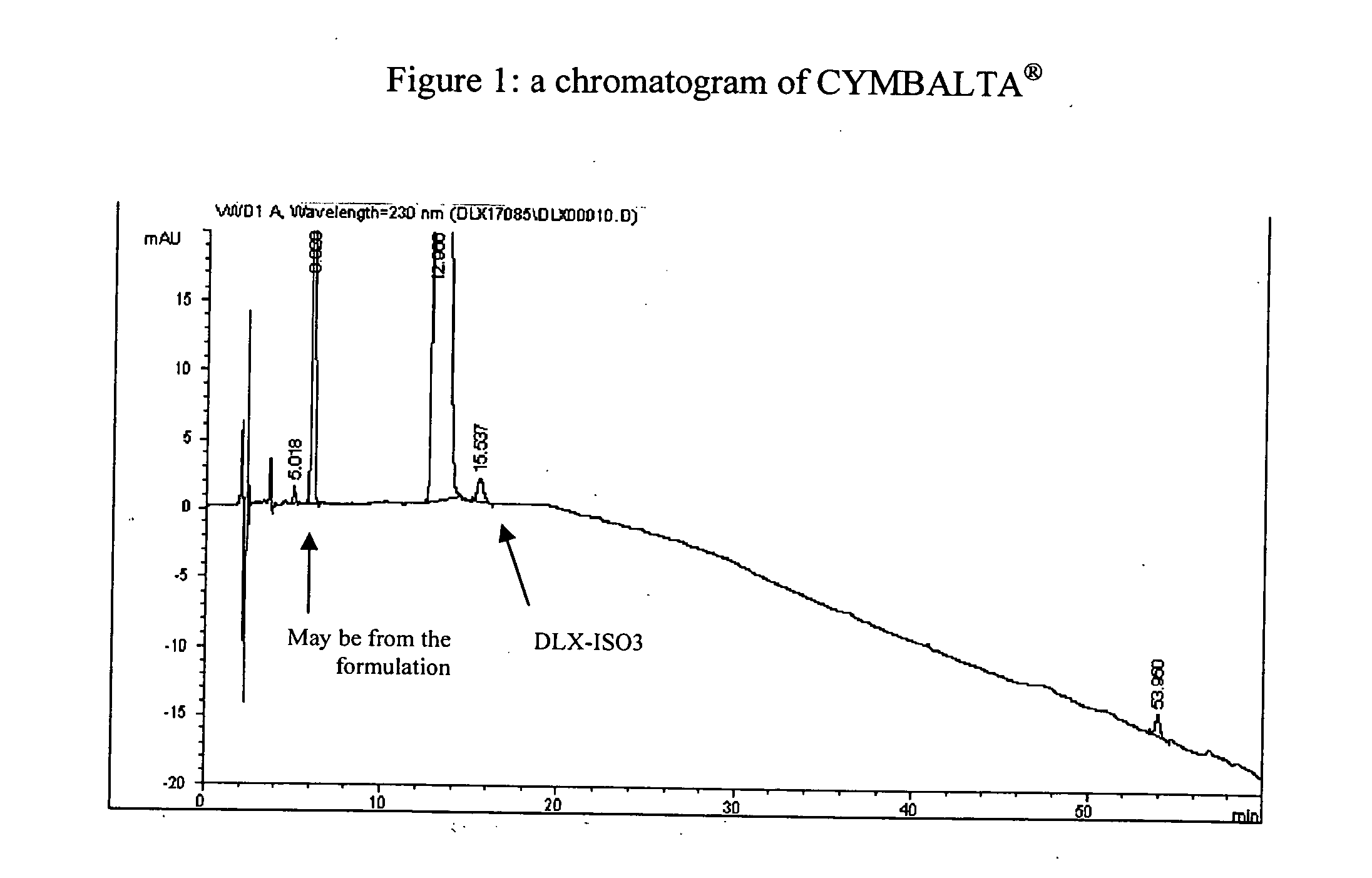
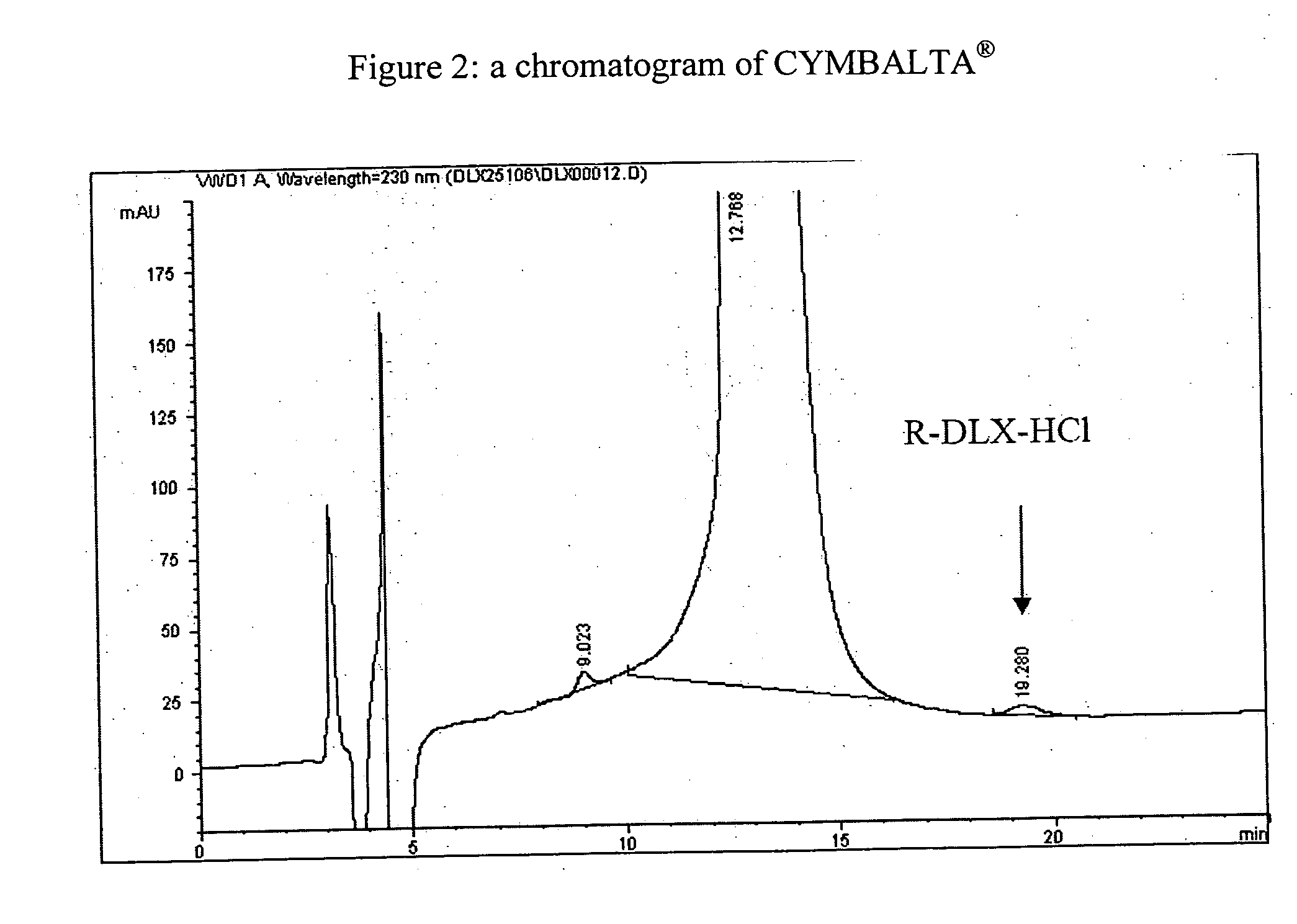
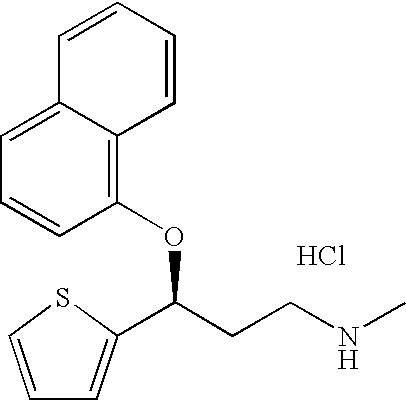
Pure duloxetine hydrochloride
a technology of duloxetine and hydrochloride, which is applied in the direction of biocide, animal repellents, drug compositions, etc., can solve the problems of reducing the level of api available in the pharmaceutical composition, impurities in duloxetine hcl or any active pharmaceutical ingredient, and harming a patien
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Purification of Duloxetine Hydrochloride in Acetone / Water
example 1a
[0063] A mixture of 20 g Duloxetine hydrochloride in 204 ml acetone / water (98:2) was heated to reflux. After the compound was dissolved, the oil bath was removed, and the solution was cooled to 15° C. overnight. The solid was filtered, washed with acetone, and dried in a vacuum oven at 45° C. for 16 hours, giving Duloxetine hydrochloride (78 percent yield), containing DLX-ISO3 (0.21 percent) and enantiomer R (<0.03 percent)
example 1b
[0064] A mixture of 13 g of the previously obtained Duloxetine hydrochloride in 130 ml acetone / water (98:1.5) was heated to reflux. After the compound was dissolved, the oil bath was removed, and the solution was cooled to 110° C. for 2 hours. The solid was filtered, washed with acetone, and dried in a vacuum oven at 45° C. for 16 hours, giving Duloxetine hydrochloride (87 percent yield), containing DLX-ISO3 (0.15 percent) and free of enantiomer R.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com