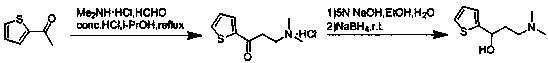
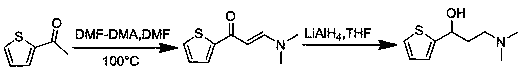
Synthesis method of key intermediate for preparing duloxetine hydrochloride from 2-acetylthiophene
A technology of duloxetine hydrochloride and acetylthiophene, which is applied in the field of synthesis of key intermediates of duloxetine hydrochloride prepared from 2-acetylthiophene, can solve problems such as volatile, expensive and unstable DMF-DMA raw materials, and achieve The effect of short reaction cycle, avoiding the use of expensive catalysts, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: Preparation of (dimethylamino)-1-(2-thienyl)-2-propene-1-one
[0031] Preparation of sodium methoxide / methanol solution: Dissolve 2.7g (50mmol) of sodium methoxide in 10mL of methanol and dissolve evenly to obtain sodium methoxide / methanol solution;
[0032] Add 18.45g (100mmol) of cyanuric chloride, 9.5mL of 1,4-dioxane, and 7.31g (100mmol) of N,N-dimethylformamide into a 250mL three-necked flask and mix well to obtain a mixed solution; solution and 12.60g (100mmol) of 2-acetylthiophene, added to the sodium methoxide / methanol solution, stirred and mixed, the temperature of the reaction solution was controlled to 20°C for the reaction, followed by TLC (petroleum ether: ethyl acetate = 2:1), the reaction ended Afterwards, the solvent was distilled off under reduced pressure, and crystals were precipitated. The crystals were recrystallized in a mixed solvent of dichloromethane:petroleum ether (V:V=1:3), and 14.12g of a light yellow solid was obtained after s...
Embodiment 2
[0034] Preparation of 3-(dimethylamino)-1-(2-thienyl)-2-propen-1-one:
[0035] Preparation of sodium methoxide / methanol solution: Dissolve 10.8g (200mmol) of sodium methoxide in 33mL of methanol and dissolve evenly to obtain sodium methoxide / methanol solution;
[0036] Add 55.35g (300mmol) of cyanuric chloride, 148mL of 1,4-dioxane, and 36.54g (500mmol) of N,N-dimethylformamide into a 250mL three-necked bottle and mix well to obtain a mixed solution; and 12.60 g (100 mmol) of 2-acetylthiophene, added to the sodium methoxide / methanol solution, stirred and mixed, controlled the temperature of the reaction solution to 40°C for the reaction, followed by TLC (petroleum ether: ethyl acetate = 2:1), after the reaction , the solvent was distilled off under reduced pressure, and crystals were precipitated. The crystals were recrystallized in a mixed solvent of dichloromethane:petroleum ether (V:V=1:3), and after suction filtration and drying, 15.31g of a light yellow solid was obtained...
Embodiment 3
[0038] Preparation of 3-(dimethylamino)-1-(2-thienyl)-2-propen-1-one:
[0039] Preparation of sodium methoxide / methanol solution: Dissolve 5.4g (100mmol) of sodium methoxide in 22mL of methanol and dissolve evenly to obtain sodium methoxide / methanol solution;
[0040] Add 7.38g (40mmol) of cyanuric chloride, 73mL of 1,4-dioxane, and 58.5g (800mmol) of N,N-dimethylformamide into a 250mL three-necked bottle and mix well to obtain a mixed solution; Add 12.60 g (100 mmol) of 2-acetylthiophene to sodium methoxide / methanol solution, stir and mix, control the temperature of the reaction solution to 50°C for reaction, TLC tracking (petroleum ether: ethyl acetate = 2:1), after the reaction , the solvent was distilled off under reduced pressure, and crystals were precipitated. The crystals were recrystallized in a mixed solvent of dichloromethane:petroleum ether (V:V=1:3), and 16.51g of a light yellow solid was obtained after suction filtration and drying, which was 3-(di Methylamino)-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com