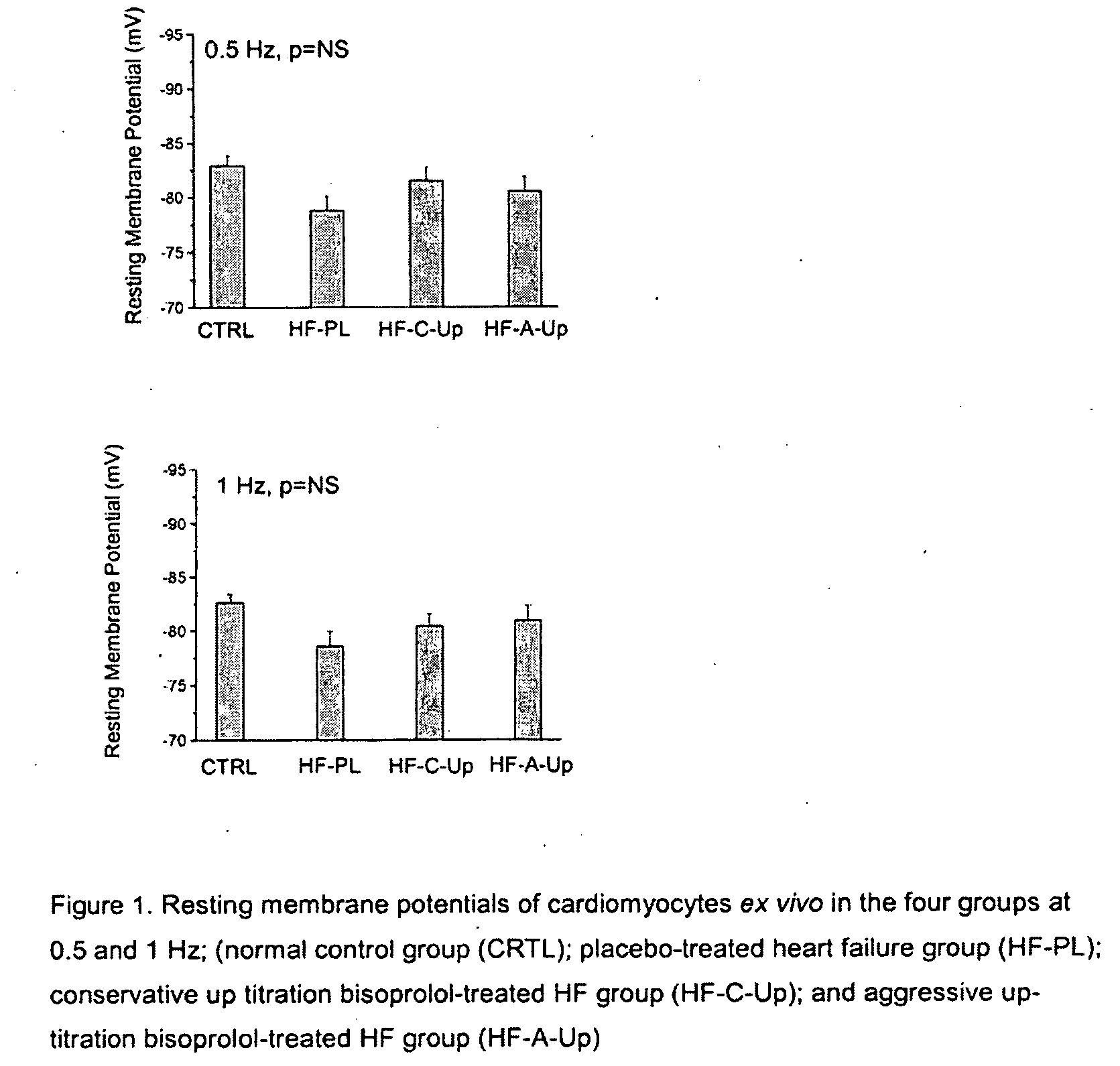
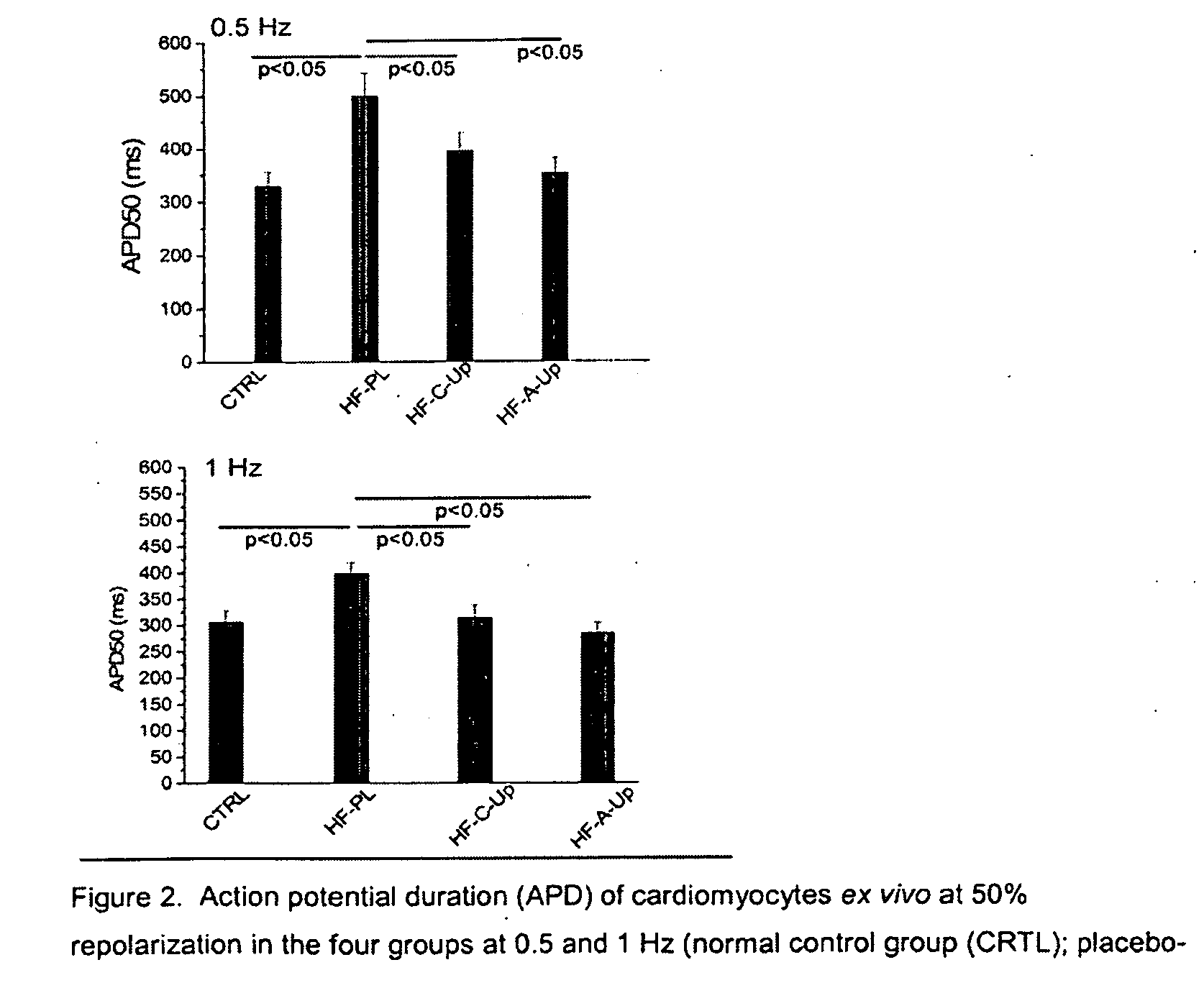
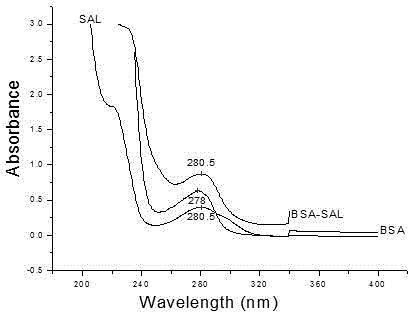
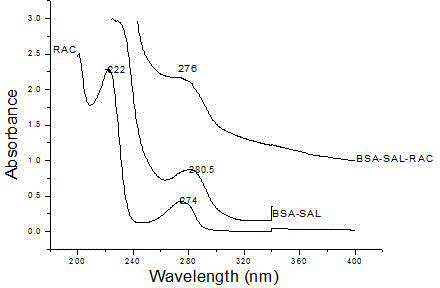
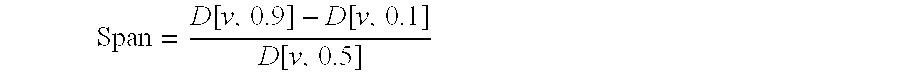Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
98 results about "Betadrenol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Betadrenol (n.) 1. ( MeSH ) An adrenergic-beta-2 antagonist that has been used for cardiac arrhythmia, angina pectoris, hypertension, glaucoma, and as an antithrombotic.
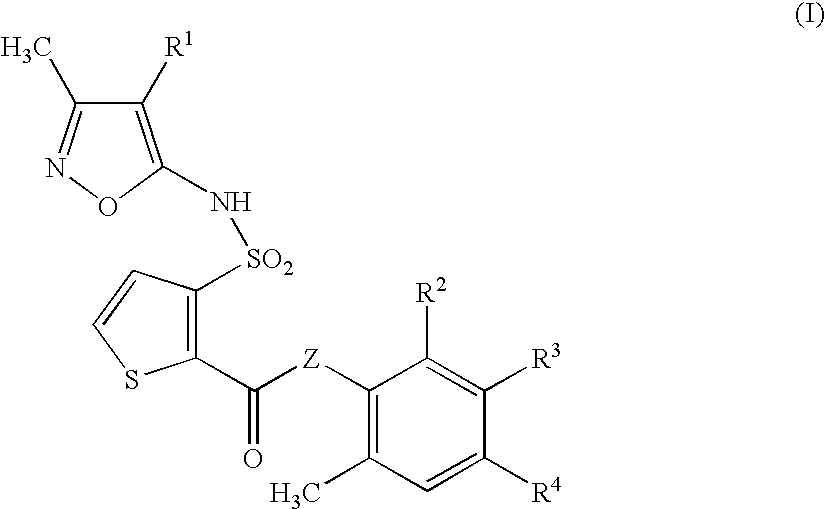
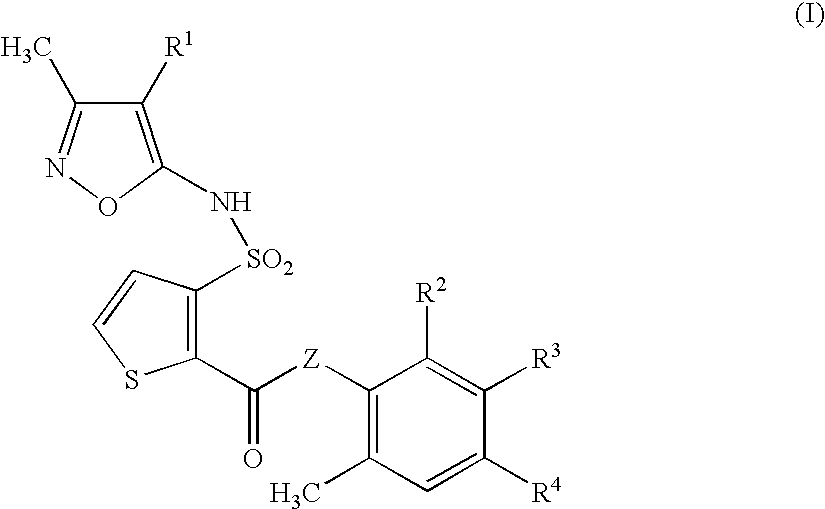
Composition and methods for treatment of neurological disorders and neurodegenerative diseases
InactiveUS6187756B1Increased formationPromote activationBiocideElcosanoid active ingredientsDiseaseGlial fibrillary acidic protein
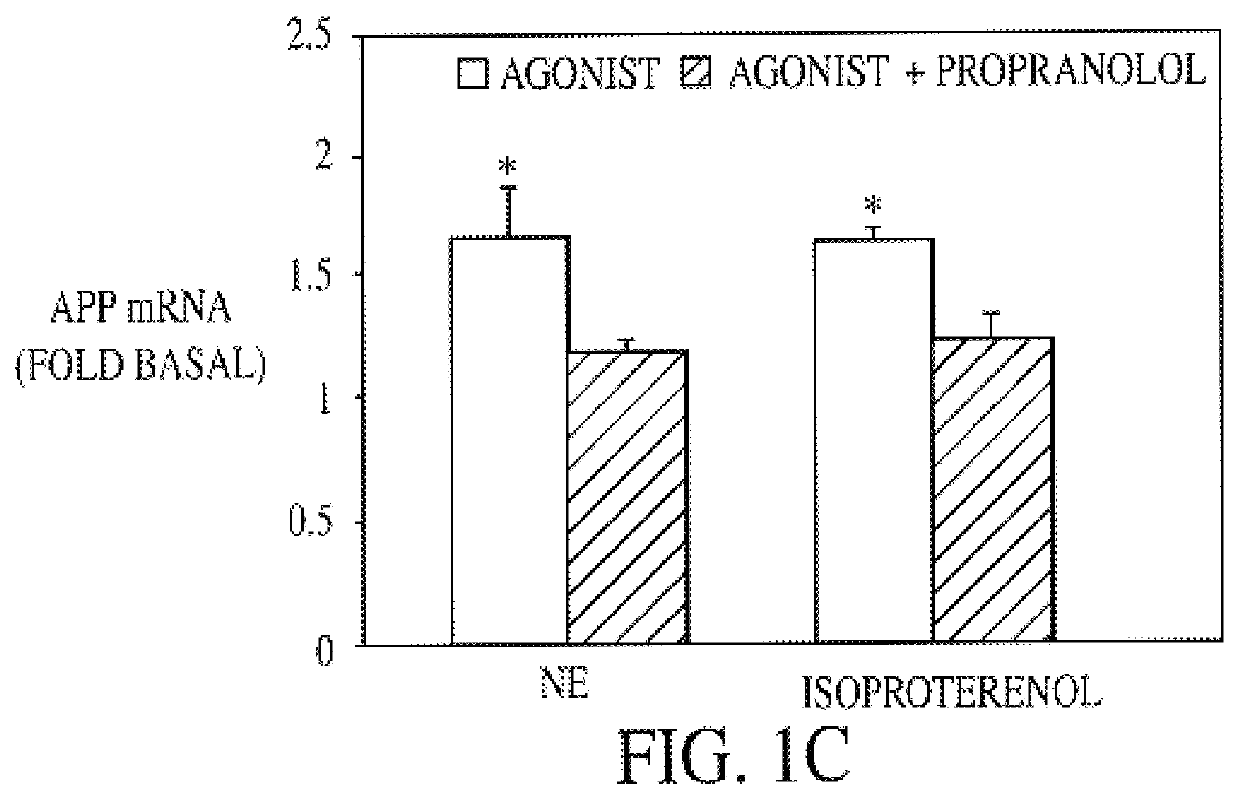
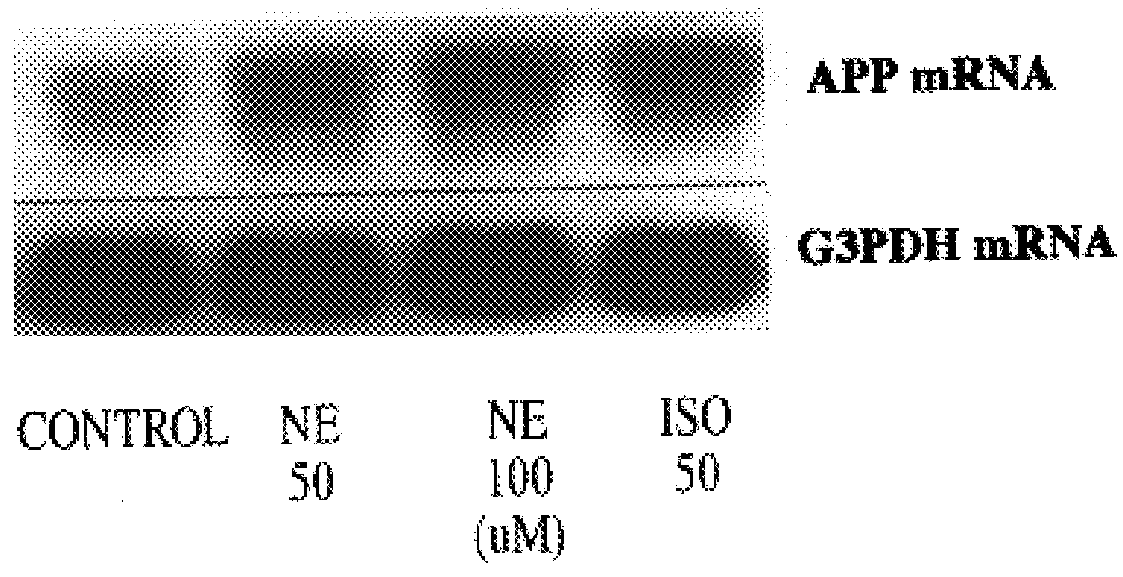
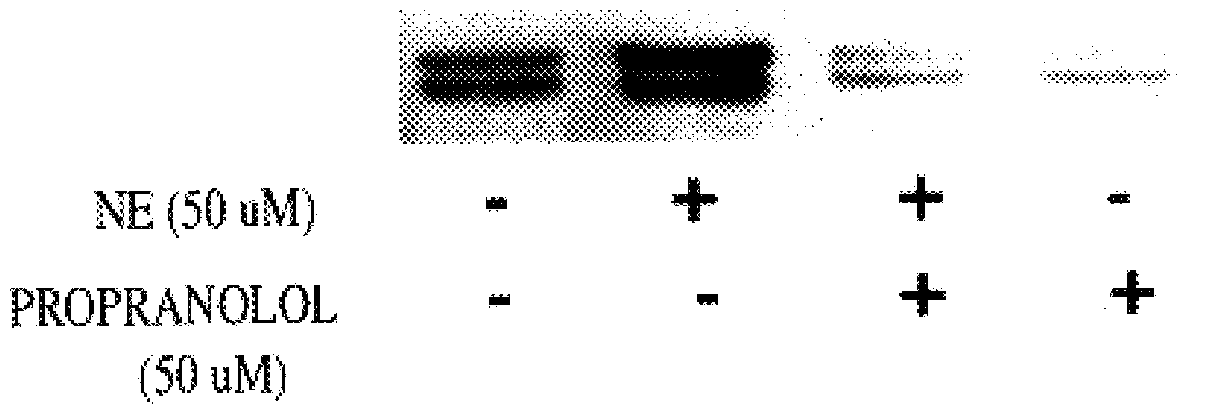
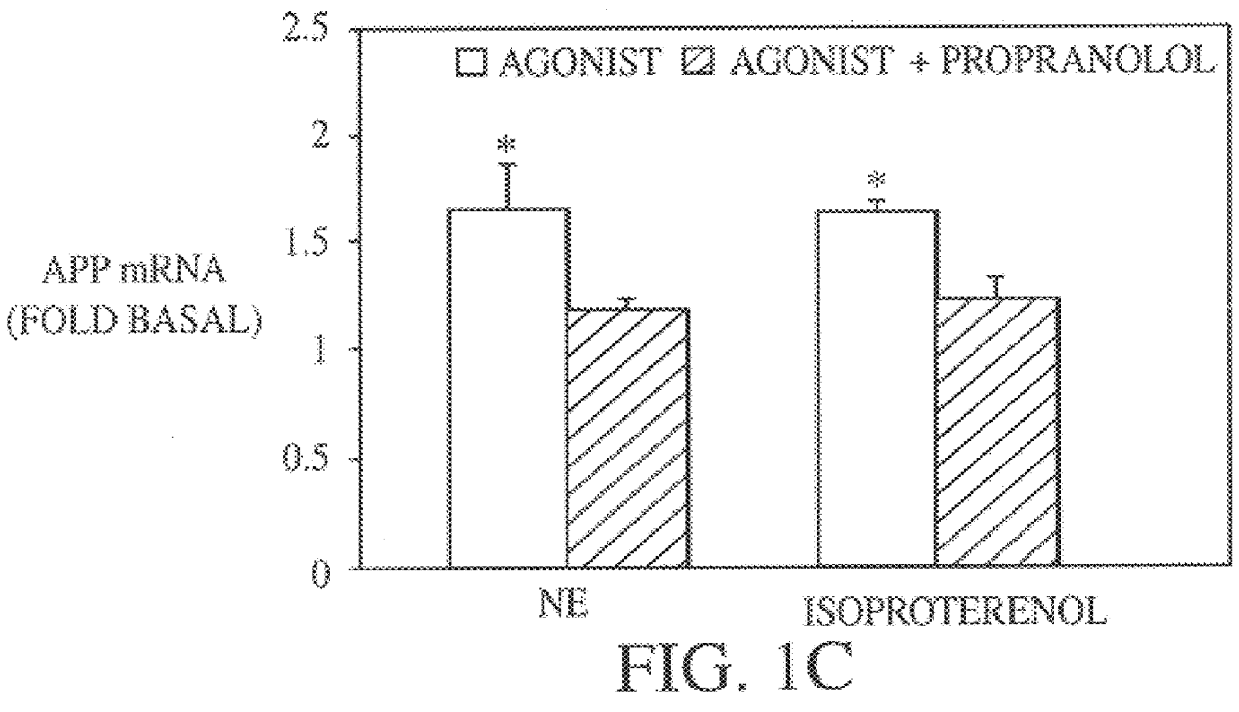
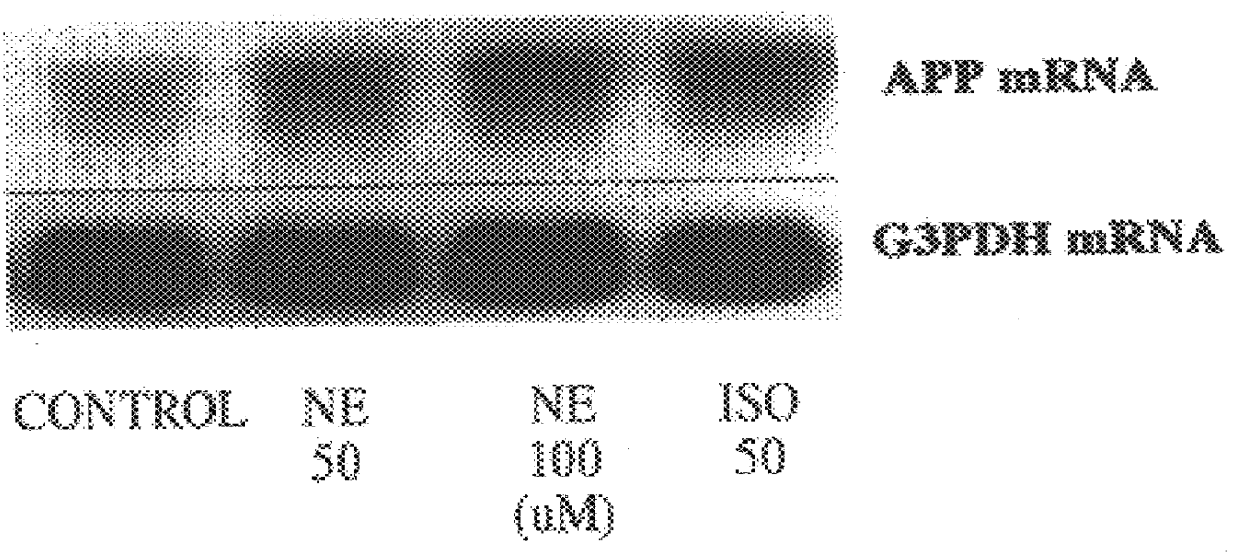
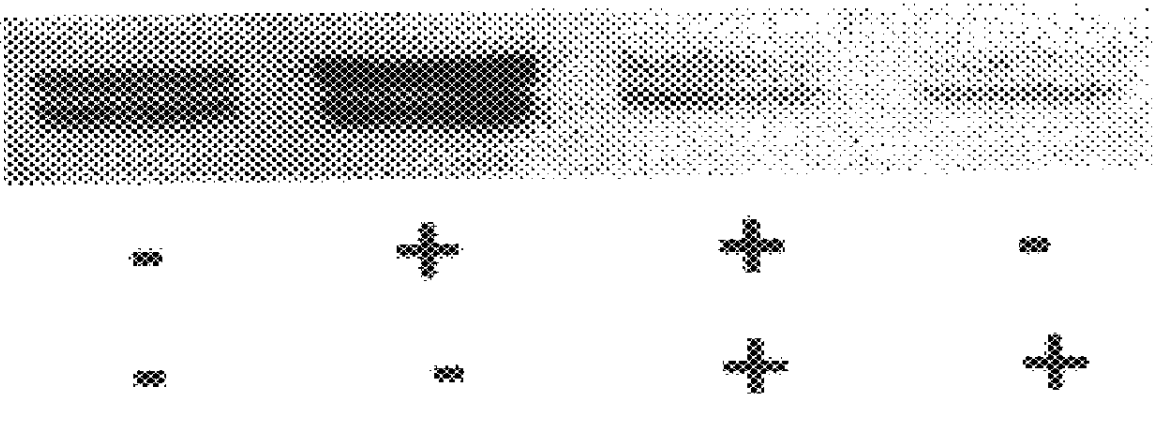
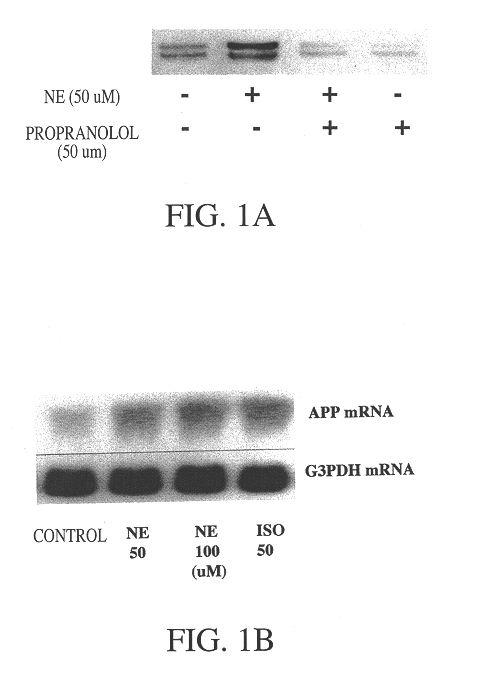
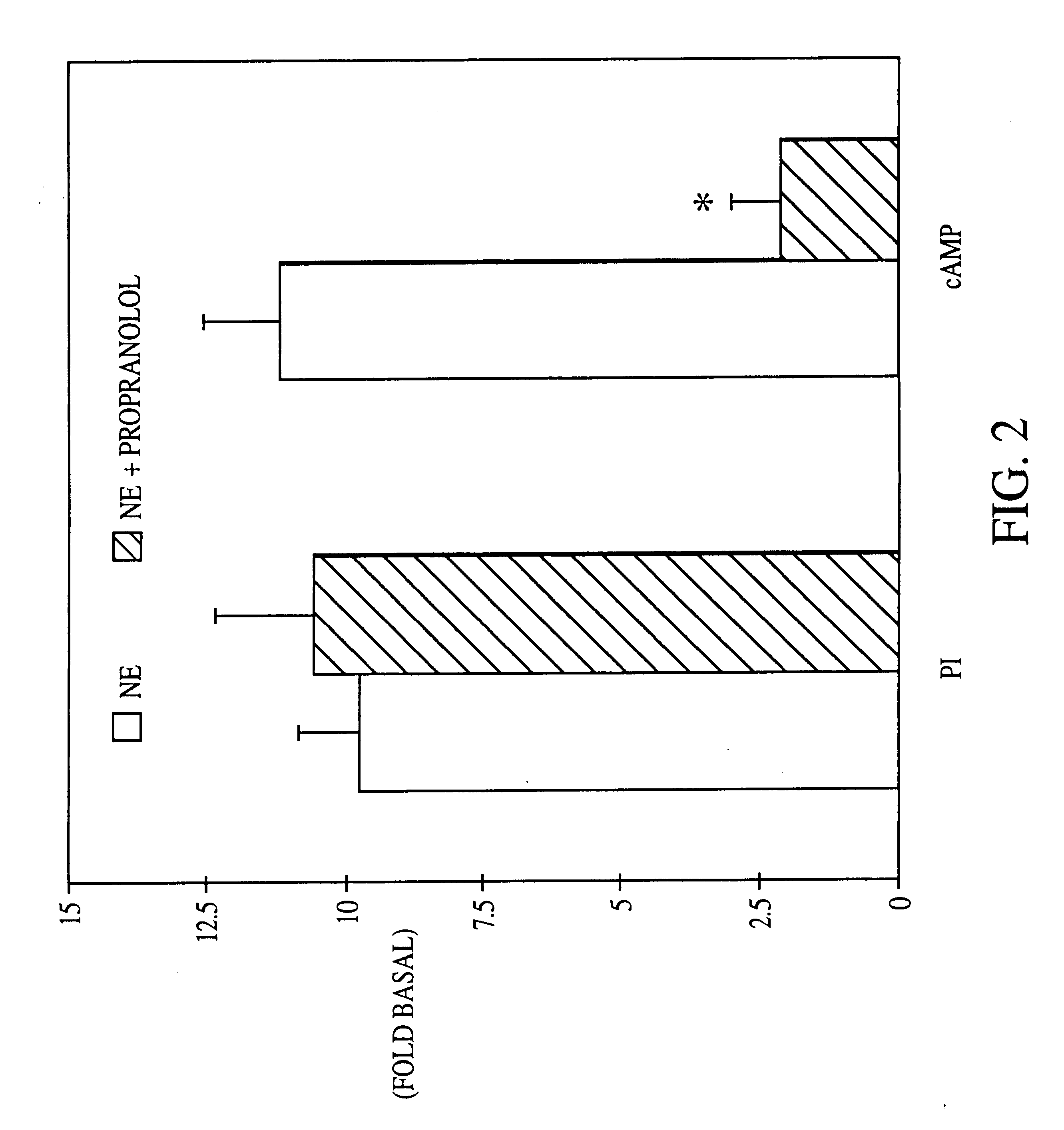
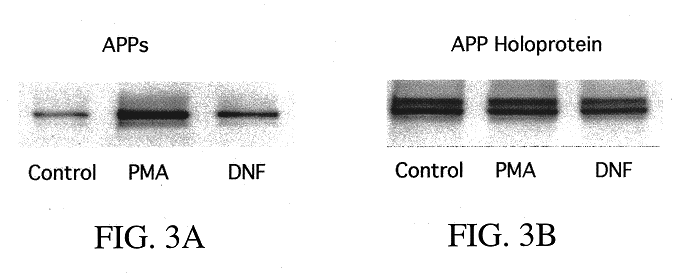
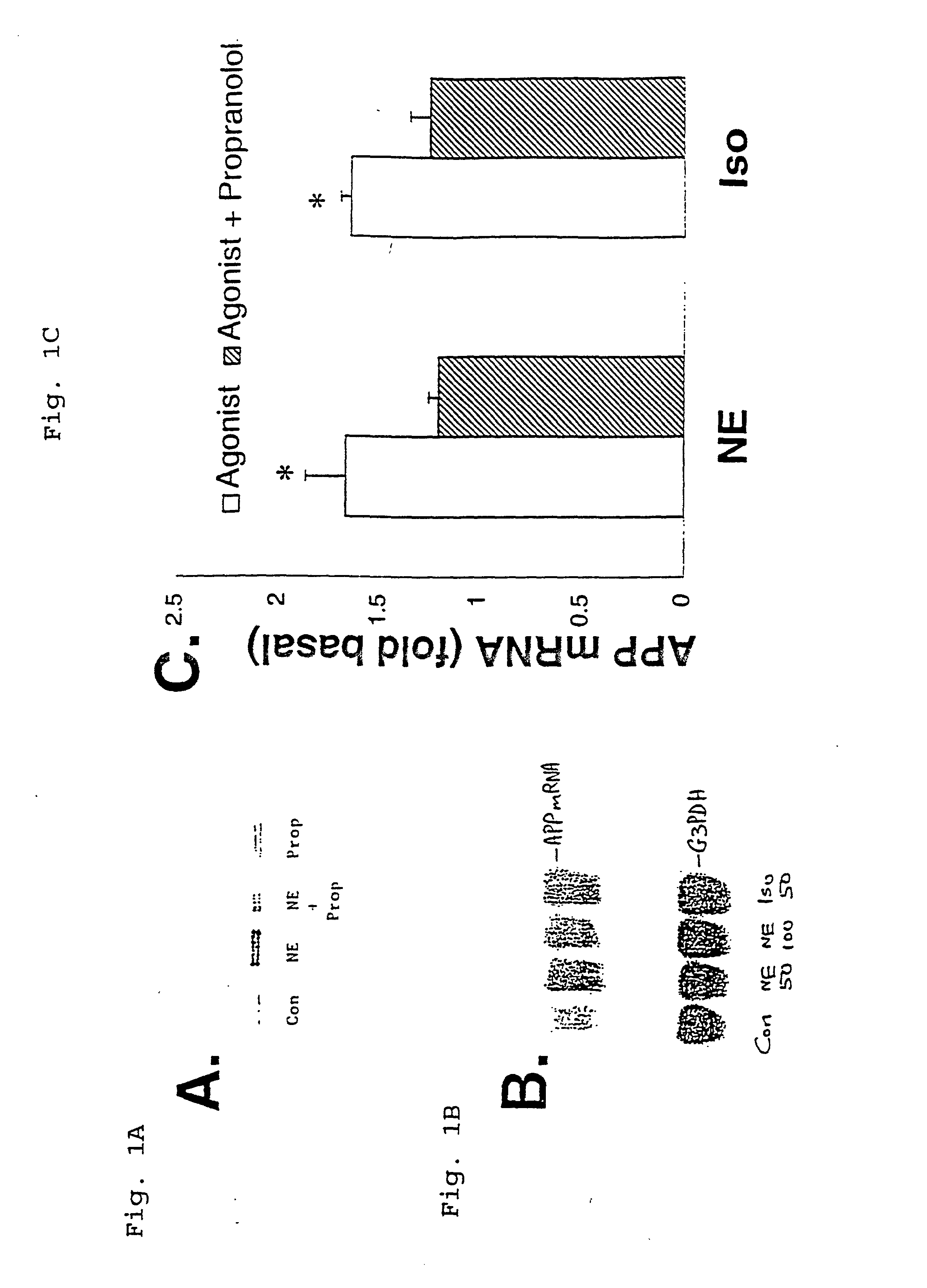
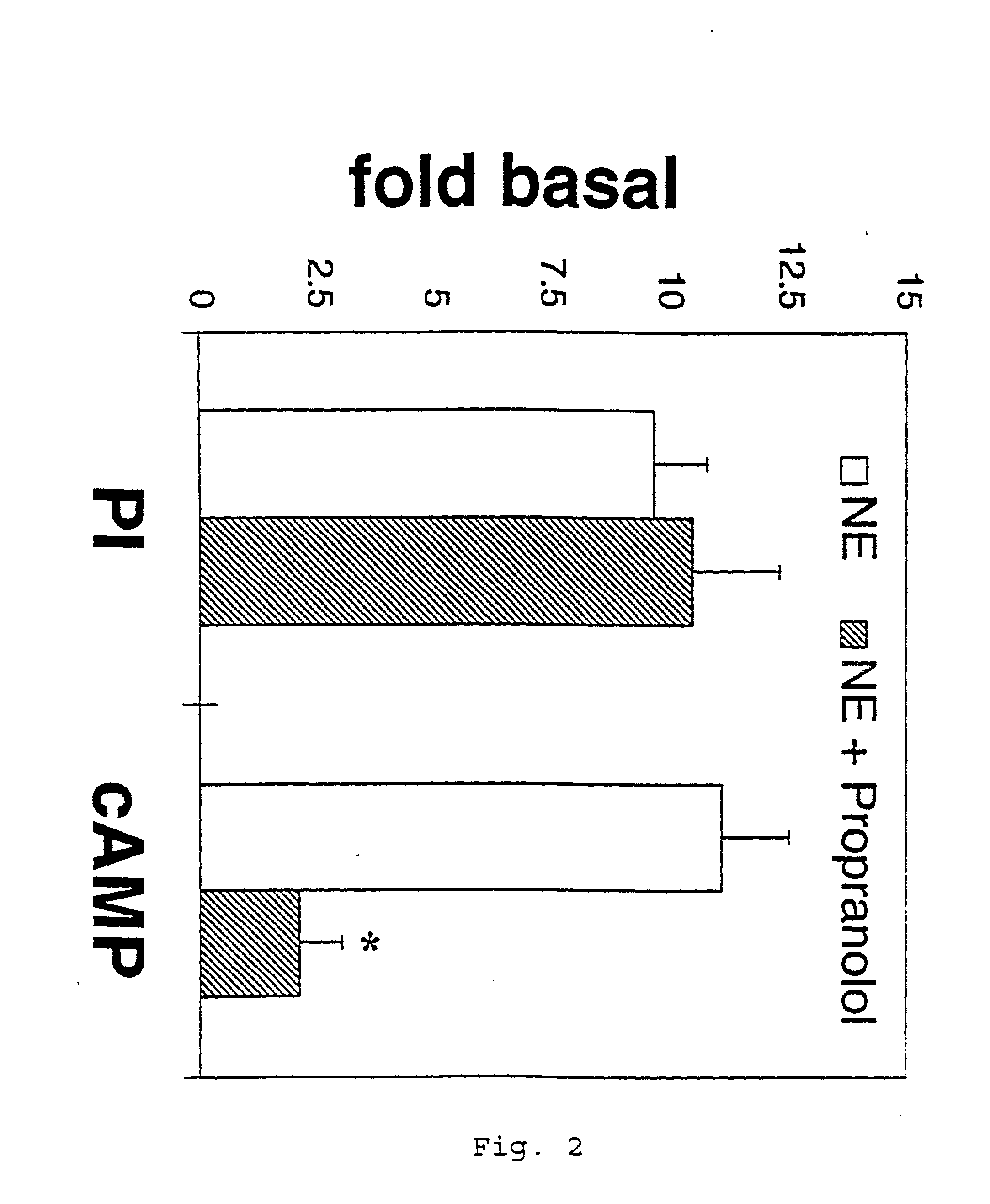
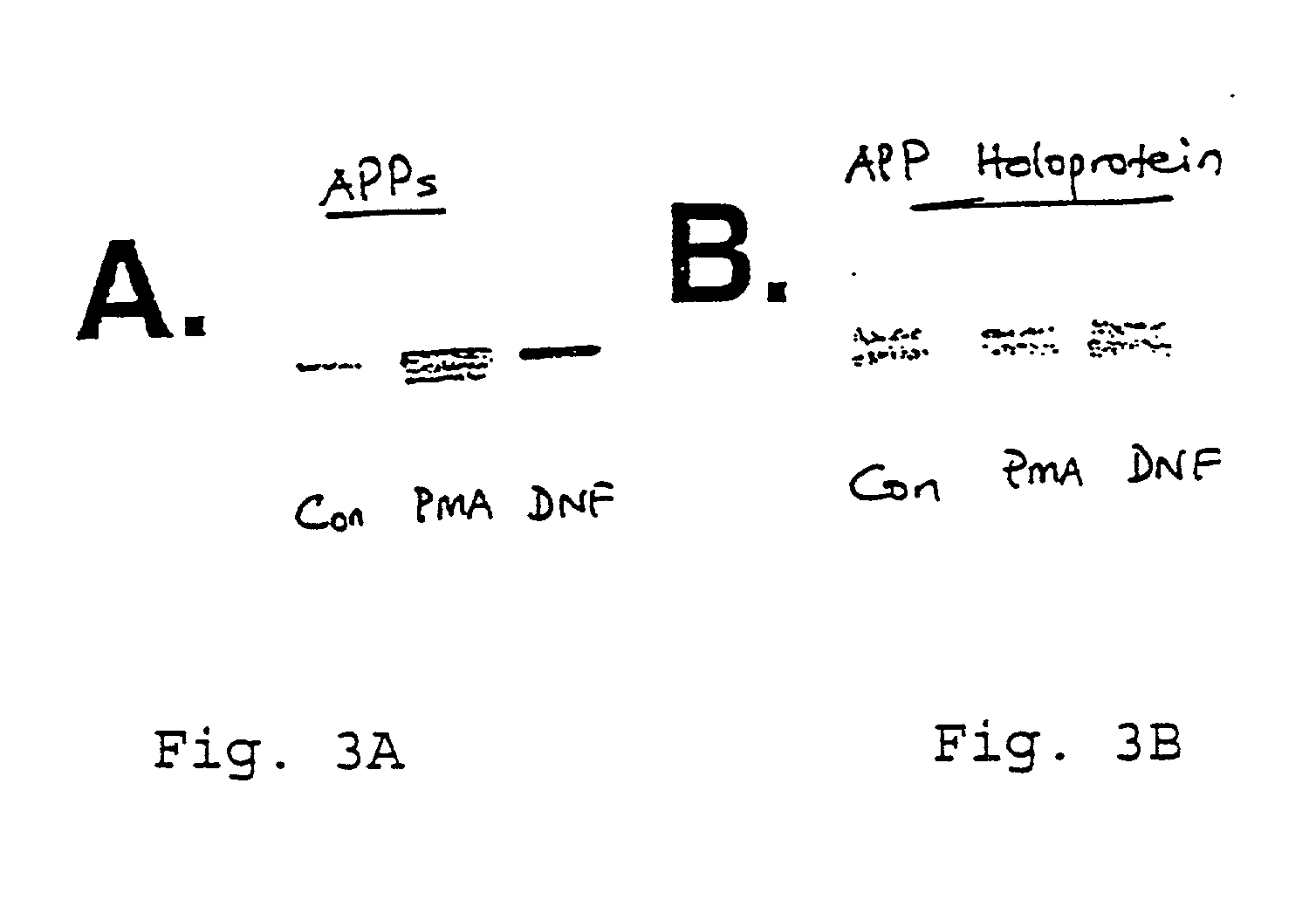
It has been discovered that the stimulation of beta-adrenergic receptors, which activate cAMP formation, give rise to increased APP and GFAP synthesis in astrocytes. Hence, the in vitro or in vivo exposure of neuronal cells to certain compositions comprising beta-adrenergic receptor ligands or agonists, including, e.g., norepinephrine, isoproterenol and the like, increases APP mRNA transcription and consequent APP overproduction. These increases are blocked by beta-adrenergic receptor antagonists, such as propranolol. The in vitro or in vivo treatment of these cells with 8Br-cAMP, prostaglandin E2 (PG E2), forskolin, and nicotine ditartrate also increased APP synthesis, including an increase in mRNA and holoprotein levels, as well as an increase in the expression of glial fibrillary acidic protein (GFAP). Compositions and methods are disclosed of regulating APP overexpression and mediating reactive astrogliosis through cAMP signaling or the activation of beta-adrenergic receptors. It has further been found that the increase in APP synthesis caused by 8Br-cAMP, PG E2, forskolin, or nicotine ditartrate is inhibited by immunosuppressants or anti-inflammatory agents, such as cyclosporin A, and FK-506 (tacrolimus), as well as ion-channel modulators, including ion chelating agents such as EGTA, or calcium / calmodulin kinase inhibitors, such as KN93. The present invention has broad implications in the alleviation, treatment, or prevention of neurological disorders and neurodegenerative diseases, including Alzheimer's Disease.
Owner:MASSACHUSETTS INST OF TECH
Compositions and methods for treatment of neurological disorders and neurodegenerative diseases
InactiveUS6043224AIncreased formationPromote activationBiocideElcosanoid active ingredientsGlial fibrillary acidic proteinDisease
It has been discovered that the stimulation of beta -adrenergic receptors, which activate cAMP formation, give rise to increased APP and GFAP synthesis in astrocytes. Hence, the in vitro or in vivo exposure of neuronal cells to certain compositions comprising beta -adrenergic receptor ligands or agonists, including, e.g., norepinephrine, isoproterenol and the like, increases APP mRNA transcription and consequent APP overproduction. These increases are blocked by beta -adrenergic receptor antagonists, such as propranolol. The in vitro or in vivo treatment of these cells with 8Br-cAMP, prostaglandin E2 (PG E2), forskolin, and nicotine ditartrate also increased APP synthesis, including an increase in mRNA and holoprotein levels, as well as an increase in the expression of glial fibrillary acidic protein (GFAP). Compositions and methods are disclosed of regulating APP overexpression and mediating reactive astrogliosis through cAMP signaling or the activation of beta -adrenergic receptors. It has further been found that the increase in APP synthesis caused by 8Br-cAMP, PG E2, forskolin, or nicotine ditartrate is inhibited by immunosuppressants or anti-inflammatory agents, such as cyclosporin A, and FK-506 (tacrolimus), as well as ion-channel modulators, including ion chelating agents such as EGTA, or calcium / calmodulin kinase inhibitors, such as KN93. The present invention has broad implications in the alleviation, treatment, or prevention of neurological disorders and neurodegenerative diseases, including Alzheimer's Disease.
Owner:MASSACHUSETTS INST OF TECH
Compositions and methods for treatment of neurological disorders and neurodegenerative diseases
InactiveUS6469055B2Increased formationPromote activationBiocideNervous disorderGlial fibrillary acidic proteinDisease
It has been discovered that the stimulation of beta-adrenergic receptors, which activate cAMP formation, give rise to increased APP and GFAP synthesis in astrocytes. Hence, the in vitro or in vivo exposure of neuronal cells to certain compositions comprising beta-adrenergic receptor ligands or agonists, including, e.g., norepinephrine, isoproterenol and the like, increases APP mRNA transcription and consequent APP overproduction. These increases are blocked by beta-adrenergic receptor antagonists, such as propranolol. The in vitro or in vivo treatment of these cells with 8Br-cAMP, prostaglandin E2 (PG E2), forskolin, and nicotine ditartrate also increased APP synthesis, including an increase in mRNA and holoprotein levels, as well as an increase in the expression of glial fibrillary acidic protein (GFAP). Compositions and methods are disclosed of regulating APP overexpression and mediating reactive astrogliosis through cAMP signaling or the activation of beta-adrenergic receptors. It has further been found that the increase in APP synthesis caused by 8Br-cAMP, PG E2, or forskolin is inhibited by immunosuppressants, immunophilin ligands, or anti-inflammatory agents, such as cyclosporin A, and FK-506 (tacrolimus), as well as ion-channel modulators, including ion chelating agents such as EGTA, or calcium / calmodulin kinase inhibitors, such as KN93. The present invention has broad implications in the alleviation, treatment, or prevention of neurological disorders and neurodegenerative diseases, including Alzheimer's Disease.
Owner:MASSACHUSETTS INST OF TECH
Compositions and methods for treatment of neurological disorders and neurodegenerative diseases
InactiveUS20020052407A1Prevent APP over-expressionInhibit overexpressionBiocideNervous disorderGlial fibrillary acidic proteinDisease
It has been discovered that the stimulation of beta-adrenergic receptors, which activate cAMP formation, give rise to increased APP and GFAP synthesis in astrocytes. Hence, the in vitro or in vivo exposure of neuronal cells to certain compositions comprising beta-adrenergic receptor ligands or agonists, including, e.g., norepinephrine, isoproterenol and the like, increases APP mRNA transcription and consequent APP overproduction. These increases are blocked by beta-adrenergic receptor antagonists, such as propranolol. The in vitro or in vivo treatment of these cells with 8Br-cAMP, prostaglandin E2 (PG E2), forskolin, and nicotine ditartrate also increased APP synthesis, including an increase in mRNA and holoprotein levels, as well as an increase in the expression of glial fibrillary acidic protein (GFAP). Compositions and methods are disclosed of regulating APP overexpression and mediating reactive astrogliosis through cAMP signaling or the activation of beta-adrenergic receptors. It has further been found that the increase in APP synthesis caused by 8Br-cAMP, PG E2, or forskolin is inhibited by immunosuppressants, immunophilin ligands, or anti-inflammatory agents, such as cyclosporin A, and FK-506 (tacrolimus), as well as ion-channel modulators, including ion chelating agents such as EGTA, or calcium / calmodulin kinase inhibitors, such as KN93. The present invention has broad implications in the alleviation, treatment, or prevention of neurological disorders and neurodegenerative diseases, including Alzheimer's Disease.
Owner:MASSACHUSETTS INST OF TECH
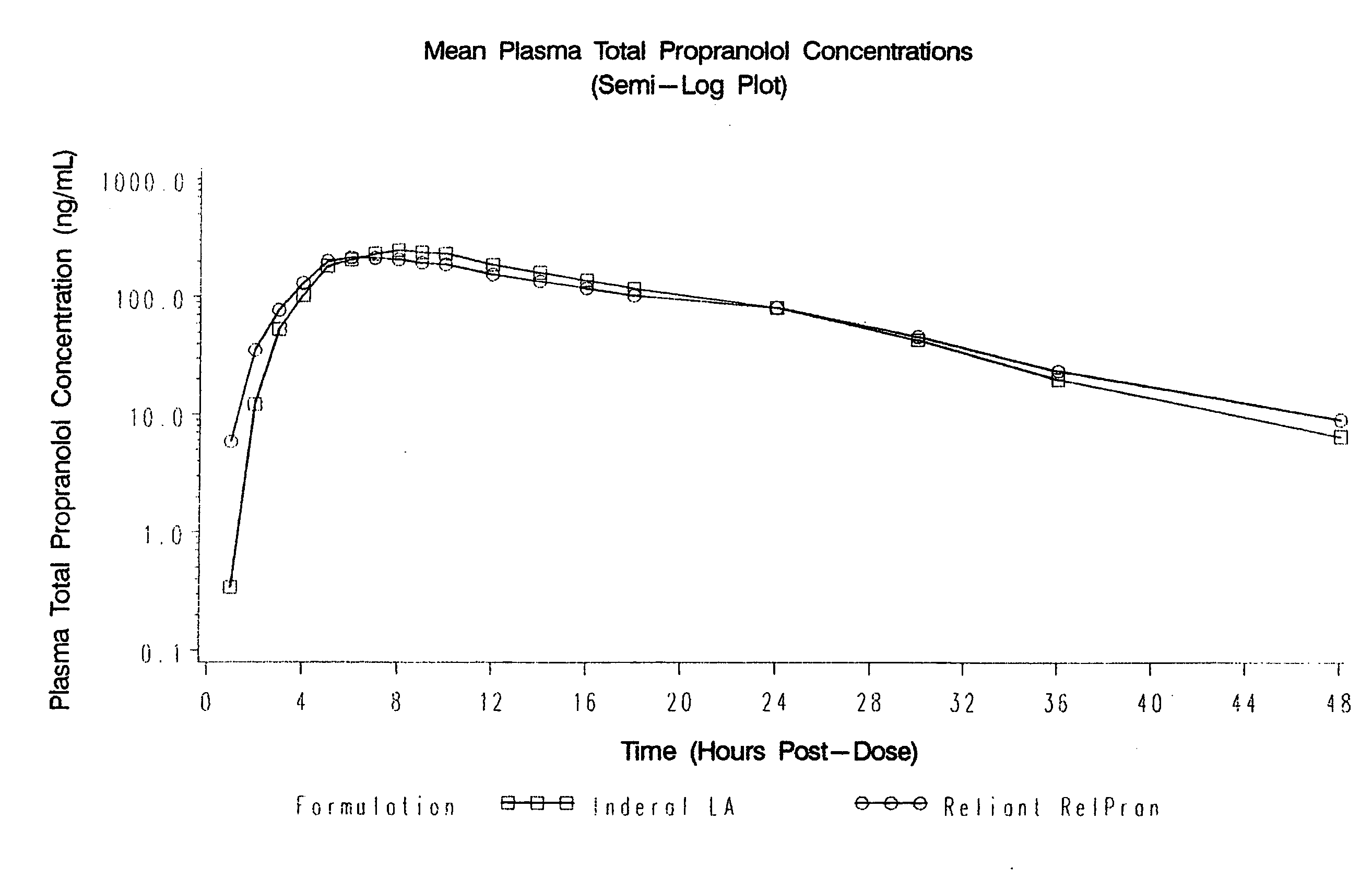
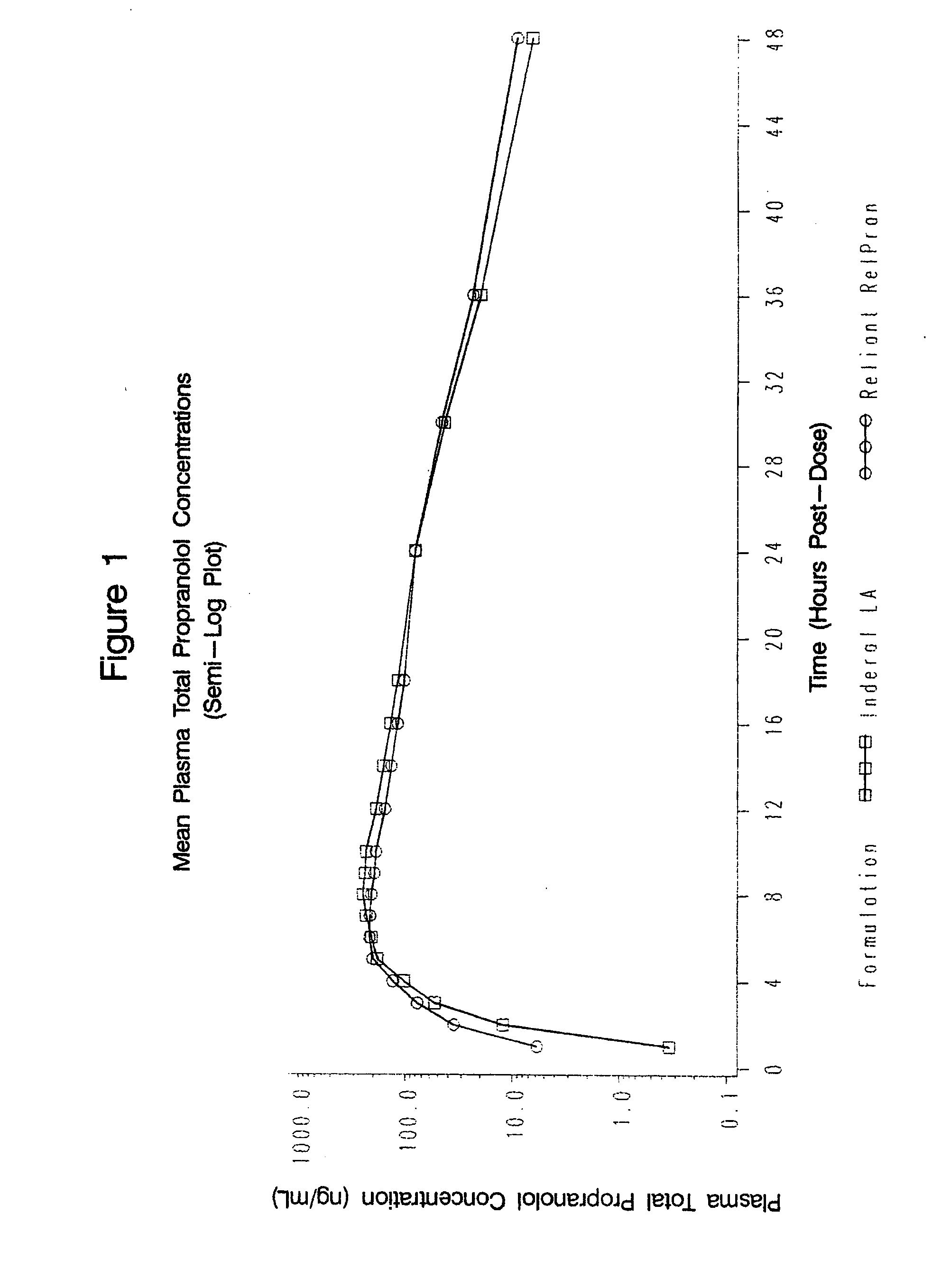
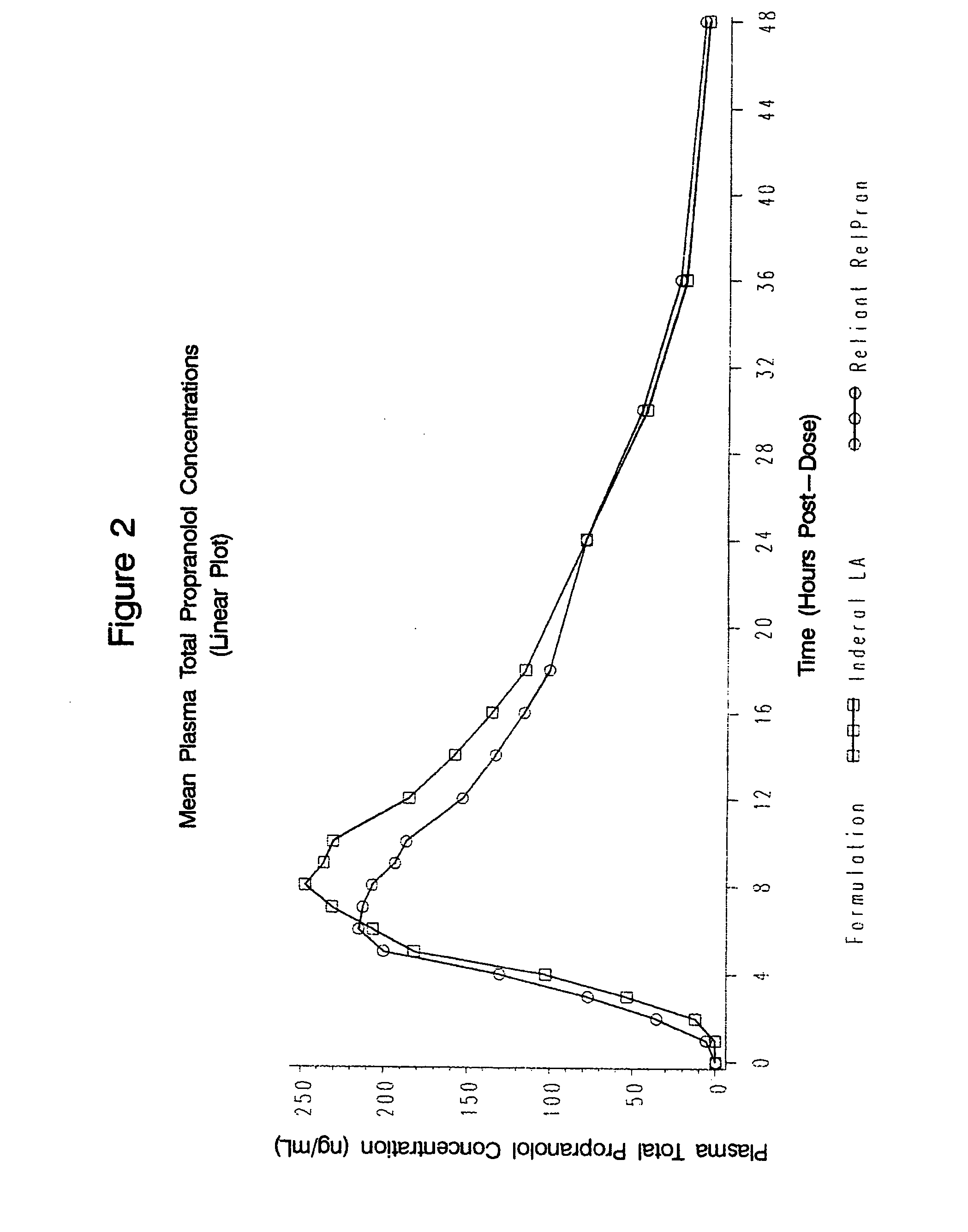
Time-sustained-release formulations comprising a beta-blocker
InactiveUS20080131517A1Providing therapyAvoid problemsPowder deliveryOrganic active ingredientsCarteololBeta blocker
The present invention relates to compositions and methods of treating human subjects with a beta-adrenergic receptor blocking agent (“beta-blocker”) provided in a time-sustained-release delivery system. The time-sustained-release drug delivery systems includes at least three populations of beads, where each population of beads includes a beta-blocker. The beads may be selected from immediate-release beads, enteric-release beads, sustained-release beads, and time-sustained-release beads. The beta-blocker may be selected from acebutolol, atenolol, betaxolol, bisoprolol, esmolol, metoprolol, nebivolol, butoxamine, carteolol, carvedilol, labetalol, nadolol, oxprenolol, penbutolol, propranolol, pindolol, sotalol, and timolol. According to presently preferred embodiments, the beta-blocker is propranolol. The dosage forms of the present invention are useful for treating conditions including hypertension, angina pectoris due to coronary atherosclerosis, hypertrophic subaortic stenosis, congestive heart failure, arrhythmias, angina, anxiety, glaucoma, migraines, esophageal varices, alcohol withdrawal syndrome, irregular heartbeat, tachycardia, tremor, and neuroleptic-induced akathisia. They are also useful in the prophylaxis of migraine headaches.
Owner:RELIANT PHARMACEUTICALS INC
Compositions and methods for the treatment of anorectal disorders
Compositions and methods for the treatment of anorectal disorders are provided in which certain combinations of NO donors, PDE inhibitors, superoxide (O2−) scavengers, β-adrenergic agonists, cAMP-dependent protein kinase activators, α1-adrenergic antagonists, L-type Ca2+ channel blockers, estrogens, ATP-sensitive K+ channel activators and smooth muscle relaxants are used.
Owner:STREHKEHN INT LTD
Aliphatic pyrazinoylguanidine sodium channel blockers with beta agonist activity
ActiveUS20100267746A1More potentLess reversibleOrganic active ingredientsSenses disorderAdrenergicChannel blocker
The present invention provides sodium channel blockers possessing beta-adrenergic receptor agonist activity.
Owner:PARION SCI DURHAM NC
Image-guided therapy of myocardial disease: composition, manufacturing and applications
InactiveUS8440168B2Ultrasonic/sonic/infrasonic diagnosticsOrganic active ingredientsEthylenediamineRadical radiotherapy
Compositions and methods for imaging and for chemotherapy and radiotherapy are disclosed. More particularly, the invention concerns agents comprising a targeting moeity comprising a beta-adrenergic receptor targeting compound conjugated or embedded with ethylenediamine. The present invention also concerns methods of application of such agents for imaging and treatment of cardiovascular diseases, and kits for preparing a radiolabeled therapeutic or diagnostic agent.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Antihypertensive therapy
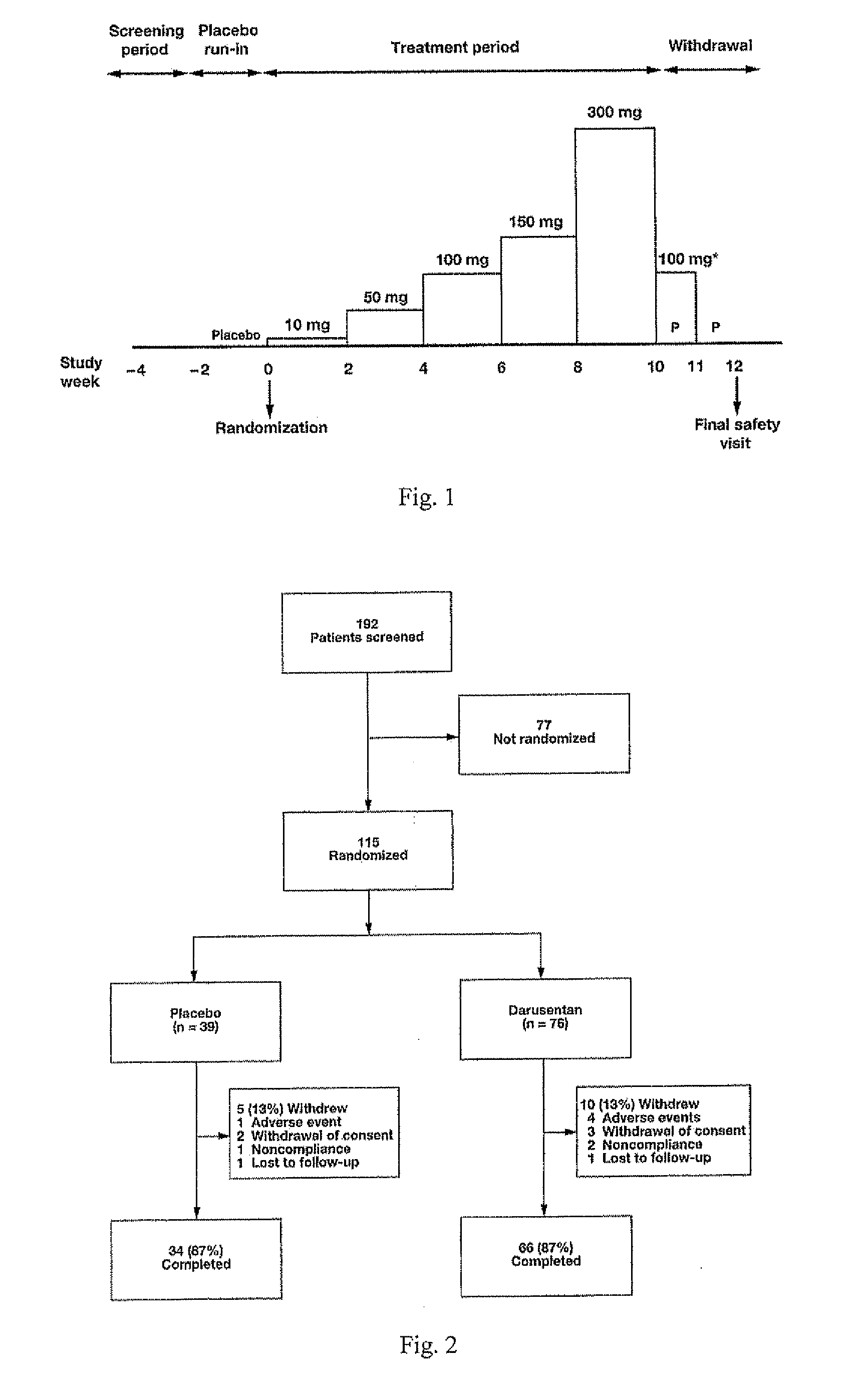
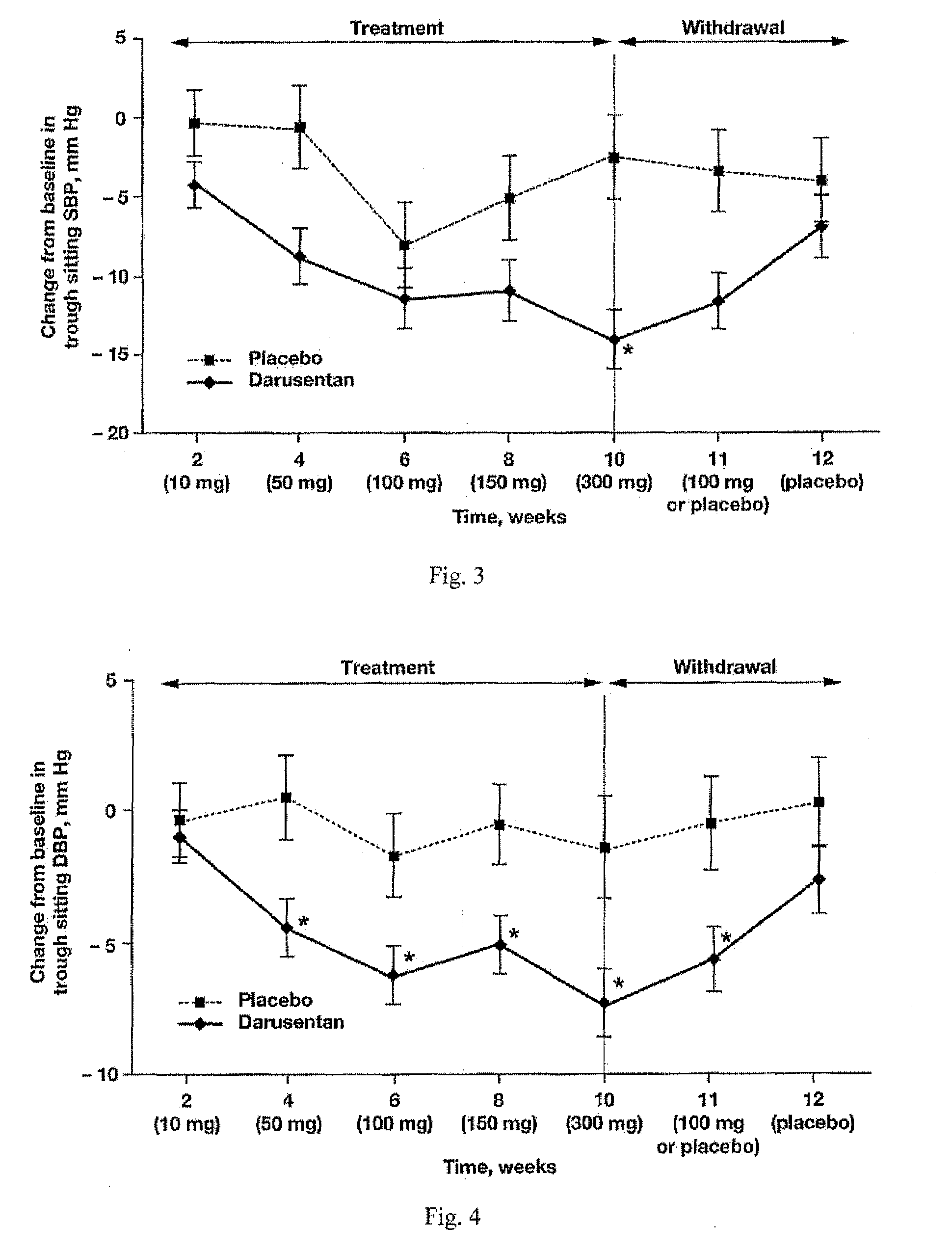
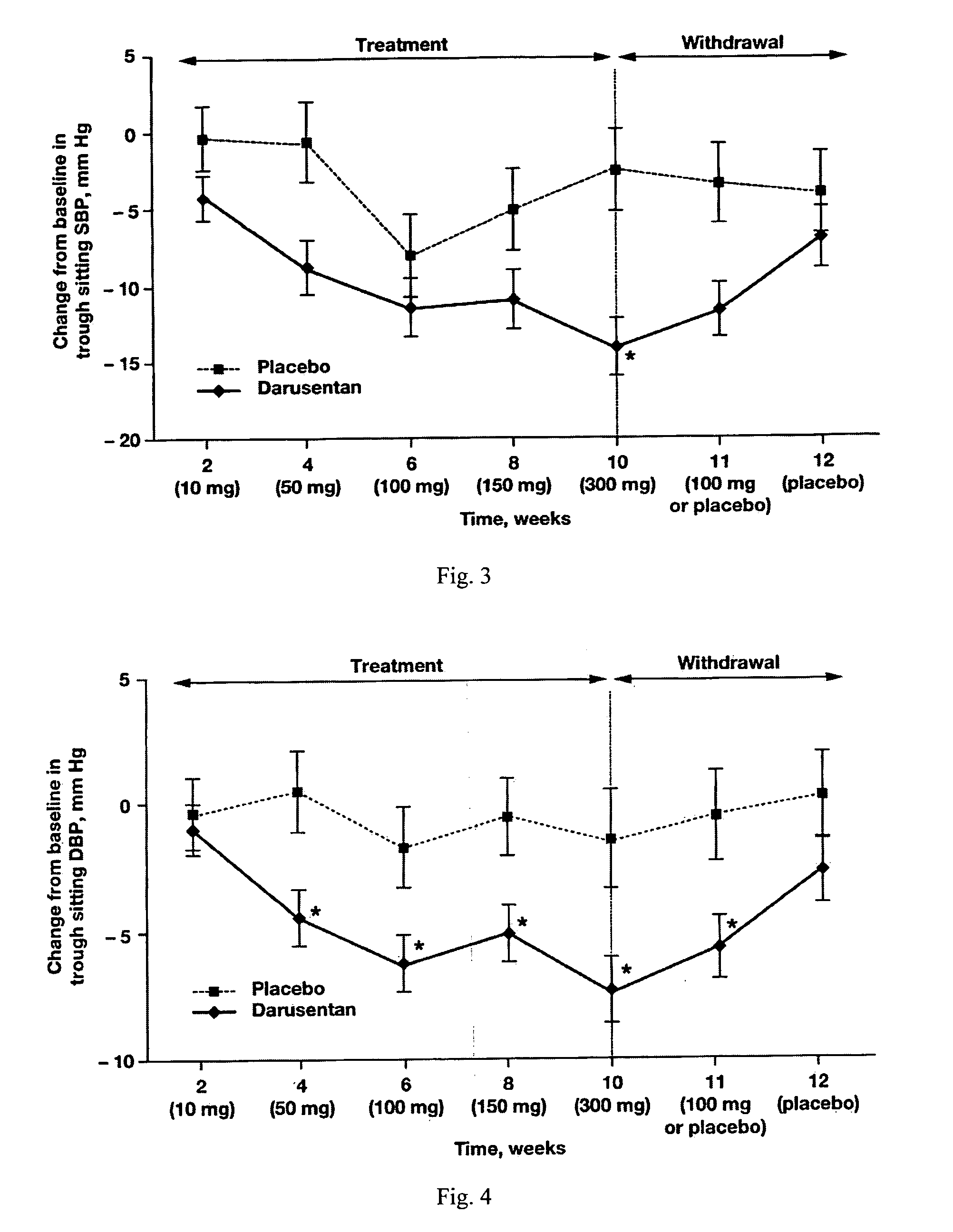
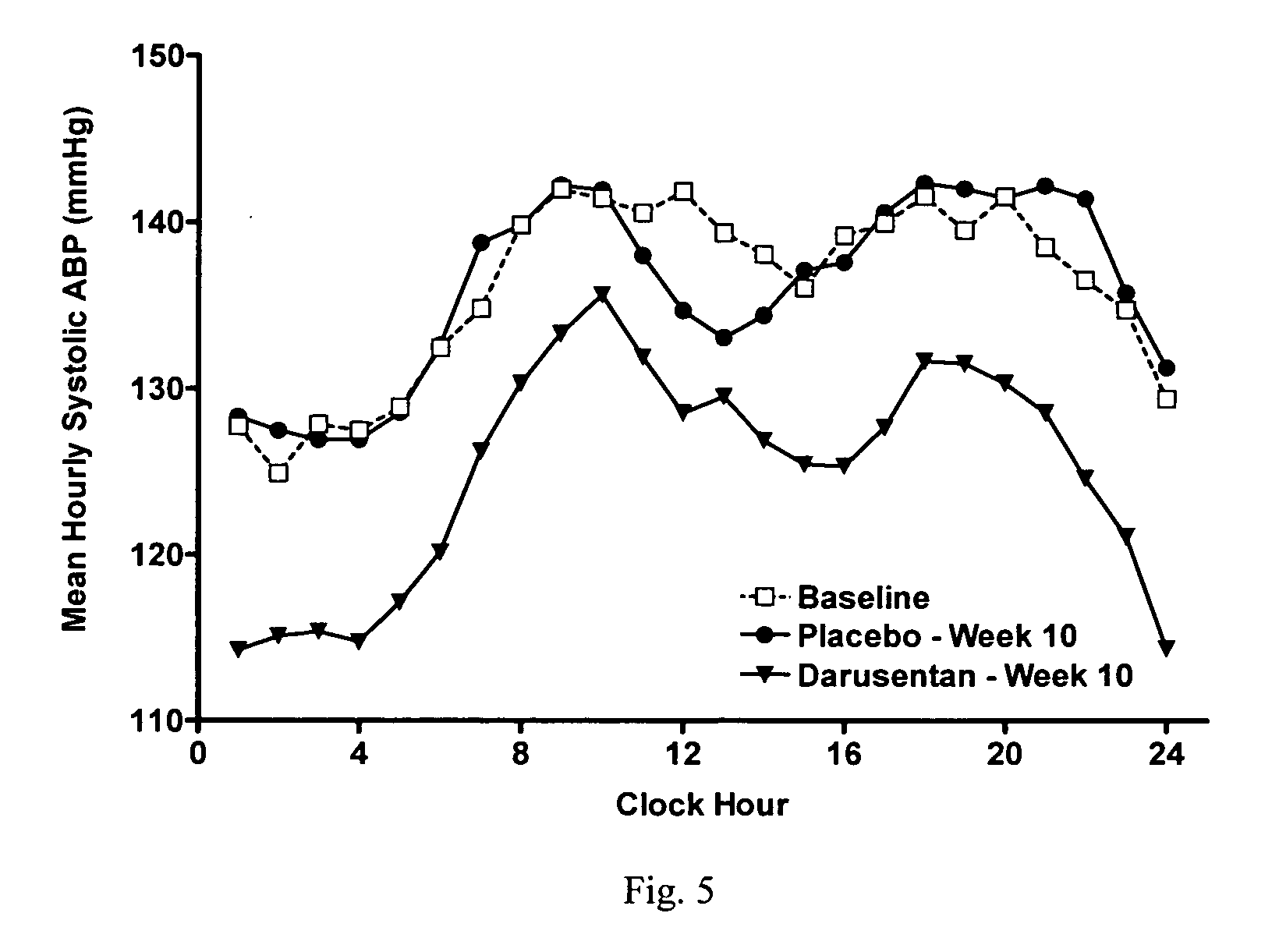
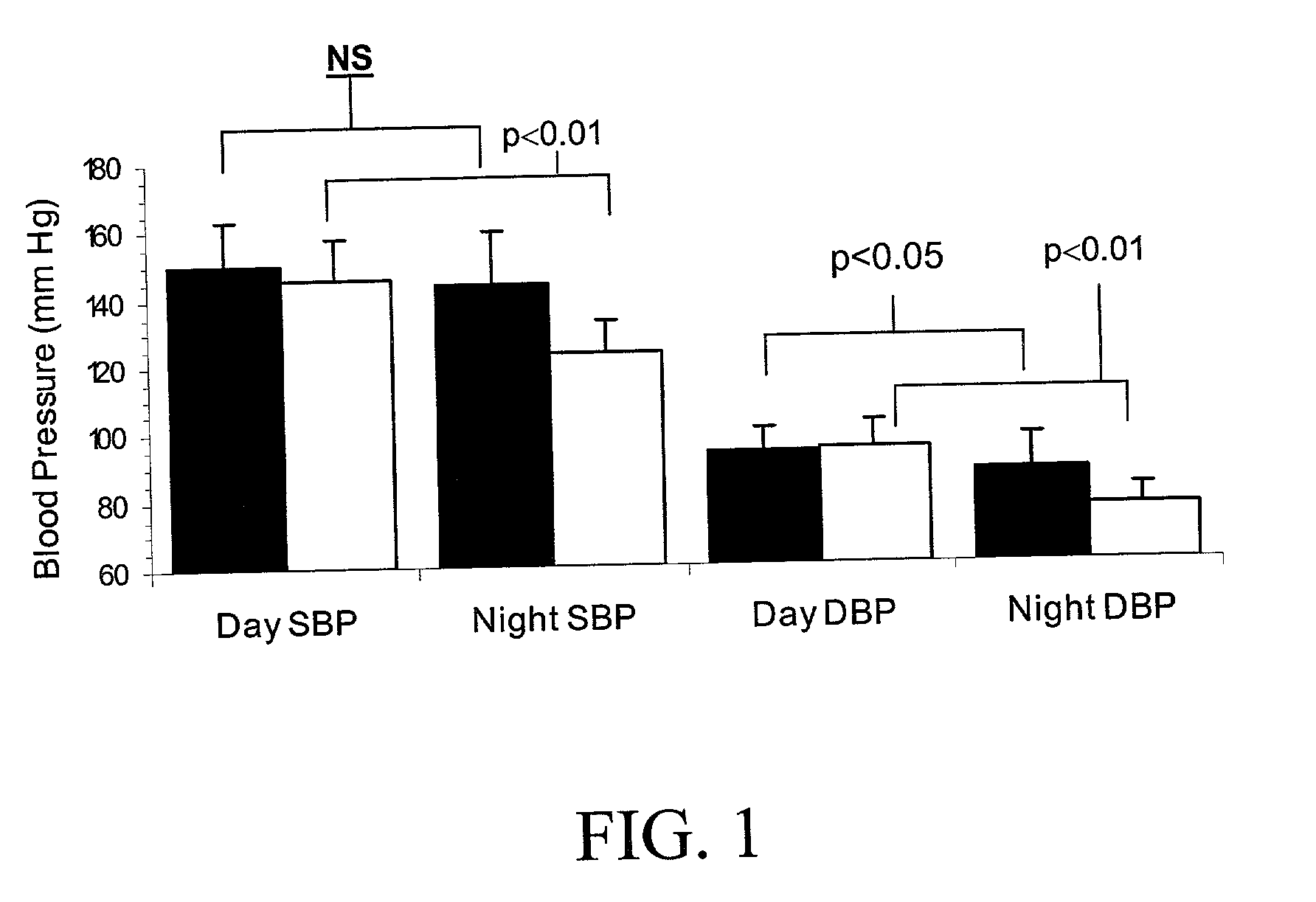
InactiveUS20090221549A1Beneficial effect on renal functionLower blood pressureBiocideAnimal repellantsAmbulatoryAnti hypertensive treatment
A new use of darusentan is provided in preparation of a pharmaceutical composition for lowering blood pressure in a patient exhibiting resistance to a baseline antihypertensive therapy with one or more drugs. The composition comprises darusentan in an amount providing a therapeutically effective daily dose; wherein (a) the composition is orally deliverable and / or (b) the daily dose of darusentan is effective to provide a reduction of at least about 3 mmHg in one or more blood pressure parameters selected from trough sitting systolic, trough sitting diastolic, 24-hour ambulatory systolic, 24-hour ambulatory diastolic, maximum diurnal systolic and maximum diurnal diastolic blood pressures. Further provided is a new use of darusentan in preparation of a pharmaceutical composition for lowering blood pressure in a patient exhibiting resistance to a baseline antihypertensive therapy, wherein the composition is administered adjunctively with at least one diuretic and at least one antihypertensive drug selected from ACE inhibitors, angiotensin II receptor blockers, beta-adrenergic receptor blockers and calcium channel blockers.
Owner:ABBOTT GMBH & CO KG
Method for treating resistant hypertension
A method is provided for lowering blood pressure in a patient having clinically diagnosed resistant hypertension. The method comprises administering darusentan to the patient adjunctively with a baseline antihypertensive regimen that comprises administration of at least one diuretic and at least two antihypertensive drugs selected from at least two of (a) ACE inhibitors and angiotensin II receptor blockers, (b) beta-adrenergic receptor blockers and (c) calcium channel blockers. The darusentan is orally administered at a dose and frequency effective, in combination with the baseline regimen, to provide a reduction of at least about 3 mmHg in one or more blood pressure parameters selected from trough sitting systolic, trough sitting diastolic, 24-hour ambulatory systolic, 24-hour ambulatory diastolic, maximum diurnal systolic and maximum diurnal diastolic blood pressures.
Owner:MYOGEN INC +1
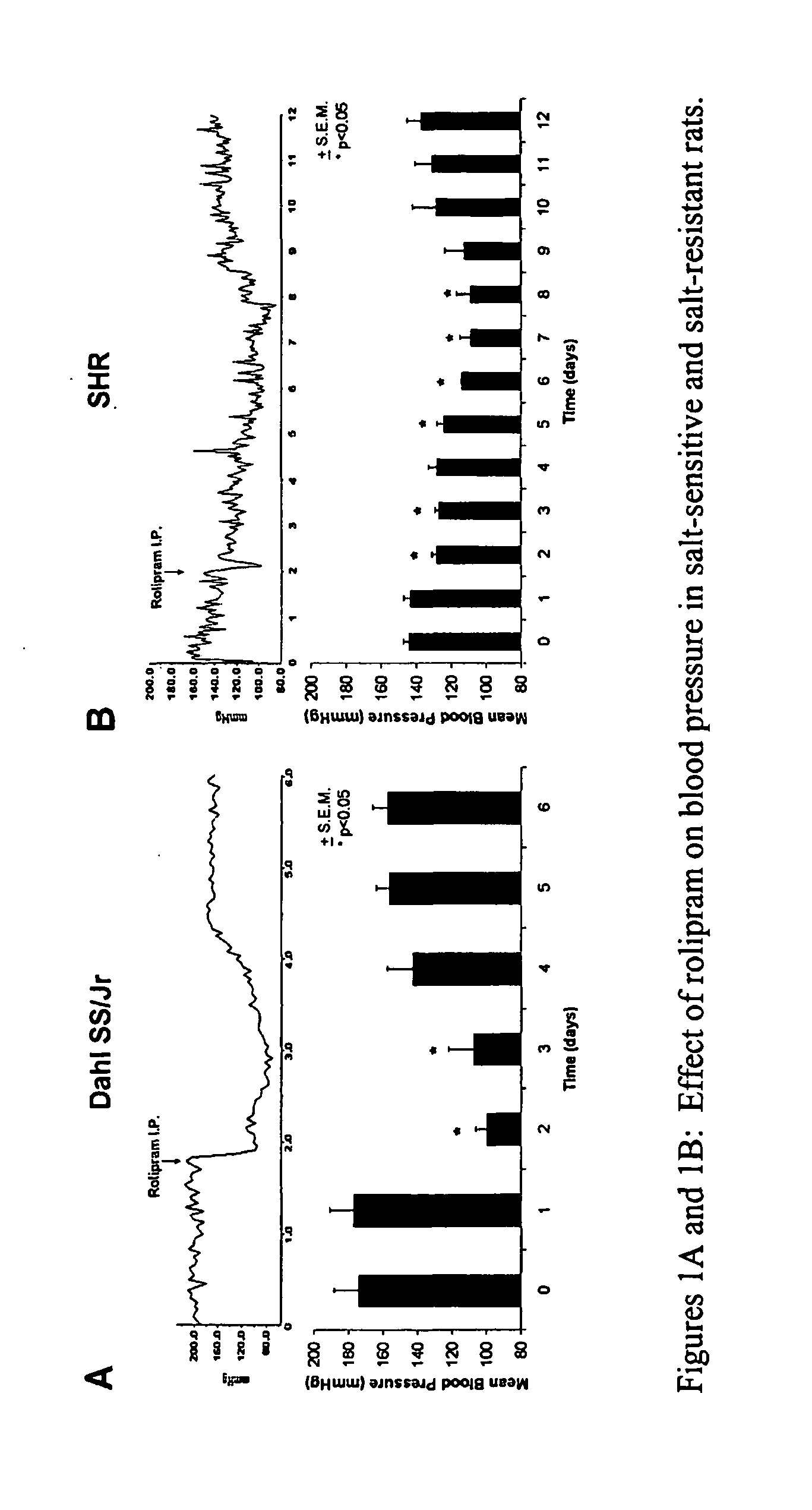
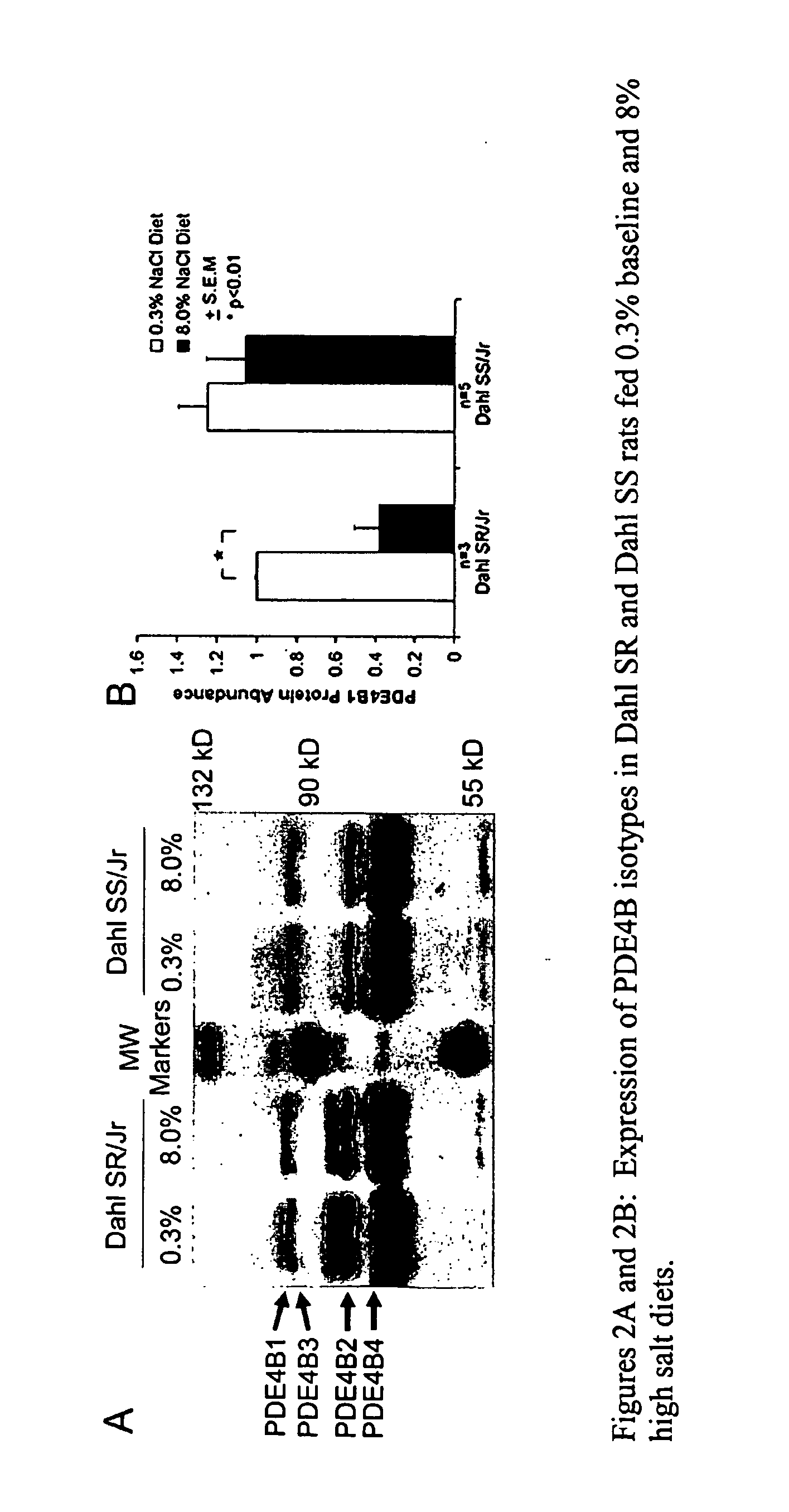
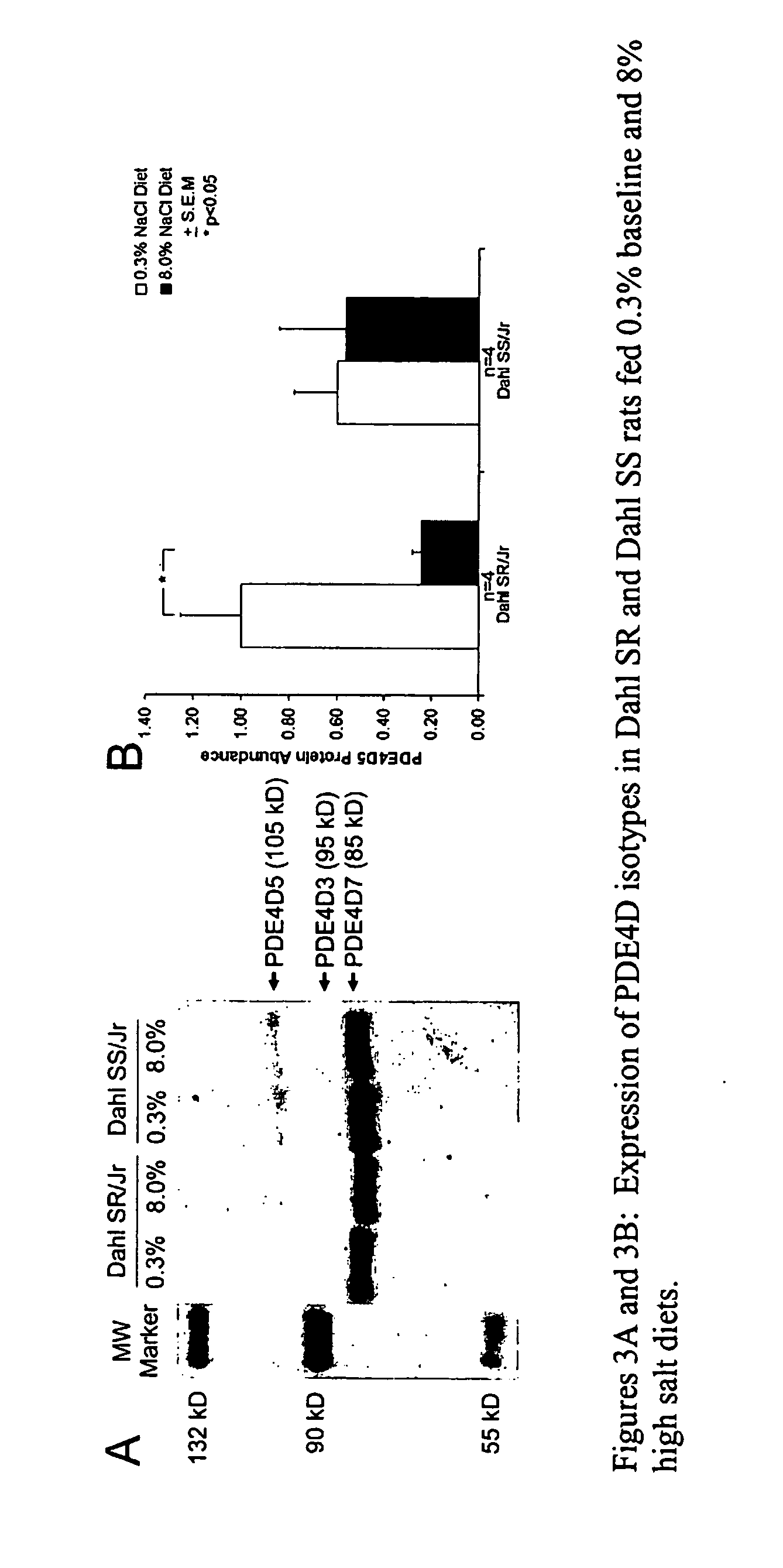
Blood Pressure Reduction in Salt-Sensitive Hypertension
InactiveUS20090042951A1Drop in blood pressureLess magnitudeBiocideAnimal repellantsBlood pressureCyclic nucleotide phosphodiesterase
This invention provides methods and compositions, most preferably pharmaceutical compositions, for treating salt-sensitive hypertension through the inhibition of certain enzymes in the beta-adrenergic pathway that are involved in the regulation of the secretion of water and sodium. These enzymes are cyclic nucleotide phosphodiesterases (PDE) that selectively hydrolyze the second messenger cAMP and, therefore, down-regulate beta-adrenergic signaling. Specifically provided are methods and pharmaceutical compositions for treating salt-sensitive hypertension by inhibiting certain members of the PDE4 family of cyclic nucleotide phosphodiesterases, particularly members of the PDE4B and PDE4D sub-families and, more particularly, the PDE4B1 and PDE4D5 isotypes thereof.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Prevention and treatment of sarcopenia
ActiveUS20120095070A1Good effectHigh prevalenceOrganic active ingredientsBiocideAdrenergicSarcopenia
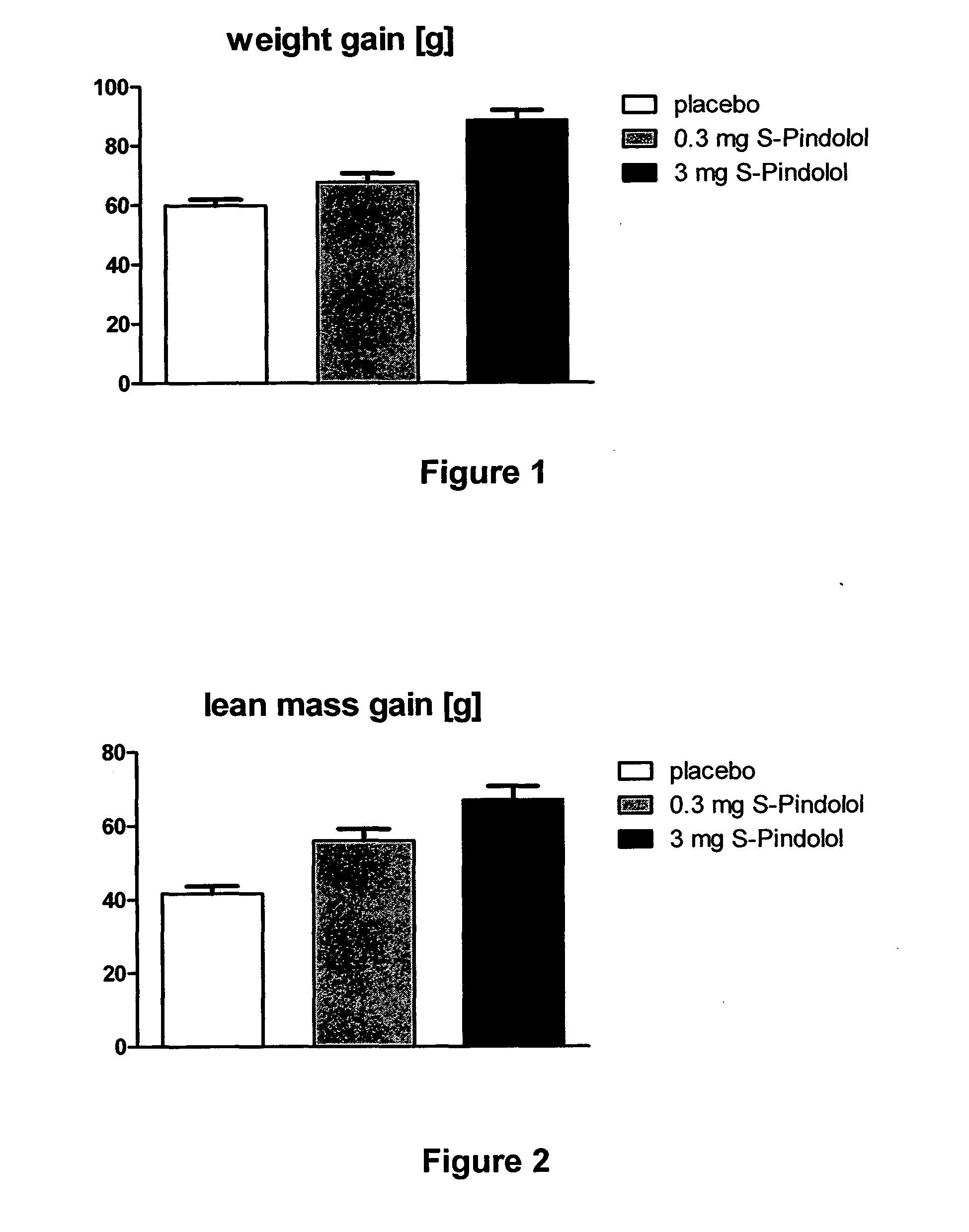
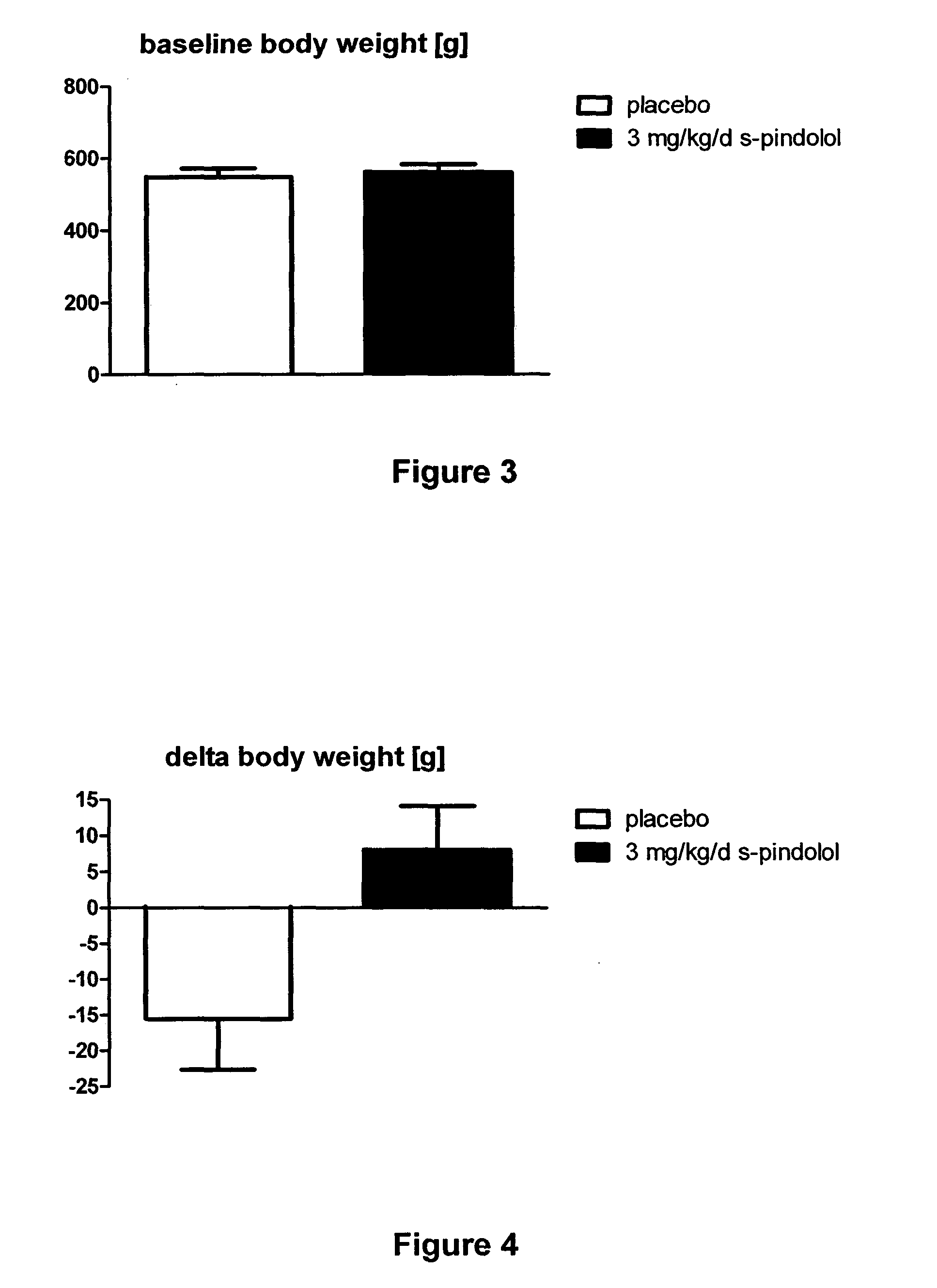
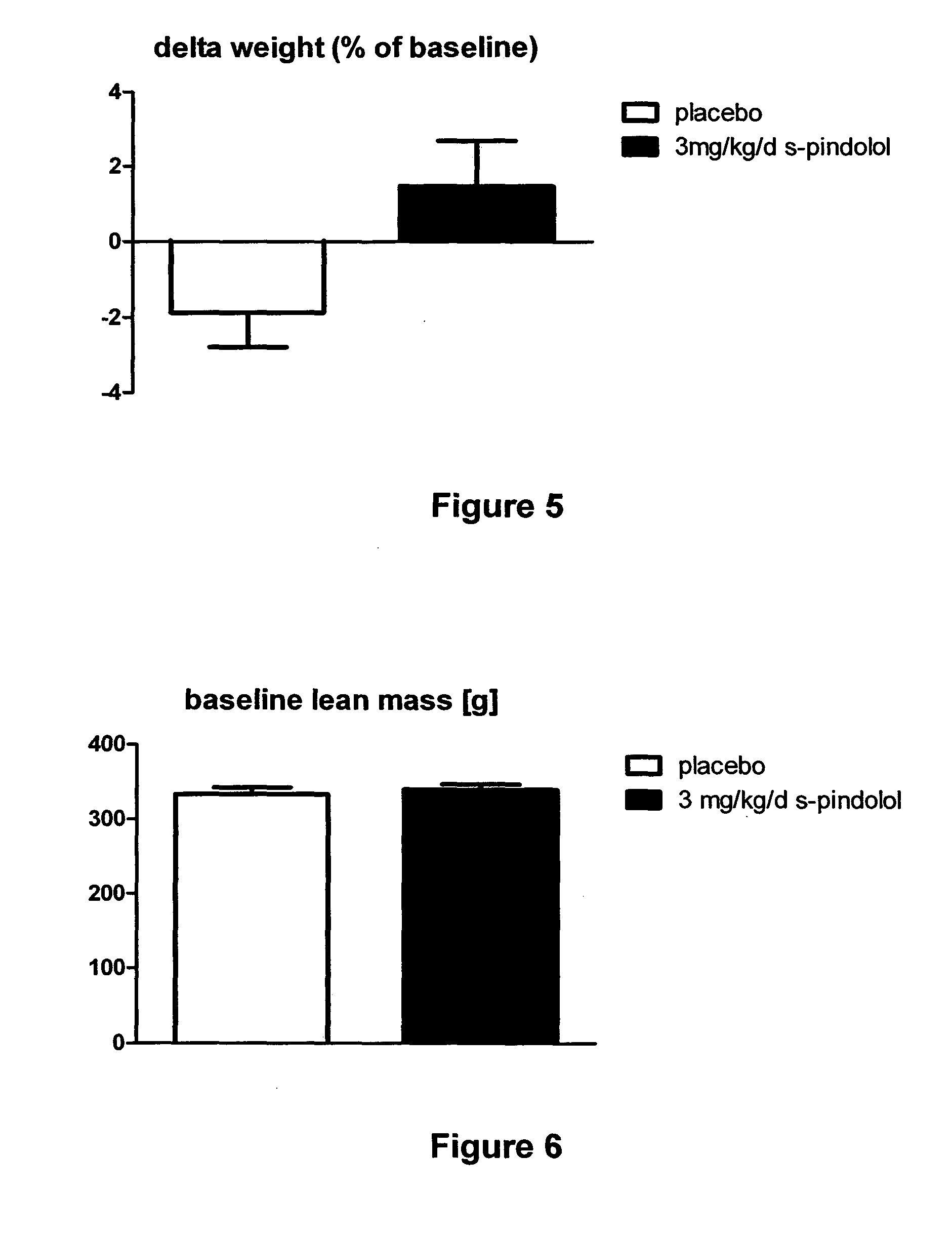
The present invention provides a means for prevention and treatment of sarcopenia by administration of a substance that both reduces the sensibility of beta-adrenergic receptors and of 5-HT1a receptors. (S)-pindolol, (S)-propanol, tertalol, or bopindolol are preferred for this purpose.
Owner:ACTIMED THERAPEUTICS LTD
Beta-adrenoceptor genetic polymorphisms and obesity
InactiveUS20020187491A1Reducing trial and error approachShorten the time limitBiocideOrganic active ingredientsHypertension medicationsBeta blocker
The present invention provides a method for identifying a subject having a risk of developing obesity, coronary microvascular dysfunction, or hypertension, comprising detection of the presence of a single nucleotide polymorphism (SNP) in a nucleic acid encoding an element of at least one beta-adrenergic receptor from the subject. The presence of the SNP is correlated with obesity, coronary microvascular dysfunction, or hypertension, and thereby identifies the subject as having a risk of developing obesity, coronary microvascular dysfunction, or hypertension. The subject invention also provides methods of identifying patients likely to benefit from the prescription of beta blocker hypertension medications. In various embodiments, the nucleic acids detected include those genes encoding ADRB1 (beta1AR), ADRB2 (beta2AR), ADRB3 (beta3AR), GNB3 (G protein beta3 subunit), or GNAS1 (GS protein alpha subunit). Methods of treating identified individuals are also provided.
Owner:FLORIDA UNIV OF A FLORIDA
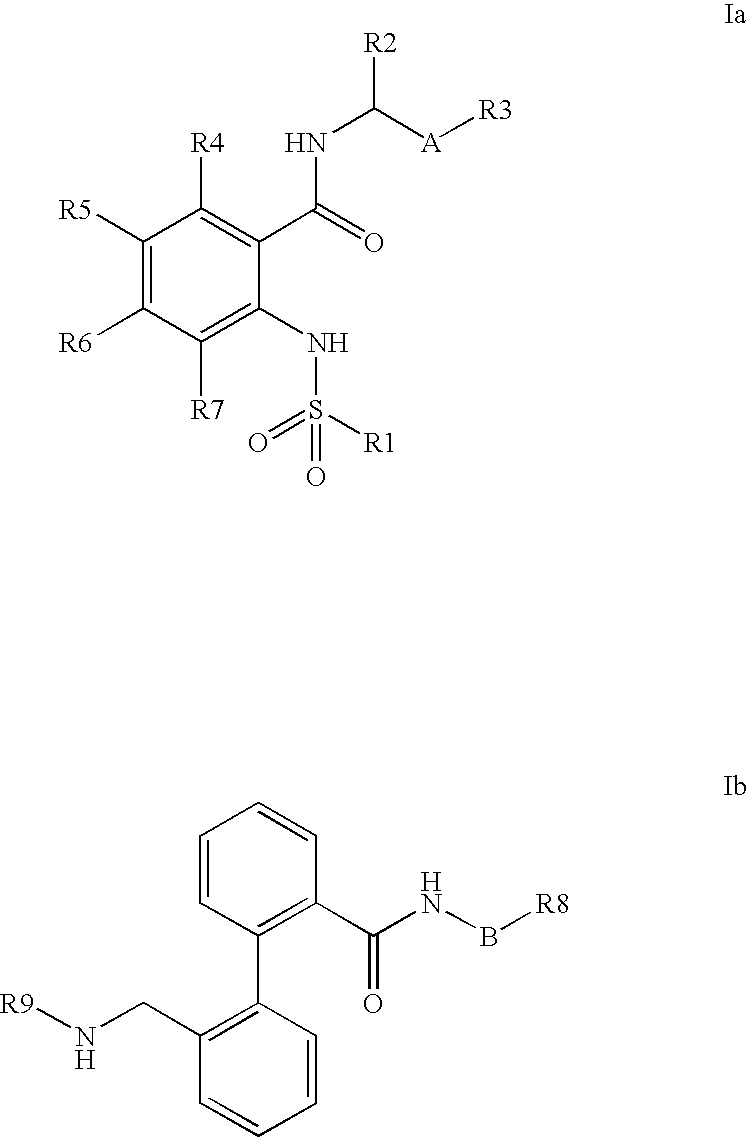
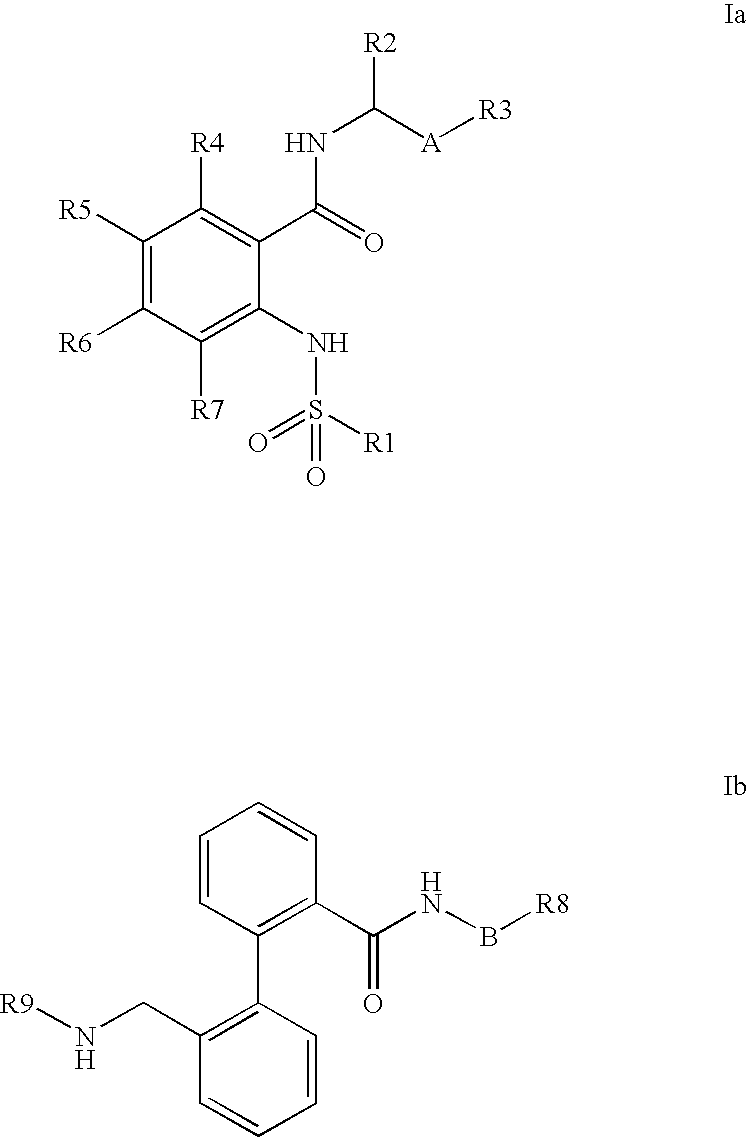
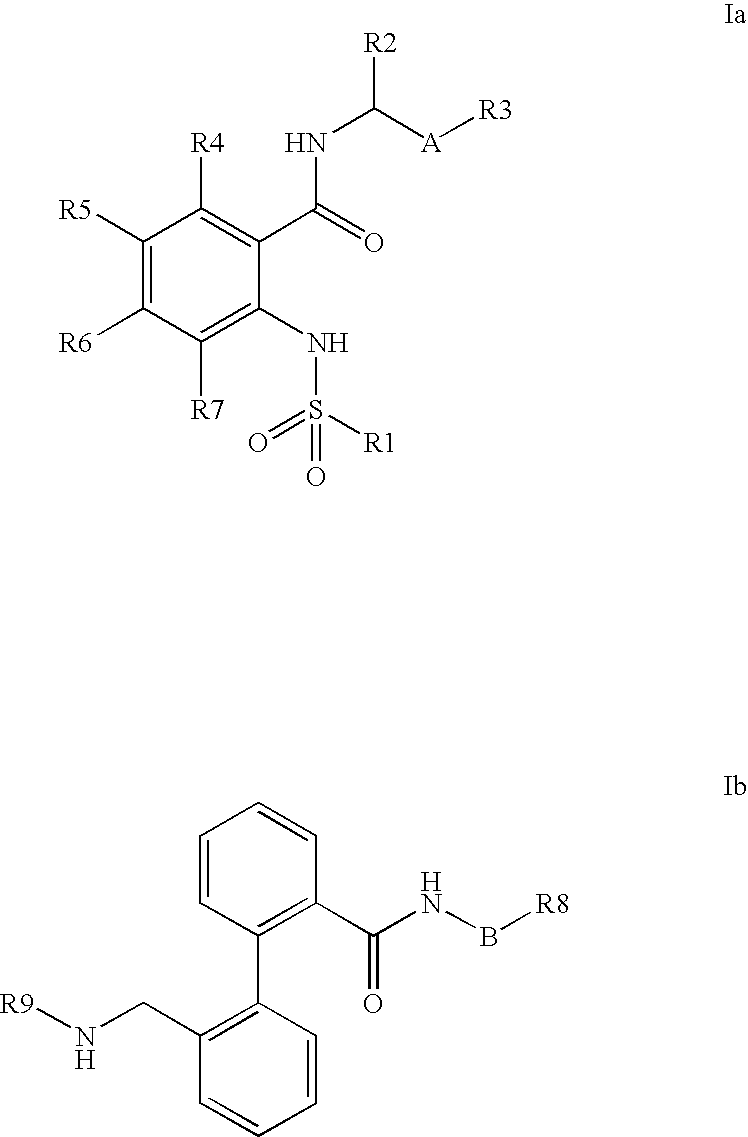
Combination of phenylcarboxylic acid amides with beta-adrenoreceptor blockers and their use for the treatment of atrial arrhythmias
The invention relates to the combination of one or more beta-blockers and of one or more Kv1.5 blockers, in particular phenylcarboxamides of the formula la and / or lb and / or physiologically tolerable salts thereof, and the use of the combination for the treatment or prophylaxis of atrial arrhythmias.
Owner:SANOFI AVENTIS DEUT GMBH
Dry powder formulation comprising an anticholinergic, a corticosteroid and a beta-adrenergic for administration by inhalation
Dry powder formulations for inhalation comprising a combination of an anticholinergic, a long-acting beta2-adrenoceptor agonist, and a corticosteroid are useful for the prevention and / or treatment of inflammatory and / or obstructive airways diseases.
Owner:CHIESI FARM SPA
Dry powder formulation comprising an anticholinergic, a corticosteroid and a beta-adrenergic for administration by inhalation
Dry powder formulations for inhalation comprising a combination of an anticholinergic, a long-acting beta2-adrenoceptor agonist, and a corticosteroid are useful for the prevention and / or treatment of inflammatory and / or obstructive airways diseases.
Owner:CHIESI FARM SPA
Beta-adrenoceptor genetic polymorphisms and obesity
InactiveUS20040033524A1Reducing trial and error approachShorten the time limitBiocideOrganic active ingredientsHypertension medicationsBeta blocker
The present invention provides a method for identifying a subject having a risk of developing obesity, coronary microvasular dysfunction, or hypertension, comprising detection of the presence of a single nucleotide polymorphism (SNP) in a nucleic acid encoding an element of at least one beta-adrenergic receptor from the subject. The presence of the SNP is correlated with obesity, coronary microvascular dysfunction, or hypertension, and thereby identifies the subject as having a risk of developing obesity, coronary microvascular dysfunction, of hypertension. The subject invention also provides methods of identifying patients likely to benefit from the prescription of beta blocker hypertension medications. In various embodiments, the nucleic acids detected include those genes encoding ADRB1 (beta1AR), ADRB2 (beta2AR), ADRB3 (beta3AR), GNB3 (G protein beta3 subunit), or GNAS1 (Gs protien alpha subunit). Methods of treating identified individuals are also provided.
Owner:FLORIDA UNIV OF A FLORIDA
Compositions and methods for treating or preventing atrial fibrillation
InactiveUS20110281853A1Preventing atrial fibrillationBiocideAmide active ingredientsAdrenergicAntagonist
The present invention provides compositions and methods for treating or preventing atrial fibrillation (AF). In particular, the present invention provides administration of muscarinic receptor antagonists (e.g., M2-selective muscarinic receptor blockers), administered alone or in combination with other therapeutic agents (e.g., beta-adrenergic receptor blockers) to treat and / or prevent atrial fibrillation.
Owner:NORTHWESTERN UNIV
Immunosensor and method for detecting various beta-adrenergic receptor stimulant residues
InactiveCN104280437AHigh sensitivityTo achieve common detectionBiological testingMaterial electrochemical variablesStimulantSpecific antibody
The invention discloses an electrochemical immunosensor capable of detecting various beta-adrenergic receptor stimulants (short for beta-stimulants). The sensor comprises a base electrode, a graphene / chitosan complex membrane and multi-determinant antigens of beta-stimulants, wherein the graphenee / chitosan complex membrane is modified on the surface of the base electrode, and the multi-determinant antigens of the beta-stimulants are fixed on the surface of the graphene / chitosan complex membrane. The multi-determinant antigens fixed on the surface of the sensor are salbutamol-ractopamine-BSA or salbutamol-ractopamine-OVA. The invention also provides an electrochemical immunodetection method for various beta-stimulant residues. The electrochemical immunosensor can be used for detecting six beta-stimulants including clenbuterol, salbutamol, ractopamine, terbutaline, mabuterol and tulobutezol through carrying out cross immune reaction on the beta-stimulants based on wide-spectrum specific antibodies of the beta-stimulants by taking K3[Fe(CN)6] as a probe.
Owner:NANJING NORMAL UNIVERSITY
Stable pharmaceutical composition for atherosclerosis
ActiveUS20120027849A1Good antihypertensive effectGood blood pressure effectBiocideAntipyreticLipid formationLipid lowering drug
The present invention relates to a stable solid oral pharmaceutical multi-component composition comprising combination of blood pressure lowering drugs with lipid lowering agent / s and optionally a platelet aggregation inhibitor in a single dosage form. The blood pressure lowering agents are selected from β-adrenergic receptor blocking agent, ACE inhibitor and diuretic. The lipid lowering agent is selected from HMG Co-enzyme-A reductase inhibitor. The pharmaceutical composition made as per present invention a) overcomes any drug-drug interactions, b) exhibits pharmacokinetic and pharmacodynamic profile of individual therapeutic agent, c) has minimal side effects. The invention provides multi-component composition (MCC) to increase adherences to therapy. The MCC as per present invention provides compositions that maintain activity of all active ingredients without significant increase in adverse event profile. The present invention further relates to a method of preparing the said pharmaceutical composition.
Owner:CADILA PHARMA
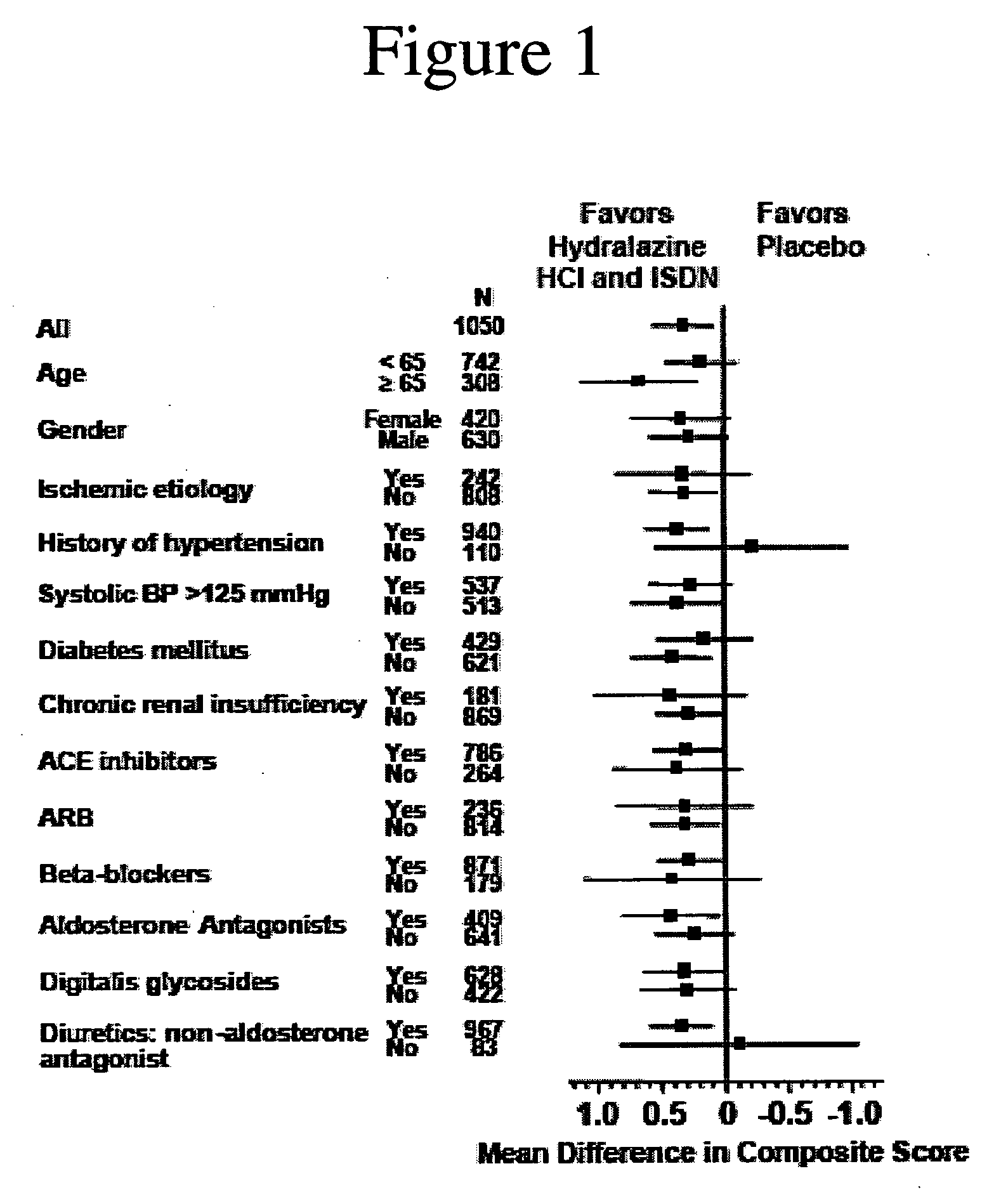
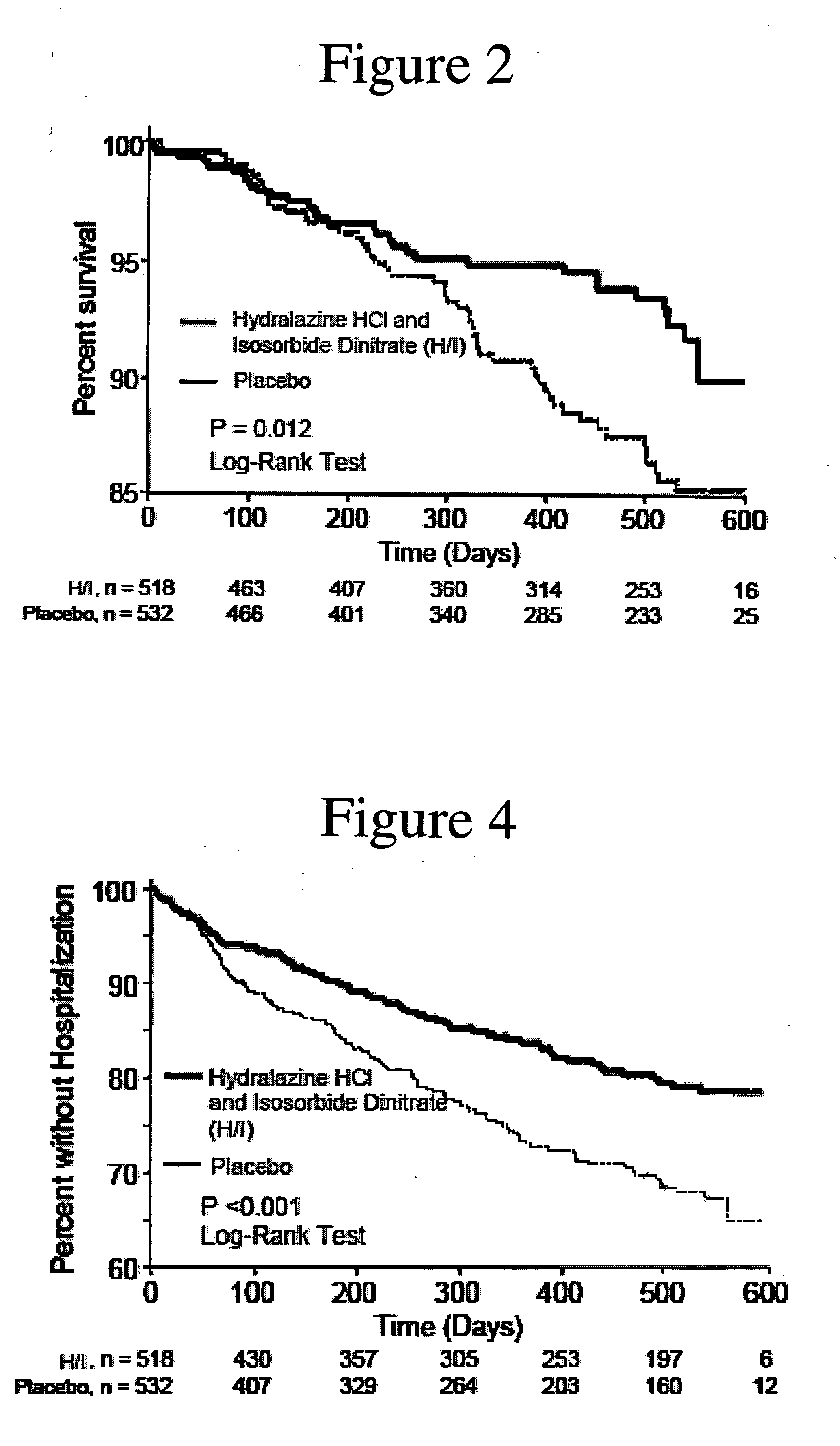
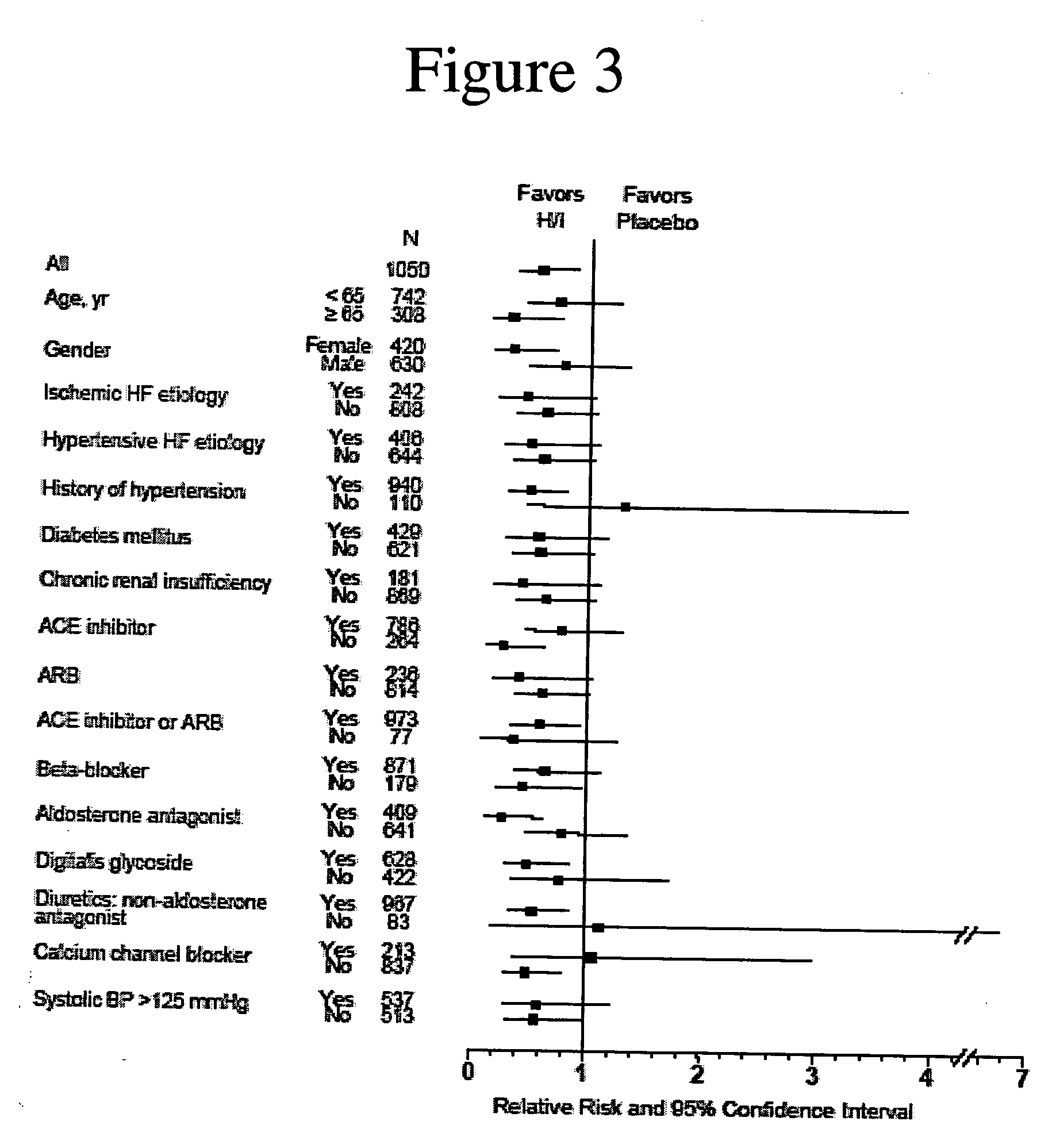
Methods for reducing hospitalizations related to heart failure
InactiveUS20060014829A1Reduce in quantityShorten the construction periodBiocideNervous disorderDigitalisMortality rate
The invention provides methods for (a) prolonging time to hospitalization for heart failure; (b) prolonging time to first hospitalization for heart failure; (c) reducing the total number of days a patient with heart failure spends in the hospital for heart failure for a single hospital stay (i.e., reducing the duration of a single hospital stay for a patient with heart failure); (d) reducing the total number of days a patient spends in the hospital for heart failure for multiple hospital stays; (e) reducing the number of hospital admissions for heart failure; and (f) reducing mortality and reducing hospitalizations for heart failure (e.g., the total number of days in the hospital and / or the number of hospital visits) in a patient in need thereof comprising administering to the patient a therapeutically effective amount of (i) a hydralazine compound or pharmaceutically acceptable salt thereof, (ii) isosorbide dinitrate and / or isosorbide mononitrate, and (iii) optionally at least one compound selected from the group consisting of angiotensin converting enzyme inhibitors, β-adrenergic antagonists, angiotensin II antagonists, aldosterone antagonists, cardiac glucosides (digitalis), and diuretic compounds.
Owner:NITROMED
Dry powder formulation comprising a corticosteroid and a beta-adrenergic for administration by inhalation
Dry powder formulations comprising a corticosteroid and a beta2-adrenergic drug in combination are useful for the prevention and / or treatment of inflammatory or obstructive airways diseases.
Owner:CHIESI FARM SPA
Antihypertensive therapy method
InactiveUS20070293552A1Improve cardiovascular functionImproved renal functionBiocideAnimal repellantsDiseaseAnti hypertensive treatment
Methods and therapeutic combinations are provided for lowering blood pressure in a subject exhibiting resistance to a baseline antihypertensive therapy with one or more drugs, or a subject having diabetes and / or chronic kidney disease. A method of the invention comprises administering, in some embodiments adjunctively with a modified baseline therapy, a compound of formula (I) as defined herein. A therapeutic combination of the invention comprises a compound of formula (I); at least one diuretic; and at least one antihypertensive drug selected from ACE inhibitors, angiotensin II receptor blockers, beta-adrenergic receptor blockers and calcium channel blockers; wherein the at least one diuretic and / or the at least one antihypertensive drug are present at substantially less than a full dose.
Owner:GILEAD COLORADO
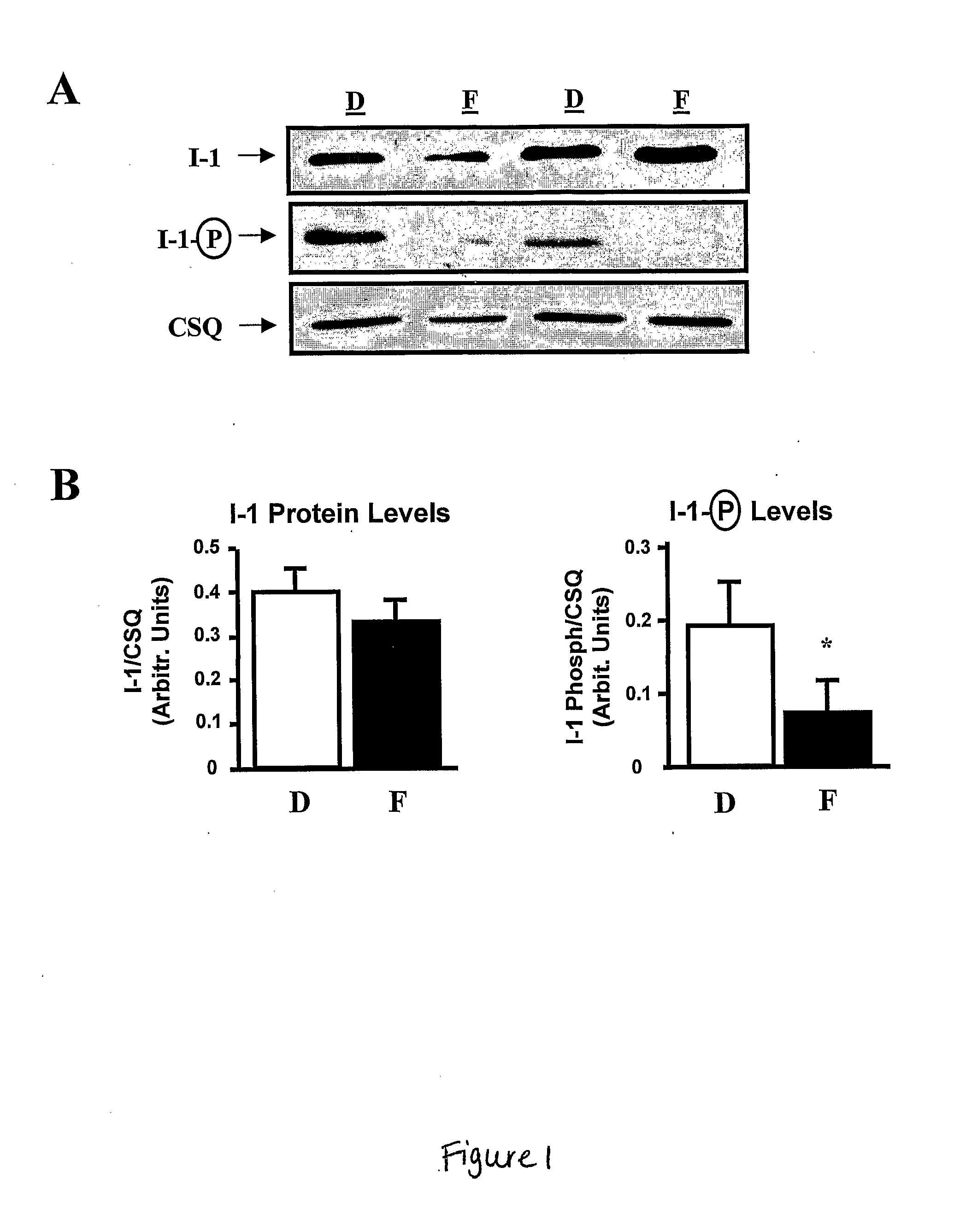
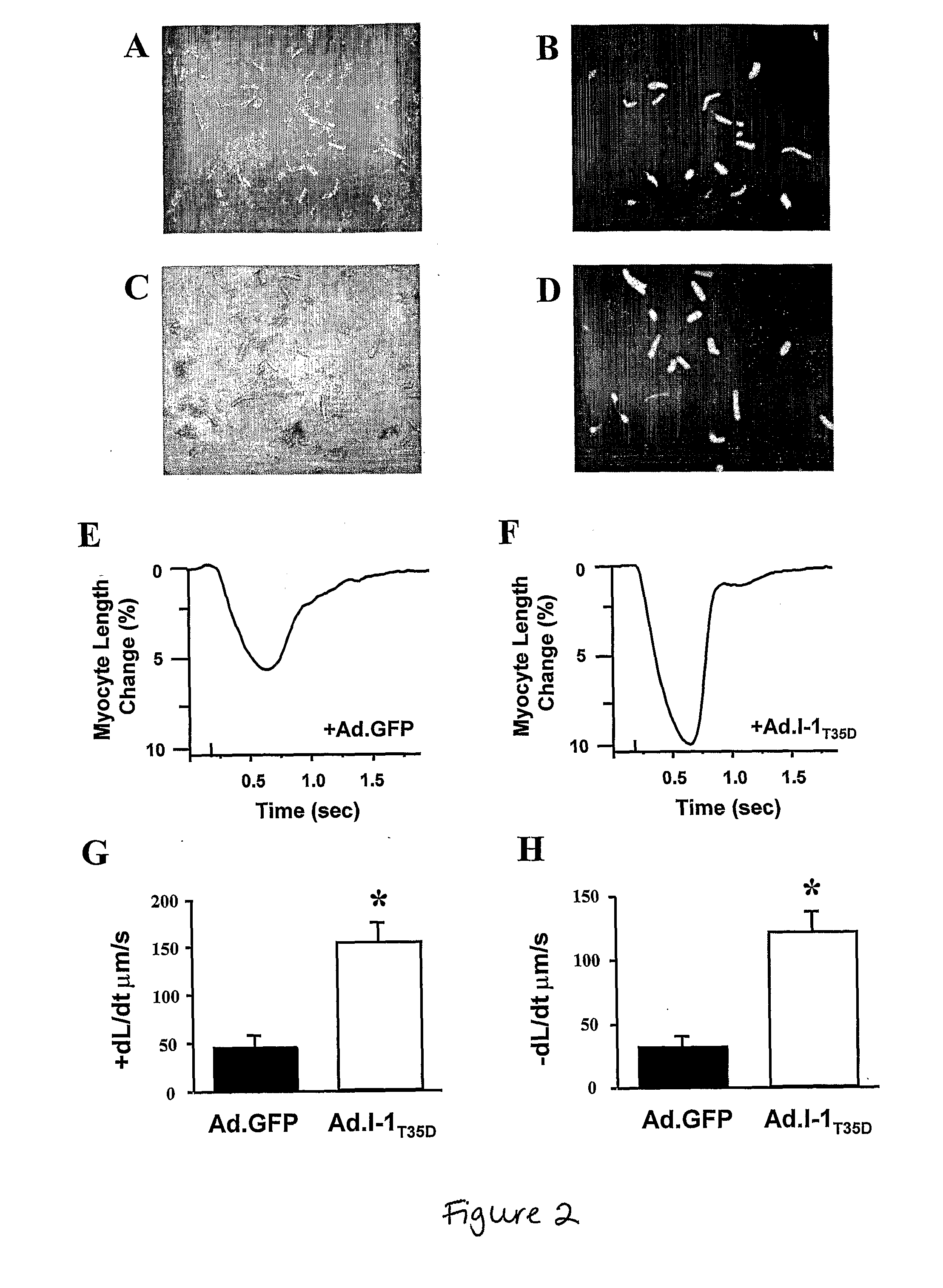
Modulating Phosphatase Activity In Cardiac Cells
ActiveUS20080125385A1Improve responsivenessDecreasing phosphatase activityVirusesMetabolism disorderHeart cellsCardiac disorders
Expression of a phosphatase inhibitor in heart cells can be used to treat cardiac disorders, e.g., heart failure. Decreasing phosphatase activity can improve β-adrenergic responsiveness.
Owner:UNIVERSITY OF CINCINNATI +1
Inhalation particles comprising a combination of an anticholinergic, a corticosteroid and a beta-adrenergic
Microparticles comprising a combination of an anticholinergic, a beta2-adrenoceptor agonist, and an inhaled corticosteroid are useful for the prevention and / or treatment of respiratory diseases.
Owner:CHIESI FARM SPA
Inhalation particles comprising a combination of an anticholinergic, a corticosteroid and a beta-adrenergic
Microparticles comprising a combination of an anticholinergic, a beta2-adrenoceptor agonist, and an inhaled corticosteroid are useful for the prevention and / or treatment of respiratory diseases.
Owner:CHIESI FARM SPA
Treatment of heart disease using beta-blockers
The present invention relates to a method of reversing the electrophysiological cardiac remodeling of animals with heart disease. More specifically, the method includes administering to an animal in need thereof a β-adrenoceptor blocker.
Owner:BAYER ANIMAL HEALTH
Multi-cluster antigen and wide-spectrum specific antibody of beta-adrenoceptor agonists and application of multi-cluster antigen and wide-spectrum specific antibody
The invention relates to a multi-cluster antigen and an antibody of beta-adrenoceptor agonists and application of the multi-cluster antigen and the antibody to detection on multi-residue of the beta-adrenoceptor agonists. The application is implemented by a detection kit and test paper. The multi-cluster antigen of the beta-agonists is salbutamol-ractopamine-bovine serum albumin (SAL-RAC-BSA) or salbutamol-ractopamine-ovalbumin (SAL-RAC-OVA), and the salbutamol-ractopamine-bovine serum albumin antibody (SAL-RAC-BSAAb) is prepared from SAL-RAC-BSA through animal immunization. The invention also discloses the kit and the test paper, which are used for detecting the multi-residue of the beta-agonists and take the SAL-RAC-BSAAb as a core agent. Based on a cross immunization reaction of the SAL-RAC-BSAAb on the beta-agonists, three beta-agonists, namely clenbuterol, SAL and RAC, can be detected.
Owner:NANJING NORMAL UNIVERSITY
Compositions and methods for modulating bone mass
InactiveUS20090202572A1Improve efficacyIncrease local deliveryBiocidePeptide/protein ingredientsDiseaseMammal
The instant invention relates to compositions and methods for treating or preventing bone diseases. In certain aspects, the invention provides compositions comprising a β-adrenergic antagonist or agonist associated to a bone-targeted molecule, as well as methods of modulating bone mass and / or growth in a mammal by administering a composition of the present invention. In other aspects, the invention provides methods of modulating bone mass and / or growth in a mammal by administering a composition comprising a β2-selective antagonist or agonist.
Owner:BAYLOR COLLEGE OF MEDICINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com