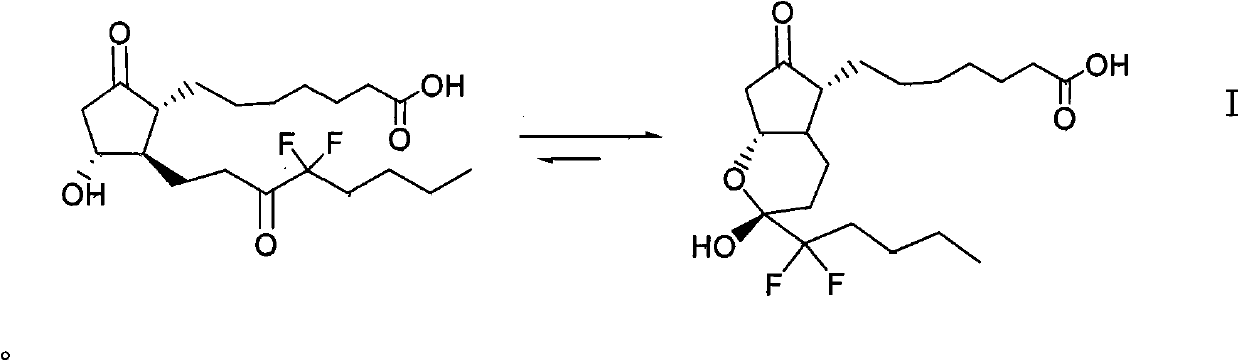
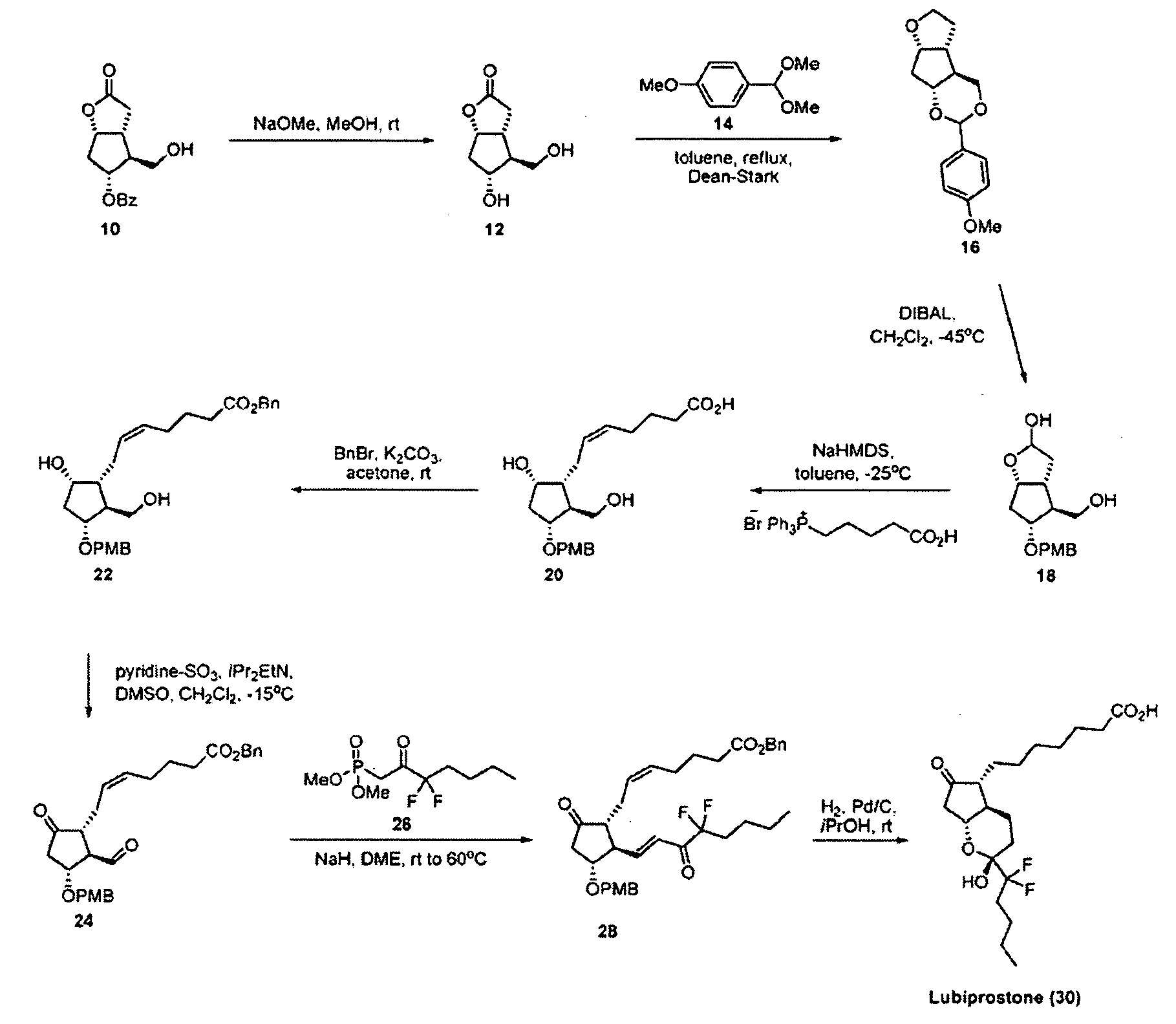
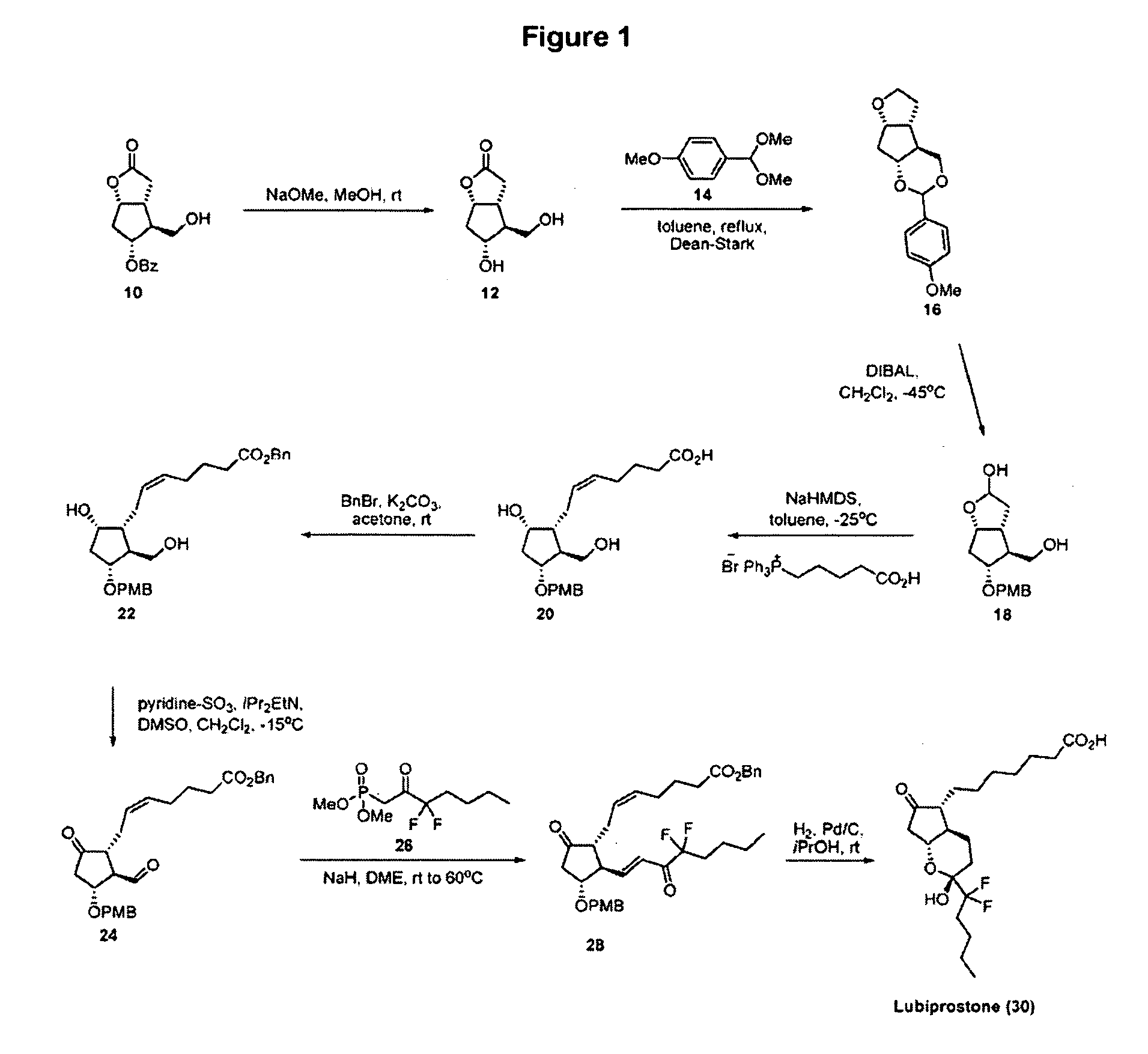
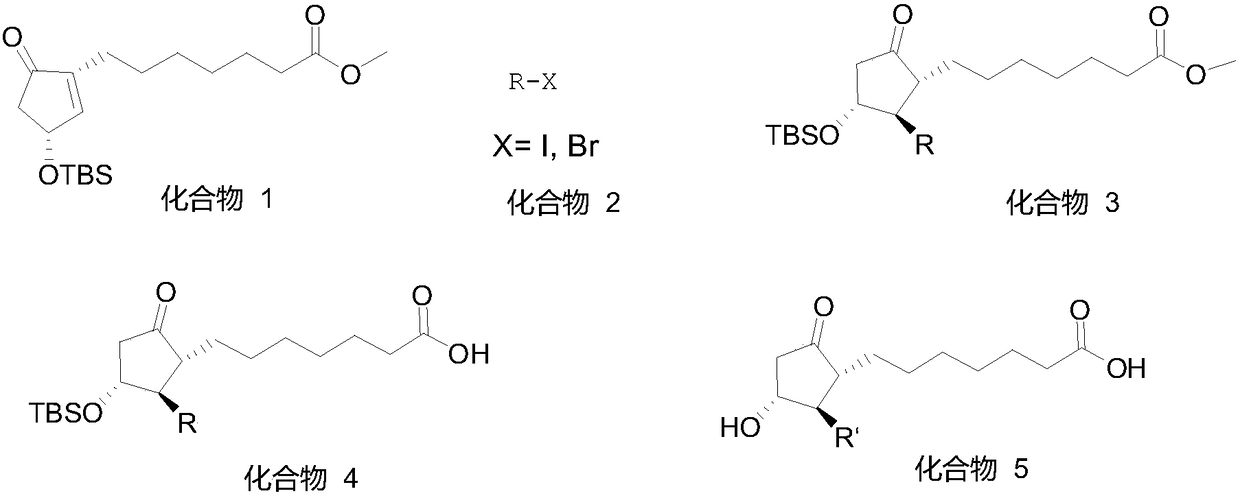
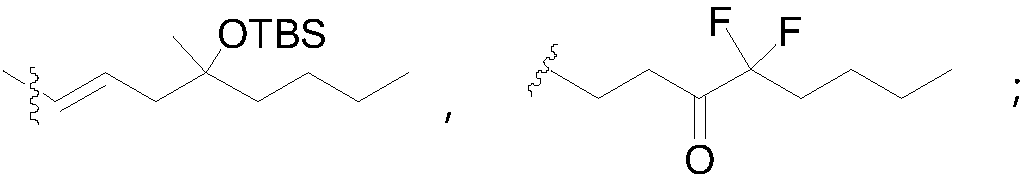
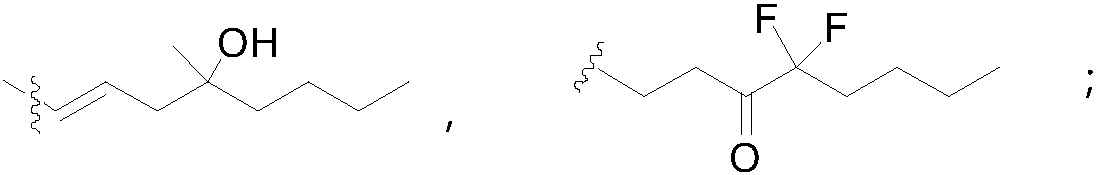
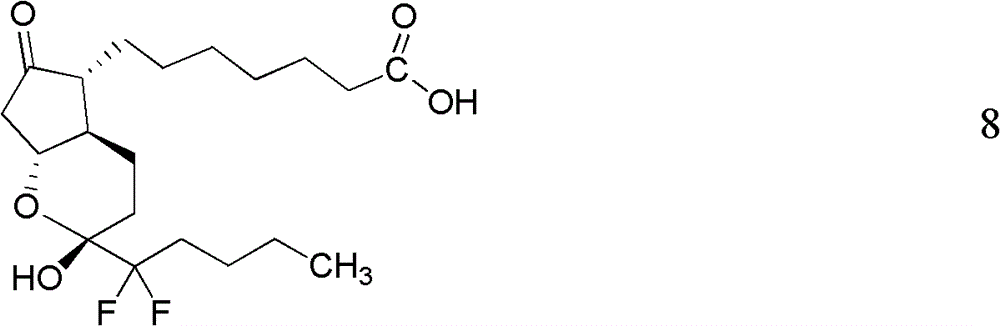
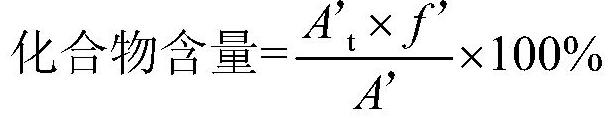Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
36 results about "Lubiprostone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
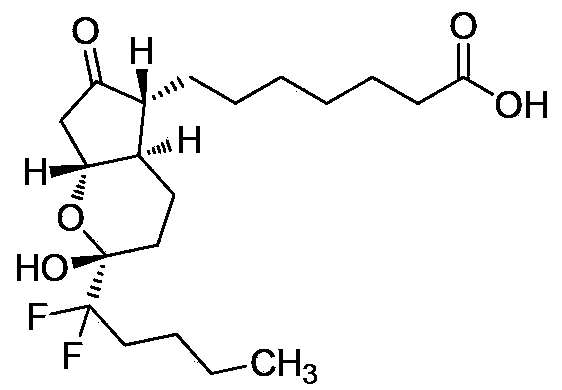
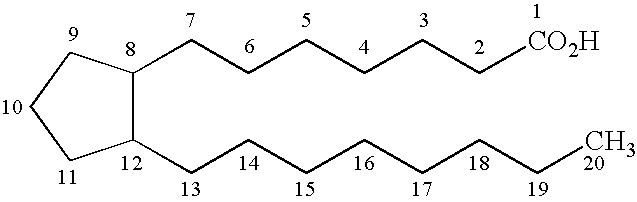
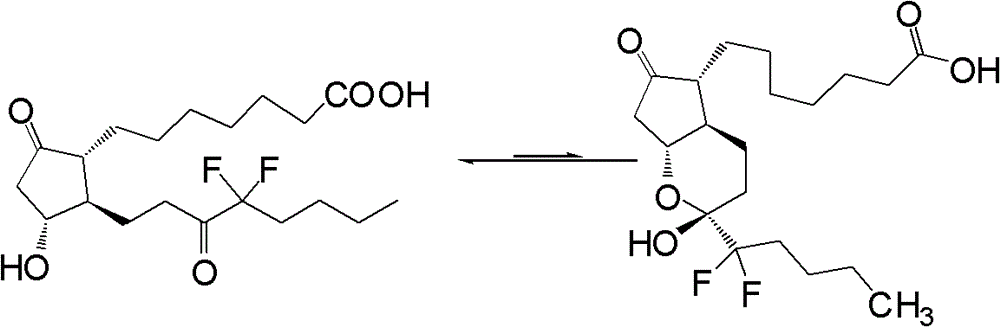
This medication is used to treat certain types of constipation (chronic idiopathic constipation, irritable bowel syndrome with constipation).
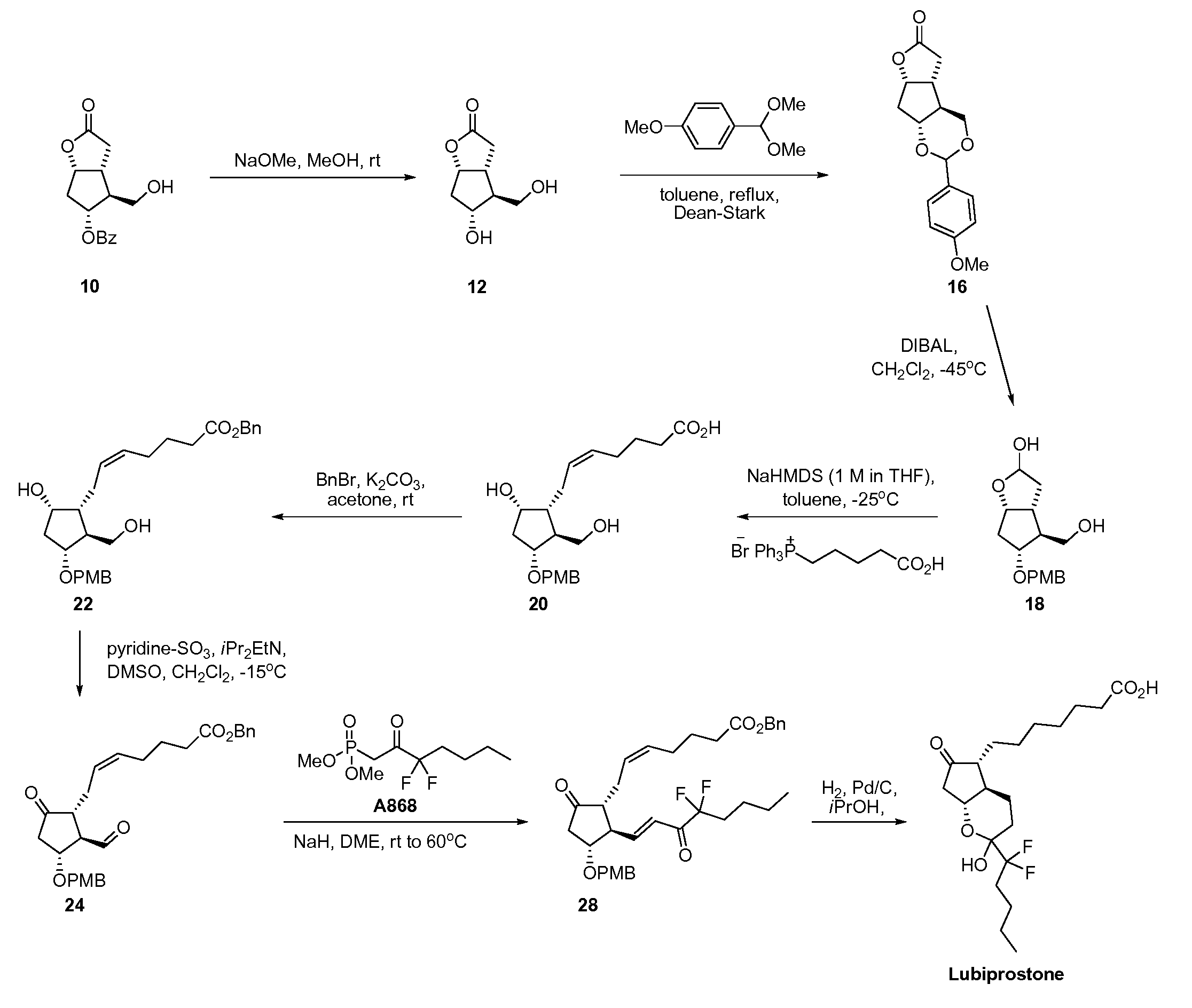
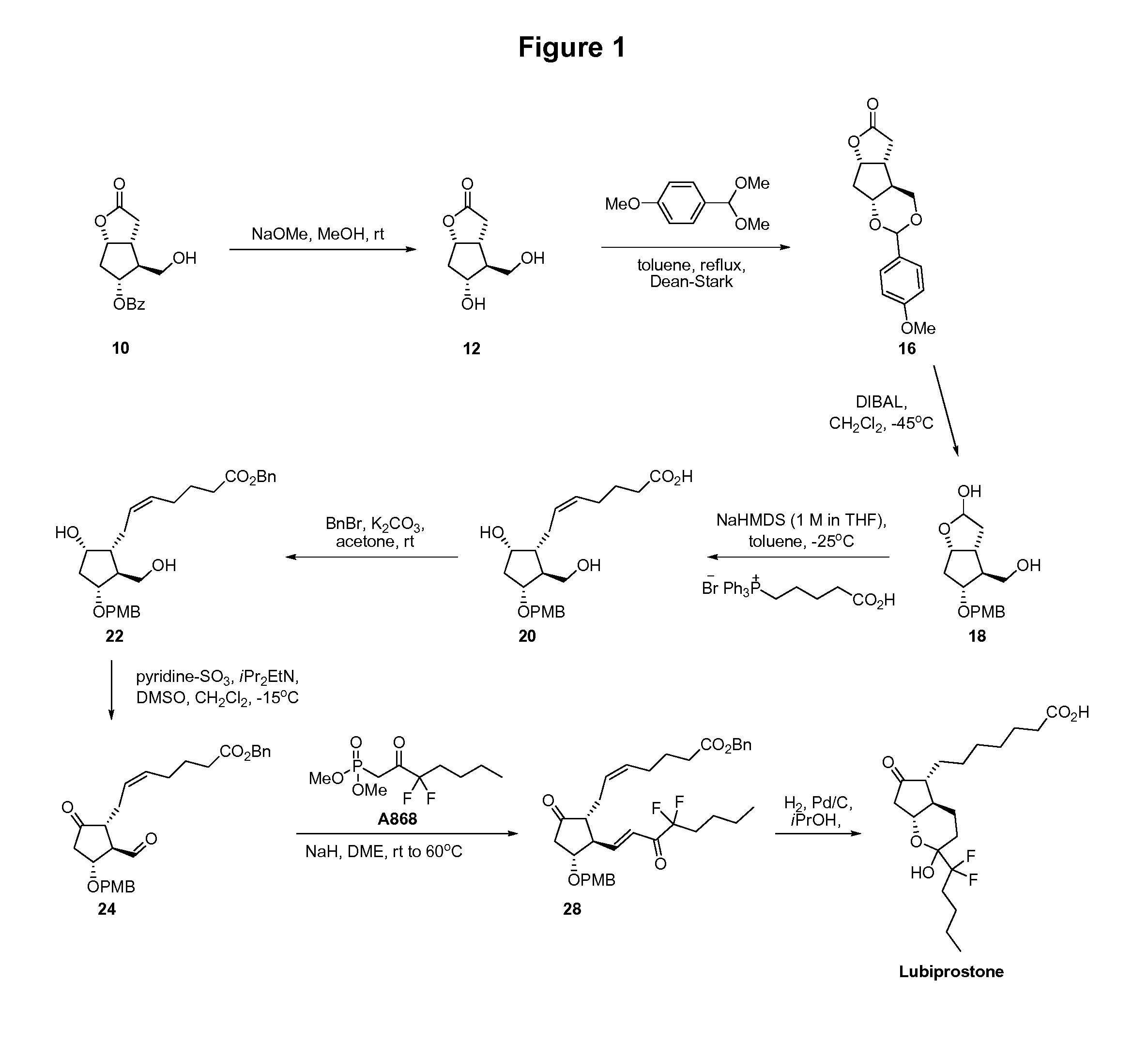
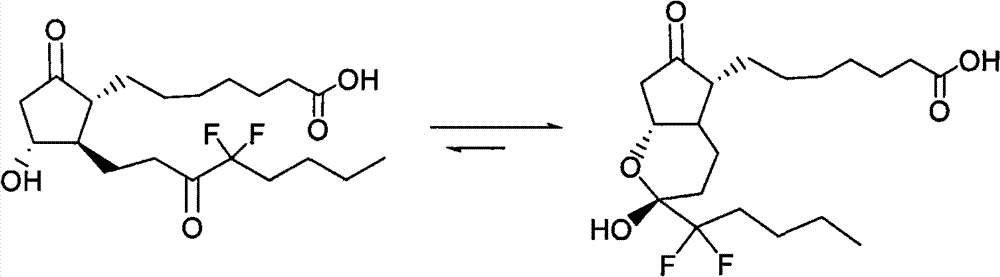
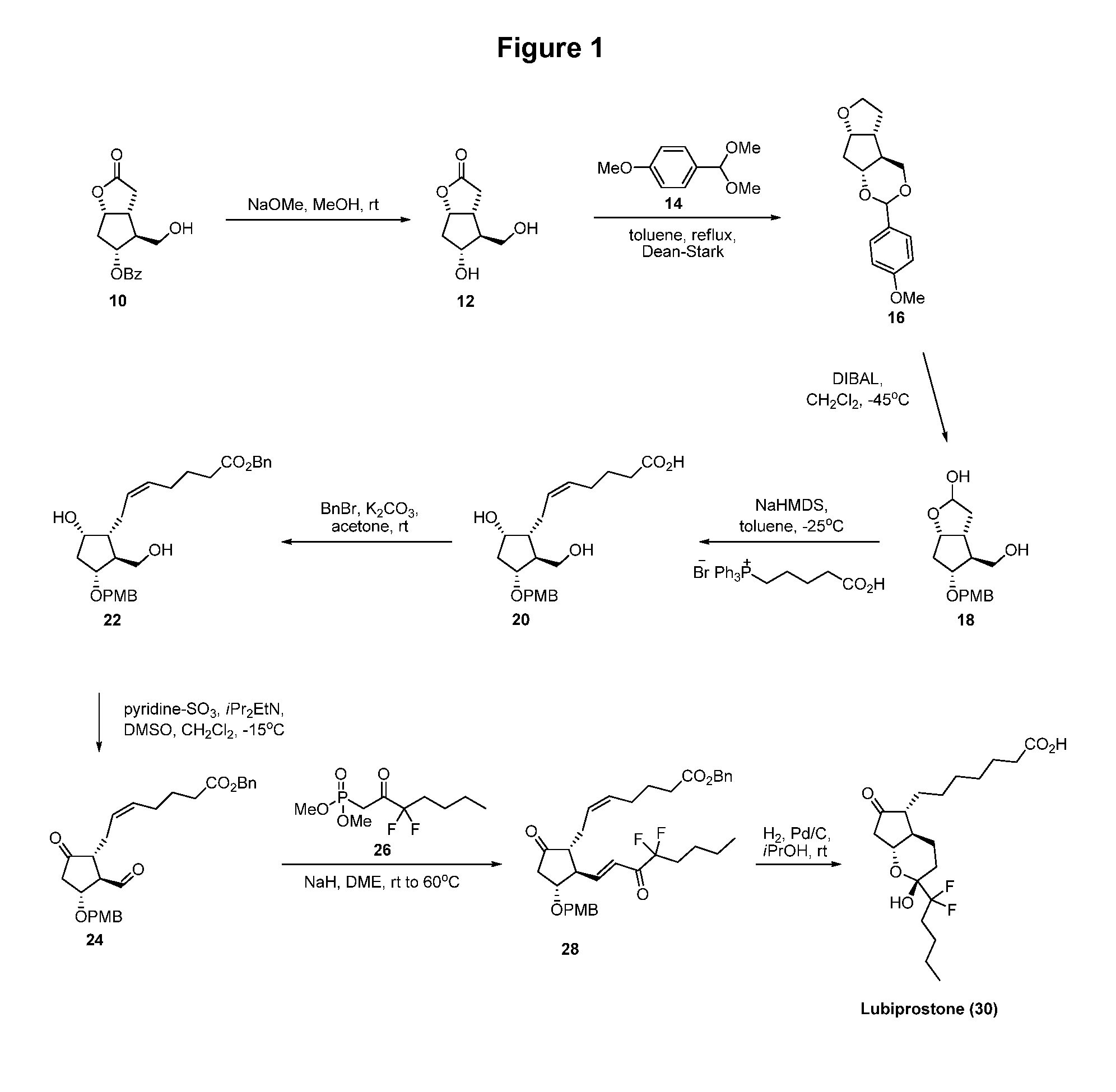
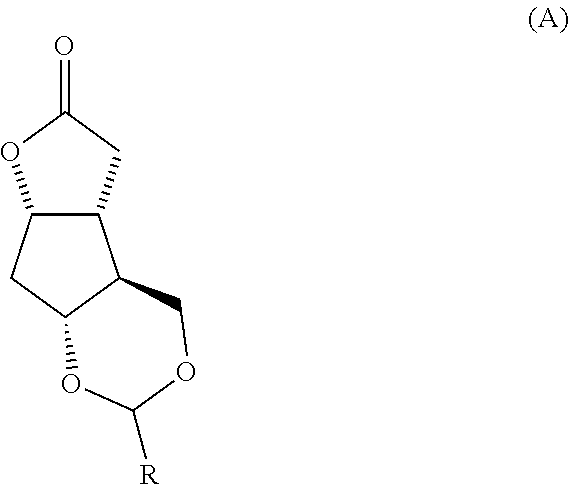
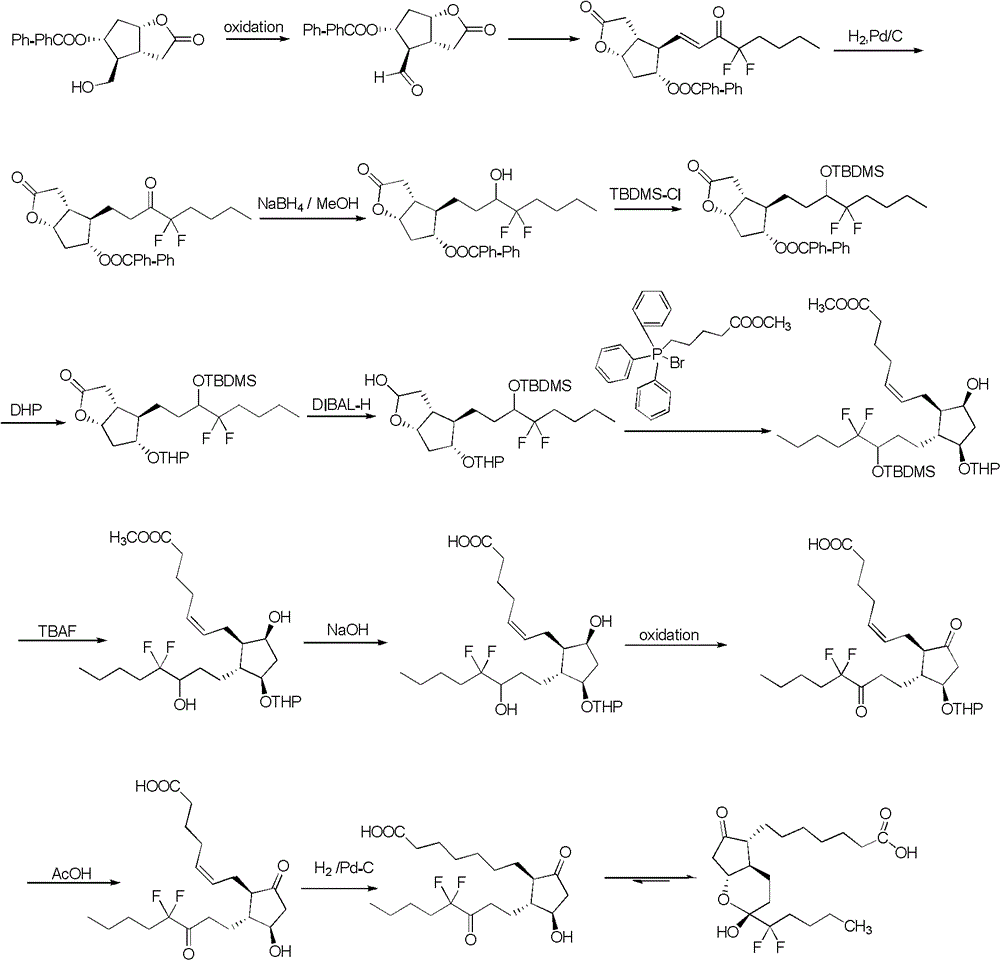
Prostaglandin synthesis and intermediates for use therein
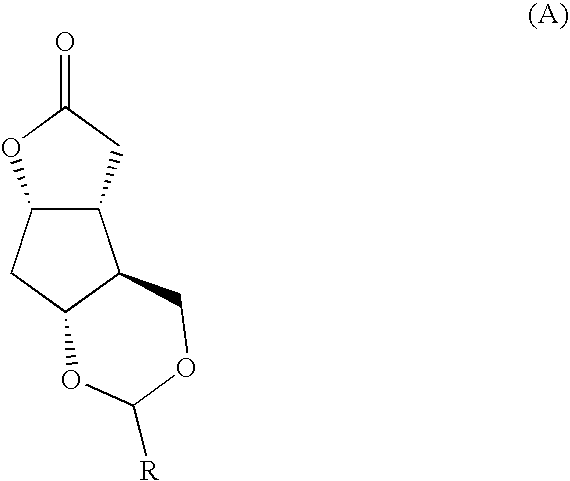
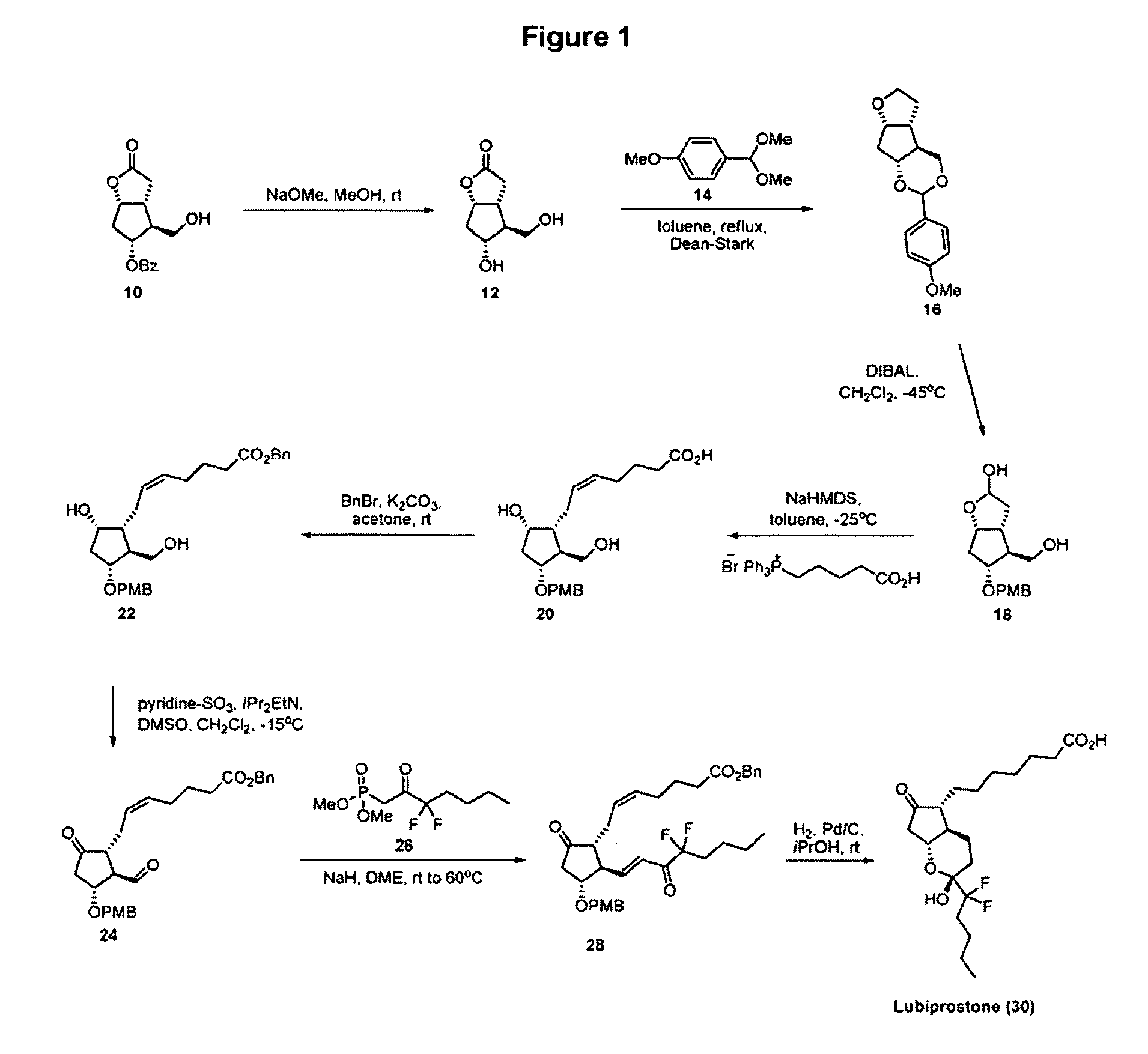
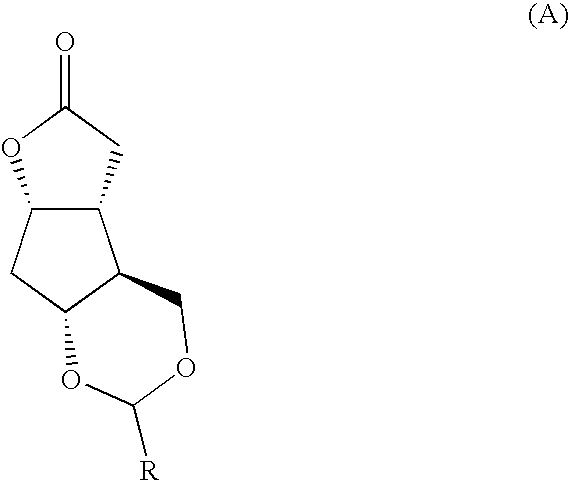
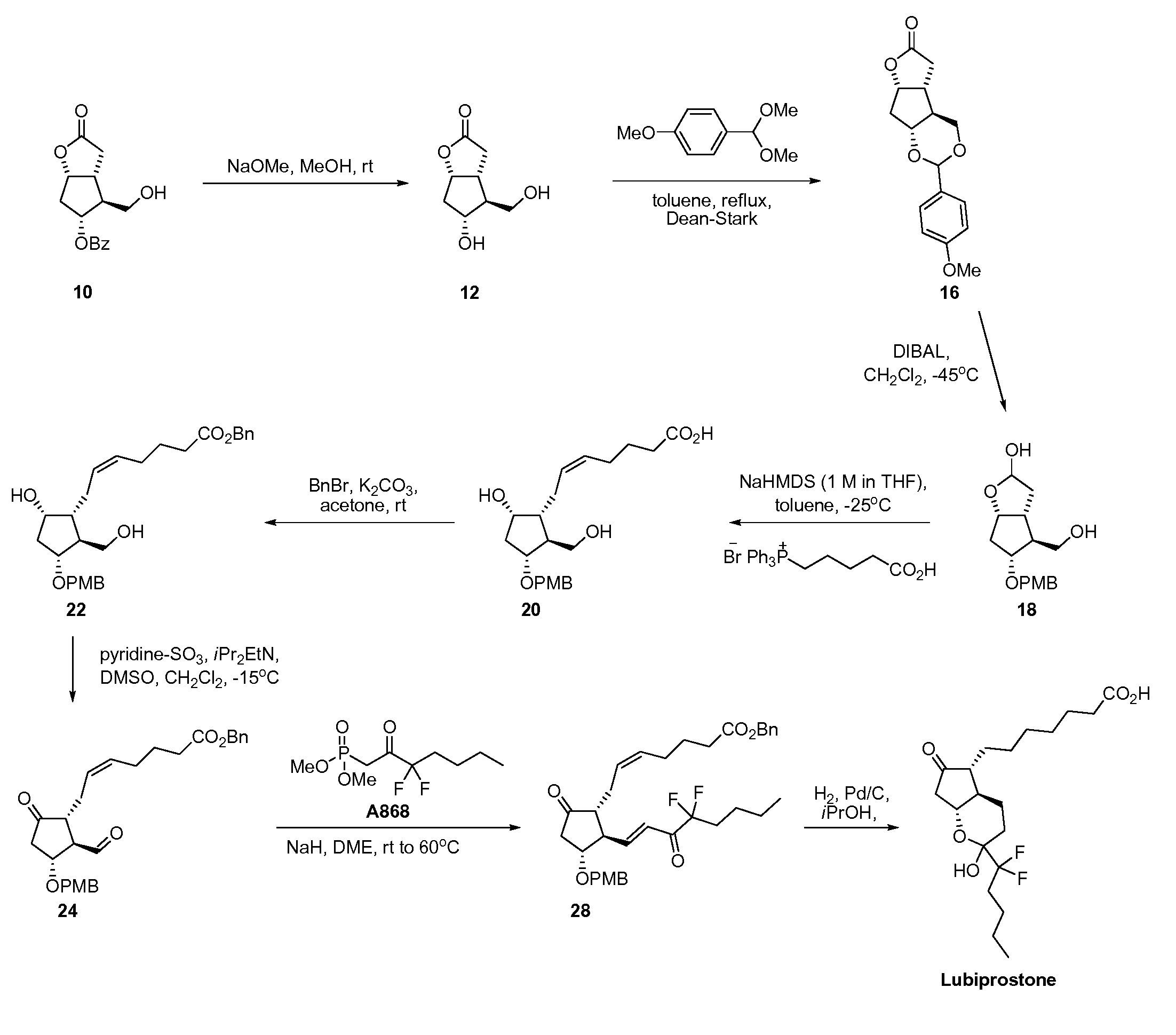
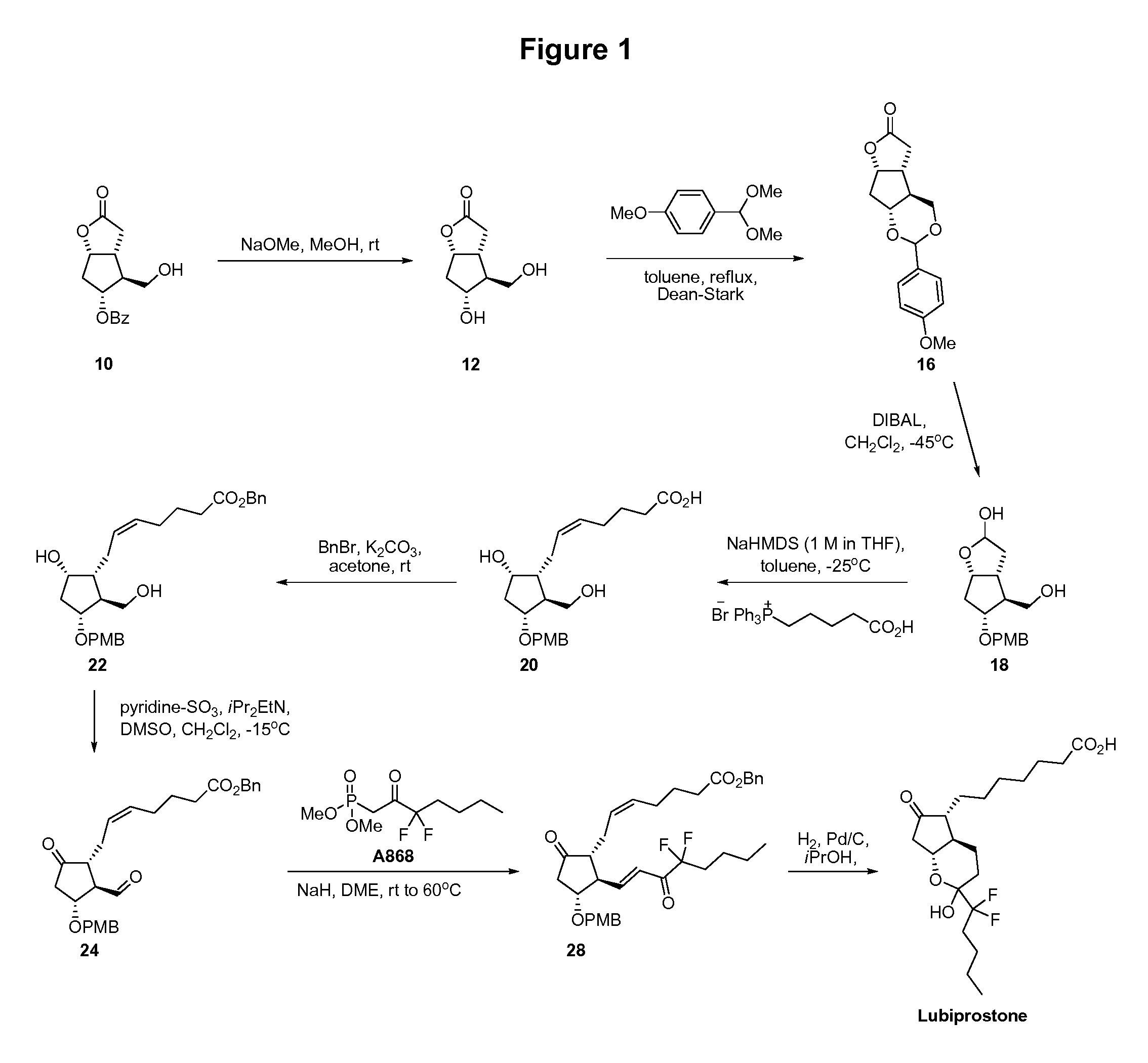
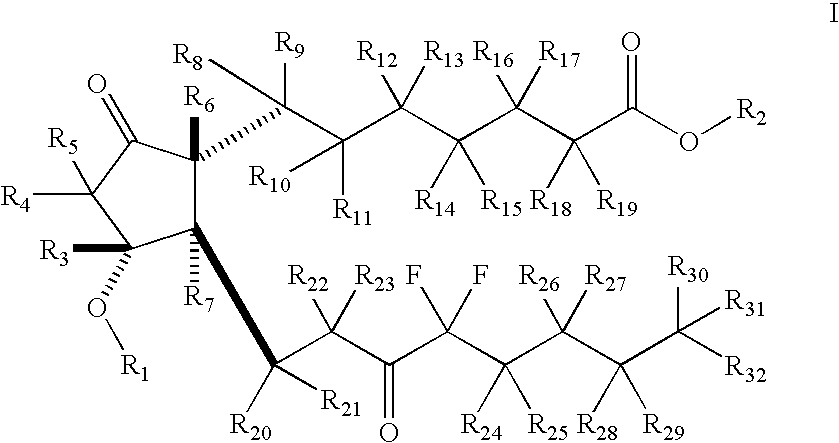
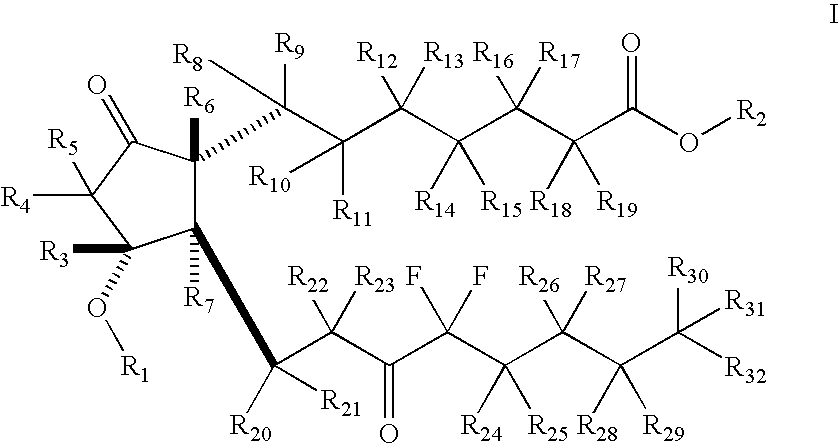
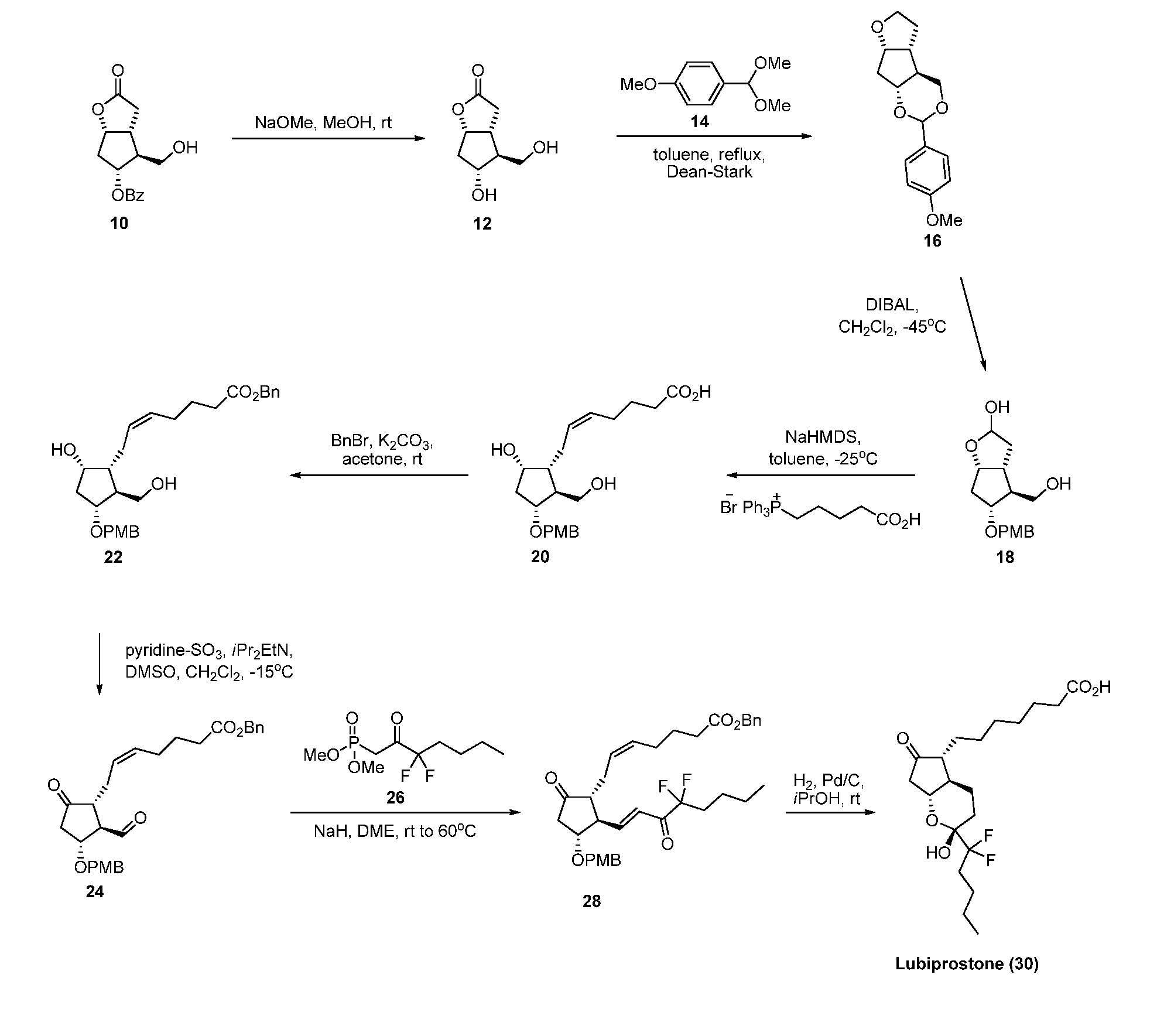
Fused cyclopentane—4-substituted 3,5-dioxalane lactone compounds useful as an intermediate in the synthesis of prostaglandin analogs are provided. The compounds have the formula A:wherein R represents an aryl group such as p-methoxyphenyl.This compound can be reacted with a lower alkyl aluminum compound to open the dioxalane ring and reduce the lactone to lactol, without over-reducing to diol. The resulting compound can be functionalized to insert chemical side groups of target prostaglandins, adding the required a-side chain and then the required ω-side chain sequentially and independently of each other. The compounds and process are particularly suitable for preparing lubiprostone.
Owner:ALPHORA RES
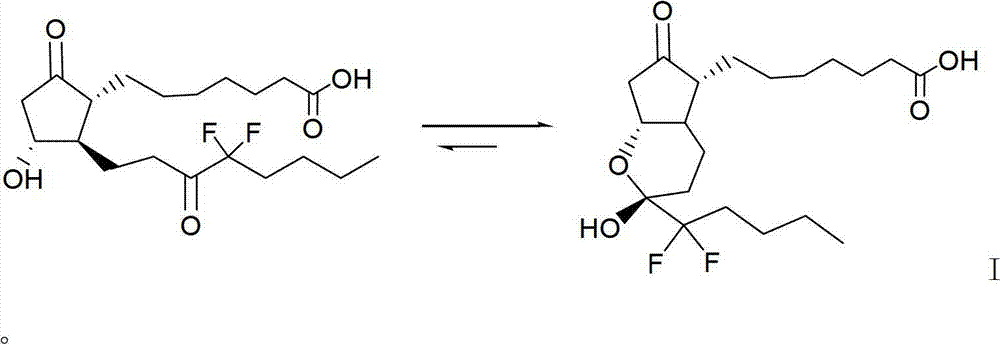
Intermediate for preparing lubiprostone, preparation method of intermediate and method for preparing lubiprostone through intermediate
ActiveCN103787942AOperational securityEasy to operateOrganic chemistryBulk chemical productionReduction treatmentCombinatorial chemistry
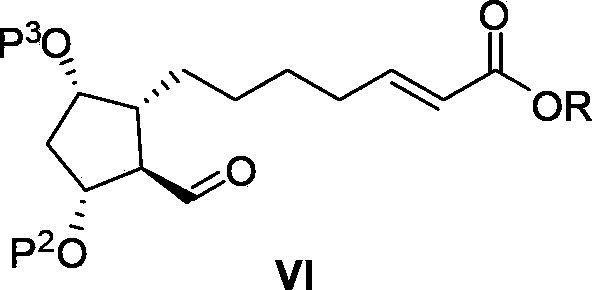
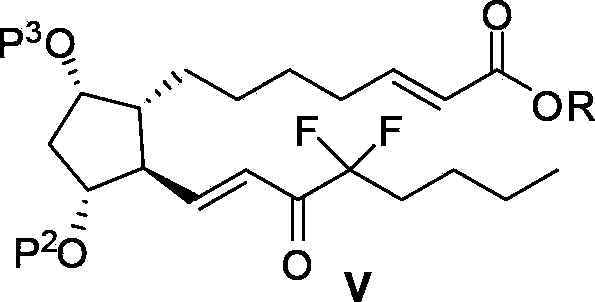
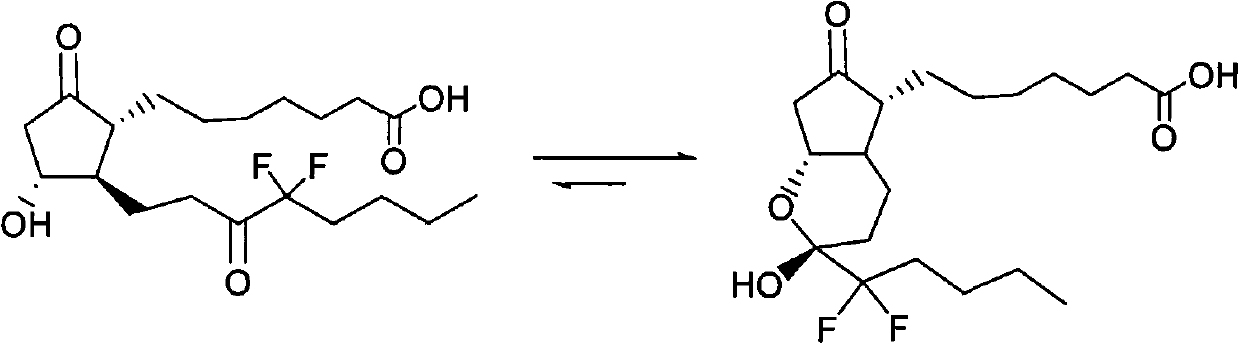
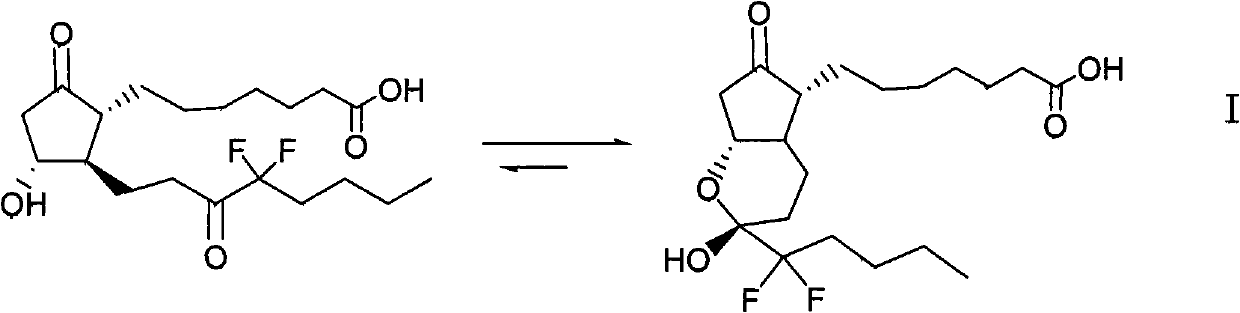
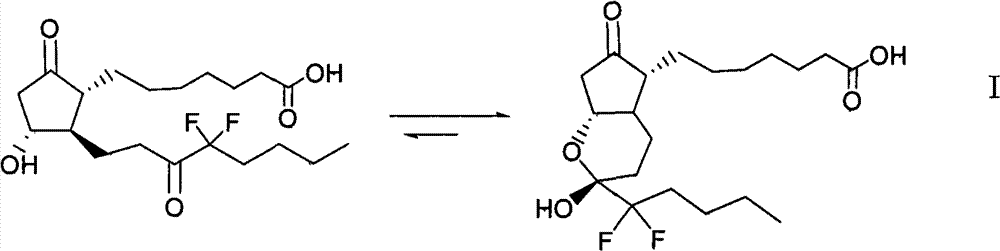
The invention relates to an intermediate for preparing lubiprostone, a preparation method of the intermediate and a method for preparing the lubiprostone through the intermediate, in particular to a compound as shown in a formula V for preparing the lubiprostone (as shown in a formula I), a preparation method of the compound and a method for preparing the lubiprostone through the compound. The method comprises the following steps: performing reduction treatment on the compound as shown in the formula V, performing selective deprotection and hydroxyl oxidation to obtain a compound as shown in a formula II, and performing hydroxyl deprotection on the compound as shown in the formula II to prepare the lubiprostone as shown in the formula I. The method is easy and convenient to operate, high in synthetic yield and suitable for large-scale production.
Owner:连云港恒运药业有限公司
High-purity lubiprostone, preparation method and application thereof
ActiveCN102020625AStable in natureLittle side effectsIon-exchange process apparatusOrganic chemistryOrganic solventStructural formula
The invention discloses high-purity lubiprostone, a preparation method and application thereof. The high-purity lubiprostone has the chemical structural formula shown in the formula I, wherein HPLC (High Performance Liquid Chromatography) purity is more than 95 percent. The invention also provides a preparation method of the high-purity lubiprostone, which comprises the steps of: 1. sampling lubiprostone crude products to a chromatographic column for preparing the HPLC; 2. eluting eluent through the chromatographic column for preparing the HPLC; and 3. crystallizing with an organic solvent to obtain the high-purity lubiprostone.
Owner:SHANGHAI TECHWELL BIOPHARMACEUTICALS CO LTD
Prostaglandin synthesis and intermediates for use therein
Fused cyclopentane—4-substituted 3,5-dioxalane lactone compounds useful as an intermediate in the synthesis of prostaglandin analogs are provided. The compounds have the formula A:wherein R represents an aryl group such as p-methoxyphenyl.This compound can be reacted with a lower alkyl aluminum compound to open the dioxalane ring and reduce the lactone to lactol, without over-reducing to diol. The resulting compound can be functionalized to insert chemical side groups of target prostaglandins, adding the required α-side chain and then the required ω-side chain sequentially and independently of each other. The compounds and process are particularly suitable for preparing lubiprostone.
Owner:ALPHORA RES
Preparation method of lubiprostone
The invention relates to a preparation method of lubiprostone, and concretely relates to a preparation method of highly pure lubiprostone represented by formula (I). The method comprises the steps of reducing an initial compound, oxidizing, and hydrolyzing in order to obtain a target compound. Compared with other methods, the method provided by the invention has the advantages of good process reappearance, simple operation, high yield, low cost, high purity of the above obtained product, suitableness for industrialized production, and very high economic benefit.
Owner:JIANGSU HANSOH PHARMA CO LTD
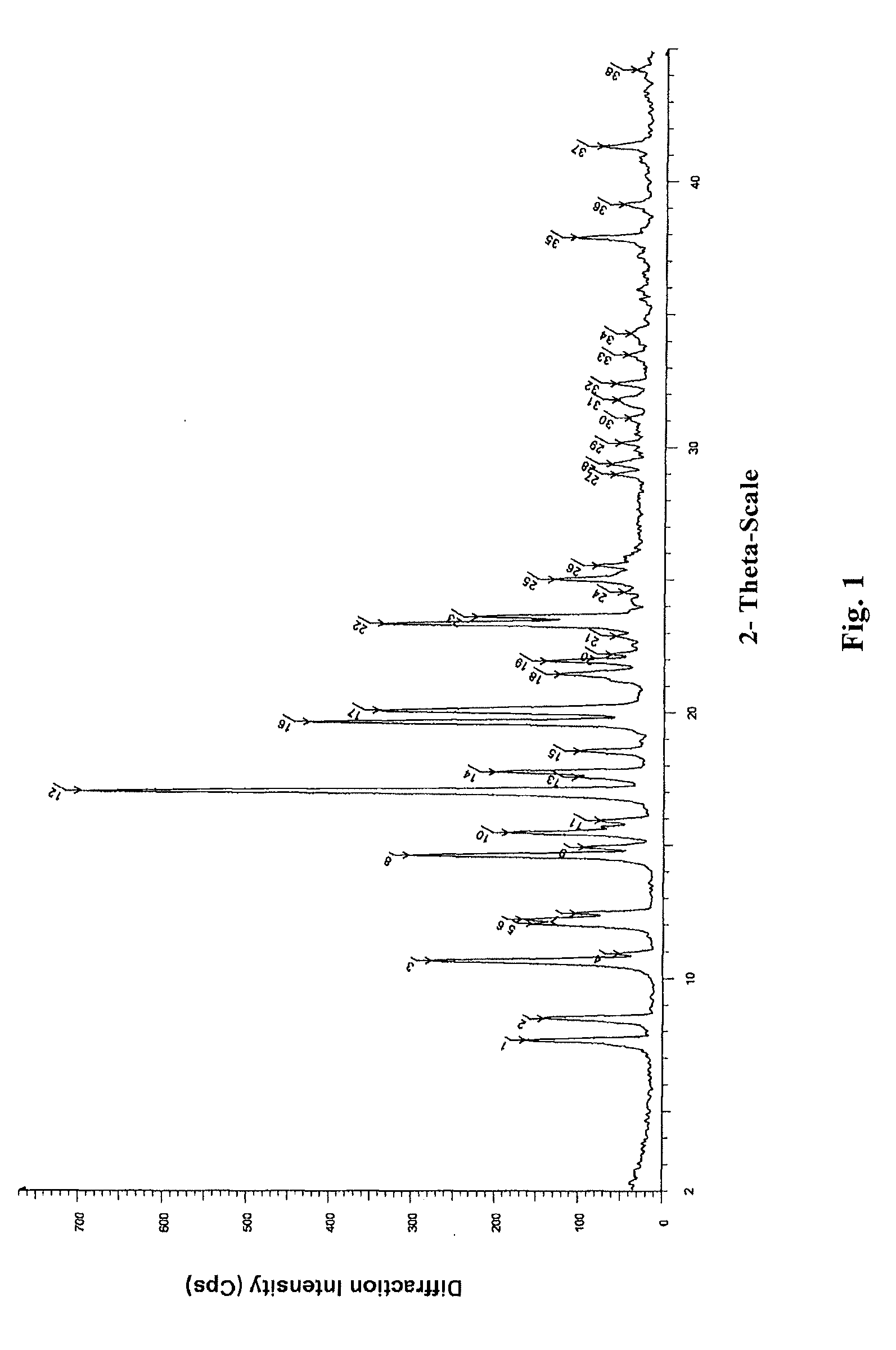
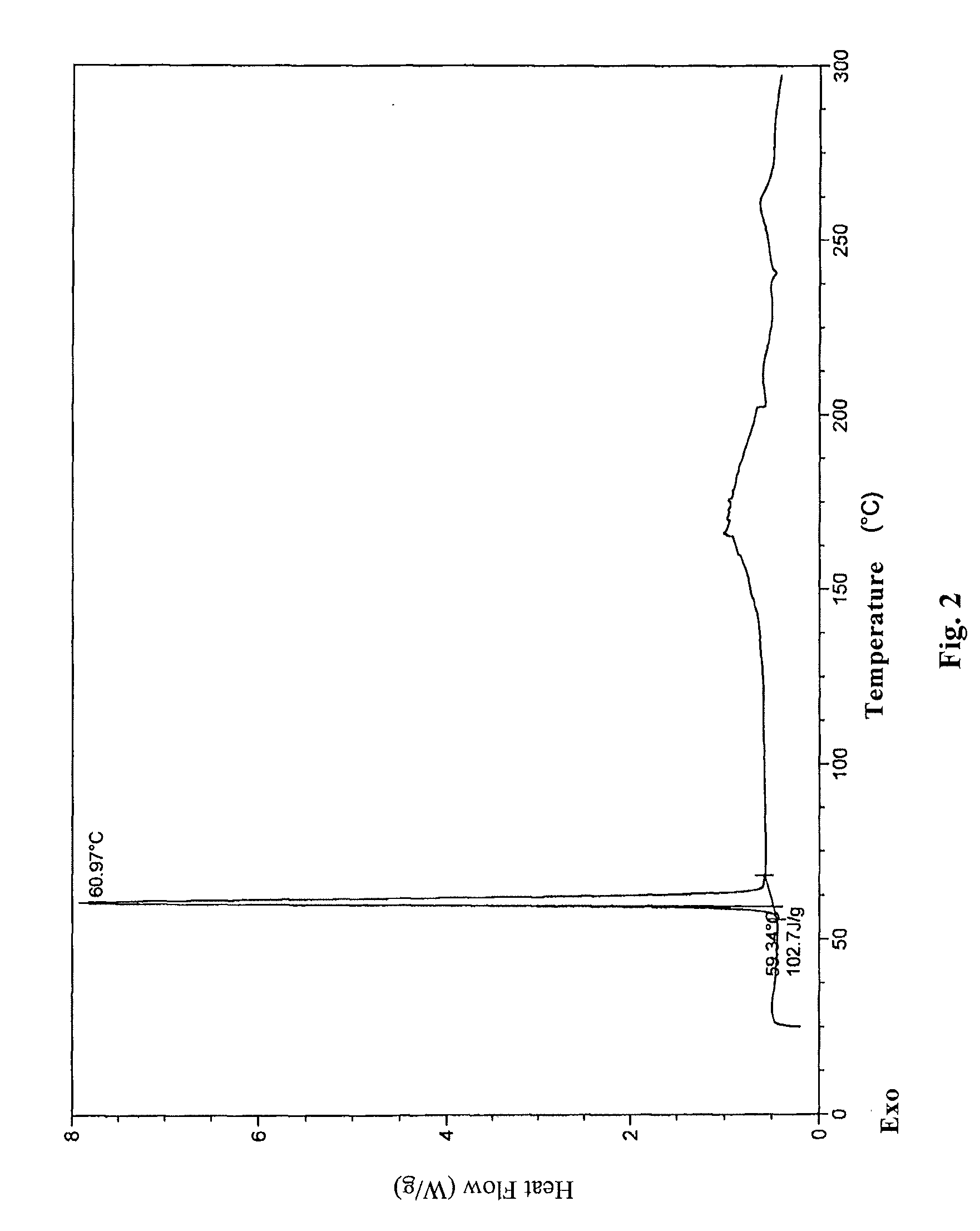
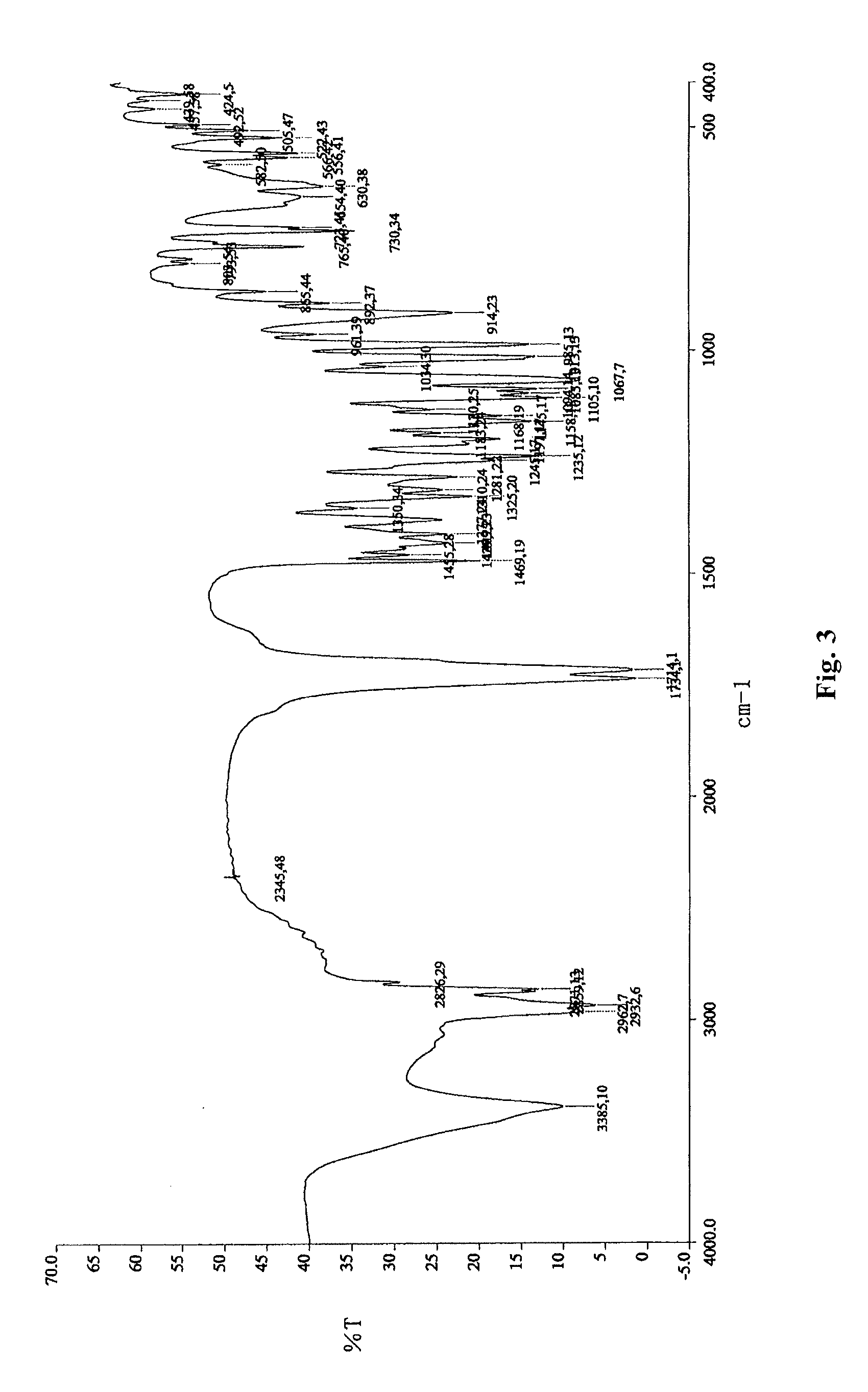
Novel crystal form of lubiprostone and preparation method of crystal form
InactiveCN104710398AHigh purityImprove stabilityOrganic chemistryElcosanoid active ingredientsX-rayPowder diffraction
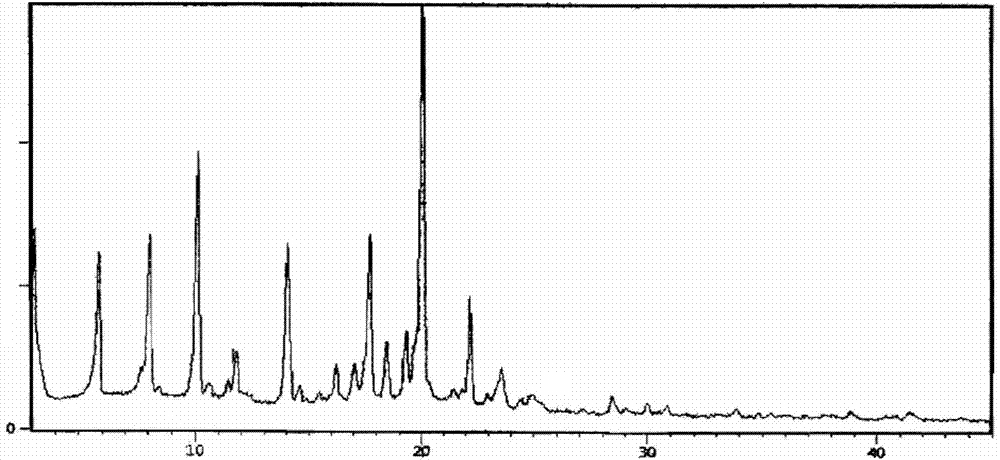
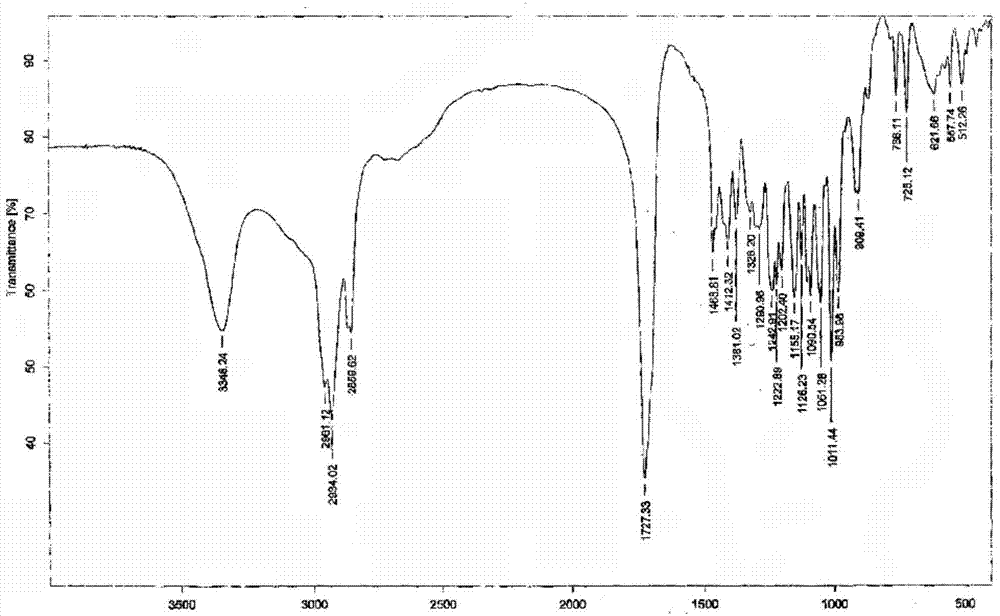
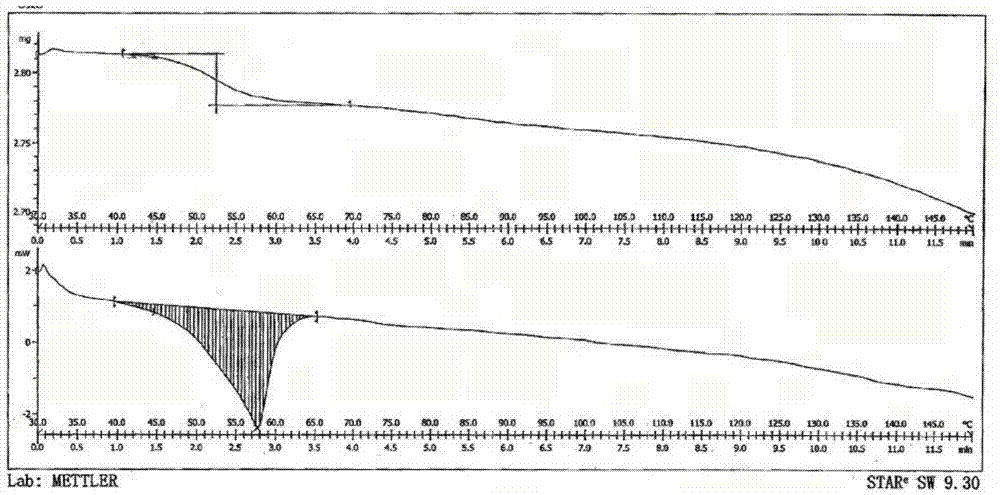
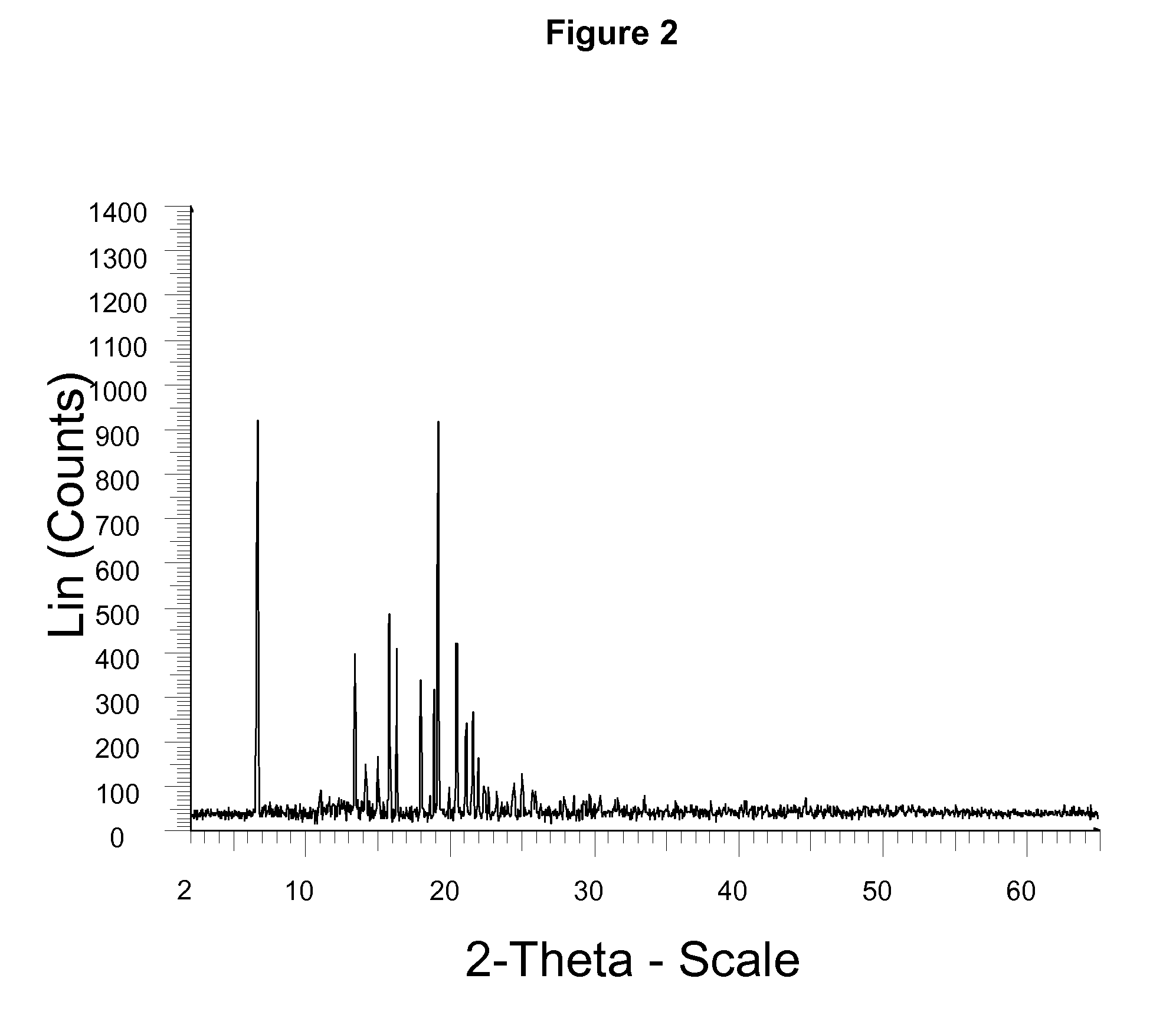
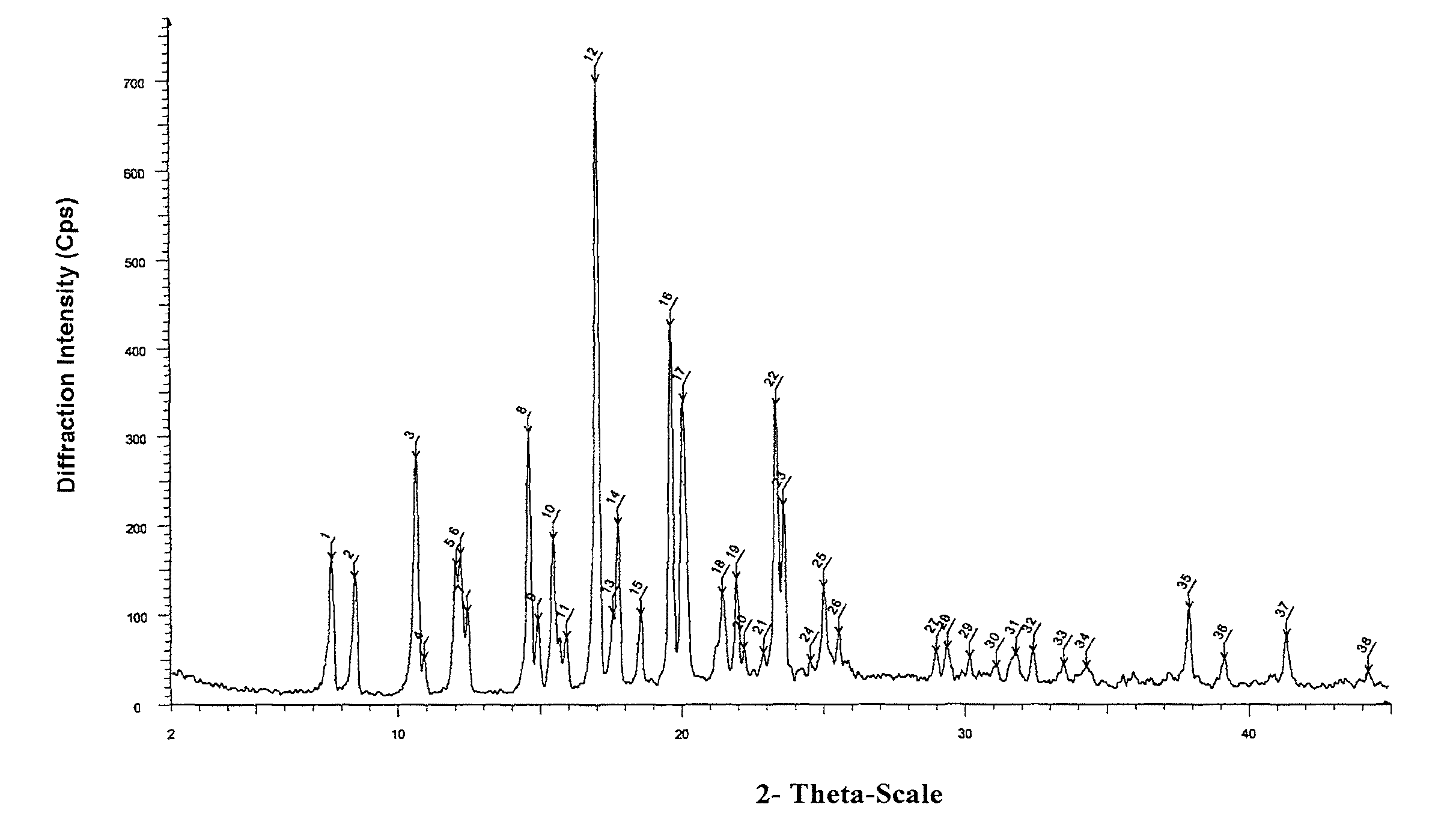
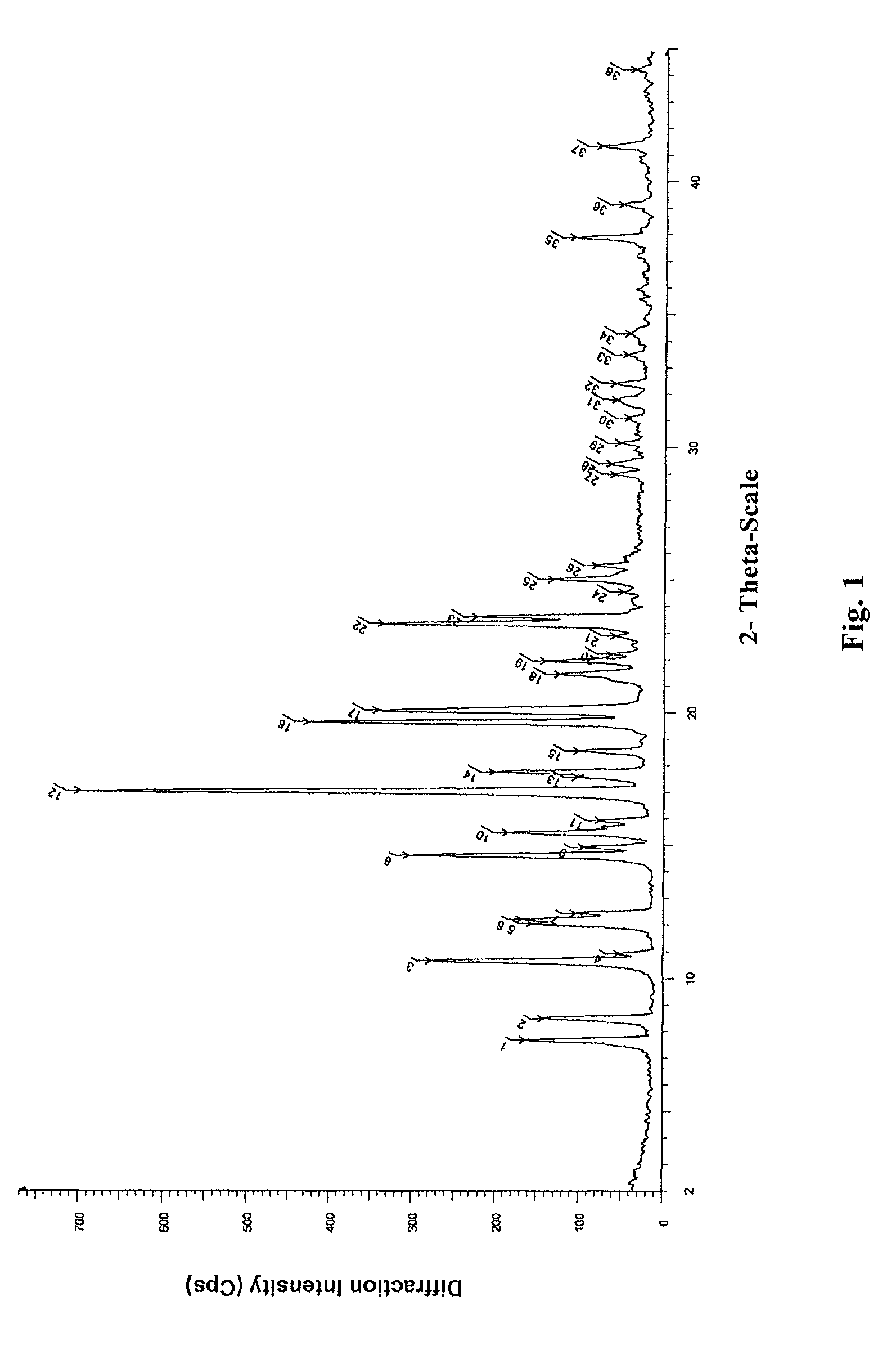
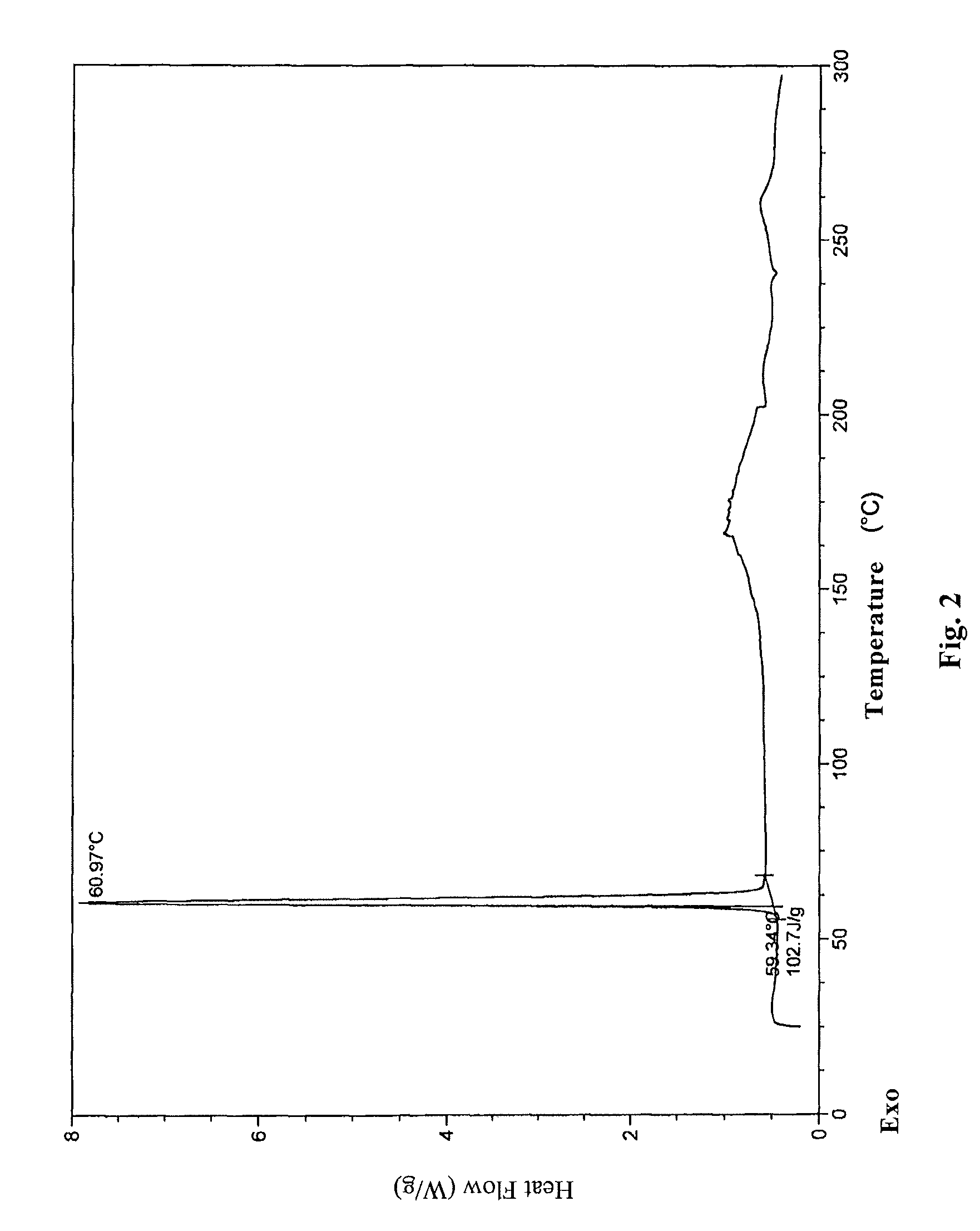
The invention belongs to the technical field of pharmaceutical chemical engineering and particularly relates to a novel crystal form of lubiprostone, a preparation method of the crystal form and the application of the crystal form to medicine preparation. The crystal form is represented through X-ray powder diffraction, infrared spectrography and differential scanning calorimetry, has the advantages of high purity, high stability and easy preparation, is more suitable for storage and use, and can be better applied to preparation of lubiprostone drug substances and preparations.
Owner:QILU PHARMA
Prostaglandin synthesis and intermediates for use therein
Fused cyclopentane—4-substituted 3,5-dioxalane lactone compounds useful as an intermediate in the synthesis of prostaglandin analogs are provided. The compounds have the formula A:wherein R represents an aryl group such as p-methoxyphenyl.This compound can be reacted with a lower alkyl aluminum compound to open the dioxalane ring and reduce the lactone to lactol, without over-reducing to diol. The resulting compound can be functionalized to insert chemical side groups of target prostaglandins, adding the required α-side chain and then the required ω-side chain sequentially and independently of each other. The compounds and process are particularly suitable for preparing lubiprostone.
Owner:ALPHORA RES
Method for preparing lubiprostone compound
ActiveCN104557845AShort reaction timeHigh reaction yieldOrganic chemistryBulk chemical productionHydrogenTriethylsilane
The invention relates to a method for preparing lubiprostone. The method comprises the following steps: by taking triethyl silicane as a hydrogen source, and taking palladium carbon or palladium hydroxide as a catalyst, performing catalytic transfer hydrogenation, removing the protecting groups, and reducing double bonds, thereby obtaining the product. Compared with a conventional catalytic hydrogenation reaction at present, the method has the advantages of simplicity in operation, mild reaction conditions, high yield and high purity, the reaction time and reaction period are obviously shortened, the production efficiency is greatly improved, the requirements on reaction equipment are reduced, and the method is suitable for industrial production.
Owner:QILU PHARMA CO LTD
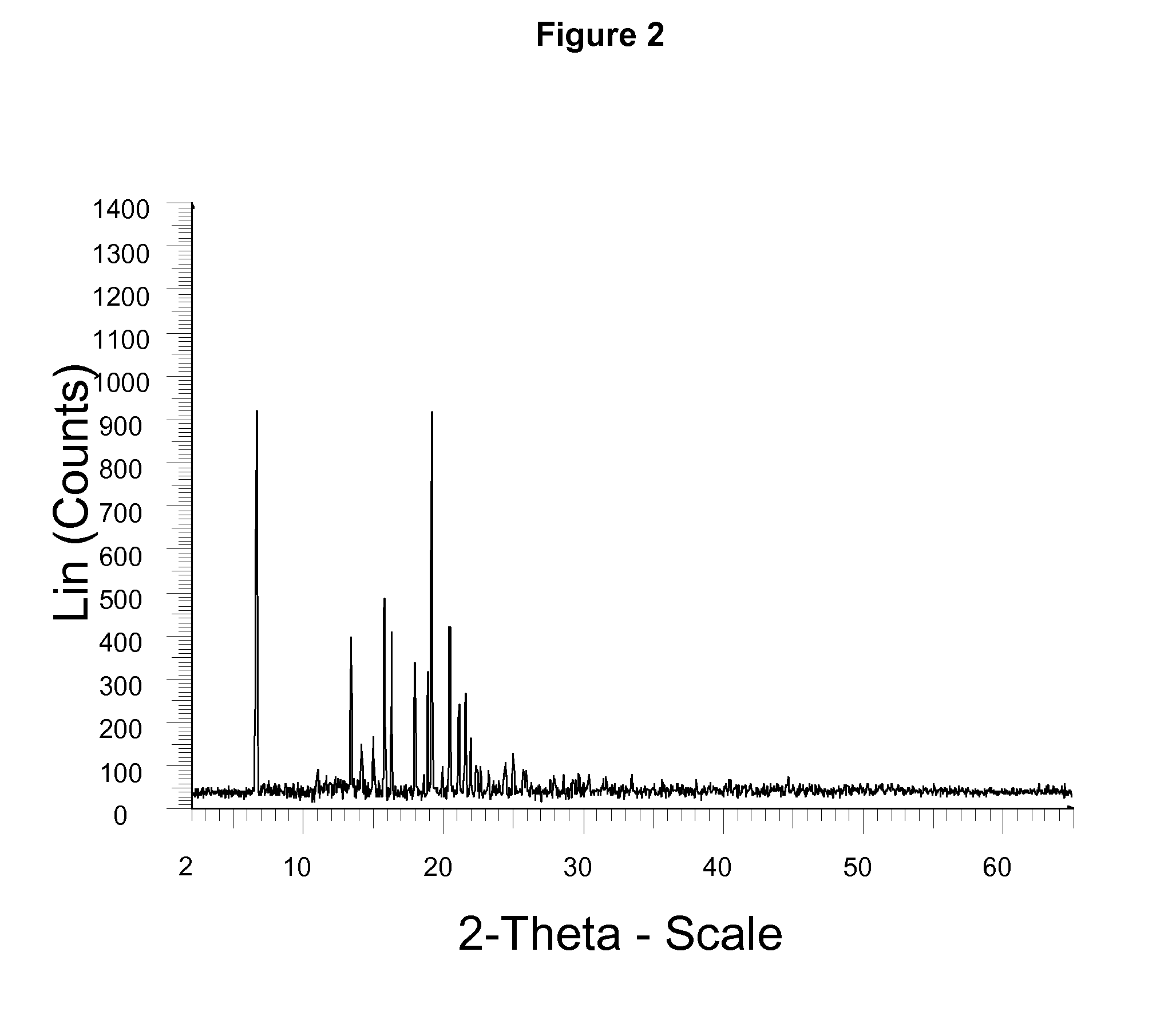
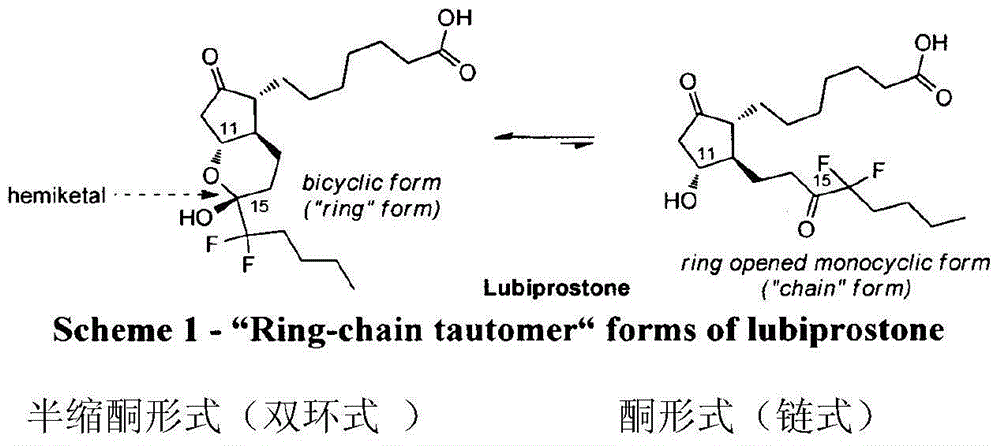
Lubiprostone crystal, the use and the method for the preparation thereof
ActiveUS20110028541A1Promote bowel movementsEasy to optimizeBiocideOrganic compound preparationDiseaseMedicine
The present invention relates to a lubiprostone crystal, the method for the preparation thereof, and a pharmaceutical composition or kit comprising the same, as well as the use of said crystal in the preparation of a medicament for the treatment of gastrointestinal tract diseases, especially constipation. The X-ray powder diffraction pattern of said crystal comprises characteristic peaks measured at the following 2θ reflection angles: 14.6±0.2°, 17.0±0.2° and 19.6±0.2°. As compared to amorphous lubiprostone, the crystal of the present invention has the advantages of relative high purity, stable properties and easy-for-storage and use.
Owner:SHANGHAI TECHWELL BIOPHARMACEUTICALS CO LTD
Medicine composition for treating constipation and/or haemorrhoids and application thereof
InactiveCN103550224AClear ingredientsDefinite curative effectElcosanoid active ingredientsDigestive systemPharmaceutical drugCurative effect
Owner:海门市凤城旅游景点开发有限公司
High-purity lubiprostone, preparation method and application thereof
ActiveCN102020625BStable in natureLittle side effectsIon-exchange process apparatusOrganic chemistryOrganic solventStructural formula
The invention discloses high-purity lubiprostone, a preparation method and application thereof. The high-purity lubiprostone has the chemical structural formula shown in the formula I, wherein HPLC (High Performance Liquid Chromatography) purity is more than 95 percent. The invention also provides a preparation method of the high-purity lubiprostone, which comprises the steps of: 1. sampling lubiprostone crude products to a chromatographic column for preparing the HPLC; 2. eluting eluent through the chromatographic column for preparing the HPLC; and 3. crystallizing with an organic solvent to obtain the high-purity lubiprostone.
Owner:SHANGHAI TECHWELL BIOPHARMACEUTICALS CO LTD
Lubiprostone refining method
InactiveCN107474033ASimple and fast operationReduce manufacturing costOrganic chemistryCrystallizationChemistry
The invention relates to the field of pharmaceutical chemistry and provides a lubiprostone refining method. In comparison with existing lubiprostone preparation methods, the preparation method of the invention can be adopted to obtain high-purity lubiprostone more simply and with high yield through a recrystallization mode.
Owner:BEIJING SHENLANHAI BIO PHARM TECH
Method for synthesizing 1R,2R,3R-substituted cyclopentanone compound
InactiveCN108503619AOvercome the disadvantage of low purityGood diastereoselectivityOrganic compound preparationCarboxylic compound preparationOrganic solventSynthesis methods
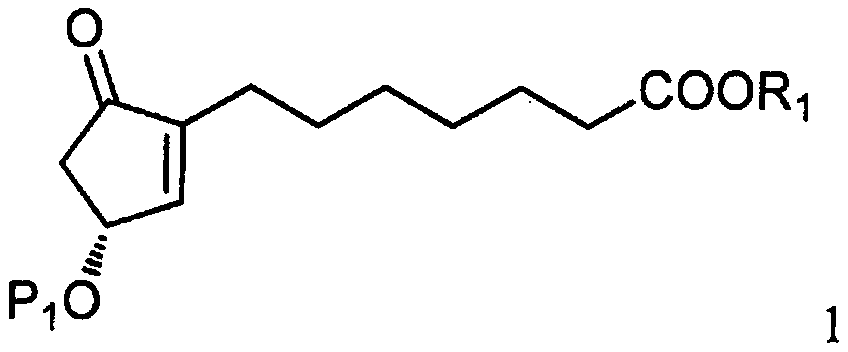
The invention relates to a method for synthesizing a 1R,2R,3R-substituted cyclopentanone compound. The method comprises: carrying out a reaction on a compound 1 and a halogenated alkane in an organicsolvent under the actions of a strong alkali and a cuprous salt to obtain a compound 3, carrying out ester hydrolysis on the compound 3 under the action of an alkali or pancreatin, and carrying out deprotection by using a fluorine reagent to obtain the product. According to the present invention, the synthesis method has characteristics of high yield, high product purity, good diastereoselectivity, easy reaction control and simple post-treatment, is suitable for industrial production, and can synthesize high-purity and high-yield alprostadil, lubiprostone, and the analogs of misoprostol and limaprost. The compound 1 is defined in the specification.
Owner:GUANGZHOU KAIMO BIOTECH CO LTD
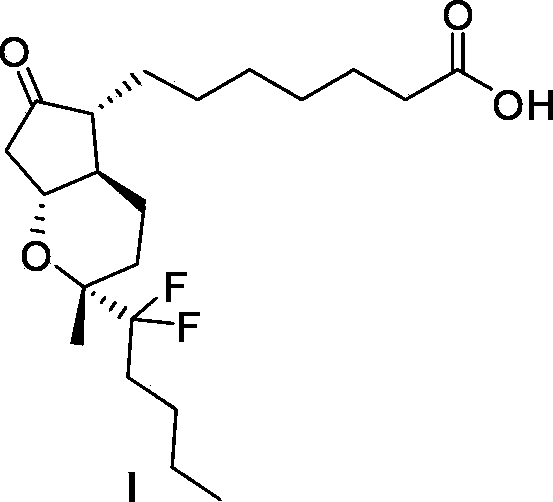
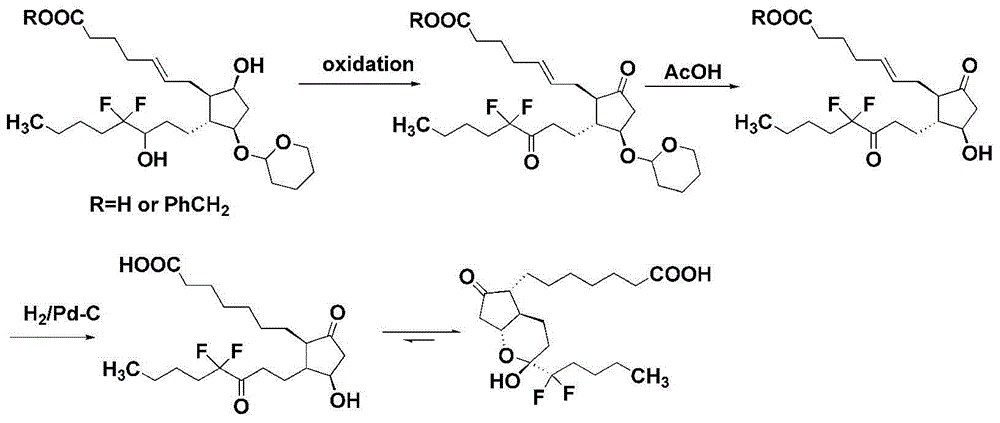
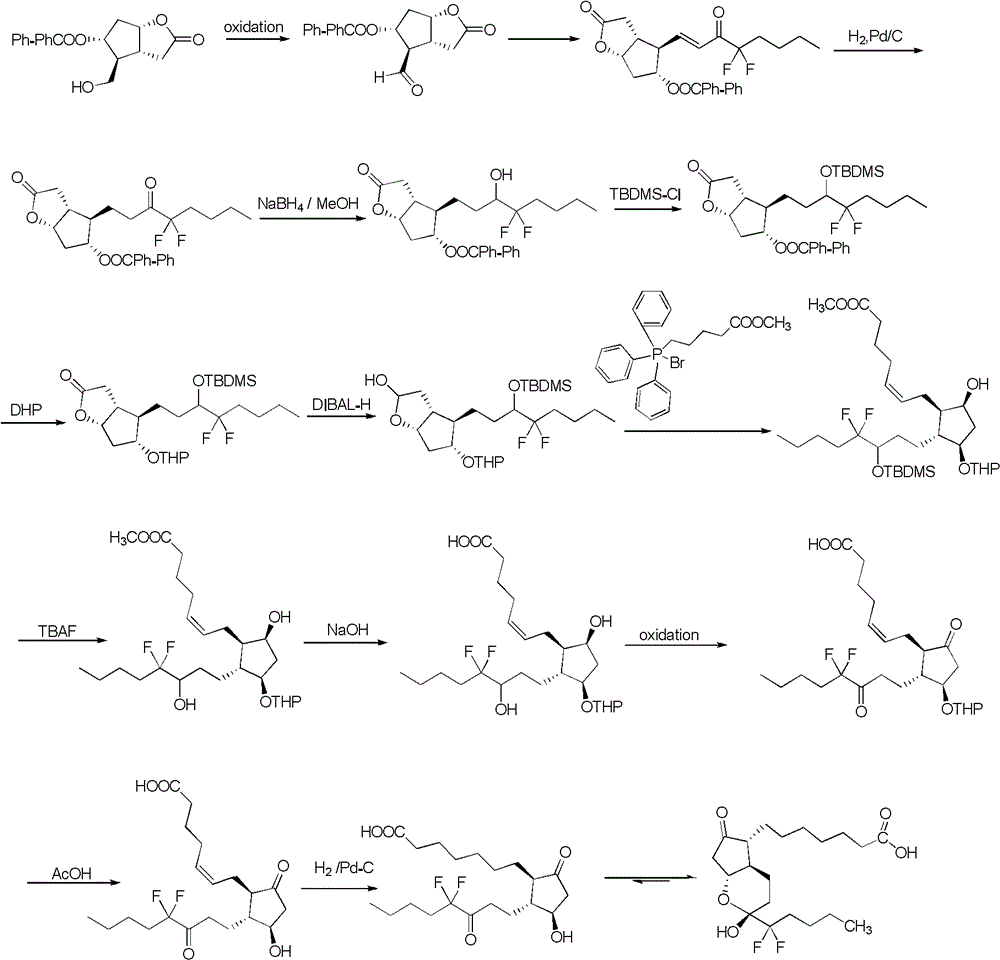
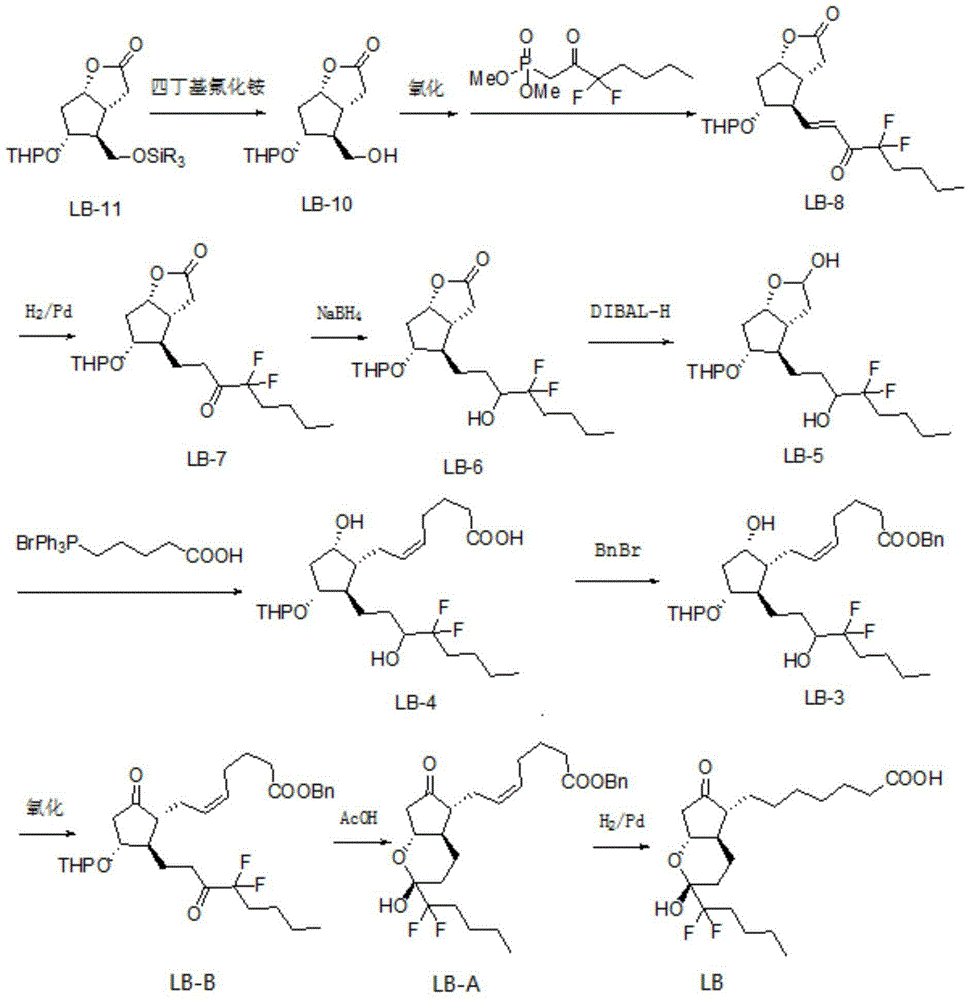
Preparation method of lubiprostone or midbody thereof
ActiveCN103058907AEasy to removeHigh yieldOrganic chemistryBulk chemical productionTert-butyldimethylsilaneWittig reaction
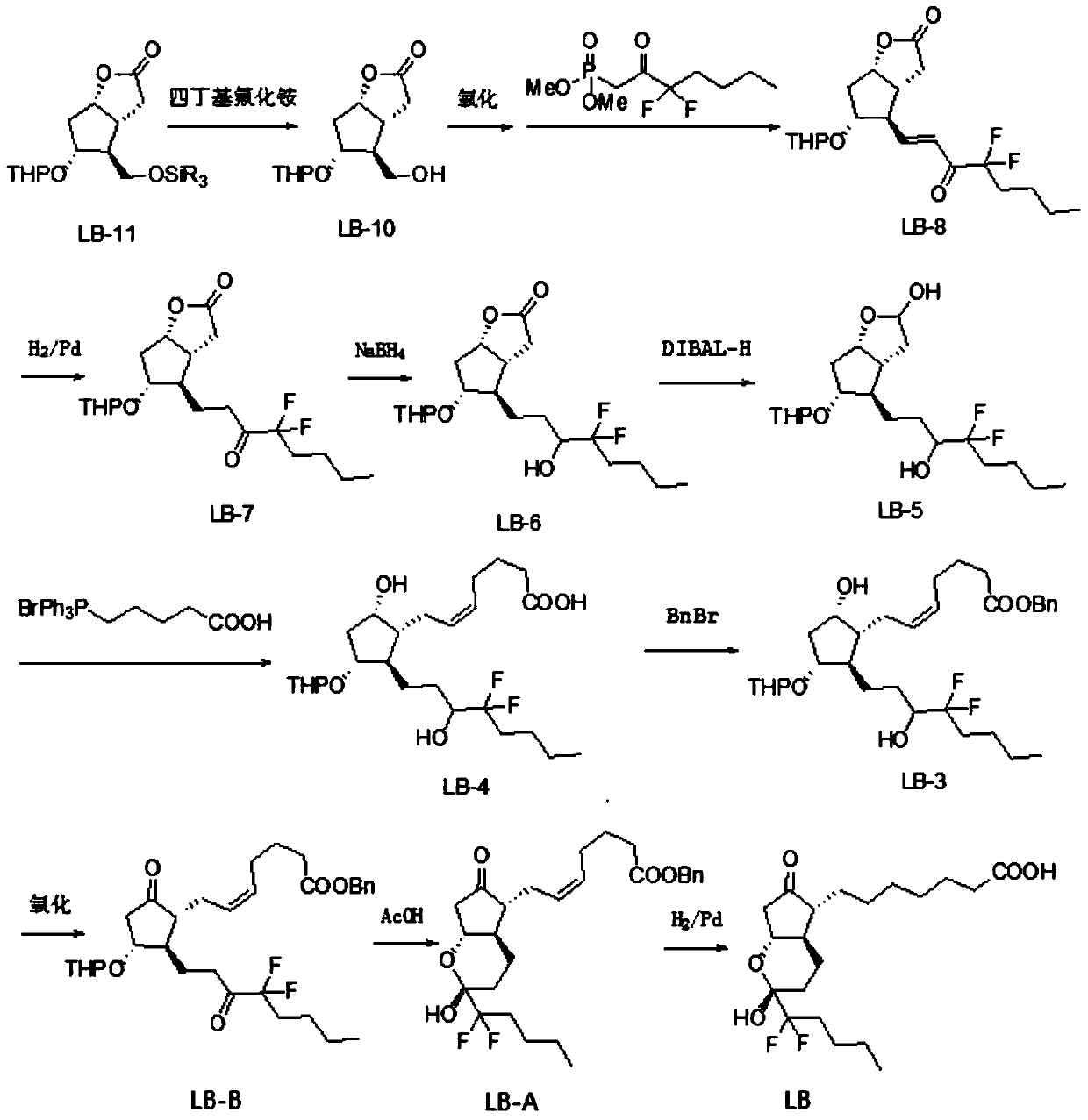
The invention discloses a novel method for preparing a lubiprostone midbody as shown in the formula 7. The method comprises the following steps: (1), a compound as shown in the formula 1 reacts with tert-butyldimethylsilyl chloride to selectively protect a primary hydroxyl group, thereby obtaining a compound shown in the formula 2; (2), a protecting group is applied to the compound 2 under the action of a catalyst, thereby obtaining a compound shown in the formula 3; (3), after the compound 3 is reduced through diisobutylaluminium hydride, a Wittig reaction is carried out on the compound 3, thereby obtaining carboxylic acid shown in the formula 4; (4), the compound 4 is protected in an acetonitrile solvent through a protecting group, thereby obtaining a compound shown in the formula 5; (5), the compound 5 is treated by using the tert-Butyldimethylsilane for removing the protecting group, thereby obtaining a compound shown in the formula 6; and (6), the compound 6 is oxidized by an oxidant and then reacts with a compound shown in the formula (10), thereby obtaining the higher-purity compound shown in the formula 7.
Owner:WUHAN QR PHARMA CO LTD
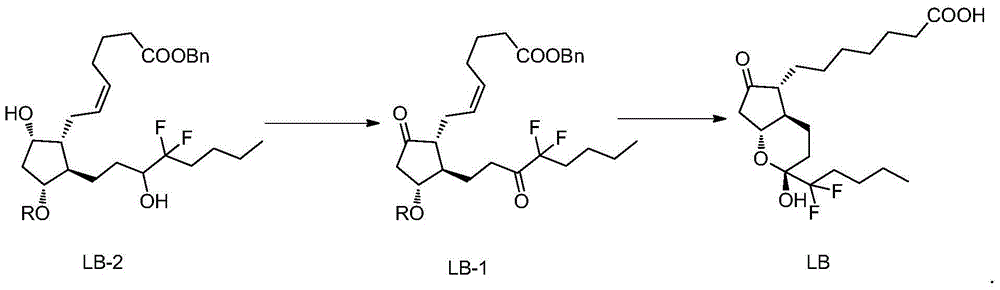
High-purity lubiprostone compound and preparation method thereof
ActiveCN105985309AHigh purityHigh reaction yieldOrganic chemistryBulk chemical productionHydrogenation reactionMedicinal chemistry
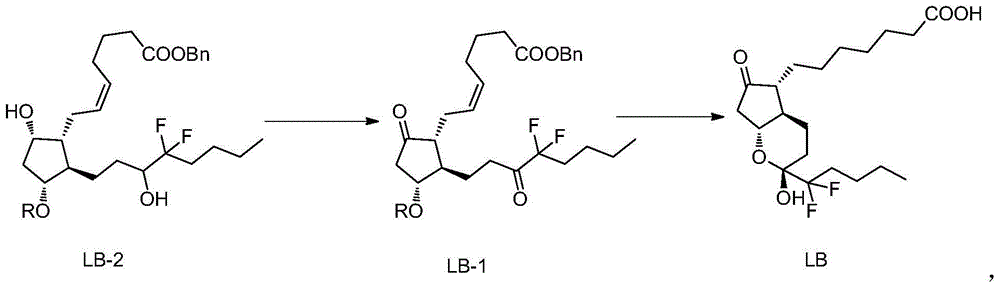
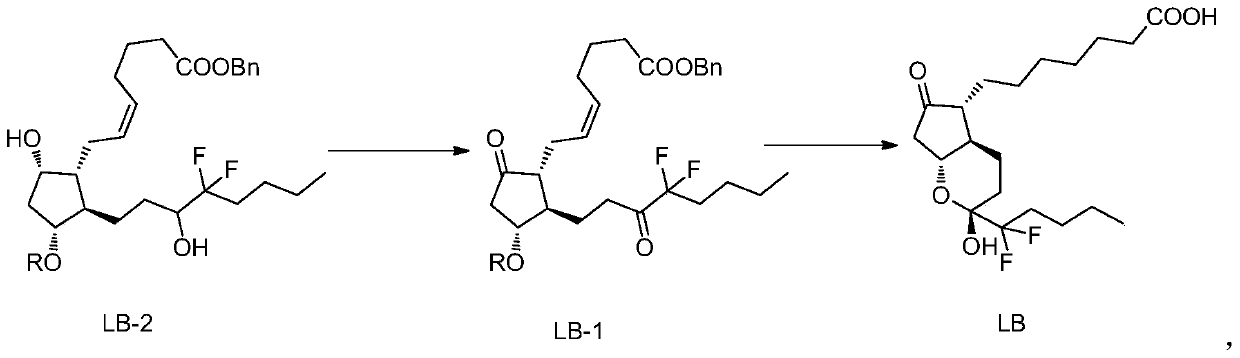
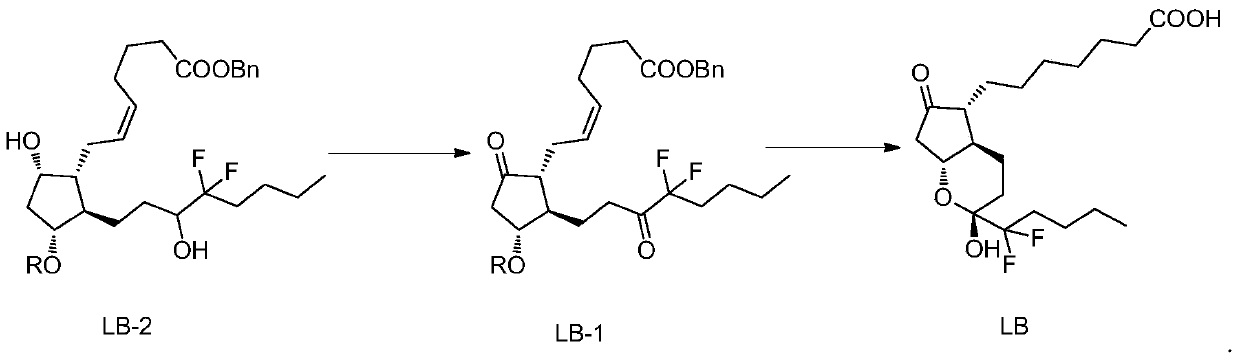
The invention belongs to the technical field of medicine, and provides a high-purity lubiprostone compound and a preparation method thereof. According to the method, a LB-2 is taken as a raw material, then is subjected to oxidation and hydrogenation reactions, and then is processed to obtain the high-purity lubiprostone compound, wherein the oxidation reaction employs a dess-martin oxidizing agent, and the hydrogenation reaction is one-step hydrogenation. According to the invention, the production process is simplified, overall yield of the reaction is high, and the high-purity lubiprostone compound is prepared.
Owner:NANJING CHIA TAI TIANQING PHARMA +1
Lubiprostone solid dispersion and preparation method thereof
InactiveCN108186597AIncrease dissolution rateSolve residual problemsElcosanoid active ingredientsDigestive systemSolubilityFreeze-drying
The present invention provides a lubiprostone solid dispersion and a preparation method thereof, wherein the lubiprostone solid dispersion comprises lubiprostone and a carrier, the carrier is a hydrophilic polymer carrier, a weight ratio of the lubiprostone to the carrier is 8:200000-400000, optionally the solid dispersion further comprises a surfactant, and a weight ratio of the lubiprostone to the carrier to the surfactant is 8:200000-400000:20000-40000. The preparation method comprises: dissolving lubiprostone, a carrier and / or a surfactant in an aqueous solvent, stirring to form a clarified solution, and carrying out freeze-drying or spray-drying to completely remove the aqueous solvent so as to prepare the solid dispersion. According to the present invention, the lubiprostone solid dispersion has advantages of improved water solubility, improved dissolution rate, promoted absorption by gastrointestinal tract and improved bioavailability.
Owner:PEKING UNIV FOUNDER GRP CO LTD +3
Prostaglandin synthesis and intermediates for use therein
Fused cyclopentane-4-substituted 3,5-dioxalane lactone compounds useful as an intermediate in the synthesis of prostaglandin analogs are provided. The compounds have the formula A:wherein R represents an aryl group such as p-methoxyphenyl.This compound can be reacted with a lower alkyl aluminum compound to open the dioxalane ring and reduce the lactone to lactol, without over-reducing to diol. The resulting compound can be functionalized to insert chemical side groups of target prostaglandins, adding the required α-side chain and then the required ω-side chain sequentially and independently of each other. The compounds and process are particularly suitable for preparing lubiprostone.
Owner:ALPHORA RES
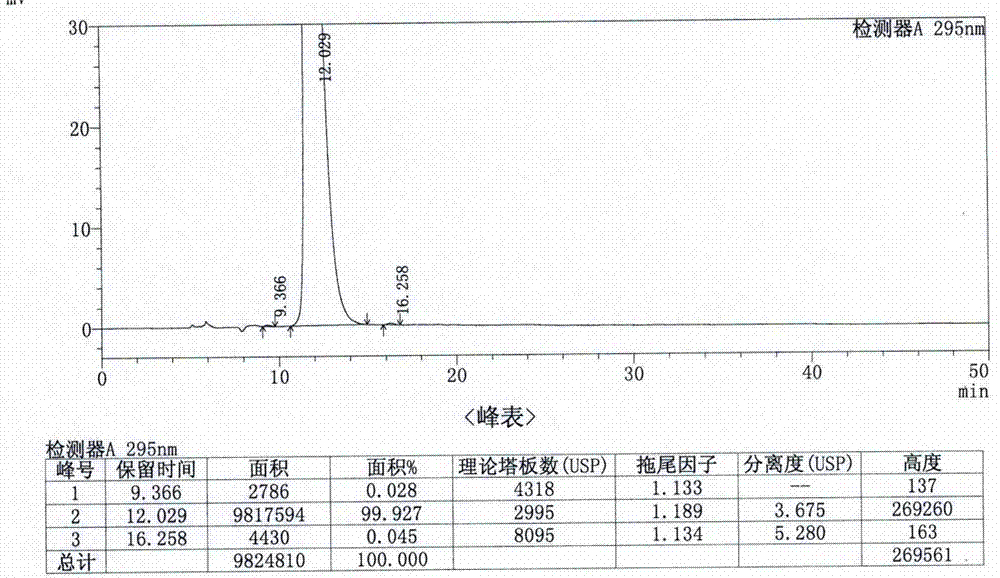
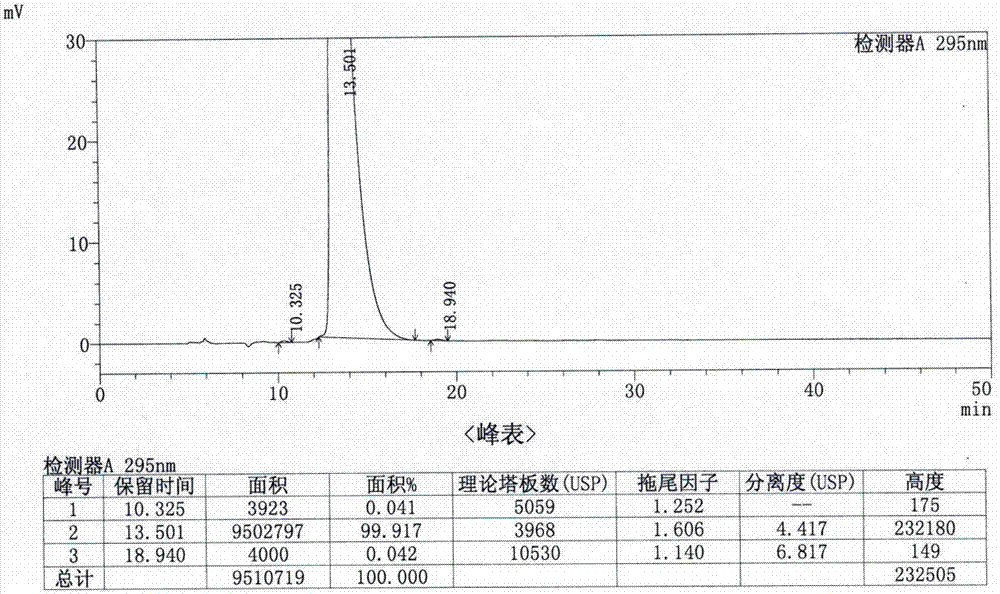
Analysis method for determining lubiprostone test sample related substances
Owner:NANJING CHIA TAI TIANQING PHARMA
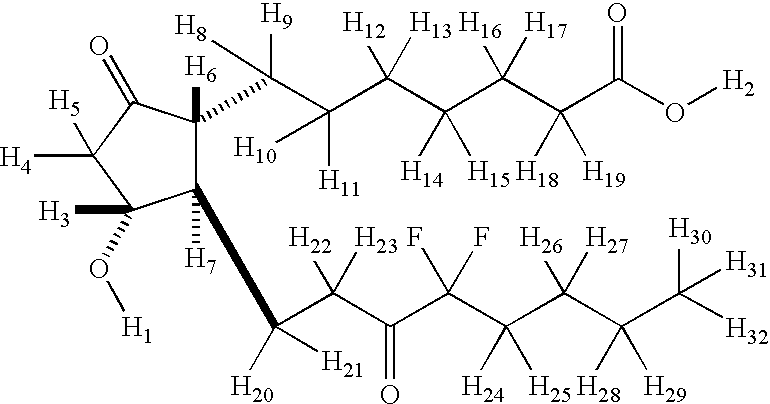
Deuterium-enriched lubiprostone
The present application describes deuterium-enriched lubiprostone, pharmaceutically acceptable salt forms thereof, and methods of treating using the same.
Owner:PROTIA LLC
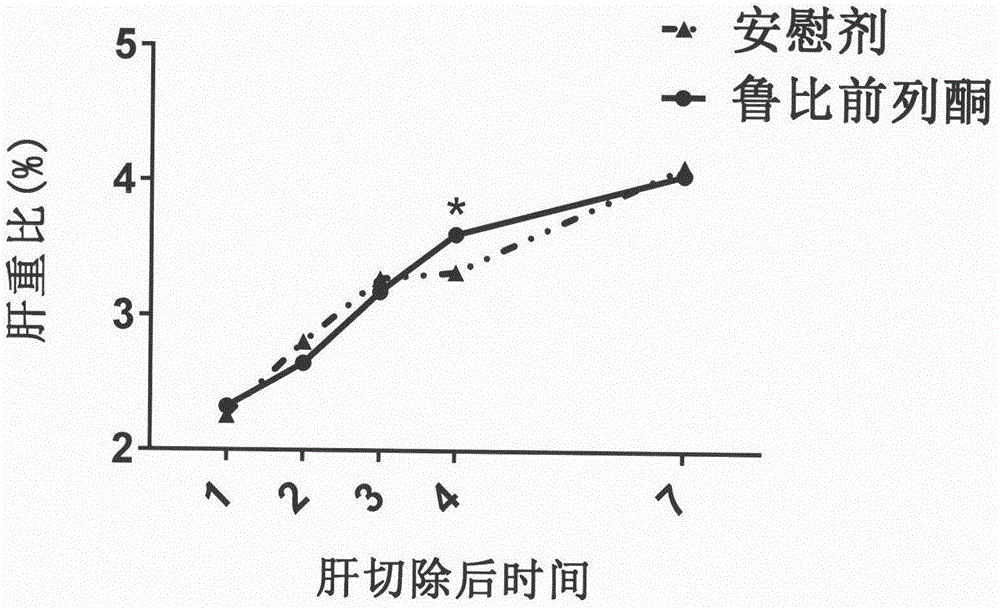
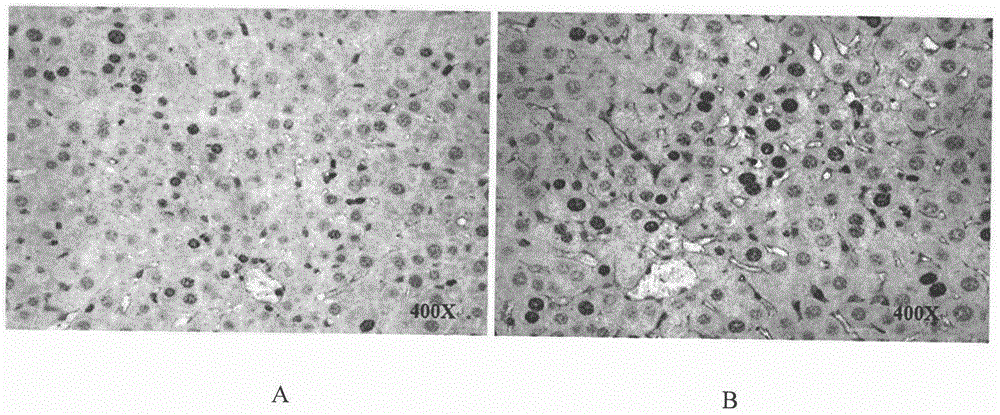
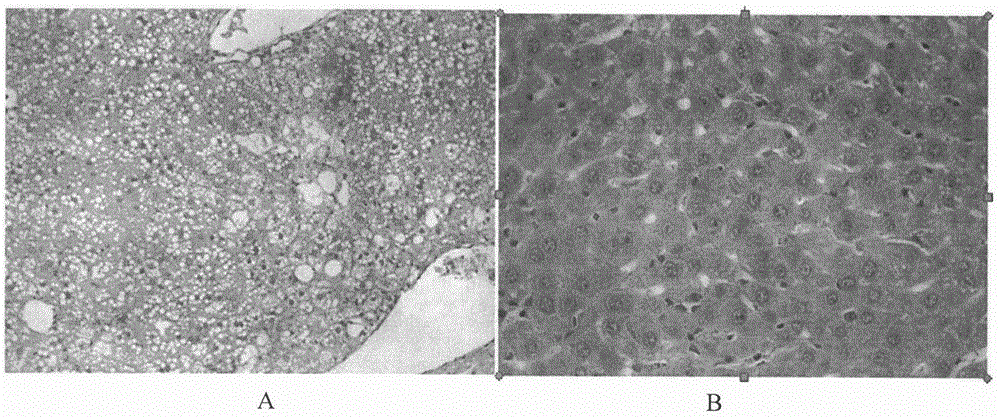
Therapeutical agent for liver regeneration
The invention provides a pharmaceutical composition which utilizes lubiprostone as a main active ingredient and is capable of inducing hepatocyte proliferation and promoting liver regeneration after liver injury. The pharmaceutical composition can be a preparation based on oral administration, preferably, the pharmaceutical composition can be an oral solid preparation based on a tablet form or a capsule form. Furthermore, the pharmaceutical composition can be prepared into a lubiprostone unit preparation suitable for oral administration.
Owner:CHINA PHARM UNIV
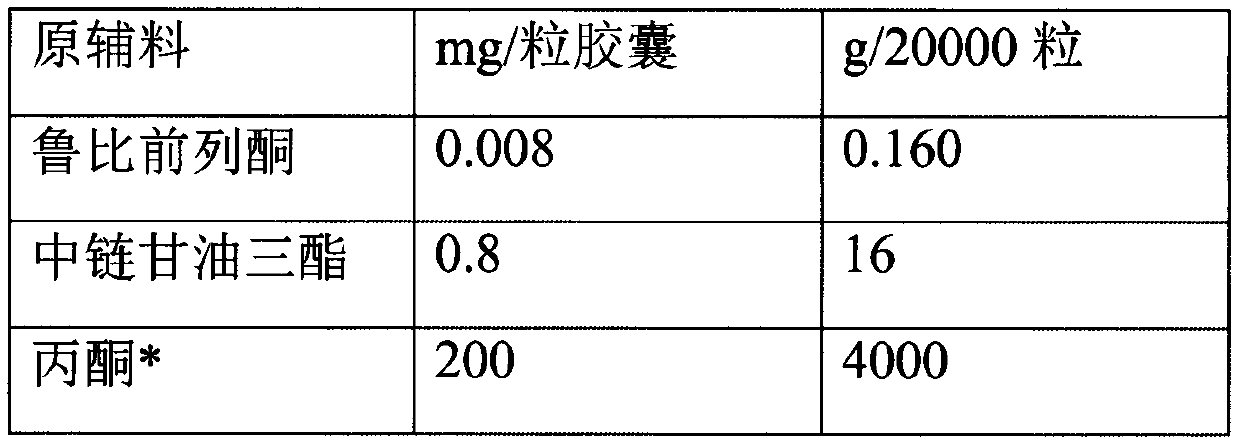
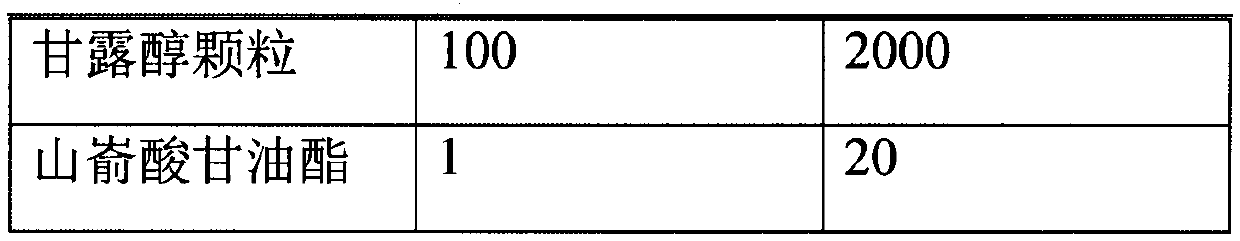
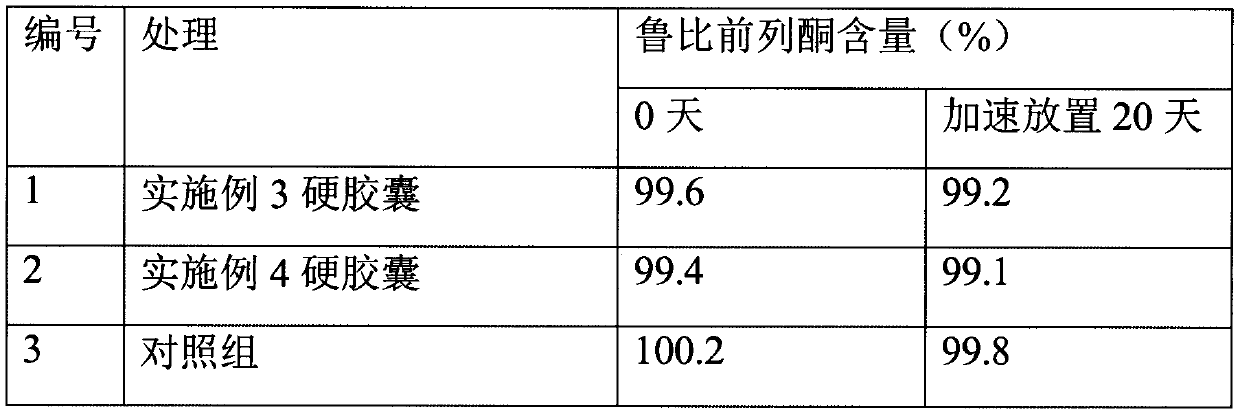
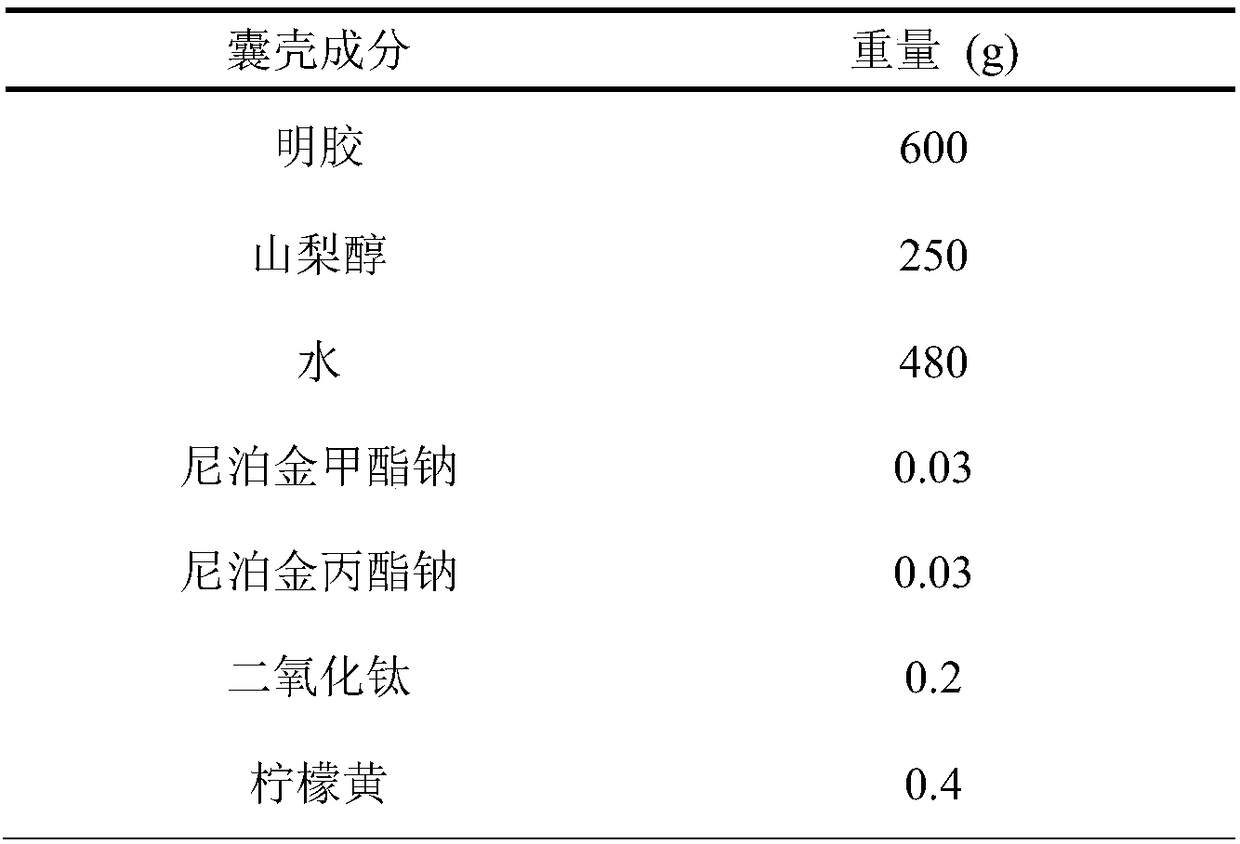
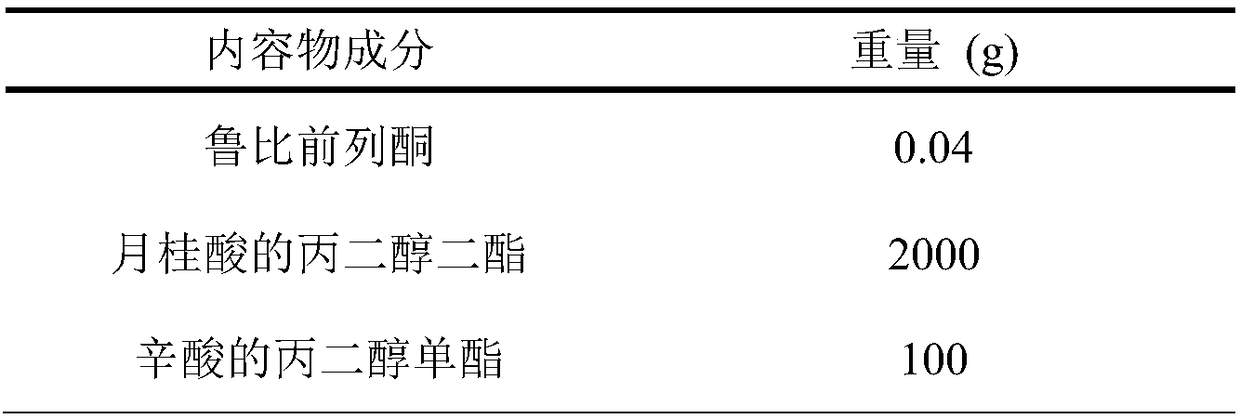
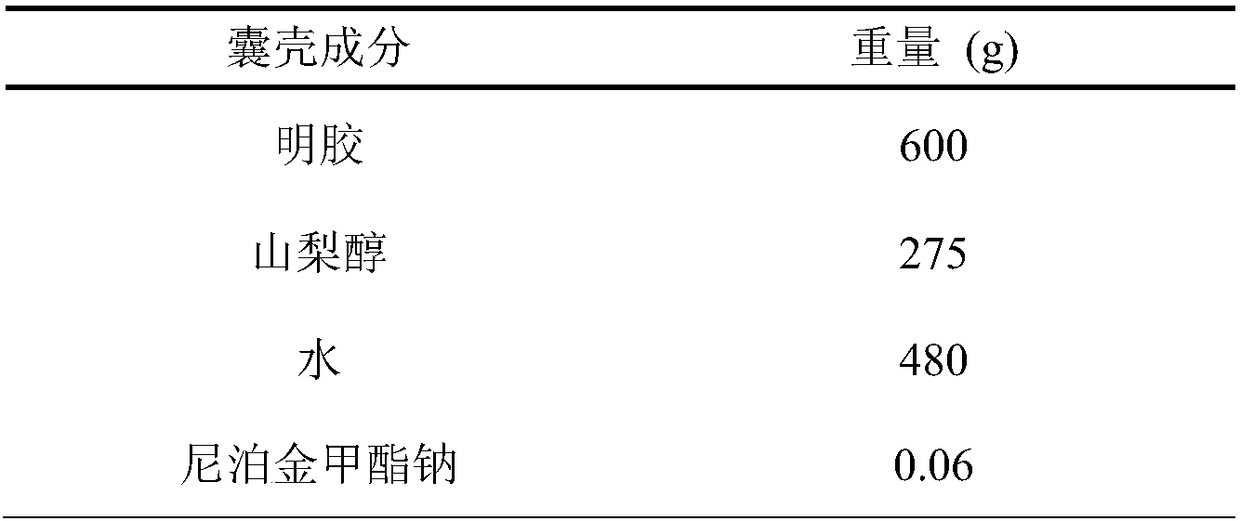
A hard capsule preparation
ActiveCN107638403BUniform contentImprove stabilityElcosanoid active ingredientsDigestive systemDrug contentHard Capsule
The invention relates to a lubiprostone hard capsule preparation and a preparation method thereof, wherein the lubiprostone hard capsule preparation comprises a gelatin capsule shell, lubiprostone-containing particles, and a lubricant. According to the present invention, the lubiprostone hard capsule preparation has advantages of uniform drug content, excellent stability, rapid dissolution in vitro and simple operation, and is particularly suitable for the industrial production of solid preparation workshops.
Owner:JIANGSU VCARE PHARMATECH
A kind of preparation method of high-purity lubiprostone compound
ActiveCN105985309BHigh purityHigh reaction yieldOrganic chemistryBulk chemical productionBiochemical engineeringHydrogenation reaction
The invention belongs to the technical field of medicine, and provides a high-purity lubiprostone compound and a preparation method thereof. According to the method, a LB-2 is taken as a raw material, then is subjected to oxidation and hydrogenation reactions, and then is processed to obtain the high-purity lubiprostone compound, wherein the oxidation reaction employs a dess-martin oxidizing agent, and the hydrogenation reaction is one-step hydrogenation. According to the invention, the production process is simplified, overall yield of the reaction is high, and the high-purity lubiprostone compound is prepared.
Owner:NANJING CHIA TAI TIANQING PHARMA +1
Prostaglandin synthesis and intermediates for use therein
Fused cyclopentane—4-substituted 3,5-dioxalane lactone compounds useful as an intermediate in the synthesis of prostaglandin analogs are provided. The compounds have the formula A:wherein R represents an aryl group such as p-methoxyphenyl.This compound can be reacted with a lower alkyl aluminum compound to open the dioxalane ring and reduce the lactone to lactol, without over-reducing to diol. The resulting compound can be functionalized to insert chemical side groups of target prostaglandins, adding the required α-side chain and then the required ω-side chain sequentially and independently of each other. The compounds and process are particularly suitable for preparing lubiprostone.
Owner:ALPHORA RES
A kind of intermediate for preparing lubiprostone, its preparation method and the method for preparing lubiprostone by it
ActiveCN103787942BOrganic chemistryBulk chemical productionReduction treatmentCombinatorial chemistry
Owner:连云港恒运药业有限公司
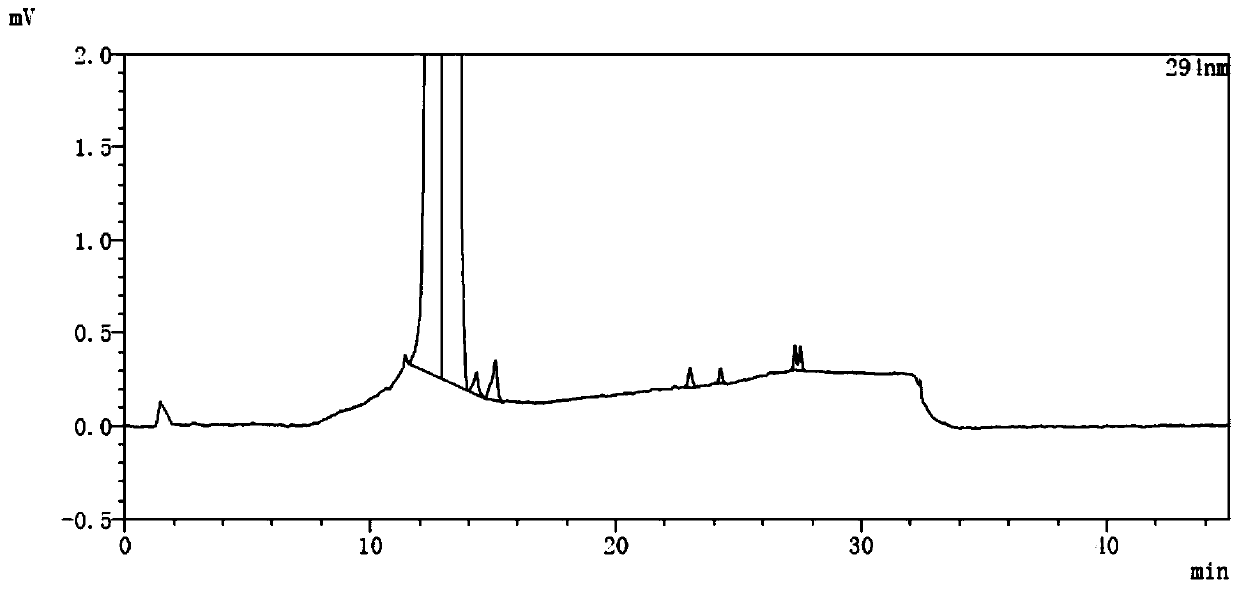
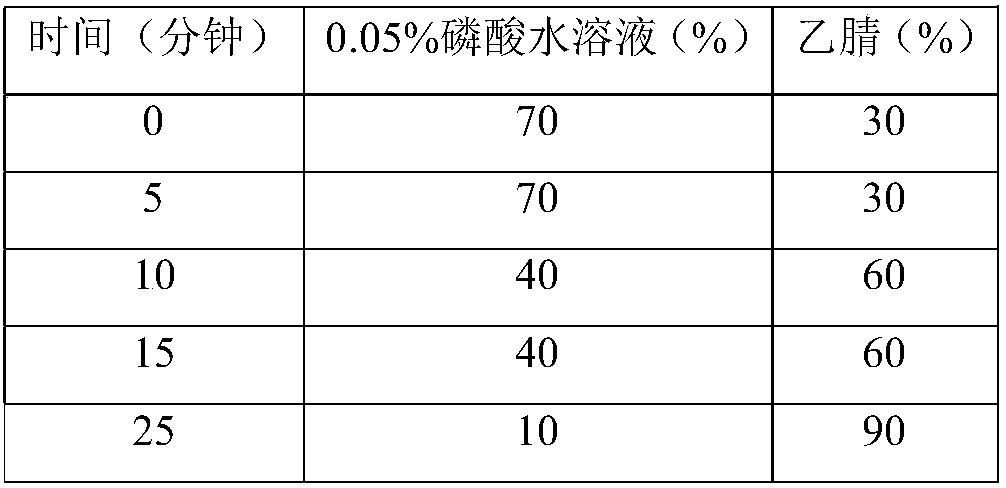
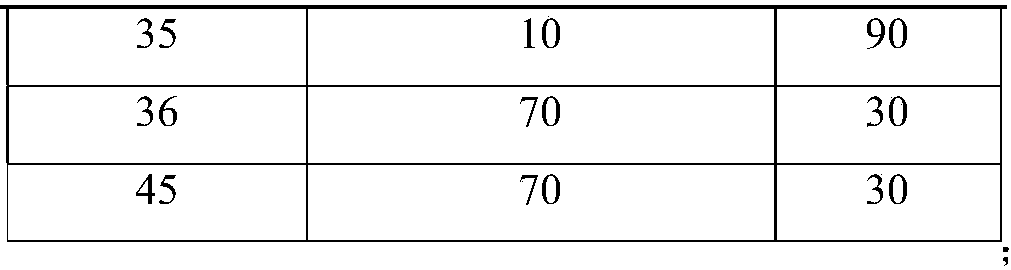
A kind of analytical method for determination of lubiprostone test substance related substances
The present invention provides an analytical method for the determination of lubiprostone test substance related substances. The method adopts reversed-phase high performance liquid chromatography to separate and determine the lubiprostone compound, the compound of formula I, the compound of formula II and the compound of formula III. Compounds, Compounds of Formula IV and Compounds of Formula V. The solvent of this method does not interfere with the detection of impurities, the specificity of the method is good, and the detection method is simple, high in sensitivity, good in repeatability and good in accuracy. Controllability of ketone quality.
Owner:NANJING CHIA TAI TIANQING PHARMA
Lubiprostone crystal, the use and the method for the preparation thereof
ActiveUS8748482B2Easy to optimizeRelieve symptomsBiocideOrganic compound preparationDiseaseGastrointestinal disorder
The present invention relates to a lubiprostone crystal, the method for the preparation thereof, and a pharmaceutical composition or kit comprising the same, as well as the use of said crystal in the preparation of a medicament for the treatment of gastrointestinal tract diseases, especially constipation. The X-ray powder diffraction pattern of said crystal comprises characteristic peaks measured at the following 2θ reflection angles: 14.6±0.2°, 17.0±0.2° and 19.6±0.2°. As compared to amorphous lubiprostone, the crystal of the present invention has the advantages of relative high purity, stable properties and easy-for-storage and use.
Owner:SHANGHAI TECHWELL BIOPHARMACEUTICALS CO LTD
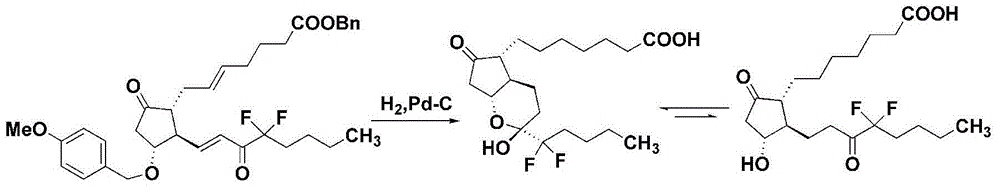
Process for the preparation of lubiprostone and intermediates thereof
ActiveCN110713477AGroup 4/14 element organic compoundsOrganic compound preparationProcess engineeringOrganic chemistry
The present invention relates to a novel process for preparing Lubiprostone and novel intermediates prepared from the process. The process of the present invention does not generate hydrogenated by-products that are difficult to be removed, and thus enables the production of Lubiprostone in an efficient and economical way.
Owner:CHIROGATE INT
Preparation method of lubiprostone or its intermediate
ActiveCN103058907BNot suitable for storageHigh yieldOrganic chemistryBulk chemical productionTert-butyldimethylsilaneWittig reaction
Owner:WUHAN QR PHARMA CO LTD
Preparation process of lubiprostone intermediate
InactiveCN109369588AHigh yieldSuitable for large-scale productionOrganic chemistryBenzoyl bromideOrganic layer
The invention discloses a preparation process of a lubiprostone intermediate. The preparation process comprises the following steps: firstly, carrying out reduction reaction on a lubi-initiator to obtain an intermediate 1; secondly, adding excessive weak base into a mixed solution of triphenylphosphonic acid bromide and absolute ether, then adding the intermediate 1 for complete reaction, then adjusting a pH value to 5 to 6, adding ethyl acetate for extracting, combining organic layers to obtain an organic phase, and carrying out vacuum concentration and drying on the organic phase to obtain an intermediate 2; thirdly, adding acetonitrile, diisopropylethylamine and benzyl bromide into the intermediate 2 for complete reaction, then carrying out vacuum concentration and drying, and adding the ethyl acetate and water for extracting and separating liquid; carrying out vacuum concentration and drying on the organic phase to obtain a crude product of an intermediate 3. The preparation process disclosed by the invention has the advantages of rapid and convenient synthesis route, high yield and relatively high economic value; the preparation process is quite suitable for scale production and provides another synthesis route with high economic benefits and high production efficiency for producing lubiprostone.
Owner:EMEISHAN HONGSHENG PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com