Medicinal coating balloon catheter
A drug-coated and balloon-catheter technology, applied in the direction of balloon-shaped catheters, catheters, coatings, etc., to achieve good safety and biocompatibility, good drug transfer effect, and accelerated release and absorption effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
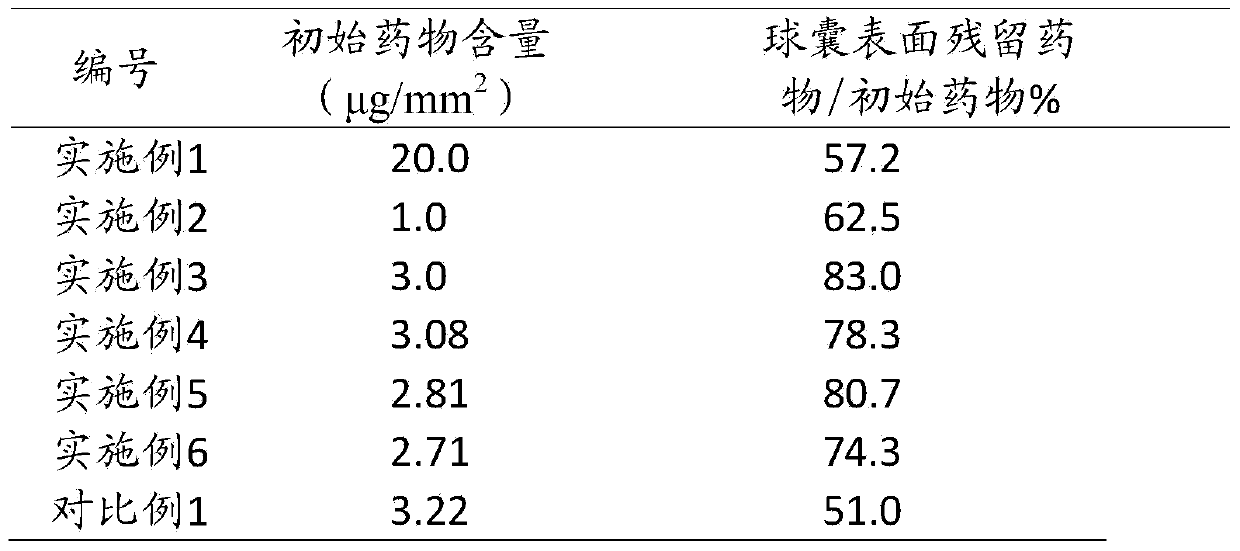
[0027] Mix 120mg of paclitaxel, 1.0mg of citrate, 0.1mg of amino alcohol, 10ml of ethanol, and 4ml of purified water to prepare a coating solution, wherein the mass ratio of the active drug to the carrier is 100; the PTCA balloon catheter (diameter 3mm, length 20mm) After the flaps were folded in three in a class 10,000 clean environment, the coating solution was drip-coated with a precision syringe (accurate to 2 μl) on the surface of the polyester balloon behind the flaps in a class 100 clean environment, and then the balloon was dried , repeated dripping until the drug concentration on the surface of the balloon reached 20 μg / mm 2 , dried for 24 hours, packaged, and ethylene oxide sterilized.
Embodiment 2
[0029] Mix 20mg of rapamycin, 17mg of lactate, 83mg of mannitol, 7ml of ethanol, and 3ml of purified water to prepare a solution, in which the mass ratio of the active drug to the carrier is 0.2; a PTCA balloon catheter (diameter 3mm, length 20mm) was placed in the After folding the wings in three in a class 10,000 clean environment, apply the coating solution onto the surface of the polyester balloon with a precision syringe (accurate to 2 μl) in a class 100 clean environment, then dry the balloon and repeat Drop coating until the drug concentration on the surface of the balloon reaches 1 μg / mm 2 , dried for 24 hours, packaged, and ethylene oxide sterilized.
Embodiment 3
[0031] Mix 120mg of paclitaxel, 36mg of sodium benzoate, 36mg of polyethylene glycol 2000, 10ml of ethanol, and 4ml of purified water to prepare a coating solution, in which the mass ratio of the active drug to the carrier is 1.67; the PTCA balloon catheter (diameter 3mm, length 20mm) After the wings were folded in three in a class 10,000 clean environment, the coating solution was sprayed onto the surface of the polyester balloon after the flaps were folded in a class 100 clean environment, so that the drug concentration on the surface of the balloon reached 3 μg / mm 2 , dried, packaged, and ethylene oxide sterilized.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com