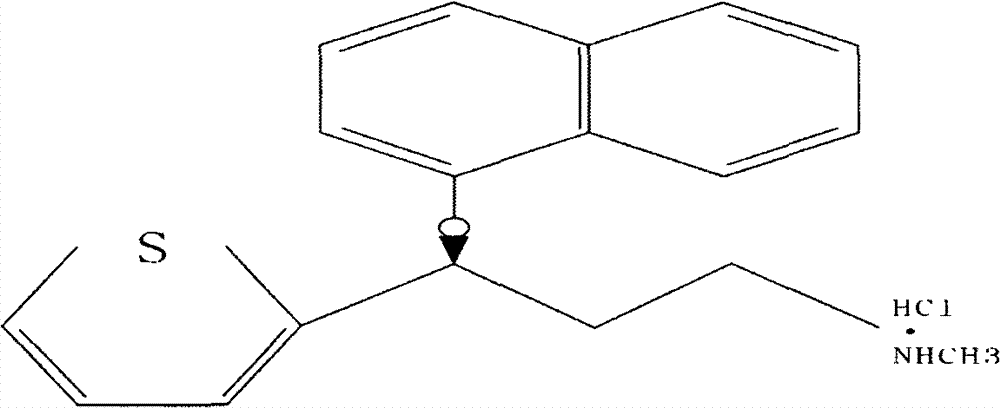
New preparation process of medicinal raw material duloxetine hydrochloride of antidepressant drug
An anti-depression and new technology technology, applied in the direction of organic chemistry and the like, can solve the problems of duloxetine hydrochloride not reaching the therapeutic effect, insufficient optical rotation of chiral compounds, and poor dealkylation reaction effect, etc. The effect of clear process, low production cost and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
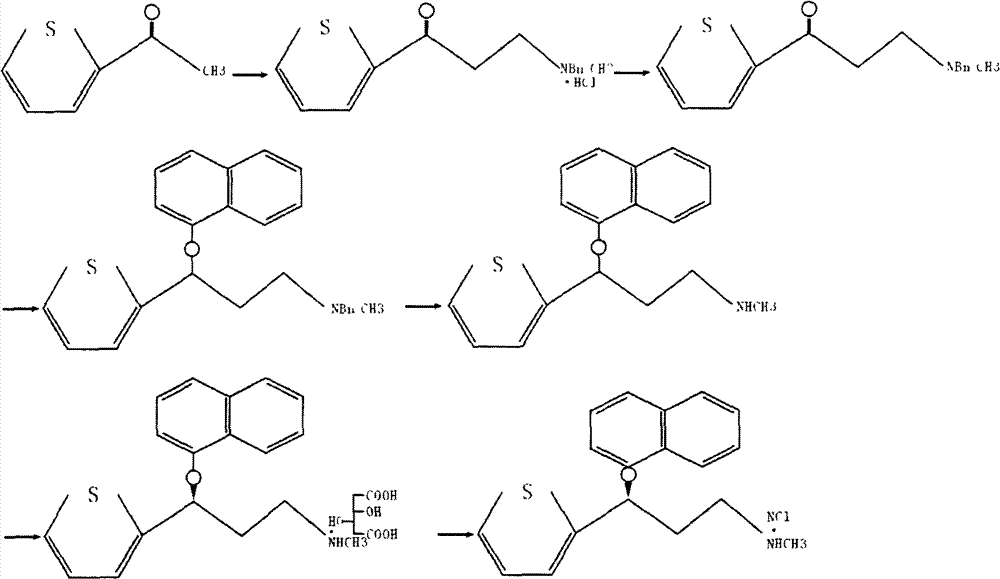
[0023] In a 1000ml three-necked flask with a reflux device, add 126.0g (1.0mol) of 2-acetylthiophene, 196.9g (1.25mol) of N-methylbenzylamine hydrochloride, and 45.0g (1.5mol) of polyaldehyde, and concentrate 10ml of hydrochloric acid, 300ml of isopropanol, heated to reflux under stirring, reacted for 8 hours, after the reaction of the raw materials was monitored by TCL, cooled to room temperature, filtered, the filter cake was washed twice with 100ml of ethanol, and the obtained solid was vacuum-dried to obtain a white crystal 274.6 g, m.p: 173-176°C, yield 94.2%.
Embodiment 2
[0025] In a 1000ml three-necked flask, 291.5g (1.0mol) of 1-(N-methyl-N-benzyl)amino-3-(2-thienyl)-3-acetone hydrochloride prepared in Example 1 was added, and 300ml Ethanol and 100ml of water to dissolve all the solids, control the temperature below 20°C, slowly add about 40g of 30% sodium hydroxide solution to pH = 11 under stirring, slowly add 37.0g (0.5mol) of sodium borohydride solution dropwise, and stir at room temperature After reacting for 3 hours, 150ml of acetone was added, stirred for 30 minutes, ethanol was distilled off under reduced pressure, and filtered by suction to obtain 231.3g of white solid, with a yield of 90%.
Embodiment 3
[0027] In a 1000ml three-necked flask, add 128.5g (0.5mol) of (RS)-(N-methyl-N-benzyl)-3-hydroxyl-3-(2-thienyl)propylamine prepared in Example 2, DMSO500ml, add Potassium hydroxide 112g (1.0mol), 1-fluoronaphthalene 73g (0.5mol), heated to 110°C and stirred for 2 hours, cooled to 20°C, filtered to obtain 162.8g of solid, yield 85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com