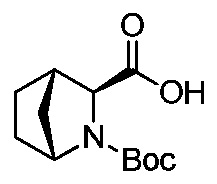
Method for reducing diastereomer impurity content in ledipasvir intermediate
A technology of diastereoisomers and enantiomers, which is applied in the field of diastereoisomer impurity content, achieves the effects of mild method, reduced production cost, and simple post-treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
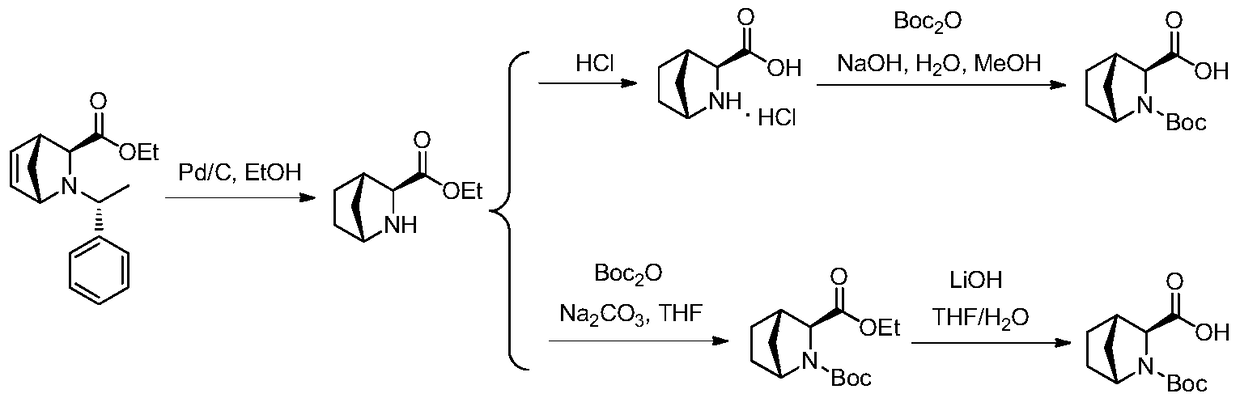
[0043] Example 1 Preparation of crude compound of formula (I)
[0044] Route 1:
[0045] Using methyl ester compound (SM) as the starting material, the specific route is as follows:
[0046]
[0047] Take the starting materials SM (500g), methanol (2.5kg) and Pd / C (5%w / w, 100g), replace with nitrogen, fill with hydrogen to 0.8-1.0MPa, react with 45-55℃ for 20 hours, concentrate, Add 1Kg methanol, 2Kg water, adjust the pH to 8 with sodium carbonate, keep the temperature below 5°C, slowly add di-tert-butyl dicarbonate (90g) dropwise, stir and react for 2 hours, naturally warm to room temperature, add DCM (dichloro Methane) extraction, collecting the organic phase, and concentrating to obtain 300 g of the crude product of compound 3.
[0048] The crude compound 3 (300g), water (1.5Kg), LiOH (50g) and tetrahydrofuran (1.5L) were added to a 5L three-necked flask and reacted at 50°C for 24 hours. The reaction solution was washed with 1L of dichloromethane. Cool the water phase to 0-10°C, ...
Embodiment 2
[0054] Example 2 Purification of the compound of formula (I)
[0055] The specific route is as follows:
[0056]
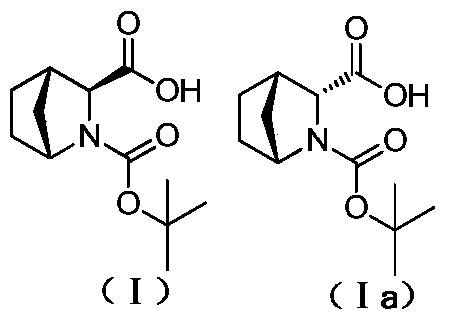
[0057] The crude product (10g) of the compound of formula (I) obtained in Example 2 was dissolved in 30mL of ethanol, the temperature was raised to 80°C, and (R)-(+)-1-phenylethylamine (5.2g) was added and stirred evenly. React for 2h, cool to 20℃, filter with suction, the resulting filter cake is dissolved in 100mL of 0.5N hydrochloric acid solution at 0℃, adjust the pH=1 to 1.5, add an equal volume of dichloromethane for extraction, take the organic phase, concentrate, and 20℃ Crystallized and filtered to obtain 8.1 g of white solid, yield 81%, de value 99.8%, content of diastereomeric impurity (Ia) 0.1%; mass spectrum: MS (M-H+): m / z 240.3. 1 HNMR (400MHz, DMSO-d6): δ 12.47 (s, 1H), 4.12-4.05 (d, 1H), 3.60 (s, H), 2.58 (s, 1H), 1.73-1.38 (m, 5H), 1.31 (s, 4H), 1.25 (s, 3H), 1.23-1.20 (t, 1H).
[0058] The diastereoisomeric impurities (Ia) (1R, 3R, 4S)-N-tert-butoxyc...
Embodiment 4
[0059] Example 4: Preparation of pure compound of formula (I)
[0060] The crude product (10g) of the compound of formula (I) obtained in Example 1 was dissolved in 80mL ethyl acetate, the temperature was raised to 70°C, (R)-(+)-1-phenylethylamine (7.5g) was added and stirred The reaction is uniform, the reaction is 2h, the temperature is lowered to 40°C, and the filter cake is filtered by suction. The resulting filter cake is dissolved in 150mL of 0.1N sulfuric acid solution at 0°C, adjusted to pH=1-1.5, and 100mL of 1,2-dichloroethane is added to the water phase. After extraction, the organic phase was taken, concentrated, crystallized at 0°C, and filtered to obtain 8.5 g of white solid, with a yield of 85%, a de value of 99.8%, and a diastereomeric impurity (Ia) content of 0.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com