Duloxetine hydrochloride enteric mini-pill preparation
A technology of loxetine intestinal and hydrochloric acid degree, which is applied in the directions of medical preparations containing active ingredients, microcapsules, organic active ingredients, etc., can solve the problems that HPMCAS is not easy to buy, unfavorable in process, and high in production cost, and achieves improved acid resistance. properties, promoting solubility, and being easily degradable
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
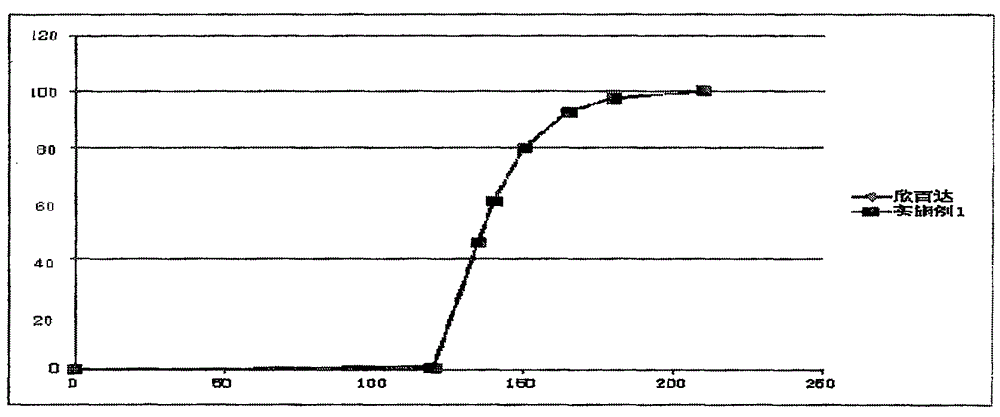
Image
Examples
Embodiment 1
[0041] Table-1 Example 1 prescription (1000 capsules)
[0042]
[0043]
[0044] Preparation Process
[0045] 1. Preparation of drug-loaded pellet cores: suspend duloxetine hydrochloride in 5% HPMC aqueous solution, and prepare drug-loaded pellets by spraying the suspension on blank sugar pill cores. Set the inlet air temperature of the bottom spray fluidized bed at 40-45°C, the atomization pressure at 1.0-1.5bar, and the air volume at 40-50m 3 / h, control the temperature of the material at 37-40°C.
[0046] 2. Preparation of isolation coat layer: After dissolving sorbitol and HPMC, add Talc to fully disperse until uniform. The suspension is sprayed onto the drug-loaded pellet core prepared in step 1 through a bottom-spray fluidized bed to obtain isolated drug-loaded pellets. Set the inlet air temperature of the bottom spray fluidized bed at 40-45°C, the atomization pressure at 1.0-1.5bar, and the air volume at 40-50m 3 / h, control the temperature of the material at ...
Embodiment 2
[0052] Table-2 embodiment 2 prescription (1000 grains)
[0053]
[0054]
[0055] Preparation process: with embodiment 1.
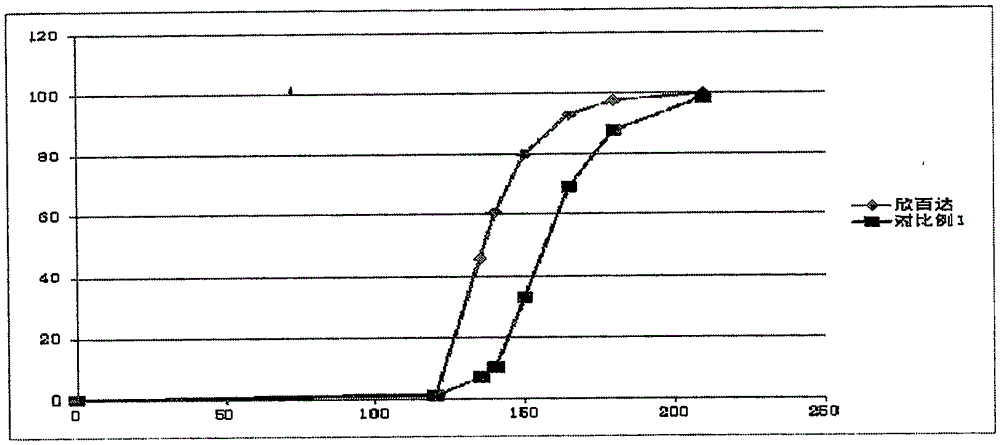
[0056] Dissolution profile see figure 2 , it can be seen that the dissolution behavior of the enteric-coated pellet preparation of Example 2 is similar to that of the positive control drug (Cymbalta), and better dissolution can be achieved.
Embodiment 3
[0058] Table-3 embodiment 3 prescription (1000 grains)
[0059]
[0060] Preparation process: with embodiment 1.
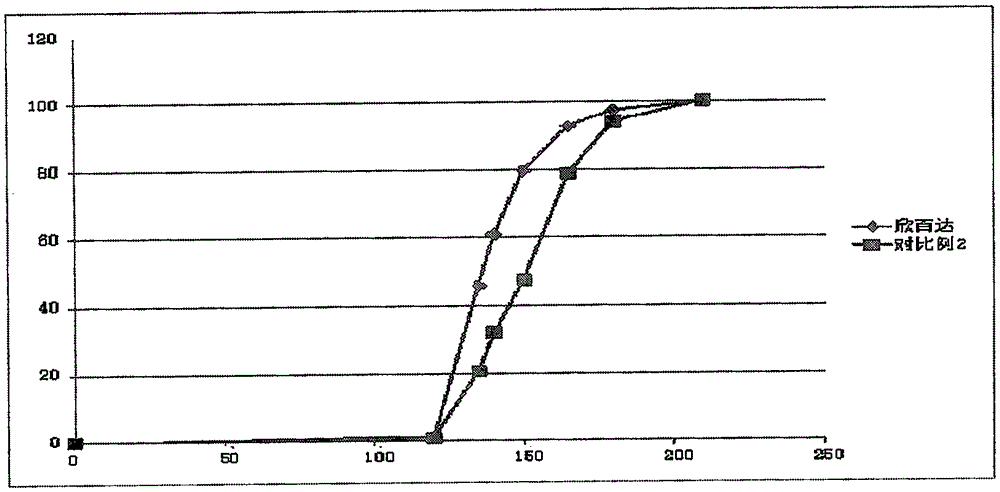
[0061] Dissolution profile see image 3 ,Depend on image 3 It can be seen that the dissolution behavior of the enteric-coated pellet preparation of Example 3 is similar to that of the positive control drug (Cymbalta), and better dissolution can be achieved.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com