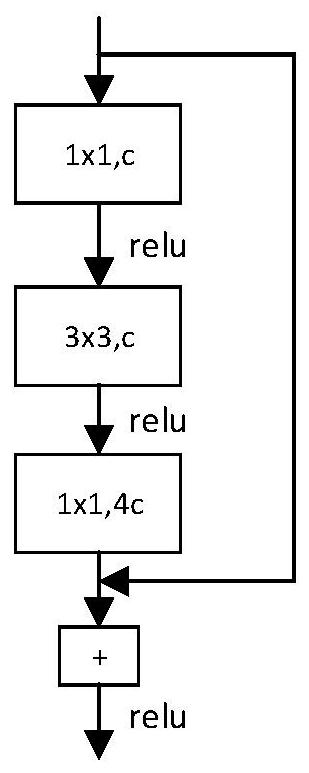
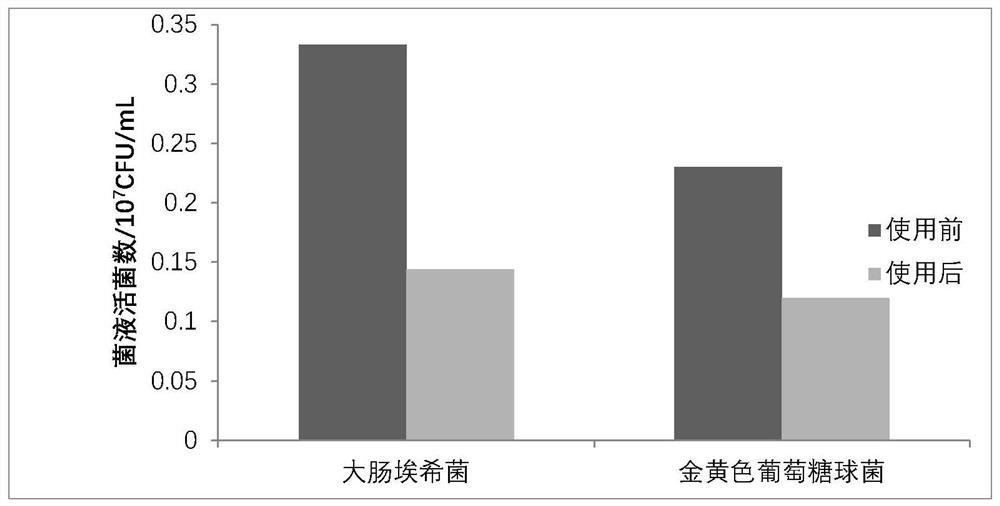
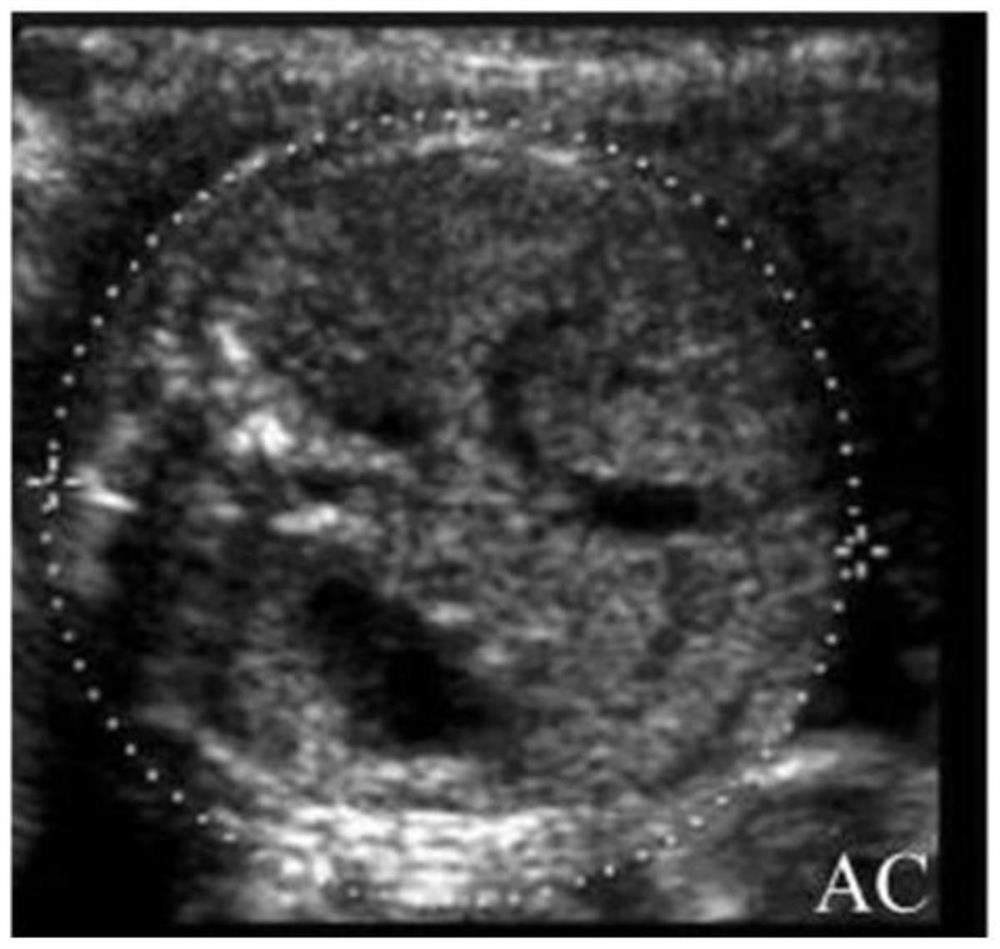
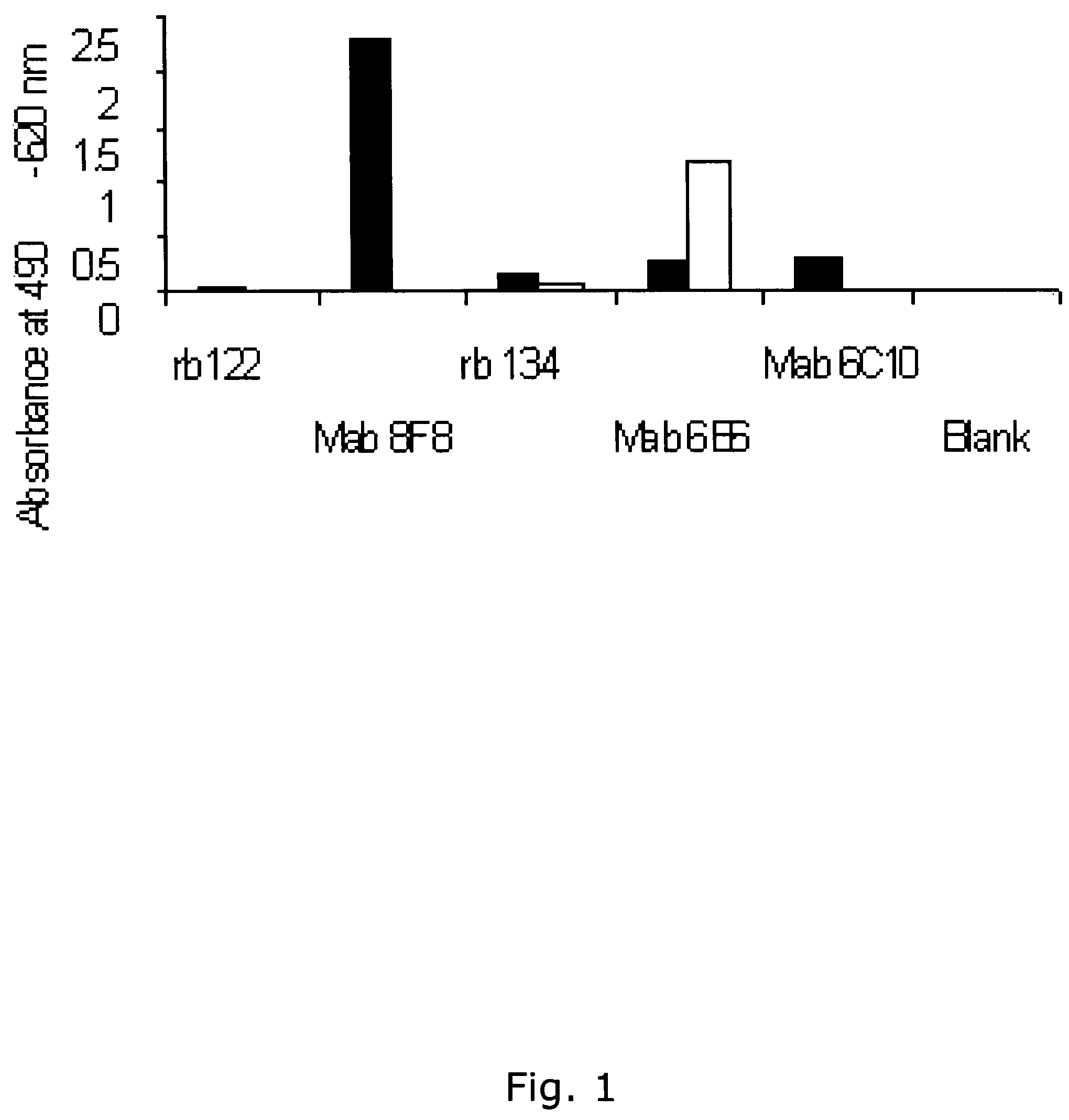
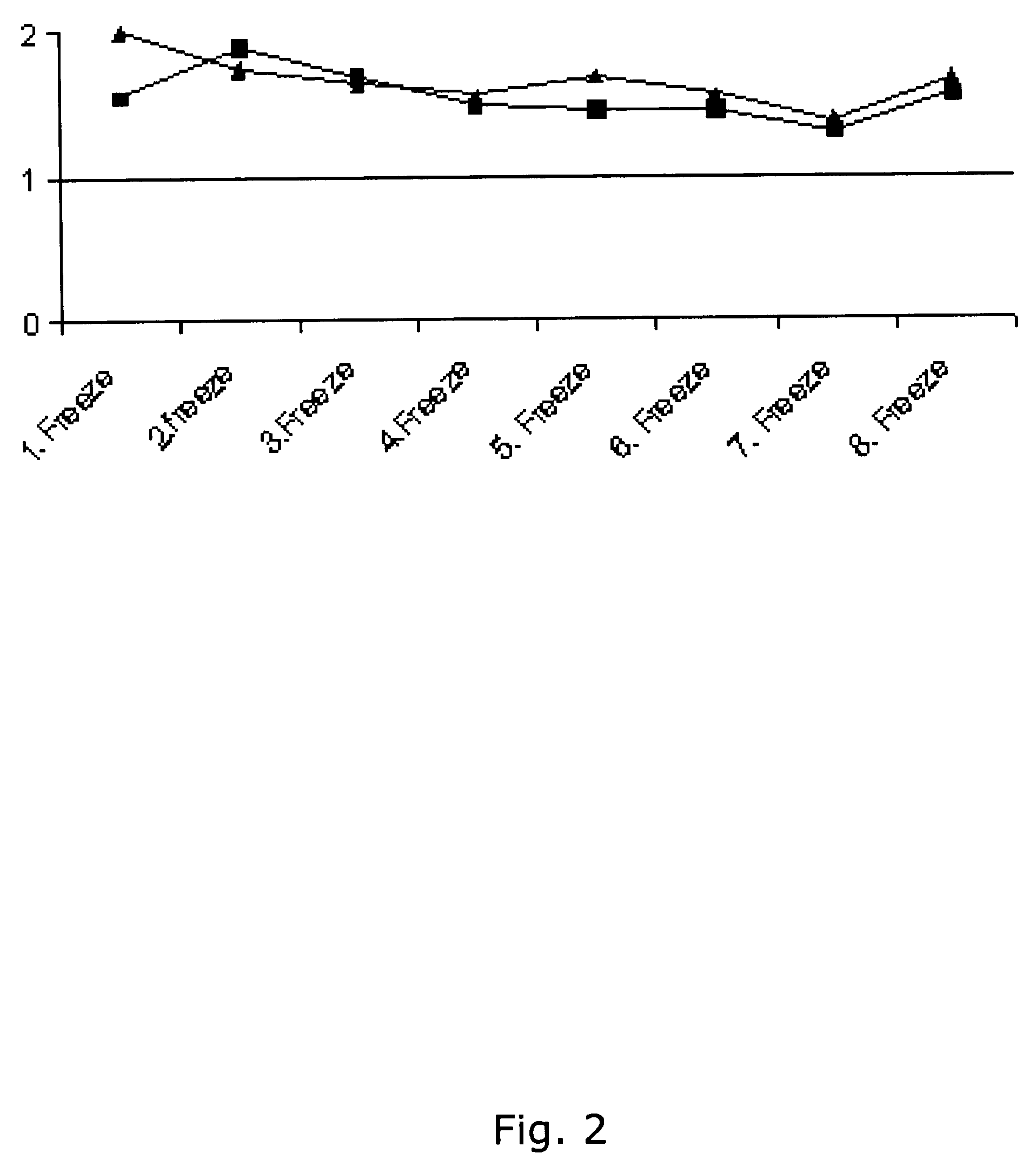
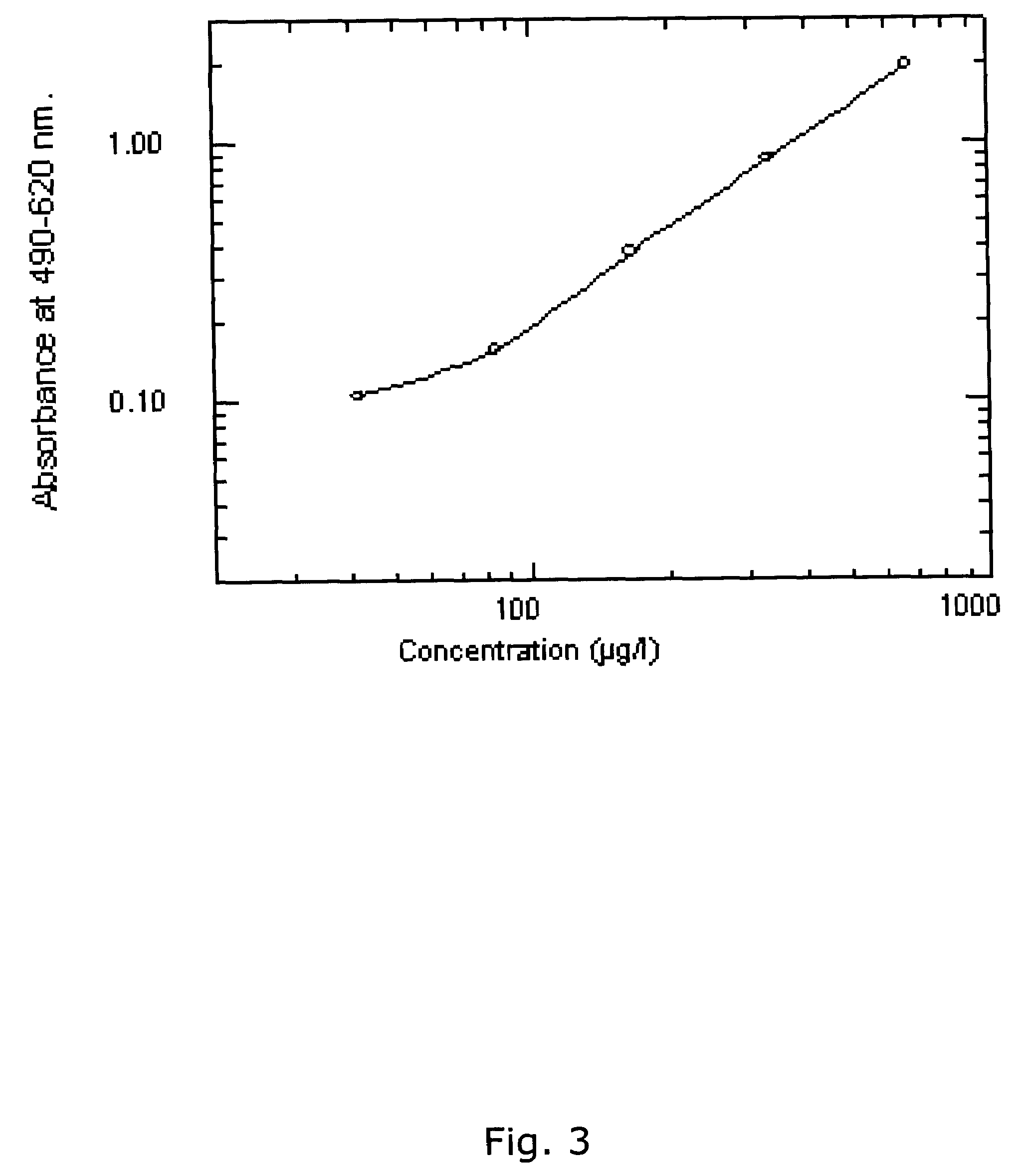
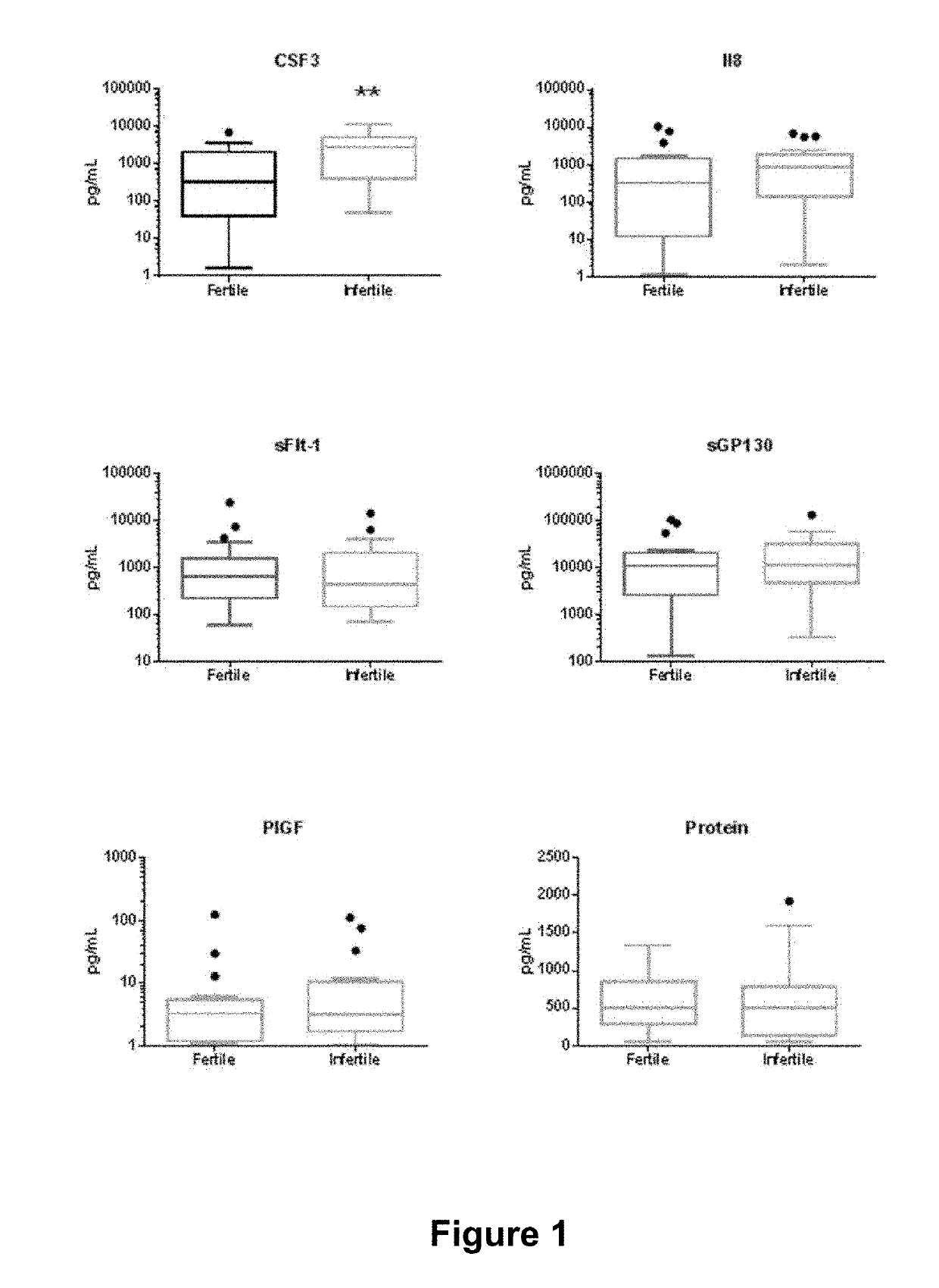
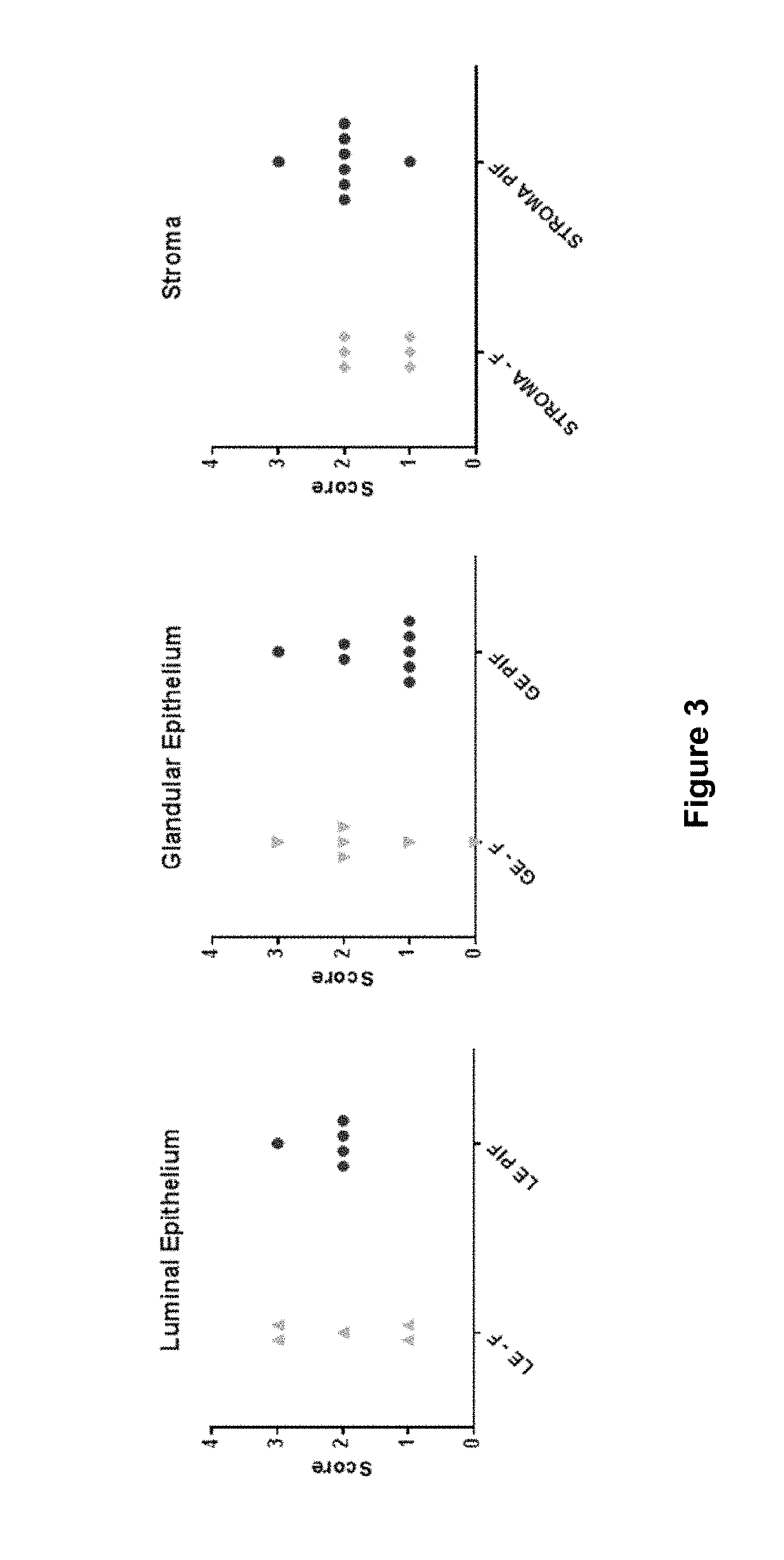
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
36 results about "Pregnancy outcomes" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pregnancy outcomes. Similar term(s): reproductive outcomes, outcomes of pregnancy, birth outcomes. Definition: Results of conception and ensuing pregnancy, such as sex ratio, birth weight, spontaneous abortion, congenital malformations, lower birth weight, preterm delivery or stillbirth.
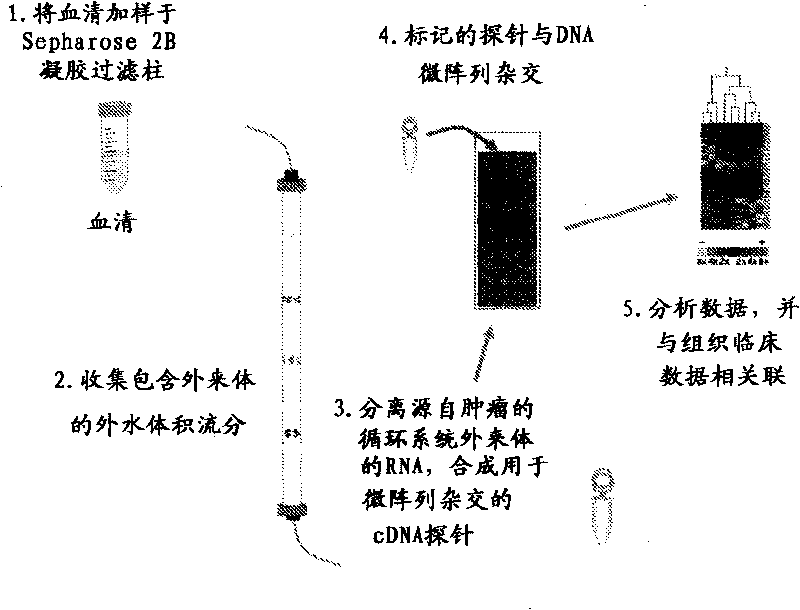
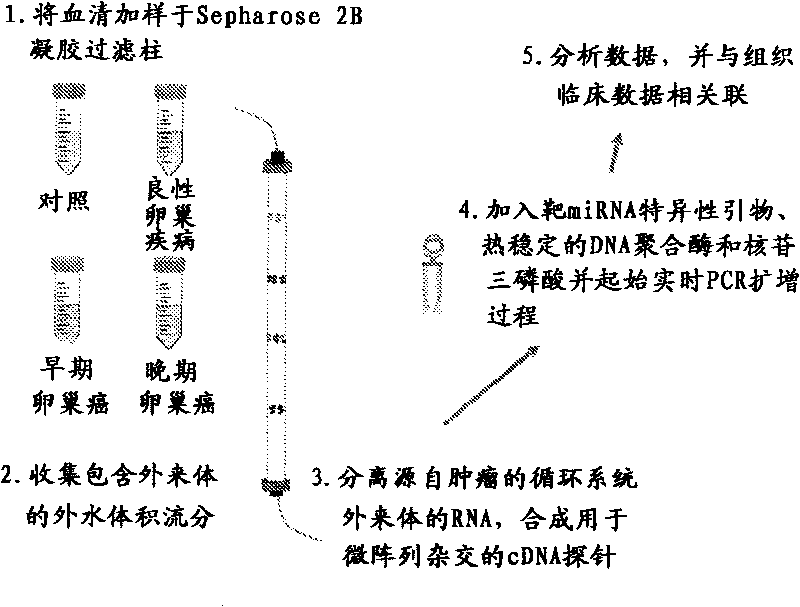
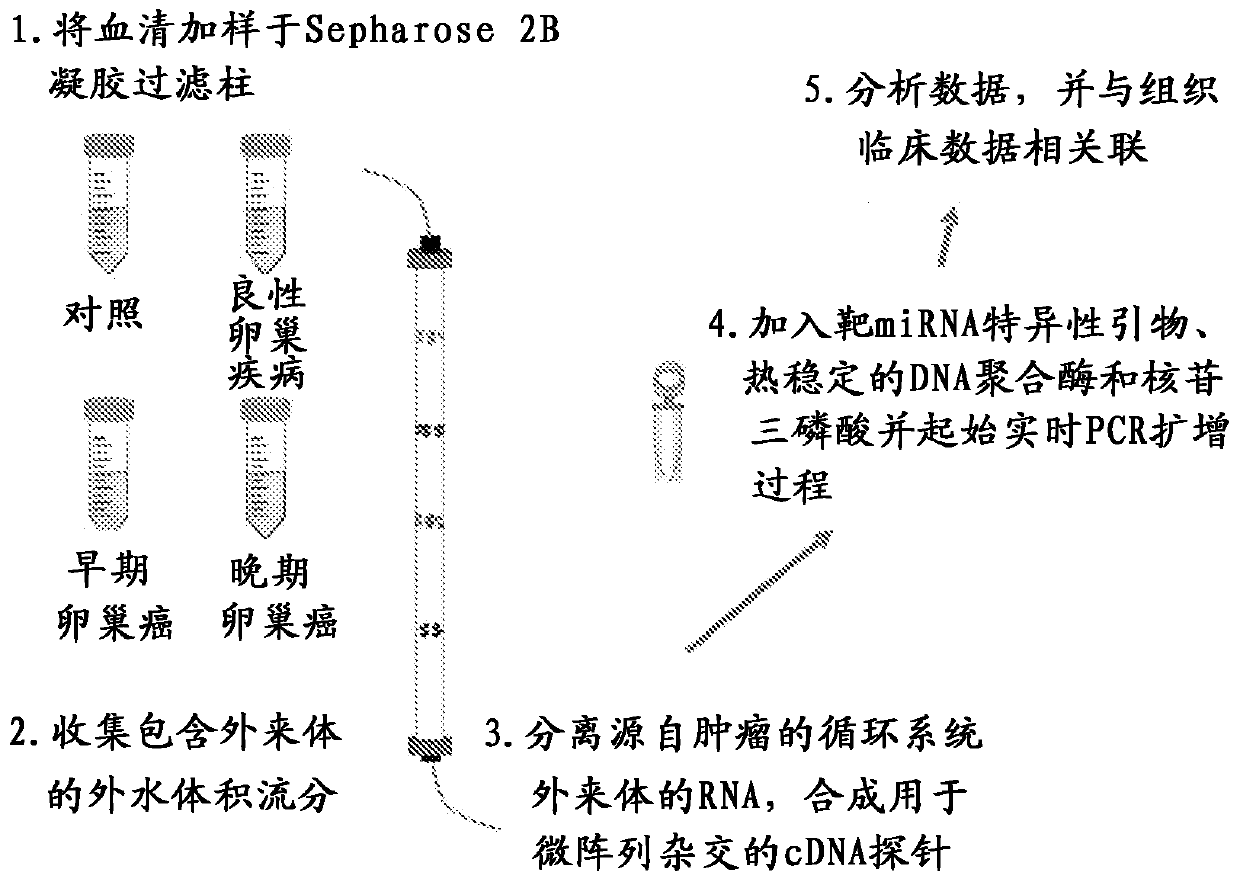
Exosome-associated microRNA as a diagnostic marker
ActiveCN101755208AMicrobiological testing/measurementApparatus for meter-controlled dispensingGynecologyPregnancy outcomes
The presently disclosed subject matter provides methods of diagnosis of cancer or adverse pregnancy outcomes in a subject by measuring amounts of one or more microRNAs present in cancer-derived exosomes isolated from a biological sample from the subject.
Owner:UNIV OF LOUISVILLE RES FOUND INC
Serum/blood plasma polypeptide marker related to gestational diabetes mellitus auxiliary early diagnosis and application thereof
ActiveCN108957011AGuaranteed stabilityFacilitate early diagnosisDisease diagnosisBiological testingSerum igePregnancy outcomes
The invention discloses a serum / blood plasma polypeptide marker related to gestational diabetes mellitus auxiliary early diagnosis and application thereof. The serum / blood plasma polypeptide marker ischaracterized in that the marker is selected from arbitrary one or more polypeptides of Q6PFW1, P35658, P32004 and Q9Y4H2. The serum / blood plasma polypeptide marker serving as a detection target is applied to preparation of gestational diabetes mellitus auxiliary early diagnosis reagents. The expression quantity of the marker for detecting pregnancy serum / blood plasma can be used in GDM auxiliaryearly diagnosis. Support is provided to clinical doctors for accurately predicting the GDM pathogenesis outcome of pregnancy and timely adopting personal prevention and treatment schemes, so that theoccurrence rate of GDM can be furthest reduced, and adverse pregnancy outcomes can be reduced.
Owner:NANJING MATERNITY & CHILD HEALTH CARE HOSPITAL
ADAM12, a novel marker for abnormal cell function
InactiveUS20060134654A1Improve the detection rateReduce false alarm rateMicrobiological testing/measurementPeptide preparation methodsDiseaseADAM12
The present invention provides a method, an assay and a kit for providing an indication of abnormal cell function. It was surprisingly found that the change in the serum ADAM12 concentration in individuals was useful as a prognostic tool to predict the clinical outcome, complications and mortality following an abnormal cell function. The present inventors describes ADAM12 as a overall general marker for abnormal cell function, and the present inventor for the first time demonstrate that ADAM12 is an important indicator of fetal chromosomal disease and placenta function. Specifically ADAM12 is a good marker for e.g. Downs's syndrome, trisomy 18, preeclampsia, Turner syndrome in both first and second trimester. The present inventors developed an enzyme-linked immunosorbent assay (ELISA) and a time-resolved immunofluorometric assay for the quantification of ADAM12 in serum. The present application demonstrates in several examples the variation of the ADAM12 level in fetal abnormality and / or adverse pregnancy outcomes correlated gestational age when compared to normal controls. It is an object of the invention to provide an improvement of the existing marker tests that exhibits a decreased false positive rate.
Owner:STATENS SERUM INST +2
Rapid method of diagnosing a normal pregnancy with high accuracy
ActiveUS7666683B1Rapidly and accurately determiningRapidly and accurately likelihoodChemiluminescene/bioluminescenceDisease diagnosisPoint of careObstetrics
The present invention relates to a novel method of determining the existence of a normal pregnancy which has a high likelihood of culminating in a term pregnancy, by measuring hyperglycosylated hCG in a pregnant woman and comparing the concentration of measured hyperglycosylated hCG with a predetermined value. A measurement of hyperglycosylated hCG above the predetermined value, for example, about 13 ng / ml, is evidence of a high likelihood of a normal pregnancy. A measurement below the predetermined value is evidence of an abnormal pregnancy (ectopic pregnancy or miscarriage). Further analysis of the patient in the event that the measurement falls below the predetermined value is made by intravaginal or abdominal ultrasound to determine whether or not the pregnancy is ectopic or will likely result in a spontaneous abortion (miscarriage). This point-of-care (POC), over-the-counter (OTC) or professional laboratory application is unexpectedly rapid and highly predictive of pregnancy outcome in women exhibiting symptoms of pregnancy.
Owner:STC UNM
Serum-based, diagnostic, biological assay to predict pregnancy disorders
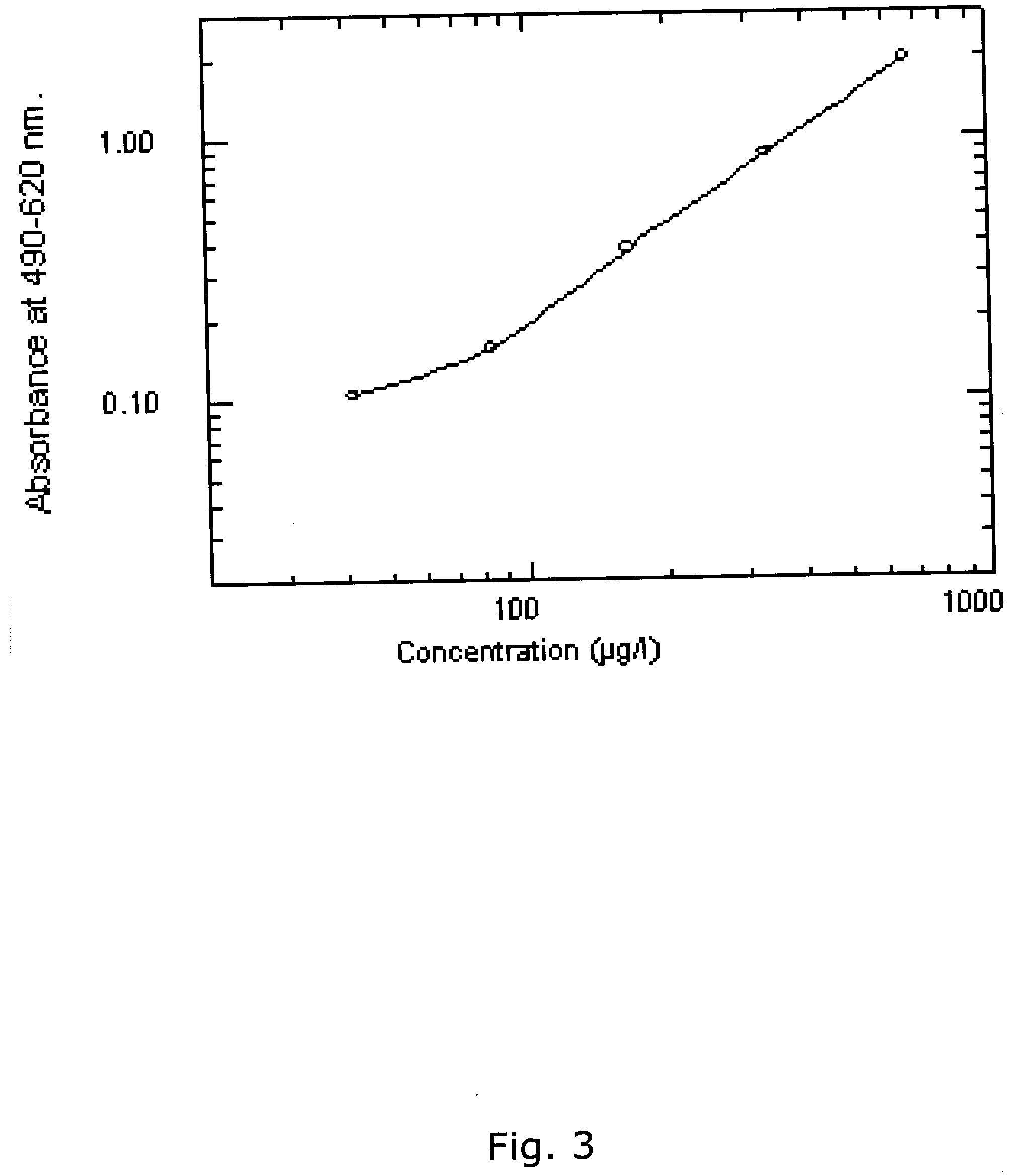
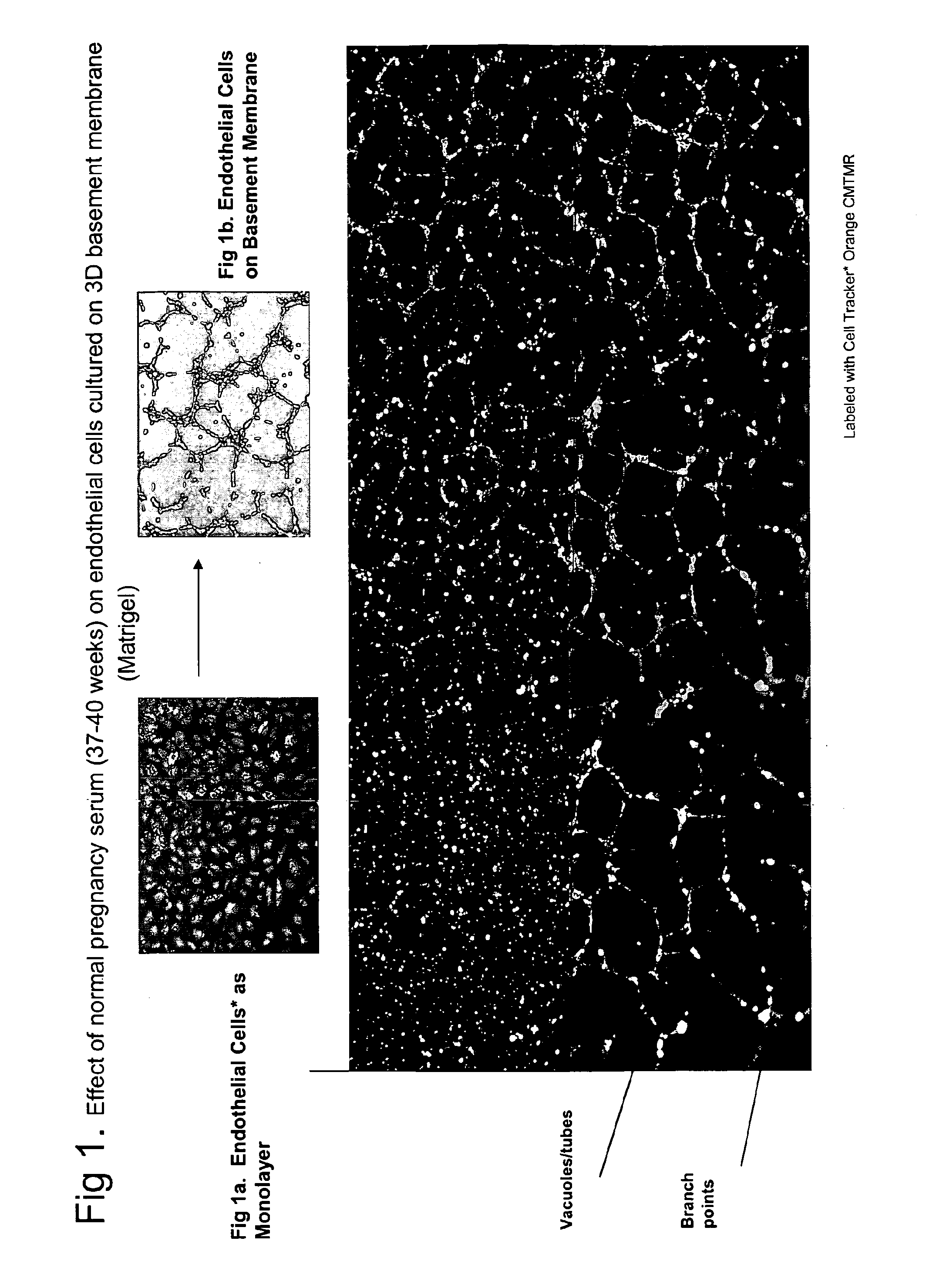
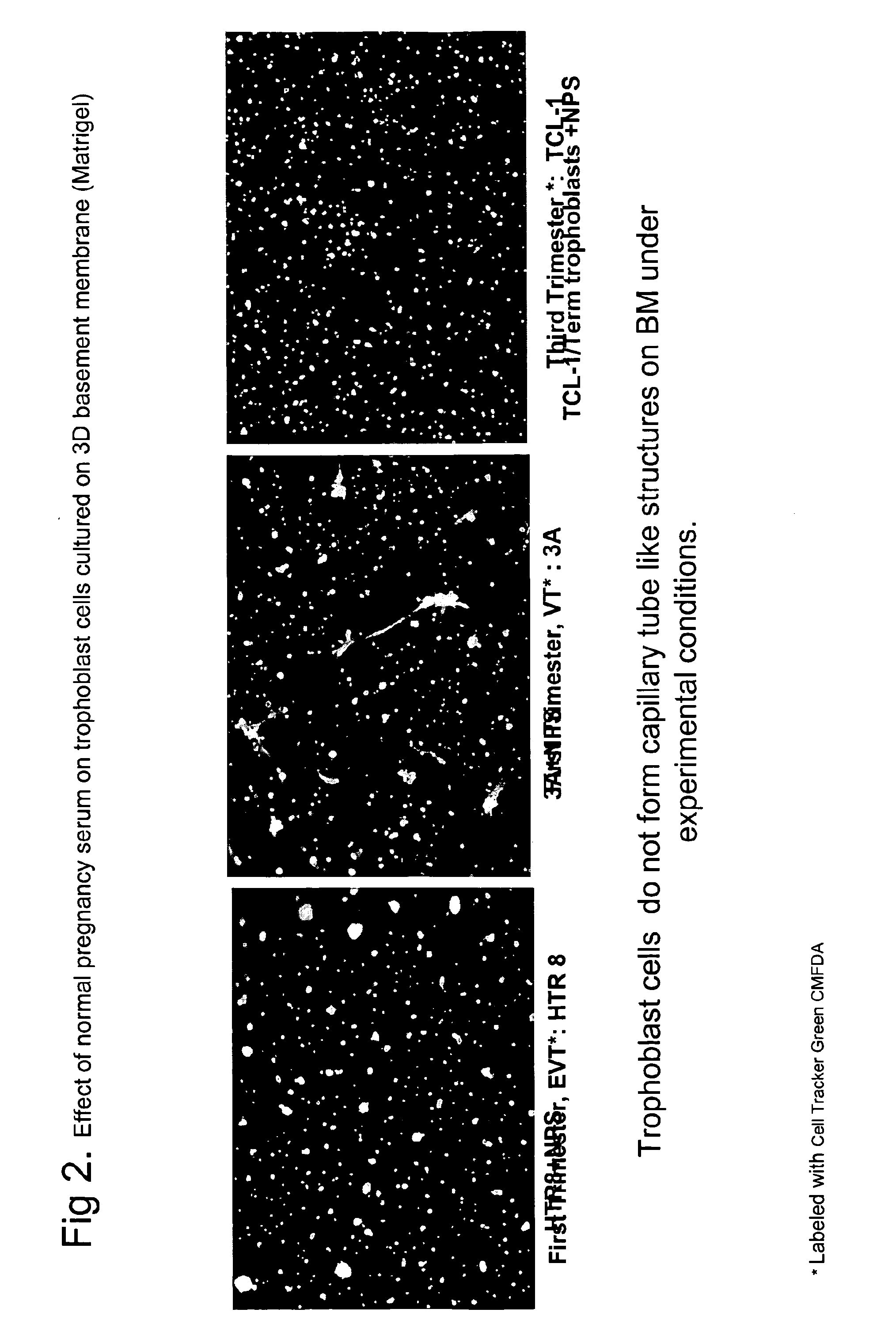
ActiveUS20110059904A1Simple, non-invasiveCost effectiveMicrobiological testing/measurementDisease diagnosisTrophoblastBiology
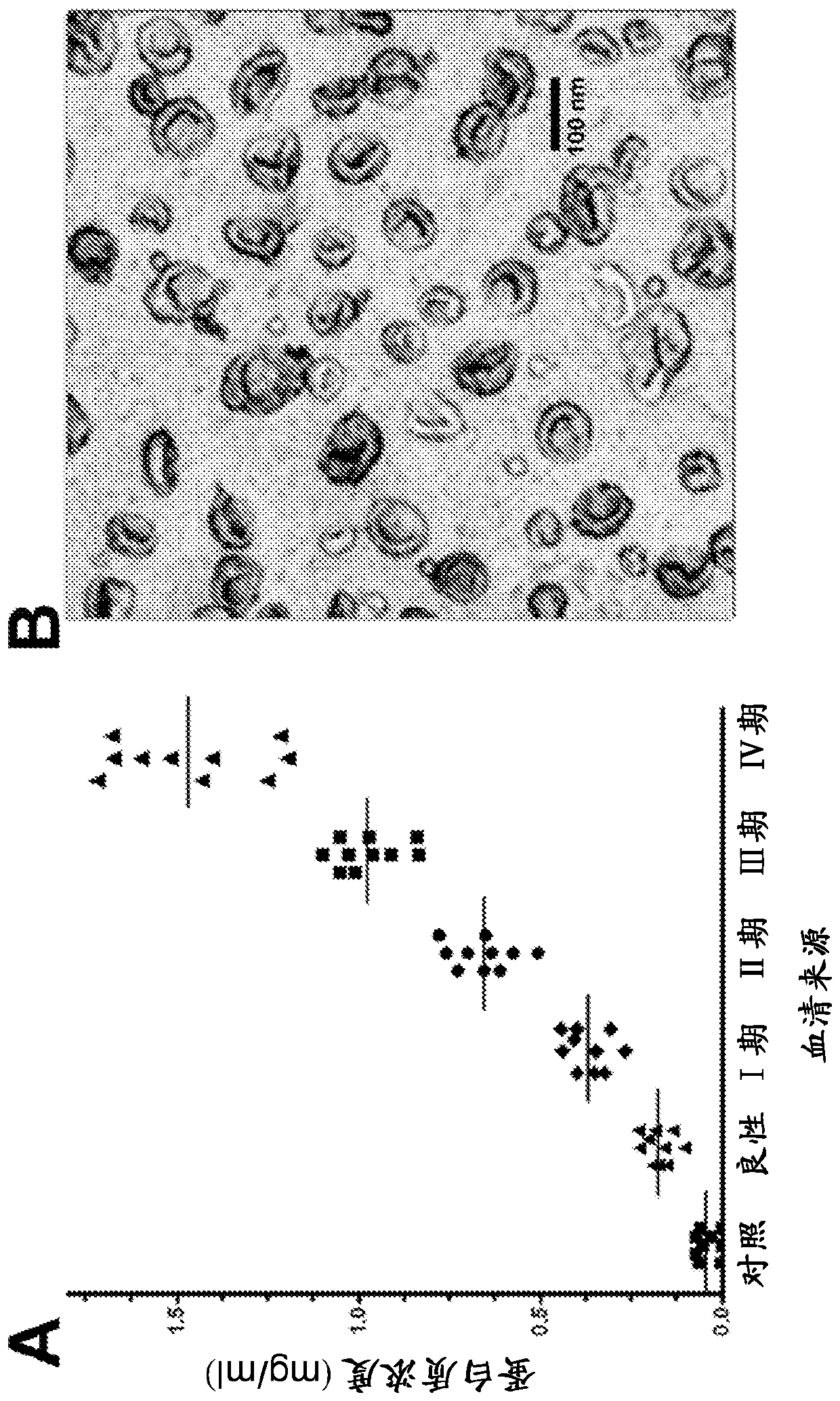
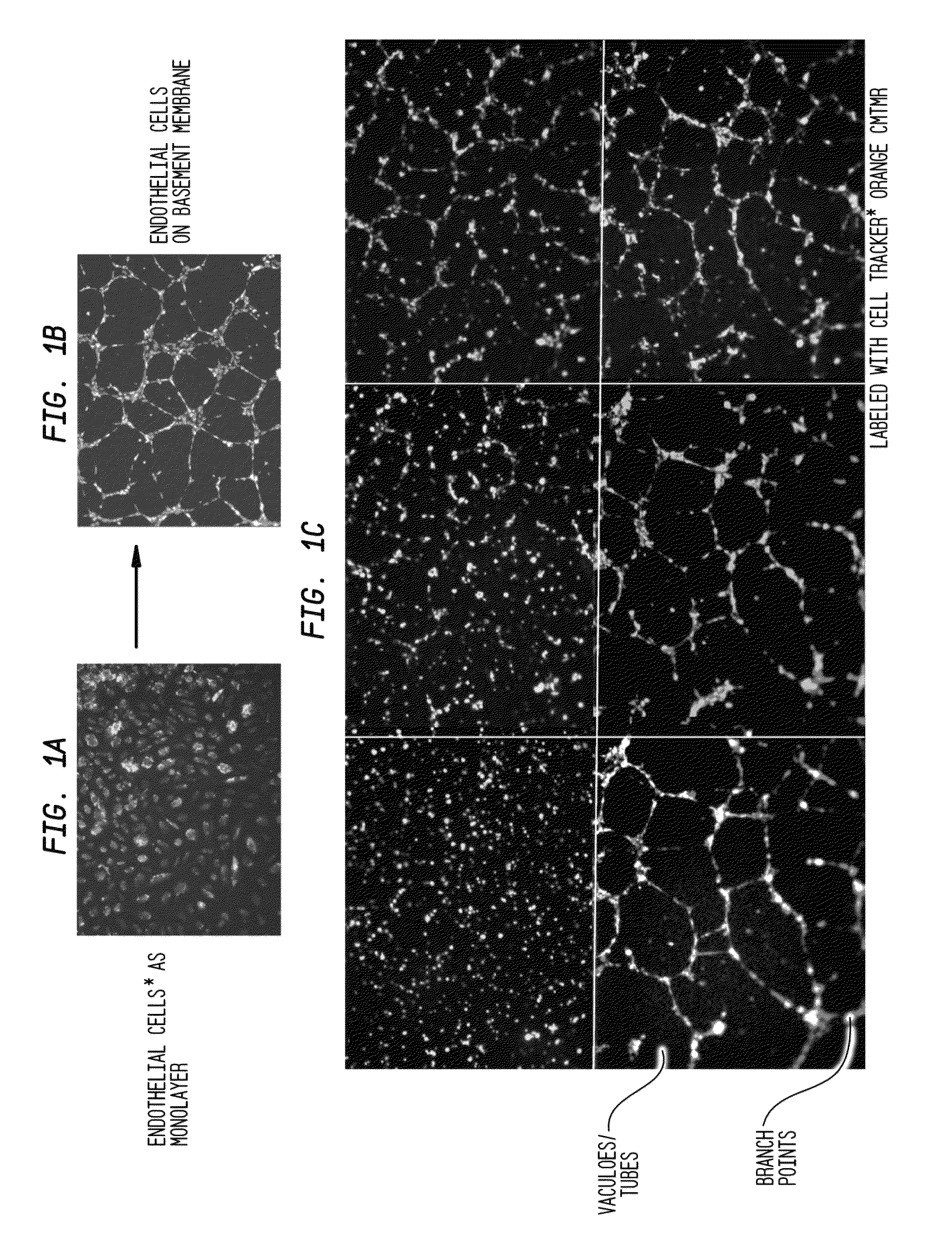
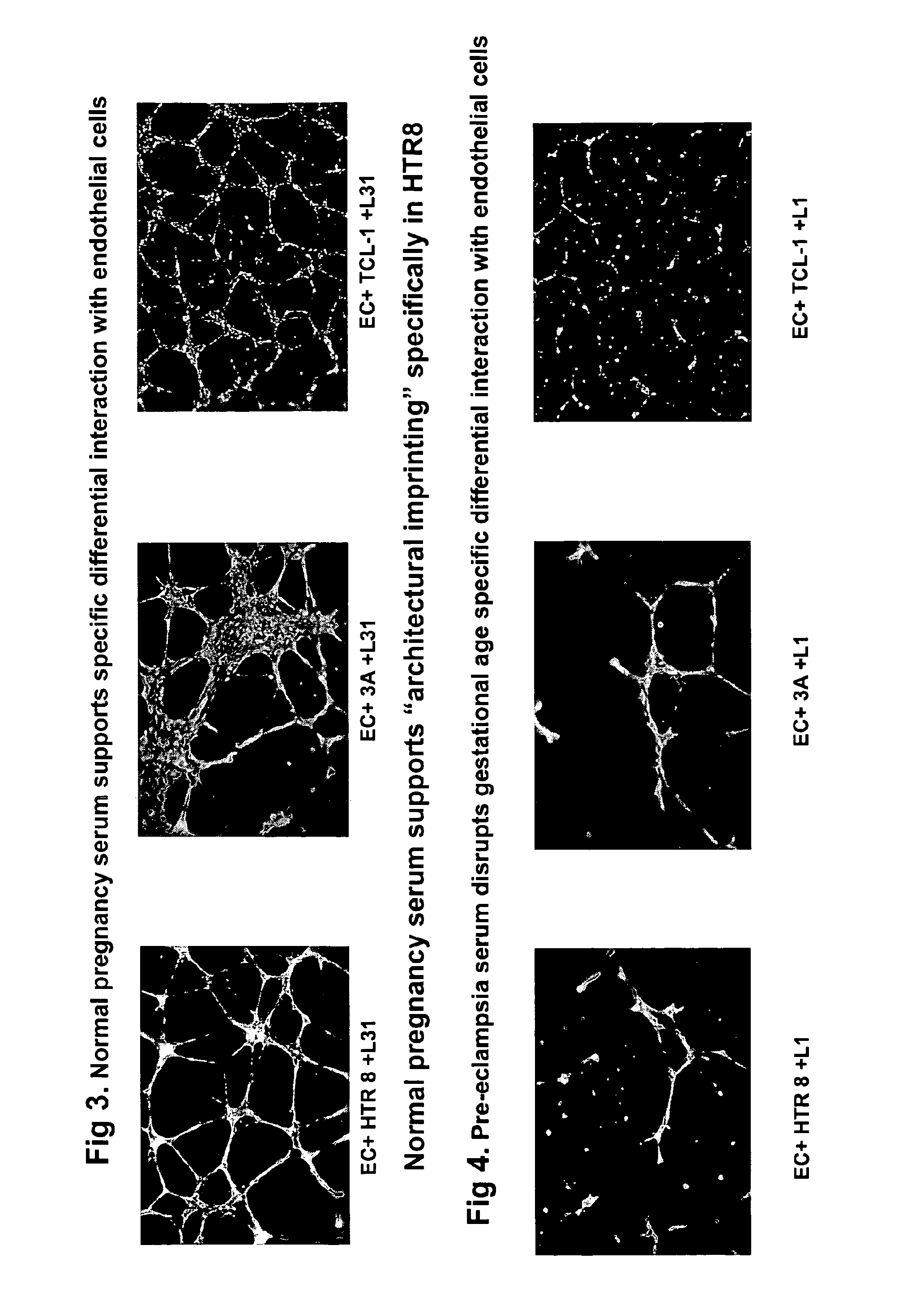
The invention provides serum-based, diagnostic, biological assays for predicting disorders of pregnancy resulting from poor trophoblast and / or placental ischemia, including preeclampsia. Serum samples from such subjects exhibit an ability to disrupt the architecture involving fetal trophoblasts and maternal endothelial cells in a three-dimensional, dual cell co-culture system provided herein, in contrast to normal pregnancy serum samples. Based on these distinctions, the assays are employed to predict pregnancy outcomes as early as first trimester.
Owner:WOMEN & INFANTS HOSPITAL OF RHODE ISLAND
Methods for assessing pregnancy outcome
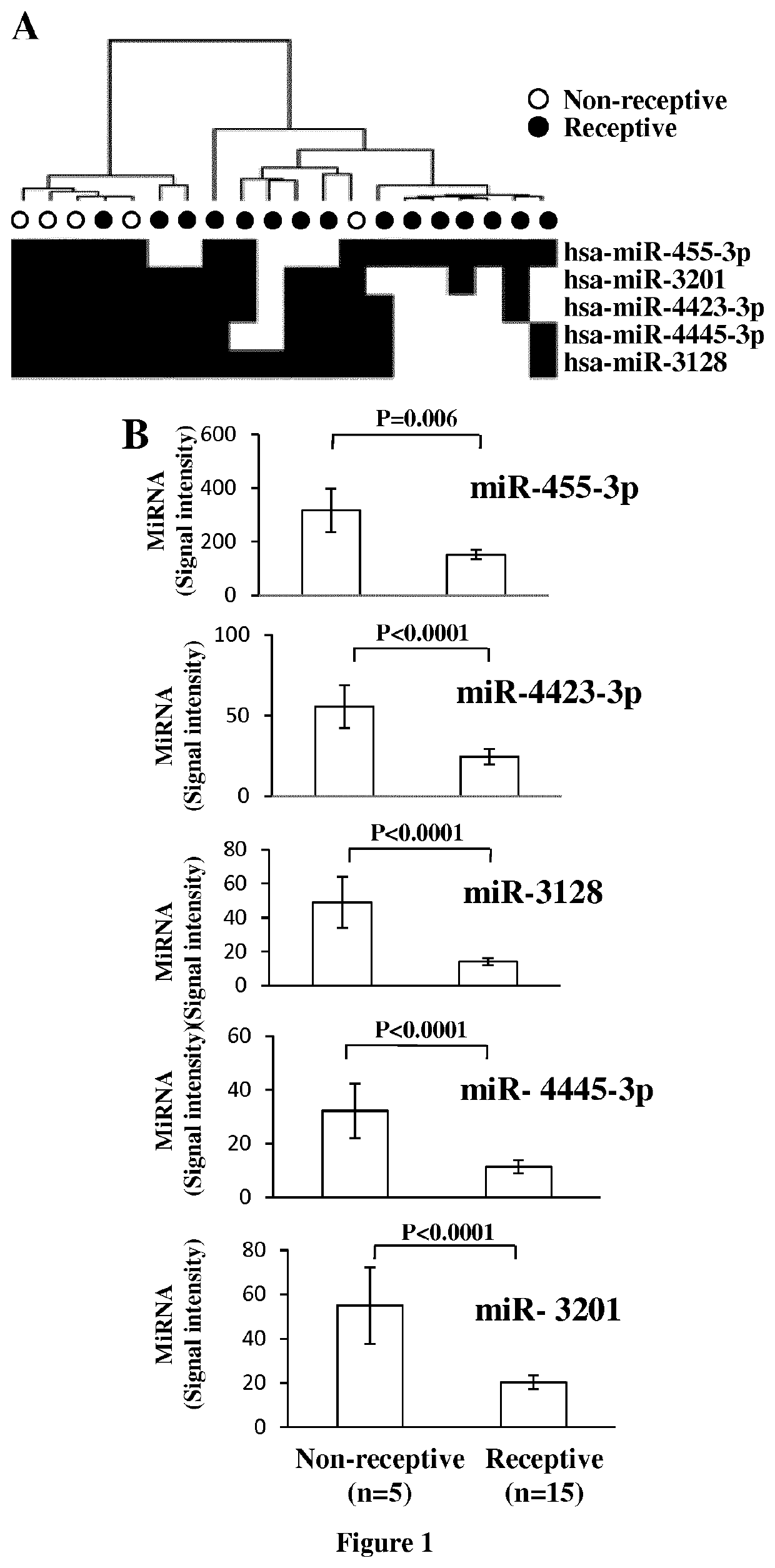
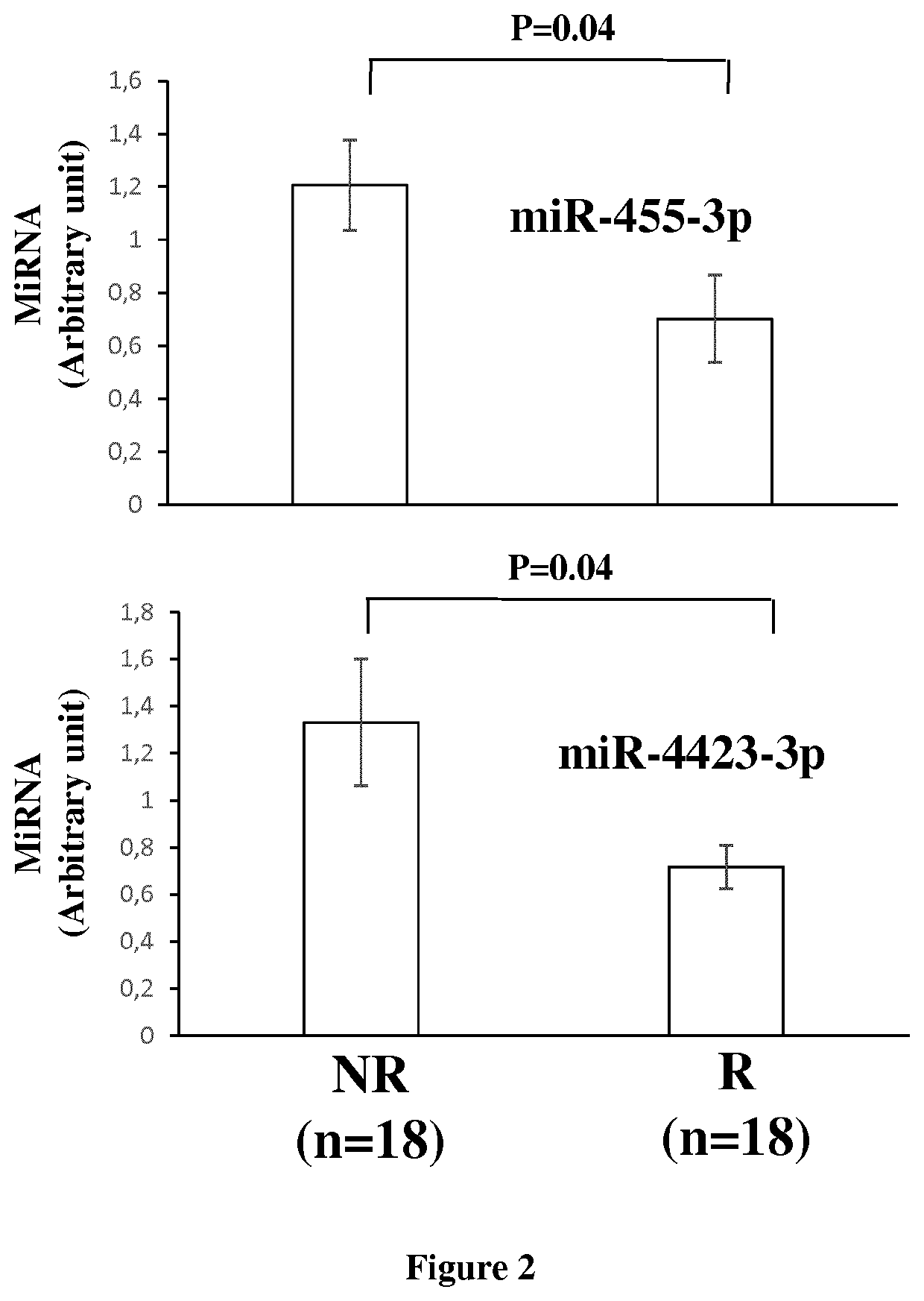
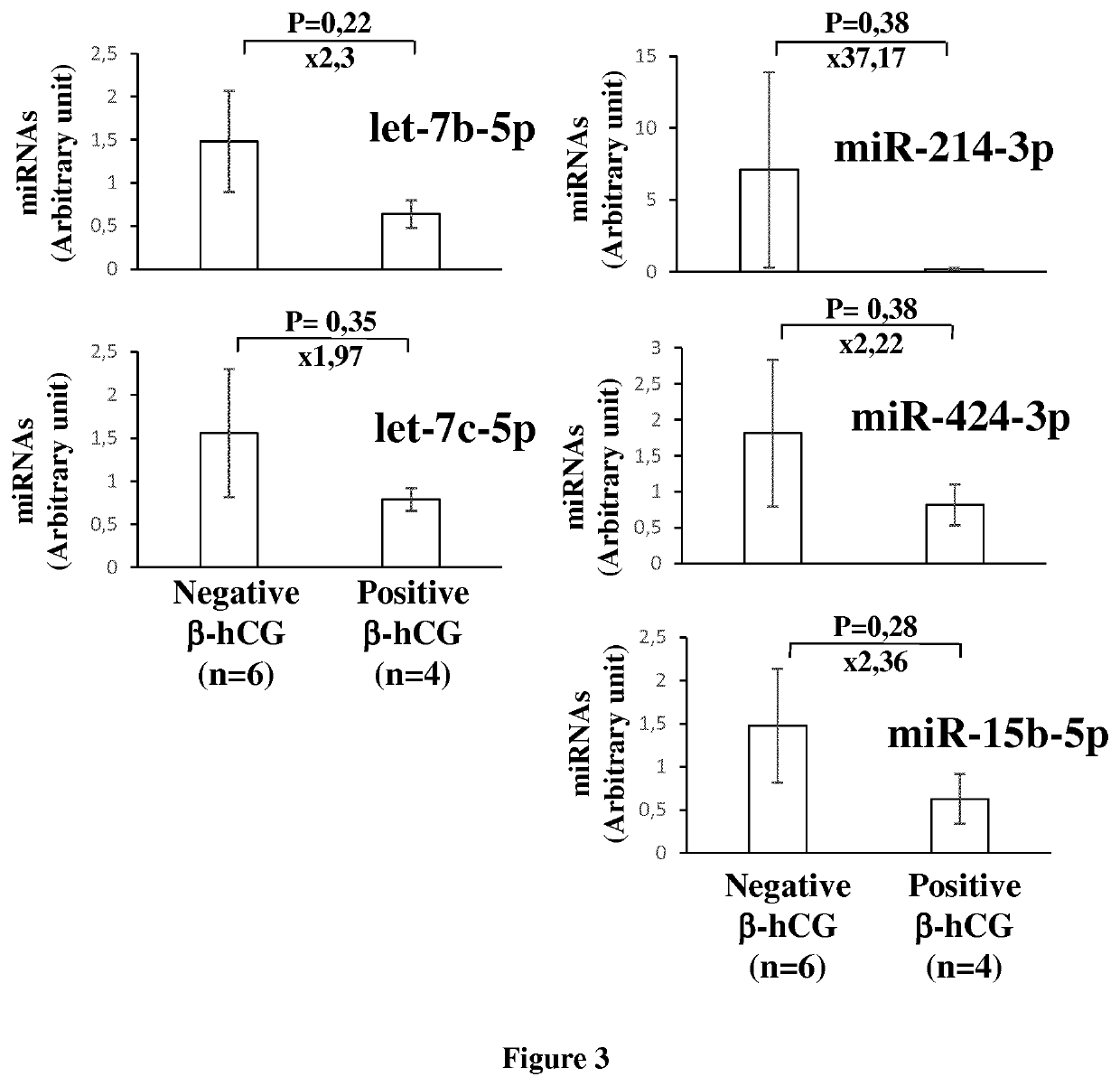
The invention relates to a method of assessing pregnancy outcome. The inventors investigated endometrial miRNAs associated with pregnancy outcome by studying miRNAs associated with endometrial receptivity, implantation failure and embryo miscarriage. They performed a miRNomic study to find miRNAs that are differentially expressed according to the endometrial receptivity status, compared the endometrial miRNome between receptive patients with negative beta-hCG and receptive patients with positive beta-hCG, and compared the endometrial miRNome between receptive patients with a miscarriage between 8-12 weeks of amenorrhoea and receptive patients with a live birth. They demonstrated miRNA differential expression in endometrial samples according to the pregnancy outcome. Thus, the invention relates to a method of assessing pregnancy outcome of a patient, comprising a step of measuring in a biological sample obtained from said patient the expression level of at least one miRNA selected from the group consisting of miR-455-3p, miR-4423-3p, miR-4445-3p, miR-3128, miR-3201, let-7b-5p, let-7c-5p, miR-4534, miR-214-3p, miR-15b-5p, miR-424-3p, miR-181a-5p, miR-574-3p, miR-92a-3p, miR-320c, let-7d-5p, miR-125a-5p, miR-320a, miR-320b and let-7f-5p.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +2
Detecting bacterial taxa for predicting adverse pregnancy outcomes
ActiveUS20170114396A1Improve stabilityIncreased riskMicrobiological testing/measurementDNA/RNA fragmentationCervical shorteningObstetrics
It provides a method for predicting the risk of an adverse pregnancy or neonatal outcome for a pregnant subject by detecting the elevated level of bacteria from one or more selected bacterial taxa (e.g., genera or species). A kit useful for such a method is also provided. In addition, it provides a method for determining the risk of having advanced cervical dilation and / or premature cervical shortening based on differentially abundant bacterial taxa.
Owner:THE CHINESE UNIVERSITY OF HONG KONG
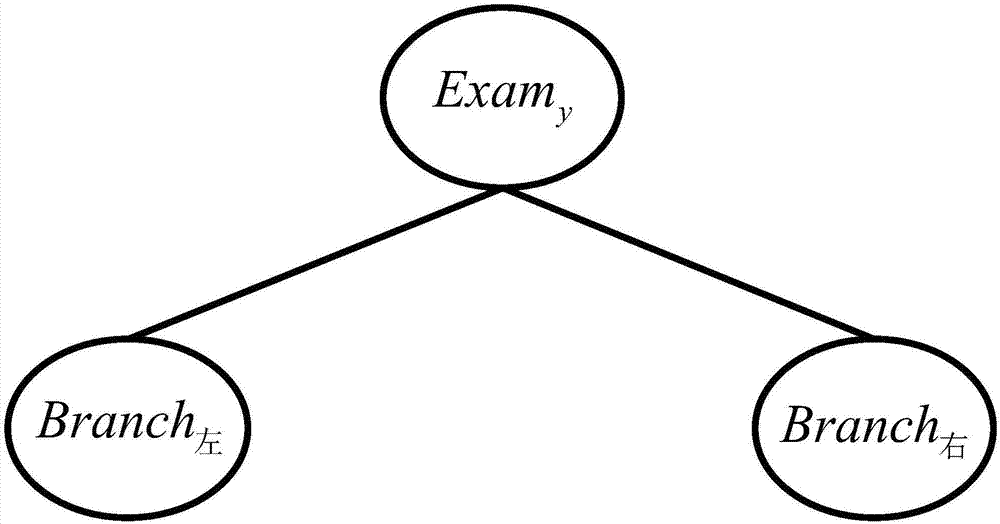
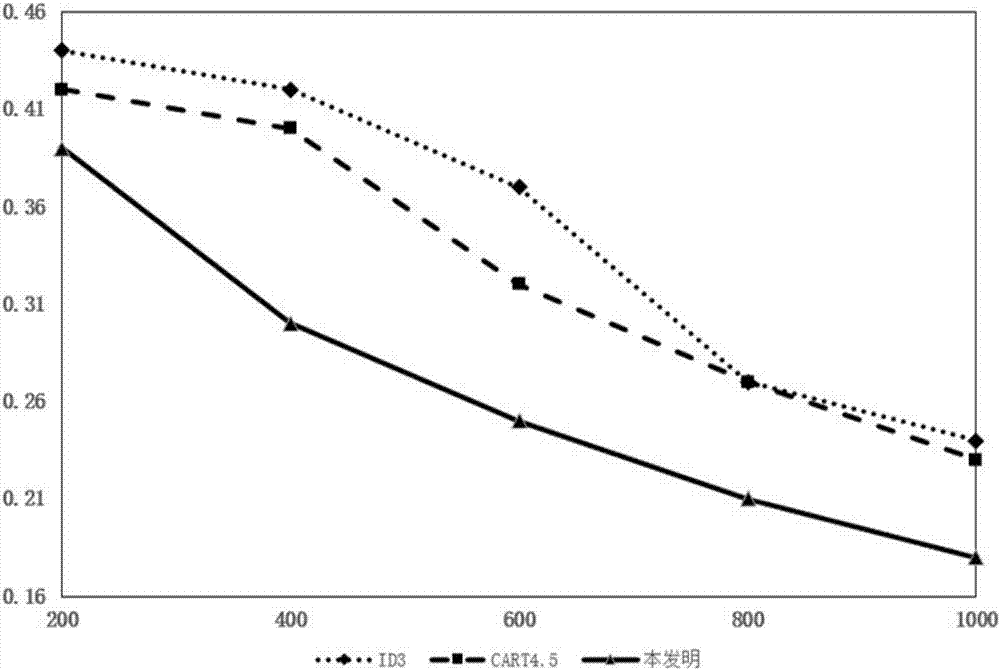
Pregnancy outcome influence factor assessment method based on relative risk decision-making tree model
ActiveCN107491656AImprove performanceImprove assessment accuracySpecial data processing applicationsNODALRelationship - Father
The invention discloses a pregnancy outcome influence factor assessment method based on a relative risk decision-making tree model. The method comprises the steps that binary digital processing is conducted on data in a national free pre-pregnant eugenic health examination item information system, then a Pg exposure value of a pre-pregnant eugenic health examination-reproductive age population exposure value multi-dimensional input matrix is obtained through establishment, and a relative risk vector RR suitable for space-time multi-dimensional condition is established according to the Pg exposure value; a pre-pregnant eugenic health examination item Examy corresponding to the maximum relative risk in the RR is selected and serves as an empty father node of the relative risk decision-making tree model TR; blade node risk factor risk serves as an empty blade node of the relative risk decision-making tree model TR. The method is applied to pregnancy outcome influence factor assessment, the assessment accuracy of pregnancy outcome influence factors and their risk factors are effectively improved, the utilization value of pre-pregnant eugenic health examination data to smart city building is improved, and the method has the important significance on promotion of social harmonious and sustainable development.
Owner:BEIHANG UNIV +1
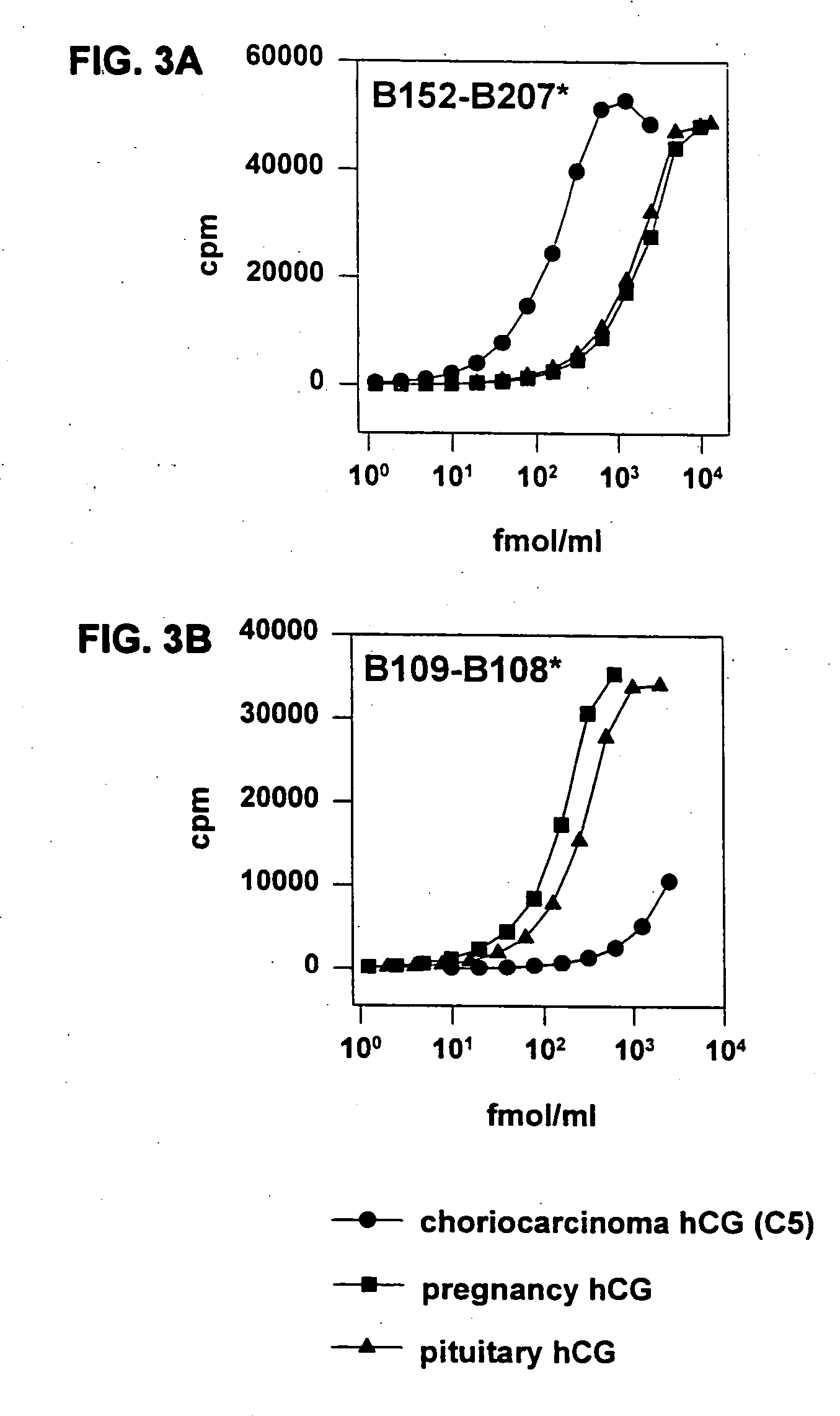
Methods for predicting pregnancy outcome in a subject by hCG assay
The present invention provides a method of predicting pregnancy outcome in a subject by determining the amount of an early pregnancy associated molecular isoform of hCG in a sample. The present invention further provides a method for determining the amount of early pregnancy associated molecular isoforms of human chorionic gonadotropin (hCG) in a sample. The present invention also provides a diagnostic kit for determining the amount of early pregnancy associated hCG in a sample. The present invention additionally provides an antibody which specifically binds to an early pregnancy associated molecular isoform of human chorionic gonadotropin. Finally, the present invention provides methods for detecting trophoblast or non-trophoblast malignancy in a sample.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
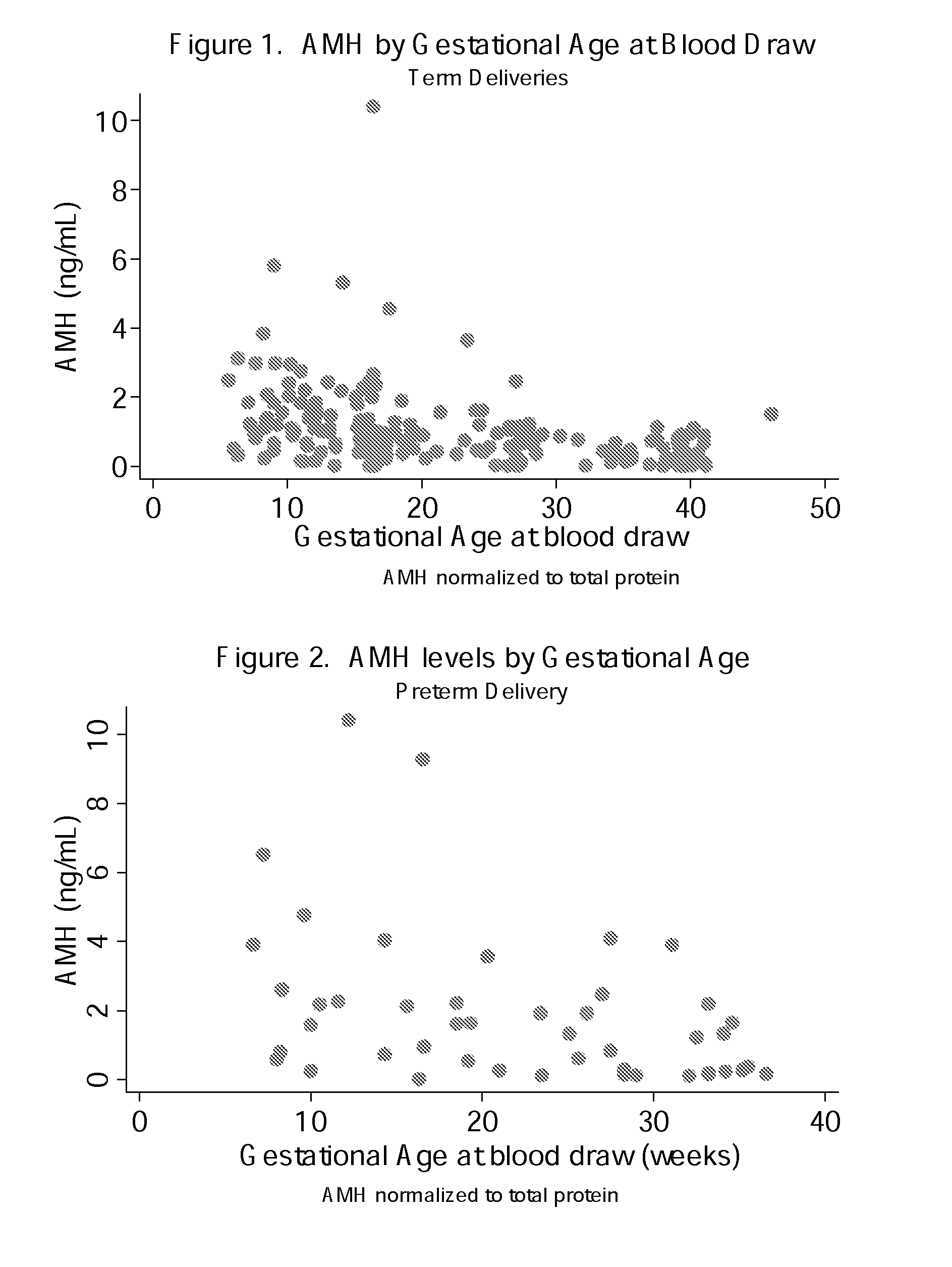
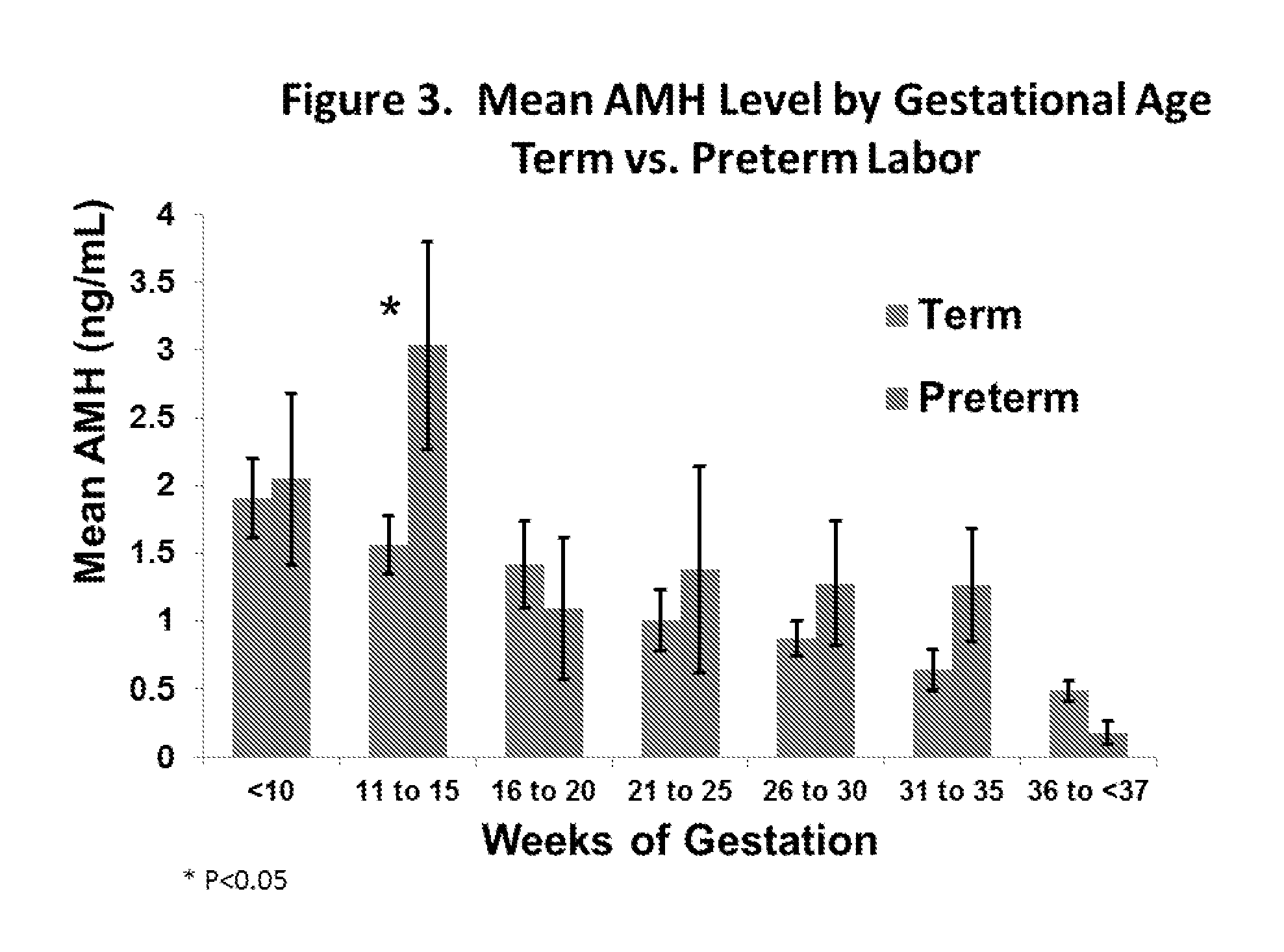
Anti-mullerian hormone changes in pregnancy and prediction of adverse pregnancy outcomes and gender
The present invention provides for methods for evaluating the risk of an adverse pregnancy outcome in a subject and methods for treating subjects evaluated as being high risk. In some aspects, the present invention provides a method of evaluating the risk of an adverse pregnancy outcome in a subject, where if the subject does have an abnormal level of AMH as compared to a predetermined normal level the subject is more likely to have an adverse pregnancy outcome, and if the subject does not have an abnormal level of AMH the subject is less likely to have an adverse pregnancy outcome. In other aspects, the present invention provides a method of determining the gender of a fetus comprising obtaining information regarding the level of AMH in a sample from a pregnant subject.
Owner:UNIV OF IOWA RES FOUND
Exosome-associated microRNA as a diagnostic marker
InactiveCN103276062AMicrobiological testing/measurementApparatus for meter-controlled dispensingGynecologyPregnancy outcomes
Owner:UNIV OF LOUISVILLE RES FOUND INC
Methods of predicting pre term birth from preeclampsia using metabolic and protein biomarkers
PendingUS20210033619A1Reduce riskImprove accuracyHealth-index calculationDisease diagnosisObstetricsMetabolite
A computer implemented method of early prediction of risk of a pregnancy outcome in a pregnant woman, comprising the steps of: inputting into a computational model values for a panel of a plurality of preeclampsia specific biomarkers comprising at least one metabolite, and optionally at least one protein or clinical risk factor, selected from Table 1, in which the values are obtained from the pregnant woman early in pregnancy; selecting a subset of inputted values comprising a value for at least one metabolite and optionally at least one protein or clinical risk factor value, based on a selected pregnancy outcome selected from pre-term preeclampsia, term preeclampsia and all preeclampsia; calculating a predicted risk of the selected pregnancy outcome based on the subset of inputted values; and outputting the predicted risk of the pregnancy outcome for the pregnant woman.
Owner:METABOLOMIC DIAGNOSTICS LEMITED
Serum-based, diagnostic, biological assay to predict pregnancy disorders
ActiveUS9176120B2Simple, non-invasiveCost effectiveMicrobiological testing/measurementDisease diagnosisPhysiologyTrophoblast
The invention provides serum-based, diagnostic, biological assays for predicting disorders of pregnancy resulting from poor trophoblast and / or placental ischemia, including preeclampsia. Serum samples from such subjects exhibit an ability to disrupt the architecture involving fetal trophoblasts and maternal endothelial cells in a three-dimensional, dual cell co-culture system provided herein, in contrast to normal pregnancy serum samples. Based on these distinctions, the assays are employed to predict pregnancy outcomes as early as first trimester.
Owner:WOMEN & INFANTS HOSPITAL OF RHODE ISLAND
Devices for pregnancy detection and corresponding methods thereof
There is disclosed a device for pregnancy detection is provided, the device comprising: a central main body comprising a first end and a second end; a pregnancy detection element that is mounted at an edge of the second opening, and an upper longitudinal crease and a lower longitudinal crease, wherein the device is opened by applying simultaneous pressure to the upper longitudinal crease and the lower longitudinal crease, wherein, when in operation, the first opening of the first end is configured to receive urine from a female user while the female user is in a standing position and enables the urine to pass through the pregnancy detection element, and wherein, when the urine comes in contact with the pregnancy detection element, the pregnancy detection element immunologically detects human chorionic gonadotropin (hCG) present in the urine of the female user and indicates positive pregnancy result if the female user is pregnant.
Owner:INSTLOO PTE LTD
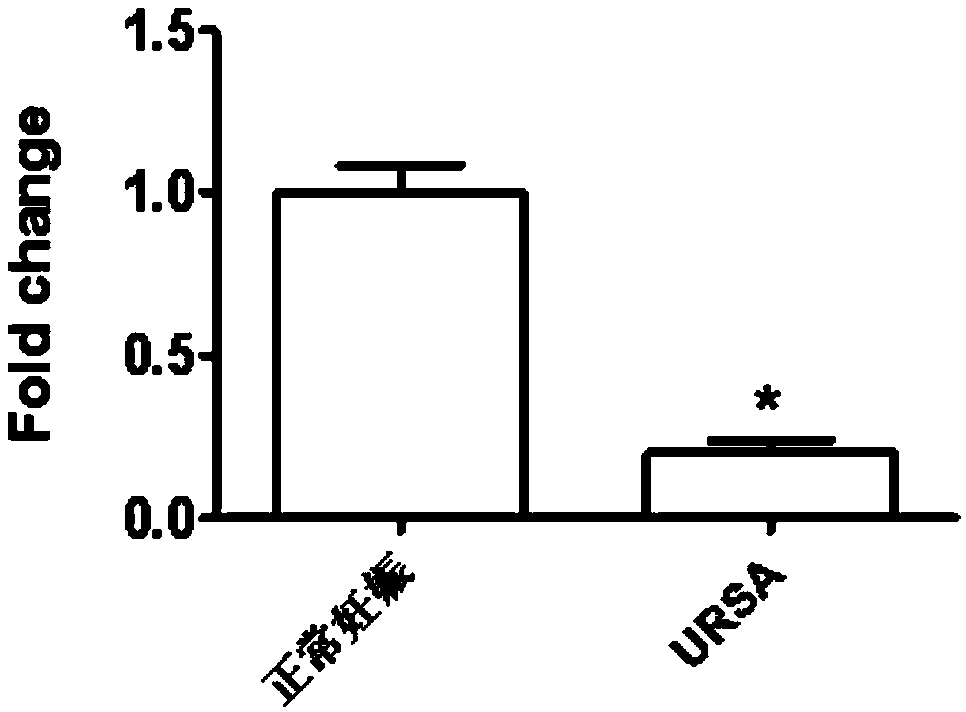
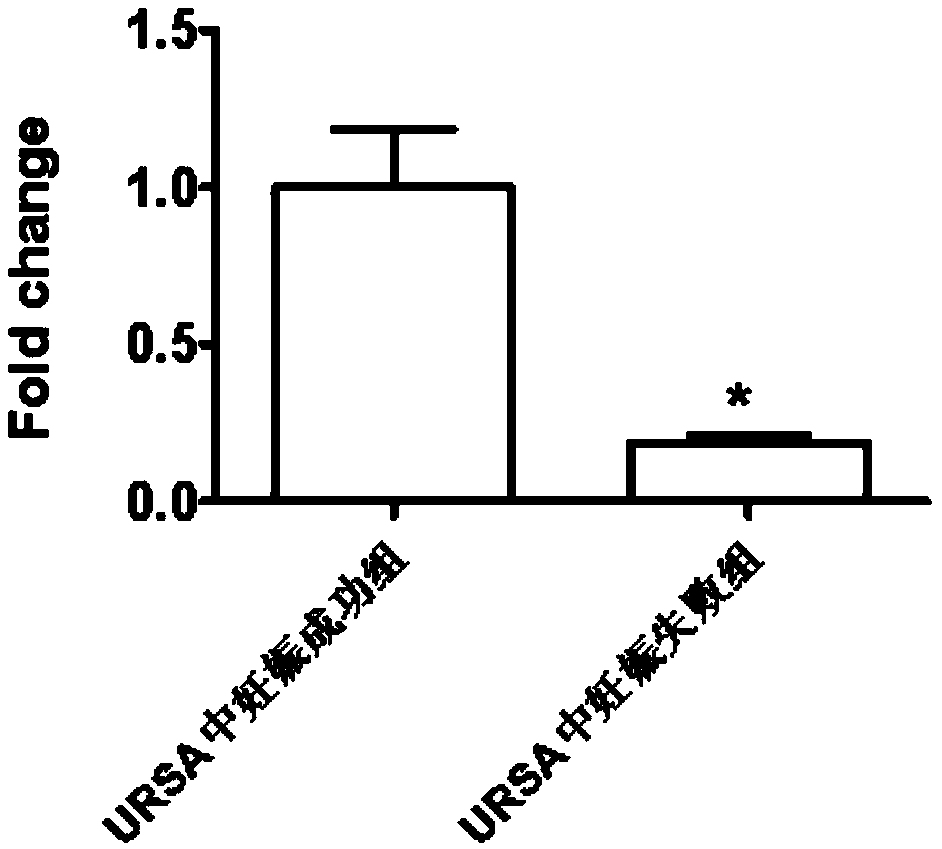
Application of LncRNA in serum as marker for URSA (unexplained recurrent spontaneous abortion) diagnosis and pregnancy outcome evaluation
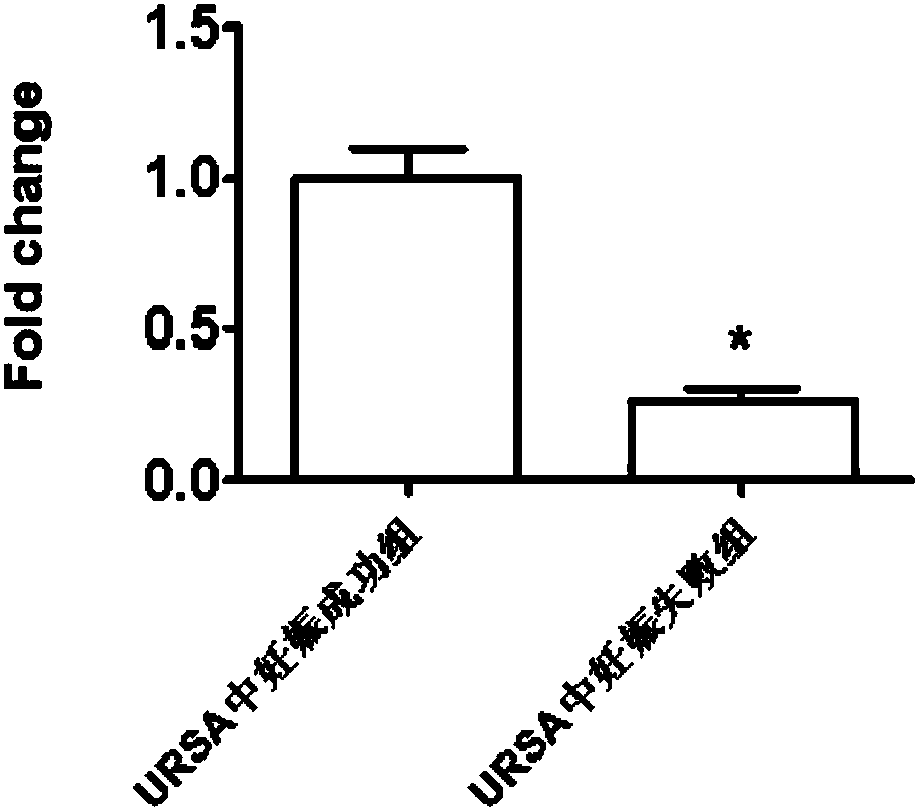
ActiveCN108707659AImprove diagnostic efficiencyEasy to detectMicrobiological testing/measurementDNA/RNA fragmentationPregnancy outcomesLegally induced abortion
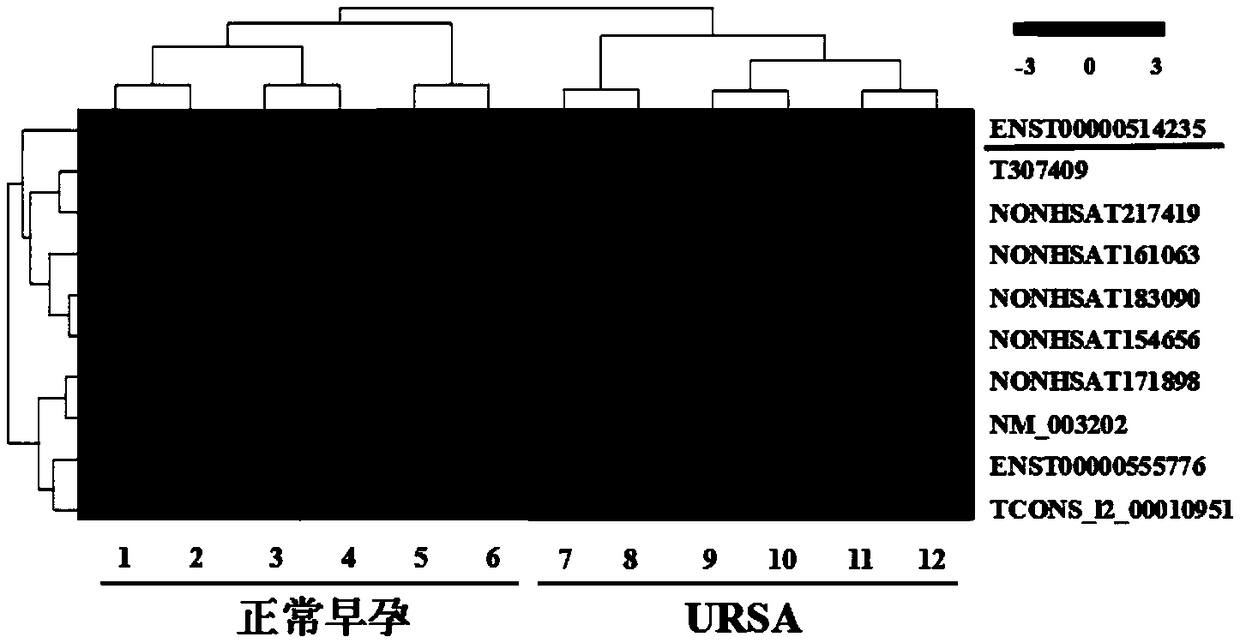
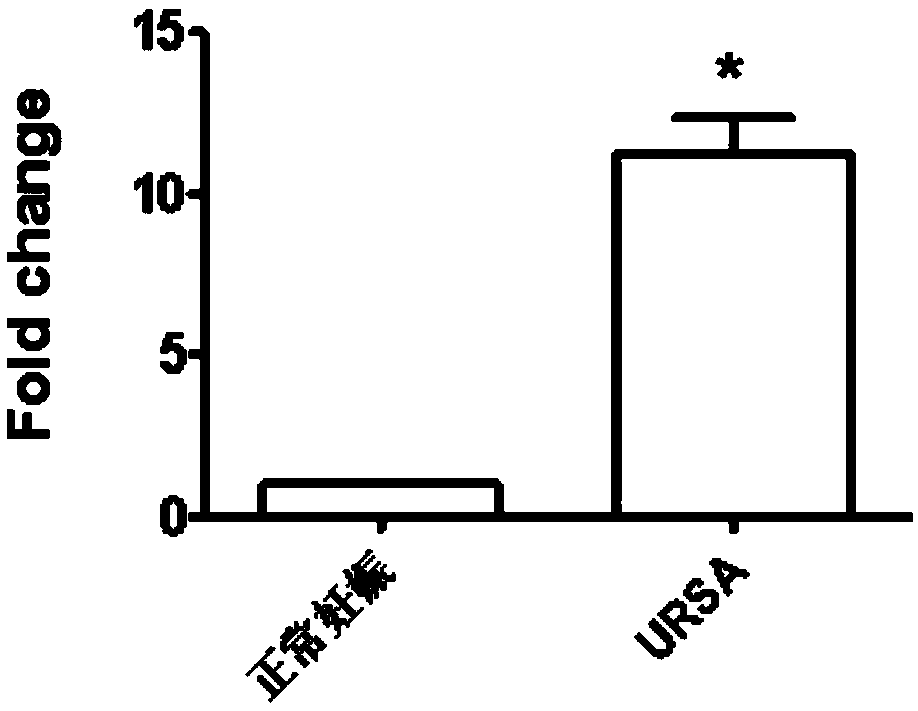
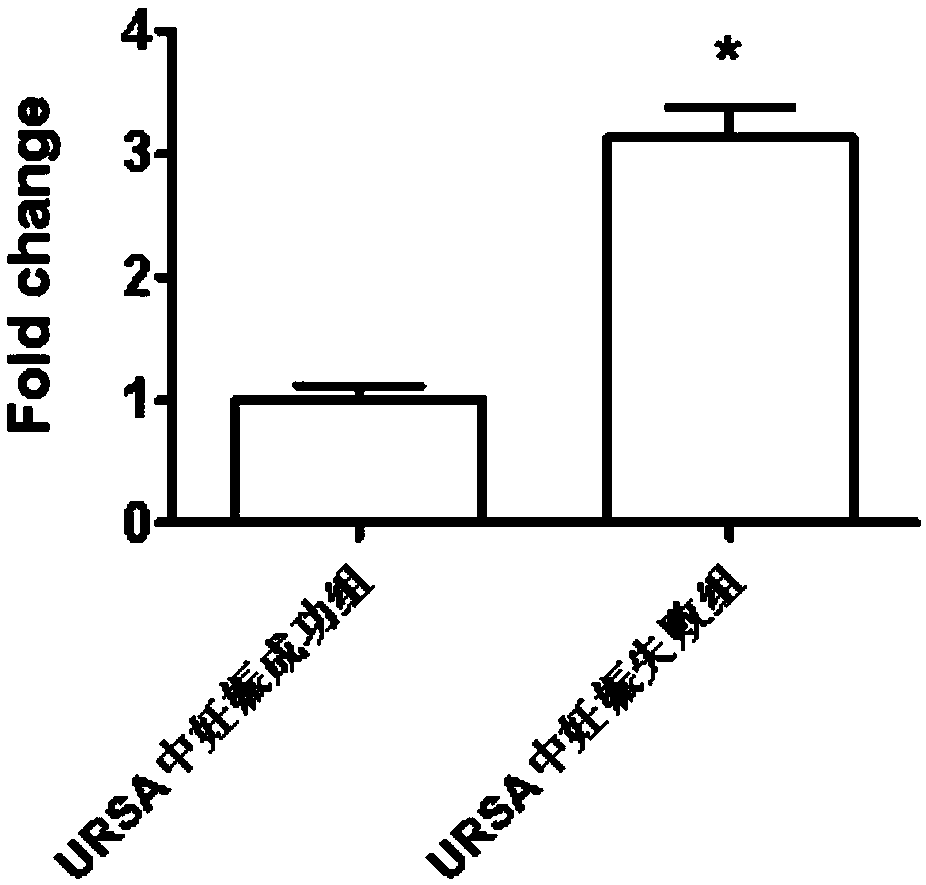
The invention discloses application of LncRNA ENST00000514235 in serum as a marker for URSA (unexplained recurrent spontaneous abortion) diagnosis and pregnancy outcome evaluation. A corresponding detection kit is developed by taking the LncRNA ENST00000514235 as the marker for URSA diagnosis and pregnancy outcome evaluation. The detection kit has the advantages of high detection sensitivity, highspecificity, convenience in detection and capability of meeting diagnosis detection requirements of patients suffering from URSA, and clinical verification shows that the diagnosis accuracy rate is high.
Owner:SHANDONG UNIV OF TRADITIONAL CHINESE MEDICINE +1
Method of treatment and prognosis
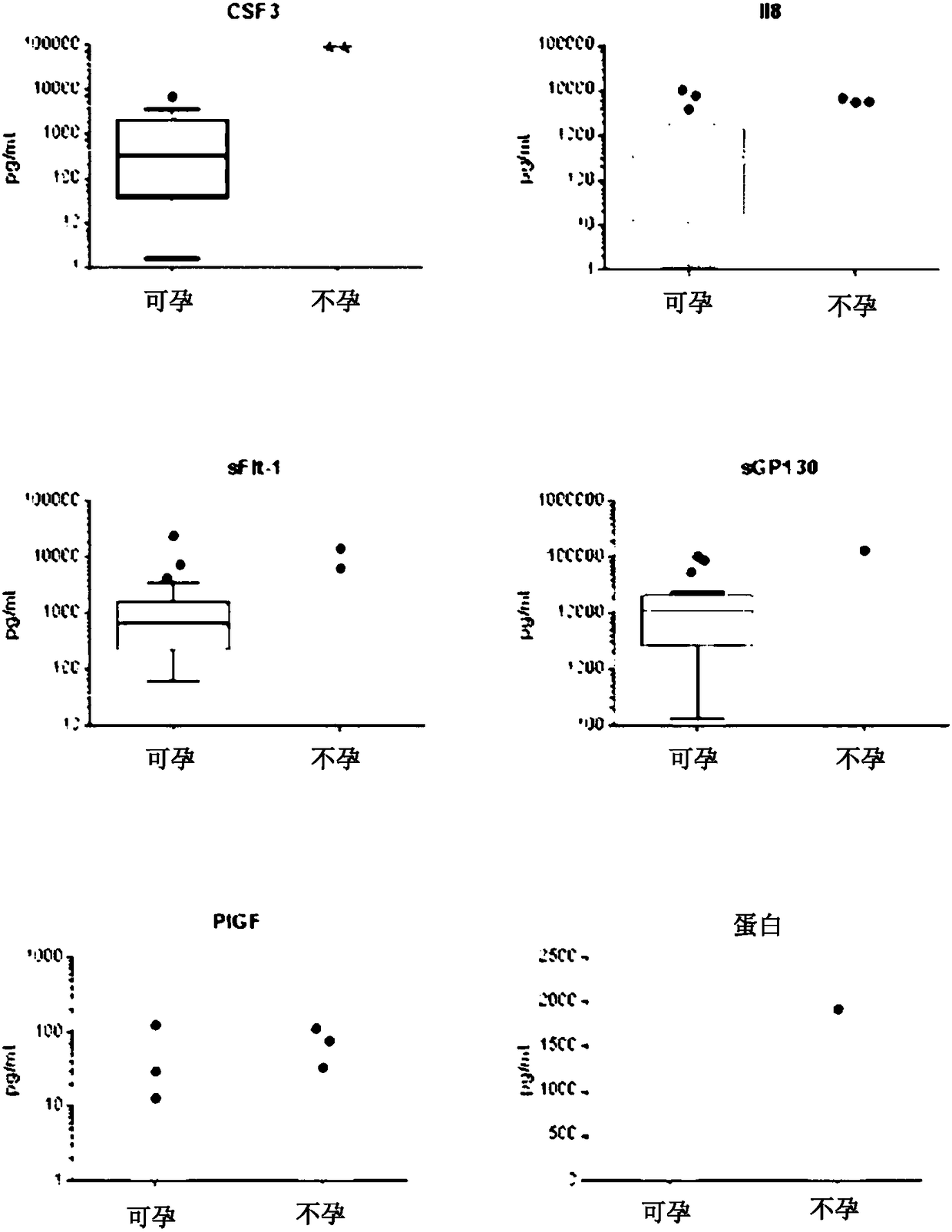
The present disclosure teaches an assay to determine the likelihood of a successful implantation of an embryo into a female subject leading to a pregnancy and a method of treatment to facilitate same.Enabled herein is an improved assisted reproduction technology protocol based on a prognostic evaluation of pregnancy outcomes and the identification of therapeutic targets. Taught herein is a composition comprising reagents required for prognostic evaluation and treatment. The present disclosure teaches an assay to determine the risk that a pregnant woman may undergo a miscarriage. Biomarkers used in the assays include CSF3, CSF3R, IL-17A or P1GF.
Owner:HUDSON INST OF MEDICAL RES
Application of circ-0079591 in serum as marker for URSA diagnosis and pregnancy outcome assessment
ActiveCN108424963AImprove diagnostic efficiencyEasy to detectMicrobiological testing/measurementDNA/RNA fragmentationOutcome assessmentPregnancy outcomes
The invention discloses application of circ-0079591 in serum as a marker for URSA diagnosis and pregnancy outcome assessment, and develops a corresponding detection kit by using the circ-0079591 as the marker for URSA diagnosis and pregnancy outcome assessment, wherein the kit has advantages of high detection sensitivity, high specificity and convenient detection, meets the detection needs of patients requiring URSA diagnosis, and has high diagnosis accuracy through clinical verification.
Owner:SHANDONG UNIV OF TRADITIONAL CHINESE MEDICINE +1
Traditional Chinese medicine health management system and method for women
InactiveCN108335752AHigh precisionRealize real-time monitoringMedical simulationHealth-index calculationHealth planningProgram planning
The invention relates to the field of health management, in particular to a traditional Chinese medicine pre-pregnancy health management system and method. The system comprises a data acquisition subsystem, a health service subsystem and a data management subsystem, wherein the data acquisition subsystem is used for carrying out data acquisition on user data and transmitting the data to the healthservice subsystem, and the user data comprises age, body temperature and headwords; the health service subsystem analyzes and evaluates the data and formulates health plans and schemes or provides consultation; the data management subsystem is used for storing the collected data, the health plans and the schemes, and is also used for tracking and predicting health conditions of users. The invention provides the traditional Chinese medicine pre-pregnancy health management system and method for women. Through traditional Chinese medicine identification of the pre-pregnancy health conditions ofwomen of childbearing age, the risk of adverse pregnancy outcomes is evaluated, and traditional Chinese medicine pre-pregnancy conditioning schemes are given with pertinence.
Owner:ZHEJIANG CHINESE MEDICAL UNIVERSITY
A multimodality-based device for predicting the outcome of embryonic pregnancy
ActiveCN109544512BImprove classification accuracyImage enhancementImage analysisAnimal scienceMidblastula
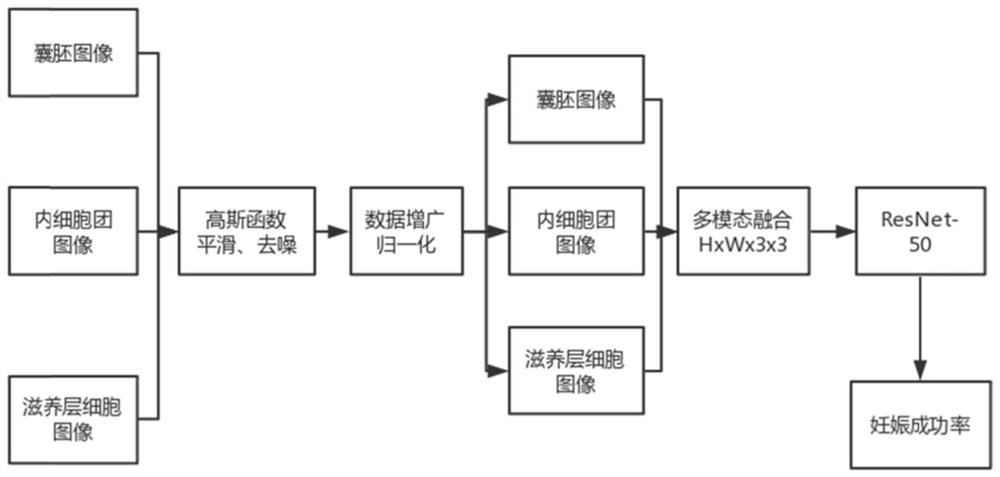
The invention discloses a device for predicting embryo pregnancy results based on multimodality, which belongs to the field of medical artificial intelligence. Firstly, the image of the embryo developed to the blastocyst stage after in vitro fertilization and the corresponding pregnancy result are obtained, and the blastocyst and inner cells of the embryo are obtained. Three images of clumps and trophoblast cells, labeled with pregnancy results, annotated data, as raw data. Then use the Gaussian kernel function to smooth the image, remove part of the noise, and then normalize the image. Afterwards, data augmentation is performed on the image as input data. Image fusion of three images using a multimodal approach such that the input image contains features for the three evaluation aspects. Pass the fused image into ResNet‑50 for training, optimize the network according to the target label, and iterate until the training is complete. After having a model, before embryo transfer, take three images, which can be imported into the model to predict the pregnancy result, and the embryo with a high success rate can be selected according to the output result, which can improve the final pregnancy success rate.
Owner:ZHEJIANG UNIV
Method for fetal urinary system malformation comprehensive evaluation
The invention discloses a method for fetal urinary system malformation comprehensive evaluation. The method specifically comprises the following steps of (1) ultrasound image acquisition and evaluation; (2) interventional sampling; (3) whole genome sequencing and evaluation; (4) umbilical cord blood Cys C, beta 2-MG level determination; (5) comprehensive evaluation. The method for the fetal urinary system malformation comprehensive evaluation has the advantages that an ultrasonic imaging technology is utilized in combination with cord blood puncture DNA high-throughput sequencing, cord blood biochemical criterion CystC and beta 2-MG level determination, the three technologies are combined to comprehensively evaluating the severity degree of abnormal fetal kidney development of the structure, gene and function of the fetal kidney, people are prompted with adverse pregnancy outcome probability or postnatal surgical risk, thereby being conductive to providing complete clinical genetic counseling.
Owner:FIRST PEOPLES HOSPITAL OF YUNNAN PROVINCE
Application of LncRNA in serum as marker to URSA diagnosis and pregnancy outcome evaluation
ActiveCN108753950AImprove diagnostic efficiencyEasy to detectMicrobiological testing/measurementDNA/RNA fragmentationPregnancy outcomesPathology
The invention discloses application of LncRNA NONHSAT167899.1 as a marker URSA diagnosis and pregnancy outcome evaluation and develops a corresponding detection kit by taking LncRNA NONHSAT167899.1 asa marker for diagnosing URSA and evaluating pregnancy outcome. The kit is high in detection sensitivity, high in specificity and convenient to detect; the detection requirements of patients with URSAcan be met; clinical experiments show that the accuracy rate of the diagnosis is high.
Owner:INST OF BASIC MEDICINE OF SAMS +1
Vaginal microbiome markers for prediction of prevention of preterm birth and other adverse pregnancy outcomes
PendingUS20220243247A1High degree of sensitivityStrong specificityMicrobiological testing/measurementDisease diagnosisVaginal microbiomeObstetrics
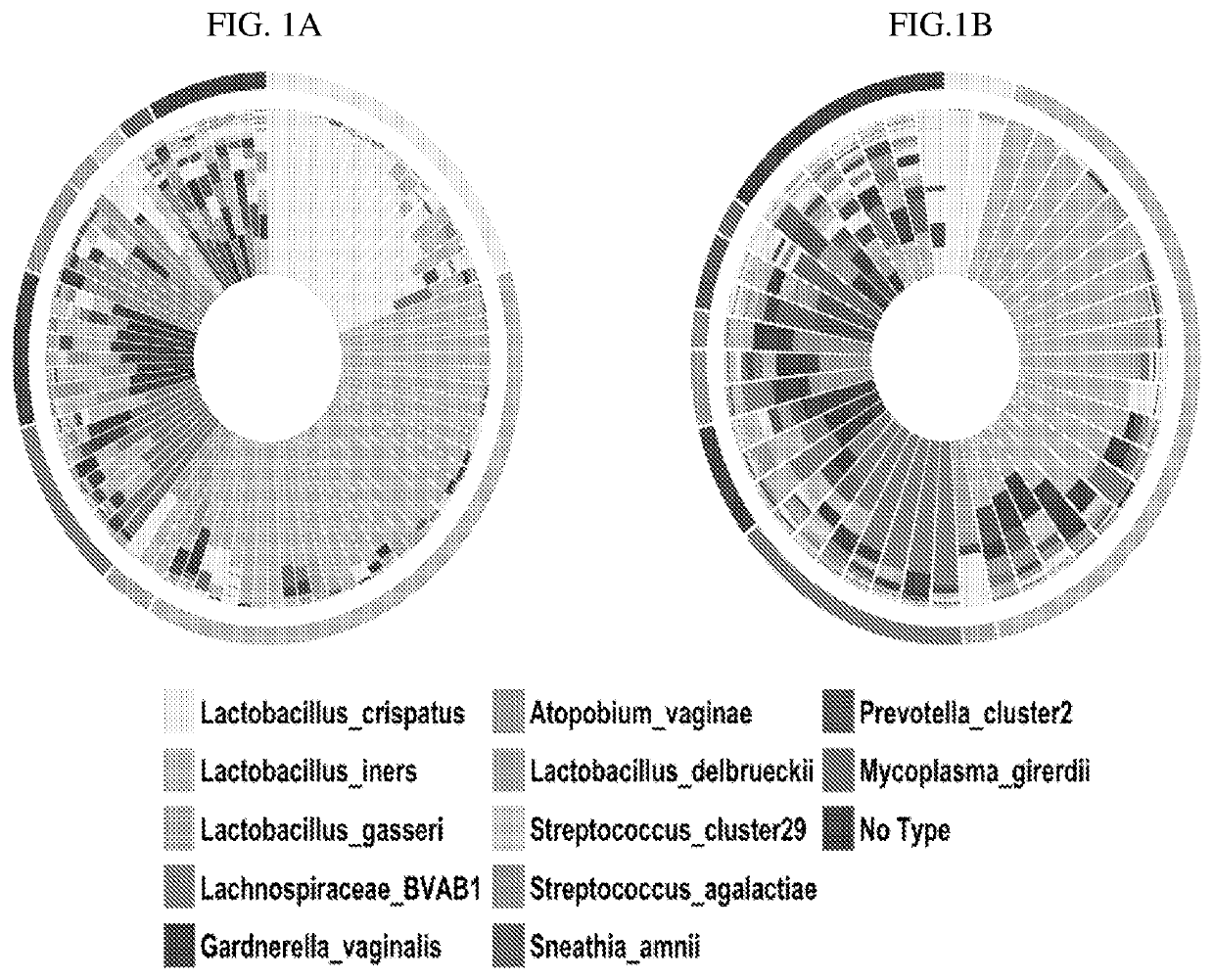
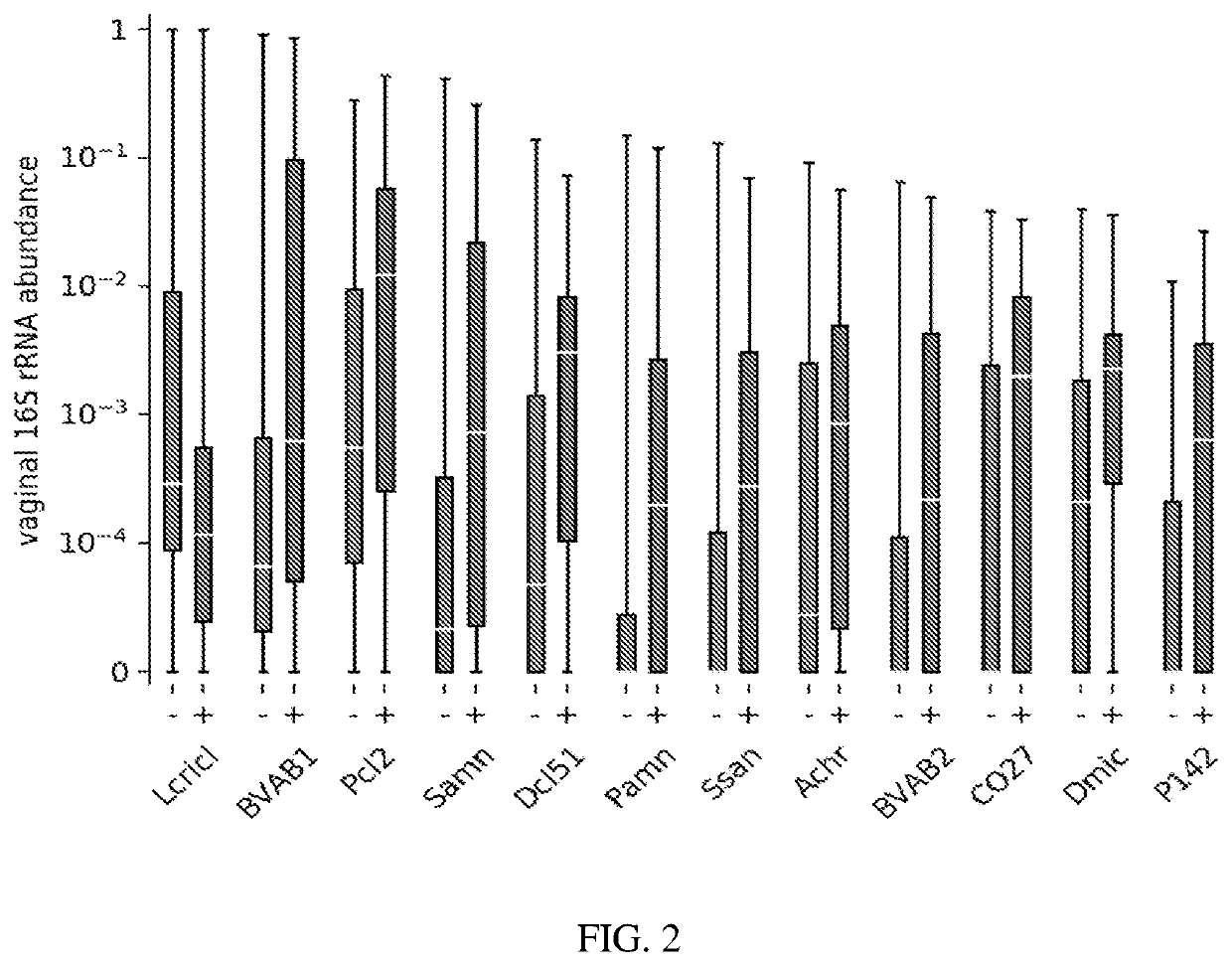
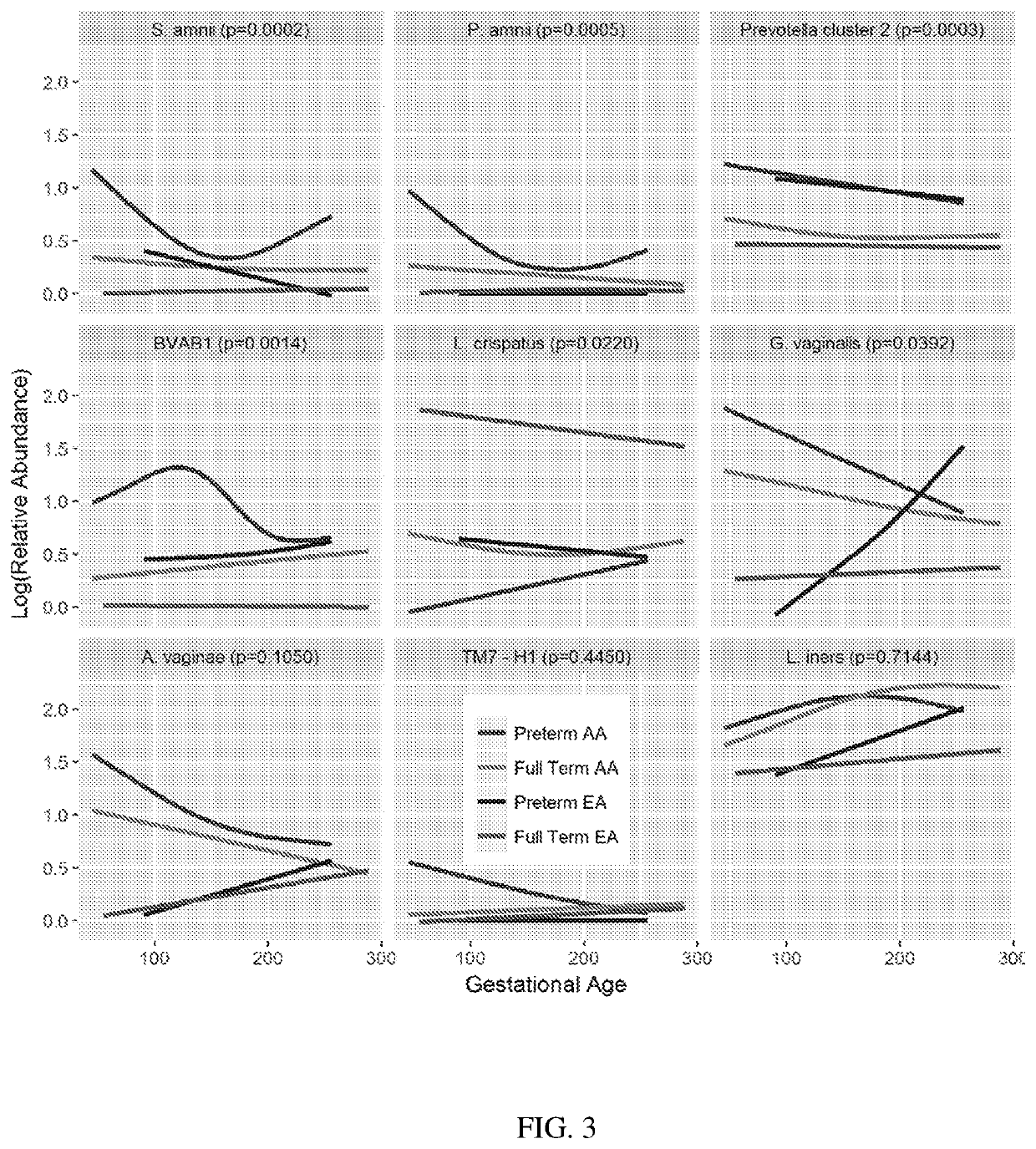
A method for determining the risk of an adverse pregnancy outcome for a woman is provided comprising the steps of measuring a level of TM7-H1 and optionally one or more of BVAB1, Sneathia amnii, and Prevotella cluster 2 in a vaginal sample obtained from the woman, and identifying the woman as having an increased risk for an adverse pregnancy outcome, or other adverse pregnancy outcomes, if the levels are increased compared to corresponding standard control levels. Methods for the prophylactic treatment of subjects identified as being at increased risk for an adverse pregnancy outcome are also provided.
Owner:VIRGINIA COMMONWEALTH UNIV
Serum/Plasma Peptide Markers Related to Aided Early Diagnosis of Gestational Diabetes Mellitus and Its Application
ActiveCN108957011BGuaranteed stabilityFacilitate early diagnosisPeptidesDisease diagnosisPregnancy outcomesNeonatal diabetes
The invention discloses a serum / blood plasma polypeptide marker related to gestational diabetes mellitus auxiliary early diagnosis and application thereof. The serum / blood plasma polypeptide marker ischaracterized in that the marker is selected from arbitrary one or more polypeptides of Q6PFW1, P35658, P32004 and Q9Y4H2. The serum / blood plasma polypeptide marker serving as a detection target is applied to preparation of gestational diabetes mellitus auxiliary early diagnosis reagents. The expression quantity of the marker for detecting pregnancy serum / blood plasma can be used in GDM auxiliaryearly diagnosis. Support is provided to clinical doctors for accurately predicting the GDM pathogenesis outcome of pregnancy and timely adopting personal prevention and treatment schemes, so that theoccurrence rate of GDM can be furthest reduced, and adverse pregnancy outcomes can be reduced.
Owner:NANJING MATERNITY & CHILD HEALTH CARE HOSPITAL
A kind of Lactobacillus gasseri and its application in the preparation of medicines for preventing premature birth
ActiveCN109402002BActive and stable biological propertiesImprove acid production performanceAntibacterial agentsBacteriaAntimicrobial peptidesBacilli
The invention discloses a female vaginal dominant bacterium Lactobacillus gasseri (Lactobacillus gasseri) H87 and its application in the preparation of medicines for preventing premature birth, belonging to the technical field of medical microorganisms. The L. gasseri H87 is a new genus of Lactobacillus gasseri isolated from the human body and has no repelling effect. The specific L. gasseri H87 antimicrobial peptide gassericin maintains the balance of the vaginal flora and has a higher resistance to The ability of vaginal epithelial cells to adhere reduces the risk of inflammation in the genitourinary region of patients, and at the same time reduces the risk of complications during pregnancy for pregnant women, and the strain can be stored for a long time to facilitate production. The lack of Lactobacillus gasseri may have adverse pregnancy outcomes for pregnant women, such as premature birth, and reshape the vaginal microenvironment of women. Therefore, this strain can better meet the actual clinical application needs and provide new ideas for reducing the premature birth rate. .
Owner:NANJING TECH UNIV
Method for predicting birth weight of full-term newborn at 21-23 weeks of pregnancy
PendingCN113855080AImprove pregnancy outcomesOrgan movement/changes detectionCharacter and pattern recognitionObstetricsPhysiology
The invention discloses a method for predicting birth weight of a full-term newborn at 21-23 weeks of pregnancy. The method comprises the following steps: S1, building a newborn weight prediction model that is EFW = 5016.51-205.71*X1+16.30*X2+102.19*X3+86.64*X4, wherein, EFW is the predicted neonatal weight in g; X1 is the accurate gestational week during the ultrasonic examination, and X2 is the body mass index (in kg / m<2>) of a pregnant woman at second trimester B-ultrasonic examination; X3 is the volume of partial thighs of a fetus at ultrasonic examination in cm<3>; and X4 is the abdominal girth (in cm) of the fetus at ultrasonic examination; S2, collecting gestational weeks and progestational body mass indexes of the pregnant woman; S3, carrying out two-dimensional ultrasonic measurement on the abdominal girth of the fetus at 21-23 weeks of pregnancy; S4, carrying out three-dimensional ultrasonic measurement on the volume of partial thighs of the fetus at 21-23 weeks of pregnancy; and S5, substituting data collected in the steps S2-S4 into the newborn weight prediction model, and calculating the predicted weight of the newborn. The method can improve the accuracy of predicting the birth weight of the full-term newborn, and be used for advancing maternal management to the second trimester, thereby scientifically improving pregnancy results.
Owner:NANFANG HOSPITAL OF SOUTHERN MEDICAL UNIV
Noninvasive evaluation method and system for embryo quality in human freeze-thaw blastocyst stage and medium
PendingCN114648488AEliminate differences in scoring methodologiesImage enhancementImage analysisEvaluation resultGynecology
The invention provides an image-based non-invasive evaluation method for embryo quality in a human blastocyst freezing and thawing stage, which comprises the following steps: acquiring a single static image of a thawed blastocyst and a corresponding pregnancy result, and inputting the single static image and the corresponding pregnancy result into a neural network for training to generate an evaluation model; and obtaining a single static image of a to-be-evaluated thawed blastocyst, inputting the single static image into the evaluation model, and obtaining an embryo evaluation result. The invention further relates to an image-based non-invasive evaluation system for the embryo quality in the human freeze-thaw blastocyst stage and a computer readable storage medium.
Owner:福建省妇幼保健院
ADAM12, a novel marker for abnormal cell function
InactiveUS7678544B2Improve the detection rateReduce false alarm rateDepsipeptidesPeptide preparation methodsDiseaseADAM12
Owner:STATENS SERUM INST +2
Method of treatment and prognosis
PendingUS20190204333A1Improve pregnancy success rateAntagonizing activitiesMicrobiological testing/measurementDisease diagnosisGynecologyPregnancy outcomes
The present disclosure teaches an assay to determine the likelihood of a successful implantation of an embryo into a female subject leading to a pregnancy and a method of treatment to facilitate same. Enabled herein is an improved assisted reproduction technology protocol based on a prognostic evaluation of pregnancy outcomes and the identification of therapeutic targets. Taught herein is a composition comprising reagents required for prognostic evaluation and treatment.
Owner:HUDSON INST OF MEDICAL RES
Lipid profiling methods for predicting positive pregnancy outcome
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD +1
Prognostic Assay for Success of Assisted Reproductive Technology
ActiveUS20170115302A1Facilitate endometrial receptivityBetter clinical outcomeMicrobiological testing/measurementDisease diagnosisPregnancy outcomesAssisted fertilization
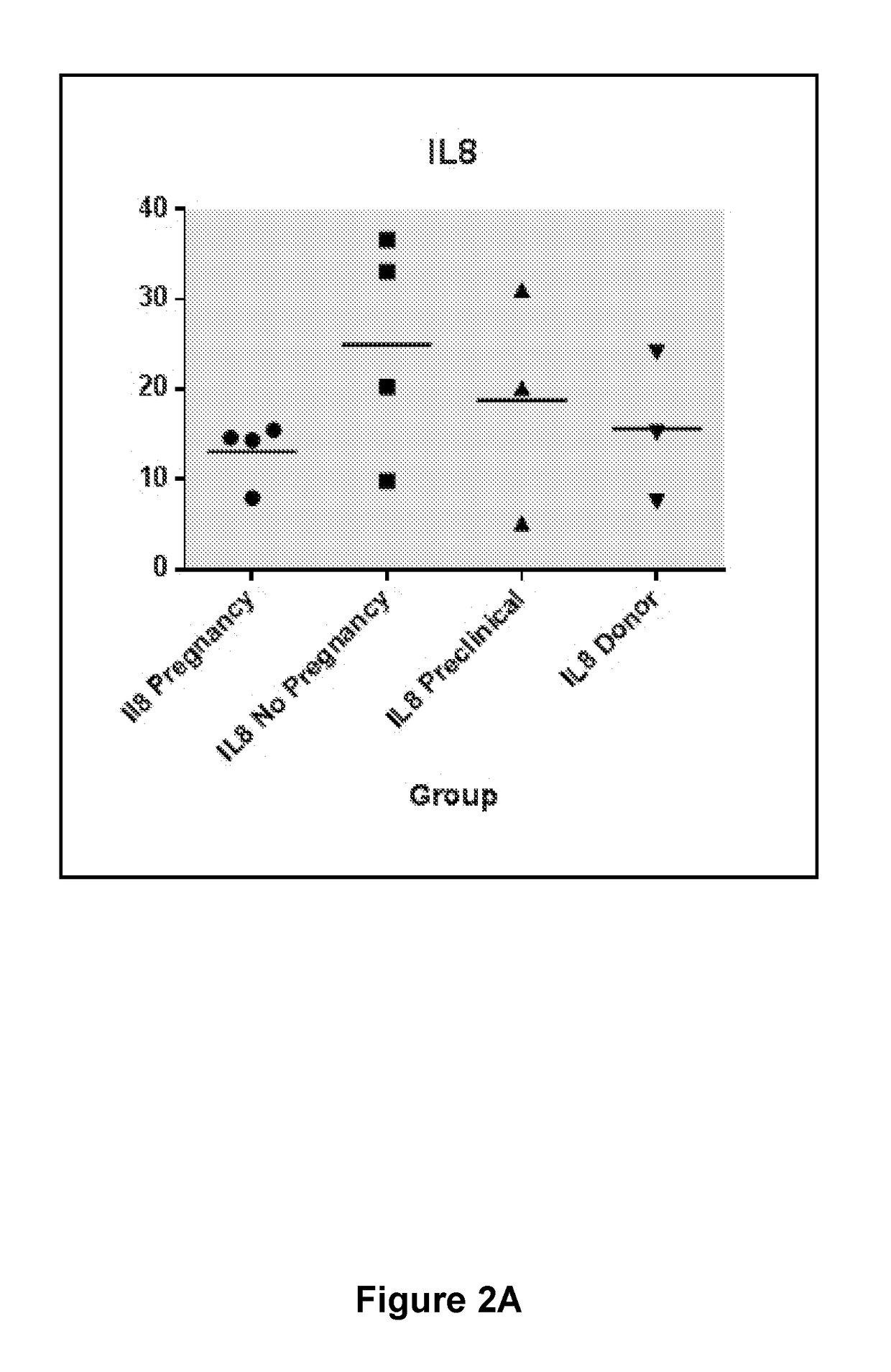
The present disclosure teaches an assay to determine the likelihood of a successful implantation of an embryo into a female subject leading to a pregnancy. Enabled herein is an improved assisted reproduction technology protocol based on a prognostic evaluation of pregnancy outcomes. Taught herein is a composition comprising reagents required for the prognostic evaluation. Taught herein are assays comprising determination of levels of the biomarkers IL-8, G-CSF and / or VEGFA in a biological fluid sample taken before embryo implantation.
Owner:HUDSON INST OF MEDICAL RES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com