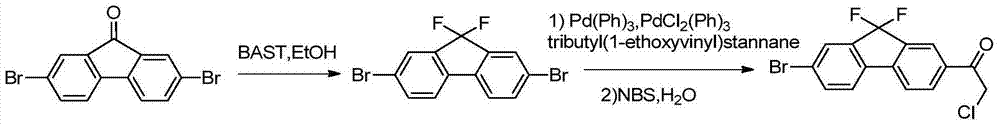
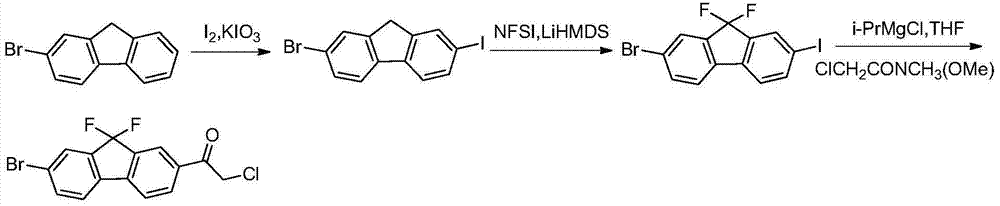
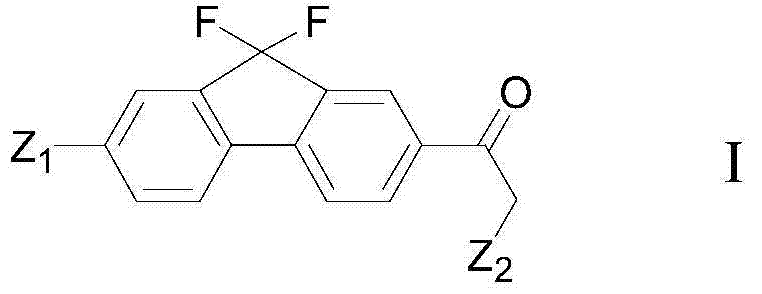
Preparation method of fluorene ethyl ketone derivative
A compound and inert solvent technology, applied in the field of preparation of ledipasvir synthesis intermediates, can solve the problems of expensive Weinreb amides, unsuitable for industrial production, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0117] The present invention also provides a preparation method of a compound of formula I, said method comprising the steps of:
[0118]
[0119] (iv) deprotecting with the compound of formula VII in an inert solvent to obtain the compound of formula I; wherein, the definition of each group is as described above.
[0120] The deprotection is preferably carried out by a hydrolysis reaction; preferably, the deprotection in the step (iv) is carried out under acid catalysis;
[0121] The acid is not particularly limited, and is preferably an acid selected from the following group: H 2 SO 4 , HX, H 3 PO 4 , polyphosphoric acid, HNO 3 、CH 3 SO 4 、CF 3 SO 4 , HOAc, CF 3 COOH, Sulfonic acid ion exchange resin, HXO 4 , or a combination thereof; where, R 6 is H or C1-C4 alkyl; X is selected from the following group: Cl, Br, I.
[0122] In the above reaction, the raw material compound of formula VII can be prepared by the method described in the present invention, and can...
Embodiment 1
[0157] Synthesis of Example 1 Compound 4-1
[0158]
[0159] In a 250ml four-necked flask, add 16.2g (123mmol, 1.5eq) aluminum trichloride, 150ml dichloromethane, start stirring, slowly add 20g compound 2 (82mmol, 1.0eq), and stir for 30 minutes until most of the Aluminum trichloride dissolves.
[0160] The reaction mixture was cooled to 0°C, and 11g (98.4mmol, 1.2eq) of chloroacetyl chloride was slowly added dropwise. After the addition was complete, it was kept at 0°C for 1 hour, then naturally warmed to room temperature, and stirred for 2 hours. TLC traced until the disappearance of the starting material.
[0161] The reaction solution was poured into 100ml of 10% ice water-hydrochloric acid mixture, stirred for 30 minutes, and the organic layer was separated. The organic phase was washed with water, 10% sodium bicarbonate solution, saturated brine, dried over anhydrous sodium sulfate, and concentrated to obtain a crude product, which was recrystallized to obtain 19.3 ...
Embodiment 2
[0162] Synthesis of Example 2 Compound 4-2
[0163]
[0164] In a 250ml four-necked flask, add 16.2g (123mmol, 1.5eq) aluminum trichloride, 150ml dichloromethane, start stirring, slowly add 20g compound 2 (82mmol, 1.0eq), and stir for 30 minutes until most of the Aluminum trichloride dissolves.
[0165] The reaction mixture was cooled to 0°C, and 7.7g (98.4mmol, 1.2eq) of acetyl chloride was slowly added dropwise. After the dropwise addition, it was kept at 0°C for 1 hour, then naturally warmed to room temperature, and continued to stir for 2 hours. TLC traced until the disappearance of the starting material.
[0166] The reaction solution was poured into 100ml of 10% ice water-hydrochloric acid mixture, stirred for 30 minutes, and the organic layer was separated. The organic phase was washed with water, 10% sodium bicarbonate solution, saturated brine, dried over anhydrous sodium sulfate, and concentrated to obtain 22.0 g of compound 4-2 (yield: 93.8%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com