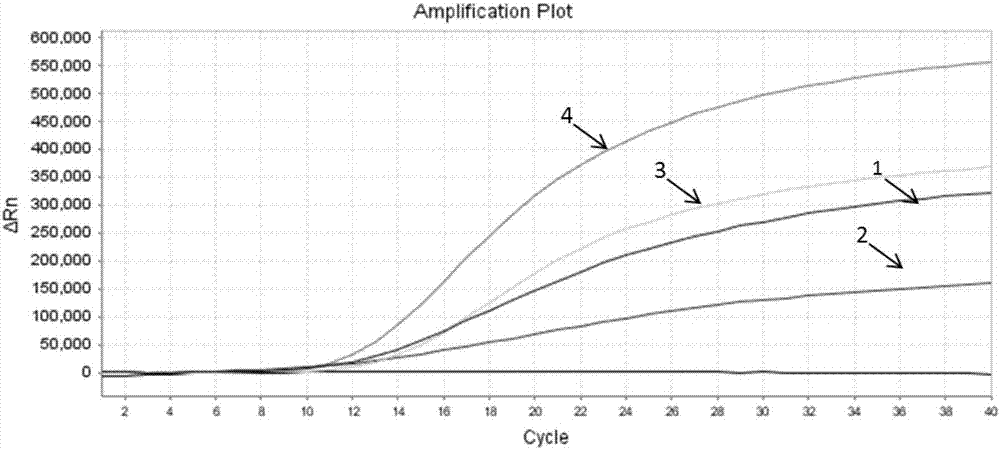
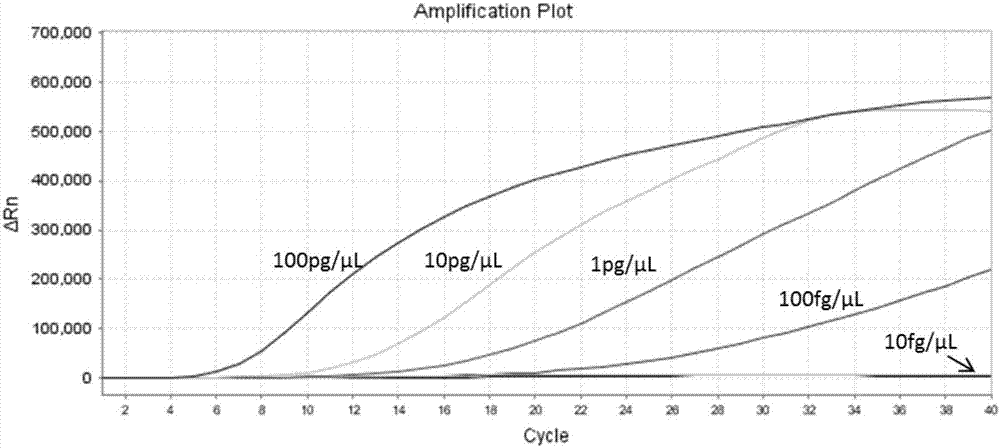
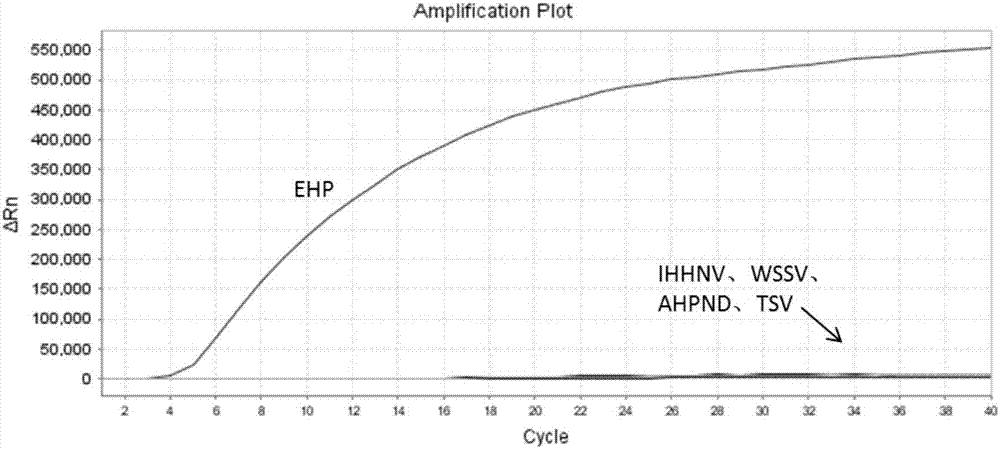
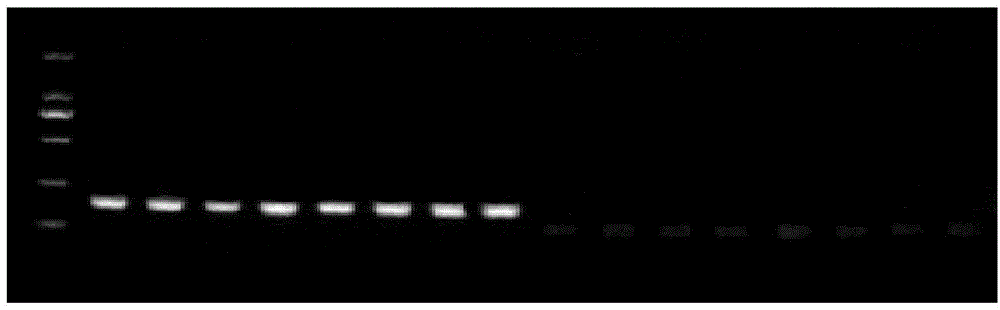
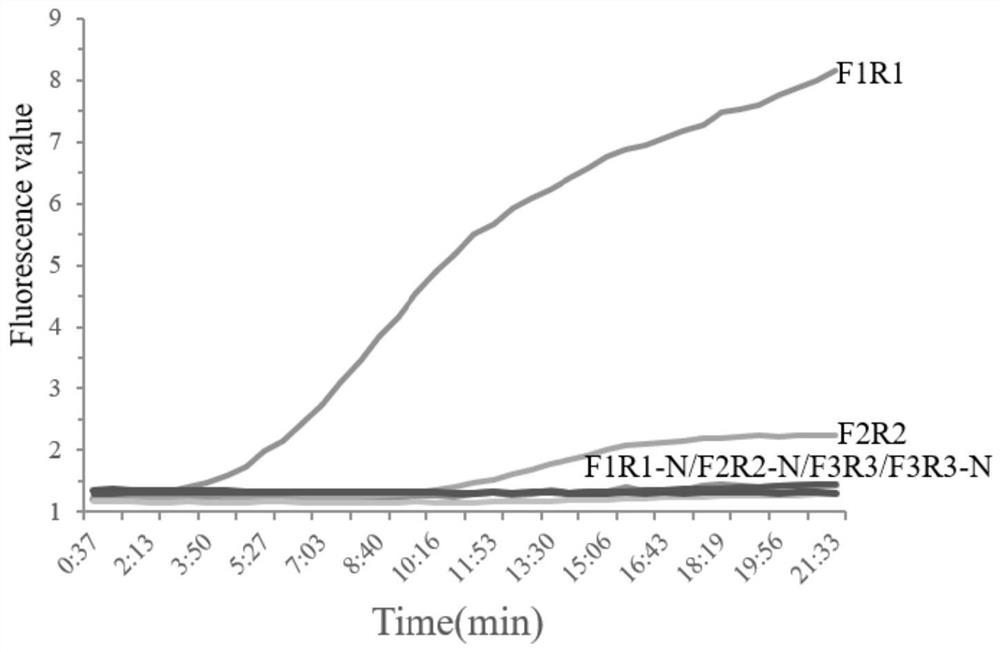
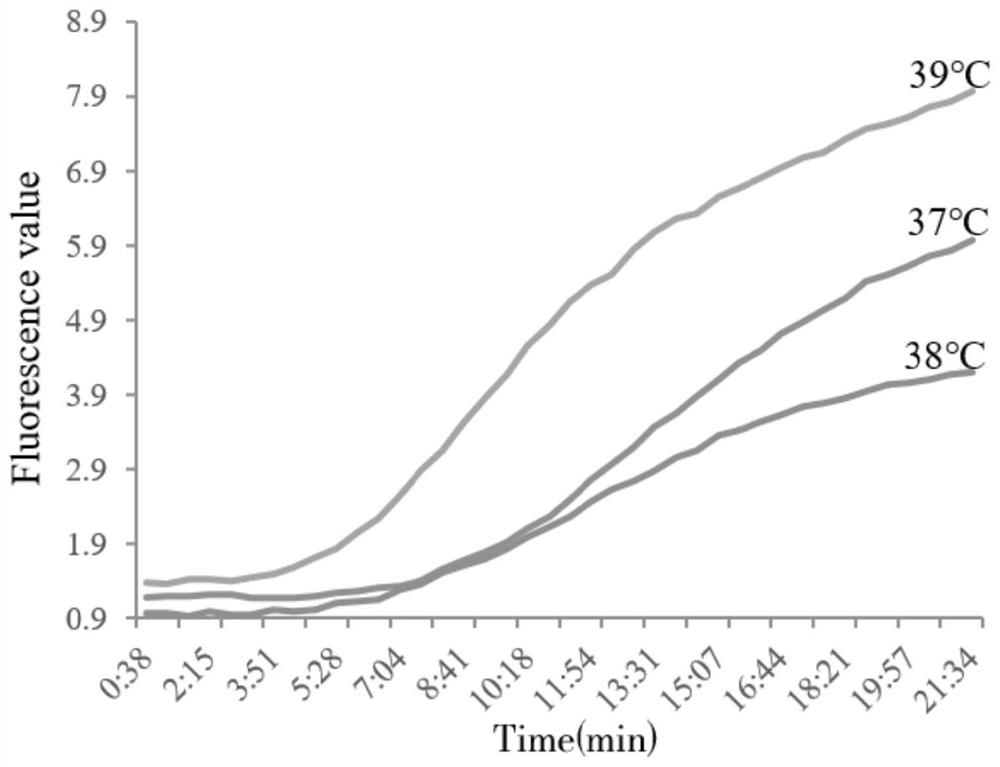
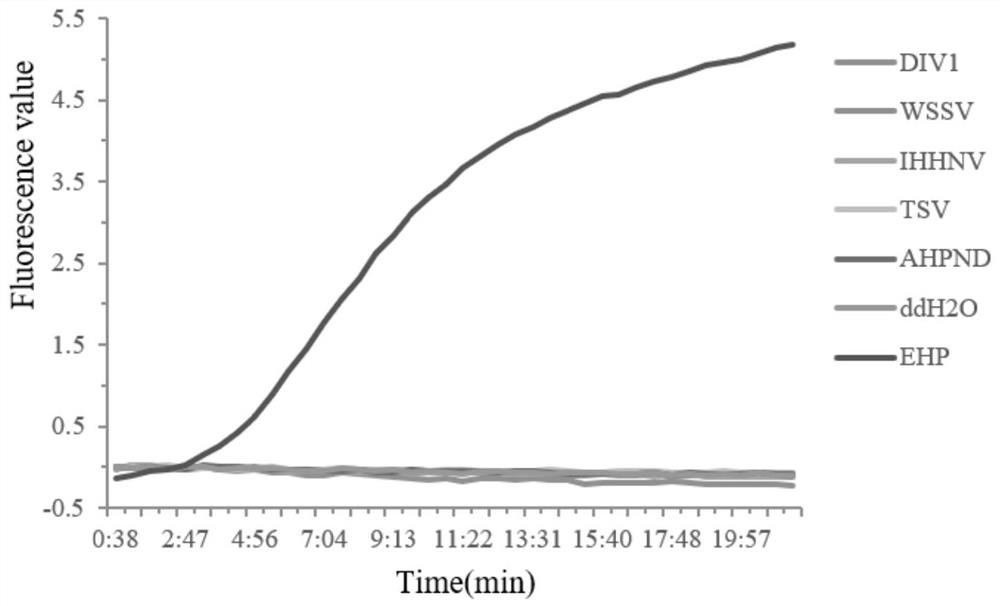
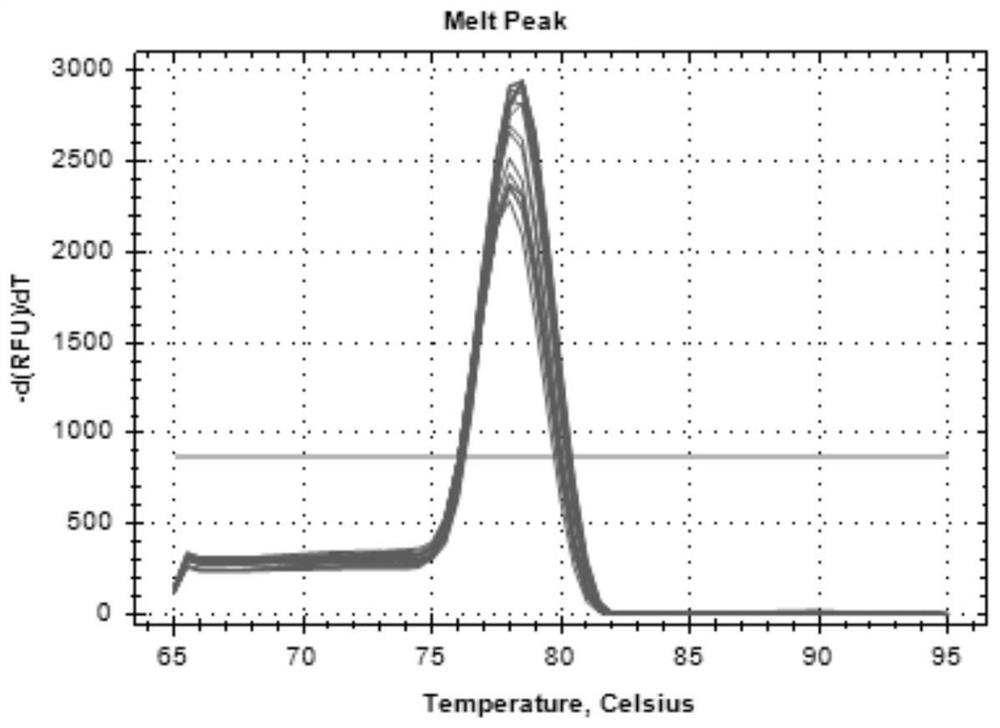
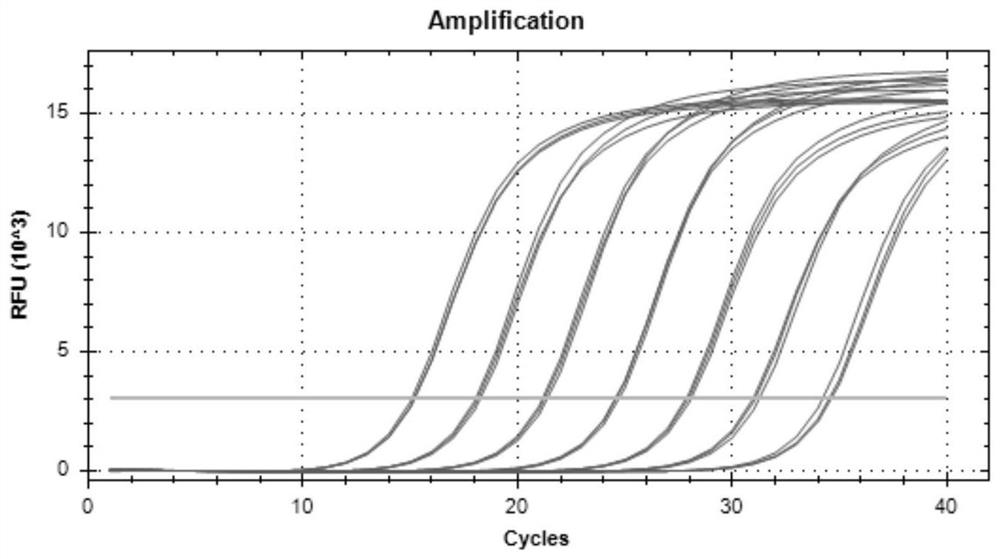
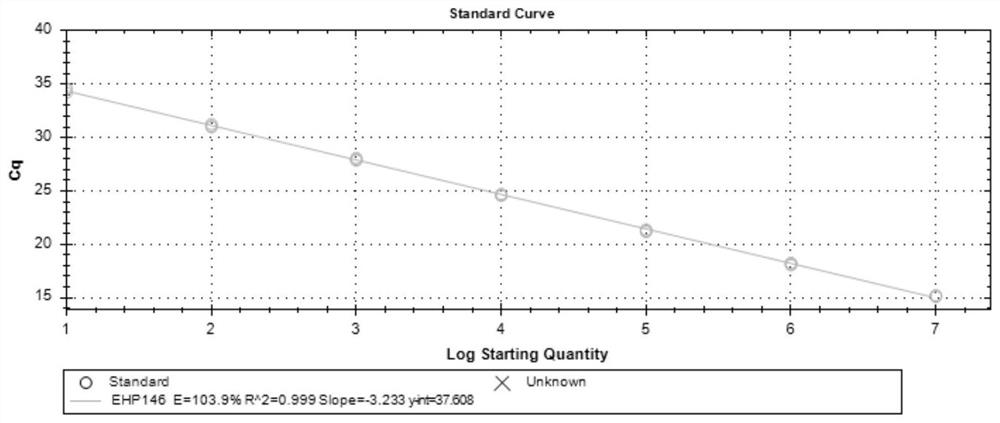
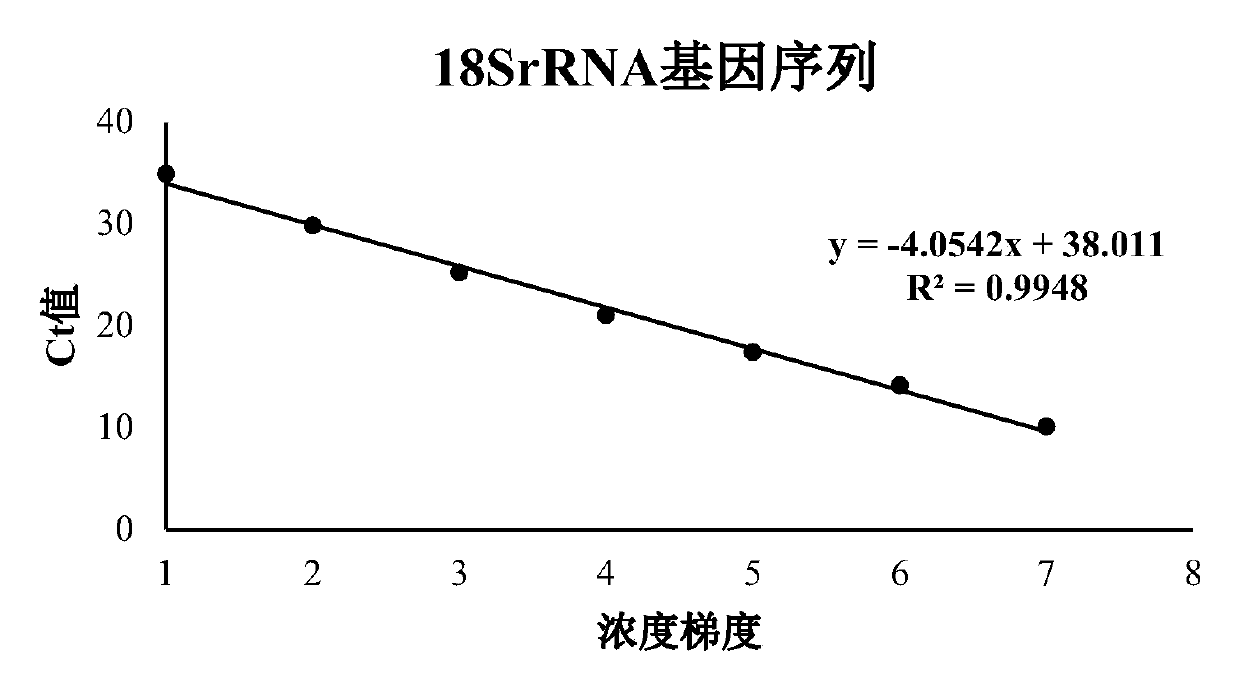
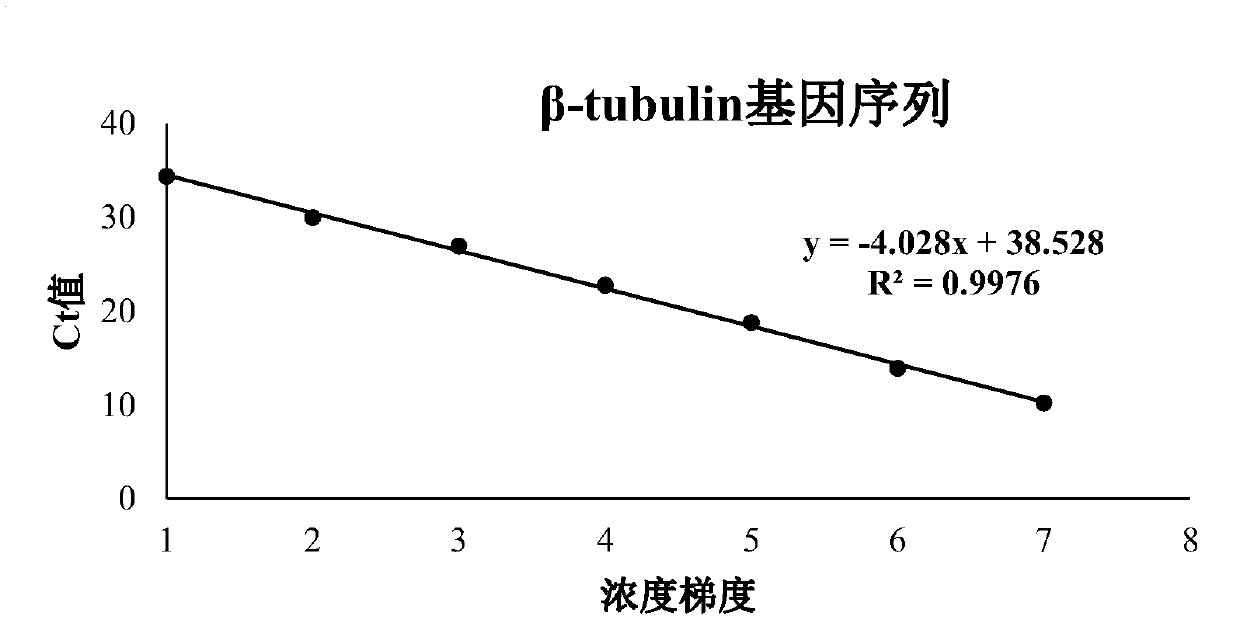
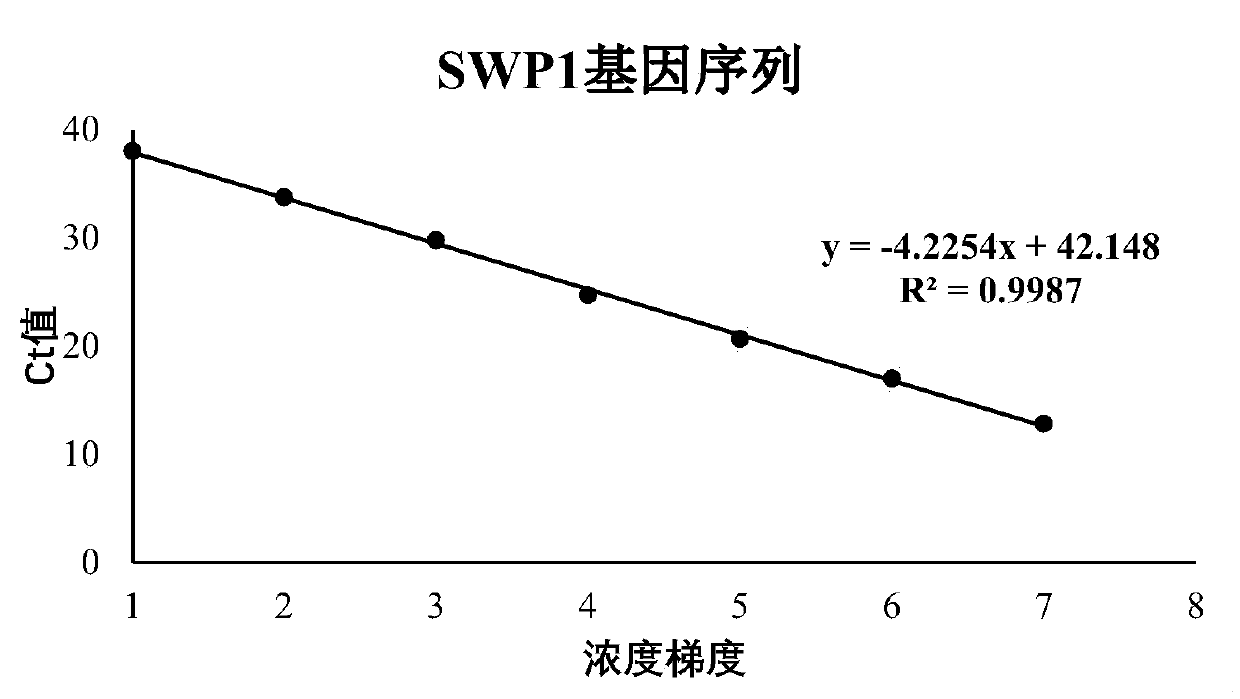
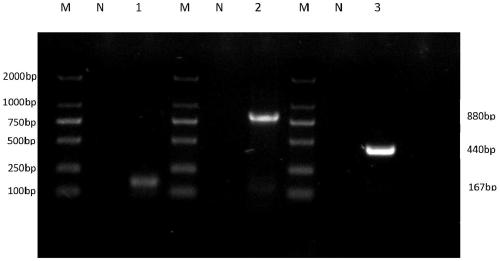
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
36 results about "Enterocytozoon hepatopenaei" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
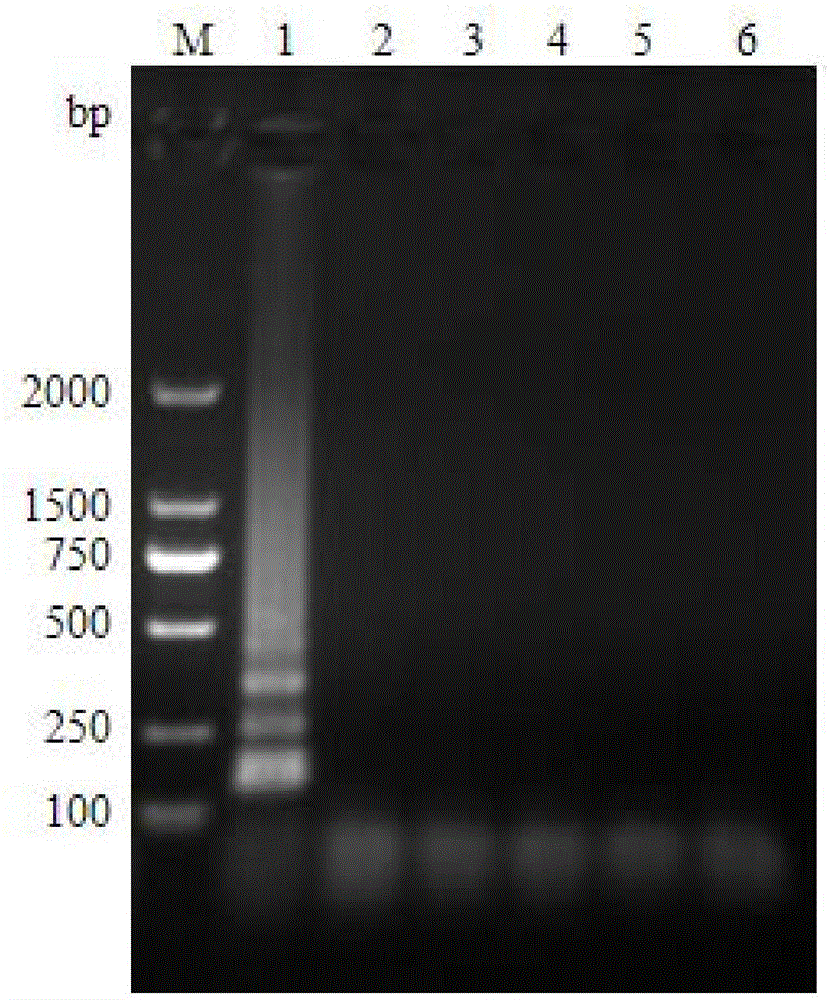
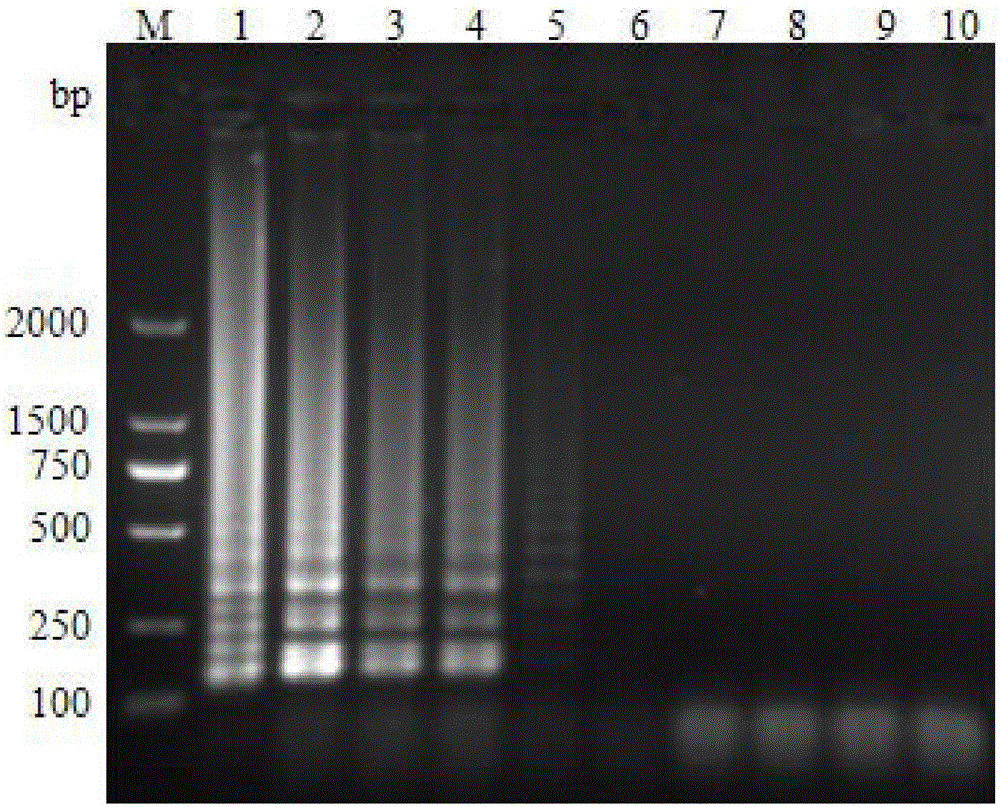
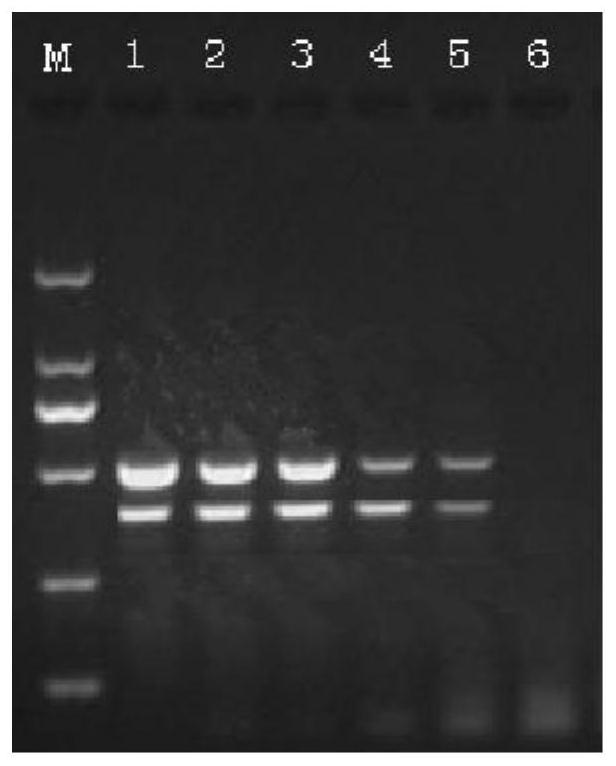
Multiple-PCR detection method and kit for simultaneously detecting WSSV (white spot syndrome virus), AHPND (acute hepatopancreatic necrosis disease), EHP (enterocytozoon hepatopenaei) and IHHNV (infectious hypodermal and hepatopancreatic necrosis virus) in prawns
ActiveCN106636471AGood repeatabilityImprove stabilityMicrobiological testing/measurementDNA/RNA fragmentationPrawnWhite spot syndrome
The invention belongs to the technical field of marine organism pathogen detection, and in particular relates to a multiple-PCR detection method and a kit for simultaneously detecting WSSV (white spot syndrome virus), AHPND (acute hepatopancreatic necrosis disease), EHP (enterocytozoon hepatopenaei) and IHHNV (infectious hypodermal and hepatopancreatic necrosis virus) in prawns. With the application of the multiple-PCR detection method, amplification of four pathogen DNAs can be simultaneously conducted in one time by virtue of special detection primers, namely WSSV F and WSSV R, AHPND F and AHPND R, EHP F and EHP R as well as IHHNV F and IHHNV R of the WSSV, the AHPND, the EHP and the IHHNV. The multiple-PCR detection method provided by the invention is convenient and rapid, low in detection limit, strong in specificity, high in sensitivity and high in accuracy rate and is capable of simultaneously detecting the white spot syndrome virus, the acute hepatopancreatic necrosis disease, the enterocytozoon hepatopenaei and the infectious hypodermal and hepatopancreatic necrosis virus in the prawns.
Owner:MARINE BIOLOGY INST OF SHANDONG PROVINCE
Fluorescence detection kit for detecting prawn enterocytozoon hepatopenaei (EHP) in China
InactiveCN107488730AHigh sensitivityHigh amplification rateMicrobiological testing/measurementDNA/RNA fragmentationPositive sampleDisease
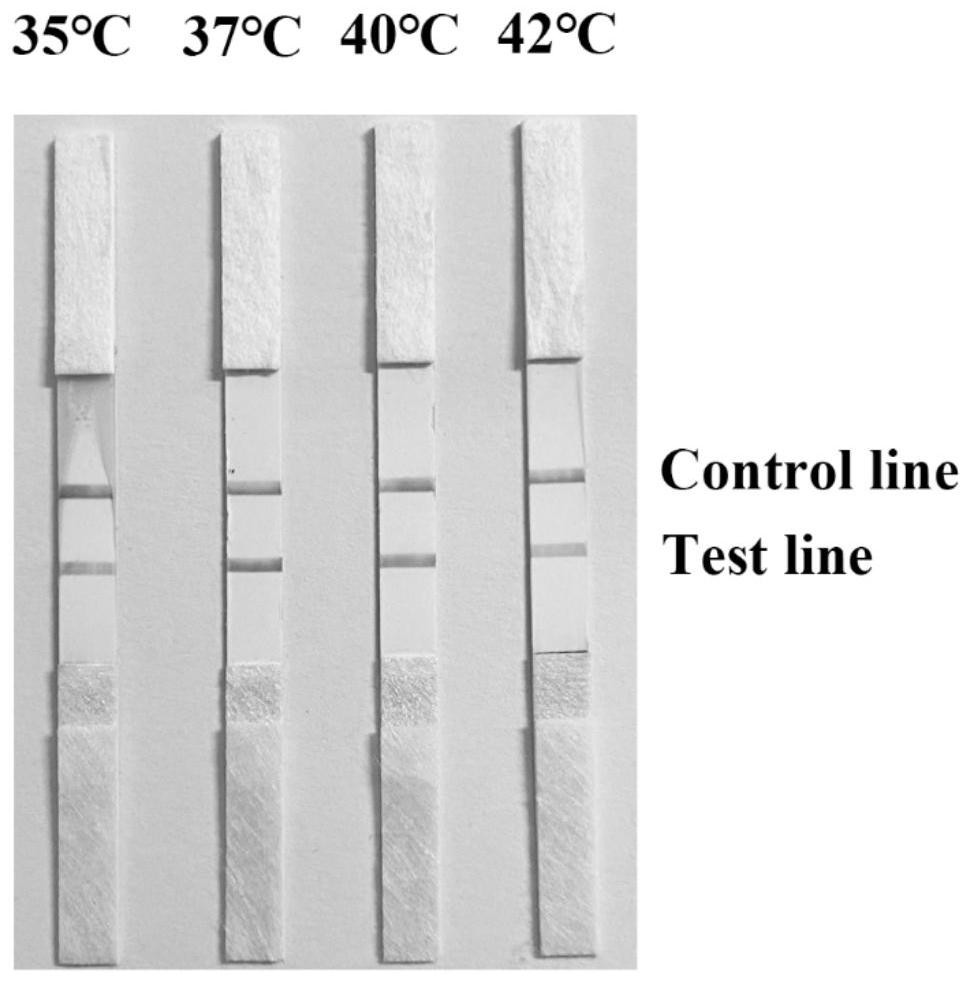
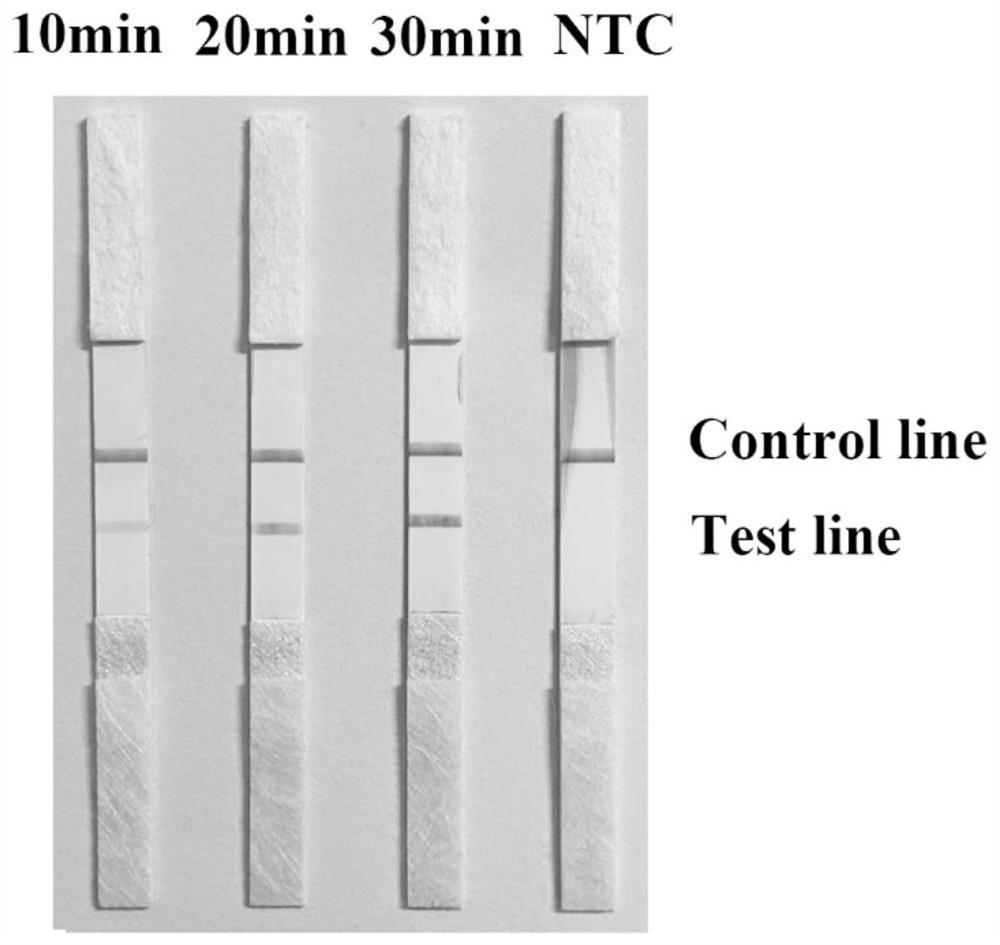
The invention discloses a fluorescence detection kit for detecting prawn enterocytozoon hepatopenaei (EHP) in China and relates to detection of prawn diseases. The fluorescence detection kit is provided with a tissue lysis solution, a constant-temperature fluorescence process reaction solution, a positive reference substance and a negative reference substance. Application of the fluorescence detection kit comprises the following steps: extraction of prawn tissue DNA; constant-temperature fluorescence amplification of prawn EHP; result judgment of the constant-temperature fluorescence detection of prawn EHP. According to the fluorescence detection kit performing EHP detection through the constant-temperature fluorescence process, a positive sample can show a high-linearity amplification curve after being amplified by a constant-temperature fluorescence amplification apparatus, and also has a higher amplification rate; the result of a sensitivity experiment indicates that the fluorescence detection kit has higher sensitivity and can be applied to a quantitative experiment. A constant-temperature fluorescence primer with strong specificity, high sensitivity and high amplification efficiency is designed and developed for the genome DNA of prawn EHP; compared with a common LAMP primer, a loop primer is added, the amplification efficiency is higher, and the detection is faster.
Owner:INSPECTION & QUARANTINE TECH CENT OF XIAMEN ENTRY EXIT INSPECTION & QUARANTINE BUREAU +1
RAA (recombinase-aid amplification) constant-temperature fluorescence detection method and reagent for EHP (enterocytozoon hepatopenaei)
ActiveCN107988325AEasy to operateMild conditionsMicrobiological testing/measurementForward primerPolymerase L
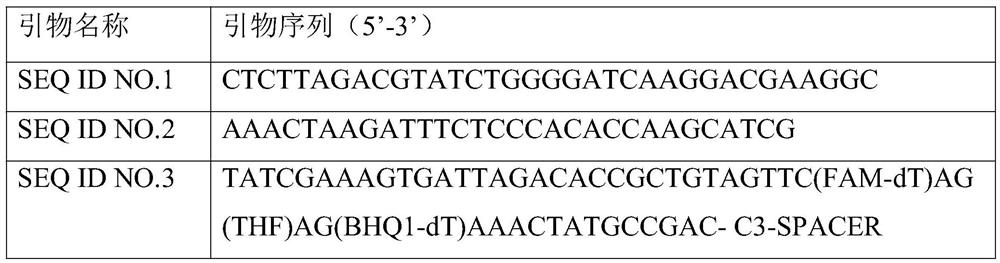
The invention dislcoses an RAA (recombinase-aid amplification) constant-temperature fluorescence detection method and a detection kit for EHP (enterocytozoon hepatopenaei). The detection kit comprisesa forward primer SEQ ID NO. 1, a reverse primer SEQ ID NO. 2, a specific fluorescence probe SEQ ID NO. 3, reaction liquid, recombinant polymerase and a reference substance. The kit has the advantagesthat the specificity is strong; the detection sensitivity is high, and can reach 100fg / mu L; high accuracy and reliability are achieved; the operation is simple and rapid, and the kit is suitable forfield detection and has wide application scenarios.
Owner:HANGZHOU ZHONGCE BIO SCI&TECH CO LTD
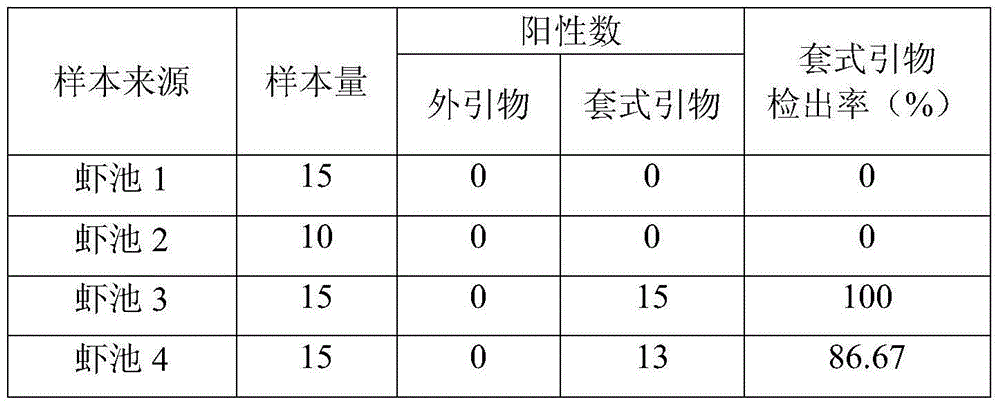
Nested primer used for early warning of enterocytozoon hepatopenaei of Chinese cultured shrimps and application of nested primer
ActiveCN104928288AShort ampliconShort fragmentMicrobiological testing/measurementDNA/RNA fragmentationShrimpTissue dose
The invention relates to a nested primer used for early warning of enterocytozoon hepatopenaei infection of Chinese cultured shrimps and an application of the nested primer. The nested primer comprises a pair of outer primers EMISF / EMISR and a pair of inner primers EMISnF / EMISnR, wherein the specific sequences are as follows: EMISF: GCGGTAATTCCAACTCCAAG; EMISR: TAAGCAGCACAATCCACTCC; EMISnF: AGTAGCGGAACGGATAGGGA; EMISnR: ACCCAGCATTGTCGGCATAG. The nested primer is very high in detection flexibility and good in specificity, suitable for monitoring enterocytozoon hepatopenaei in young shrimps with extremely low content and very small tissue dose, suitable for early monitoring and early warning of trace infection or latent infection of the enterocytozoon hepatopenaei of the Chinese cultured shrimps, and has good application prospect.
Owner:EAST CHINA SEA FISHERIES RES INST CHINESE ACAD OF FISHERY SCI
High-sensitivity target gene for enterocytozoon hepatopenaei disease, primer pair, kit and detection method
ActiveCN109486976AStrong specificityMicrobiological testing/measurementMicroorganism based processesDiseasePrawn
The invention belongs to the technical fields of agriculture and animal quarantine and particularly relates to a high-sensitivity target gene for enterocytozoon hepatopenaei disease, a primer pair, akit and a detection method. The sequence of the high-sensitivity target gene is represented by SEQ ID NO:1. The invention further provides the primer pair for detecting the target gene represented bySEQ ID NO:1. The invention further provides a high-sensitivity detection method for the enterocytozoon hepatopenaei disease. The detection method comprises the following steps: (1) extracting total DNA from a to-be-detected prawn; (2) carrying out PCR amplification through the primer pair by taking DNA as a template; and (3) detecting a PCR product obtained in the step (2) by virtue of 1.5% agarose gel electrophoresis, and if a target strip is generated, determining that the prawn is infected by enterocytozoon hepatopenaei. Primers are designed based on a sporoderm protein gene (SWP) of EHP, have strong specificity, have a definite corresponding relation with pathogen EHP and are suitable for being taken as detection primers for detecting enterocytozoon hepatopenaei by virtue of a PCR method.
Owner:ZHEJIANG ACADEMY OF AGRICULTURE SCIENCES
LAMP detection kit for Enterocytozoon hepatopenaei
InactiveCN106591474AStrong specificityHigh sensitivityMicrobiological testing/measurementAgainst vector-borne diseasesDisease monitoringBetaine
The invention discloses an LAMP detection kit for Enterocytozoon hepatopenaei. The LAMP detection kit comprises a tissue lysate and an LAMP amplification reaction solution, wherein the LAMP amplification reaction solution comprises 1.4 mM LAMP inner primer, 0.2 mM LAMP outer primer, 20 mM Tris-HC, 10 mM potassium chloride, 15 mM ammonium sulfate, 8 mM magnesium sulfate, 0.1% Triton X-100, 0.6 M betaine, 1.4 mM dNTP, 2.5 U / [mu]L Bst DNA polymerase large fragment, 1.25 mM calcein, 25 mM MnCl2, and the balance of water. The detection kit of the present invention has advantages of strong specificity, high sensitivity and quick and easy operation, can overcome the incapable application problem of the existing detection method in the breeding disease monitoring, and is suitable for the screening and the prevention of Enterocytozoon hepatopenaei.
Owner:ZHEJIANG INST OF FRESH WATER FISHERIES
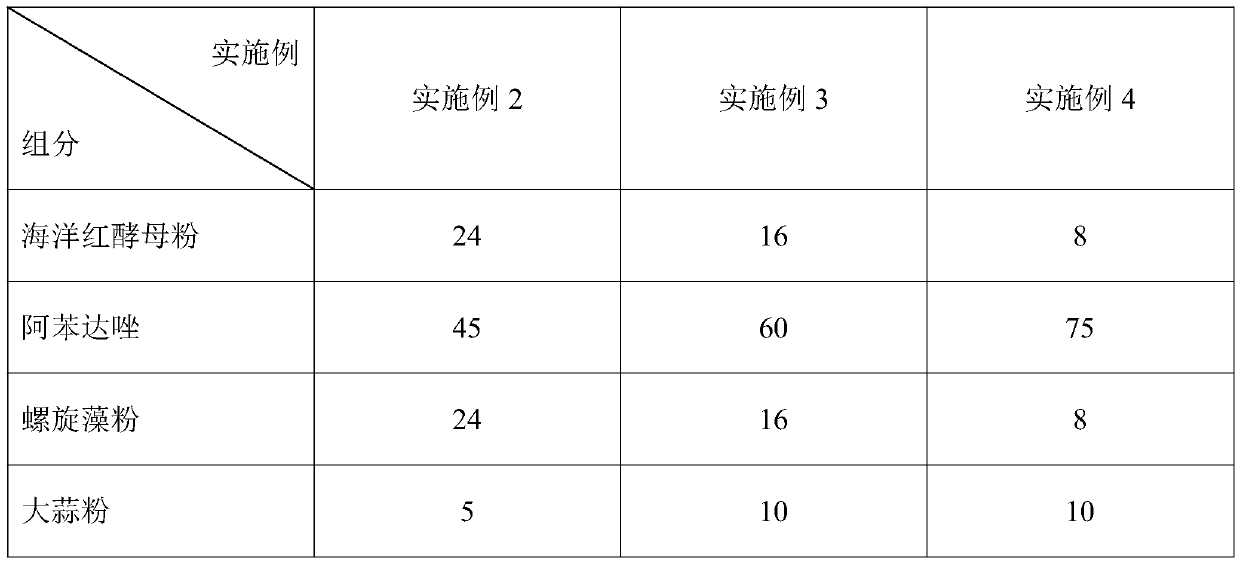
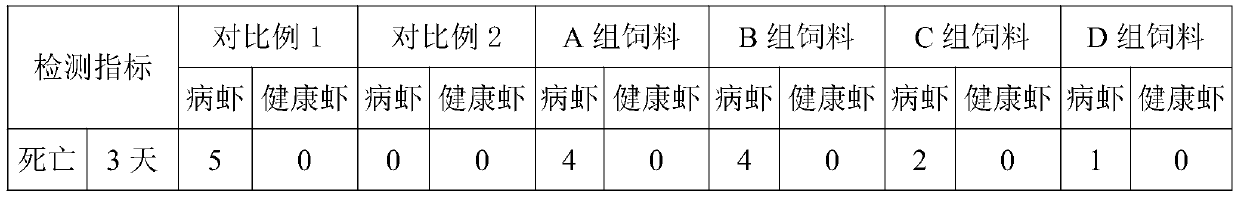
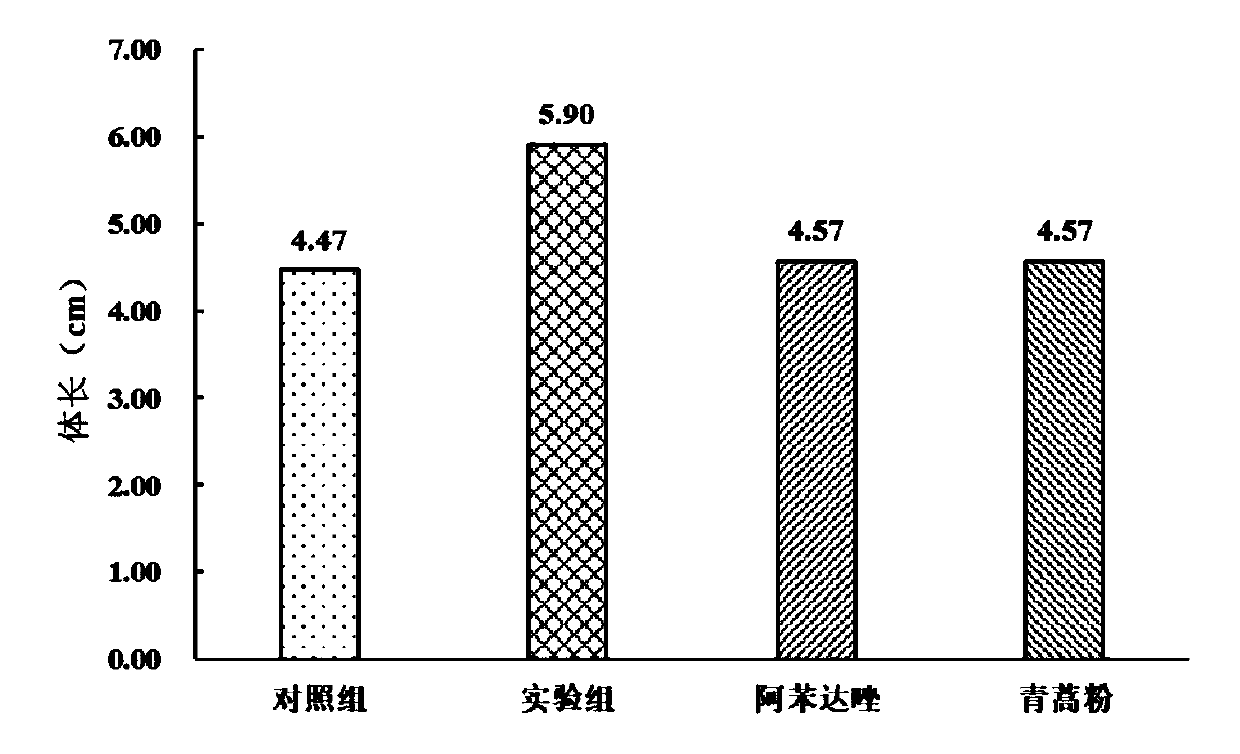
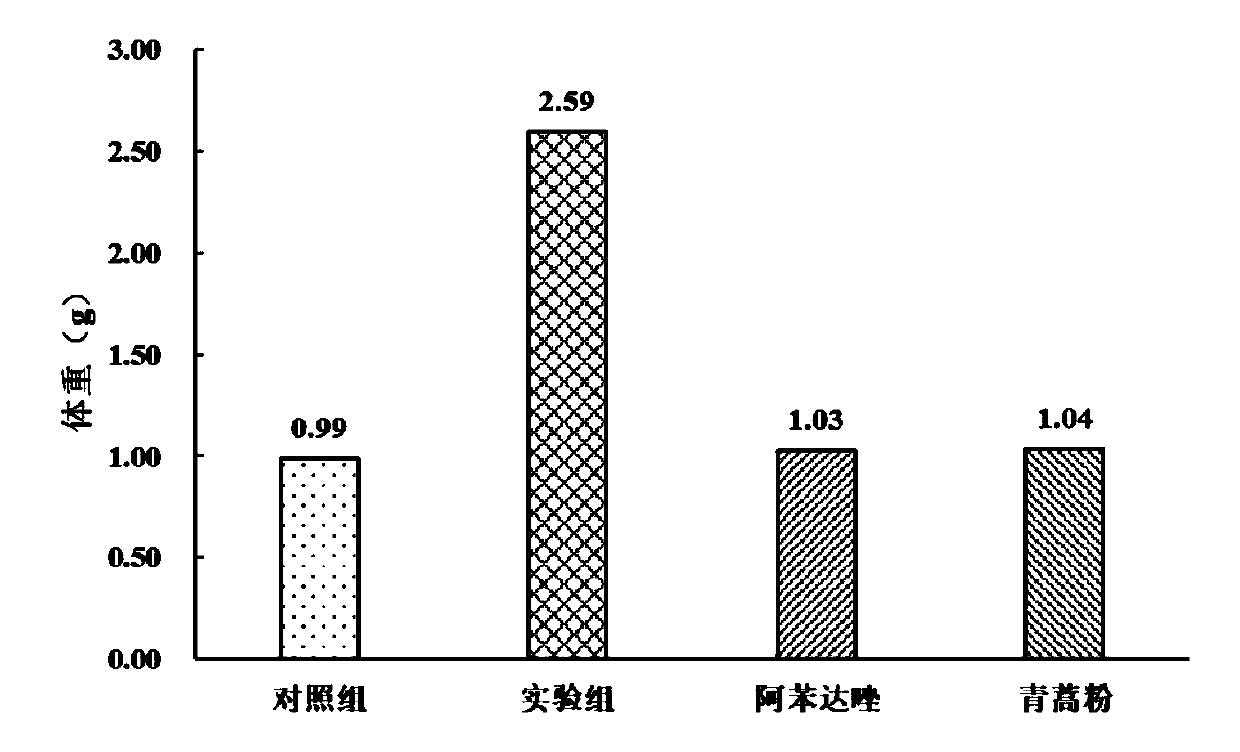
Medicinal bait additive for preventing and treating enterocytozoon hepatopenaei of litopenaeus vannamei
InactiveCN110178973AGuaranteed healthy growthHigh in proteinOrganic active ingredientsClimate change adaptationPrawnGARLIC POWDER
The present invention discloses a medicinal bait additive for preventing and treating enterocytozoon hepatopenaei of litopenaeus vannamei. The medicinal bait additive comprises the following components in parts by weight: 8-32 parts of marine red yeast powder, 30-75 parts of albendazole, 8-32 parts of spirulina powder and 5-10 parts of garlic powder. The medicinal bait additive is used in a compound feed for the litopenaeus vannamei and has an addition amount of only 6-12%; and the medicinal bait additive can also be mixed with the feed at a weight ratio of 1:10 for feeding before feeding. Themedicinal bait additive uses an internal administration route, so that a medicine internal environment is formed in a host body, the medicinal bait additive is subjected to blood circulation to reachtarget organs and parasites cannot survive. The medicinal bait additive can effectively kill the enterocytozoon hepatopenaei in the litopenaeus vannamei body, is non-toxic to the litopenaeus vannameibody, does not cause medicine residues in the litopenaeus vannamei body, at the same time also does not cause pollution to the breeding environment, and can effectively play a therapeutic role in thediseased litopenaeus vannamei infected by the enterocytozoon hepatopenaei.
Owner:SOUTH CHINA SEA INST OF OCEANOLOGY - CHINESE ACAD OF SCI
Fluorescent quantitative PCR detection primer group and kit for enterocytozoon hepatopenaei based on TaqMan-MGB probe
PendingCN111635953AHigh amplification efficiencyGood linear relationshipMicrobiological testing/measurementDNA/RNA fragmentationPcr methodBiochemistry
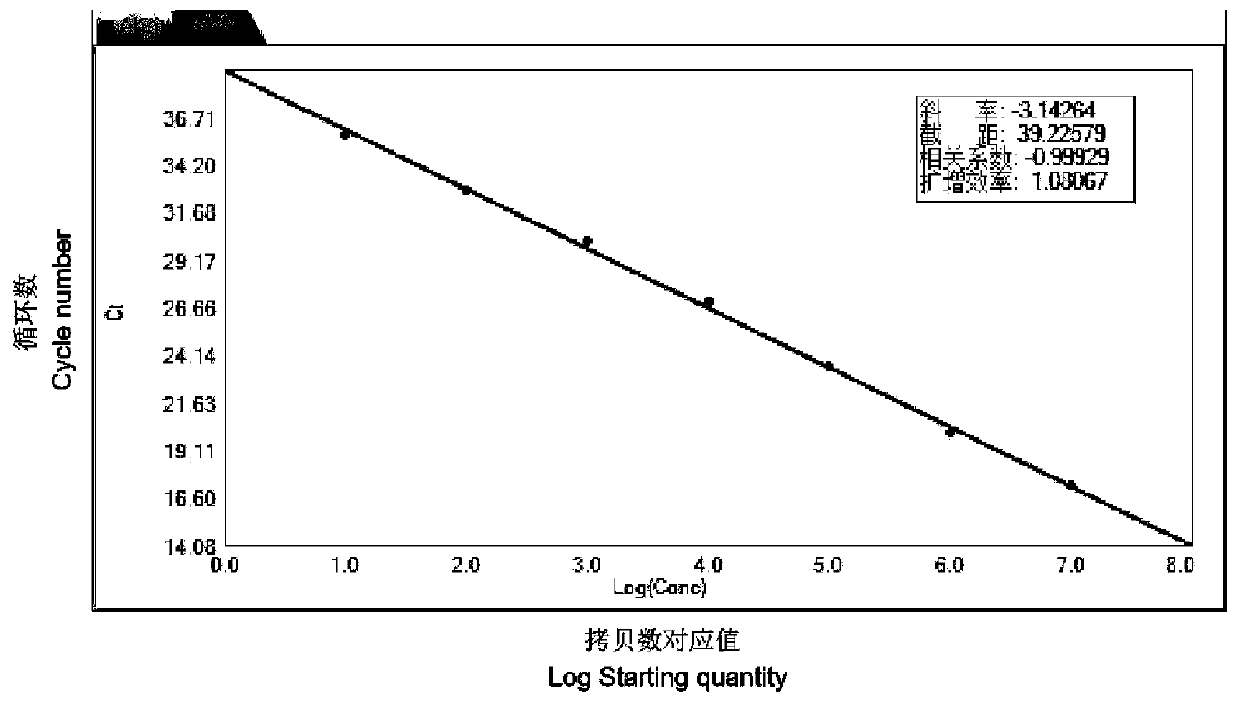
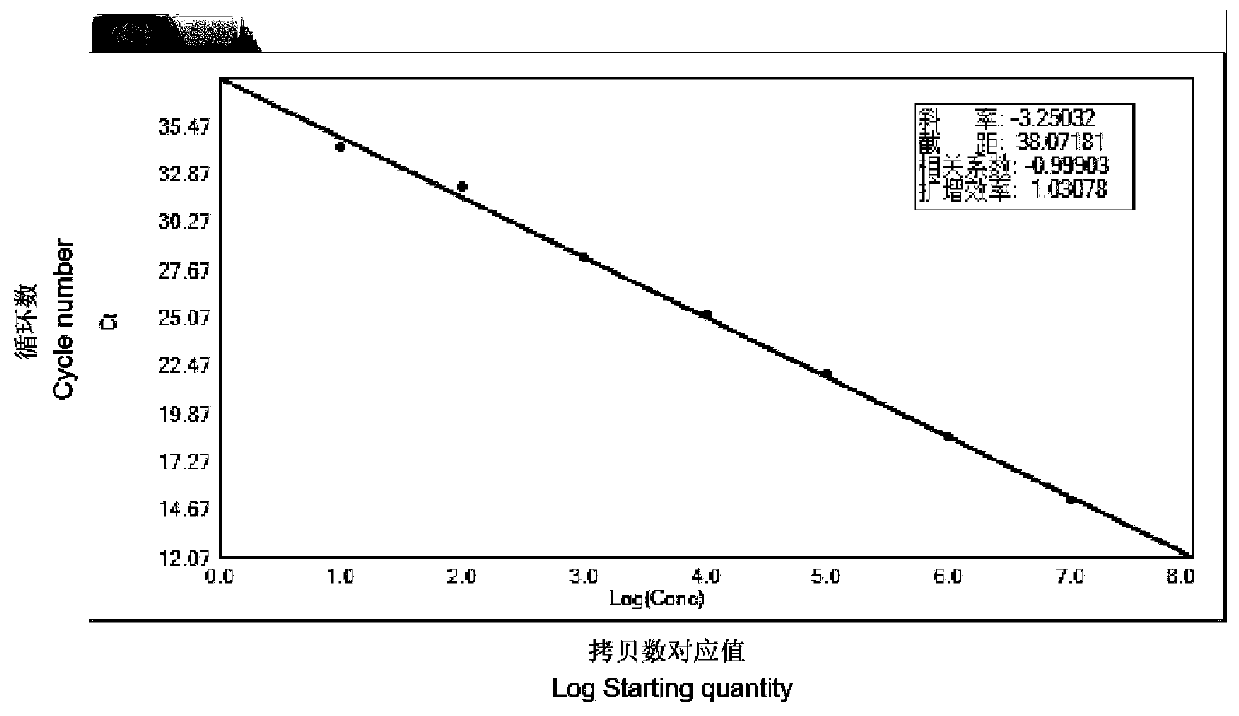
The invention discloses a fluorescent quantitative PCR detection primer group and kit for enterocytozoon hepatopenaei based on a TaqMan-MGB probe, and belongs to the technical field of virus detection. The detection primer group specifically comprises a primer for detecting enterocytozoon hepatopenaei (EHP) and a TaqMan-MGB probe, and the invention also provides the kit and a detection method based on the detection primer and the probe. According to the designed specific primer and TaqMan-MGB probe, a recombinant plasmid standard substance pMD18-T-SSUEHP is prepared, and the TaqMan-MGB probe fluorescent quantitative PCR method for detecting EHP is established. The detection primer group and kit have the advantages of strong specificity, high sensitivity, good repeatability, wide quantitative range, simplicity, rapidness and the like, are suitable for quantitative detection of EHP of shrimp samples, and can provide a new technical means for rapid quantitative detection and real-time monitoring of EHP.
Owner:GUANGXI ACADEMY OF FISHERY SCI
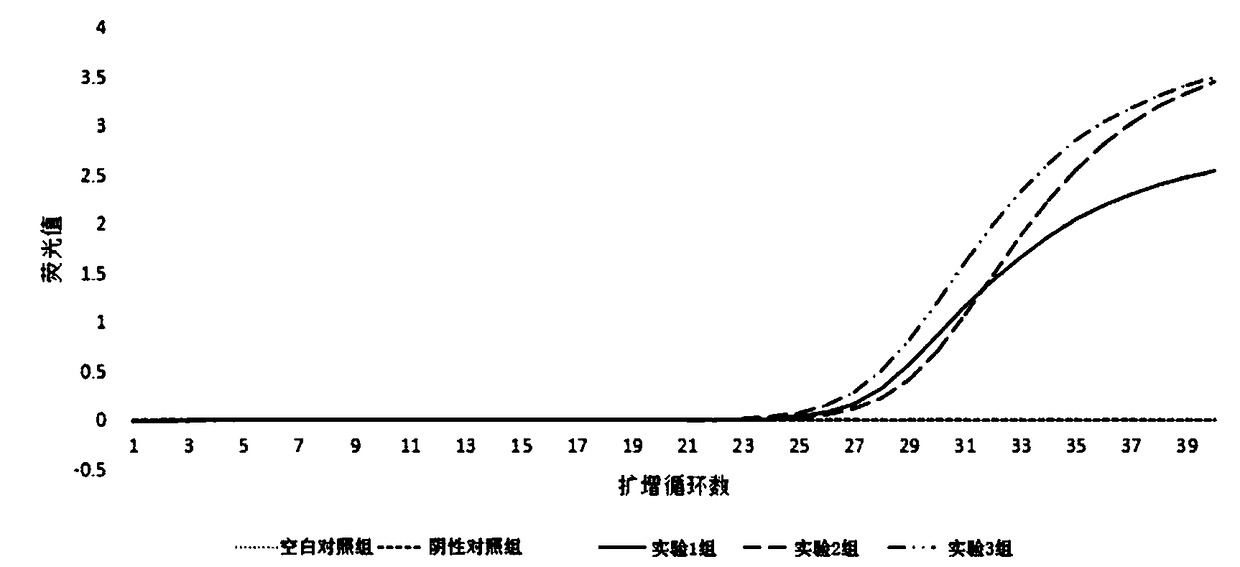
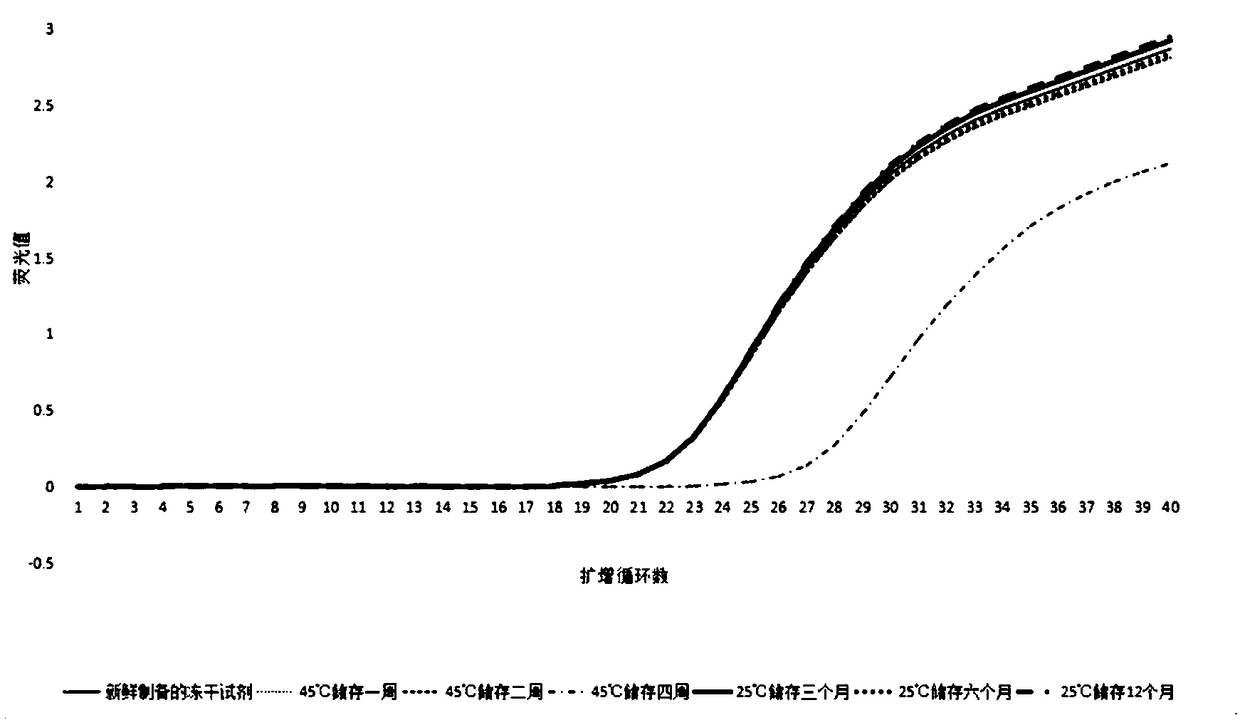
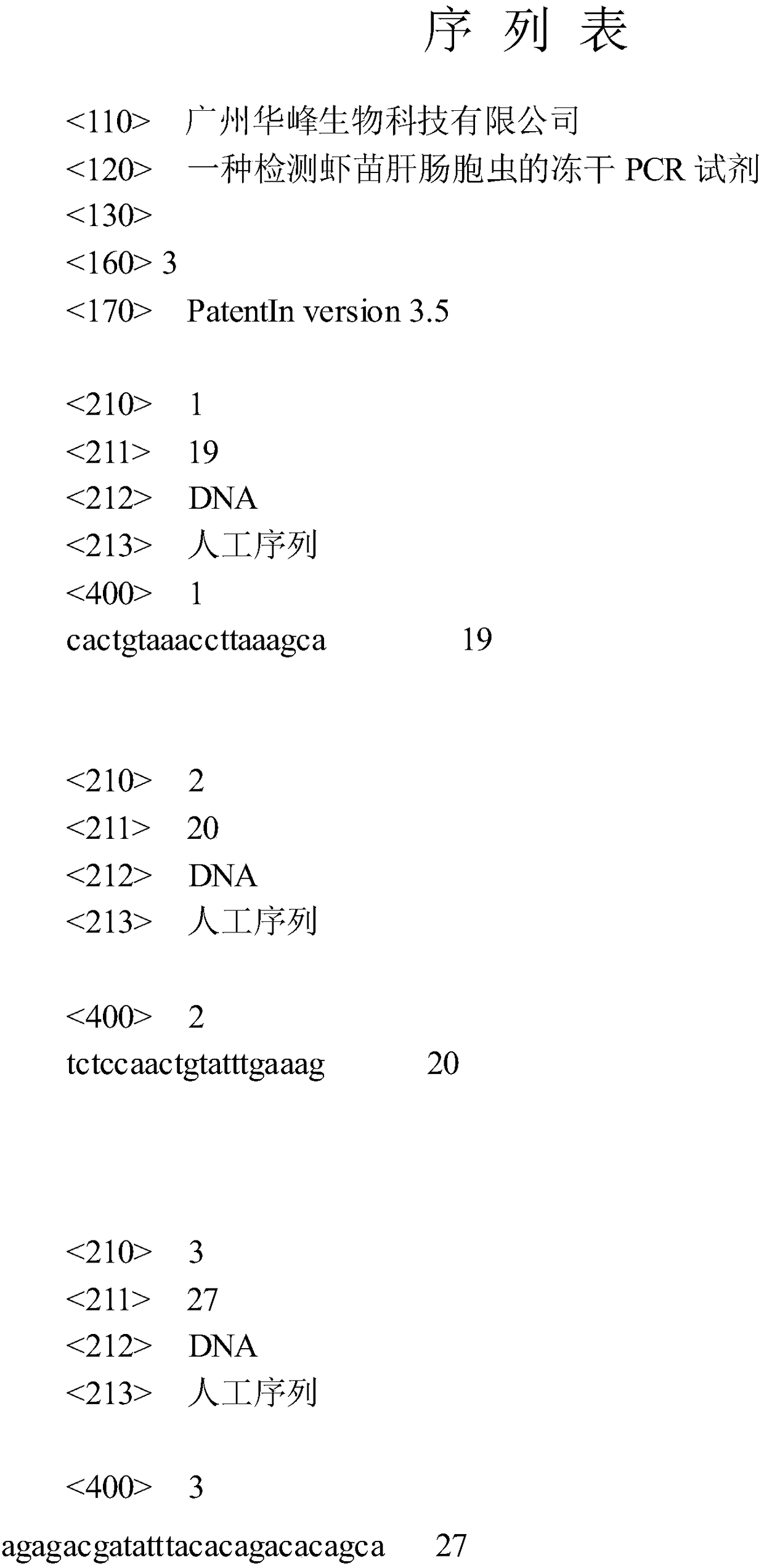
Freeze-drying PCR reagent for detecting shrimp seed enterocytozoon hepatopenaei
InactiveCN109457039AReduce the possibilityAmplification will notMicrobiological testing/measurementAgricultural scienceShrimp
The invention discloses a freeze-drying PCR reagent for detecting shrimp seed enterocytozoon hepatopenaei. The freeze-drying PCR reagent comprises TaqDNA polymerase, a freeze-drying protective agent,an oligonucleotide upstream primer, an oligonucleotide downstream primer and an oligonucleotide fluorescence probe. A sequence of the oligonucleotide upstream primer is 5'-CACTGTAAACCTTAAAGCA-3', a sequence of the oligonucleotide downstream primer is 5'-TCTCCAACTGTATTTGAAAG-3', and a sequence of the oligonucleotide fluorescence probe is 5'-AGAGACGATATTTACACAGACACAGCA-3'. The freeze-drying PCR reagent for detecting the shrimp seed enterocytozoon hepatopenaei can tolerate a higher temperature, is convenient in transportation and storage, and capable of realizing sensitive, specific, rapid and convenient detection on the shrimp seed enterocytozoon hepatopenaei, and has a good application prospect.
Owner:GUANGZHOU HUAFENG BIOTECH
Composite preparation for killing prawn enterocytozoon hepatopenaei and application method of composite preparation
InactiveCN106266381AEfficient killingMeeting the Needs of Controlling Shrimp Enterococcus HepaticaOrganic active ingredientsClimate change adaptationBiotechnologyBetel nuts
The invention discloses a composite preparation for killing prawn enterocytozoon hepatopenaei and an application method of the composite preparation. The composite preparation is prepared from the following raw materials by mass percent: 4.5 to 6.0 percent of albendazole, 5.0 to 8.0 percent of betel nut extract, and 86.0 to 90.5 percent of corn starch auxiliary materials. The betel nut extract is extracted in a laboratory and obtained by virtue of boiling, concentrating and freeze-drying, and one kilogram of betel nuts can be prepared into 100 grams of extract. The composite preparation has the beneficial effects that the composite preparation is safe to a target animal; by using the composite preparation, the prawn enterocytozoon hepatopenaei can be effectively killed, and the requirement for preventing the enterocytozoon hepatopenaei in the cultivation process of prawns can be met; and the preparation process is simple, the use is convenient, and the application prospect is good.
Owner:EAST CHINA SEA FISHERIES RES INST CHINESE ACAD OF FISHERY SCI
Shrimp health system visual rapid detection kit based on LAMP technology
ActiveCN112048572AAvoid influenceSuitable for useMicrobiological testing/measurementMicroorganism based processesDiseaseWhite spot syndrome
The invention discloses a shrimp health system visual rapid detection kit based on an LAMP technology, and belongs to the technical field of aquatic pathogen detection reagent development. The kit comprises an internal reference quality control primer group and a to-be-detected pathogen primer group. The kit is designed for common shrimp pathogenic microorganisms of IHHNV virus, white spot syndrome virus, enterocytozoon hepatopenaei, shrimp hemocyte iridescent virus, shrimp acute hepatopancreatic necrosis disease-vibrio parahaemolyticus and the like; the kit can be used for simultaneously detecting various pathogens; by adding the internal reference quality control primer group, the influence of failure of a reaction reagent and nucleic acid extraction quality of an amplification templateon a detection result can be eliminated; the accuracy of the detection result is ensured; and the kit is suitable for screening and purchasing young shrimps and parent shrimps by grass-roots farmers and customs rapid quarantine customs clearance.
Owner:FISHERIES RES INST OF FUJIAN
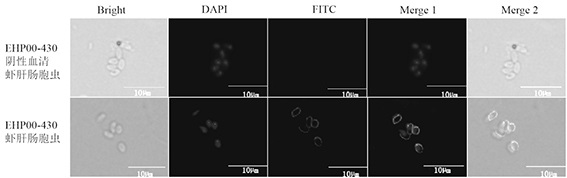
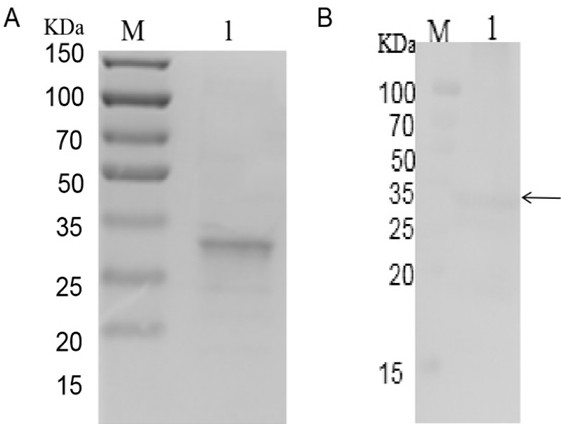
Enterocytozoon hepatopenaei spore wall protein as well as preparation and application of polyclonal antibody thereof
InactiveCN111909253AAccurate detectionSerum immunoglobulinsAntibody ingredientsBiotechnologySporeling
The invention provides an enterocytozoon hepatopenaei spore wall protein as well as preparation and application of a polyclonal antibody thereof, and belongs to the technical field of animal quarantine. The enterocytozoon hepatopenaei spore wall protein (EHP00_430) is positioned on the surfaces of enterocytozoon hepatopenaei spores in the infection period, has high abundance, plays an important role in the process that enterocytozoon hepatopenaei infects prawns, and is an important target for detection, prevention and control of enterocytozoon hepatopenaei. The enterocytozoon hepatopenaei spore wall protein provided by the invention is used for preparing a polyclonal antibody for resisting the EHP00_430 protein; and the polyclonal antibody is high in specificity, can be used for accuratelydetecting enterocytozoon hepatopenaei in the infection period, and can be applied to detection of enterocytozoon hepatopenaei pathogens.
Owner:CHONGQING NORMAL UNIVERSITY
Primer pair, amplification reagent, amplification kit and detection method for detecting Enterocytozoon hepatopenaei of penaeus vannamei and application
PendingCN113278718ASuitable for useSimple structureMicrobiological testing/measurementMicroorganism based processesFluoProbesMelicertus
The invention relates to a primer pair, amplification reagent, amplification kit and detection method for detecting Enterocytozoon hepatopenaei of penaeus vannamei and application. The primer pair for detecting the Enterocytozoon hepatopenaei is designed and is composed of primers EHP-F and EHP-R; and a fluorescent probe EHP-P is designed. Amplification of a target gene of the Enterocytozoon hepatopenaei based on a RPA technology can be successfully achieved; the detection method disclosed by the invention is based on the RPA technology and can be used for specifically detecting the Enterocytozoon hepatopenaei; and the method is simple and convenient in operation, high in sensitivity, mild in reaction conditions and short in reaction time and can meet the primary needs of a farm on site detection.
Owner:湛江海关技术中心
Special killing drug of Enterocytozoon hepatopenaei in prawns and preparation method and application of special killing drug
ActiveCN111110718APrevent proliferationInfection controlOrganic active ingredientsAnimal feeding stuffBiotechnologyPhyllanthus urinaria
The invention discloses a special killing drug of Enterocytozoon hepatopenaei in prawns and a preparation method and application of the special killing drug. The special killing drug of Enterocytozoonhepatopenaei in prawns includes the following components: in percents by weight, 30-60% of albendazole, 10-30% of bile acid, 5-15% of Phyllanthus urinaria powder, 10-20% of artemisia apiacea powder and 5-15% of vitamin C. Application of the special killing drug comprises the steps: in percent by weight, taking the drug, mixing 1-2% of the drug with feed evenly, then spraying a diluted adhesive, performing drying for use, and performing application continuously for 2 weeks or above, wherein the drug can also be used as a feed additive, and be added into the feed at a ratio of 0.5-1% by weight.The combination and matching of the drug are reasonable and scientific, and hepatopancreas of the prawns can be protected through the synergy of the pesticide effects of the components, so that Enterocytozoon hepatopenaei in the prawns is killed, growth of the prawns is accelerated, and the immunity of the prawns is improved.
Owner:SOUTH CHINA SEA INST OF OCEANOLOGY - CHINESE ACAD OF SCI
SWP2 protein-resistant monoclonal cell strain and application thereof
The invention discloses an SWP2 protein-resistant monoclonal cell strain and an application thereof, and belongs to the technical field of agricultural and animal quarantine. On one hand, the invention provides a novel SWP2 protein-resistant monoclonal cell strain and a SWP2 monoclonal antibody secreted by the monoclonal cell strain, and on the other hand, the invention provides the application ofthe monoclonal cell strain and the SWP2 monoclonal antibody in preparing a reagent for detecting enterocytozoon hepatopenaei diseases. The monoclonal antibody generated by the SWP2 protein-resistantmonoclonal cell strain provided by the invention can be combined with an SWP2 antigen with high specificity, has high affinity, and can provide accurate results for judging whether shrimp bodies are infected with enterocytozoon hepatopenaei (EHP) or not.
Owner:ZHEJIANG ACADEMY OF AGRICULTURE SCIENCES
Specific primer pair for rapidly detecting Enterocytozoon hepatopenaei by utilizing RPA-LFS, detection kit and application of detection kit
InactiveCN111979342AAvoid detection methodMicrobiological testing/measurementAgainst vector-borne diseasesBiotechnologyBiochemistry
The invention discloses a specific primer pair for rapidly detecting Enterocytozoon hepatopenaei by utilizing RPA-LFS, a detection kit and application of the detection kit. The detection kit comprisesthe specific primer pair and a probe, wherein an upstream primer is ACAATTTCAAACACTGTAAACCTTAAAGCA, a downstream primer is Bioten-TCATTCATTTTCCTTTTATCTTCTGATATG, and the probe is FITC-TAAAAAGAGACGATATTTACACAGACACAG[THF]ATTTGTAGGATATGA-C3Spacer. According to the specific primer pair, the detection kit and the application, disclosed by the invention, the Enterocytozoon hepatopenaei (Ehp) can be rapidly detected on site under a room-temperature environment through the RPA-LFS, a traditional long-time high-temperature detection method that a nest needs to depend on temperature control equipment is avoided, a result can be judged and read by naked eyes, more convenience is obtained, and the detection kit is suitable for diagnosing aquaculture animal diseases.
Owner:JIANGSU OCEAN UNIV +1
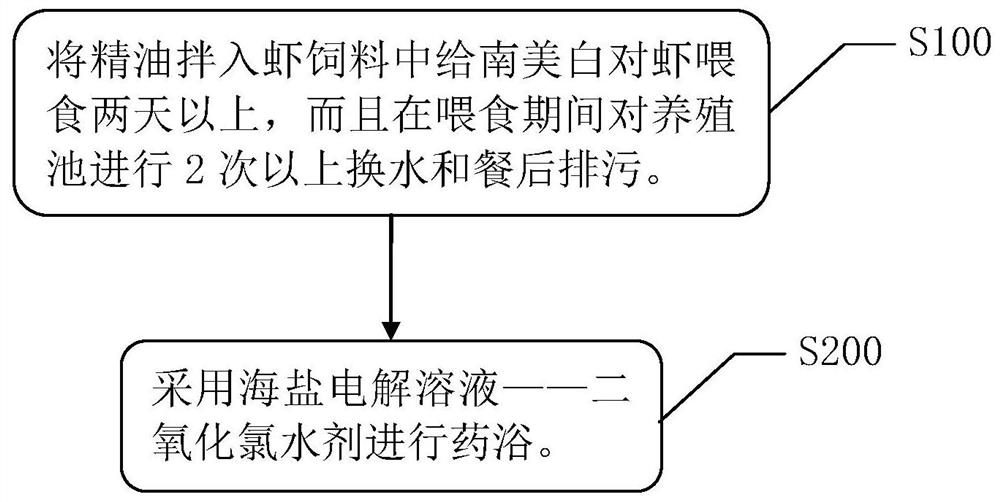
Method for killing EHP (Enterocytozoon hepatopenaei) on Penaeus vannamei
InactiveCN111616089AFast killing effectEasy to operateClimate change adaptationPisciculture and aquariaMelicertusChlorine dioxide
The invention relates to a method for killing EHP (Enterocytozoon hepatopenaei) on Penaeus vannamei. The method comprises the steps: mixing essential oil into shrimp feed firstly, feeding Penaeus vannamei for two days or above, performing water change and pollution discharge after feeding on a culture pond two times or above in the feeding period, and performing medicated bath by using a sea saltelectrolytic solution-aqueous chlorine dioxide preparation so as to kill and eliminate EHP on Penaeus vannamei successfully. The domestic and foreign EHP treatment technology is supplemented, the economic benefits are high, the killing effect on EHP is fast, the operation is simple, and promotion is quick and convenient.
Owner:珠海市海盟水产养殖应用研发有限公司
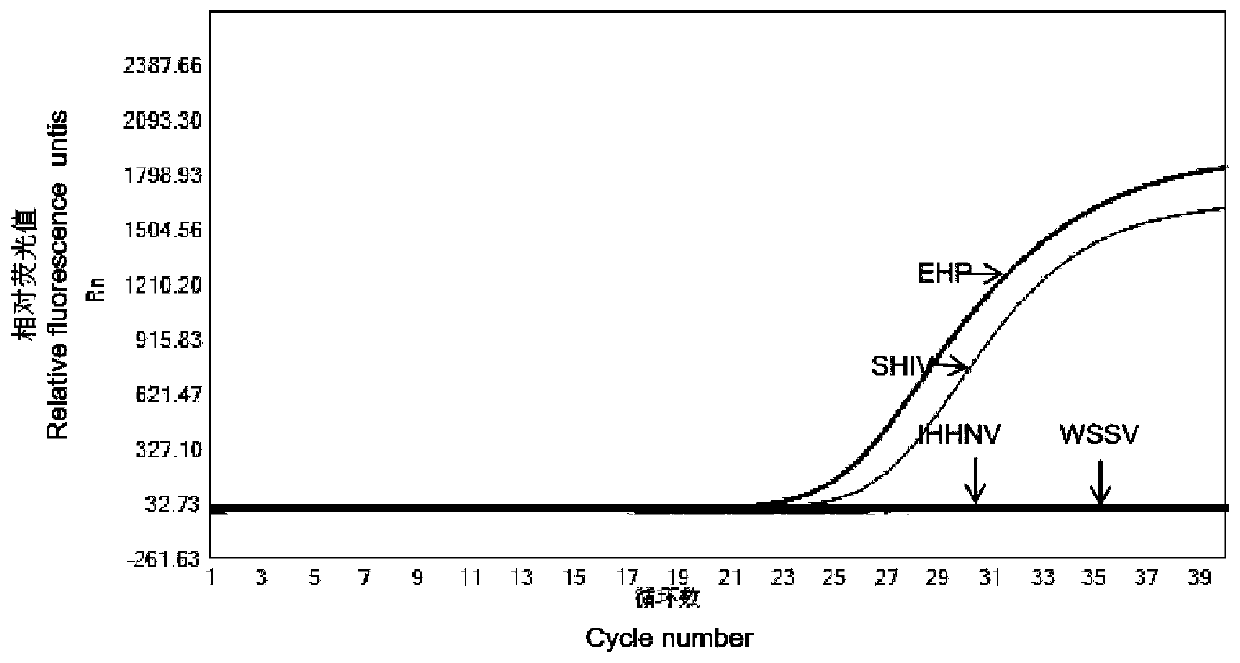
EHP and SHIV dual real-time fluorescent quantitative PCR detection primer and probe combination and kit
InactiveCN110117678AStrong specificityIncreased sensitivityMicrobiological testing/measurementMicroorganism based processesAquatic animalIridovirus
The invention provides an EHP and SHIV dual real-time fluorescent quantitative PCR detection primer and probe combination and kit, and belongs to the technical field of aquatic animal pathogen detection. The primer and probe combination adopts a dual real-time fluorescent quantitative PCR detection method, and amplification of two kinds of pathogenic DNA can be conducted simultaneously at a time by means of specialized detection primers and probes of EHP and SHIV. According to the detection kit, convenience, rapidness and low detection limit are achieved, the specificity is high, the sensitivity is high, the accuracy is high, shrimp enterocytozoon hepatopenaei and shrimp hemocyte iridovirus can be detected simultaneously through dual real-time fluorescent quantitative PCR detection, and the combination and kit are suitable for early monitoring of cover infection of shrimps.
Owner:珠海科艺普检测科技有限公司
PCR (polymerase chain reaction) Detection method for Enterocytozoon hepatopenaei infection in penaeid shrimps
InactiveCN108374049AHigh synthesis rateStrong specificityMicrobiological testing/measurementForward primerPositive control
The invention discloses a PCR (polymerase chain reaction) detection method for Enterocytozoon hepatopenaei infection in penaeid shrimps and a detection kit. The method comprises: (1) designing primers; (2) providing a PCR system; (3) carrying out PCR. The detection kit includes a box body and a box cover; a PCR buffer tube filled with 2*PCR buffer is rested on a support plate; a primer tube filledwith forward and revers primers of Enterocytozoon hepatopenaei, wherein a forward primer sequence is 5'-ATT AGA CAC CGC TGT AGT TC-3', and a reverse primer sequence is 5'-GTT ATT GCC TTC TCC CTC T-3'; a deionized water tube filled with aseptic deionized water; a negative control tube filled with SPF (specified-pathogens free) nucleic acid of penaeid shrimp; a positive control tube filled with 106-copy cDNA of Enterocytozoon hepatopenaei. The PCR (polymerase chain reaction) detection method for Enterocytozoon hepatopenaei infection in penaeid shrimps and the detection kit have the advantages of high sensitivity, good specificity, good operational simplicity and high speed.
Owner:LUDONG UNIVERSITY
RPA primer, probe, kit and method for detecting enterocytozoon hepatopenaei
ActiveCN113430274AStrong specificityReal-time monitoring of reaction resultsMicrobiological testing/measurementAgainst vector-borne diseasesCell wallPlasmid
The invention discloses an RPA primer, a probe, a kit and a method for detecting enterocytozoon hepatopenaei. A pair of RPA primers and probes with high specificity is designed and screened by taking the cell wall protein gene of the enterocytozoon hepatopenaei as a target gene, and a Real-time RPA method for detecting EHP is established on the basis of the RPA primers and the probes. The method is simple in operation, is high in detection sensitivity, is capable of detecting plasmid DNA molecules of 10 copies / mu L at least, has good repeatability, and has a reliable detection result. The method is suitable for detection and identification of EHP pathogens in litopenaeus vannamei culture, and is an attempt of applying Real-time RPA to quantitative detection of EHP for the first time.
Owner:SUN YAT SEN UNIV
Visual rapid detection kit for enterocytozoon hepatopenaei (EHP)
InactiveCN109337990AShorten the timeEasy to operateMicrobiological testing/measurementMagnetic beadLoop-mediated isothermal amplification
The invention discloses a visual rapid detection kit for enterocytozoon hepatopenaei (EHP). The visual rapid detection kit comprises a reagent A, a reagent B, a reagent C, a reagent D, a reagent E, areagent F, a reagent G and a detection tube loaded with a reagent H. The kit provided by the invention is high in detection specificity and detection sensitivity, and magnetic beads are adopted for extracting nucleic acid, thereby adequately extracting the nucleic acid out of a sample to be tested, avoiding the use of large-scale instruments, simplifying the complicated operation process and greatly shortening the time spent in nucleic acid extraction. The use of dye calcein can enable a user to strictly follow the principle of not opening a lid during a loop-mediated isothermal amplification(LAMP) reaction, thereby greatly reducing the false positive rate. The kit provided by the invention is simple and convenient in the whole operation process, and the detection can be finished within 1.5-2 hours without adopting high-end instruments.
Owner:天津市水生动物疫病预防控制中心
Detection and application of prawn enterocytozoon hepatopenaei trehalose-6-phosphate synthase gene
ActiveCN107119062ASimple designHigh expressionMicrobiological testing/measurementMicroorganism based processesDiseaseTrehalose-6-phosphate synthase
The invention provides detection and application of a prawn enterocytozoon hepatopenaei trehalose-6-phosphate synthase gene and relates to prawn enterocytozoon hepatopenaei. Reverse transcription is performed through extracting the total RNA of a prawn infected with the prawn enterocytozoon hepatopenaei, and then the one trehalose-6-phosphate synthase gene is identified through specific primer amplification. According to gene sequence information, an optimal primer is designed and used for a real-time quantitative PCR method, so that a basis is provided for detecting a transcription expression level of the gene in a prawn. The transcription level of the prawn enterocytozoon hepatopenaei trehalose-6-phosphate synthase gene in the prawn is detected through the real-time quantitative PCR method, and the real-time quantitative PCR method can be used in the detection of prawn enterocytozoon hepatopenaei diseases. The method has the characteristics of being high in sensitivity and good in specificity, and the information and the method are provided for prawn disease prevention and control.
Owner:THIRD INST OF OCEANOGRAPHY MINIST OF NATURAL RESOURCES
Primer and method for fluorescent quantitative PCR detection of Enterocytozoon hepatopenaei (EHP)
PendingCN113512607AAvoiding the False Positive ProblemStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationMicrobiologyTarget gene
The invention relates to a method for fluorescent quantitative PCR detection of Enterocytozoon hepatopenaei (EHP). According to the method, a pair of specific primers taking EHP spore wall protein SWP as a target gene and an SWP standard substance kit are used. The standard substance kit comprises an SWP standard plasmid diluted by 10 times of gradient and a bacterial suspension of an SWP standard strain. A TBGreen fluorescent dye method is adopted, the EHP infection of cultured prawns is quantitatively detected by detecting the EHP SWP, high sensitivity and specificity are achieved, the method can be used for rapid detection and diagnosis of EHP, and a foundation is laid for monitoring and early prevention and control of EHP infection.
Owner:杭州市农业技术推广中心
Nucleic acid sequence combination, kit and detection method for LAMP-CRISPR isothermal detection of prawn enterocytozoon hepatopenaei
PendingCN111808980ASimple and fast operationMild conditionsBioreactor/fermenter combinationsBiological substance pretreatmentsNucleic acid sequencingDNA
The invention discloses a nucleic acid sequence combination, a kit and a detection method for LAMP-CRISPR isothermal detection of prawn enterocytozoon hepatopenaei. The nucleic acid sequence combination comprises a primer group for LAMP detection, crRNA and a probe. The detection method uses the kit, and the detection method comprises the following steps: (1) extracting DNA of a to-be-detected sample; (2) taking the DNA extracted in the step (1) as a template, and carrying out LAMP isothermal amplification reaction by using the primer group in the claim 1; (3) carrying out CRISPR isothermal detection reaction by using the crRNA and the probe in the claim 1 by taking an LAMP isothermal amplification reaction product in the step (2) as a template; and (4) analyzing the CRISPR isothermal detection result obtained in the step (3) through an isothermal amplification instrument, and if an amplification curve occurs, the result is positive, otherwise, the result is negative. The detection method is rapid, efficient, simple and convenient to operate, high in specificity, high in sensitivity and simple and convenient to identify.
Owner:HANGZHOU ALLSHENG INSTR
Duplex PCR (polymerase chain reaction) detection method and kit for simultaneously detecting shrimp enterocytozoon hepatopenaei and tetrapod iridovirus 1
PendingCN114717358AGood repeatabilityImprove stabilityMicrobiological testing/measurementAgainst vector-borne diseasesDuplex pcrIridovirus
The invention belongs to the technical field of marine organism pathogen detection, and particularly relates to a dual PCR (polymerase chain reaction) detection method and kit for simultaneously detecting shrimp enterocytozoon hepatopenaei and decapod iridovirus 1. The invention provides a duplex PCR (polymerase chain reaction) detection method for simultaneously detecting enterocytozoon hepatopenaei and tetrapod iridovirus 1, used PCR primers are shown as SEQ ID NO: 1 and SEQ ID NO: 2, and detected pathogenic gene segments do not need to be cloned, sequenced and subjected to sequence alignment. Whether a sample to be detected contains one or two of the two pathogens or not can be detected through one experiment. And a plurality of limitations of complicated operation, low sensitivity, low applicability and the like of the conventional single detection are overcome.
Owner:日照市海洋与渔业研究院(日照市海域使用动态监视监测中心日照市水生野生动物救护站)
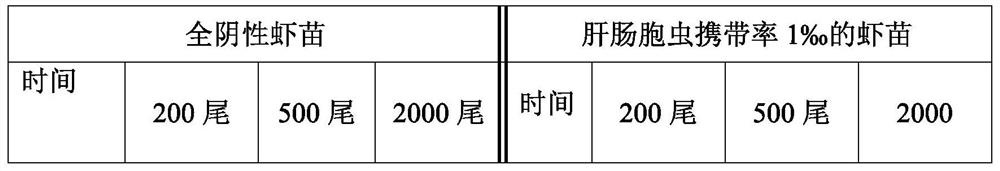
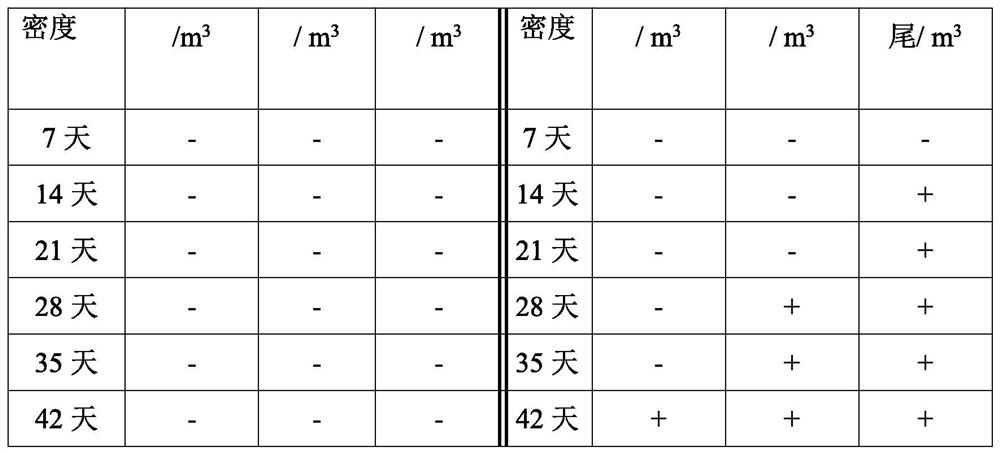
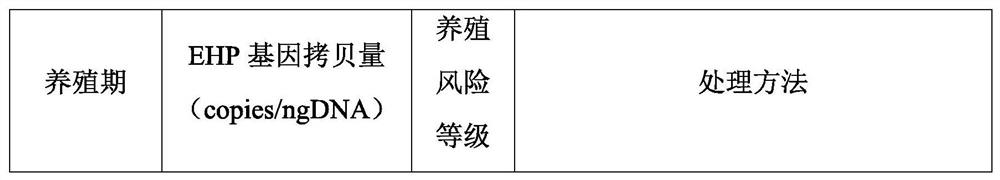
Penaeus vannamei breeding method based on detection of enterocytozoon hepatopenaei infection dose
ActiveCN114342850ALow costReduce the risk of later breedingMicrobiological testing/measurementClimate change adaptationAnimal scienceMelicertus
The invention discloses a penaeus vannamei breeding method based on detection of enterocytozoon hepatopenaei infection dose, and belongs to the technical field of penaeus vannamei breeding. According to the method, the infection dose of enterocytozoon hepatopenaei is regularly monitored in the whole culture period of the penaeus vannamei boone, and different culture measures are adopted for different infection doses. According to the method, high-quality shrimp seeds are selected for centralized thickening, pathogen indexes including enterocytozoon hepatopenaei are monitored regularly, and the shrimp seeds are cultivated in different pools after the shrimp seeds are subjected to thickening about one month and each item is detected to be qualified, so that compared with the technology of directly putting the shrimp seeds, on one hand, the cost is reduced through centralized management, and on the other hand, the risk of later-stage cultivation caused by missing detection of low disease-carrying rate of the shrimp seeds can be reduced. The vigor and ingestion growth conditions of the shrimps are observed at any time in the culture period, enterocytozoon hepatopenaei pathogens are monitored regularly, real-time fluorescent quantitative detection is supplemented if positive results are detected, treatment is carried out according to the method disclosed by the invention according to the culture period, the specification state of the shrimps and quantitative detection data, and the economic loss is reduced.
Owner:INST OF OCEANOLOGY & MARINE FISHERIES JIANGSU
Method for purifying enterocytozoon hepatopenaei of prawns
ActiveCN109628314AHigh purityLess impuritiesProtozoaMicroorganism based processesCentrifugationPrawn
A method for purifying enterocytozoon hepatopenaei of prawns comprises the steps of taking hepatopancreas of a plurality of prawns infected with enterocytozoon hepatopenaei, removing membranes by grinding; diluting them to 35-40 ml with sterile water; conducting alternative centrifugation at 1500-2000 rpm and at 500-600 rpm till no precipitate is observed by the naked eyes; centrifuging the supernatant without precipitate at the high speed of 3000-3500 rpm, and taking and dissolving a precipitate in sterile water to serve as a crude extracting solution; adopting different concentrations of Percoll cell separation solutions for gradient separation; adding 1-1.5 ml of crude extracting solution at the topmost of the liquid level with set gradient; using a centrifugal machine to carry out centrifugation under the conditions of 15 DEG C and 9000-10000 rpm; and obtaining a purified enterocytozoon hepatopenaei suspension by washing. The purified enterocytozoon hepatopenaei has high purity andless impurities, and the operation is simple.
Owner:SHENYANG AGRI UNIV
Preparation for preventing and treating prawn enterocytozoon hepatopenaei disease as well as preparation method and application thereof
ActiveCN112957392AGrowth inhibitionInhibition of killingPowder deliveryAntiparasitic agentsBiotechnologyAnimal science
The invention discloses a preparation for preventing and treating prawn enterocytozoon hepatopenaei disease as well as a preparation method and application thereof. The preparation is prepared from the following raw materials: rosemary oil, anise oil, talcum powder and auxiliary materials. The preparation comprises following components in percentage by mass: 3.0 to 3.5 percent of rosemary oil, 1.0 to 2.0 percent of anise oil, 15.0 to 18.0 percent of talcum powder and 78.0 to 80.0 percent of auxiliary materials. The talcum powder is feed-grade talcum powder, and the auxiliary material is corn starch. The invention also provides a preparation method and application of the preparation. The preparation is added into a prawn feed for use, which is mixed with the feed and is orally taken for feeding, 2-3g of the preparation is added into per kilogram of the prawn feed, and the preparation is continuously used for 5-7 days. The compound preparation prepared by the invention is safe to target animals, can effectively inhibit the growth of the enterocytozoon hepatopenaei and kill the enterocytozoon hepatopenaei, and is simple in preparation process, convenient to use, non-toxic and free of drug residue risk.
Owner:上海联旺水产科技有限公司 +2
Fluorescent quantitative PCR (polymerase chain reaction) method for detecting enterocytozoon hepatopenaei of prawns and corresponding kit
PendingCN111485017AHigh amplification efficiencyAchieving correct detectionMicrobiological testing/measurementPrawnMolecular biology
The invention discloses a fluorescent quantitative PCR (Polymerase Chain Reaction) method for detecting enterocytozoon hepatopenaei of prawns and a corresponding kit. Specific gene detection is ingeniously applied to distinguish prawn enterocytozoon hepatopenaei from other bacterial genera, and accurate bacterial genus information is obtained through comprehensive determination. Compared with an existing mainstream detection kit, the kit for detecting prawn enterocytozoon hepatopenaei has the advantages of being high in sensitivity, rapid, convenient to use, good in specificity, rigorous and accurate in judgment and the like, and has good application prospects and market value.
Owner:广东美格基因科技有限公司
Primer set for simultaneously detecting three kinds of microsporidia and application of primer set
ActiveCN111269997AIncreased sensitivityImprove featuresMicrobiological testing/measurementAgainst vector-borne diseasesShrimpMicrosporidium
The invention discloses a primer set for simultaneously detecting three kinds of microsporidia and application of the primer set. The primer set comprises a first specific primer pair for detecting enterocytozoon hepatopenaei, a second specific primer pair for detecting enterocytozoon artemia and a third specific primer pair for detecting enterocytozoon eriocheir. The first specific primer pair aims at a shrimp enterocytozoon hepatopenaei ptp2 gene, the second specific primer pair aims at an enterocytozoon artemia STPK gene, and the third specific primer pair aims at an enterocytozoon eriocheir swp7 gene. According to the invention, the infection conditions of enterocytozoon hepatopenaei, enterocytozoon artemia and enterocytozoon eriocheir in shrimps and crabs can be rapidly and accuratelydetected through multiple PCR reactions, and the sensitivity and specificity are better.
Owner:SHENYANG AGRI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com