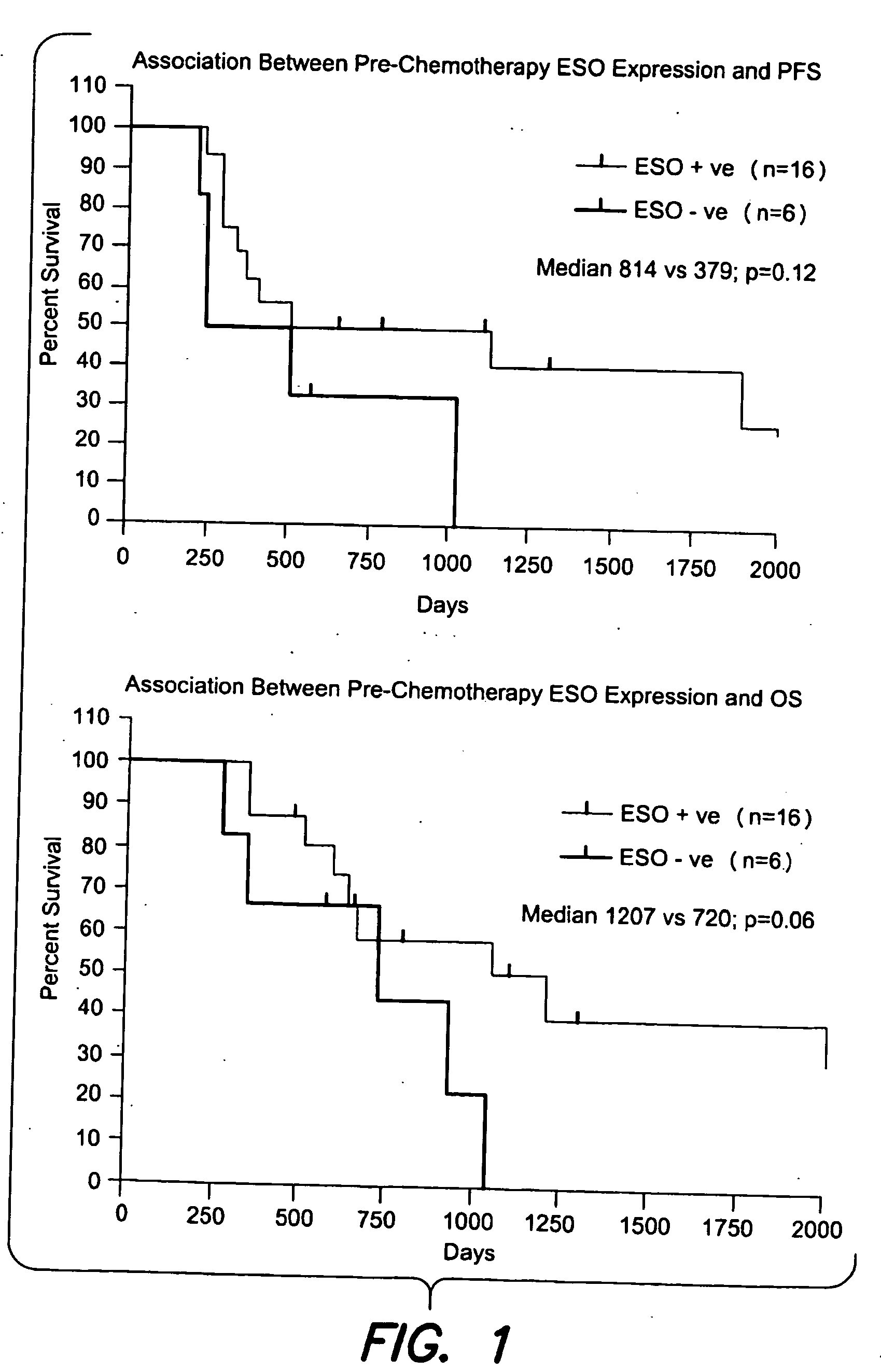
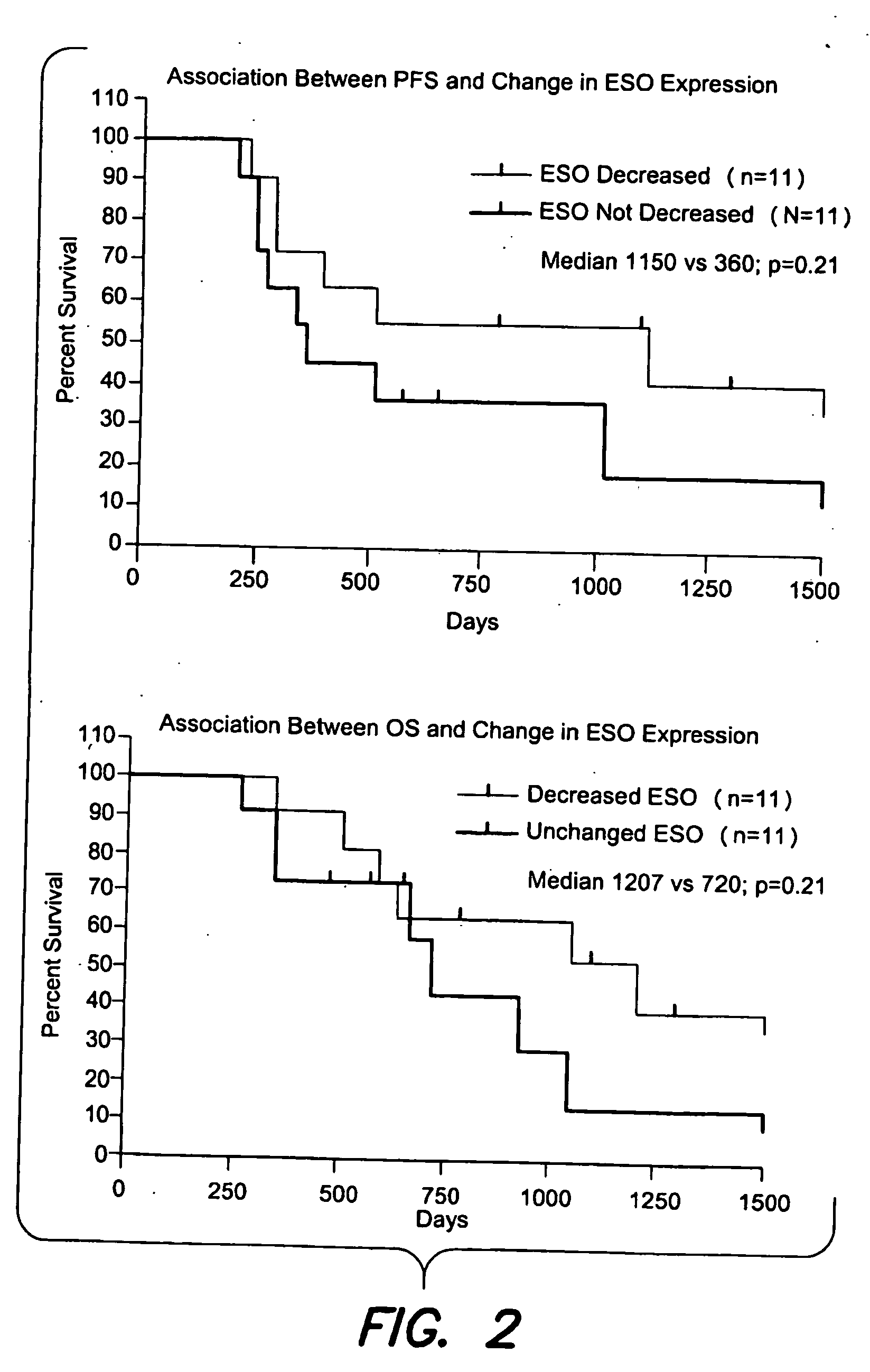
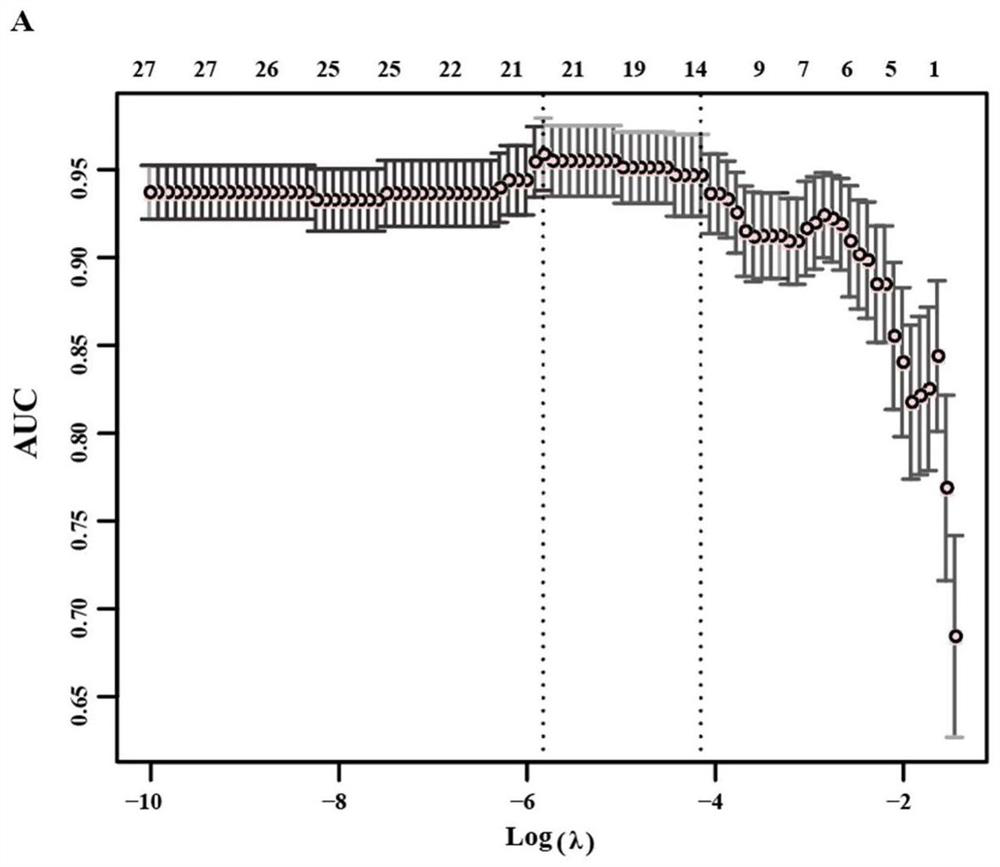
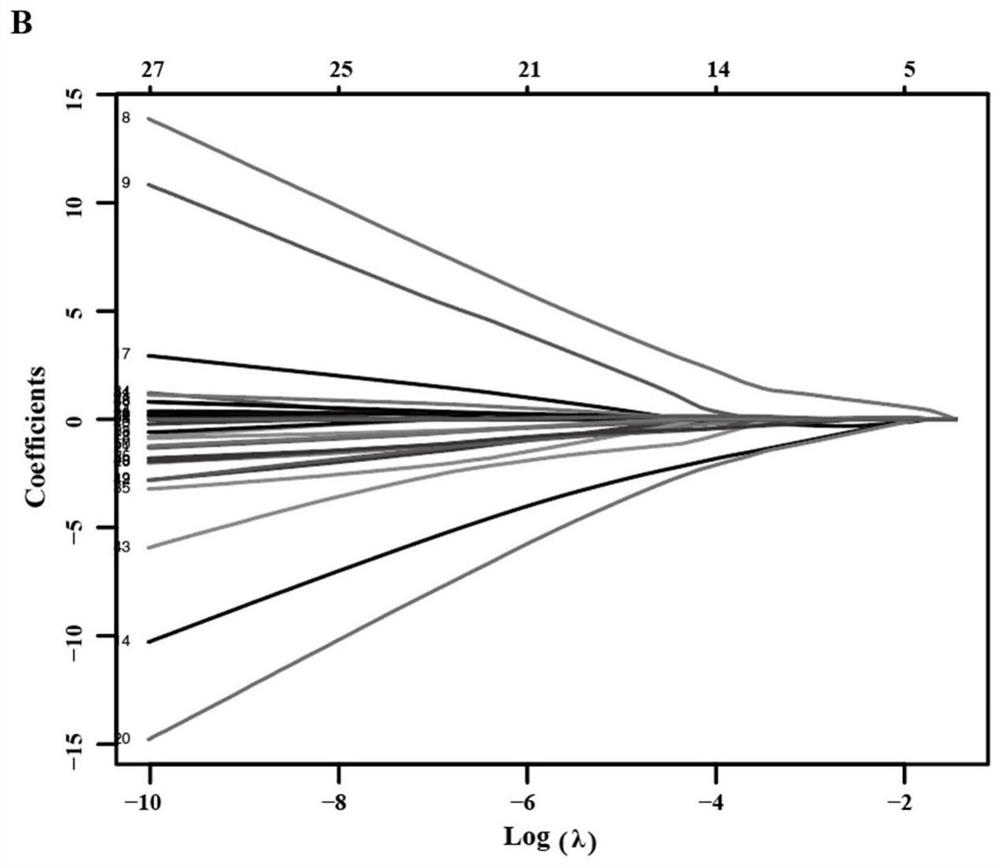
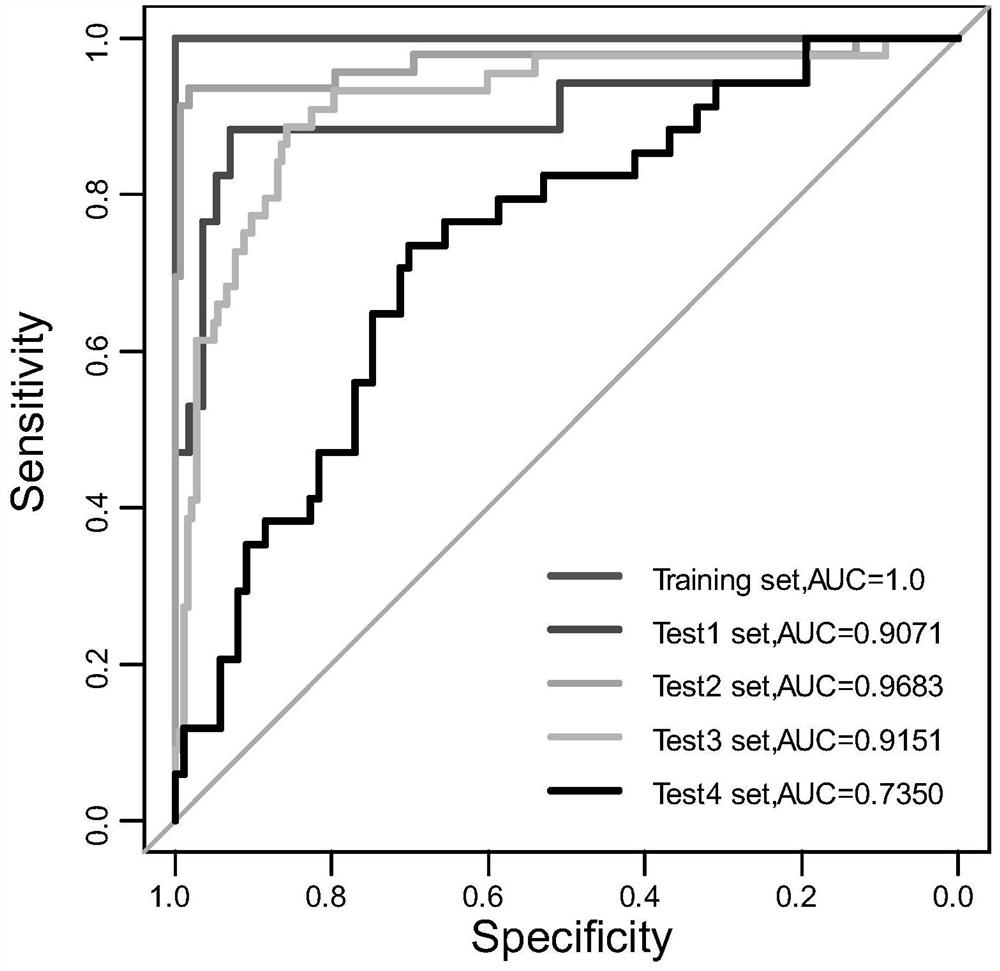
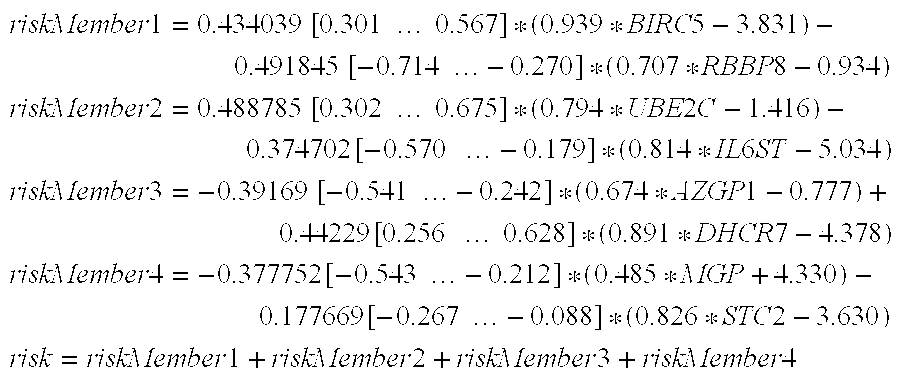Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
41 results about "Neo adjuvant chemotherapy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Neo-adjuvant chemotherapy. Neo-adjuvant chemotherapy (sometimes called preoperative chemotherapy), is also given in addition to other treatments, but it is usually given before surgery in order to shrink the tumor and make it easier to remove or treat with radiation.
Cancer testis antigens as biomarkers in non-small cell lung cancer
InactiveUS20130011496A1Increase opportunitiesReduce riskBiocideHeavy metal active ingredientsAntigenAbnormal tissue growth
A cancer testis antigen biomarker useful to determine whether a non- small cell lung cancer tumor is likely to respond to neoadjuvant chemotherapy is provided. Methods of using the biomarker in the diagnosis, treatment and prognosis of non-small cell lung cancer also are provided.
Owner:LUDWIG INST FOR CANCER RES
System for predicting prognosis of gastric cancer in subjects
ActiveCN111724903AImproved prognosisMedical data miningHealth-index calculationGastric carcinomaOncology
The invention provides a system for predicting prognosis of gastric cancer of subjects. The system comprises: a data acquisition module, which is used for acquiring clinical characteristic data of thesubjects, immune marker characteristic data of the subjects and protein expression data of the subjects; a data processing module, which is used for further processing the data acquired in the data acquisition module; and a module for calculating the prognosis risk of the gastric cancer of the subjects, wherein the module utilizes the data processed in the data processing module to calculate theprognosis risk values of the gastric cancer of the subjects, and groups the subjects based on the risk values. According to the system and a method provided by the invention, eight characteristics, including five immunomarkers (CD3, CD4, PDL1, PAX5, and GZMB), an EMT protein marker (CDH1) and two clinical characteristics (pTNM and age), are selected to develop the system and the method that can significantly improve prognostic ability of gastric cancer patients. The system and the method may be applicable to patients with or without neoadjuvant chemotherapy and exhibit predicted values, and the patients may benefit from post-operative adjuvant chemotherapy.
Owner:BEIJING CANCER HOSPITAL PEKING UNIV CANCER HOSPITAL
Biomarker for predicting neoadjuvant chemotherapy curative effect of breast cancer and application of biomarker
The invention discloses a biomarker for predicting neoadjuvant chemotherapy curative effect of breast cancer and application of the biomarker. The biomarker for predicting neoadjuvant chemotherapy curative effect of breast cancer is a long non-coding RNA and has a sequence shown in SEQ ID NO:1 or has a corresponding DNA sequence. The biomarker can be used for preparing a kit having one or more than one of functions of prediction of neoadjuvant chemotherapy curative effect of breast cancer, clinical diagnosis, drug screening and treatment assessment. The kit is an RT-qPCR kit or a sequencing kit for quantitatively or qualitatively detecting the long non-coding RNA. The application of the biomarker for predicting the neoadjuvant chemotherapy curative effect of breast cancer is clear, the difference (P is less than 0.05) of the biomarker in pCR group and NpCR group fresh breast cancer tissues is displayed, the expression in the NpCR group fresh breast cancer tissues is obviously higher than that in the pCR group, the biomarker can be used for predicting the neoadjuvant chemotherapy curative effect, and the blindness of the neoadjuvant chemotherapy is avoided.
Owner:ZHEJIANG CANCER HOSPITAL
Diagnostic methods for determining prognosis of non-small cell lung cancer
InactiveUS20090258350A1Accurate identificationEnhanced informationMicrobiological testing/measurementFavorable prognosisNucleic Acid Probes
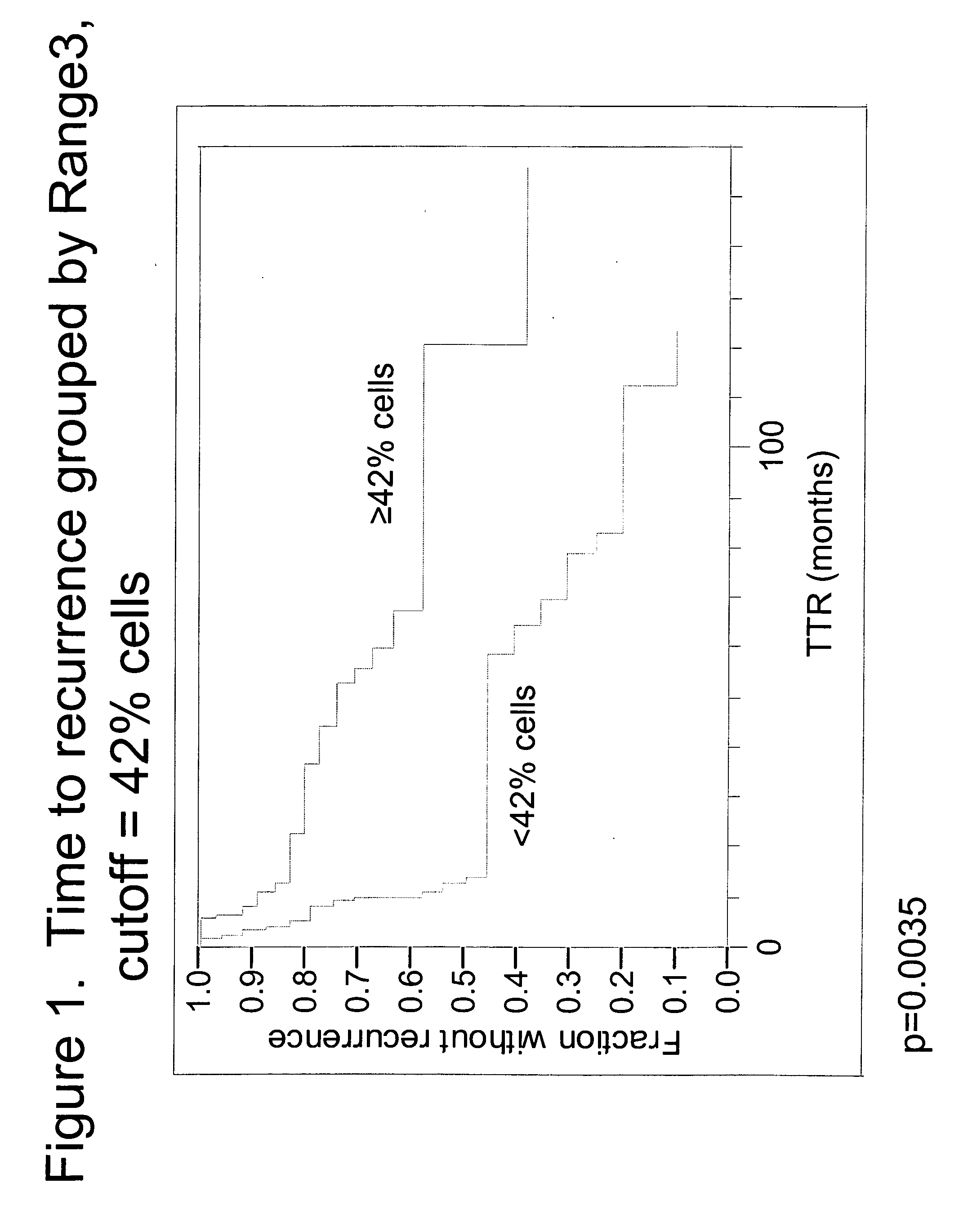
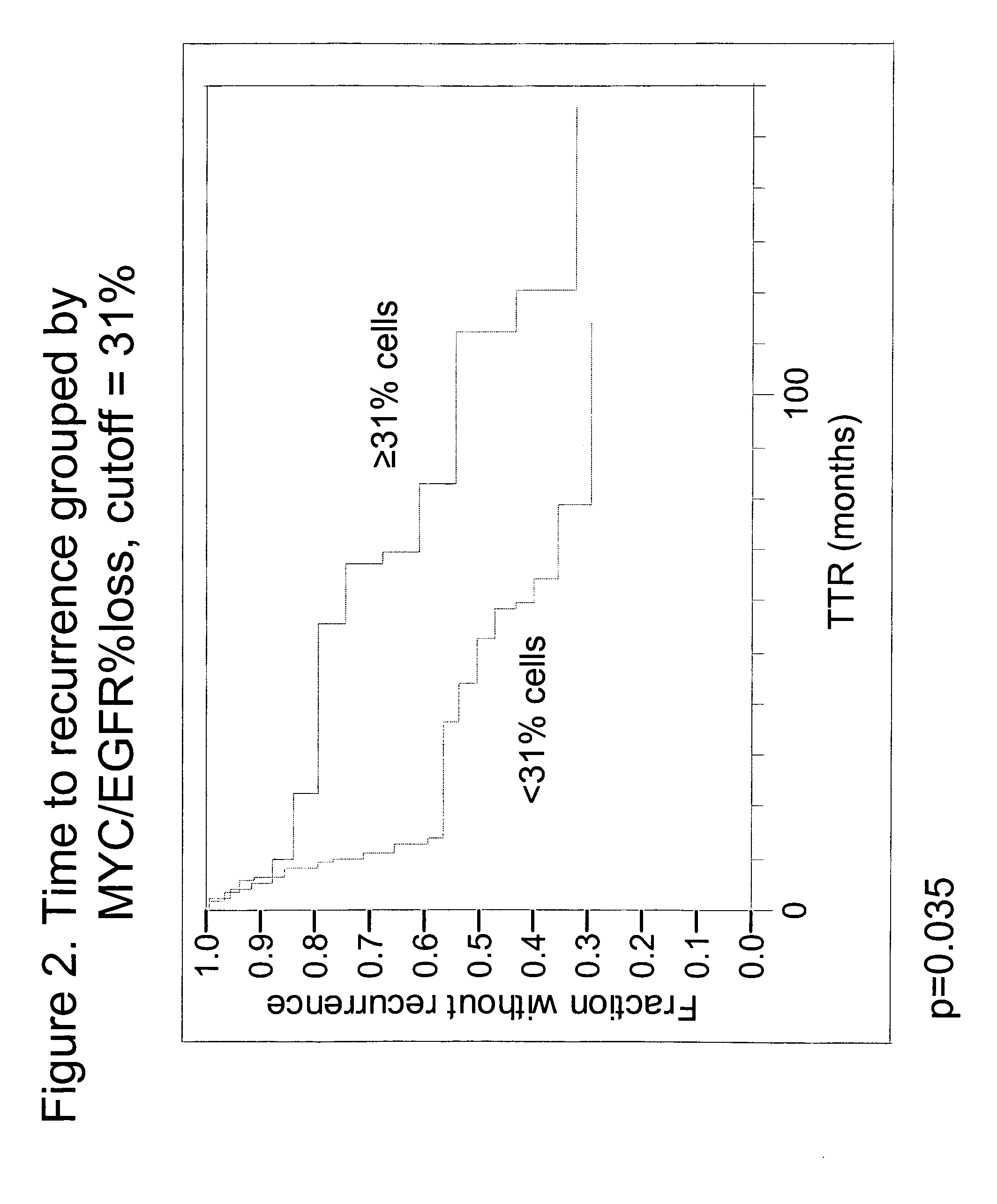
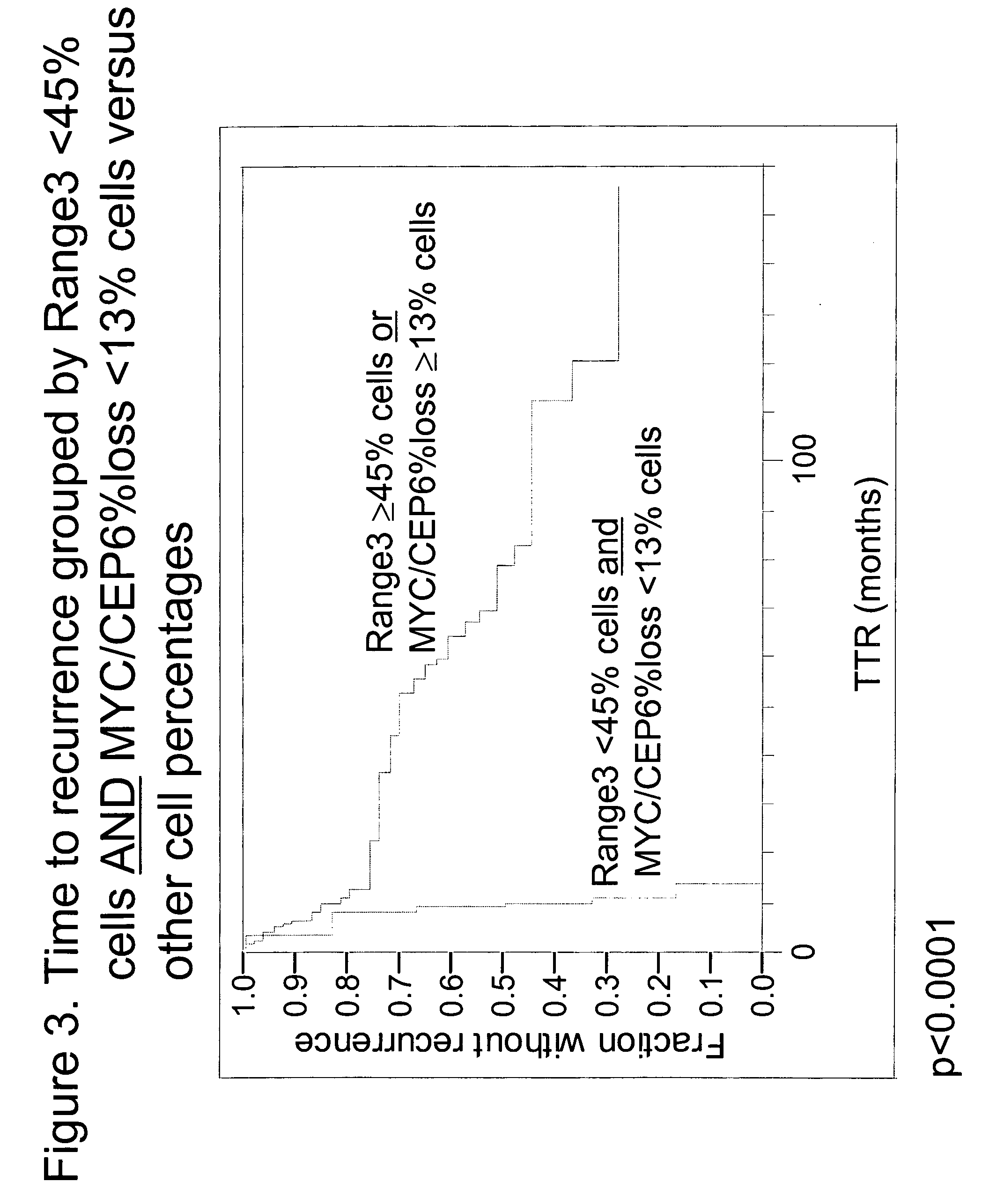
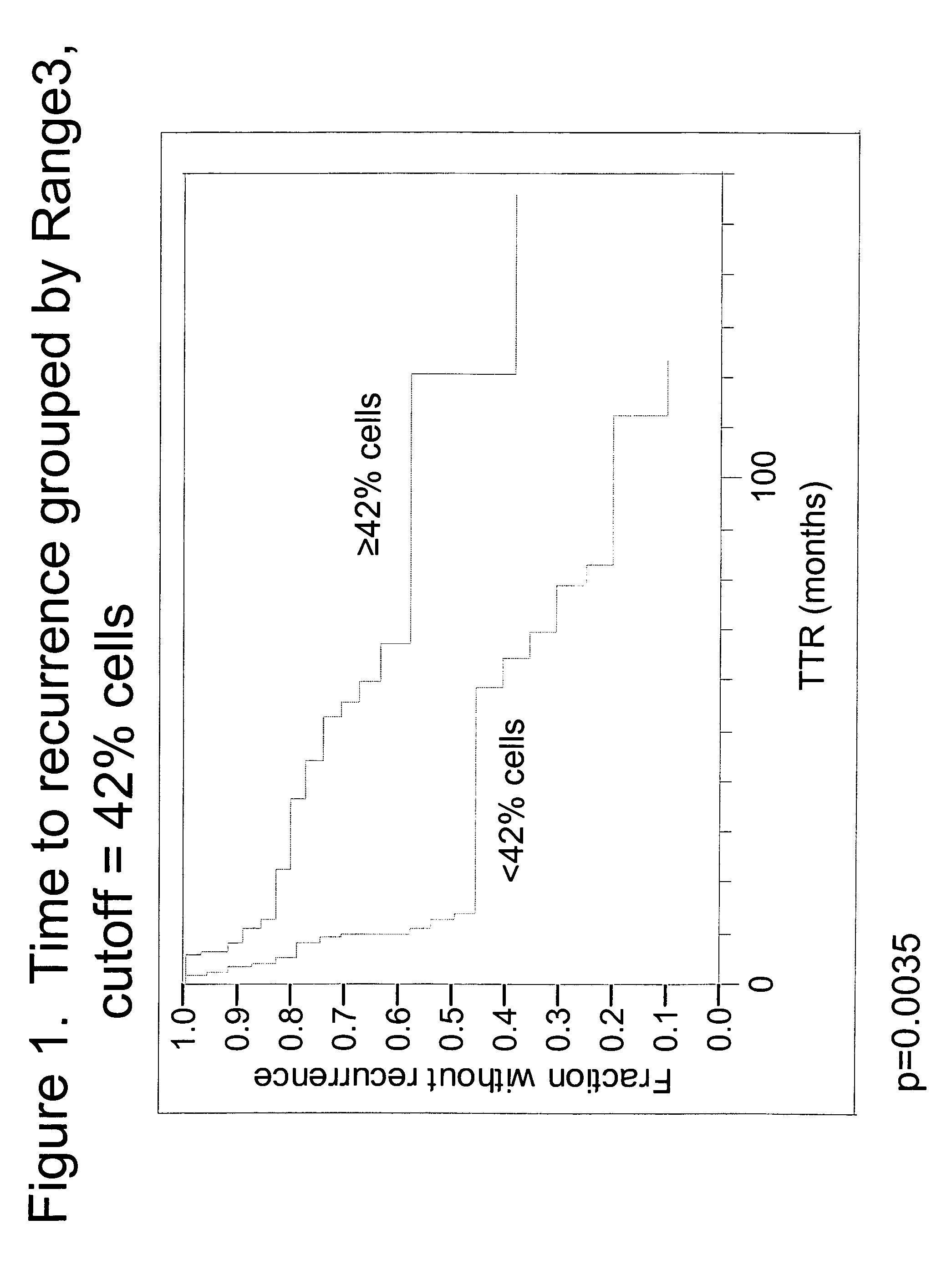
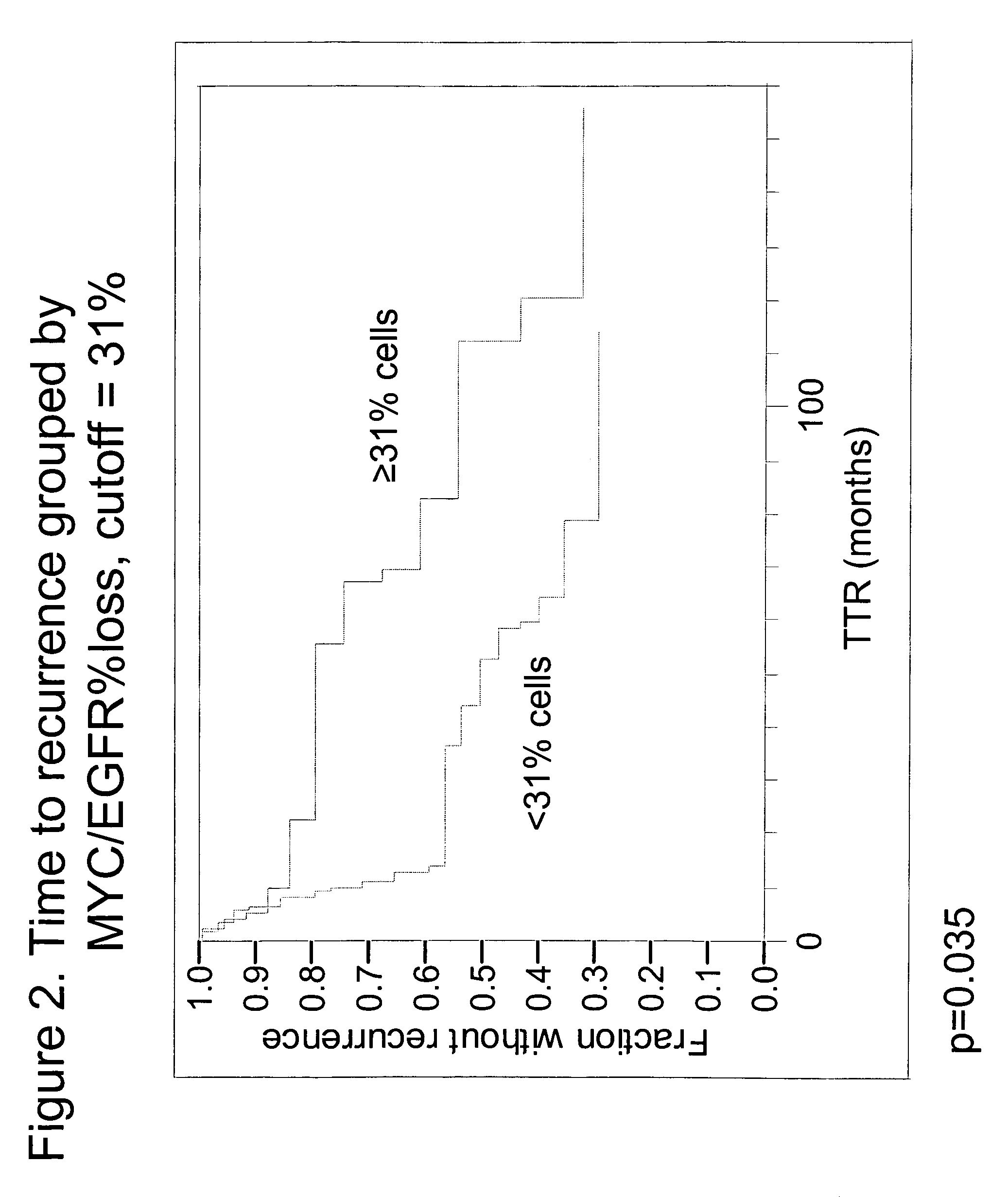
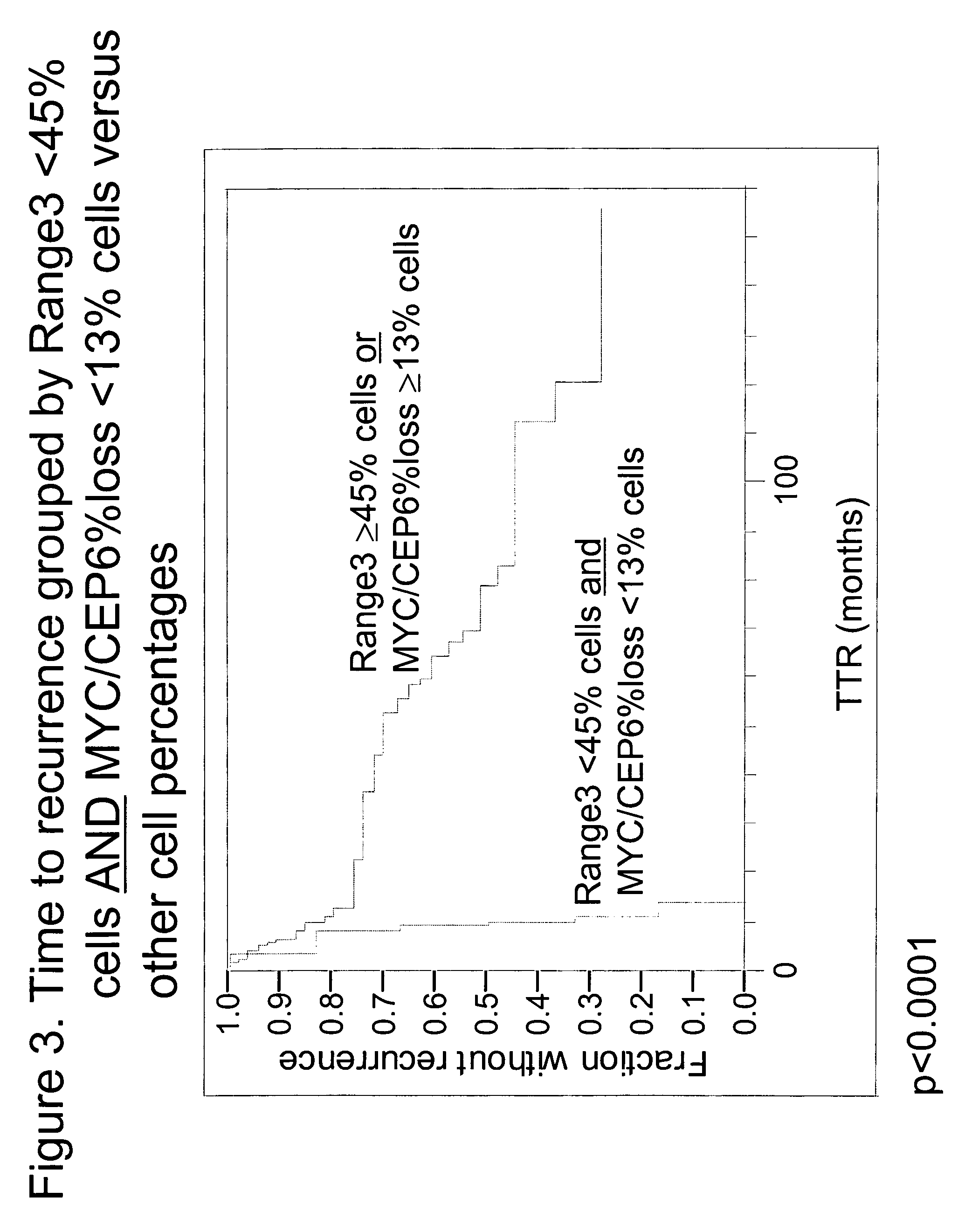
The invention provides methods for identifying early stage non-small cell lung cancer (NSCLC) patients who will have a favorable prognosis for the recurrence of lung cancer after surgical resection. The invention is based on the discovery that assessment of chromosomal copy number abnormalities at two or more of chromosome 5p15, 7p12, 8q24 and centromere 6 can be used for prognostic classification. The invention preferably uses fluorescence in situ hybridization with fluorescently labeled nucleic acid probes to hybridize to patient samples to quantify the chromosomal copy number of the these genetic loci. Assessment of the copy number abnormality patterns using four classifiers produced statistically significant prognostic classification for NSCLC: (i) the Range3 pattern of cells showing a difference on a cell by cell basis, of at least three FISH probe signals between the FISH signals at the chromosomal locus with the largest number of FISH signals minus the FISH signals at the chromosomal locus with the lowest number of FISH signals; (ii) the MYC / EGFR % loss pattern assessing the percentage of cells showing fewer MYC FISH probe signals than EGFR FISH probe signals; (iii) a combination of the Range3 pattern and the MYC / CEP6 ratio pattern of a percentage of cells showing a relative loss of MYC FISH probe signals to the FISH probe signal for CEP6; (iv) the combination of the MYC / 5p15 ratio pattern showing the relative ratio of MYC and 5p15 locus signals of ≧0.80 and the 5p15 / CEP6 ratio pattern assessing percentage of cells having a relative ratio of 5p15 FISH probe signals to CEP6 FISH probe signals ≧1.1 versus MYC / 5p15 ratio of <0.80 or 5p15 / CEP6<1.1; and (v) a combination of the average range of probe signal differences of equal to or greater than about 2.5 with the Range3 pattern in a percentage of the cells. The invention can be used to identify those early stage NSCLC patients at higher risk of recurrence who should be treated with neoadjuvant chemotherapy before surgery or with adjuvant chemotherapy after surgery.
Owner:ABBOTT MOLECULAR INC
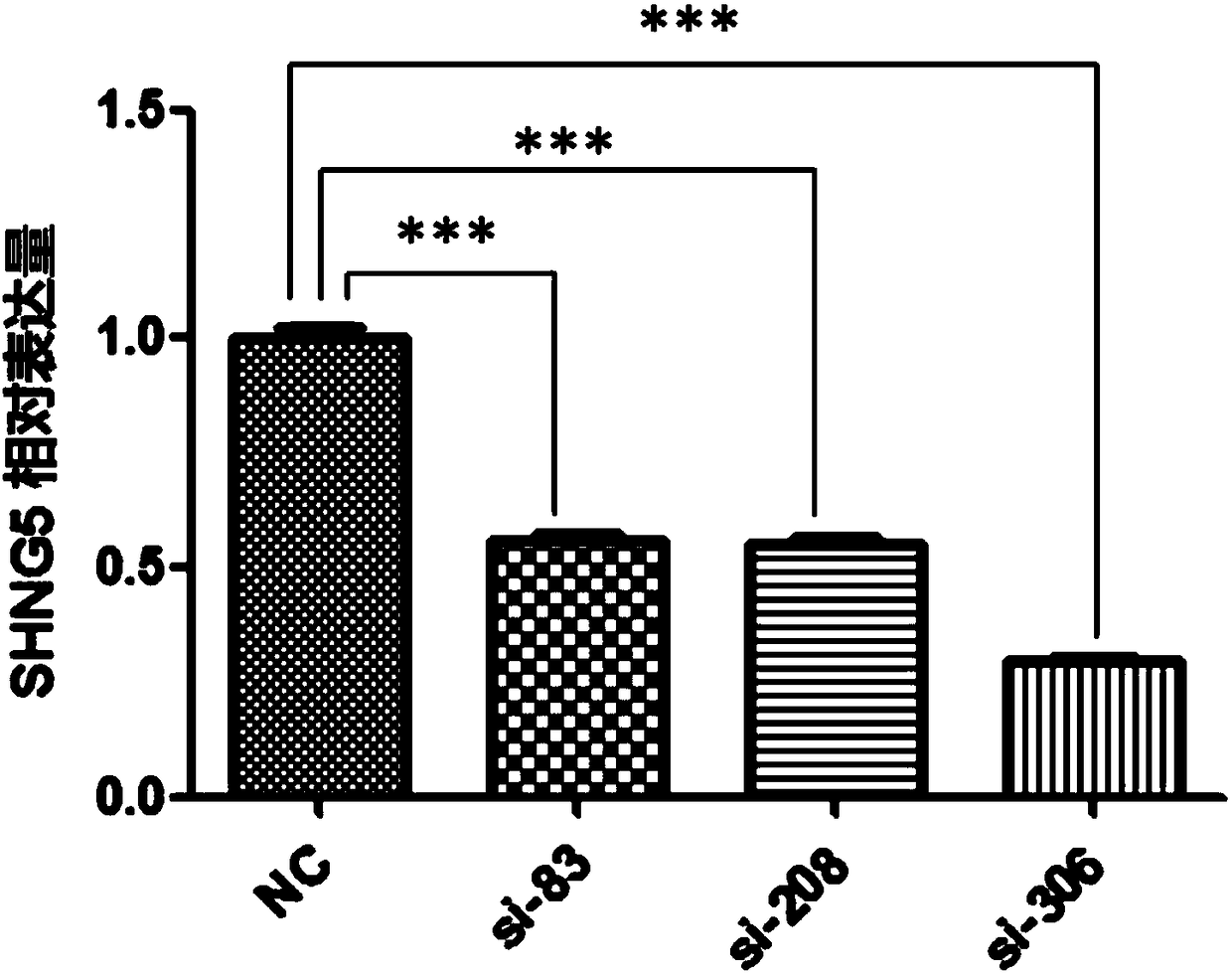
Application of SNHG5 (small nucleolar RNA host gene 5) in diagnosis, evaluation and efficacy prediction of breast cancer
InactiveCN108467891AHigh degree of malignancyMicrobiological testing/measurementApoptosisWilms' tumor
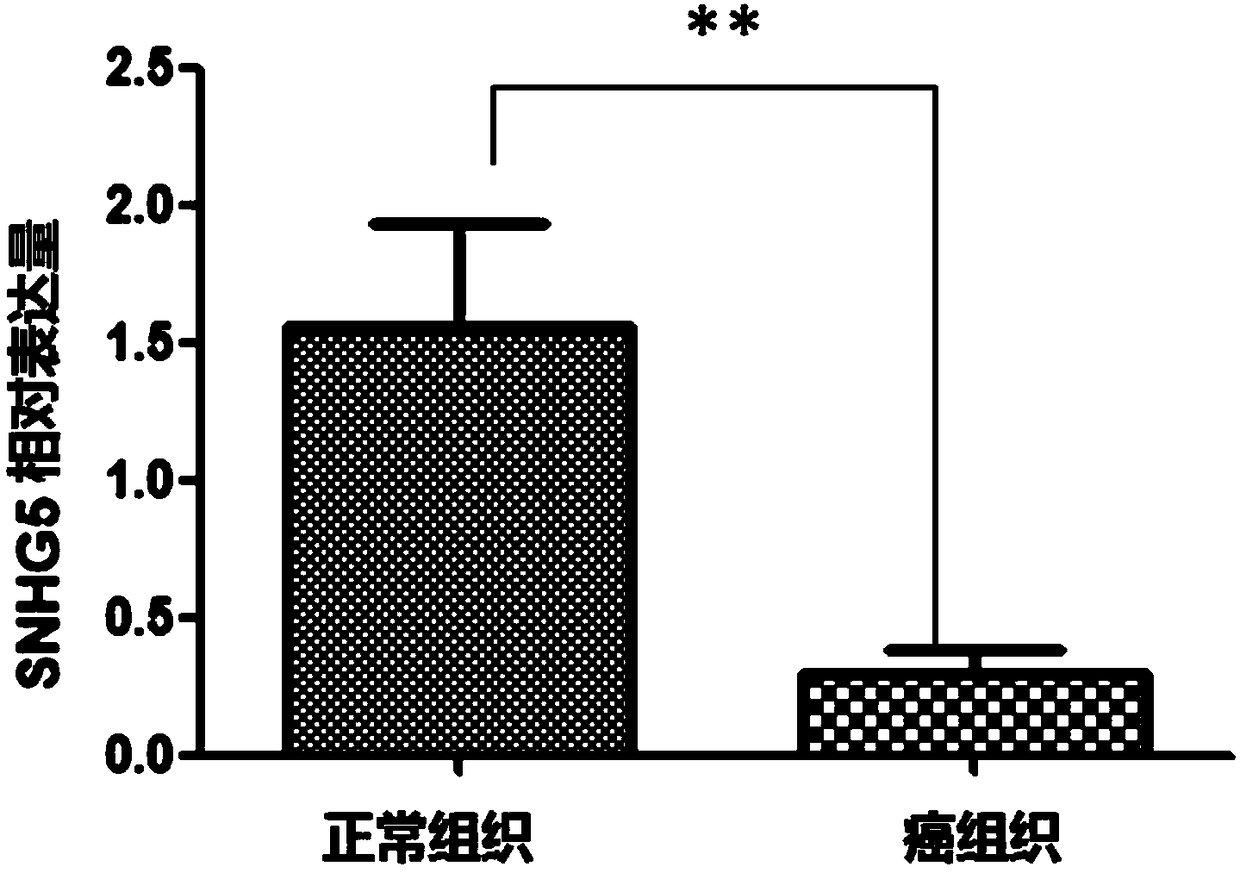
The invention discloses an application of SNHG5 (small nucleolar RNA host gene 5) in diagnosis, evaluation and efficacy prediction of breast cancer. It is found that lncRNA SNHG5 has significant low expression in breast cancer tissue and breast cancer cell lines, knockout of the lncRNA SNHG5 gene can enhance proliferation and migration of breast cancer cells and inhibit apoptosis, and thus, the expression level of long-chain non-coding RNA SNHG5 is closely related with diagnosis, transfer and relapse of tumor, patient prognosis and neoadjuvant chemotherapy effect are closely related, which indicates that the lncRNA SNHG5 gene can be used as a marker for diagnosis, prognosis evaluation or effect prediction. A breast cancer cell model which has low expression of SNHG5 and is established by transfecting breast cancer cell lines with small interference RNA can be used in a preparation method of a preparation for preventing relapse and transfer of breast cancer.
Owner:TONGDE HOSPITAL OF ZHEJIANG PROVINCE
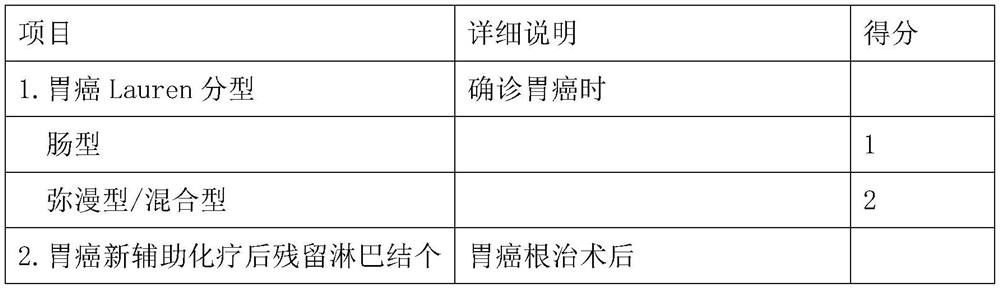
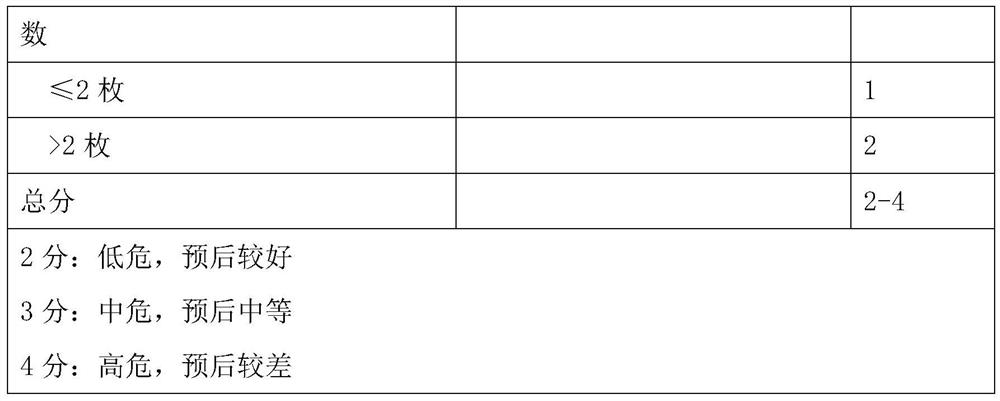
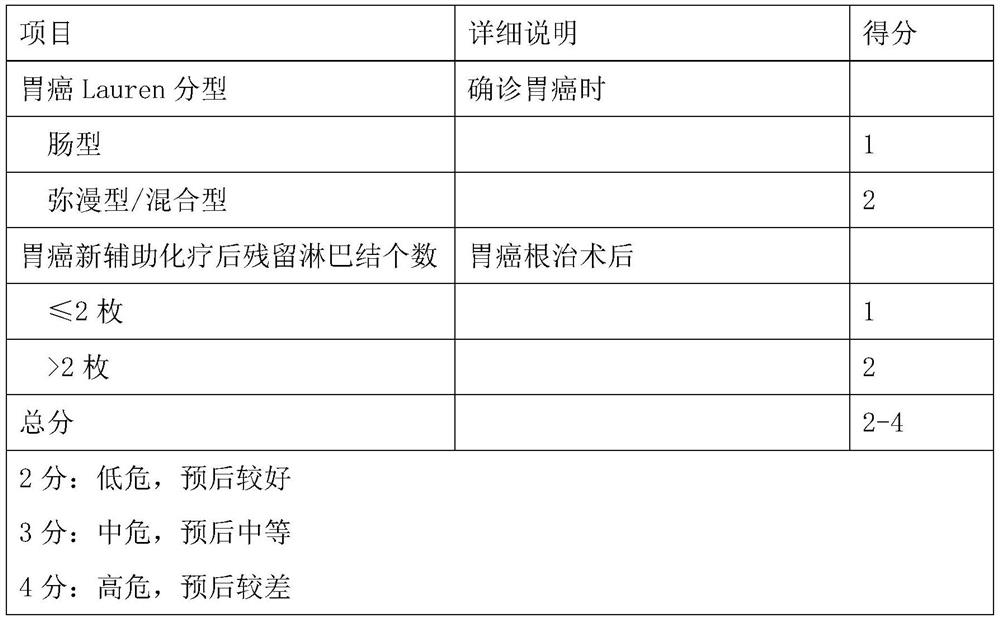
Model for evaluating gastric cancer prognosis based on Lauren typing and postoperative residual lymph nodes and application
PendingCN112837815AEfficient communicationEffective predictionMechanical/radiation/invasive therapiesHealth-index calculationPhysician patient communicationOncology
The invention relates to a model for evaluating gastric cancer prognosis based on Lauren typing and postoperative residual lymph nodes and application, and belongs to the technical field of biomedical detection. According to the invention, the number of residual lymph nodes after Lauren typing and neoadjuvant chemotherapy is comprehensively used as a prognosis evaluation index after neoadjuvant chemotherapy of gastric cancer, and a prognosis evaluation model for patients after neoadjuvant chemotherapy of gastric cancer is established. Through clinical verification, the evaluation model can effectively pre-judge disease outcome after neoadjuvant chemotherapy of gastric cancer, and is more accurate than the traditional Japan JGCA standard and German Becker standard. The invention provides reference information for prognosis of tumor patients, so that clinical doctors are helped to formulate the most reasonable diagnosis and treatment plan for the tumor patients, and timely and effective doctor-patient communication is facilitated.
Owner:ZHONGSHAN HOSPITAL FUDAN UNIV
System for predicting chemotherapy sensitivity of patient with bladder cancer
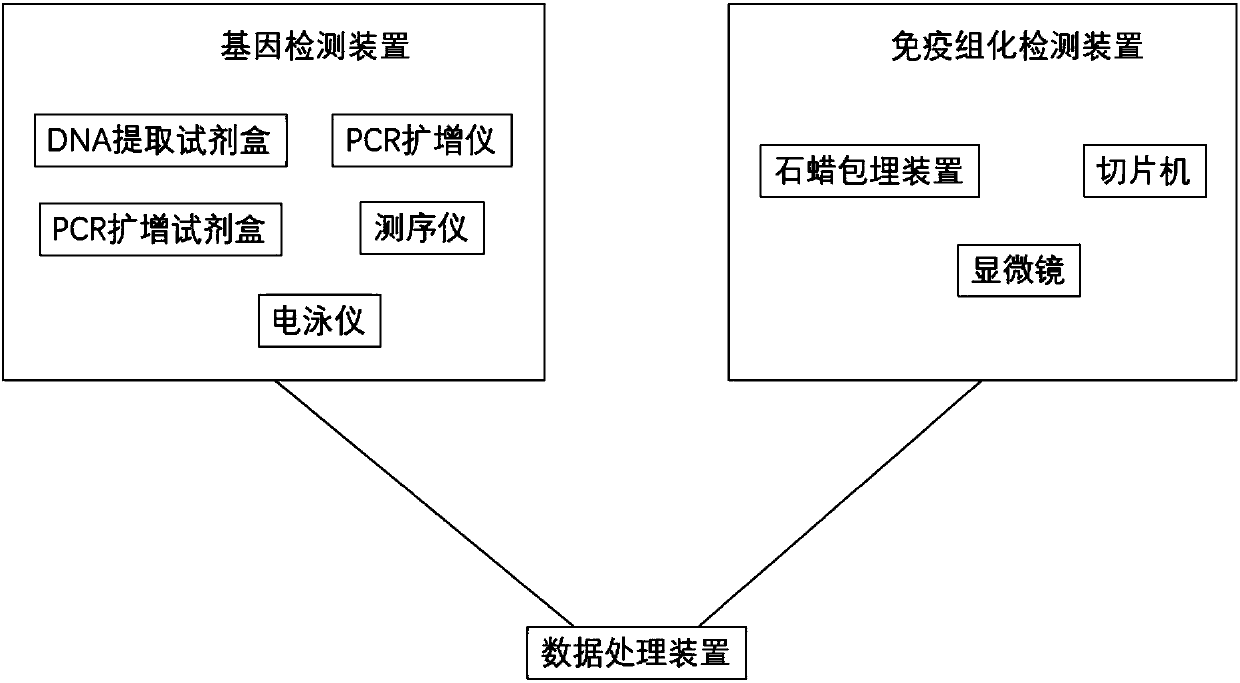
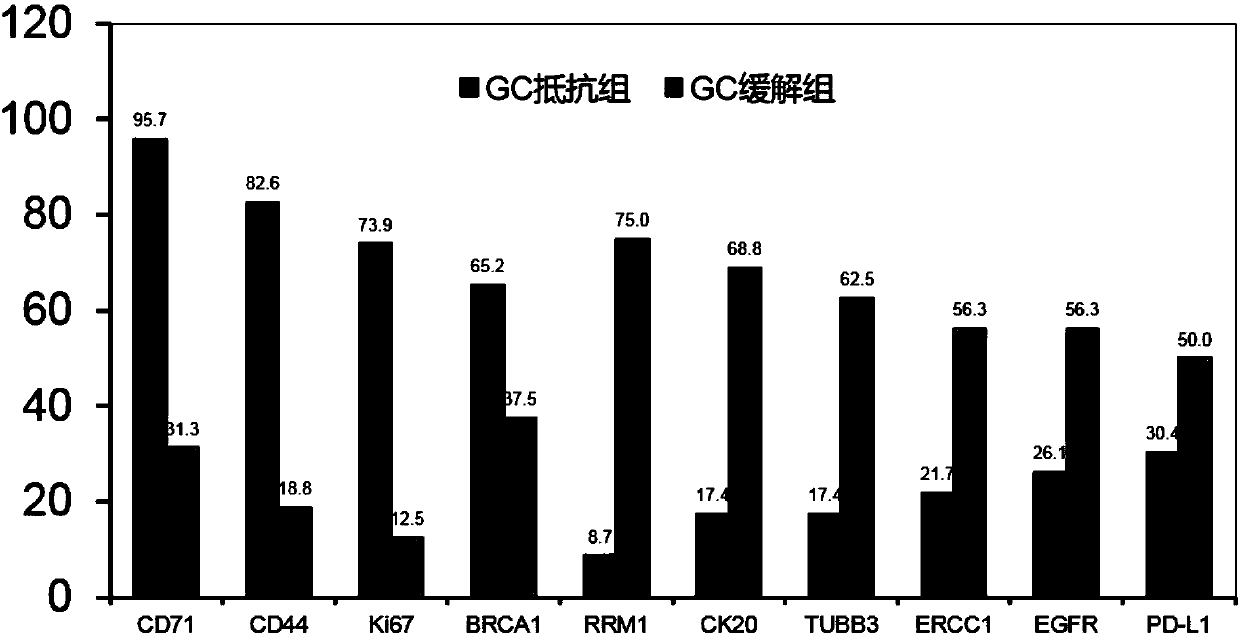
The invention discloses a system for predicting the chemotherapy sensitivity of a patient with bladder cancer. The system comprises a gene detection device, an immunohistochemical detection device anda data processing device, wherein results of the gene detection device and the immunohistochemical detection device are analyzed and processed by virtue of the data processing device; the gene detection device comprises a DNA extraction kit, a primer group, a PCR amplification instrument, a PCR purification kit, a sequencer and an electrophoresis instrument. According to the system, correspondingspecific primers and antibodies are designed and prepared by virtue of bladder cancer mutant genes including FGFR3, HRAS, TERT promoters, TP53, TSC1, PIK3CA and HER2 as well as abnormal expression proteins including EGFR, ERCC1, CK20, BRCA1, CD44, RRM1, PD-L1, TUBB3, Ki67 and CD71, so as to meet the detection requirements of molecular pathological indexes; and the system is simple, convenient, economic and used for guiding the implementation of neoadjuvant chemotherapy, and the prediction accuracy of the chemotherapy sensitivity of the bladder cancer can be greatly improved.
Owner:李翀
Application of reagent for detecting gene expression level and construction method of NAC (neoadjuvant chemotherapy) curative effect prediction model for breast cancer patients
ActiveCN109439753AChemotherapy is effectiveImprove survival rateMicrobiological testing/measurementChemotherapy combinationsGene expression level
The invention discloses an application of a reagent for detecting gene expression level and a construction method of an NAC (neoadjuvant chemotherapy) curative effect prediction model for breast cancer patients, and relates to the technical field of breast cancer. Particularly, the prediction model can predict the curative effect of anthracyclines and taxus drugs in NAC, so as to provide a basis for whether the breast cancer patients accept NAC regimens or choose which type of NAC regimens. The patients who are sensitive to chemotherapy regimens and may reach pCR can seize the opportunity of NAC by predicting the curative effect, so that effective NAC is conducted, and the breast-conserving rate or survival rate is increased; however, the patients who are insensitive to chemotherapy regimens or may cause tumor progression can choose other effective treatments in time.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
Tumor marker and application thereof
ActiveCN112795647AImproved prognosisOptimize treatment planMicrobiological testing/measurementGenetic engineeringTumor responseGenes proteins
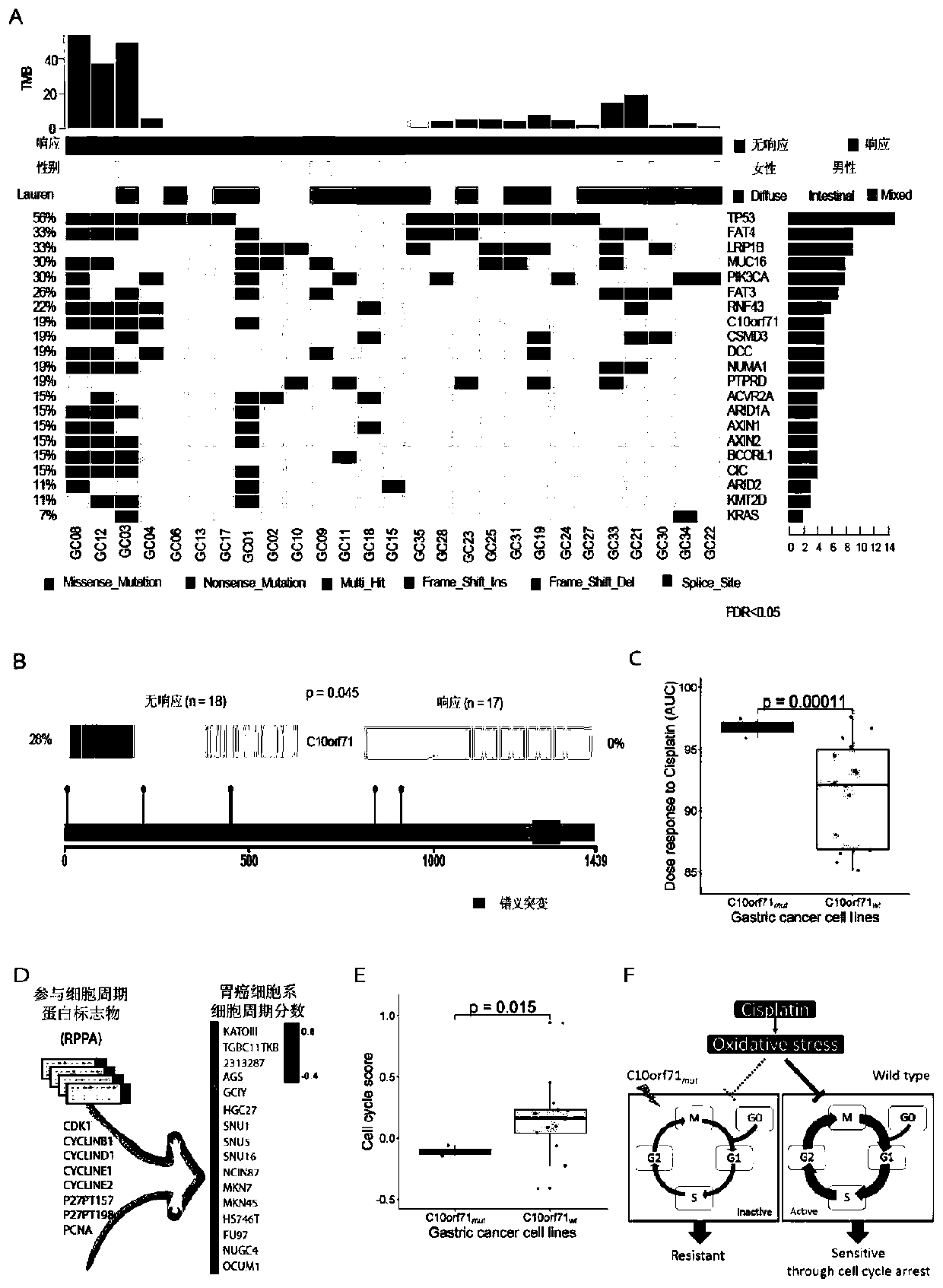
The invention relates to a tumor marker and an application thereof, in particular to an application of at least one of a C10orf71 gene, a C10orf71 protein and a C10orf71 gene detection primer kit in preparation of a product for predicting response of a cancer patient to neoadjuvant chemotherapy. According to the invention, the C10orf71 gene mutation is determined to be a key molecular characteristic of tumor response to neoadjuvant chemotherapy for the first time, and based on the technical scheme of the invention, the neoadjuvant chemotherapy has advantages in improvement of prognosis and determination of responsive patients before treatment, so that a better treatment scheme can be formulated.
Owner:BEIJING CANCER HOSPITAL PEKING UNIV CANCER HOSPITAL +1
Antitumor lymphatic metastasis function of mitoxantrone and pharmaceutical preparation of mitoxantrone
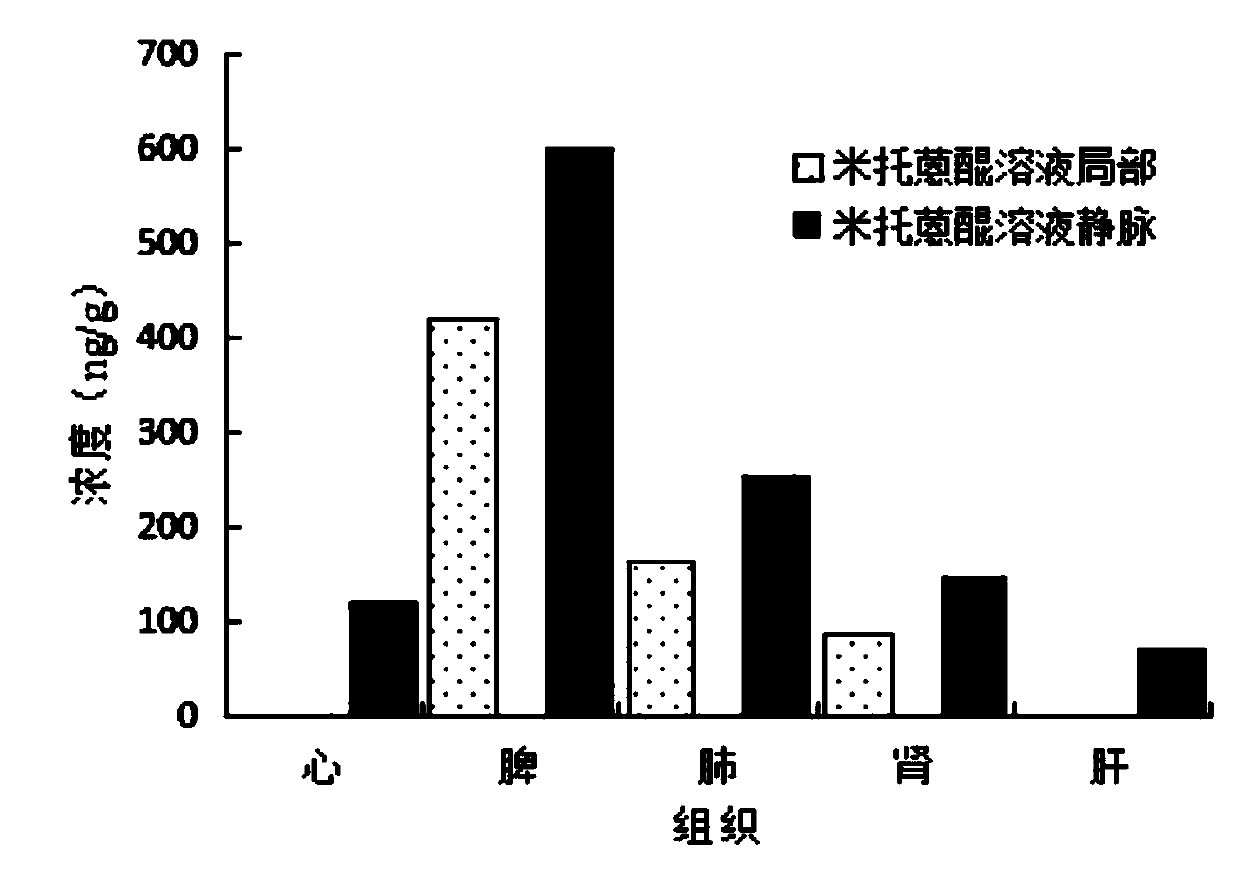
InactiveCN109718228AImprove the quality of lifeInhibit transferOrganic active ingredientsAerosol deliveryLymphatic vesselSide effect
The present invention relates to the technical field of medicine and particularly relates to an antitumor lymphatic metastasis function of mitoxantrone and a pharmaceutical preparation of the mitoxantrone. The mitoxantrone is administered locally, such as intratumoral administration and peritumoral and tumor subcutaneous administration. The mitoxantrone can be prepared into the clinically acceptable pharmaceutical preparation with a pharmaceutically acceptable carrier. The pharmaceutical preparation is a parenteral administration preparation. The parenteral administration preparation is selected from a solution, a liposome, a dendritic macromolecule, a nanoparticle, a nanocrystal, a microcrystal, a microsphere and a gel agent. The mitoxantrone is used for local chemotherapy to increase anti-tumor activity, reduces toxic and side effects, targets a lymphatic system, inhibits tumor lymphatic metastasis, can reduce tumor volume and is convenient for subsequent surgical resection when usedfor neoadjuvant chemotherapy, can also kills tumor cells metastasized in lymphatic vessels, reduces risks of postoperative tumor recurrence, and improves quality of life of patients.
Owner:SHENYANG PHARMA UNIVERSITY
Method for predicting the response to chemotherapy in a patient suffering from or at risk of developing recurrentbreast cancer
InactiveUS20140228241A1Simple methodAvoid unnecessary adjuvantNucleotide librariesMicrobiological testing/measurementRna expressionTumor Sample
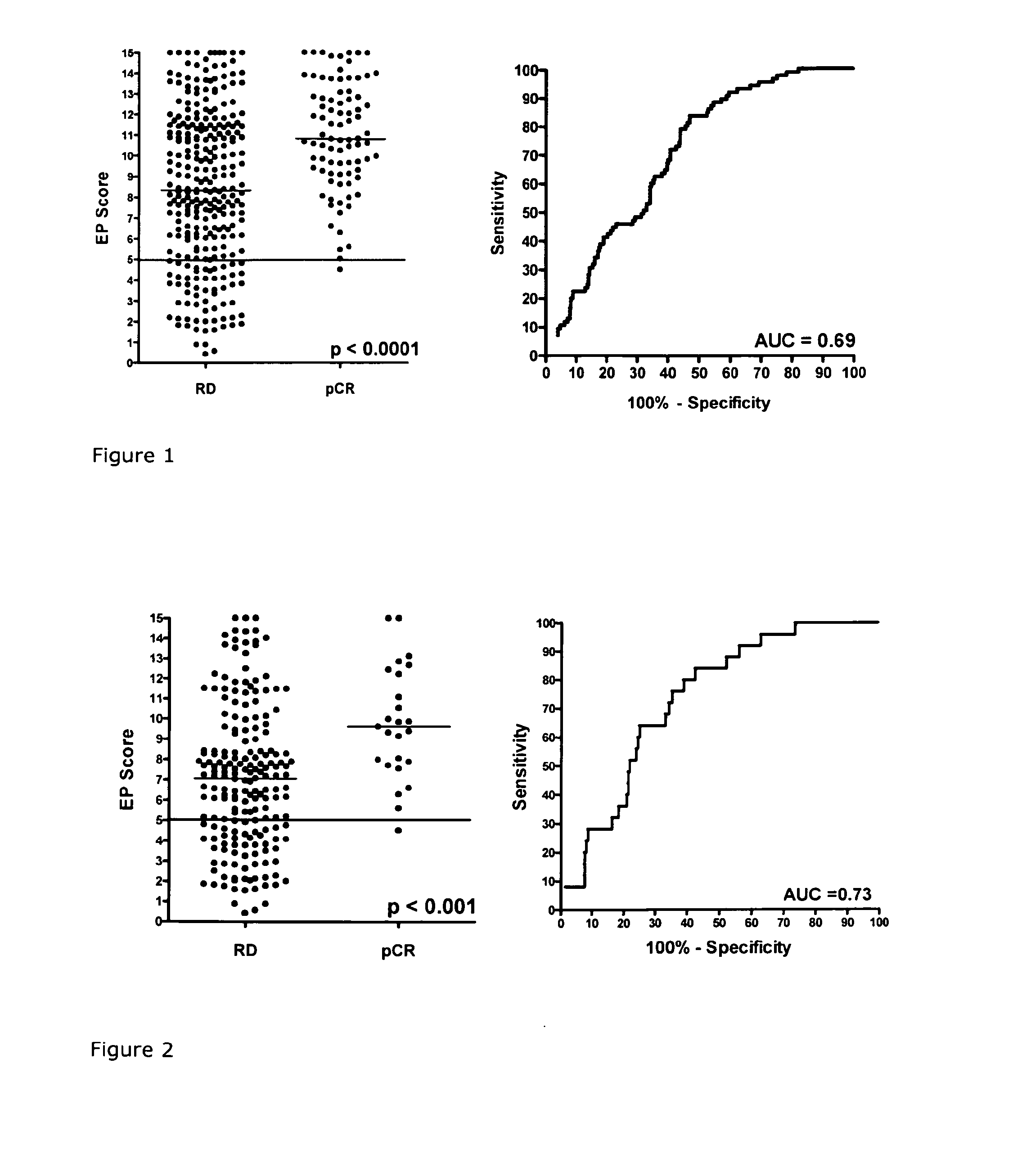
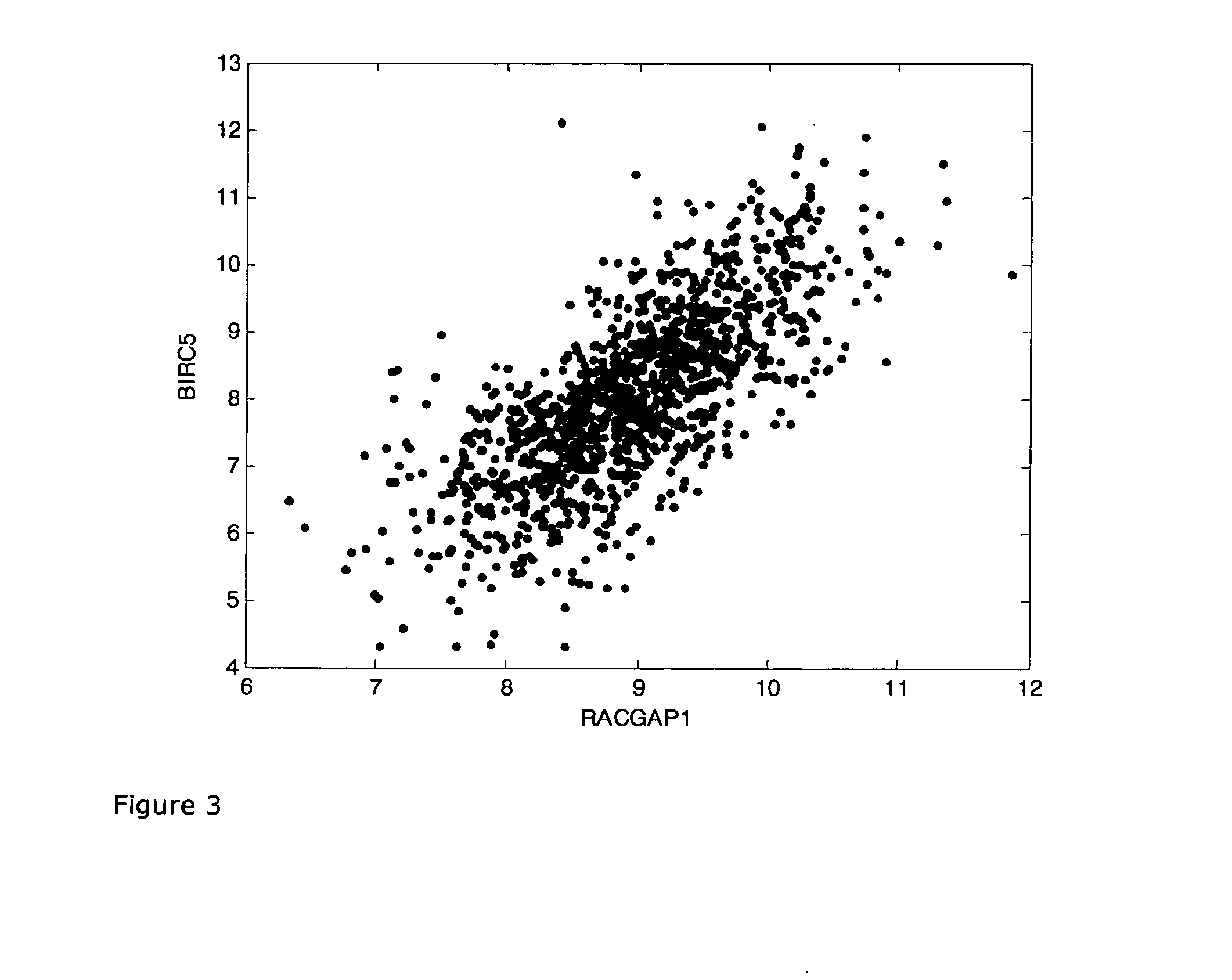
A method for predicting a response to and / or benefit of chemotherapy, including neoadjuvant chemotherapy, in a patient suffering from or at risk of developing recurrent neoplastic disease, in particular breast cancer, said method comprising the steps of: (a) determining in a tumor sample from said patient the RNA expression levels of the following 8 genes: UBE2C, RACGAP1, DHCR7, STC2, AZGP1, RBBP8, IL6ST, and MGP, indicative of a response to chemotherapy for a tumor, or (b) determining in a tumor sample from said patient the RNA expression levels of the following 8 genes: UBE2C, BIRC5, DHCR7, STC2, AZGP1, RBBP8, IL6ST, and MGP; indicative of a response to chemotherapy for a tumor (c) mathematically combining expression level values for the genes of the said set which values were determined in the tumor sample to yield a combined score, wherein said combined score is predicting said response and / or benefit of chemotherapy.
Owner:SIVIDON DIAGNOSTICS
Biomarker for forecasting new auxiliary chemotherapy curative effect of breast cancer and fluorescence quantitative immune PCR kit
InactiveCN105039319AAvoid blindnessMicrobiological testing/measurementDNA/RNA fragmentationCCL2Fluorescence
The invention discloses a biomarker for forecasting the new auxiliary chemotherapy curative effect of breast cancer and a fluorescence quantitative immune PCR kit. The biomarker is at least one of a CCL2 gene, a CCR1 gene, a CXCL10 gene, a CXCL11 gene and an IL2RG gene. The five genes of the biomarker are efficiently expressed in a pCR group; the IL2RG gene has an extremely obvious expression difference (P is less than 0.01) in the pCR group and the NpCR group, and the other four genes have an obvious expression difference (P is less than 0.05) in the pCR group and the NpCR group; the five genes are indicated to be biomarkers for forecasting the new auxiliary chemotherapy curative effect of the breast cancer, and the blindness of new auxiliary chemotherapy is avoided.
Owner:ZHEJIANG SCI-TECH UNIV
Gene tag for predicting breast cancer neoadjuvant chemotherapy sensitivity and application thereof
ActiveCN113061655AGood predictive abilityGood forecastMicrobiological testing/measurementProteomicsChemotherapy combinationsLogistische regression
The invention belongs to the technical field of tumor gene detection, and particularly relates to a gene tag for predicting breast cancer paclitaxel and anthracycline neoadjuvant chemotherapy sensitivity and application thereof. In the invention, based on LASSO logistic regression, a gene tag consisting of 25 genes related to breast cancer neoadjuvant chemotherapy sensitivity is obtained, and a score containing a gene expression quantity is calculated and predicted, so that the sensitivity of a breast cancer patient using paclitaxel and anthracycline neoadjuvant chemotherapy can be accurately predicted, the response of the patient to treatment is predicted, and whether the patient benefits from chemotherapy is discriminated, and thus selection of neoadjuvant chemotherapy regimens for breast cancer is guided, excessive treatment is avoided, and the medical cost is reduced.
Owner:HEFEI INSTITUTES OF PHYSICAL SCIENCE - CHINESE ACAD OF SCI +1
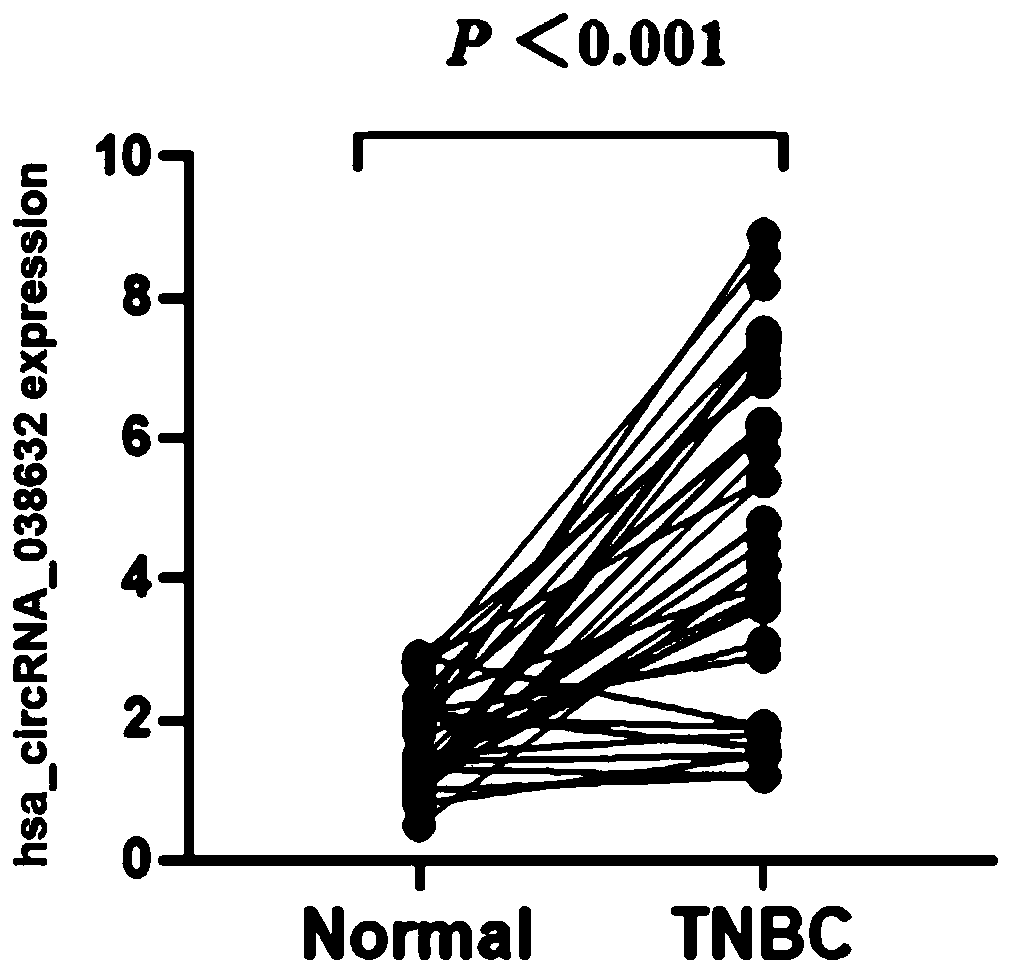
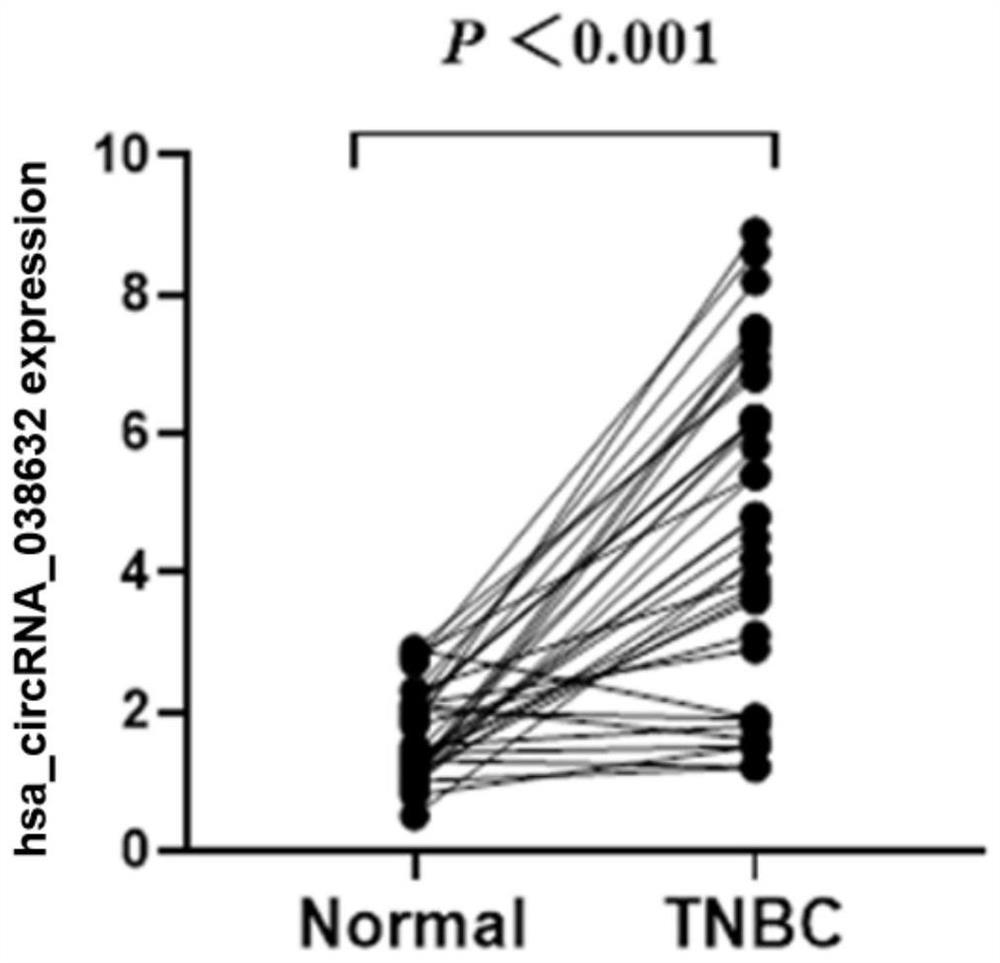
CircRNA detection kit for predicting neoadjuvant chemotherapy reactivity of triple-negative breast cancer (TNBC)
ActiveCN110923321AResponsive PredictionHelp determineMicrobiological testing/measurementDNA/RNA fragmentationNucleotideTnbc patient
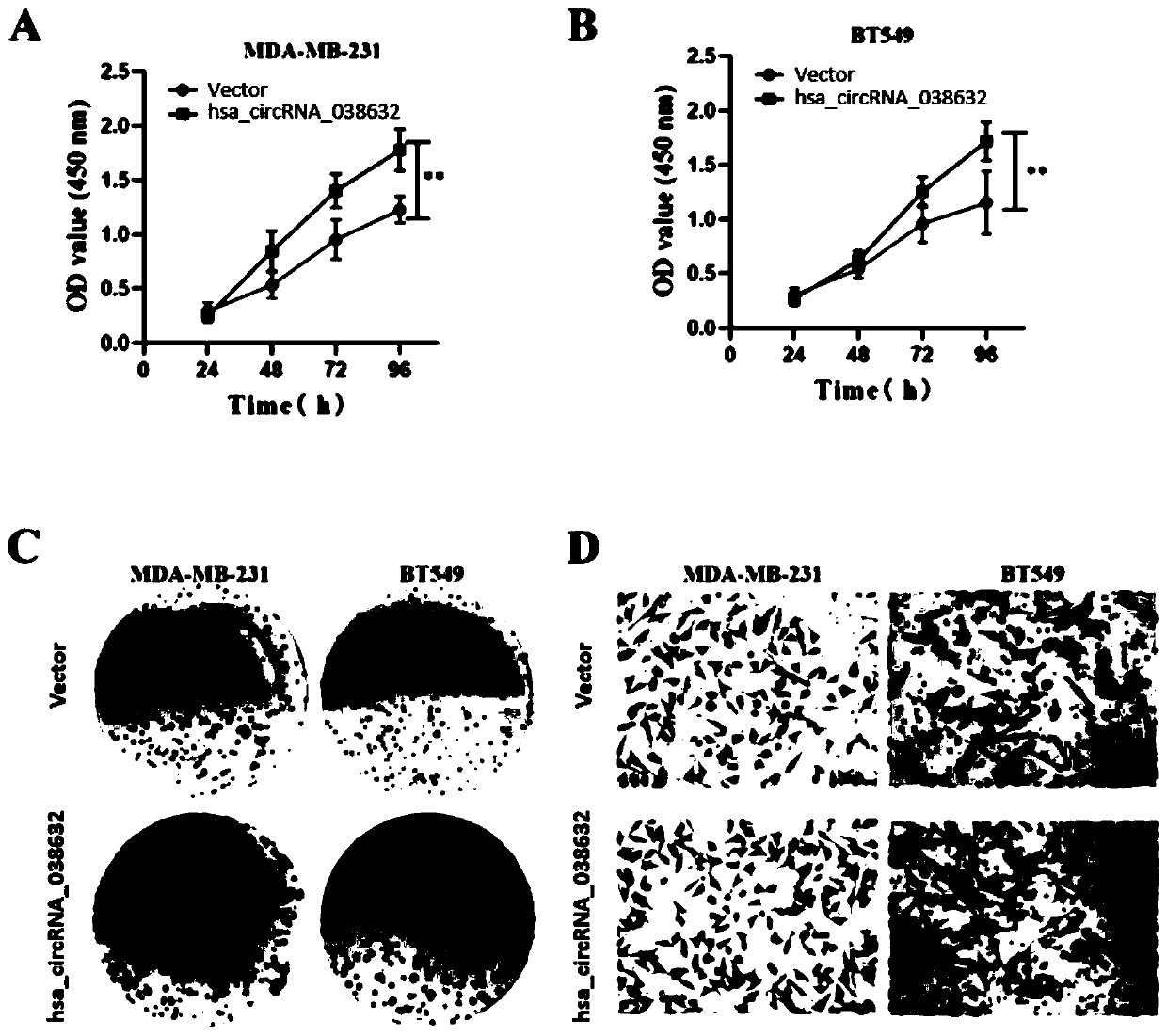
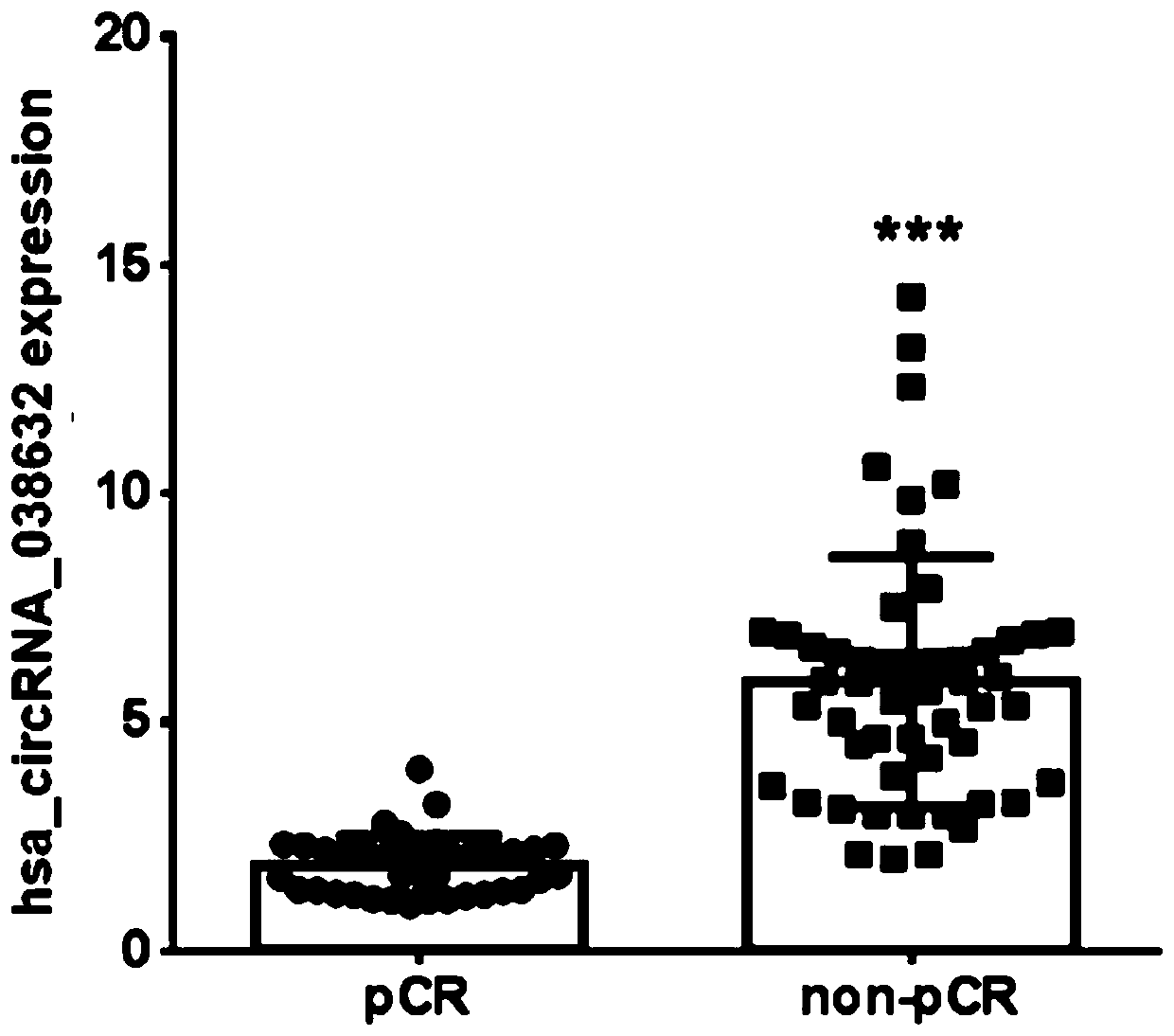
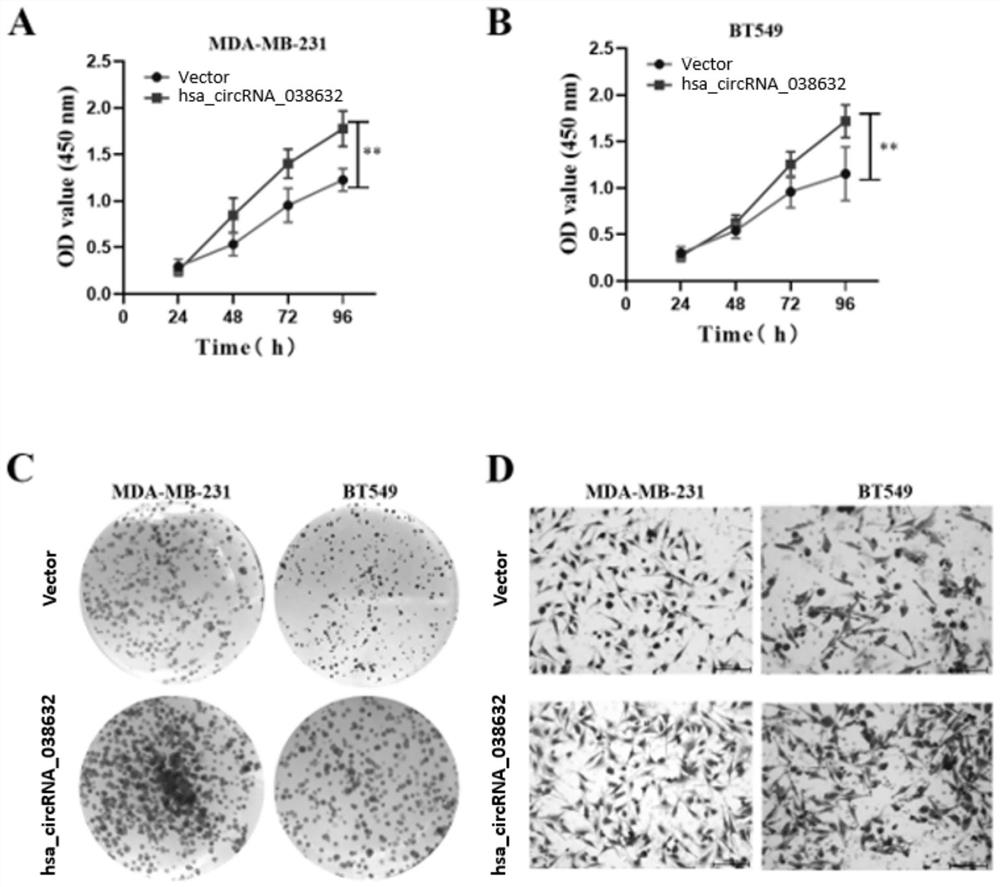
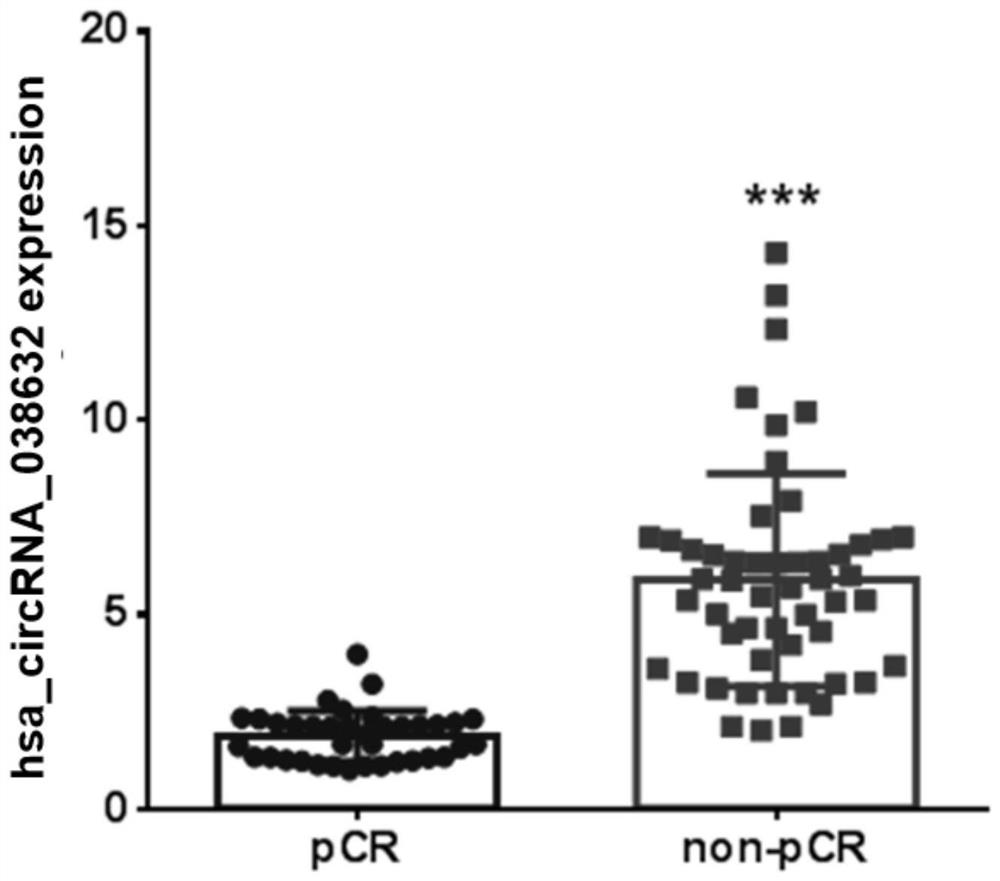
The invention provides circRNA for predicting neoadjuvant chemotherapy sensitivity of triple-negative breast cancer (TNBC). The circRNA is hsa_circRNA_038632, with a nucleotide sequence shown in SEQ ID NO: 1. The invention further provides a kit for predicting neoadjuvant chemotherapy sensitivity of TNBC. The invention finds the correlation between the hsa_circRNA_038632 and reactivity of TNBC patients to neoadjuvant chemotherapy for the first time, and the reactivity of the TNBC patients to the neoadjuvant chemotherapy can be predicted by detecting the expression level of the hsa_circRNA_038632, so that determination of chemotherapy regimens for the TNBC patients is facilitated.
Owner:GUANGDONG GENERAL HOSPITAL
Marker for predicting efficacy of neoadjuvant chemotherapy for breast cancer patients and application of marker
ActiveCN110923318ADetermine chemotherapy regimenHelp determineMicrobiological testing/measurementDNA/RNA fragmentationDocetaxel-PNPDocetaxel
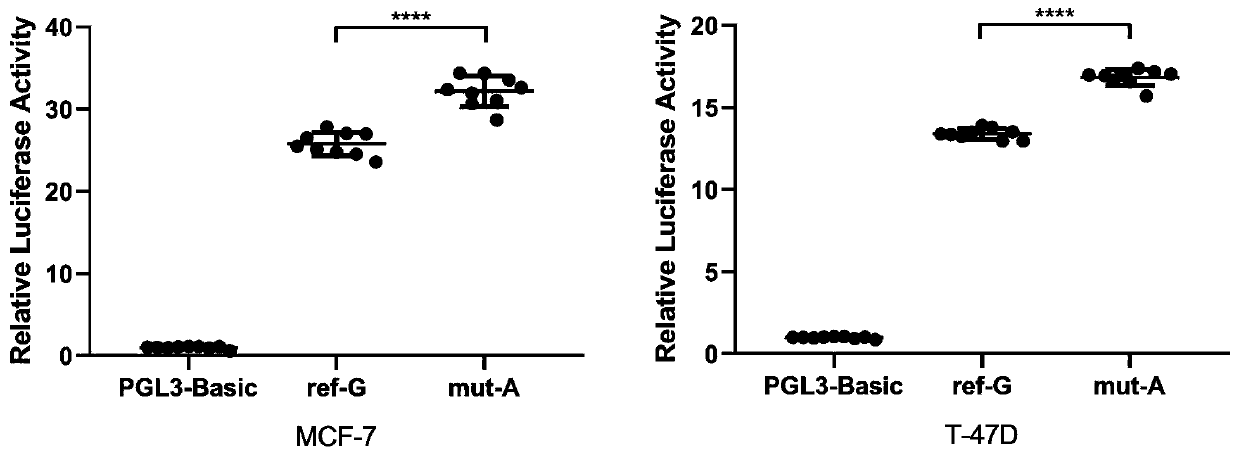
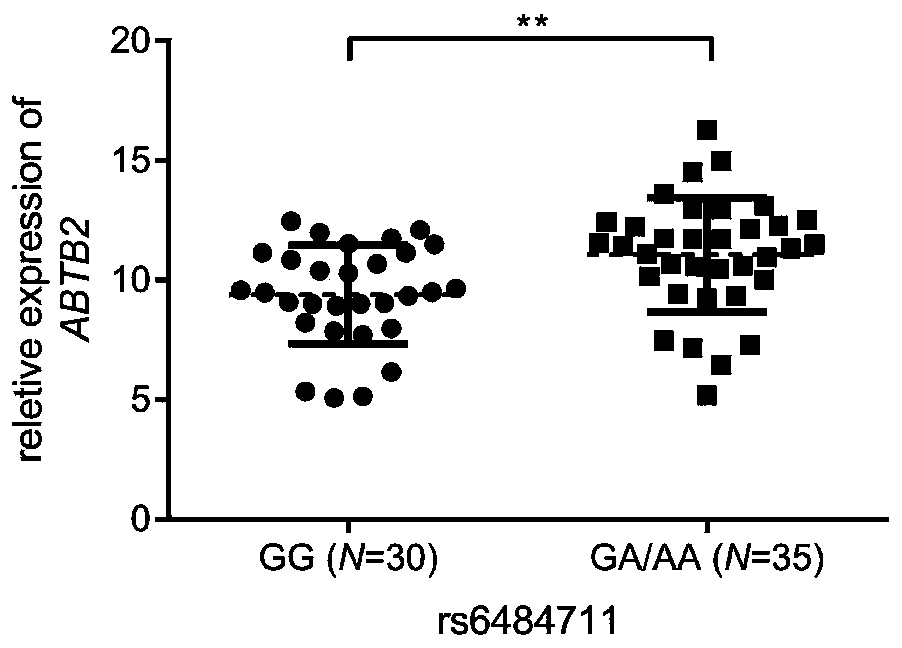
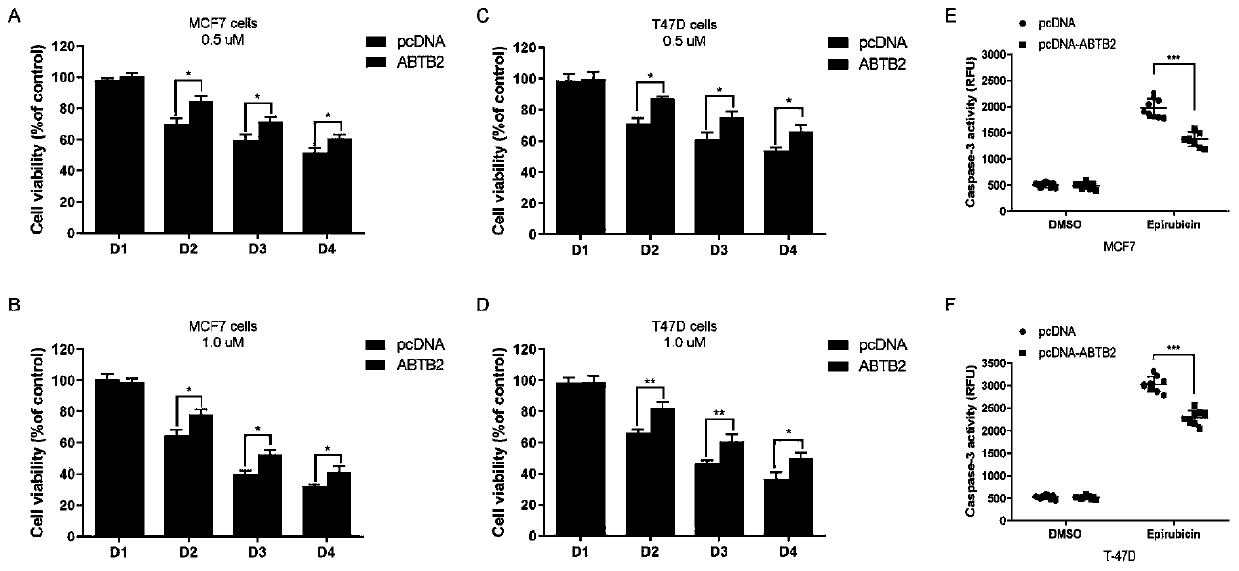
The invention provides rs6484711 as a marker for predicting the efficacy of neoadjuvant chemotherapy for breast cancer. Results prove that the rs6484711 variant participates in tumor cell resistance to the neoadjuvant chemotherapy containing epirubicin and docetaxel by regulating the expression of ABTB2, so that the marker is helpful for personalized treatment of the neoadjuvant chemotherapy and is expected to be a target for development of novel therapeutic drugs.
Owner:CANCER INST & HOSPITAL CHINESE ACADEMY OF MEDICAL SCI
Predictive and prognostic methods in breast cancer
PendingCN113383091AOrganic active ingredientsMicrobiological testing/measurementComplete remissionIV Chemotherapy
Owner:百欧恩泰诊断有限责任公司 +1
Methods for selecting active agents for cancer treatment
InactiveUS8187800B2Accurate scoreAccurate assessmentMicrobiological testing/measurementMaterial analysisActive agentPositive response
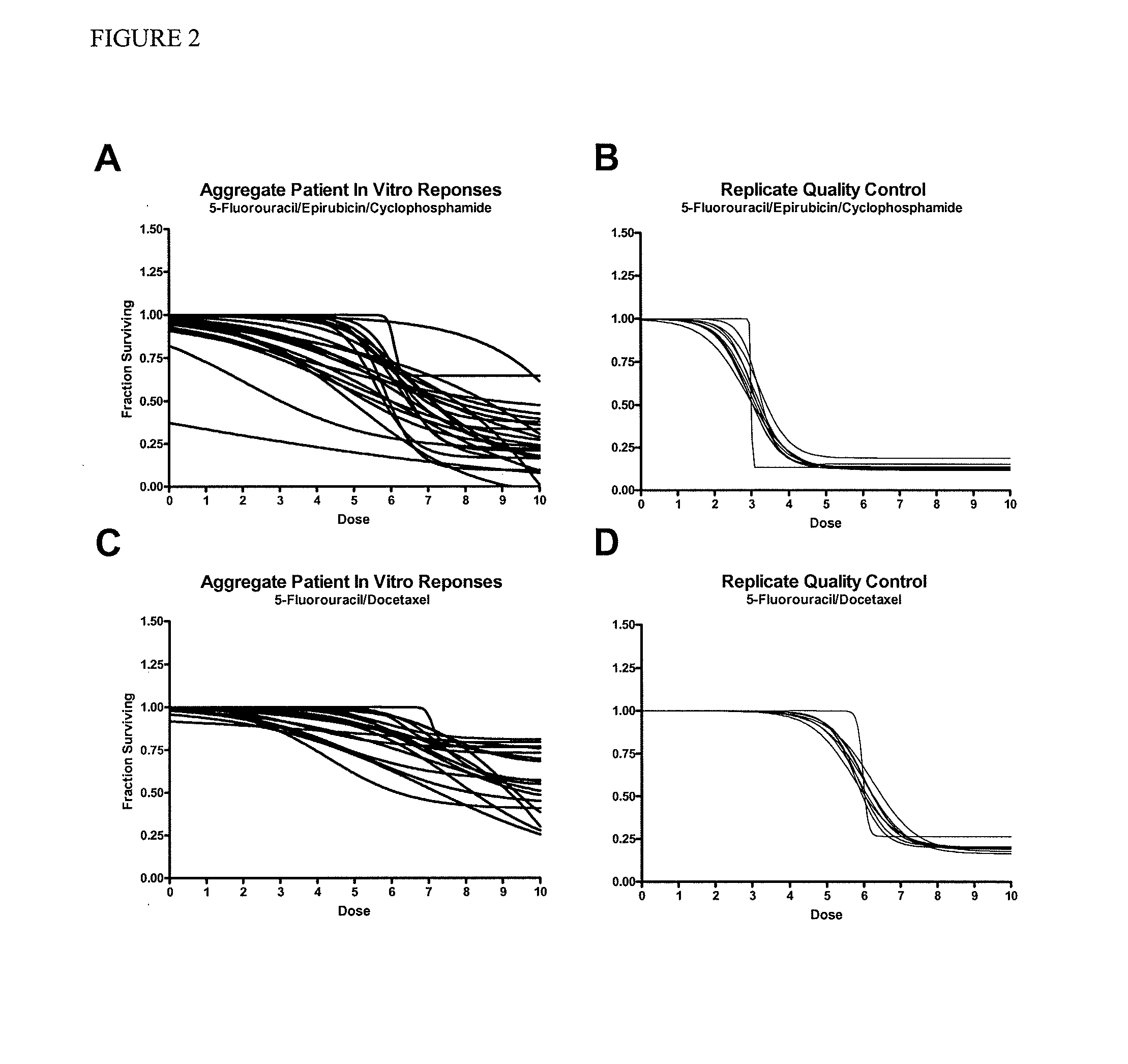
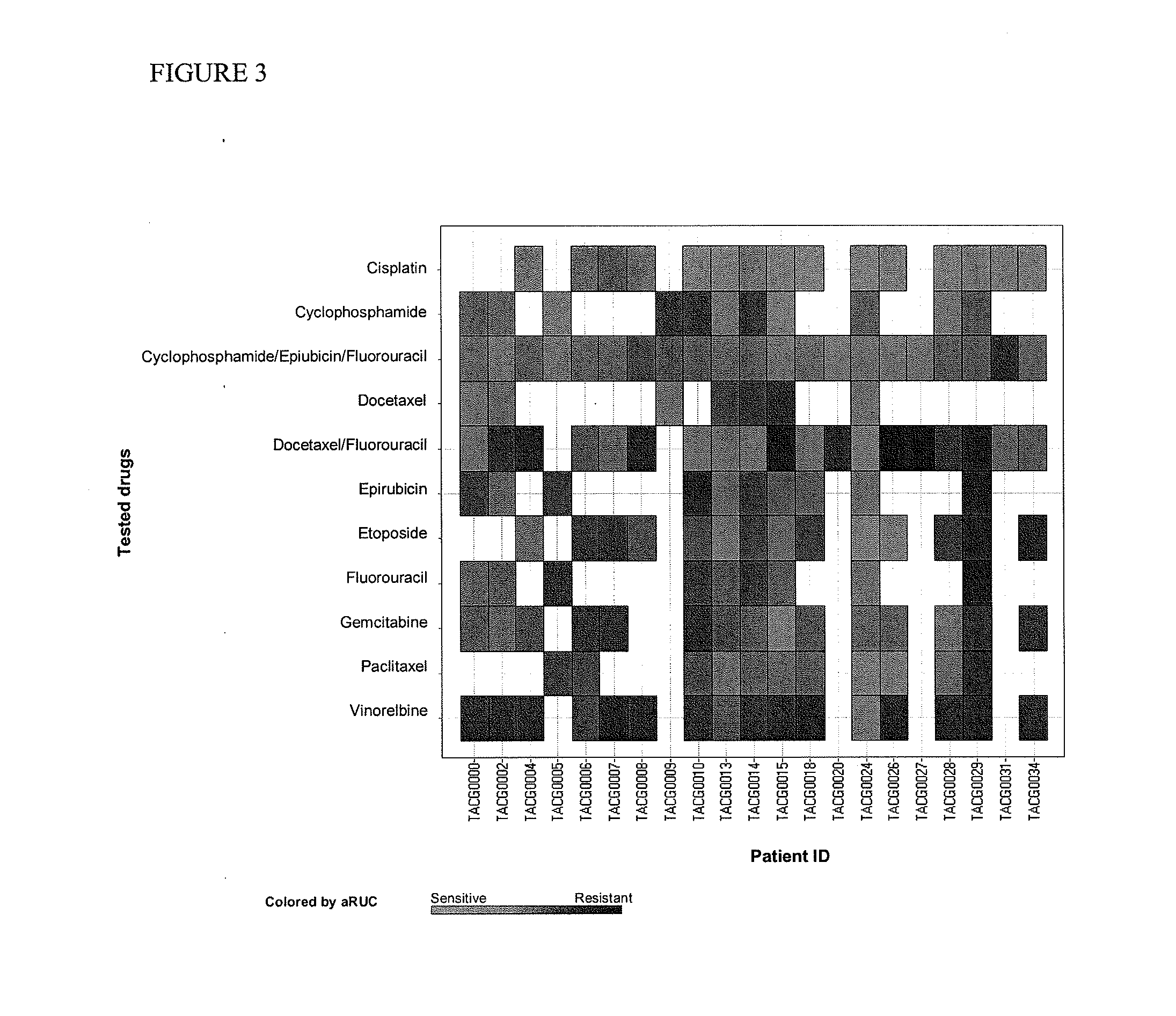
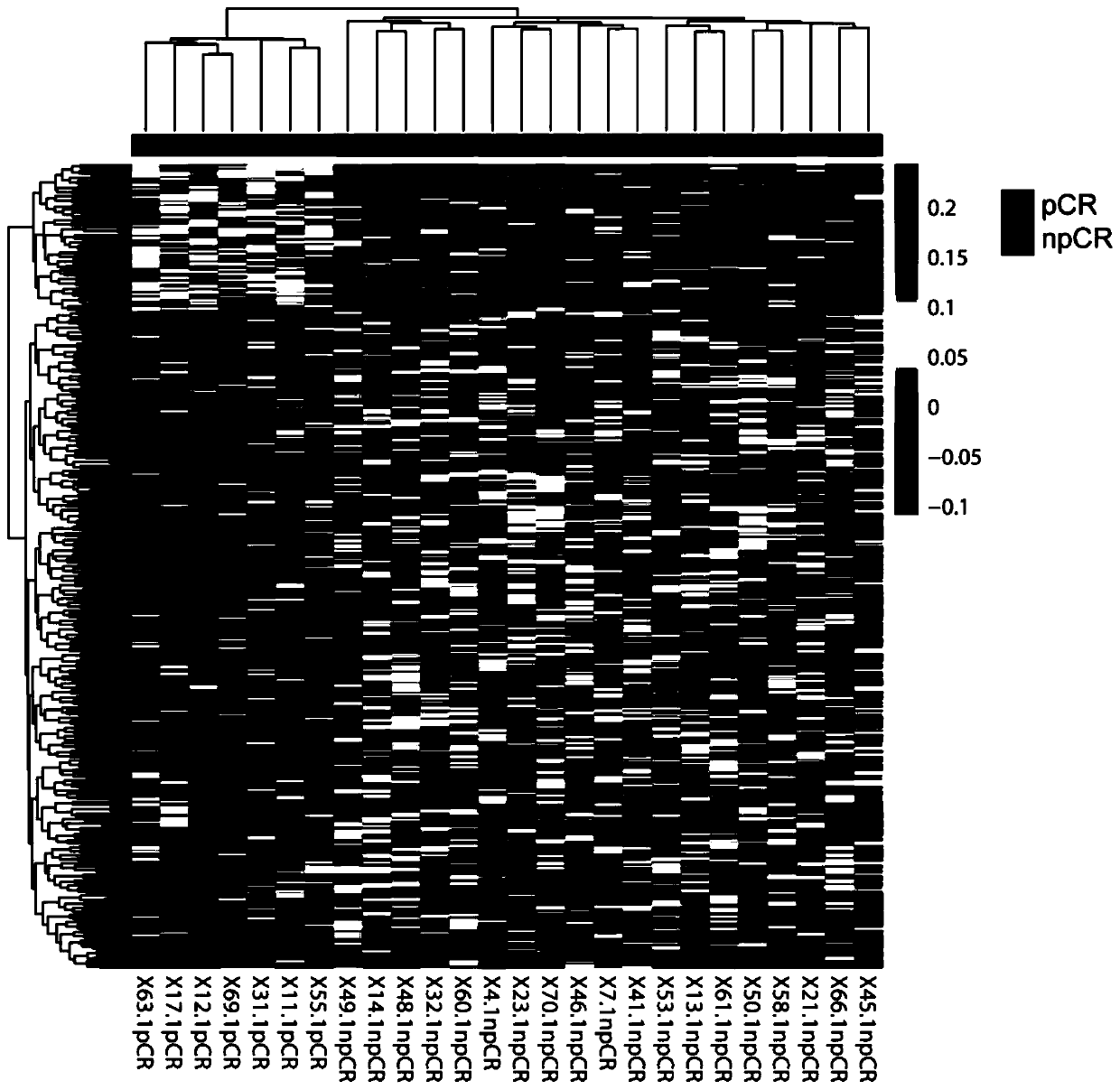
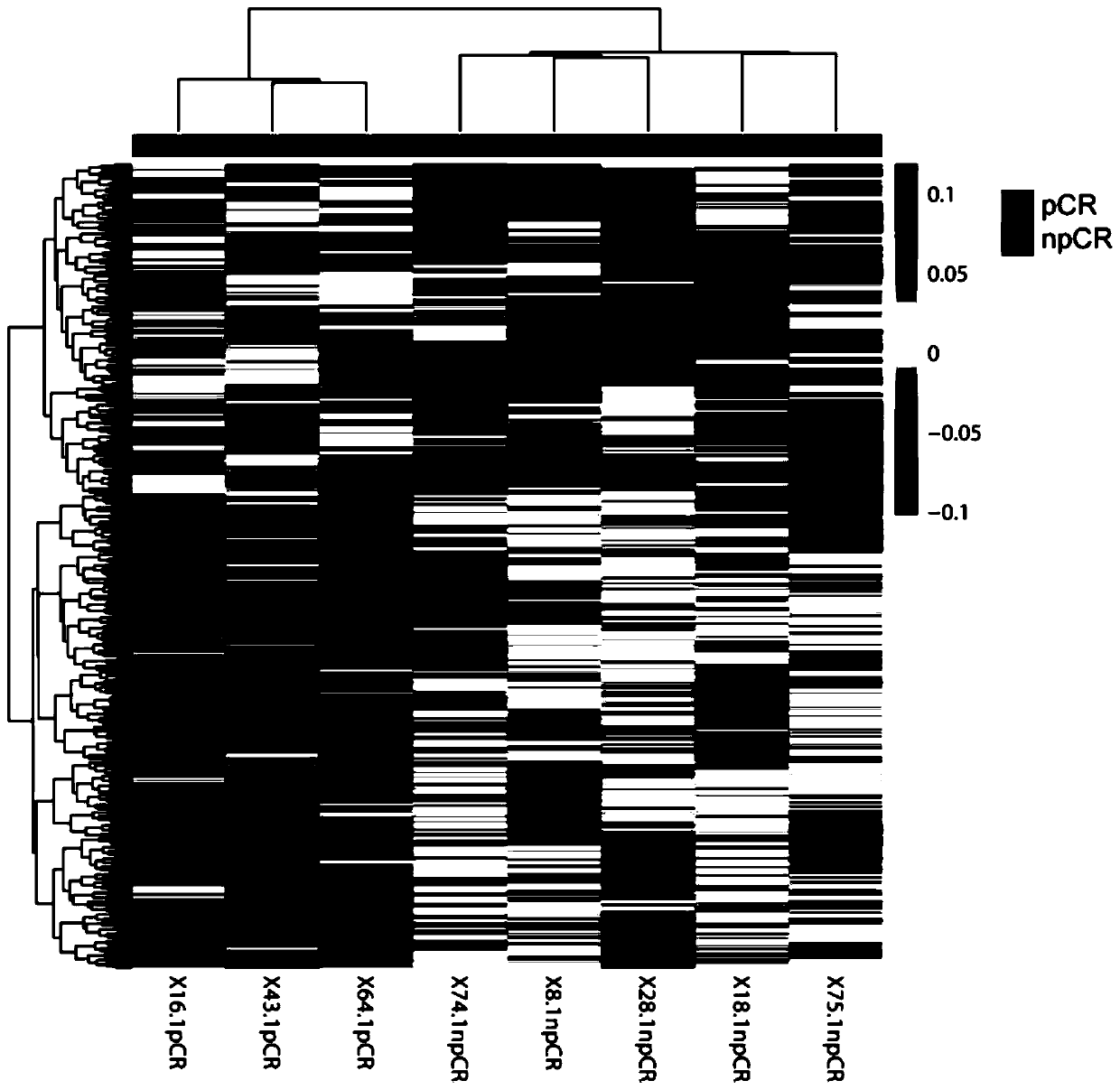
The present invention provides methods for individualizing chemotherapy, and particularly methods for individualizing neoadjuvant chemotherapy. The present invention provides methods for predicting a cancer patient's response to neoadjuvant chemotherapy, including assessing the probability of a positive response upon treatment with candidate agents prior to surgery. In various aspects, the invention involves culturing a monolayer of malignant cells from an explant of a patient's biopsy specimen, such as a transcutaneous biopsy-sized specimen, and testing the malignant cells for resistance or sensitivity to one or a plurality of candidate agents for neoadjuvant therapy. In other aspects, the invention provides methods for accurately scoring and interpreting such assays, and discloses in vitro chemoresponse results that are predictive of a patient's pathological complete response (pCR) upon receiving the corresponding treatment regimen.
Owner:PRECISION THERAPEUTICS
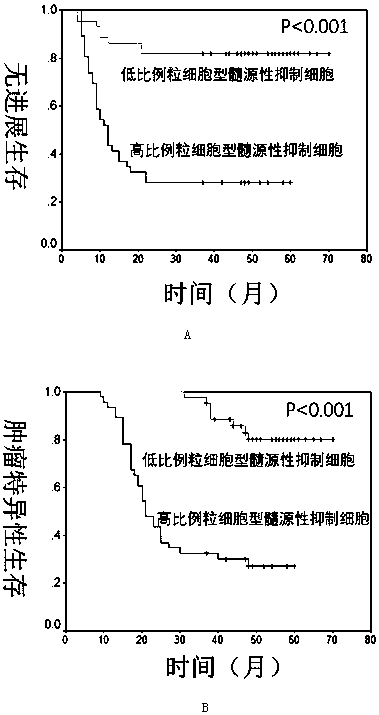
Use of granulocyte type myeloid derived suppressor cell as diagnostic biomarker
InactiveCN108254301ASignificant technological progressImprove survival rateIndividual particle analysisBladder cancer patientImproved survival
The invention provides use of a granulocyte type myeloid derived suppressor cell in the preparation of a diagnostic biomarker for diagnosing and predicting a neoadjuvant chemosensitivity effect of muscle-invasive bladder cancer. The invention further provides use of the granulocyte type myeloid derived suppressor cell in the preparation of a kit for diagnosing and predicting the neoadjuvant chemosensitivity effect of the muscle-invasive bladder cancer. The invention further provides a kit for diagnosing and predicting the neoadjuvant chemosensitivity effect of the muscle-invasive bladder cancer. The kit contains a reagent for detecting the granulocyte type myeloid derived suppressor cell. The invention discovers the granulocyte type myeloid derived suppressor cell as a novel biomarker fordiagnosing and predicting the neoadjuvant chemosensitivity effect of the muscle-invasive bladder cancer. A muscle-invasive bladder cancer patient with low-content granulocyte type myeloid derived suppressor cells can be sequentially subjected to neoadjuvant chemosensitivity and total cystectomy operation, so that the survival rate is increased, the excessive treatment is avoided, and the operationopportunity is not delayed.
Owner:RENJI HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Diagnostic methods for determining prognosis of non-small cell lung cancer
InactiveUS8951725B2Accurate identificationEnhanced informationBioreactor/fermenter combinationsBiological substance pretreatmentsFavorable prognosisFluorescence
The invention provides methods for identifying early stage non-small cell lung cancer (NSCLC) patients who will have a favorable prognosis for the recurrence of lung cancer after surgical resection. The invention is based on the discovery that assessment of chromosomal copy number abnormalities at two or more of chromosome 5p15, 7p12, 8q24 and centromere 6 can be used for prognostic classification. The invention preferably uses fluorescence in situ hybridization with fluorescently labeled nucleic acid probes to hybridize to patient samples to quantify the chromosomal copy number of the these genetic loci. Assessment of the copy number abnormality patterns using four classifiers produced statistically significant prognostic classification for NSCLC: (i) the Range3 pattern of cells showing a difference on a cell by cell basis, of at least three FISH probe signals between the FISH signals at the chromosomal locus with the largest number of FISH signals minus the FISH signals at the chromosomal locus with the lowest number of FISH signals; (ii) the MYC / EGFR % loss pattern assessing the percentage of cells showing fewer MYC FISH probe signals than EGFR FISH probe signals; (iii) a combination of the Range3 pattern and the MYC / CEP6 ratio pattern of a percentage of cells showing a relative loss of MYC FISH probe signals to the FISH probe signal for CEP6; (iv) the combination of the MYC / 5p15 ratio pattern showing the relative ratio of MYC and 5p15 locus signals of ≧0.80 and the 5p15 / CEP6 ratio pattern assessing percentage of cells having a relative ratio of 5p15 FISH probe signals to CEP6 FISH probe signals ≧1.1 versus MYC / 5p15 ratio of <0.80 or 5p15 / CEP6<1.1; and (v) a combination of the average range of probe signal differences of equal to or greater than about 2.5 with the Range3 pattern in a percentage of the cells. The invention can be used to identify those early stage NSCLC patients at higher risk of recurrence who should be treated with neoadjuvant chemotherapy before surgery or with adjuvant chemotherapy after surgery.
Owner:ABBOTT MOLECULAR INC
Gastric cancer marker and application thereof
ActiveCN110607371AImproved prognosisOptimize treatment planMicrobiological testing/measurementWilms' tumorStomach cancer
The invention relates to a gastric cancer marker and application thereof, in particular to application of at least one of a C10orf71 gene, a C10orf71 protein and a C10orf71 gene detection primer kit in the preparation of a product for predicting the response of a GC patient to neoadjuvant chemotherapy. It is determined for the first time that C10orf71 gene mutation is a key molecular feature withcancer in response to neoadjuvant chemotherapy. Based on the technical scheme, neoadjuvant chemotherapy has advantages in improving prognosis and determining responsive patients before treatment begins, and better therapeutic schemes can be developed. The scheme is an important step for optimizing therapeutic strategies for individual patients with GC.
Owner:BEIJING CANCER HOSPITAL PEKING UNIV CANCER HOSPITAL +1
Methods for selecting active agents for cancer treatment
InactiveUS20120219982A1Accurate scoreAccurate assessmentMicrobiological testing/measurementMaterial analysisActive agentPositive response
The present invention provides methods for individualizing chemotherapy, and particularly methods for individualizing neoadjuvant chemotherapy. The present invention provides methods for predicting a cancer patient's response to neoadjuvant chemotherapy, including assessing the probability of a positive response upon treatment with candidate agents prior to surgery. In various aspects, the invention involves culturing a monolayer of malignant cells from an explant of a patient's biopsy specimen, such as a transcutaneous biopsy-sized specimen, and testing the malignant cells for resistance or sensitivity to one or a plurality of candidate agents for neoadjuvant therapy. In other aspects, the invention provides methods for accurately scoring and interpreting such assays, and discloses in vitro chemoresponse results that are predictive of a patient's pathological complete response (pCR) upon receiving the corresponding treatment regimen.
Owner:HELOMICS CORP
Method for constructing breast cancer neoadjuvant chemotherapy effect classification model
PendingCN110570951ADetermine treatment strategyOvercome experienceCharacter and pattern recognitionProteomicsLymphatic SpreadTreatment strategy
The invention discloses a method for constructing a breast cancer neoadjuvant chemotherapy effect classification model. The method comprises obtaining the plasma cfDNA sequencing data of a patient with a known curative effect and performing TSSs area coverage analysis; determining a coverage difference and then performing cluster analysis to obtain a classification model. The model constructed bythe method of the invention can realize the effect prediction of neoadjuvant chemotherapy for a breast cancer patient, determines the treatment strategy of the breast cancer, and guides the breast cancer neoadjuvant chemotherapy for. The data required by the model of the present invention derives from peripheral blood and belongs to the category of noninvasive detection. The method solves the poorclinical experience, the incapability of solving tumor heterogeneity, the puncture contraindications, and the risk of tumor spread and metastasis caused by the need for invasive puncture sampling ofan existing material for predicting the efficacy of the breast cancer neoadjuvant chemotherapy,.
Owner:广州市雄基生物信息技术有限公司
Method for improving neoadjuvant chemotherapy
Disclosed is a method and composition for optimizing the efficiency of breast cancer neoadjuvant chemotherapy, depending on the particular constitutional genotype characteristics of the gene BRCA1 in each patient. Generally, the invention concerns a new method to improve neoadjuvant therapy depending on a particular constitutional genotype. Subject of invention allow to synthesize DNA and identification of germline BRCA1 genetic abnormalities which are correlated with a significantly decreased clinical response to neoadjuvant chemotherapy based on taxane-derived cytostatics in breast cancer patients.
Owner:BYRSKI TOMASZ +4
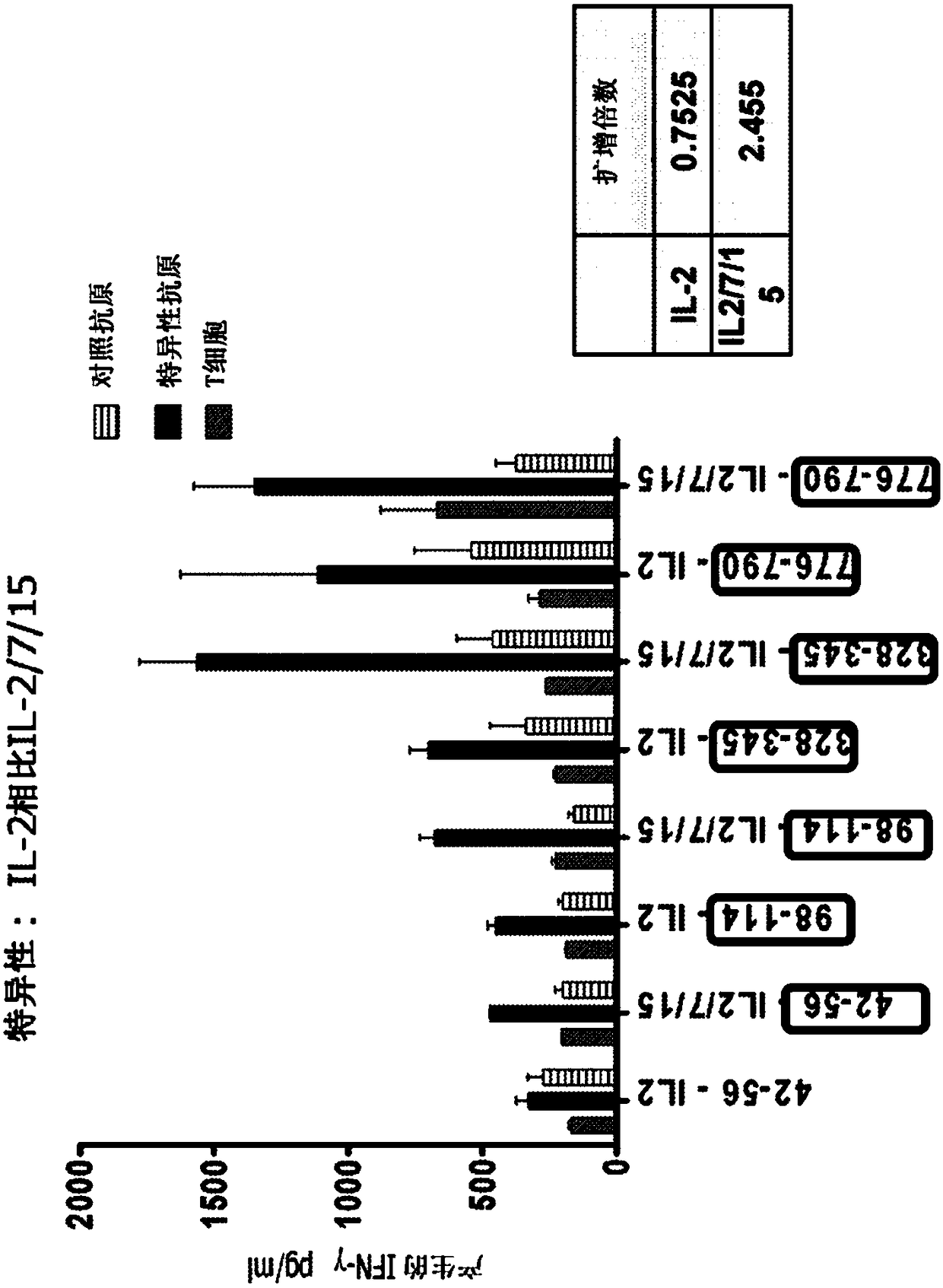
In vitro artificial lymph node for sensitization and expansion of t cells for therapy and epitope mapping
InactiveCN108289910AMammal material medical ingredientsBlood/immune system cellsCulture expansionInvasive breast carcinoma
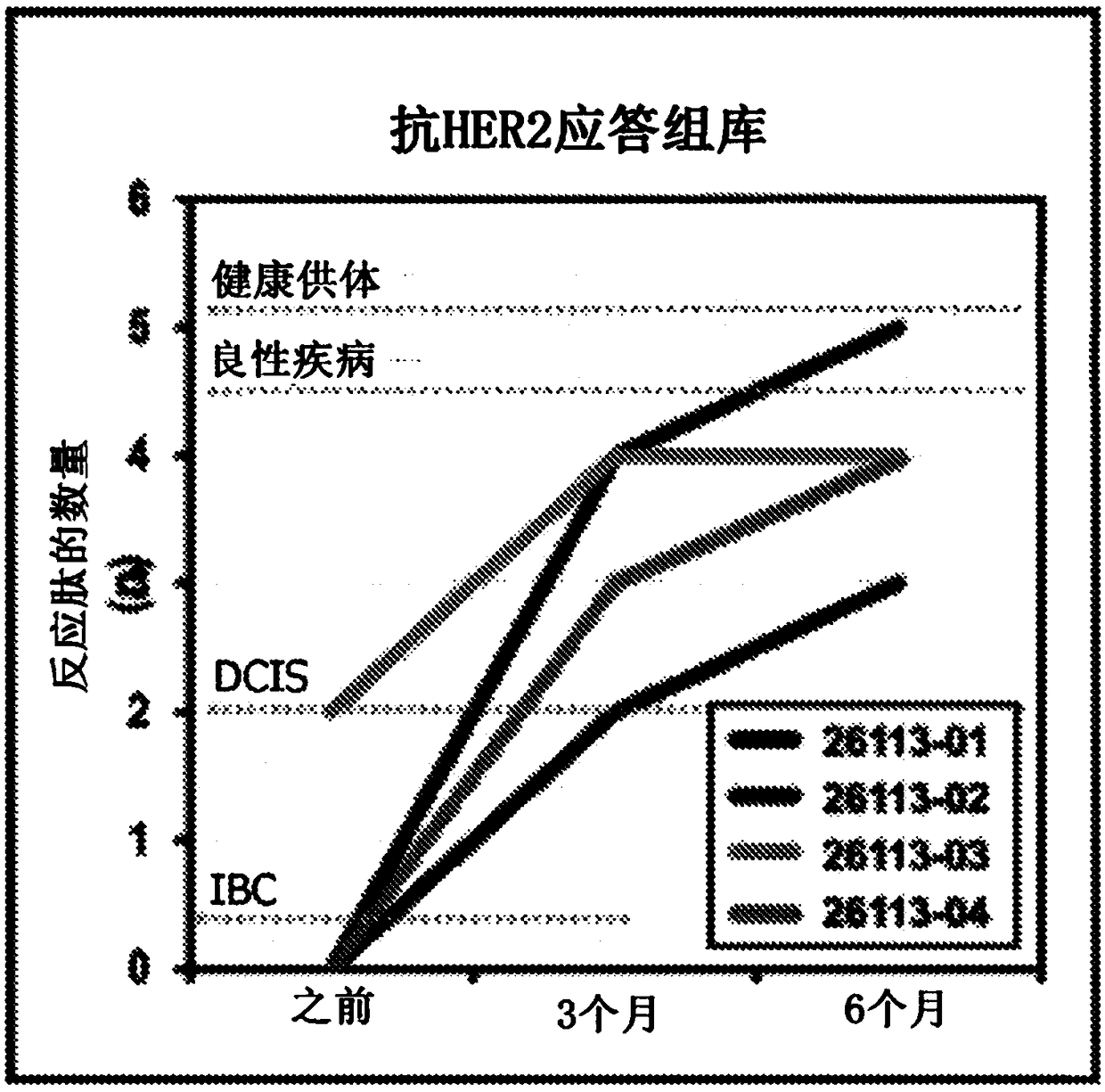
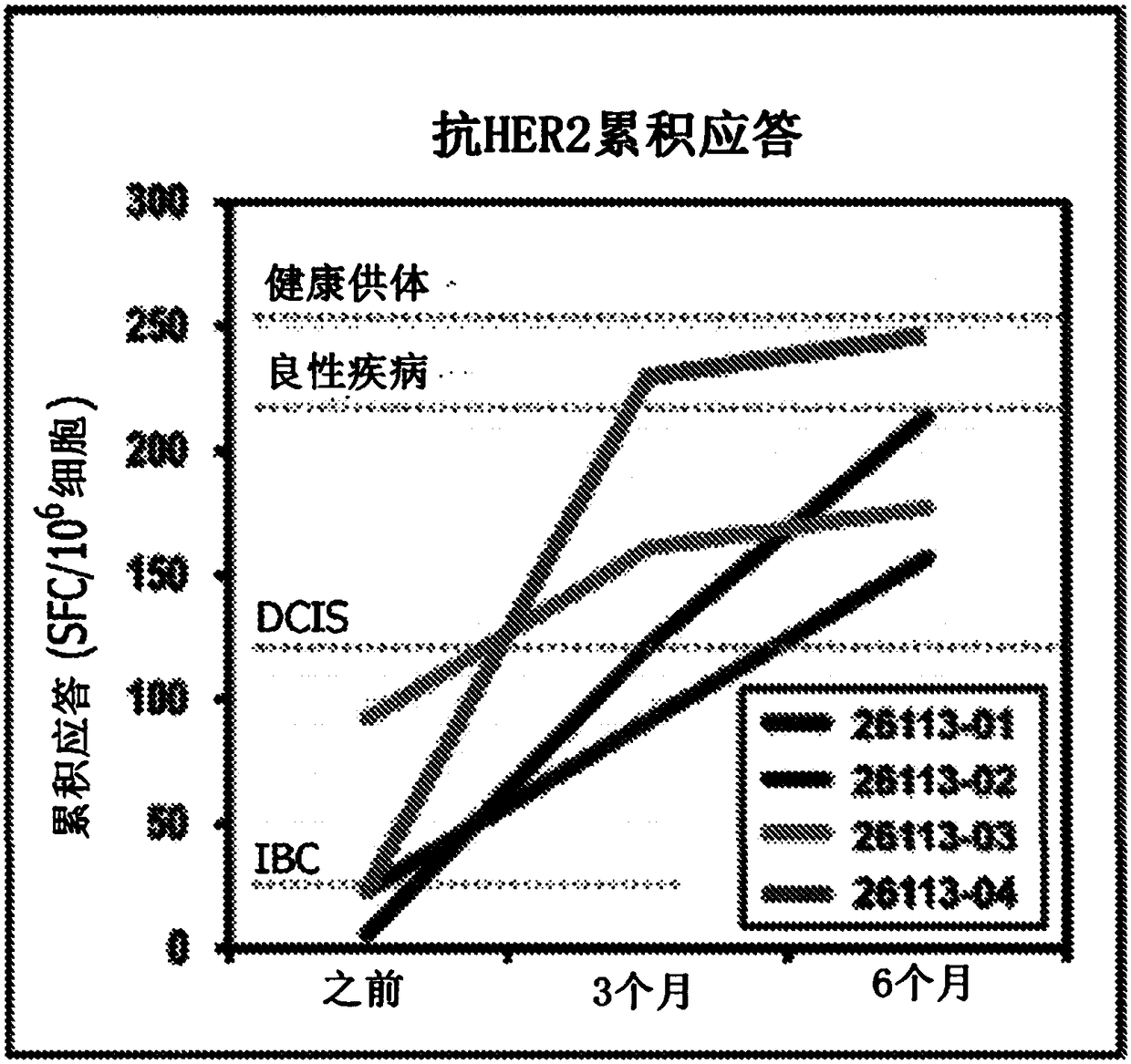
HER2+ invasive breast cancer (IBC) patients with residual disease following neoadjuvant chemotherapy have an anti-HER2 Type 1 T helper (Th1) cell immune deficit and a significant risk of recurrent disease. It has been shown that anti-HER2 CD4+ T-cell responses incrementally decrease along the breast cancer continuum - a robust response in healthy donors and patients with benign disease, a depressed response in patients with HER2+ ductal carcinoma in situ, and a nearly absent response in patients with HER2+ IBC. This invention relates to a method of creating a microenvironment for culture expansion of T cells. The expanded T cells can be used for a variety of therapeutic and research purposes.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
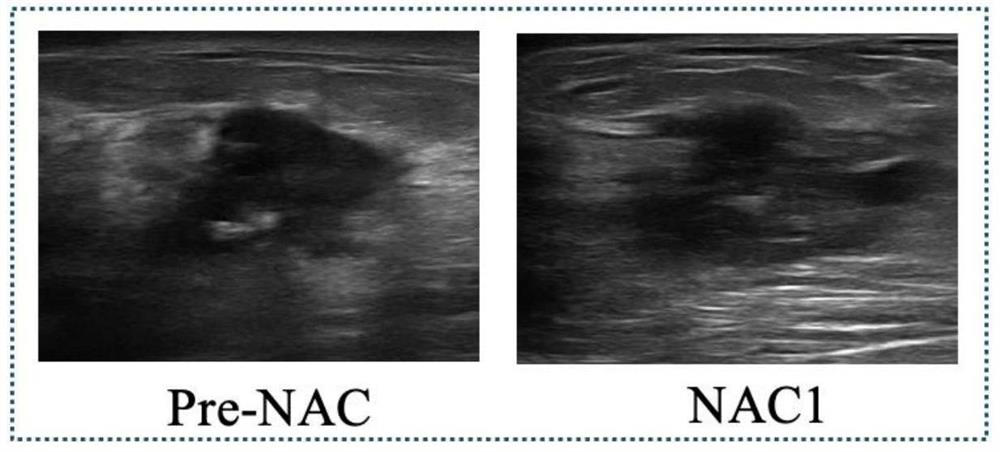
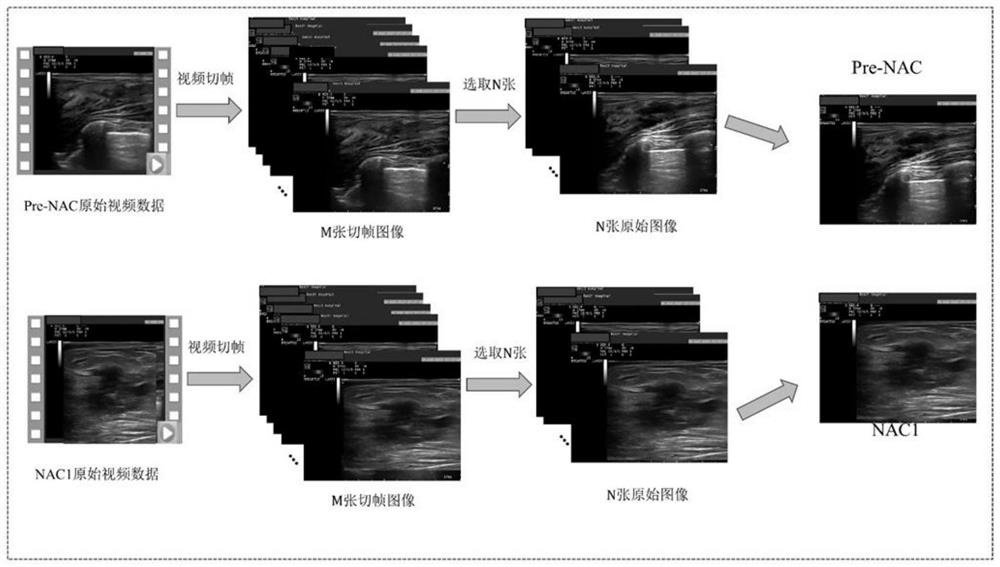
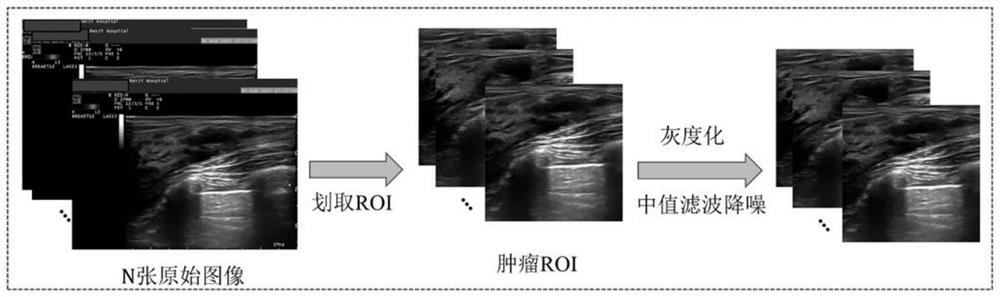
Early prediction method for curative effect of cancer chemotherapy based on two-channel convolutional neural network
The invention discloses a cancer chemotherapy curative effect early prediction method based on a two-channel convolutional neural network, and the method comprises the steps: respectively inputting image data before chemotherapy and image data after first-stage chemotherapy into two channels for layer-by-layer convolution operation, and carrying out the feature fusion between the two channels, the features output by the last layer of the two channels are subjected to weighted fusion to obtain an output result, each channel has 9 convolution layers, and four times of feature fusion and three times of pooling are carried out. According to the method, the relation between the data in different chemotherapy stages is considered, the relation characteristics between the data can be effectively utilized, in addition, a channel weight analysis strategy is added, a priori knowledge guiding model is introduced for training, the relation between the data in different chemotherapy stages is fully considered, and the accuracy of the method is improved. The prediction task can be completed only by using the ultrasonic image data of the first-stage neoadjuvant chemotherapy, and the precision and the speed are high.
Owner:SHANGHAI UNIV
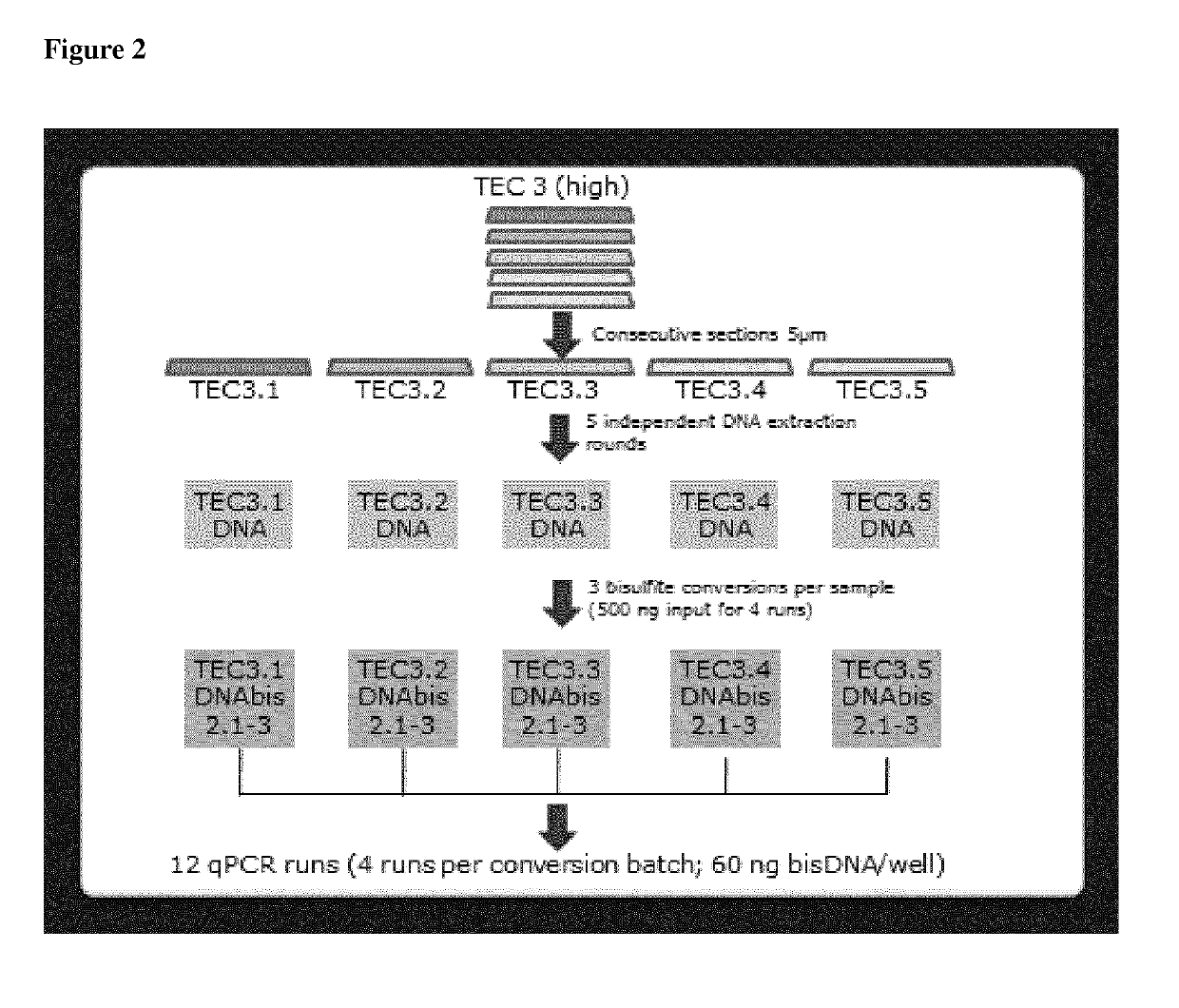
Methods for assessing the treatment response of tnbc patients to neo-adjuvant chemotherapy by analysing cpg methylation
Owner:THERAWIS DIAGNOSTICS GMBH
Methods for assessing the treatment response of TNBC patients to neo-adjuvant chemotherapy by analysing CpG methylation
The present invention relates to methods for predicting the efficacy of anthracycline-based neo-adjuvant chemotherapy in triple-negative breast cancer. This is achieved by determining epigenetic changes within the PITX2 gene. Detection of the methylation state of Cp G sites in a genomic sequence of PITX2 allows an estimate of the response or failure of an individual breast cancer patient to neo-adjuvant therapy.
Owner:THERAWIS DIAGNOSTICS GMBH
Predictive and Prognostic Methods in Breast Cancer
PendingUS20210363594A1Increase probabilityOrganic active ingredientsMicrobiological testing/measurementComplete remissionIV Chemotherapy
Owner:BIONTECH DIAGNOSTICS +1
A circular RNA detection kit predicts responsiveness to neoadjuvant chemotherapy in triple-negative breast cancer
ActiveCN110923321BResponsive PredictionHelp determineMicrobiological testing/measurementDNA/RNA fragmentationNucleotideChemotherapy combinations
Owner:GUANGDONG GENERAL HOSPITAL
The use of pdl1 SNP genotype as a marker for predicting the efficacy of neoadjuvant chemotherapy in breast cancer
ActiveCN108315430BSignificant technological progressProlong survival timeMicrobiological testing/measurementDNA/RNA fragmentationComplete remissionChemotherapy effects
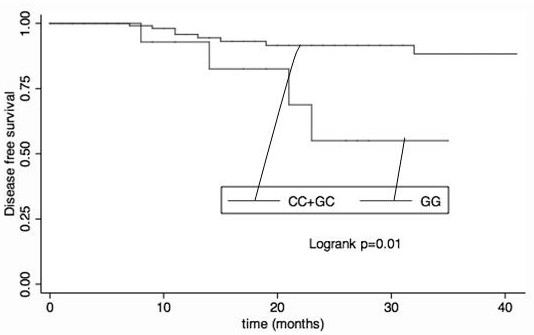
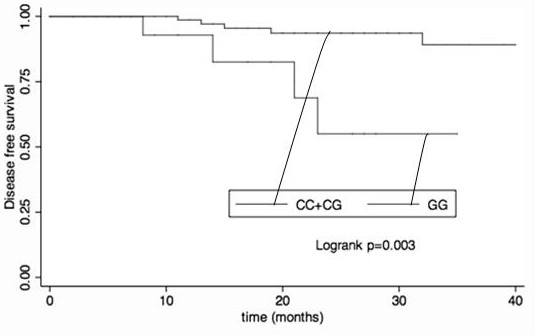
The invention provides the use of the PDL1 SNP genotype in the preparation of markers for diagnosis and prediction of the curative effect of neoadjuvant chemotherapy for breast cancer. Also provided is the use of the PDL1 SNP genotype as a marker in the preparation of reagents for diagnosing and predicting the curative effect of neoadjuvant chemotherapy for breast cancer. Also provided is a kit for detecting the PDL1 SNP genotype, which contains the primers shown in SEQ ID NO.1-18. The present invention finds that in all breast cancer populations and HR-positive populations, after the SNP rs4143815 C to G change in the non-coding region of PDL1 gene, patients with GC genotype are more likely to achieve pCR than patients with GG and CC genotypes. Through the detection of the SNP in the non-coding region of the PDL1 gene in the patient's blood, patients with the GC genotype can be detected as early as possible, and neoadjuvant chemotherapy can be performed in order to achieve complete remission of the pathology and improve the survival time.
Owner:RENJI HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com