Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
31 results about "Solifenacin Succinate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
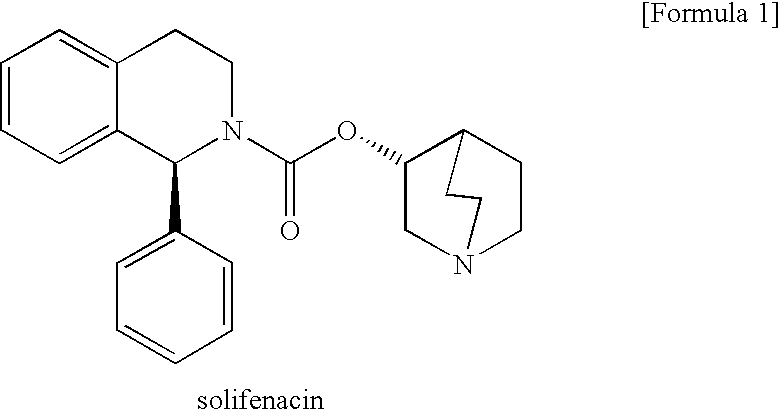
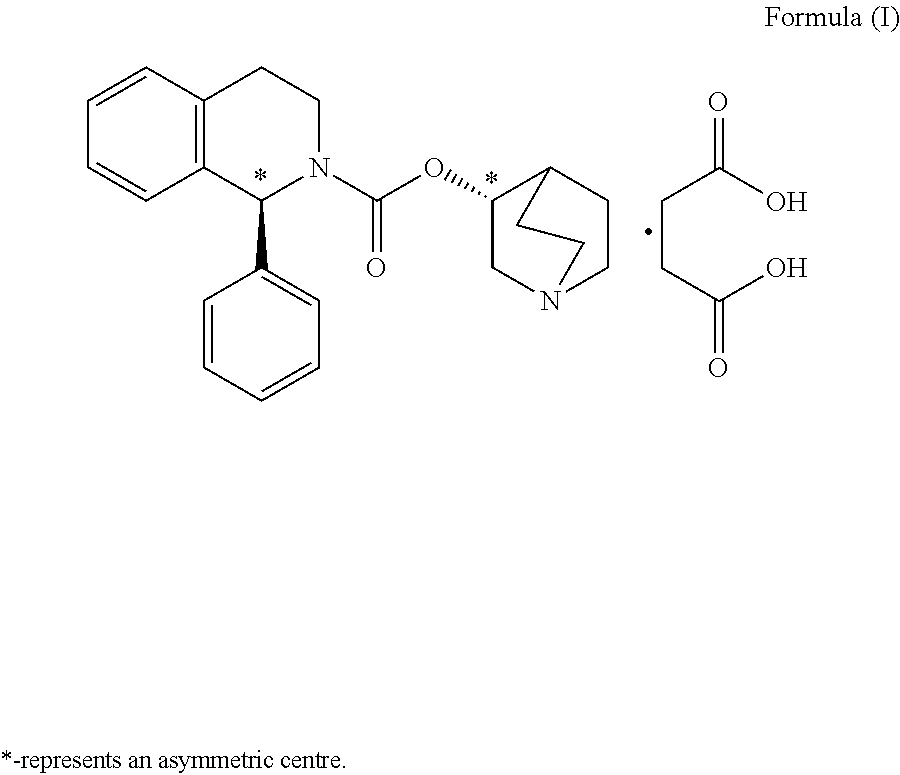
A quinuclidine and tetrahydroisoquinoline derivative and selective M3 MUSCARINIC ANTAGONIST. It is used as a UROLOGIC AGENT in the treatment of URINARY INCONTINENCE.
Solifenacin compositions
InactiveUS20100137358A1Minimize the possibilityMaintaining crystal formBiocidePowder deliverySolifenacin SuccinateMedicine
Compositions and / or formulations comprising solifenacin or a salt thereof and processes for preparing the same. Certain compositions and formulations contain a stable amorphous form of solifenacin succinate.
Owner:DR REDDYS LAB LTD +1
Solifenacin succinate-containing orally disintegrating tablet and preparation method thereof
InactiveCN103585123AQuality assuranceImprove liquidityPill deliveryUrinary disorderSolifenacin SuccinateOrally disintegrating tablet
The invention belongs to the field of pharmaceutical preparations, and particularly relates to a solifenacin succinate-containing orally disintegrating tablet and a preparation method thereof. The orally disintegrating tablet is clinically used for treating symptoms such as frequent micturition, urgent urination and urinary incontinence caused by the overactive bladder.
Owner:BEIJING VENTUREPHARM BIOTECH
Preparation technology of solifenacin succinate
ActiveCN102875544AImprove conversion rateReverse reaction facilitationOrganic chemistrySolifenacin SuccinateCondensation process
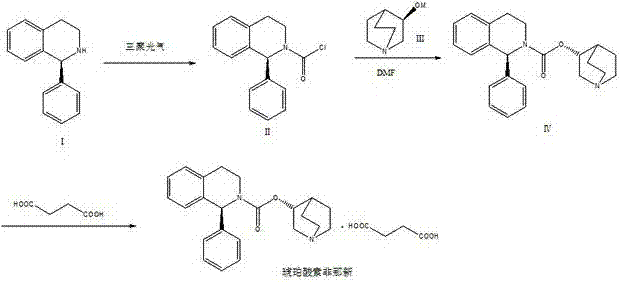
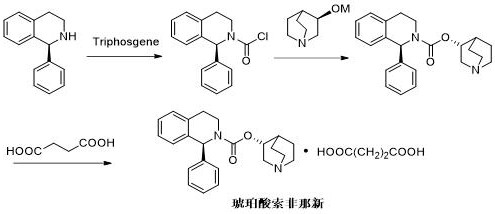
The invention relates to a preparation technology of solifenacin succinate. According to the preparation technology, (S)-1-phenyl-1, 2, 3, 4-tetrahydroisoquinoline carbamyl chloride (II) and (R)-3-quinuclidine alcohol metal salt (III) are reacted to generate solifenacin alkali, and then the solifenacin alkali is prepared into solifenacin succinate. With the adoption of the preparation technology provided by the invention, nucleophilic species which lead to a reverse reaction can be avoided during condensation process, namely, the reverse reaction cannot be generated in the preparation technology, therefore, a transformation rate of the solifenacin succinate is improved, a reaction is greatly promoted, a reaction process is quickly carried out under a mild condition, and the preparation technology is suitable for large-scale production.
Owner:CHENGDU SINO STRONG PHARMA
Pharmaceutical composition containing solifenacin succinate
ActiveCN104940152AUrinary disorderPharmaceutical non-active ingredientsSolifenacin SuccinateSuccinic acid
The invention relates to a pharmaceutical composition containing solifenacin succinate. The pharmaceutical composition is characterized by simultaneously containing direct compression lactose, sodium carboxymethyl starch, a binder and a lubricating agent. The invention also discloses a preparation method, namely a direct powder compression method of the pharmaceutical composition. The pharmaceutical composition and the preparation method thereof are not influenced by water in the preparation process, the pharmaceutical composition is low in impurity content and better in drug dissolubility, and the preparation method is more suitable for industrial production.
Owner:SICHUAN GOWELL PHARMA
Pharmaceutical agent comprising solifenacin
InactiveUS20090131469A1Easy to useImprovement of urinary urgencyBiocideNervous disorderDiseaseSolifenacin Succinate
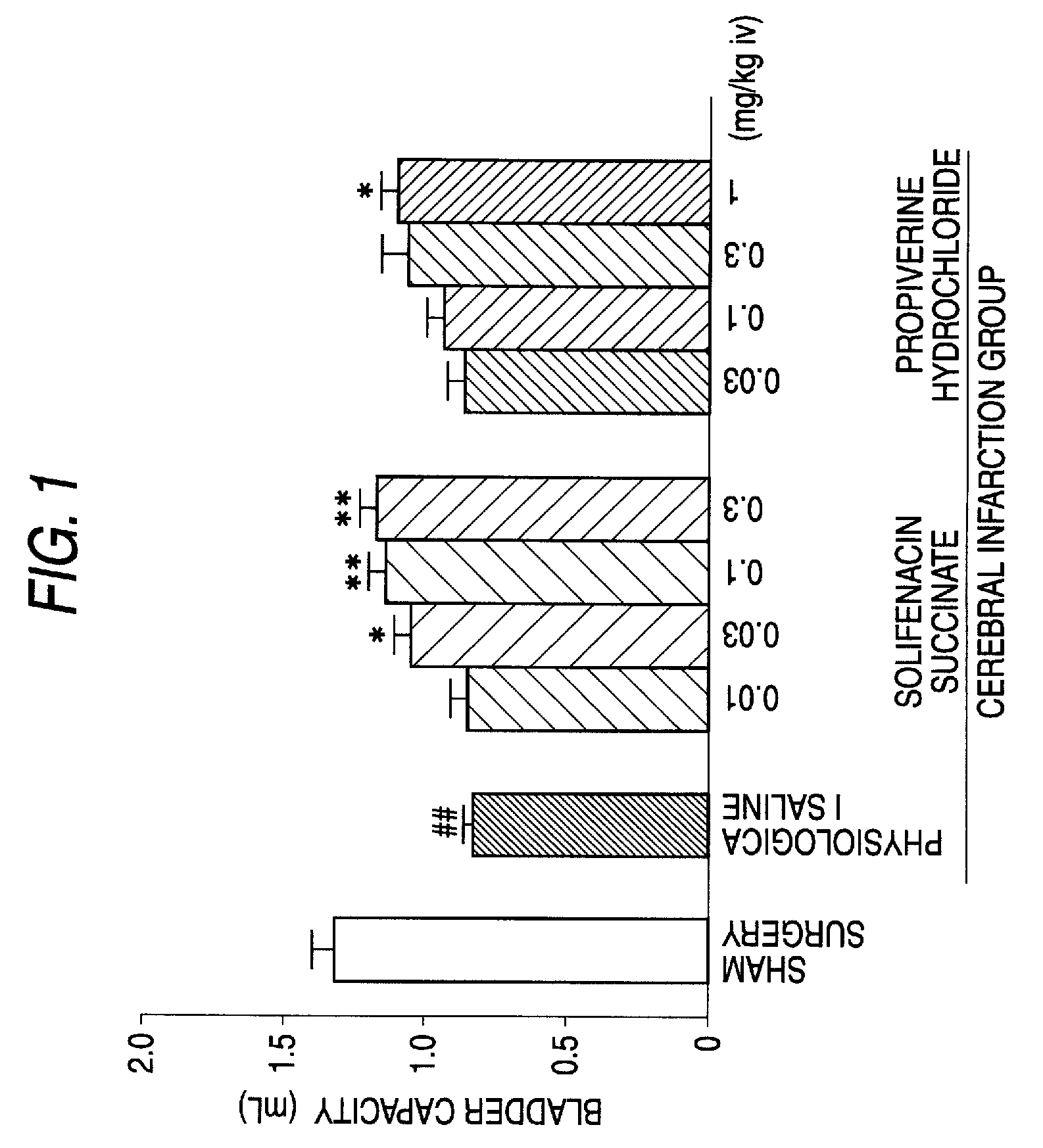
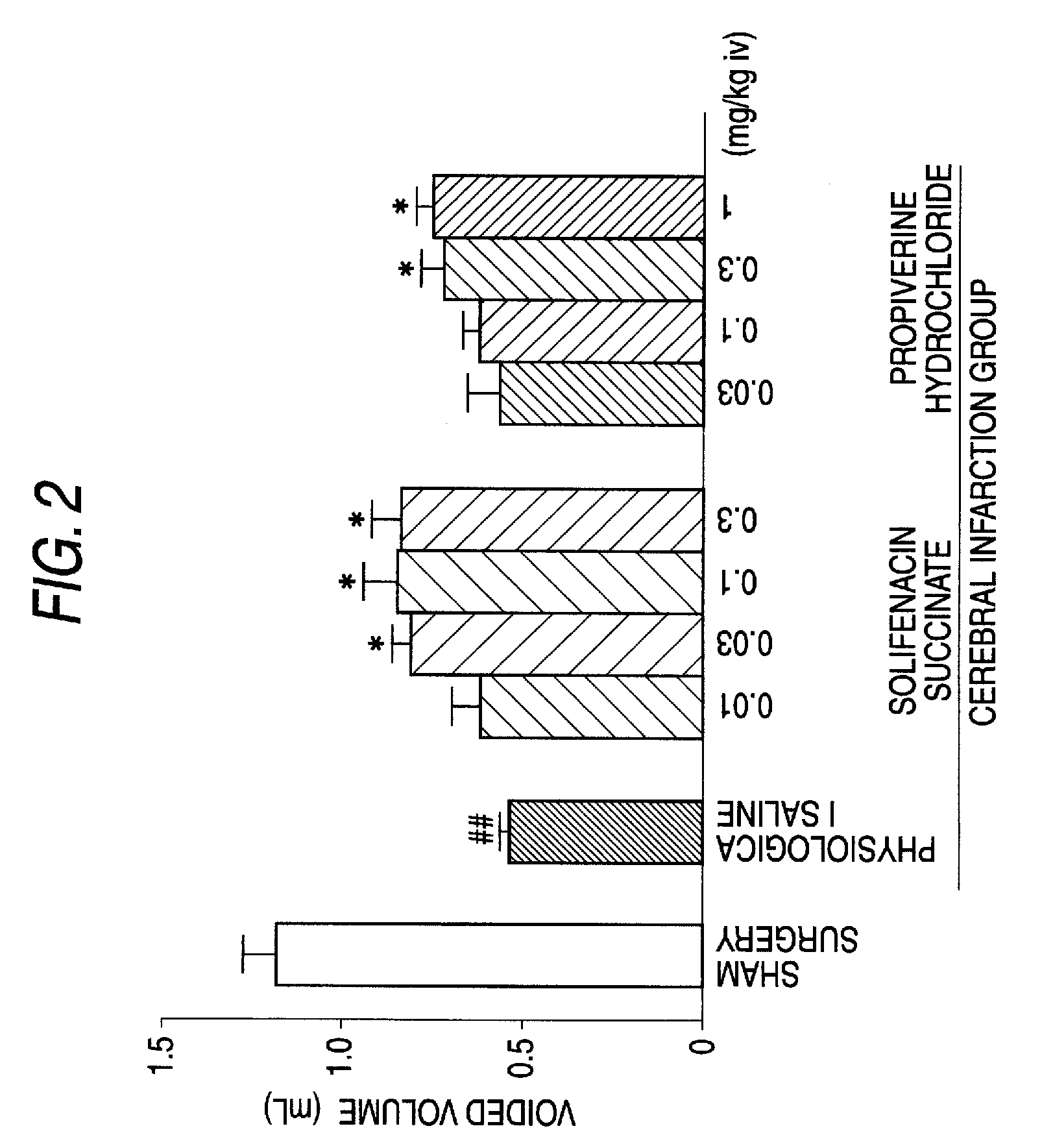
There is provided an agent comprising solifenacin succinate in an amount of 5 mg to 10 mg or solifenacin or a pharmaceutically acceptable salt thereof in an amount equimolar to 5 mg to 10 mg of solifenacin succinate for improvement of urinary urgency, pollakiuria and urinary incontinence due to neurogenic bladder caused by neurodegenerative diseases such as cerebrovascular disease or cerebral infarction, brain or spinal cord injury due to trauma, multiple sclerosis, Parkinson's disease, congenital malformation of the nerve system, peripheral neuropathy, and various spine lesions, that is, spinal cord compression and injury due to fracture, cervical and lumbar spondylosis, spondylosis deformans, spondylolisthesis, spinal stenosis, vertebral disk hernia and the like.
Owner:ASTELLAS PHARMA INC
Package
InactiveUS20130153459A1Improve moisture resistanceGood hygroscopicitySmall article dispensingPharmaceutical containersSolifenacin SuccinateAluminum foil
Owner:ASTELLAS PHARMA INC +1
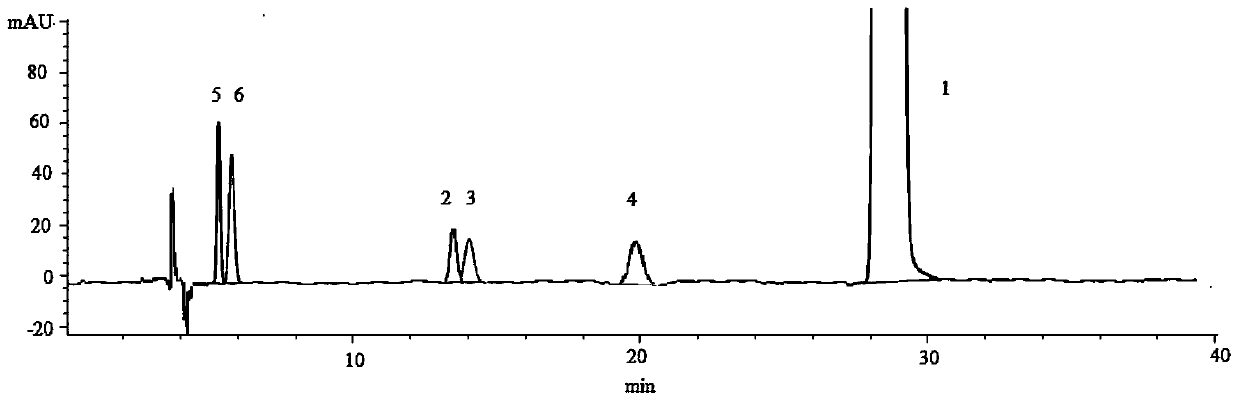
Analysis method for determining stereoisomers and intermediates in solifenacin succinate
ActiveCN107167530AEfficient determinationGuarantee product qualityComponent separationSolifenacin SuccinateCarbamate
The invention relates to an analysis method for determining stereoisomers and intermediates in solifenacin succinate by adopting high-performance liquid chromatography. The analysis method is characterized in that high-performance liquid chromatography (HPLC) is adopted, and isocratic elution is carried out on an amylose-tris(3,5-dimethylphenyl carbamate) bonded silica gel chiral HPLC column; a mobile phase is n-hexane-absolute ethyl alcohol-isopropyl alcohol-diethylamine solution (65:15:20:0.1) (volume ratio); and the mobile phase is adopted to dissolve a sample. The method can be used for simultaneously determining enantiomers and a diastereoisomer of the solifenacin succinate and intermediates as mutual enantiomers, thus providing a more reliable guarantee for the research on new synthesis process routes for the solifenacin succinate and quality control.
Owner:SHANDONG ACADEMY OF PHARMACEUTICAL SCIENCES +1
Stable pharmaceutical composition and preparation method thereof
ActiveCN105012961AStable contentSubstance reductionPill deliveryPharmaceutical non-active ingredientsSolifenacin SuccinateHypromellose
The invention provides a stable pharmaceutical composition and its preparation method. The pharmaceutical composition contains solifenacin succinate and hydroxypropyl methylcellulose, wherein viscosity of hydroxypropyl methylcellulose is 1-4mPa.s.
Owner:燃点(南京)生物医药科技有限公司
Preparation method for impurity of Solifenacin succinate
The invention relates to a preparation method for an impurity of Solifenacin succinate, i.e., [2-(2-benzoyl-phenyl)-ethyl]-1-aza-bicyclo[2.2.2]octyl-3-carbamate. The impurity is prepared through carrying out oxidation ring-opening on Solifenacin succinate and then carrying out separation and purification. According to the preparation method, through preparing the impurity of Solifenacin succinate, a reference substance for qualitative and quantitative analysis on Solifenacin succinate is provided, the quality of Solifenacin succinate is effectively controlled, and thus the safety of clinical medication is guaranteed.
Owner:天津市医药集团技术发展有限公司 +1
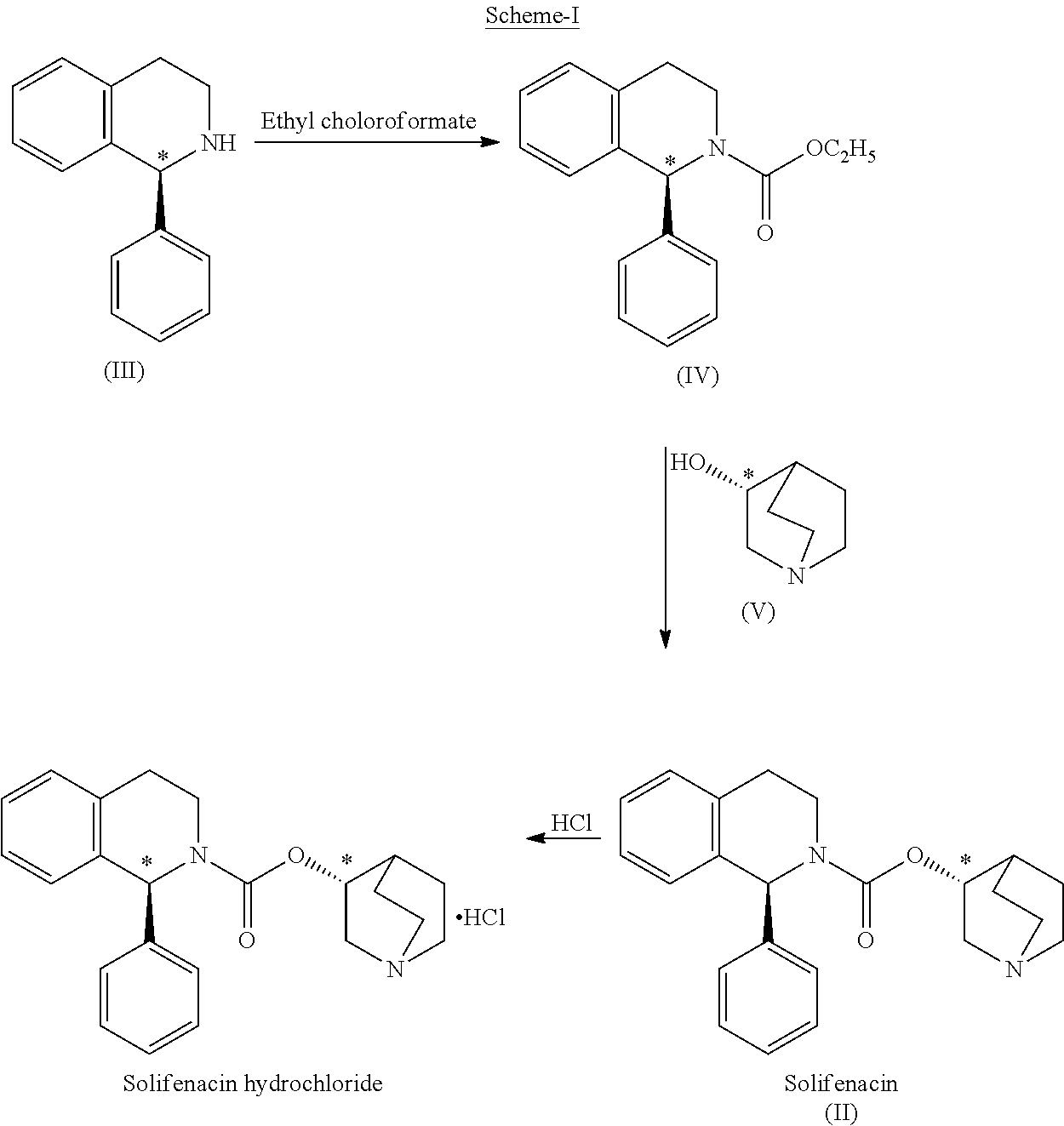
Method for preparing solifenacin succinate
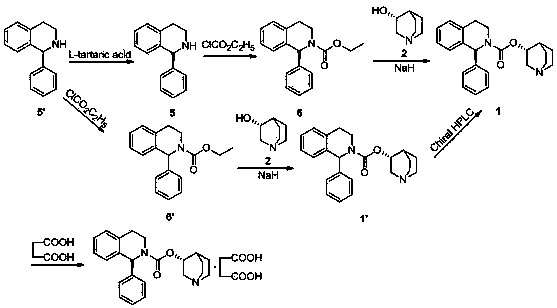
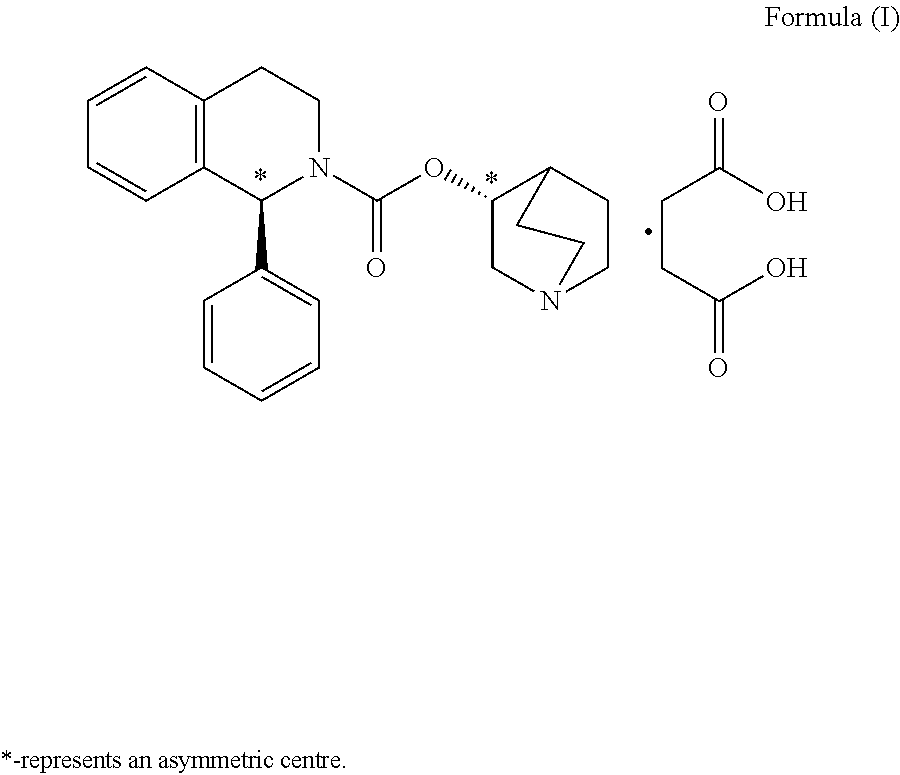
The invention relates to a method for preparing a solifenacin succinate ((3R)-1-azabicyclo [2.2.2] octane-3-yl (1S)-1-phenyl-3,4-dihydroisoquinoline-2-(1H)-carboxylate succinate). The method comprises the following steps: 1) preparing a compound (S)-1-phenyl-1,2,3,4-tetrahydroisoquinoline carboxylic acid ethyl ester of formula III from a compound (S)-1-phenyl-1,2,3,4-tetrahydroisoquinoline, serving as a raw material, of formula IV; 2) synthesizing a compound (3R)-1-azabicyclo [2.2.2] octane-3-yl (1S)-1-phenyl-3,4-dihydroisoquinoline-2-(1H)-carboxylate of formula II in an ionic liquid by using the compound (S)-1-phenyl-1,2,3,4-tetrahydroisoquinoline carboxylic acid ethyl ester of formula III; and 3) salifying the compound (3R)-1-azabicyclo [2.2.2] octane-3-yl (1S)-1-phenyl-3,4-dihydroisoquinoline-2-(1H)-carboxylate of formula II in an organic solvent to obtain the compound solifenacin succinate ((3R)-1-azabicyclo [2.2.2] octane-3-yl (1S)-1-phenyl-3,4-dihydroisoquinoline-2-(1H)-carboxylate succinate) of formula I. The preparation method has the advantages that the process is simple, the method is suitable for industrialized production, the yield of the product is high and the purity of the product is high.
Owner:CHONGQING KERUI PHARMA GRP
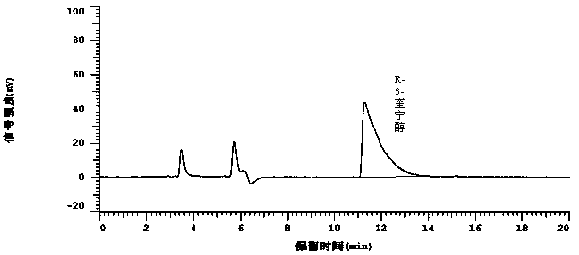
Method for detecting R-3-quinuclidinol in solifenacin succinate
ActiveCN105510458AEasy to detectAccurate detectionComponent separationSolifenacin SuccinateUltraviolet detectors
The invention provides an analysis method for detecting R-3-quinuclidinol in solifenacin succinate by high performance liquid chromatography. The method is characterized in that high performance liquid chromatography is adopted, and a chromatographic column adopting aminopropyl bonded silica gel as a filling agent is employed; an ultraviolet detector which has the detection wavelength of 210 nm is used; the mobile phase is an n-hexane-ethanol-methanol-diethylamine (600:300:100:1) solution; a sample is dissolved by the mobile phase and has the concentration of 20 mg / ml, and quantitative analysis is made according to peak area by the external standard method. The method has the advantages of being good in selectivity, high in sensitivity and accuracy, excellent in repeatability, and simple, convenient and quick, and provides a guarantee for accurate detection of R-3-quinuclidinol in solifenacin succinate.
Owner:迪嘉药业集团股份有限公司
Synthesis method of solifenacin succinate
InactiveCN104447734AReduce usageAvoid Azeotropic Separation OperationsOrganic chemistrySolifenacin SuccinateOrganic base
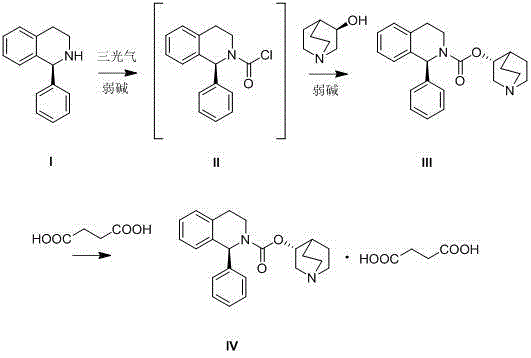
The invention discloses a synthesis method of solifenacin succinate. The synthesis method comprises the following processing steps: (1) respectively dissolving (S)-1-phenyl-1,2,3,4-tetrahydroisoquinoline, organic alkali and triphosgene into a proper amount of high-boiling-point solvent, dropwise adding the (S)-1-phenyl-1,2,3,4-tetrahydroisoquinoline and a solution of the organic alkali to the solution of the triphosgene, so as to obtain an intermediate (S)-1-phenyl-3,4-dihydro-1H-isoquinoline-2-formyl chloride, and finally adding a proper amount of organic alkali and high-boiling-point solvent, adding (R)-3-quinuclidinol, heating to 100-200 DEG C and carrying out temperature control reaction, so as to prepare solifenacin; and (2) dissolving the solifenacin into a mixed solvent of ethyl acetate and ethyl alcohol, adding new seed crystal of the solifenacin succinate, stirring and crystallizing, so as to prepare the solifenacin succinate. The synthesis method of the solifenacin succinate has the advantages that two-step condensation reaction is carried out by virtue of a one-pot operation in the synthesis method, so that the troublesome azeotropic separation operation reported in the literature is avoided; and homogeneous catalysis is carried out by selecting soluble organic alkali, so that the reaction efficiency is improved.
Owner:JINGCHU UNIV OF TECH
Solifenacin succinate raw medicine synthesis process
InactiveCN108329309AEasy to operateMeet the requirements of mass productionOrganic chemistry methodsSolifenacin Succinate3-quinuclidinone
The invention discloses a solifenacin succinate raw medicine synthesis process. 2-phenylethylamine and 3-quinuclidinone hydrochloride are respectively used as starting raw materials for synthesizing afragment A and a fragment B; then, condensation reaction occurs to generate solifenacin; through salt formation, the solifenacin succinate is obtained. The process is characterized in that straight-chain paraffin and water are used as reaction solvents; alkali metal hydroxides or carbonate and bicarbonates of the alkali metal hydroxides are used as acid-binding agents; phenylethylamine and benzoyl chloride take acylation reaction to generate midbodies 1 of solid precipitation fragments A insoluble in reaction solvents; in the post treatment process, filtering is directly performed; isomers ofthe fragment A are subjected to catalytic racemization through alkali metal hydroxides by using dimethylsulfoxide as a solvent, so that the byproduct isomers can be recovered and utilized; in the second-step reaction post treatment of the fragment B, a conventional pressure reduced distillation method is used for obtaining high-purity and high-yield 3-acetoxyquinine acetate. The invention provides a novel synthesis process with the advantages of high yield and economic and environment-friendly effects, and is suitable for industrial mass production.
Owner:江西博雅欣和制药有限公司
A kind of preparation method of solifenacin succinate
The present invention relates to a kind of sorenacin succinate ((3R)-1-azabicyclo[2.2.2]octane-3-yl(1S)-1-phenyl-3,4-dihydroisoquinone The preparation method of phenoline-2-(1H)-carboxylate succinate) comprises the following steps: 1) Formula IV compound (S)-1-phenyl-1,2,3,4-tetrahydroisoquinone Phenyl is used as raw material to prepare ethyl ester of formula III compound (S)-1-phenyl-1,2,3,4-tetrahydroisoquinolinecarboxylate; 2) formula III compound (S)-1-phenyl Synthesis of Formula Ⅱ Compound (3R)-1-azabicyclo[2.2.2]octane-3-yl(1S) from Ethyl-1,2,3,4-tetrahydroisoquinolinecarboxylate in Ionic Liquid -1-phenyl-3,4-dihydroisoquinoline-2-(1H)-carboxylate; 3) Formula II compound (3R)-1-azabicyclo[2.2.2]octane- 3-base (1S)-1-phenyl-3,4-dihydroisoquinoline-2-(1H)-carboxylate is salified in organic solvent, obtains formula I compound sorenacin succinate (( 3R)-1-azabicyclo[2.2.2]octane-3-yl(1S)-1-phenyl-3,4-dihydroisoquinoline-2-(1H)-carboxylate succinic acid salt); the preparation method has the advantages of simple process, suitability for industrial production, high product yield and high product purity.
Owner:CHONGQING KERUI PHARMA GRP
Novel process for the preparation of solifenacin succinate
The present invention relates to a process for the preparation of Solifenacin succinate by condensing a compound of formula (IVb) with (RS)-3-quinuclidinol, wherein, R represents methyl, ethyl, isopropyl; to produce a diastereomeric mixture of (1S)-3,4-dihydro-1-phenyl-2(1H)-isoquinolinecarboxylic acid (3RS)-1-azabicyclo[2.2.2]oct-3-yl ester, which is treated with succinic acid in a solvent or mixture of solvents to produce optically pure Solifenacin succinate, Formula (X).
Owner:AUROBINDO PHARMA LTD
Stable pharmaceutical composition and its preparation method
ActiveCN105012961BStable contentSubstance reductionPharmaceutical non-active ingredientsUrinary disorderSolifenacin SuccinateCellulose
Owner:燃点(南京)生物医药科技有限公司
A kind of pharmaceutical composition containing solifenacin succinate
The invention relates to a pharmaceutical composition containing solifenacin succinate. The pharmaceutical composition is characterized by simultaneously containing direct compression lactose, sodium carboxymethyl starch, a binder and a lubricating agent. The invention also discloses a preparation method, namely a direct powder compression method of the pharmaceutical composition. The pharmaceutical composition and the preparation method thereof are not influenced by water in the preparation process, the pharmaceutical composition is low in impurity content and better in drug dissolubility, and the preparation method is more suitable for industrial production.
Owner:SICHUAN GOWELL PHARMA
Tablets comprising mirabegron and solifenacin
InactiveUS20190307696A1Organic active ingredientsPill deliverySolifenacin SuccinateControlled release
The present invention relates to a pharmaceutical multi layer tablet comprising a controlled release part with mirabegron and an immediate release part wherein the immediate release formulation comprises: solifenacin succinate and a water insoluble diluent in an amount of 50 to 99% w / w relative to the total weight of the immediate release part of the tablet. The invention further relates to the use of said composition as a medicament, particularly in the treatment of urinary incontinence.
Owner:SYNTHON BV
An analytical method for determining stereoisomers and intermediates in solifenacin succinate
ActiveCN107167530BEfficient determinationGuarantee product qualityComponent separationSolifenacin SuccinateCarbamate
The invention relates to an analysis method for determining stereoisomers and intermediates in solifenacin succinate by using high performance liquid chromatography. It is characterized in that: high performance liquid chromatography is used to carry out isocratic elution on amylose-three (3,5-xylylcarbamate) bonded silica gel chiral chromatographic column; mobile phase is n-hexane- Absolute ethanol-isopropanol-diethylamine solution (65:15:20:0.1) (volume ratio); the sample is dissolved in mobile phase. This method can simultaneously determine the enantiomers, diastereomers and two intermediates of solifenacin succinate which are enantiomers, which is the research on the synthetic process route of solifenacin succinate And quality control to provide more reliable guarantee.
Owner:SHANDONG ACADEMY OF PHARMACEUTICAL SCIENCES +1
Process for the preparation of solifenacin succinate
Owner:AUROBINDO PHARMA LTD
Determination method of r-3-quinine alcohol in solifenacin succinate
ActiveCN105510458BEasy to detectAccurate detectionComponent separationSolifenacin SuccinateSilica gel
The invention provides an analysis method for detecting R-3-quinuclidinol in solifenacin succinate by high performance liquid chromatography. The method is characterized in that high performance liquid chromatography is adopted, and a chromatographic column adopting aminopropyl bonded silica gel as a filling agent is employed; an ultraviolet detector which has the detection wavelength of 210 nm is used; the mobile phase is an n-hexane-ethanol-methanol-diethylamine (600:300:100:1) solution; a sample is dissolved by the mobile phase and has the concentration of 20 mg / ml, and quantitative analysis is made according to peak area by the external standard method. The method has the advantages of being good in selectivity, high in sensitivity and accuracy, excellent in repeatability, and simple, convenient and quick, and provides a guarantee for accurate detection of R-3-quinuclidinol in solifenacin succinate.
Owner:迪嘉药业集团股份有限公司
Synthesis process of solifenacin succinate
PendingCN113200979AHigh yieldReduce reversible reactionsOrganic compound preparationCarboxylic acid salt preparationSolifenacin SuccinateSuccinic acid
The invention discloses a synthesis process of solifenacin succinate, and relates to the technical field of medicines. The preparation method comprises the following steps: step 1, reacting (R)-quinuclidin-3-ol (B) with a reaction substance to generate (R)-quinuclidin-3-yl carbonochloridate (C); step 2, enabling (S)-1-phenyl-1,2,3,4-tetrahydroisoquinoline (A) to react with (R)-quinuclidin-3-yl carbonochloridate (C) to generate solifenacin (D); and step 3, using the solifenacin (D) to prepare the solifenacin succinate. After the technical scheme is adopted, the method has the beneficial effects that in the synthesis process, the problem of low conversion rate caused by direct ester exchange reaction is avoided, meanwhile, the probability of reversible reaction caused by nucleophilic intermediate products generated in the condensation process is also reduced, and the reaction is greatly promoted, so that the yield of solifenacin succinate is increased, the reaction conditions are mild, the operation is simple, the operation procedures are reduced, and the method is suitable for large-scale production.
Owner:上海予君生物科技发展有限公司
Solifenacin succinate tablet and preparation method thereof
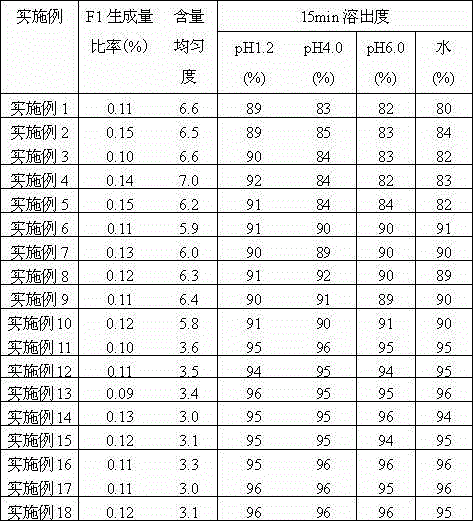
ActiveCN114099452AReduce moisture absorption performanceReduce reunionUrinary disorderPharmaceutical non-active ingredientsSolifenacin SuccinateUse medication
The invention provides a solifenacin succinate tablet and a preparation method thereof. The solifenacin succinate tablet comprises the following components: solifenacin succinate, trehalose, a disintegrating agent and a hydrophobic adhesive. The solifenacin succinate tablet disclosed by the invention is relatively good in uniformity and relatively high in medication safety.
Owner:YANGTZE RIVER PHARM GRP GUANGZHOU HAIRUI PHARM CO LTD +1
A kind of aftertreatment of solifenacin and the method for preparing solifenacin succinate
The invention discloses an after-treatment method for solifenacin. The method comprises the steps that strong acid is slowly dripped into total synthesis liquid of solifenacin, after a pH value is adjusted to be 1.0-2.0, temperature is raised, water is added, and an extracting agent is used for extraction; after a water phase is cooled, a saturated sodium carbonate solution is added to alkalinity, dichloromethane is added to carry out graded extraction, extract liquor is washed with water, and a drying agent is added for drying, so that a dry product is obtained; the dry product is steamed at low temperature to be dry to obtain an oily substance solifenacin. By optimizing the after-treatment technology, isopropyl ether and dichloromethane are only used in the after-treatment technology of solifenacin, types of solvent are reduced, operation and recycle are facilitated, and cost is saved greatly. Strong base or strong acid is not used in the alkali efflorescence process of hydrochloric acid solifenacin, a saturated NaCO3 solution is used, the reaction is mild, temperature can be controlled easily, most importantly, generation of isomers is greatly reduced, and the content of the isomers of a non-structure type (1S and 3R) is reduced.
Owner:成都华宇制药有限公司
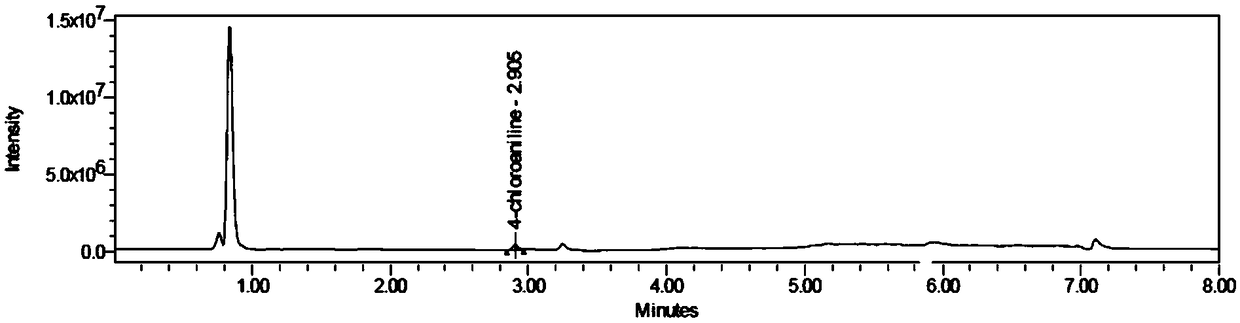
Method for determining aniline-type genotoxic impurities in novel solifenacin succinate crude drug
The invention provides a method for determining aniline-type genotoxic impurities in a novel solifenacin succinate crude drug. The method comprises the steps that 4-chloroaniline and (-)-4'-chloroaniline tartramide acid are used as reference substances, a 50% of acetonitrile water solution is used as a solvent, reference substance solutions of the 4-chloroaniline and the (-)-4'-chloroaniline tartramide acid are prepared, the novel solifenacin succinate crude drug is obtain and is dissolved with 50% of acetonitrile water solution to obtain a test solution; detection is performed with a UPLC-MSmethod, the peak areas of the 4-chloroaniline and the (-)-4'-chloroaniline tartramide acid in the reference substance solutions are respectively obtained, and respective response factors are calculated; the test solution is detected by the UPLC-MS method, the peak area of the test solution is obtained, and the content of the 4-chloroaniline and the (-)-4'-chloroaniline tartramide acid in the testsolution is calculated according to the response factors. The method has the advantgaes of being short in detection time, good in accuracy, high in precision and good in repeatability.
Owner:上海明捷医药科技有限公司
Solifenacin succinate new preparation method
The present invention relates to a solifenacin succinate new preparation method which mainly comprises the following steps: 1) formula III compound (R)-(-)-quinuclidin-3-ol and formula IV compound N,N,-N,N-carbonyldiimidazole are reacted to obtain formula V compound (R)-imidazole-1-carboxylic acid-1-azabicyclo[2,2,2] octyl ester; 2) the formula V compound (R)-imidazole-1-carboxylic acid-1-azabicyclo[2,2,2] octyl ester is reacted with formula VI compound (S)-1-phenyl-1,2,3,4-tetrahydroisoquinoline to obtain a formula II solifenacin alkaline body; 3) the formula II solifenacin alkaline body isreacted with succinic acid, refined and purified to obtain formula I solifenacin succinate. The preparation method has the advantages that the yield of the solifenacin succinate is high and the purityis high, and the process is simple and suitable for industrial production.
Owner:BEIJING LABWORLD BIO MEDICINE TECH
Preparation technology of solifenacin succinate
ActiveCN102875544BImprove conversion rateReverse reaction facilitationOrganic chemistryCondensation processSolifenacin Succinate
The invention relates to a preparation technology of solifenacin succinate. According to the preparation technology, (S)-1-phenyl-1, 2, 3, 4-tetrahydroisoquinoline carbamyl chloride (II) and (R)-3-quinuclidine alcohol metal salt (III) are reacted to generate solifenacin alkali, and then the solifenacin alkali is prepared into solifenacin succinate. With the adoption of the preparation technology provided by the invention, nucleophilic species which lead to a reverse reaction can be avoided during condensation process, namely, the reverse reaction cannot be generated in the preparation technology, therefore, a transformation rate of the solifenacin succinate is improved, a reaction is greatly promoted, a reaction process is quickly carried out under a mild condition, and the preparation technology is suitable for large-scale production.
Owner:CHENGDU SINO STRONG PHARMA
Method for Determination of Aniline Genotoxic Impurities in Solide Sodium Succinate New API
ActiveCN109374808BPrecise positioningQuick responseComponent separationSolifenacin SuccinateSuccinic acid
The invention provides a method for determining aniline-type genotoxic impurities in a novel solifenacin succinate crude drug. The method comprises the steps that 4-chloroaniline and (-)-4'-chloroaniline tartramide acid are used as reference substances, a 50% of acetonitrile water solution is used as a solvent, reference substance solutions of the 4-chloroaniline and the (-)-4'-chloroaniline tartramide acid are prepared, the novel solifenacin succinate crude drug is obtain and is dissolved with 50% of acetonitrile water solution to obtain a test solution; detection is performed with a UPLC-MSmethod, the peak areas of the 4-chloroaniline and the (-)-4'-chloroaniline tartramide acid in the reference substance solutions are respectively obtained, and respective response factors are calculated; the test solution is detected by the UPLC-MS method, the peak area of the test solution is obtained, and the content of the 4-chloroaniline and the (-)-4'-chloroaniline tartramide acid in the testsolution is calculated according to the response factors. The method has the advantgaes of being short in detection time, good in accuracy, high in precision and good in repeatability.
Owner:上海明捷医药科技有限公司
A kind of preparation method of solifenacin succinate impurity
The present invention relates to a kind of solifenacin succinate impurity [2-(2-benzoyl-phenyl)-ethyl]-carbamic acid 1-aza-bicyclo[2.2.2]oct-3-yl ester method of preparation. It is obtained from solifenacin succinate through oxidative ring opening and separation and purification. The invention provides a reference substance for the qualitative and quantitative analysis of solifenacin succinate through the preparation of the impurity solifenacin succinate, effectively controls the quality of solifenacin succinate, and ensures the safety of clinical medication.
Owner:天津市医药集团技术发展有限公司 +1
Preparation process of solifenacin succinate tablet composition
InactiveCN111407735AEasy to prepareSimple processUrinary disorderPharmaceutical non-active ingredientsSolifenacin SuccinateCellulose
The invention discloses a preparation process of a solifenacin succinate tablet composition. The preparation process comprises the following specific preparation steps: step 1, pretreatment: sieving solifenacin succinate and lactose by a 40-mesh sieve for later use; step 2, weighing: according to a batch prescription, re-checking and weighing the solifenacin succinate, the lactose, corn starch andhydroxypropyl methylcellulose by two persons; step 3, solution preparation: preparing 460g of 8% hydroxypropyl methylcellulose solution from the weighed hydroxypropyl methylcellulose; and step 4, preheating and primary mixing: according to the batch prescription, after the feeding amount is checked by two persons, sequentially adding the lactose, the solifenacin succinate (more different in process, directly adding or adding together with an adhesive) and the corn starch into a multifunctional fluidized bed in sequence, and preheating and mixing for 12min. The preparation effect is more outstanding by utilizing the cooperation of multiple steps, and the process is simple compared with the existing preparation technology, large-batch production can be carried out, and the production cost is greatly reduced.
Owner:NANJING MEIRUI PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com