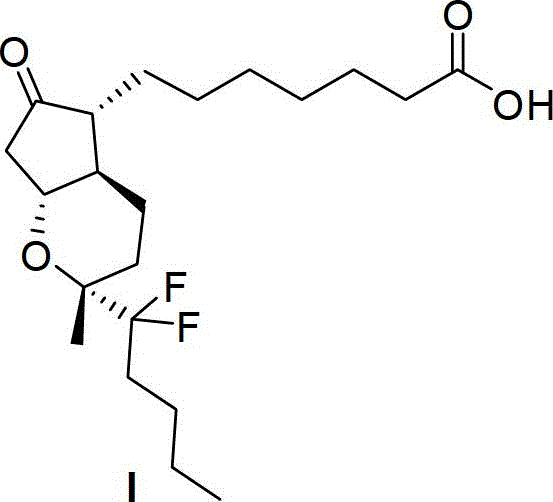
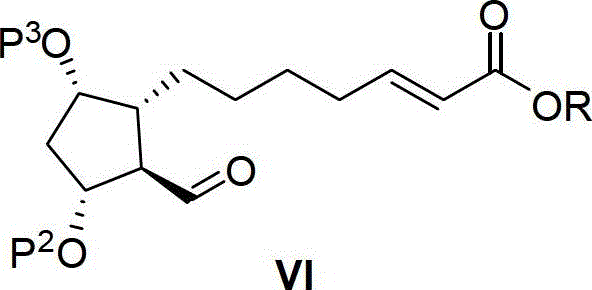
A kind of intermediate for preparing lubiprostone, its preparation method and the method for preparing lubiprostone by it
A technology for lubiprostone and compound, which is applied in the field of preparing lubiprostone, can solve problems such as complicated synthesis process operation, and achieves the effects of safe operation, high synthesis efficiency, significant social and economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Example 1: Preparation of Compound VIa (prepared according to the same method as in Examples 1 and 2 of Chinese Patent Application 201210338692.1)
[0063] step 1):
[0064] N 2 Under protection, Corey lactone XVII (1.72g, purchased from Taizhou Aoxiang Pharmaceutical Technology Co., Ltd.) was suspended in dichloromethane (60mL), added 4 dimethylaminopyridine (122mg), triethylamine (13.4mL), After cooling to -20°C, a solution of tert-butyldimethylchlorosilane (1.48g) in dichloromethane (20mL) was slowly added dropwise, and then raised to 20°C for 24 hours. After the reaction, add methyl tert-butyl ether (50mL) and saturated ammonium chloride (50mL), separate the layers, wash the organic phase with saturated brine, anhydrous Na 2 SO 4 Dry, filter and concentrate to give a white solid. The white solid (2.86g) was dissolved in dichloromethane (30mL), PPTS (500mg) and DHP (4.6mL) were added and reacted at 20°C for 3 hours. After the reaction, add methyl tert-butyl ethe...
Embodiment 2
[0089] Embodiment 2: the preparation of compound Va
[0090] Compound VIIa (1.7g, synthesized according to patent US4187381) was dissolved in 20mL of methyl tert-butyl ether, under nitrogen protection, lithium hydroxide monohydrate (253mg) was added, and reacted at 20°C for 1 hour. A solution of compound VIa (1.7 g) in methyl tert-butyl ether (10 mL) was added, and then 0.9 mL of water was added, and the temperature of the reaction system was raised to 45° C. for 36 hours. Add 20 mL of water to the reaction system, stir, extract three times with ethyl acetate, combine the organic phases, dry over anhydrous sodium sulfate, filter, and concentrate to obtain 2.04 g of compound Va, which is directly put into the next reaction. Yield: 86%.
[0091] Va: 1 H-NMR (400MHz, CDCl 3 )δ=7.13-7.03(m,1H),6.98-6.90(m,1H),6.71-6.62(m,1H),5.81(d,J=16Hz,1H),5.16(t,J=4.4Hz, 1H),4.59-4.52(m,1H),4.15-4.02(m,1H),3.85-3.70(m,4H),3.47-3.41(m,1H),2.83-2.68(m,1H),2.63- 2.56(m,1H),2.17-1.27(m,26H),0...
Embodiment 3
[0092] Embodiment 3: the preparation of compound Iva
[0093] Compound Va (1.4g) was dissolved in 20mL ethyl acetate, and 500mg 10%Pd / C (50%H 2 O), 20 ℃ normal pressure reaction for 2 hours. Palladium-carbon was filtered out through celite, the filter cake was washed with ethyl acetate, and the filtrate was concentrated under reduced pressure to obtain 1.4 g of compound IVa, which was directly put into the next step reaction. Yield: 100%.
[0094] IVa: 1 H-NMR (400MHz, CDCl 3 )δ=5.09(s,1H),4.58-4.52(m,1H),3.97-3.82(m,1H),3.68(s,3H),3.50-3.46(m,1H),2.82(m,1H) ,2.33-2.29(m,3H),2.1-1.2(m,32H),0.94(m,3H)ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com