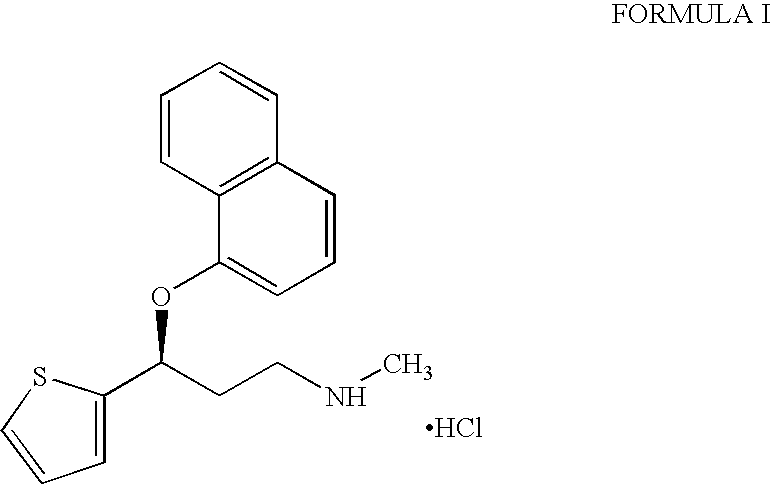
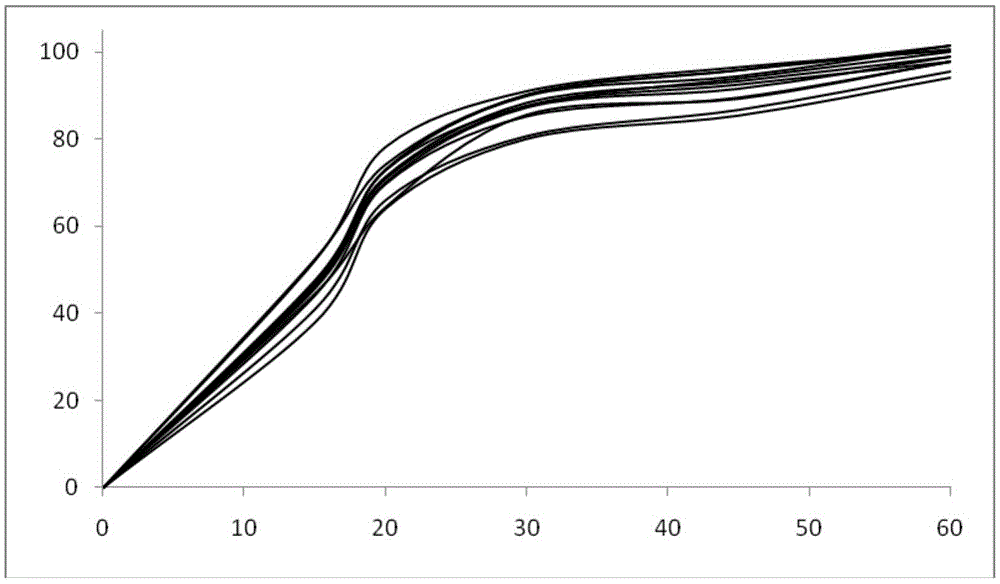
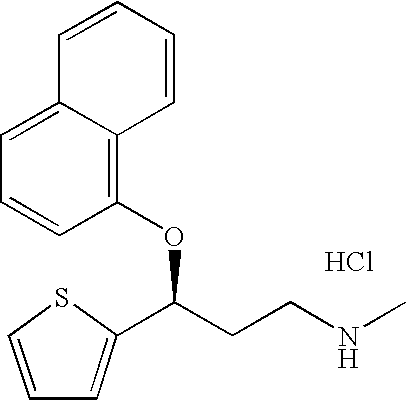
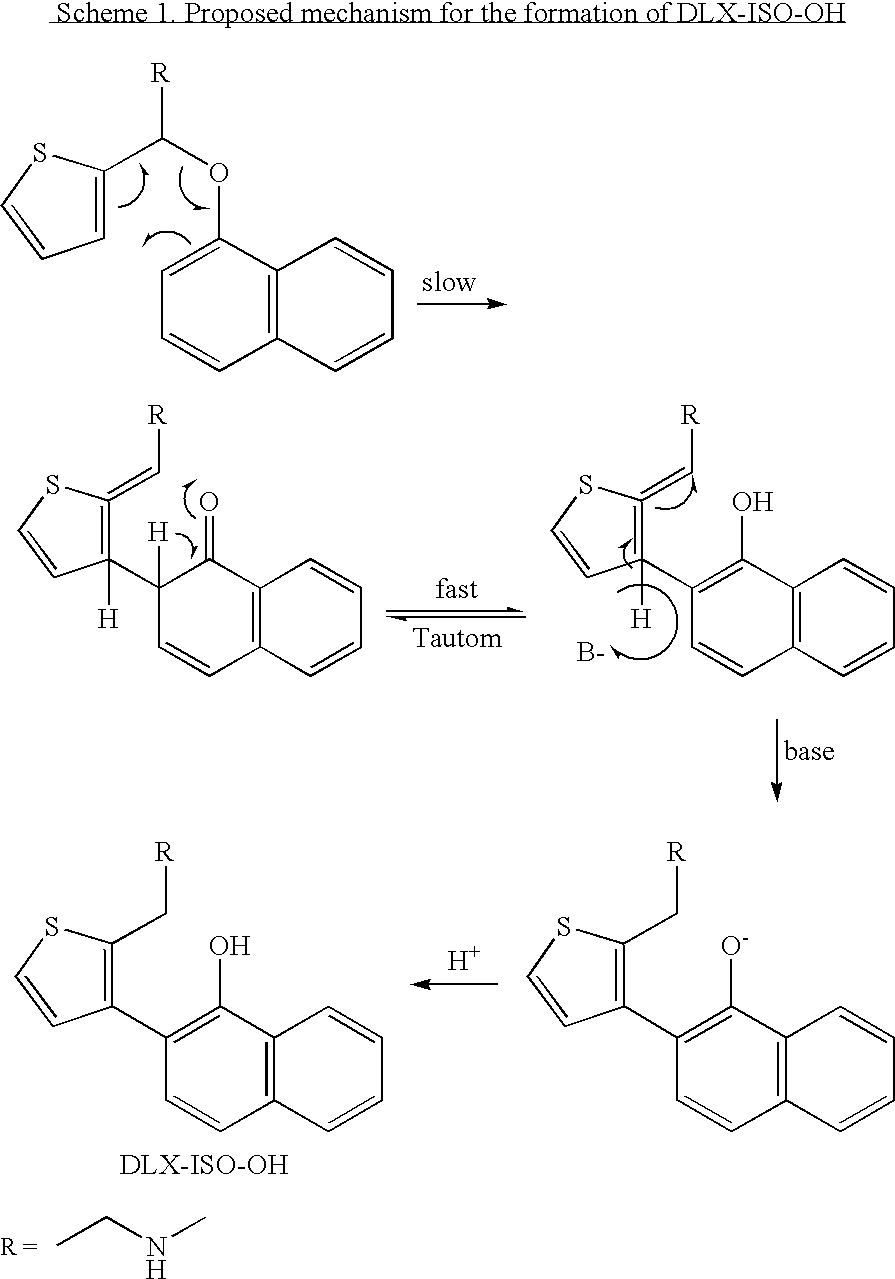
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
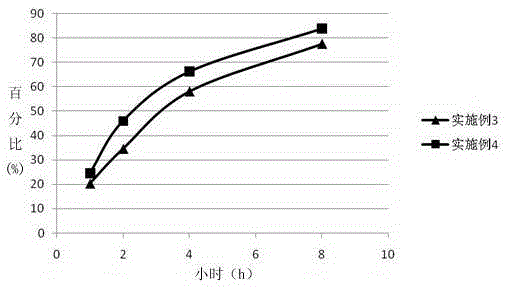
67 results about "Duloxetine Hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
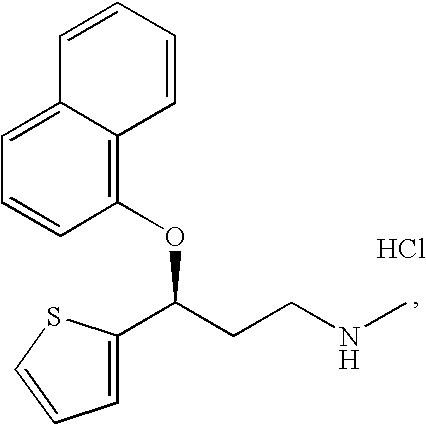
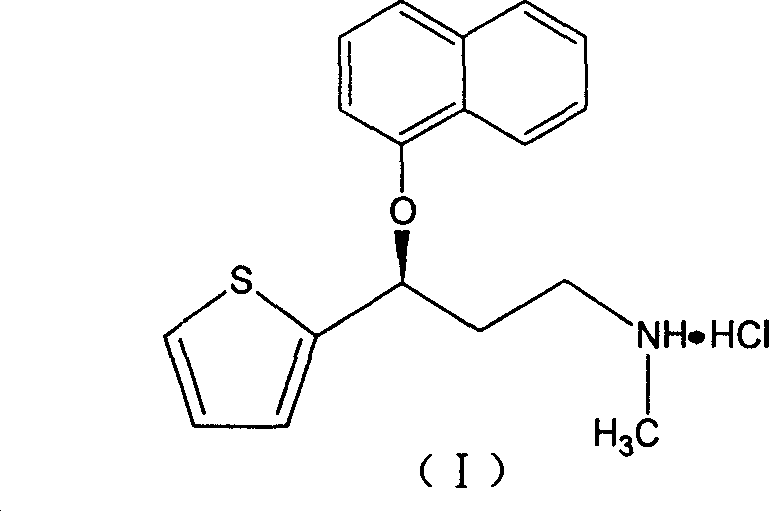
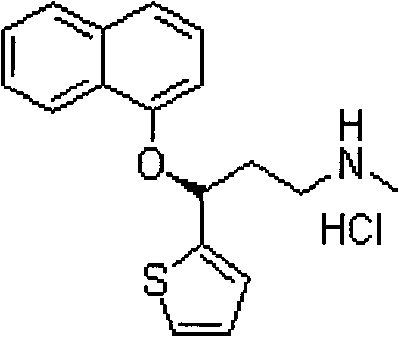
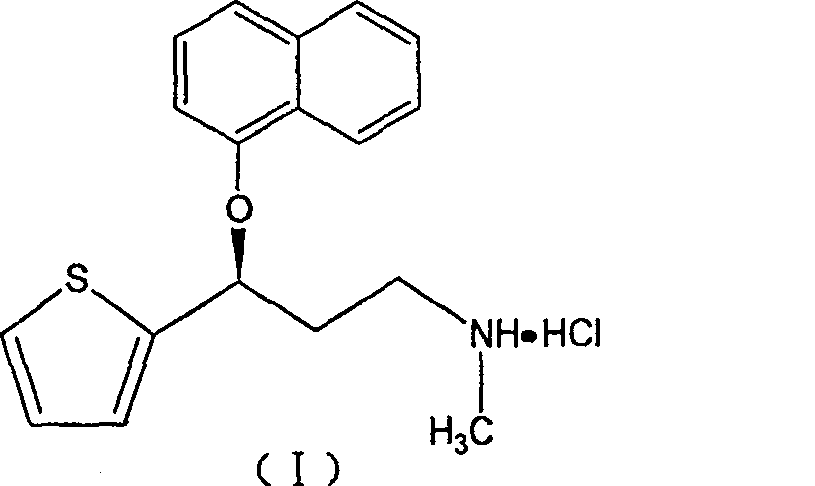
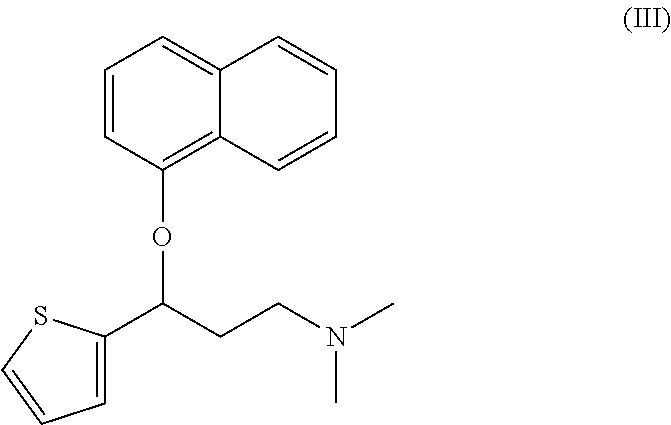
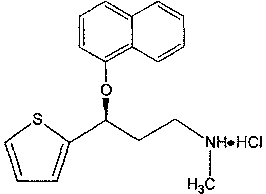
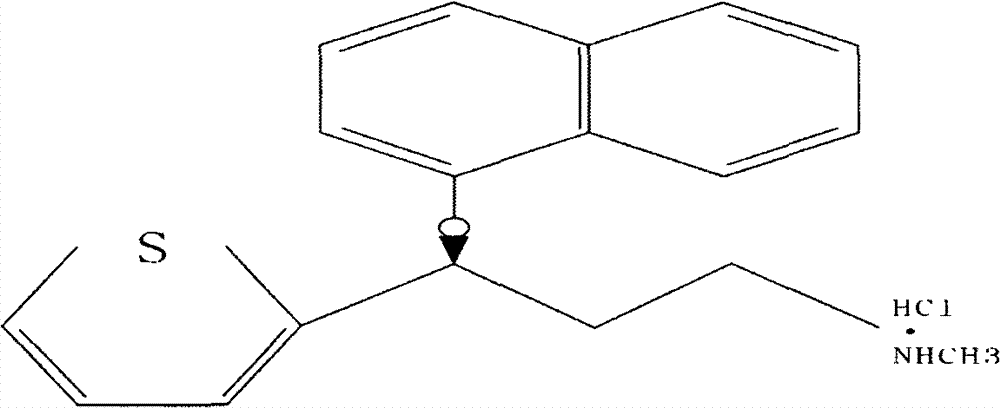
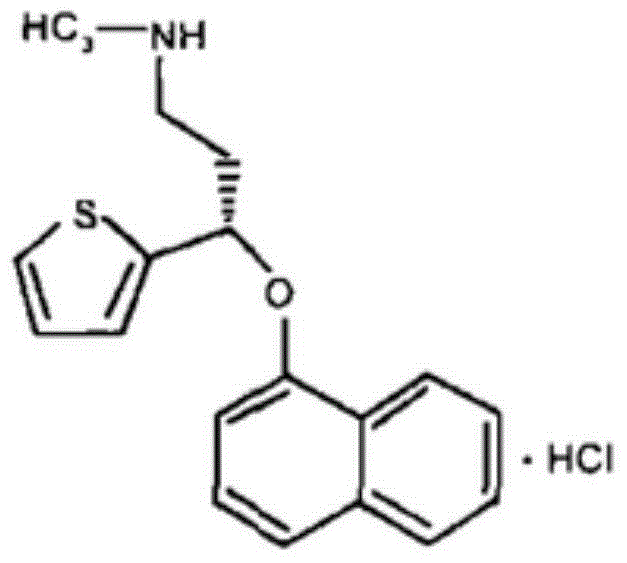
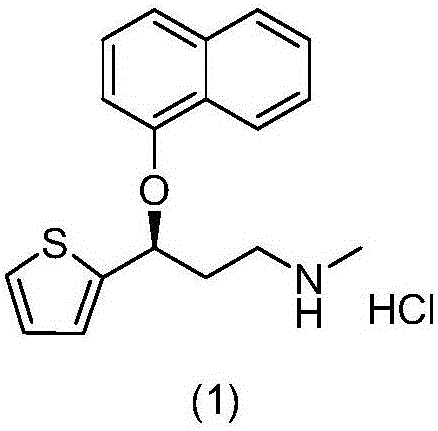
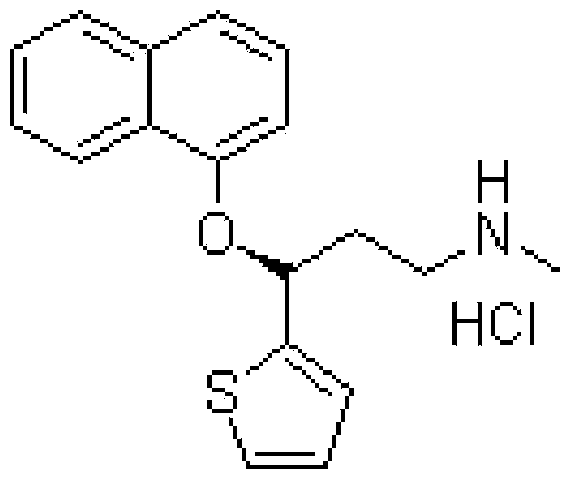
A thiophene derivative and selective NEUROTRANSMITTER UPTAKE INHIBITOR for SEROTONIN and NORADRENALINE (SNRI). It is an ANTIDEPRESSIVE AGENT and ANXIOLYTIC, and is also used for the treatment of pain in patients with DIABETES MELLITUS and FIBROMYALGIA.
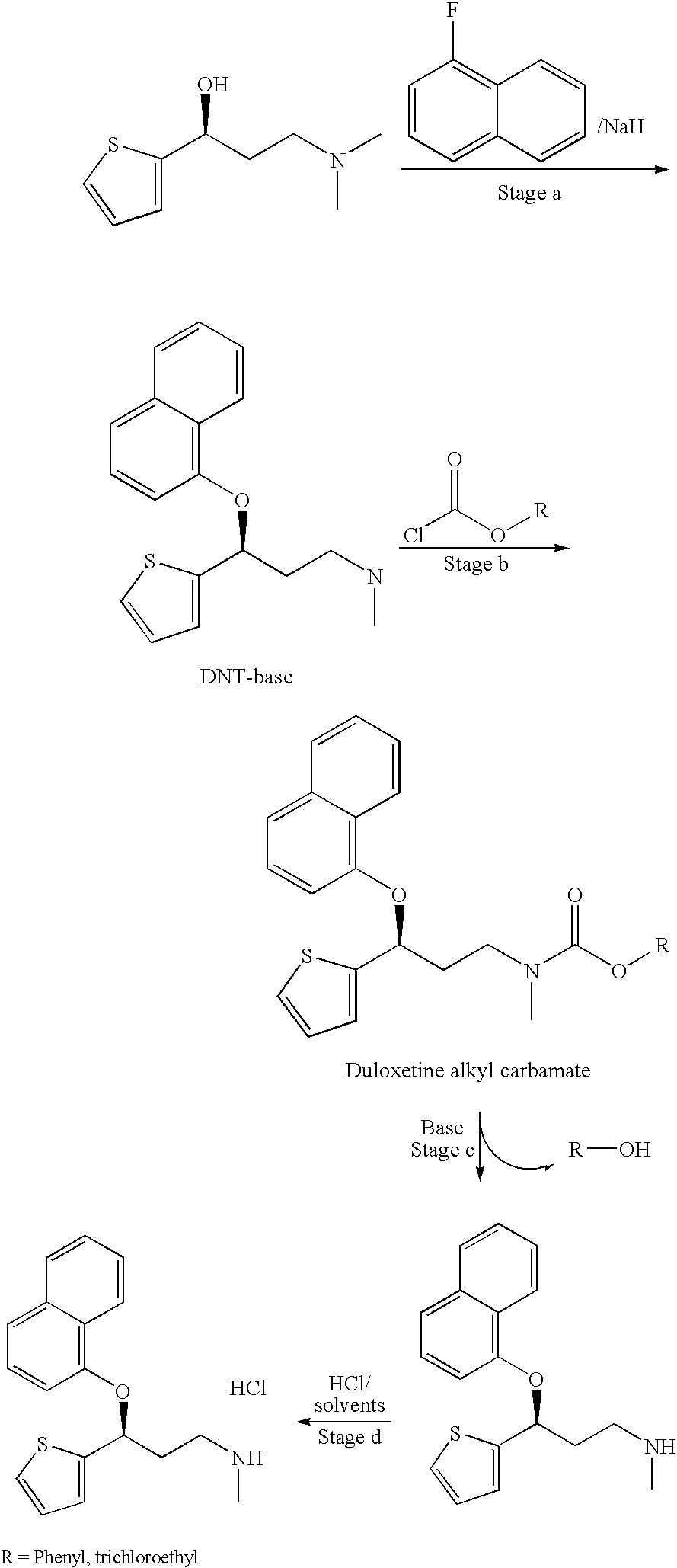
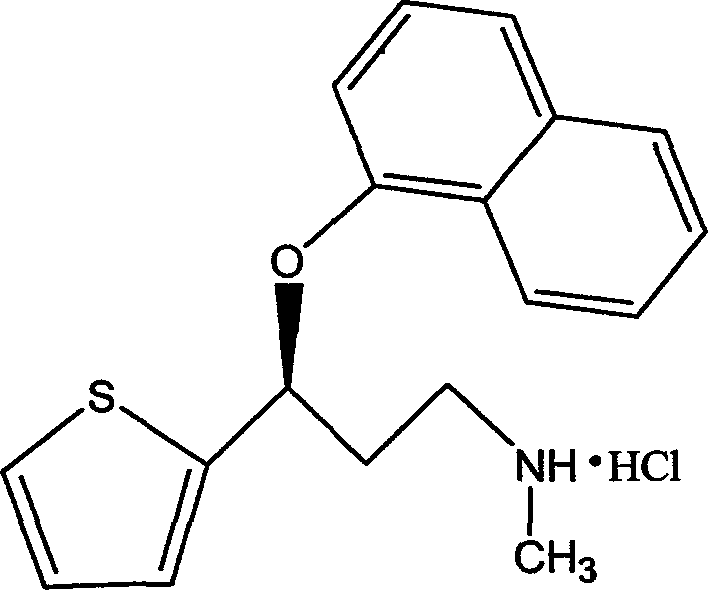
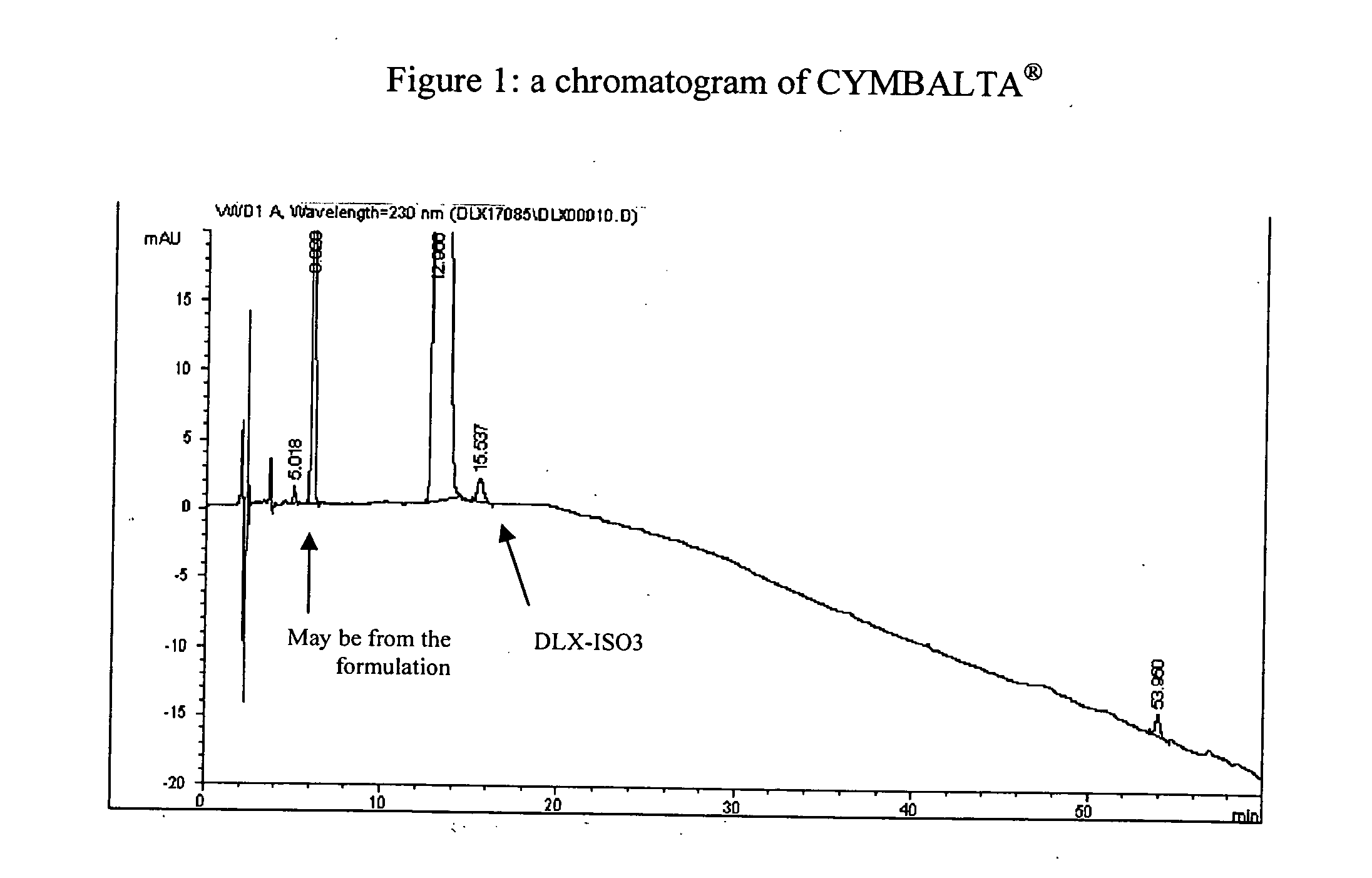
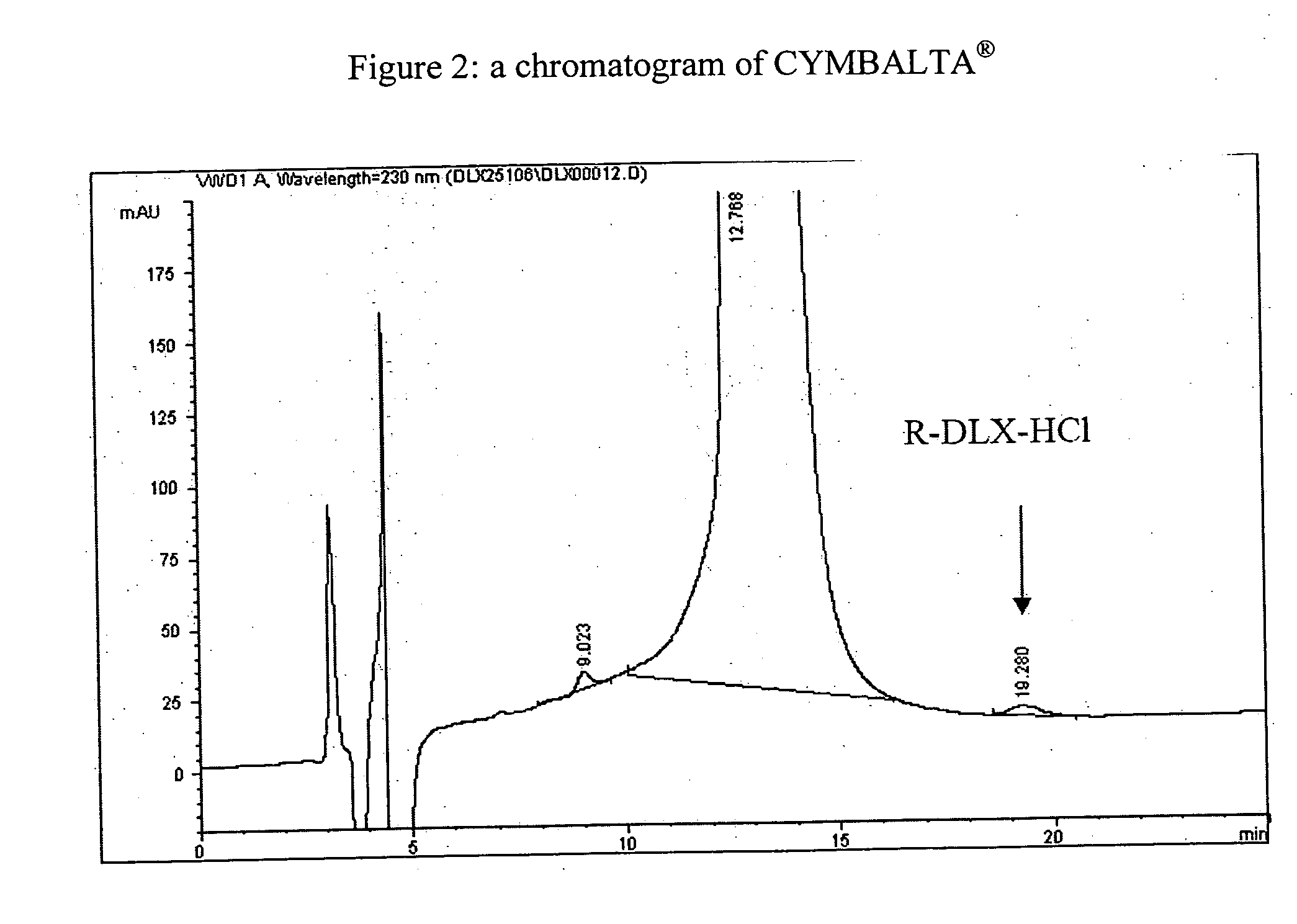
Process for preparing pharmaceutically acceptable salts of duloxetine and intermediates thereof
Processes for preparing DNT-base, duloxetine alkyl carbamate, duloxetine-base and duloxetine hydrochloride, are provided. Also provided, are processes for converting DNT-base, duloxetine alkyl carbamate and duloxetine-base into pharmaceutically acceptable salts of duloxetine.
Owner:TEVA PHARM USA INC
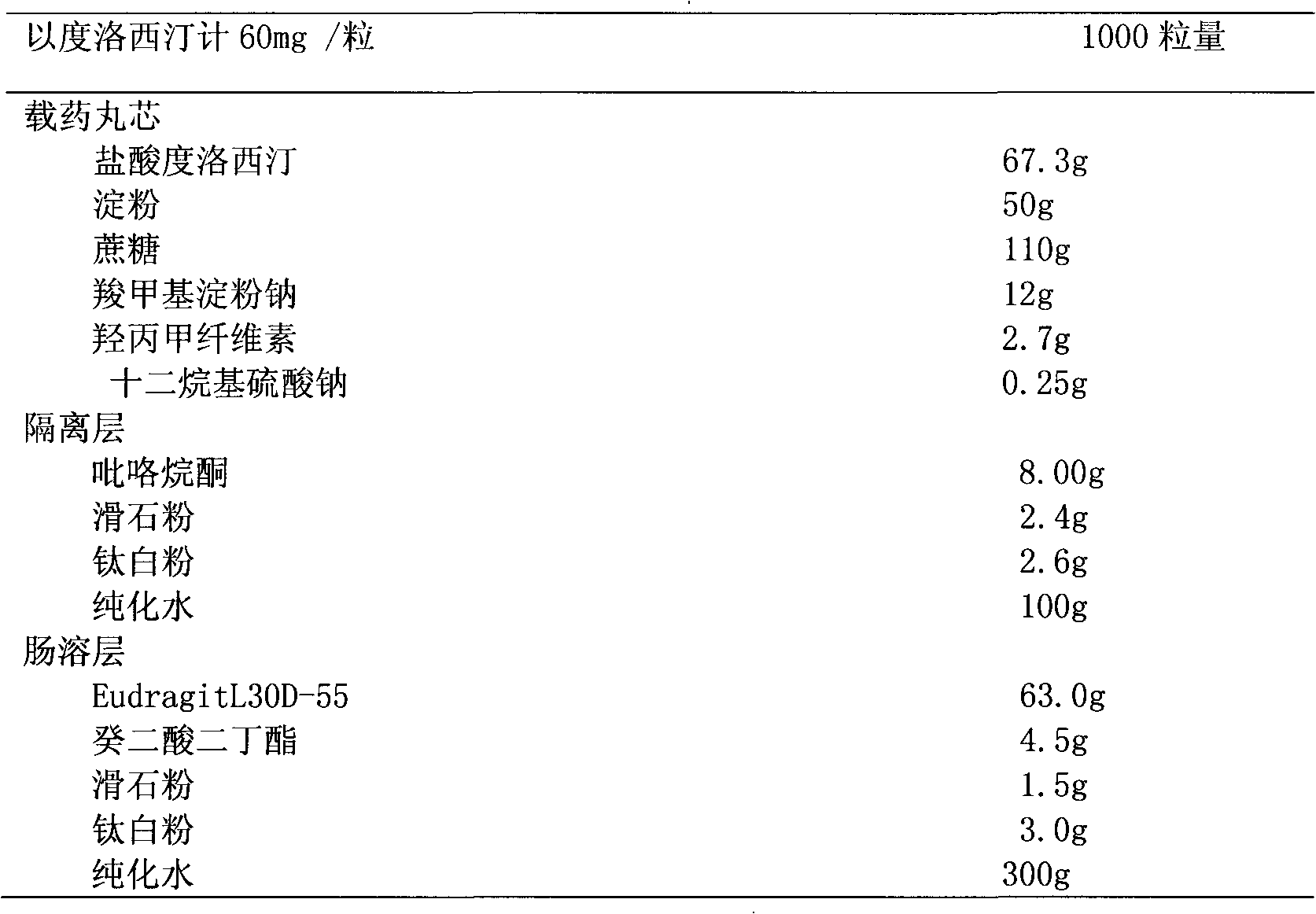
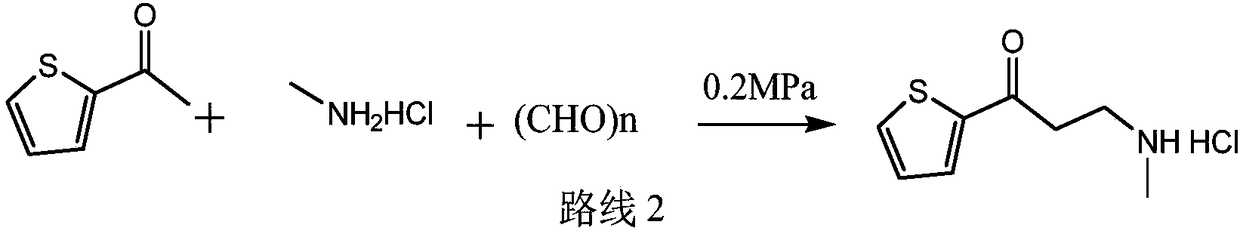
Medicinal preparations containing duloxetine hydrochloride and preparation method thereof
ActiveCN101190208AImpact releaseImprove toleranceOrganic active ingredientsNervous disorderIsolation layerPharmaceutical formulation
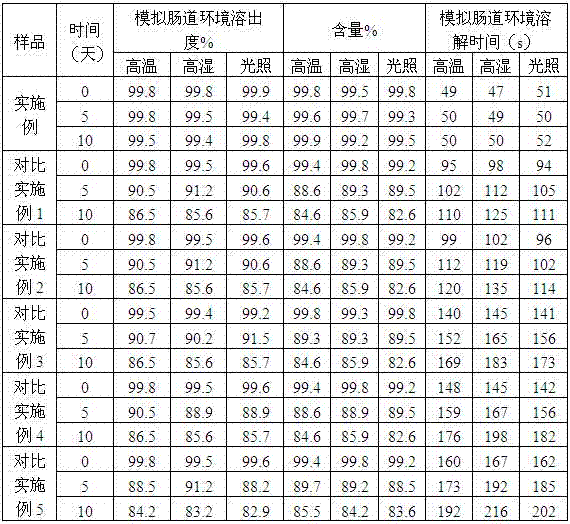
The invention relates to an enteric-coated tablet containing duloxetine hydrochloride and a preparation method thereof. The enteric-coated tablet of duloxetine hydrochloride consists of three parts: a tablet core, a gastric-coated isolation layer and an enteric-coated layer. The technical proposal of the invention can effectively avoid the cross reaction between the medicine and enteric coating material occurring during the releasing process of the medicine and affecting the release of the medicine; therefore, the stability of the medicine can be effectively improved. The preparation method is simple and is easy to be operated, which is suitable for industrialized production.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD +1
Duloxetine hydrochloride delayed release formulations
Delayed release formulations of duloxetine hydrochloride and methods for its manufacture are described. A preferred formulation includes an inert core, a drug layer comprising duloxetine hydrochloride, a separating layer and an enteric layer comprising at least one of methacrylic acid copolymer and hydroxypropyl methyl cellulose phthalate.
Owner:TEVA PHARM USA INC
Duloxetine hydrochloride enteric mini-pill preparation
InactiveCN105534949AImprove stabilityEasy to buyOrganic active ingredientsNervous disorderPositive controlAcrylic resin
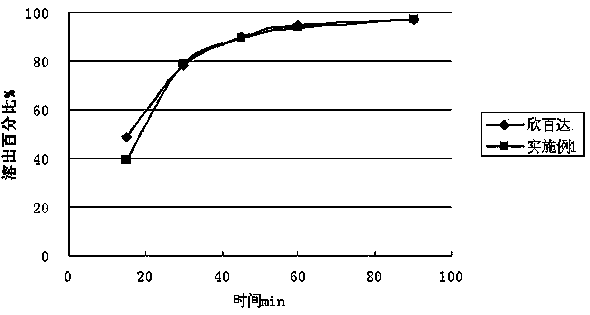
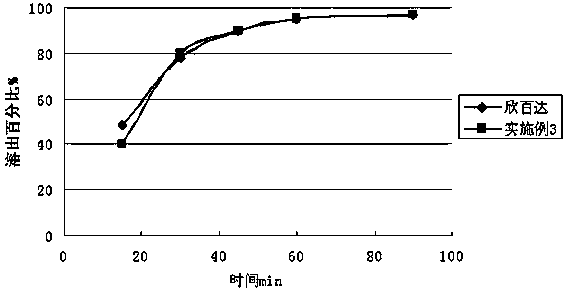
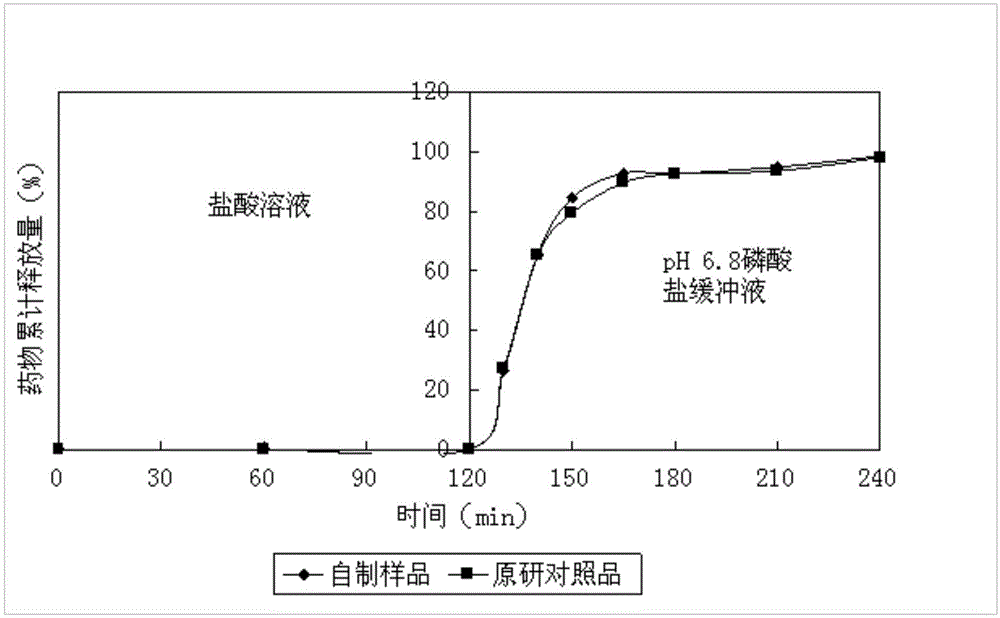
The invention relates to a duloxetine hydrochloride enteric mini-pill preparation. Mini-pills include medicine-carrying pill cores, isolation coating layers, enteric coating layers and dispensable modification layers. When a relatively common methyl acrylic copolymer is adopted as an enteric material in a prescription, the purpose that an in-vitro dissolution curve of active ingredients is consistent to positive control medicine Cymbalta is achieved by adding sorbitol into the isolation layers. The duloxetine hydrochloride enteric mini-pill preparation adopts medicinal acrylic resin which is marked with medicinal registration batch number and easy to obtain as an enteric material. An adopted conventional preparation method and a process are simple, the duloxetine hydrochloride enteric mini-pill preparation is suitable for industrial production, and a medicine reasonable in price and reliable in treating effect is provided for patients suffering from depression.
Owner:北京修正创新药物研究院有限公司
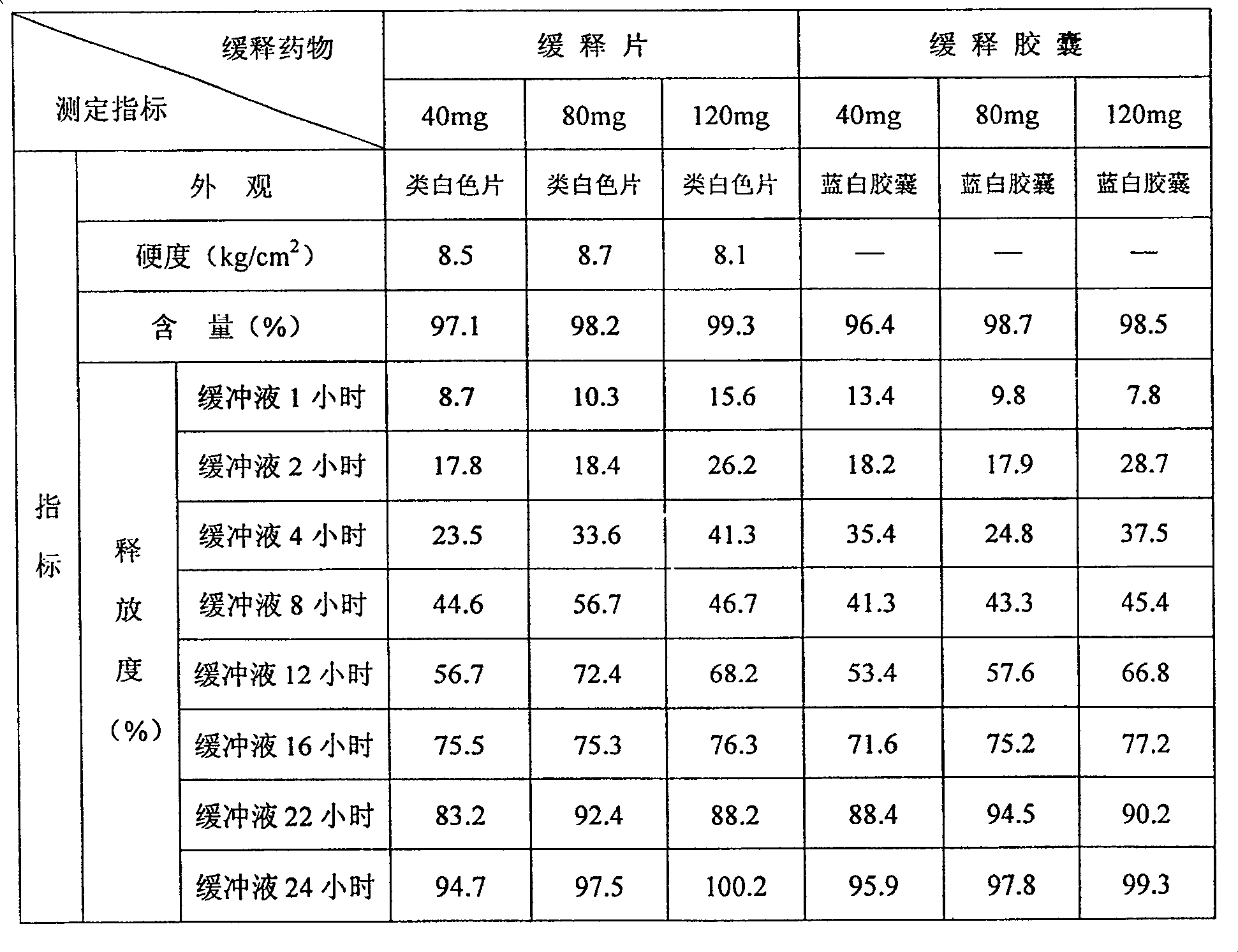
Duloxetine hydrochloride sustained release medicine
ActiveCN101164532AEase of industrial productionThe manufacturing process is simple and controllableOrganic active ingredientsNervous disorderOral medicineSolubility
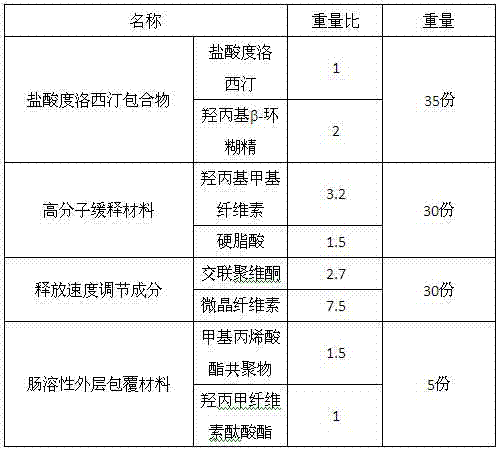
The present invention relates to a duloxetine hydrochloride slow-released medicine. Said slow-released medicine is formed from duloxetine hydrochloride and acceptable auxiliary components in oral medicine, in which the duloxetine hydrochloride content is 15-65% of total weight of said medicine. The described auxiliary components at least include (by wt%) 1%-80% of high-molecular skeleton slow-release material, 0.1%-50% of releasing speed regulation component and 1.5%-10% of enteric solubility external coating material.
Owner:CHONGQING SHENGHUAXI PHARMA CO LTD
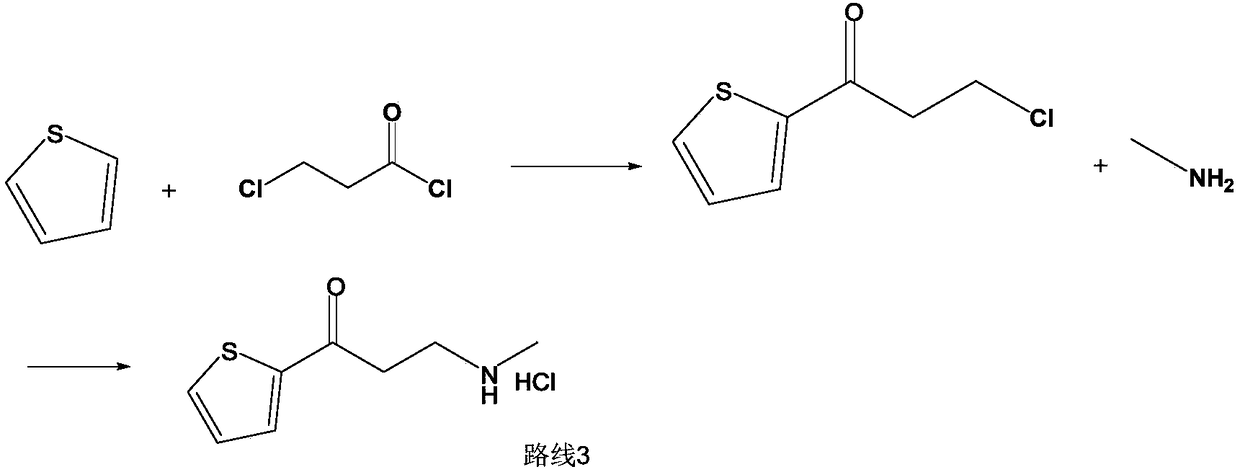
Process for synthesizing duloxetine hydrochloride
InactiveCN102070602AOvercoming low optical purityInhibit side effectsOrganic chemistryButanoneHydrochloride
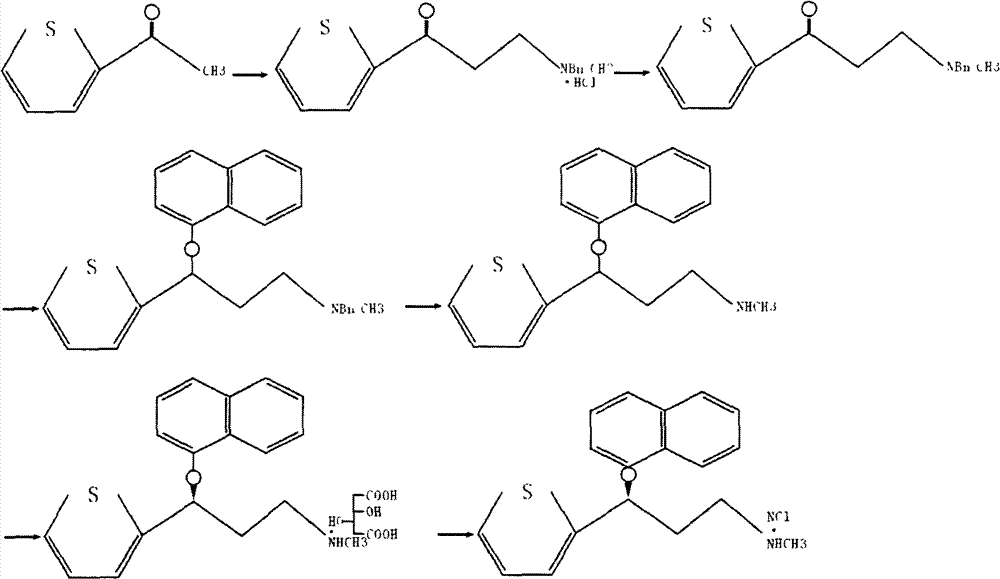
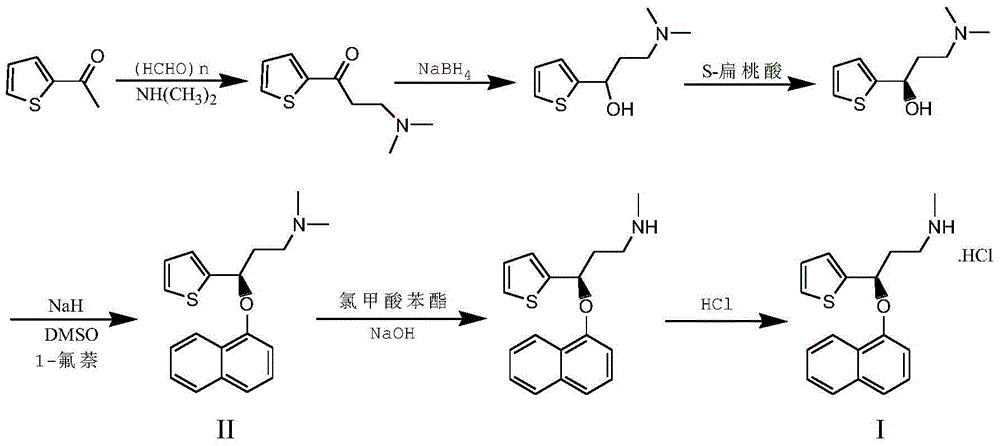
The invention belongs to the field of medicine synthesis, relates to a process for synthesizing duloxetine hydrochloride, and solves the technical problem to provide a new process for synthesizing the duloxetine hydrochloride, which makes raw materials dissolved completely and reduced thoroughly. The synthetic process comprises the following steps of: 1, preparing 2-thienyl-2-dimethylaminomethyl butanone hydrochloride; 2, preparing N,N-dimethyl-3-hydroxy-3-(2-thienyl)propanamine; 3, preparing (S)-N,N-dimethyl-3-hydroxy-3-(2-thienyl)propanamine; 4, preparing (S)-N,N-dimethyl-3-(1-naphthalenyloxy)-3-(2-thienyl)propanamine; 5, preparing (S)-N-methyl-3-(1-naphthalenyloxy)-3-(2-thienyl)propanamine; and 6, preparing (S)-N-methyl-3-(1-naphthalenyloxy)-3-(2-thienyl)propanamine hydrochloride. By the six steps of the process, the total synthetic yield is 24.1 percent.
Owner:王念军
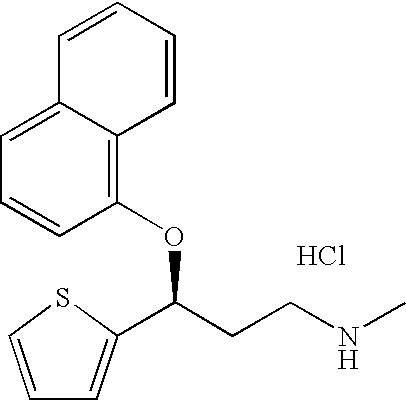
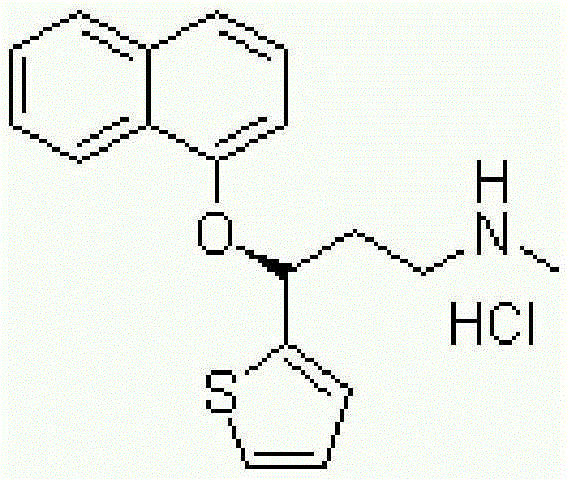
Pure duloxetine hydrochloride
Chemically and / or enantiomerically pure duloxetine HCl and process for preparing chemically and / or enantiomerically pure duloxetine HCl are provided.
Owner:TEVA PHARM USA INC
Duloxetine hydrochloride enteric capsules and preparation method thereof
InactiveCN102908331AGood reproducibilityUniform contentOrganic active ingredientsNervous disorderForeign - SimilarPharmaceutical formulation
The invention relates to the technical field of medicinal preparations, in particular to duloxetine hydrochloride-containing enteric capsules and a preparation method thereof. The enteric capsules consist of capsule shells and coated pellets, wherein the coated pellets also comprise duloxetine hydrochloride-containing medicine carrying pellet cores and coating layers; and the medicine carrying pellet cores contain a disintegrating agent. In preparation of the medicine carrying pellet cores, the duloxetine hydrochloride raw material is subjected to super micronization, so that the particle size distribution that the diameter of 99 percent is not greater than 50 mum, the diameter of 90 percent is not greater than 35 mum, the diameter of 50 percent is not greater than 20 mum and the diameter of 5 percent is not greater than 10 mum is achieved; and after the disintegrating agent is added into the medicine carrying pellet cores, the release speed of the medicine in intestines is increased. According to the duloxetine hydrochloride-containing enteric capsules and the preparation method thereof, a method of directly preparing the medicine carrying pellet cores is adopted, so that the complexity for loading a medicine on blank pellets of the existing domestic and foreign similar products is reduced, the technological process is simplified, and the duloxetine hydrochloride raw material is firstly crushed to a certain particle size range and then prepared into the medicine carrying pellet cores; and the prepared capsules have uniform medicine content and high reproducibility and are suitable for industrial production.
Owner:ZHEJIANG JIUZHOU PHARM SCI & TECH CO LTD
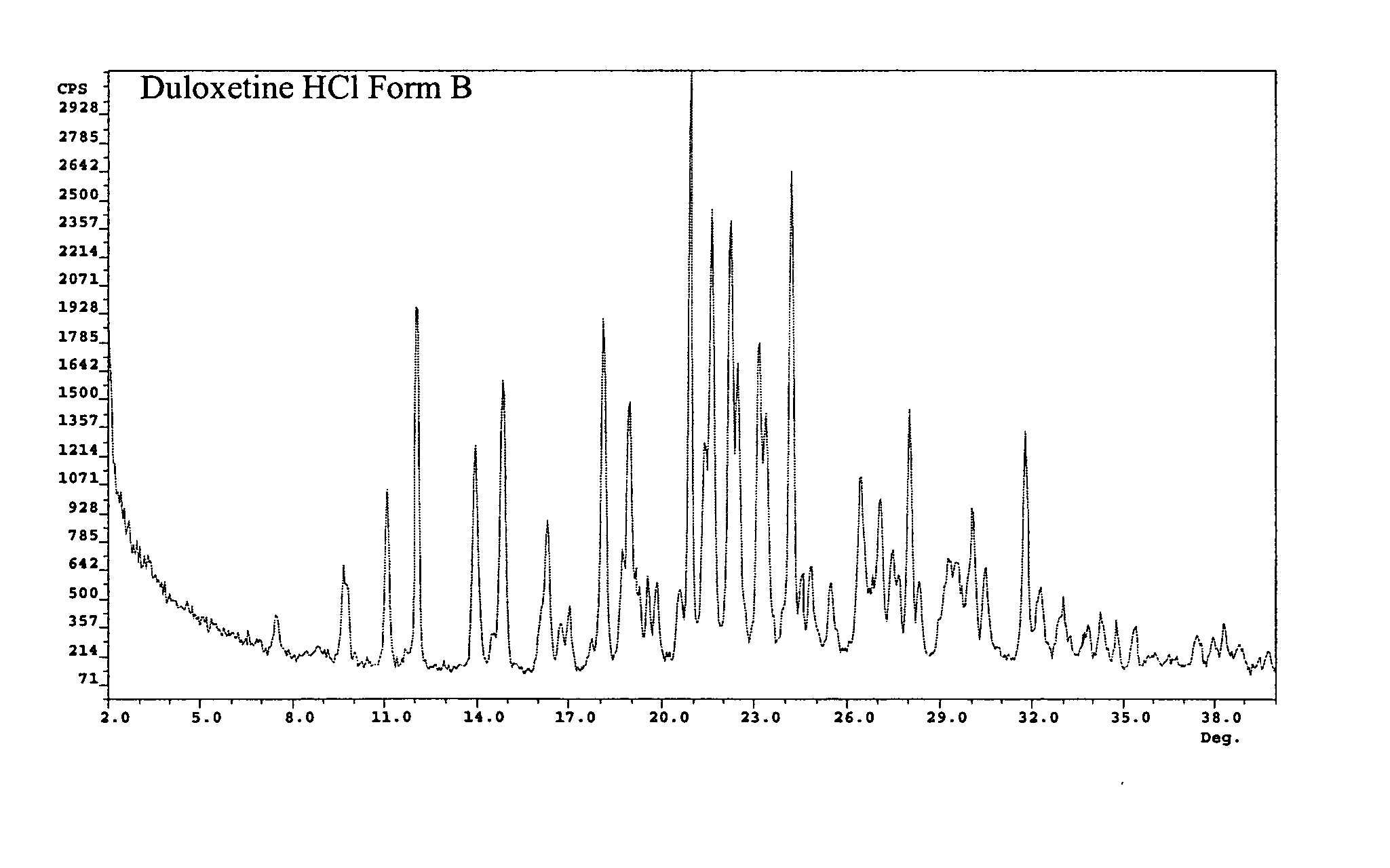
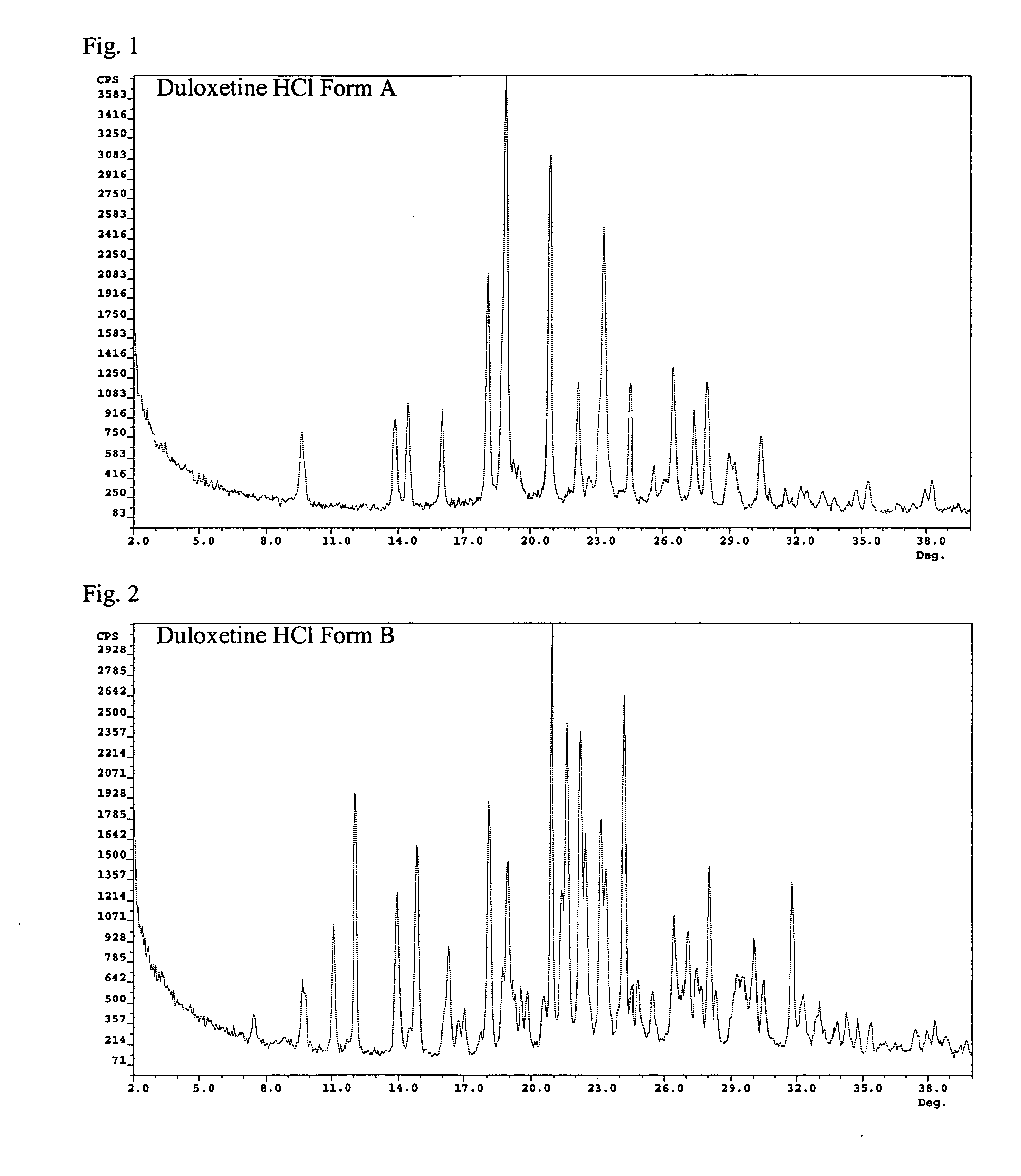
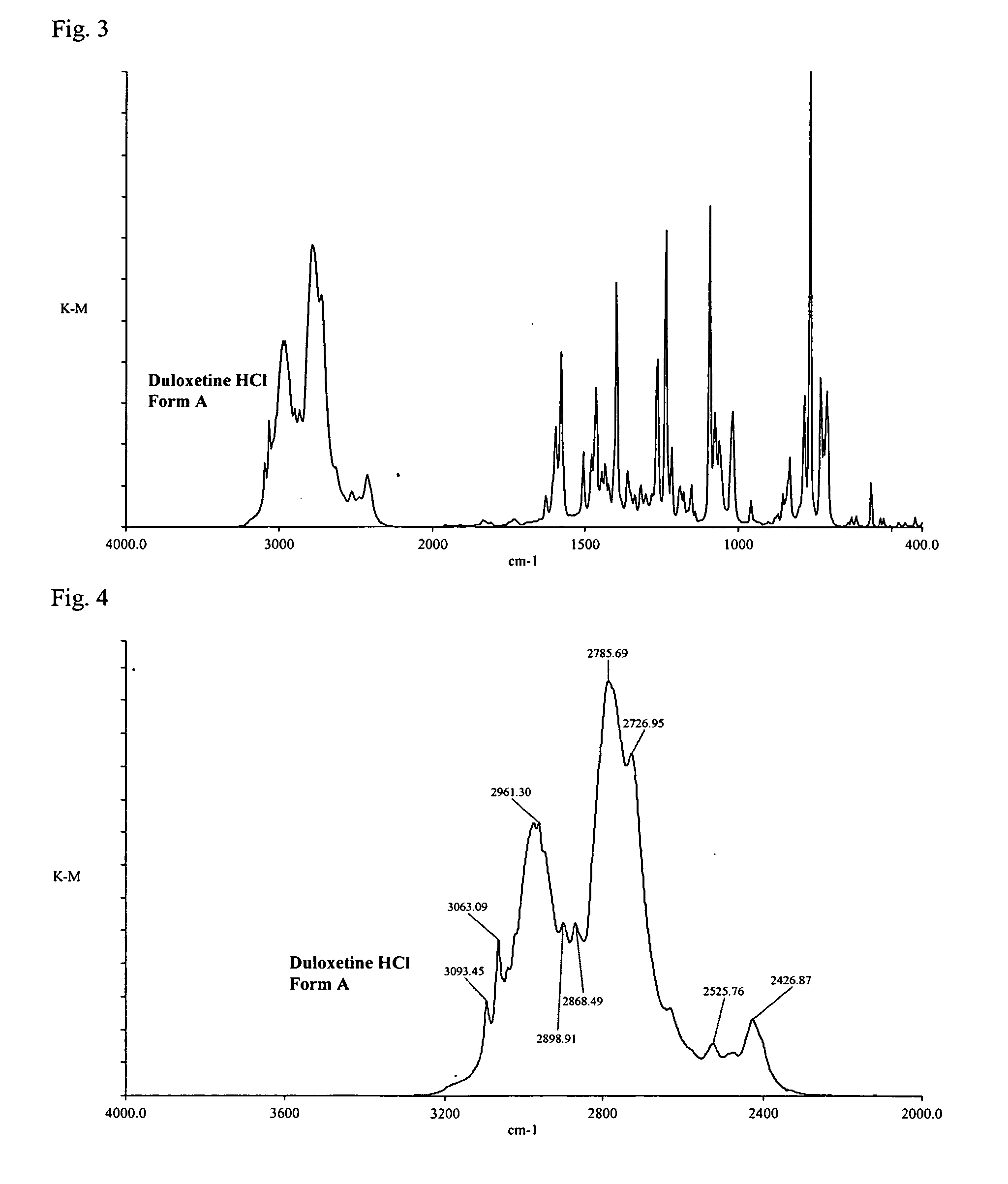
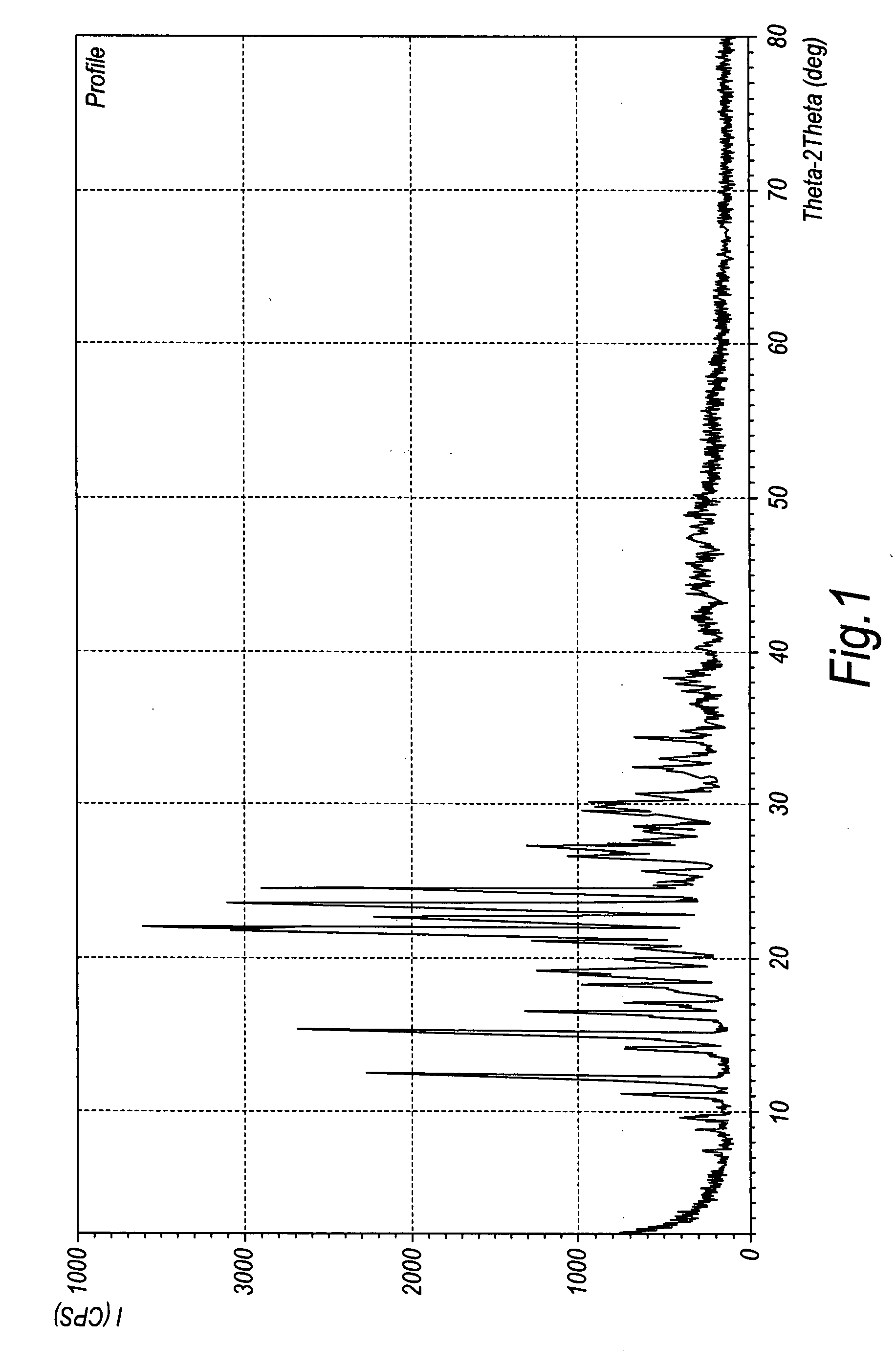
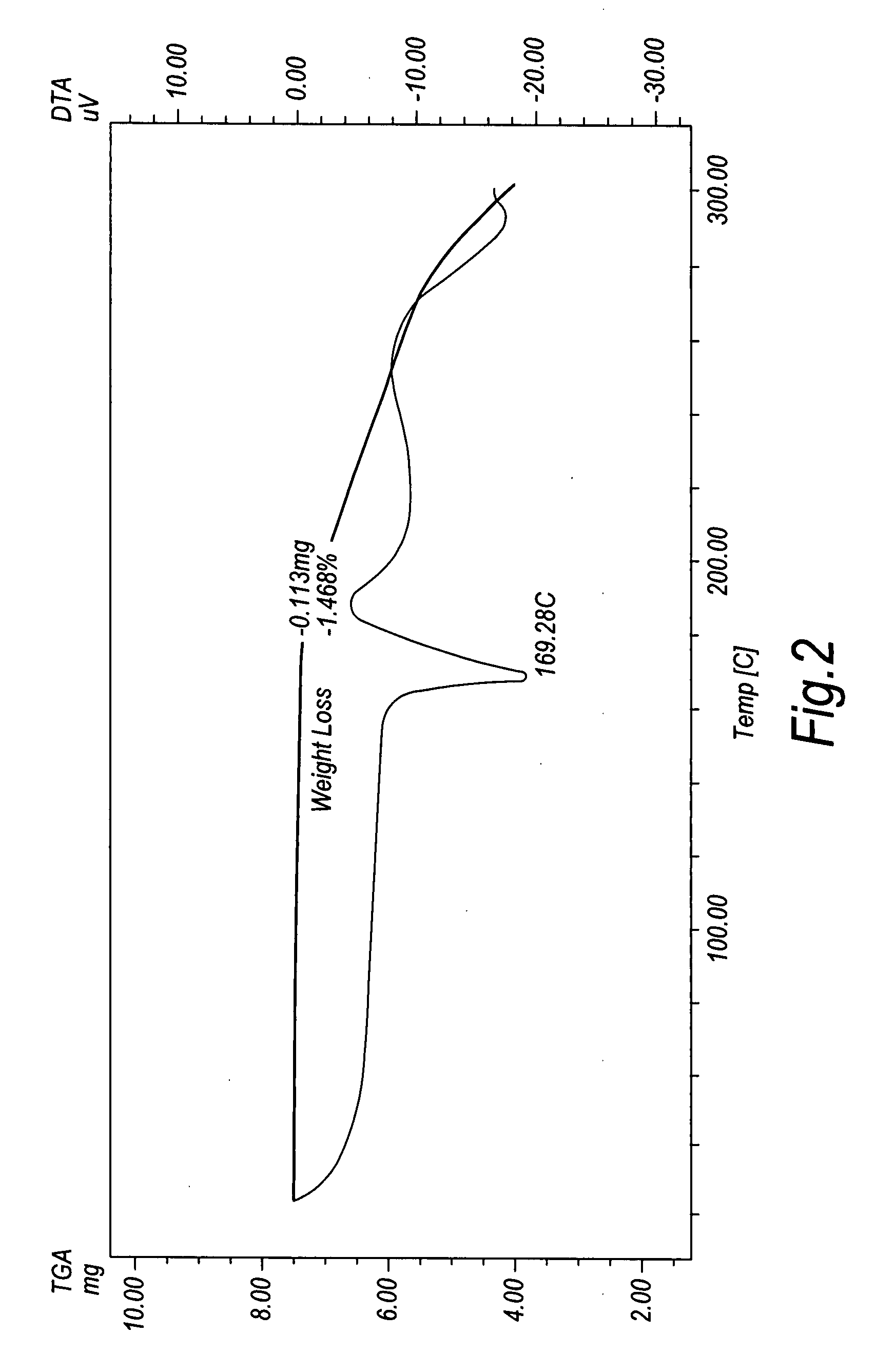
Duloxetine HCl polymorphs
A crystalline form of duloxetine hydrochloride, pharmaceutical compositions of the crystalline form of duloxetine hydrochloride, and methods of preparing the crystalline form of duloxetine hydrochloride are provided.
Owner:TEVA PHARM USA INC
Duloxetine hydrochloride sustained release medicine
ActiveCN100525760CHas enteric sustained-release functionQuick effectOrganic active ingredientsNervous disorderSustained release drugAdditive ingredient
Owner:CHONGQING SHENGHUAXI PHARMA CO LTD
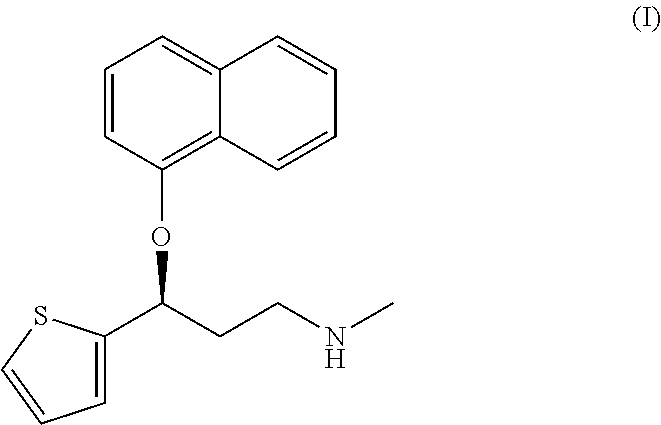
Method for the preparation of (s)-n-methyl-3-(1-naphthyloxy)-3-(2-thienyl)propylamine hydrochloride (duloxetine)
A method for the preparation of a duloxetine hydrochloride salt from a duloxetine base, comprising the steps of: reacting duloxetine base with concentrated hydrochloric acid in ethylmethylketone; and crystallizing duloxetine hydrochloride salt from said concentrated hydrochloric acid in ethylmethylketone.
Owner:ZENTIVA AS
Duloxetine hydrochloride enteric pellet and preparation method thereof
ActiveCN103565749AOvercome the problem of broken powderSmall particle sizeOrganic active ingredientsNervous disorderSugarProtection layer
The invention relates to a duloxetine hydrochloride enteric pellet and a preparation method thereof. The pellet comprises a blank cane sugar pellet core, a protection layer, a drug layer, an isolating layer, an enteric layer and an optional modification layer. The method comprises the following steps: coating the blank pellet core with the protection layer; and then carrying out coating by successively using the drug layer, the isolating layer, the enteric layer and the optional modification layer so as to prepare the duloxetine hydrochloride enteric pellet. According to the invention, the blank cane sugar pellet core with a small particle size is employed, coating with the protection layer is carried out at first, so the problems of fragmentation and powder falling of the pellet core in the process of coating are effectively overcome; the prepared enteric pellet has a small particle size, a capsule filled with the enteric pellet has good content uniformity, and industrial mass production of the enteric pellet is facilitated.
Owner:YAOPHARMA CO LTD
Duloxetine hydrochloride enteric micropill preparation
ActiveCN103505421AEasy to buyLow costOrganic active ingredientsNervous disorderAlcohol sugarsPharmaceutical drug
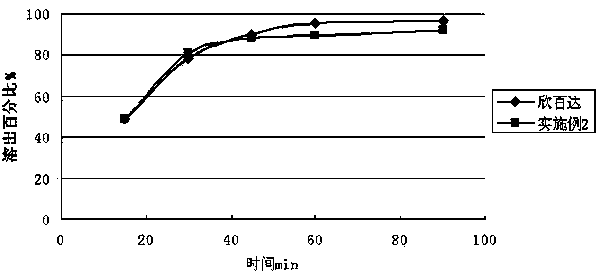
The invention relates to a duloxetine hydrochloride enteric micropill preparation which is composed of a drug-carrying pill core, an isolating coating layer and an enteric coating layer. When the enteric materials in the prescription adopt common methacrylic acid copolymers, sugar alcohol can be added into the isolating layer to achieve the goal that the active component in-vitro dissolution curve is consistent with a positive control drug (Cymbalta of Eli Lilly and Company). The product has the advantages of high acid resistance, high dissolution speed, high rate and the like; the methacrylic acid copolymers used as the enteric material are easy to purchase and low in cost; and the adopted conventional preparation method is simple in technique and suitable for industrial production, thereby providing a drug with reasonable price and reliable curative effect for patients with melancholia.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD +1
Process for the preparation of pure duloxetine hydrochloride
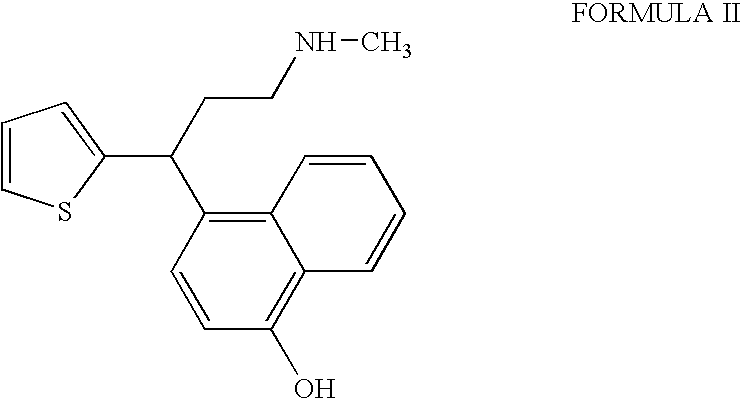
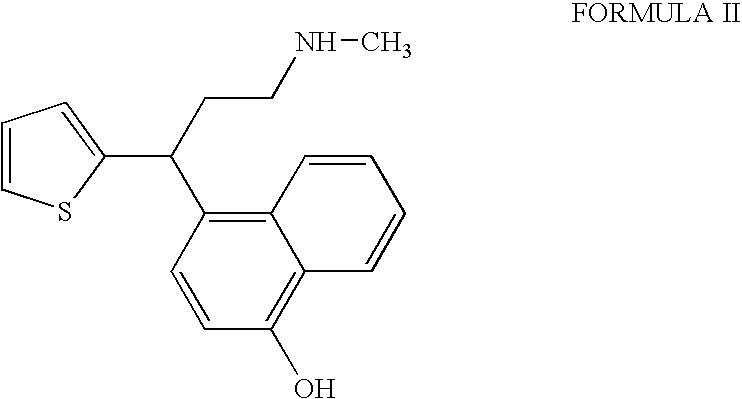
InactiveUS20090275760A1Increase contentFormation of the compound of Formula II can be minimizedNervous disorderOrganic chemistryDuloxetine HydrochlorideChemistry
The present invention relates to a process for the preparation of pure Duloxetine hydrochloride. The present invention further relates to duloxetine hydrochloride substantially free of residual hydrochloric acid.
Owner:SUN PHARMA INDS
Gastro-resistant pharmaceutical oral compositions comprising duloxetine or its pharmaceutically acceptable derivatives
The present invention relates to a pharmaceutical composition comprising an active core comprising duloxetine or its pharmaceutically acceptable derivatives, a separating layer comprising a pH modifier and a gastro-resistant coating comprising a gastro-resistant polymer selected from methacrylic acid copolymers and optionally an over-coating layer. The present invention also relates to a process for the preparation of duloxetine hydrochloride. It also relates to a packaged medicament.
Owner:KRKA D D NOVO MESTO
Pharmaceutic preparation of duloxetine hydrochloride and preparation method thereof
The invention belongs to the technical field of medicines, and in particular relates to a preparation method of duloxetine hydrochloride enteric coated drugs. The duloxetine hydrochloride is prepared by wrapping four layers of coating on a blank mini-pill, wherein the four layers of coatings are respectively a drug carrier layer, an isolation layer, a enteric coating layer and a film coating layer.
Owner:BEIJING WANQUAN SUNSHINE MEDICAL TECH CO LTD
New preparation process of medicinal raw material duloxetine hydrochloride of antidepressant drug
InactiveCN103360365AMild reaction conditionsClear workmanshipOrganic chemistryTreatment effectMannich reaction
The invention relates to a new preparation process of the medicinal raw material duloxetine hydrochloride of an antidepressant drug and belongs to the technical field of drug synthesis. The new preparation process is characterized in that N-methyl benzylamine hydrochloride but not dimethylamine hydrochloride is used in Mannich reaction; because benzyl is easier to remove as compared with methyl, dealkylation in a subsequent step has better effect and higher yield; an expensive chiral catalyst or a phase-transfer catalyst is not used; a better solvent crystallizing and removing method is adopted, so that the harm of a residual crystallizing solvent to a human body is prevented; the splitting of a chiral compound is carried out after dealkylation, and a mixture obtained after splitting is separated by adopting a unique recrystallization technology to prepare (S)-N-methyl-3-(1-naphthoxy)-3-(2-thienyl) propylamine / tartrate with high purity and high optical activity, so that a finally obtained duloxetine hydrochloride product can achieve better treatment effect. The new preparation process disclosed by the invention has the advantages of mildness in reaction condition, clear process, good easiness and convenience in operation and low production cost and is extremely favorable for industrial production.
Owner:李晓红
Method for preparing intermediate of duloxetine hydrochloride
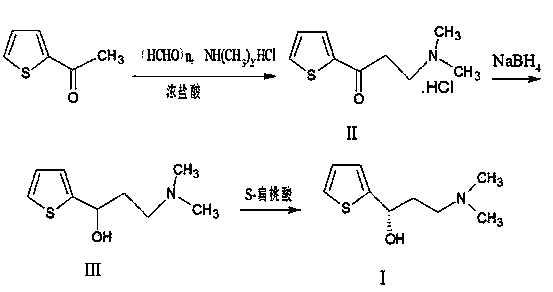
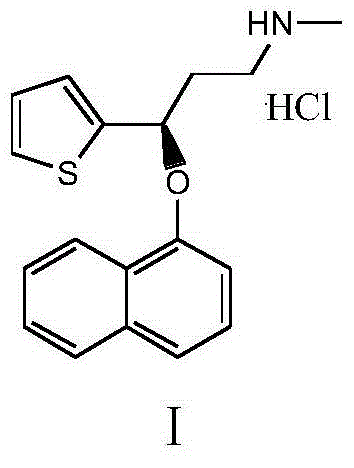
InactiveCN103508998AShorten the reduction reaction timeImprove reduction efficiencyOrganic chemistryPropylamineMandelic acid
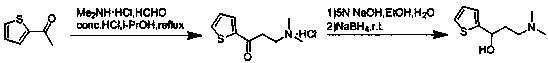
The invention relates to a method for preparing an intermediate of duloxetine hydrochloride. The method comprises the following steps: adding 2-acetylthiophene into concentrated hydrochloric acid for reacting, preparing a compound of a formula II, adding NaBH4 for reacting, and refining to prepare a compound of a formula III; taking the compound of the formula III and methyl tertiary butyl ether, adding S-mandelic acid for reacting, filtering, spraying, drying to obtain a white granular solid, dissolving the white granular solid in water, regulating a pH value, extracting by using dichloromethane, drying, and preparing S-(-)-N,N-dimethyl-3-hydroxy-3-(2-thienyl) propylamine (a compound of a formula I) after drying. The invention provides a simple method, so that the raw materials are completely dissolved, namely the consumption of NaBH4 is reduced, a reduction reaction can be completely carried out, and the maximum reaction rate can be 95.6 percent. Meanwhile, the invention provides a method for improving resolution yield and purity, and the maximum yield can be 47.6 percent. According to the improved process, the reaction time is greatly reduced, the operation is simplified, and the total yield of the compound of the formula I which is synthesized in three steps is more than 36 percent.
Owner:SHANDONG LUYAO PHARMA
Preparation of duloxetine hydrochloride
ActiveCN104829587AReduce salt forming stepsShorten the production cycleOrganic chemistryChloroformatePhenyl chloroformate
The invention belongs to the fields of organic chemistry and pharmaceutical chemistry, and particularly relates to a synthesis technique of duloxetine hydrochloride. By converting the R configuration compound into S configuration, compared with the duloxetine hydrochloride prepared from the single S-configuration compound, the total yield is enhanced by nearly 47%, and the production cost is lowered. 1-chloroethylchloroformate is used instead of phenyl chloro-formate to directly generate the duloxetine hydrochloride during demethylation, thereby reducing the salification step, shortening the production cycle and saving the cost.
Owner:SHANGHAI WANXIANG PHARMA
Duloxetine hydrochloride enteric micro-pellet capsule and preparation method thereof
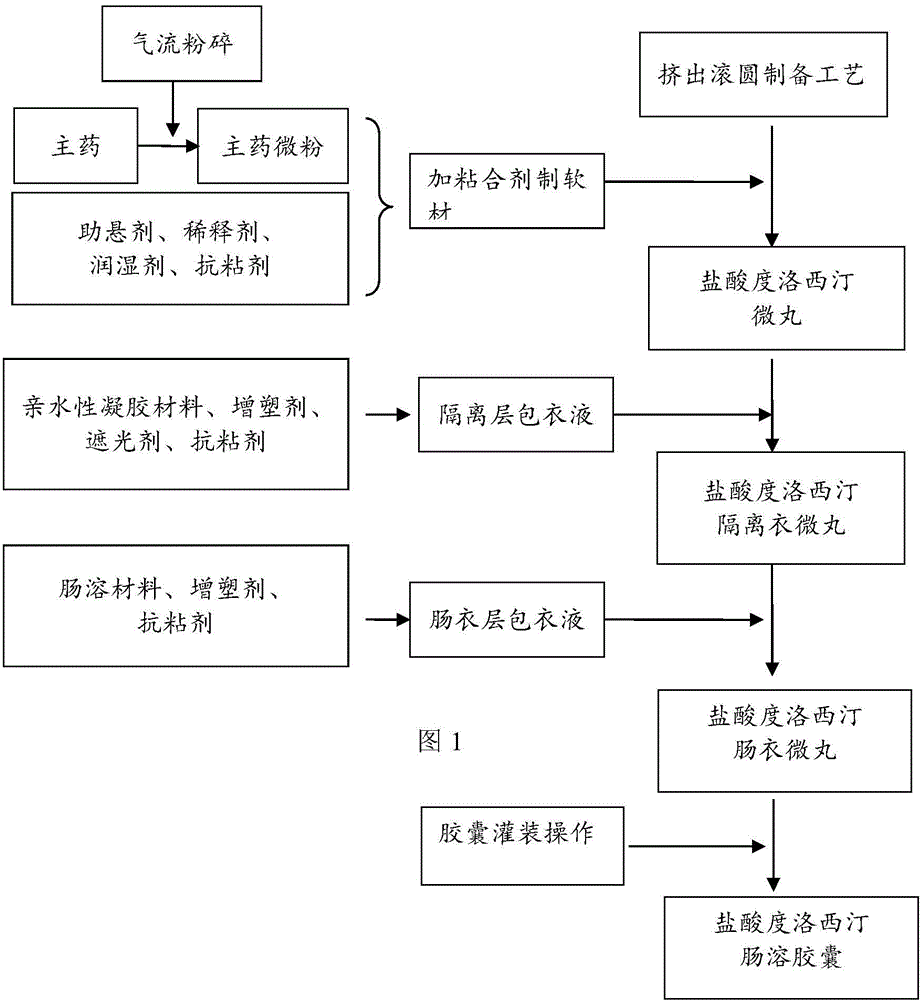
The invention provides a preparation method of a duloxetine hydrochloride enteric micro-pellet capsule. The preparation method comprises following steps: (1) performing air jet pulverization to duloxetine hydrochloride to obtain duloxetine hydrochloride micro-particles; (2) mixing the duloxetine hydrochloride micro-particles with a medicinal excipient and preparing duloxetine hydrochloride micro-pellets through an extrusive granulation technology; (3) wrapping the duloxetine hydrochloride micro-pellets with an isolating layer for reducing mutual influence between main drugs and an enteric coating material; and (4) wrapping the isolating layer on the duloxetine hydrochloride micro-pellets by an enteric coating layer for achieving the enteric effect.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH +1
Enteric sustained release granules containing duloxetine hydrochloride and preparation method of enteric sustained release granules
InactiveCN107412198ATo achieve a sustained release effectAvoid degradationOrganic active ingredientsNervous disorderBlood drug concentrationDrug effect
The invention discloses enteric sustained release granules containing duloxetine hydrochloride and a preparation method of the enteric sustained release granules. According to the preparation disclosed by the invention, duloxetine hydrochloride is prepared into a clathrate compound, and then the clathrate compound is prepared into enteric granules. The enteric sustained release granules comprise the duloxetine hydrochloride clathrate compound and acceptable auxiliary materials, wherein the auxiliary materials at least comprise a macromolecular sustained-release material, a release speed regulating ingredient and an enteric outer-layer coating material, the medicine has erosion and release in the intestinal tract, the action time is lasting, the side reaction occurrence rate caused by rapid release of the medicines after medicine taking is remarkably reduced, so that the blood drug concentration and the drug effect are stable and durable, and the medication compliance and clinical treatment effect of patients are enhanced.
Owner:BEIJING VENTUREPHARM BIOTECH
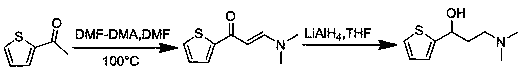
Synthesis method of key intermediate for preparing duloxetine hydrochloride from 2-acetylthiophene
The invention provides a synthesis method of a key intermediate for preparing duloxetine hydrochloride from 2-acetylthiophene and belongs to the technical field of chemical synthesis. The synthesis method comprises the following steps: reacting cyanuric chloride and N,N-dimethylformamide with 2-acetylthiophene to obtain 3-(dimethylamino)-1-(2-thienyl)-2-propenyl-1-one; reducing 3-(dimethylamino)-1-(2-thienyl)-2-propenyl-1-one through lithium aluminum hydride to obtain a duloxetine hydrochloride intermediate which is (R,S)-N,N-dimethyl-3-hydroxy-3-(2-thienyl)propylamine. The synthesis method iscapable of synthesizing a target product which is a key intermediate of duloxetine hydrochloride through two-step reaction, is cheap in raw materials, simple in process, simple and convenient to operate, mild in reaction condition, short in reaction period and free of expensive catalysts, is environmentally friendly, and is suitable for industrial production; the prepared products are high in yield and purity.
Owner:ZHEJIANG UNIV OF TECH
A kind of duloxetine hydrochloride pharmaceutical composition, enteric-coated sustained-release preparation and preparation method
ActiveCN103637997BAvoid destructionGuaranteed stabilityOrganic active ingredientsNervous disorderMedicineDrug release
Owner:QINGDAO HUANGHAI PHARM CO LTD
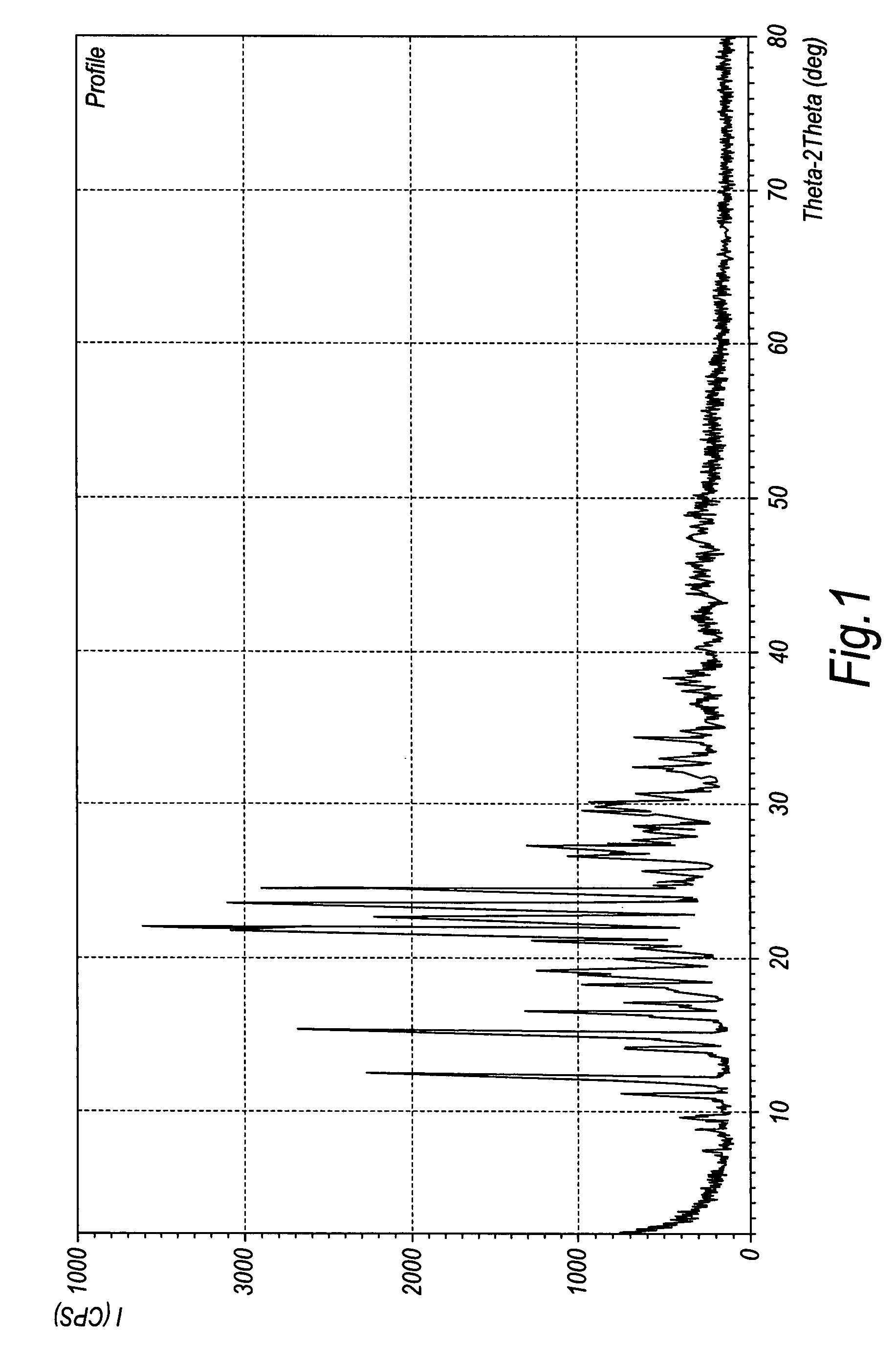
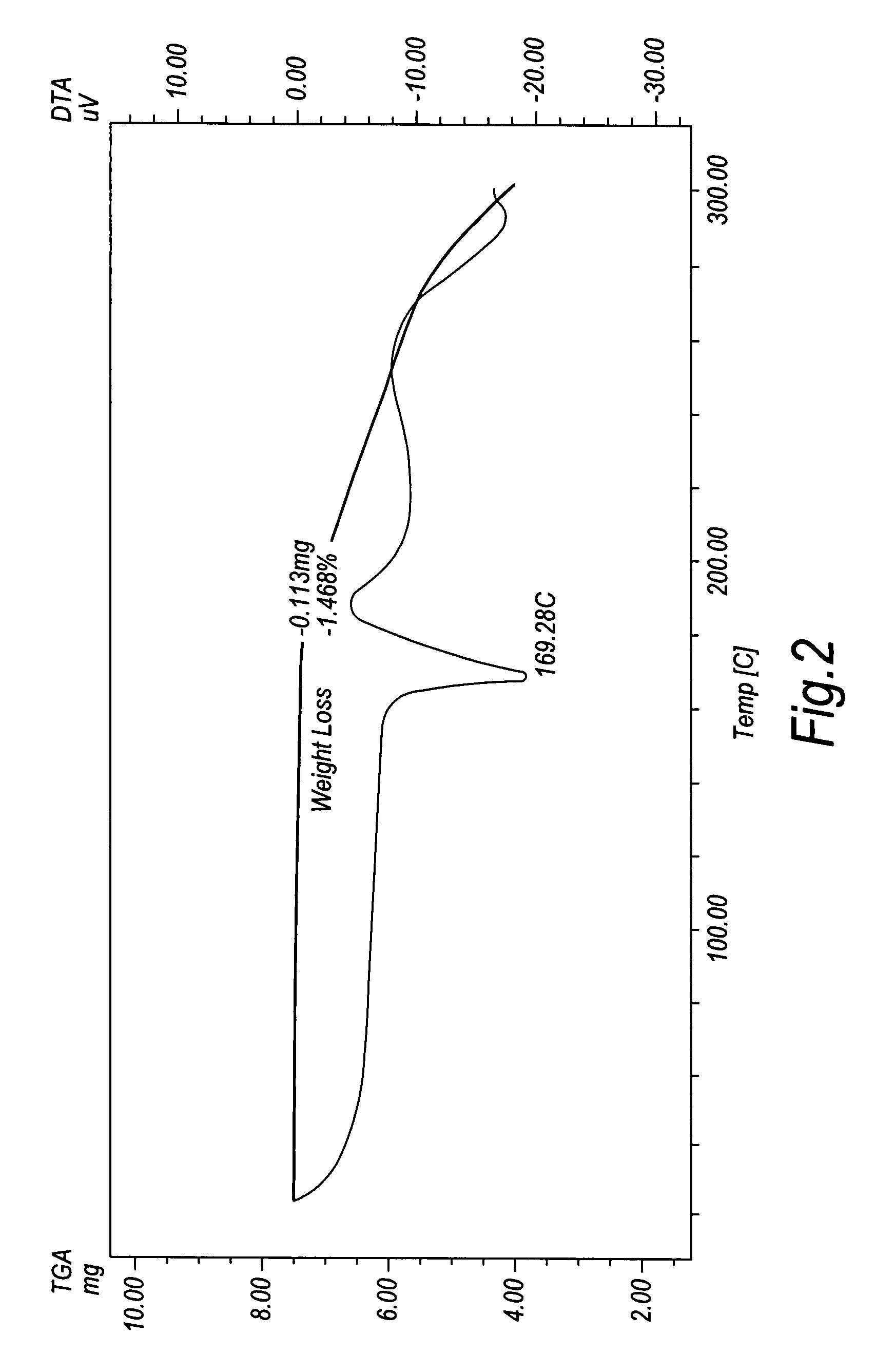
Crystalline duloxetine hydrochloride
InactiveUS7795454B2Improve purification effectCheaper and more convenient to perform crystallisation on a large scaleBiocideNervous disorderCrystallographyDuloxetine Hydrochloride
Owner:ARROW INT INC
Purification method for preparing high-purity duloxetine hydrochloride intermediate
The invention relates to a purification method for preparing high-purity duloxetine hydrochloride intermediate (S)-(-)-N,N-dimethyl-3-hydroxy-3-(2-thienyl)propanamine; (S)-(-)-N,N-dimethyl-3-hydroxy-3-(2-thienyl)propanamine is subjected to program-controlled recrystallization in a solvent to obtain a purified product, wherein the solvent is a mixed solvent of methanol and water, and program-controlled recrystallization includes the specific steps of heating and refluxing at 72-74 DEG C for 5-60 min, stirring at a speed of 0.5-15 r / min, cooling for crystallization at a temperature range of 72-80 DEG C for 120-150 min, and stirring at a speed of 6-15 r / min. The purification method is simple to perform, purity of isomer impurity is less than 0.03%, and the purification method is suitable for industrial production.
Owner:苏州正济药业有限公司
Duloxetine hydrochloride medicament composition, enteric sustained release preparation and preparation method thereof
ActiveCN103637997AAvoid destructionGuaranteed stabilityOrganic active ingredientsNervous disorderMedicineDrug release
The invention provides a duloxetine hydrochloride medicament composition, an enteric sustained release preparation and a preparation method thereof. The enteric sustained release preparation comprises the following components in parts by weight: 5-40 parts of duloxetine hydrochloride, 35-65 parts of sodium alginate, 5-20 parts of calcium salt, 15-35 parts of filler and 0.5-20 parts of lubricant. The medicament composition and the enteric sustained release preparation provided by the invention are hardly released under the gastric acid condition, so that a main medicine is prevented from being damaged in a stomach, no functional sustained release coating is needed to control medicine release, no enteric sustained release material is needed, only high-velocity sodium alginate and other medical auxiliaries are used, the enteric effect is achieved by adjusting the ratio, meanwhile the sustained release function is achieved, the medicine is effectively prevented from being damaged by the gastric acid environment, and the stability of the medicament inside a body in the absorption process is maintained.
Owner:QINGDAO HUANGHAI PHARM CO LTD
Chrystalline duloxetine hydrochloride
InactiveUS20080021090A1Improve purification effectCheaper and more convenient to perform crystallisation on a large scaleBiocideNervous disorderDuloxetine HydrochlorideChemistry
Crystalline duloxetine hydrochloride, compositions containing the same and methods for the production thereof.
Owner:ARROW INT INC
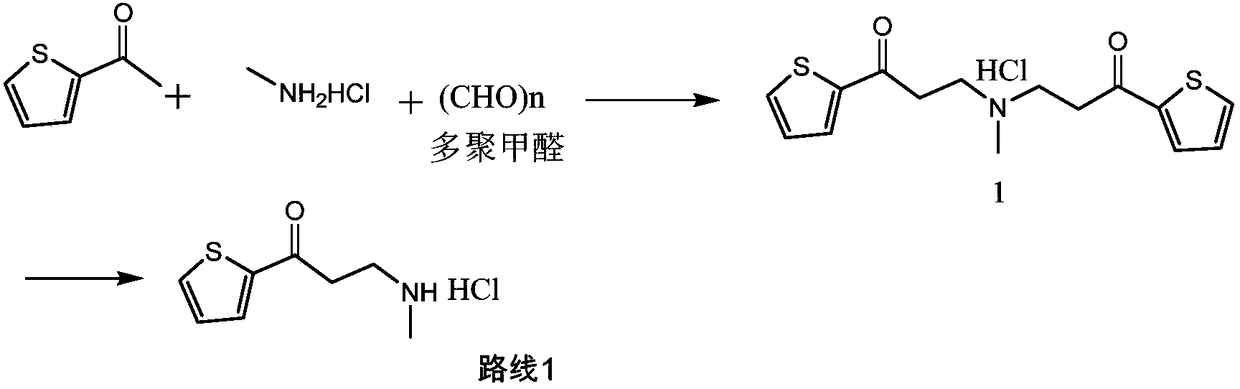
Synthetic method of 3-methylamino-1-(2-thienyl)-1-acetone hydrochloride
ActiveCN109134427AReduce pressure on environmental protectionNo irritating smellOrganic chemistryEnvironmental resistanceHigh pressure
The invention discloses a synthetic method of a compound 3-methylamino-1-(2-thienyl)-1-acetone hydrochloride, namely an intermediate of duloxetine hydrochloride, and relates to the field of drug synthesis. According to the method, a compound II, a compound III and a compound IV are taken as raw materials, and the compound I is obtained through the effect of a catalyst in a polar solvent. The usedcatalyst is one or more of silver trifluoromethanesulfonate and indium chloride. The maximum improvement characteristic of the synthetic method lies in that the product can be obtained at high yield without the conditions of high pressure and high temperature. Compared with the prior art, the raw materials used in the synthetic method are relatively low in environmental protection pressure and free of pungent smell; and moreover, the synthetic method is simple in technology, relatively low in requirement for equipment, simple and convenient in aftertreatment and high in yield.
Owner:ZHEJIANG LEPU PHARMA CO LTD
Duloxetine hydrochloride enteric-coated tablets and preparation method thereof
InactiveCN106619556AFully absorbedImprove bioavailabilityOrganic active ingredientsNervous disorderFood deliveryBioavailability
The invention provides duloxetine hydrochloride enteric-coated tablets. The tablets are prepared from components in parts by weight shown in the specification. The duloxetine hydrochloride enteric-coated tablets are seldom affected by food delivery rhythm of digestive tracts, the distribution area on intestinal tract surfaces is increased, so the drugs are completely absorbed, the bioavailability of the drugs is increased, and the plasma concentration fluctuation is reduced. The method is suitable for industrial production and has higher application value.
Owner:HUNAN DONGTING PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com