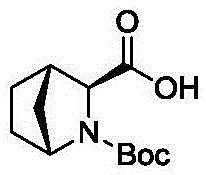
Preparation method of high-purity Ledipasvir intermediate
An intermediate and high-purity technology, applied in the field of medicine, to achieve safe production process, high reaction yield, and improve the effect of drug safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
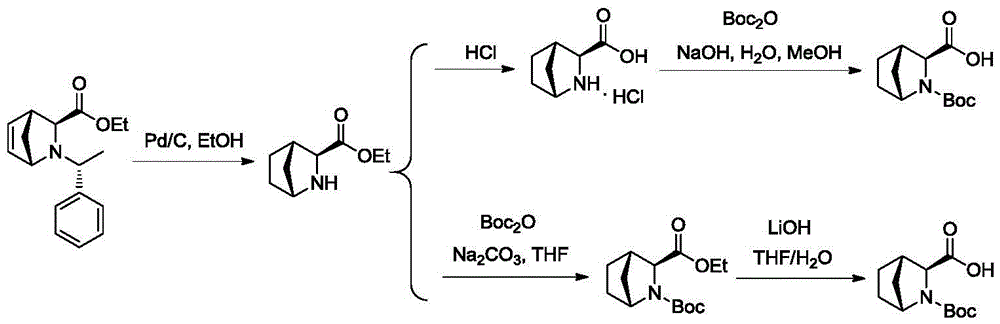
[0060] 1) Preparation of (1R,3S,4S)-N-tert-butoxycarbonyl-2-azabicyclo[2.2.1]heptane-3-carboxylic acid: take compound 1 (50g), methanol (250g) and Pd / C (5% w / w, 9.8g), replaced with nitrogen, filled with hydrogen to 0.8-1.0MPa, reacted at 45-55°C for a period of time (2-4 hours), concentrated, added 100g methanol, 200g water, and used carbonic acid Adjust the pH to 8 with sodium, keep the temperature below 5°C, slowly add di-tert-butyl dicarbonate (86g) dropwise, and stir for half an hour to react. Rise naturally to room temperature, add DCM for extraction, collect the organic phase, concentrate to obtain compound 3 (28g, yield=77%, de is 80-86%), repeat three times, the results of the three batches are as follows: The table below Show:
[0061]
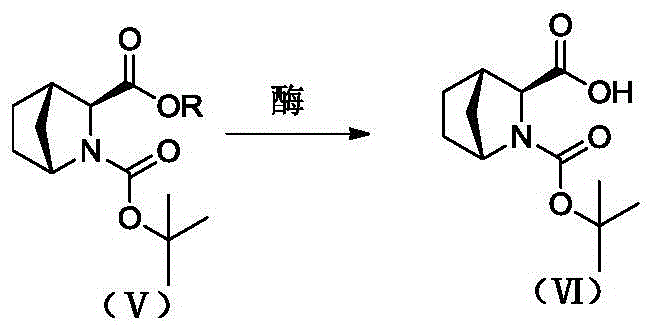
[0062] 2) Preparation of optically pure (1R,3S,4S)-N-tert-butoxycarbonyl-2-azabicyclo[2.2.1]heptane-3-carboxylic acid: Compound 3 (10g, 39mmol), water (50mL) , triethylamine (1.2 mL), and porcine pancreatic lipase (50 mg) were...
Embodiment 2
[0069] Compound 3 (10g, 39mmol, de 83%), water (50mL), triethylamine (6mL), and porcine pancreatic lipase (55mg) were added to a 100mL three-neck flask, and reacted at 55°C for 24 hours. After the reaction is complete, extract the reaction liquid with dichloromethane (50 mL), collect the water phase, continue to add an equal volume of dichloromethane to the water phase, cool down to 0-10 ° C, add a certain amount of 6N HCl, and adjust the pH to about 2 . The organic phase was collected, concentrated until a solid precipitated, cooled to room temperature, crystallized by adding petroleum ether (5 mL), suction filtered, and dried to obtain a white solid 6 (7.18 g). The yield was 69.2%, and the de was 99.7%. The isomer impurity content was 0.17%. The white solid was confirmed to be the target product by structural characterization, and the specific data are as follows:
[0070] Mass Spectrum: MS (M-H+): m / z 240.3.
[0071] 1 HNMR(400MHz,DMSO-d6):δ12.45(s,1H),4.12-4.05(d,1H),3...
Embodiment 3
[0072] The screening of embodiment 3 enzymes
[0073] Compound 3 (10g), water (50mL), and triethylamine (1.2mL) were added to a 100mL three-necked flask, and different hydrolytic enzymes were added, and reacted at 50°C for 24 hours, and the reaction results as table 1 .
[0074] Table 1 Hydrolysis of compound 3 catalyzed by different hydrolases
[0075]
[0076] The results show that the above hydrolases can effectively obtain products with high de value, and the de value is 98.6%-99.7%. Among them, porcine pancreatic lipase and Novozymes 435 are better, and the yields are both greater than 70%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com