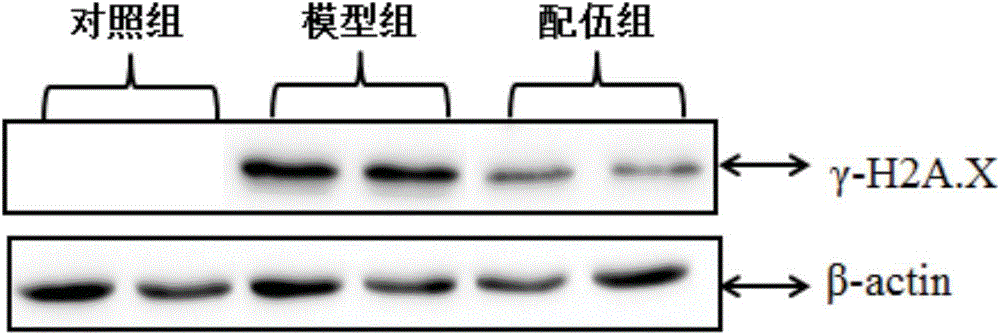
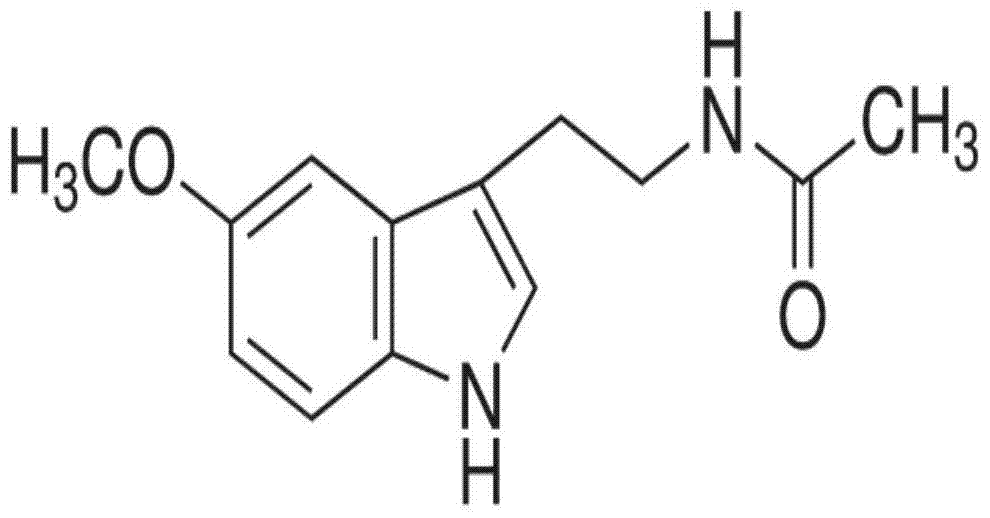
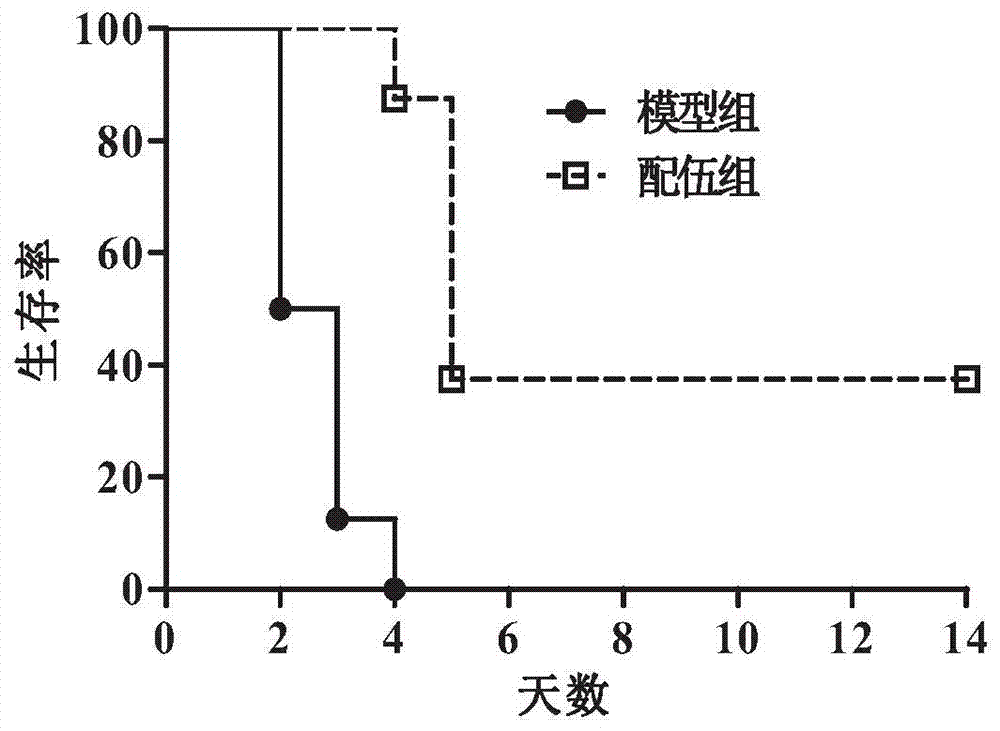
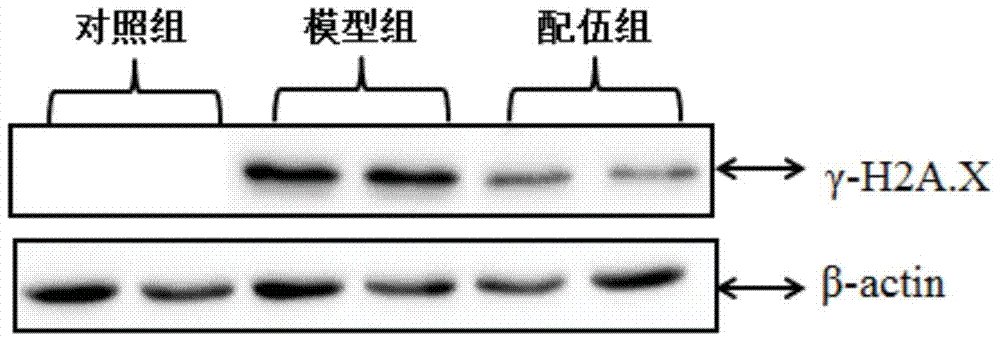
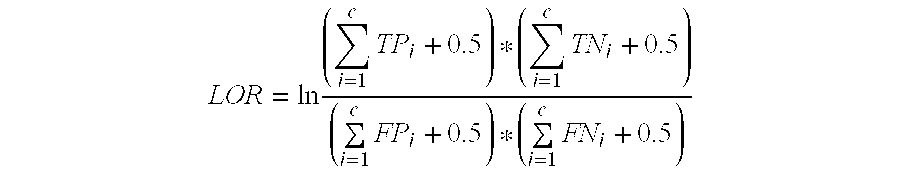Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
34 results about "Sub acute" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The term "sub acute" is used in contrast to acute which indicates very sudden onset or rapid change and chronic which indicates indefinite duration or virtually no change. A chronic condition is one lasting 3 months or more, by the definition of the U.S. National Center for Health Statistics.
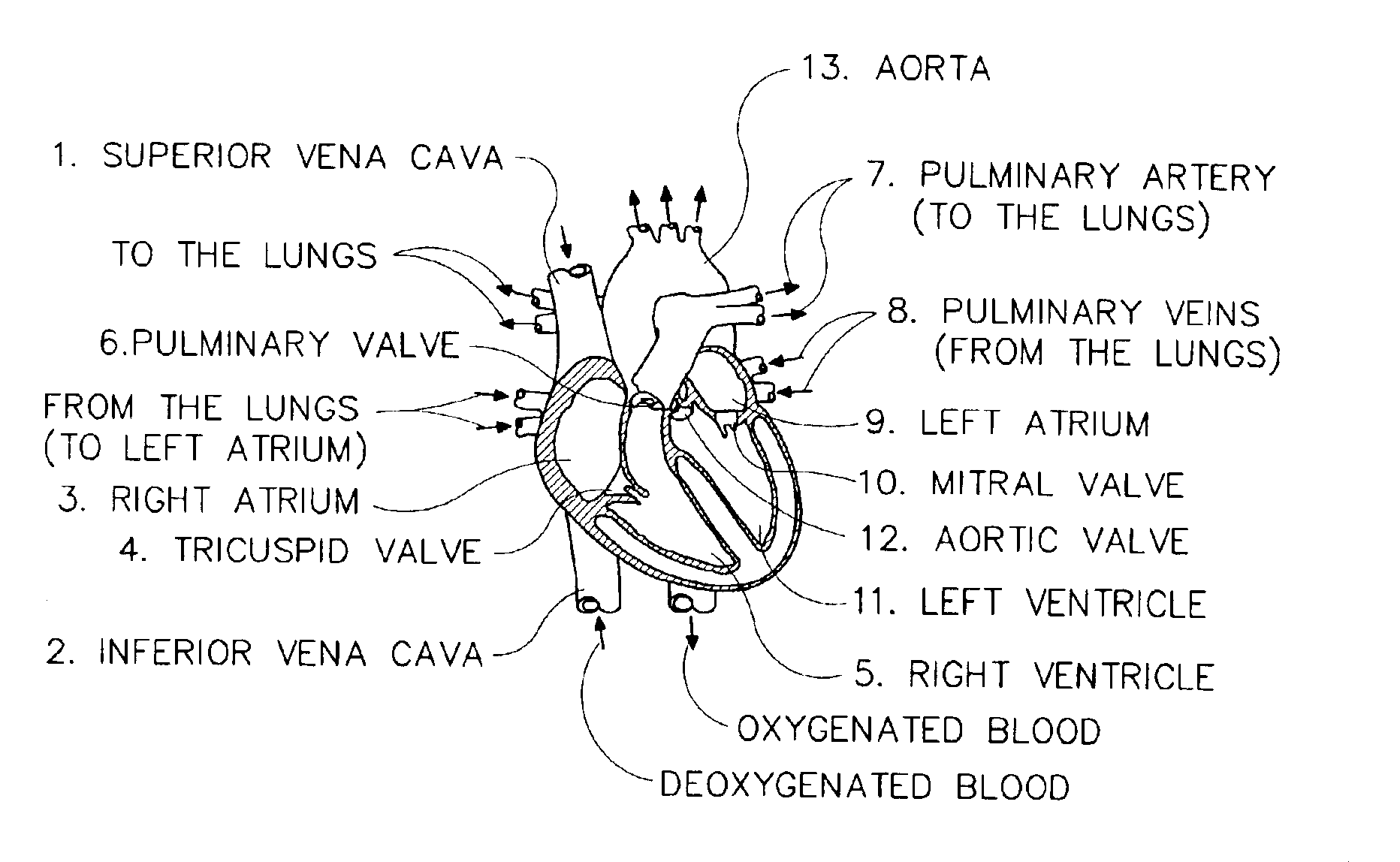
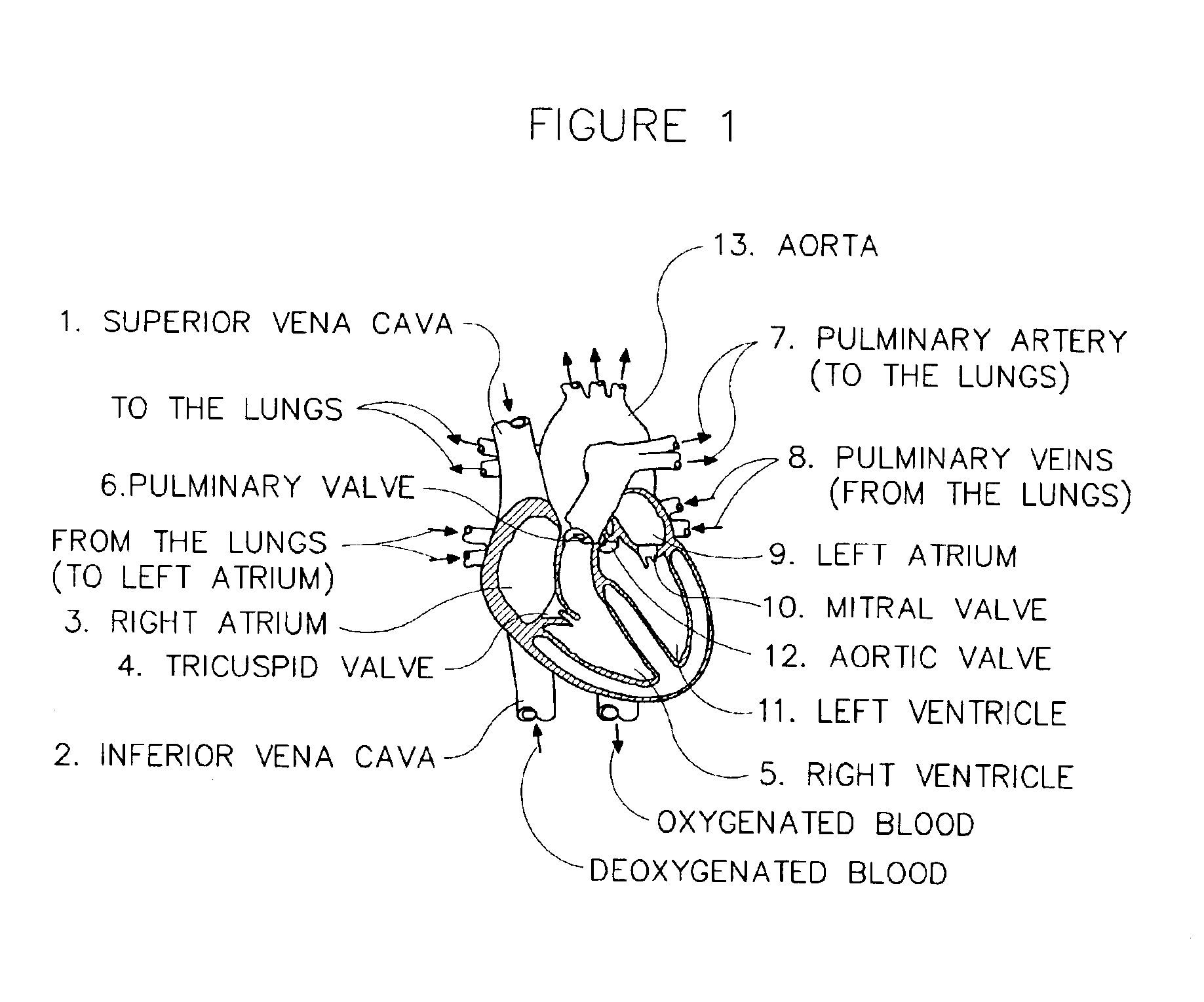
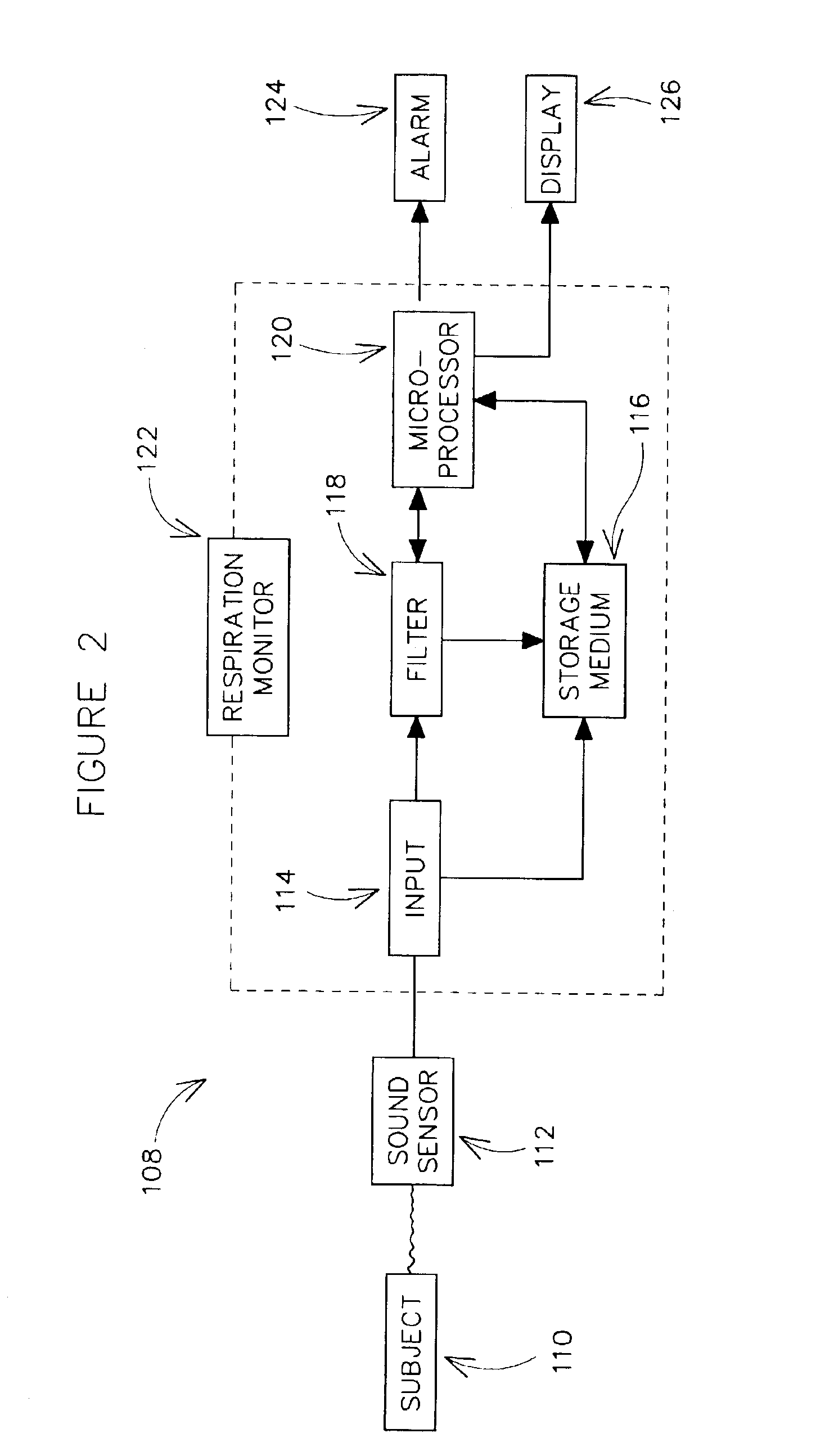
Methods and systems for monitoring respiration
A method for determining respiration rate in a patient can include various parts. The respiration rate can be determined by measuring the heart's S2 split. The S2 split can be identified by observing the timing of the heart sounds. Other respiration related information, such as respiration phase and the occurrence of apnea, can be identified as well. A respiration monitor of this type may be useful for monitoring sub-acute patients, and outpatients. A sensor for the respiration monitor and an electrode for an ECG monitor may be combined into a single probe.
Owner:GE MEDICAL SYST INFORMATION TECH
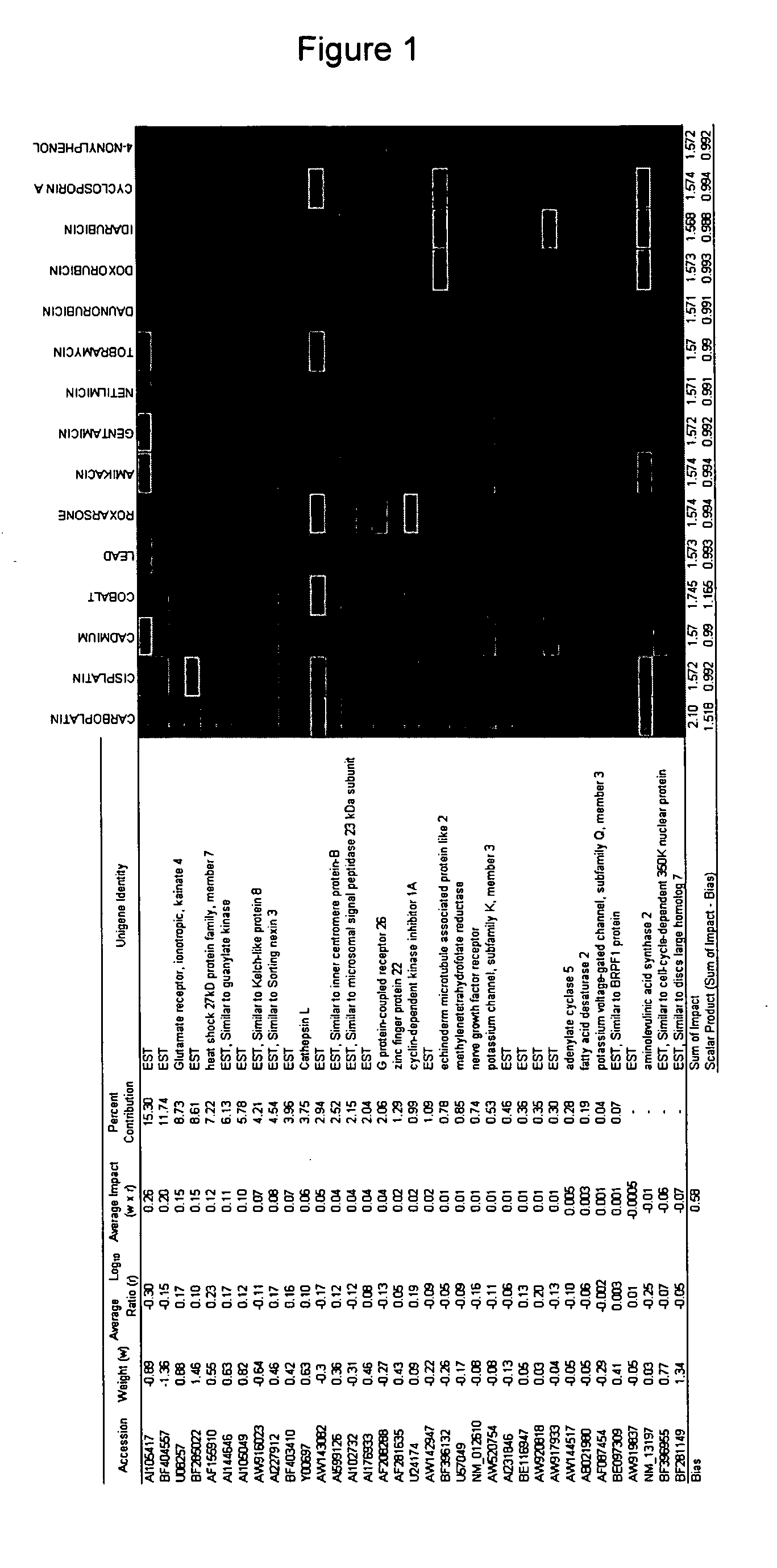
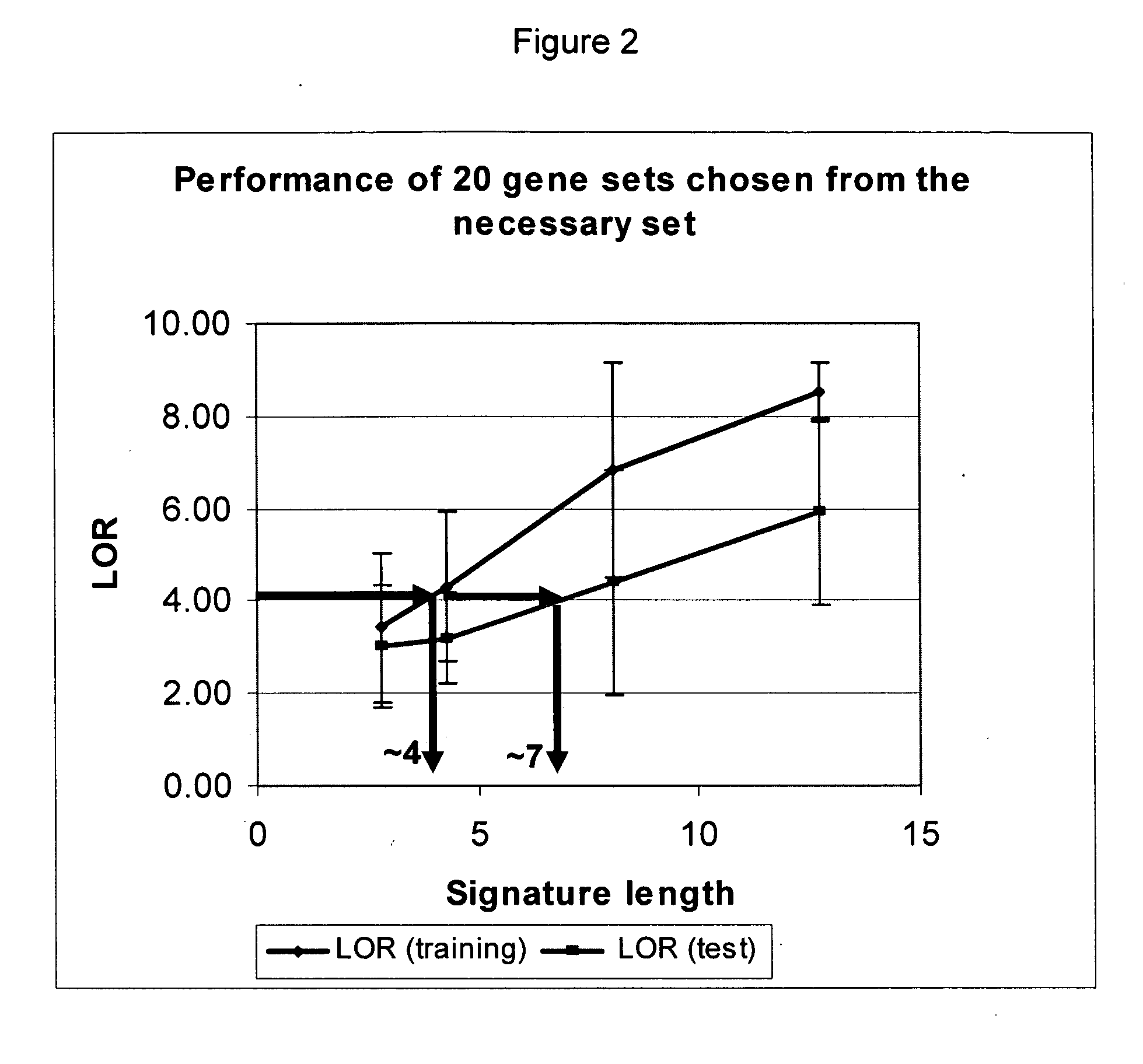
Reagent sets and gene signatures for renal tubule injury
The invention discloses reagent sets and gene signatures for predicting onset of renal tubule injury in a subject. The invention also provides a necessary set of 186 genes useful for generating signatures of varying size and performance capable of predicting onset of renal tubule injury. The invention also provides methods, apparatuses and reagents useful for predicting future renal tubule injury based on expression levels of genes in the signatures. In one particular embodiment the invention provides a method for predict whether a compound will induce renal tubule injury using gene expression data from sub-acute treatments.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Antithrombotic and Anti-restenotic drug eluting stent
ActiveUS20080188925A1Increasing effective wall thicknessAdversely impactingStentsSurgeryDiseasePercent Diameter Stenosis
An expandable medical device includes a plurality of elongated struts, forming a substantially cylindrical device which is expandable from a cylinder having a first diameter to a cylinder having a second diameter. A plurality of different beneficial agents may be loaded into different openings within the struts for delivery to the tissue. For treatment of conditions such as restenosis, different beneficial agents are loaded into different openings in the device to address different biological processes involved in restenosis and are delivered at different release kinetics matched to the biological process treated. The different beneficial agents may also be used to address different diseases, such as restenosis and acute myocardial infarction from the same drug delivery device. In addition, anti-thrombotic agents may be affixed to at least a portion of the surfaces of the medical device for the prevention of sub-acute thrombosis.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
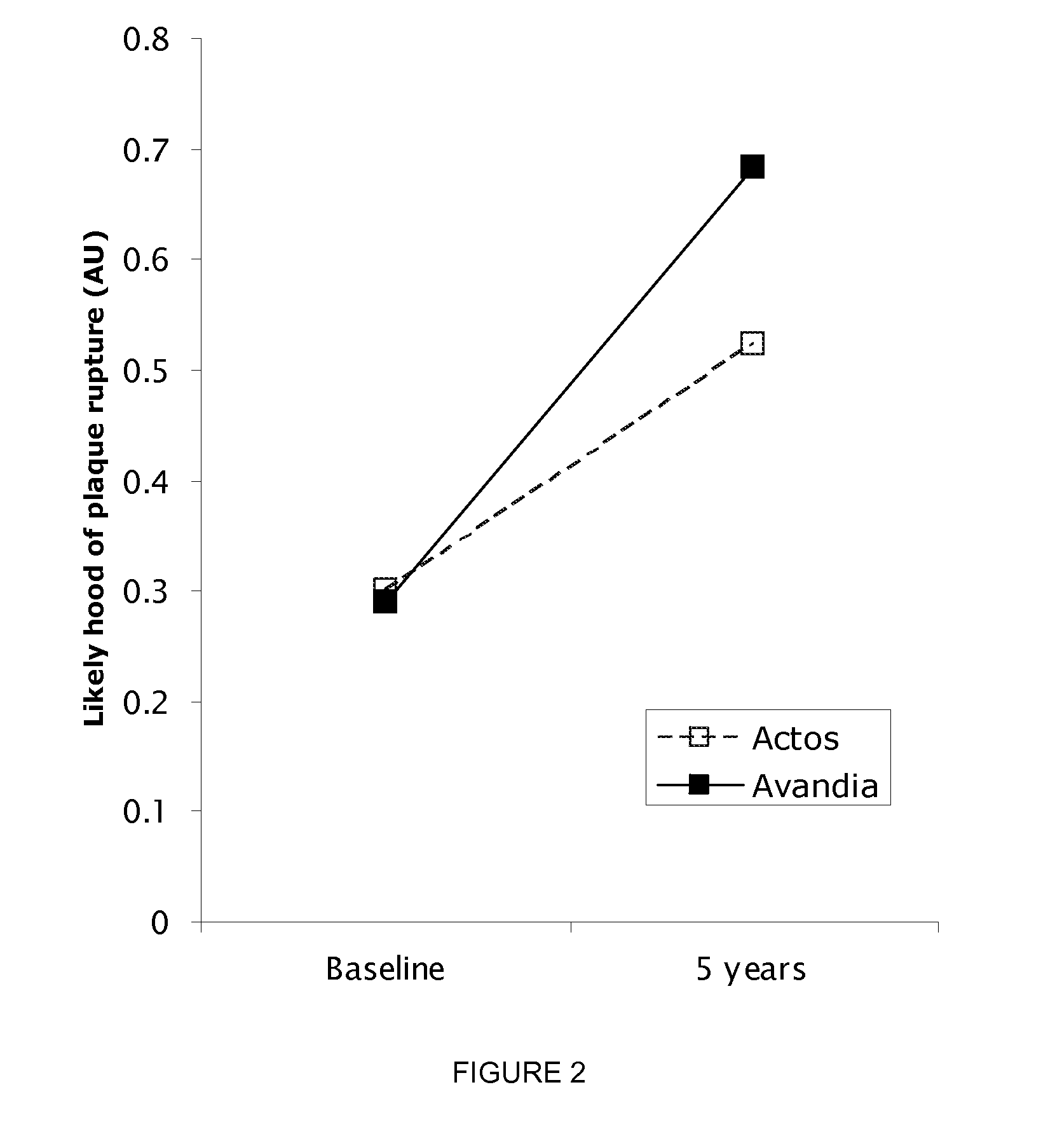
Biomarkers For Assessing Altherosclerotic Potential
The invention also provides methods, apparatuses and reagents useful for predicting future atherosclerosis based on expression levels of genes selected from the set of 68 genes with differential expression in response to pioglitazone and rosiglitazone. The invention also discloses reagent sets and biomarkers for predicting progression of atherosclerosis induced by anti-diabetic therapy in a subject. In one particular embodiment the invention provides a method for predict whether a compound will induce atherosclerosis using gene expression data from sub-acute treatments.
Owner:ENTELOS HLDG
Novel base material for pharmaceutical and/or cosmetic cream (herbal composition for itchy or infected skin)
InactiveUS20070014749A1Reduce impactPromoting faster healingBiocideCosmetic preparationsDiseaseFreeze-drying
The present invention relates to the preparation and use of compositions for the treatment of skin disorders itchy and / or infected skin such as impetigo, acne (on face, forehead scalp and on the back of the body) and fungal infection of skin and nails. Whether the infection may be acute or chronic or sub-acute or acute on chronic. As well as for the promotion of non carrier state of the human beings and animals from some pathogenic bacteria such as staphylococci. The compositions are based on extracts from the plants Cassia tora, Centratherum anthelminticum and / or Melia azadirachta. A variety of other herbal extracts may be included and the composition may take the form of a freeze dried or a spray dried powder or presence of this in a cream or ointment based on Ghee, or in a cream or ointment form developed with any other vehicle, or they may be in a powdered form without spray drying. That spray dried or freeze dried powder may be suspended in a suitable mouth wash or nasal drops. The herbs may be in a powdered form of suitable for preparing decoctions in hot water. Laboratory results are of Specimen Example 2. Specimen Example 1 I have found working on the patients clinically as broad spectrum covering pustules as well as fungal infections.
Owner:VIRAJ SHAH VARION
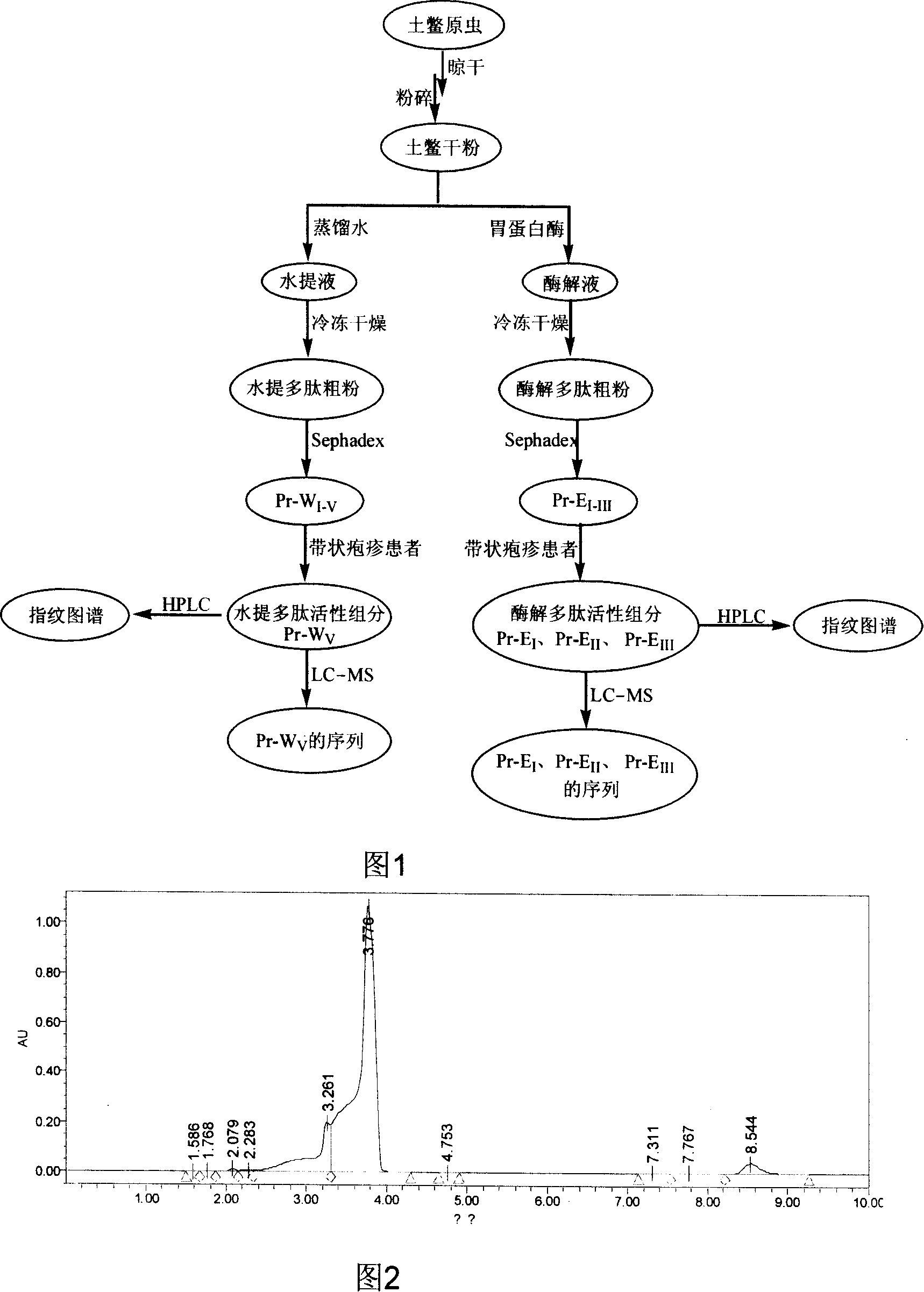
Preparation of aqueous extract and zymolyte of woodlousedry powder, and application of the same in medicine
ActiveCN101152557ASafe for clinical useAnthropod material medical ingredientsPeptide/protein ingredientsClinical efficacyHplc esi ms
The invention discloses a process of extracting an effective fraction for curing herpes zoster from ground beetle dry powder, and a series of polypeptide components obtained by the extracting process. The invention also discloses HPLC, Maldi-TOF and HPLC-ESI-MS finger prints and amino acid sequences of the polypeptide components, and application of the polypeptide components in curing herpes zoster. The extracting process of the invention includes the following procedures: water extract liquid and enzymolysis liquid of the ground beetle dry powder respectively receive chromatography by SephadexG25 column, to obtain a series of polypeptide components; and then the polypeptide components after separation are prepared into freeze-dry powder by the freeze-dry process. The clinical effect observation has shown the series of polypeptide components of the invention have obvious effect in curing the herpes zoster. The results of animal acute toxicity and sub-acute toxicity tests have shown, the polypeptide components of the invention are safe, without toxicity, and safe in clinical application.
Owner:ZHEJIANG MEDICINE CO LTD XINCHANG PHAMACEUTICAL FACTORY
Construction method for clinic information quantification system
InactiveCN106227996AGuaranteed stabilityFacilitate communicationMedical automated diagnosisSpecial data processing applicationsCoronary arteriesClinical information
The invention provides a construction method for a clinic information quantification system. Clinic common parameters such as clinic essential characteristics of KD child patients, blood routine examination indexes and blood biochemical indexes as quantification indexes; a quantification method for assessing IVIG resistance, acute and sub-acute coronary artery lesions, coronary artery continuous lesions and recovery phase coronary artery aneurysms is established through the multi-factor logistic regression analysis method; the probability of occurrence of IVIG resistance and coronary artery lesions of the KD child patients and an assessment result (area under the curve, sensitivity and specificity) can be obtained, and then clinic treatment is directed, and follow-up visit is performed. The prediction efficiency for IVIGR of the quantification method is optimized obviously in comparison with the conventional methods; the quantification method predicts KD recovery phase coronary artery continuous lesions and recovery phase coronary artery aneurysms; the clinic parameters required by the quantification method come from the clinic essential characteristics, the blood routine examination indexes and the blood biochemical indexes, thereby facilitating application in primary hospital and exchange and promotion.
Owner:ZHEJIANG UNIV
Amyloid protein intra-membrane segment for treating Alzheimer disease and application thereof
InactiveCN102229651AImprove cognitive functionOvercoming the disadvantages of subacute meningitisNervous disorderPeptide/protein ingredientsAcute meningococcaemiaSide effect
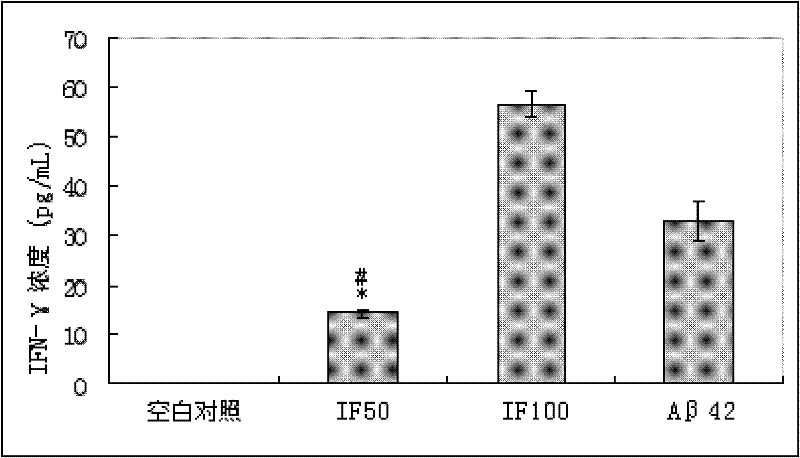
The invention relates to an amyloid protein intra-membrane segment for treating Alzheimer disease and an application thereof, in particular to the application of the amyloid protein intra-membrane segment in the preparation of a vaccine medicament for treating Alzheimer disease. The problem of treating Alzheimer disease can be efficiently solved. The technical scheme provided by the invention is to choose an intra-membrane segment (IF-A beta segment), which is called as AIIGLMVGGVVIA for short, from amino acid sequences of amyloid proteins (A beta42) of 42 amino acids. In a use process, after the synthesized IF-A beta and a Freund adjuvant are mixed and injected to an immunized AD (Alzheimer Disease) model mouse, the A beta42 plaques can be efficiently eliminated, the cognitive function of the AD mouse can be improved and the defect of the sub-acute meningitis caused by using A beta42 as an immunogen can be overcome, thereby developing a therapeutic medicament for efficiently treating AD without side effect.
Owner:CENT SOUTH UNIV
Deer fetus-pearl capsule
InactiveCN1931289AMeet the requirementsIn line with the development trendMetabolism disorderDigestive systemSide effectAcute toxicity testing
The present invention discloses one kind of deer fetus-pearl capsule with the functions of beautifying, nursing face and delaying senility. The deer fetus-pearl capsule is prepared with red deer fetus powder, red sage, pearl powder and grape seed extract as material. Acute toxicity test and sub-acute toxicity test show that the deer fetus-pearl capsule has no any toxic side effect. Regularly taking the deer fetus-pearl capsule can activate the microcirculation of skin, activate cell regeneration, eliminate chloasma and delay women's climacteric period. It is suitable for women with qi and blood deficiency, pale face, insomnia and dreaminess, etc.
Owner:INNER MONGOLIA JIANYUAN DEER IND
Reagent sets and gene signatures for renal tubule injury
InactiveUS20060199205A1Improve performanceBioreactor/fermenter combinationsCompound screeningRenal Tubule EpitheliumRenal tubule
The invention discloses reagent sets and gene signatures for predicting onset of renal tubule injury in a subject. The invention also provides a necessary set of 186 genes useful for generating signatures of varying size and performance capable of predicting onset of renal tubule injury. The invention also provides methods, apparatuses and reagents useful for predicting future renal tubule injury based on expression levels of genes in the signatures. In one particular embodiment the invention provides a method for predict whether a compound will induce renal tubule injury using gene expression data from sub-acute treatments.
Owner:ENTELOS INC
Application of saxifrage for preparing medicaments for treating hyperplasia of mammary glands, lipoma and thyroid gland carcinoid
The invention discloses an application of saxifrage for preparing medicaments for treating hyperplasia of mammary glands, lipoma and thyroid gland carcinoid. The medicaments for the treating hyperplasia of mammary glands, the lipoma and the thyroid gland carcinoid have good effect. An acute and sub-acute toxicity experiment shows that the medicament has no toxic and side effect.
Owner:朱彤
Use of long-acting recombinant human soluble tumor necrosis factor alpha receptor in manufacture of a medicament for the treatment and/or prophylaxis of hepatic failure
ActiveUS20090176702A1Decreases IL- levelPrevention of acutePeptide/protein ingredientsMetabolism disorderTreatment effectHalf-life
The present invention belongs to the field of the application of genetic engineering and gene function, and it is directed to a new medical use of the gene encoding the recombinant soluble tumor necrosis factor α receptor (HusTNFR). The present invention made intervention to fulminant hepatic failure in mice by use of the long-acting recombinant human soluble tumor necrosis factor α receptor and the classic animal models of acute and sub-acute hepatic failure. The results showed that the long-acting soluble tumor necrosis factor αreceptor of the present invention has a half-life extended more than 10 times, and it significantly decreased the mortality of model animals and has superior therapeutic effect for the treatment and / or prophylaxis of acute and sub-acute hepatic failure in model animals. These receptors have a noticeable therapeutic effect for the treatment and / or prophylaxis of acute and sub-acute hepatic failure in comparison with the non-long-acting HusTNFR.
Owner:LI ZHENYI
Therapy for Constipation
A method is disclosed of treating acute, sub-acute or chronic constipation in a patient having a condition requiring such treatment. The method includes administering a lipase inhibitor.
Owner:2294719 ONTARIO
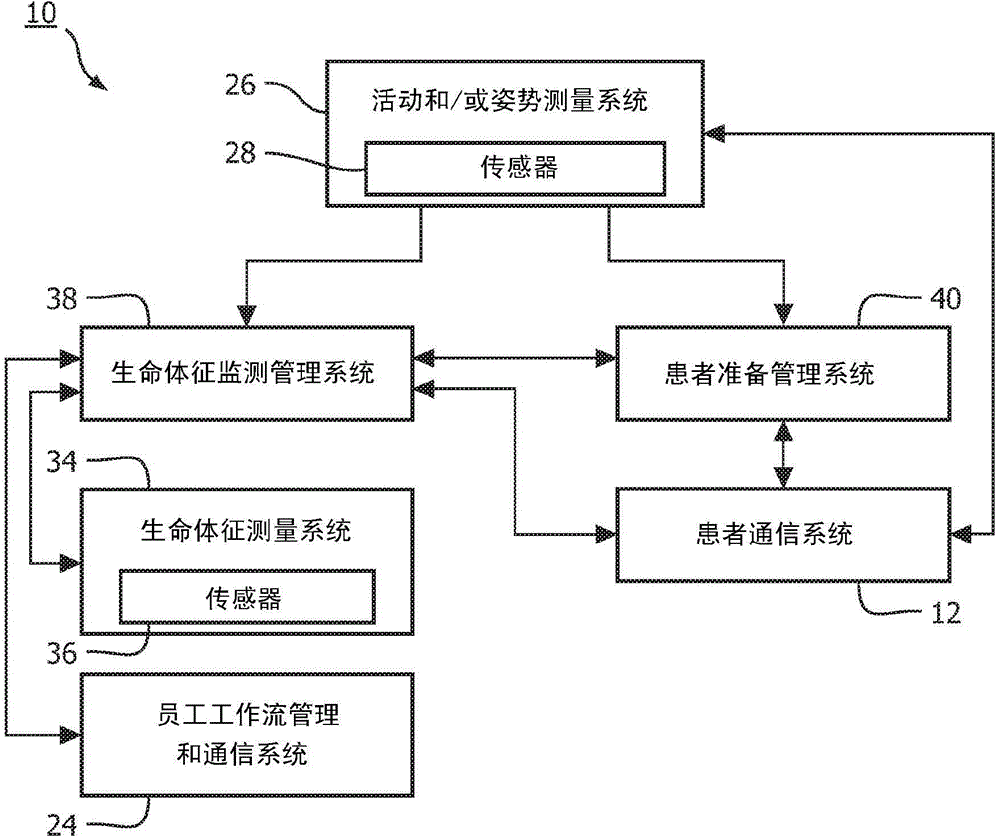
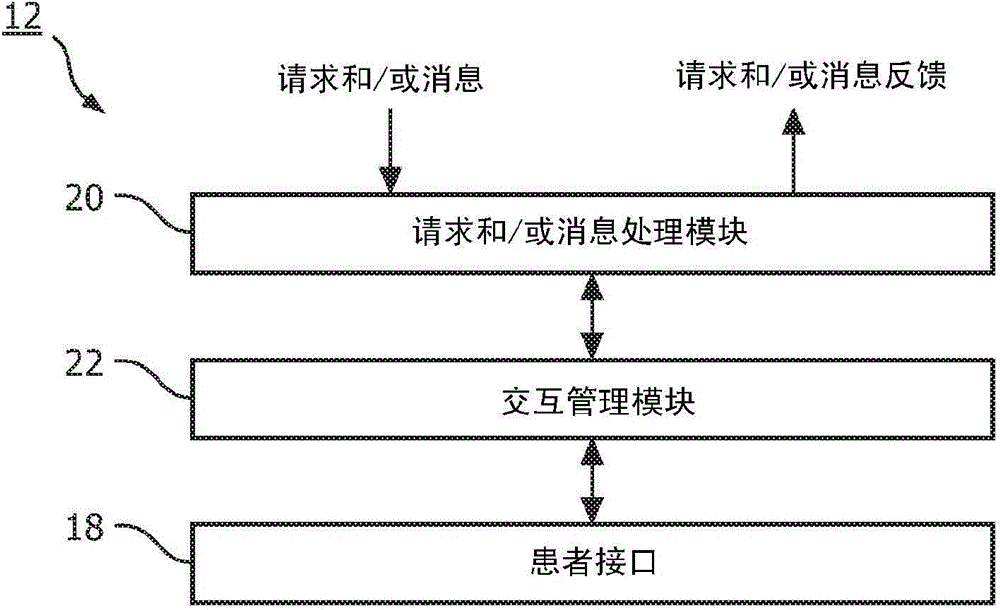
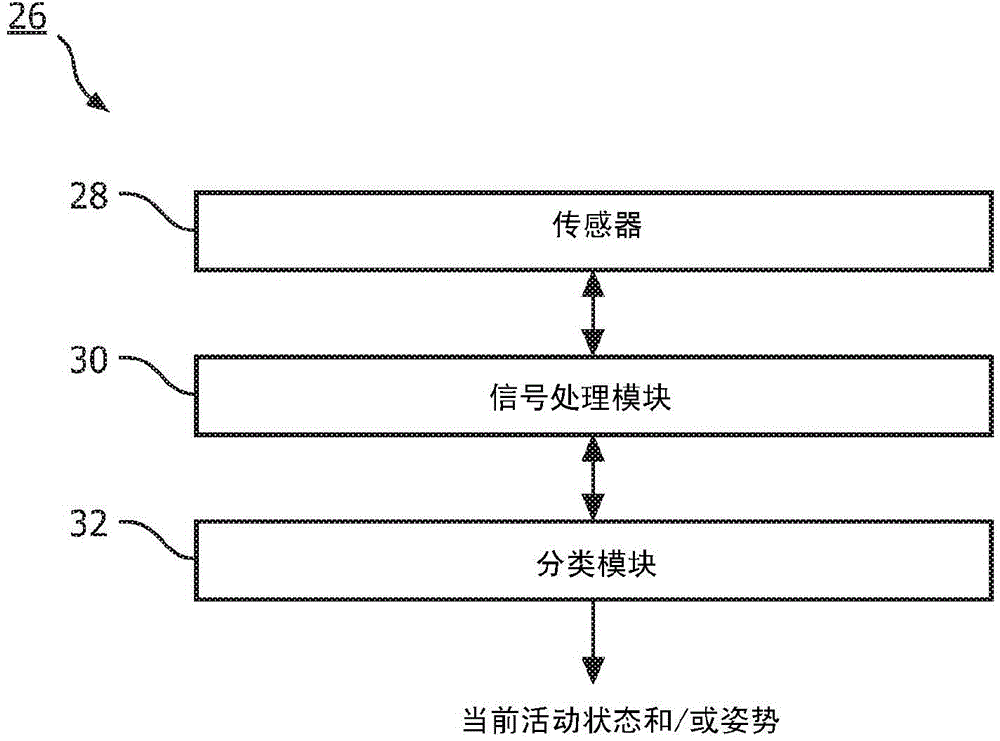
Patient monitoring for sub-acute patients based on activity state and posture
ActiveCN104883962AReliable monitoringReduce workloadHealth-index calculationMedical automated diagnosisEmergency medicineVital signs
A method, and corresponding system (300), for monitoring patients based on activity state and posture. Activity state and / or posture of a patient are measured. Further, one or more vital signs of the patient are measured according to a schedule. Based on the measured activity state and / or posture of the patient and the measured one or more vital signs, the schedule is adjusted and / or patient deterioration is monitored for.
Owner:KONINKLJIJKE PHILIPS NV
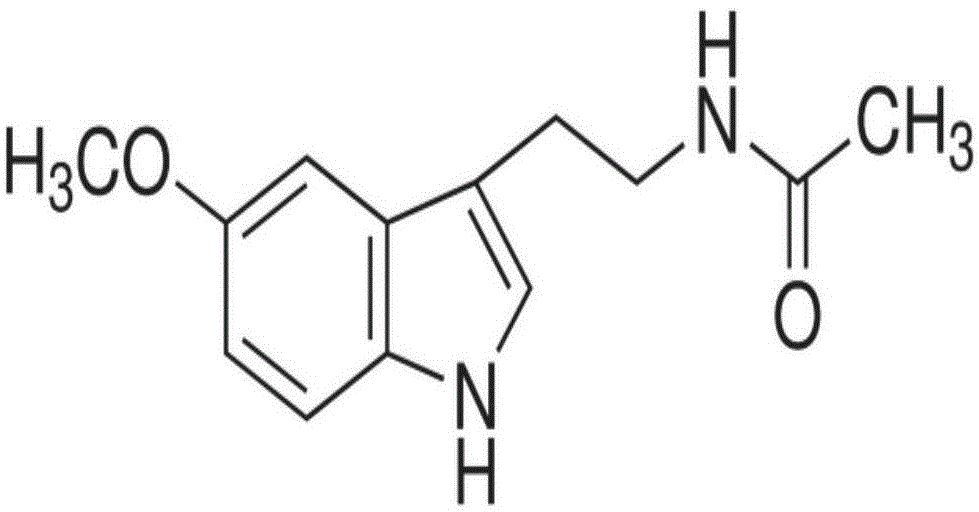
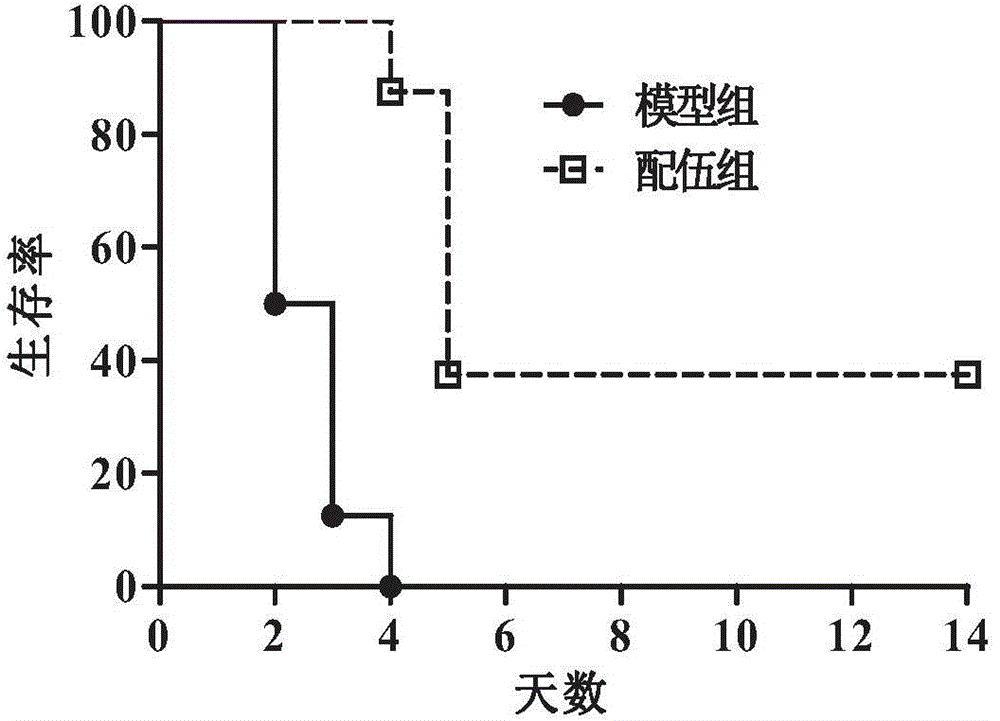
Composition containing melatonin and catechin as well as application of composition
ActiveCN104784172AReduce lethal toxicityPharmacological effects are not affectedOrganic active ingredientsMetabolism disorderSide effectPhysiology
The invention relates to composition containing melatonin and catechin as well as an application of the composition, and in other words, composition used for foods, drugs or healthcare products contains melatonin and catechin. The combination use of melatonin and catechin in the composition can reduce toxic and side effects of catechin and greatly improve the safety of catechin, and the pharmacological action of catechin is not affected. The invention further relates to a novel application of melatonin, in particular to an application of melatonin to the preparation of the composition used for the healthcare products, the drugs or the foods; the composition is characterized by further containing catechin, wherein melatonin can reduce toxic and side effects of catechin, lethal toxicity of catechin and acute hepatotoxicity and sub-acute liver injury caused by catechin.
Owner:ANHUI AGRICULTURAL UNIVERSITY
Medicine for treating sub-acute eczema
InactiveCN105362561APharmaceutical delivery mechanismPteridophyta/filicophyta medical ingredientsPronephriumCoreopsis
The invention belongs to the technical field of medicines and particularly relates to a medicine for treating sub-acute eczema. The medicine is prepared from, by weight, 4-6 parts of Chinese fanpalm seeds, 4-6 parts of a fewflower lysionotus herb, 4-6 parts of roots of candolle pimpinella, 4-6 parts of roots of champion pentasacme, 4-6 parts of tubers of serrate sikoku jackinthepulpit, 4-6 parts of nude pronephrium rhizomes, 4-6 parts of all-grass of winkled marshweed, 4-6 parts of perny germander roots, 4-6 parts of a pilose bushclover herb and 4-6 parts of lance coreopsis leaves. The medicinal materials are used in a combined mode and jointly have the effects of clearing away heat and toxic materials, invigorating blood circulation, eliminating swelling, regulating qi-flowing, removing stasis, dispersing blood stasis and relieving pain, and the medicine is used for treating the sub-acute eczema and has the advantages of being high in cure rate and obvious effective rate.
Owner:JINAN XINSHIDAI MEDICINE SCI & TECH
Therapy for constipation
A method is disclosed of treating acute, sub-acute or chronic constipation in a patient having a condition requiring such treatment. The method includes administering a lipase inhibitor. Also provided is a method of treating chronic pain in a patient which includes administration of a lipase inhibitor, with or without pain medication.
Owner:2294719 ONTARIO
A composition containing melatonin and catechin and its application
ActiveCN104784172BReduce lethal toxicityPharmacological effects are not affectedOrganic active ingredientsMetabolism disorderSide effectPhysiology
The present invention relates to a composition containing melatonin and catechin and its application, in other words, a composition used for food, medicine or health products, containing melatonin and catechin. The combination of melatonin and catechin in the composition can reduce the toxic and side effects of the catechin and greatly improve the safety of the catechin without affecting the pharmacological action of the catechin. The present invention also relates to the new application of melatonin, specifically to the application of melatonin in the preparation of compositions for health products, medicines or foods, characterized in that the composition also contains catechins, wherein melatonin Catechin can reduce the toxic and side effects of catechin, reduce the lethal toxicity of catechin, and reduce the acute liver toxicity and subacute liver injury caused by catechin.
Owner:ANHUI AGRICULTURAL UNIVERSITY
Traditional Chinese medicine washing liquor for controlling eczema and preparation method thereof
InactiveCN104689117AAntibacterialPromote blood circulationAnthropod material medical ingredientsDermatological disorderTaraxacum mongolicumAlligator
The invention discloses a traditional Chinese medicine washing liquor for controlling eczema, and a preparation method thereof. The traditional Chinese medicine washing liquor for controlling eczema comprises the following raw materials by parts of weight: 15-20 parts of radix sophorae flavescentis, 15-20 parts of gallnut, 15-30 parts of taraxacum mongolicum, 200-300 parts of fresh radish leaves, 100-200 parts of fresh alligator alternanthera, and 30-50 parts of indicalamus leaves. The traditional Chinese medicine washing liquor for controlling eczema has functions of resisting bacterium, strengthening skin blood circulation, and repairing damaged skin. The traditional Chinese medicine washing liquor for controlling eczema takes effect on acute, sub-acute and chronic skin damages.
Owner:浙江纤丽菒健康科技有限公司
Establishing method for rat central type superior mesenteric vein thrombosis model
InactiveCN105055041AReduce experiment costThe method is simpleSurgical veterinaryVeinInferior mesenteric vein
The invention relates to an establishing method for a rat central type superior mesenteric vein thrombosis model. The model is established by establishing an acute central type superior mesenteric vein thrombosis model, ligaturing the trunk root of a superior mesenteric vein at a time through a 7-0 no-damage nylon suture at the position 0.2 cm below the joint of the free superior mesenteric vein and a splenic vein, and conducting abdomen closing. A sub-acute central type superior mesenteric vein thrombosis model is established by placing a metal stick with the diameter of 1 mm and a superior mesenteric vein in parallel at the position 0.2 cm below the joint of the trunk of the free superior mesenteric vein and the splenic vein, ligaturing the superior mesenteric vein and the metal stick together through a 7-0 no-damage nylon suture, retreating the metal stick after ligation so that opened calibers of all rats can be kept basically consistent, and conducting abdomen closing. Food and water are freely fed in the next day. Abdomen opening is conducted again 48 hours later, the superior mesenteric vein is completely ligatured, and abdomen closing is conducted. Food and water are freely fed in the next day after the second operation. The rat central type superior mesenteric vein thrombosis model is suitable for superior mesenteric vein thrombosis intestinal pathology and pathophysiology research.
Owner:NORTH CHINA UNIVERSITY OF SCIENCE AND TECHNOLOGY
Method of preventing acute or sub-acute hepatic failure in a subject by administering a soluble human tumor necrosis factor alpha receptor fusion protein
ActiveUS8227404B2Good effectEfficiently preventBiocideOrganic active ingredientsHuman tumorAcute hepatic failure
The present invention belongs to the field of the application of genetic engineering and gene function, and it is directed to a new medical use of the gene encoding the recombinant soluble tumor necrosis factor α receptor (HusTNFR). The present invention made intervention to fulminant hepatic failure in mice by use of the long-acting recombinant human soluble tumor necrosis factor α receptor and the classic animal models of acute and sub-acute hepatic failure. The results showed that the long-acting soluble tumor necrosis factor α receptor of the present invention has a half-life extended more than 10 times, and it significantly decreased the mortality of model animals and has superior therapeutic effect for the treatment and / or prophylaxis of acute and sub-acute hepatic failure in model animals. These receptors have a noticeable therapeutic effect for the treatment and / or prophylaxis of acute and sub-acute hepatic failure in comparison with the non-long-acting HusTNFR.
Owner:LI ZHENYI
Preparation method of medicine for treating acute and sub-acute eczema
InactiveCN107661321AStrong penetrating powerSignificant effectOrganic active ingredientsPharmaceutical non-active ingredientsSkin penetrationIrritation
The invention discloses a preparation method for treating acute and subacute eczema, which comprises the following steps: 1) taking 20g of loratadine and 10g of neomycin sulfate and adding them to 250g of ethanol, stirring and dissolving to obtain a mixed solution for use ; 2) Sprinkle 4g chitosan evenly to make it swell naturally; 3) Add 1.0g arbutin, keep stirring, and add ethanol to 1000mL; 4) Adjust the pH to 6.0~7.0, stir well, that is It can be used to treat acute and subacute eczema. In the present invention, loratadine and neomycin sulfate have obvious effects on inhibiting bacteria, and loratadine and neomycin sulfate are prepared into medicines. Due to the laurocapram transdermal technology, the penetration of the skin and limbs of the medicine is increased. , showing the superior curative effect of the coating agent on chronic eczema; the medicine for treating acute and subacute eczema made of chitosan has no greasy feeling, is easy to spread, has strong adhesion, and has a certain tear resistance. Convenient and non-irritating to the skin.
Owner:CHENGDU SHENGSHI GUANGHUA BIOTECH CO LTD
Reservoir eluting stent
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
A drug eluting balloon
ActiveCN105105892BPromote absorptionPrevent thrombosisStentsMedical devicesVascular endotheliumThrombus
Owner:DONGZHIMEN HOSPITAL OF BEIJING UNIV OF CHINESE MEDICINE
Deer fetus-pearl capsule
InactiveCN100396303CActivate microcirculationActivate regenerationMetabolism disorderDigestive systemSide effectAcute toxicity testing
The present invention discloses one kind of deer fetus-pearl capsule with the functions of beautifying, nursing face and delaying senility. The deer fetus-pearl capsule is prepared with red deer fetus powder, red sage, pearl powder and grape seed extract as material. Acute toxicity test and sub-acute toxicity test show that the deer fetus-pearl capsule has no any toxic side effect. Regularly taking the deer fetus-pearl capsule can activate the microcirculation of skin, activate cell regeneration, eliminate chloasma and delay women's climacteric period. It is suitable for women with qi and blood deficiency, pale face, insomnia and dreaminess, etc.
Owner:INNER MONGOLIA JIANYUAN DEER IND
Medicine for treating severe sub-acute hepatitis
InactiveCN100490886CGood treatment effectSmall side effectsDigestive systemAntiviralsCurcuma aromaticaCurative effect
The invention relates to a medicinal composition for treating severe hepatitis which comprises the active constituents of oriental wormwood 30g, cape jasmine 20g, yellow-corktree bark 20g, baikal skullcap root 15g, oriental water plantain rhizome 20g, radix paeoniae rubrathe 50g, root of red rooted saliva 30g, curcuma aromatica 20g, root of herbaceous peony 30g, fruit of barbary wolf berry 15g, prepared rhizome of rehmannia 30g, schisandra fruit 15g, cornus officinalis 10g, creeping euphorbia 30g, Chinese dates 15g, licorice root 5g and notoginseng powder 3g.
Owner:李召忠
Application of calcium-activated chloride channel antagonist
InactiveCN105497901AAnti-denervation and demyelination symptoms are goodNervous disorderHeterocyclic compound active ingredientsDiseaseSubacute sclerosing panencephalitis
The invention belongs to the field of medicine and particularly relates to application of a calcium-activated chloride channel antagonist, in particular to application of the calcium-activated chloride channel antagonist in preparing medicine for treating nerve demyelination diseases. According to the application, it is proposed for the first time that the calcium-activated chloride channel antagonist is used for preparing the medicine for treating nerve demyelination diseases, and the diseases such as multiple sclerosis, acute infectious polyradiculoneuritis, multifocal leukoencephalitis, sub-acute sclerosing panencephalitis, pons central type myelin disintegrative disorder, progressive subcortical ischemic encephalopathy, senile dementia and schizophrenia can be safely, efficiently and stably treated.
Owner:WUHAN WORLDNER UNITED PHARMA
Film coating agent for treating acute and sub-acute eczema
InactiveCN107661332AStrong penetrating powerSignificant effectHydroxy compound active ingredientsAntipyreticIrritationLoratadine
The invention discloses a coating agent for treating acute and subacute eczema, expressed by weight, consisting of 15-42 g of loratadine, 7-23 g of cetirizine, 2-8 g of chitosan, and bearberry Glycoside 0.5~2.1g, ethanol 500~900g composition. In the present invention, loratadine and cetirizine have obvious effects on inhibiting bacteria, and loratadine and cetirizine are prepared into medicines. Due to the transdermal technology of arbutin, the penetrating power of medicine skin limbs has been increased. , showing the superior curative effect of the coating agent on chronic eczema; the medicine for treating acute and subacute eczema made of chitosan has no greasy feeling, is easy to spread, has strong adhesion, and has a certain tear resistance. Convenient and non-irritating to the skin.
Owner:CHENGDU SHENGSHI GUANGHUA BIOTECH CO LTD
Application of saxifrage for preparing medicaments for treating benign tumor of thyroid
The invention discloses an application of saxifrage for preparing medicaments for treating thyroid gland carcinoid. The medicaments for the treating hyperplasia of mammary glands, the lipoma and the thyroid gland carcinoid have good effect. An acute and sub-acute toxicity experiment shows that the medicament has no toxic and side effect.
Owner:朱彤
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com