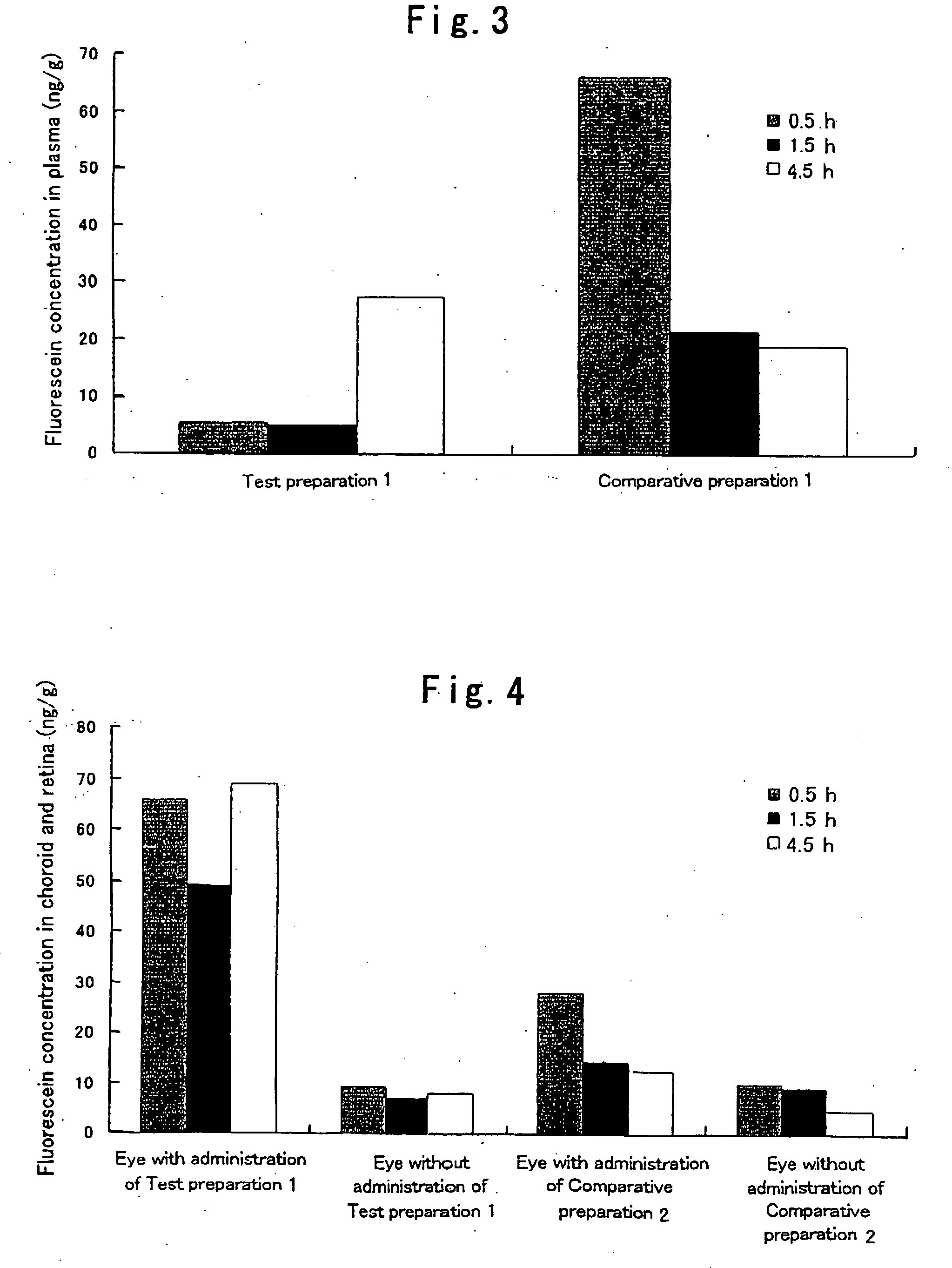
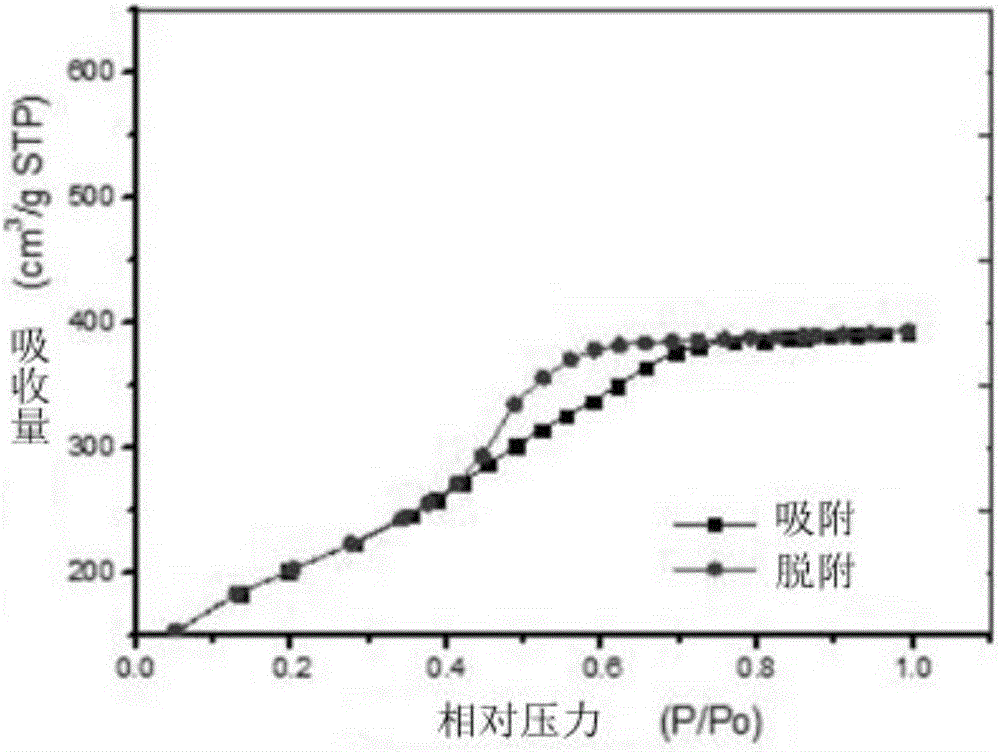
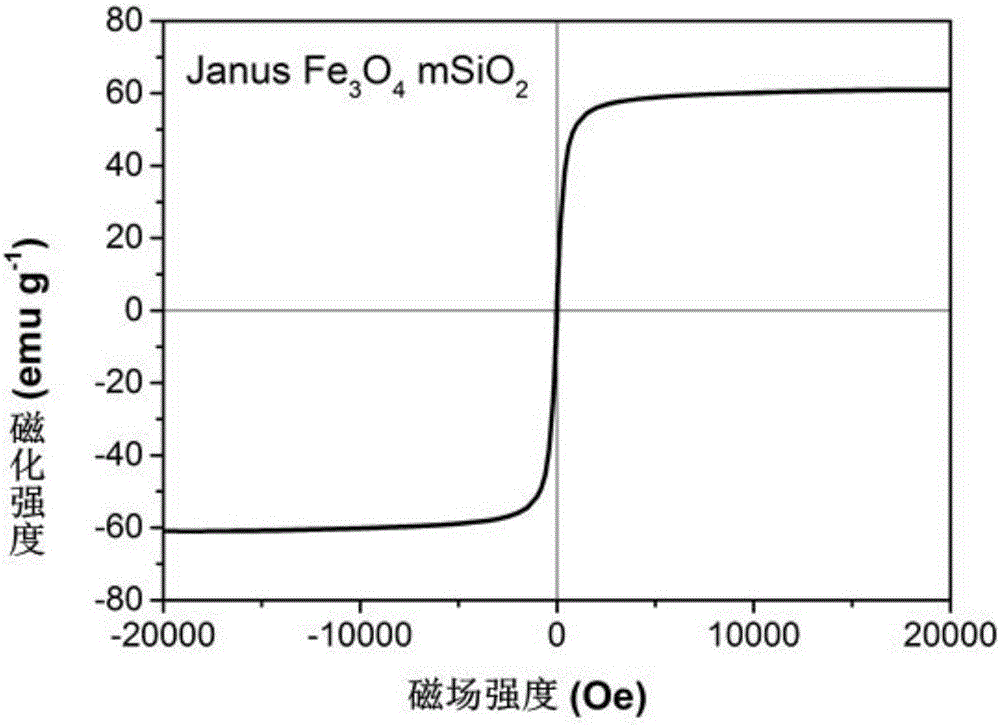
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
111 results about "Drug transfer" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
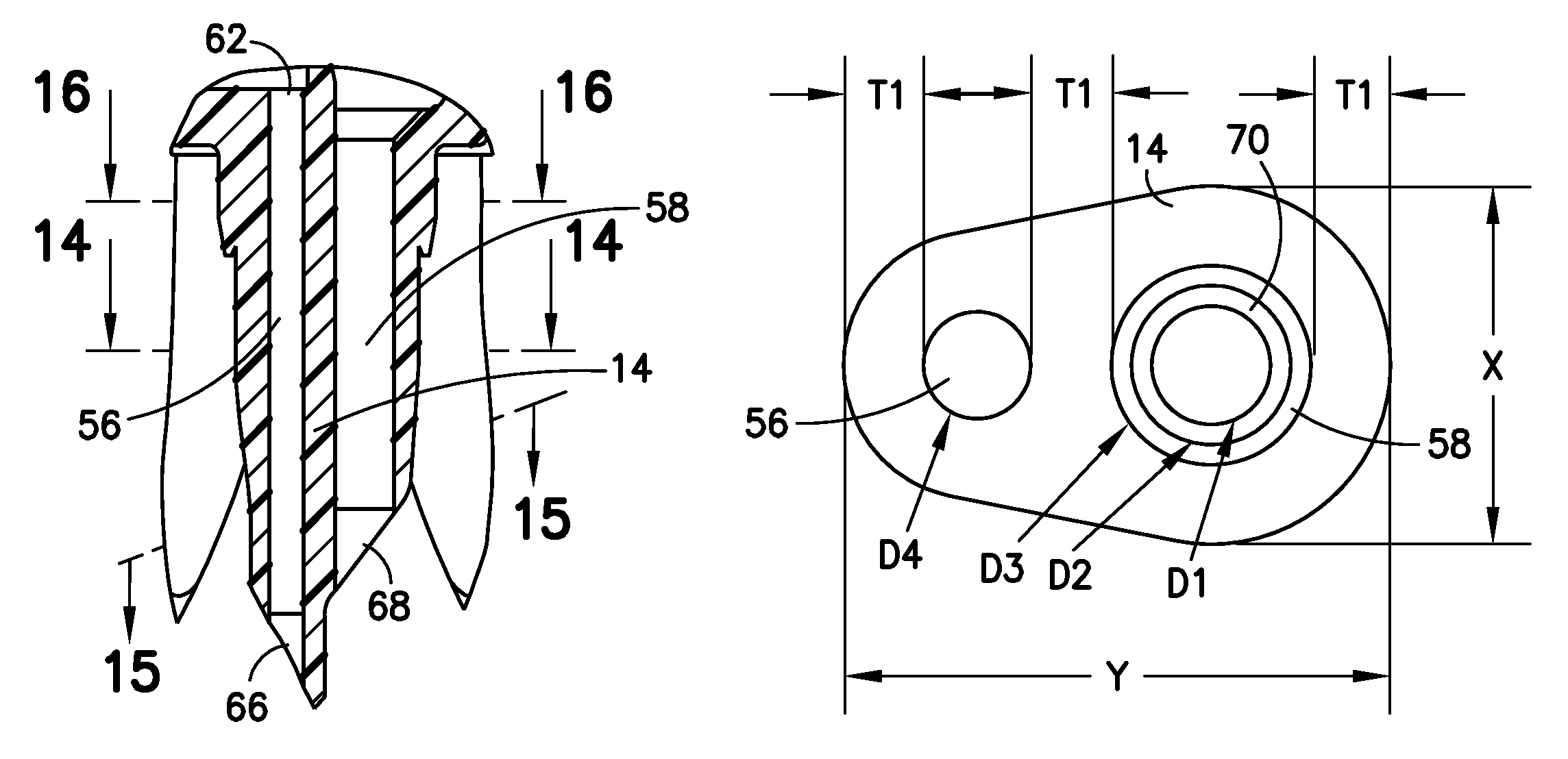
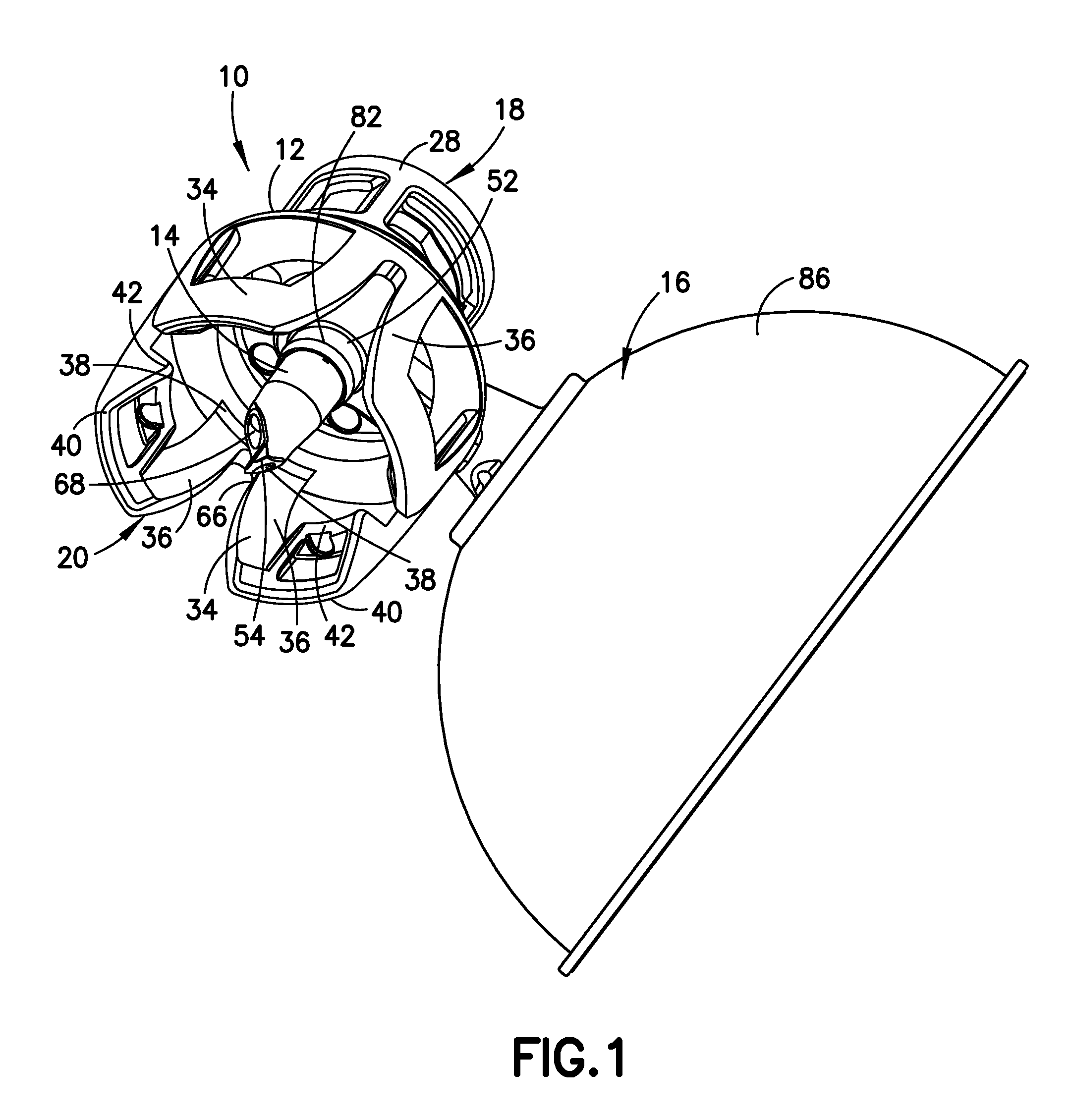
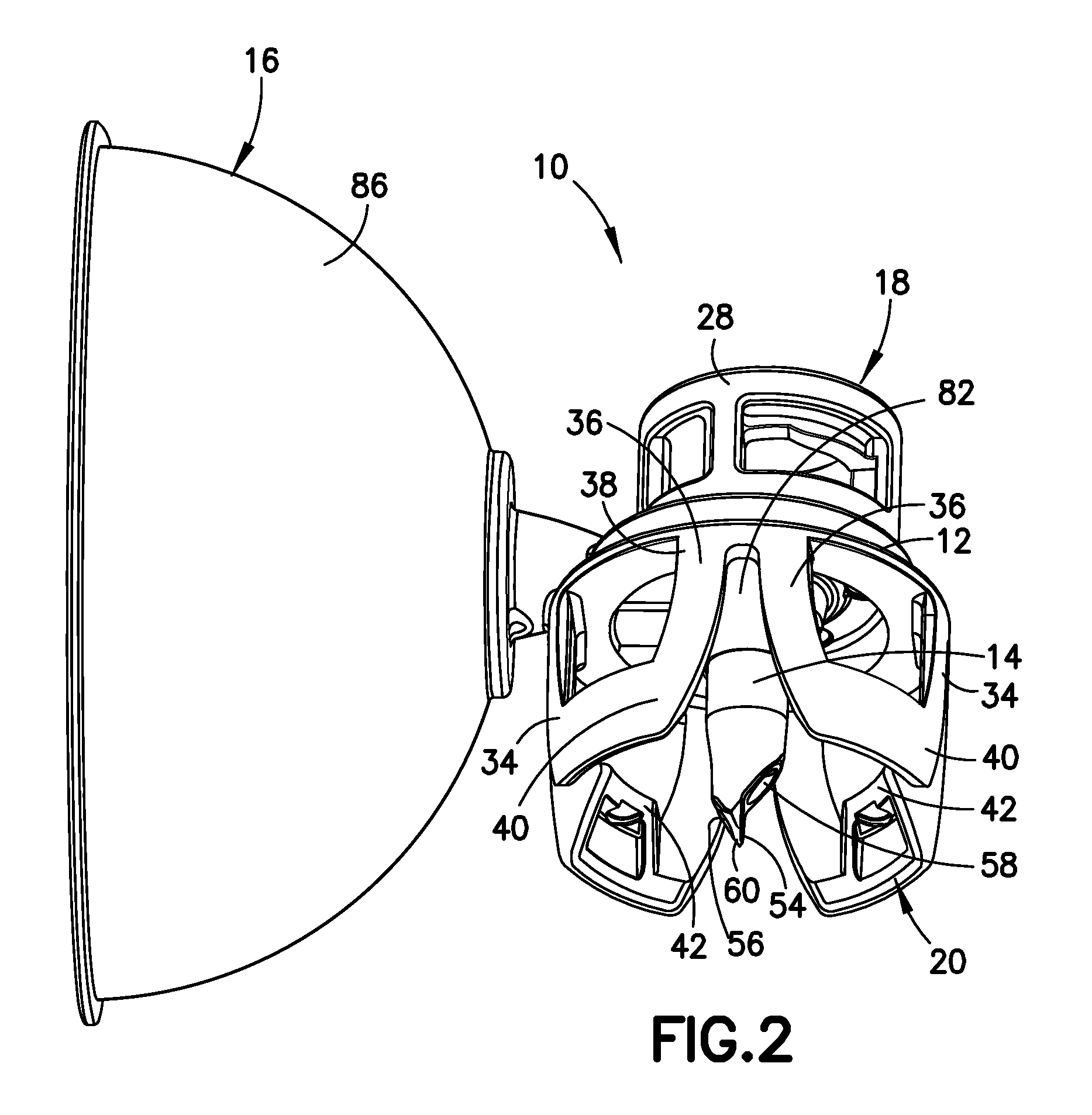
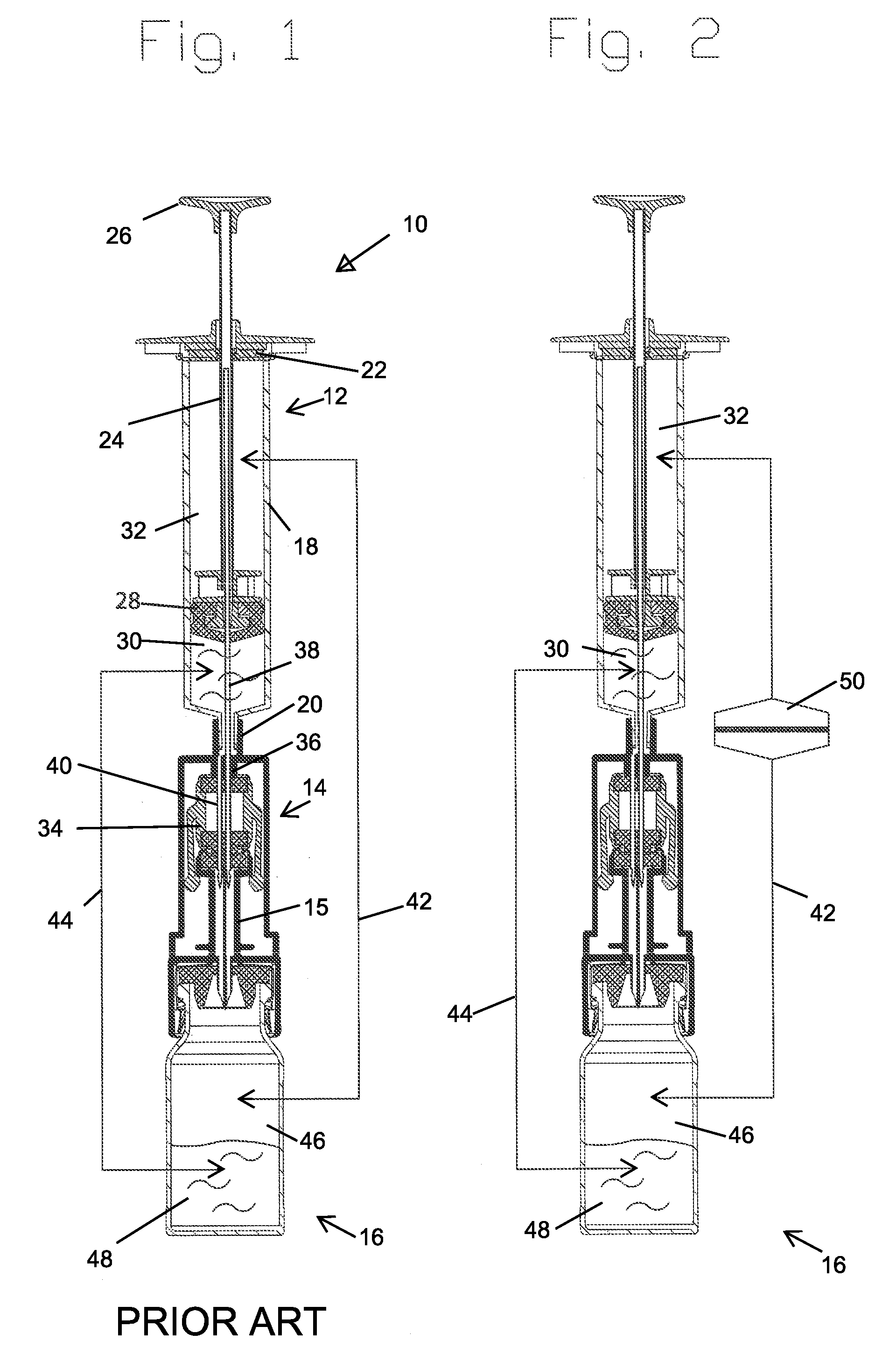
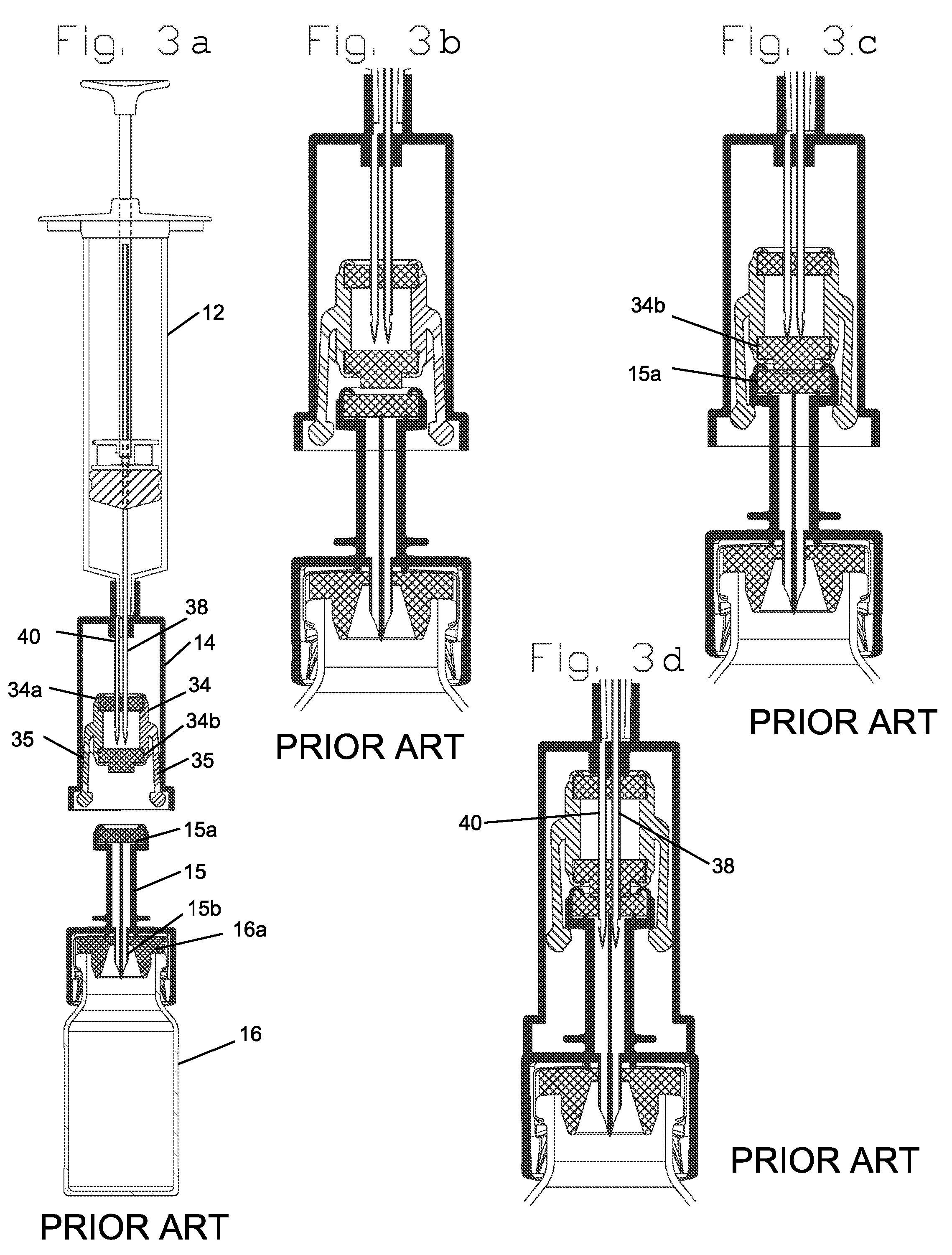
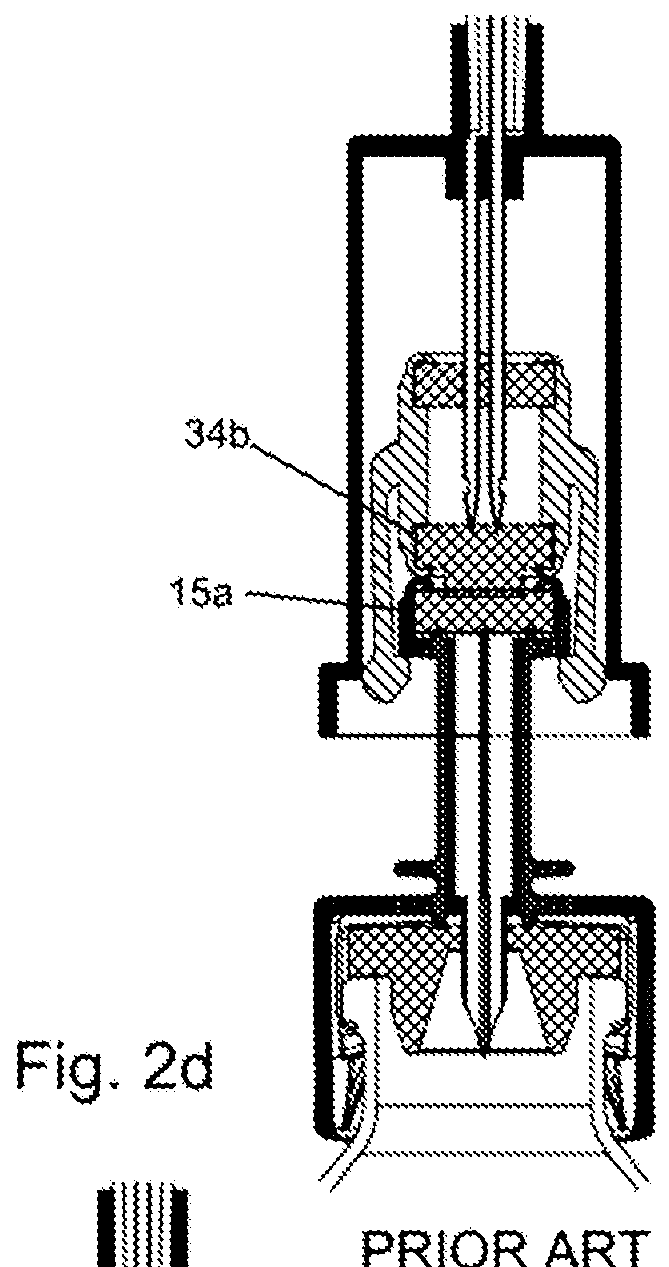
A closed system drug transfer device or "CSTD" is a drug transfer device that mechanically prohibits the transfer of environmental contaminants into a system and the escape of hazardous drug or vapor concentrations outside the system. Open versus closed systems are commonly applied in medical devices to maintain the sterility of a fluid pathway.
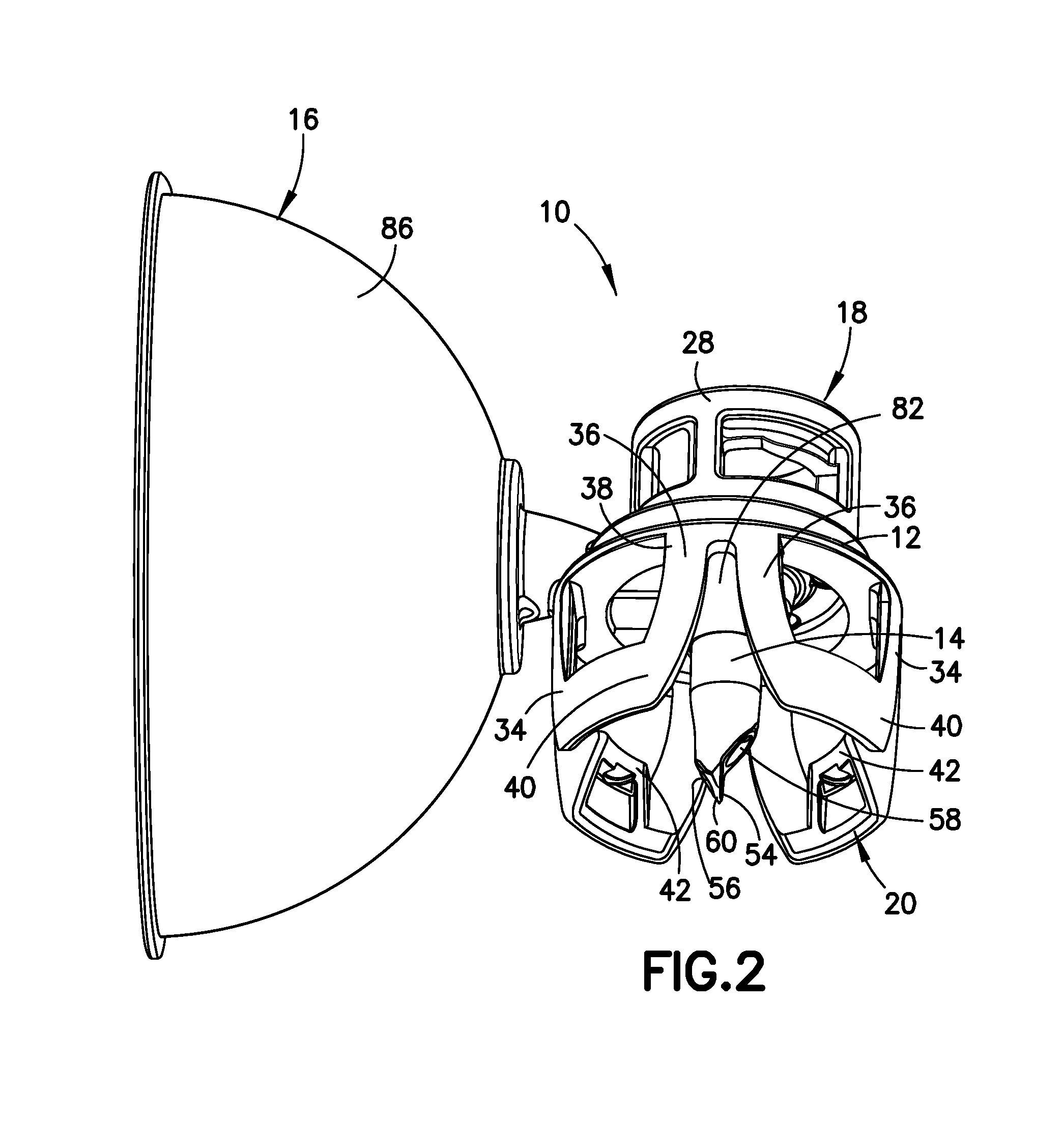
Balloon coating with drug transfer control via coating thickness
InactiveUS20100285085A1Improved coating transfer efficiencyPromote absorptionBiocideGlovesMedical deviceBlood vessel
Owner:ABBOTT CARDIOVASCULAR
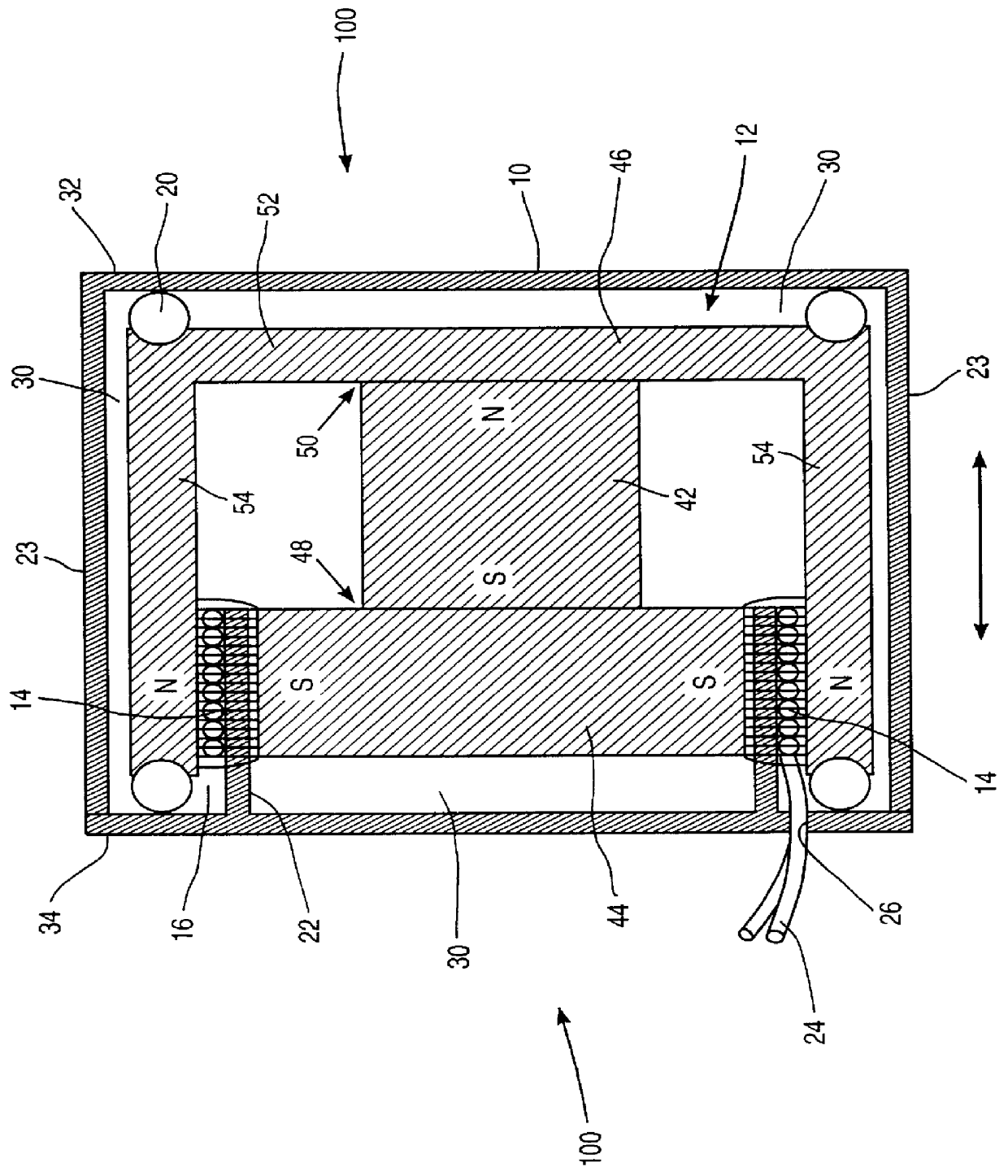
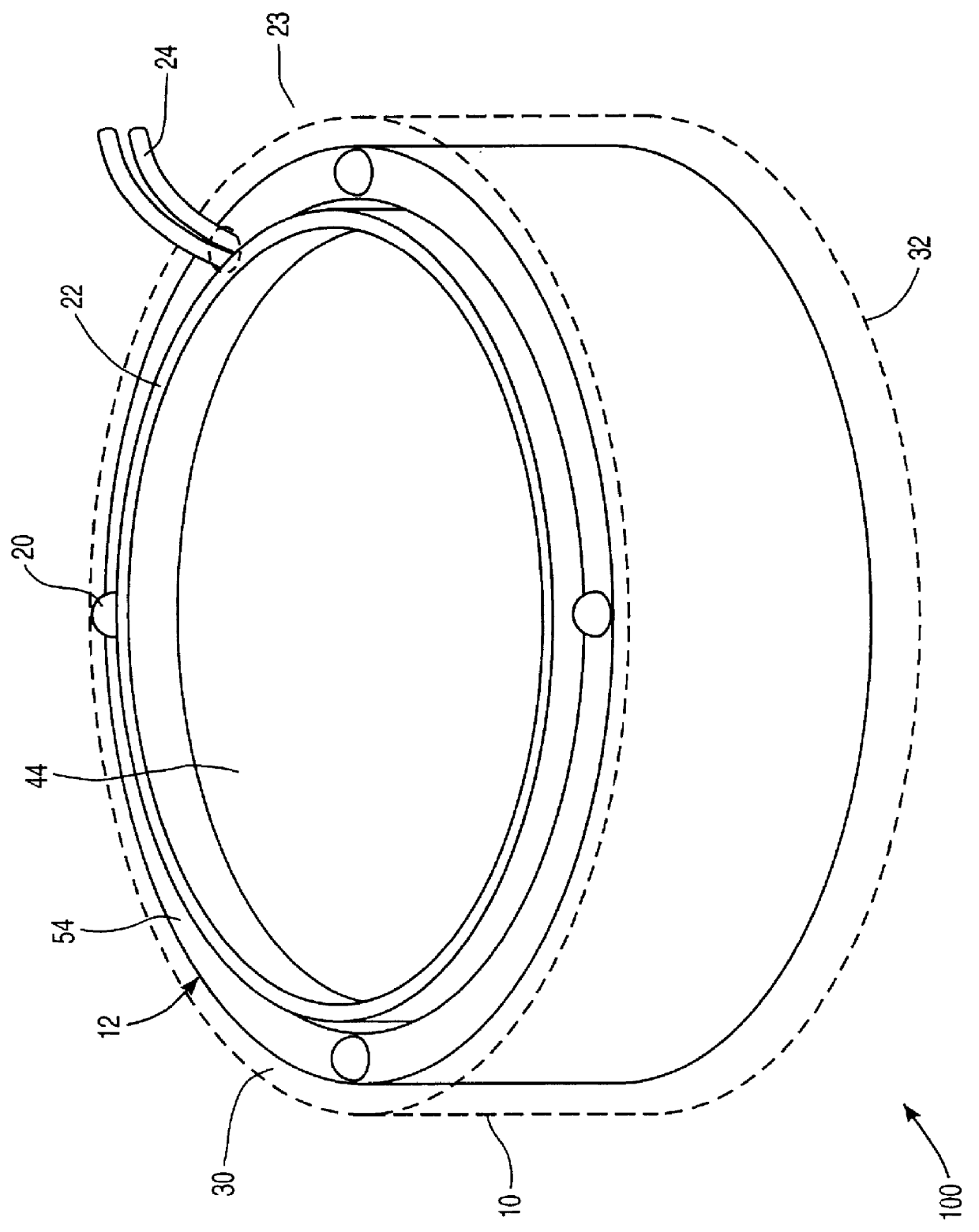
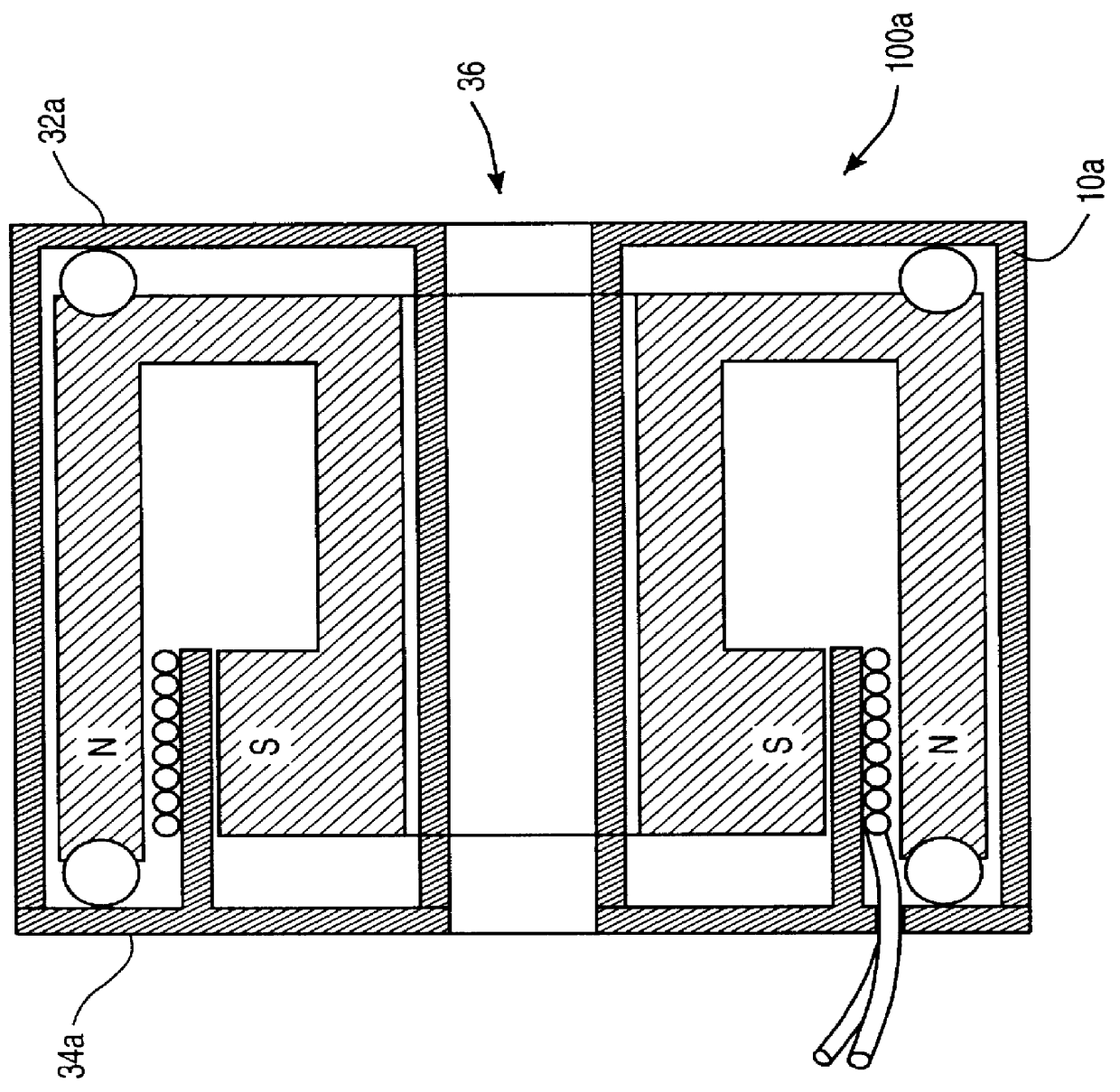
Apparatus and method for sonically enhanced drug delivery
Apparatus for delivering medicament media into tissue comprises a medicament supply assembly and an oscillatory drive assembly. The medicament supply assembly includes a medicament transfer surface, and the oscillatory drive assembly includes a housing, a coil mounted within the housing, and a magnet suspended within the housing. By applying an electrical drive signal to the coil, the housing can be oscillated to phonophoretically enhance delivery of medicament from the medicament transfer surface into tissue.
Owner:MED EL ELEKTROMEDIZINISCHE GERAETE GMBH +1
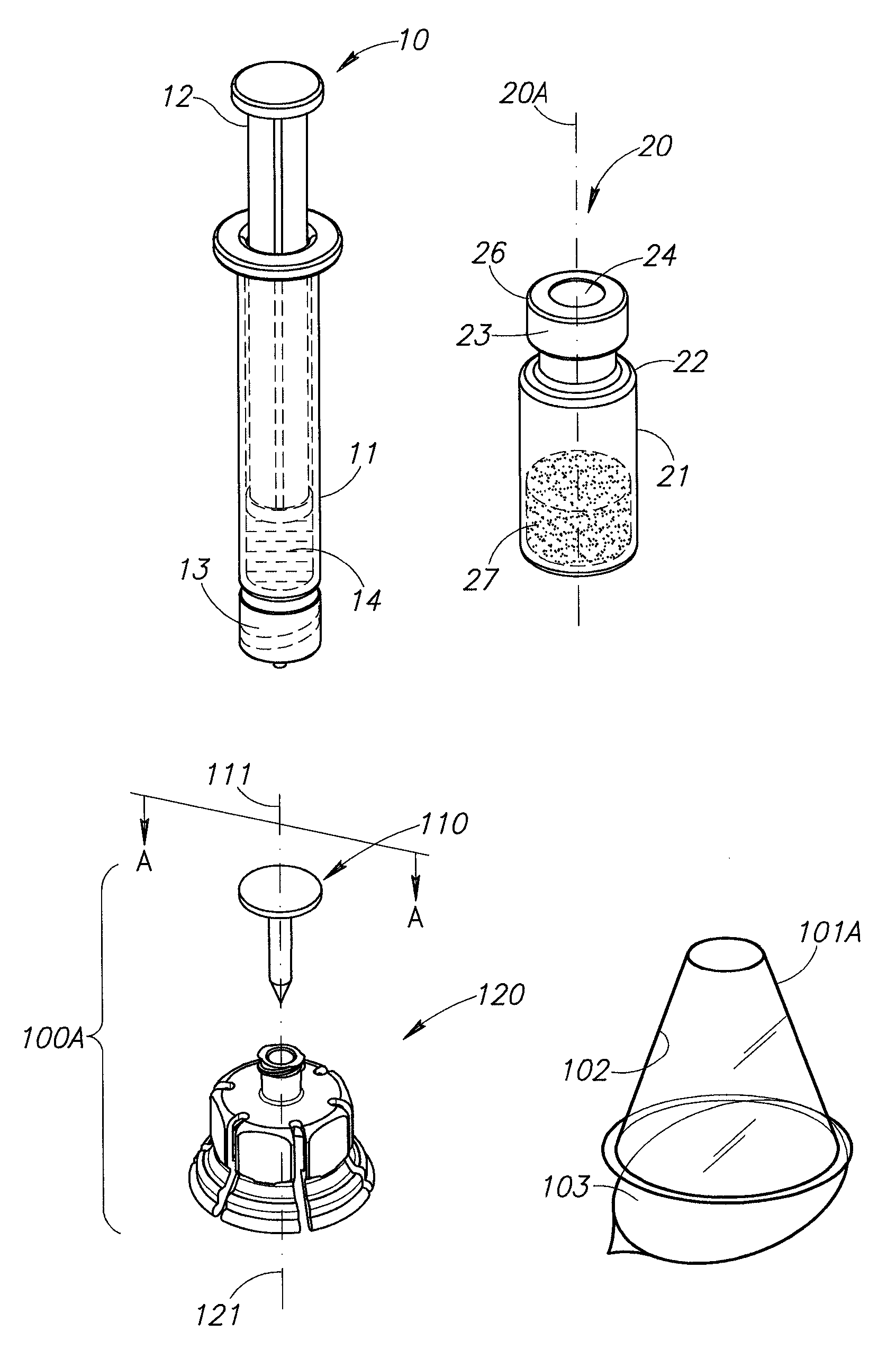
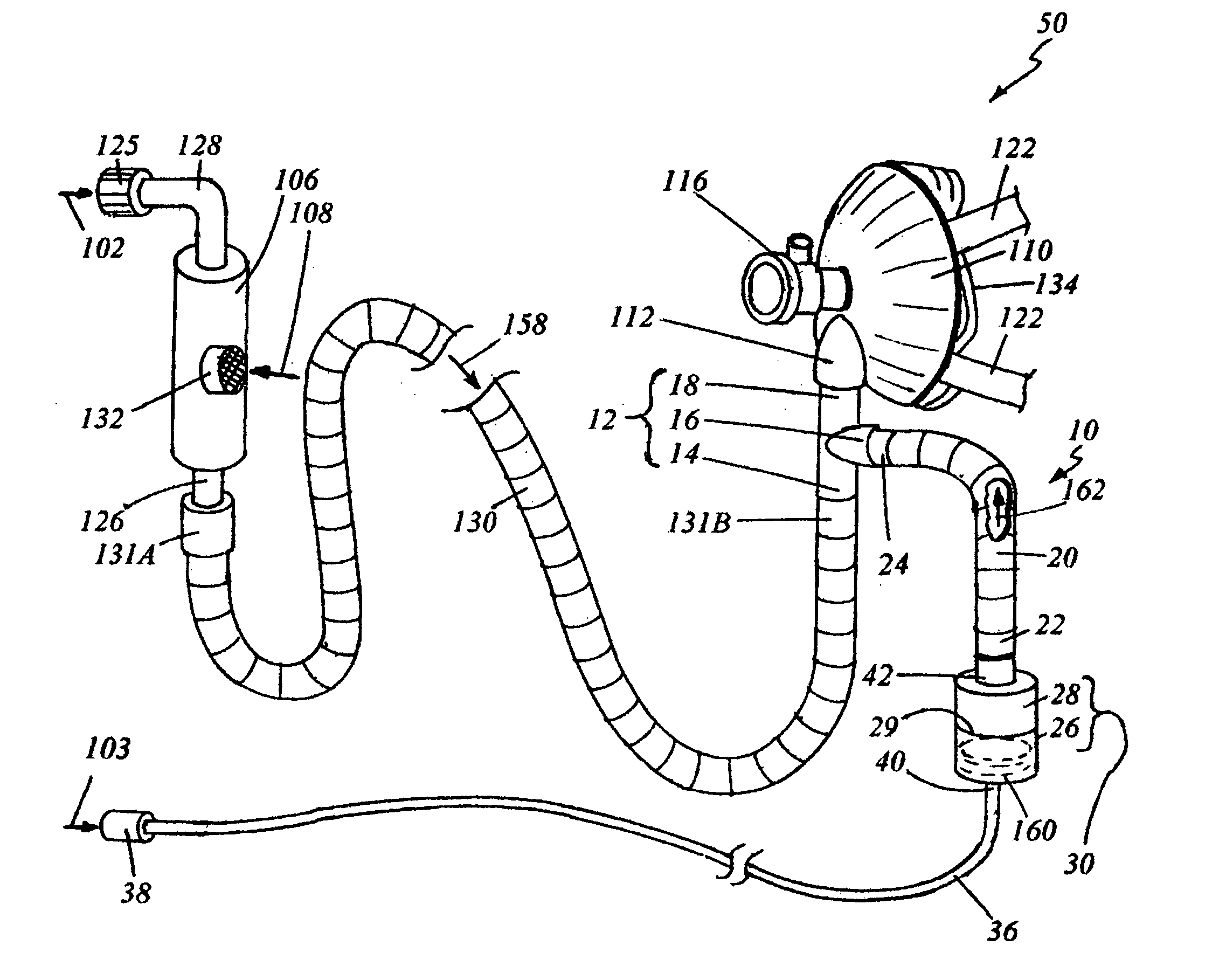
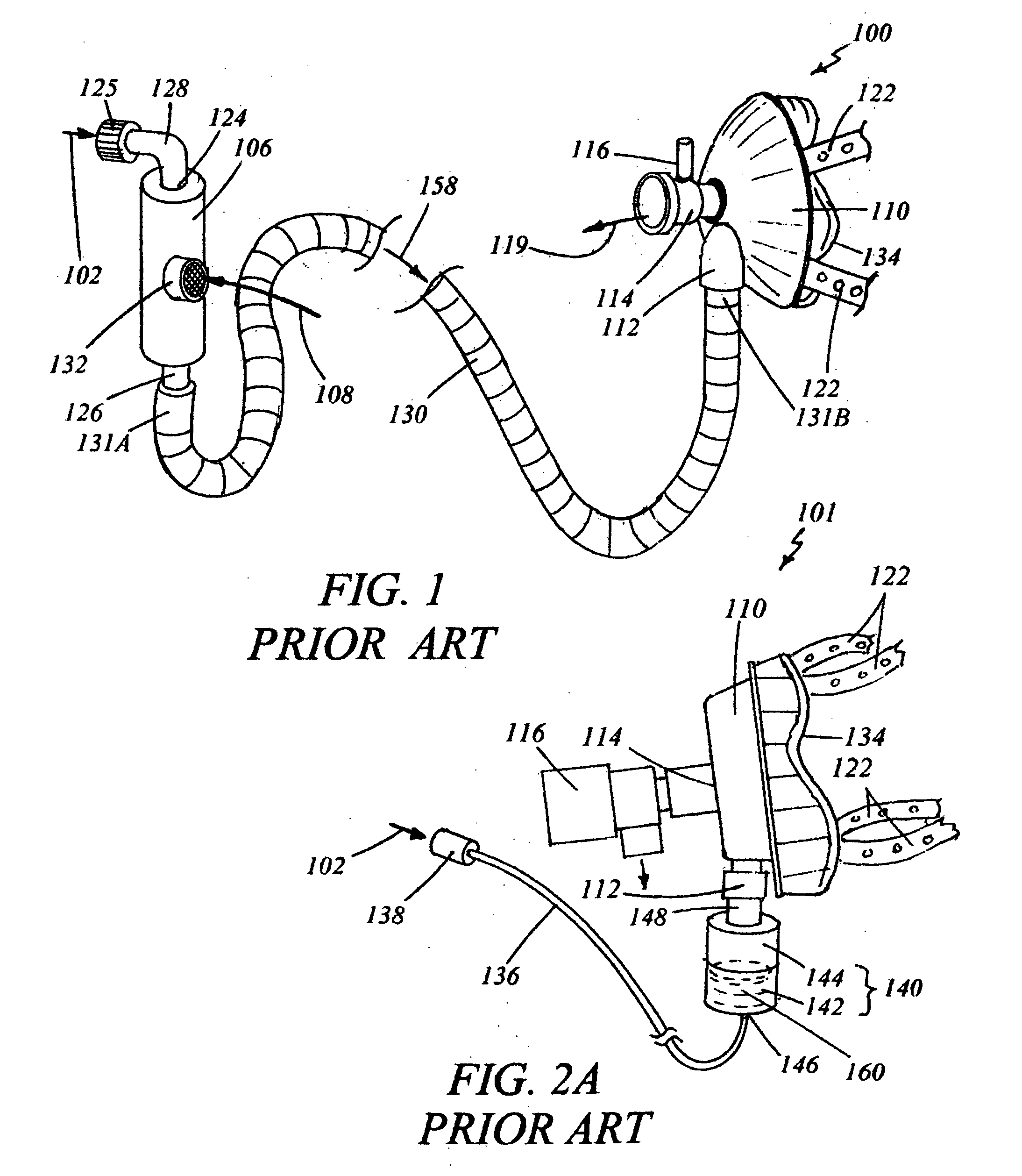
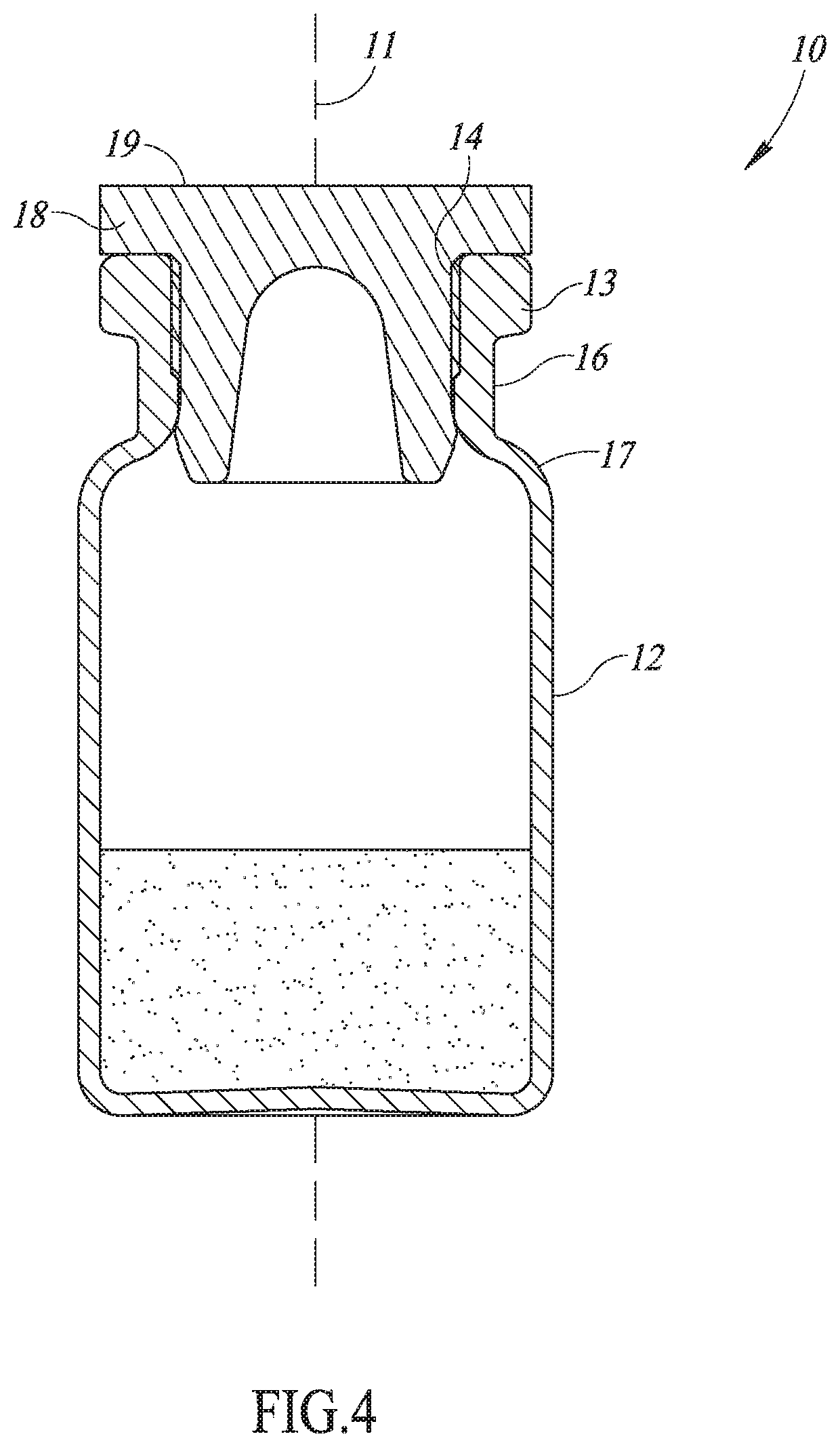
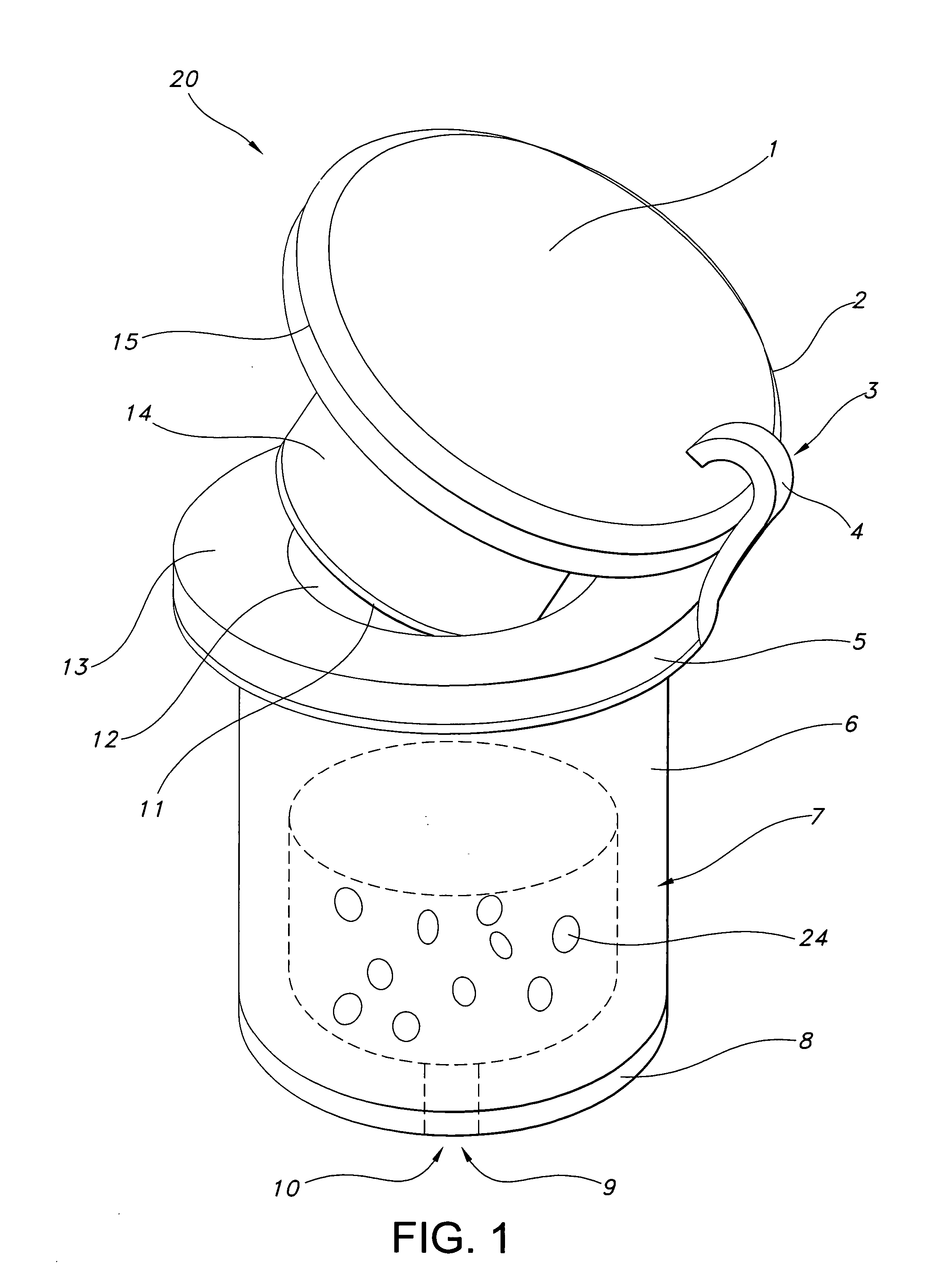
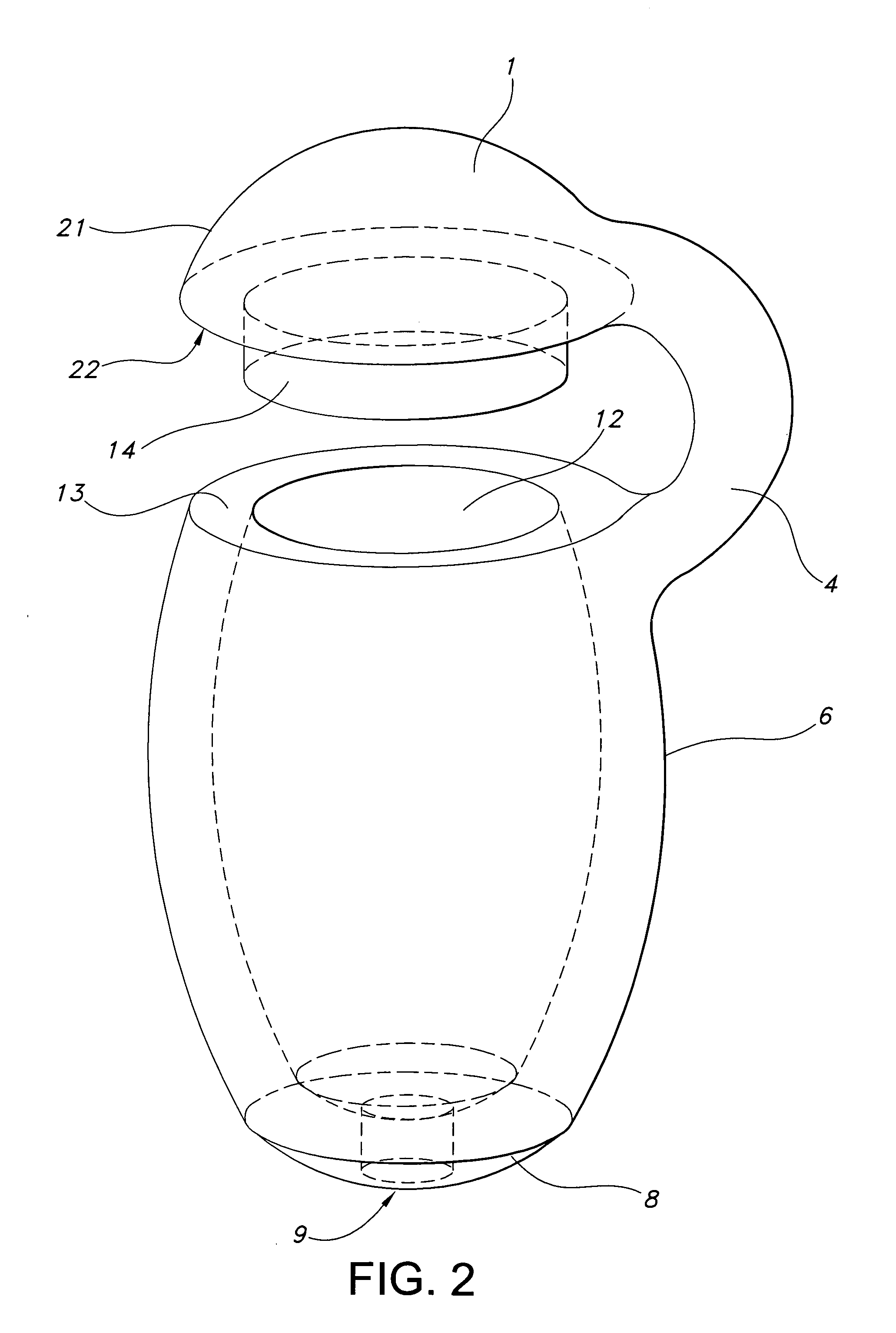
Medical vial access device with pressure equalization and closed drug transfer system and method utilizing same
Owner:BECTON DICKINSON & CO LTD
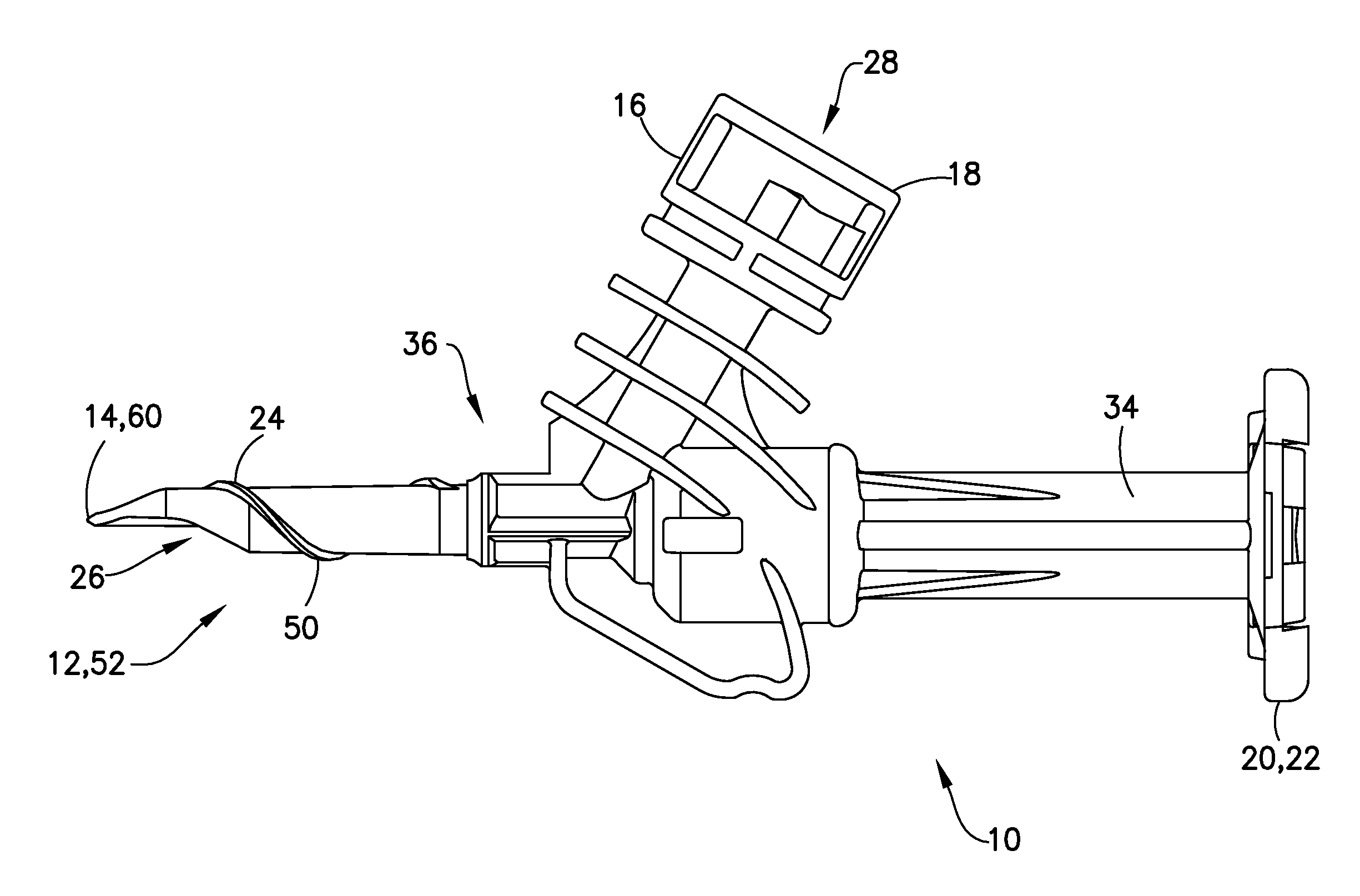
Liquid drug transfer assembly
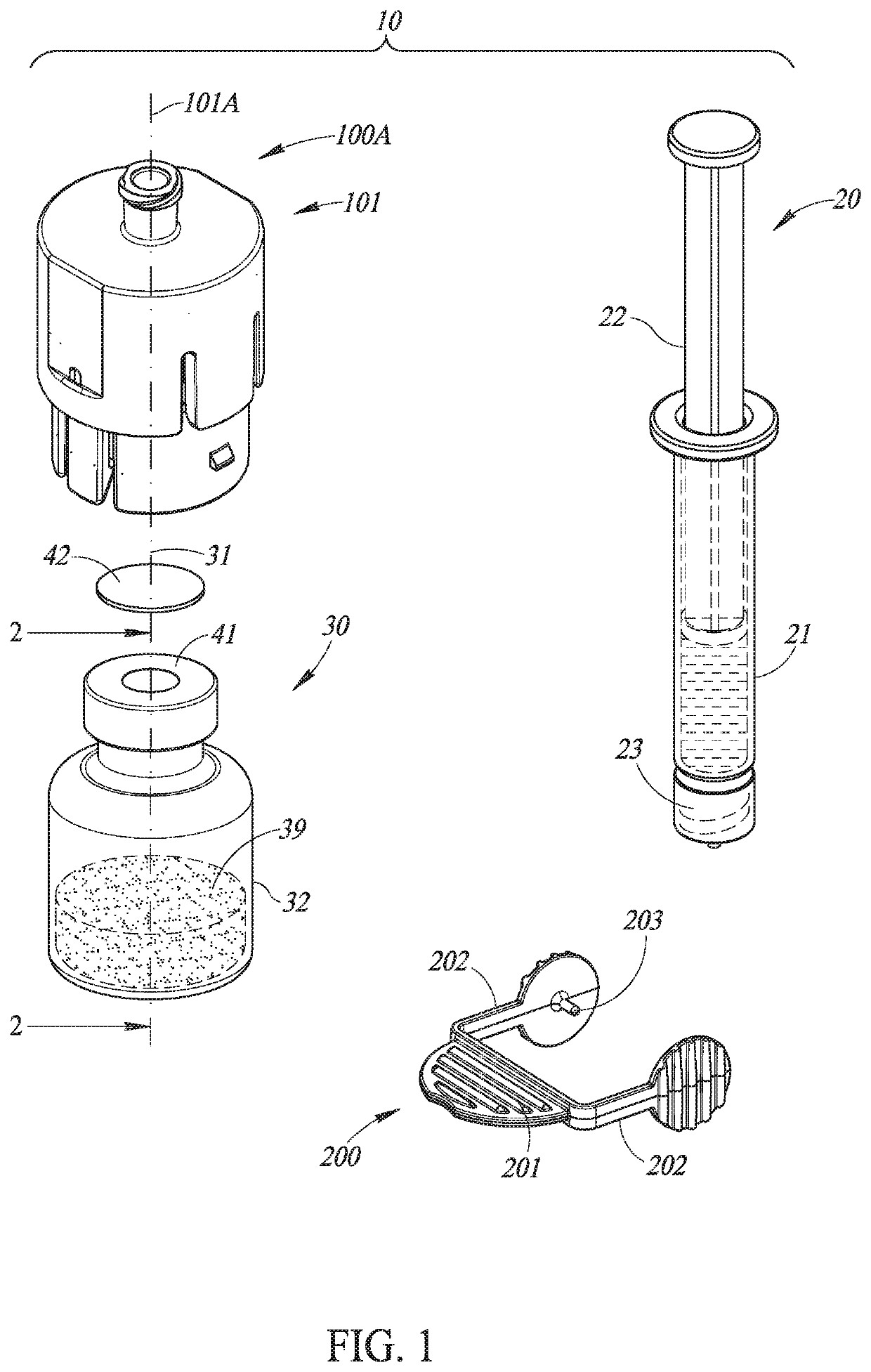
Liquid drug transfer assemblies for use with a drug vial having a drug vial opening stopped by a drug vial stopper. The liquid drug transfer assemblies include a drug vial stopper puncturing member for puncturing a drug vial stopper. The liquid drug transfer assemblies also include a drug vial adapter having a drug vial adapter skirt, an upright drug vial adapter port and a drug vial adapter sleeve downward depending opposite the upright drug vial adapter port and in flow communication therewith. The drug vial adapter is slidingly disposed on the drug vial stopper puncturing member such that on mounting the liquid drug transfer assembly on the drug vial, the drug vial stopper puncturing member punctures the drug vial stopper to form a throughgoing puncture bore and the drug vial adapter sleeve lines the puncture bore.
Owner:WEST PHARM SERVICES IL LTD
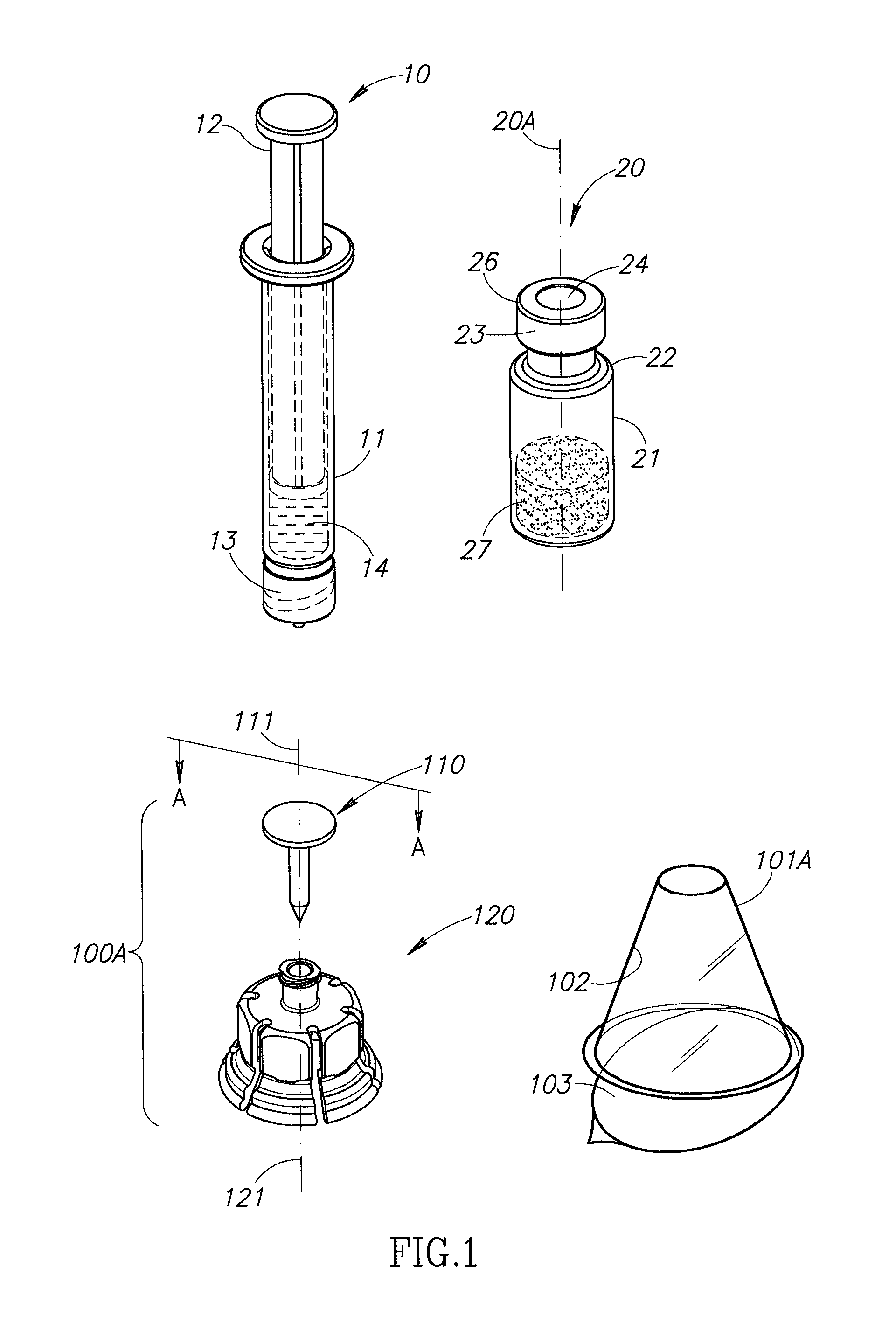
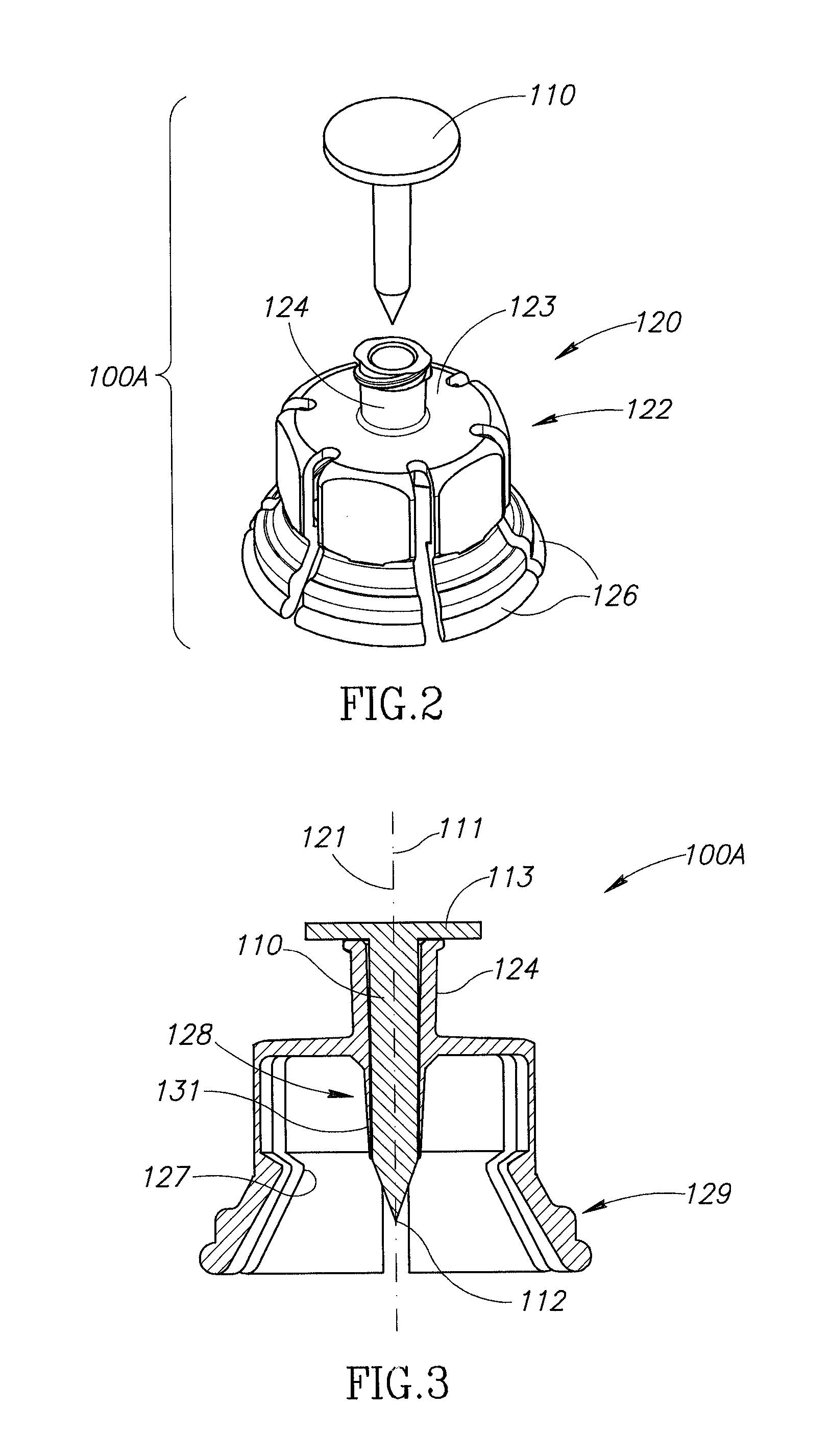
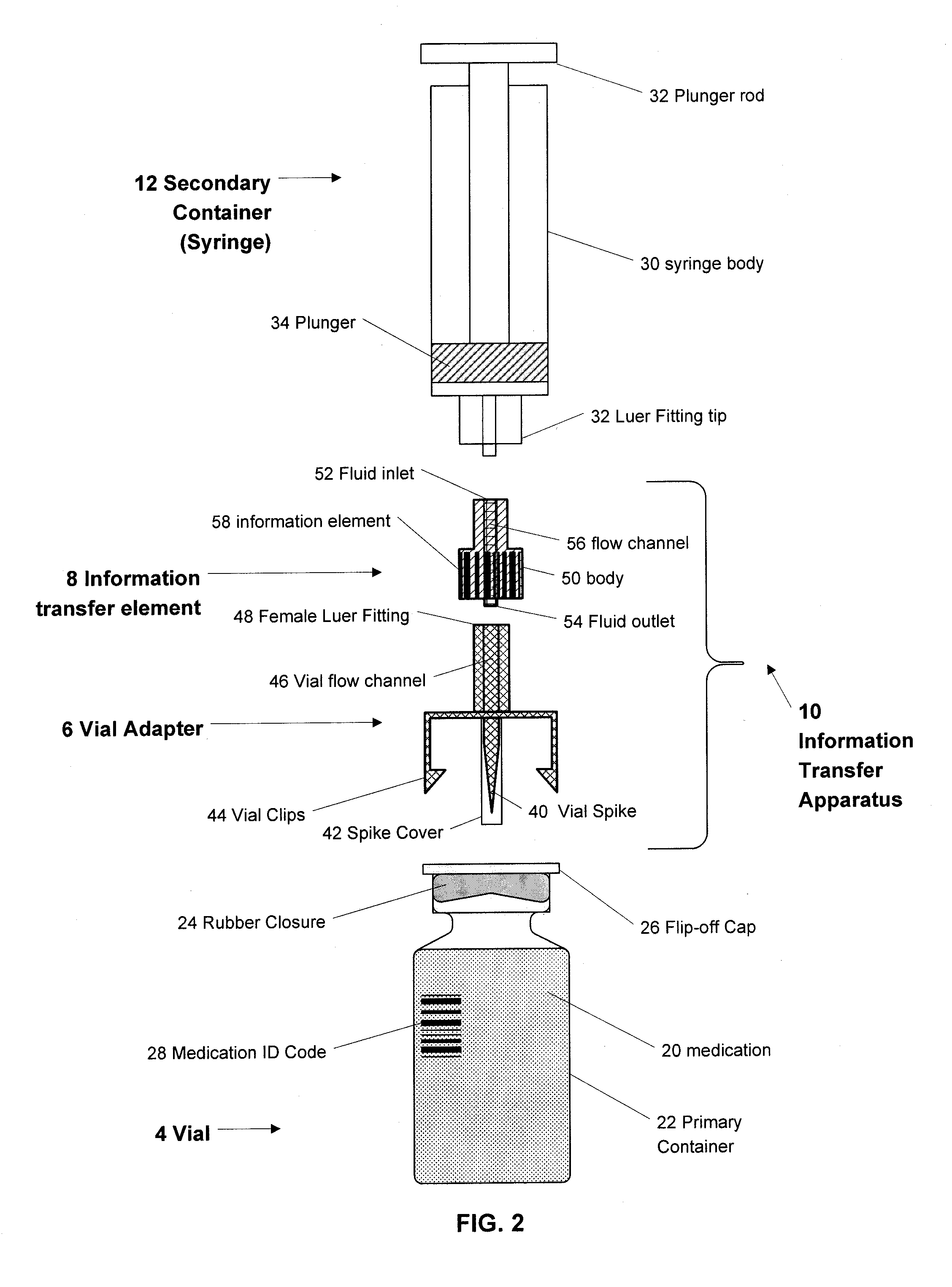
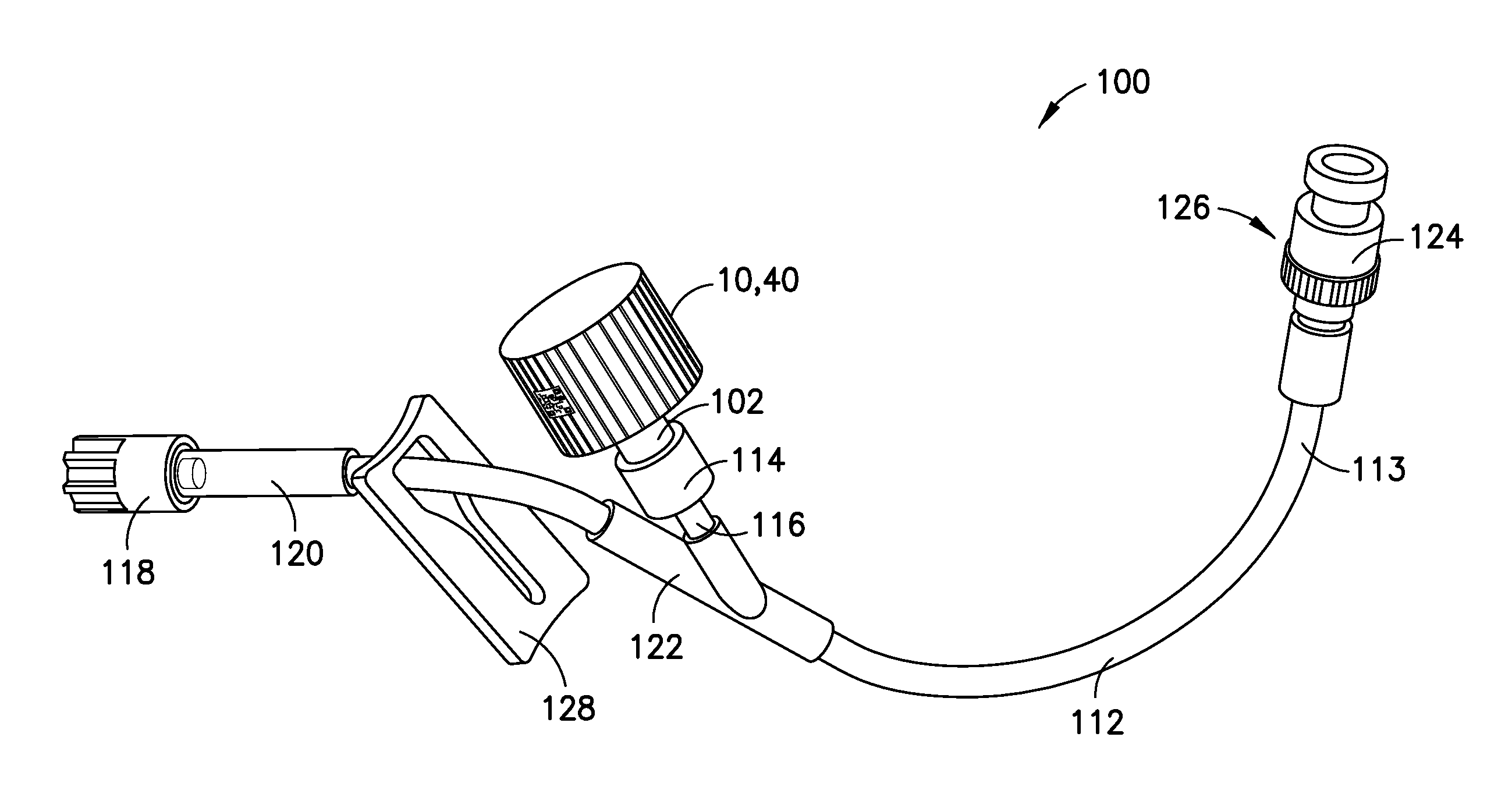
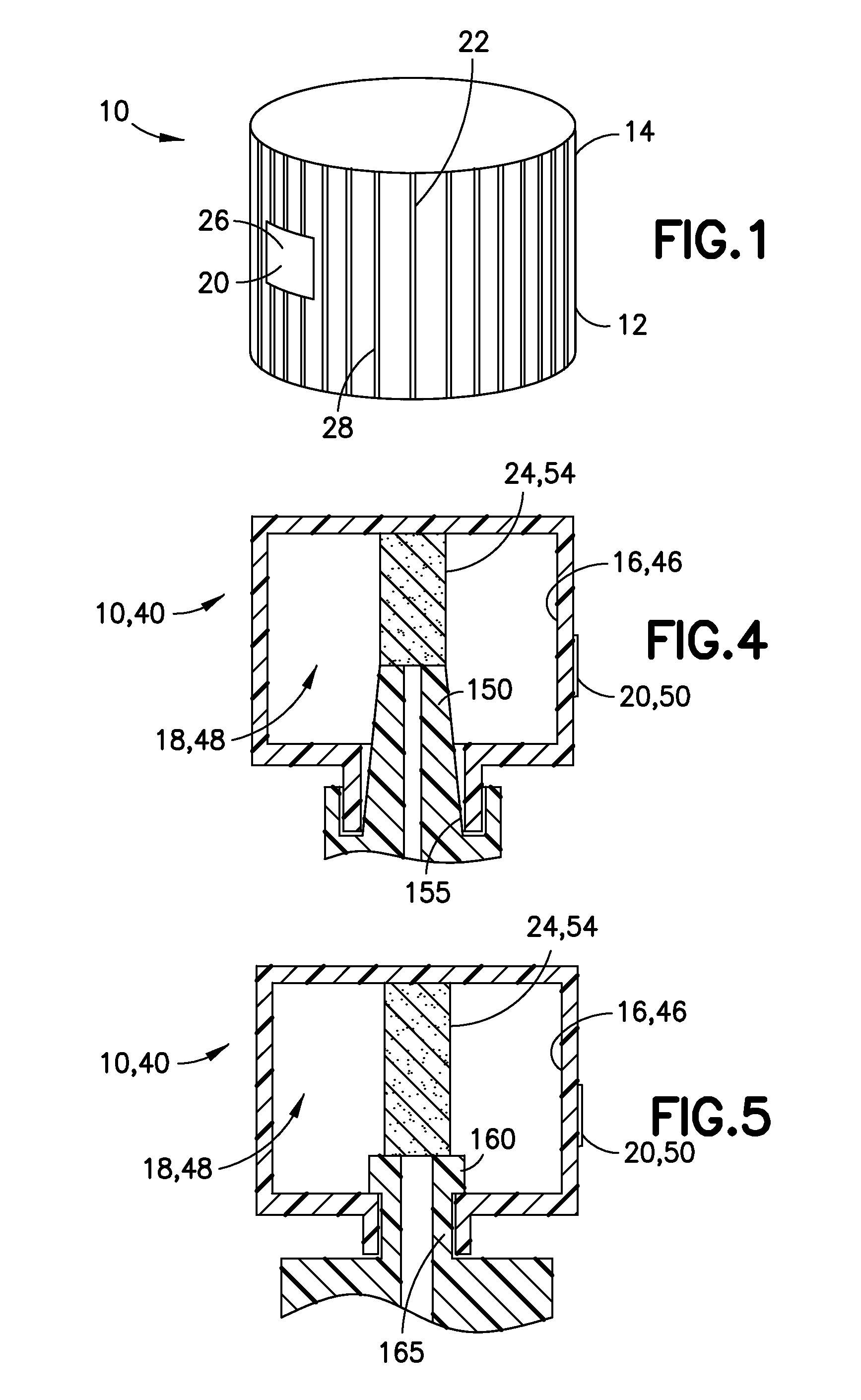
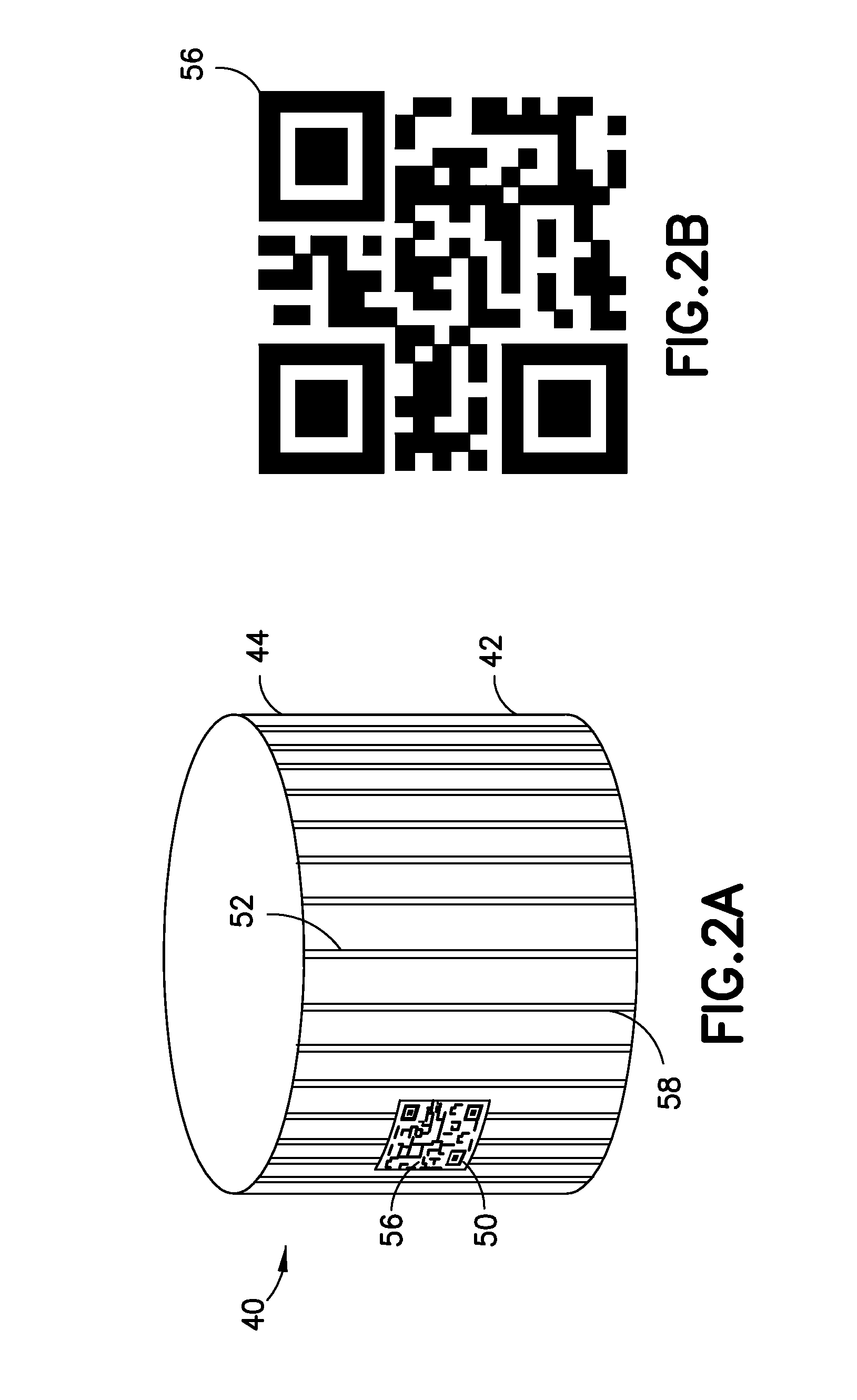
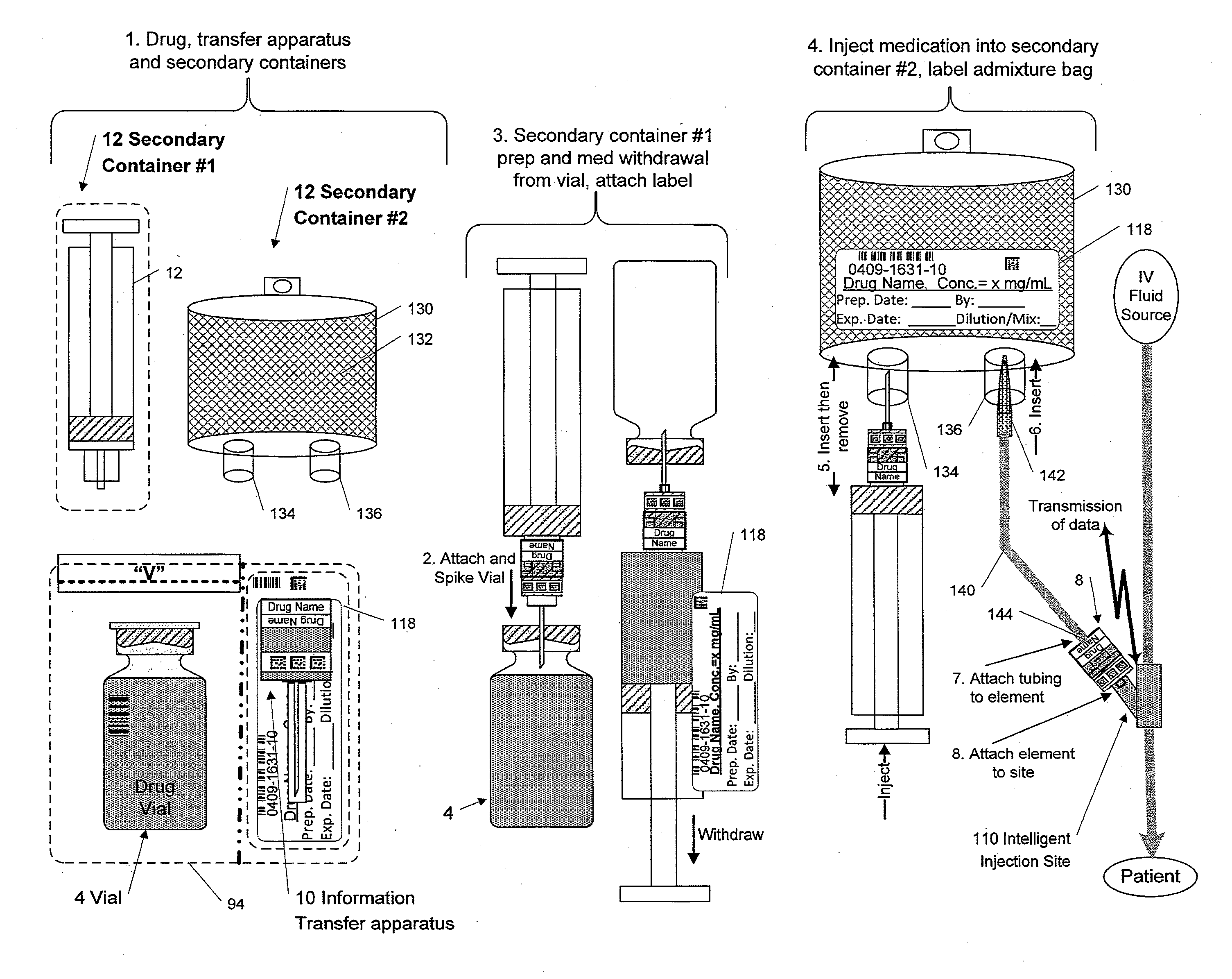
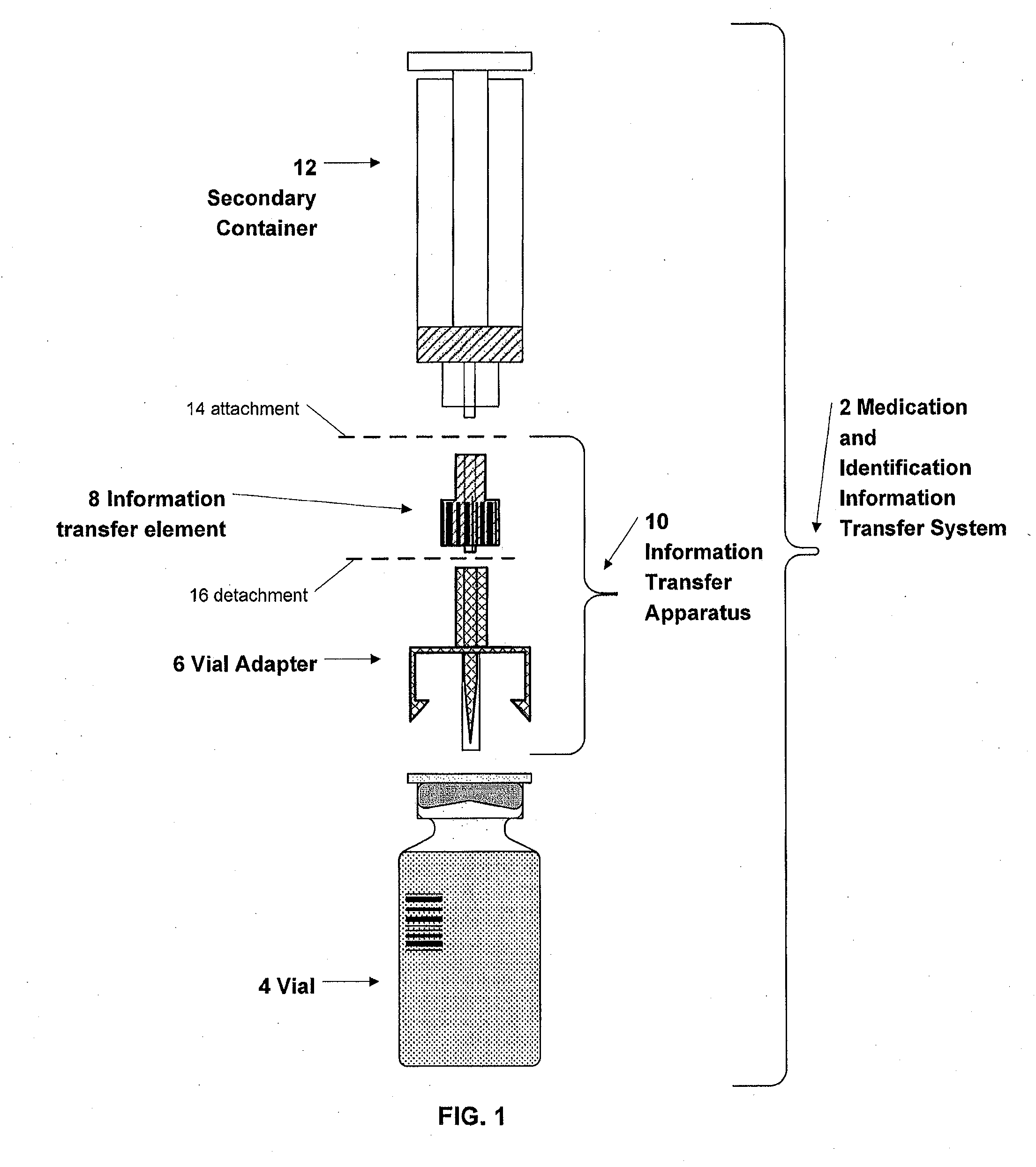
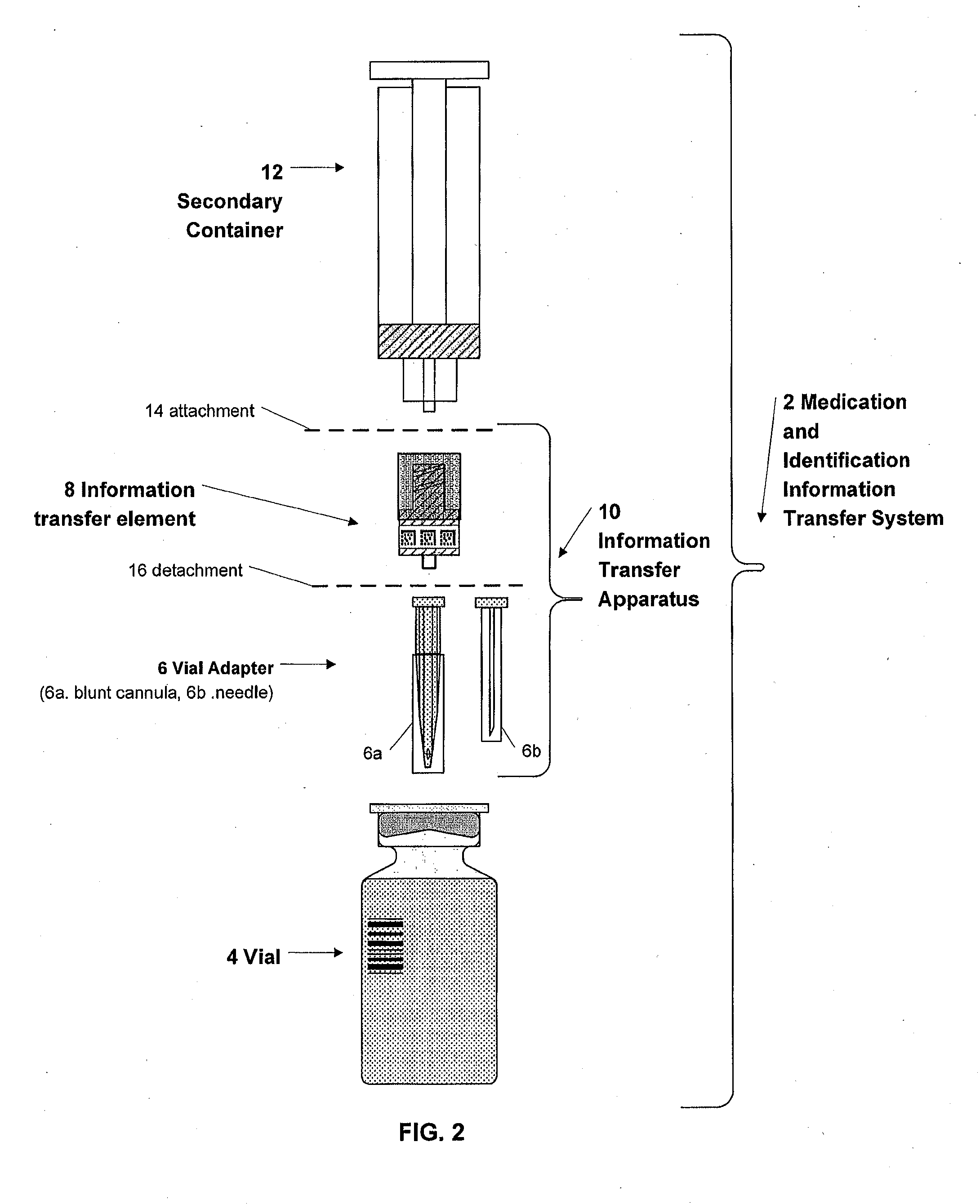
Medication and identification information transfer apparatus
A medication and identification information transfer system is provided that includes a medication vial, a secondary medication container (syringe) and a medication information transfer apparatus. The medication information transfer apparatus, when coupled to a vial, can transfer information indicative of the contents of the vial to an intelligent injection site. The medication information transfer apparatus has a shape and size enabling it to be connected to a vial adapter for removal of medication from the vial transfer it to a syringe for delivery to an injection site while simultaneously transferring information about the medication in the vial to the injection site. In some implementations, the medication injection site can be placed on a fluid delivery line for infusion into a patient. Related apparatus, systems, and kits are also disclosed.
Owner:CRISI MEDICAL SYST
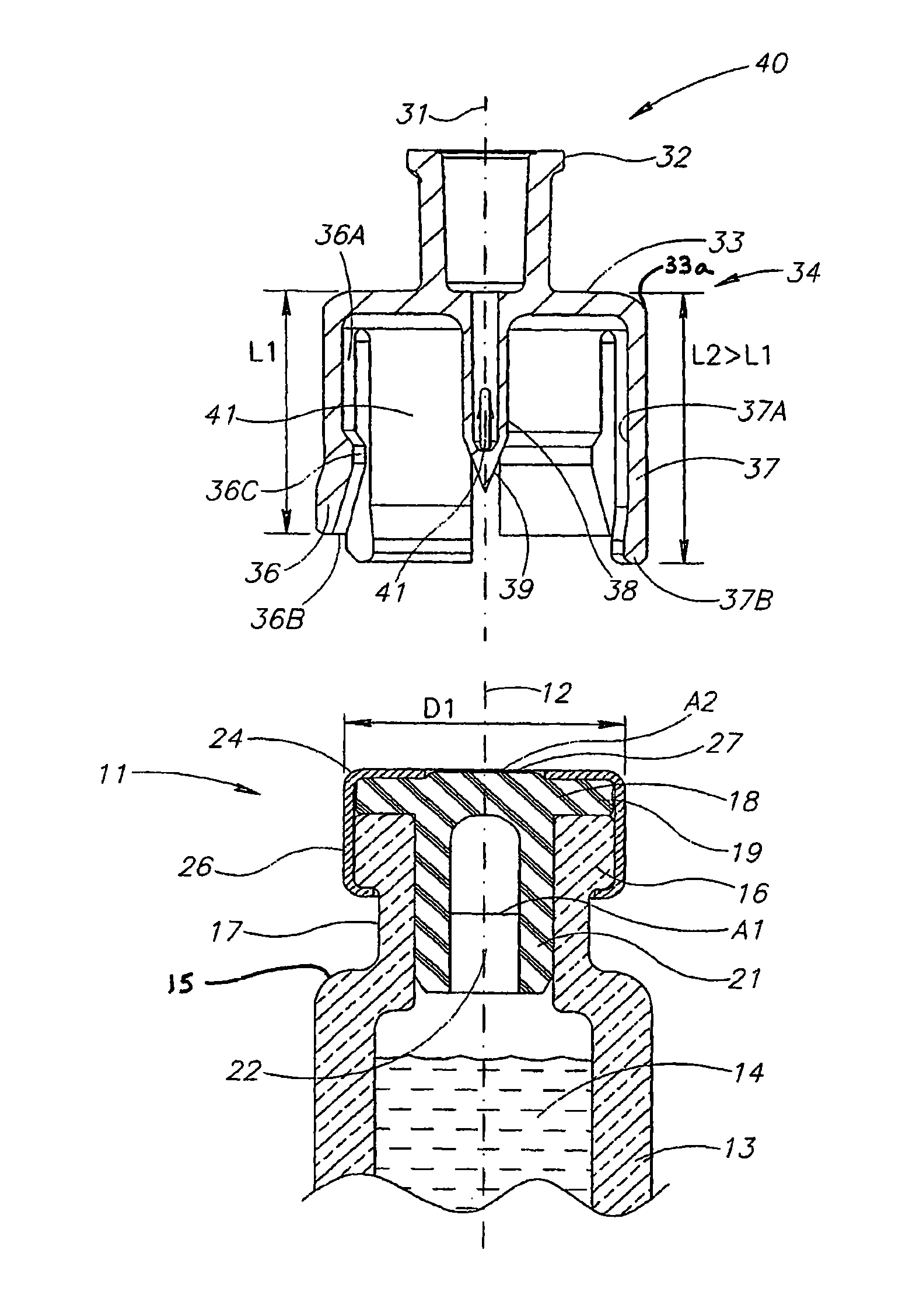
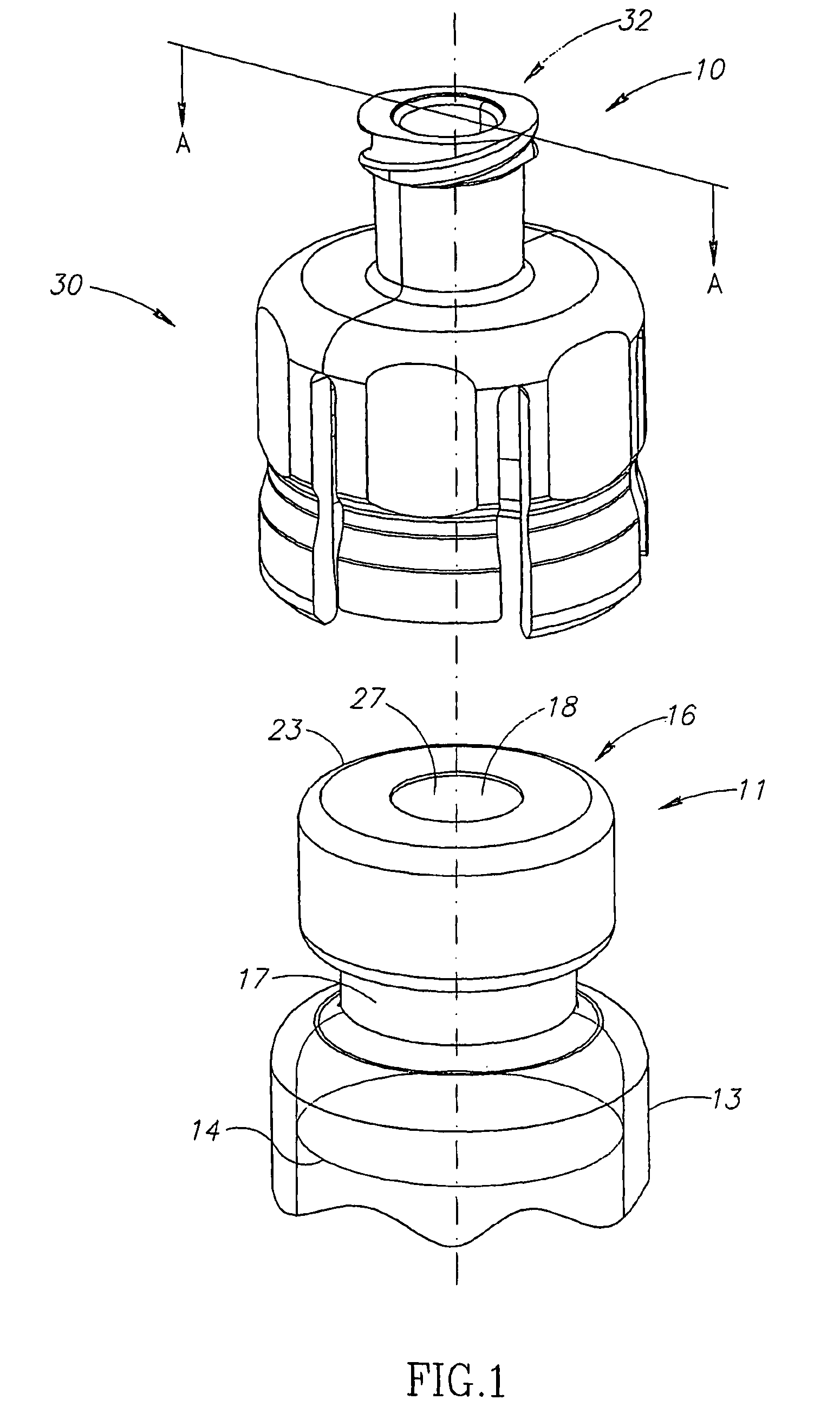
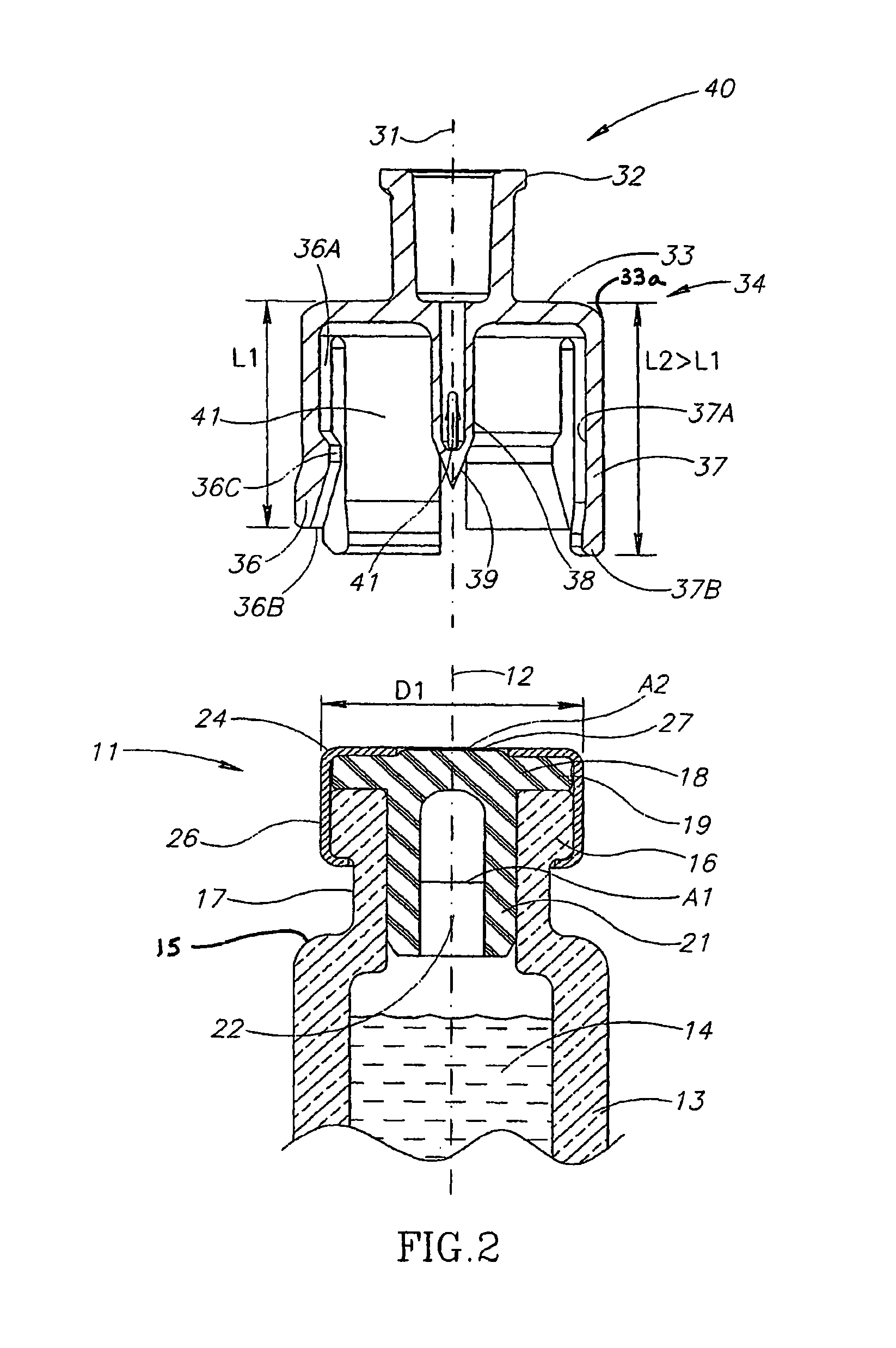
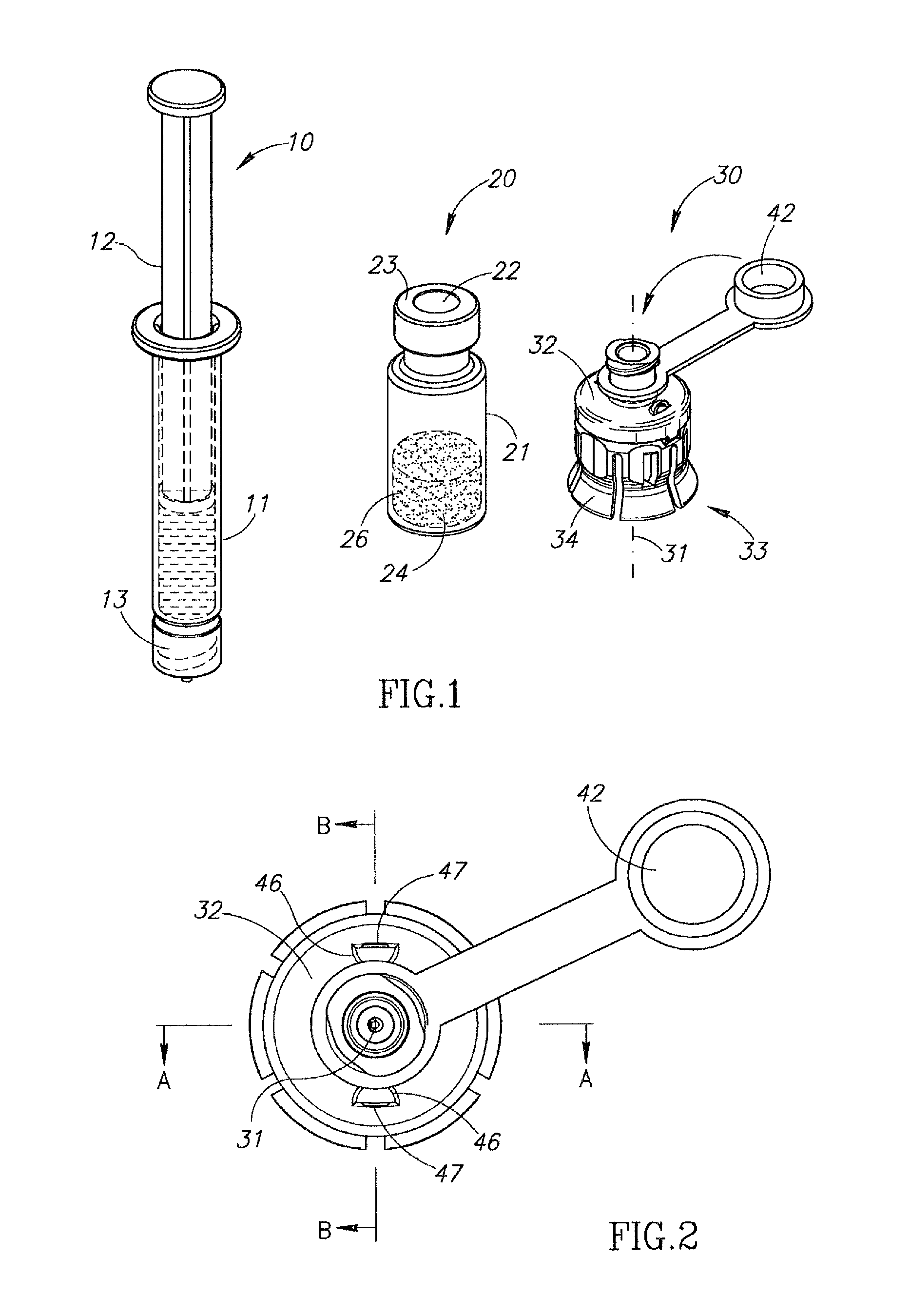
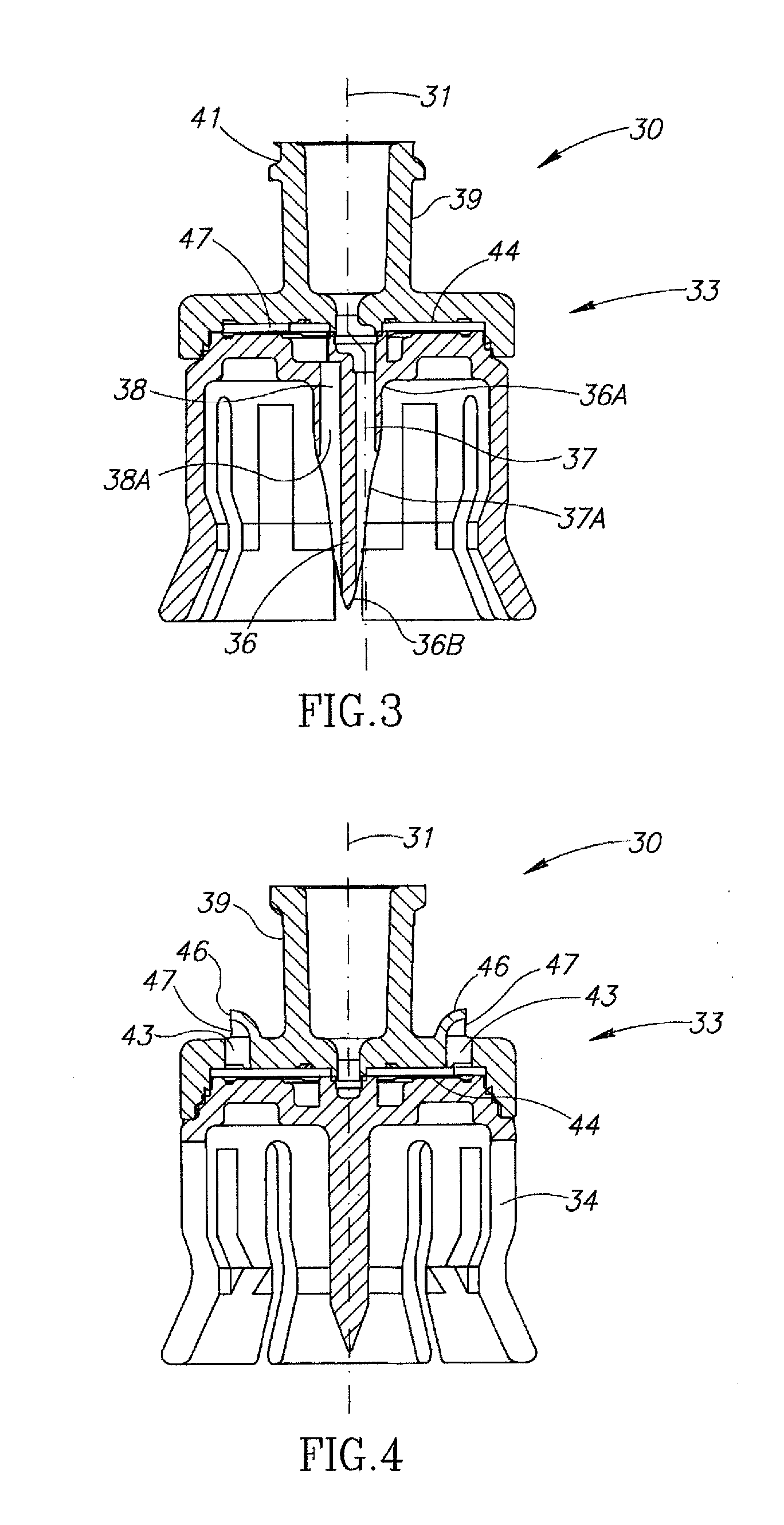
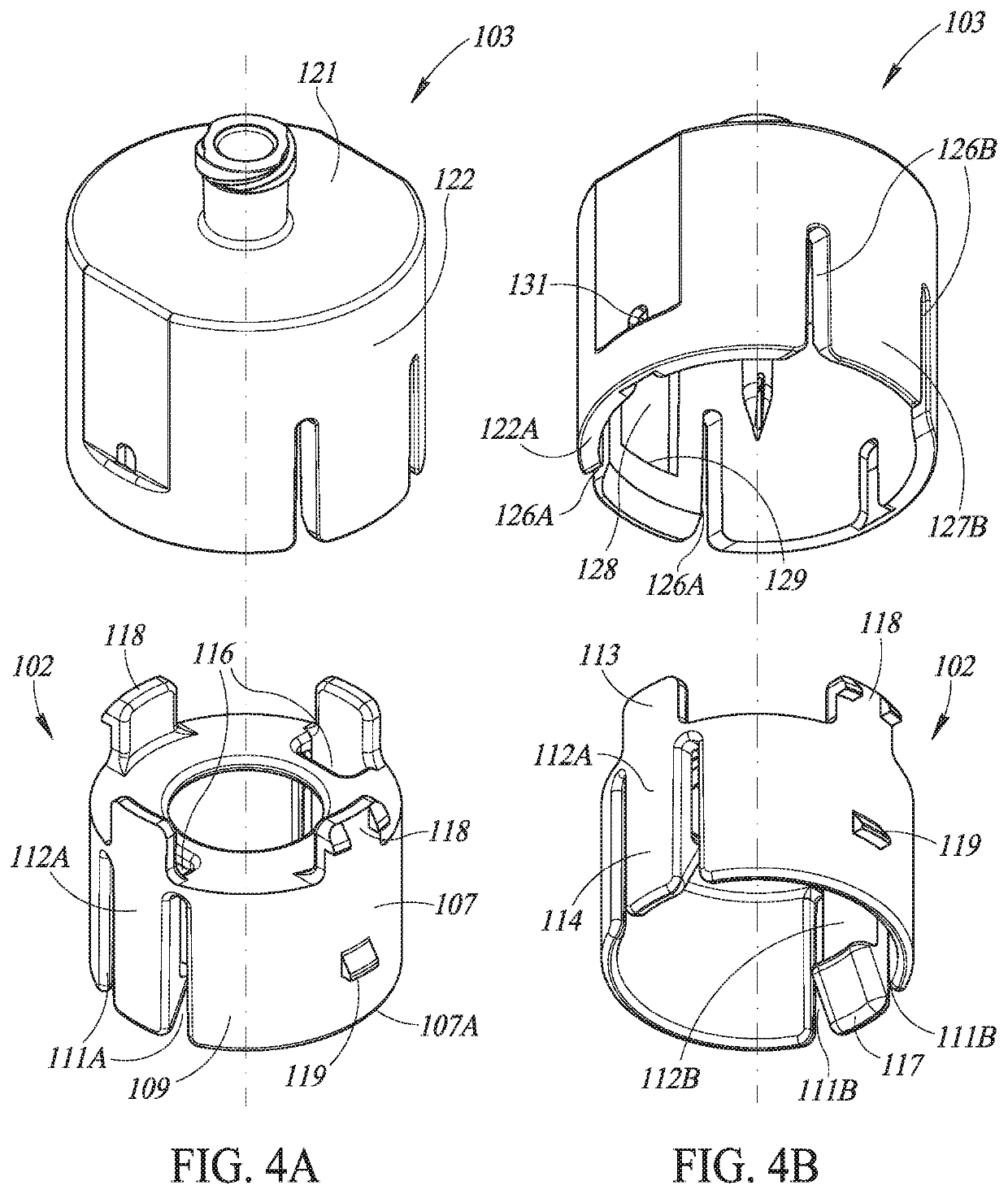
Liquid drug transfer devices for failsafe correct snap fitting onto medicinal vials
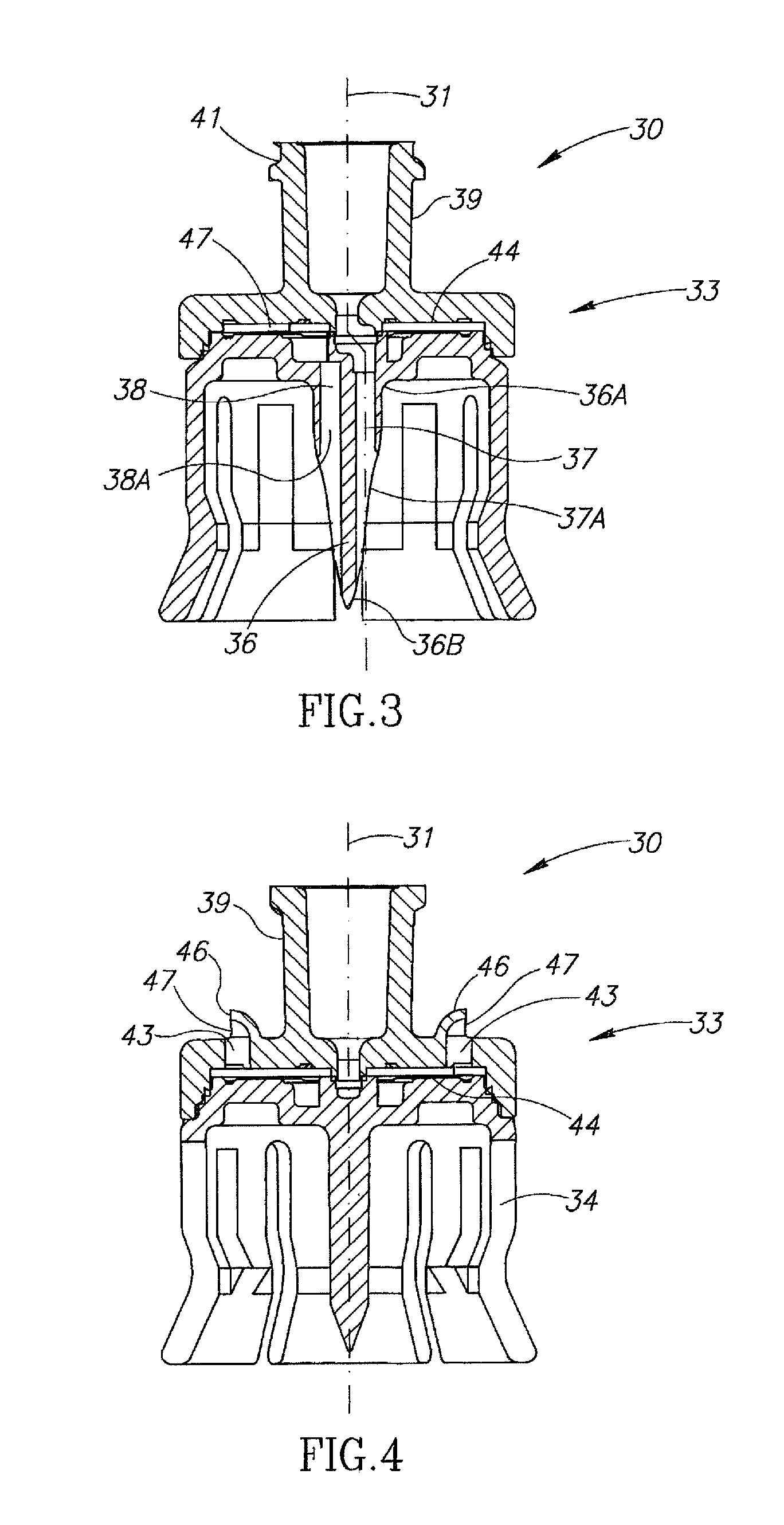
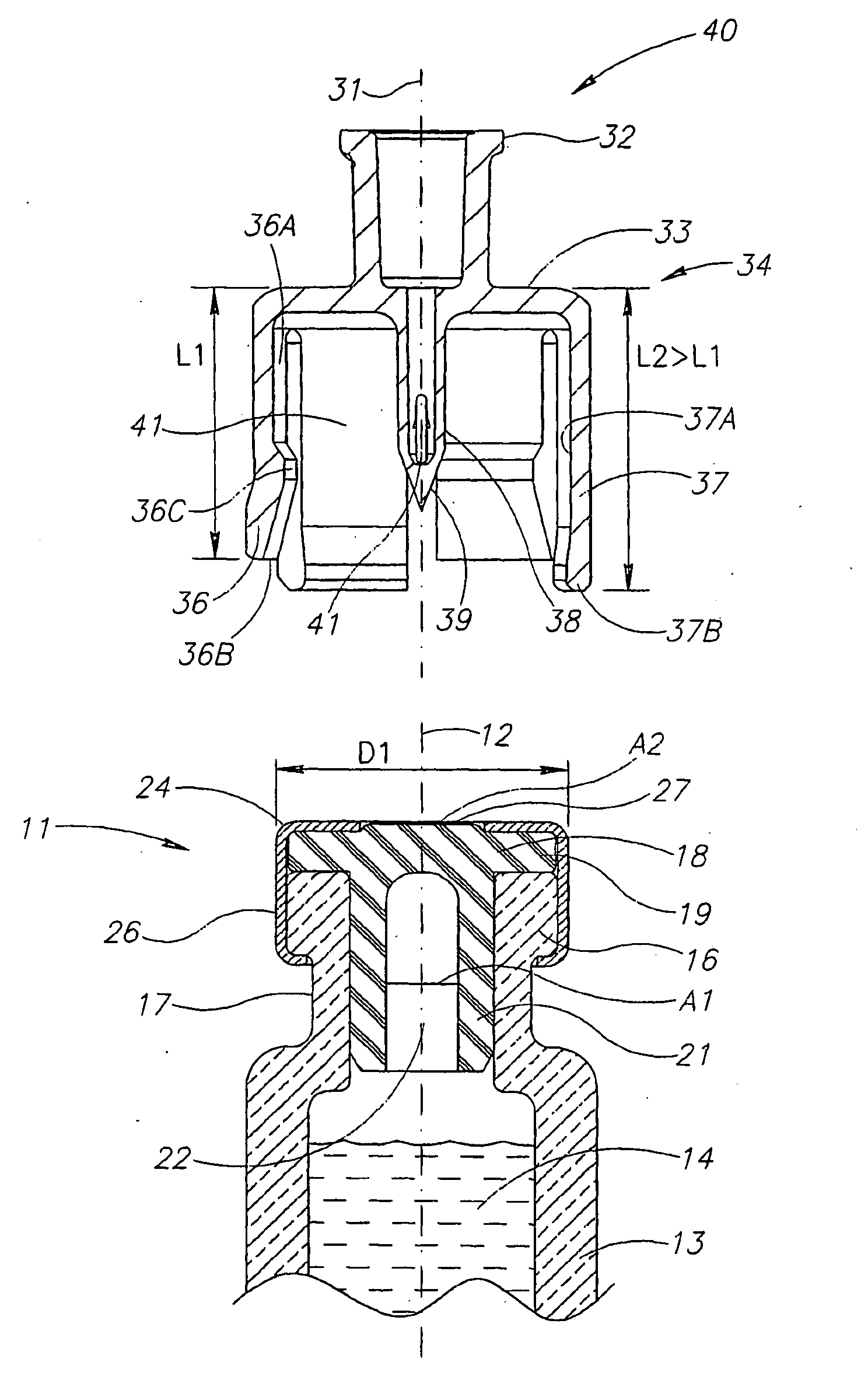
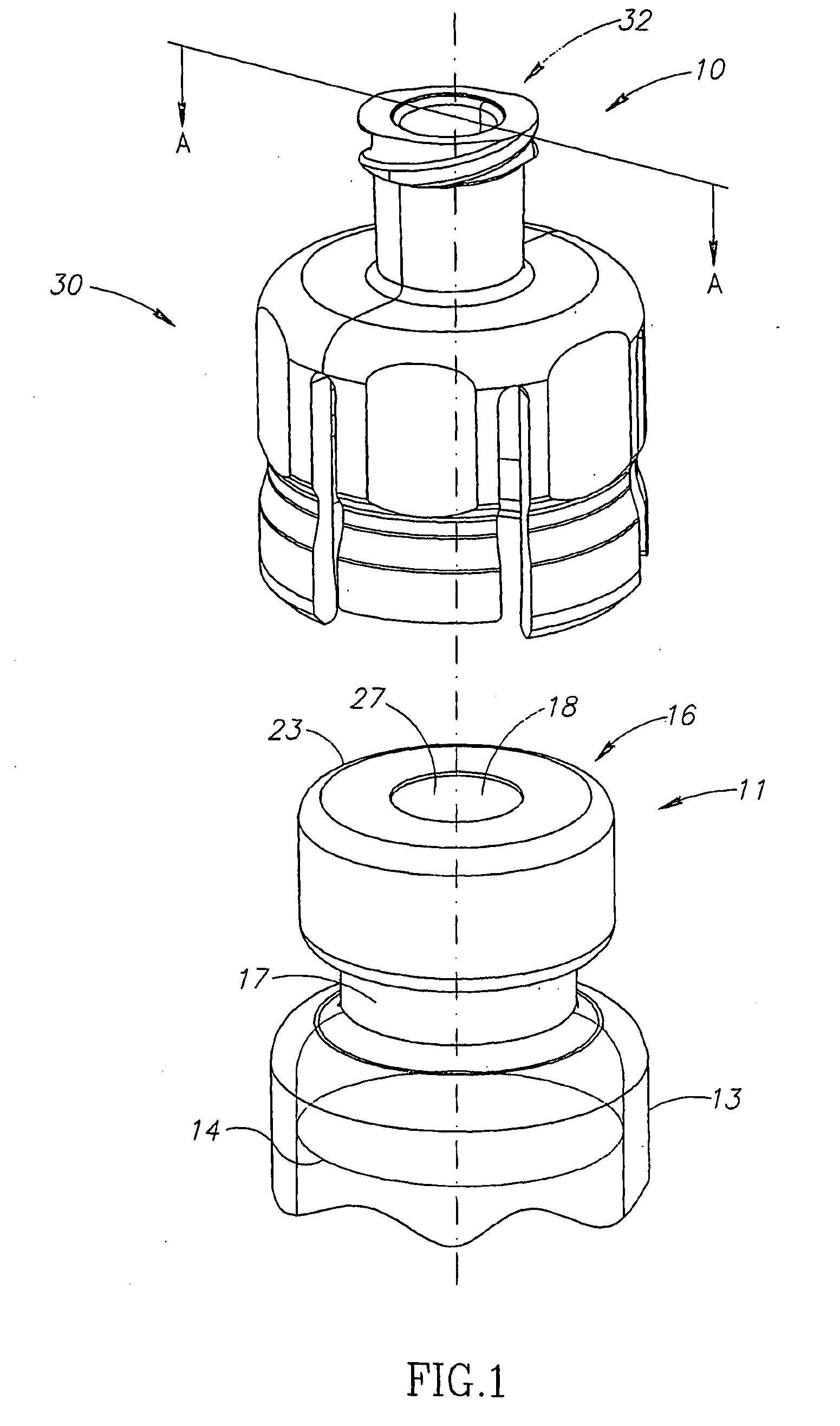
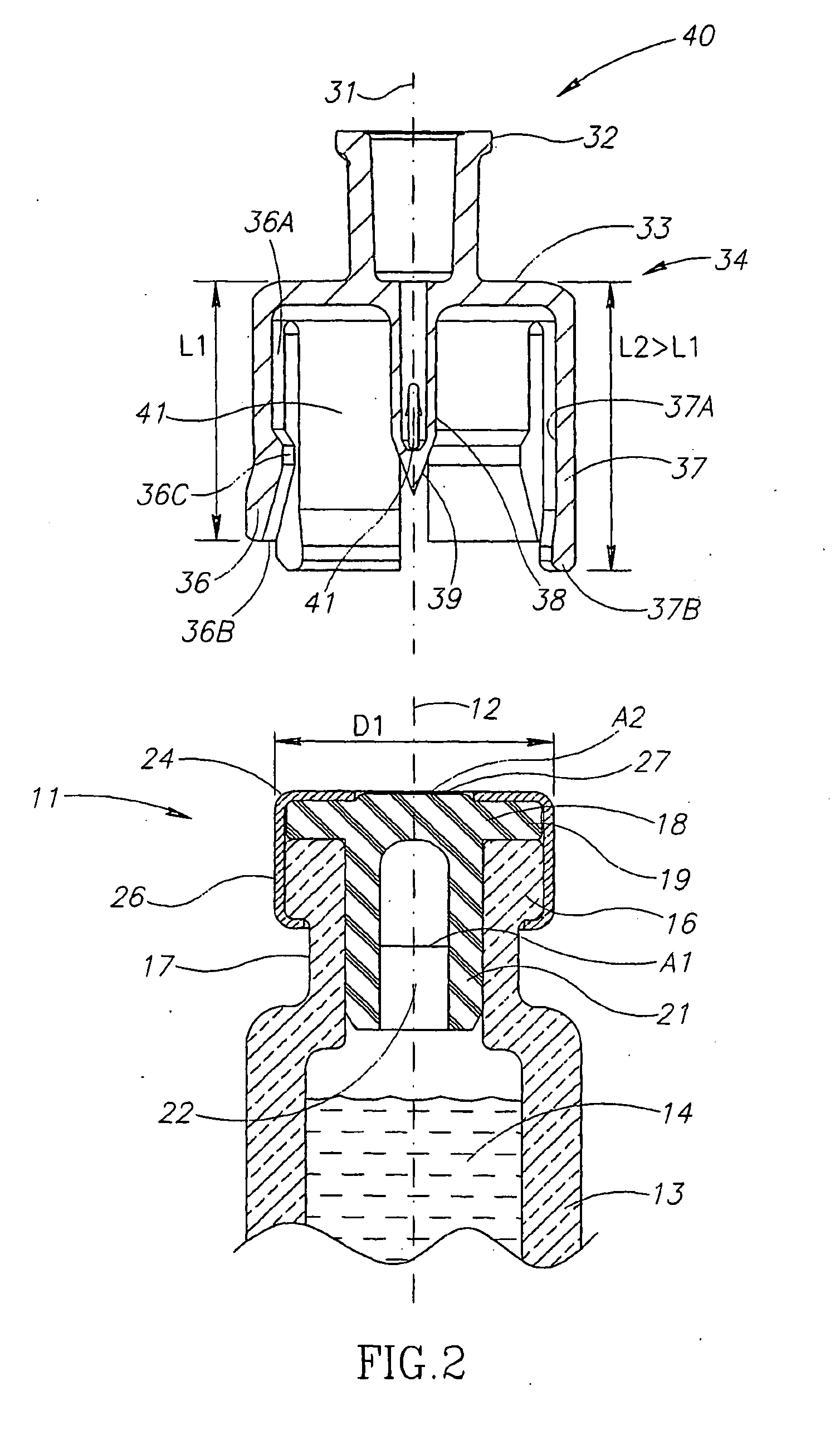
Liquid drug transfer devices including a vial adapter designed for failsafe correct snap fitting on a medicinal vial for ensuring flow communication with the vial's interior. The vial adapters include at least two non-adjacent vial retention flex members for snap fitting over a vial opening for vial retention purposes and at least two non-adjacent vial guidance flex members longer than their counterpart vial retention flex members for guiding a vial adapter with respect to a vial prior to snap fitting the vial adapter thereon.
Owner:WEST PHARM SERVICES IL LTD
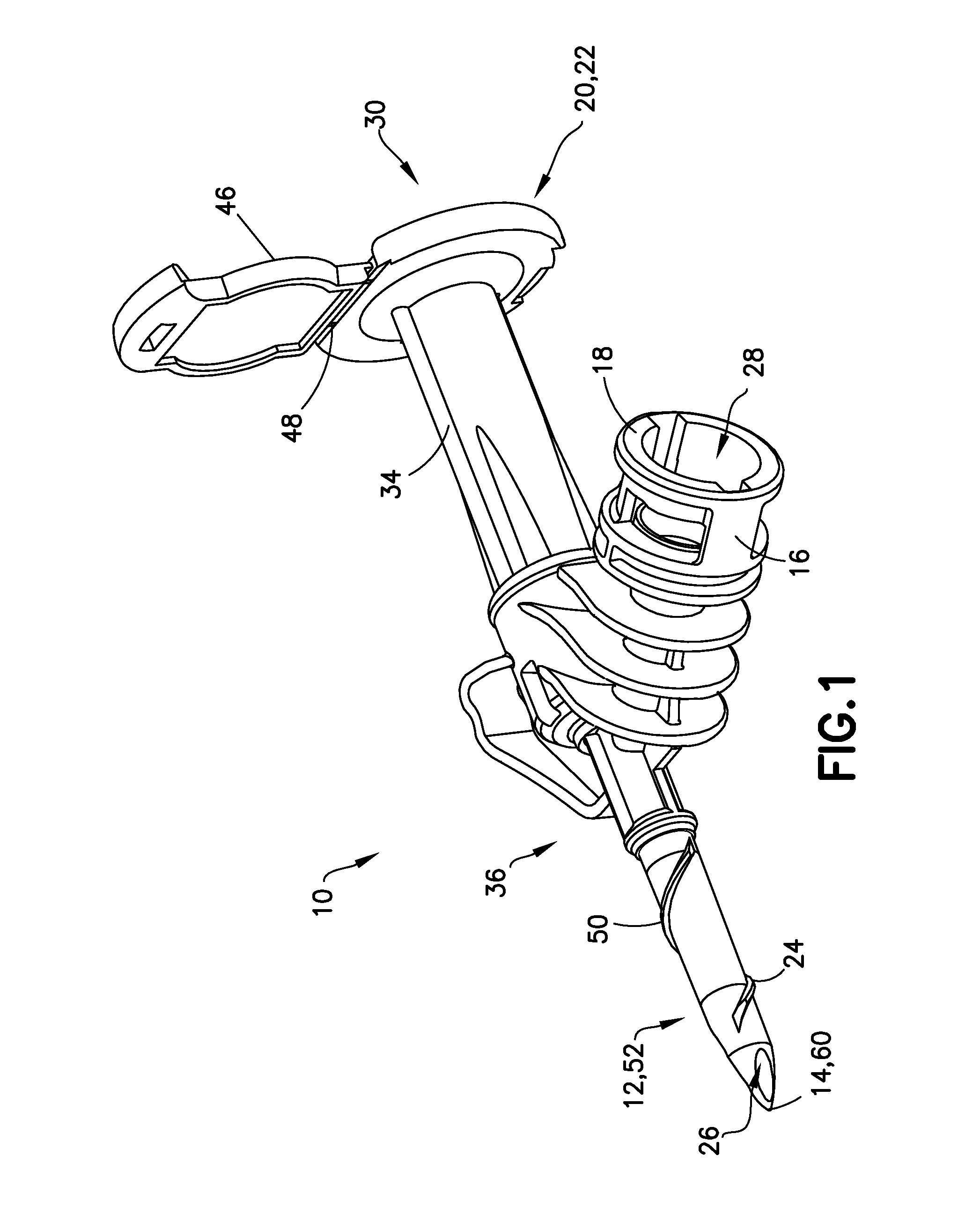
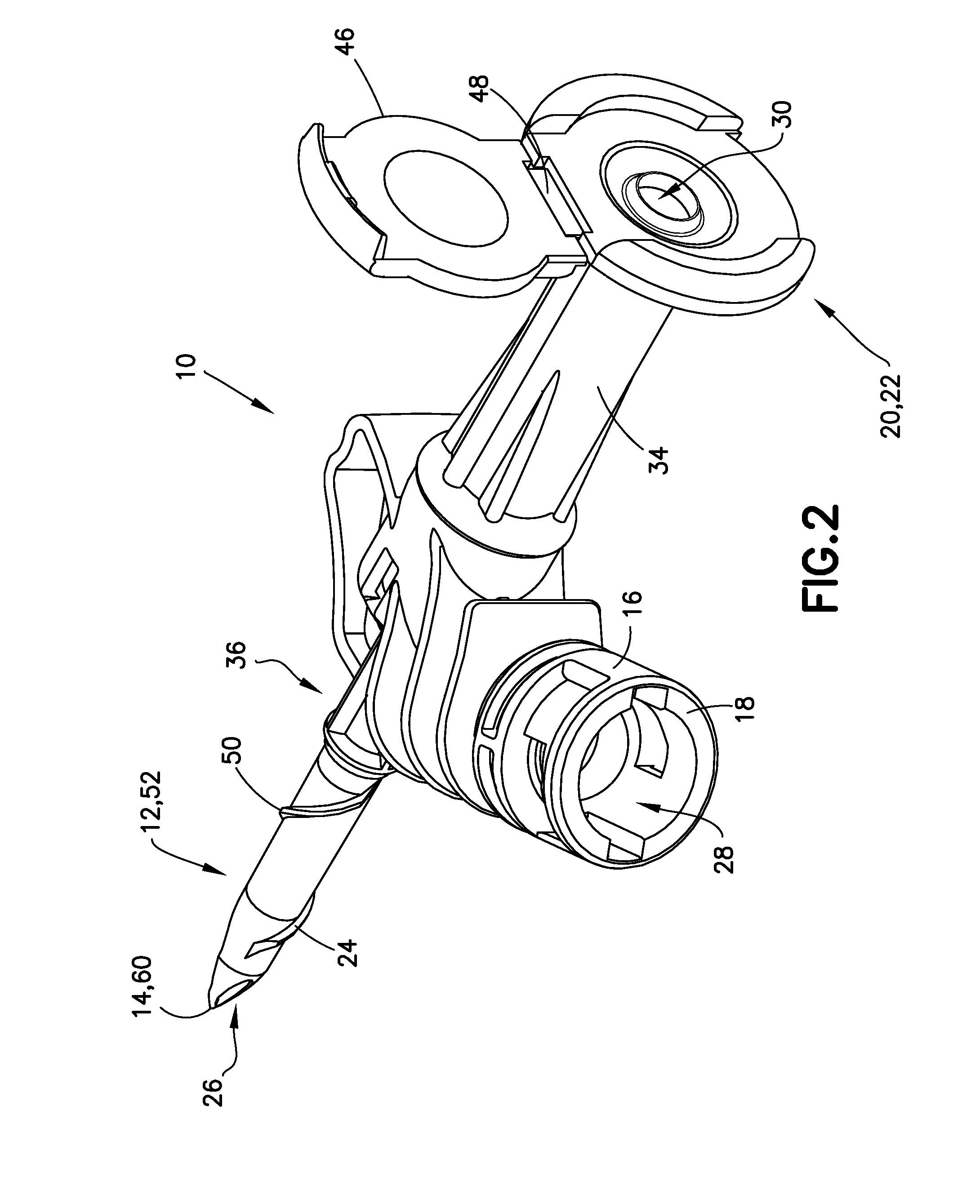
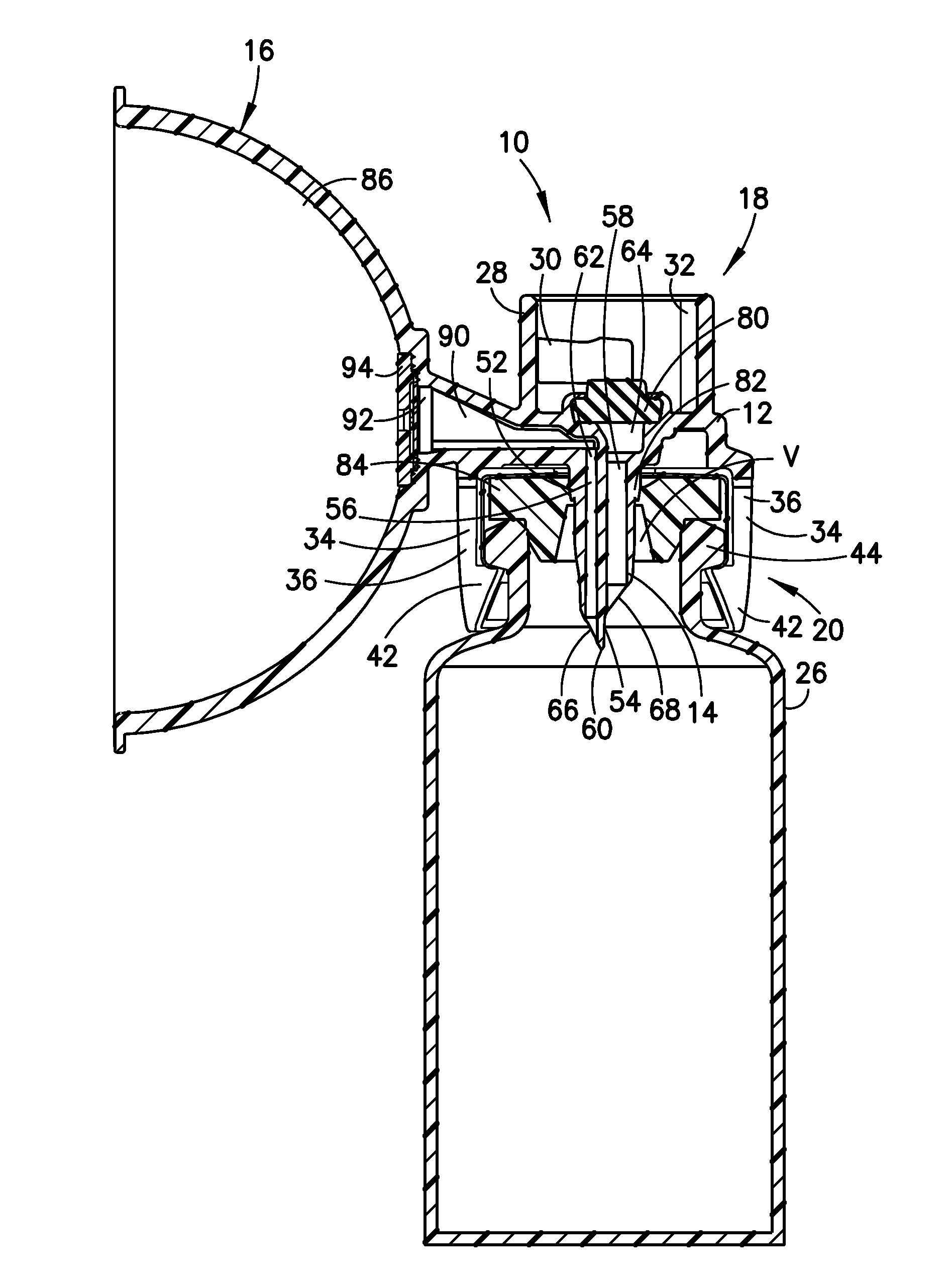
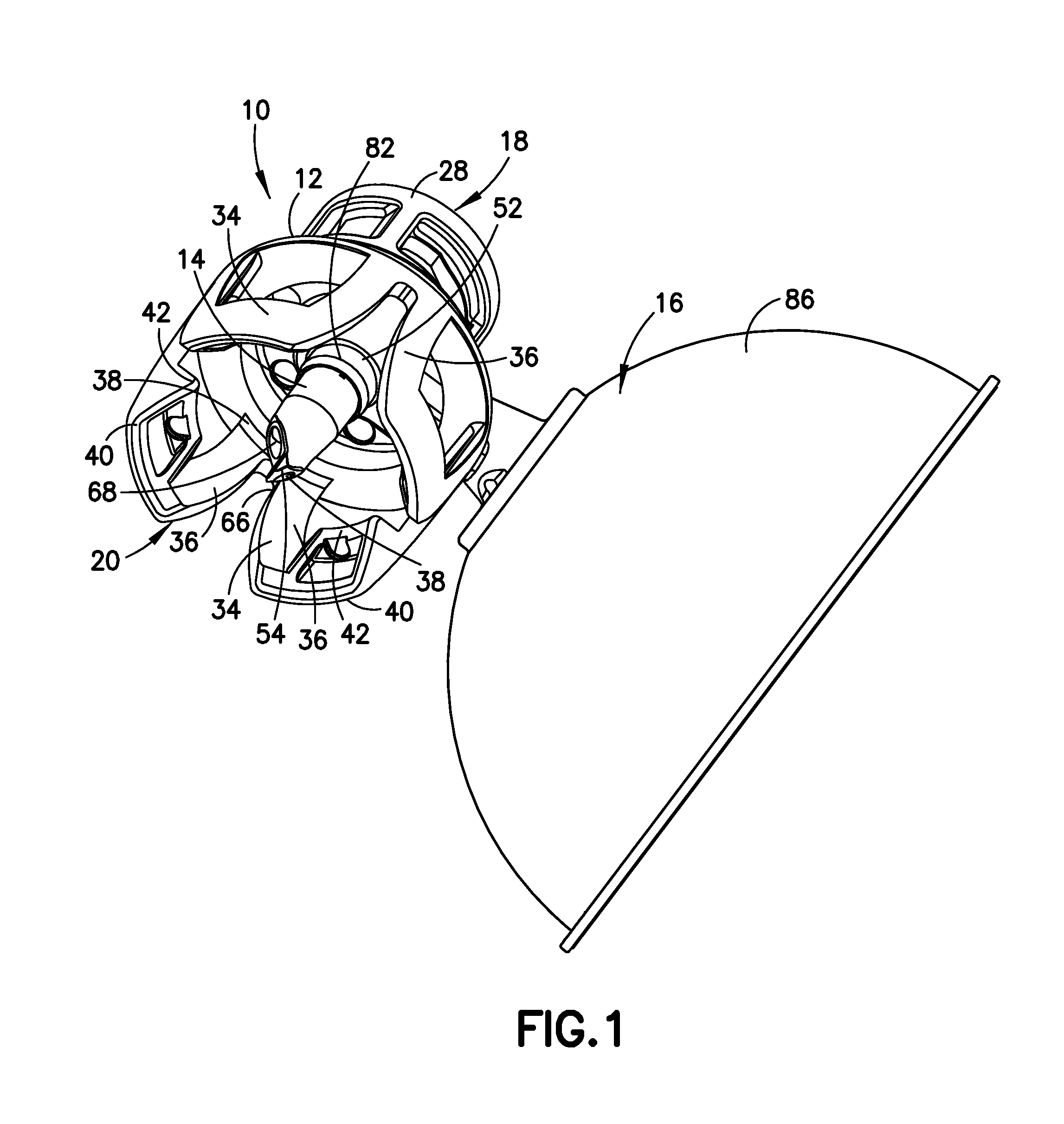
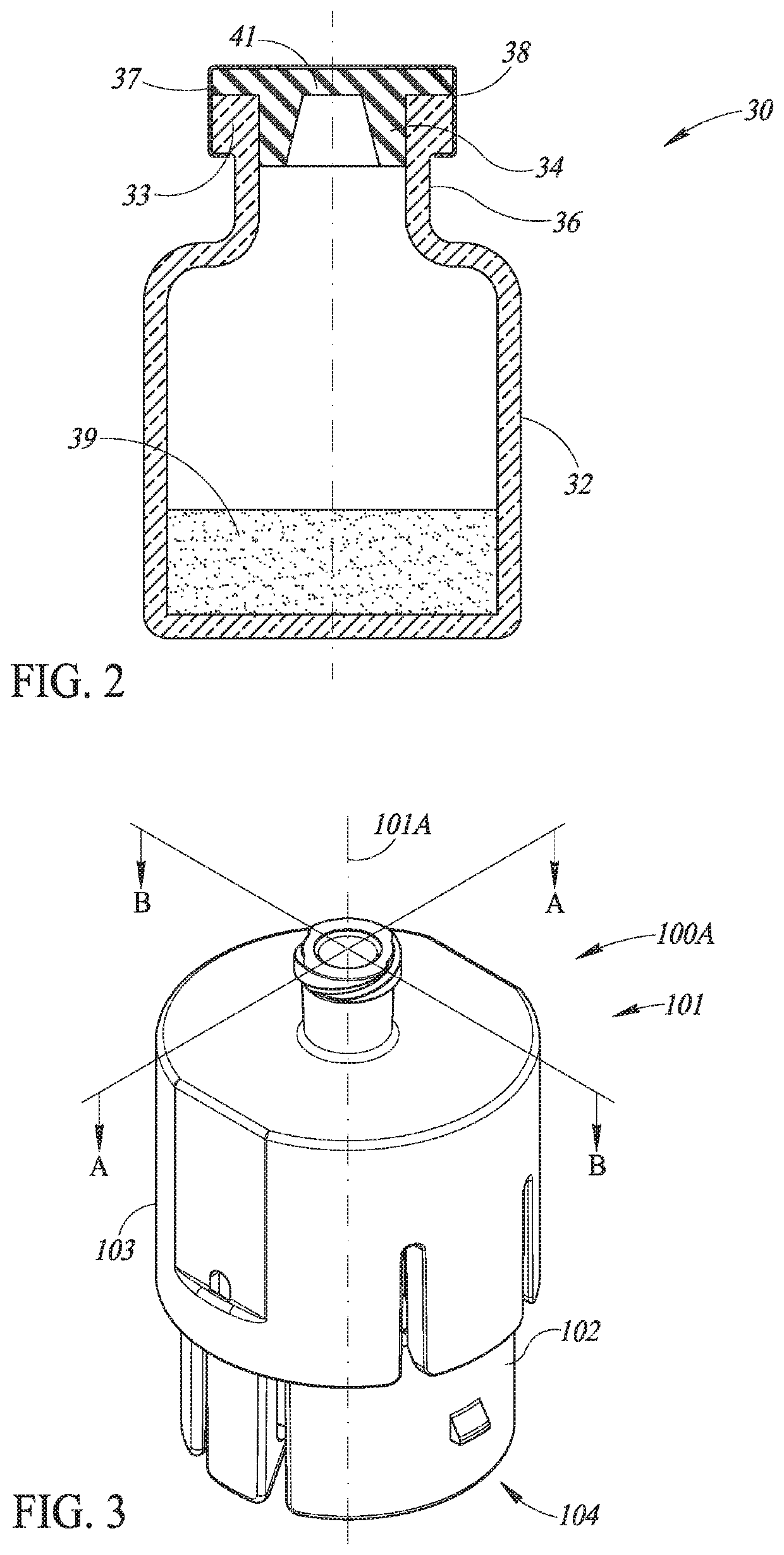
Liquid drug transfer device with vented vial adapter
Liquid drug transfer devices including a vented vial adapter having a top wall, a downward depending skirt, and a dual lumen puncturing spike. The top wall includes vent apertures in flow communication with an underlying air filter and protective hoods for covering the vent apertures from splashes. The hood-like hoods are preferably quarter sphere shaped with hood apertures facing radial outwards.
Owner:WEST PHARM SERVICES IL LTD
Liquid Drug Transfer Devices for Failsafe Correct Snap Fitting Onto Medicinal Vials
Liquid drug transfer devices including a vial adapter designed for failsafe correct snap fitting on a medicinal vial for ensuring flow communication with the vial's interior. The vial adapters include at least two non-adjacent vial retention flex members for snap fitting over a vial opening for vial retention purposes and at least two non-adjacent vial guidance flex members longer than their counterpart vial retention flex members for guiding a vial adapter with respect to a vial prior to snap fitting the vial adapter thereon.
Owner:WEST PHARM SERVICES IL LTD
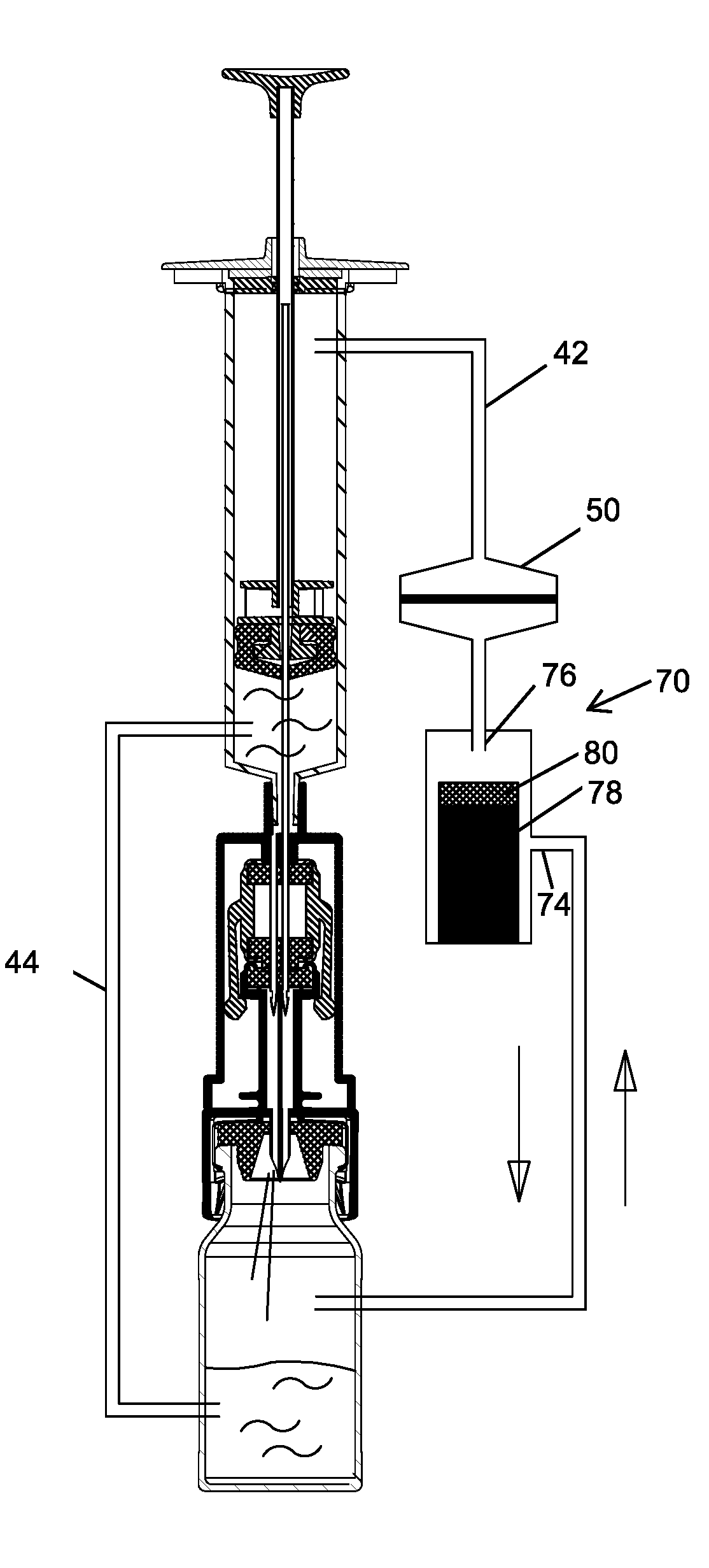
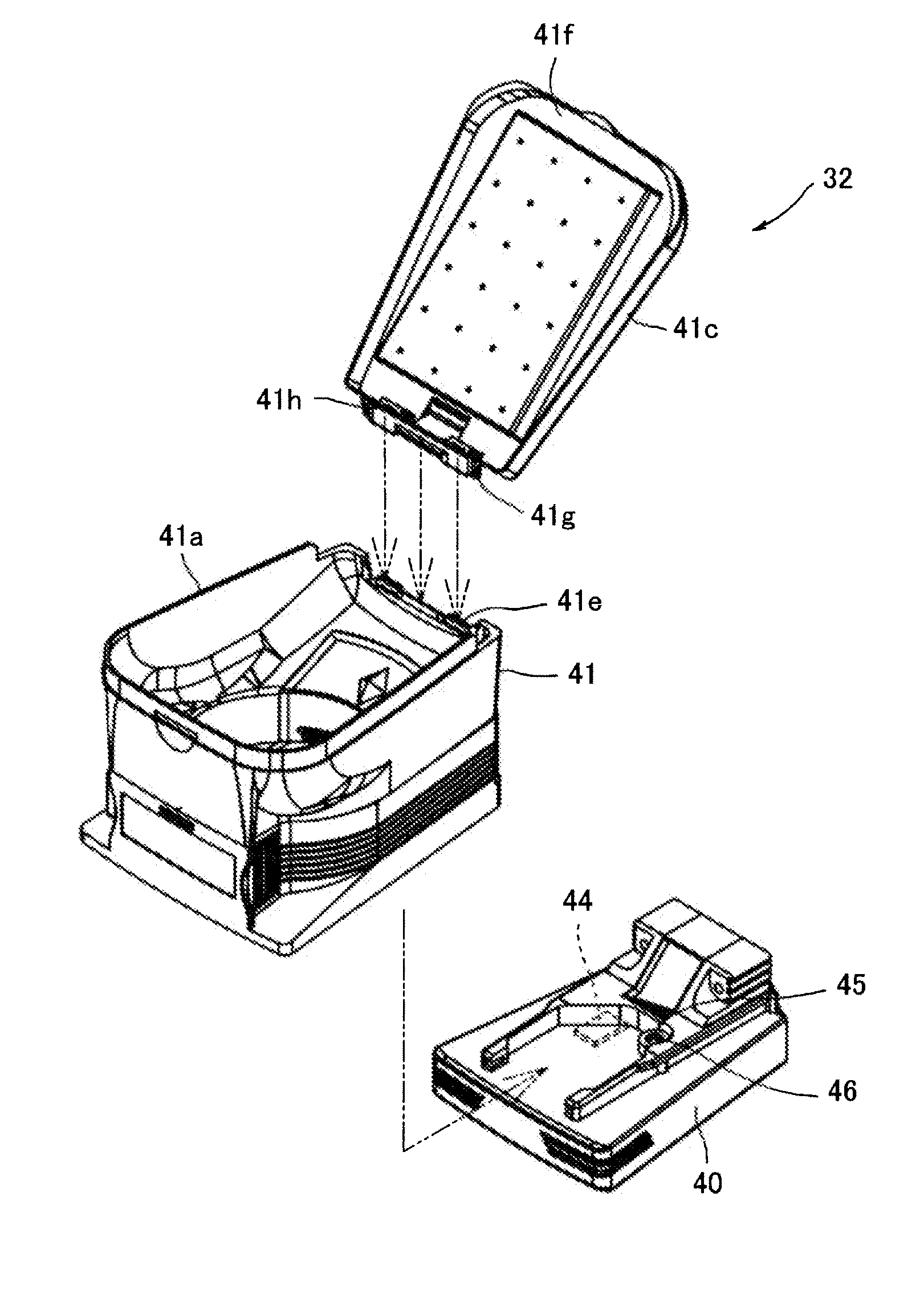
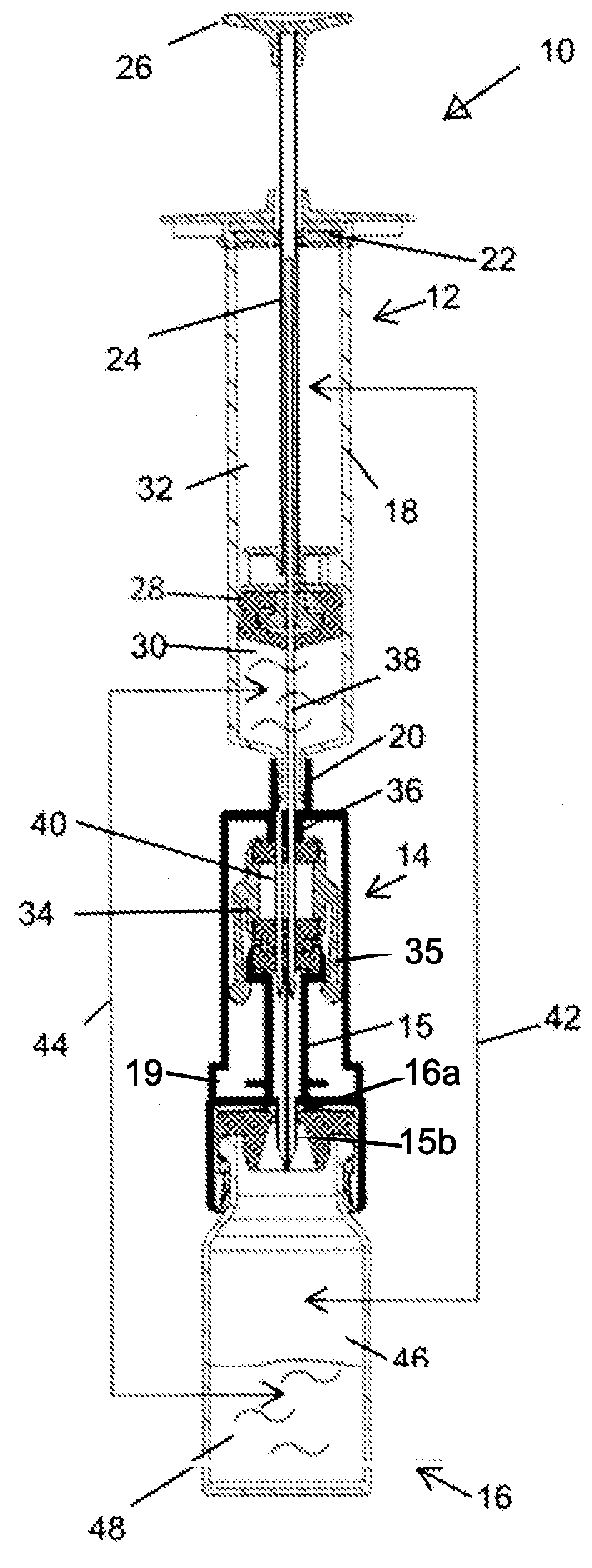
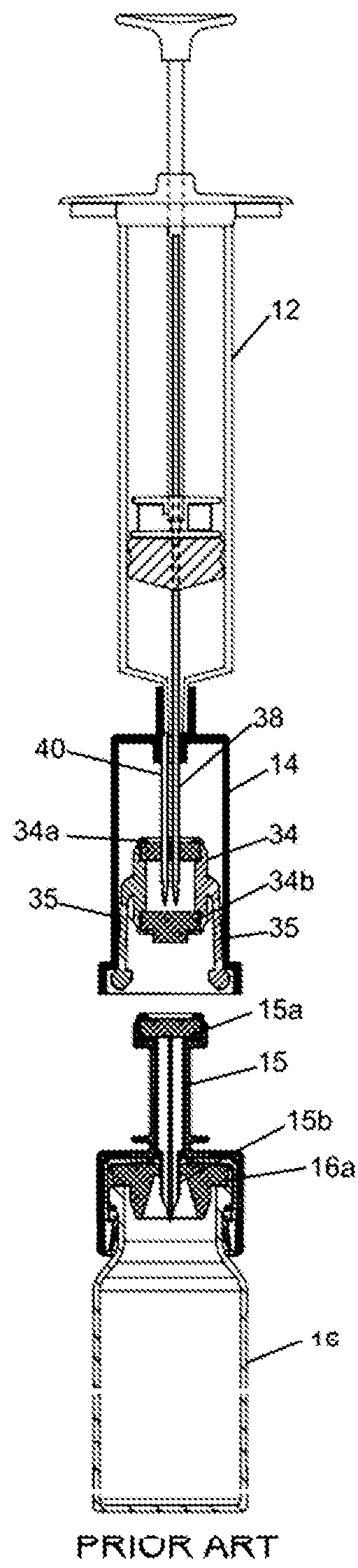
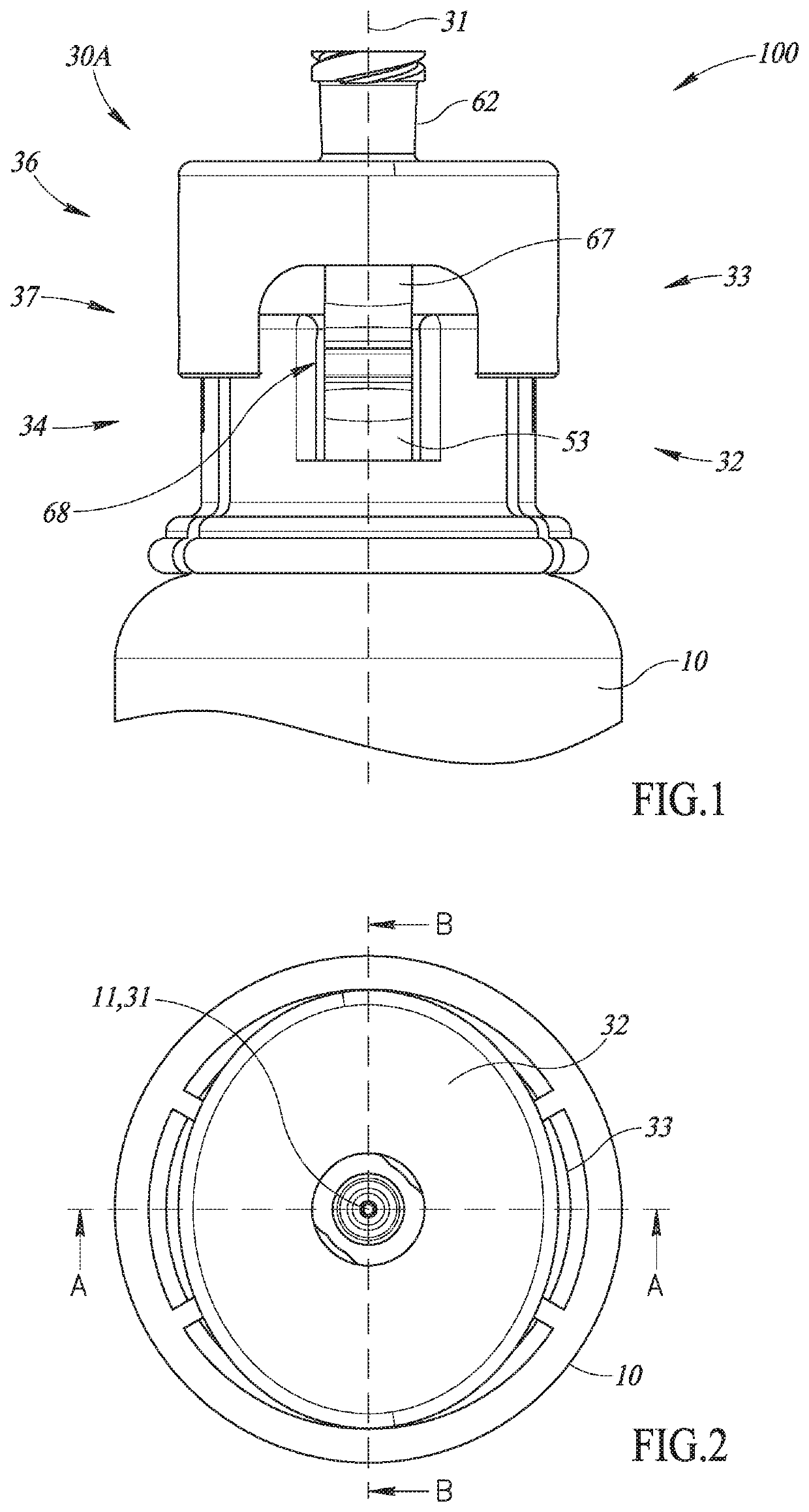
Closed drug transfer system
The application describes innovative designs for components of a drug transfer apparatus that overcome problems encountered in daily use of prior art apparatuses. Specifically described are components configured to prevent liquid from entering the air chamber of syringes coupled to the apparatus and to prevent tearing of the rubber stopper of drug vials when they are attached to the apparatus.
Owner:EQUASHIELD MEDICAL
Infusion Adapter for Drug Transfer Assembly
ActiveUS20140150911A1Little strengthAvoid disconnectionInfusion devicesCouplingsInjection portBiomedical engineering
An infusion adapter for connection with an infusion fluid container includes a connection portion including an anchor component for connecting to an injection port of the infusion fluid container, and a first port adapted for connection with a syringe assembly containing a medication fluid. The first port is in fluid communication with the connection portion. The anchor component is configured to securely connect the infusion adapter to the infusion fluid container to substantially prevent disconnection of the infusion adapter from the infusion fluid container once the infusion adapter is connected to the infusion fluid container.
Owner:BECTON DICKINSON & CO
Medical Vial Access Device with Pressure Equalization and Closed Drug Transfer System and Method Utilizing Same
ActiveUS20140014210A1Prevent coringPharmaceutical containersMedical packagingEqualizationBiomedical engineering
A vial access device includes a housing having first and second connectors. The first connector is configured to be secured to a first container and the second connector is configured to be secured to a second container. The vial access device further includes a spike member extending from the housing and having a proximal end and a distal end. The spike member defines a vent lumen and a fluid lumen spaced from the vent lumen with each of the vent lumen and the fluid lumen having a distal opening. A shape defined by a circumference of the spike member is only symmetric about one axis at a position between the proximal end of the spike member and the distal opening of the fluid lumen.
Owner:BECTON DICKINSON & CO
Medicine Dispensing System and Medicine Dispensing Device
ActiveUS20100287880A1Sure easyDrug and medicationsSolid materialDrug dispensingBiomedical engineering
Owner:YUYAMA MFG CO LTD
Medical Device Cap for Drug Transfer Assembly
ActiveUS20160089530A1Improve complianceEasy to monitorMedical devicesCatheterDocumentation procedureRelevant information
A medical device cap that protectively surrounds a medical device component for disinfection purposes and includes an identification element that can record and transmit pertinent information of the medical device cap and the medical device component is disclosed. The medical device cap enables documentation of instances when the cap is used and promotes stronger compliance with the use of medical device caps for applications involving disinfection of a medical device component such as an IV access port or a luer tip. Use of the medical device cap results in better compliance and monitoring of the use of such caps and leads to reduced incidence of CRBSI infections related to medical device component contamination. In one embodiment, the medical device cap enables automated real-time electronic documentation of when the cap is applied, while also documenting duration of application and tracking of device for inventory management.
Owner:BECTON DICKINSON & CO
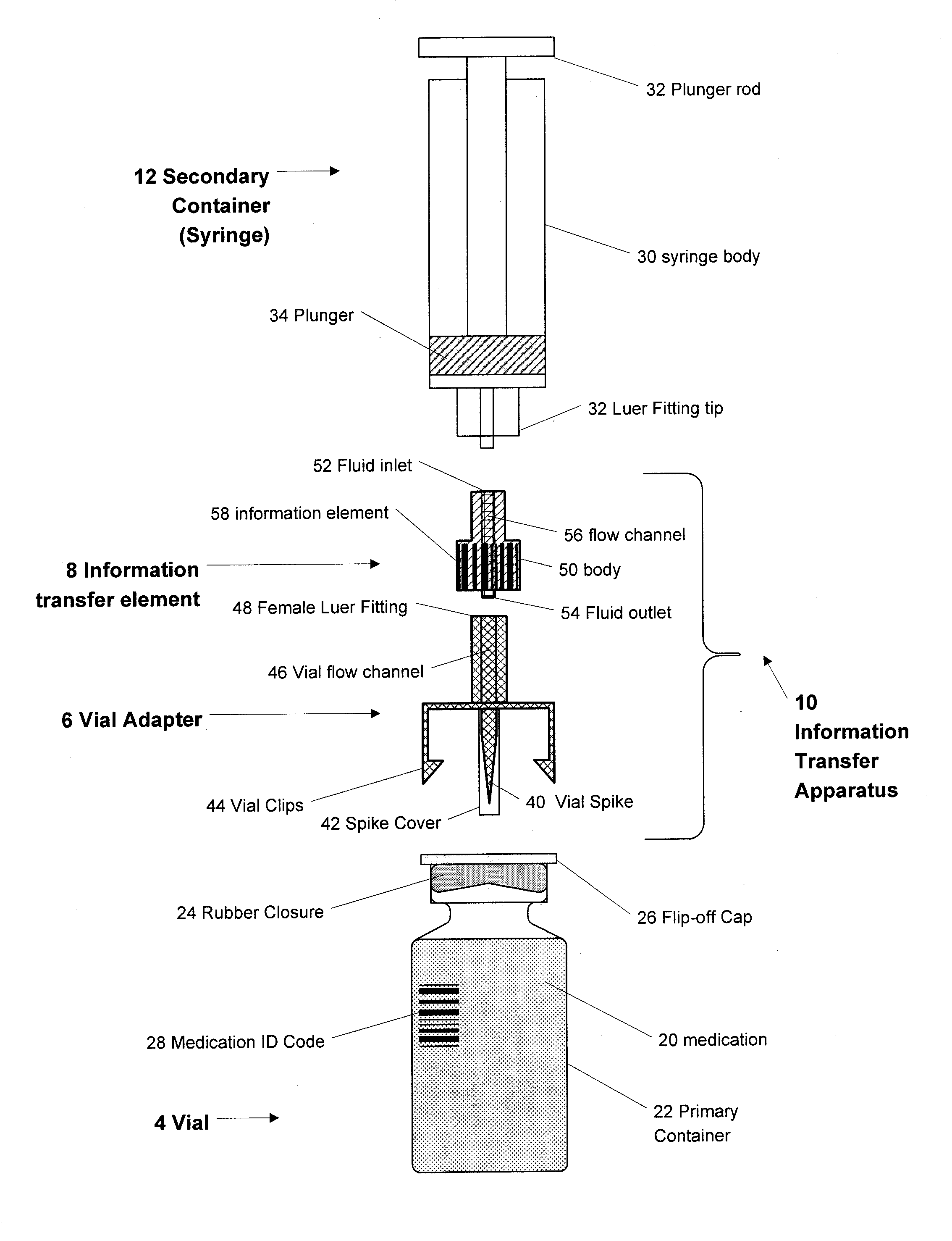
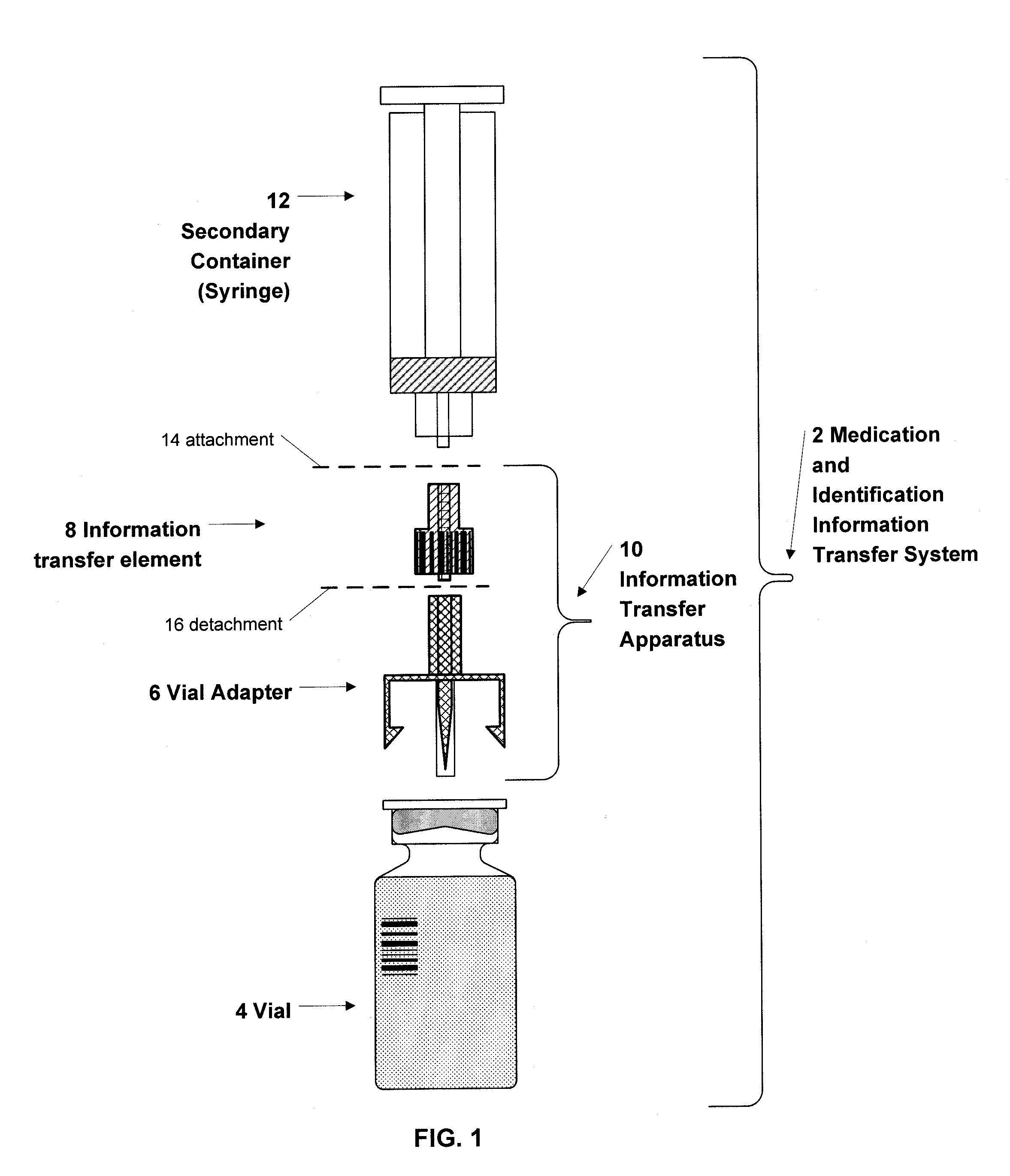
Medication and Identification Information Transfer Apparatus
A medication and identification information transfer system is provided that includes a primary medication container, a secondary medication container, a secondary container label and a medication information transfer apparatus. The medication information transfer apparatus, when coupled to the primary medication container, can transfer information indicative of the contents of the primary medication container to a medication delivery device such as an intelligent injection site. The medication information transfer apparatus has a shape and size enabling it to be connected to an adapter for removal of medication from the primary medication container which enables transfer of the medication to a secondary container while simultaneously transferring information about the medication in the primary medication container to the injection site. In some implementations, the medication injection site can be placed on a fluid delivery line for infusion into a patient. Related apparatus, systems, methods and kits are also disclosed.
Owner:CRISI MEDICAL SYST
Medication delivery device and method
InactiveUS20070049841A1Highly controllableIncrease chanceRespiratory masksBreathing masksEmergency medicineOxygen
A medication delivery apparatus and method for a continuous positive airway pressure (CPAP) system such as used in emergency treatment of severe respiratory distress. The apparatus of the invention includes a 3-port Tee fitting, one port of which is connected to the inlet port of a CPAP face mask operable at an elevated pressure. A second port is connected to a CPAP gas conduit supplying an oxygen-containing gas at a pressure above atmospheric. The third port is connected to a flexible tube receiving aerosolized medication from an upper outlet of an openable / refillable lightweight nebulizer. The flexible tubing is only long enough to bend vertically downward to support a lightweight nebulizer in a vertical attitude, whereby full nebulization takes place, no medication is spilled, and the tubing length is minimized for maximal transfer of medication to a patient. In addition, cutting of the CPAP gas supply tube and placement of a T fitting between the cut ends, with its concomitant wastage of condensed medication, is avoided. The patient head does not need to be in an upright position. The patient's airway is continuously maintained at an elevated pressure to maintain an open airway and oxygenate the patient, permitting repeated doses of nebulized medications at independently controlled nebulization rates, minimizing downtime of both the pressurized oxygen and aerosolized medication.
Owner:LEPEL PAMELA
Medicinal coating balloon catheter
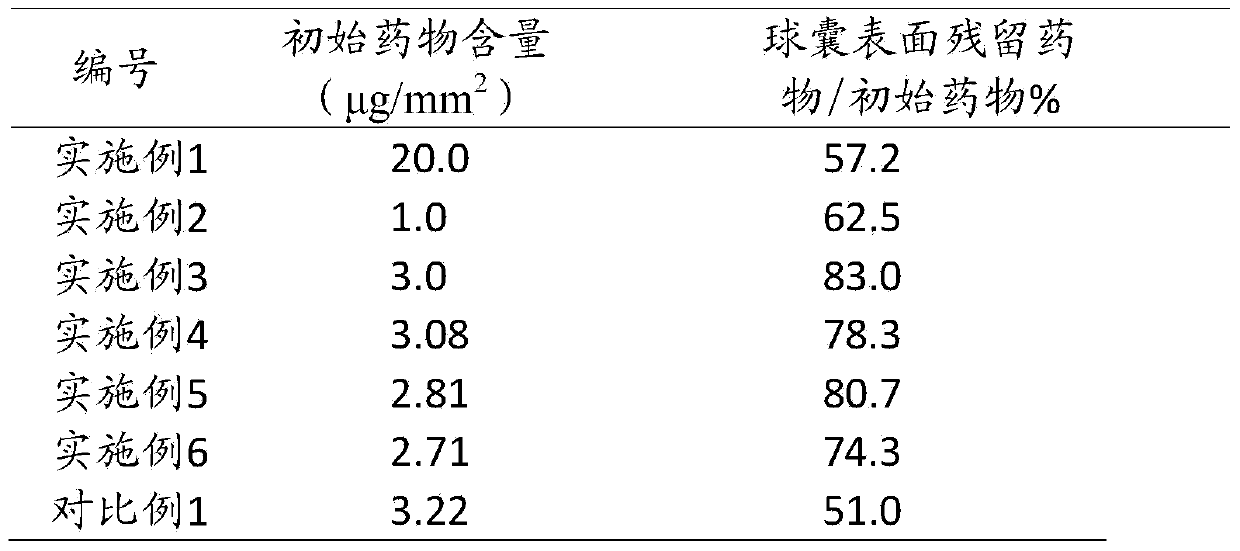
ActiveCN103736154AQuick releaseDrug transfer effect is goodBalloon catheterSurgeryOrganic acidAlcohol
The invention relates to a medicinal coating balloon catheter. The balloon catheter comprises a balloon and a medicinal coating, wherein the surface of the balloon is coated with the medicinal coating; the medicinal coating comprises an active medicine and a carrier, the active medicine is paclitaxel, sirolimus, paclitaxel derivative or sirolimus derivative; the carrier comprises organic acid salt and polyhydric alcohols; the mass ratio of the active medicine to the carrier in the medicinal coating is 0.2-100, and the mass ratio of the organic acid to polyhydric alcohols is (0.2-5):1. The organic acid salt and the polyhydric alcohols in the medicinal coating play effects together, so that premature drug release of the balloon catheter before the balloon catheter is implanted to a target point can be prevented, the medicine is accelerate to quickly release from the surface of the balloon and to be absorbed by a target tissue, drug loss in the transporting process can be reduced, and the balloon catheter has a good drug transfer effect.
Owner:LIFETECH SCI (SHENZHEN) CO LTD
Liquid drug transfer device with vented vial adapter
Liquid drug transfer devices including a vented vial adapter having a top wall, a downward depending skirt, and a dual lumen puncturing spike. The top wall includes vent apertures in flow communication with an underlying air filter and protective hoods for covering the vent apertures from splashes. The hood-like hoods are preferably quarter sphere shaped with hood apertures facing radial outwards.
Owner:WEST PHARM SERVICES IL LTD
Improved components of a fluid transfer apparatus
ActiveUS20180161245A1Eliminate deformationInfusion syringesInfusion devicesDrug transferElectrical and Electronics engineering
Described are improvements to components of fluid transfer apparatuses comprising a first component, e.g. a syringe, a connector component configured to connect between the first component and an adapter component that is configured to allow connection of the connector component to a second component of the drug transfer apparatus, e.g. to a drug vial. The improvements include inter alia changes to the sealing elements that seal the proximal end of the syringe, redesign of a septum holder inside the connector component and corresponding redesign of the housing of the connector component; changes to the structure of the end of the connector component that connects to the first component to allow the first component to swivel relative to the connector component; and changes to the design of the adapter component to a second component of the drug transfer apparatus to allow it to mate with the redesigned housing of the connector component.
Owner:EQUASHIELD MEDICAL
Adapter Cap for Drug Transfer Assembly
ActiveUS20140074038A1Reduce frictionMedical devicesInfusion needlesBiomedical engineeringDrug transfer
A drug transfer assembly including a connector connectable to a portion of an intravenous line adapted for connection to a patient's bloodstream and an adapter cap removably connectable with the connector is disclosed. The connector is formed of a rigid material and the adapter cap is formed of a pliable material. With the adapter cap connected to the connector, the adapter cap protectively surrounds and shields the connector. The adapter cap provides a cushioning surface which prevents rubbing of the connector against the skin of a patient. In this manner, the adapter cap prevents the connector of a drug transfer assembly from causing irritation and / or infection to the skin of a patient. Furthermore, the adapter cap provides a protective shield which prevents the connector of a drug transfer assembly from becoming contaminated with undesirables.
Owner:BECTON DICKINSON & CO
Sealed medicine transfer device
InactiveCN104800079APrevent splashAvoid wastingPharmaceutical containersMedical packagingNeedle Free InjectionSputtering
The invention discloses a sealed medicine transfer device, which comprises a clamping cover and a joint, wherein the clamping cover is tightly clamped with a bottle opening of a penicillin bottle, the joint is used for being connected with a liquid outlet of a needleless injector, when the clamping cover is connected with a penicillin bottle and the joint is connected with the liquid outlet of the needleless injector, a liquid inlet and outlet medicine liquid passage is formed in an inner cavity of the medicine transfer device, after the needleless injector is separated from the joint, the medicine liquid passage is closed, and meanwhile, the sealed medicine transfer device is also provided with an air passage used for balancing inside and outside pressure of the penicillin bottle. The sealed medicine transfer device is applied to medicine preparation, the outward sputtering of the medicine can be avoided, certain protection effects can be achieved on medical personnel, no needle is used in the medicine preparing process, and the puncture injury of medical personnel by an injection needle is avoided; after the medicine preparation is completed, the penicillin bottle is in a sealed state, the sealed storage can be realized so that other patients can use the medicine, and the medicine waste is avoided.
Owner:SHANDONG WEIGAO GROUP MEDICAL POLYMER
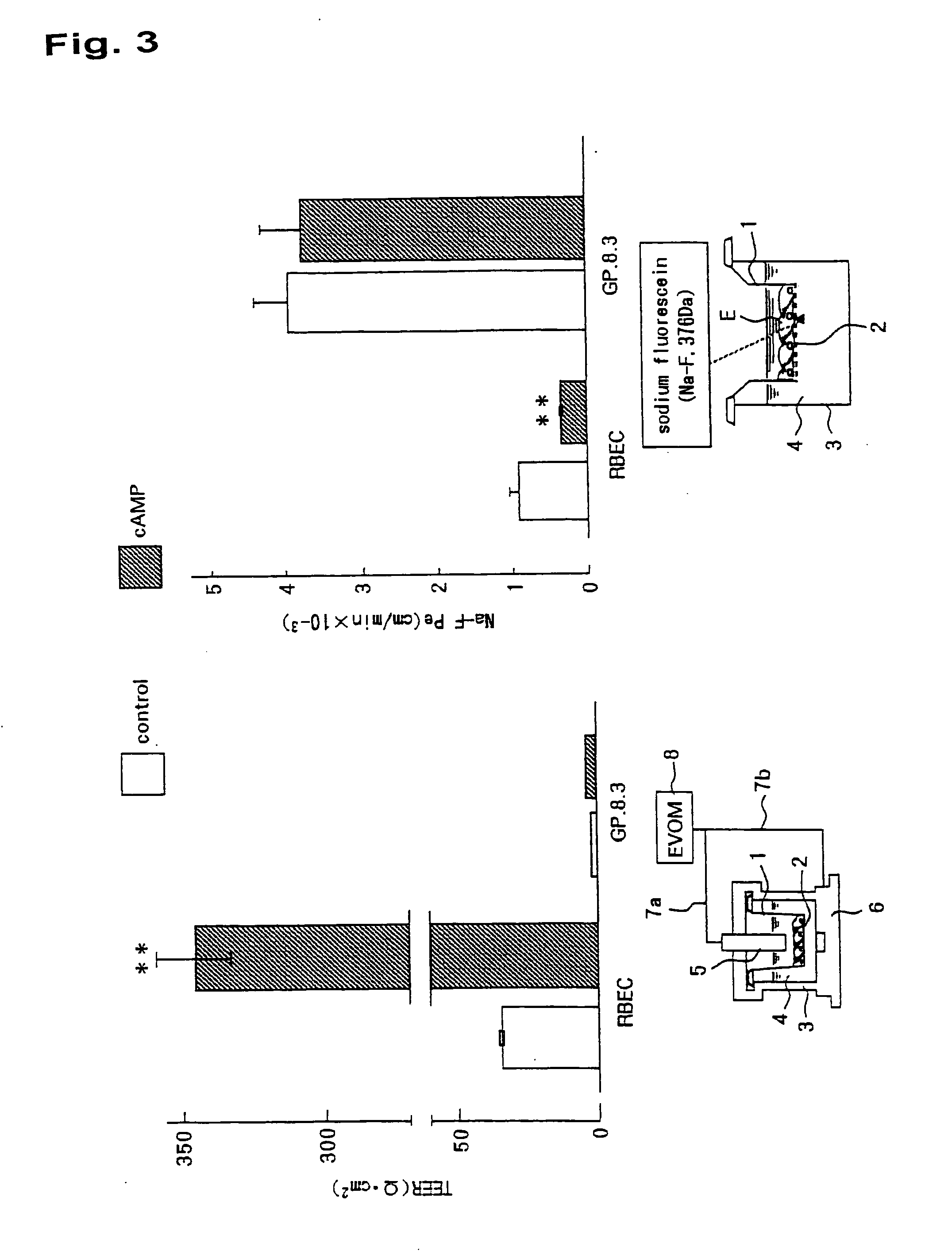
In-Vitro Model of Blood-Brain Barrier, In-Vitro Model of Diseased Blood-Brain Barrier, and Drug Screening Method, Analysis Method for Functions of Diseased Blood-Brain Barrier, and Analysis Method for Pathogenesis Using the Same
InactiveUS20100273200A1Efficient developmentMicrobiological testing/measurementArtificial cell constructsDiseaseScreening method
It is intended to provide a screening system for a centrally acting drug transported across the blood-brain barrier, a drug acting on the blood-brain barrier itself, or a drug transferred into the brain without being expected to centrally act. Moreover, another object of the present invention is to achieve pathogenesis analysis study or the screening in a diseased state by applying various diseased environments to this screening system. The present invention provides an in-vitro model of blood-brain barrier obtained by using a three-dimensional culture apparatus comprising: a culture solution; a plate holding the culture solution; and a filter immersed in the culture solution and placed in no contact with the inside bottom of the plate, the filter having plural pores of 0.35 to 0.45 μm in diameter, and by comprising: seeding primary cultured brain capillary endothelial cells onto the upper surface of the filter; seeding primary cultured brain pericytes onto the under surface of the filter; seeding primary cultured astrocytes onto the inside surface of the plate; and coculturing these cells in a normal culture solution.
Owner:PHARMACO CELL
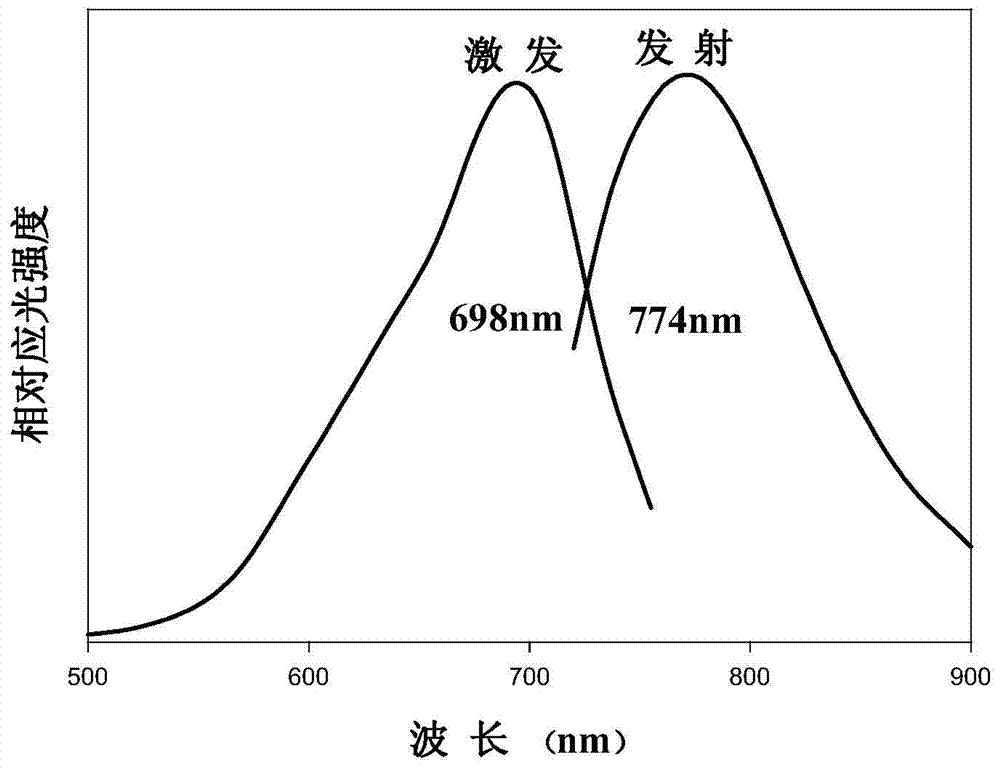
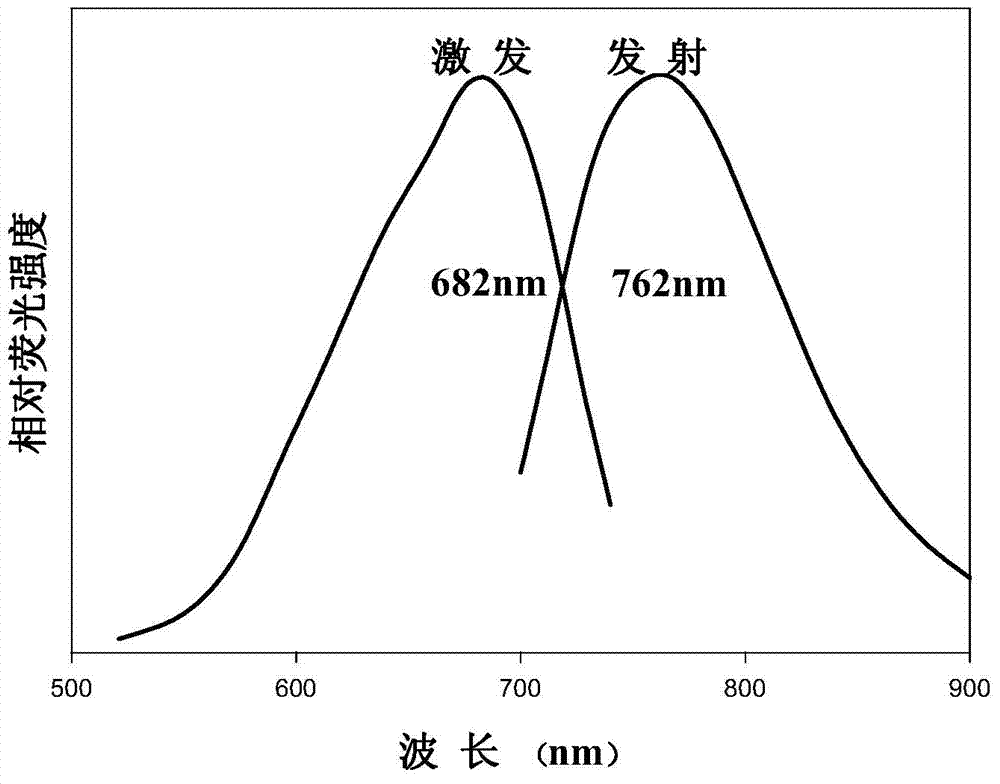
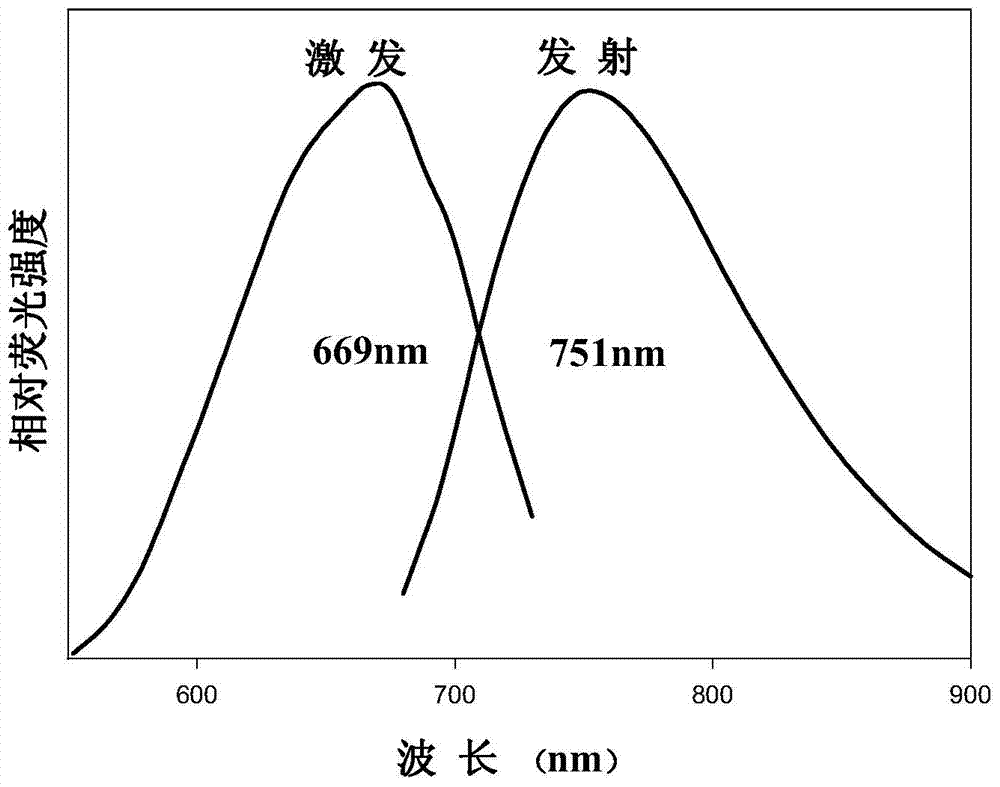
Method for preparing near-infrared carbon quantum dots by using fuchsin as carbon source
InactiveCN104495782AStable in natureHigh fluorescence intensityLuminescent compositionsReaction temperatureBiological imaging
The invention discloses a method for preparing near-infrared carbon quantum dots by using fuchsin as a carbon source. The method comprises the following steps: weighing 1.0000g of yellow ginger extraction residues, putting into a 50mL hydrothermal reaction kettle, adding 40mL of secondary water, putting in a drying box, reacting at 200 DEG C for 2 hours, after the reaction finishes, cooling to room temperature, and filtering to obtain the carbon quantum dot solution; dissolving 0.01507g of fuchsin, and adding the solvent to a constant volume in a 50mL volumetric flask to obtain a 1.0*10<-3> mol / L fuchsin solution; adding the fuchsin solution and carbon quantum dot solution into a 50mL reaction kettle, uniformly mixing, adding secondary water until the total reaction volume is 40mL, putting in a drying box, and reacting at 220 DEG C for 4 hours; and after the reaction finishes, cooling to room temperature, and filtering to obtain the near-infrared carbon quantum dots. The near-infrared carbon quantum dots prepared by the method have the advantages of stable properties and high fluorescence intensity, and have application prospects in the fields of biological imaging, sensing, drug transfer, photocatalysis, biological living imaging and the like.
Owner:HUNAN UNIV OF SCI & TECH
Liquid drug transfer devices for use with intact discrete injection vial release tool
ActiveUS20190343725A1PowerfulSimple taskPharmaceutical containersMedical packagingVIAL ADAPTERBiomedical engineering
Liquid drug transfer devices including an integral telescopic vial adapter for telescoping from an initial pre-compacted state to a final compacted state. In the initial pre-compacted state, the vial adapter telescopically snap fits on an initially non-punctured intact discrete injection vial leaving its injection vial stopper non-punctured. In the final compacted state, the vial adapter punctures the injection vial stopper. The liquid drug transfer device is used with an intact discrete injection vial release tool for applying a pincers-like compression for convenient release of a non-punctured intact discrete injection vial in the initial pre-compacted state. The vial adapter includes a clamping arrangement for irreversibly clamping same in the final compacted state. The vial adapter precludes release of a punctured discrete injection vial in its final compacted state.
Owner:WEST PHARM SERVICES IL LTD
User actuated liquid drug transfer devices for use in ready-to-use (RTU) liquid drug transfer assemblages
Liquid drug transfer device for use in a Ready-To-Use liquid drug transfer assemblage for establishing flow communication between a liquid source and an injection vial. The liquid drug transfer device includes an injection vial adapter for mounting on an intact injection vial having an injection vial stopper without puncturing same, a liquid source adapter for attachment to a liquid source, a dual ended liquid transfer member for flow communication with the liquid source and puncturing an injection vial stopper, a safety catch mechanism requiring an initial manual linear sliding extension for priming the liquid drug transfer device, an extension limit arrangement for limiting the linear sliding extension, and a snap fit securing arrangement for securing the liquid source adapter on the injection vial adapter after actuation.
Owner:WEST PHARM SERVICES IL LTD
Noninvasive Drug Delivery System To Tissue of Posterior Segment of Eye Using Solid Composition
InactiveUS20090036552A1Easy transferRaise transfer toBiocideOrganic active ingredientsConjunctivaConjunctival sac
Owner:SANTEN PHARMA CO LTD
Nano drug-carrying system, and preparation and application thereof
ActiveCN106822921AGood biocompatibilityLow cytotoxicityEnergy modified materialsGenetic material ingredientsPolyethylene glycolMesoporous silica
The invention relates to the technical field of medicine, and provides a nano drug-carrying system. The nano drug-carrying system comprises a magnetic-mesoporous silicon dioxide nanorod carrier, a pro-drug GCV (ganciclovir) carried on the carrier, and a graft copolymer PLL-g-PEG (poly-L-lysine graft polyethylene glycol) formed by PLL and PEG, wherein the PLL-g-PEG also carries a suicide gene TK. The magnetic-mesoporous silicon dioxide nanorods are used as the magnetic targeted carrier to carry the suicide gene TK / pro-drug GCV to enter cells, thereby implementing the time and space consistency of the suicide gene and pro-drug transfer, and further implementing accurate drug release. The magnetic-mesoporous silicon dioxide silicon dioxide nanorods and suicide gene / pro-drug treatment method are effectively combined, thereby enhancing the combined treatment effect on the liver cancer. The preparation method of the nano drug-carrying system is simple in technique and suitable for large-scale industrial production.
Owner:SUZHOU INST OF BIOMEDICAL ENG & TECH CHINESE ACADEMY OF SCI
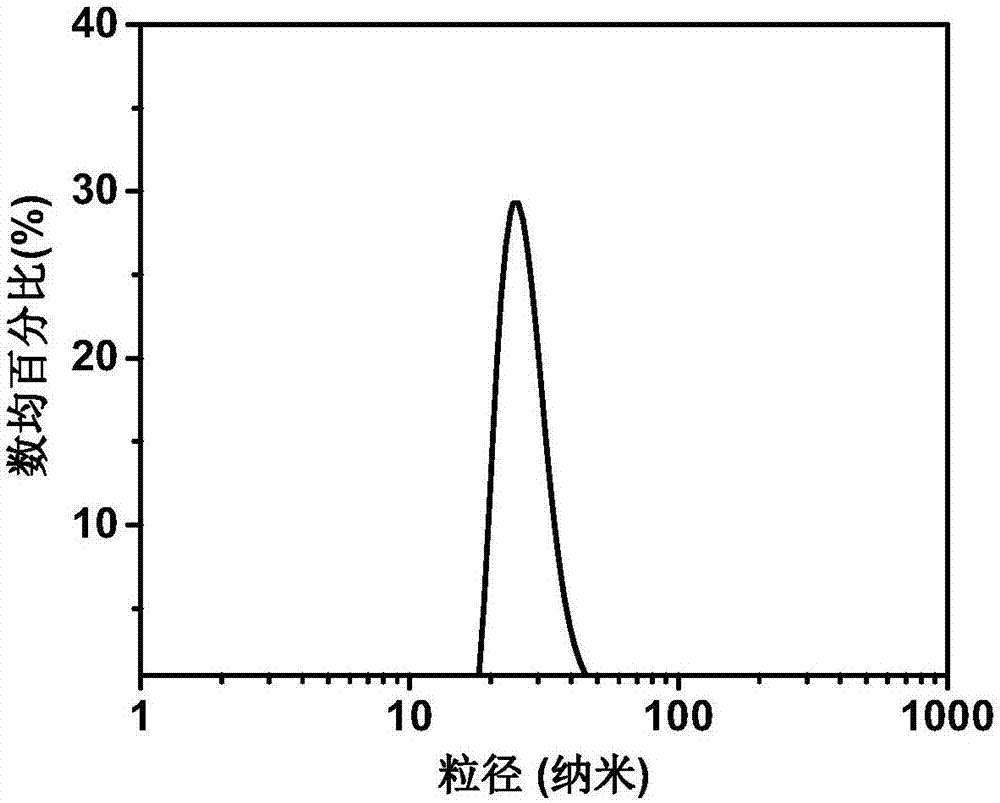
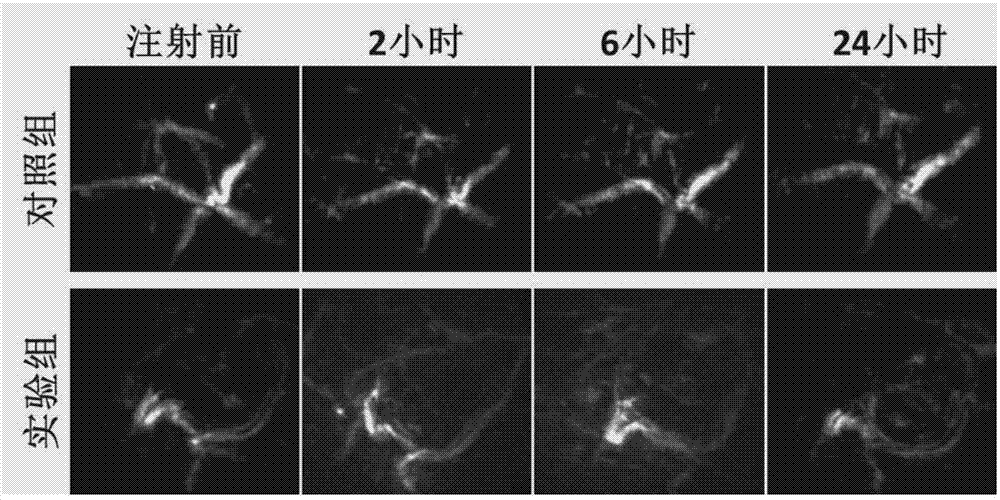
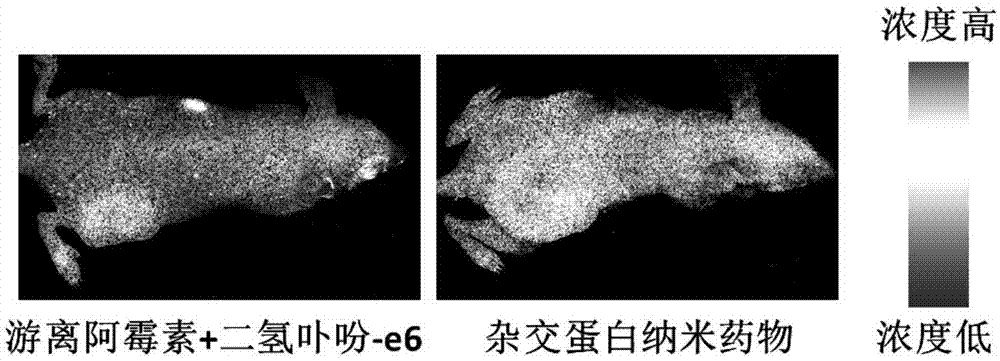
Preparation method of drug-loading and oxygen-loading hybrid protein nanoparticle, drug-loading and oxygen-loading hybrid protein nanoparticle and application
ActiveCN107952072AEasy to prepareSuitable for large scale preparationOrganic active ingredientsPowder deliveryHigh concentrationSide effect
The invention provides a preparation method of a drug-loading and oxygen-loading hybrid protein nanoparticle, the drug-loading and oxygen-loading hybrid protein nanoparticle and application and relates to the technical field of biological pharmacy. The preparation method comprises the following steps: firstly, mixing a reducing agent with serum albumin, homogenizing and carrying out reduction reaction to obtain reduced serum albumin; secondly, mixing the reduced serum albumin, hemoglobin and a drug under the aerobic condition, and homogenizing to prepare the drug-loading and oxygen-loading hybrid protein nanoparticle. According to the drug-loading and oxygen-loading hybrid protein nanoparticle, the technical problems that oxygen cannot be conveyed into tumors by adopting an existing inhalation high-pressure oxygen mode for supplying oxygen, and lung and a central nervous system suffer from oxygen poisoning caused by high-concentration oxygen are alleviated; the oxygen and a drug are conveyed into the tumors by the drug-loading and oxygen-loading hybrid protein nanoparticle in a targeted manner, and drug enrichment is improved; besides, reversible release of oxygen molecules can berealized according to oxygen concentration of a micro-environment; in addition, no side effects are generated; the technical effects that integration of tumor oxygen increase and drug transfer is realized, and the bioavailability of the drug is improved are achieved.
Owner:SHENZHEN INST OF ADVANCED TECH
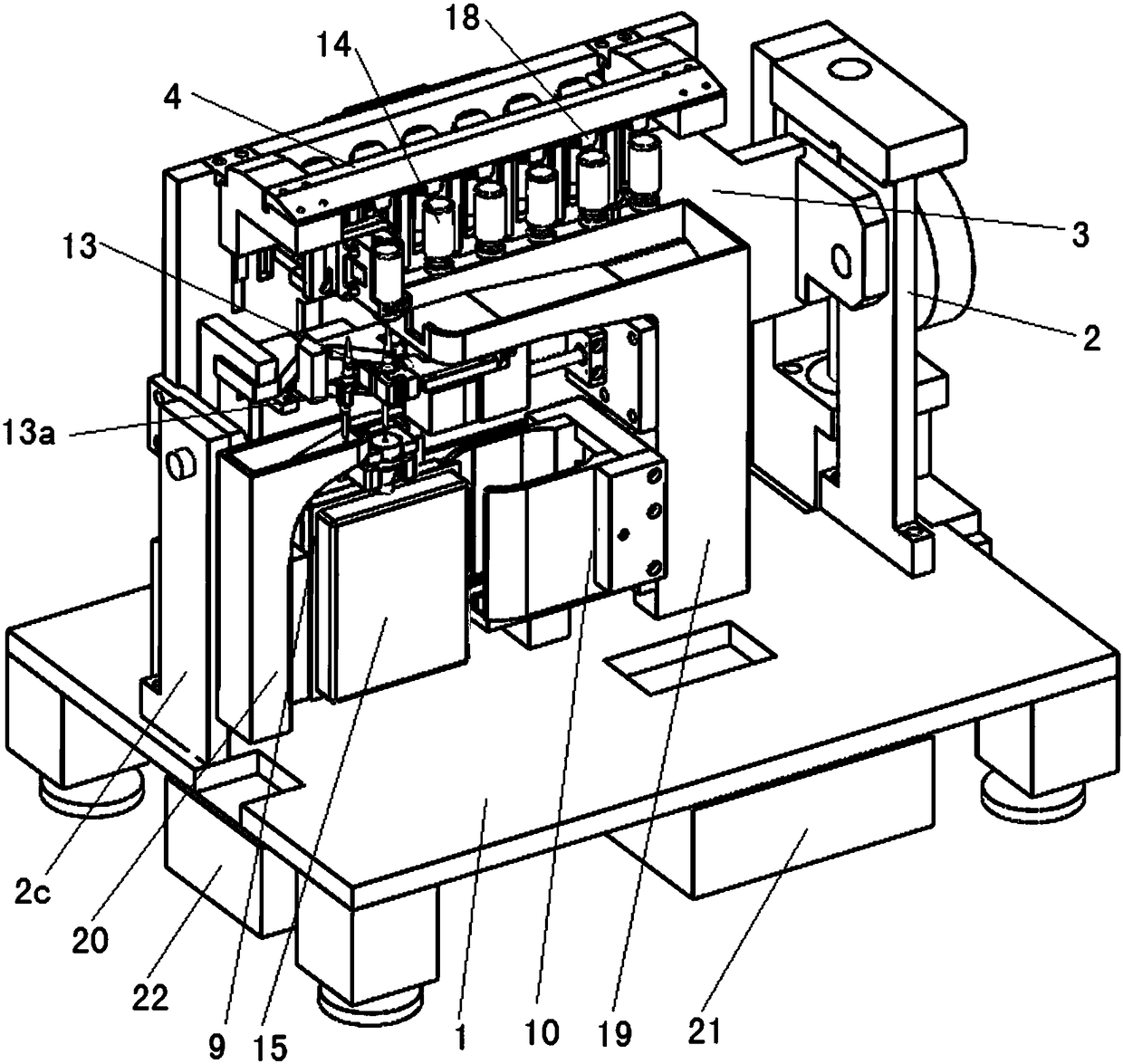
Automatic drug liquid preparation machine for injection
PendingCN108852832AImprove configuration qualityReduce wastePharmaceutical containersMedical packagingMedical equipmentEngineering
The invention discloses an automatic drug liquid preparation machine for injection, and relates to the field of medical equipment. The machine comprises a platform. A rotation plate is rotationally arranged above the platform through a rotating driving mechanism, a containing bottle placement uniform mixing assembly and a vertical sliding plate are arranged on the rotation plate, the vertical sliding plate can vertically move relative to the rotation plate through a first displacement driving mechanism, a horizontal sliding plate is arranged on the vertical sliding plate through a second displacement driving mechanism in a transverse motion mode, an infusion bag placement assembly and an infusion bag clamping assembly are arranged on the horizontal sliding plate, the infusion bag placementassembly can vertically move relative to the horizontal sliding plate through a third displacement driving mechanism, the infusion bag clamping assembly can transversely move relative to the horizontal sliding plate through a fourth displacement driving mechanism, and a liquid drug transfer assembly is arranged on the horizontal sliding plate. The machine is compact and reasonable in structure, and easy and convenient to operate, integrates various functions of negative boosting suction, injection, uniform mixing and the like, has the good human computer interaction, high integration level and high efficiency, and can be widely applied to a medical health system.
Owner:天津硕华医药科技有限公司 +1
User actuated liquid drug transfer devices for use in ready-to-use (RTU) liquid drug transfer assemblages
Liquid drug transfer device for use in a Ready-To-Use liquid drug transfer assemblage for establishing flow communication between a liquid source and an injection vial. The liquid drug transfer device includes an injection vial adapter for mounting on an intact injection vial having an injection vial stopper without puncturing same, a liquid source adapter for attachment to a liquid source, a dual ended liquid transfer member for flow communication with the liquid source and puncturing an injection vial stopper, a safety catch mechanism requiring an initial manual linear sliding extension for priming the liquid drug transfer device, an extension limit arrangement for limiting the linear sliding extension, and a snap fit securing arrangement for securing the liquid source adapter on the injection vial adapter after actuation.
Owner:WEST PHARM SERVICES IL LTD
Capsule for encasing tablets for surgical insertion into the human body
A drug delivery device for placement in a body includes a drug core comprising a pharmaceutically active agent, and a holder that holds the drug core. The holder is made of a material impermeable to passage of the active agent and includes at least one opening for passage of the pharmaceutically agent therethrough to eye tissue. The holder is continuous with the top through a continuous loop. The continuous loop may be reinforced and sized to provide a point from which the device can be anchored when implanted.
Owner:BAUSCH & LOMB INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com