Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
50 results about "Ledipasvir" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
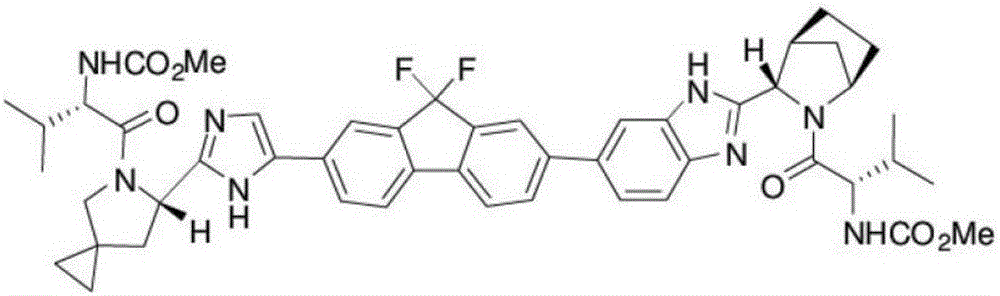
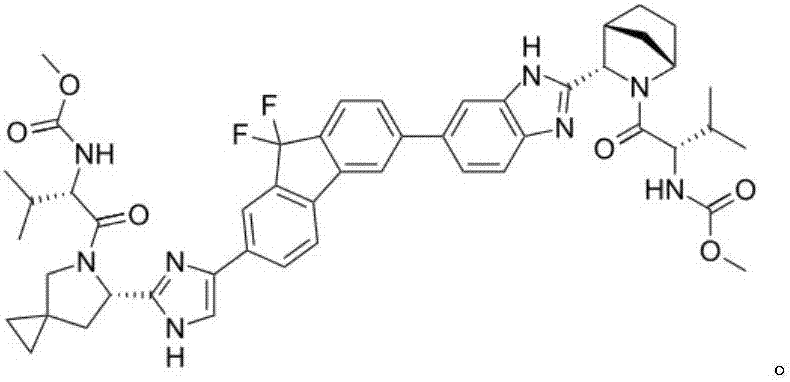
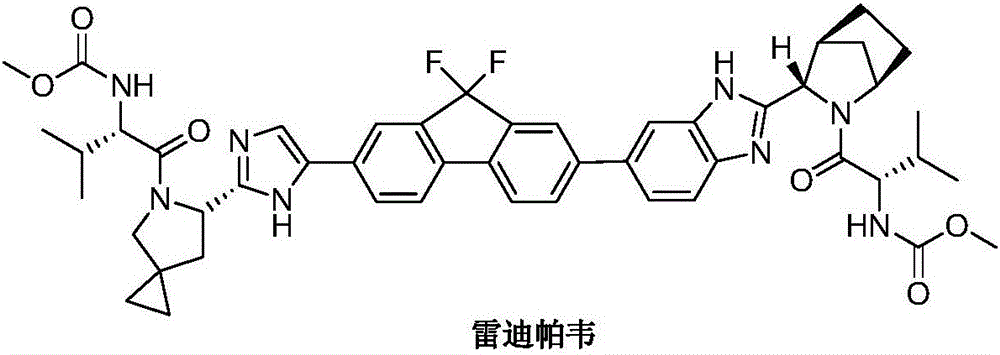
Ledipasvir is a drug for the treatment of hepatitis C that was developed by Gilead Sciences. After completing Phase III clinical trials, on February 10, 2014 Gilead filed for U.S. approval of a ledipasvir/sofosbuvir fixed-dose combination tablet for genotype 1 hepatitis C. The ledipasvir/sofosbuvir combination is a direct-acting antiviral agent that interferes with HCV replication and can be used to treat patients with genotypes 1a or 1b without PEG-interferon or ribavirin.
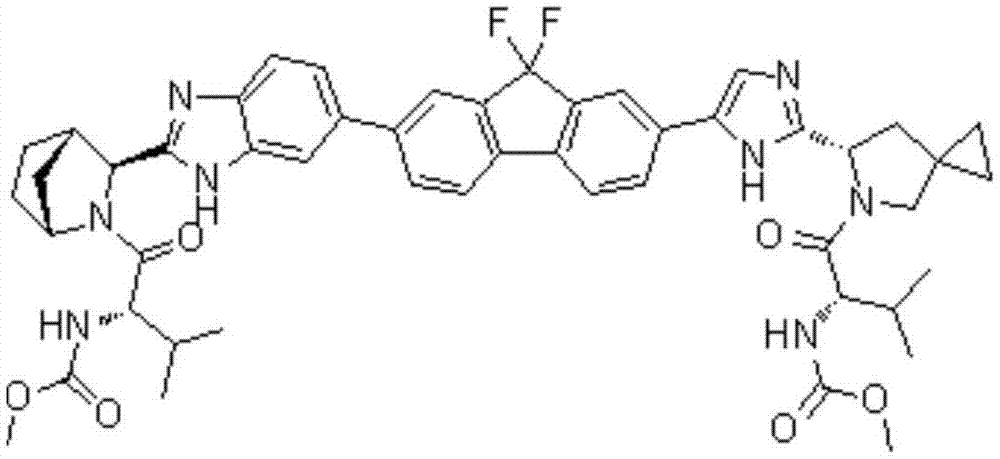
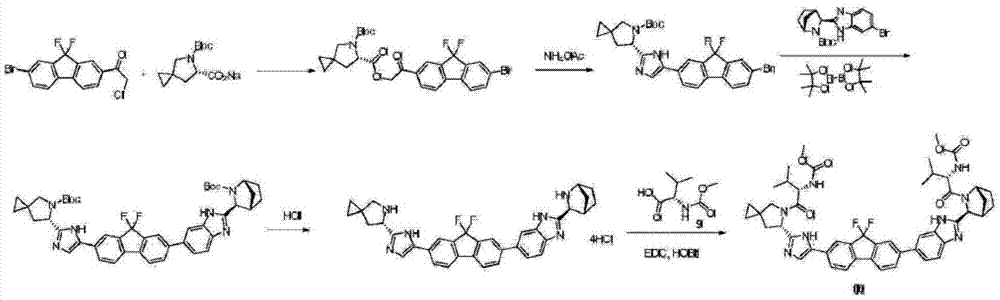
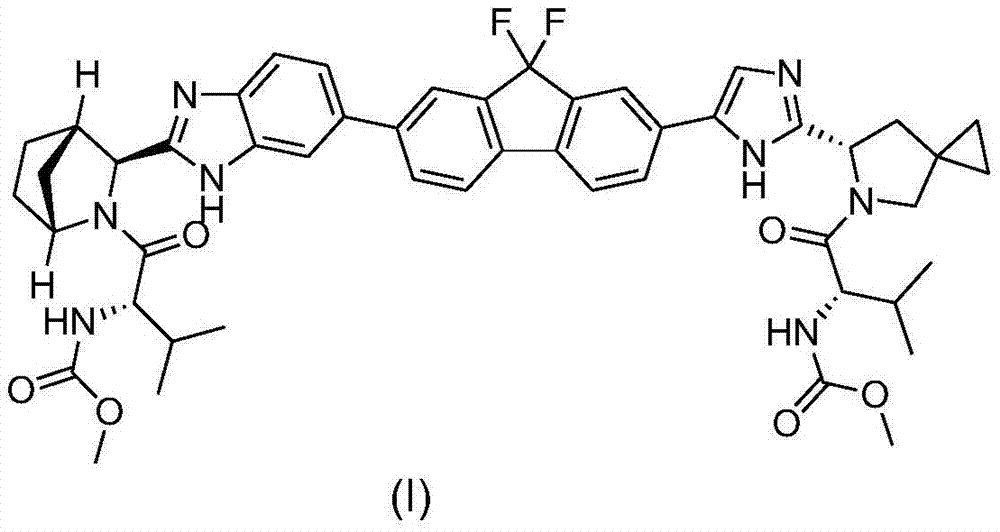
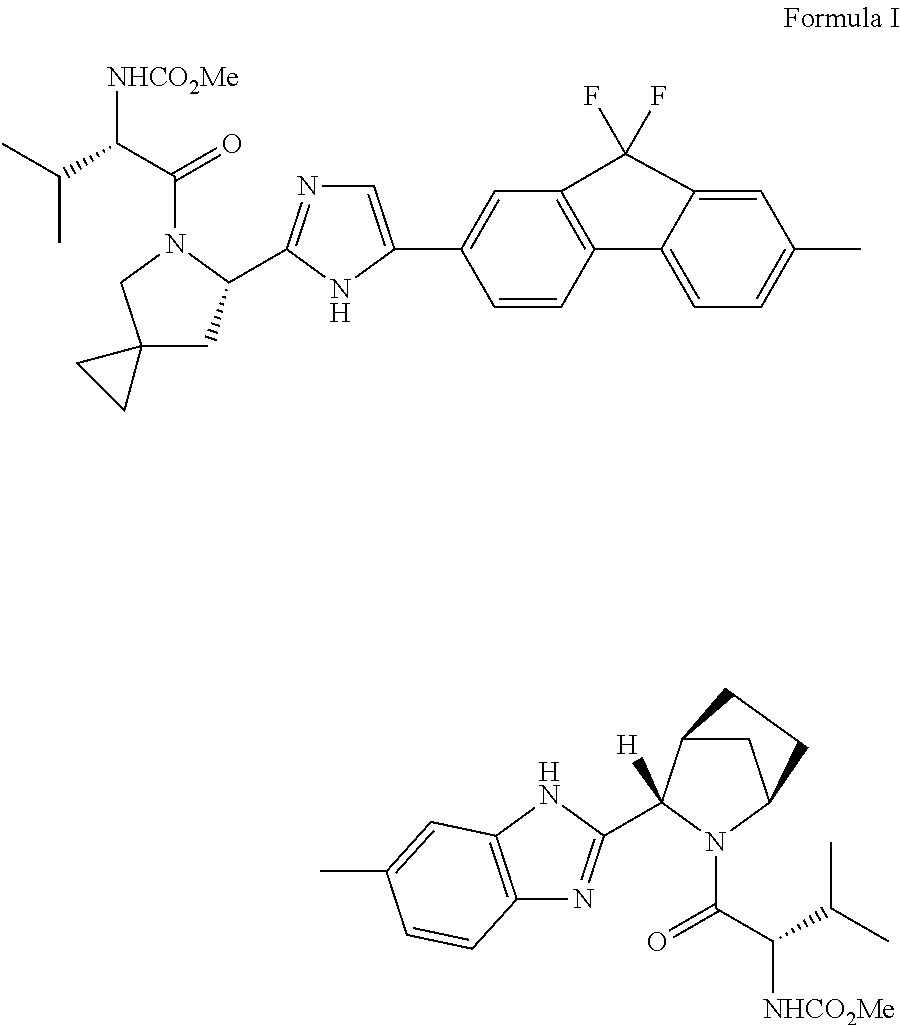
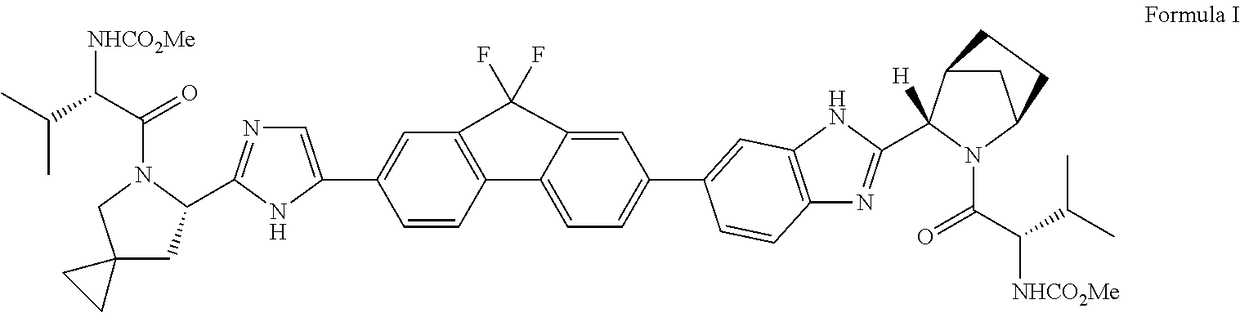
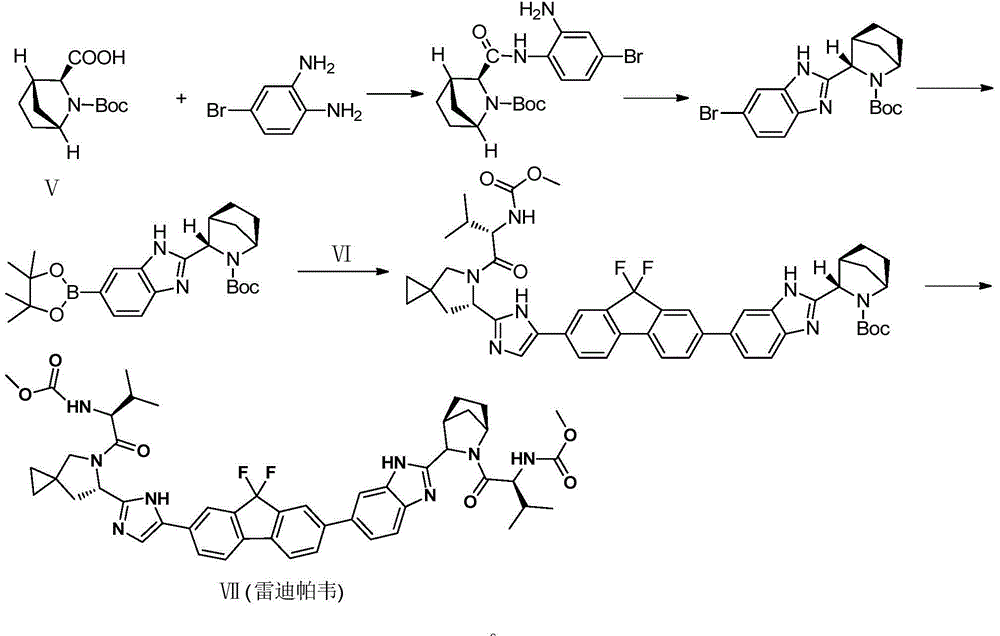
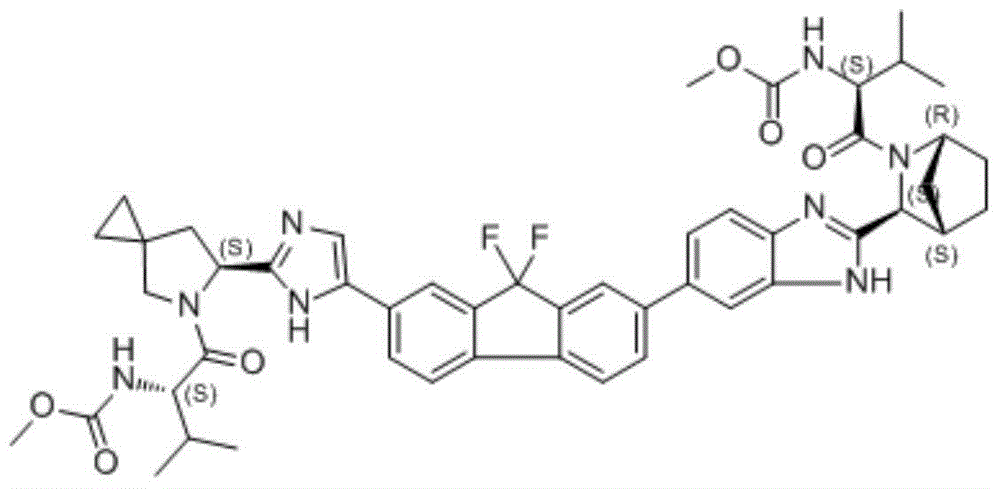
Preparation method of ledipasvir and derivative thereof, and intermediate compound for preparing the ledipasvir
The invention relates to a preparation method of ledipasvir and a derivative thereof, and an intermediate compound for preparing the ledipasvir, and in particular, the invention discloses a preparation method of a compound represented as the formula I, and a series of new preparation methods of the ledipasvir. The methods are simple and high-effective and are excellent in application prospect.
Owner:SHANGHAI FOREFRONT PHARMA CO LTD
Combination formulation of two antiviral compounds
Disclosed are pharmaceutical compositions having an effective amount of substantially amorphous ledipasvir and an effective amount of substantially crystalline sofosbuvir.
Owner:GILEAD PHARMASSET LLC
Ledipasvir intermediate preparation method
ActiveCN104478877AEasy to operateEasy to separate and purifyOrganic chemistryTert-Butyloxycarbonyl protecting groupCarboxylic acid
The invention relates to a method for preparing (1R,3S,4S)-N-tert-butyloxycarbonyl-2-azabicyclo[2,2,1]heptane-3-carboxylic acid (formula V), wherein the formula V is defined in the instruction. The method comprises: dissolving glyoxylate in a solvent, cooling to a temperature of -10-0 DEG C, adding a dewatering agent and (R)-phenyl ethylamine, and at unit time intervals, sequentially adding an alcohol solvent, an additive, cyclopentadiene and palladium carbon to carry out catalytic hydrogenation and adding an inorganic alkali aqueous solution and Boc anhydride to carry out a reaction. Compared with the method in the prior art, the method of the present invention has the following significant advantages that: the reaction operation is simple, the multi-step reaction is changed into the one-pot method, the production period is shortened, the separation purification is simple, the single configuration target product can be obtained only requiring the recrystallization, the yield is high, the reaction is efficient, the atom economy is reflected, -78 DEG C and other harsh reaction conditions are abandoned, and the method is expected to be used in mass production.
Owner:SUNSHINE LAKE PHARM CO LTD
Method for preparing Ledipasvir and intermediates of method for preparing Ledipasvir
InactiveCN104530016AHigh molecular weightPromote formationOrganic chemistryBulk chemical productionCombinatorial chemistryLedipasvir
The invention relates to a new preparing method of hepatitis c medicine Ledipasvir (I) and new intermediates used in the preparing method. The chemical formula of the Ledipasvir (I) is shown in the specification.
Owner:PHARMA SHANGHAI
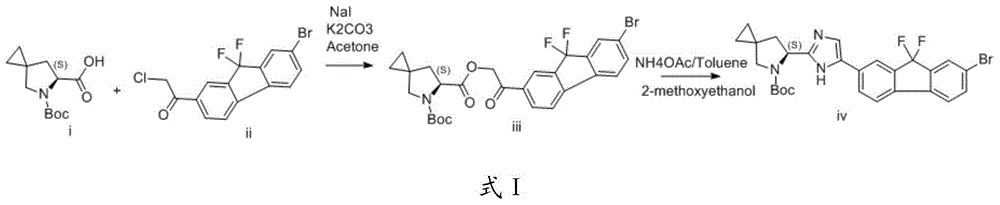
Preparation method of fluorene ethyl ketone derivative
InactiveCN104513223AOrganic compound preparationCarbonyl compound preparation by condensationKetoneReaction conditions
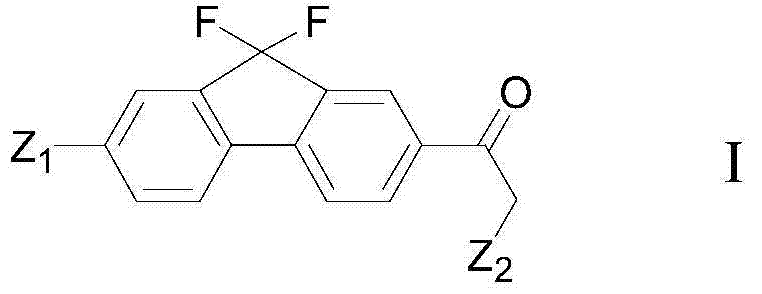
The present invention provides a preparation method of a fluorene ethyl ketone derivative, and particularly provides a preparation method of compounds of formula I, definition of each group is as described in the specification. The compound can be used as an intermediate for preparation of ledipasvir, and the intermediate is used for the preparation of a ledipasvir synthesis key intermediate and further preparation of ledipasvir. The method is low in cost, mild in reaction conditions, and suitable for industrialized production.
Owner:SHANGHAI FOREFRONT PHARMA CO LTD
Preparation method of Ledipasvir
The invention discloses a preparation method of Ledipasvir. The method has no need for chromatographic column separation, reduces the cost of raw materials, and can acquire a high purity product by a simple precipitation or crystallization purification means, thus providing feasible technical conditions for large-scale industrial production.
Owner:厦门蔚嘉制药有限公司
Preparation method for novel NS5A inhibitor medicine
InactiveCN104926796AEasy to controlShort process routeOrganic chemistryBulk chemical productionPtru catalystAzabicyclane
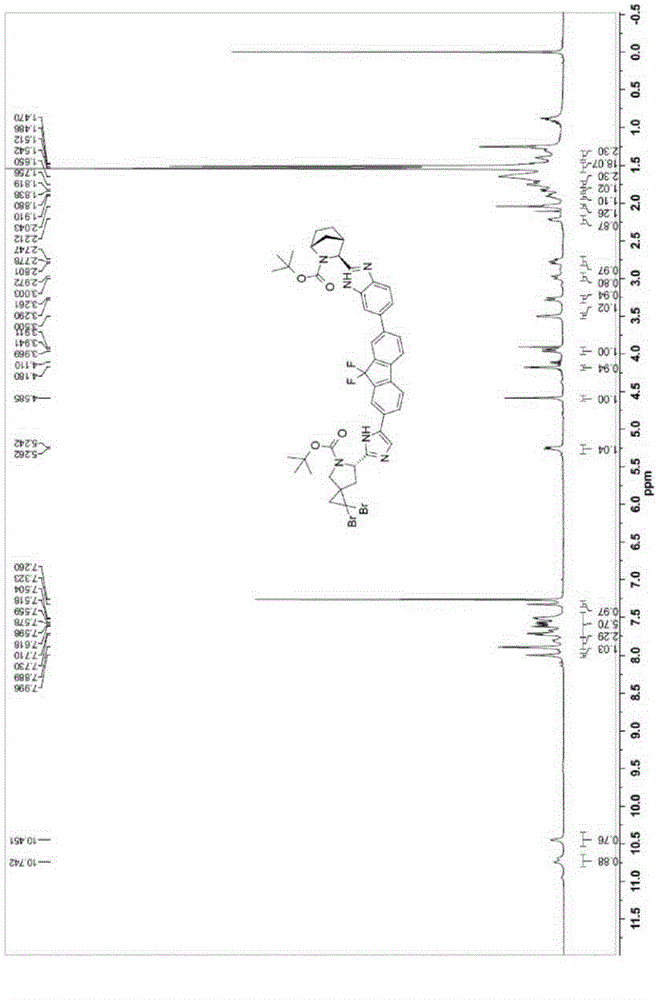
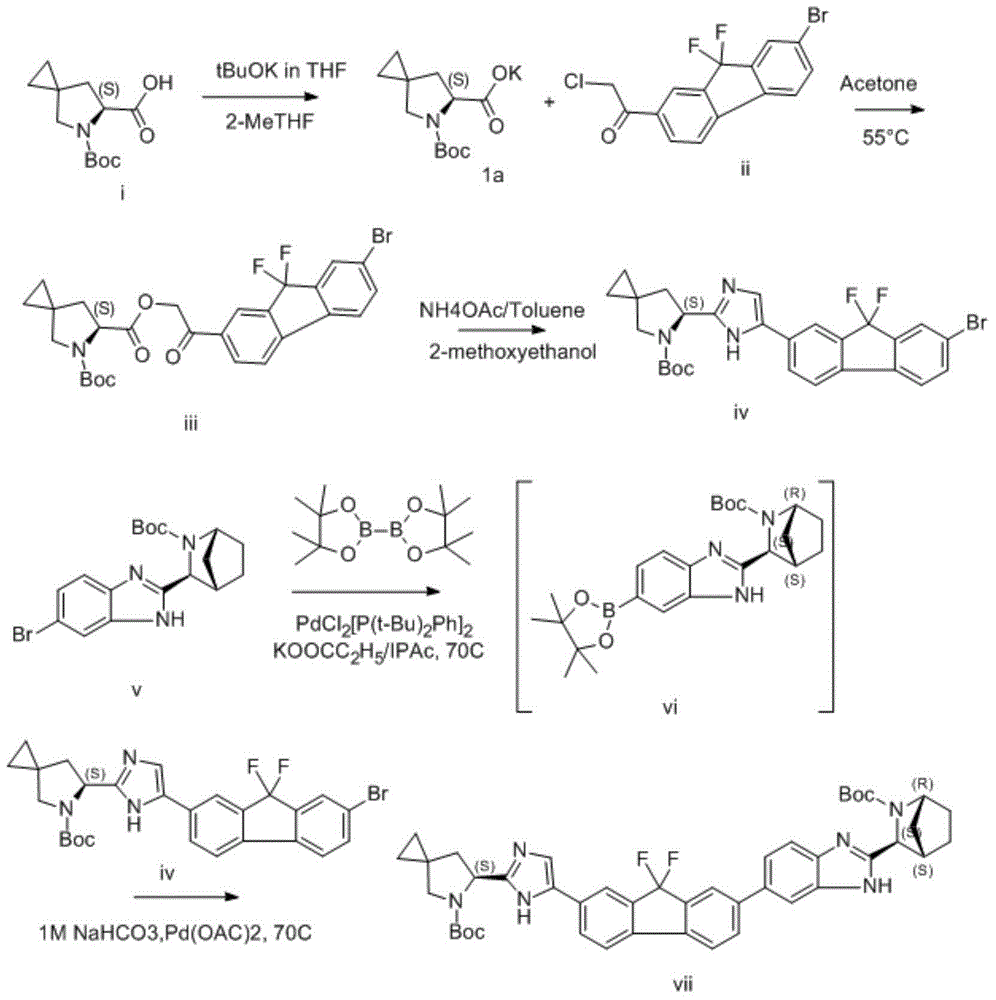
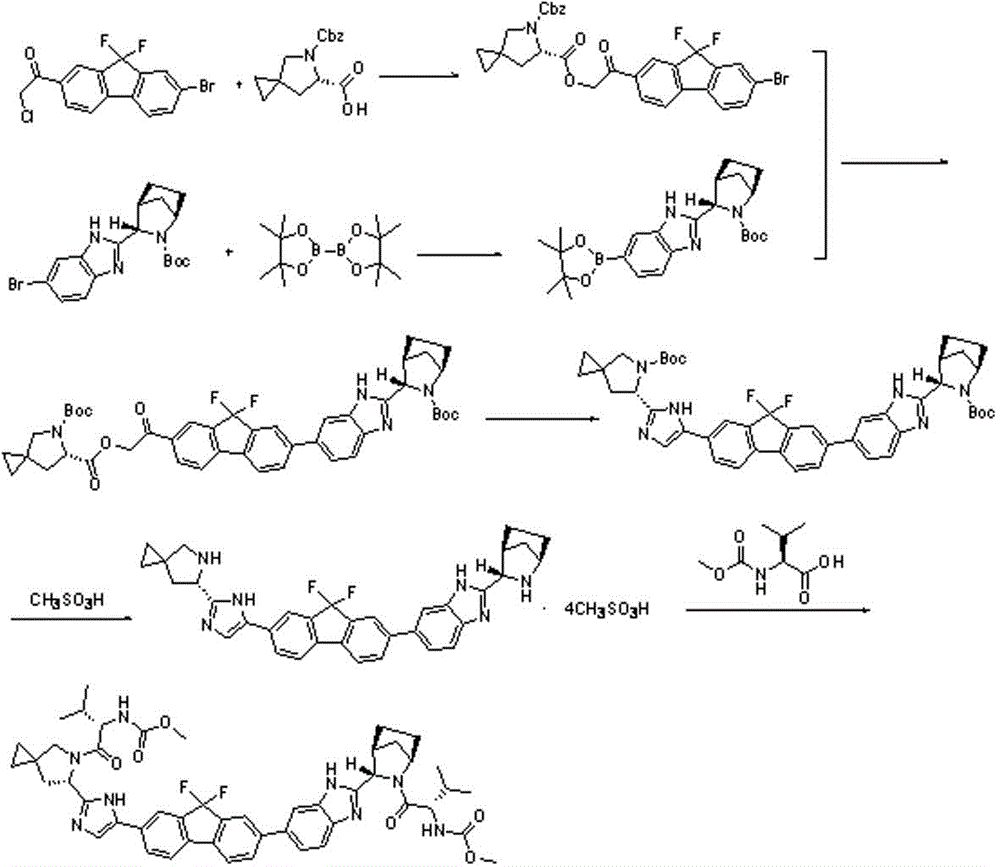
The invention discloses a preparation method for novel NS5A inhibitor medicine. The preparation method includes the steps that step1, (1R, 3S, 4S)-3-(6-bromine-1H-benzimidazole-2-base)-2- azabicyalo (2.2.1) heptane-2-carboxylic acid tert-butyl ester serves as raw materials and reacts with bis (pinacolato) diboron under the action of a metal catalyst to obtain an intermediate L1; 1-(7-bromine-9,9-difluoro-9H-fluorene-2-base)-2-phenacyl chloride and (6S)-5-azaspiro (2.4) heptane-5,6-dicarboxylic acid 5-benzyl ester react under the action of a base to obtain an intermediate L2; the intermediate L1 and the intermediate L2 react under the action of a metal catalyst to obtain an intermediate L3; the intermediate L3 and an amine reagent react in a cyclization mode to obtain an intermediate L4; a protecting group is removed from the intermediate L4 under the action of methanesulfonic acid, so that an intermediate L5 in a mesylate form is obtained; the intermediate L5 and MOC valine react under the action of a condensing agent to obtain a Ledipasvir finished product. The process route is short, the preparation technology is simple, the intermediates are easy to control, and the reaction yield is high.
Owner:NANTONG CHANGYOO PHARMATECH CO LTD
Preparation method of ledipasvir intermediate
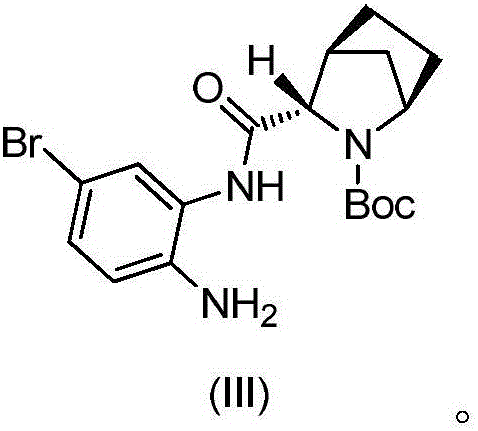
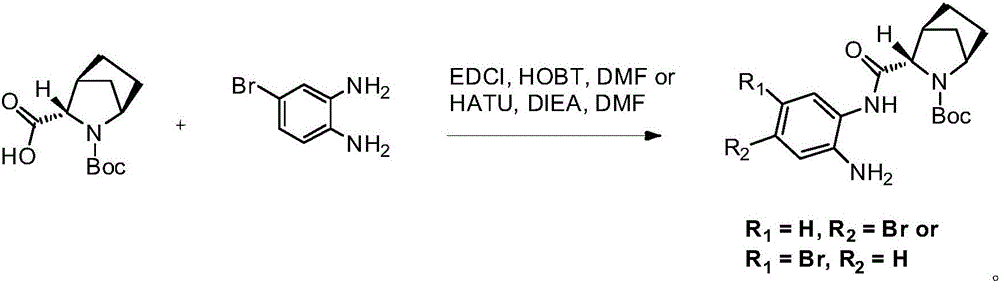
ActiveCN105968040ANot easy to oxidize and change colorEase of industrial productionOrganic chemistry methodsTert-Butyloxycarbonyl protecting groupBromine
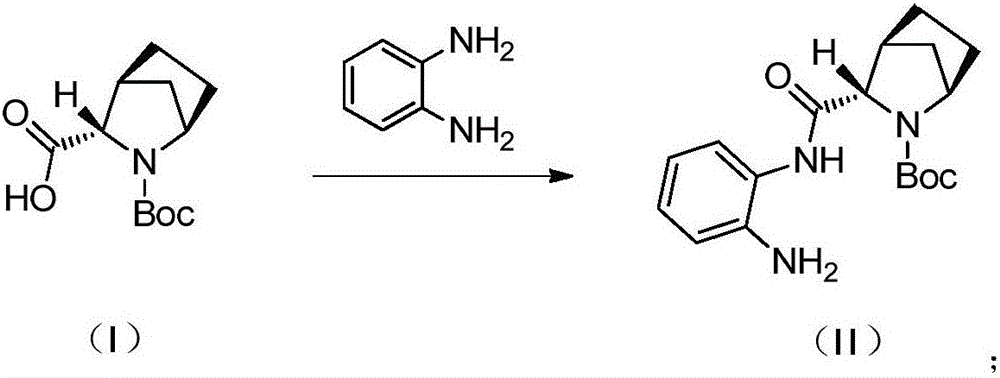
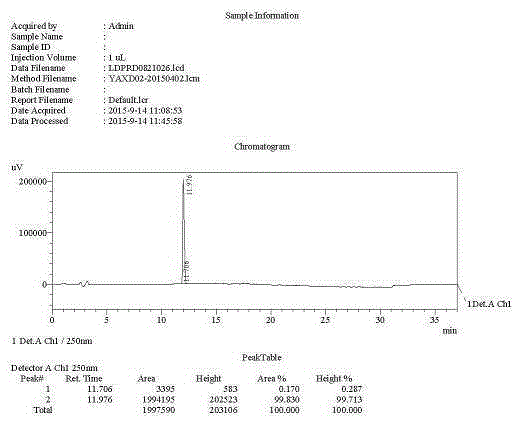
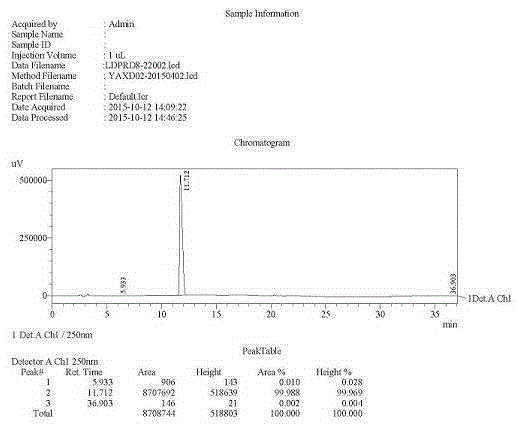
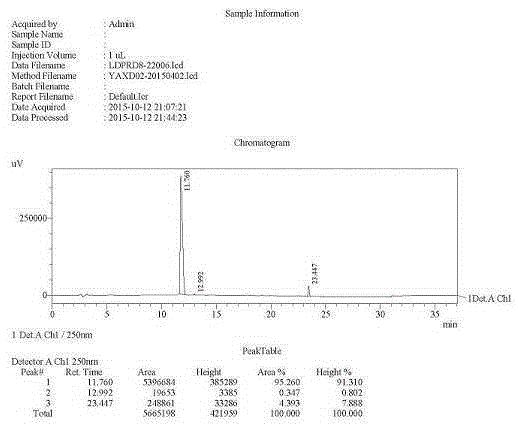
The invention discloses a preparation method of a ledipasvir intermediate shown as formula (III). The method is characterized by taking (1R, 3S,4S)-N-t-butyloxycarbonyl-2-azabicyalo[2.2.1] heptane-3-carboxylic acid and o-phenylenediamine as raw materials and synthesizing (1R, 3S,4S)-3-(6-bromine-1H-benzimidazole-2-yl)-2-azabicyalo[2.2.1] heptane-2-carboxylic acid tert-butyl ester by using an anhydride mixing method. The whole route is low in production cost and high in yield; the formed monoamide is easy to purify; the generation and the residual of impurities are reduced; the o-phenylenediamine used as the raw material is cheap and easily-available, is hard to oxidize and discolor and can be stored in bulks; the industrial production is facilitated. The formula (III) is described in the description.
Owner:ASTATECH CHENGDU BIOPHARM CORP
Preparation method of ledipasvir key intermediate
The invention relates to a preparation method of a ledipasvir key intermediate, and concretely relates to a preparation method of t-butyl (1R,3S,4S)-3-(5-halo-1H-benzimidazolyl-2-yl)-2-azabicyclo[2. 2. 1]heptane-carboxylate. The method has the advantages of low price and massive commercial purchasing of a raw material, and complete avoiding of generation of disubstituted impurities.
Owner:WISDOM PHARM CO LTD
Ledipasvir and sofosbuvir compound coating tablet preparation and preparation method thereof
InactiveCN104546886AGood compressibilitySmooth and translucent appearanceOrganic active ingredientsDigestive systemPolyethylene glycolBULK ACTIVE INGREDIENT
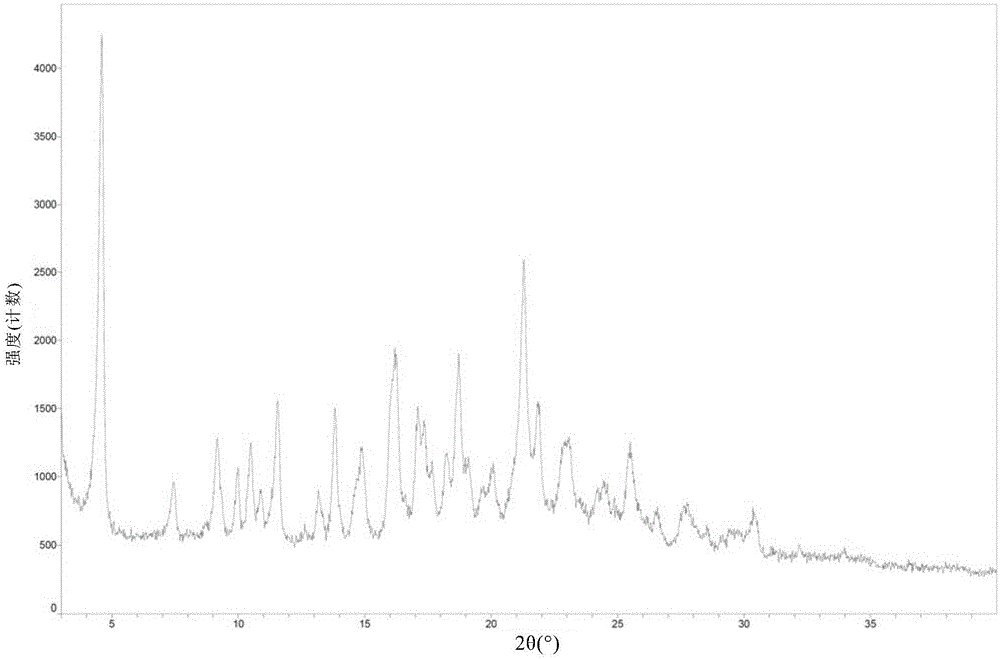
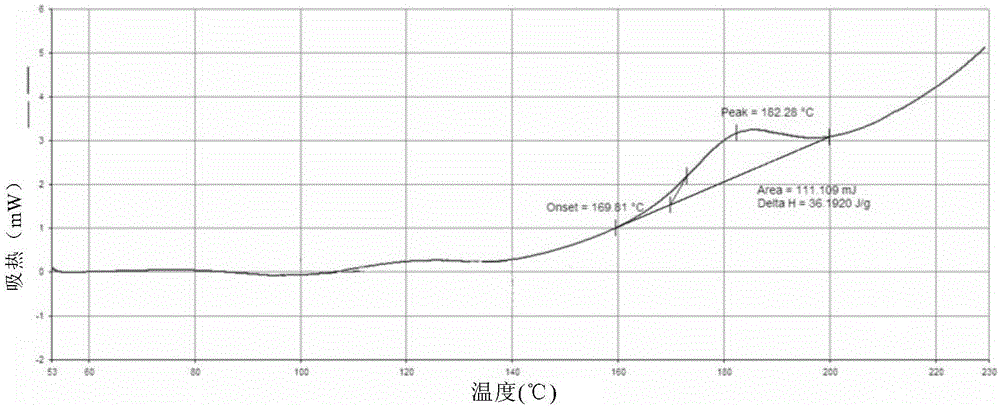
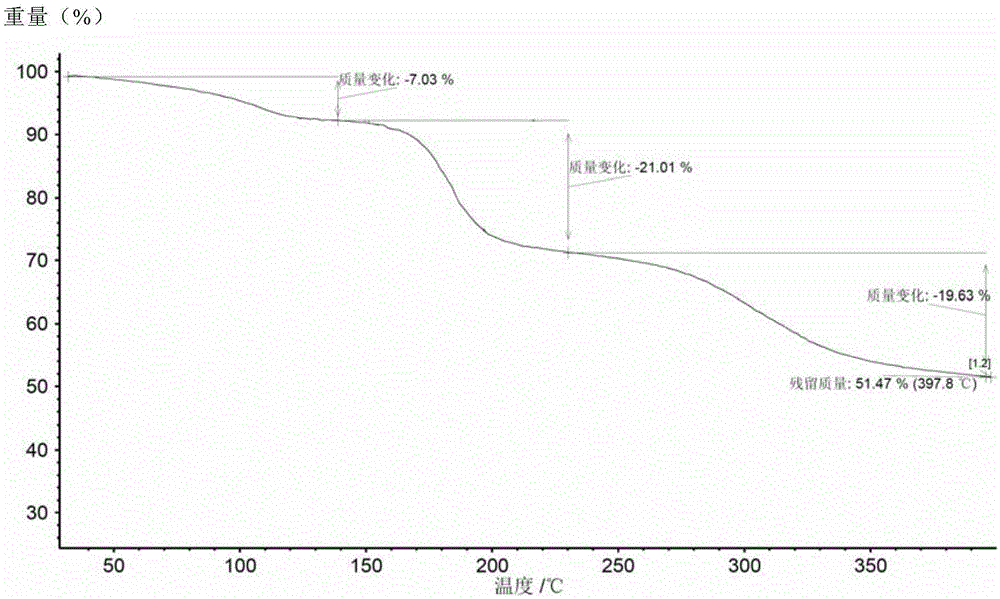
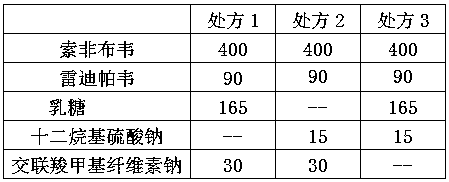
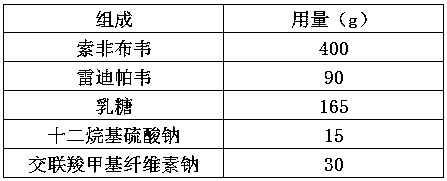
The invention discloses a ledipasvir and sofosbuvir compound coating tablet preparation. The preparation is prepared from the following active ingredients in percentage by mass: 45.0-55.0 percent of ledipasvir and sofosbuvir, 2.0-8.0 percent of a disintegrating agent, 30-40 percent of a diluting agent and 0.5-1.5 percent of a lubricating agent, wherein the disintegrating agent is one or more of povidone, croscarmellose sodium, hydroxypropyl methylcellulose and sodium carboxymethyl starch; the diluting agent is one or more of microcrystalline cellulose, mannitol, lactose and polyethylene glycol; the lubricating agent is one or more of magnesium stearate, talcum powder, aerosil and calcium stearate. The ledipasvir and sofosbuvir compound coating tablet preparation is prepared by coating with a rhombic buccal tablet, and the tablet content has good uniformity. The preparation has the advantages of simple process, high yield, good stability and the like, and is easy for large-scale industrial production.
Owner:ANHUI YELLEN PHARMA
Preparation method of high-purity Ledipasvir intermediate
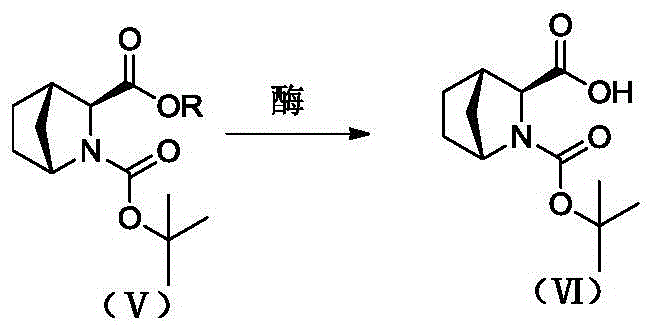
ActiveCN105461606AHigh reaction yieldNot generatedOrganic chemistryFermentationEnzymatic hydrolysisTert-Butyloxycarbonyl protecting group
The invention discloses a preparation method of a high-purity Ledipasvir intermediate (1R, 3S and 4S)-N-t-butylcarbonyl-2-azabicyalo[2.2.1] heptane-3-carboxylic acid. According to the method, (1R, 3S and 4S)-N-t-butylcarbonyl-2-azabicyalo[2.2.1] heptane-3-carboxylate serves as an initial raw material, and the Ledipasvir intermediate is obtained through enzymatic hydrolysis. A test proves that the high-purity Ledipasvir intermediate is obtained and a feasible path is provided for reducing production cost and improving drug use safety. Meanwhile, the method has the advantages that operation is easy, environment friendliness is achieved, the yield is high, selectivity is high and cost is low; large-scale production can be achieved, and industrial application and popularization are facilitated.
Owner:ASTATECH CHENGDU BIOPHARM CORP
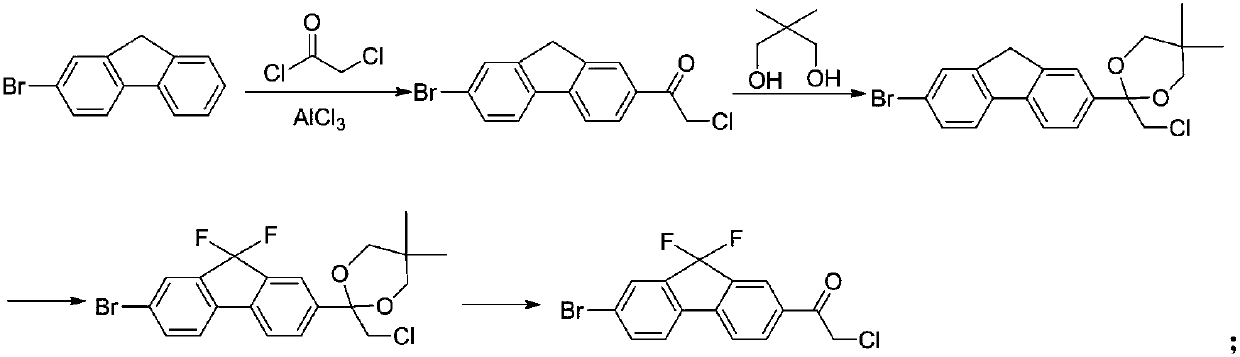
Preparation method of key intermediate of anti-hepatitis C drug ledipasvir
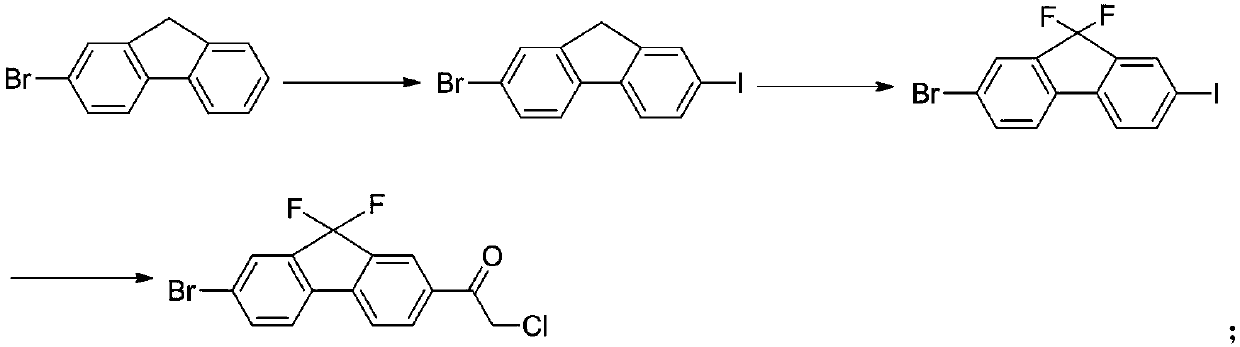
InactiveCN109678686ARaw materials are easy to getLow priceOrganic compound preparationCarboxylic acid esters preparationN-methylacetamideIobenzamic acid
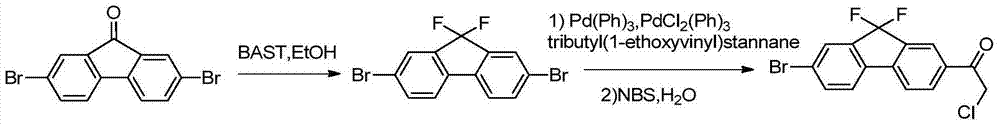
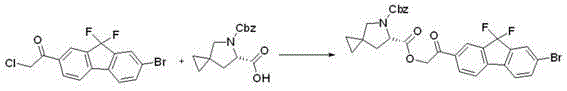
The invention provides a preparation method of a key intermediate 1-(7-bromo-9,9-difluoro-9H-fluoren-2-yl)-2-chloroethanone. The method comprises the steps as follows: 2-amino-5-bromobenzoic acid is taken as a raw material, and subjected to diazotization, iodination,synthesis of 5-bromo-2-iodobenzoic acid, methylation, coupling reaction with phenylboronic acid, ester hydrolysis, acyl chlorination,intramolecular Friedel-Crafts alkylation, carbonyl reduction, iodization, fluorination and final reaction with 2-chloro-N-methoxy-N-methylacetamide to prepare the target product. The process adopts easily available starting raw materials, is low in price and free of hazardous process and has mild reaction conditions..
Owner:IANGSU COLLEGE OF ENG & TECH
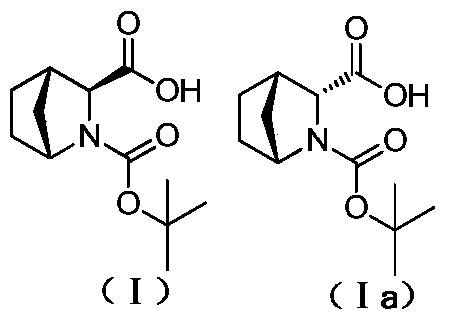
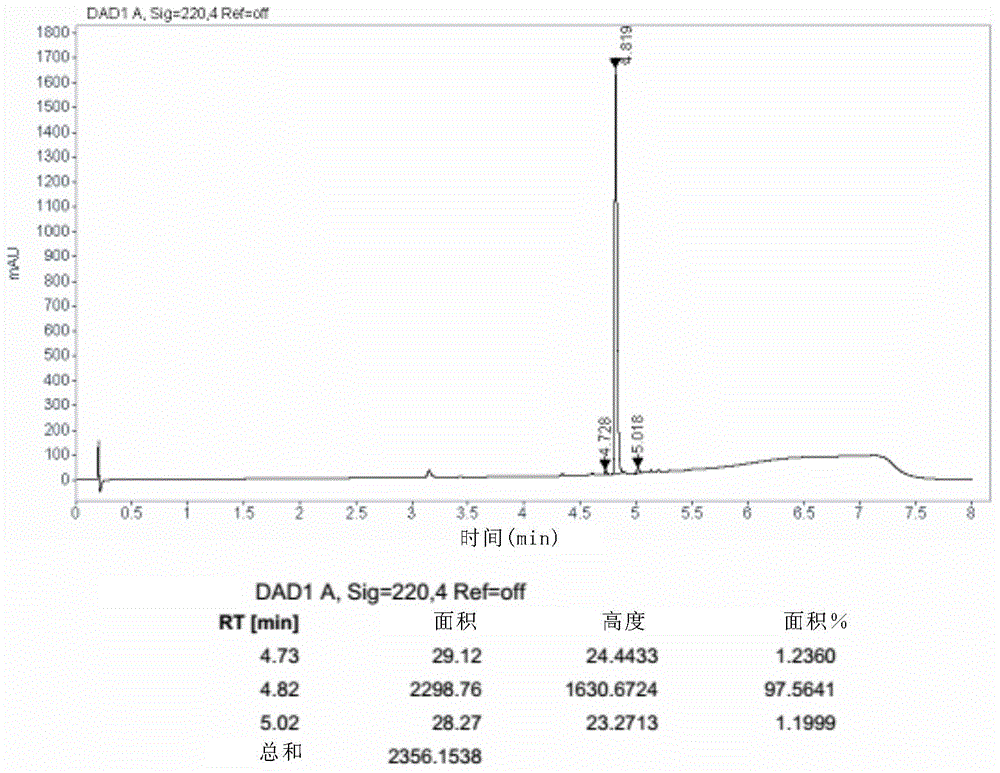
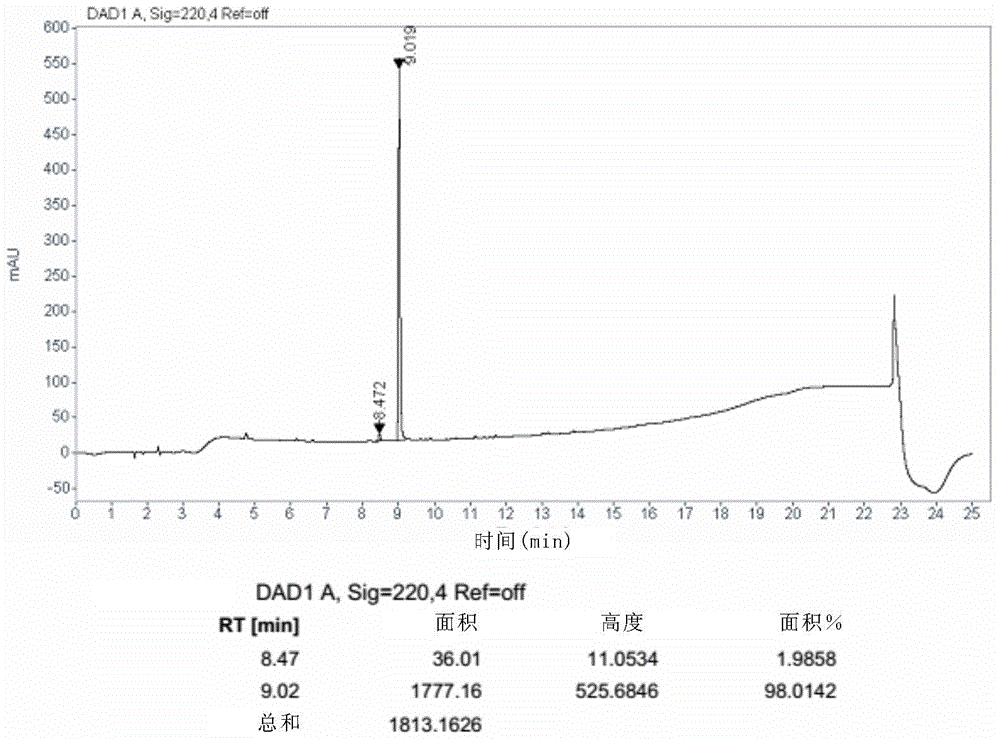
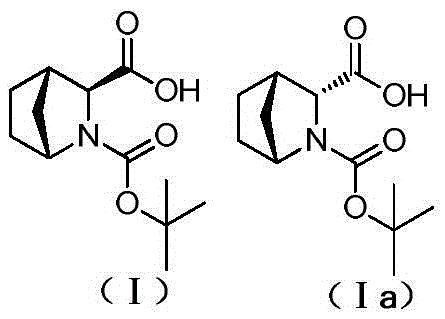
Method for reducing content of diastereoisomer impurity in Ledipasvir intermediate
ActiveCN105418477AGentle method of purificationReduce manufacturing costOrganic chemistryOrganic solventTert-Butyloxycarbonyl protecting group
The invention discloses a method for reducing content of a diastereoisomer impurity (Ia) in a Ledipasvir intermediate (1R,3S,4S)-N-t-butyloxycarboryl-2-azabicyalo[2.2.1]heptane-3-carboxylic acid (I). The method comprises the steps of firstly taking a crude product of a compound shown as a formula (I) and containing the diastereoisomer impurity (Ia), dissolving in an organic solvent, adding alkaline organic amine to react with the crude product, separating out a solid, and filtering to obtain an amine salt of the compound shown as the formula (I); acidizing the obtained amine salt in an aqueous phase solution; extracting an obtained aqueous phase, separating out a solid, and separating to obtain the high-purity Ledipasvir intermediate (I). The product obtained by the method has a de value up to more than 99.5 percent, and the yield of more than 80 percent; reaction conditions in the method are mild, raw materials are easy to get, and the method is suitable for industrial application.
Owner:ASTATECH CHENGDU BIOPHARM CORP
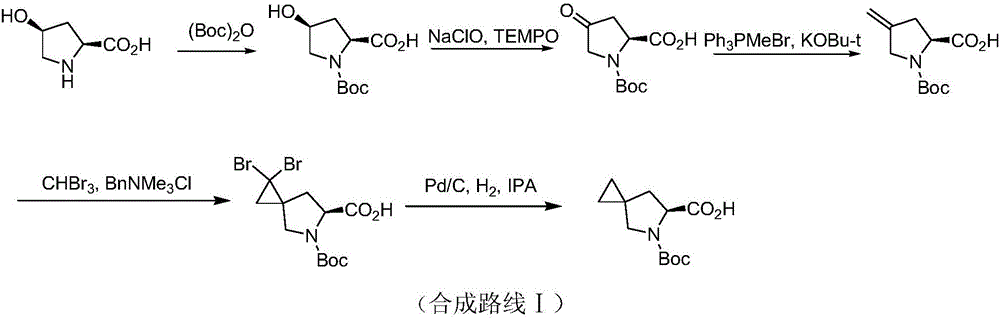
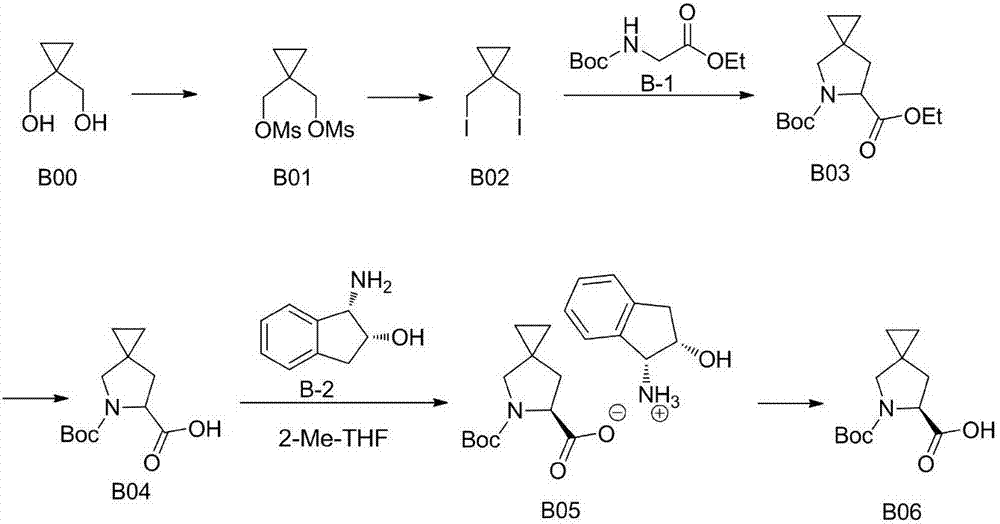
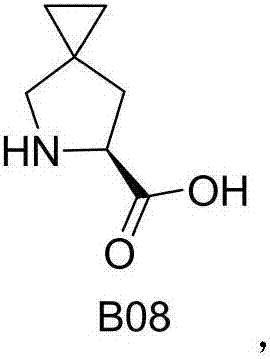
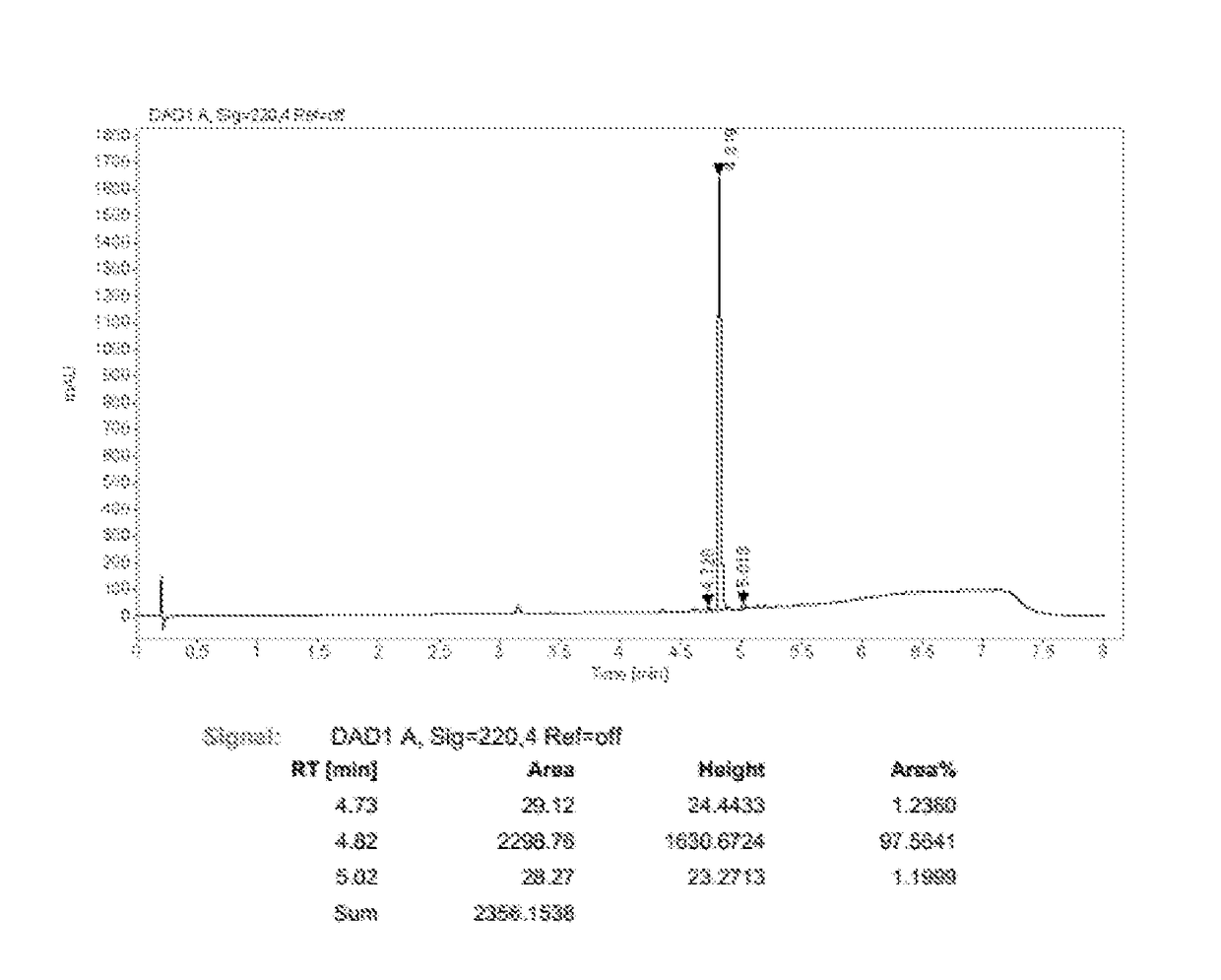
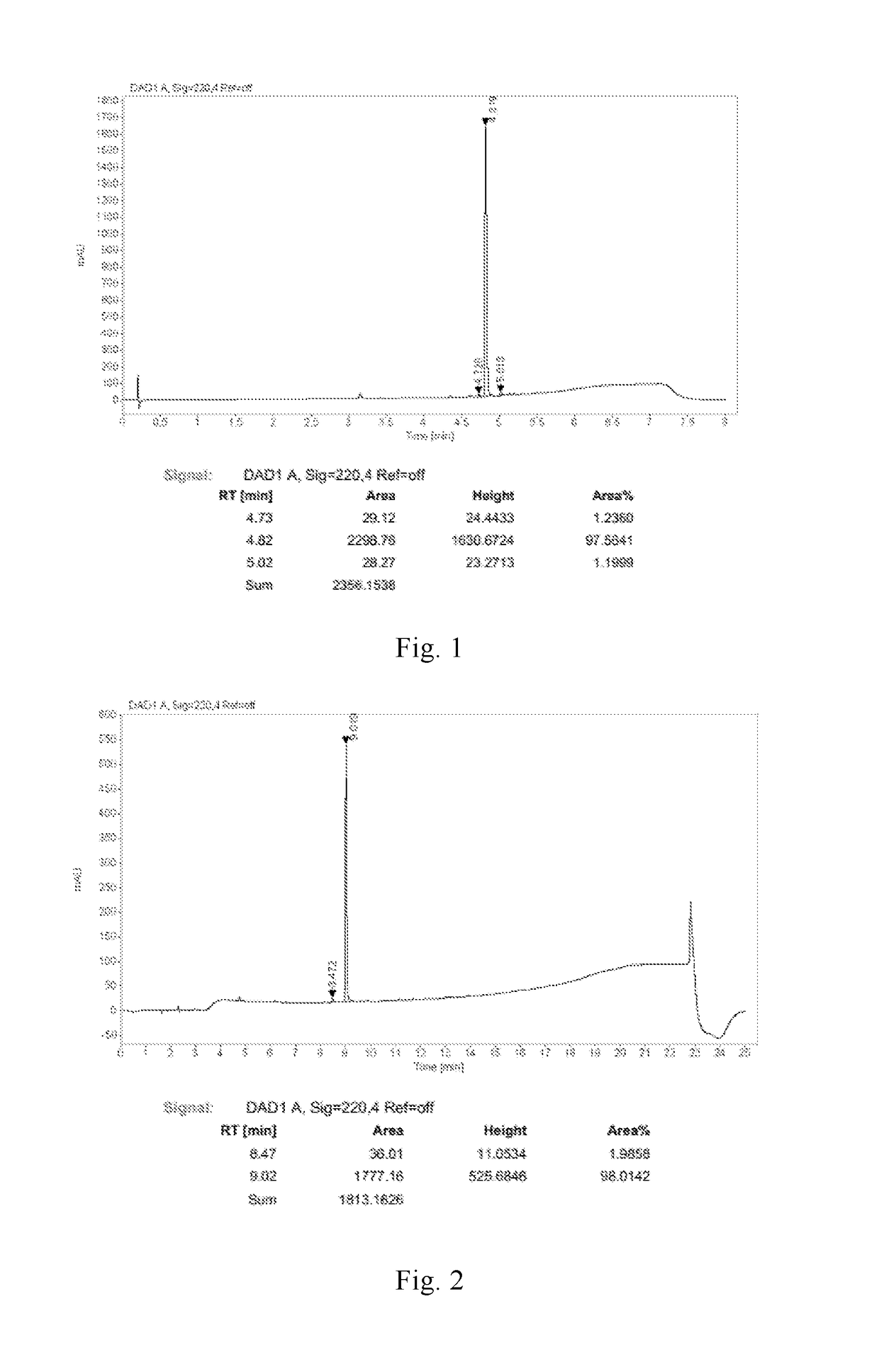
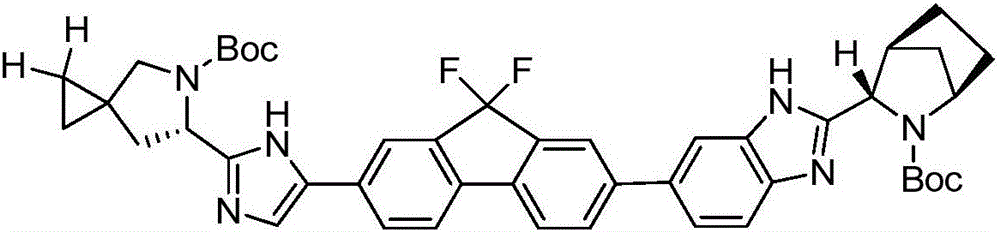
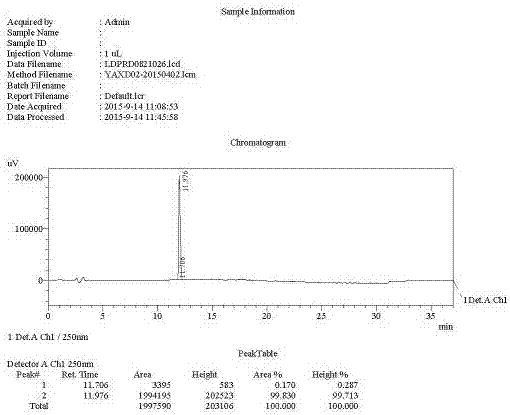
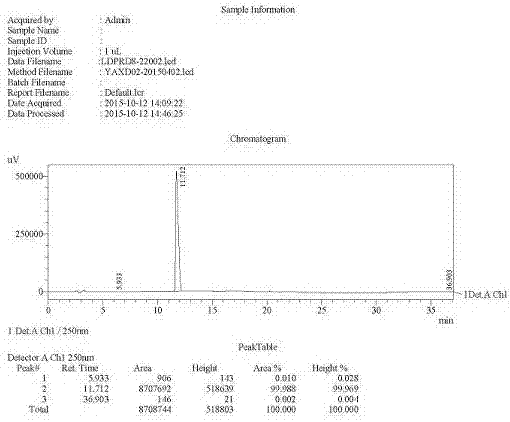
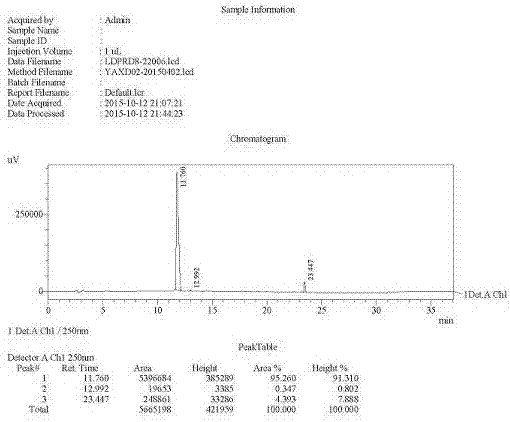
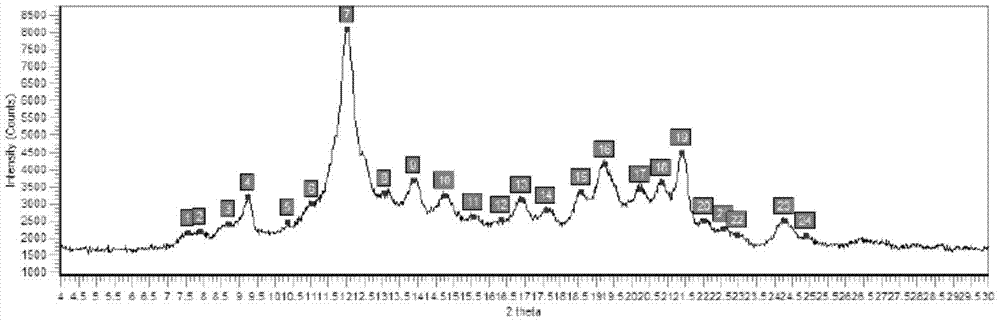
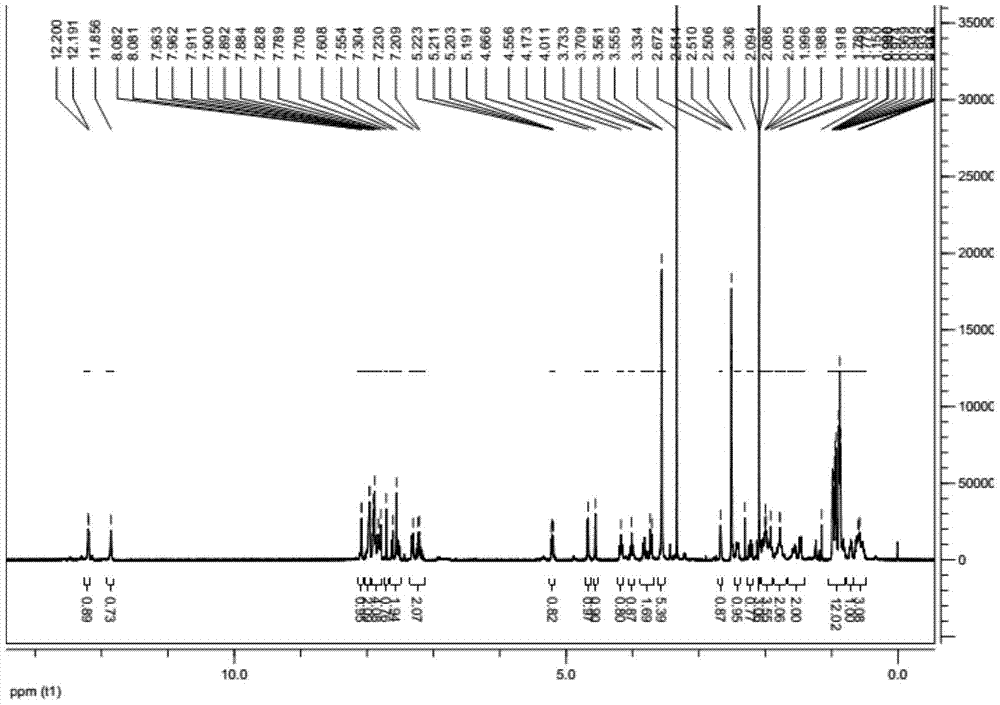
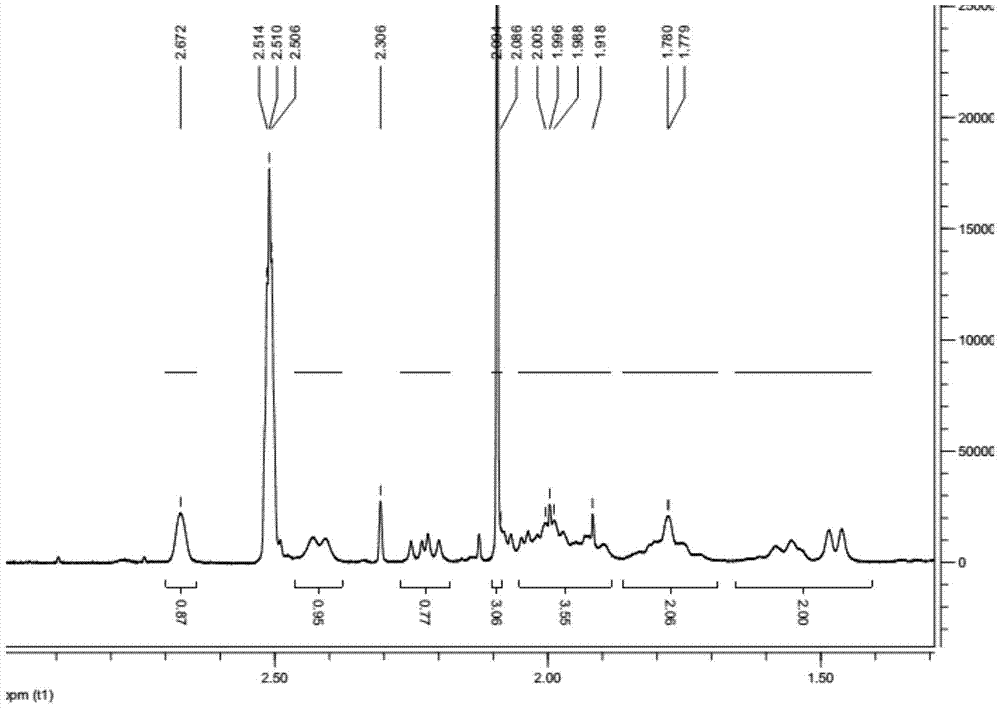
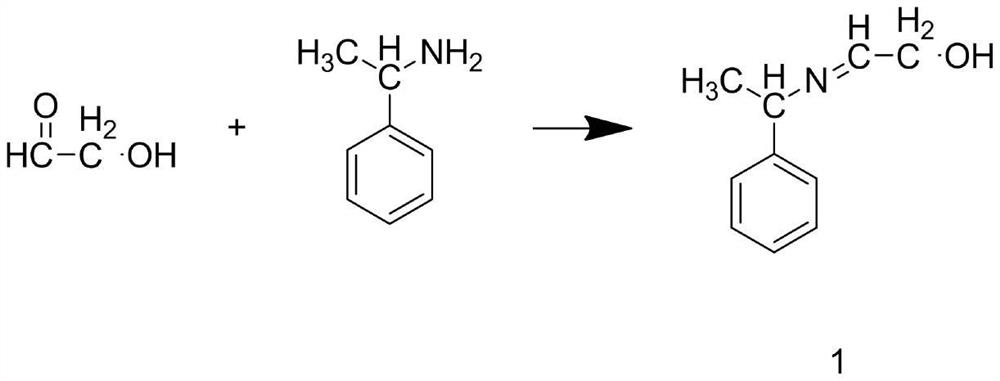
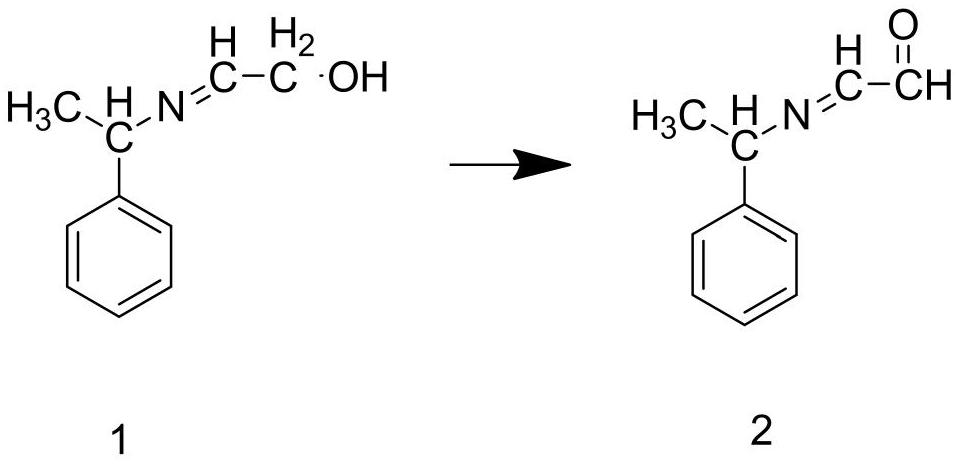
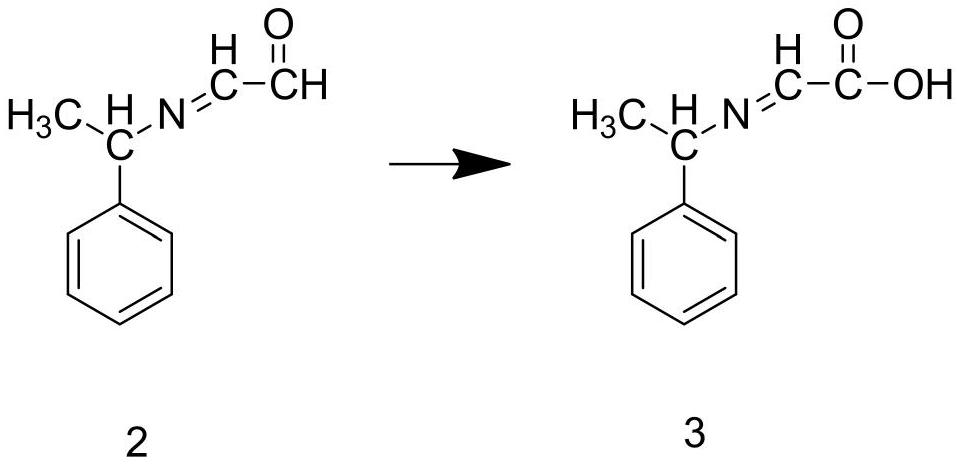
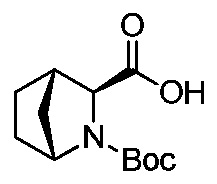
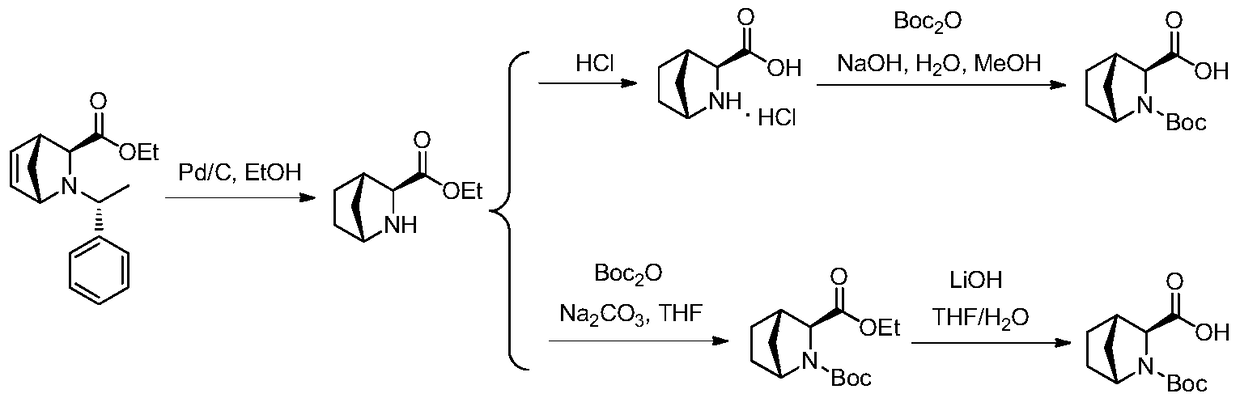
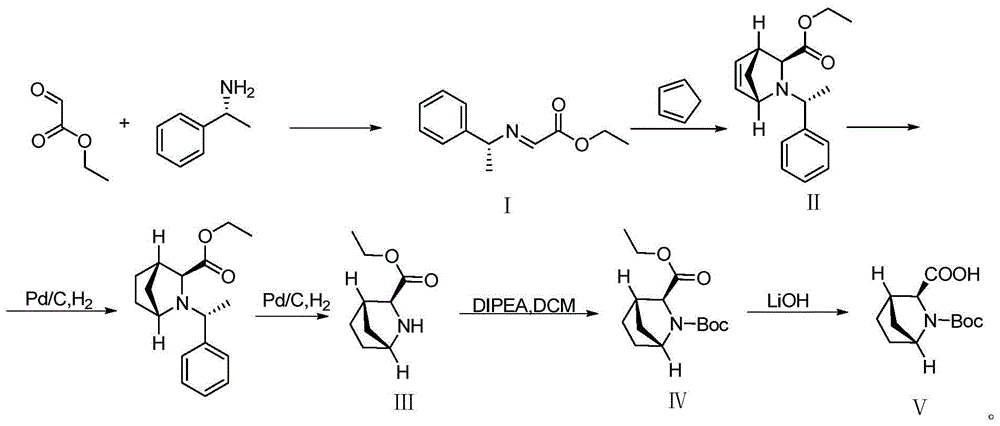
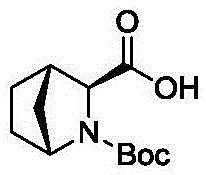
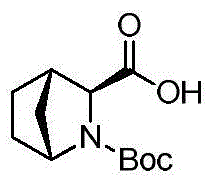
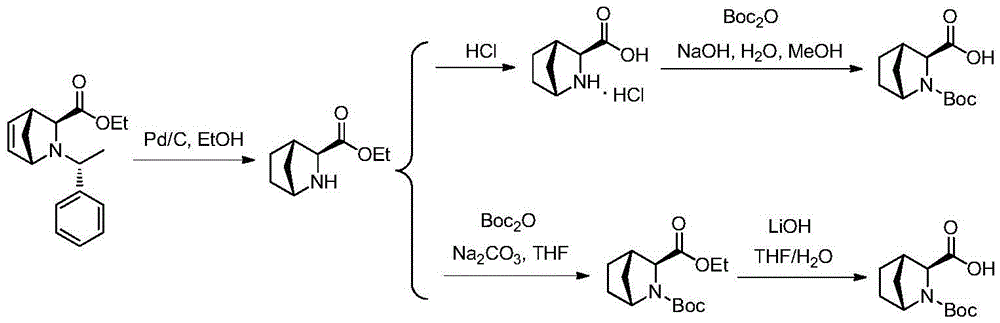
Preparation method of ledipasvir intermediate (1R,3R,4R)-2-Boc-2-azabicyalo [2,2,1] pentane-3-carboxylic acid
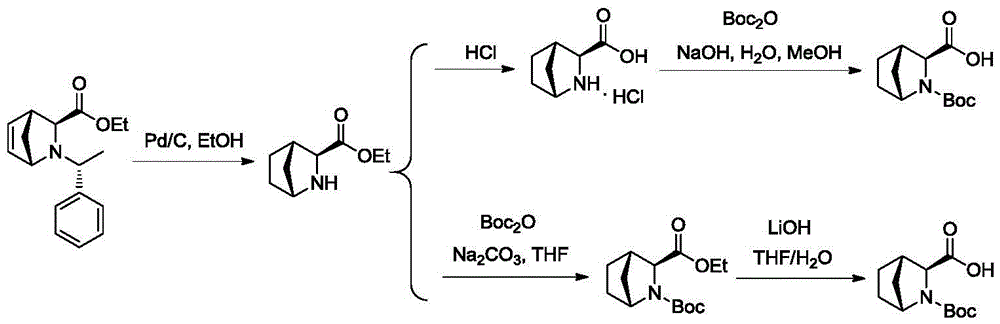
The invention discloses a preparation method of a ledipasvir intermediate (1R,3R,4R)-2-Boc-2-azabicyalo [2,2,1] pentane-3-carboxylic acid. Benzylamine and glyoxylic ester are adopted as raw materials, under the effect of a chiral catalyst, the raw materials react with cyclopentadiene to generate (1S,3S,4R)-2-benzyl-2-azabicyalo [2,2,2] penta-5-alkene-3-carboxylic ethyl ester in a high-selective mode, the (1S,3S,4R)-2-benzyl-2-azabicyalo [2,2,2] penta-5-alkene-3-carboxylic ethyl ester is subjected to debenzylation and Boc protection and then hydrolyzed to obtain ledipasvir intermediate (1R,3R,4R)-2-Boc-2-azabicyalo [2,2,1] pentane-3-carboxylic acid. The method is good in selectivity, easy to implement and environmentally friendly.
Owner:启东东岳药业有限公司 +1
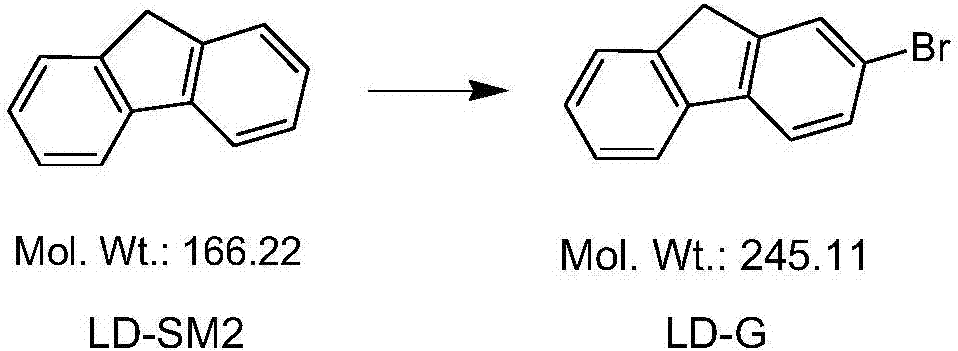
Ledipasvir key intermediate and preparation method thereof
InactiveCN107879908AStable and mature processImprove product qualityCarbonyl compound preparation by condensationHalogenated hydrocarbon preparationCombinatorial chemistryLedipasvir
The invention discloses a ledipasvir key intermediate LD-J, a structure of the ledipasvir key intermediate LD-J and a preparation method of the ledipasvir key intermediate LD-J. The preparation methodcomprises the following steps: (A1) preparing LD-G; (A2) preparing LD-H; (A3) preparing LD-I; (A4) preparing a Grignard reagent; and (A5) preparing LD-J. The ledipasvir key intermediate LD-J and thepreparation method thereof have the advantages that the process is mature and stable, the product is stable in quality, the production process is safe and reliable, and the preparation method is suitable for industrial production.
Owner:安徽诺全药业有限公司
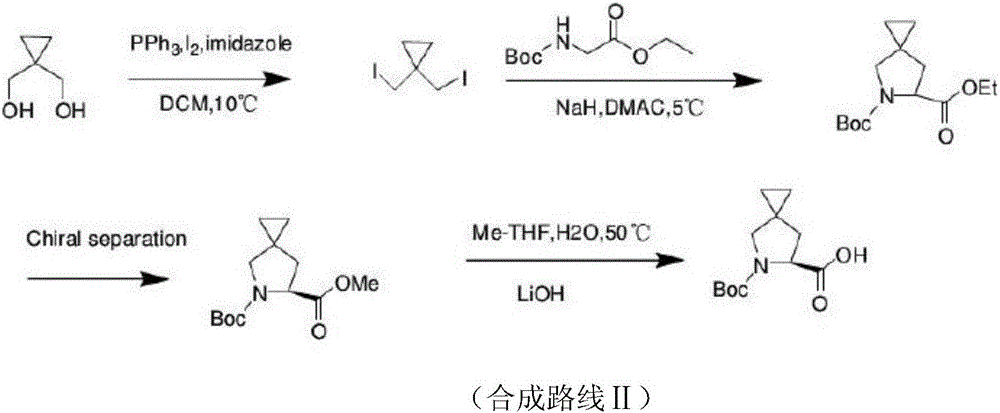
New method for synthesizing Ledipasvir chiral intermediate
ActiveCN106008316ARaw materials are easy to getLow costOrganic chemistryBulk chemical productionCarboxylic acidEthyl ester
The invention discloses a new method for synthesizing a Ledipasvir chiral intermediate. The specific synthetic method includes the following steps that 1,1-dihalo-methyl cyclopropane and N-Boc-glycine ethyl ester are subjected to cyclization in an alkaline environment, and a spiro-compound is obtained; the spiro-compound is subjected to saponification hydrolysis and BOC deprotection, and a 5-diazaspiro[2,4]heptane-6-carboxylic acid raceme is obtained; the 5-diazaspiro[2,4]heptane-6-carboxylic acid raceme is subjected to asymmetric resolution, and S-5-diazaspiro[2,4]heptane-6-carboxylic acid is obtained. By means of the new method for synthesizing the Ledipasvir chiral intermediate, the atom economy is improved, the production cost is reduced, synthesis is easy, preparation is convenient, and large-scale industrial production is promoted.
Owner:CHENGDU BAISHIXING SCI & TECH IND
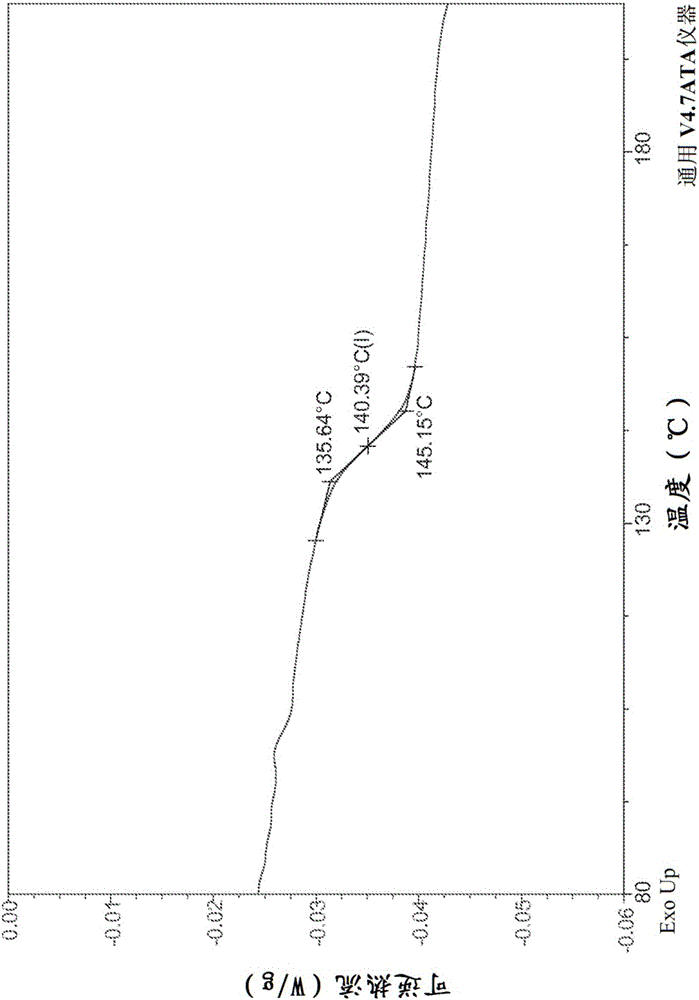
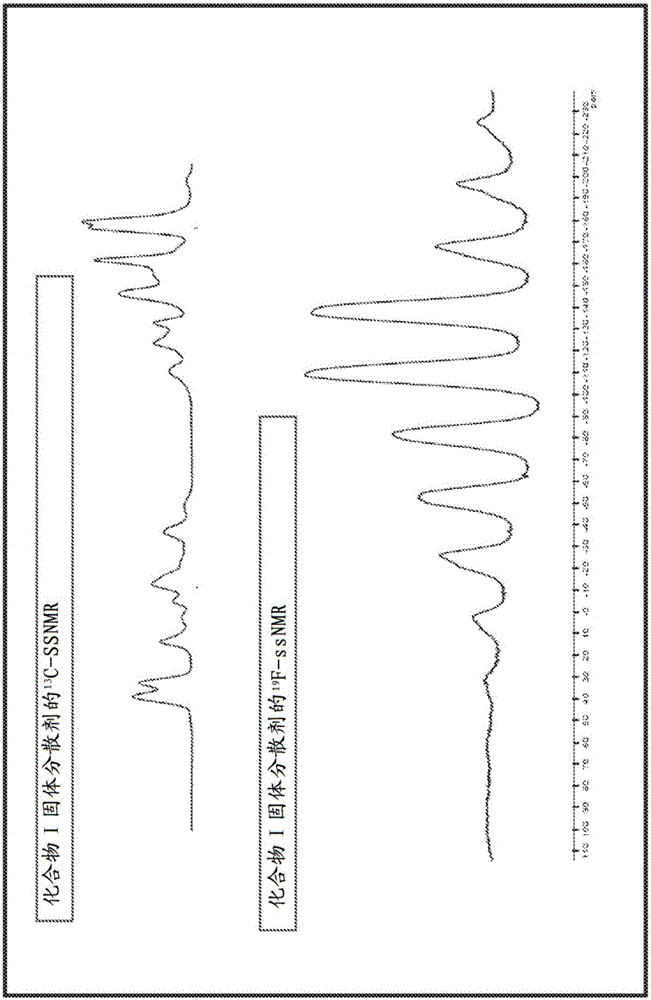
Ledipasvir intermediate monosulfate, crystalline form thereof and preparation method therefor
InactiveCN105017228AOrganic active ingredientsOrganic chemistry methodsSulfateCombinatorial chemistry
The invention discloses a ledipasvir intermediate monosulfate, a crystalline form thereof and a preparation method therefor, and specifically discloses a compound (crystal-form or amorphous) shown in a formula I, a preparation method therefor and application thereof. According to the preparation method provided by the invention, the crystal form I of the prepared compound shown in the formula I is good in light stability.
Owner:SHANGHAI FOREFRONT PHARMA CO LTD
Sofosbuvir and ledipasvir compound preparation
PendingCN108125915APromote dissolutionSimple methodOrganic active ingredientsDigestive systemDissolutionLactose
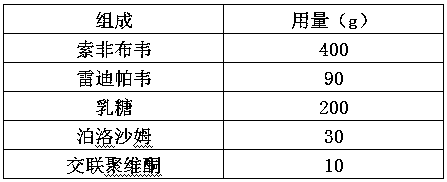
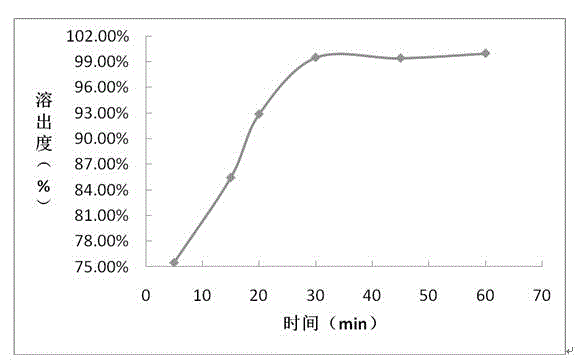
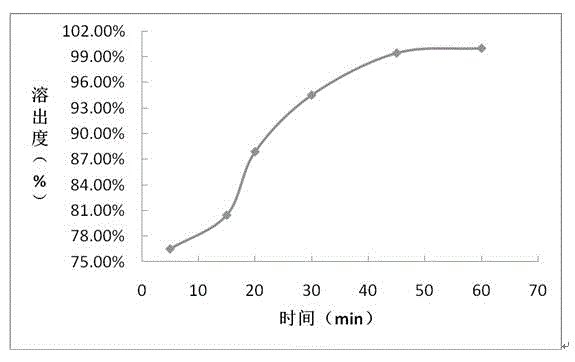
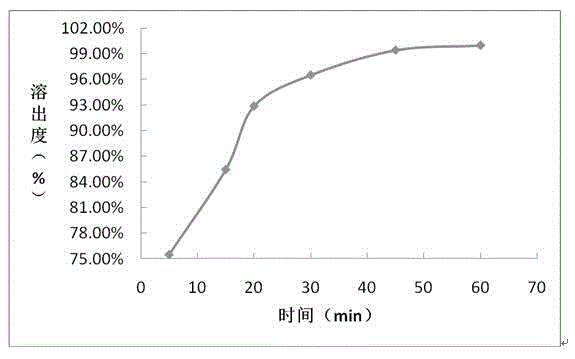
The invention provides a sofosbuvir and ledipasvir compound preparation. The compound preparation is prepared from sofosbuvir, ledipasvir, lactose, a solubilizer and a disintegrant, wherein the particle size d of ledipasvir is not larger than 55 mu m. With combined application of lactose, the solubilizer and the disintegrant in the compound preparation, dissolution of sofosbuvir and ledipasvir canbe obviously improved. The invention further provides a method for preparing the sofosbuvir and ledipasvir compound preparation. The method is simple and easy to operate, production requirements canbe met by conventional equipment, quality is controllable, and the method is suitable for industrial large-scale production.
Owner:BEIJING WINSUNNY PHARMA CO LTD
Improved method for preparing ledipasvir optical intermediate
InactiveCN107216278AHigh optical purityHigh yieldOrganic chemistry methodsTert-Butyloxycarbonyl protecting groupCarboxylic acid
The invention relates to an improved method for preparing a ledipasvir optical intermediate, and belongs to the field of pharmaceutical chemical engineering. The method adopts 5-azaspiro[2,4]heptane-6-carboxylic acid, a crystallization induced asymmetric conversion method is used to conduct chiral separation, so that an (S)-5-azaspiro[2,4]heptane-6-carboxylic acid or a salt thereof is obtained; and the (S)-5-azaspiro[2,4]heptane-6-carboxylic acid or the salt thereof is transformed into the ledipasvir optical intermediate which is (S)-5-(tert-butoxycarbonyl)-5-azaspiro[2,4]heptane-6-carboxylic acid. The method has a low price, is simple and convenient, improves the utilization rate of the raw materials, and is suitable for large industrialized production.
Owner:SUNSHINE LAKE PHARM CO LTD
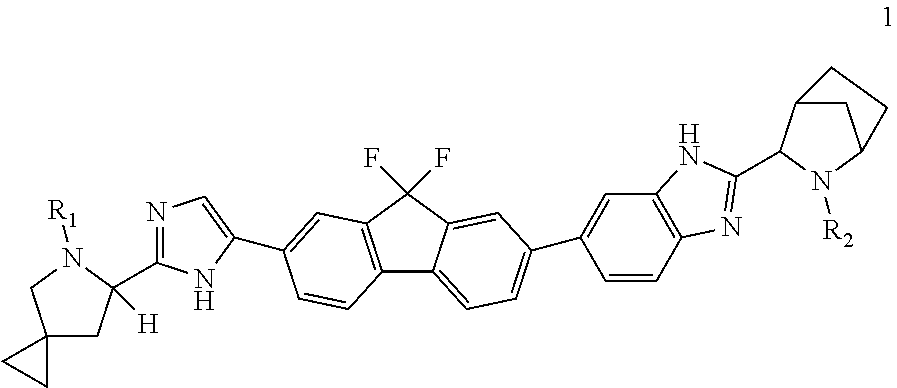
Method of preparation for ledipasvir and derivative thereof, and intermediate compound for preparation of ledipasvir
Methods of preparing Ledipasvir and derivatives thereof, and intermediate compounds used in the preparation of Ledipasvir are provided. Specifically, a method for preparing the compounds of formula 1 and a series of preparation methods of preparing Ledipasvir are provided. The methods described herein are simple and efficient, and have better application prospects.
Owner:SHANGHAI FOREFRONT PHARMA CO LTD
Preparation method of ledipasvir
InactiveCN106892905AShort process routeSimple and fast operationOrganic chemistryMetal catalystLedipasvir
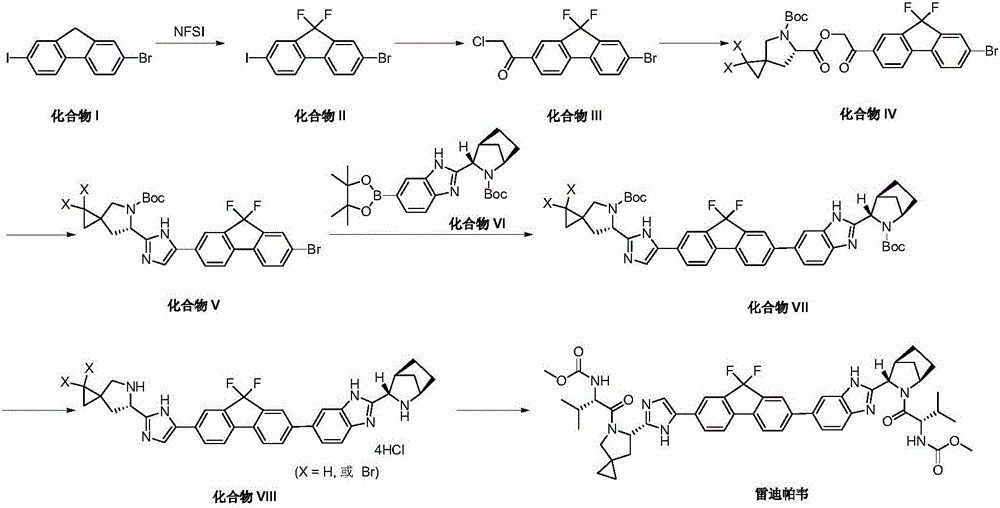
The invention belongs to the drug synthesis field and particularly relates to a preparation method of ledipasvir. The preparation method comprises the following steps: reacting by virtue of a compound (II) and a compound (III) under the action of alkali, so as to obtain a compound (IV); reacting by virtue of the compound (IV) and a compound (V) under the action of a metal catalyst, so as to obtain a compound (VI); reacting by virtue of the compound (VI) and an amine reagent, so as to obtain a compound (VII); reacting by virtue of the compound (VII) under the action of acid, so as to obtain a compound (VIII); and reacting by virtue of the compound (VIII) and Moc-L-valine under the action of a condensing agent, so as to obtain the ledipasvir represented by a chemical formula (I). The preparation method has the beneficial effects that the process route is short, the operation is simple and convenient, and the reaction yield is high.
Owner:常州市勇毅生物药业有限公司
Combination formulation of two antiviral compounds
Disclosed are pharmaceutical compositions having an effective amount of substantially amorphous ledipasvir and an effective amount of substantially crystalline sofosbuvir.
Owner:GILEAD SCI INC
Preparation method of ledipasvir intermediate and intermediate compound
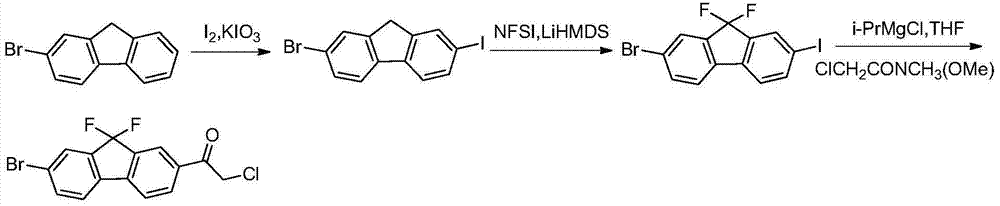
ActiveCN106349229ALow costAtom economy is highGroup 3/13 element organic compoundsLedipasvirFluorine containing
The invention provides a novel method for preparing a ledipasvir intermediate compound VII. According to the method, a compound X is taken as a starting raw material and is subjected to three-step reactions by virtue of fluorine-containing compounds so as to obtain the compound VII; meanwhile, the total yield of three metal-catalyzed reactions is obviously increased, so that the atomic economic efficiency of fluorine is improved, and strong support is provided for the lowering of the cost of ledipasvir; and the reaction route is as follows: formula (shown in the description).
Owner:ANHUI TWISUN HI TECH PHARM CO LTD
Preparation of key intermediates of ledipasvir
The invention relates to a preparation method of a ledipasvir key intermediate, and concretely relates to a preparation method of t-butyl (1R,3S,4S)-3-(5-halo-1H-benzimidazolyl-2-yl)-2-azabicyclo[2. 2. 1]heptane-carboxylate. The method has the advantages of low price and massive commercial purchasing of a raw material, and complete avoiding of generation of disubstituted impurities.
Owner:WISDOM PHARM CO LTD
An improved process for the preparation of hcv inhibitor
The present invention provides a novel process for the preparation of Ledipasvir of Formula I and its pharmaceutically acceptable salts.
Owner:VETUKURI PRASAD RAJU VNKV +4
Method for recycling precious metal catalyst in preparation process of ledipasvir midbody
InactiveCN110041373AReduce manufacturing costSimple processOrganic-compounds/hydrides/coordination-complexes catalystsGroup 8/9/10/18 element organic compoundsRecovery methodOrganic solvent
The invention relates to a method for recycling a precious metal catalyst in the preparation process of a ledipasvir midbody. The method comprises the following steps that a, a compound 1, bisdiboron,the catalyst palladium, dichlorobis(di-tert-butylphenylphosphine) and potassium carbonate are stirred and added into tetrahydrofuran in a reactor at the temperature of 50-60 DEG C, the reaction fluidis filtered so as to remove the residual potassium carbonate after the reaction is end, the filtrate is concentrated to be dry, then an organic solvent is added, the mixture is subjected to beating for 5-6 hours at the temperature of 60-70 DEG C, then the product is centrifugated when the temperature is decreased to be 20-30 DEG C, and a compound 2 is obtained; and b, the residual mother liquor being centrifugated in the last step is collected and concentrated to be dry, tetrahydrofuran, di-tert-butylphenylphosphine and an assistant with the weight being 5 times of the concentrated crude product are added to react for 1-2 hours at the temperature of 50-65 DEG C, and the catalyst palladium, dichlorobis(di-tert-butylphenylphosphine) can be obtained. The preparation technology is simple, thereaction yield is high, the production cost is lowered, and the method is suitable for industrialized production.
Owner:NANTONG WANNIANCHANG PHARMA
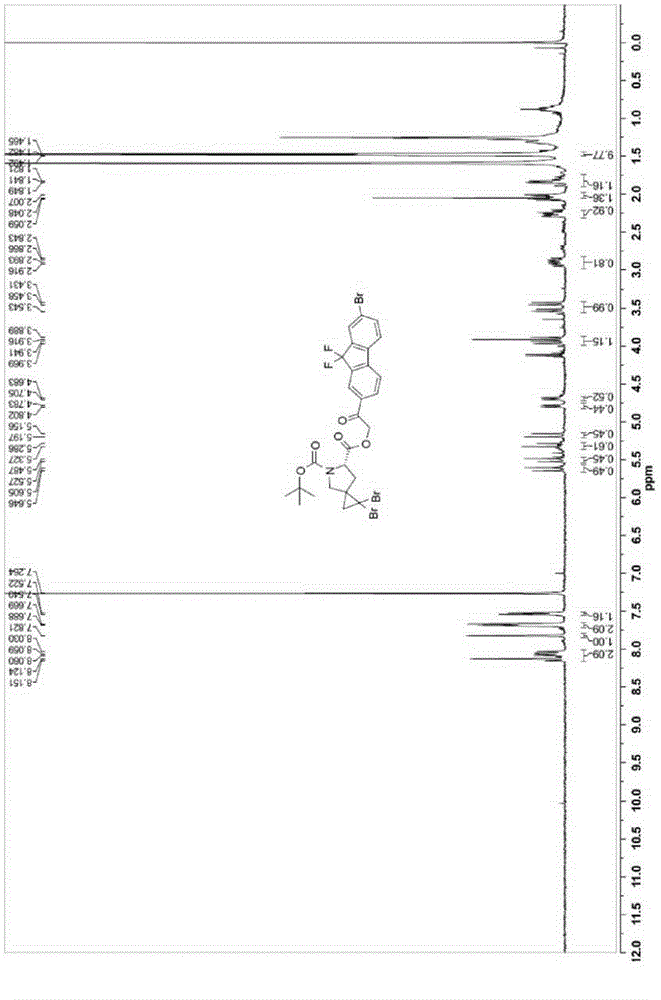
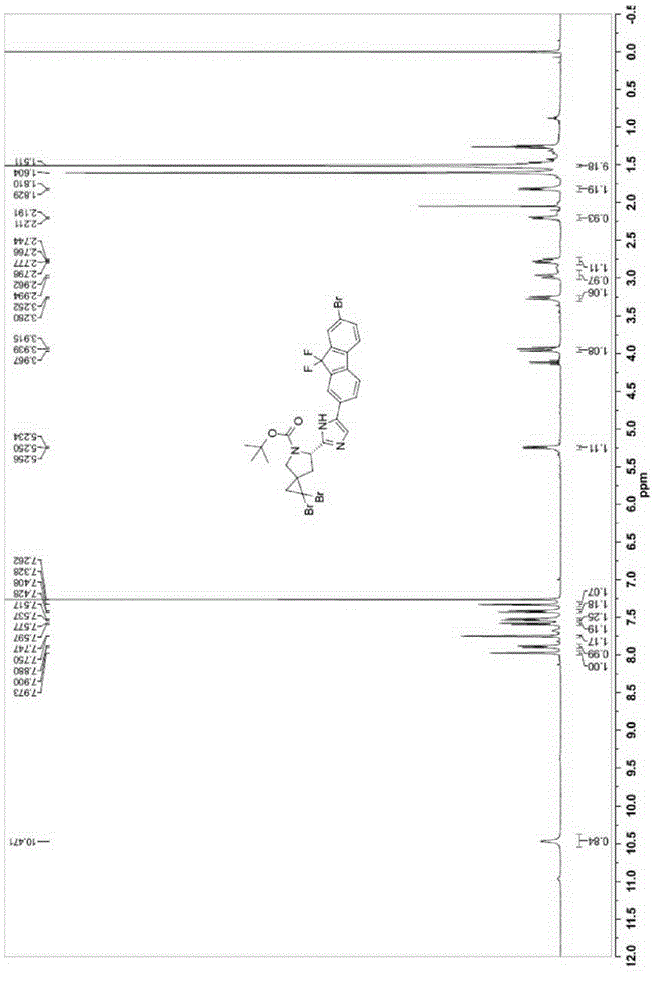
Synthesis technology for ledipasvir intermediate
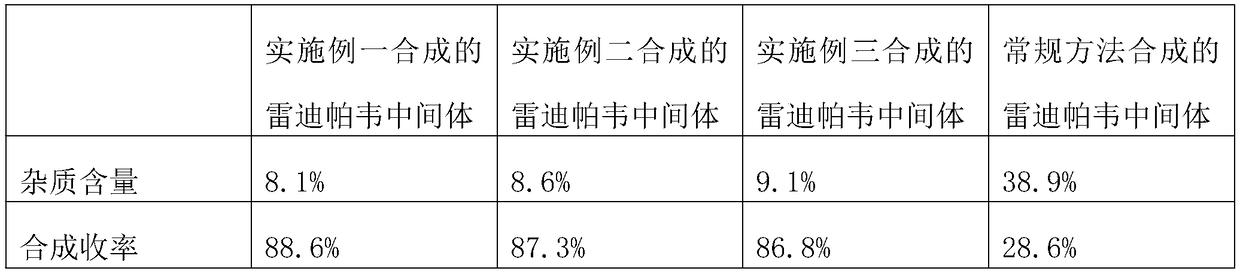
The invention discloses a synthesis technology for a ledipasvir intermediate. The synthesis technology is carried out through the steps of raw material selection and ledipasvir intermediate synthesis.The synthesis technology has the beneficial effects that synthesis steps are simple, impurity contents generated during the ledipasvir intermediate synthesis can be reduced so as to bring conveniencefor subsequent reaction, the synthesis efficiency of a ledipasvir finished product is improved, the yield of ledipasvir is improved, and therefore, the synthesis technology is favorable for realizingmass production and is also favorable for promotion, has a good use effect, and cost is lowered.
Owner:慎终(上海)生物科技有限公司
Crystalline ledipasvir compound and process for its preparation
InactiveCN105237517BCrystal stableEasy to transportOrganic active ingredientsOrganic chemistry methodsLedipasvirActive ingredient
Owner:NANJING CHIA TAI TIANQING PHARMA +1
Synthesis process of ledipasvir intermediate
ActiveCN112851545AAffect yieldHigh yieldPhysical/chemical process catalystsOrganic chemistry methodsPtru catalystModified carbon
The invention discloses a synthesis process of a ledipasvir intermediate, wherein the intermediate is prepared by taking alpha-phenylethylamine and o-nitroaniline as raw materials, the two raw materials are low in price and easy to store, the alpha-phenylethylamine is further made into an intermediate 4, enamine is effectively prevented from reverse reaction under an acidic condition, and then the yield of the ledipasvir intermediate is affected; in addition, a catalyst is prepared in the process of preparing the ledipasvir intermediate, the catalyst takes a carbon nanotube as a carrier, the surface of the carbon nanotube contains a large number of active groups under the action of mixed acid, and the modified carbon nanotube is prepared; the modified carbon nanotube is soaked in an ethanol solution of palladium chloride and ruthenium chloride, so that the surface of the modified carbon nanotube contains a large amount of palladium and ruthenium; and compared with a traditional catalyst, the catalyst can be repeatedly used and is more convenient to separate from a reaction solution, the treatment difficulty of industrial wastewater is greatly reduced, and environmental pollution is prevented.
Owner:日照巴洛特药业有限公司
Method for reducing diastereomer impurity content in ledipasvir intermediate
ActiveCN105418477BGentle method of purificationReduce manufacturing costOrganic chemistryOrganic solventTert-Butyloxycarbonyl protecting group
The invention discloses a method for reducing content of a diastereoisomer impurity (Ia) in a Ledipasvir intermediate (1R,3S,4S)-N-t-butyloxycarboryl-2-azabicyalo[2.2.1]heptane-3-carboxylic acid (I). The method comprises the steps of firstly taking a crude product of a compound shown as a formula (I) and containing the diastereoisomer impurity (Ia), dissolving in an organic solvent, adding alkaline organic amine to react with the crude product, separating out a solid, and filtering to obtain an amine salt of the compound shown as the formula (I); acidizing the obtained amine salt in an aqueous phase solution; extracting an obtained aqueous phase, separating out a solid, and separating to obtain the high-purity Ledipasvir intermediate (I). The product obtained by the method has a de value up to more than 99.5 percent, and the yield of more than 80 percent; reaction conditions in the method are mild, raw materials are easy to get, and the method is suitable for industrial application.
Owner:ASTATECH CHENGDU BIOPHARM CORP
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
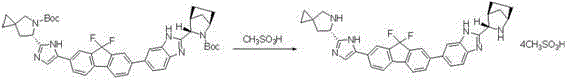
![Preparation method of ledipasvir intermediate (1R,3R,4R)-2-Boc-2-azabicyalo [2,2,1] pentane-3-carboxylic acid Preparation method of ledipasvir intermediate (1R,3R,4R)-2-Boc-2-azabicyalo [2,2,1] pentane-3-carboxylic acid](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/f289626a-4120-4239-9635-706b9dcb9343/BDA0001021092410000011.PNG)
![Preparation method of ledipasvir intermediate (1R,3R,4R)-2-Boc-2-azabicyalo [2,2,1] pentane-3-carboxylic acid Preparation method of ledipasvir intermediate (1R,3R,4R)-2-Boc-2-azabicyalo [2,2,1] pentane-3-carboxylic acid](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/f289626a-4120-4239-9635-706b9dcb9343/BDA0001021092410000021.PNG)
![Preparation method of ledipasvir intermediate (1R,3R,4R)-2-Boc-2-azabicyalo [2,2,1] pentane-3-carboxylic acid Preparation method of ledipasvir intermediate (1R,3R,4R)-2-Boc-2-azabicyalo [2,2,1] pentane-3-carboxylic acid](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/f289626a-4120-4239-9635-706b9dcb9343/BDA0001021092410000031.PNG)