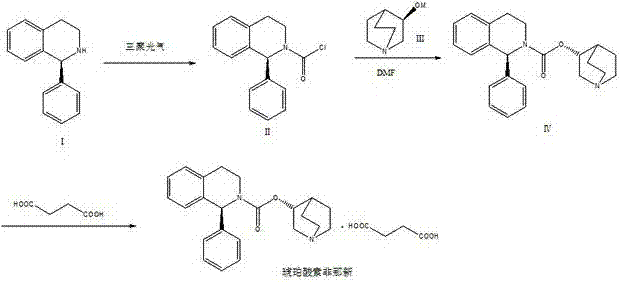
Preparation technology of solifenacin succinate
A technology of solifenacin succinate and preparation process, applied in the field of medicine, can solve the problems of helpless conversion rate, low conversion rate of transesterification reaction, difficult industrialized production, etc. The effect of conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] The preparation method of Solifenacin Succinate of the present embodiment is as follows:
[0022] 1. First add 10.0g (S)-1-phenyl-1,2,3,4-tetrahydroisoquinoline and 6.3mL triethylamine into 40mL THF (tetrahydrofuran), and drop into the dissolved Add 6.0g of triphosgene to 20mL of toluene solution, add dropwise under ice-water bath, remove the ice-water bath after dropwise addition, and continue to react for 30 minutes. The organic layer was washed twice with 50 mL of water, and concentrated to obtain 12 g of oily (S)-1-phenyl-1,2,3,4-tetrahydroisoquinolinecarbamoyl chloride;
[0023] 2. Add 5.6g (R)-3-quinuclidinol to 50mL DMF (dimethylformamide), stir to dissolve, then add 1.2g sodium hydride in an ice-water bath, stir for 5 minutes after adding, remove Ice-water bath, and continue stirring for 1 hour to obtain a DMF solution of sodium (R)-3-quinuclidinate. Dissolve 12g (S)-1-phenyl-1,2,3,4-tetrahydroisoquinolinecarbamoyl chloride oil in 50mL DMF, and drop the soluti...
Embodiment 2
[0029] The preparation method of Solifenacin Succinate of the present embodiment is as follows:
[0030] 1. First add 10.0g (S)-1-phenyl-1,2,3,4-tetrahydroisoquinoline and 6.3mL N-methylmorpholine into 40mL tetrahydrofuran, then drop into the dissolved Add 6.0g of phosgene to 20mL of toluene solution, add dropwise under ice-water bath, remove the ice-water bath after dropwise addition, and continue to react for 30 minutes. The organic layer was washed twice with 50 mL of water, and concentrated to obtain 12 g of oily (S)-1-phenyl-1,2,3,4-tetrahydroisoquinolinecarbamoyl chloride;
[0031] 2. Add 5.6g (R)-3-quinuclidinol into 50mL tetrahydrofuran, stir to dissolve, add 2.0g potassium hydride under ice-water bath, stir for 5 minutes after adding, remove the ice-water bath, and continue stirring for 1 hour A DMF solution of potassium (R)-3-quinuclidinate was obtained. Dissolve 12g (S)-1-phenyl-1,2,3,4-tetrahydroisoquinolinecarbamoyl chloride oil in 50mL DMF, and drop the solutio...
Embodiment 3
[0037] The preparation process steps in Example 1 were adopted, but the reaction solvent therein was replaced for experimentation, and one solvent component was changed each time.
[0038] (1) (S)-1-phenyl-1,2,3,4-tetrahydroisoquinoline carbamoyl chloride (Ⅱ) reacts with (R)-3-quinuclidinol metal salt (Ⅲ) to form a Phenacin base reaction solvent adopts THF, DMF, dioxane, dichloromethane respectively, and the productive rate of the Soferapine succinate finally prepared is respectively THF (42%), DMF (58%), dioxane Cyclo(37%), Dichloromethane (31%).
[0039] (2) Synthesis of (R)-3-quinuclidinol metal salt (Ⅲ) was obtained by reacting sodium hydride, potassium hydride and (R)-3-quinuclidinol respectively, and the final prepared Sophirasine succinate Yields were consistent. When adopting DMF as solvent to carry out condensation, productive rate is all about 58%.
[0040] (3) Reaction of (S)-1-phenyl-1,2,3,4-tetrahydroisoquinoline with phosgene, diphosgene or triphosgene to gene...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com