Enteric sustained release granules containing duloxetine hydrochloride and preparation method of enteric sustained release granules
A technology of duloxetine hydrochloride and loxetine intestine, which is applied in the direction of pharmaceutical formulas, medical preparations with no active ingredients, medical preparations containing active ingredients, etc., can solve the problem of strict technical parameters for the preparation of enteric-coated pellets It is unfavorable for large-scale industrial production and long preparation time, and achieves the effects of improving medication compliance, reducing the number of medications, and reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
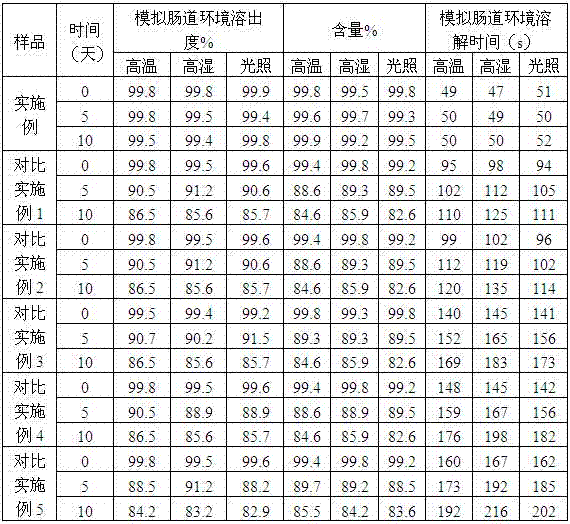
Examples
Embodiment
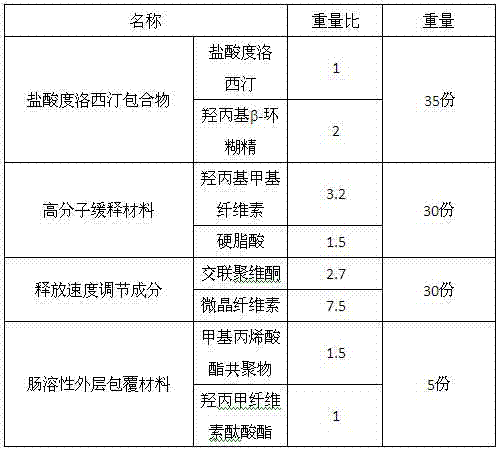
[0018] Duloxetine hydrochloride: 1:2 hydroxypropyl β-cyclodextrin
[0019]
[0020] Preparation Process:
[0021] In an aqueous medium containing 50% ethanol, react duloxetine hydrochloride with hydroxypropyl β-cyclodextrin according to the prescription ratio, filter the resulting solution through a microporous membrane until clarified, separate the clathrate from the mixture Pass the duloxetine hydrochloride inclusion compound through a 100-mesh sieve, and crush the rest of the auxiliary materials through a 80-mesh sieve; accurately weigh the raw and auxiliary materials in the prescribed amount, mix them evenly, and add an appropriate amount of water to make the mixture into a wet material. Sieve and granulate, dry at 60°C, and coat the obtained granules with enteric-coated material in a fluidized bed to obtain enteric-coated sustained-release granules.
[0022] In this application, specific excipients were selected to prepare duloxetine hydrochloride inclusion compound e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com