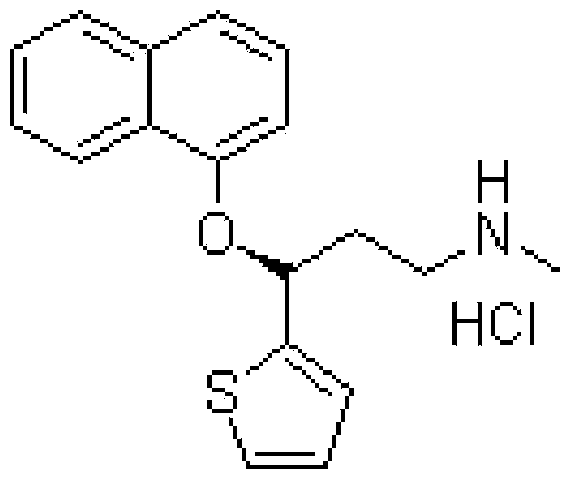
Duloxetine hydrochloride medicament composition, enteric sustained release preparation and preparation method thereof
A duloxetine hydrochloride, sustained-release preparation technology, applied in the field of medicine, can solve problems such as complicated steps and inconvenient operation, and achieve the effects of taking convenience, maintaining stability, and avoiding damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
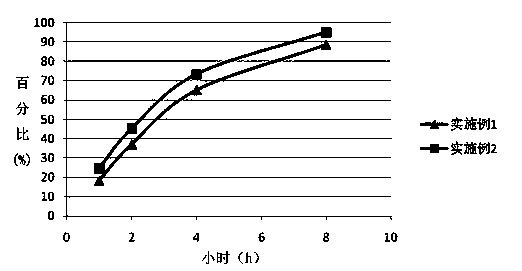
Embodiment 1
[0031] The preparation method of duloxetine hydrochloride enteric-coated sustained-release preparation described in the present embodiment comprises the following steps:
[0032] 1. Take 18 parts of duloxetine hydrochloride, crush them through a 100-mesh sieve, and mix them with 51 parts of sodium alginate, 12 parts of calcium gluconate and 17 parts of lactose in a wet mixing granulator to prepare mixture A. spare;
[0033] 2. Dissolve the adhesive PVP K30 in 95% ethanol solution, stir evenly and add it to the mixture A, stir evenly, and prepare soft materials. Wet soft materials use a swinging granulator to pass through a 20-mesh sieve to obtain wet granules. Dry in bed at 45°C to obtain dry granules, mixture B;
[0034] 3. After the prepared mixture B is granulated through a 18-mesh sieve of a granulator, a lubricant magnesium stearate is added and mixed uniformly in a multi-directional motion mixer to obtain a mixture C.
[0035] 4. After sampling and measuring the conten...
Embodiment 2
[0041] The preparation method of duloxetine hydrochloride enteric-coated sustained-release preparation described in the present embodiment comprises the following steps:
[0042] 1. Take 15 parts of duloxetine hydrochloride, crush it through a 100-mesh sieve, put it into a wet mixing granulator with 48 parts of sodium alginate, 11 parts of calcium hydrogen phosphate, 8 parts of mannitol, and 16 parts of microcrystalline cellulose Mix well, prepare mixture A, set aside;
[0043] 2. Add the binder hydroxypropyl methylcellulose E5 to the 50% ethanol solution, stir evenly, add it to the mixture A, stir evenly, and prepare soft materials. Wet soft materials are made by passing through a 20-mesh sieve with a swinging granulator Wet granules are dried in an oven at 50°C to obtain dry granules, namely mixture B;
[0044] 3. After the prepared mixture B is granulated through a 18-mesh sieve of a granulator, a lubricant magnesium stearate is added and mixed uniformly in a multi-directi...
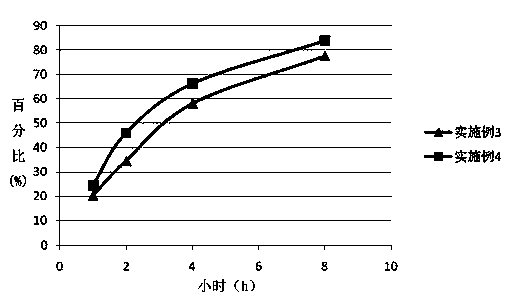
Embodiment 3
[0050] The preparation method of duloxetine hydrochloride enteric-coated sustained-release preparation described in the present embodiment comprises the following steps:
[0051] 1. Take 20 parts of duloxetine hydrochloride, crush them through a 100-mesh sieve, put 54 parts of sodium alginate, 10 parts of calcium citrate, and 14 parts of starch into a wet mixing granulator and mix evenly to prepare mixture A ,spare;
[0052] 2. Add the adhesive PVP K90 to the 95% ethanol solution, stir evenly and add it to the mixture A, and stir evenly to prepare soft materials. The wet and soft materials are passed through a 20-mesh sieve by a swinging granulator to obtain wet granules. Dry at 45°C to obtain dry granules, namely mixture B;
[0053] 3. After the prepared dry granules are sized by a 18-mesh sieve in a granulator, the lubricant magnesium stearate and micropowder silica gel are added and mixed uniformly in a multi-directional motion mixer to obtain mixture C.
[0054] 4. After...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com