Method for preparing intermediate of duloxetine hydrochloride
A technology of duloxetine hydrochloride and intermediates is applied in the field of preparation of intermediates, and can solve the problems of affecting reduction yield and purity, incomplete dissolution of raw materials, affecting chiral resolution, etc., so as to shorten the reduction reaction time and improve the Reduction efficiency, effect of reducing dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
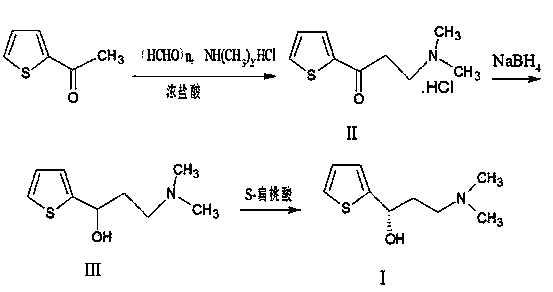
[0027] (a) Preparation of Formula II Compound
[0028] Add 100 g of 2-acetylthiophene, 86 g of dimethylamine hydrochloride, 35.6 g of paraformaldehyde, and 300 mL of isopropanol into the reaction flask, add 10 mL of concentrated hydrochloric acid while stirring, and slowly raise the temperature to reflux. After reflux reaction for 8 hours, cool down to 0°C, continue to stir for 2 hours, filter, beat and wash once with cold ethanol, rinse twice, and dry to obtain 163.5 g of bright white flaky crystals, with a yield of 94%.
[0029] (b) Preparation of the compound of formula III
[0030] Add 132.7g of the compound of formula II and 400mL of ethanol to the reaction flask, adjust the pH to 12 with 10% sodium hydroxide under ice bath, and add 14g of NaBH in batches under control of the temperature not exceeding 30°C 4 After the addition and stirring for 15 minutes, the temperature was slowly raised to 60° C. for 4 hours. Filtrate, wash the filter cake with an appropriate amount o...
Embodiment 2
[0035] (a) Preparation of Formula II Compound
[0036] Add 100 g of 2-acetylthiophene, 70.1 g of dimethylamine hydrochloride, 28.5 g of paraformaldehyde, and 300 mL of isopropanol into the reaction flask, add 3 mL of concentrated hydrochloric acid while stirring, and slowly raise the temperature to reflux. After reflux reaction for 20 hours, cool down to 0°C, continue to stir for 2 hours, filter, beat and wash with cold ethanol once, rinse twice, and dry to obtain 160 g of bright white flaky crystals with a yield of 92%.
[0037] (b) Preparation of the compound of formula III
[0038]Add 132.7g of the compound of formula II and 400mL of ethanol to the reaction flask, adjust the pH to 12 with 3% sodium hydroxide under ice bath, and add 11.5g of NaBH in batches under the control temperature not exceeding 30°C 4 After the addition and stirring for 15 minutes, the temperature was slowly raised to 70° C. for 10 hours. Filtrate, wash the filter cake with an appropriate amount of e...
Embodiment 3
[0042] (a) Preparation of Formula II Compound
[0043] Add 100 g of 2-acetylthiophene, 102 g of dimethylamine hydrochloride, 47.5 g of paraformaldehyde, and 300 mL of isopropanol into the reaction flask, add 13 mL of concentrated hydrochloric acid while stirring, and slowly raise the temperature to reflux. After 6 hours of reflux reaction, cool down to 0°C, continue to stir for 2 hours, filter, beat and wash once with cold ethanol, rinse twice, and dry to obtain 158.4 g of bright white flaky crystals, with a yield of 91.1%.
[0044] (b) Preparation of the compound of formula III
[0045] Add 132.7g of the compound of formula II and 400mL of ethanol to the reaction flask, adjust the pH to 12 with 15% sodium hydroxide under ice bath, and add 29.8g of NaBH in batches under control of the temperature not exceeding 30°C 4 After the addition and stirring for 15 minutes, the temperature was slowly raised to 40° C. for 2 hours. Filtrate, wash the filter cake with an appropriate amou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com