Predictive and prognostic methods in breast cancer
A breast cancer, breast tumor technology, applied in biochemical equipment and methods, microbial determination/inspection, pharmaceutical formulations, etc., can solve the problems of difficult standardization and different implementation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0428] Example 1: Using Protocol to Isolate Total RNA from FFPE Samples
[0429] Formalin-fixing of tumor tissue and subsequent embedding in paraffin is a standard procedure in clinical pathology, allowing for long-term preservation of samples. Due to the chemical modification of nucleic acids in FFPE samples, special protocols are required to extract amplifiable nucleic acids. Three steps are required for this: (1) removal of the paraffin, (2) lysis of the tissue and release of the RNA (demodification of the nucleic acid if necessary), (3) purification of the RNA by several washing steps.
[0430] The kit (BioNTech Diagnostics GmbH, Maniz, Germany) allows purification without the use of organic solvents, which can be performed in a single reaction vessel.
[0431] In the first step, the paraffin contained in the FFPE sections is liquefied in an optimized lysis buffer. Subsequent addition of proteinase K lyses the tissue and releases the cellular nucleic acids (RNA and D...
Embodiment 2
[0432] Example 2: Using Kits measure gene expression levels of biomarkers
[0433] The kit (BioNTech Diagnostics GmbH, Maniz, Germany) allows determination of the expression levels of selected biomarkers at the mRNA level by reverse transcription quantitative PCR (RT-qPCR).
[0434] In order to determine the expression level of a biomarker at the transcriptional level by PCR, the RNA must first be transcribed into complementary DNA (cDNA) by reverse transcriptase (so-called first-strand synthesis). The marker-specific cDNA is then amplified by DNA polymerase, and the amplification is detected in real-time in PCR using fluorescently labeled hydrolysis probes. RT-qPCR in The assay is performed as a one-step reaction, ie reverse transcription of RNA followed by PCR of DNA is performed sequentially in the same reaction mixture. In addition to enzymes (reverse transcriptase and DNA polymerase), the enzyme mix contains dNTPs as well as salts and PCR additives. for RT-qPCR ...
Embodiment 3
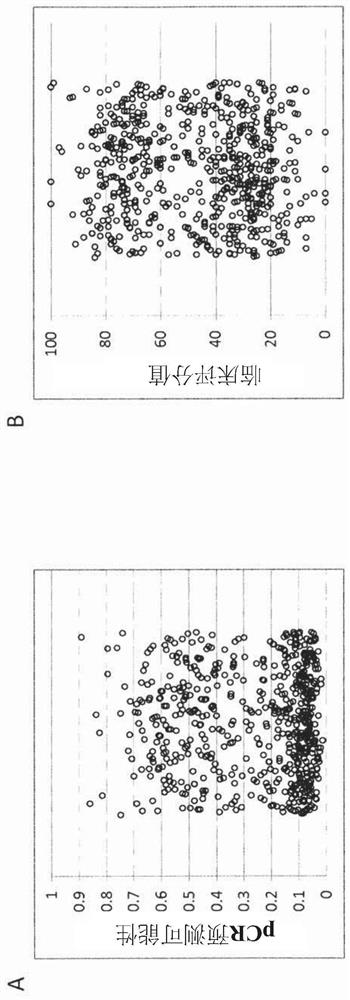
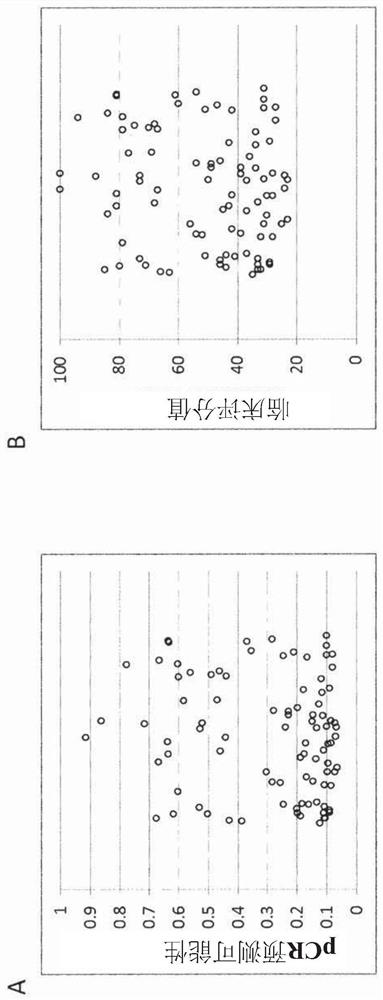
[0449] Example 3: Training for Unscaled Scoring (Scoring 1)
[0450] The unscaled score was trained on a set of routine FFPE biopsy samples from patients who underwent neoadjuvant chemotherapy at the Erlangen University Clinic (Germany) between 2000 and 2015. After selecting a sample with enough tissue available for sectioning (20% minimum tumor cell content and sufficient for RNA tested (valid outcome), a total of 598 samples were included in the study. Use the nucleic acid isolation kit according to the manufacturer's instructions RNA extracted from 10 μm curls of each sample, performed according to manufacturer's instructions Test (BioNTech Diagnostics GmbH, Maniz, Germany). measurement is at 480II (Roche Diagnostics). Samples in the cohort also met these inclusion / exclusion criteria.
[0451] Inclusion criteria
[0452]-Female patient in the Department of Gynecology at Erlangen University Clinic (Germany)
[0453] -Age: at least 18 years old
[0454] - Diagno...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| PCR efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com