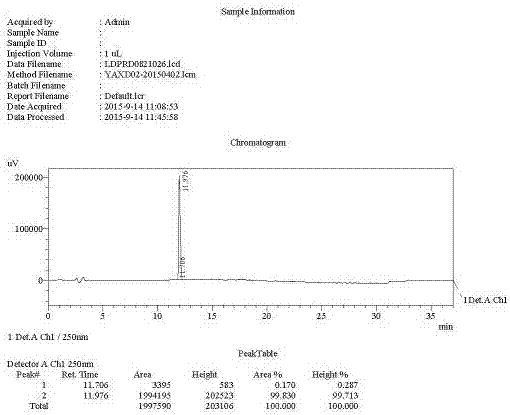
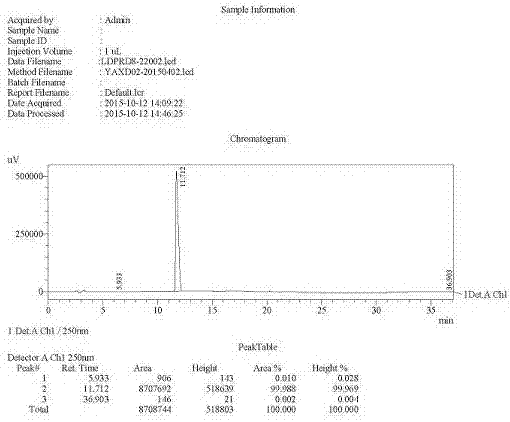
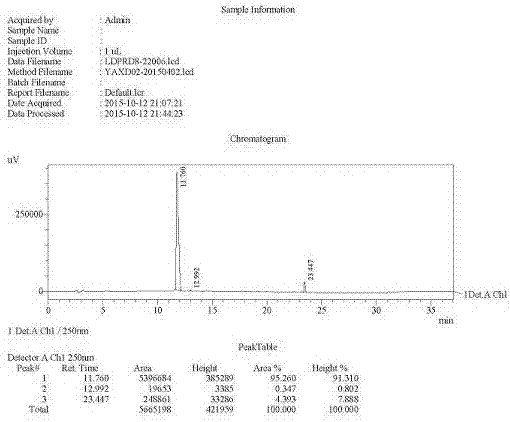
Preparation of key intermediates of ledipasvir
A Chinese-style and systematic technology, applied in organic chemistry and other directions, can solve problems such as affecting the quality of finished drugs, difficult to remove disubstituted impurities, and unstable compounds.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Preparation of compound (X) (X = Br): Add IV (10.0g, 0.041mol, 1.0eq) and anhydrous CH to a clean four-neck flask 2 Cl 2 (50 mL), turn on magnetic stirring (nitrogen protection). Add DMAP (20mg, 0.16mmol) and 4-bromo-2-nitroaniline (9.8g, 0.045mol), stir well after adding, add hydroxybenzotriazole (HOBT) (6.1g, 0.045mol) and 1- Ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC .HCl) (8.6 g, 0.045 mol). Stir evenly after the addition, cool the reaction system to 5°C, then slowly add triethylamine (TEA) (10.4g, 0.10mol), and keep the reaction temperature not higher than 10°C during the dropwise addition. After the dropwise addition, the reaction solution was stirred at room temperature for 20 h. After the reaction was completed, the reaction solution was added with H 2 The reaction was quenched with O (50 mL) and the organic phase was separated. CH for aqueous phase 2 Cl 2 (3×40 mL) for extraction; the organic phases were combined, washed with 1M HCl (...
Embodiment 2
[0027] Preparation of compound (X) (X = Cl): add anhydrous CH to a 250mL three-neck flask 2 Cl 2 (90 mL), methyl chloroformate (7.5 g, 0.079 mol) and IV (18.0 g, 0.075 mol, 1.0 eq). After the addition, stir until it dissolves. Slowly cool the system down to 0°C, then slowly add diisopropylethylamine (DIPEA, 10.7g, 0.083mol), and keep the system temperature below 5°C during the dropwise addition. After the dropwise addition, slowly add 4-chloro-2-nitroaniline (14.2g, 0.083mol) in CH 2 Cl 2 (90mL) solution, keep the temperature of the system not exceeding 10°C during the dropwise addition, and it takes 1h. After the dropwise addition, the system was naturally raised to room temperature and stirred for 3 h. Add H2O (100mL) to the reaction system to quench the reaction, let stand to separate the organic phase, and the aqueous phase with CH 2 Cl 2 (3 x 90 mL) extraction. The organic phases were combined and washed with 1M HCl (100 mL) and then with saturated brine, and th...
Embodiment 3
[0029] Preparation of compound (XI) (X = Br): Add DMF (60mL), 5-bromo-2-nitroaniline (8.4g, 0.039mol), IV (8.5g, 0.035mol, 1.0eq) and N-methylmorpholine (7.8g, 0.078mol). After the reaction system was cooled to 0°C with ice-brine, 2-(7-azobenzotriazole)-N,N,N',N'-tetramethyluronium hexafluorophosphate (HATU, 14.6g, 0.038mol ). Subsequently, the reaction was naturally raised to room temperature for 3 h. After the reaction system was concentrated under reduced pressure, the residue was added to H 2 O (50mL) and CH 2 Cl 2 (50mL). Separate the organic phase and use CH for the aqueous phase 2 Cl 2 (3 x 50 mL) extraction. The organic phases were combined and washed with 1M HCl (30 mL) and then with saturated brine, and the organic phase was dried over anhydrous sodium sulfate. After filtration, the filtrate was concentrated to dryness under pressure at 40°C, and the residue was purified by silica gel column chromatography (petroleum ether / ethyl acetate, 5 / 1, then 2 / 1) to o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com