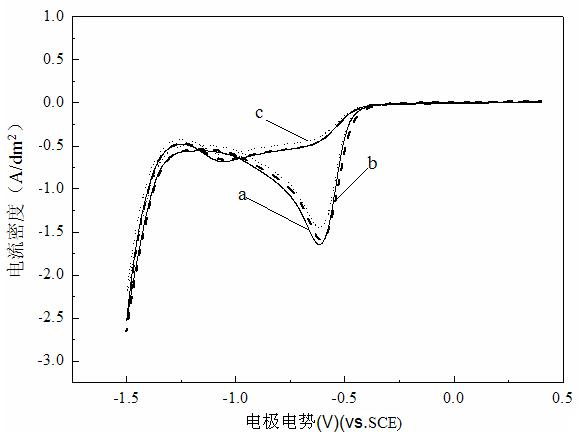
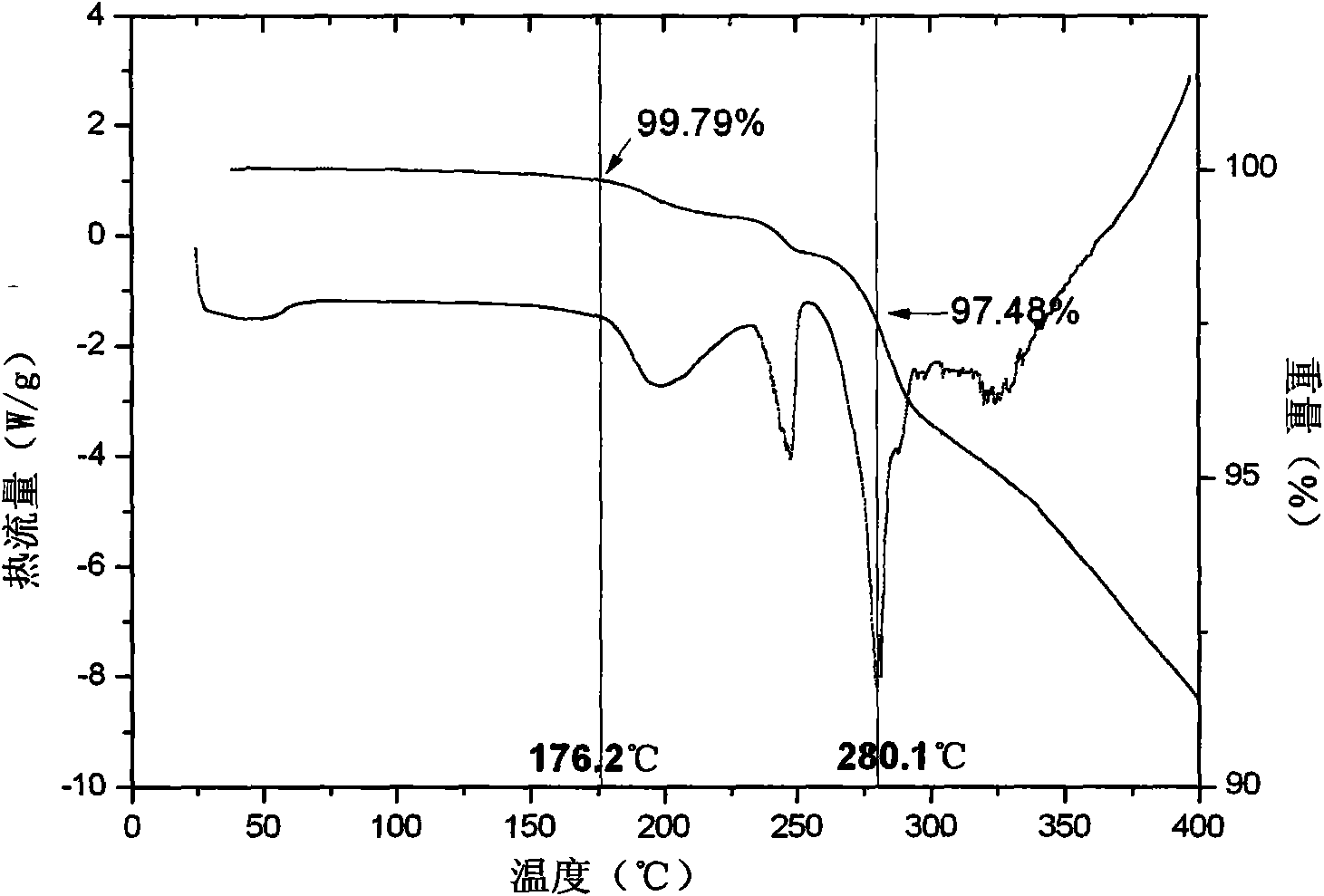
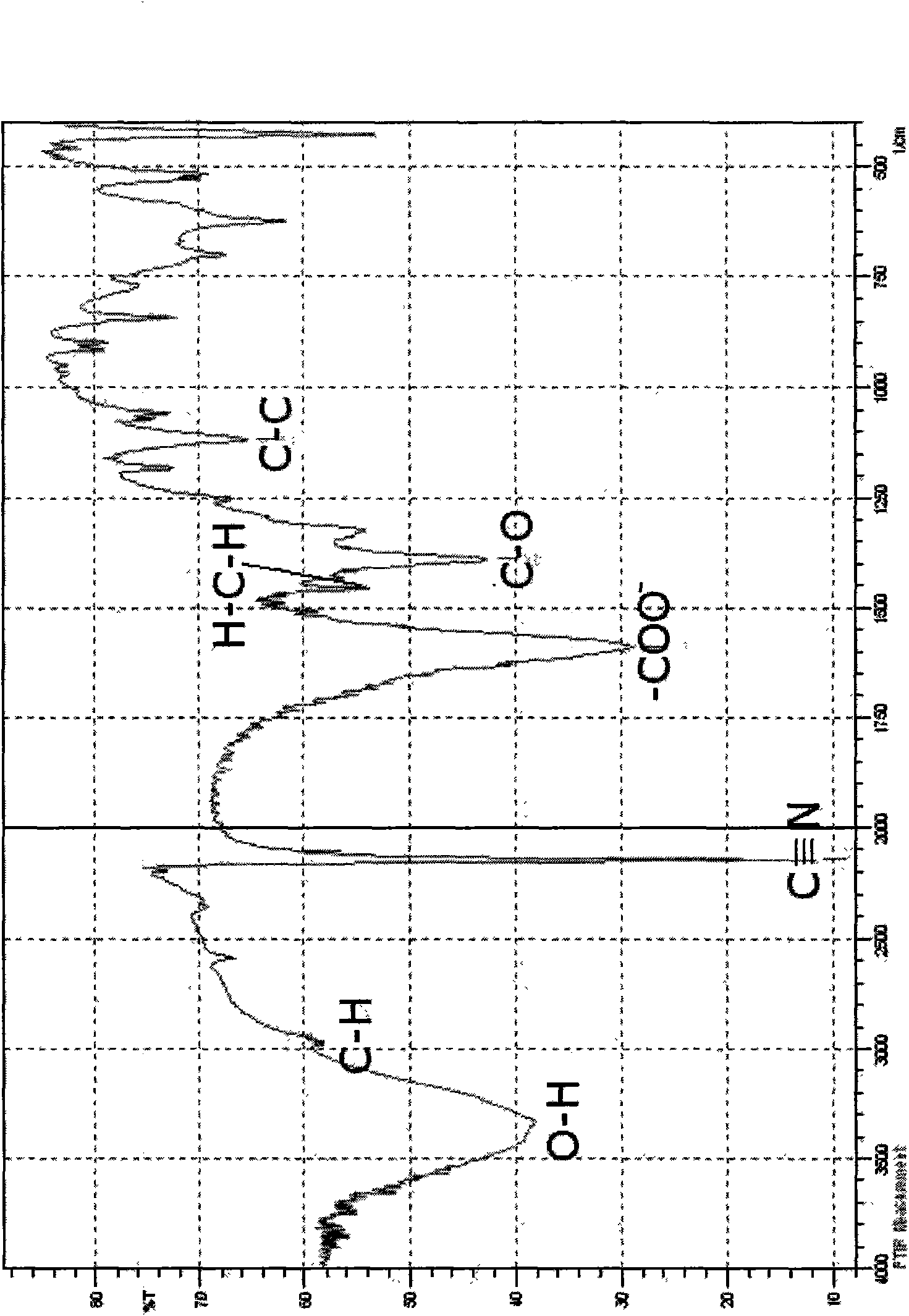
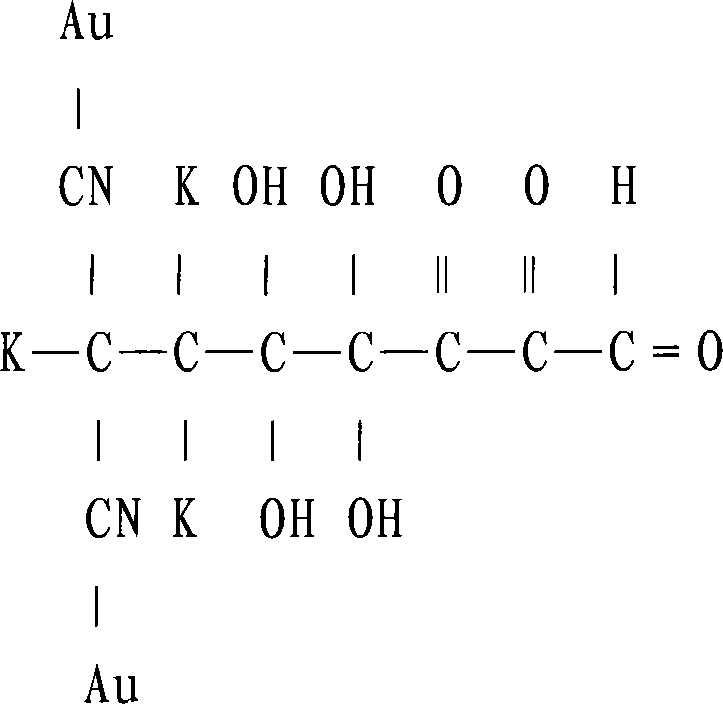Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
46 results about "Gold(III) chloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Gold(III) chloride, traditionally called auric chloride, is a chemical compound of gold and chlorine. With the molecular formula Au₂Cl₆, the name gold trichloride is a simplification, referring to the empirical formula, AuCl₃. The Roman numerals in the name indicate that the gold has an oxidation state of +3, which is common for gold compounds. There is also another related chloride of gold, gold(I) chloride (AuCl). Chloroauric acid, HAuCl₄, the product formed when gold dissolves in aqua regia, is sometimes referred to as "gold chloride" or "acid gold trichloride". Gold(III) chloride is very hygroscopic and highly soluble in water as well as ethanol. It decomposes above 160 °C or in light.
Cyanogens-free gold plating solution and method for plating gold by adopting same
The invention provides cyanogens-free gold plating solution and a method for plating gold by adopting the cyanogens-free gold plating solution, relating to a plating solution and a gold electroplating method. The invention solves the problems that cyanide is toxic and the stability of sulphite gold plating solution is poor. The cyanogens-free gold plating solution of the invention is composed of coordinating agent, auric chloride, potassium carbonate, potassium pyrophosphate and compound additive. The gold plating method of the invention includes the following steps: sodium hydroxide is used for regulating the pH value of cyanogens-free gold plating solution, then constant current mode is adopted, and plating is carried out under the conditions that the distance of cathode and anode is 5-20cm and the temperature is 30-60 DEG C. The cyanogens-free gold plating solution of the invention contains no highly toxic substance, and the stability of plating solution is good, the plating solution is not muddy or changed in colour when being used in 30 days (including plating and ingredient compensation). Meanwhile 10ml plating solution is used for continuous plating and 0.15Ah of electric quantity passes through, and a coating in good surface state still can be obtained.
Owner:WUXI HAITE NEW MATERIAL RES INST
Gold potassium lemon acid for gold plating and method for producing the same
ActiveCN101172946BReduce processing costsReduce pollutionCarboxylic acid salt preparationGold contentPotassium
The invention discloses citric acid gold potassium used for gold plating and the preparation method thereof. The molecular formular of the citric acid gold potassium is K3Au2C9H5O7N2. The method comprises the steps as follows: gold trichloride is dissolved in water of certain temperature, is concentrated and diluted under the temperature for multiple times, and is reacted with potassium citrate, ethylene diamine tetraacetic acid and malononitrile in a reactor under the certain temperature and the certain time to produce the organic gold salt which has low toxicity and contains no free cyanogen. When the product has the same gold content with the potassium gold cyanide, the total CN- is 5 to 6 percent, being 50 percent lower than that of the CN- in the potassium gold cyanide. The waste liquid after gold plating contains free the CN- in small amount. The invention reduces the cost of waste water treatment for the electroplating plants.
Owner:HENGSHENG TECH R&D CO LTD
Citric acid gold potassium for gilding and preparation method thereof
The invention relates to citric acid gold potassium for gilding and a preparation method thereof. The molecular formula of the citric acid gold potassium is KAu2N4C12H11O8. The citric acid gold potassium is prepared by dissolving auric chloride in water, concentrating and diluting for several times, and reacting the obtained liquid with sodium potassium, edetic acid and malononitrile in a reactor.The citric acid gold potassium has the advantages of low toxicity and no free cyanogens in the product. Compared with gold potassium cyanide, the total CN<-> content of the citric acid gold potassiumis 5-6 percent in case of the same gold content, and is above 50 percent lower than that in the gold potassium cyanide. The waste liquid after gilding only contains the micro-free CN<->, thereby thewaste water processing cost in electroplating enterprises is reduced.
Owner:HENGSHENG TECH R&D CO LTD
Gold potassium lemon acid for gold plating and method for producing the same
ActiveCN101172946AReduce processing costsReduce pollutionCarboxylic acid salt preparationGold contentPotassium
The invention discloses citric acid gold potassium used for gold plating and the preparation method thereof. The molecular formular of the citric acid gold potassium is K3Au2C9H5O7N2. The method comprises the steps as follows: gold trichloride is dissolved in water of certain temperature, is concentrated and diluted under the temperature for multiple times, and is reacted with potassium citrate, ethylene diamine tetraacetic acid and malononitrile in a reactor under the certain temperature and the certain time to produce the organic gold salt which has low toxicity and contains no free cyanogen. When the product has the same gold content with the potassium gold cyanide, the total CN- is 5 to 6 percent, being 50 percent lower than that of the CN- in the potassium gold cyanide. The waste liquid after gold plating contains free the CN- in small amount. The invention reduces the cost of waste water treatment for the electroplating plants.
Owner:HENGSHENG TECH R&D CO LTD
Molten gold resisting high-temp sinter and its preparing process
A gold solution resisting high-temp sinter for ceramic decoration contains Au (10-15 wt.%), Th (2.2-3.0), Rh (0.1-0.3), Sb (0.1-0.8), Pb (0.3-0.4), Bi (1.0-1.4) and mixed solvent. It is prepared through preparing resinates of thorium nitrate, RhO3 and SbCl3, mixing them together; preparing gold ammonium percloride from AuCl3, reaction on balsam sulfide to obtain gold resinate; preparing resinatesof lead nitrate and bismuth nitrate, mixing them together; mixing said three components together, and diluting with mixed solvent.
Owner:NANJING UNIV
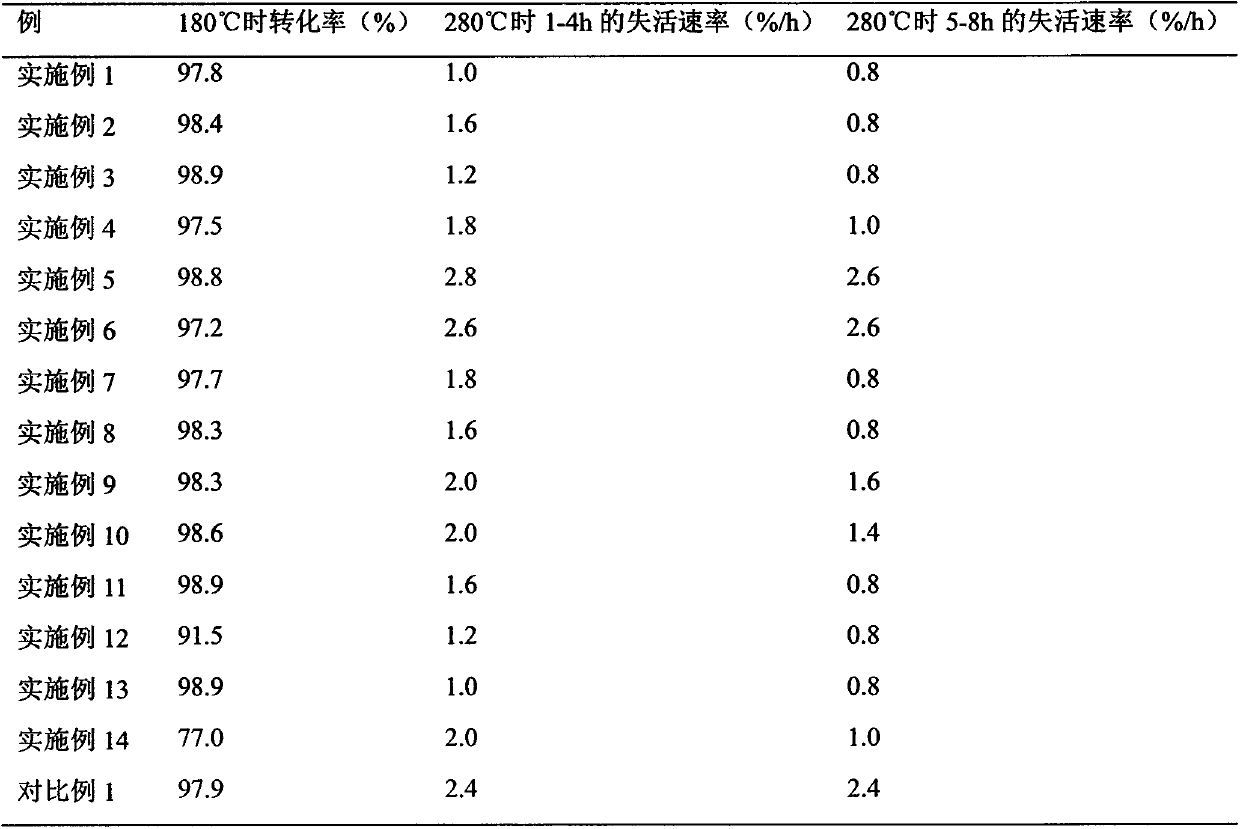
Preparation method of high-temperature inactivation resistant gold-based catalyst for synthesizing chlorethylene
ActiveCN104001549AThe optimal dosage is lessImprove thermal stabilityPreparation by halogen halide additionOrganic-compounds/hydrides/coordination-complexes catalystsActivated carbonChloride
The invention discloses a preparation method of a high-temperature inactivation resistant load type gold catalyst for synthesizing chlorethylene in acetylene hydrochlorination. The catalyst takes active carbon as a carrier, takes aminothiopropionic acid, cystine, methionine and thiocarbamide as active carbon organic surface modifiers and takes auric chloride as an activity center. During the reaction of synthesizing chlorethylene in acetylene hydrochlorination, the active carbon surface modified load type gold catalyst has a high-temperature inactivation resistant performance better than that of an unmodified active carbon load type gold catalyst.
Owner:内蒙古海驰高科新材有限公司
Cyanide-free gold electroplating liquid
The invention relates to cyanide-free gold electroplating liquid, and belongs to the field of surface modification of metal materials. The cyanide-free gold electroplating liquid has the advantages that: the electroplating liquid is non-toxic, simple in a formula and high in stability, does not have the phenomenon of turbidity and color change after being stood at normal temperature and still can be used. The cyanide-free gold electroplating liquid comprises the following main ingredients: gold trichloride, sodium sulfite or potassium sulfite serving as a main complexing agent, ethylene diamine tetraacetic acid (EDTA) or sodium citrate serving as an auxiliary complexing agent and sodium chloride or potassium chloride serving as inorganic salt. In the cyanide-free gold electroplating liquid, a new formula proportion is adopted, the cost is low, a plating layer is high in bonding force and bright, and the preparation of the functional gold plating layer is realized. The popularization and application of the cyanide-free gold electroplating liquid can create economic and social benefit.
Owner:BEIJING UNIV OF TECH
Cyanogens-free gold plating solution and method for plating gold by adopting same
The invention provides cyanogens-free gold plating solution and a method for plating gold by adopting the cyanogens-free gold plating solution, relating to a plating solution and a gold electroplating method. The invention solves the problems that cyanide is toxic and the stability of sulphite gold plating solution is poor. The cyanogens-free gold plating solution of the invention is composed of coordinating agent, auric chloride, potassium carbonate, potassium pyrophosphate and compound additive. The gold plating method of the invention includes the following steps: sodium hydroxide is used for regulating the pH value of cyanogens-free gold plating solution, then constant current mode is adopted, and plating is carried out under the conditions that the distance of cathode and anode is 5-20cm and the temperature is 30-60 DEG C. The cyanogens-free gold plating solution of the invention contains no highly toxic substance, and the stability of plating solution is good, the plating solution is not muddy or changed in colour when being used in 30 days (including plating and ingredient compensation). Meanwhile 10mL plating solution is used for continuous plating and 0.15Ah of electric quantity passes through, and a coating in good surface state still can be obtained.
Owner:WUXI HAITE NEW MATERIAL RES INST
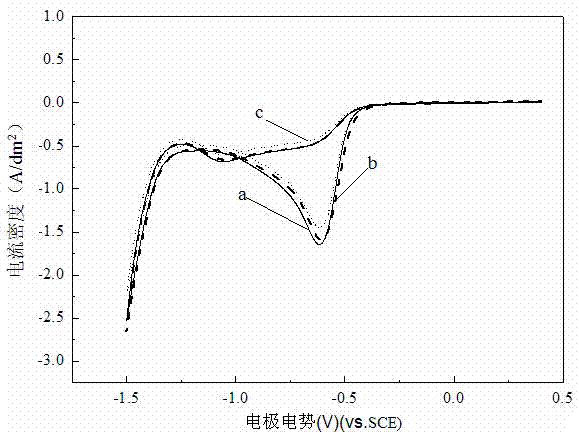
Method for enhancing gold precipitation grade in gold electrorefining process
InactiveCN103590071AHigh densityStrong resistance to hydrochloric acid corrosionPhotography auxillary processesDiaphragmsElectrolysisMetal impurities
The invention provides a method for enhancing the gold precipitation grade in a gold electrorefining process. According to the method, a coarse gold plate is adopted as an anode, a pure gold plate is adopted as a cathode, and a gold chloride solution is taken as an electrolyte solution to perform electrorefining. Specifically, the outside of the anode is sleeved with a pocket shaped diaphragm made of polypropylene fiber, and the mesh opening of the diaphragm is 800-850 meshes. The cathode is made by the method of: taking the coarse gold plate as the anode, adopting a titanium alloy plate as the cathode, and using a gold chloride solution as an electrolyte solution to conduct electrolysis, precipitating out a pure gold layer on both sides of the titanium alloy plate, and stripping the pure gold layer, thus obtaining a cathode plate used for gold electrorefining. The method provided by the invention effectively avoids adsorption of AgCl and part of insoluble metal impurities on the cathode, thereby enhancing the gold precipitation grade and ensuring the stability of gold electrolysis production. At the same time, by improving the quality of a gold starting sheet, the gold precipitation grade is further improved.
Owner:BAIYIN NONFERROUS GROUP
Organic gold conductor paste
ActiveCN113658742AReduce square resistanceImprove adhesionNon-conductive material with dispersed conductive materialPrintingMeth-Electrical conductor
The invention discloses organic gold conductor paste which comprises the following components in percentage by mass: 40%-60% of resin acid gold, 2%-10% of a metal organic matter mixing agent, 30%-60% of an organic carrier and 1%-4% of an oily gold nano solution, wherein the resin acid gold is prepared by reacting sulfurized balsam and gold trichloride; the metal organic matter mixing agent is a mixture of lead caprylate, bismuth 2-hexylhexane, rhodium caprylate, silicon 2-ethylhexanoate, vanadium iso-octoate and trimethyl boron; the concentration of gold in the oily gold nano solution is 40-60 mug / mL, and the diameter is 5-40 nm. By adding the oily gold nano solution, the sheet resistance of the conductor paste is greatly reduced, the adhesive force and the scratch resistance are improved, meanwhile, a sintered film is compact and flat in surface, low in roughness, resistant to acid and alkali, bright yellow and free of light leakage, the roughness Ra is lower than 0.05 mum, the sheet resistance is about 20 momega / square, the adhesive force reaches 64 N / mm<2> or above, and the scratch resistance reaches 3B.
Owner:西安宏星电子浆料科技股份有限公司
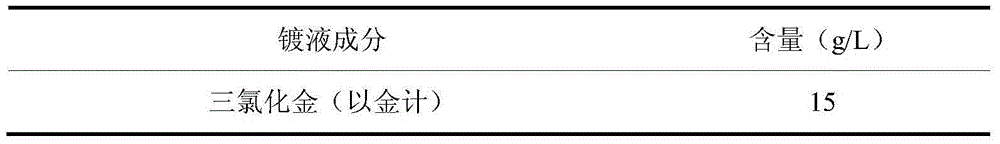
Electroplate liquid and electroplate method for cyanide-free gold electroplating of sulfite
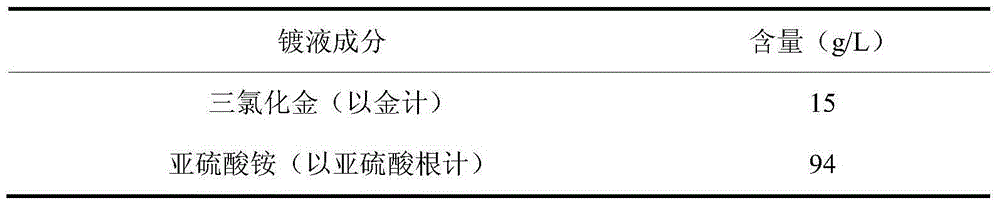
The invention discloses electroplate liquid and an electroplate method for cyanide-free gold electroplating of sulfite. The electroplate liquid is composed of 10-15 g / L of gold chloride in terms of gold, 78-94 g / L of sulfite in terms of sulfite, and 14-20 g / L of alkali metal mercaptopropionic sulfonate in terms of mercaptopropionic sulfonate radicals. According to the electroplate liquid, the sulfite serves as main coordination agents, the alkali metal mercaptopropionic sulfonate in terms of mercaptopropionic sulfonate radicals serves as auxiliary coordination agents, and the gold chloride serves as main gold salt, so that the obtained electroplate liquid is good in dispersion capacity and covering capacity, cathode currents are high in efficiency, and the electroplate liquid is excellent in performance. By the adoption of the electroplate liquid, plating obtained through electroplating under the alkaline condition is low in porosity, high in brightness and good in quality.
Owner:无锡永发电镀有限公司
Method for preparing cage-shaped gold nanoparticles through lanreotide acetate template
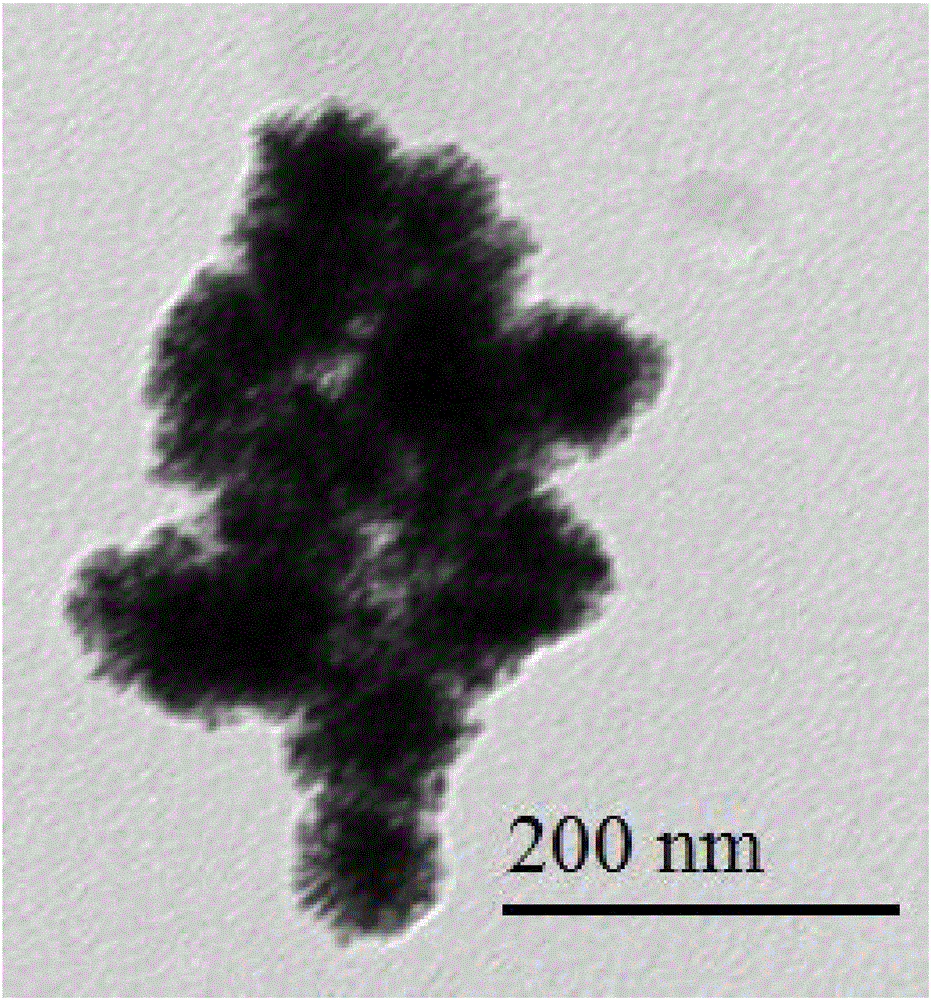
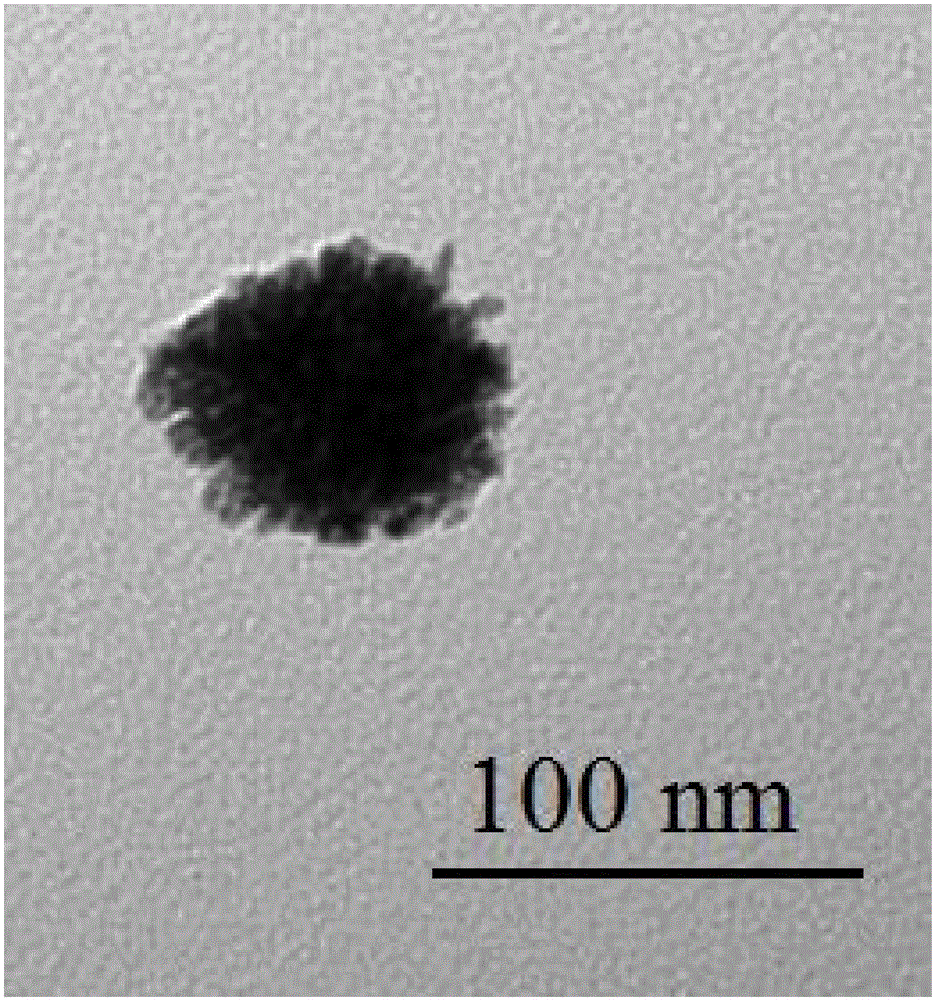
ActiveCN105458287ASimple compositionEasy to analyzeTransportation and packagingMetal-working apparatusWater bathsNanoparticle
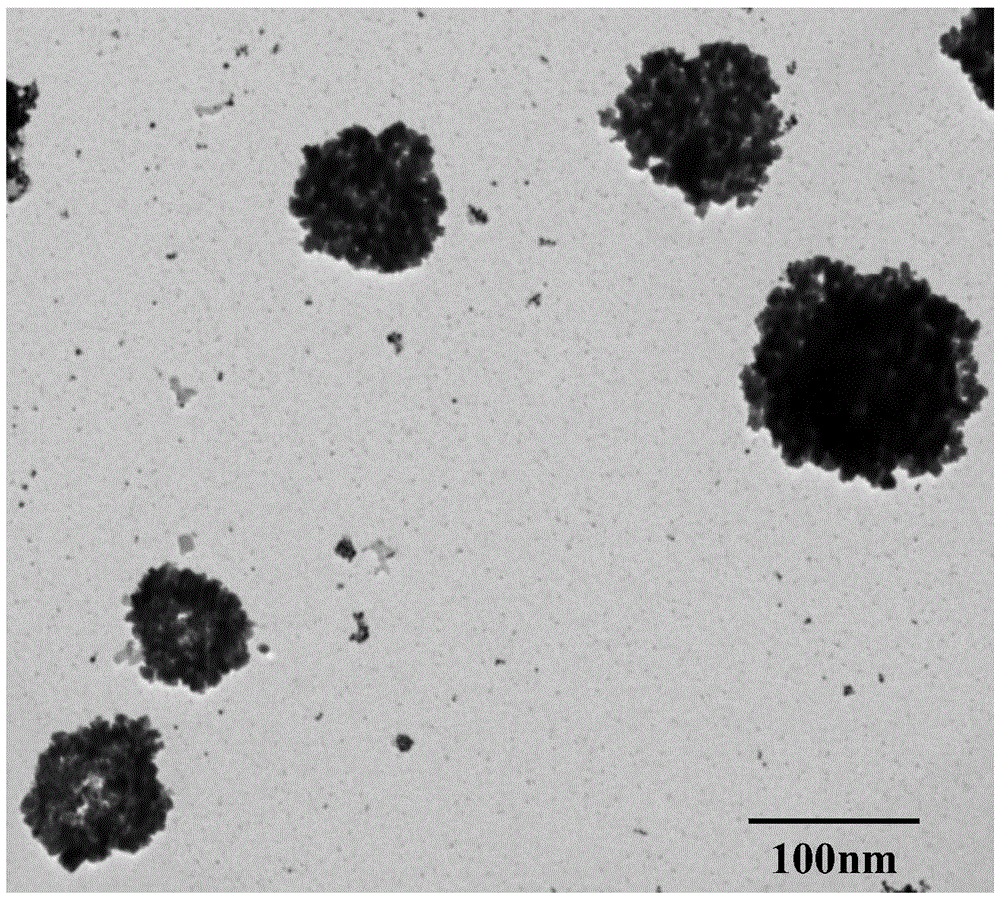
The invention discloses a method for preparing cage-shaped gold nanoparticles through a lanreotide acetate template. Mainly, according to the proportion that 0.5-1.0 mg of lanreotide acetate is added in each milliliter of a hydrochloric acid solution with the pH of 2-6, a lanreotide acetate solution is prepared and placed in a metal bath at the temperature of 70 DEG C for 20-40 min, and ultrasonic treatment is carried out; an auric chloride solution is added in the lanreotide acetate solution, the molar ratio of the auric chloride solution and the lanreotide acetate solution is 1:4-8, the mixture is placed in a water bath constant-temperature oscillator, the rotating speed is 100-150 rpm, and incubation at the temperature of 25-30 DEG C is conducted for 16-24 h; reducing agent sodium borohydride is added in the incubated solution, the molar ratio of the reducing agent sodium borohydride to the auric chloride solution is 1:2-4, the dripping speed is controlled at 2 drops / min, the reaction temperature is about 22-25 DEG C, the reaction time is 5-15 min, and the cage-shaped gold nanoparticles with the grain size of 80-120 nm and good dispersibility are obtained. The method has the advantages that raw materials are low in price and easy to obtain, the device is simple, operation is easy, the reaction conditions are moderate, the appearance is controllable, and repeatability is high.
Owner:YANSHAN UNIV
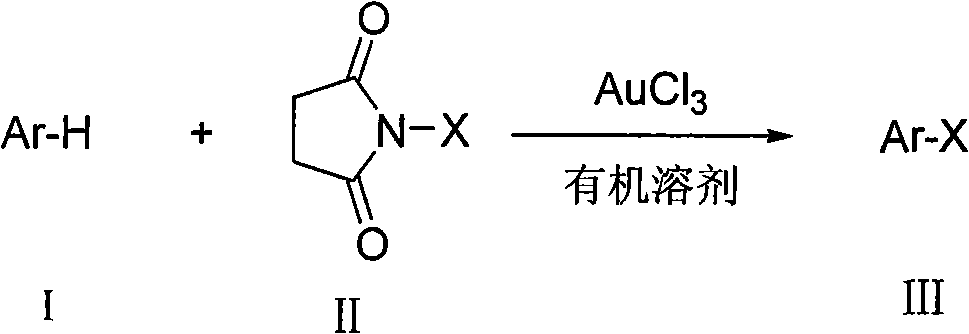
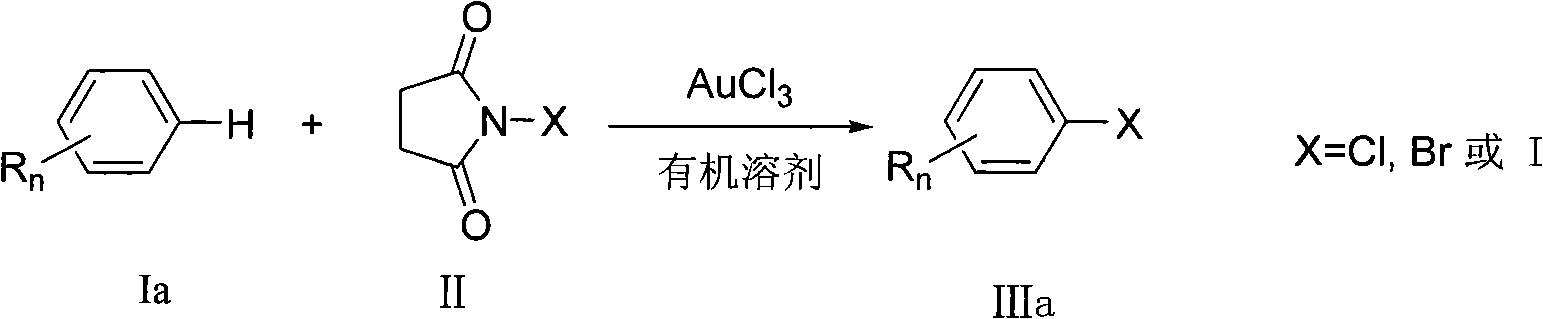
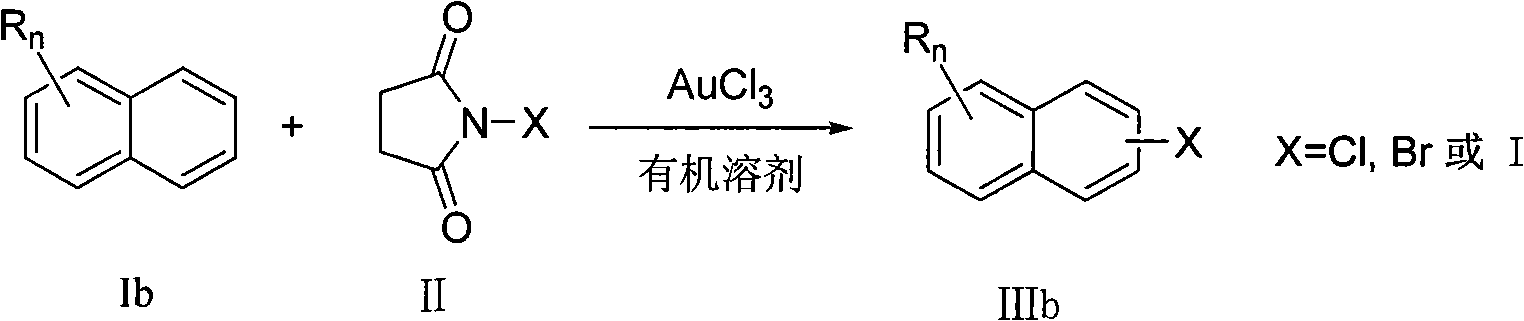
Preparation method of aromatic halogenated compound
ActiveCN101993318AEasy to operateImprove tolerancePhysical/chemical process catalystsOrganic compound preparationArylOrganic solvent
The invention discloses a preparation method of an aromatic halogenated compound. Arene Ar-H and N-halogenated succimide are reacted in an organic solvent under the catalysis of auric chloride to obtain an aromatic halogenated compound Ar-X, wherein Ar represents substituted or unsubstituted non-heterocycle aryl and X represents chlorine, bromine or iodine. The reaction in the method does not need strict water-free and oxygen-free condition and occurs successfully in air. The invention has the advantages of good tolerance and universality to functional groups, little use amount of the catalyst and lower reaction cost, is simple and convenient for operation, and can be widely used for preparing the aromatic halogenated compound.
Owner:PEKING UNIV
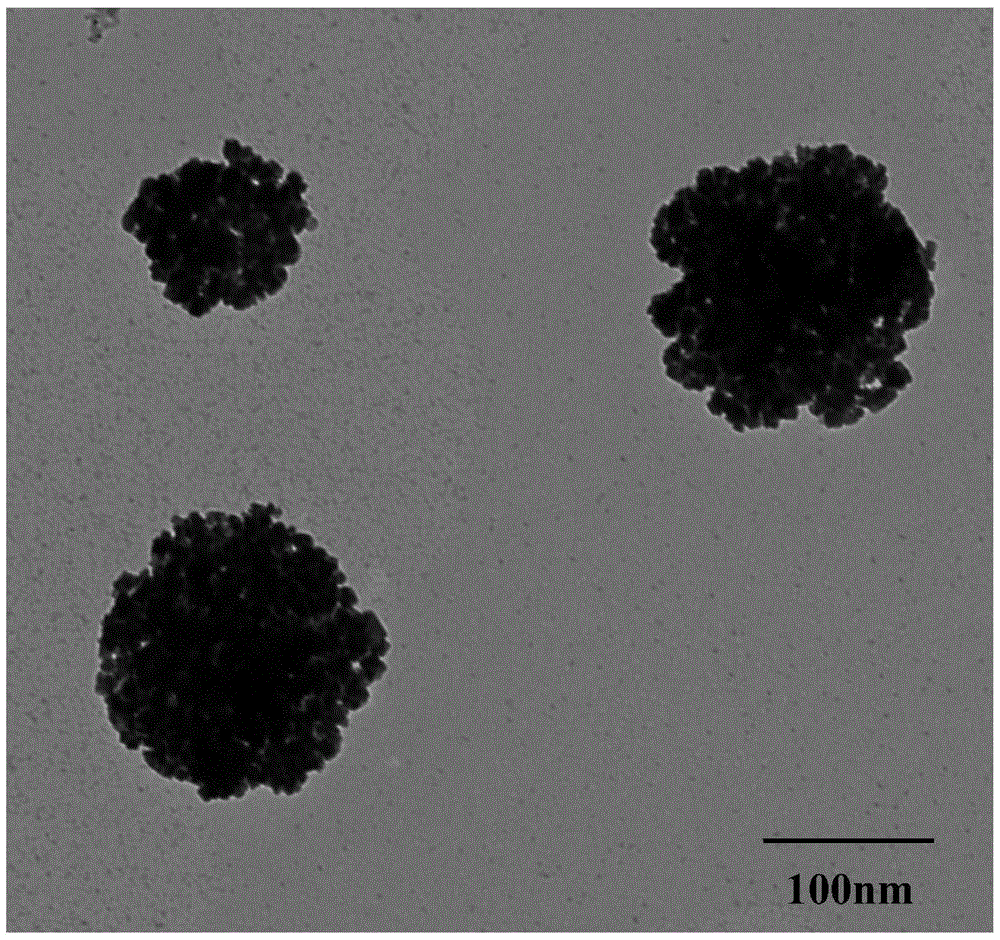
Method for self-assembling and synthesizing spherical gold nanoparticles by using bacitracin as template
ActiveCN105880624AEasy to controlPrecisely madeMaterial nanotechnologyTransportation and packagingReaction temperatureSodium borohydride
A method for self-assembling and synthesizing spherical gold nanoparticles by using bacitracin as a template mainly comprises the following steps: feeding bacitracin into a hydrochloric acid solution to obtain 0.35 to 0.70mM acidic bacitracin solution, then thermally processing the acidic bacitracin solution through metal bath at the temperature of 40 to 60 DEG C for 1 to 2 hours, and ultrasonically processing for 30 seconds; feeding a gold perchloride solution into the bacitracin solution, and then transferring into a double-layer gas bath oscillator to incubate for 20 to 30 hours at the speed of 100 to 150 rpm and under the temperature of 20 to 25 DEG C; feeding 60 to 90 microlitre sodium borohydride as a reducing agent into the incubated solution, and controlling the reaction temperature to be 21 to 23 DEG C to react for 40 to 60 minutes, thus obtaining the spherical gold nanoparticles of which the particle is 80 to 90nm. The method has the advantages that the process is simple, the shape and the appearance can be controlled, and the reaction conditions are green and mild; the prepared spherical gold nanoparticles are high in specific surface area, high in dispersibility, and high in metal load rate; the phenomenon of agglomerating of the gold nanoparticles in a traditional preparation process can be overcome.
Owner:YANSHAN UNIV
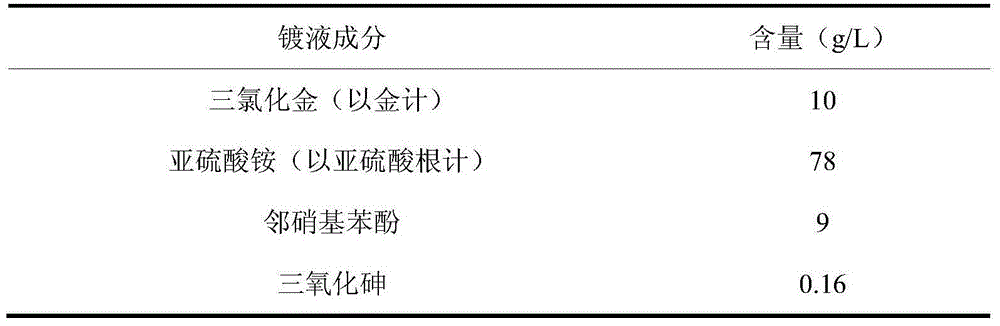
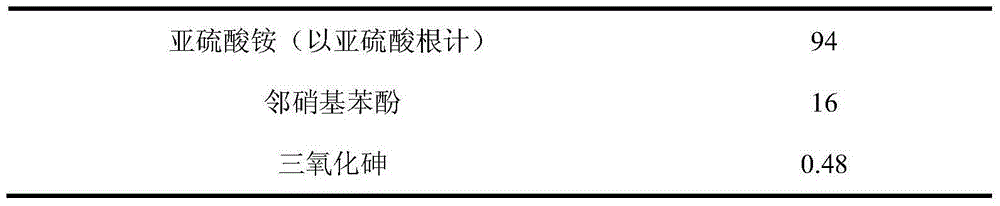
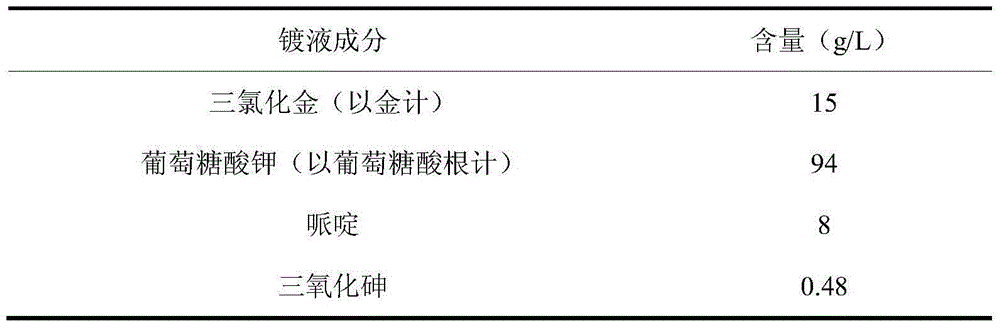
Arsenic-contained sulfite cyanide-free plated gold electroplating liquid and electroplating method
The invention discloses arsenic-contained sulfite cyanide-free plated gold electroplating liquid and an electroplating method. The electroplating liquid comprises 10-15 g / L of gold trichloride calculated by gold, 78-94 g / L of sulfite calculated by sulfurous acid radical, 9-16 g / L of nitrophenol and 0.16-0.48 g / L of arsenic trichloride. The electroplating liquid uses the sulfite as a main coordination agent, the nitrophenol as a stable positioning agent, the arsenic trichloride as a brightening agent and the gold trichloride as gold main salt, so that the electroplating liquid is better in dispersibility and deep plating capacity, high in cathode current efficiency and excellent in plating liquid performance. A plating layer electroplated by the plating liquid under the alkaline condition is low in porosity, high in brightness and excellent in plating layer quality.
Owner:无锡永发电镀有限公司
Manufacturing method of high-purity gold
InactiveCN102925679AEliminate the steps of decontaminationHigh purityProcess efficiency improvementOxalateCacodylic acid
The invention relates to a manufacturing method of high-purity gold. The manufacturing method includes the following steps: (1) gold sheets are dissolved, and concentration, purification and extraction are performed to obtain a gold chloride solution; and (2) high-purity gold is obtained through reduction, oxalic acid is added in the solution obtained in the step (1) under the condition of continuous stirring to perform reduction so as to obtain gold sediment, the gold sediment is washed by distilled water for 5-6 times, the distilled water is pumped, the gold sediment is arranged in a drying oven to be dried at the temperature of 80-100 DEG C, and the high-purity gold is obtained. The gold chloride solution is extracted by using high-purity diethyl ether so as to remove impurities to the optimum effect, and the high-purity oxalic acid is used as a reducing agent for restoring the gold chloride to obtain the high-purity gold. Due to the fact that a by-product of the oxalic acid using as the reducing agent is gas CO2, the step of impurity removal is omitted.
Owner:TIANJIN CHEM REAGENT RES INST
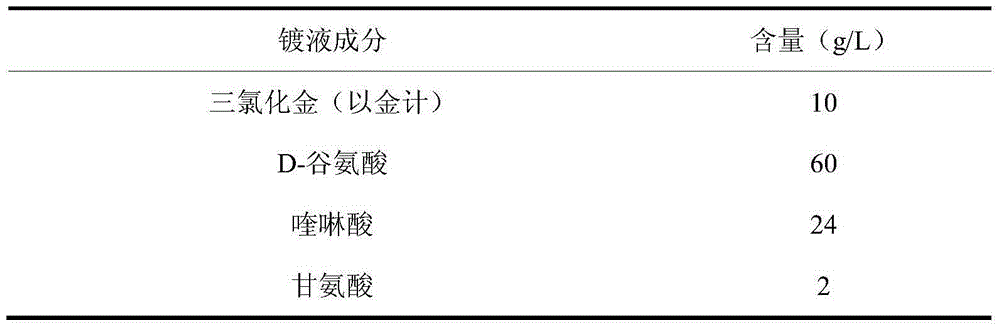
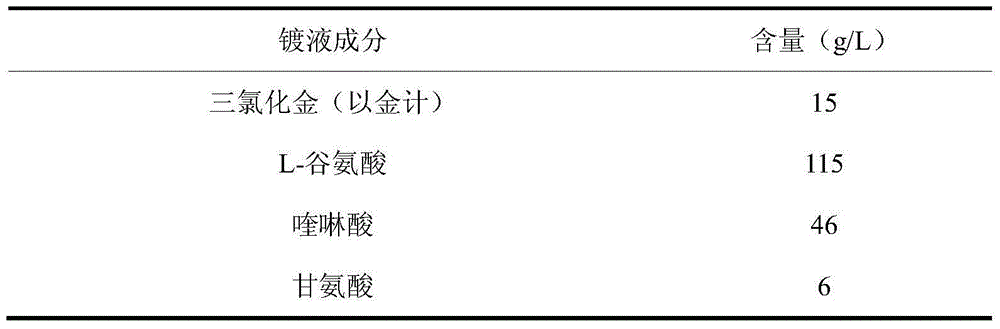
Glutamic-acid cyanide-free gold-plating electroplating liquid and electroplating method thereof
The invention discloses a glutamic-acid cyanide-free gold-plating electroplating liquid and an electroplating method thereof. The electroplating liquid comprises 10-15 g / L of gold trichloride calculated based on gold, 60-115 g / L of glutamic acid, 24-46 g / L of quinaldinic acid and 2-6 g / L of glycine. Glutamic acid is employed as a coordination agent and quinaldinic acid is employed as an auxiliary coordination agent, glutamic acid and quinaldinic acid give play to synergic effect for improving the coordination capability of gold. Glycerin is employed as a stabilizing agent and prevents the electroplating liquid from being oxidized under an acidic condition. Gold trichloride is employed as a gold main salt, and thus the obtained electroplating liquid possesses relatively good dispersing force and covering power, and is high in cathode current efficiency and excellent performances. A plating layer obtained by employing the electroplating liquid for performing electroplating under an alkaline condition is low in porosity, high in brightness and good in plating-layer quality.
Owner:无锡杨市表面处理科技有限公司
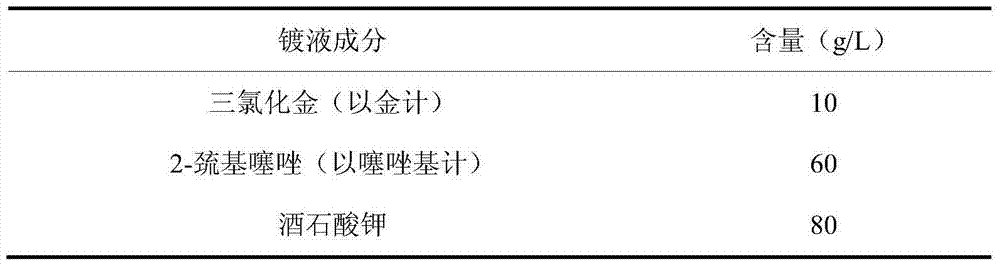
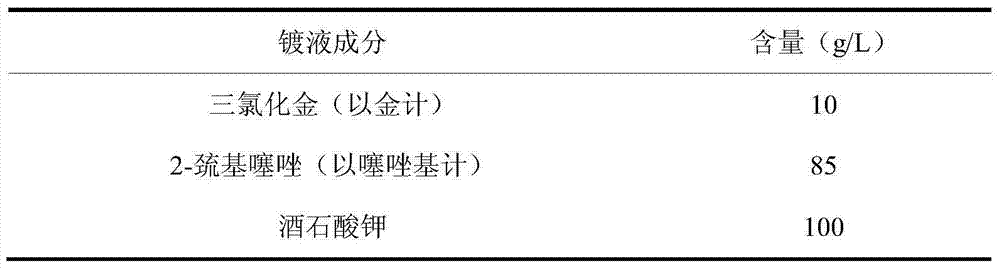
Electroplating liquid for non-cyanide plating gold by thiazole and electroplating method thereof
The invention discloses an electroplating liquid for non-cyanide plating gold by thiazole and an electroplating method thereof. The electroplating liquid comprises 8-12g / L auric chloride (measured by gold), 60-90g / L of thiazolyl compounds (measured by thiazolyl) ad 80-120 g / L citric acid alkali metal salt. According to the electroplating liquid and the preparation method thereof, the thiazolyl compounds are adopted as a complexant for gold ions and the citric acid alkali metal salt is adopted as an auxiliary complexant, the two materials act synergistically to improve complexing capacity with gold, and the auric chloride is adopted as a main salt of gold, and therefore, the electroplating liquid is good in dispersion force and deep plating capacity, high in cathode current efficiency and excellent in performance. The plated layer obtained through electroplating by using the electroplating liquid under the alkaline condition is low in porosity, high in brightness and good in quality.
Owner:华文蔚
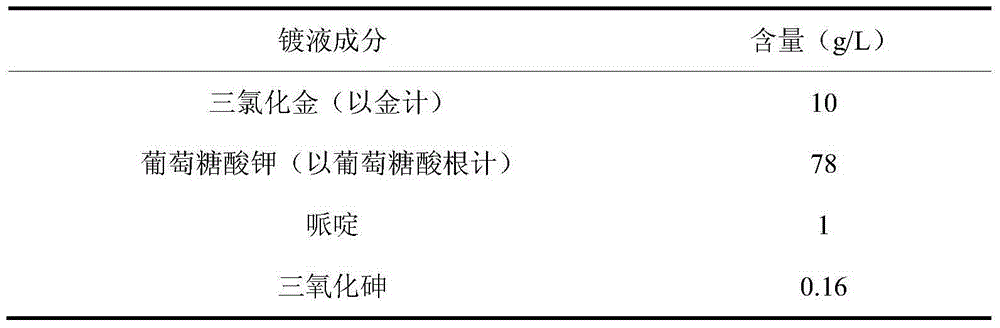
Gluconic acid cyanide-free gold-electroplating solution and electroplating method thereof
The invention discloses a gluconic acid cyanide-free gold-electroplating solution and an electroplating method thereof. The gold-electroplating solution comprises 10-15 g / L by gold of auric chloride, 78-94 g / L by a gluconic acid group of gluconic acid or gluconate, 1-8 g / L of piperidine, and 0.16-0.48 g / L of arsenic trioxide. Gluconic acid or gluconate is taken as a complexant, a complex of piperidine and arsenic trioxide is taken as a brightener, and auric chloride is a main gold salt. The obtained gold-electroplating solution has preferable dispersion and deep electroplating performance; the cathodic current efficiency is high; and performance of the solution is excellent. Through adoption of the gold-electroplating solution in an alkaline condition, an obtained electroplated layer is low in porosity, high in brightness, and excellent in quality.
Owner:无锡杨市表面处理科技有限公司
Supported foamy copper catalyst used for removing acrylonitrile through supercritical water oxidation
InactiveCN106311277AImprove removal efficiencyImprove stabilityWater treatment compoundsWater contaminantsAcrylonitrileSupercritical water oxidation
The invention discloses a supported foamy copper catalyst used for removing acrylonitrile through supercritical water oxidation. A preparation method of the catalyst comprises the following steps: cleaning foamy copper and then modifying foamy copper with AgNO3, thus preparing a substance M; modifying the substance M with mixed liquor prepared from polyvinylpyrrolidone, methanol, palladium dichloride, gold chloride, concentrated hydrochloric acid and sodium borohydride, thus preparing a substance N; modifying the substance N with mixed liquor prepared from PbCl2, SrCl2, concentrated nitric acid, urea and ammonium fluoride, thus preparing a substance O; modifying the substance O with mixed liquor prepared from tetra-n-butyl titanate, tetraethyl orthosilicate and acetylacetone, thus preparing a substance P; modifying the substance P with mixed liquor prepared from ZrCl4, CdCl2 and AlCl3, thus preparing a substance Q; modifying the substance Q with mixed liquor prepared from ZnCl2, BiCl3, NaOH, Na2CO3 and CuCl2, thus obtaining a substance, namely the supported foamy copper catalyst used for removing acrylonitrile through supercritical water oxidation.
Owner:北京清水润土环保科技有限公司
Electroplate liquid and electroplate method for cyanide-free gold plating of thallium-contained sulfite
The invention discloses electroplate liquid and an electroplate method for cyanide-free gold plating of thallium-contained sulfite. The electroplate liquid is composed of 10-15 g / L of gold chloride in terms of gold, 78-94 g / L of sulfite in terms of sulfite radicals, 1-4 g / L of glycerinum, 0.30-0.65 g / L of organic phosphorus compounds and 0.05-0.14 g / L of thallous salt in terms of thallium. According to the electroplate liquid, the sulfite serves as main coordination agents, the glycerinum serves as stabilizers, the organic phosphorus compounds serve as electron accelerators, the thallous salt serves as brightening agents, and the gold chloride serves as main gold salt, so that the obtained electroplate liquid is good in dispersion capacity and covering capacity, cathode currents are high in efficiency, and the electroplate liquid is excellent in performance. By the adoption of the electroplate liquid, plating obtained through electroplating under the alkaline condition is low in porosity, high in brightness and good in quality.
Owner:无锡永发电镀有限公司
Plating solution for mercapto sulfonic acid non-cyanogen gold plating and plating method thereof
The invention discloses a plating solution for mercapto sulfonic acid non-cyanogen gold plating and a plating method thereof. The plating solution comprises 10-15g / L of gold trichloride calculated by gold, 35-55g / L mercapto sulfonic acid compound calculated by mercapto group, 11-17g / L citric acid and salts thereof calculated by citrate, and 50-70g / L EDTA. The plating solution uses mercapto sulfonic acid compound as the main complexing agent, uses citric acid and its salts, and EDTA as the auxiliary complexing agent, and uses gold trichloride as the main gold salt, so that the obtained plating solution has good dispersion force, deep plating capability power, high cathodic current efficiency, and excellent performance. The plating layer obtained under basic conditions by using the plating solution has the advantages of low porosity, high brightness and good plating layer quality.
Owner:无锡永发电镀有限公司
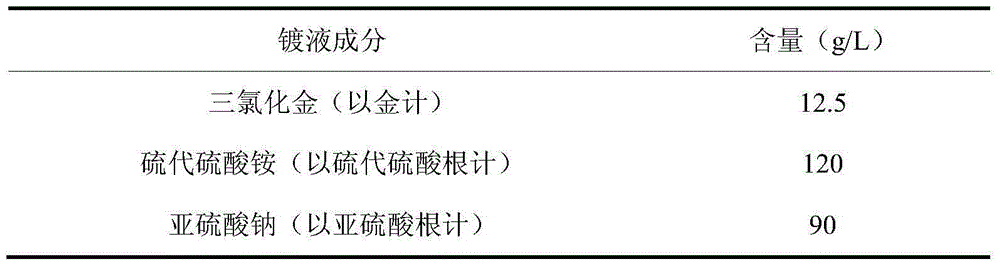
Propylene-glycol-added thiosulfate cyanide-free gold-electroplating solution and electroplating method
The invention discloses a propylene-glycol-added thiosulfate cyanide-free gold-electroplating solution and an electroplating method. The gold-electroplating solution comprises 10-15 g / L by gold of gold trichloride, 100-140 g / L by thiosulfate radical of thiosulfate, 78-94 g / L by sulfite radical of sulfite, 0.8-3.4 g / L of propylene glycol, 0.30-0.65 g / L of an organic phosphorus compound, and 0.05-0.14 g / L by thallium of thallium (I) salt. In the gold-electroplating solution, thiosulfate is taken as a main complexant; propylene glycol is taken as a stabilizing agent; the organic phosphorus compound is taken as an electron accelerant; thallium (I) salt is taken as a brightener; and gold trichloride is taken as main gold salt. Therefore, the obtained gold-electroplating solution is relatively good in dispersion and deep plating ability, high in cathodic current efficiency, and excellent in electroplating solution performance. Through adopting the gold-electroplating solution in an alkaline condition, a plated layer obtained through electroplating is low in porosity, high in brightness, and excellent in plating quality.
Owner:无锡杨市表面处理科技有限公司
Preparation of silver-gold alloy nanometer spherical shell based on vapreotide acetate
ActiveCN106694901AEasy to makeMild reaction conditionsMaterial nanotechnologyTransportation and packagingDispersityWater baths
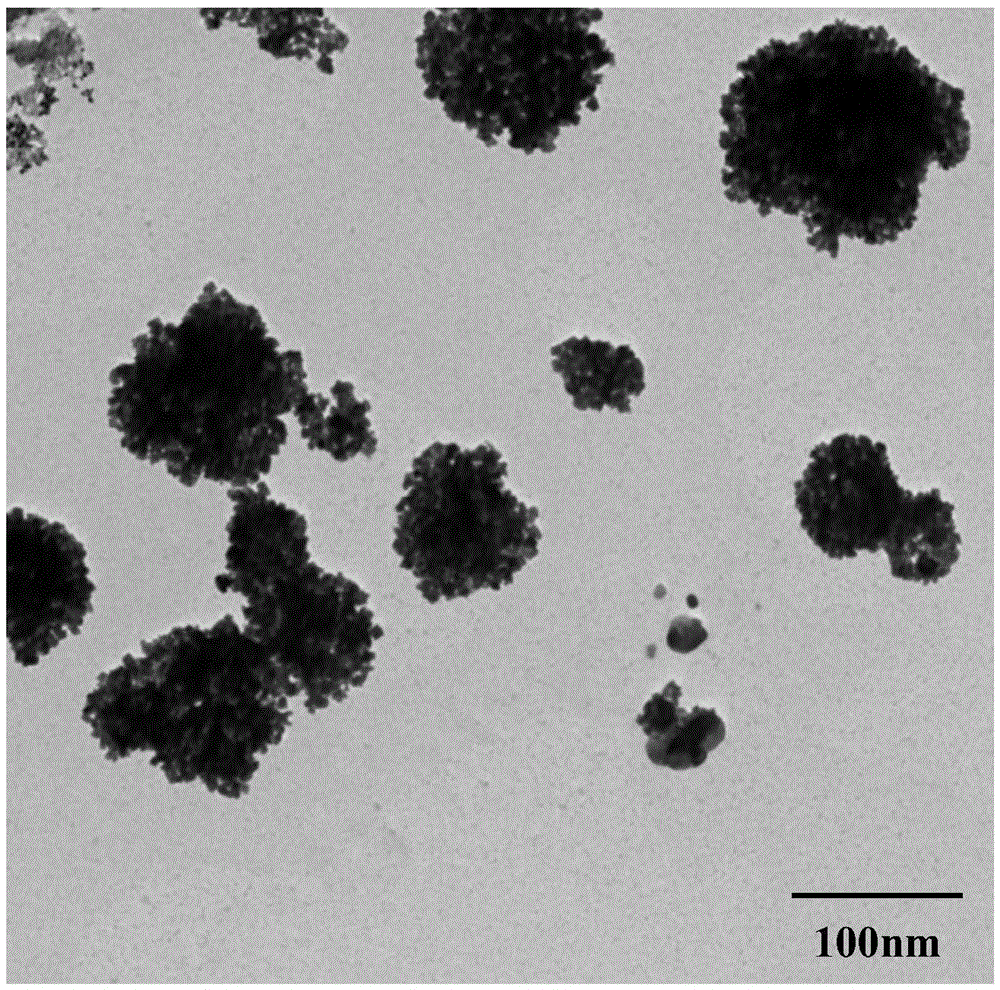
Provided is a preparation method of a silver-gold alloy nanometer spherical shell based on vapreotide acetate. The preparation method mainly comprises the steps that according to the proportion that 0.91-1.13 mg of vapreotide acetate is added to 1 ml of a hydrochloric acid solution, a vapreotide acetate solution is prepared, and treatment is conducted for 20-24 min at the temperature of 65-67 DEG C; a silver nitrate solution is added according to the ratio of 1 to 3-9, a mixture is put into a water bath constant-temperature oscillator, and incubation is conducted for 24-26 h at the temperature of 24 DEG C; according to the molar ratio of 1 to 1-2 of silver nitrate and sodium borohydride, reducing agent sodium borohydride is added dropwise, and a reaction is conducted for 16-18 min at the temperature of 24 DEG C; the mixture is put into a 100-DEG C boiling water bath, 10 min later, a auric chloride solution is added according to the molar ratio of 1 to 25-45 of auric chloride and the silver nitrate, the mixture is put into the 100-DEG C boiling water bath for 2-4 min, and the silver-gold alloy nanometer spherical shell with the grain size of 90-110 nm is prepared. By the adoption of the preparation method, the morphology is easy to control, the operation is simple, environmental protection is achieved, the reaction condition is gentle, and prepared silver-gold alloys are uniform in grain size and good in dispersity.
Owner:YANSHAN UNIV
Method for preparing gold nano-flowers
ActiveCN106475574ASmall molecular weightSimple structureMaterial nanotechnologyTransportation and packagingWater bathsDispersity
The invention discloses a method for preparing gold nano-flowers. The method mainly comprises steps as follows: a vapreotide acetate solution with the concentration being 0.5-0.7 mM is prepared from a hydrochloric acid solution, is placed in a metal bath at 60-64 DEG C for heat treatment for 40-44 min and is subjected to 80-Hz ultrasonic treatment for 30-50 s; then a gold chloride aqueous solution is added with the molar ratio of vapreotide acetate to the gold chloride aqueous solution being 1:(10-13), and the mixture is evenly mixed and is placed into a water-bath thermostatic oscillator for incubation at 110-120 rpm at 23 DEG C for 20-22 h; and sodium borohydride is added to the incubated solution with the molar ratio of sodium borohydride to the gold chloride aqueous solution being 1:(2-5) for a reaction at the reaction temperature of 23 DEG C for 20-24 min until the solution is slowly changed from faint yellow to bluish violet, and the gold nano-flowers with the particle size ranging from 120 nm to 150 nm are prepared. With the method, the preparation technology is simple, reaction conditions are mild, and the prepared gold nano-flowers are uniform in particle size, relatively high in metal loading capacity, regular in shape and good in dispersity.
Owner:YANSHAN UNIV
Loaded type foam aluminum catalyst for removing acrylonitrile through supercritical water oxidation
InactiveCN106391012AImprove removal efficiencyImprove stabilityWater treatment compoundsWater contaminantsAcrylonitrileSupercritical water oxidation
The invention discloses a loaded type foam aluminum catalyst for removing acrylonitrile through supercritical water oxidation. Foam aluminum is cleaned, is then prepared into a substance M through AgNO3 modification; the substance M is prepared into a substance N through the modification on a mixed solution prepared from polyvinylpyrrolidone, methyl alcohol, gold chloride, concentrated hydrochloric acid and sodium borohydride; the substance N is prepared into a substance O through the modification on a mixed solution prepared from CoCl2, CuCl2, concentrated nitric acid, urea and ammonium fluoride; the substance O is prepared into a substance P through the modification on a mixed solution prepared from tetra-n-butyl titanate, tetraethoxysilane and acetylacetone; the substance P is prepared into a subsequent Q through the modification on a mixed solution prepared from SnCl4, PbCl2 and CeCl3; the subsequent Q is prepared into a substance through the modification on a mixed solution prepared from SrCl2, InCl3, NaOH, Na2CO3 and NiCl2, and the substance is the loaded type foam aluminum catalyst for removing acrylonitrile through supercritical water oxidation.
Owner:北京清水润土环保科技有限公司
Load-type foamed aluminum catalyst for removing dimethyl formamide by use of supercritical water oxidization
InactiveCN106423210AImprove removal efficiencyImprove stabilityWater contaminantsMetal/metal-oxides/metal-hydroxide catalystsSupercritical water oxidationSubstance M
The invention discloses a load-type foamed aluminum catalyst for removing dimethyl formamide by use of supercritical water oxidization. The load-type foamed aluminum catalyst for removing dimethyl formamide by use of supercritical water oxidization is prepared by virtue of the following steps: washing foamed aluminum, and then modifying the washed foamed aluminum with AgNO3, thereby obtaining a substance M; modifying a mixed solution prepared from polyvinyl pyrrolidone, methanol, gold trichloride, concentrated hydrochloric acid and sodium borohydride by the substance M, thereby obtaining a substance N; modifying a mixed solution prepared from SrCl2, CoCl2, concentrated aqua fortis, urea and ammonium fluoride by the substance N, thereby obtaining a substance O; modifying a mixed solution prepared from tetra-n-butyl titanate, tetraethyl orthosilicate and acetylacetone by the substance O, thereby obtaining a substance P; modifying a mixed solution prepared from ZrCl4, MgCl2 and InCl3 by the substance P, thereby obtaining a substance Q; and modifying a mixed solution prepared from PbCl2, BiCl3, NaOH, Na2CO3 and CdCl2 by the substance Q, thereby obtaining a substance, namely the load-type foamed aluminum catalyst for removing the dimethyl formamide by use of supercritical water oxidization.
Owner:北京清水润土环保科技有限公司
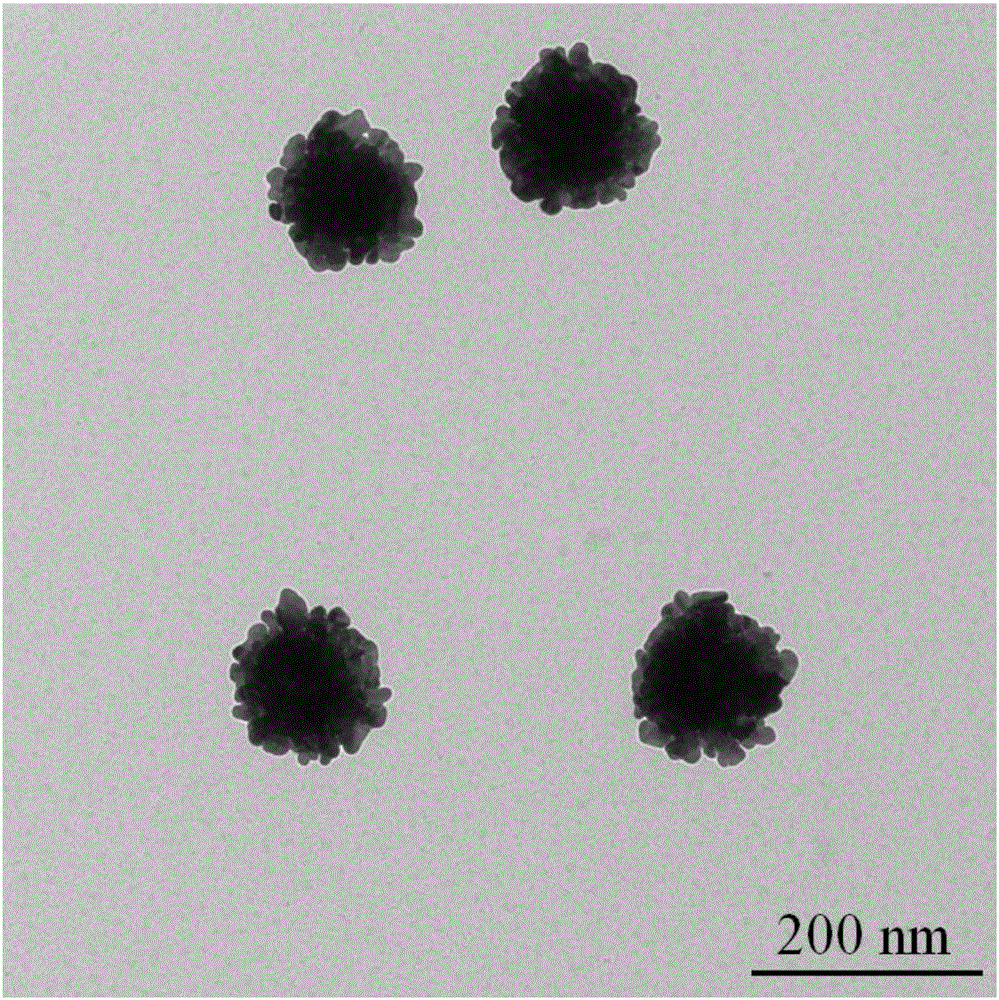
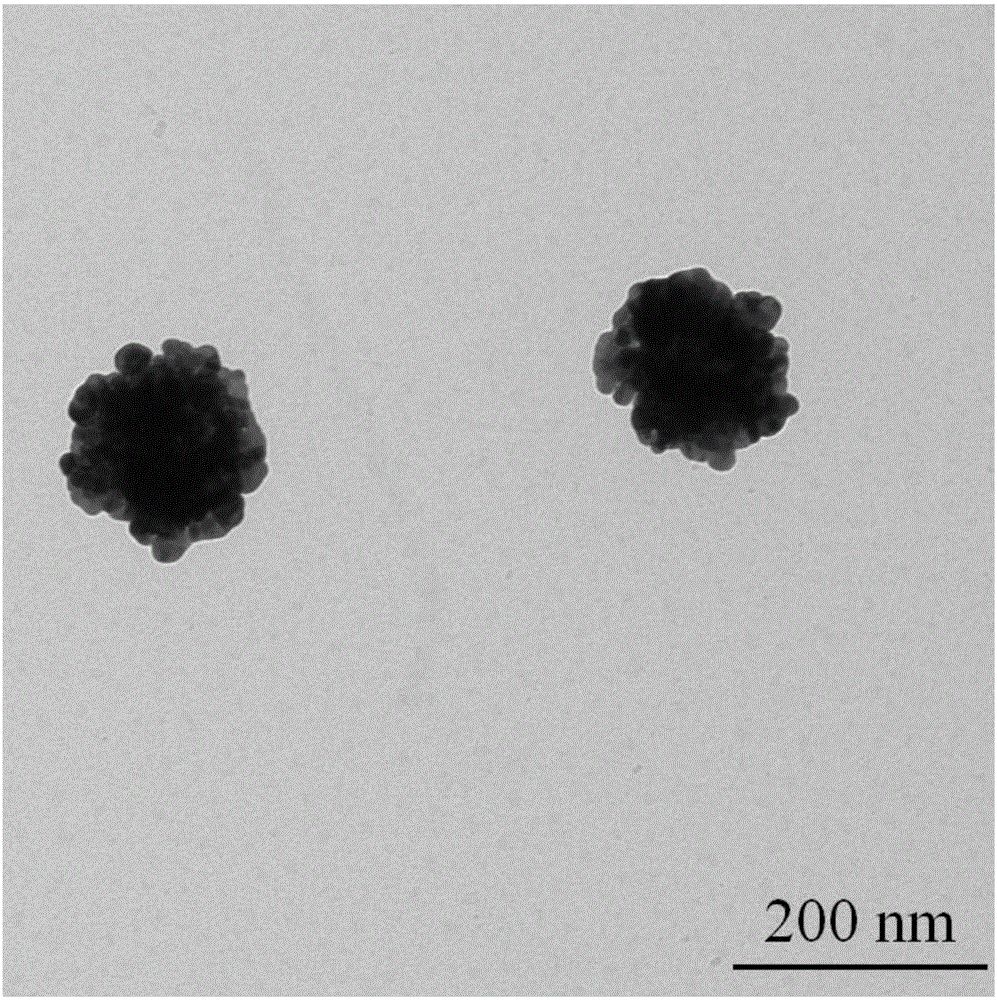
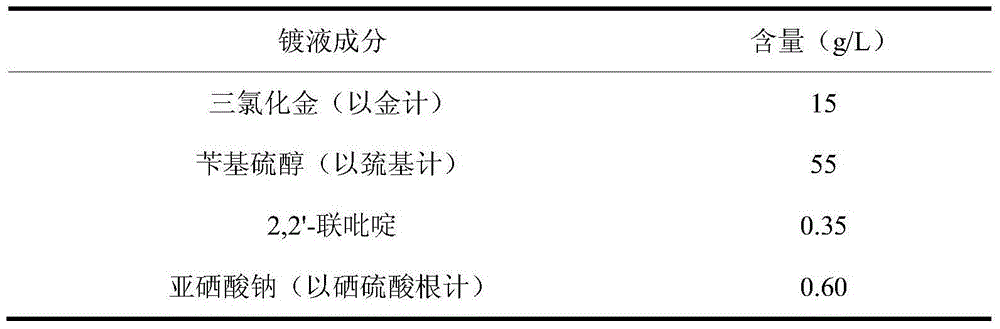
Aromatic mercaptan cyanide-free gilding electroplating solution and method
The invention discloses an aromatic mercaptan cyanide-free gilding electroplating solution and method. The electroplating solution comprises 10 g / L to 15 g / L of auric chloride by gold, 35 g / L to 55 g / L of aromatic mercaptan by sulfydryl, 0.10 g / L to 0.35 g / L of a dipyridyl compound and 0.30 g / L to 0.60 g / L of selenite by selenious acid radical. The aromatic mercaptan serves as a main coordination agent, the dipyridyl compound serves as an electron acceleration agent, the selenite serves as a brightening agent, and the auric chloride serves as main auric salt, so that the obtained electroplating solution has the good dispersion force and the deep-plating capacity, cathode current efficiency is high, and the performance of the electroplating solution is excellent. The electroplating solution is adopted for electroplating under the alkaline condition, so that an obtained plated layer is low in porosity, high in brightness and good in quality.
Owner:无锡永发电镀有限公司
Molten gold resisting high-temp sinter and its preparing process
A gold solution resisting high-temp sinter for ceramic decoration contains Au (10-15 wt.%), Th (2.2-3.0), Rh (0.1-0.3), Sb (0.1-0.8), Pb (0.3-0.4), Bi (1.0-1.4) and mixed solvent. It is prepared through preparing resinates of thorium nitrate, RhO3 and SbCl3, mixing them together; preparing gold ammonium percloride from AuCl3, reaction on balsam sulfide to obtain gold resinate; preparing resinates of lead nitrate and bismuth nitrate, mixing them together; mixing said three components together, and diluting with mixed solvent.
Owner:NANJING UNIV
Micro relay contacts, reed gold plating process
The invention discloses a gold-plating process method for contacts and reeds of miniature relays. The materials and the composition thereof are: gold trichloride of 6-14g / L in terms of gold content; anhydrous sodium nitrite 100-200g / L ; Potassium citrate 30-100g / L; Potassium chloride 30-90g / L; EDTA 5-15g / L; Cobalt sulfate heptahydrate 0.5-5g / L; The electroplating of silver nitrate 0.01-0.3g / L Put the electroplating workpiece into the solution for electroplating. Compared with the prior art, in terms of structural features of the present invention, the density is higher than that of the original cyanide gold-plated cobalt alloy gold plating, the purity of the gold coating reaches 99.99%, and the hardness is (110-120) HV, which is higher than the original cyanide gold-plated cobalt alloy gold plating The hardness of the gold layer (85-100) HV is high, which is lower than that of the original cyanide gold-plated nickel alloy (180-220) HV; in terms of technical improvement, the stability of the electrolyte and the dispersion ability are greatly improved, and the compactness of the coating layer is improved; quality; In terms of improvement, the contact resistance failure rate of its products decreased by 95%, the bonding failure rate decreased by 70%, and the consistency of coating quality increased by 80%.
Owner:贵州振华群英电器有限公司(国营第八九一厂)
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com