Novel crystal form of lubiprostone and preparation method of crystal form
A lubiprostone and crystal form technology, which is applied to medical preparations containing active ingredients, pharmaceutical formulas, digestive systems, etc., can solve the problems of inconvenient production and storage of preparations, and achieve easy operation, simple preparation methods, and high purity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example
[0037] Preparation example: Preparation of lubiprostone oily substance
[0038] (Z)-7-[(1R,2R,3R)-2-(4,4-difluoro-3-oxooctyl)-3-hydroxy-5-oxocyclopentyl]heptanoic acid-5 -Enbenylate (2.5g) is added to 20mL of ethanol, palladium hydroxide charcoal is added, 3g of triethylsilane is added to 20mL of ethanol, diluted and added to the above solution, and reacted at 20-25℃ for 30 minutes After filtration, the filtrate is concentrated to obtain 2.0 g of oily substance, which is lubiprostone oily substance. The reaction formula is as follows:
[0039]
Embodiment 1
[0040] Example 1 Preparation of new crystal form of lubiprostone
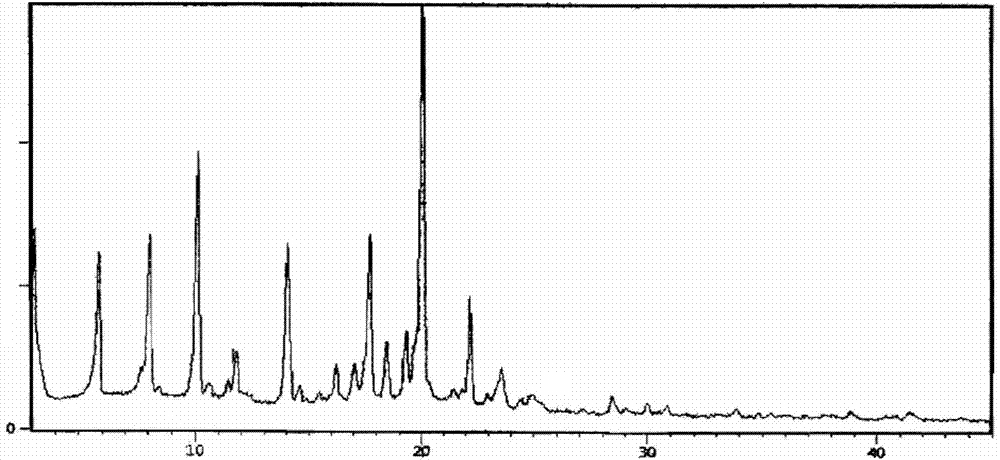
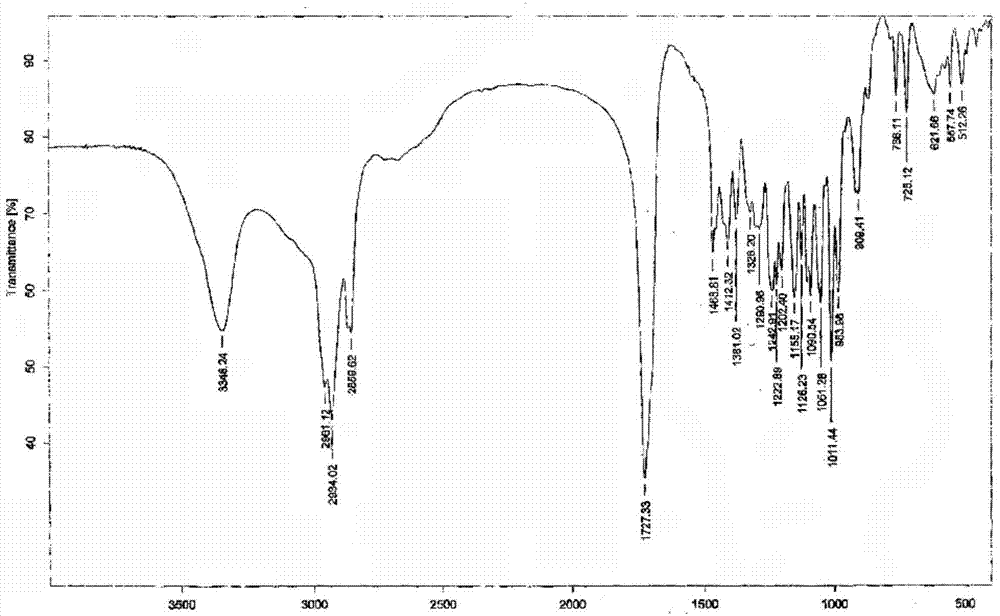
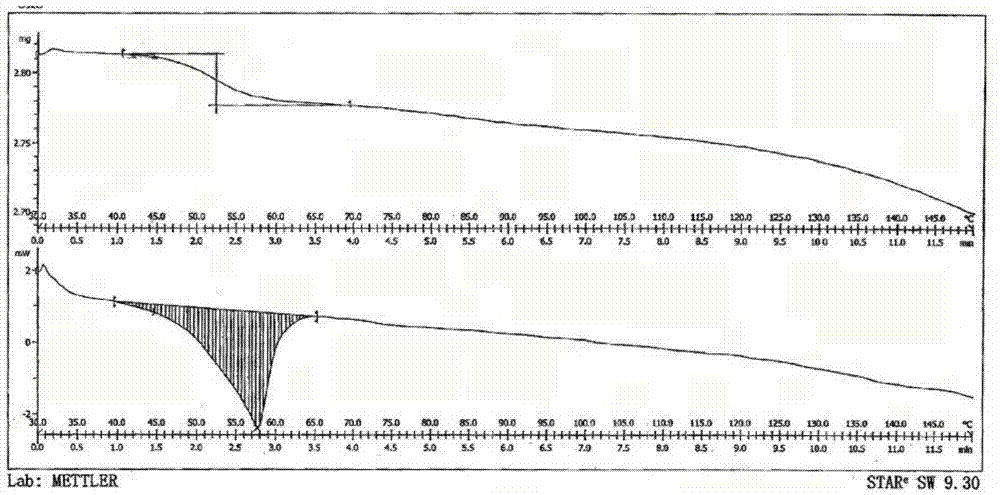
[0041] 1g of lubiprostone oil prepared by the method of Preparation Example 1 was dissolved in 5mL of isopropyl acetate, stirred to dissolve, cooled to 0-10°C, 75mL of n-heptane was added dropwise, stirred for 2h, and a white solid precipitated slowly. Filtration with suction, drying under reduced pressure, to obtain 0.9g of white solid, which is a new crystal form of lubiprostone, with a purity of 99.96% and a yield of 90.0%. The X-RPD spectrum is as follows figure 1 As shown, its infrared absorption spectrum is as figure 2 As shown, its DSC spectrum is as image 3 Shown.
Embodiment 2
[0042] Example 2 Preparation of new crystal form of lubiprostone
[0043] 0.5g of lubiprostone oil obtained according to the method of Preparation Example 1 was dissolved in 2mL of propyl acetate, stirred to dissolve, cooled to 0~10℃, 25mL of n-hexane was added dropwise, stirred for 2h, a white solid precipitated slowly, and filtered with suction And dried under reduced pressure to obtain 0.44 g of white solid, which is a new crystal form of lubiprostone, with a purity of 99.88% and a yield of 88.0%. After determination, its X-RPD spectrum is figure 1 The infrared spectrum is basically the same as figure 2 Basically the same, its DSC spectrum is image 3 Basically the same.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com