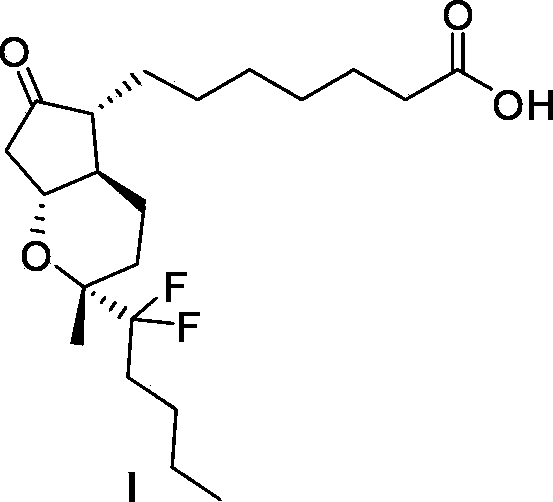
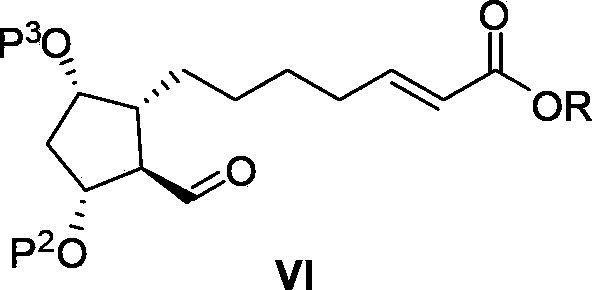
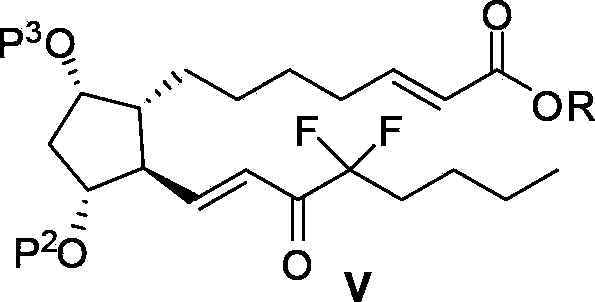
Intermediate for preparing lubiprostone, preparation method of intermediate and method for preparing lubiprostone through intermediate
A technology for lubiprostone and compounds, which is applied in the field of preparing lubiprostone, can solve problems such as cumbersome synthesis process operations, and achieve the effects of safe operation, high synthesis efficiency, and significant social and economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Example 1: Preparation of compound VIa (prepared according to the same method as Examples 1 and 2 of Chinese Patent Application 201210338692.1)
[0063] step 1):
[0064] N 2 Under protection, Corey lactone XVII (1.72g, purchased from Taizhou Aoxiang Pharmaceutical Technology Co., Ltd.) was suspended in dichloromethane (60mL), and 4 dimethylaminopyridine (122mg), triethylamine (13.4mL) were added, After cooling to -20°C, a solution of tert-butyldimethylchlorosilane (1.48g) in dichloromethane (20mL) was slowly added dropwise, and then raised to 20°C to react for 24 hours. After the reaction, add methyl tert-butyl ether (50mL) and saturated ammonium chloride (50mL), separate the layers, wash the organic phase with saturated brine, and anhydrous Na 2 SO 4 Dry, filter and concentrate to obtain a white solid. The white solid (2.86 g) was dissolved in dichloromethane (30 mL), PPTS (500 mg), DHP (4.6 mL) were added, and the reaction was carried out at 20°C for 3 hours. After the...
Embodiment 2
[0089] Example 2: Preparation of Compound Va
[0090] Compound Vila (1.7g, synthesized according to patent US4187381) was dissolved in 20mL of methyl tert-butyl ether, protected by nitrogen, added with lithium hydroxide monohydrate (253mg), and reacted at 20°C for 1 hour. A solution of compound Via (1.7 g) in methyl tert-butyl ether (10 mL) was added, and 0.9 mL of water was added. The reaction system was heated to 45° C. for 36 hours. 20 mL of water was added to the reaction system, stirred, and extracted with ethyl acetate three times, the organic phases were combined, dried over anhydrous sodium sulfate, filtered, and concentrated to obtain 2.04 g of compound Va, which was directly put into the next step reaction. Yield: 86%.
[0091] Va: 1 H-NMR (400MHz, CDCl 3 )δ=7.13-7.03(m,1H), 6.98-6.90(m,1H), 6.71-6.62(m,1H), 5.81(d,J=16Hz,1H), 5.16(t,J=4.4Hz, 1H), 4.59-4.52(m,1H), 4.15-4.02(m,1H), 3.85-3.70(m,4H), 3.47-3.41(m,1H), 2.83-2.68(m,1H), 2.63 2.56(m,1H),2.17-1.27(m,26H),0.94(...
Embodiment 3
[0092] Example 3: Preparation of Compound Iva
[0093] Compound Va (1.4g) was dissolved in 20mL ethyl acetate, 500mg 10%Pd / C(50%H 2 O), react at normal pressure at 20°C for 2 hours. The palladium carbon was filtered through Celite, the filter cake was washed with ethyl acetate, and the filtrate was concentrated under reduced pressure to obtain 1.4 g of compound IVa, which was directly put into the next step reaction. Yield: 100%.
[0094] IVa: 1 H-NMR (400MHz, CDCl 3 )δ=5.09(s,1H), 4.58-4.52(m,1H), 3.97-3.82(m,1H), 3.68(s,3H), 3.50-3.46(m,1H), 2.82(m,1H) ,2.33-2.29(m,3H),2.1-1.2(m,32H),0.94(m,3H)ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com