Ledipasvir intermediate monosulfate, crystalline form thereof and preparation method therefor
A kind of intermediate, sulfate technology, applied in its crystal form and amorphous and its preparation, ledipasvir intermediate monosulfate field, can solve the problems such as poor crystallinity of formula II compound, difficult to form salt and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0078] The present invention provides a method for preparing the compound of formula I, comprising preparing the compound of formula I in amorphous and crystalline forms.
[0079] Since the crystal form has higher purity (eg ≥99%), it is preferred to prepare the crystal form in the present invention and use this crystal form as the raw material of ledipasvir.
[0080] One way to prepare the crystal form is to prepare the amorphous compound of formula I first, for example, those skilled in the art can prepare the amorphous compound of formula I with reference to the teachings of the present invention, and then use the amorphous compound to prepare the compound of formula I Crystalline raw materials to obtain crystalline products, such as crystal form I.
[0081] Another method for preparing crystal forms is to react the compound of formula II with an appropriate amount of sulfuric acid, and directly form the compound of formula I in crystal form during or after the reaction.
...
Embodiment 1
[0102] The preparation of embodiment 1 formula I compound (amorphous substance)
[0103] Weigh 37mg of the compound of formula II into a 1.5ml centrifuge tube, add 0.1mL of THF, sonicate until dissolved, add 0.1mL of 0.5M sulfuric acid in acetonitrile, immediately a white solid precipitates.
[0104] Part of the white solid precipitate was taken and identified by a polarizing microscope and confirmed to be an amorphous form.
Embodiment 2
[0105] Preparation of Embodiment 2 Formula I Compound (Crystal Form I)
[0106] (1) Weigh 37mg of the compound of formula II into a 1.5ml centrifuge tube, and add 0.1mL of ethanol. Sonicate until dissolved. Add 0.1 mL of 0.5M sulfuric acid in ethanol. The reaction mixture was left at room temperature for 6 hours and a solid precipitated out. Part of the solid precipitate was taken and identified as crystal by polarizing microscope.
[0107] (2) Place the centrifuge tube containing the centrifuge in a centrifuge, centrifuge at 12,000 rpm for 5 minutes, remove the supernatant, and dry the separated solid at room temperature for 1 hour.
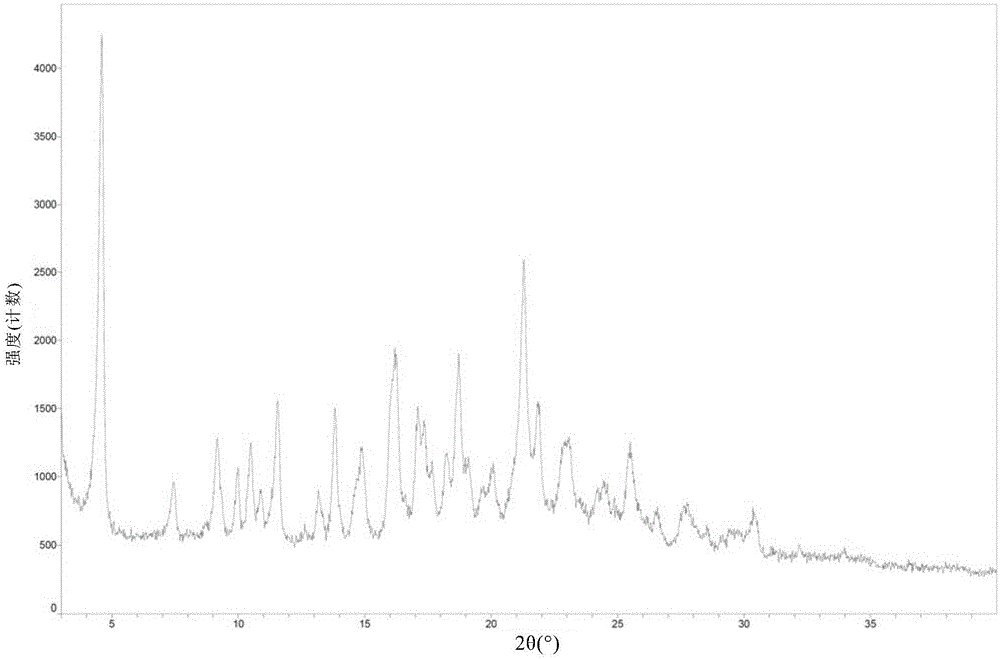
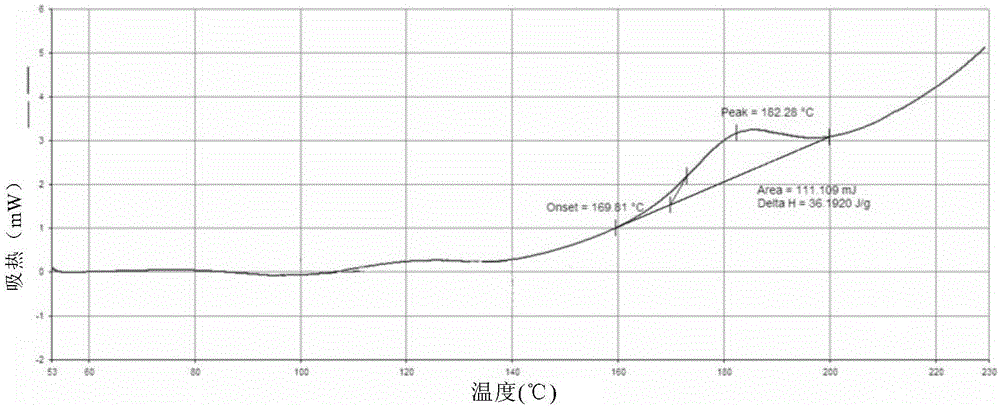
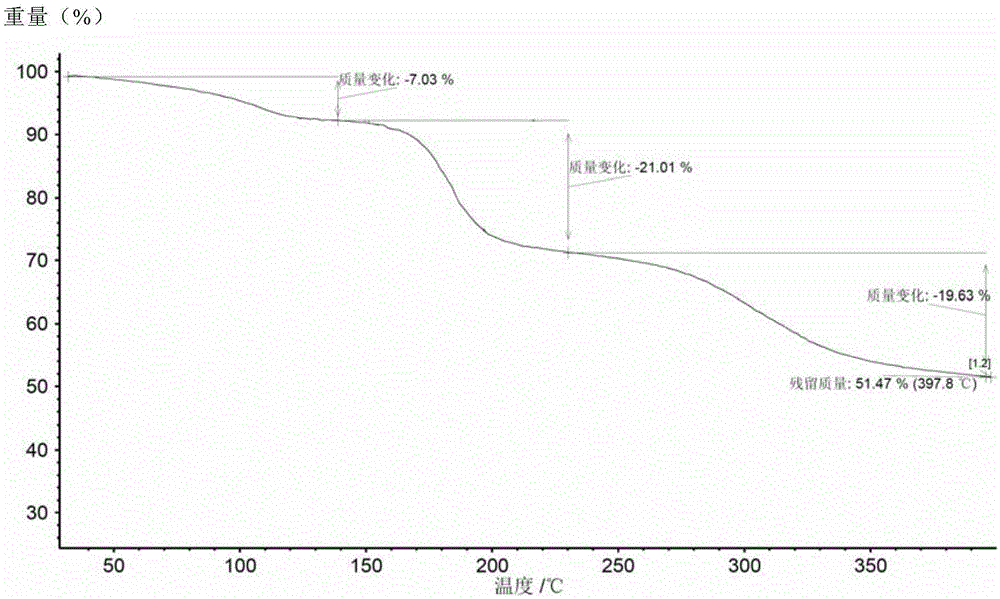
[0108] (3) Test the sulfate content of the dried solid by ion chromatography, and characterize the solid form by XRPD, DSC and TGA.
[0109] result
[0110] (1) The ion chromatographic analysis results show that the sulfate content in the crystal is basically consistent with the theoretical content (11.0 wt%) of the sulfate in the compound ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com