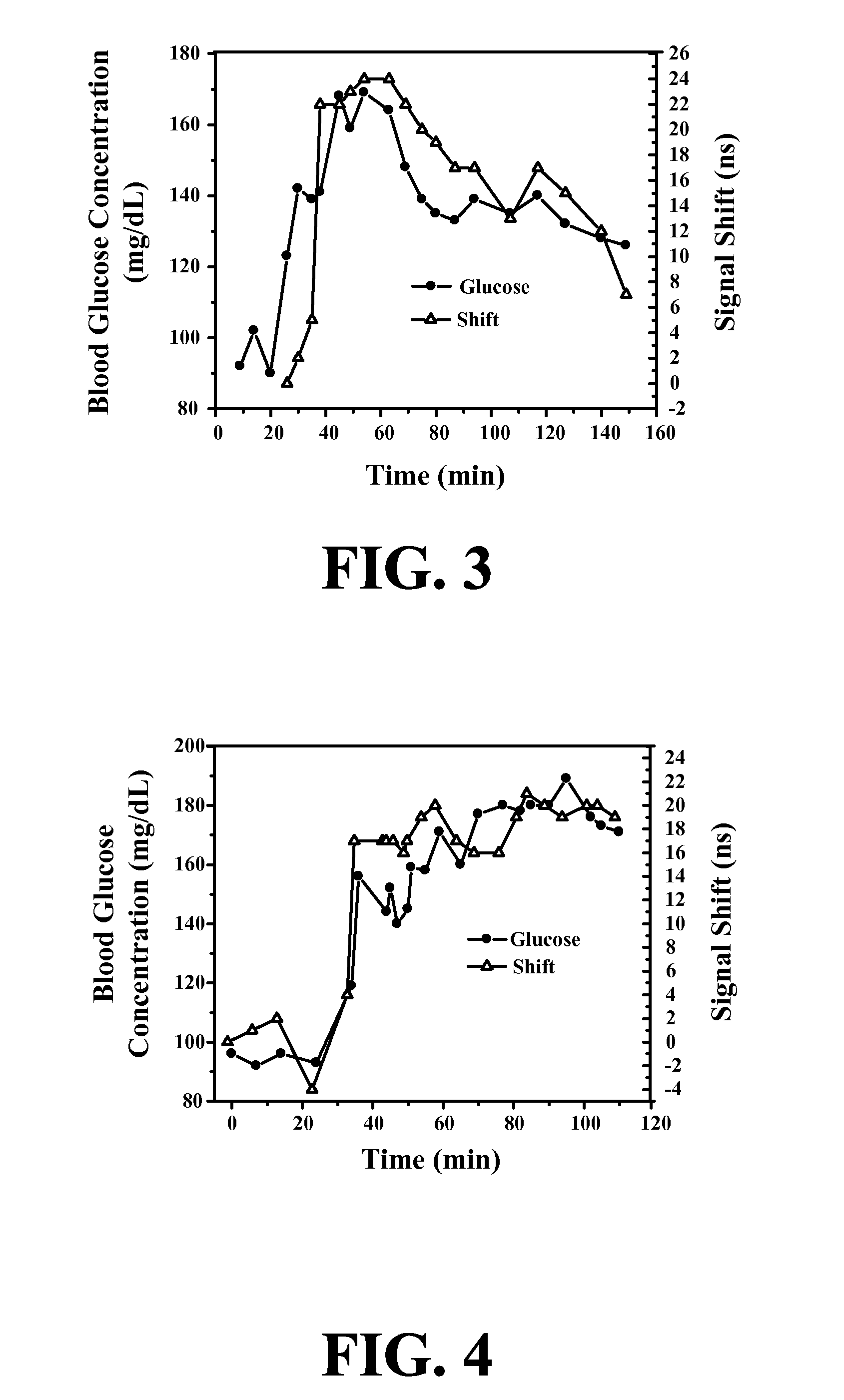
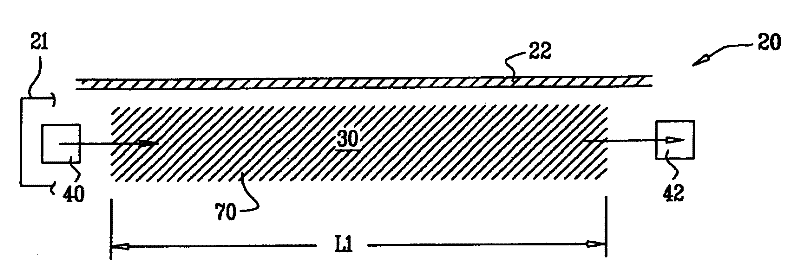
ActiveUS20070255141A1Reduce complicationsReduce mortalityUltrasonic/sonic/infrasonic diagnosticsInfrasonic diagnosticsMedicineD-Glucose
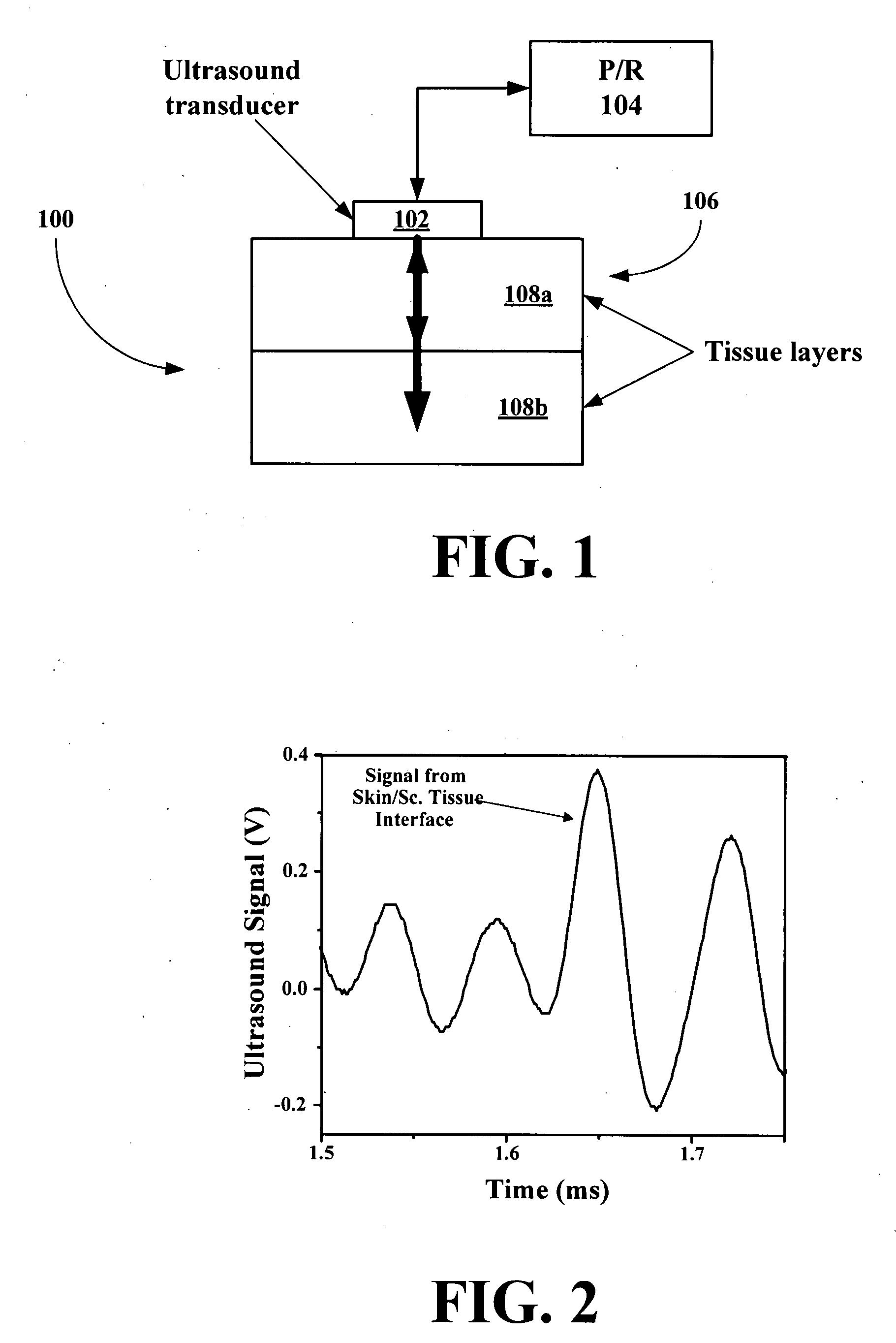
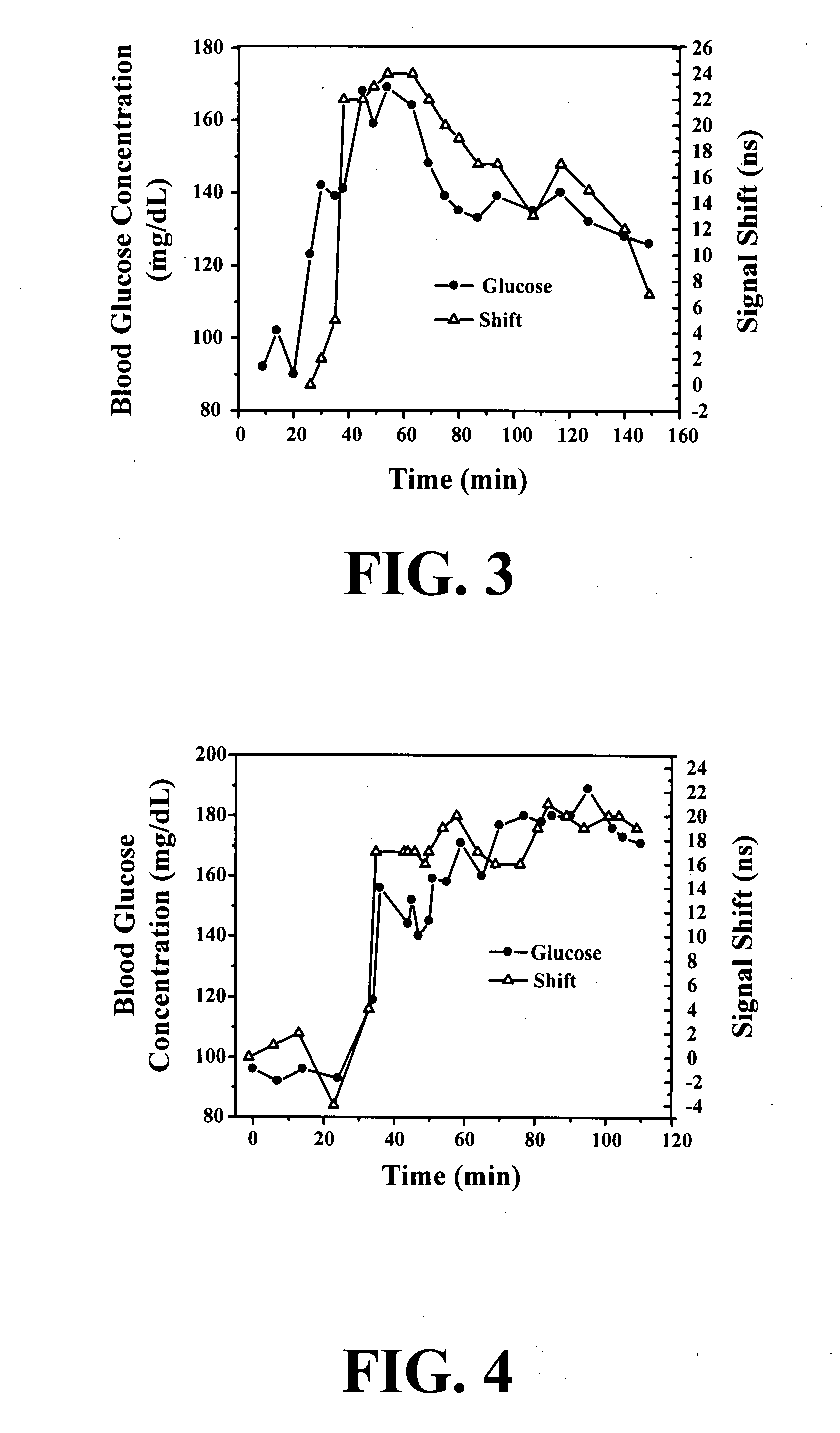
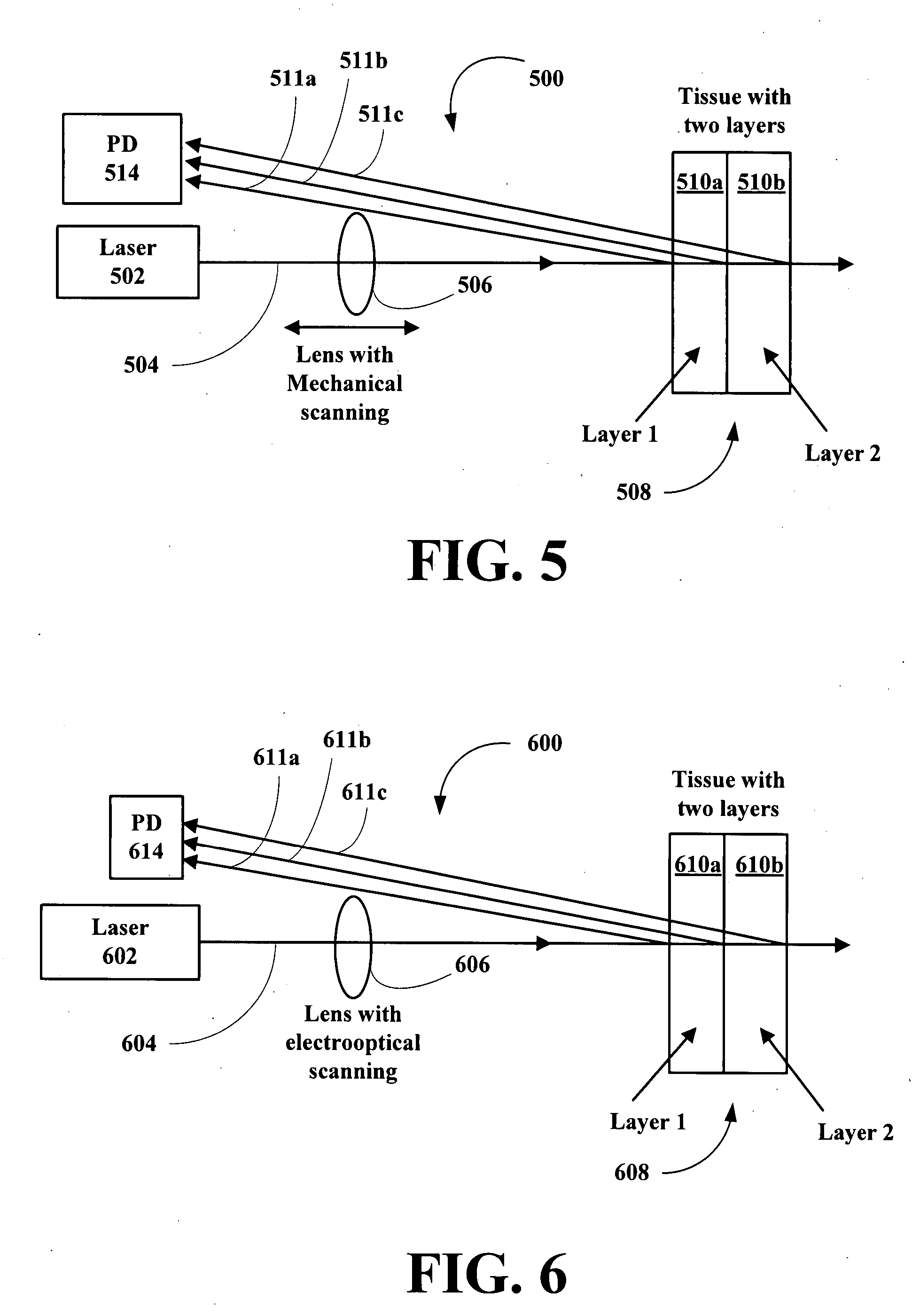
New methods and systems for noninvasive glucose monitoring and sensing with electromagnetic waves or ultrasound are disclosed. The methods are based on absolute or relative measurement of tissue dimensions (or changes in the dimensions) including, but not limited to: thickness, length, width, diameter, curvature, roughness as well as time of flight of ultrasound and optical pulses and optical thickness, which change with changing blood glucose concentrations. By measuring noninvasively absolute or relative changes in at least one dimension of at least one tissue or tissue layer or absolute or relative changes in time of flight of ultrasound or optical pulses, one can monitor blood glucose concentration noninvasively.
Owner:ESENALIEV RINAT O +1
Method and apparatus for analyte sensing
InactiveUS7226414B2Reducing dispersion viscosityLow viscosityCatheterDiagnostic recording/measuringRare-earth elementEngineering
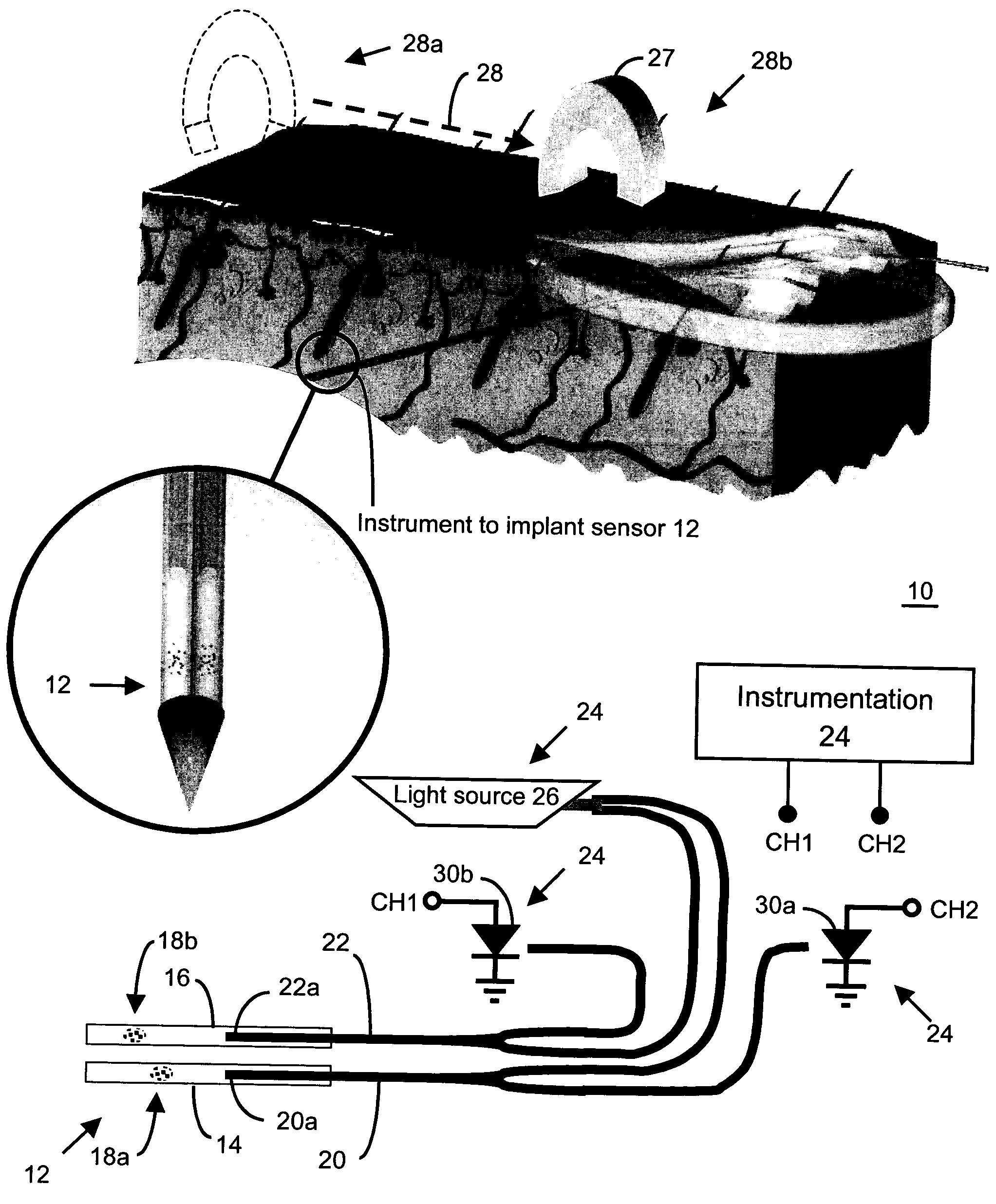
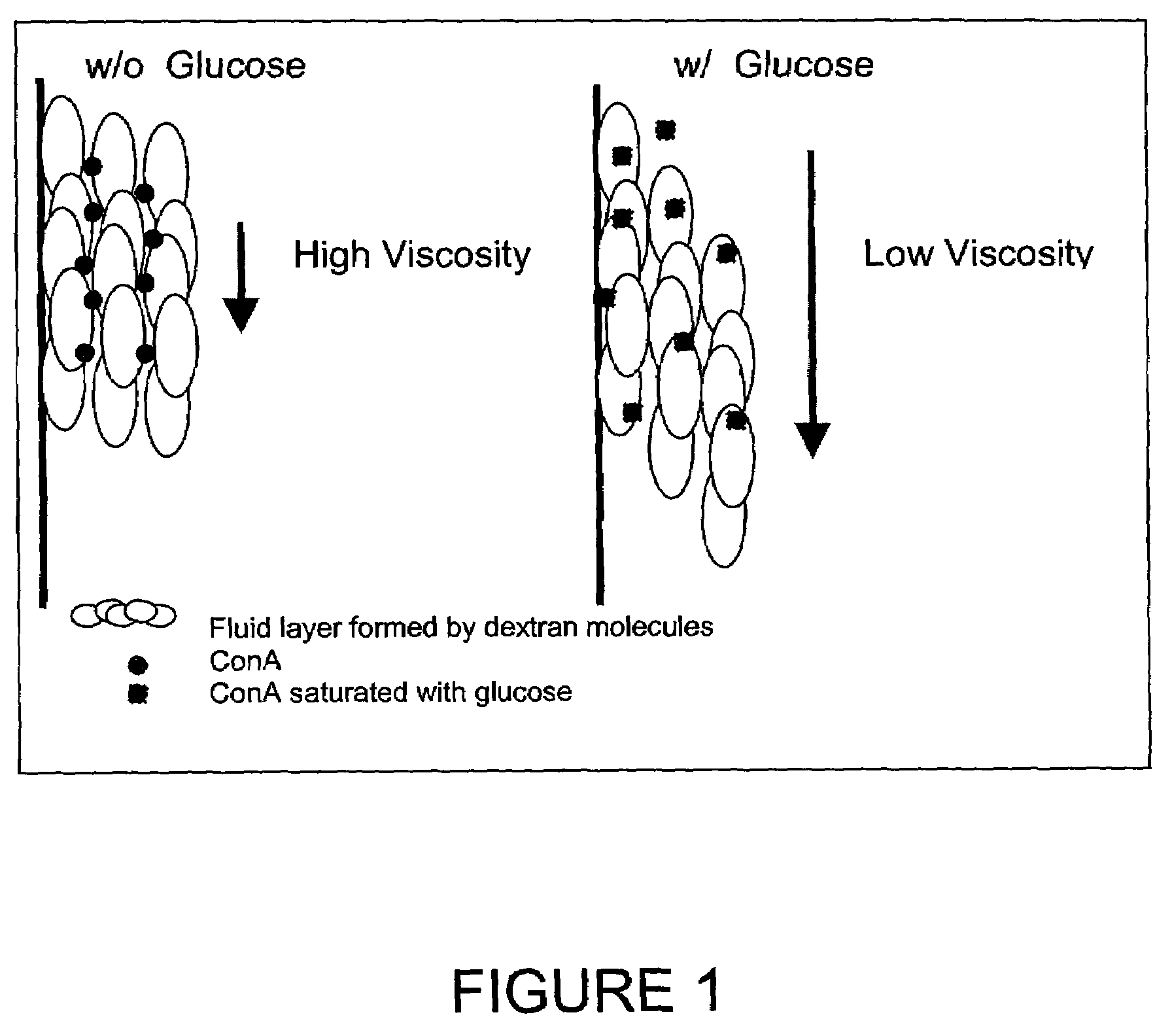
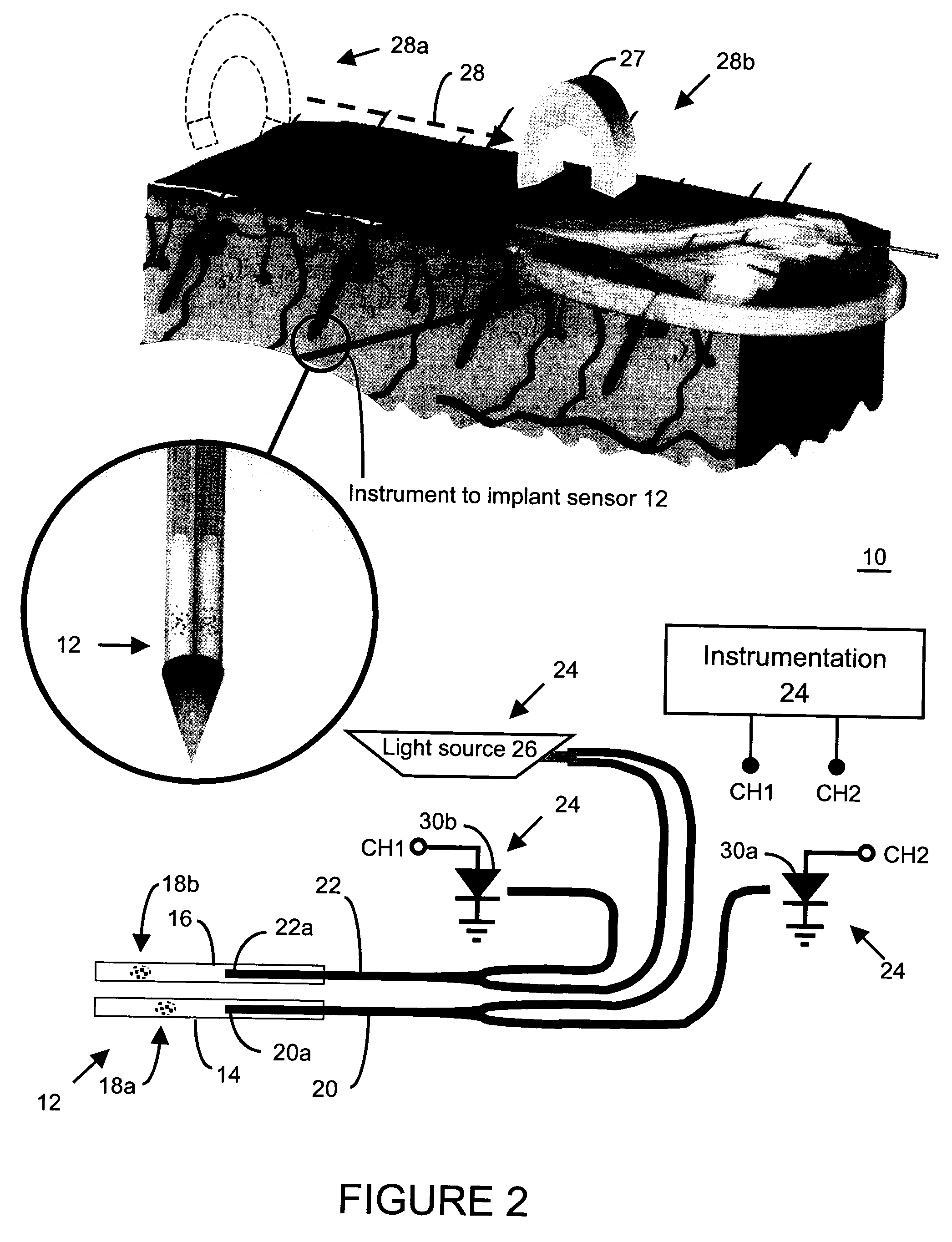
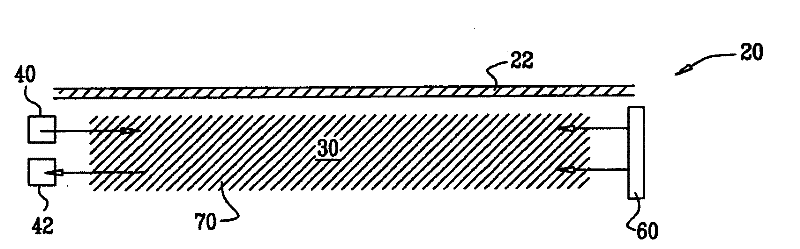
In one aspect, the present invention is directed to a glucose sensing device for implantation within subcutaneous tissue of an animal body. In one embodiment, the glucose sensing device includes a first chamber containing first magnetic particles and a hydrocolloid solution (for example, ConA-dextran hydrocolloid) wherein the first magnetic particles are dispersed in the hydrocolloid solution. In operation, glucose within the animal may enter and exit the first chamber and the hydrocolloid solution changes in response to the presence or concentration of glucose within the first chamber. The sensing device also includes a reference chamber containing second magnetic particles and a reference solution wherein the second magnetic particles are dispersed in the reference solution. The reference solution (for example, oil or alcohol compounds) includes a known or fixed viscosity. The reference solution may also be a hydrocolloid solution (for example, ConA-dextran hydrocolloid). The first and / or second magnetic particles may include amine-terminated particles, at least one rare earth element (for example, neodymium or samarium), and / or a ferromagnetic material.
Owner:BIOTEX
Method of and system for stabilization of sensors
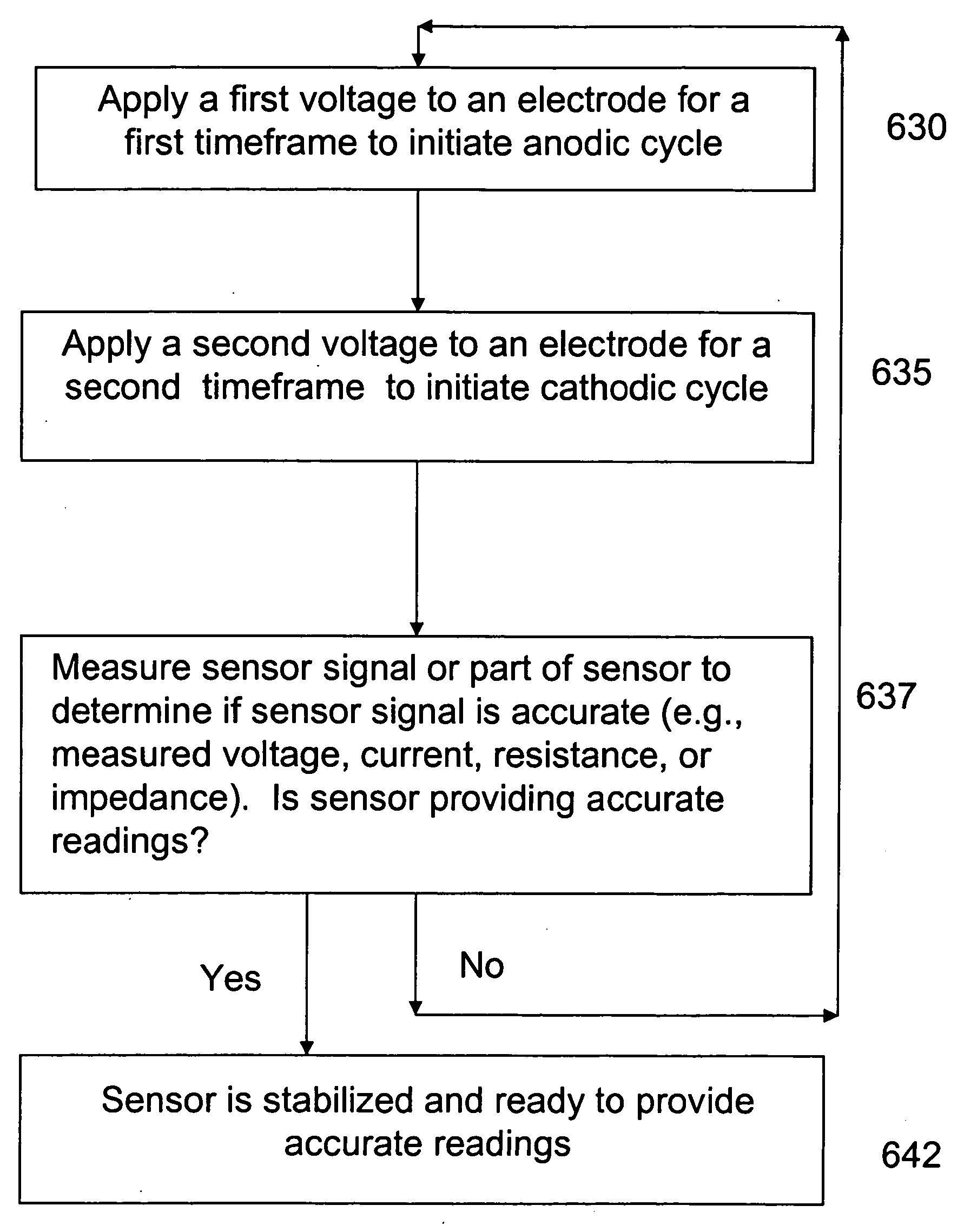
InactiveUS20070173712A1Faster run-in timeLess background currentWave based measurement systemsMaterial analysis by electric/magnetic meansElectronVoltage
A blood glucose sensing system includes a sensor and a sensor electronics device. The sensor includes a plurality of electrodes. The sensor electronics device includes stabilization circuitry. The stabilization circuitry cases a first voltage to be applied to one of the electrodes for a first timeframe and causes a second voltage to be applied to one of the electrodes for a second timeframe. The stabilization circuitry repeats the application of the first voltage and the second voltage to continue the anodic-cathodic cycle. The sensor electronics device may include a power supply, a regulator, and a voltage application device, where the voltage application device receives a regulator voltage from the regulator, applies a first voltage to an electrode for the first timeframe, and applies a second voltage to an electrode for the second timeframe.
Owner:MEDTRONIC MIMIMED INC
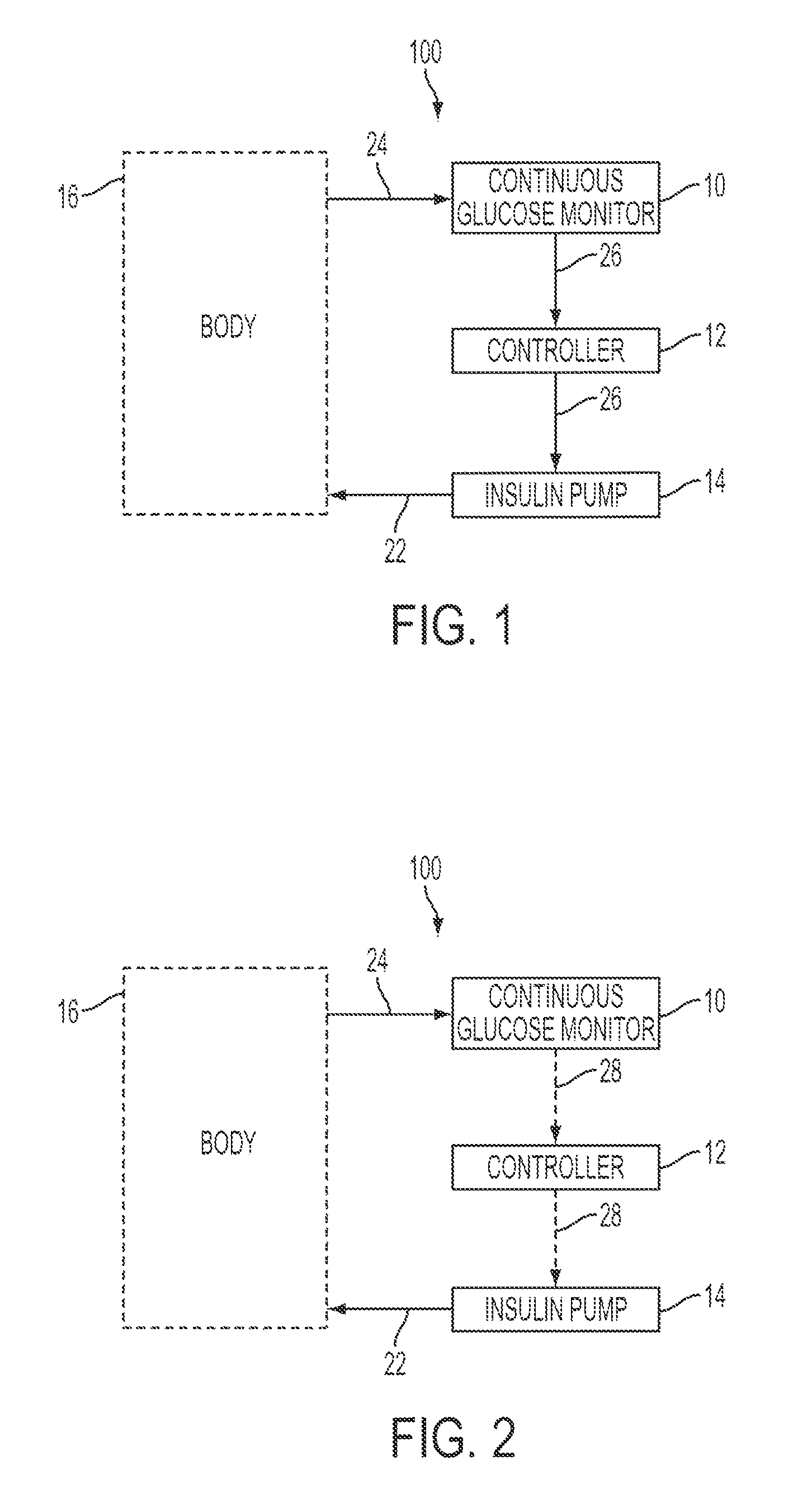
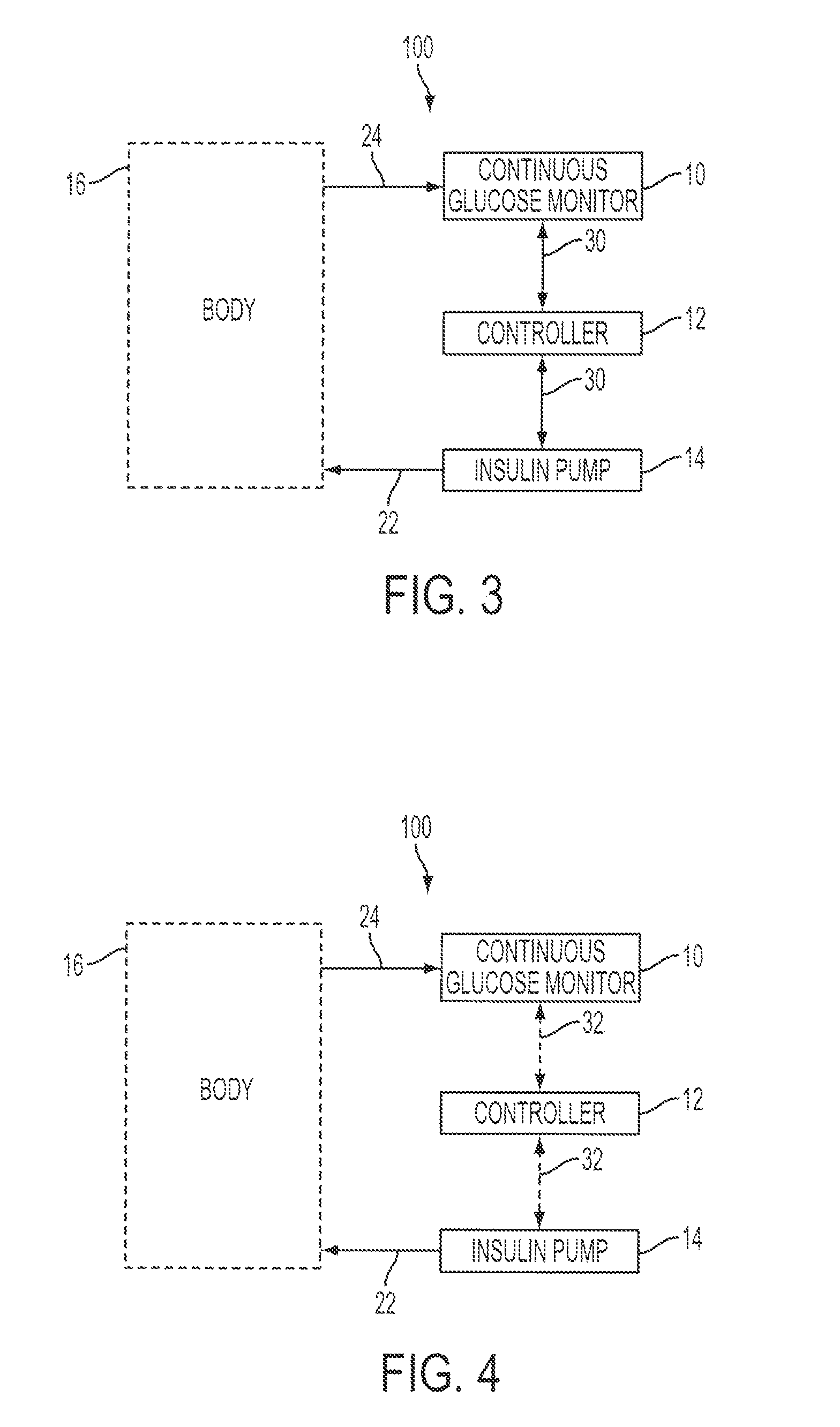
Predictive control based system and method for control of insulin delivery in diabetes using glucose sensing
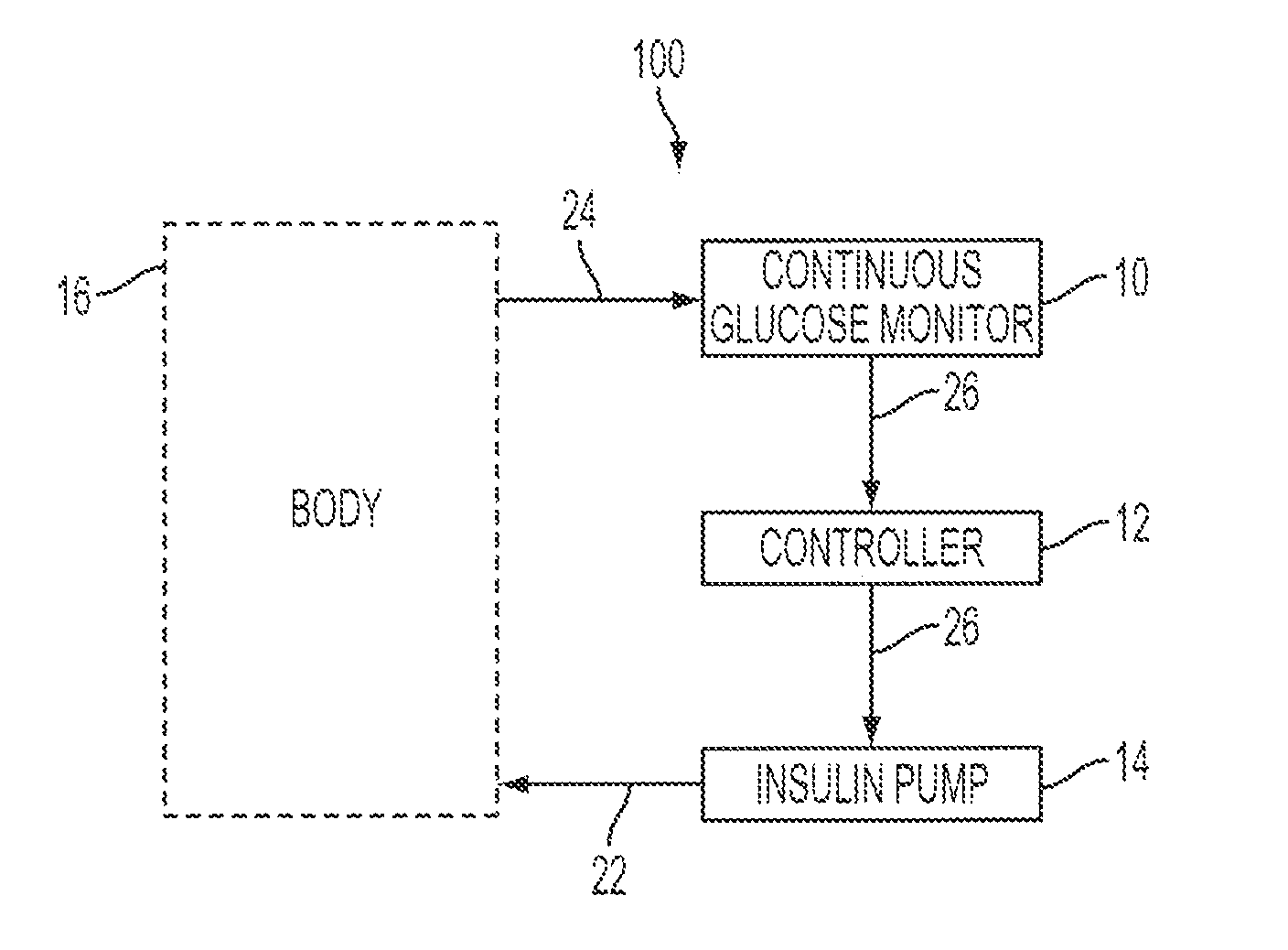
A system and method for providing optimal insulin injections to a subject, using a controller, a continuous glucose monitor, and an insulin delivery unit is disclosed. The controller possesses a discrete-time, linear model predictive control law, means for sending information to the insulin delivery unit, and means for receiving information from the CGM. The control law implemented is derived from a discrete-time model of glucose insulin dynamics and an aggressiveness parameter. The result is that using only glucose measurements obtained from sensor readings and, prior values of external insulin infusion and meal and exercise announcement the optimal insulin injection necessary to safely regulate blood glucose can be calculated.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
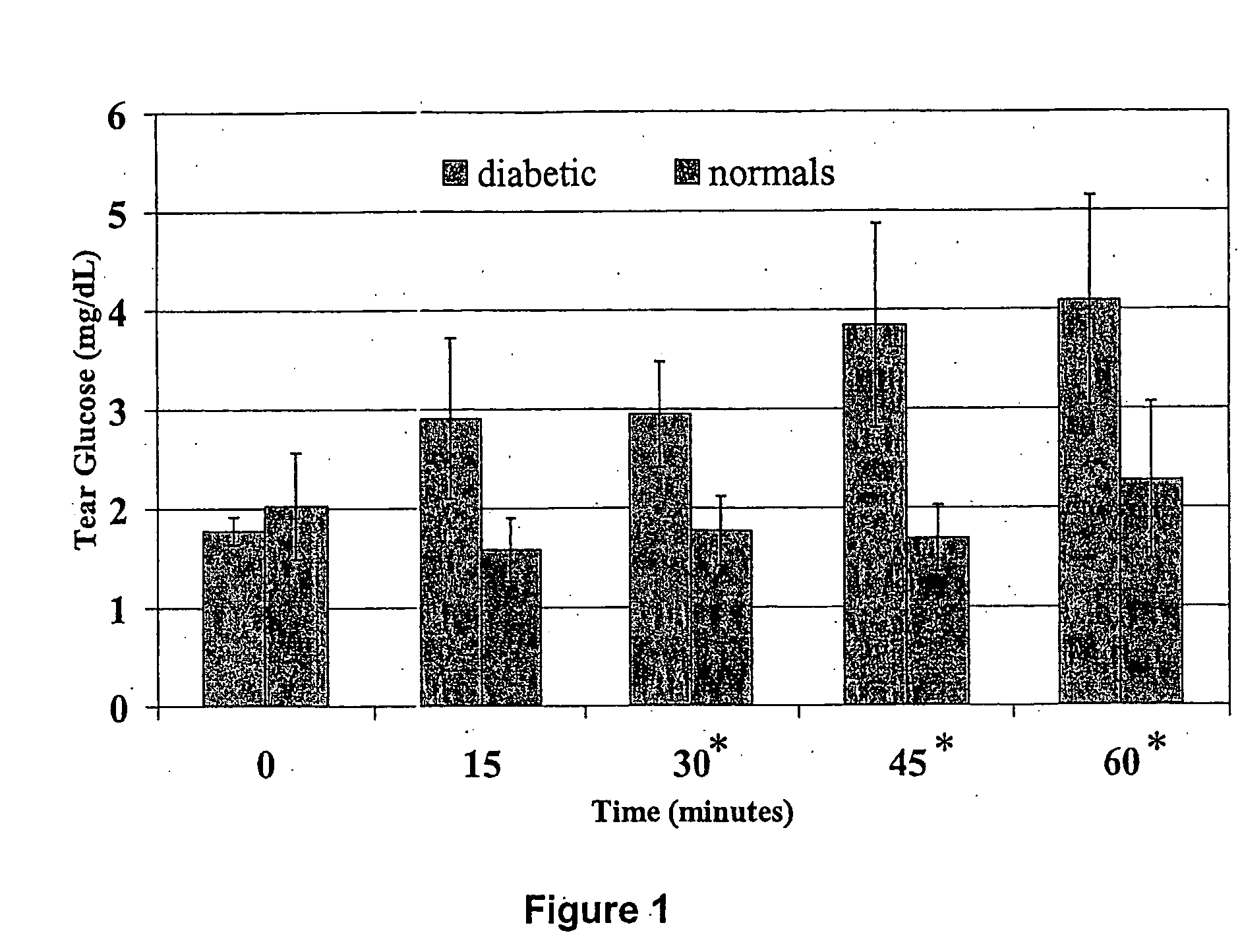
Methods and kits for assays of rapid screening of diabetes
InactiveUS20050038329A1Microbiological testing/measurementDisease diagnosisAssayConcentrations glucose
The invention provides an in vivo screening assay and an in vitro screening assay for rapid screening of diabetes. A method of the invention includes determining a first glucose concentration in an ocular fluid of a patient; administering orally a load of carbohydrate to the patient; determining a second glucose concentration in an ocular fluid of the patient at a period of time of less than 50 minutes after orally administering of the load of carbohydrate; comparing the second glucose concentration with the first glucose concentration to determine if the patient is likely to be a diabetic. The method of the invention is performed by using a kit of the invention. The kit comprises: (1) a glucose-sensing ophthalmic device and instructions for using the glucose-sensing ophthalmic device to screen for diabetes; or (2) two or more tear-collecting devices, and a testing agent composition which specifically reacts with glucose to form a detectable signal. The glucose-sensing ophthalmic device comprises a testing agent composition which specifically and reversibly interacts with glucose to form a detectable optical signal which changes in a concentration-dependent manner.
Owner:MORRIS CAROL ANN +2
Optical device and method for non-invasive real-time testing of blood sugar levels
A device and method for non-invasive real-time testing of blood sugar levels in a diabetic patient. Specifically, this invention is directed to an optical device comprising a contact lens having a glucose-sensing optical pattern imprinted, marked, coated or otherwise disposed on or incorporated within the contact lens. The indicator pattern is further comprised of a glucose-sensing coating containing a boronic acid derivative, which reacts in the presence of glucose to create a readable pattern, which can then be correlated to a pre-determined or pre-calibrated blood glucose level. A polarized light source is one method that may be used to read the indicator pattern. The invention is also directed to methods for quantifying blood glucose levels using the inventive optical device and manufacturing methods for disposing the glucose-sensing coating onto, or incorporating it into, the contact lens material.
Owner:THE UNIVERSITY OF AKRON
Method and apparatus for non-invasive glucose sensing through the eye
InactiveUS6885882B2Polarisation-affecting propertiesDiagnostic recording/measuringAqueous humorD-Glucose
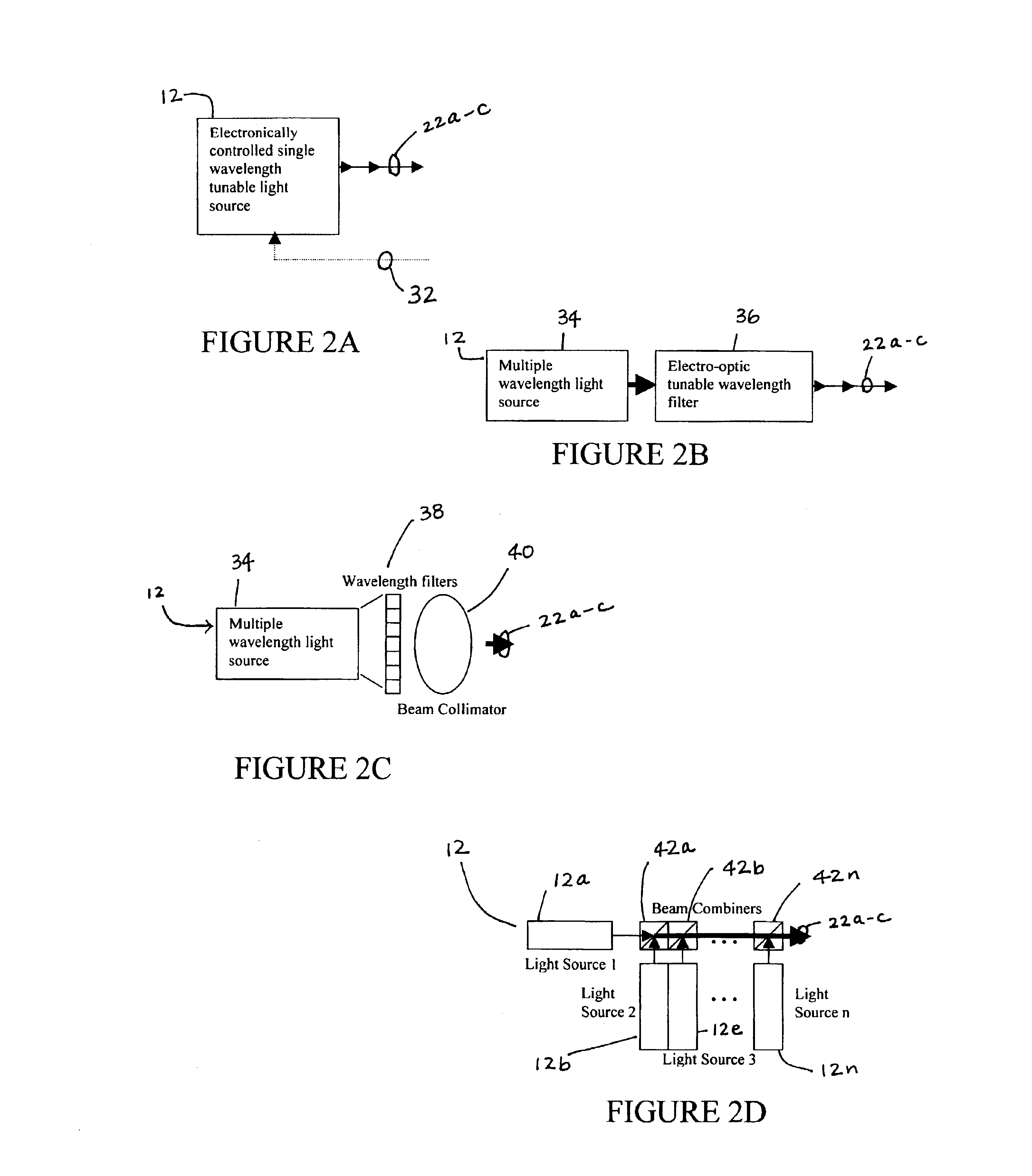
There are many inventions described and illustrated herein. In one aspect, the present invention is a system and technique for non-invasively measuring, monitoring, inspecting, characterizing, determining and / or evaluating the blood glucose level in the aqueous humor of the eye of a patient (for example, a diabetic). In one embodiment, the present invention employs a plurality of wavelengths of light (for example, more than three) to measure, monitor, characterize, determine and / or evaluate the blood glucose level or concentration of a patient. The plurality of wavelengths of light may be directed into the aqueous humor of the eye and the reflected light (i.e., the light reflected by the eye) is detected and analyzed to provide information which is representative of the blood glucose level or concentration of the patient.
Owner:COTE GERARD L +1
Contact lens integrated with a biosensor for the detection of glucose and other components in tears
ActiveUS8385998B2Safe and convenientEfficient and non-invasiveOptical articlesDiagnostic recording/measuringFluorescenceMonitors blood glucose
The present invention provides contact lens with integrated biosensor for the continuous, non-invasive monitoring of physiological glucose by employing biocompatible nanostructure-laden lens materials. These contact lenses can be worn by diabetics who can colorimetrically see changes in their contact lens color or other fluorescence-based properties, giving an indication of tear and blood glucose levels. This invention for the glucose biosensor based on the new disposal contact lens provides a safe, convenient and non-expensive glucose sensing device. The sensing device disclosed herein provides an efficient and noninvasive solution for monitoring blood glucose.
Owner:ZHANG JIN +1
Glucose dependent release of insulin from glucose sensing insulin derivatives
InactiveUS7316999B2Facilitated releasePeptide/protein ingredientsMetabolism disorderGlucose sensorsConcentrations glucose
Insulin derivatives having a built-in glucose sensor, capable of delivering insulin from a depot as a function of the glucose concentration in the surrounding medium (e.g. tissue), such that the rate of insulin release from the depot increases with an increased glucose concentration and decreases with a decreased glucose concentration.
Owner:NOVO NORDISK AS
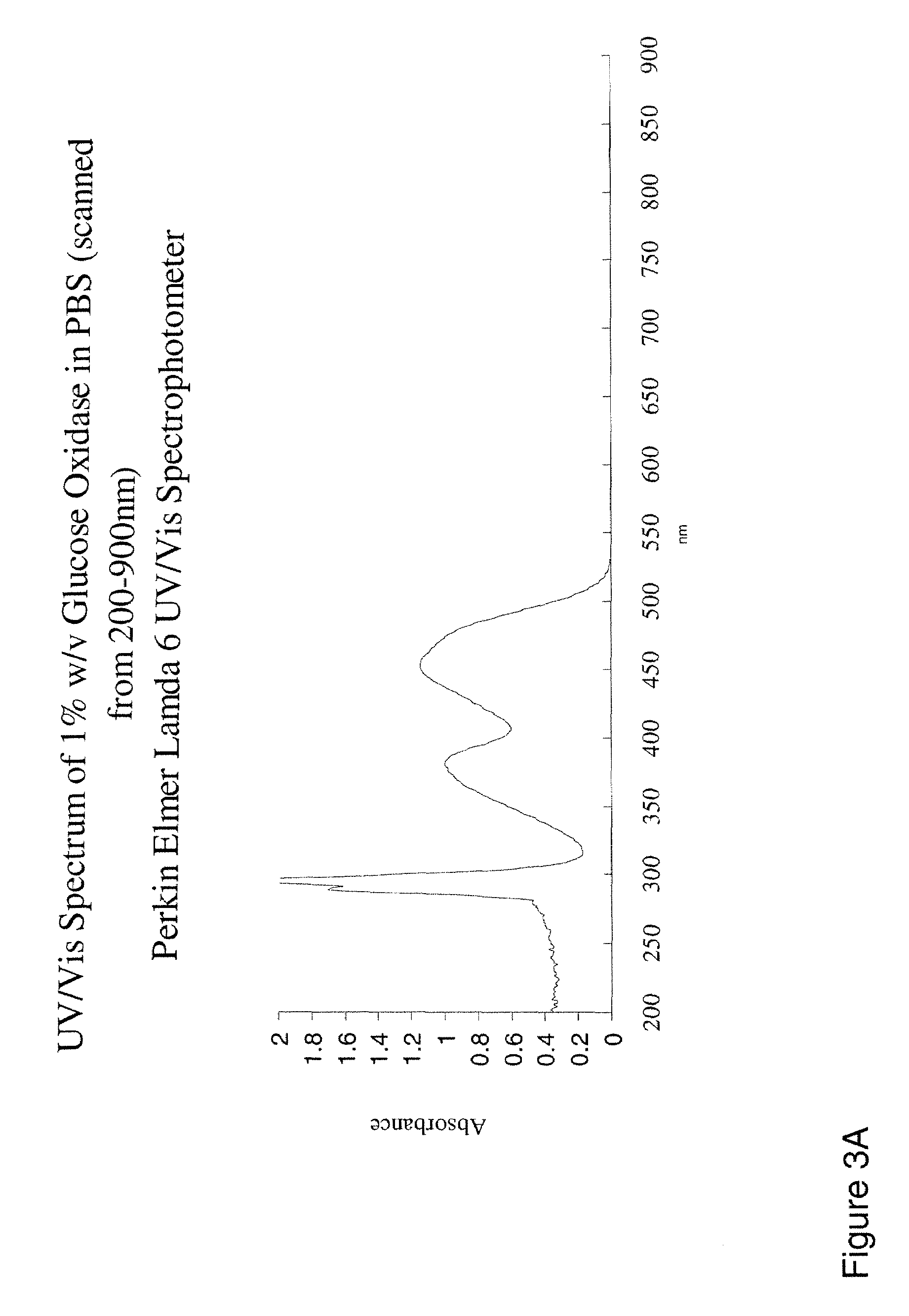
Electrochemical biosensor
ActiveUS20100285514A1Promote resultsImprove electrochemical performanceBioreactor/fermenter combinationsBiological substance pretreatmentsElectrochemical biosensorFluorescence
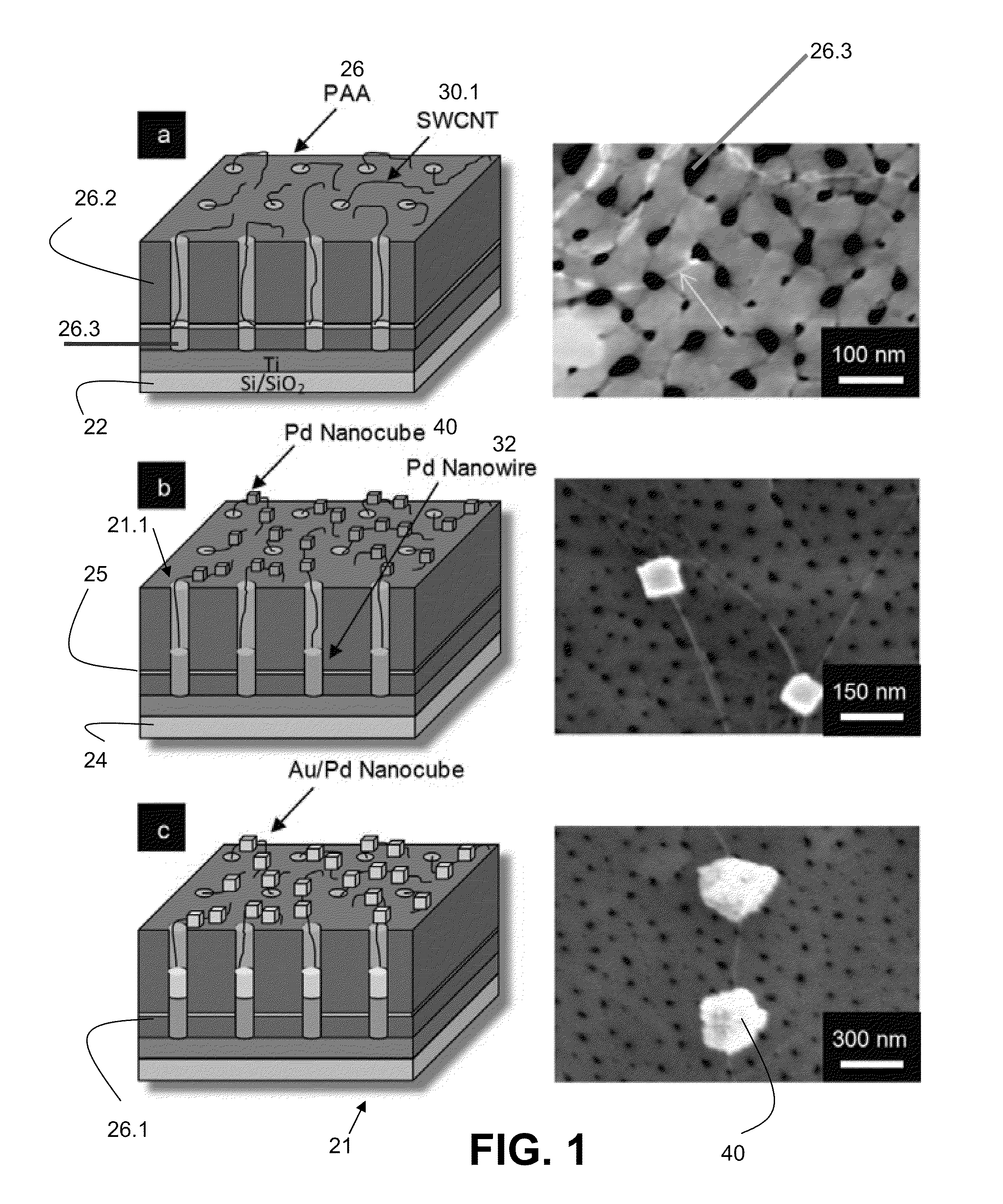
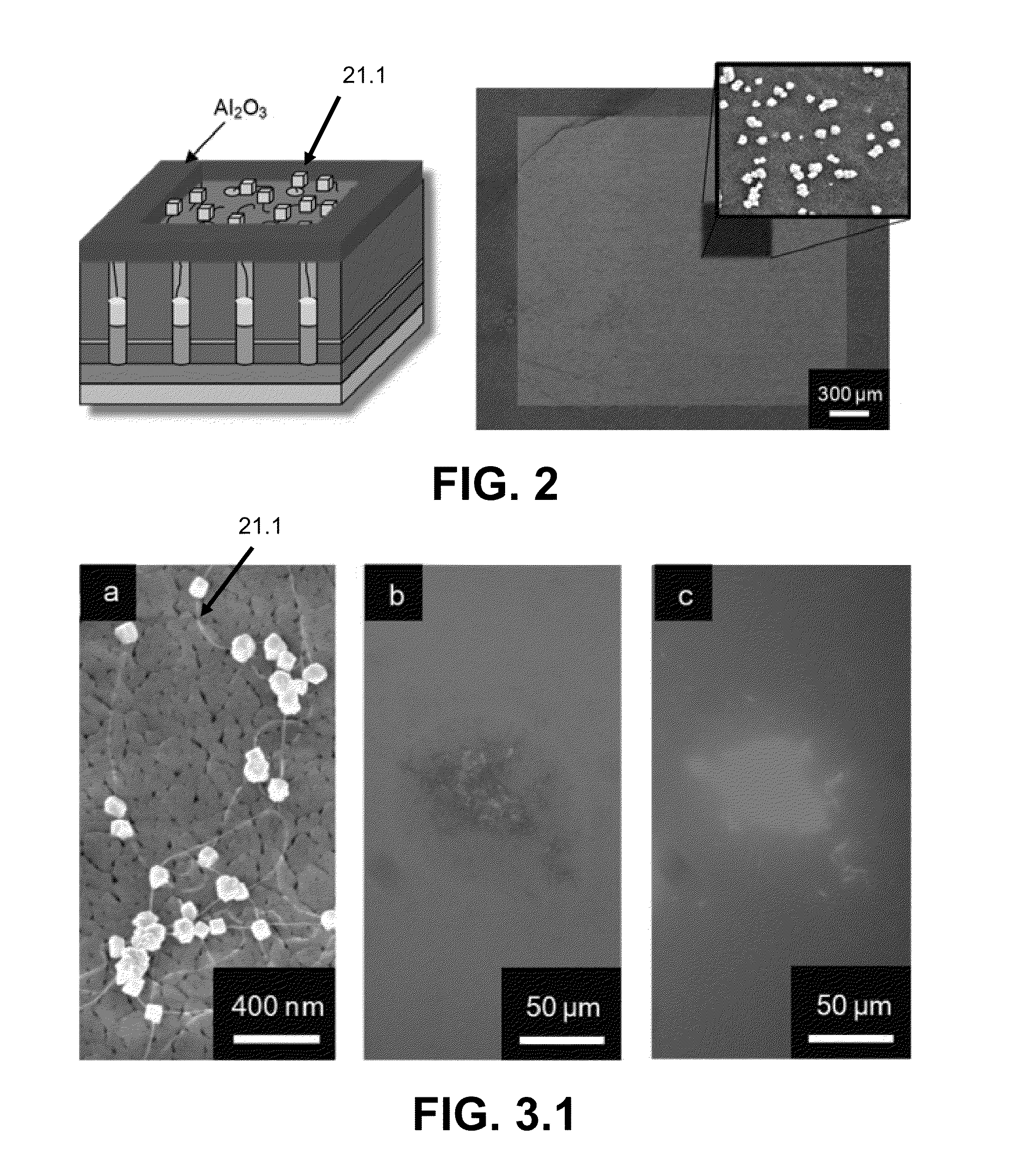
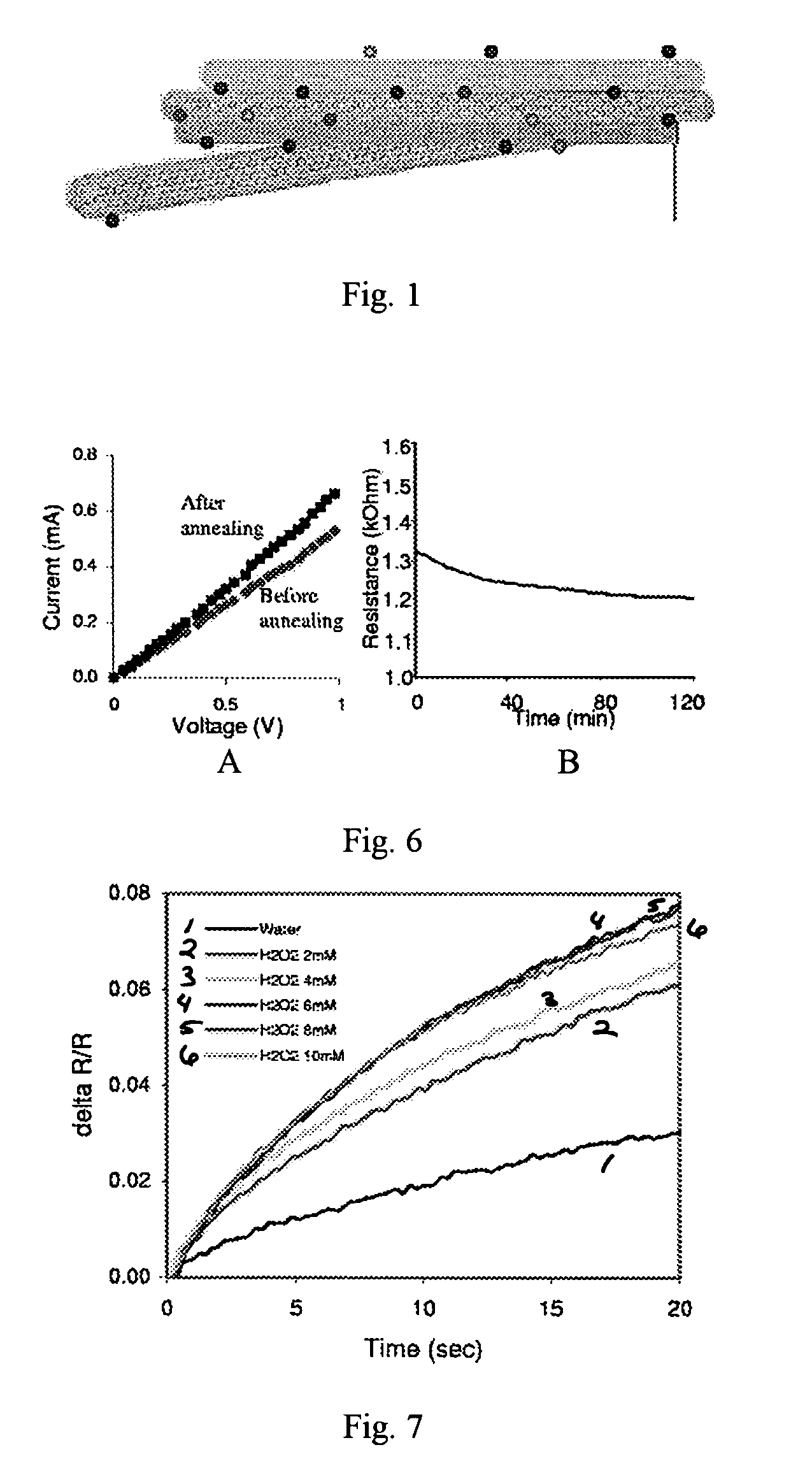
Networks of single-walled carbon nanotubes (SWCNTs) decorated with Au-coated Pd (Au / Pd) nanocubes are employed as electrochemical biosensors that exhibit excellent sensitivity (2.6 mA mM−1 cm−2) and a low estimated detection limit (2.3 nM) at a signal-to-noise ratio of 3 (S / N=3) in the amperometric sensing of hydrogen peroxide. Biofunctionalization of the Au / Pd nanocube-SWCNT biosensor is demonstrated with the selective immobilization of fluorescently labeled streptavidin on the nanocube surfaces via thiol linking. Similarly, glucose oxidase (GOx) is linked to the surface of the nanocubes for amperometric glucose sensing. The exhibited glucose detection limit of 1.3_M (S / N=3) and linear range spanning from 10 μM to 50 mM substantially surpass other CNT-based biosensors. These results, combined with the structure's compatibility with a wide range of biofunctionalization procedures, would make the nanocube-SWCNT biosensor exceptionally useful for glucose detection in diabetic patients and well suited for a wide range of amperometric detection schemes for biomarkers.
Owner:PURDUE RES FOUND INC
Materials containing multiple layers of vesicles
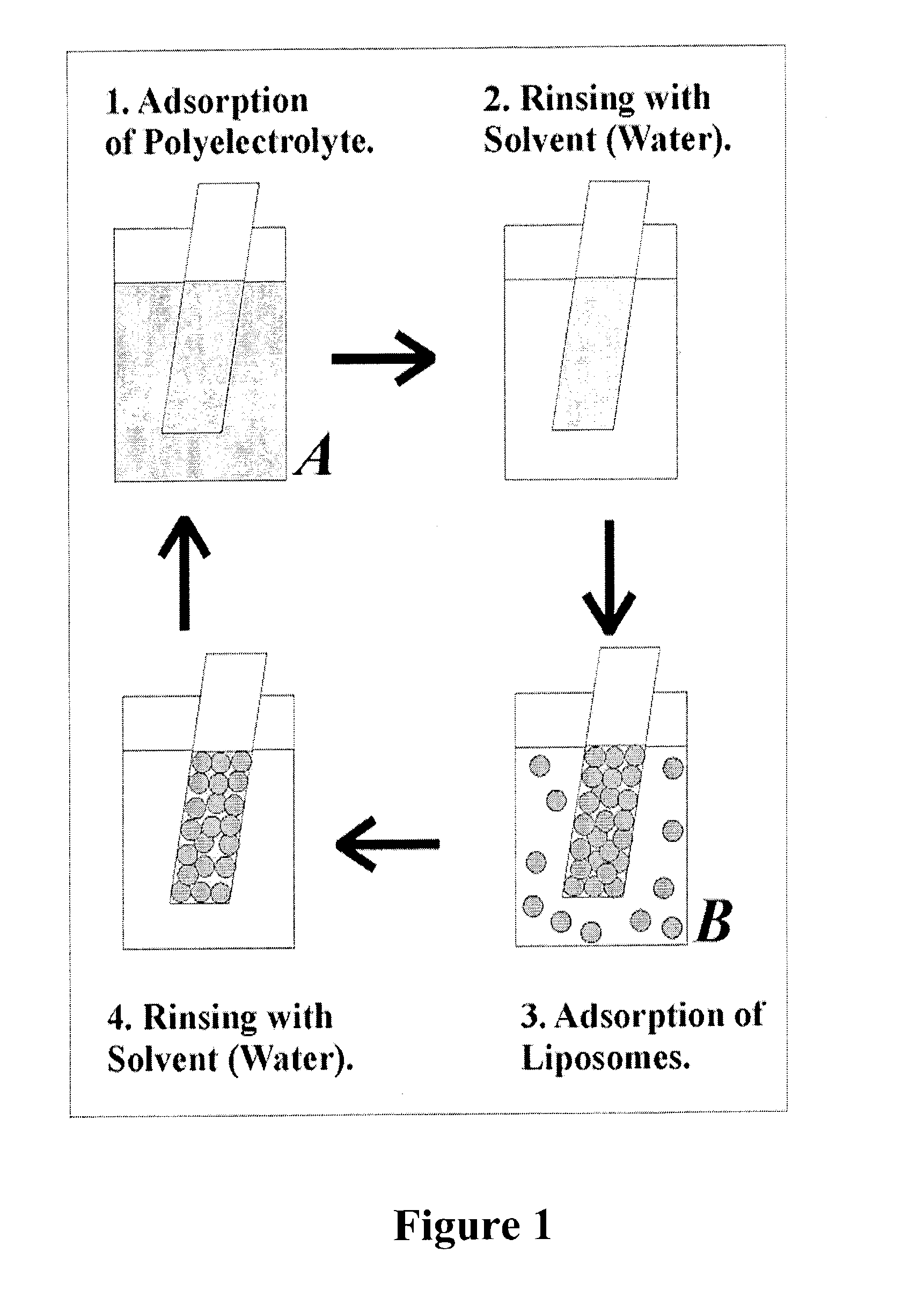
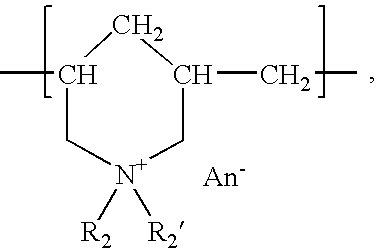
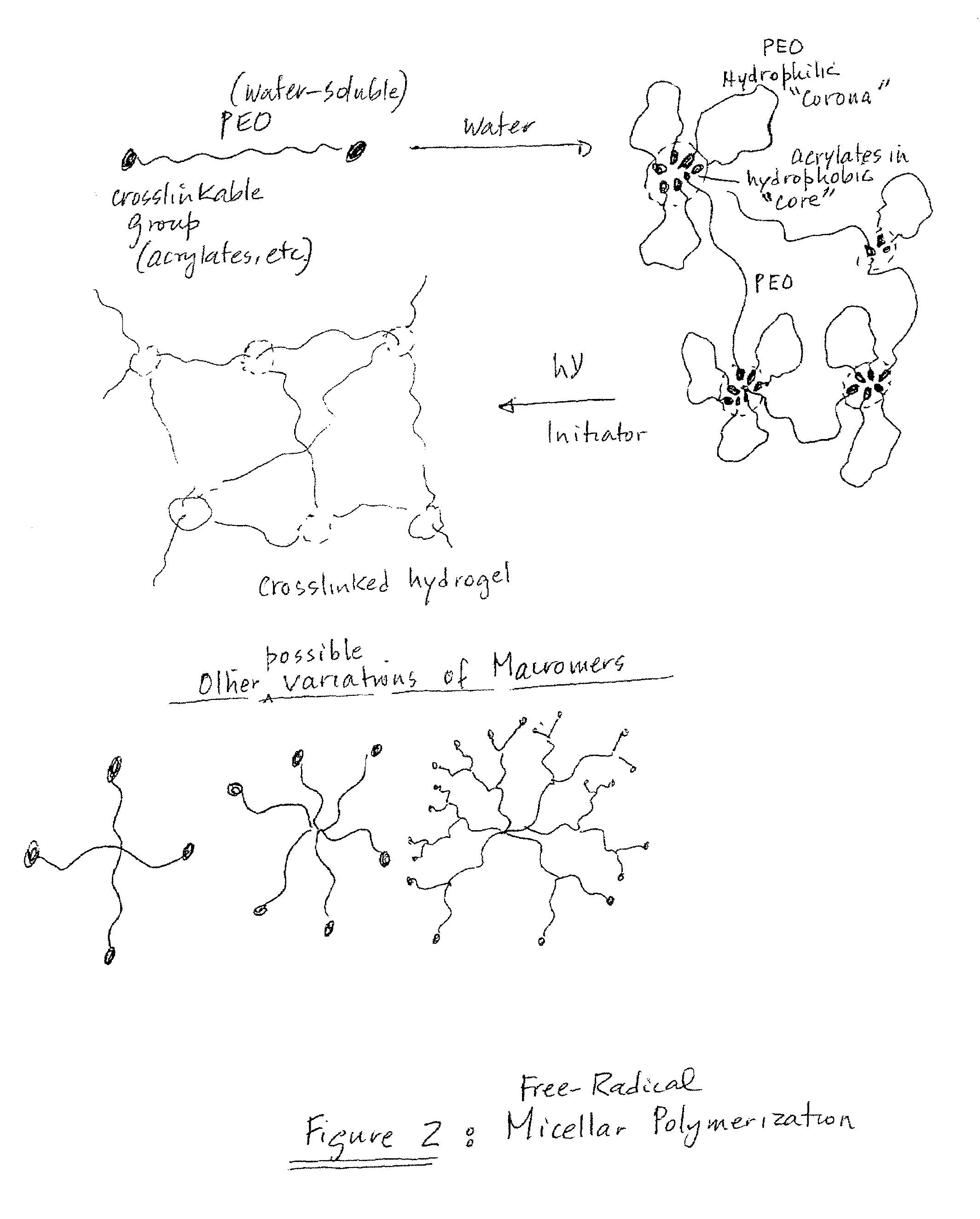
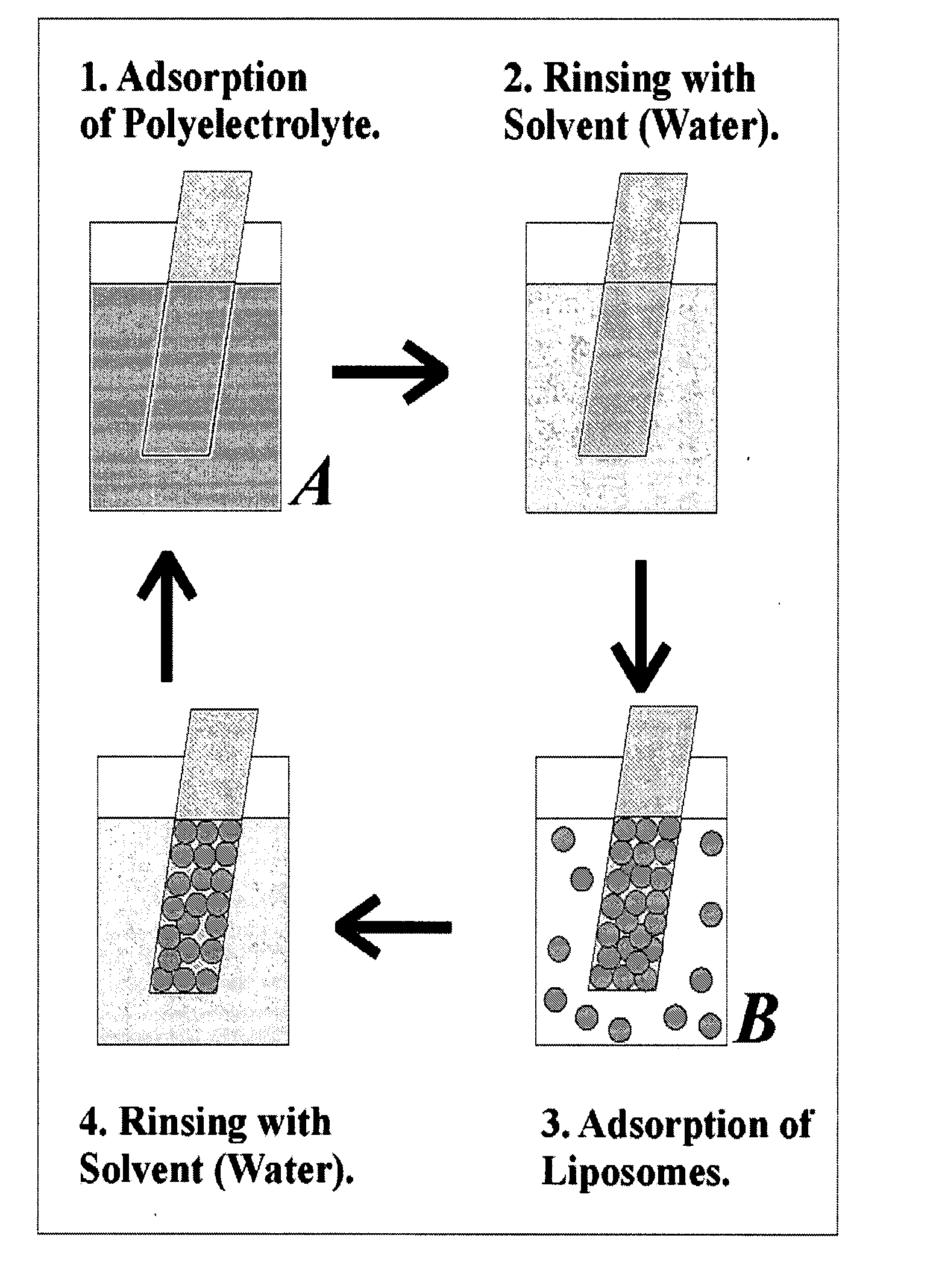
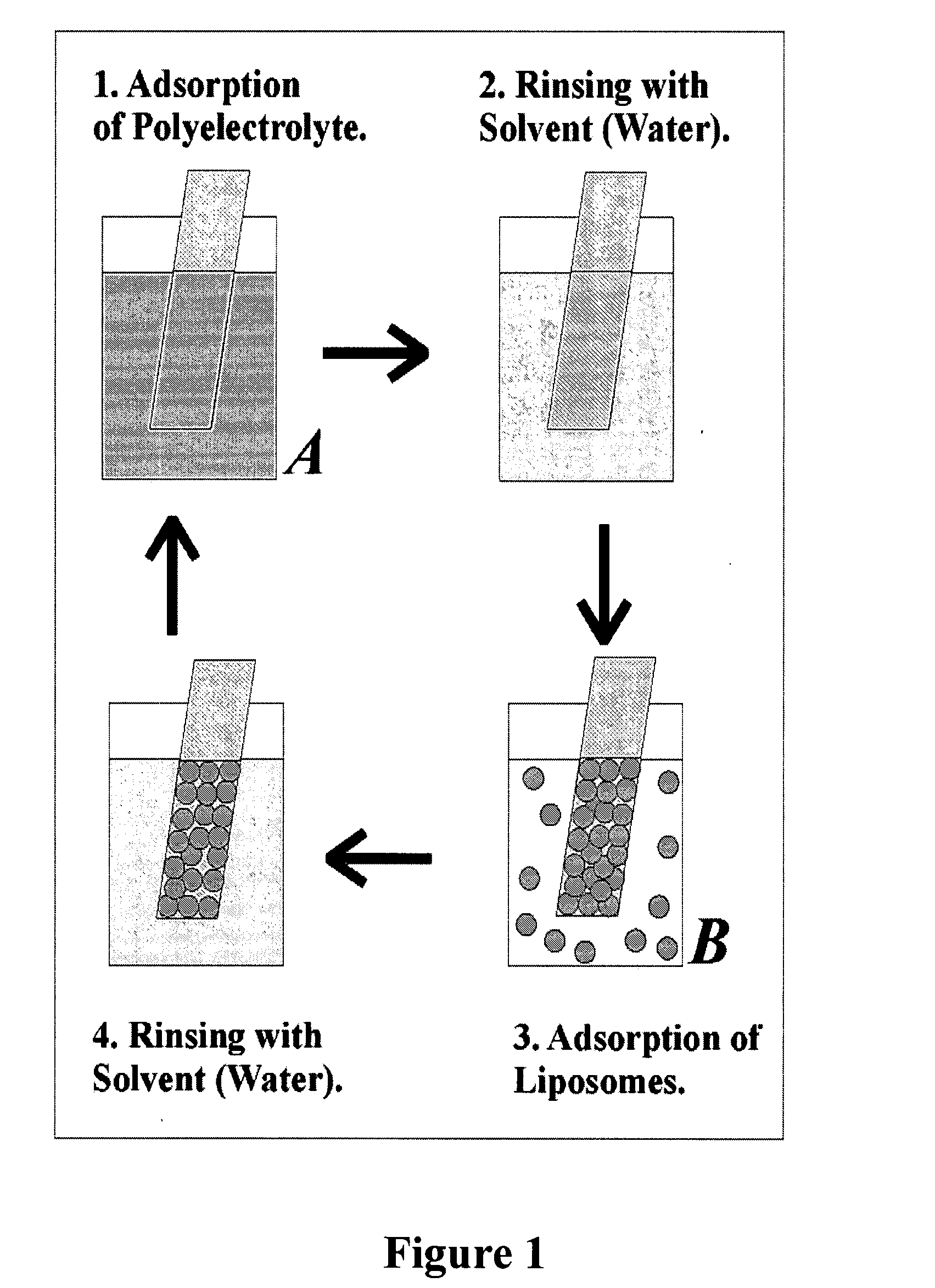
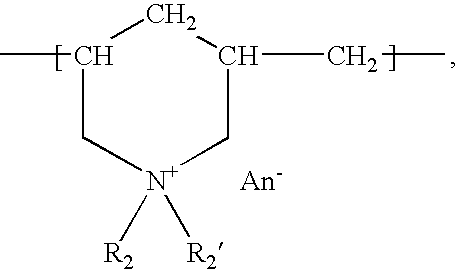
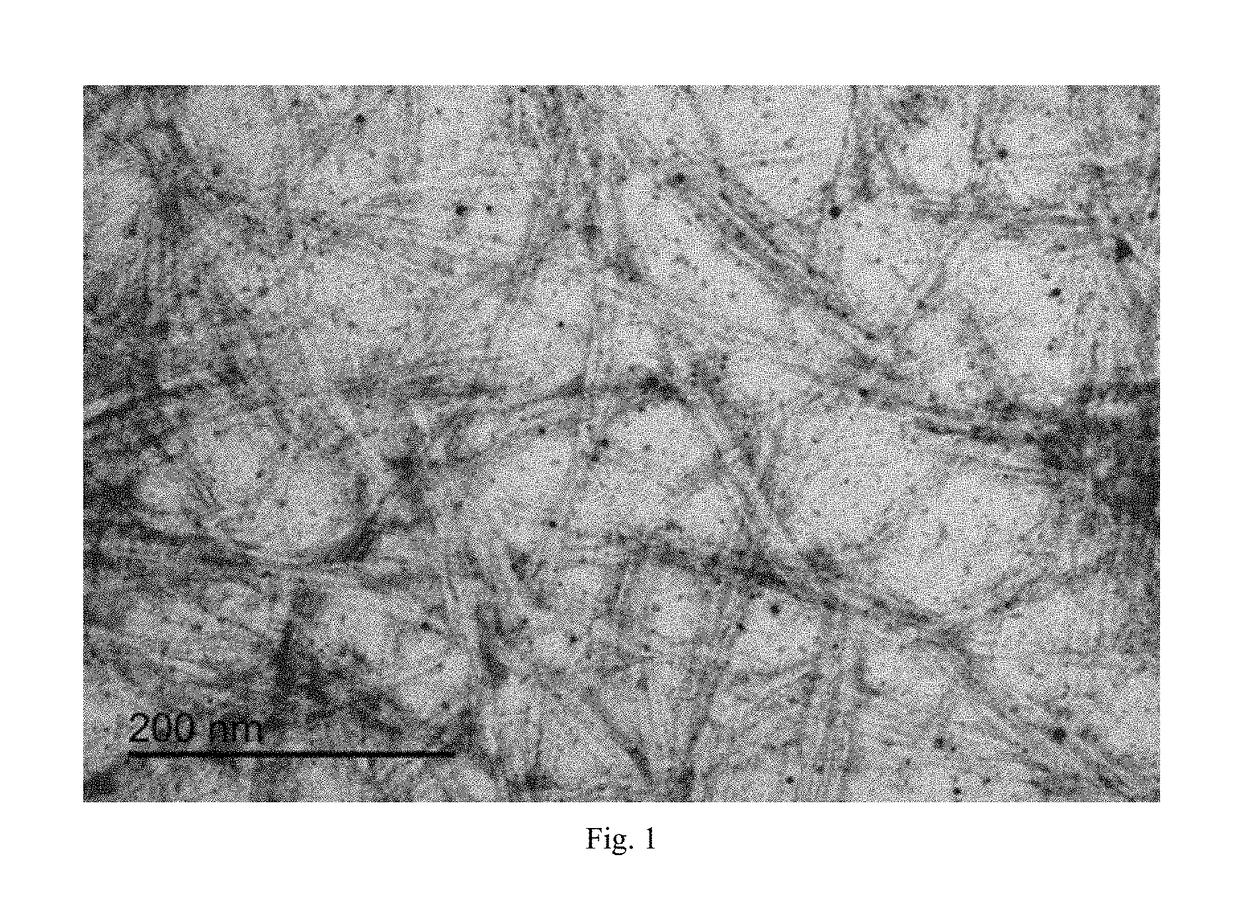
The present invention provides a composite material, preferably an ophthalmic device, more preferably a contact lens, which comprises a vesicle-containing coating including at least one layer of a vesicle and one layer of a polyionic material having charges opposite the charges of the vesicle. Such composite material can find use in biomedical applications, for example, a device for localized drug delivery and an in vivo analyte sensor such as glucose sensing contact lens. By lifting off the vesicle-containing coating from a substrate, a self-standing membrane (film) capable of encapsulating a wide variety of guest materials can be prepared. In addition, the invention provides methods for making vesicle-containing composite and film materials of the present invention.
Owner:ALCON INC
Micro/nano-fabricated glucose sensors using single-walled carbon nanotubes
InactiveUS20050124020A1Strong specificityEasy to detectMaterial nanotechnologyBioreactor/fermenter combinationsHydrogenGlucose sensors
A novel glucose sensor utilizing hydrogen-specific gas sensing capability of single walled carbon nanotubes assembled on microelectrodes. Highly specific glucose sensing was demonstrated using buffered sample solutions with clinically significant concentrations. The approach enables a simple but powerful bio-sensor reliably operating with a completely new principle, and opens up novel device applications where functional nano-components can be integrated into a bioMEMS device.
Owner:NORTHWESTERN UNIV
Implantable optical glucose sensing
InactiveUS20100160749A1CatheterDiagnostic recording/measuringOptical measurementsOptical communication
Apparatus is provided, including a support configured to be implanted within a body of a subject and a sampling region coupled to the support. The apparatus is configured to passively allow passage through the sampling region of at least a portion of fluid from the subject. The apparatus also comprises an optical measuring device in optical communication with the sampling region. The optical measuring device comprises at least one light source configured to transmit light through at least a portion of the fluid, and at least one sensor configured to measure a parameter of the fluid by detecting light passing through the fluid. Other embodiments are also described.
Owner:GLUSENSE LTD
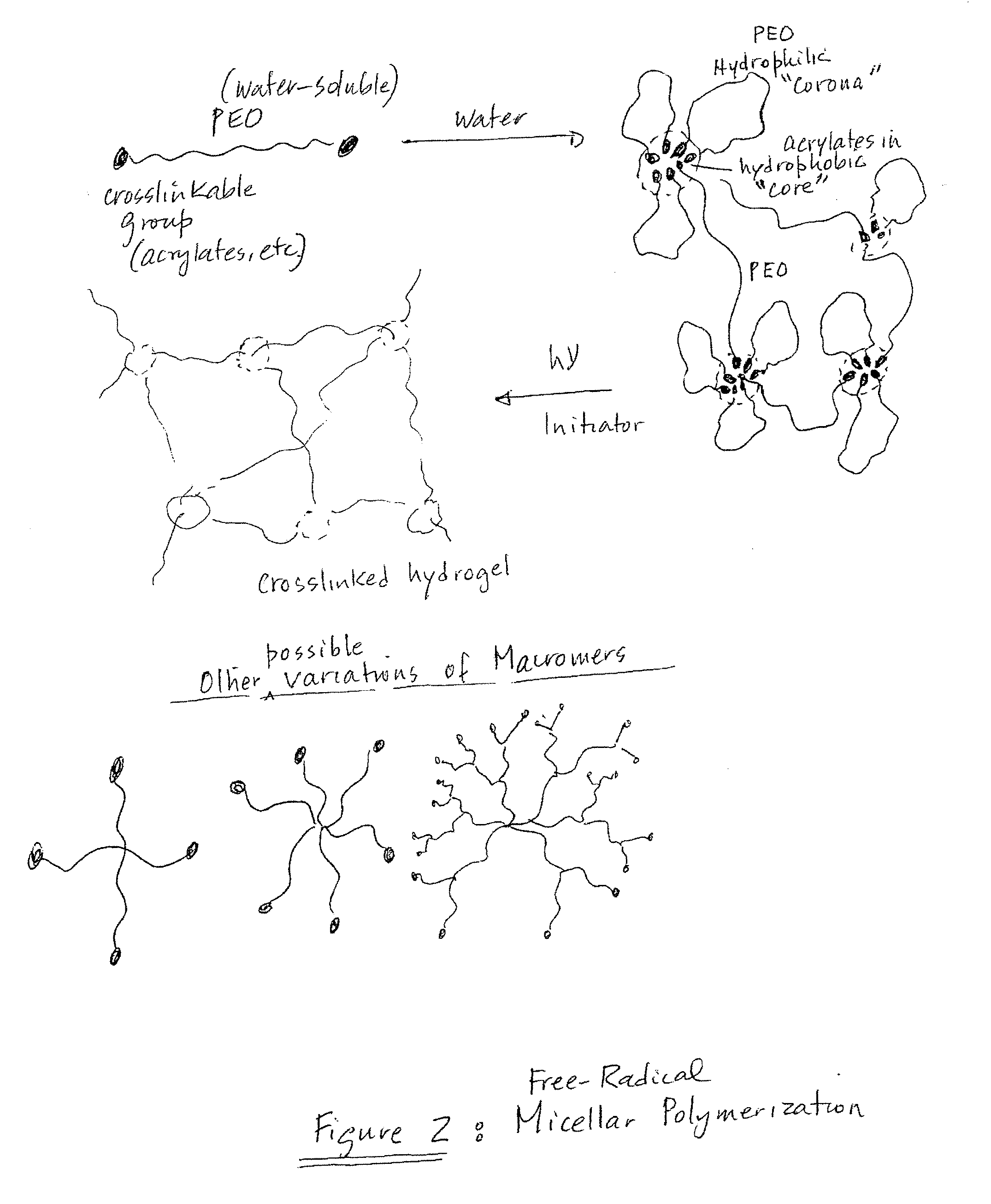
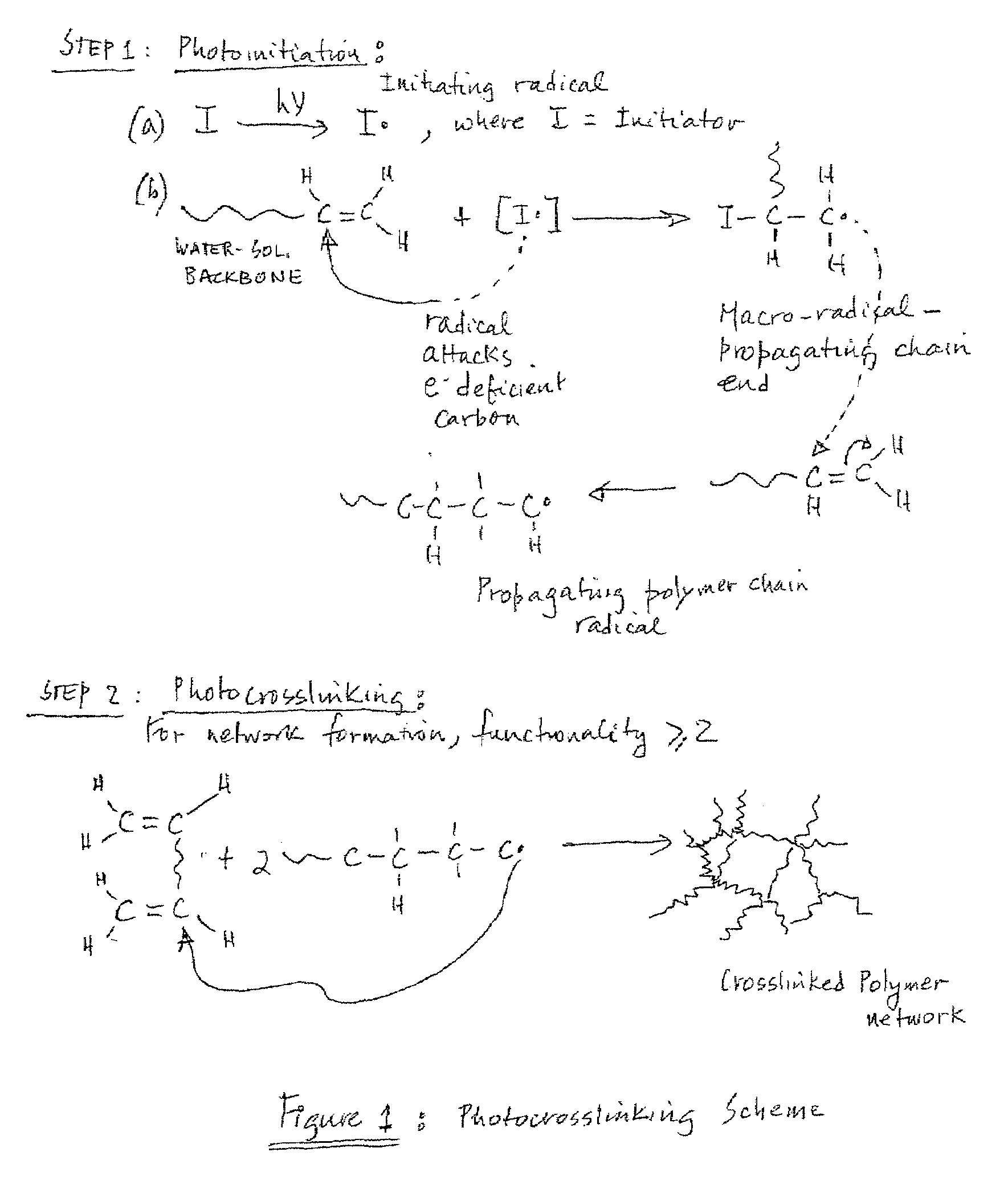
Biocompatible chemically crosslinked hydrogels for glucose sensing
InactiveUS20070128681A1Advanced technologyImprove the level ofCosmetic preparationsImpression capsActive componentSensing applications
An hydrogel incorporating a biologically active component is provided that is useful as an interface material for transdermal sensing applications. Specifically, the hydrogel has a crosslinked gel structure and a biologically active component incorporated into the crosslinked gel structure, the biologically active component being capable of converting glucose into a compound having potentiometric activity.
Owner:ECHO THERAPEUTICS INC
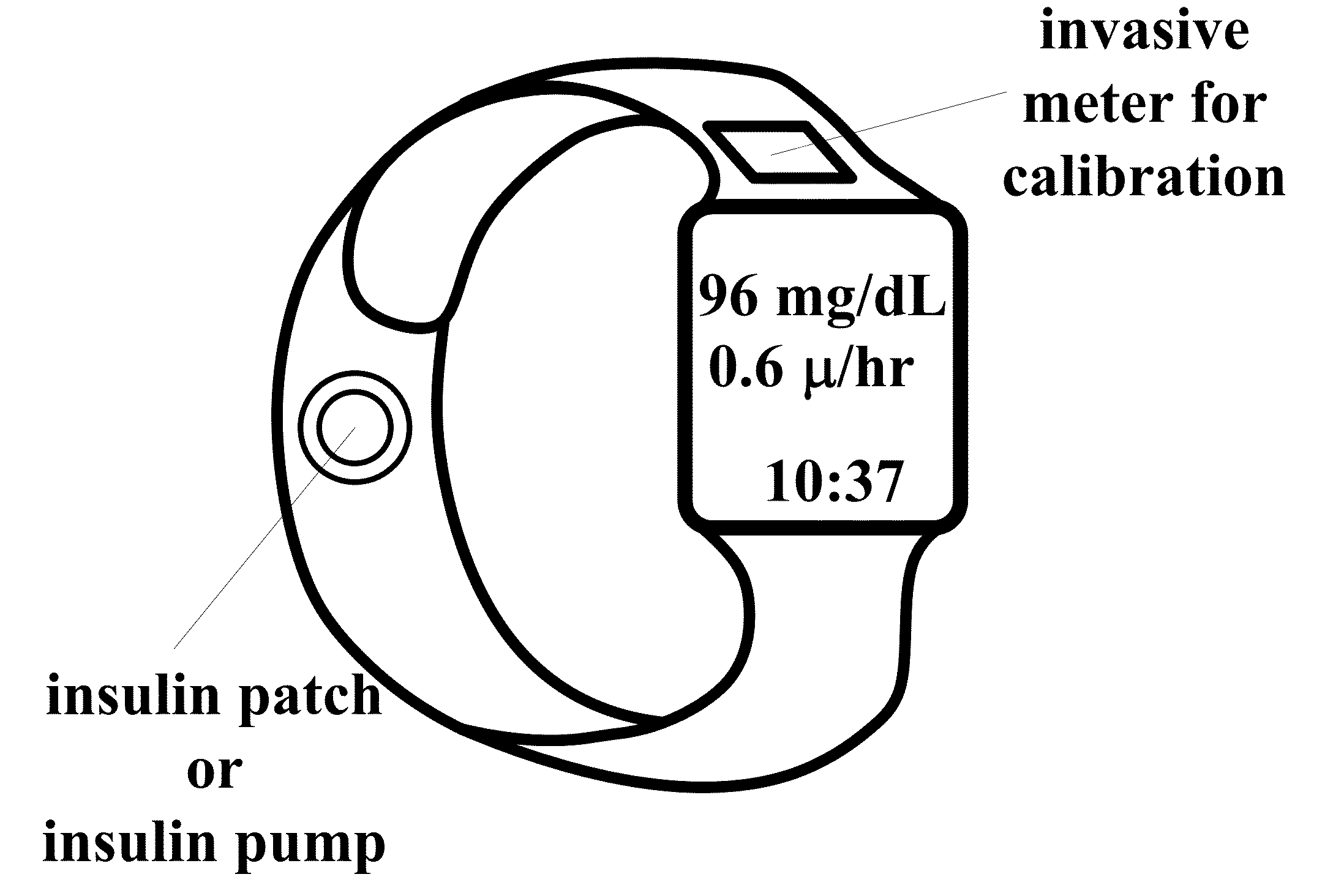
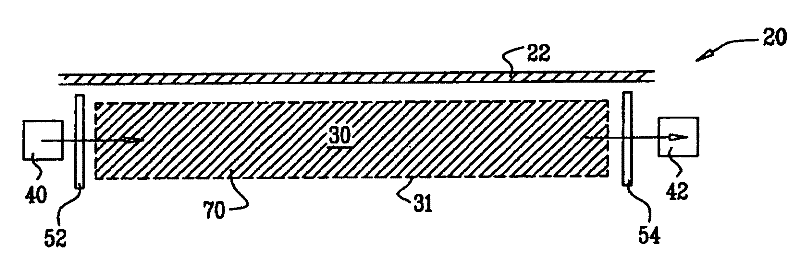
Wearable, noninvasive glucose sensing methods and systems
ActiveUS20160192867A1Improve ease and safetyReduce morbidityDiagnostics using lightHealth-index calculationGlucose polymersUltrasonic testing
New wearable systems for noninvasive glucose sensing include an ultrasound generator, an ultrasound detector and a feedback unit. Methods for noninvasive glucose sensing using a wearable device include measuring a thickness (geometrical and / or optical) of a target tissue or a time of flight of ultrasound or optical pulses in the target tissue and determining a glucose value from the thickness of the target tissue or the time of flight in the target tissue in accordance with a target tissue thickness (geometrical and / or optical) or time of flight versus glucose calibration curve.
Owner:ESENALIEV RINAT O
Glucose biosensor and method
ActiveUS7955484B2The result is accurateMinimize interference from dissolved oxygenImmobilised enzymesBioreactor/fermenter combinationsD-GlucoseGlucose polymers
A system for more accurately measuring glucose in a sample includes a first glucose-sensing electrode incorporating a quantity of glucose oxidase, a second glucose-sensing electrode incorporating a quantity of PQQ-glucose dehydrogenase, a reference electrode, and means for selecting between a first glucose measurement made with the first glucose-sensing electrode and a second glucose measurement made with the second glucose-sensing electrode.
Owner:NOVA BIOMEDICAL
Biocompatible chemically crosslinked hydrogels for glucose sensing
InactiveUS7432069B2Advanced technologyImprove the level ofCosmetic preparationsImpression capsActive componentSensing applications
An hydrogel incorporating a biologically active component is provided that is useful as an interface material for transdermal sensing applications. Specifically, the hydrogel has a crosslinked gel structure and a biologically active component incorporated into the crosslinked gel structure, the biologically active component being capable of converting glucose into a compound having potentiometric activity.
Owner:ECHO THERAPEUTICS INC
Noninvasive glucose sensing methods and systems
ActiveUS8135450B2Reduce complicationsReduce mortalityUltrasonic/sonic/infrasonic diagnosticsInfrasonic diagnosticsMedicineGlucose polymers
New methods and systems for noninvasive glucose monitoring and sensing with electromagnetic waves or ultrasound are disclosed. The methods are based on absolute or relative measurement of tissue dimensions (or changes in the dimensions) including, but not limited to: thickness, length, width, diameter, curvature, roughness as well as time of flight of ultrasound and optical pulses and optical thickness, which change with changing blood glucose concentrations. By measuring noninvasively absolute or relative changes in at least one dimension of at least one tissue or tissue layer or absolute or relative changes in time of flight of ultrasound or optical pulses, one can monitor blood glucose concentration noninvasively.
Owner:ESENALIEV RINAT O +1
Implantable optical glucose sensing
Owner:GLUSENSE LTD
Micro/nano-fabricated glucose sensors using single-walled carbon nanotubes
InactiveUS7118881B2Strong specificityEasy to detectMaterial nanotechnologyBioreactor/fermenter combinationsHydrogenGlucose sensors
A novel glucose sensor utilizing hydrogen-specific gas sensing capability of single walled carbon nanotubes assembled on microelectrodes. Highly specific glucose sensing was demonstrated using buffered sample solutions with clinically significant concentrations. The approach enables a simple but powerful bio-sensor reliably operating with a completely new principle, and opens up novel device applications where functional nano-components can be integrated into a bioMEMS device.
Owner:NORTHWESTERN UNIV
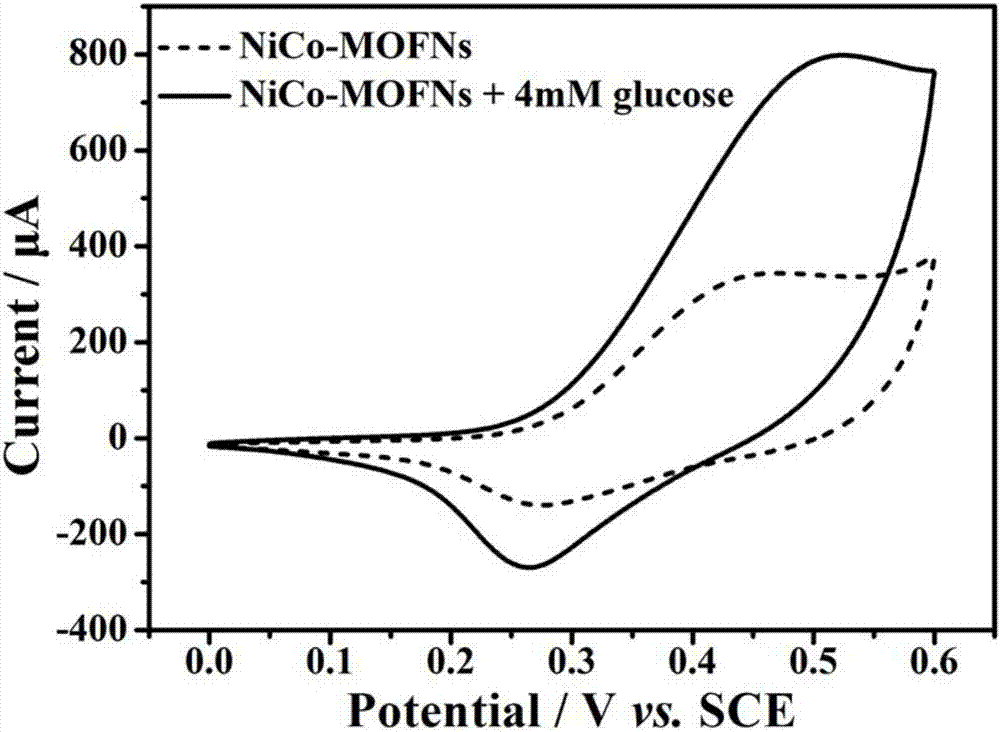
Two-dimensional nickel and cobalt MOFs (metal organic frameworks) nano-sheet and application of nano-sheet to glucose detection
ActiveCN107238650ALarge specific surface areaImprove Sensing PerformanceMaterial electrochemical variablesGlucose sensorsElectrochemistry
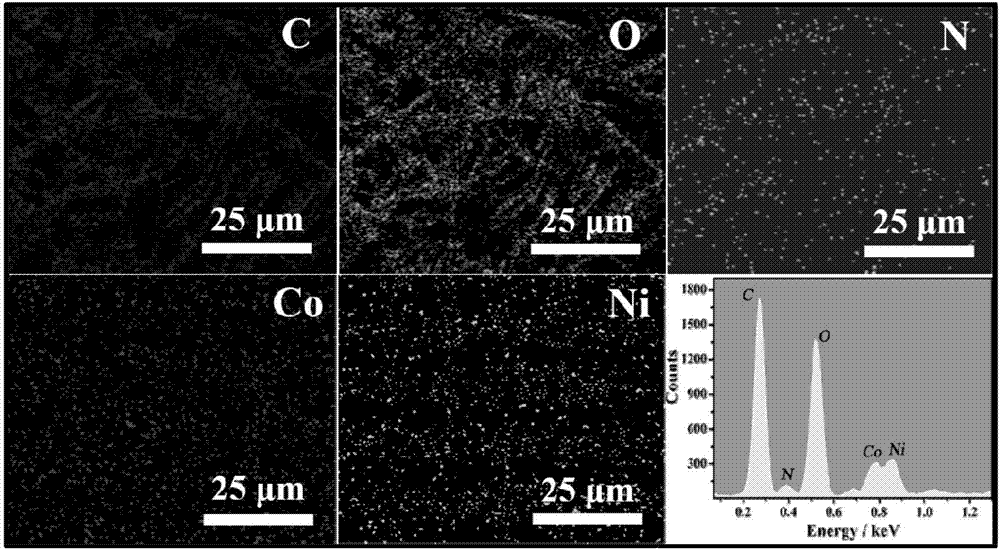
The invention discloses a two-dimensional nickel and cobalt MOFs (metal organic frameworks) nano-sheet and an application of the nano-sheet to glucose detection. The two-dimensional nickel and cobalt MOFs nano-sheet is synthesized on substrates such as nano-porous gold needles, carbon paper and gold sheets by a one-step hydrothermal method from bottom to top, serves as an electrode and is applied to a non-enzyme electrochemical glucose sensor. A two-dimensional nickel and cobalt MOFs nano-sheet material has the advantages that more active sites are exposed, nickel and cobalt are cooperated, charge transfer capacity is high and the like relative to a traditional spherical polyhedral MOFs material, so that glucose sensing properties of the nano-sheet material is effectively improved.
Owner:JILIN UNIV
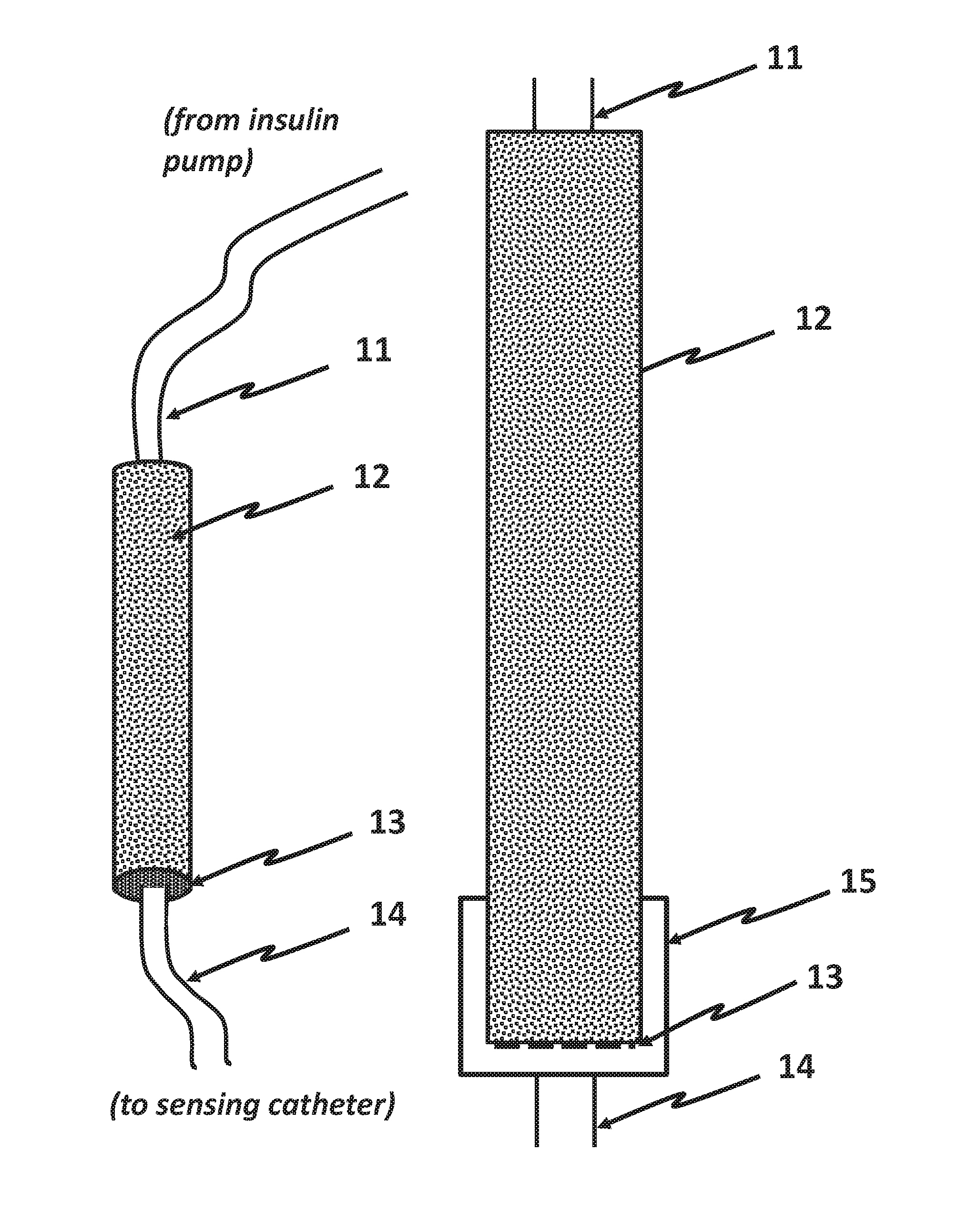
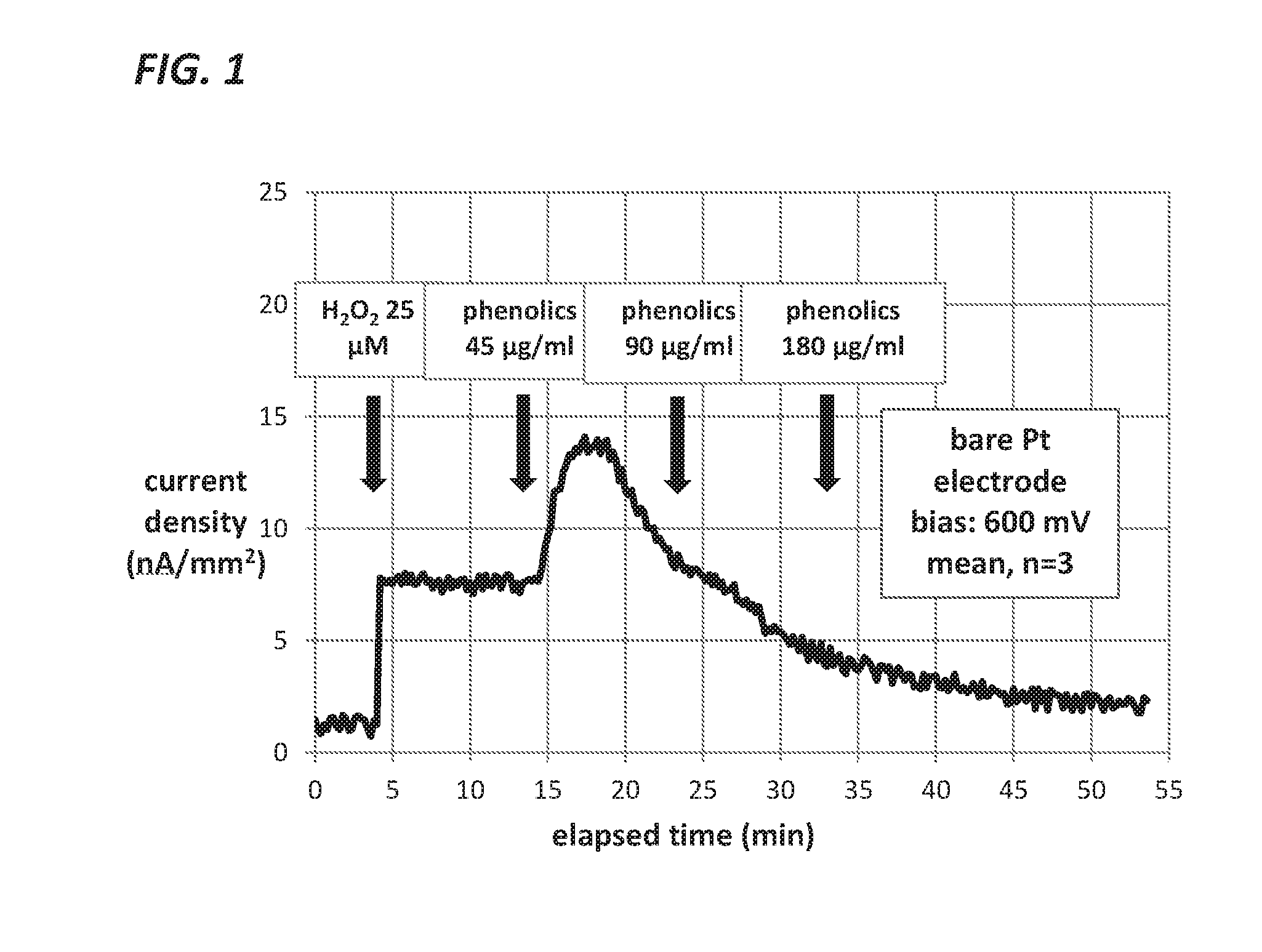
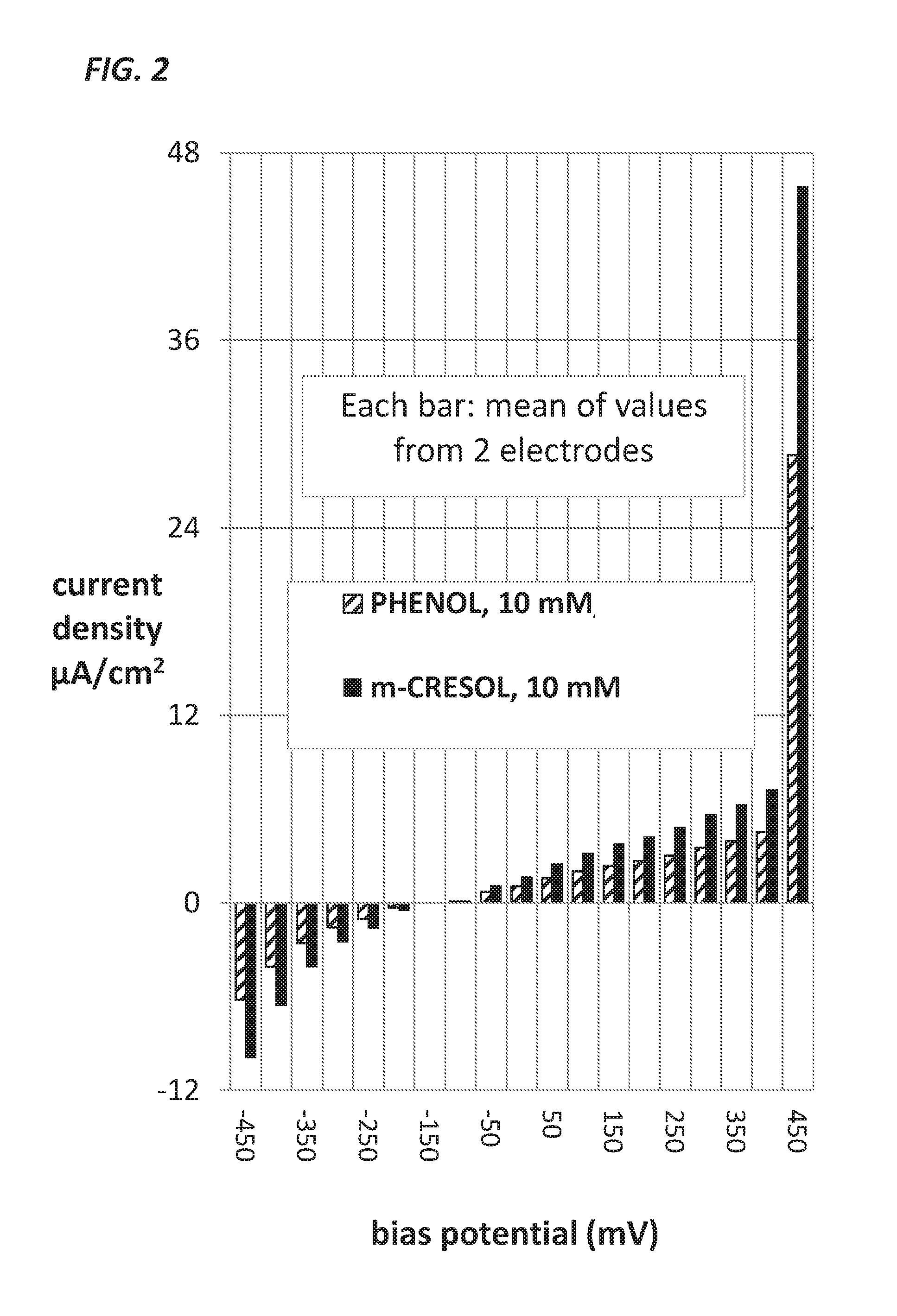
Measurement of Glucose in an Insulin Delivery Catheter by Minimizing the Adverse Effects of Insulin Preservatives
ActiveUS20160354542A1Avoid VulnerabilityLow costFiltering accessoriesMedical devicesFiltrationPharmaceutical formulation
This disclosure teaches the concept, and method of creating, a dual use device intended for persons who take insulin. In one embodiment, the novel device is an insulin delivery cannula, the outer wall of which contains electrodes, chemical compounds and electrical interconnects that allow continuous glucose sensing and delivery of data to a remote device. Heretofore, the main problem in attempting to sense glucose at the site of insulin delivery has been the high current resulting from oxidation by the sensor of the preservatives in the insulin formulations. One means of eliminating these interferences is to poise the indicating electrode(s) of the sensor at a bias sufficiently low to avoid the signal from oxidation of the preservatives. One way of obtaining a glucose signal at a low bias is to use an osmium-ligand-polymer complex instead of conventional hydrogen peroxide sensing. Another is to use a size exclusion filter located in line with the insulin delivery tubing in order to remove the smaller phenolic preservative molecules while allowing the larger insulin molecules to pass unimpeded. These filtration concepts can also be more broadly applied, that is, the general concept of removal of unwanted drug formulation excipients from a drug delivery system.
Owner:PACIFIC DIABETES TECH INC
Materials containing multiple layers of vesicles
Owner:ALCON INC
Metal Nanoparticles/Nanocellulose Composites-Based Non-Enzymatic Electrochemical glucose sensor and Preparation Method Thereof
ActiveUS20170276640A1Good biocompatibilityLow priceMaterial electrochemical variablesGlucose sensorsMaterials science
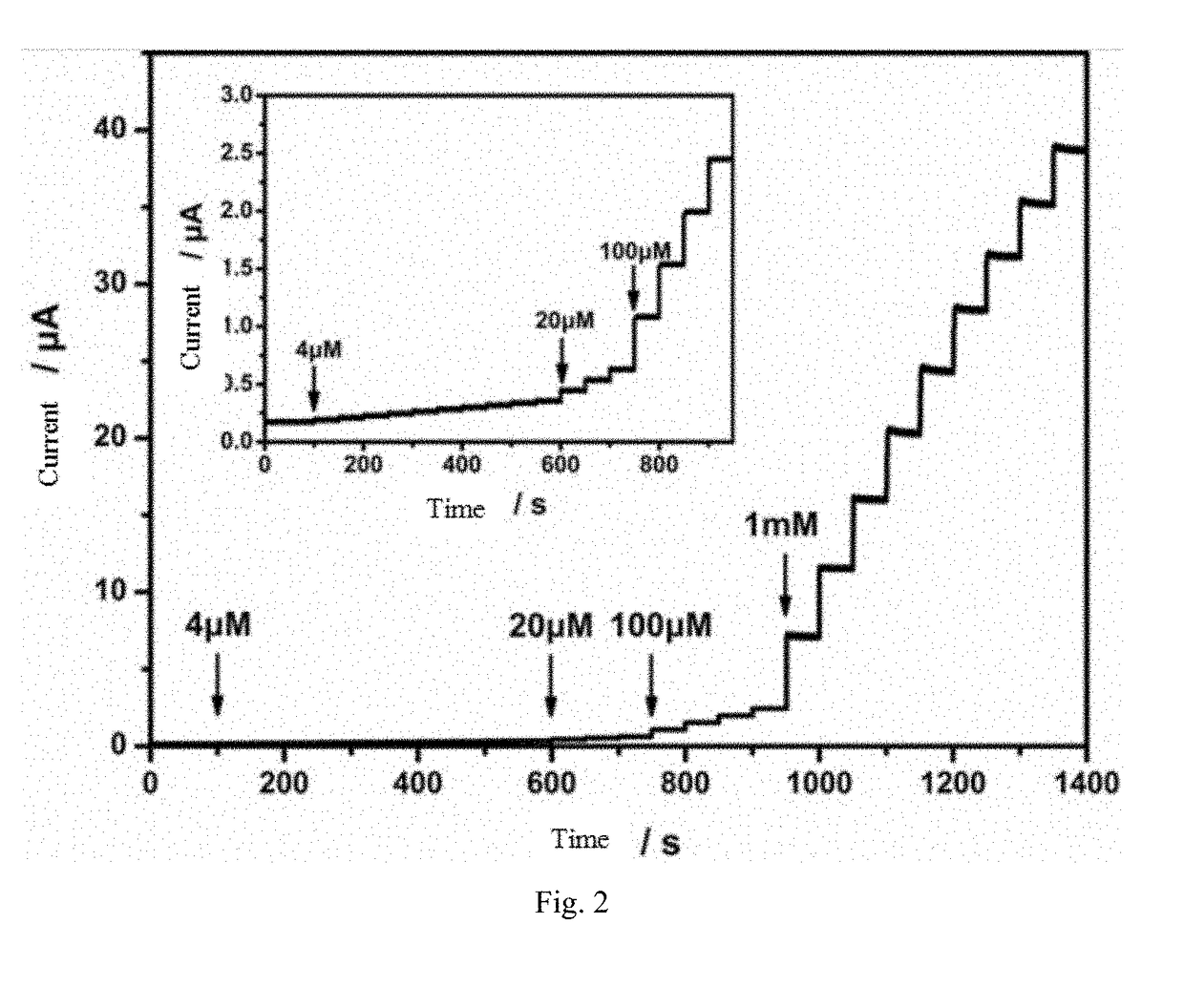
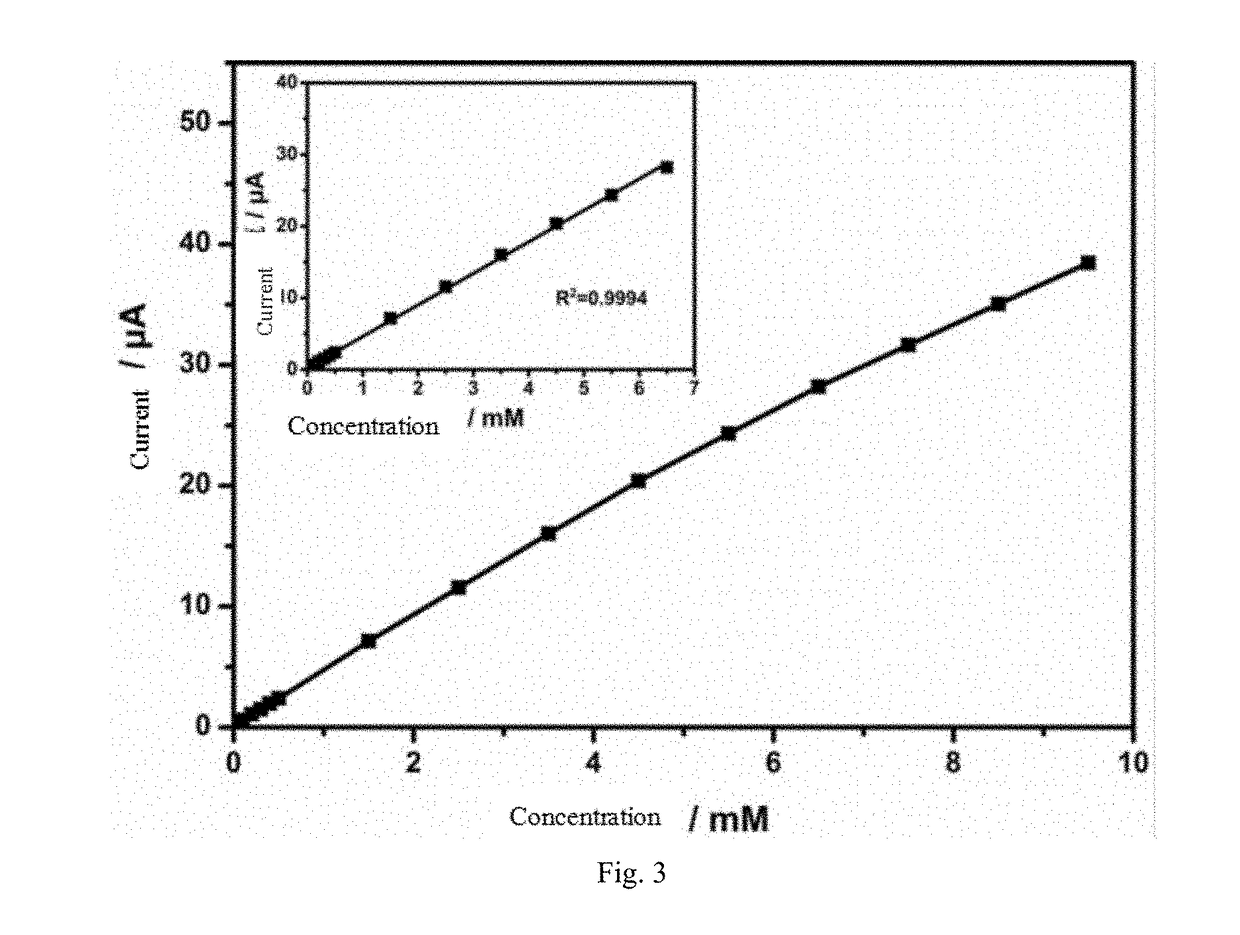
The invention discloses a metal nanoparticles / nanocellulose composites-based non-enzymatic electrochemical glucose sensor, comprising a three-electrode system composed of a working electrode, a counter electrode, and a reference electrode. The surface of the working electrode is coated with a metal nanoparticles / nanocellulose composites. The surface of the nanocellulose is modified with a strong cationic conducting polymer. The invention promotes the sensitivity and selectivity of glucose sensing with the linear range of 4 μM-15 mM and the detection limit of 1.4 μM. Therefore, the sensor possesses high sensitivity, high response speed, stable performance and high anti-interference ability. The preparation method of the metal nanoparticles / nanocellulose composites-based non-enzymatic electrochemical glucose sensor is simple and the cost is low. And enzyme is introduced into the preparation process.
Owner:ENERGY RES INST CO LTD HENAN ACADEMY OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com