Method for preparing Ledipasvir and intermediates of method for preparing Ledipasvir
A process method and a technology of chemical formula, applied in the field of pharmaceutical synthesis, to achieve the effects of easy purification operation process, high product quality and yield, and easy recrystallization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
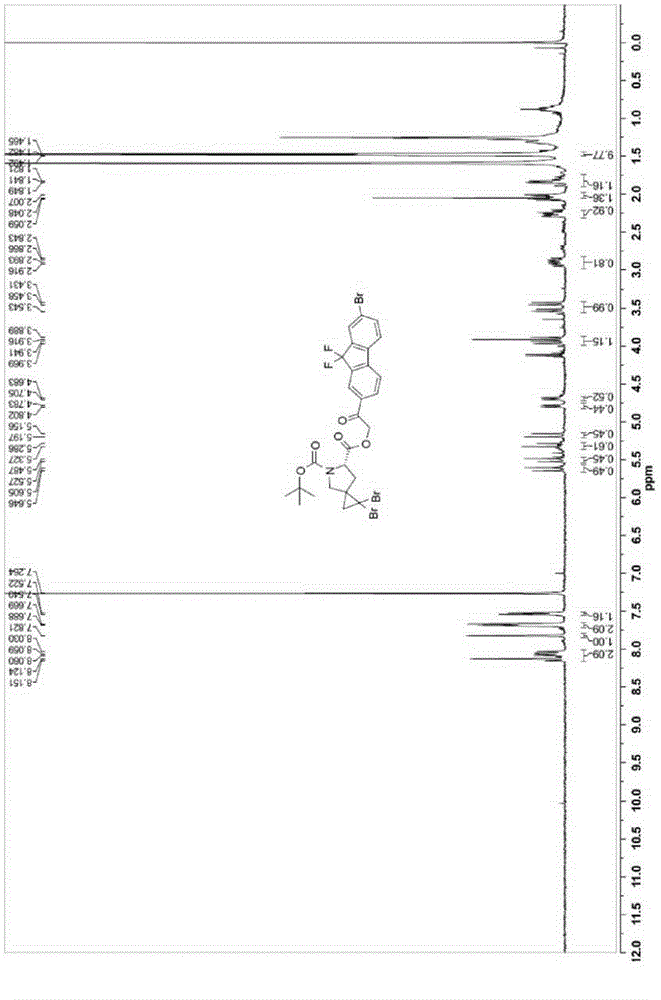
[0064] (6S)-6-[2-(7-Bromo-9,9-difluoro-9H-fluoren-2-yl)-2-oxoethyl]-5-tert-butyl-1,1-dibromo- Preparation of 5-azaspiro[2.4]heptane-5,6-dicarboxylate (4):
[0065]
[0066] Compound 2 (200 mg, 0.5 mmol) and compound 3 (180 mg, 0.5 mmol) were dissolved in acetone (5 mL), and sodium bicarbonate (130 mg, 1.5 mmol) was added. The reaction was stirred at 60°C overnight. Filter and concentrate the filtrate. The obtained concentrate was dissolved in ethyl acetate (20 mL), washed with water (15 mL) and saturated brine (15 mL), dried over sodium sulfate, and filtered. The filtrate was concentrated. The crude product was recrystallized from ethyl acetate and n-hexane (volume ratio 1:10 to 1:1) to obtain target compound 4. Yield: 280 mg (77%, 90:10 diastereomers). MS(M+1): 722. 1 H-NMR (400MHz, CDCl 3 ),δ8.15-8.03(m,2H),7.82(s,1H), 7.68(d,J=7.6Hz,1H), 7.54(d,J=7.2Hz,1H), 5.64(d,J= 16.4Hz,0.5H), 5.52(d,J=16.4Hz,0.5H), 5.32(d,J=16.4Hz,0.5H), 5.19(d,J=16.4Hz,0.5H), 4.80(d, J=7.6Hz,0.5H)...
Embodiment 2
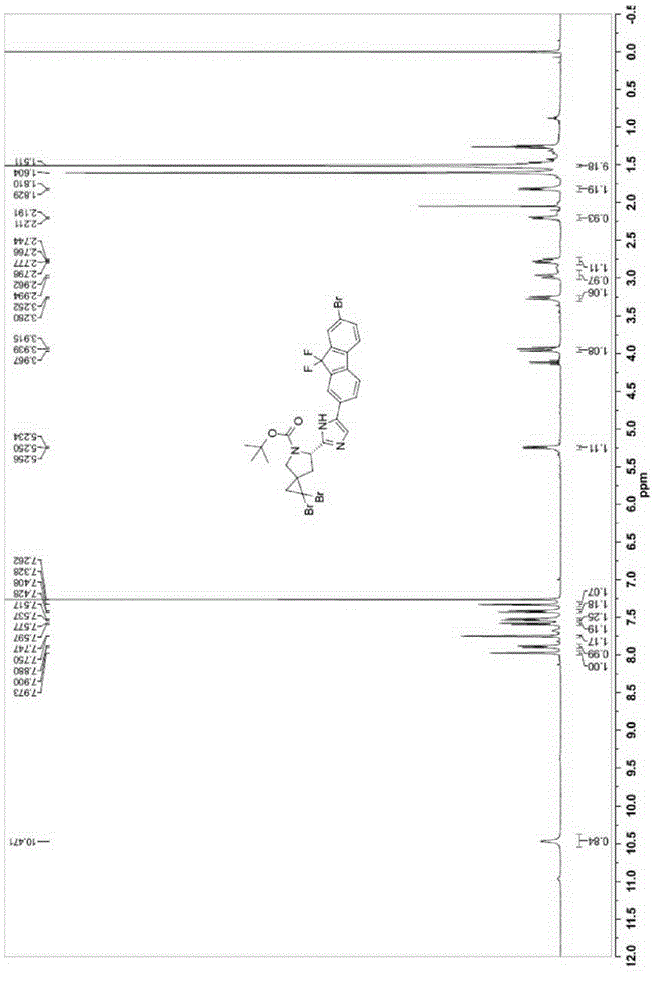
[0068] (6S)-tert-butyl-1,1-dibromo-6-[5-(7-bromo-9,9-difluoro-9H-fluoren-2-yl)-1H-imidazol-2-yl)- Preparation of 5-azaspiro[2.4]heptane-5-carboxylate (5):
[0069]
[0070] Compound 4 (1.65 g, 2.3 mmol) was dissolved in toluene (17 mL), and ammonium acetate (1.06 g, 13.75 mmol) was added. The reaction mixture was heated to 90°C and reacted overnight for about 16 hours. TLC showed that compound 4 was completely converted. Cool to room temperature, add ethyl acetate (50 mL) to dilute, and wash with water (50 mL) and saturated brine (50 mL) respectively. Dry with anhydrous sodium sulfate and filter. The filtrate was concentrated. The obtained crude product was recrystallized with ethyl acetate and n-hexane (volume ratio 1:10 to 1:1) to obtain the target compound 5. Yield: 1.2 g (75%, 90:10 diastereomers). MS(M+1): 702. 1 H-NMR (400MHz, CDCl 3 ),δ10.47(br.1H),7.97(s,1H),7.90(d,J=8Hz,1H),7.75(s,1H),7.59(d,J=8Hz,1H),7.53(d ,J=8Hz,1H),7.42(d,J=8Hz,1H),7.32(s,1H), 5.25(d,J=8.8Hz,1H...
Embodiment 3
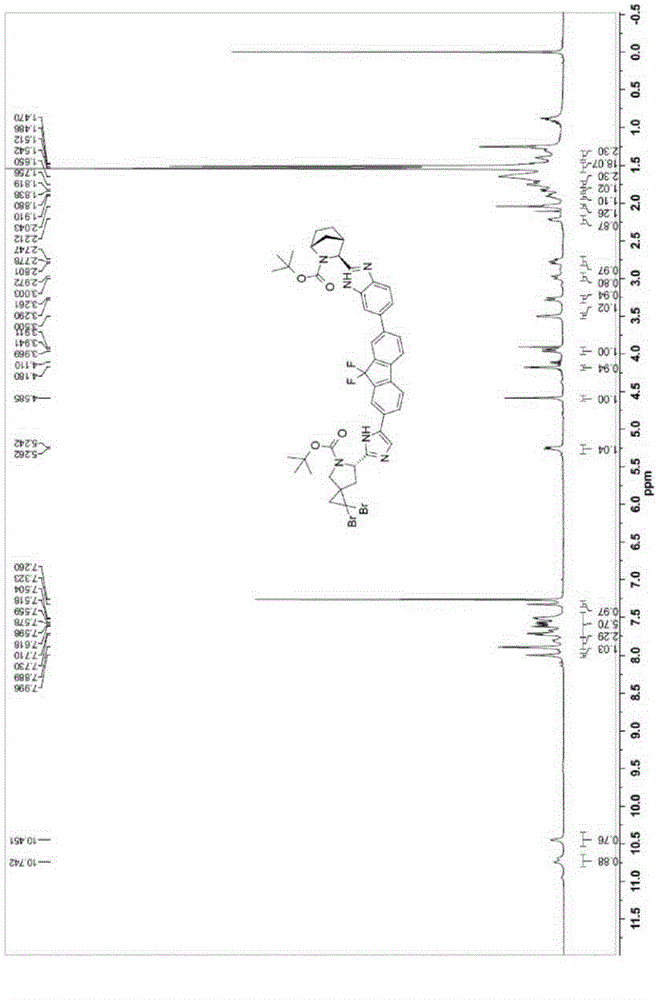
[0072] (1R,3S,4S)-tert-butyl-3-(6-(7-(2-((6S)-1,1-dibromo-5-(tert-butoxycarbonyl)-5-azaspiro[ 2.4)Heptane-6-yl)-1H-imidazol-5-yl)-9,9-difluoro-9H-fluoren-2-yl)-1H-benzimidazol-2-yl)-2-aza Preparation of bicyclo[2.2.1]heptane-2-carboxylate (7):
[0073]
[0074] Compound 5 (1.5g, 2.14mmol), compound 6 (1.14g, 2.57mmol), bis(di-tert-butylphenylphosphine) palladium dichloride (118mg, 0.19mmol) were dissolved in 15mL of isopropyl acetate , 1M aqueous potassium phosphate solution (7.5 mL) was added. The reaction system was replaced with nitrogen and heated to 75-80°C for 2 hours. The reaction mixture was cooled to room temperature and extracted with ethyl acetate (50 mL×2). The organic layers were combined, washed with saturated brine (50 mL), and dried over sodium sulfate. Filter and concentrate the filtrate. The crude product was recrystallized with ethyl acetate and n-hexane (volume ratio 1:10 to 1:1) to obtain target compound 7. Yield: 1.15 g (57.5%, 90:10 diastereomers). M...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com