Novel coronavirus SARS-CoV-2 mRNA vaccines and preparation method and application thereof
A sars-cov-2, sequence technology, applied in the fields of biomedicine and virology, can solve the problem of lack of specific drugs and vaccines, and achieve the effect of improving immune protection, high yield and reducing immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Embodiment 1: mRNA vaccine preparation
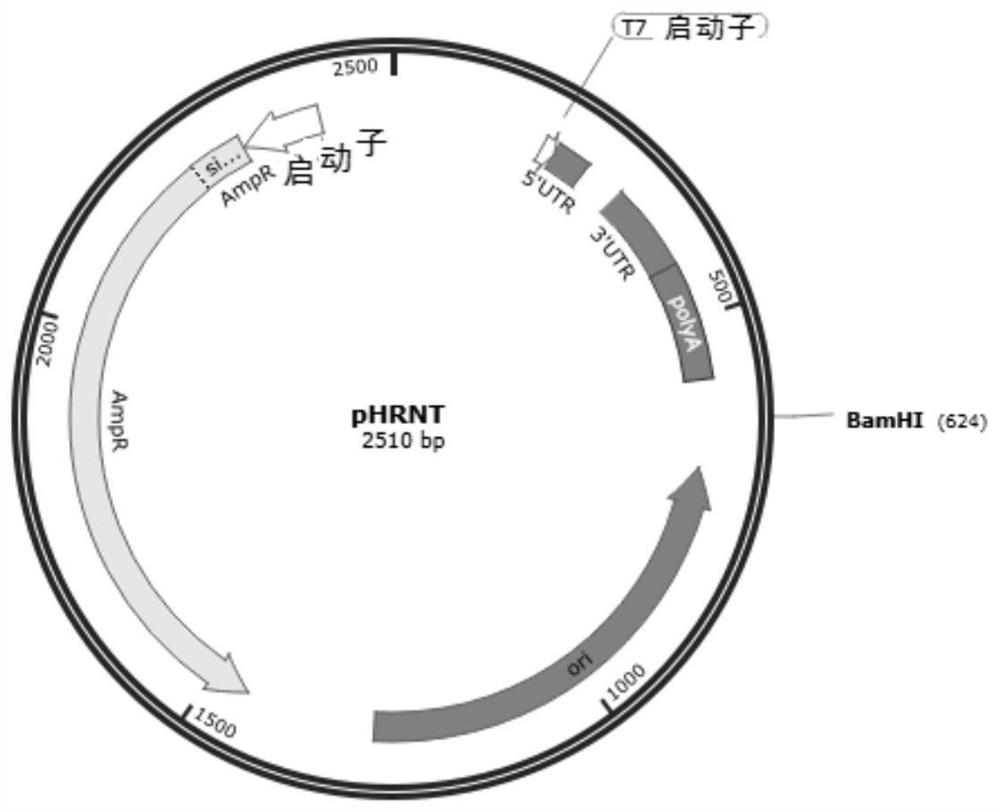
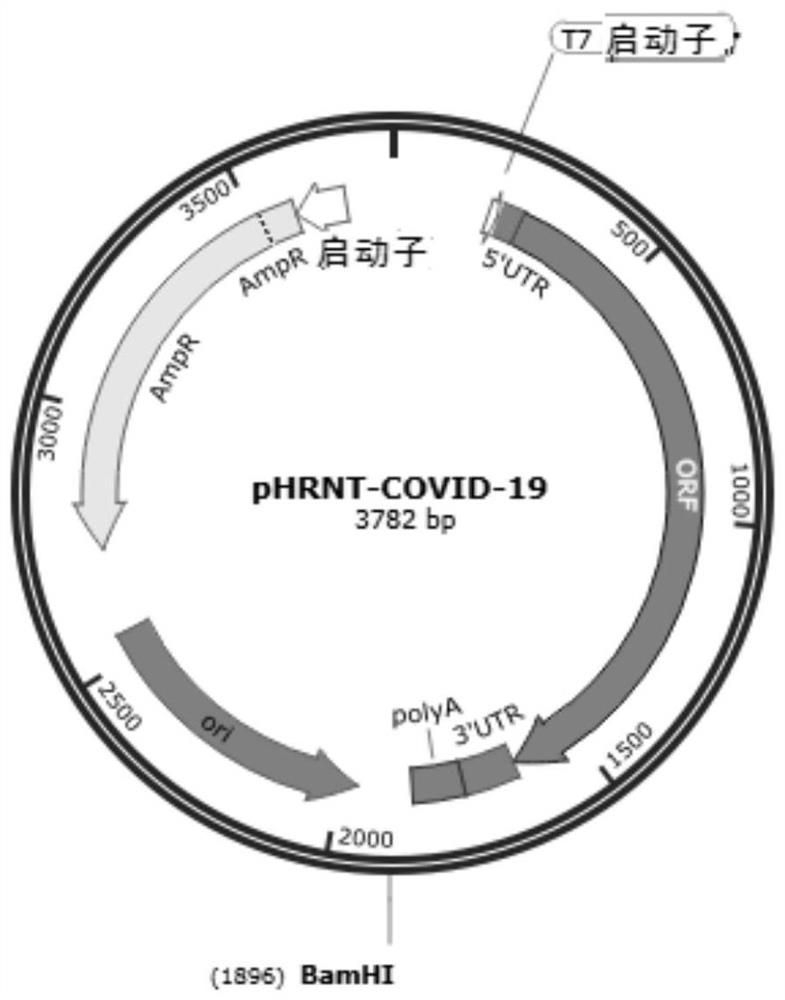
[0082] (1) Acquisition of target genes: The full-length amino acid sequences of SARS-CoV-2 receptor binding domain RBD, spike protein S1 subunit, and spike protein S were obtained from Genebank MN908947. After codon optimization, the nucleic acid sequences are as follows: Shown in SEQ ID NO: 2, 6 and 8, synthesized by Beijing Qingke Xinye Biotechnology Co., Ltd. and respectively cloned into the mRNA transcription template pHRNT vector (for example, purchased from Beijing Qingke Xinye Biotechnology Co., Ltd.) ( figure 1 ), obtain mRNA transcription template plasmid pHRNT-RBD, pHRNT-S1 and pHRNT-S ( figure 2 ).
[0083] (2) mRNA transcription
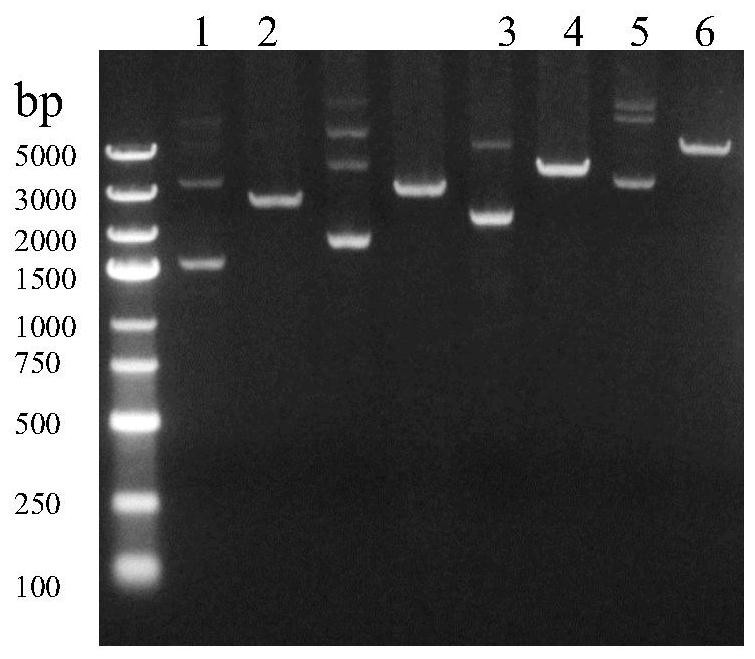
[0084] Restriction endonuclease BamHI was used to linearize the mRNA transcription templates pHRNT-RBD, pHRNT-S1 and pHRNT-S respectively, and run agarose gel electrophoresis ( image 3 ), to confirm whether the linearization is complete, and then use the gel extraction kit to recover th...
Embodiment 2
[0107] Example 2: Evaluation of Vaccine-Induced Humoral Immune Response
[0108] Divide 36 female BALB / c mice aged 6-8 weeks into 6 groups, 6 in each group, and inject placebo intramuscularly (the packaging method is the same as that of the vaccine group but lipid nanoparticles coated with polycytidylic acid, in which polycytidylic acid Cytidylic acid was purchased from Sigma), RBD (0.3 μg, 2 μg and 15 μg), S1 (15 μg) or S (15 μg) mRNA vaccine, and the blood was collected at the 4th week after immunization, and the serum was separated at 4°C and sterilized at 56°C Store at -80°C for 30 minutes before use.
[0109] (1) Determination of antigen-specific antibody titer
[0110] Dilute the SARS-CoV-2S extracellular segment protein with ELISA coating solution to 1 μg / ml, add 100 μl to each well of a 96-well plate, and place overnight at 4°C. The next day, the ELISA plate was closed, and the mouse serum was diluted in a 2-fold gradient, added to the ELISA plate and incubated at 37...
Embodiment 3
[0123] Example 3: Evaluation of Vaccine-Induced Cellular Immune Response
[0124] Divide 12 female C57BL / 6 mice aged 6-8 weeks into 2 groups, 6 mice in each group, inject placebo and RBD (15 μg) intramuscularly, and euthanize the mice at the 4th week after immunization, and take the spleen Placed in pre-cooled 1640 medium. Put the 40μm sieve on the 50ml centrifuge tube, use a 5ml syringe to grind the spleen, add 1640 medium to drop the cells into the 50ml tube, and filter out impurities and large cell clusters. Transfer all the cells to a 15ml centrifuge tube, collect the cells by centrifugation at 2000 rpm at room temperature, add 12ml of 1640 medium to wash again, and centrifuge again to collect the cells. Add 4ml of erythrocyte lysate, suspend the cells, place at room temperature for 5-10 minutes, then add 8ml of 1640 medium, collect the cells by centrifugation, add 12ml of 1640 medium to wash once, and centrifuge again to collect the cells. Add 10ml of 1640 medium to sus...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com