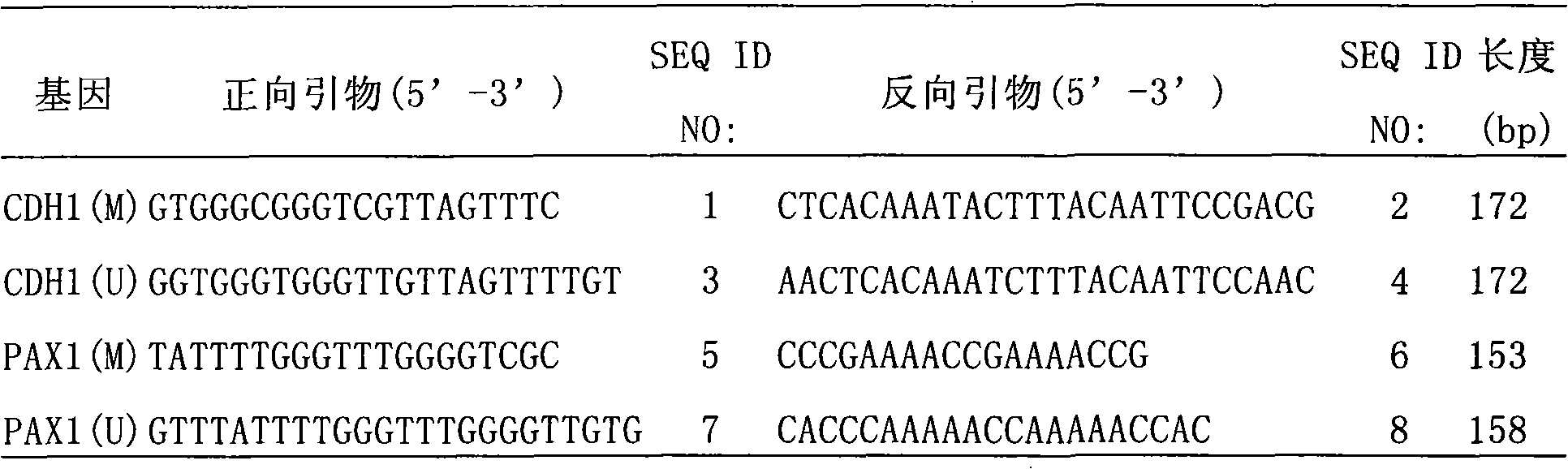
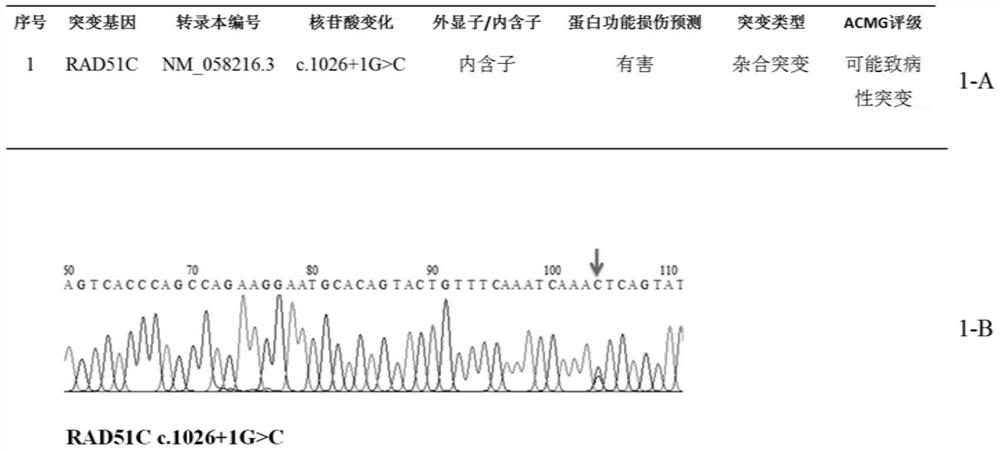
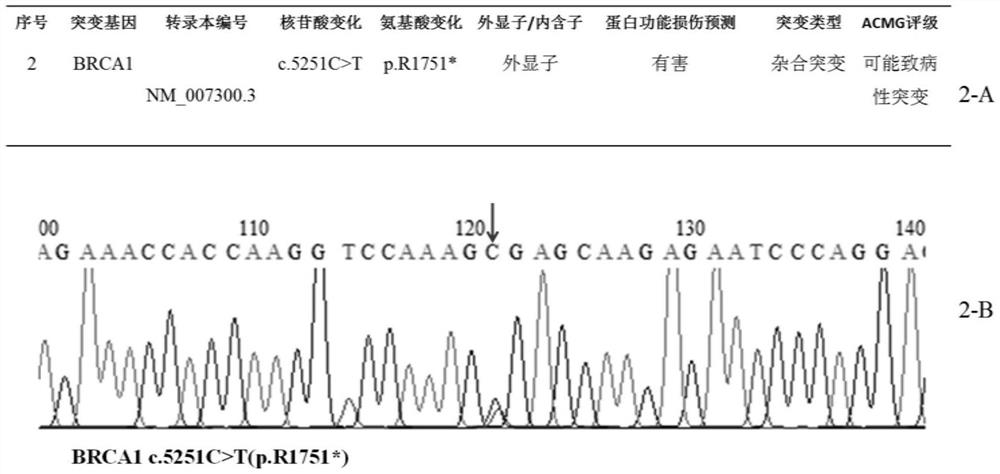
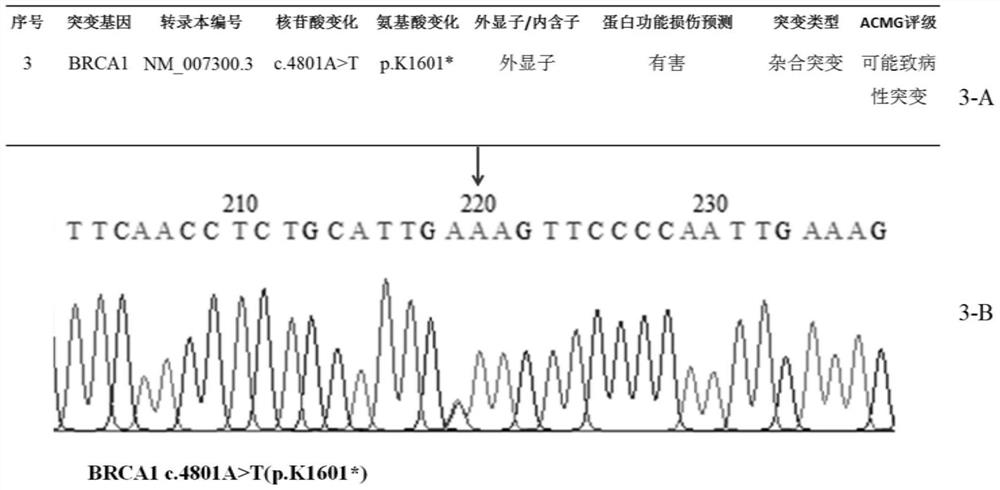
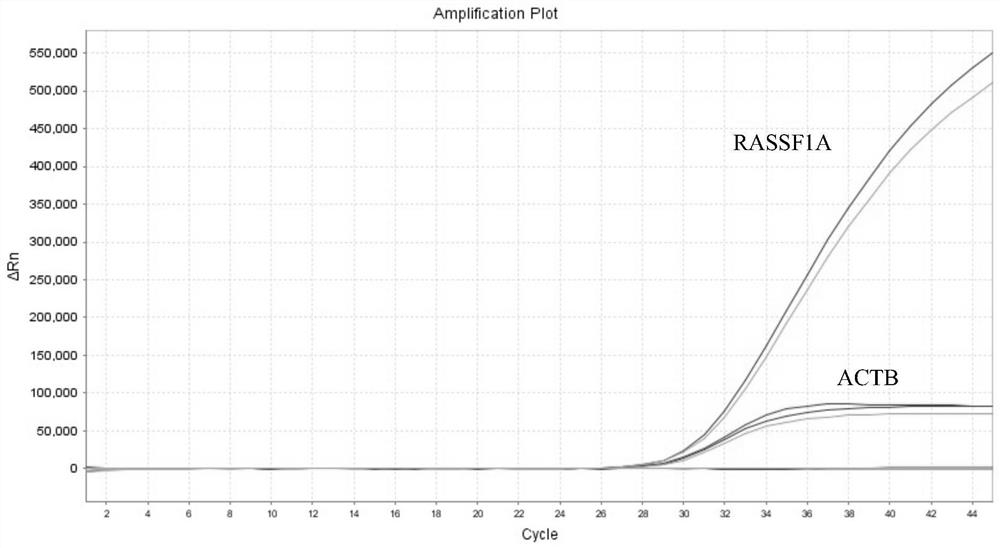
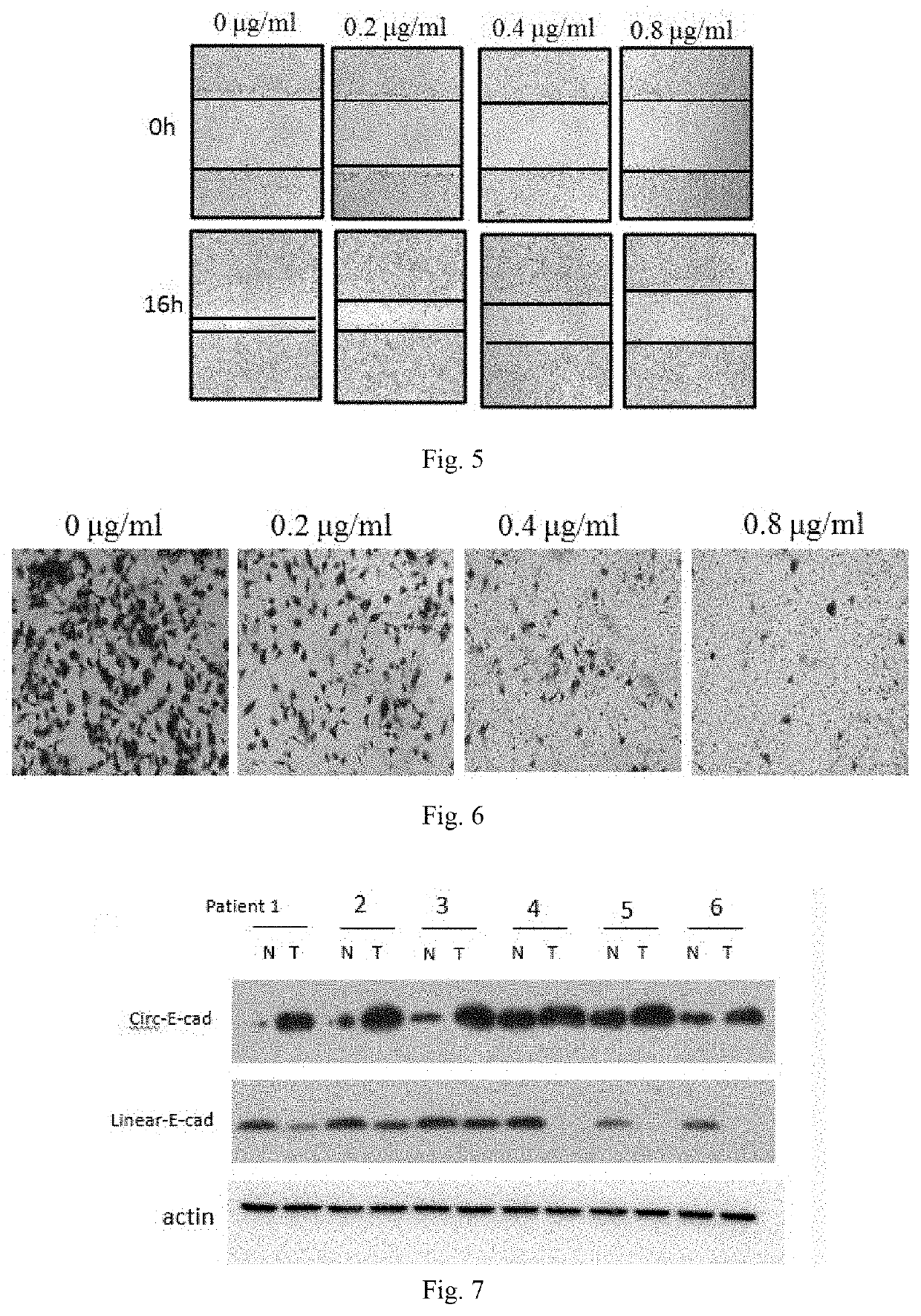
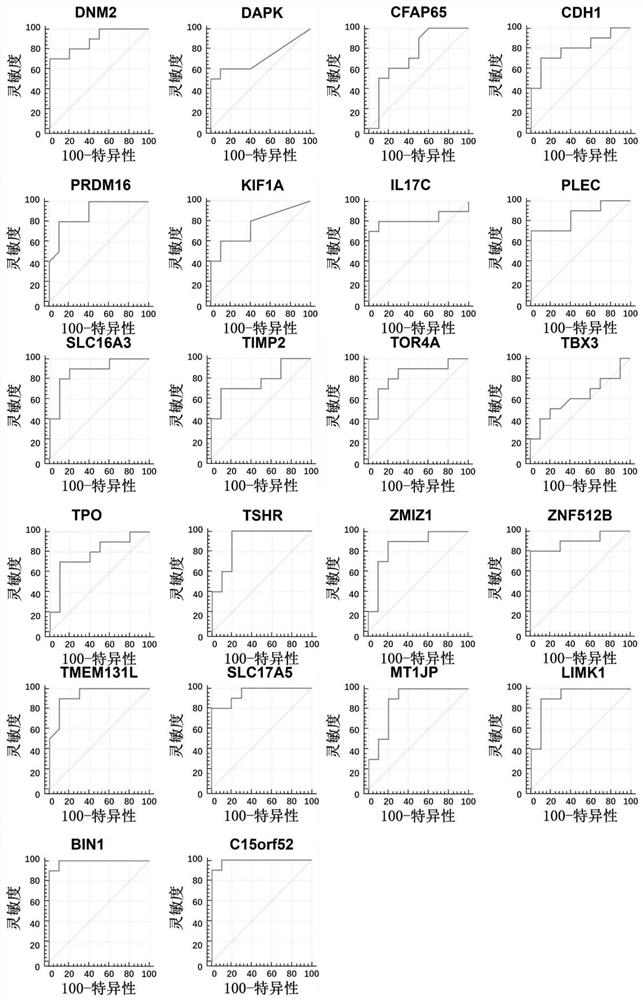
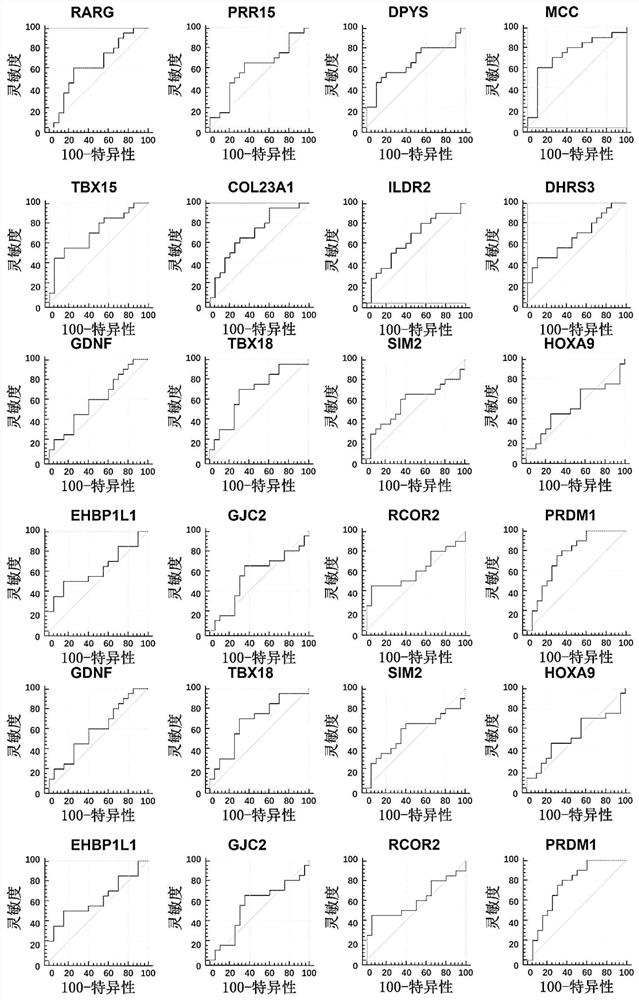
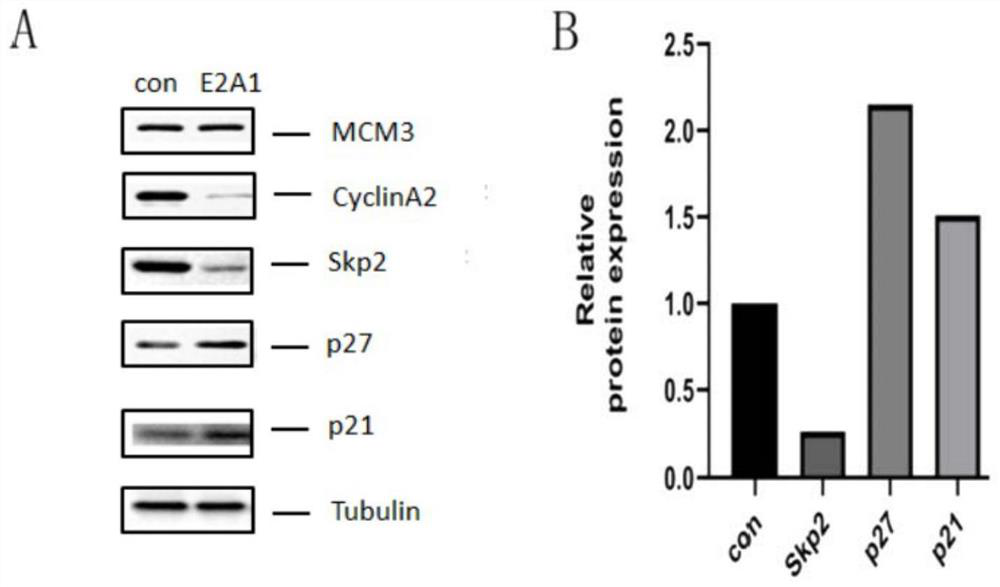
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
41 results about "CDH1" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cadherin-1 also known as CAM 120/80 or epithelial cadherin (E-cadherin) or uvomorulin is a protein that in humans is encoded by the CDH1 gene. CDH1 has also been designated as CD324 (cluster of differentiation 324). It is a tumor suppressor gene.
Mutant gene group for mammary cancer risk assessment and detection kit thereof
InactiveCN103981273AImprove detection efficiencyImprove the detection rateMicrobiological testing/measurementDNA/RNA fragmentationBrca1 geneCvd risk
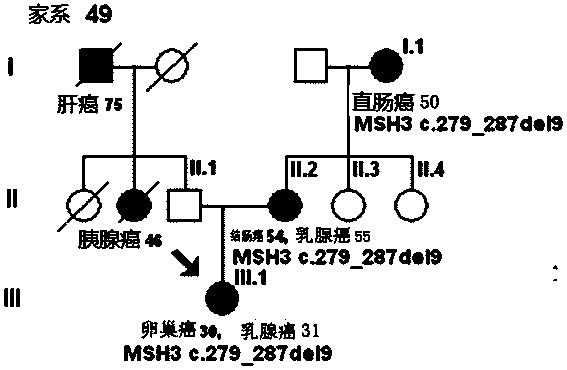
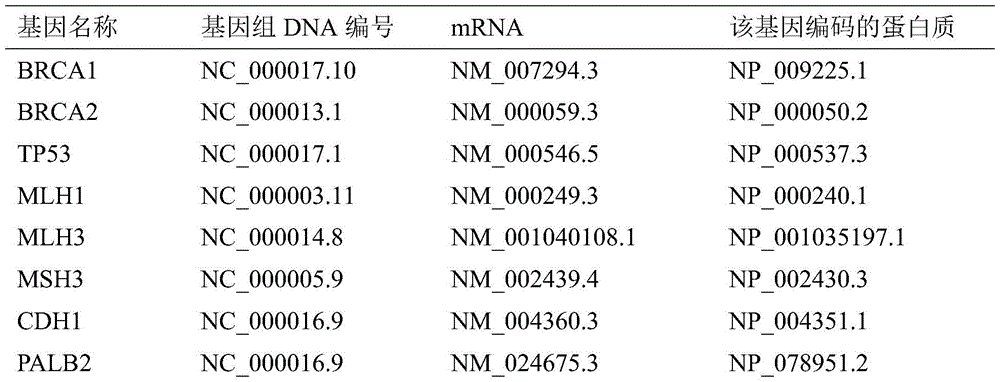
The invention discloses a mutant gene group for mammary cancer risk assessment and a detection kit thereof. The mutant gene group is a mutant gene set comprising the following seven genes: BRCA1 gene, BRCA2 gene, TP53 gene, MLH1 gene, MLH3 gene, MSH3 gene and CDH1 gene. The mutant gene group of the seven genes totally carries 25 mutant sites disclosed as Table 3. The invention also discloses a detection kit of the mutant gene group for mammary cancer risk assessment, which comprises probes or primers for detecting the mutant genes in the mutant gene group. The detection kit disclosed by the invention has the advantages of high detection efficiency, high detection speed, high detectable rate and the like.
Owner:FUDAN UNIV SHANGHAI CANCER CENT
Method and kit for detecting cervical carcinoma by using PAX1 and CDH1 gene methylation
InactiveCN101831490AStrong complementarityWide coverageMicrobiological testing/measurementBiologyCDH1
The invention relates to a method and a kit for detecting cervical carcinoma by using PAX1 and CDH1 gene methylation. Particularly, the invention provides a kit, which contains (a) a primer pair aiming at methylation specificities and non-methylation specificities of a POX1 gene and a CDH1 gene, or contains (b) a methylation sensitivity restriction endonuclease, and a primer pair aiming at the specificities of the POX1 gene and the CDH1 gene. The kit can effectively separate the cervical carcinoma from cervicitis and HPV infection.
Owner:上海市第八人民医院
System for predicting prognosis of gastric cancer in subjects
ActiveCN111724903AImproved prognosisMedical data miningHealth-index calculationGastric carcinomaOncology
The invention provides a system for predicting prognosis of gastric cancer of subjects. The system comprises: a data acquisition module, which is used for acquiring clinical characteristic data of thesubjects, immune marker characteristic data of the subjects and protein expression data of the subjects; a data processing module, which is used for further processing the data acquired in the data acquisition module; and a module for calculating the prognosis risk of the gastric cancer of the subjects, wherein the module utilizes the data processed in the data processing module to calculate theprognosis risk values of the gastric cancer of the subjects, and groups the subjects based on the risk values. According to the system and a method provided by the invention, eight characteristics, including five immunomarkers (CD3, CD4, PDL1, PAX5, and GZMB), an EMT protein marker (CDH1) and two clinical characteristics (pTNM and age), are selected to develop the system and the method that can significantly improve prognostic ability of gastric cancer patients. The system and the method may be applicable to patients with or without neoadjuvant chemotherapy and exhibit predicted values, and the patients may benefit from post-operative adjuvant chemotherapy.
Owner:BEIJING CANCER HOSPITAL PEKING UNIV CANCER HOSPITAL
Kit for identification of gastric cancer and/or gastric polyps and application thereof
InactiveCN108977544AGuaranteed correctnessGuaranteed reliabilityMicrobiological testing/measurementGastric PolypTrue positive rate
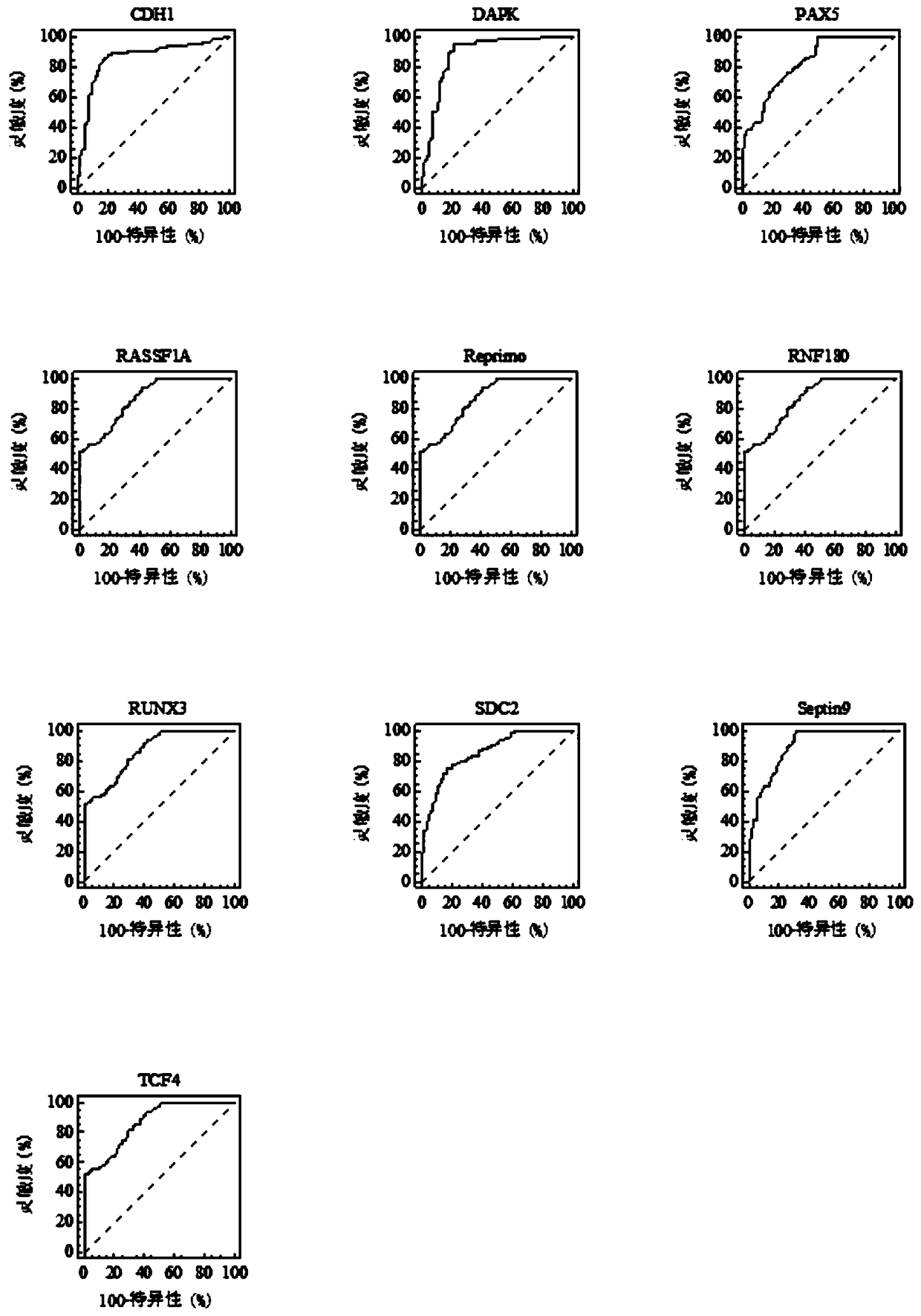
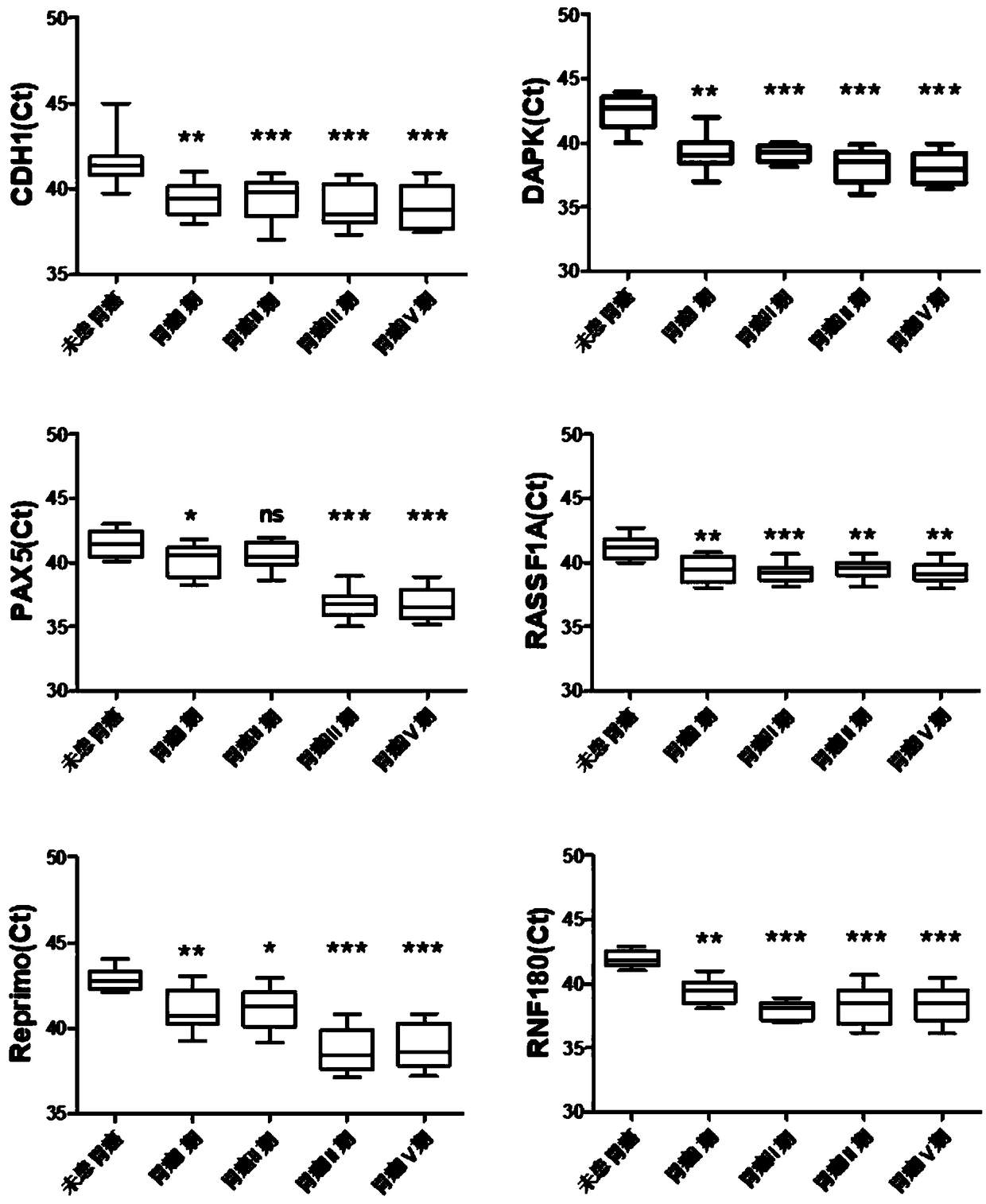
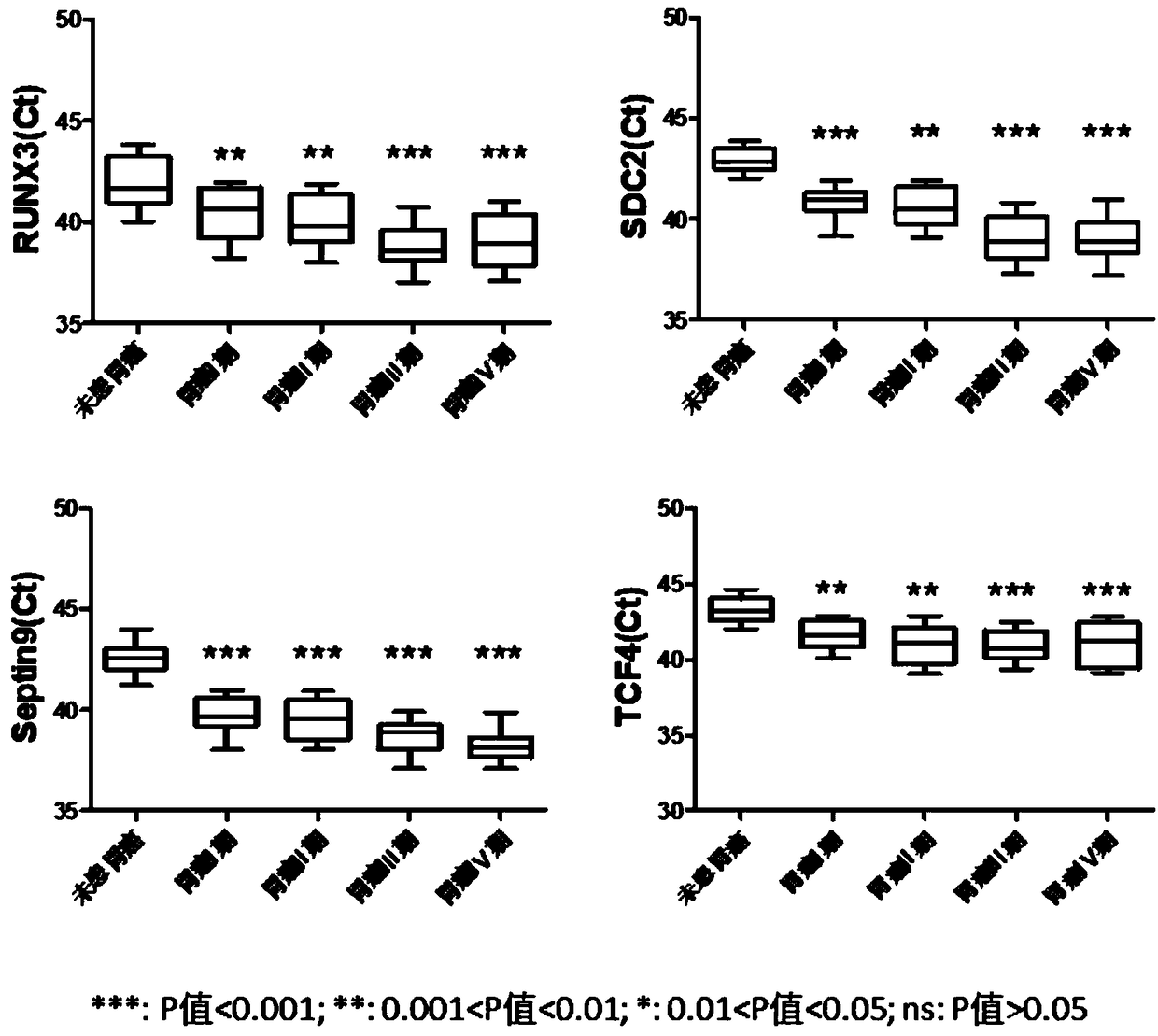
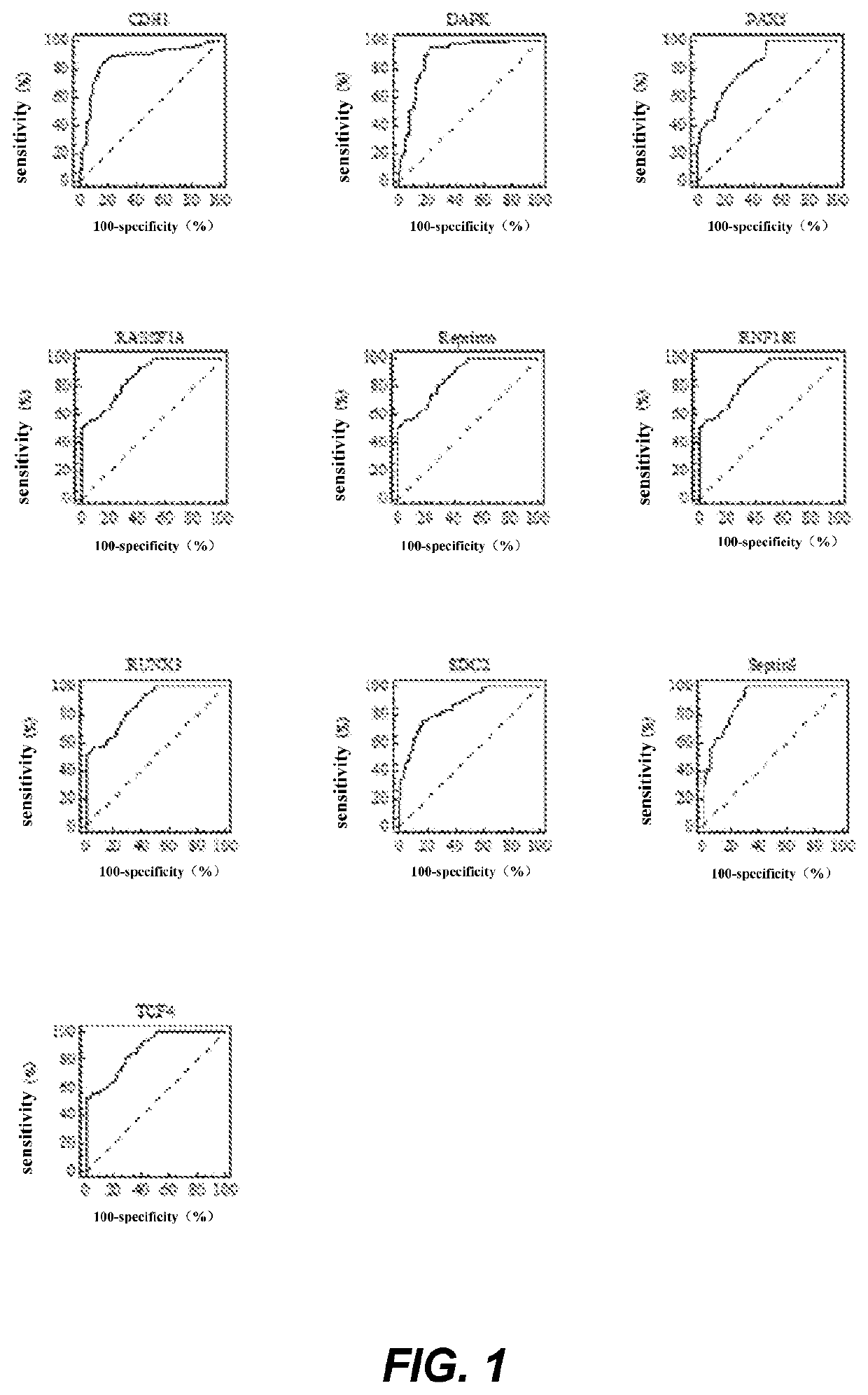
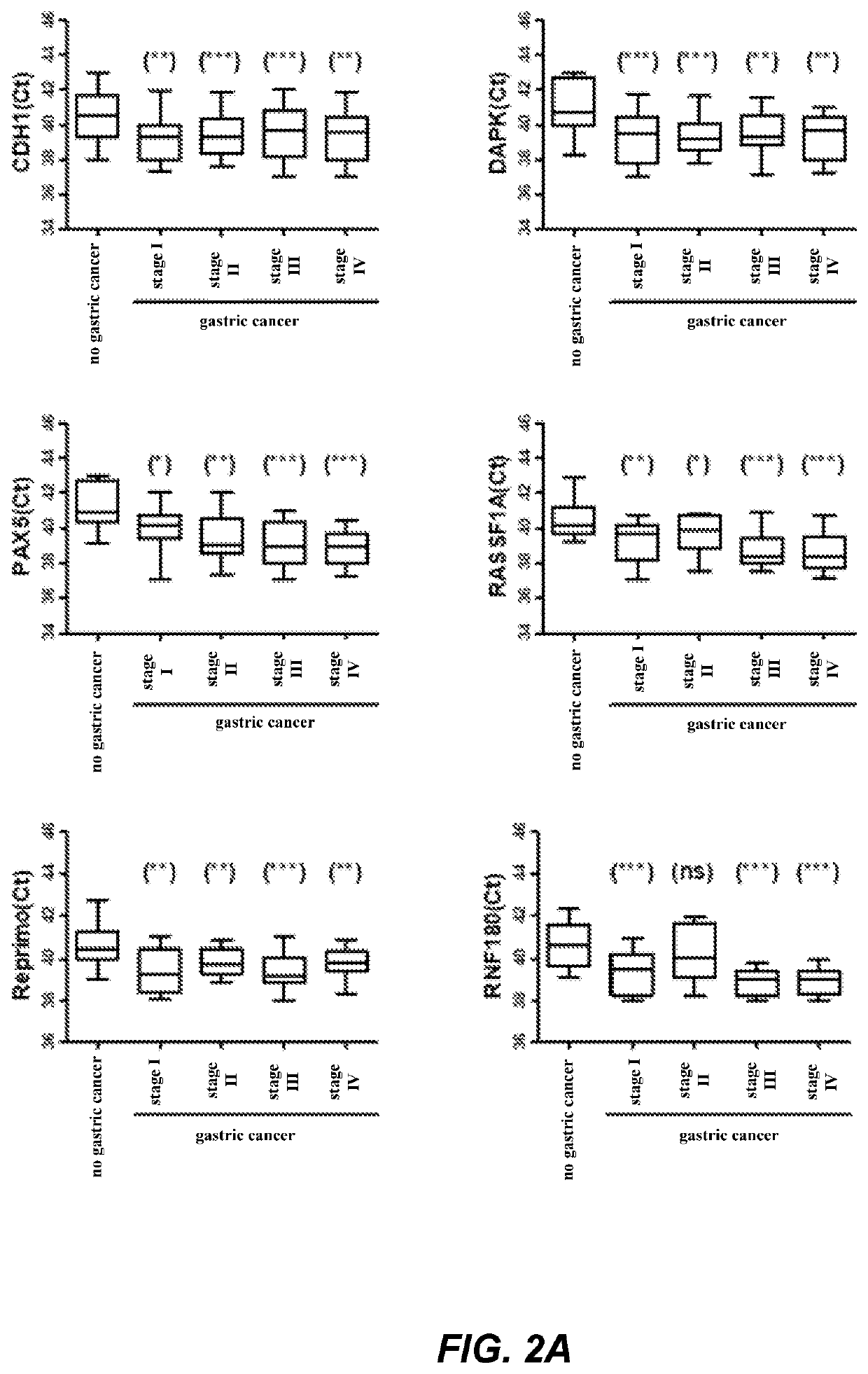
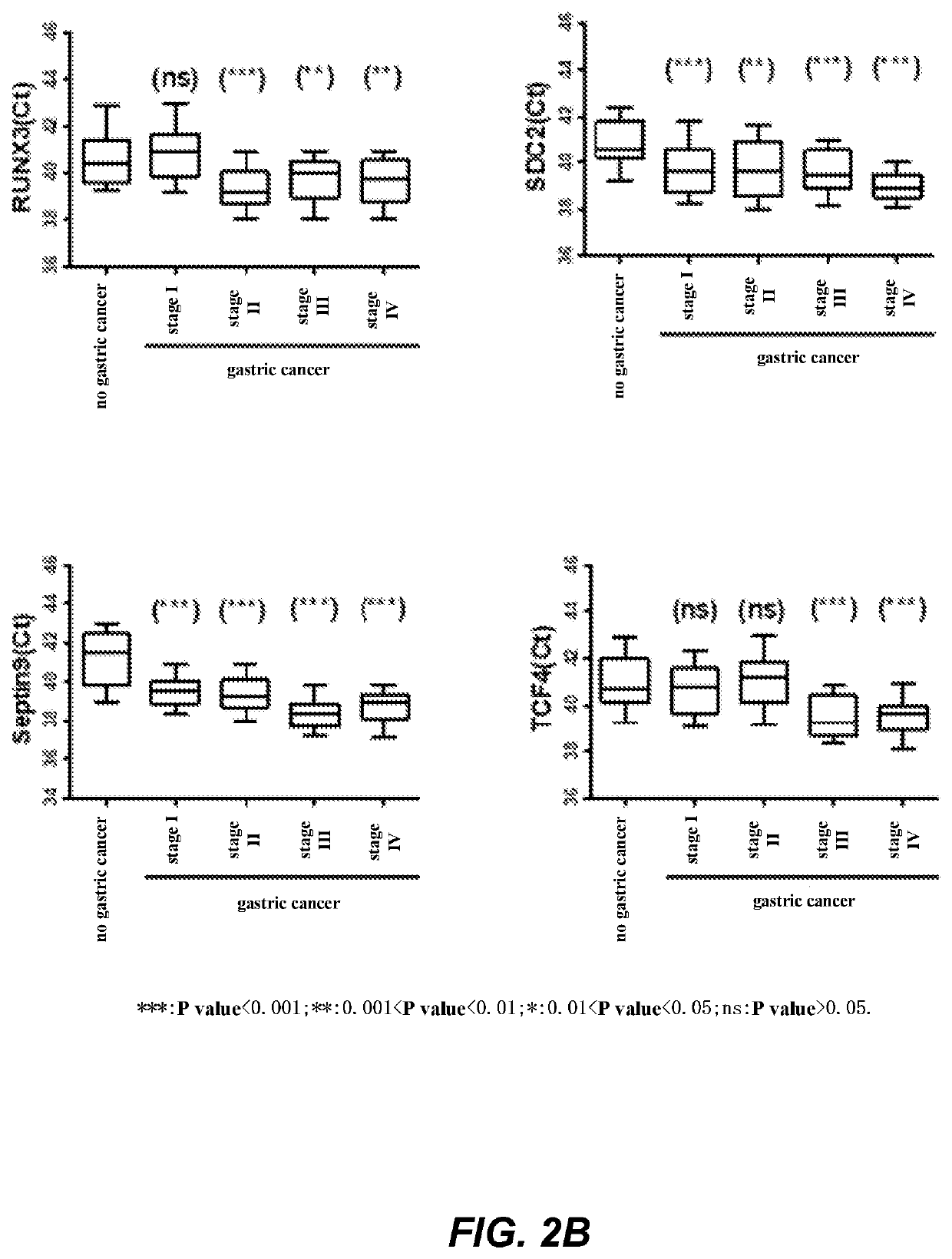
The invention relates to a kit for the identification of gastric cancer and / or gastric polyps and application thereof. The kit comprises a primer pair, wherein the primer pair is used for detecting the methylation level of a biological marker gene or corresponding segments in a biological sample of a tester; the primer pair is used for performing PCR (polymerase chain reaction) amplification reaction by using the biological marker gene or segments thereof treated by bisulfite as a template; the biological marker gene is selected from one or multiple of CDH1, DAPK, PAX5, RASSF1A, Reprimo, RNF180, RUNX3, SDC2, Septin9 and TCF4. The kit has the advantage that by jointly detecting the methylation level of the biological marker gene or corresponding segments, the sensitivity and specificity indetection of the gastric cancer and / or gastric polyps is improved, so that the correctness and reliability of the detection results are guaranteed, and a quick, reliable and accurate novel path is provided for the predicting, diagnosis and evaluation on the gastric cancer and / or gastric polyps.
Owner:BEIJING EXELLON MEDICAL TECH CO LTD
Analytical method for excavating key lncRNA in process of differentiating chick embryo stem cells into male germ cells
PendingCN105838791AShort cycleImprove efficiencyMicrobiological testing/measurementTotal rnaPlant Germ Cells
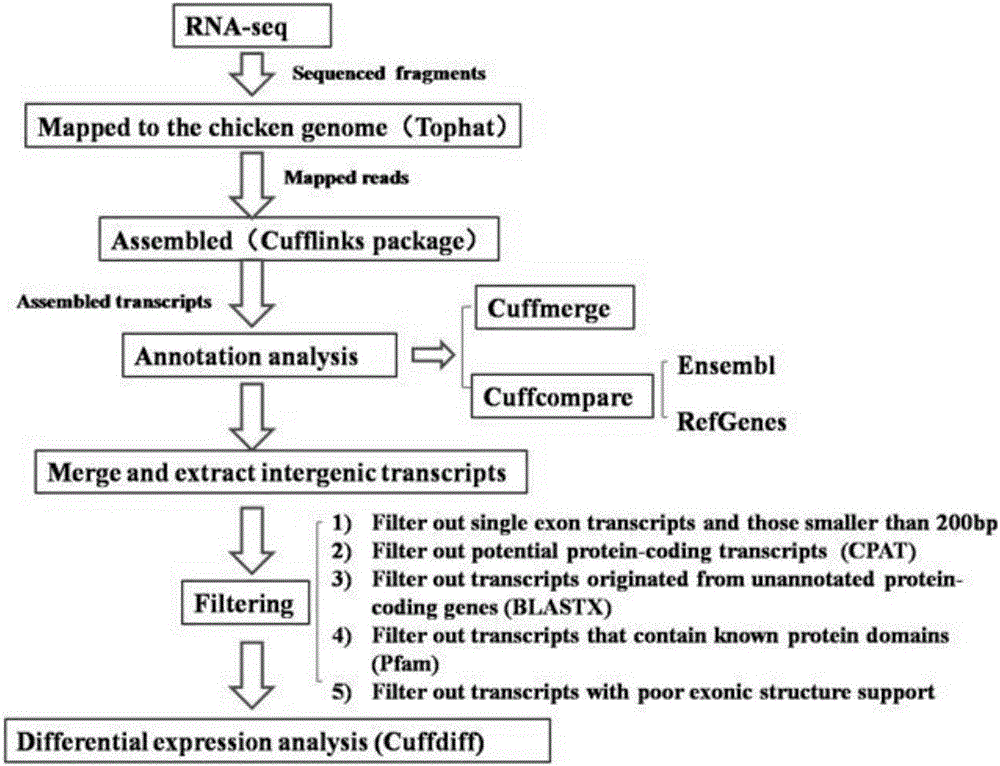
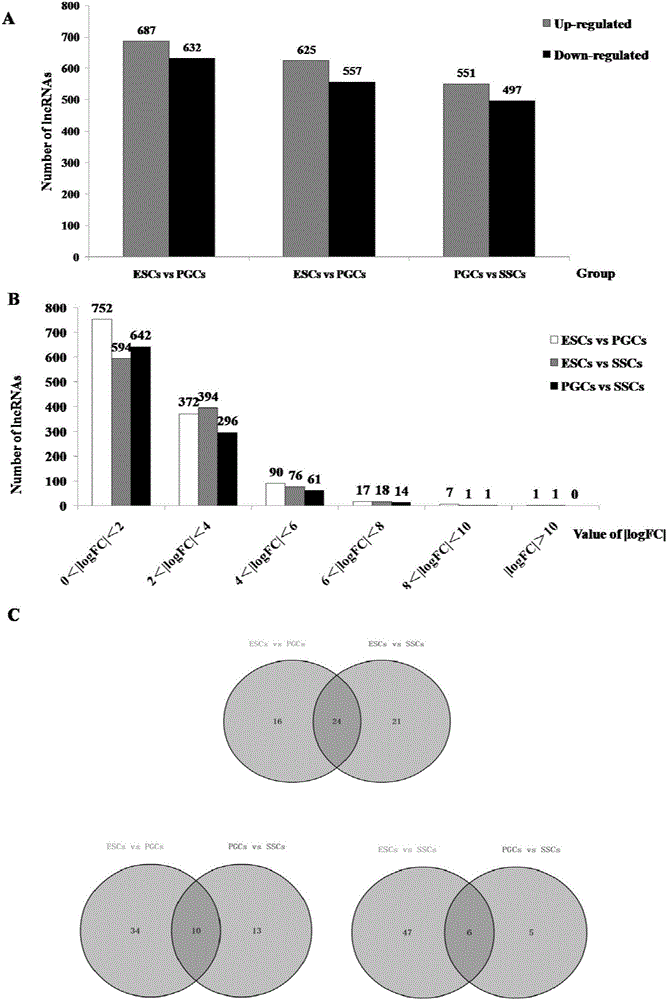
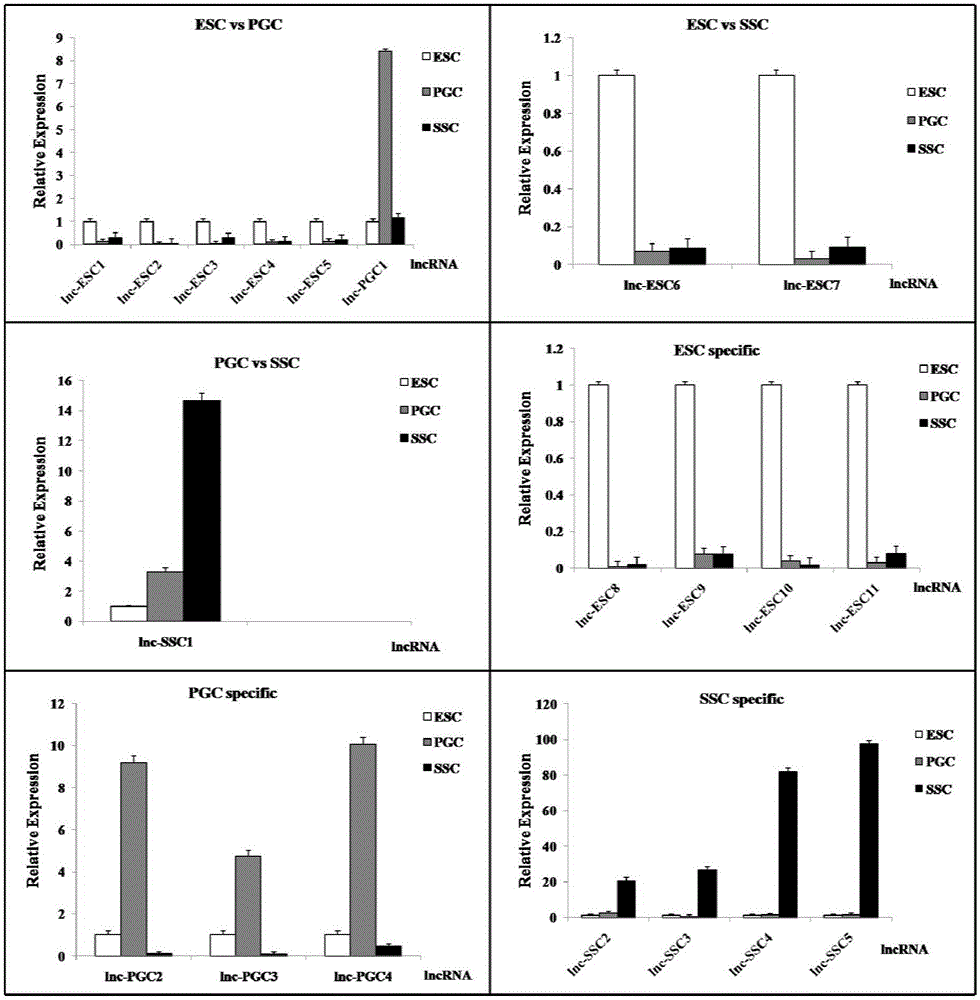
The invention discloses an analytical method for excavating key lncRNA in a process of differentiating chick embryo stem cells into male germ cells. The method comprises the following steps: firstly, separating chick ESCs, PGCs and SSCs from an embryonic disc of a fresh fertilized egg of a Rugao yellow chicken, a germinal ridge of the 19th stage (72h) and a testicle of the 18th day, performing sex determination by using a PCR amplification method according to a design specificity of a CDH1 gene, sorting antibody labeled ESCs, PGCs and SSCs, extracting cell total RNA from positive cells, detecting the quality of RNA after purification, and performing RNA-Seq sequencing after passing the detection; screening a transcript of assembled RNA from a sequencing result; separating chicken ESCs, PGCs and SSCs, extracting total RNA, and reversely transcribing the total RNA into cDNA; performing predictive analysis of Trans and Cis target genes on candidate lncRNA, searching a GO function item of the lncRNA by virtue of a DAVID database, annotating functions of the lncRNA by virtue of GO analysis, and performing functional analysis on the lncRNA; and drawing an interaction network diagram of the relationship between the key lncRNA, a target gene thereof and related signal channels.
Owner:YANGZHOU UNIV
Epithelial type cadherin expressed gene CDH1 mutation detection kit and application thereof
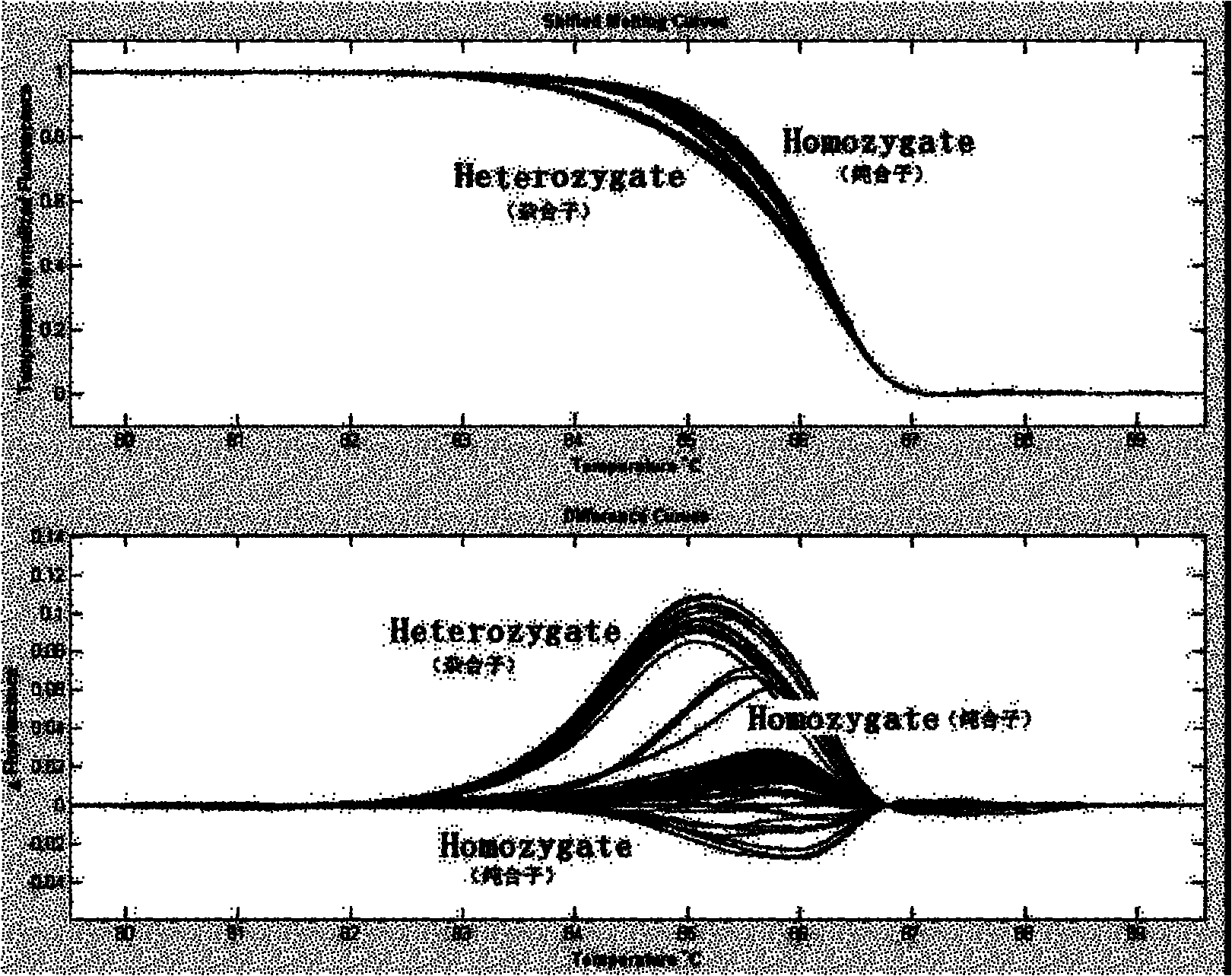
The invention relates to a CDH1 mutation detection kit and an application thereof. The kit mainly comprises the following main ingredients: (1) 19 pairs of CDH1 gene PCR amplifying and sequencing primers; and (2) PCR reagent for amplification. The PCR amplification product obtained by the kit can screen gene micromutation by a high-resolution dissolved curve process or carries out polymorphism analysis on restrictive segment length. The kit has high-efficiency mutation detection and functional evaluation actions. The invention is used for CDH1 gene mutation detection.
Owner:NANJING UNIV
Prognostic and treatment response predictive method
PendingUS20200239968A1Avoiding unwanted side effectAggressive treatmentMicrobiological testing/measurementDisease diagnosisDPYDPredictive methods
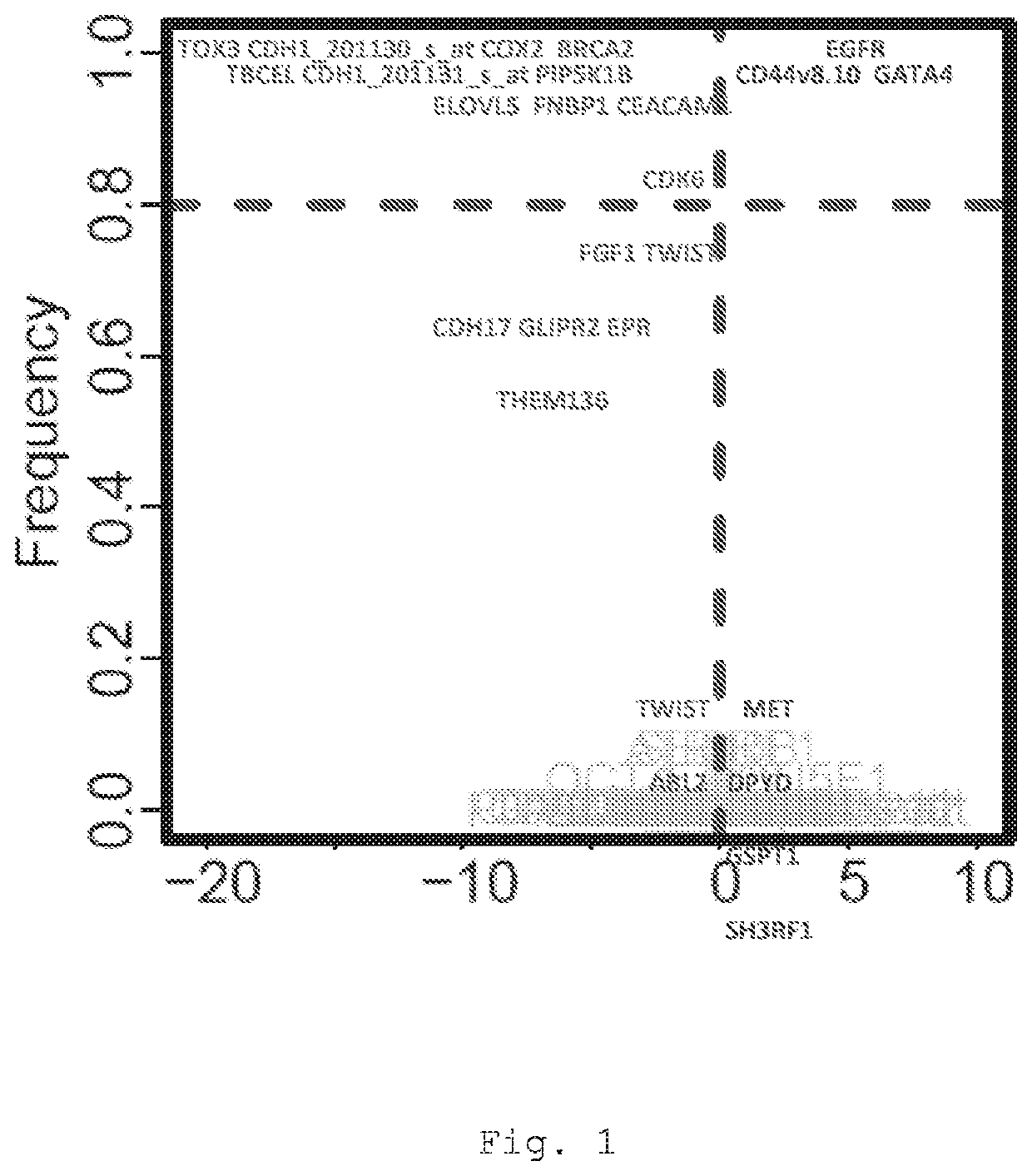
The present invention provides a method for predicting the treatment response of a human gastroesophageal cancer patient, the method comprising: a) measuring the gene expression of at least 3 of the following genes: CDH1, CDK6, COX2, ELOVL5, GATA4, EGFR, TBCEL, FGF7, CDH17, FNBP1, PIP5K1B, TWIST, CD44, MET, CEACAM1, TOX3, GLIPR2, GSTP1, RON, TMEM136, MYB, BRCA2, FGF1, POU5F1, EPR, DPYD, ABL2 and SH3RF1 in a sample obtained from the gastroesophageal tumour of the patient to obtain a sample gene expression profile of at least said genes; and b) making a prediction of the treatment response and / or prognosis of the patient based on the sample gene expression profile. Also provided are related computer-implemented methods and methods of treatment of gastroesophageal cancer.
Owner:THE INST OF CANCER RES ROYAL CANCER HOSPITAL +2
Method and kit for identifying lung cancer status
PendingUS20210032704A1Fast and reliable and accurate newMicrobiological testing/measurementCancer researchBioinformatics
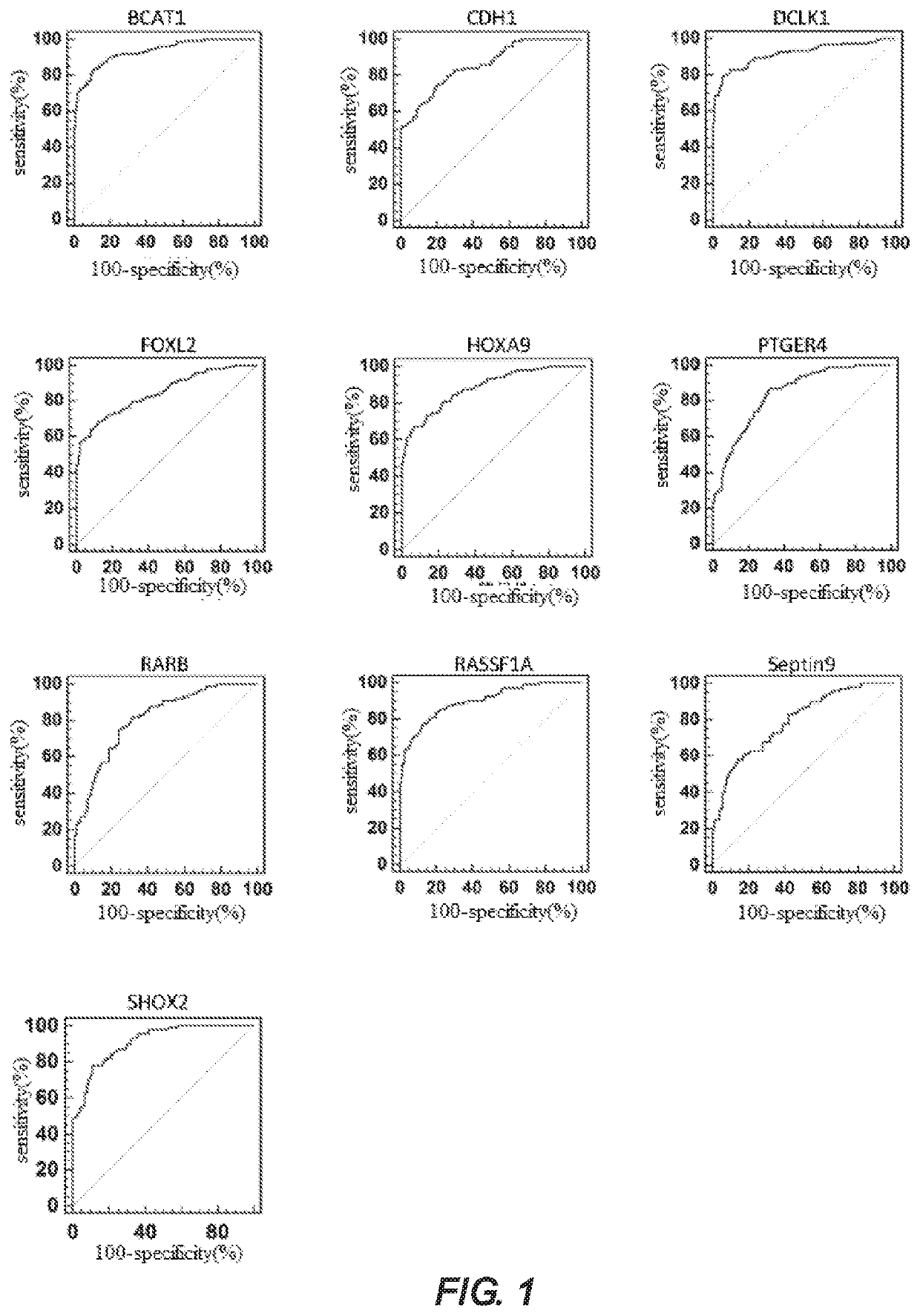
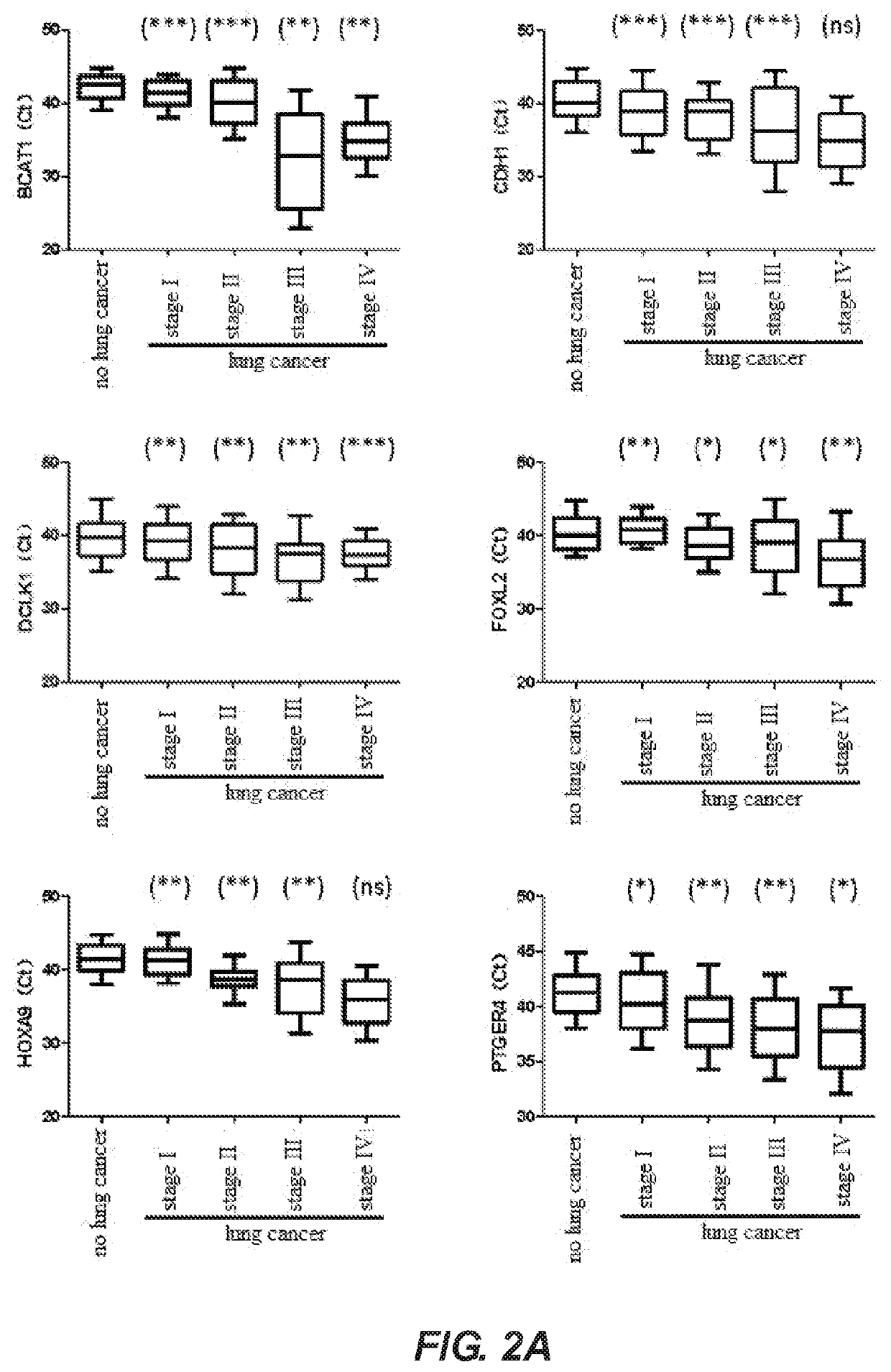
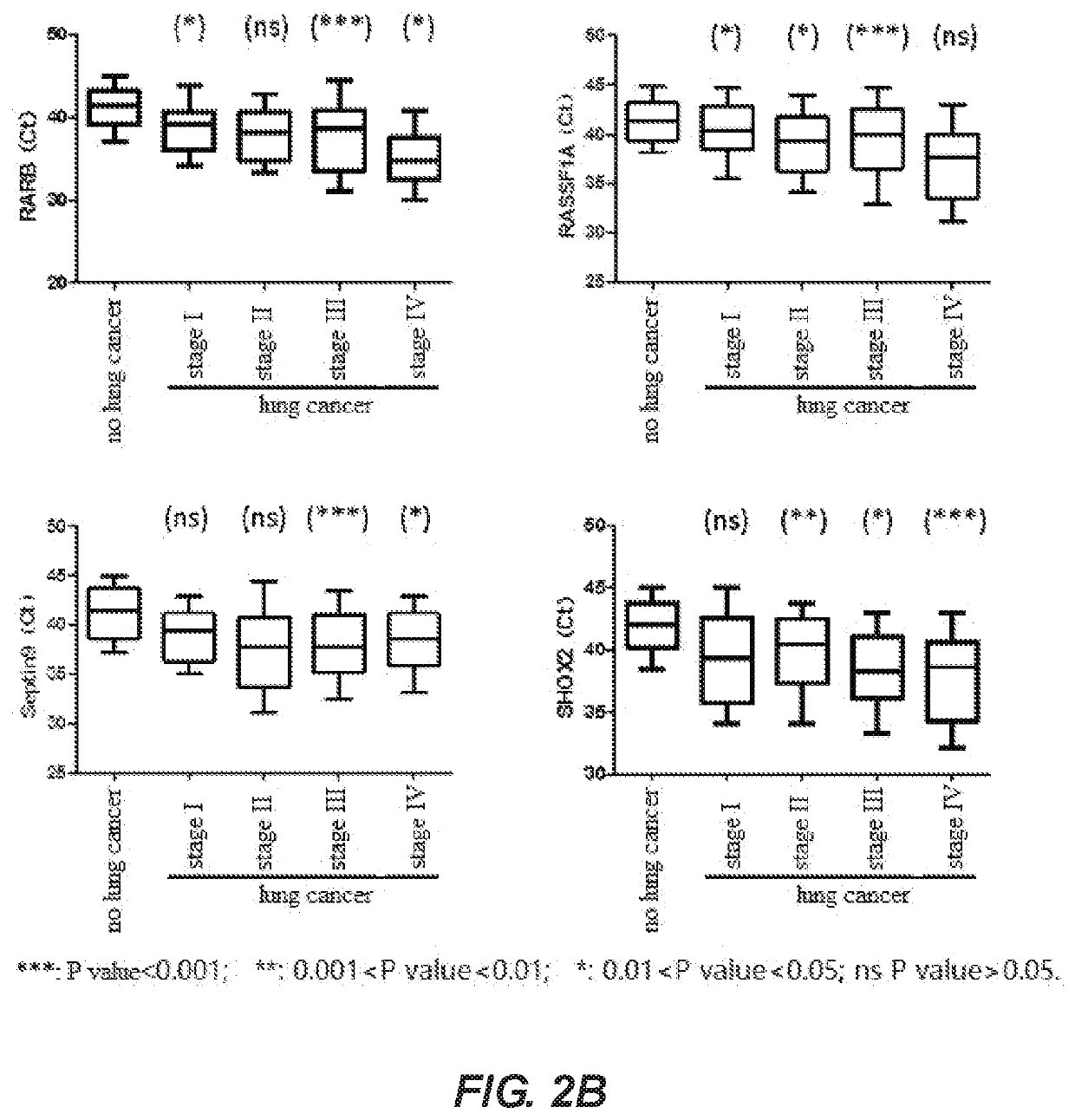
A method for identifying lung cancer status in a subject comprising: collecting a biological sample from the subject; detecting the methylation level of a biomarker gene in the biological sample, the biomarker gene being one or more selected from the following genes: BCAT1, CDH1, DCLK1, FOXL2, HOXA9, PTGER4, RARB, RASSF1A, Septin9, and SHOX2; and comparing the detected methylation levels with a normal methylation level of a corresponding biomarker gene in the population to determine the lung cancer status in the subject. Also provided herein is a kit for identifying lung cancer status in a subject.
Owner:EXCELLEN MEDICAL
Composition for noninvasive screening of early lung cancer, application, kit and sample treatment method
InactiveCN111808963AImprove the detection rateImprove accuracyMicrobiological testing/measurementDNA/RNA fragmentationDNA methylationPromoter
The invention relates to a composition for noninvasive screening of early lung cancer, application, a kit and a sample treatment method. The composition comprises a detection reagent for specific detection of DNA methylation of a human CDH1 gene; and a detection reagent for specifically detecting DNA methylation of the human SHP-1 gene. The kit for detecting methylation sites of the CDH1 gene andthe SHP-1 gene not only comprises a promoter region of the gene, but also comprises a coding region and a non-coding region of the gene, so that the target sequences of the CDH1 gene and the SHP-1 gene which are highly related to the early lung cancer can be found through multi-region detection, meanwhile, the detection accuracy and specificity can be improved, and the detection rate of methylation of the CDH1 gene and the SHP-1 gene is increased. The kit has strong pertinence on early screening of lung cancer at the molecular level, and has the advantages of good detection specificity, high sensitivity, good reliability and the like, so that early possible lung cancer patients can be prevented as soon as possible, and corresponding treatment measures can be taken.
Owner:上海纳奥生物科技有限公司
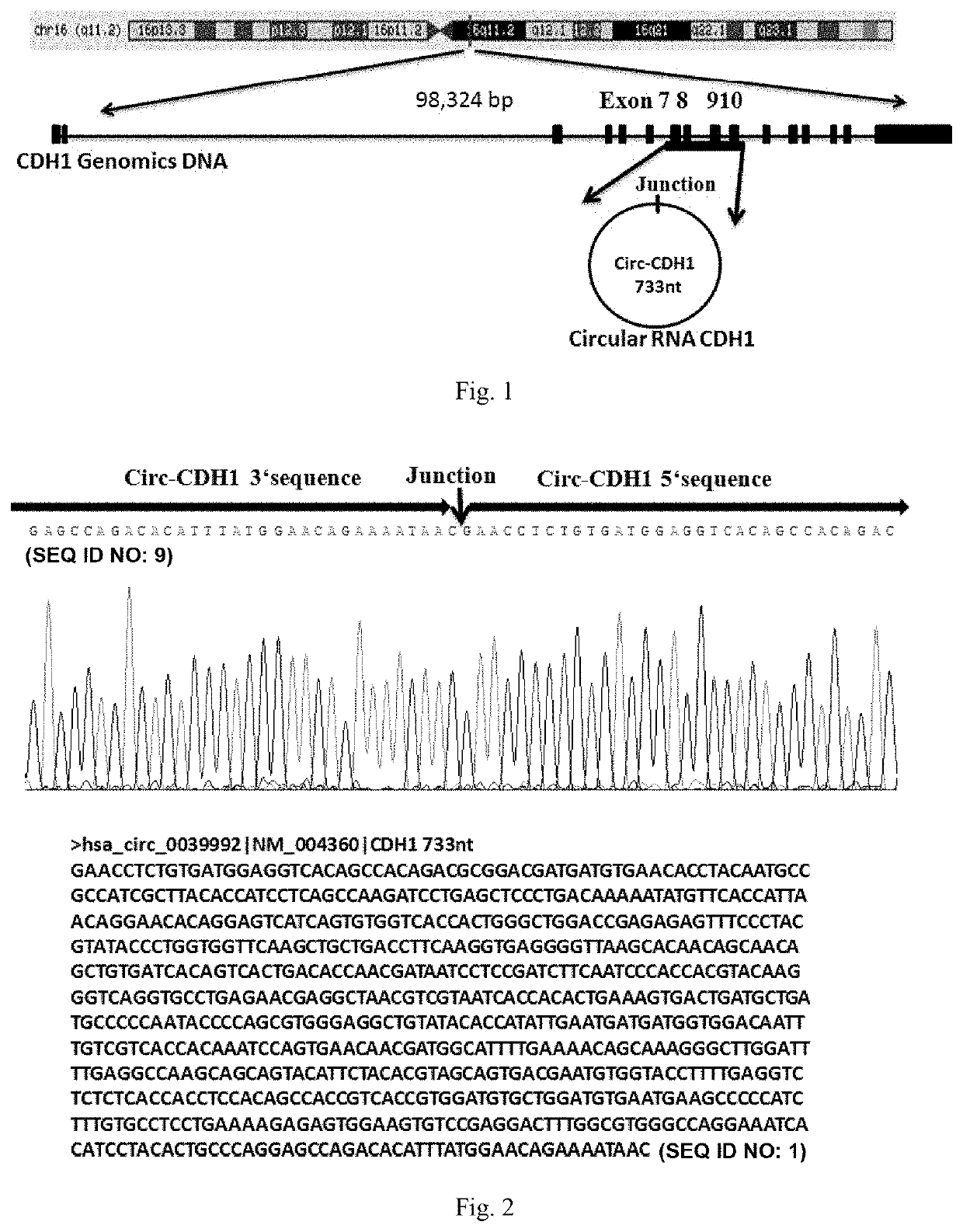
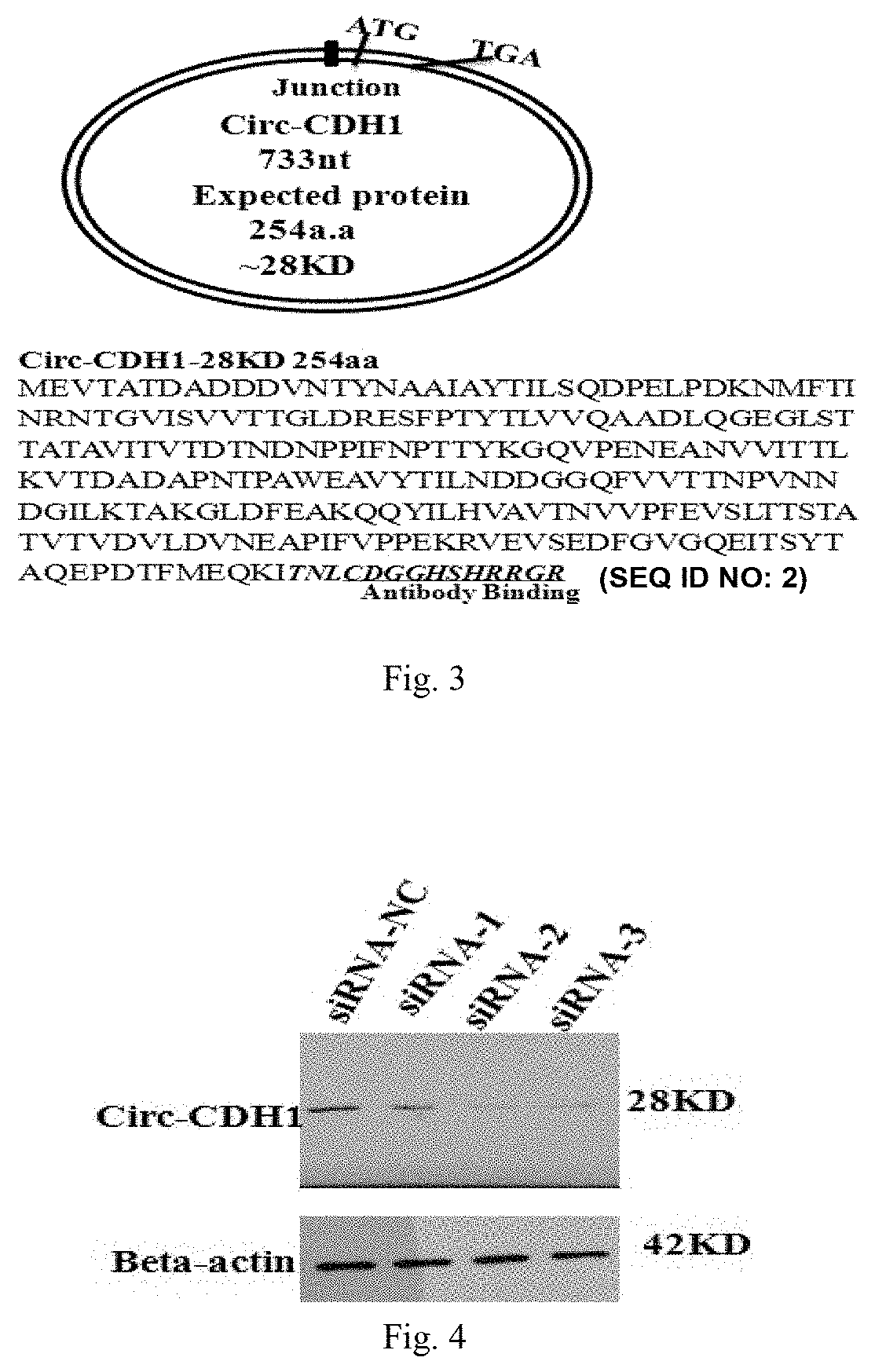
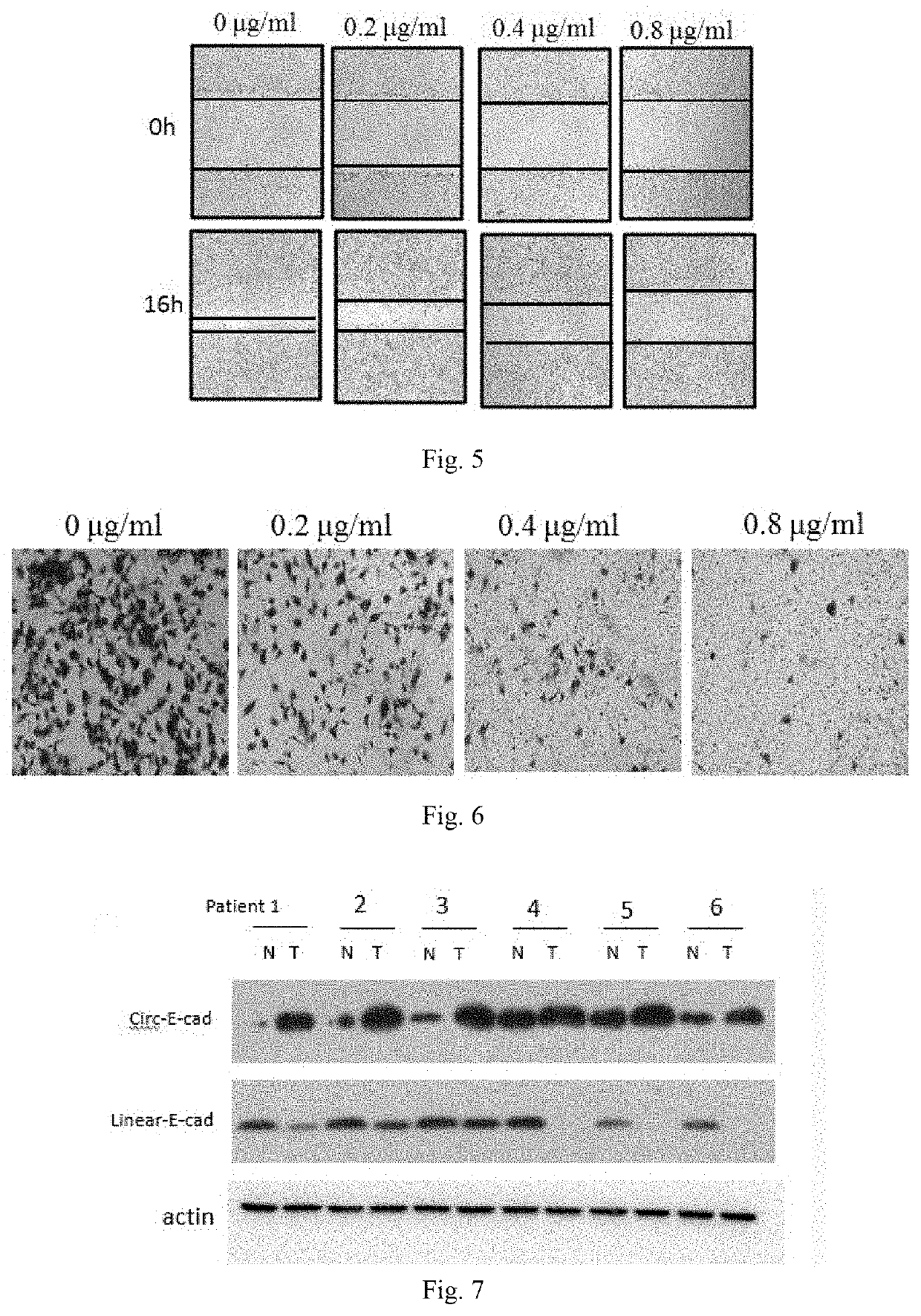
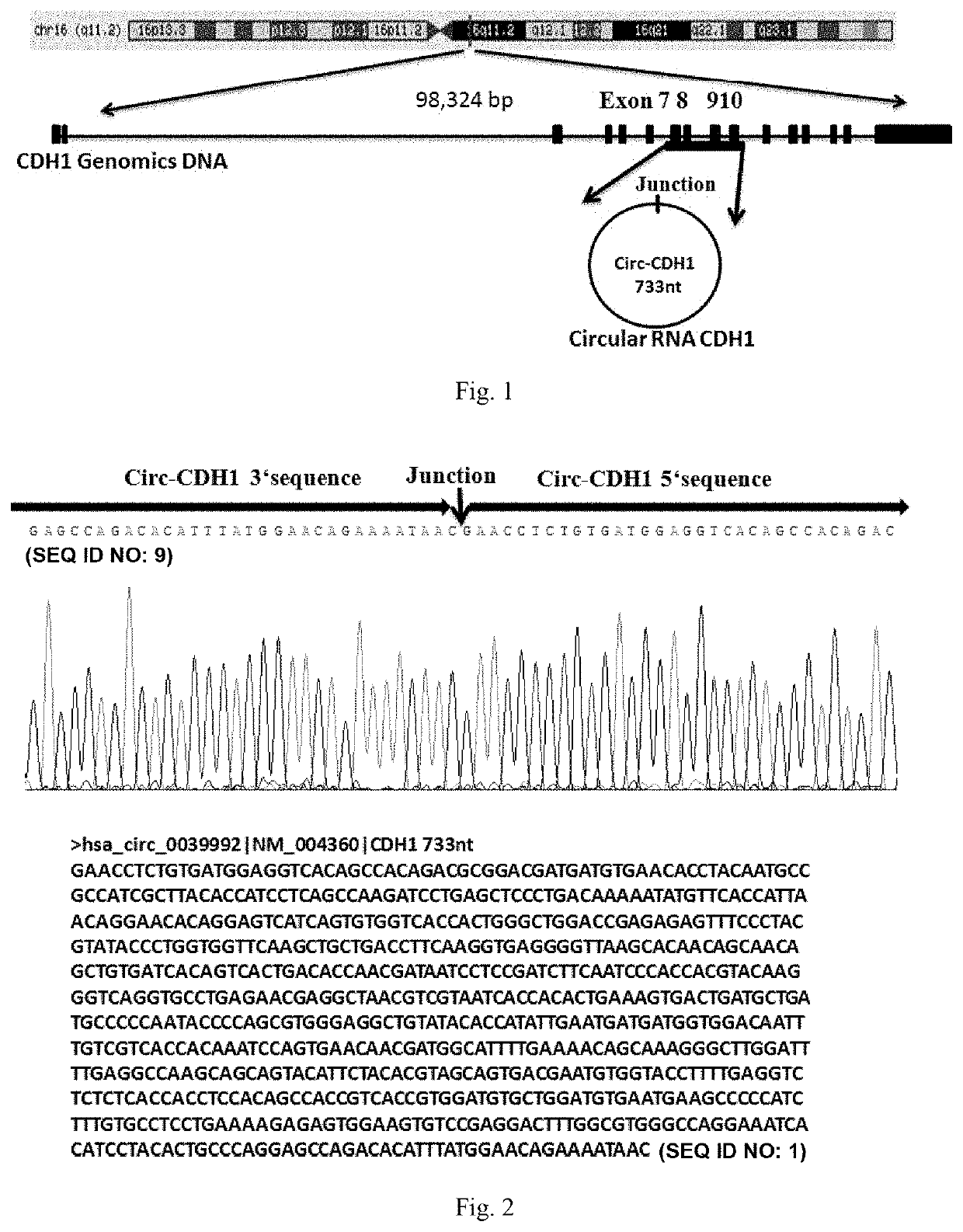
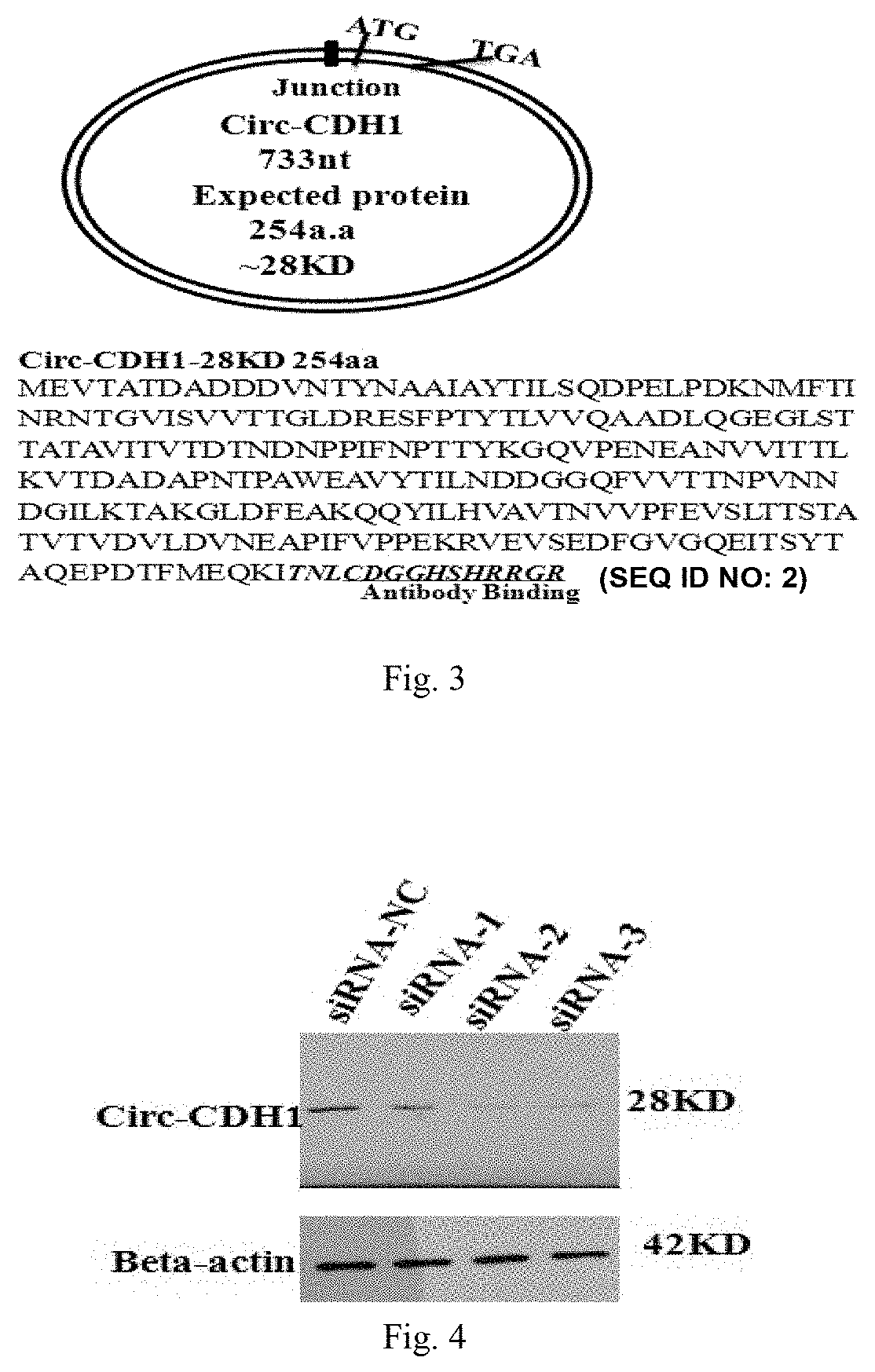
Use of Circ-CDH1 Inhibitors
ActiveUS20200231695A1Growth inhibitionImmunoglobulins against cell receptors/antigens/surface-determinantsAntineoplastic agentsAntiendomysial antibodiesOncology
The invention belongs to the field of research and development of genetic engineering antibody drugs, in particular, relates to a method for treating a tumor by administering Circ-CDH1. The Circ-CDH1 is a circular RNA Circ-CDH1 nucleic acid molecule or the protein Circ-CDH1-28 KD expressed by the circular RNA Circ-CDH1 nucleic acid molecule. In particular, a monoclonal antibody Anti-Circ-CDH1 is designed against Circ-CDH1-28 KD, which can specifically detect the content of a protein encoded by endogenous circular RNA Circ-CDH1, can remarkably inhibit invasion and metastasis of cells from tumors such as glioma, breast cancer and the like, and has wide application prospects in clinical detection of tumors and invasion and metastasis treatment.
Owner:THE FIRST AFFILIATED HOSPITAL OF SUN YAT SEN UNIV
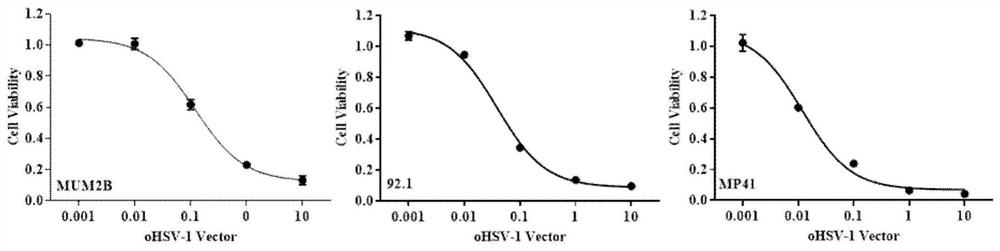
Application of oncolytic virus in treatment on uveal melanoma, marker of treatment effect and detection reagent thereof
ActiveCN111850126AEnhanced cell killingIncrease lethalityOrganic active ingredientsPeptide/protein ingredientsCytosine deaminaseCD44
The invention discloses a preparation for treating, preventing and / or slowing down uveal melanoma, the preparation comprises oncolytic herpes simplex virus type 1 capable of expressing cytosine deaminase genes and 5-fluorocytosine, and the oncolytic herpes simplex virus type 1 and the 5-fluorocytosine are combined to significantly reduce the volume of the uveal melanoma and improve the lifetime ofa subject. The invention further discloses a marker for evaluating the treatment effect of uveal melanoma through the preparation, and the marker is selected from markers capable of representing theepithelial-mesenchymal transition degree and mainly comprises IL-6, DPD, TWIST1, ZEB1, CD44 and CDH1.
Owner:BEIJING NEUROSURGICAL INST
Biomarker for breast cancer typing and application thereof
PendingCN114277148AThe diagnosis is correctFacilitate precise treatmentMicrobiological testing/measurementProteomicsLuminal bBiologic marker
The invention relates to a biomarker for breast cancer typing, and relates to the technical field of medical detection. When the biomarker is used for typing diagnosis of breast moistening ductal carcinoma or breast moistening lobular carcinoma, the biomarker comprises at least five genes such as CDH1, TP53, GATA3, CBFA2T3, MYC and the like, and the diagnostic power AUC can reach 0.8696; when the biomarker is used for breast cancer Luminal A type, breast cancer Luminal B type, breast cancer HER-2 overexpression type or basal sample breast cancer typing diagnosis, the biomarker comprises at least five genes such as TP53, ERBB2, PWWP2A, SPOP and RARA, the diagnostic power AUC can reach 0.8001, the biomarker has excellent diagnostic power, a molecular level-based discrimination method for different pathologies and molecular subtypes is provided, and the biomarker can be applied to diagnosis of breast cancer Luminal A type, breast cancer Luminal B type, breast cancer HER-2 overexpression type or basal sample breast cancer typing. And mutual verification is provided for pathological diagnosis results, so that case diagnosis results are ensured to be correct, and subsequent precise treatment is facilitated.
Owner:深圳康华君泰生物科技有限公司
Method and kit for identifying gastric cancer status
PendingUS20210222260A1Fast and reliable and accurateMicrobiological testing/measurementGastric carcinomaBiologic marker
A method for identifying the gastric cancer status of a subject, comprising: 1) collecting a biological sample from the subject; 2) detecting methylation level of a biomarker gene in the biological sample, wherein the biomarker gene is selected from one or more of the following genes: CDH1, DAPK, PAX5, RASSF1A, Reprimo, RNF180, RUNX3, SDC2, Septin9 and TCF4; and 3) comparing the detected methylation level from step 2) with a normal methylation level of a corresponding biomarker gene in a population to determine the gastric cancer status in the subject. Also provided is a kit for identifying the gastric cancer status of a subject. The method and the kit provided herein provide a new way for fast, reliable and accurate prediction, diagnosis and evaluation of gastric cancer.
Owner:BEIJING EXELLON MEDICAL TECH CO LTD
Methods for detection of respiratory diseases
ActiveUS9952225B2Disease diagnosisBiological testingObstructive Pulmonary DiseasesPulmonary circulation diseases
Owner:NAT JEWISH HEALTH
Mutant gene group, library and kit for evaluating risk of female malignant tumor
PendingCN111635942AImprove accuracyEarly interventionNucleotide librariesMicrobiological testing/measurementOncologyBiomedicine
The invention discloses a mutant gene group, library and kit for evaluating female malignant tumor risk, and belongs to the technical field of biomedical molecular detection. The mutant gene group forevaluating the risk of female malignant tumors comprises one or more selected from a group consisting of the following 25 genes: ATM, BRCA1, BRCA2, BRIP1, CDH1, CHEK2, EPCAM, MLH1, MSH2, MSH6, NBN, NF1, PALB2, PMS2, PTEN, RAD51C, RAD51D, STK11, TP53, BARD1, MRE11A, MUTYH, RAD50, XRCC2 and FANCC5. According to the invention, female malignant tumor related genetic markers can be widely screened andinspected, accuracy is higher, and a basis is provided for prediction, prevention and diagnosis of female malignant tumor onset risks.
Owner:AIR FORCE MEDICAL UNIV
Nucleic acid composition, kit and detection method for detecting methylation of breast cancer related genes
ActiveCN113215257AGuaranteed Quality ControlEasy to useMicrobiological testing/measurementAgainst vector-borne diseasesCancer researchGSTP1 Gene
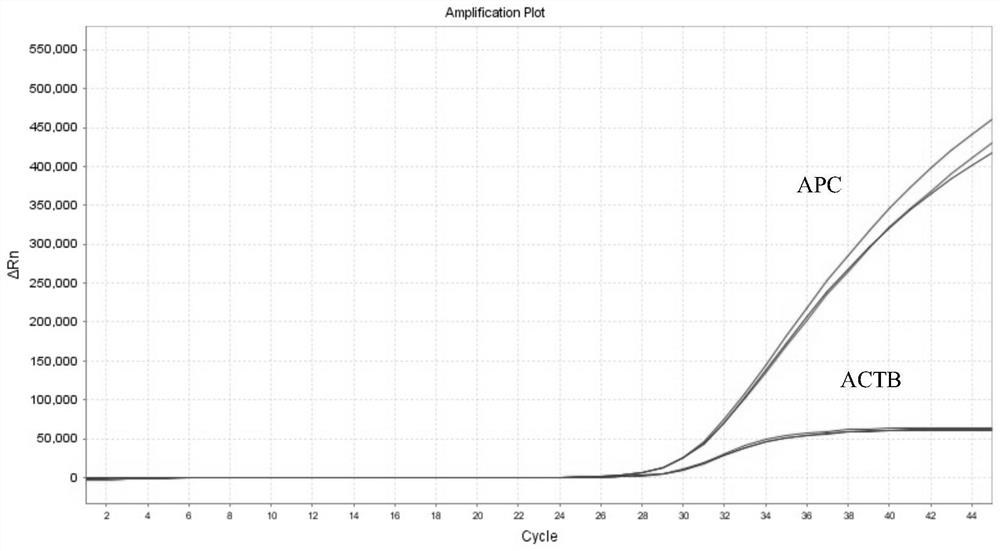
The invention discloses a nucleic acid composition, a kit and a detection method for detecting methylation of breast cancer related genes. The nucleic acid composition comprises a methylation specific primer and a probe of a target site of an RASSF1A gene; a methylation specific primer and a probe of a target site of the APC gene; a methylation specific primer and a probe of a target site of the GSTP1 gene; a methylation specific primer and a probe of a CDH1 gene target site; and a specific primer and a probe of an ACTB reference gene; The kit prepared from the nucleic acid composition disclosed by the invention has the advantages of accurate methylation result of breast cancer related genes, rapidness, high throughput, simplicity in operation, high accuracy and sensitivity and the like.
Owner:杭州圣庭医疗科技有限公司
Method and kit for identifying state of prostate cancer
PendingCN113234820AMicrobiological testing/measurementDNA/RNA fragmentationProstate cancerBiologic marker
Provided herein is a method of identifying the state of prostate cancer in a subject, and the method comprises the steps: 1) detecting the methylation level of a biomarker gene in a biological sample from the subject wherein the biomarker gene is selected from one or more of the following genes: APC, CCND2, CDH1, GSTP1, MCAM, PENK, PITX2, PTGS2, RARB, and RASSF1A; 2) comparing the methylation level detected in the step 1) with the normal methylation level of the corresponding biomarker gene in the population to determine the state of prostate cancer in the subject. Also provided herein are kits for identifying the state of prostate cancer in a subject. The method and the kit provided by the invention provide a rapid, reliable and accurate new way for prediction, diagnosis and evaluation of prostate cancer.
Owner:BEIJING EXELLON MEDICAL TECH CO LTD
A group of mutation gene groups and detection kits for assessing breast cancer risk
InactiveCN103981273BImprove detection efficiencyImprove the detection rateMicrobiological testing/measurementDNA/RNA fragmentationBrca1 geneCvd risk
The invention discloses a mutant gene group for mammary cancer risk assessment and a detection kit thereof. The mutant gene group is a mutant gene set comprising the following seven genes: BRCA1 gene, BRCA2 gene, TP53 gene, MLH1 gene, MLH3 gene, MSH3 gene and CDH1 gene. The mutant gene group of the seven genes totally carries 25 mutant sites disclosed as Table 3. The invention also discloses a detection kit of the mutant gene group for mammary cancer risk assessment, which comprises probes or primers for detecting the mutant genes in the mutant gene group. The detection kit disclosed by the invention has the advantages of high detection efficiency, high detection speed, high detectable rate and the like.
Owner:FUDAN UNIV SHANGHAI CANCER CENT
Kit for rapidly detecting malignant transformation of passage stem cells and application of kit
PendingCN111926079AEasy to operateImprove accuracyMicrobiological testing/measurementMalignancyMalignant transformation
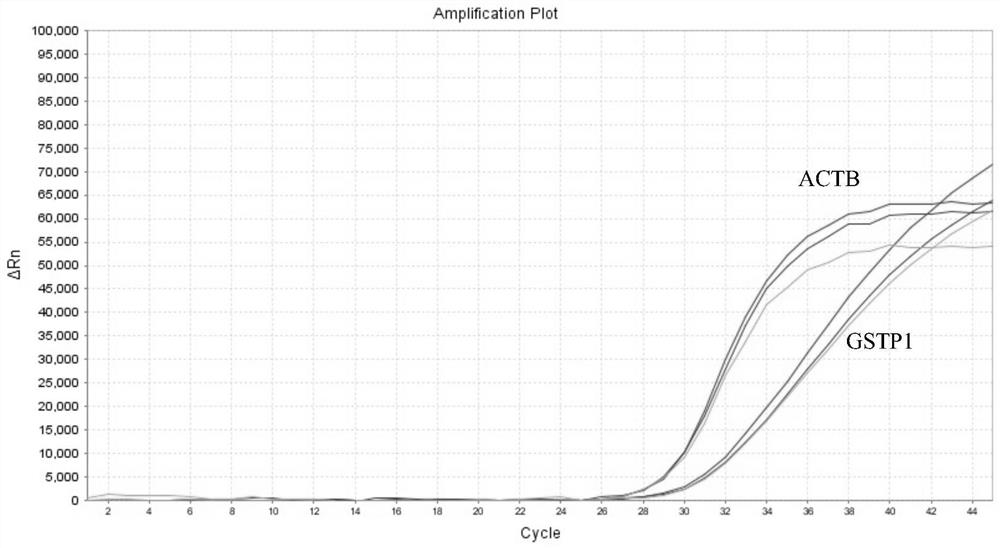
The invention provides a kit for rapidly detecting malignant transformation of passage stem cells and application of the kit, belongs to the technical field of stem cells, the kit comprises primers corresponding to four target genes of P16, RASSF1A, GSTP1 and CDH1, and a reference gene beta-actin primer, the kit is applied to rapid detection of malignant transformation of passage stem cells, and has the advantages of simple operation, good accuracy and high sensitivity in detection of malignant transformation of stem cells.
Owner:成都睿杰森生物科技有限公司
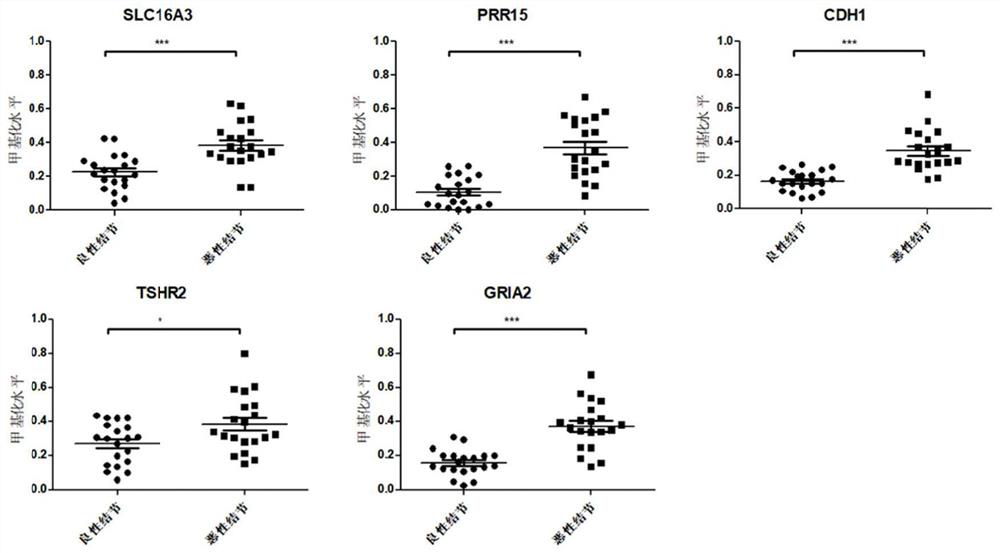
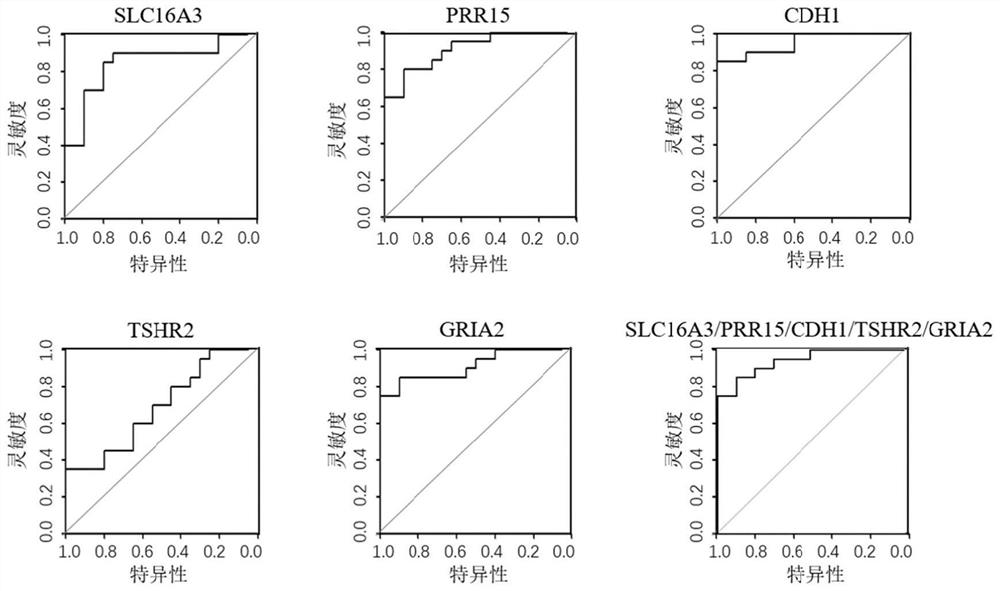
Thyroid nodule benign and malignant related marker and application thereof
PendingCN114277115AHigh sensitivityImprove featuresMicrobiological testing/measurementSequence analysisReference genesMalignancy
The invention provides a composition for identifying benign and malignant thyroid nodules. The composition comprises a nucleotide sequence of an SLC16A3 gene, a nucleotide sequence of a PRR15 gene, a nucleotide sequence of a CDH1 gene, a nucleotide sequence of a TSHR gene, a nucleotide sequence of a GRIA2 gene, a nucleotide sequence of a reference gene ACTB and a nucleotide sequence of an optional reference gene in methylation regions related to benign and malignant thyroid nodules in a sample. By analyzing the methylation level of the methylation marker in tissue or plasma, benign and malignant thyroid nodules of a subject can be diagnosed.
Owner:SINGLERA GENOMICS (SHANGHAI) LTD
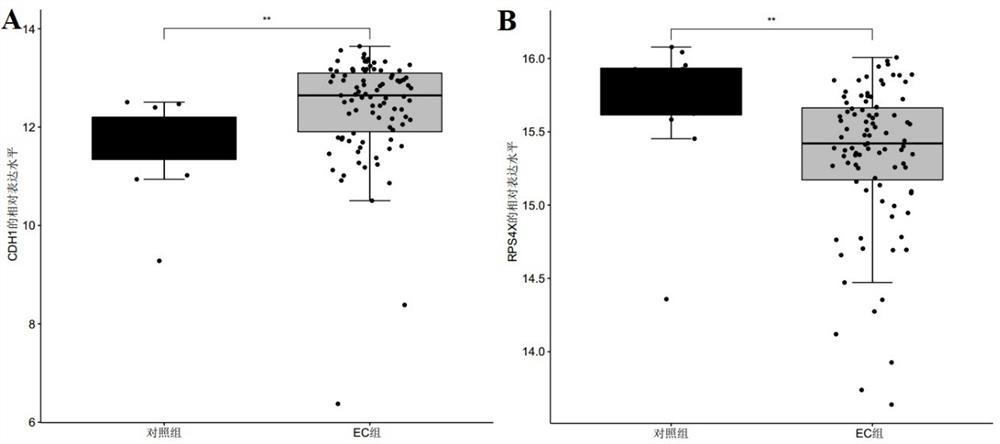
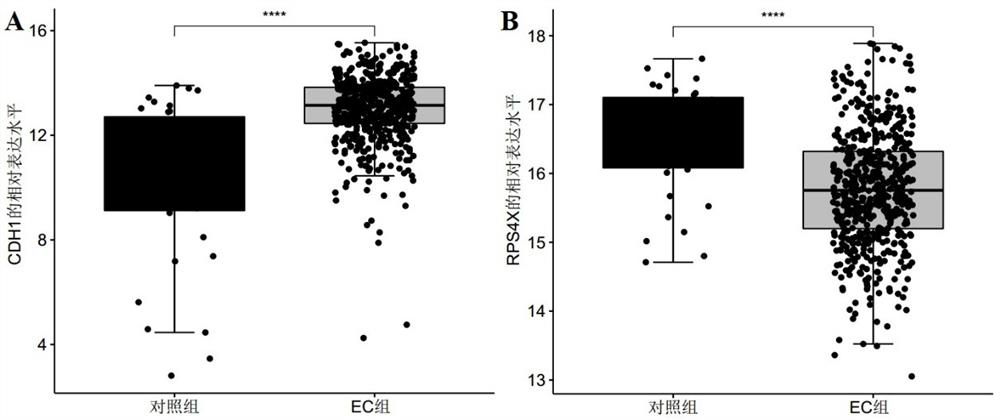
Endometrial cancer-related marker molecules and their application in the diagnosis of endometrial cancer
ActiveCN113493838BMicrobiological testing/measurementMaterial analysisEndometrial CarcinomasBiologic marker
Owner:BEIJING MEDINTELL BIOMED CO LTD
Use of Circ-CDH1 inhibitors
ActiveUS11332540B2Growth inhibitionImmunoglobulins against cell receptors/antigens/surface-determinantsAntineoplastic agentsAntiendomysial antibodiesOncology
The invention belongs to the field of research and development of genetic engineering antibody drugs, in particular, relates to a method for treating a tumor by administering Circ-CDH1. The Circ-CDH1 is a circular RNA Circ-CDH1 nucleic acid molecule or the protein Circ-CDH1-28 KD expressed by the circular RNA Circ-CDH1 nucleic acid molecule. In particular, a monoclonal antibody Anti-Circ-CDH1 is designed against Circ-CDH1-28 KD, which can specifically detect the content of a protein encoded by endogenous circular RNA Circ-CDH1, can remarkably inhibit invasion and metastasis of cells from tumors such as glioma, breast cancer and the like, and has wide application prospects in clinical detection of tumors and invasion and metastasis treatment.
Owner:THE FIRST AFFILIATED HOSPITAL OF SUN YAT SEN UNIV
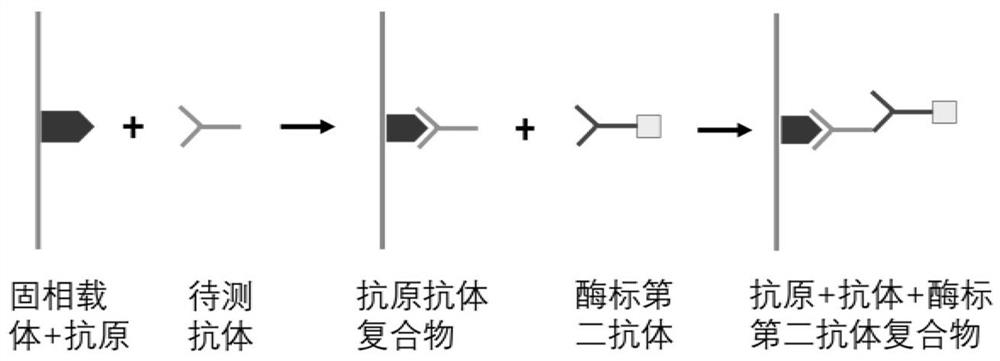
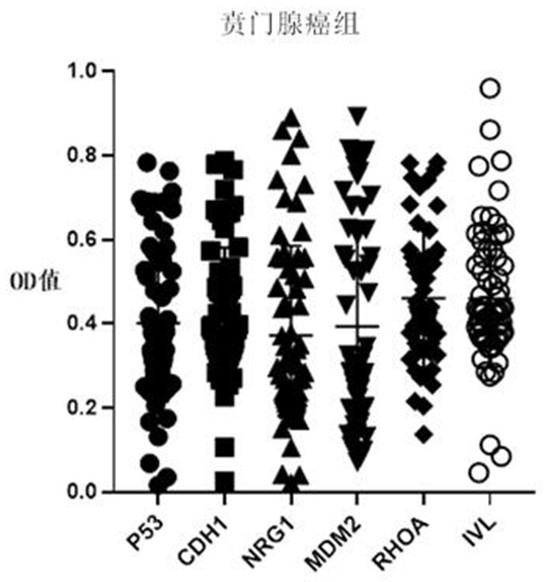
Autoantibody joint detection ELISA kit for early cardia adenocarcinoma screening
ActiveCN111323587AEfficient detectionHigh detection sensitivityMaterial analysisAutoantibodyElisa kit
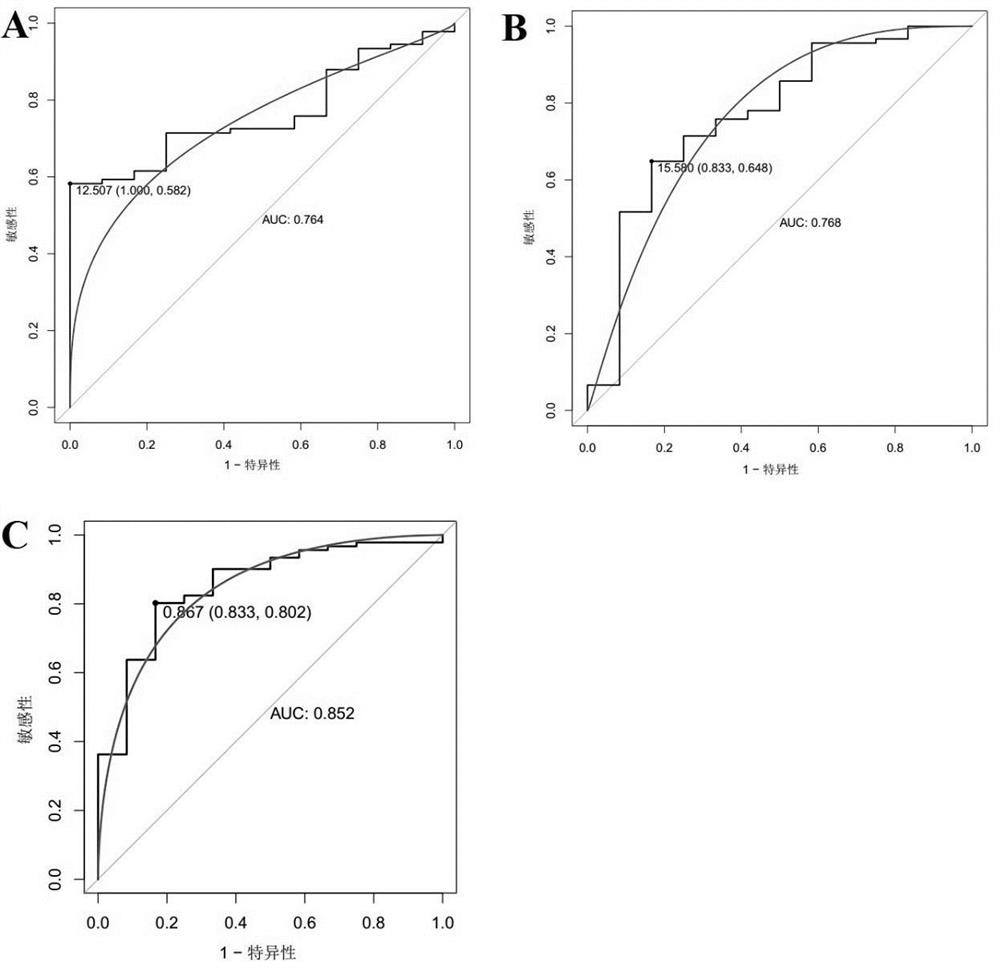
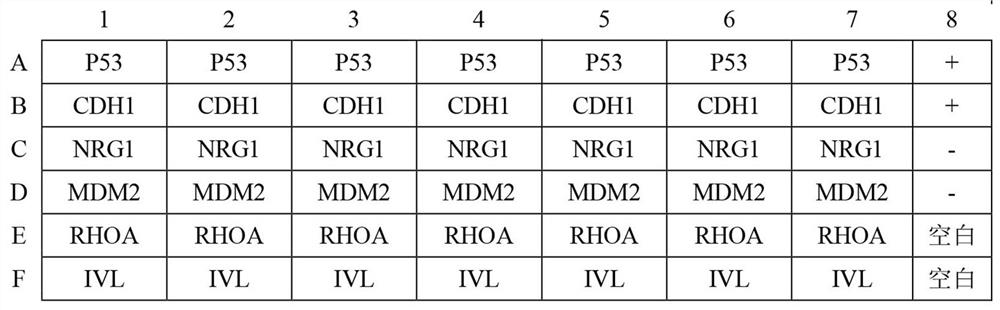
The invention belongs to the technical field of medical biology, and specifically discloses an autoantibody joint detection ELISA kit for early cardia adenocarcinoma screening. The kit comprises a solid phase carrier and a tumor-associated antigen coated on the solid phase carrier, and the tumor-associated antigen is composed of P53, CDH1, NRG1, MDM2, RHOA and IVL. Furthermore, the kit also comprises a sample diluent, a second antibody, a second antibody diluent, positive control serum, negative control serum, a developing solution, a stop solution and a washing solution. The ELISA kit provided by the invention can effectively detect cardia adenocarcinoma, especially early cardia adenocarcinoma, has detection sensitivity as high as 86% and specificity as high as 88%, can be used for large-scale screening of asymptomatic people in cardia adenocarcinoma high-incidence areas, and is beneficial to screening and early discovery of asymptomatic high-risk people.
Owner:THE FIRST AFFILIATED HOSPITAL OF ZHENGZHOU UNIV
Reagent for detecting DNA methylation and application
PendingCN113122633AMicrobiological testing/measurementDNA/RNA fragmentationDNA methylationNodular lesion
The invention discloses a method for identifying the property of a thyroid nodule. The method comprises the step of detecting the DNA methylation levels of regions selected from the following (1) and (2) in a sample: (1) fragments of one or more genes selected from the following: SLC16A3, DNM2, IL17C, CDH1, TSHR, BIN1 and SLC17A5, and (2) nucleic acid regions within the upstream and downstream 10Kb of the genes described in (1).
Owner:SINGLERA GENOMICS (SHANGHAI) LTD
Reagent for detecting DNA methylation and application
PendingCN113122632AMicrobiological testing/measurementDNA/RNA fragmentationDNA methylationNodular lesion
The invention discloses a method for identifying the property of a thyroid nodule. The method comprises the step of detecting the DNA methylation levels of regions selected from the following (1) and (2) in a sample: (1) fragments of one or more genes selected from the following: RARG, PRR15, DPYS, MCC, TBX15, TSHR, DAPK and CDH1, and (2) nucleic acid regions within the upstream and downstream 10Kb of the genes described in (1).
Owner:SINGLERA GENOMICS (SHANGHAI) LTD
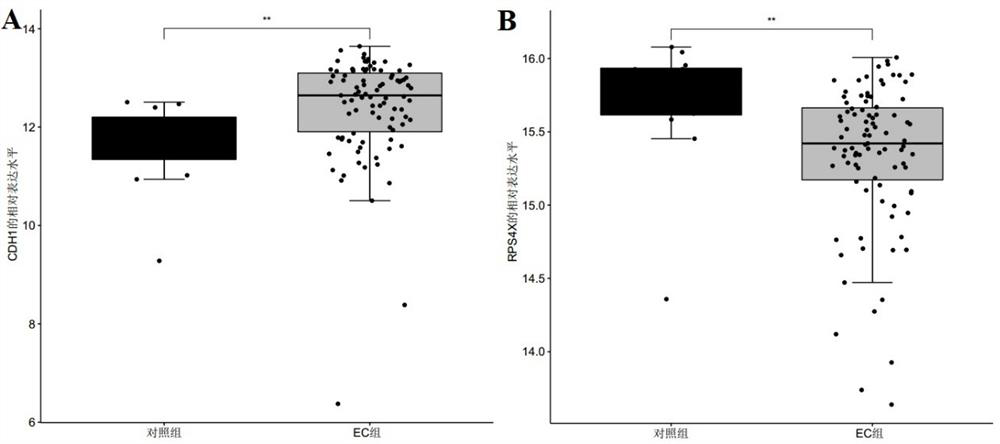
Marker molecule related to endometrial cancer and application thereof in diagnosis of endometrial cancer
ActiveCN113493838AMicrobiological testing/measurementMaterial analysisEndometrial CarcinomasBiologic marker
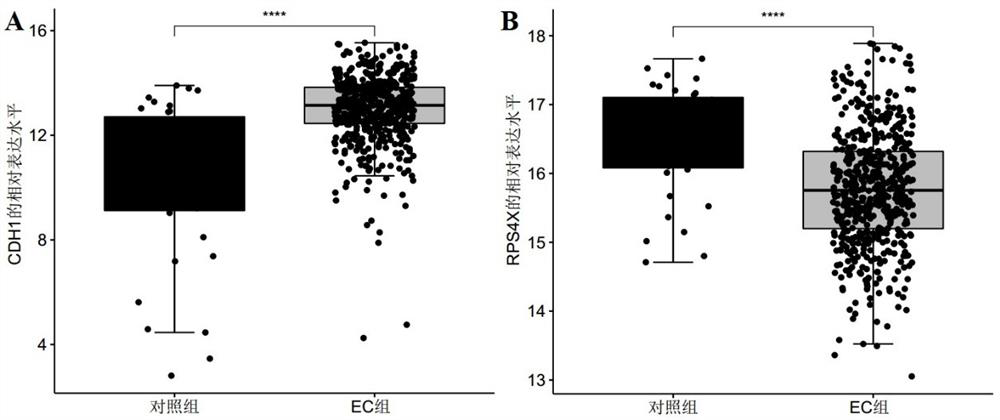
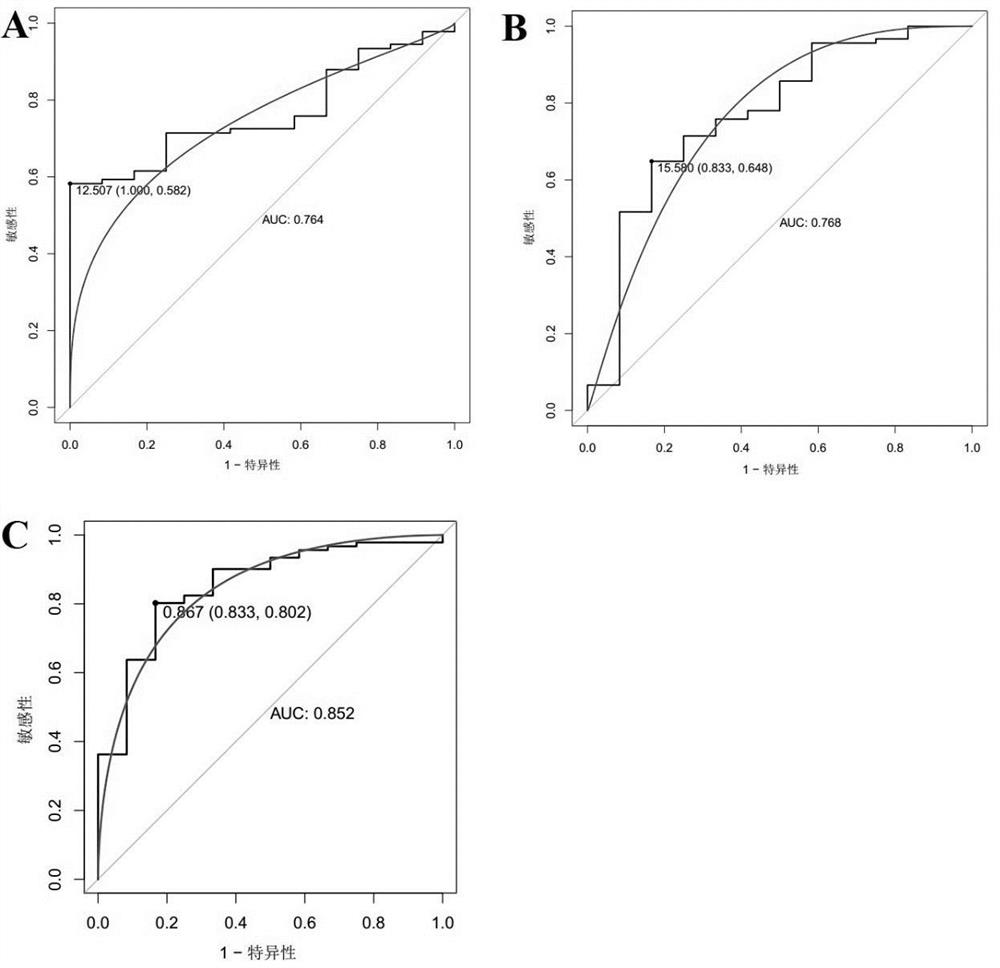
The invention discloses a marker molecule related to endometrial cancer and application thereof in diagnosis of the endometrial cancer. Biomarkers include CDH1 and RPS4X, and the CDH1 and RPS4X are combined to have good diagnosis efficiency, high sensitivity and high specificity on the endometrial cancer. The invention further provides a product and a diagnosis system for diagnosing the endometrial cancer.
Owner:BEIJING MEDINTELL BIOMED CO LTD
Construction method of gene mutation library for hormone receptor positive breast cancer recurrence monitoring
The invention discloses a hormone receptor-positive breast cancer recurrence monitoring gene mutation library construction method, which is characterized that the library covers the total 1357 somatic cell mutations on human genes such as KRAS, JAK3, AKT1, CREBBP, PTEN, RB1, TP53, CTNNB1, TSC2, TSC1, ERBB2, PIK3CA, SF3B1, JAK2, CDH1, SMAD4, GATA3, MAP2K4, MAP3K1 and ESR1. According to the present invention, a plurality of the target sequences are subjected to single tube amplification with the construction method to rapidly complete the library construction, wherein the whole library construction process takes only 2-3 h and the manual time only needs 45 min, such that the difficulty that the multi-gene and multi-target detection of somatic cells on the basis of the small amount of the peripheral blood sample is required in the clinical breast cancer recurrence monitoring is required can be effectively solved, and the lost is low.
Owner:XIAMEN SPACEGEN BIOTECH CO LTD
An ELISA kit for combined detection of autoantibodies for early-stage cardia adenocarcinoma screening
ActiveCN111323587BEfficient detectionHigh detection sensitivityMaterial analysisAutoantibodyElisa kit
Owner:THE FIRST AFFILIATED HOSPITAL OF ZHENGZHOU UNIV
The beauveria bassiana analogue is applied as micromolecular agonist of APC/C
ActiveCN112569341AWide variety of sourcesEasy to expand and cultureCyclic peptide ingredientsAntineoplastic agentsDiseaseAgonist
The invention discloses an application of a beauveria bassiana analogue as a small molecular agonist of APC / C. The invention discloses the function of activating APC / C of the beauveria bassiana analogue with the natural source as shown in the formula I for the first time, and the beauveria bassiana analogue is a powerful agonist of a late-stage promoter compound (APC / C-Cdh1) or a late-stage promoter compound (APC / C-Cdc20). As a small molecular agonist of APC / C, the compound has important application value in the research fields of cell physiology change caused by APC / C protein activity disorder, targeted activation of APC / C to control cell proliferation disorder diseases and the like.
Owner:FUZHOU UNIV
Reagent for detecting DNA methylation and application
PendingCN113122635AMicrobiological testing/measurementDNA/RNA fragmentationDNA methylationNodular lesion
The invention discloses a method for identifying the property of a thyroid nodule. The method comprises the step of detecting the DNA methylation levels of regions selected from the following (1) and (2) in a sample: (1) fragments of one or more genes selected from the following: NEURL1, RUSC1, IL17C, TSHR, DAPK, PAX5, IRX4 and CDH1, and (2) nucleic acid regions within the upstream and downstream 10Kb of the genes described in (1).
Owner:SINGLERA GENOMICS (SHANGHAI) LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com