Prostaglandin synthesis and intermediates for use therein
a technology of prostaglandin and analog, which is applied in the field of prostaglandin analog and its synthesis, can solve the problems of difficult synthesizing of prostaglandin analog, limited range of industrially acceptable reagents, solvents, catalysts, etc., which can be used in their synthesis, and complications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0031]
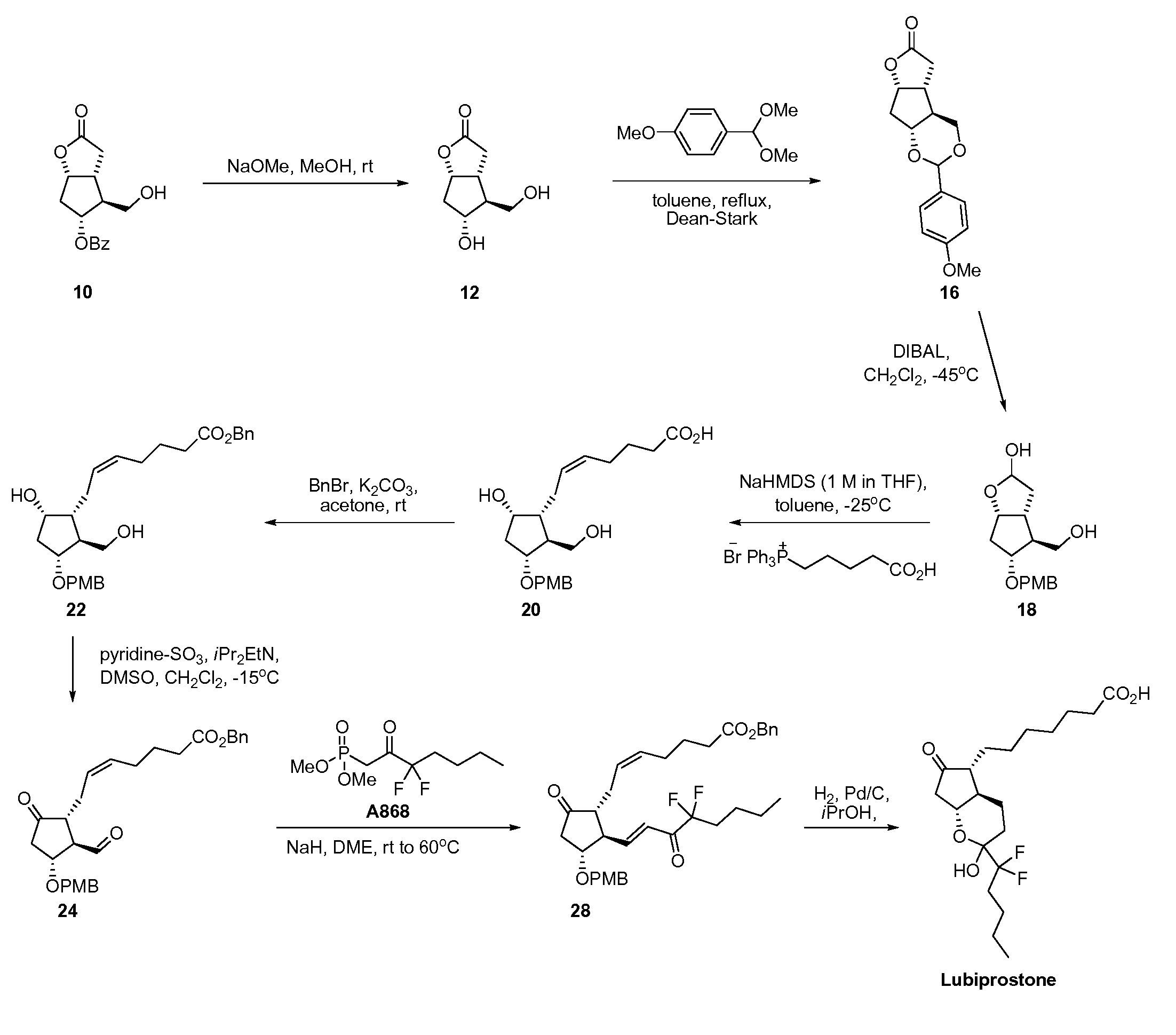
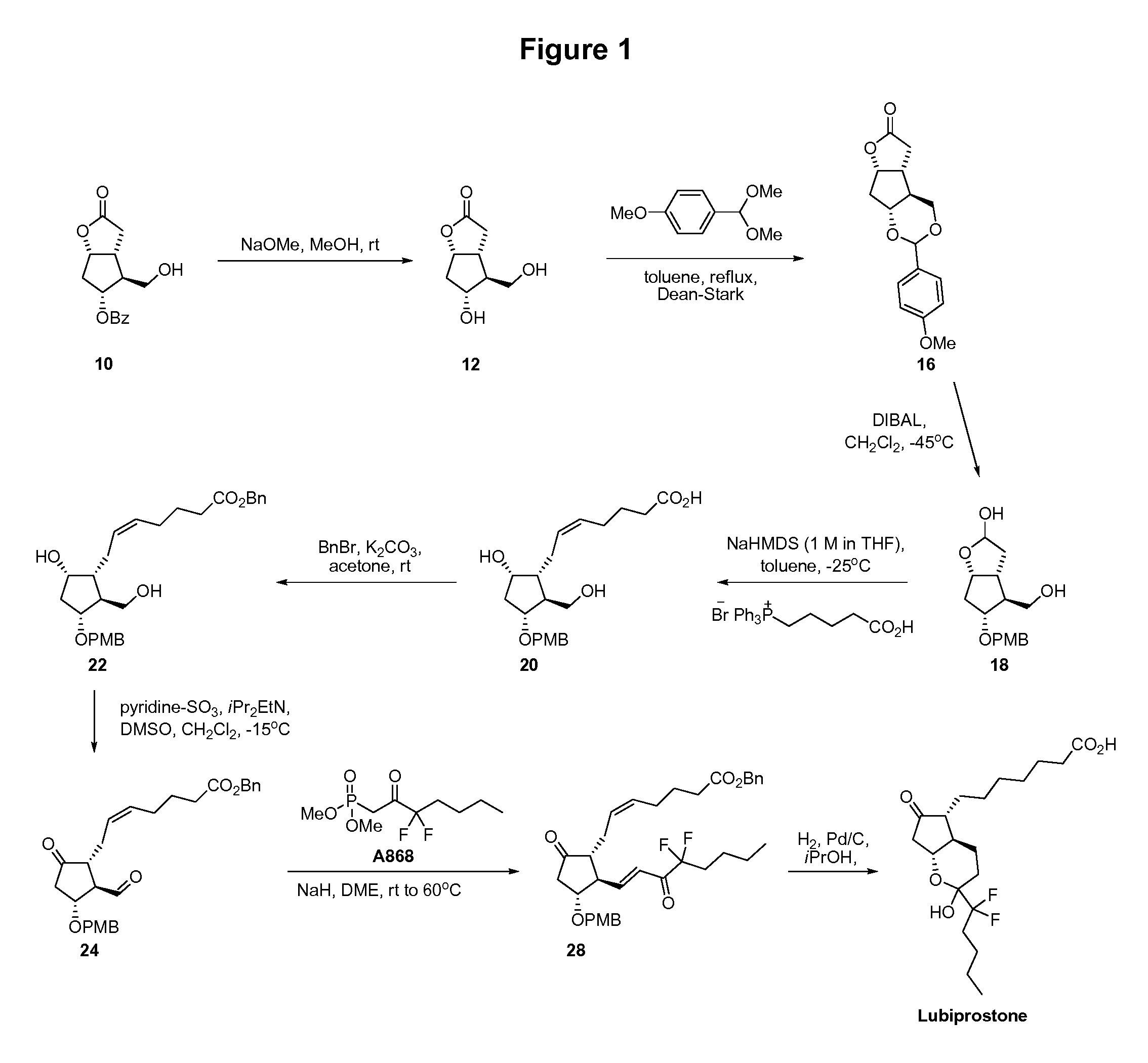
[0032]Corey Lactone Diol 12. To a suspension of 10 (15 g, 54 mmol, 1 equiv) in methanol (75 mL) was added sodium methoxide (25% wt in methanol, 1.2 mL, 5.4 mmol, 0.1 equiv). The mixture was stirred at room temperature for 1.5 h and then hydrochloric acid solution (4 M in dioxane, approximately 1 mL) was added until the pH was 3-4. The solution was stirred at room temperature for 10 min and then concentrated to dryness under vacuum on a rotary evaporator. The resulting white solid was suspended in methyl tert-butyl ether (150 mL) and stirred at room temperature for 1 h. The solid was filtered, washed with methyl tert-butyl ether, and dried under vacuum for 10 min to afford 9.1 g of 12 (97%) as a white solid.
[0033]Protected Diol 16. To a suspension of 12 (5.0 g, 29 mmol, 1 equiv) in toluene (100 mL) was added anisaldehyde dimethyl acetal (14) (7.4 mL, 44 mmol, 1.5 equiv) and p-methoxy benzoic acid (44 mg, 0.29 mmol, 0.01 equiv). A condenser and a Dean-Stark apparatus were attach...
example 2
[0044]Protected lubiprostol compound 28, prepared as described in Example 1, was deprotected by hydrogenation in mixed ethanol / 2-propanol, and crystallized from isopropyl acetate.
[0045]To a thick walled clear Pyrex Reaction Bottle was added under a flow of nitrogen palladium on carbon (10% wt on carbon, 50% wt in water, 1.07 g, 0.1 equiv, 0.503 mmol). Ethanol / 2-propanol (1:4 v / v, 18 mL, 6 parts) was added under a flow of nitrogen. A mixture of compound 28 (3 g, 5.03 mmol, 1 equiv) in ethanol / 2-propanol (1:4 v / v, 51 mL, 17 parts) was added under a flow of nitrogen. The flask was rinsed with ethanol / 2-propanol (1:4 v / v, 6 mL, 2 parts). The mixture was shaken in a Parr shaker at 40 psi at room temperature for 24 h. The mixture was purged with nitrogen, filtered through Celite (15 g, 5 parts), and washed with ethanol / 2-propanol (1:4 v / v, 75 mL, 25 parts). The solution was concentrated to dryness under vacuum on a rotary evaporator at 45° C. The resulting yellow oil was dissolved in dich...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperatures | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com