Sofosbuvir and ledipasvir compound preparation
A technology of compound preparations and solubilizers, which is applied in the direction of medical preparations of non-active ingredients, antiviral agents, pill delivery, etc. It can solve the problems of cumbersome preparation methods, unsuitability for large-scale industrial production, poor dissolution rate of ledipasvir, etc. problems, achieve quality control, overcome dissolution problems, and improve initial dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
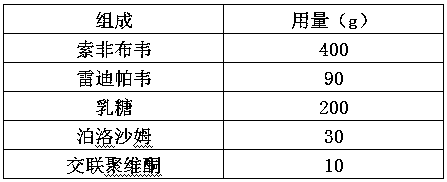
Examples
Embodiment 1
[0027]
[0028] Redipavir d0.9=20μm
[0029] Preparation:
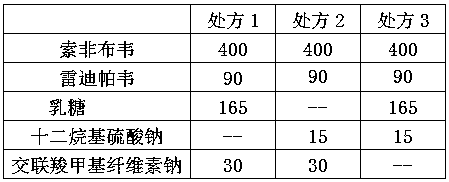
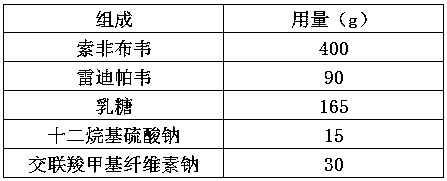
[0030] a. Mix sofosbuvir, ledipasvir, lactose, sodium lauryl sulfate, and croscarmellose sodium evenly to prepare a mixture;
[0031] b. dry granulating the mixture in step a to obtain drug granules;
[0032] c. Compressing the drug granules into tablets.
Embodiment 2
[0034]
[0035] Redipavir d0.9=25μm
[0036] The preparation method is the same as in Example 1.
Embodiment 3
[0038]
[0039] Redipavir d0.9=30μm
[0040] The preparation method is the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com