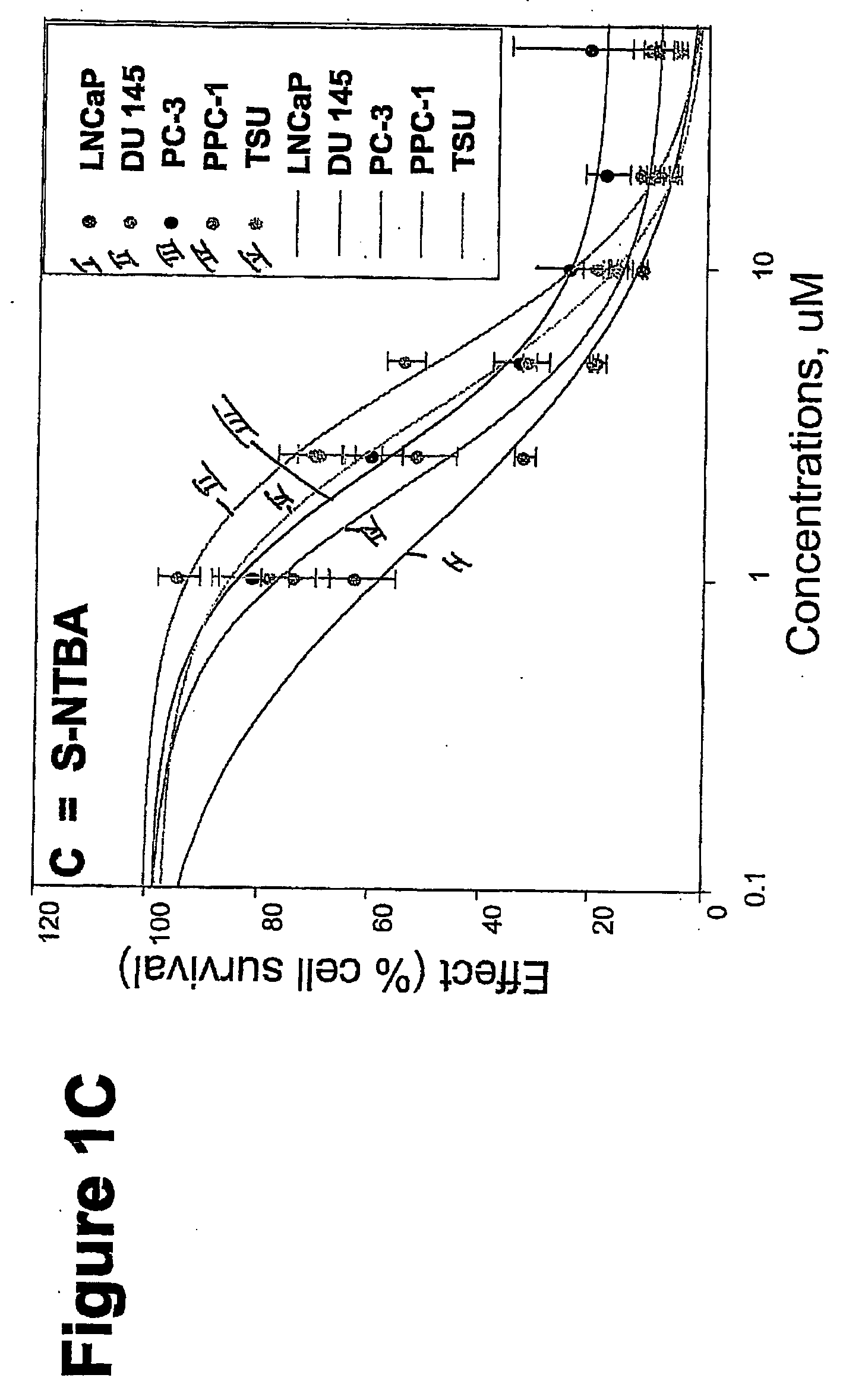
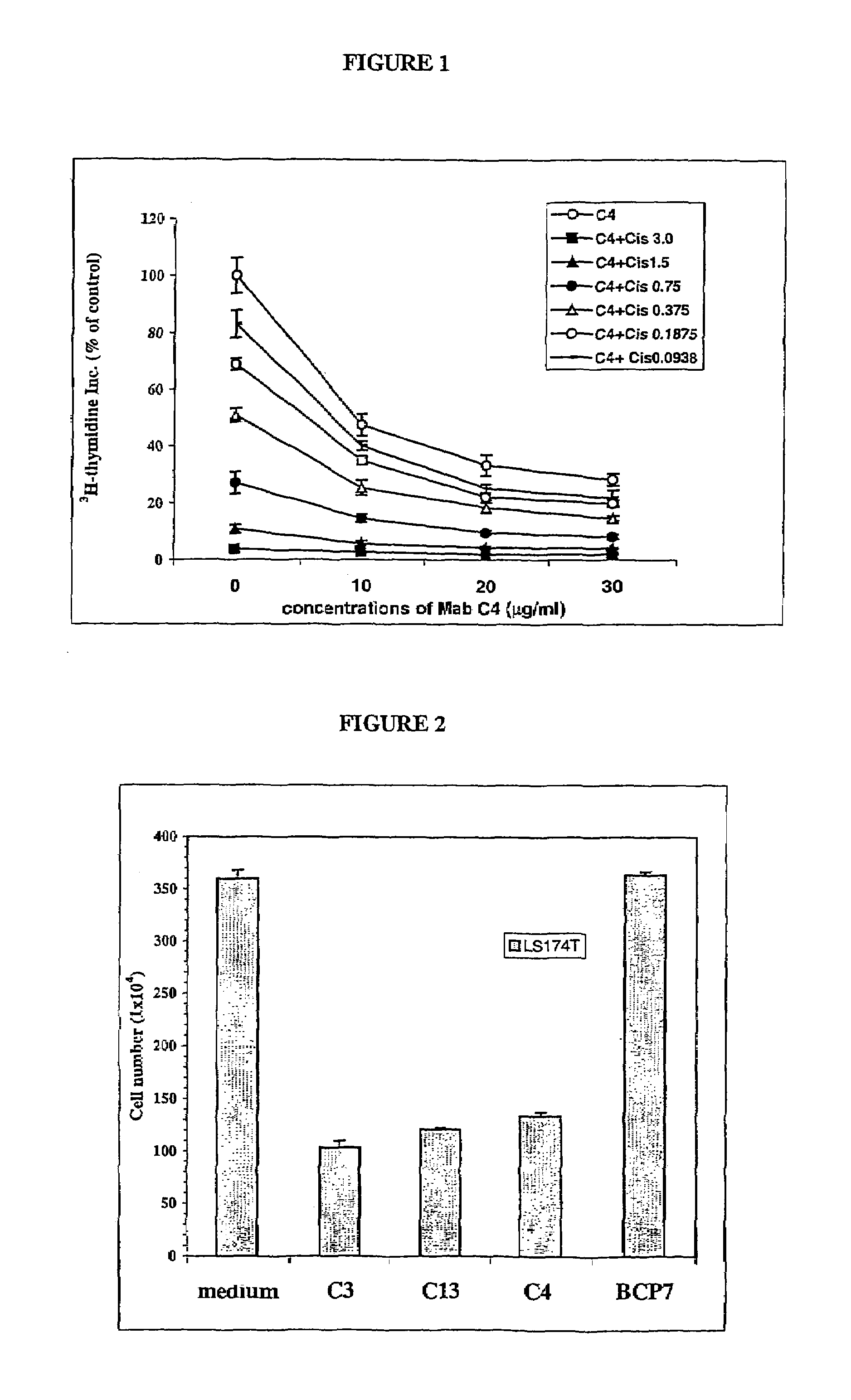
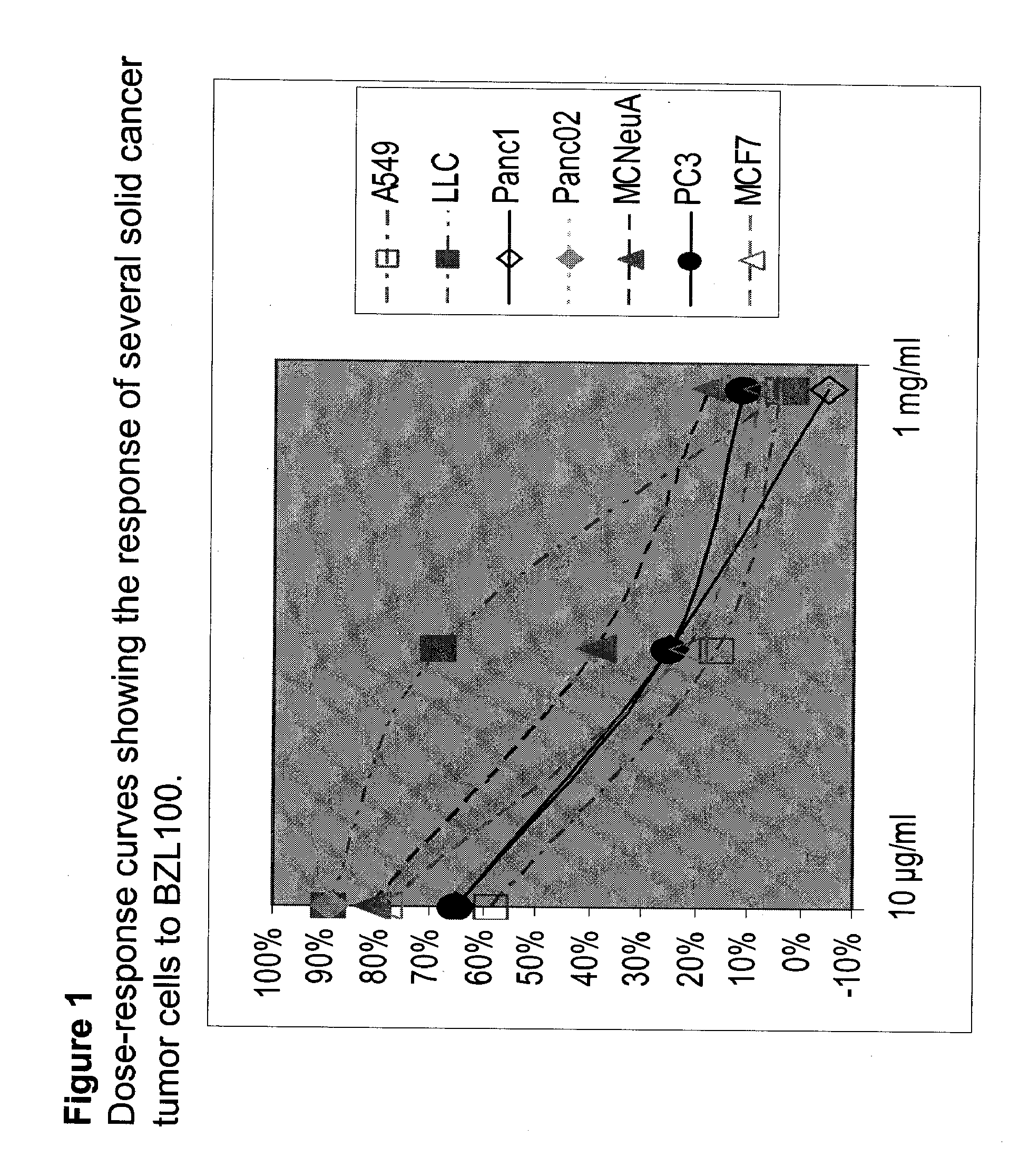
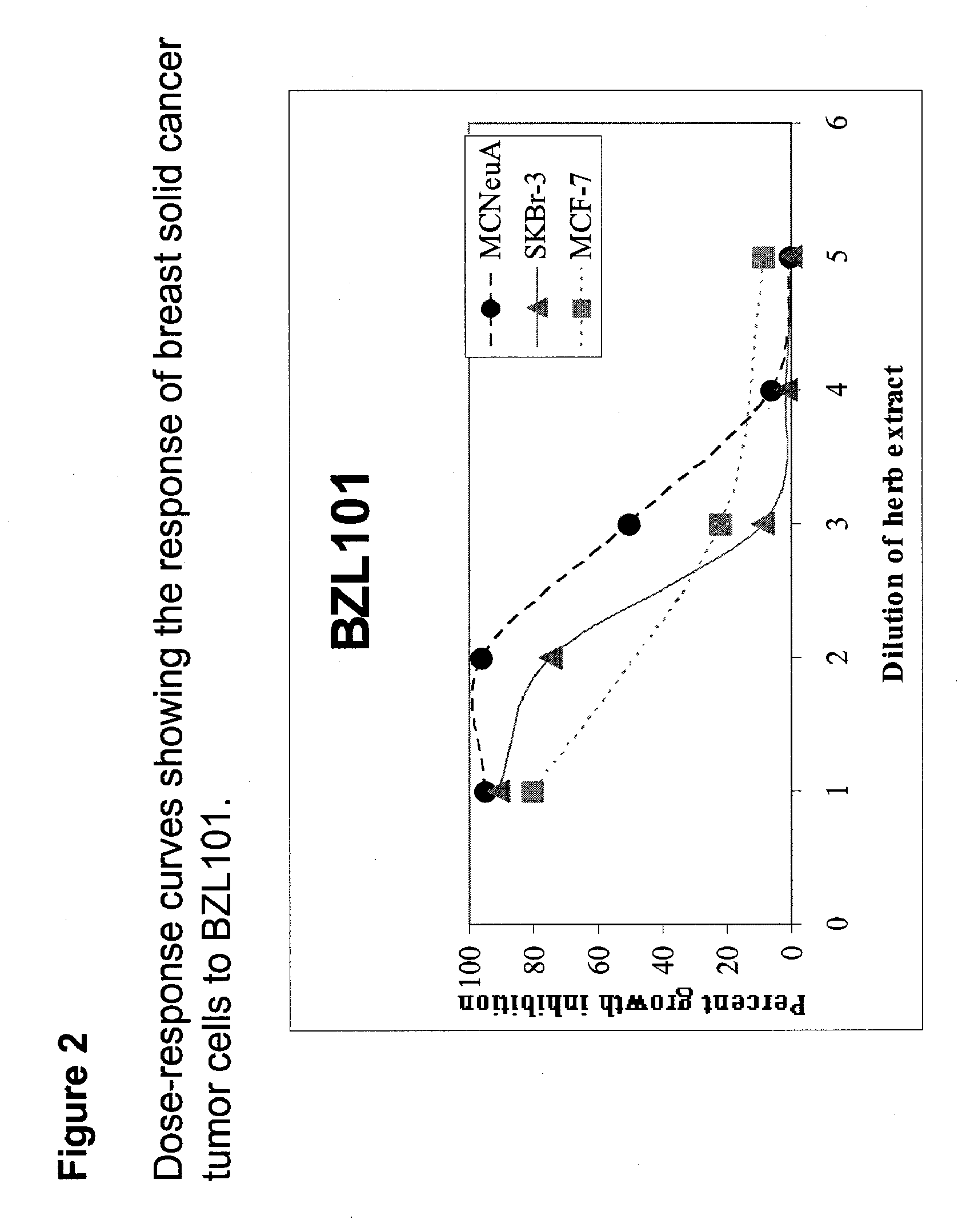
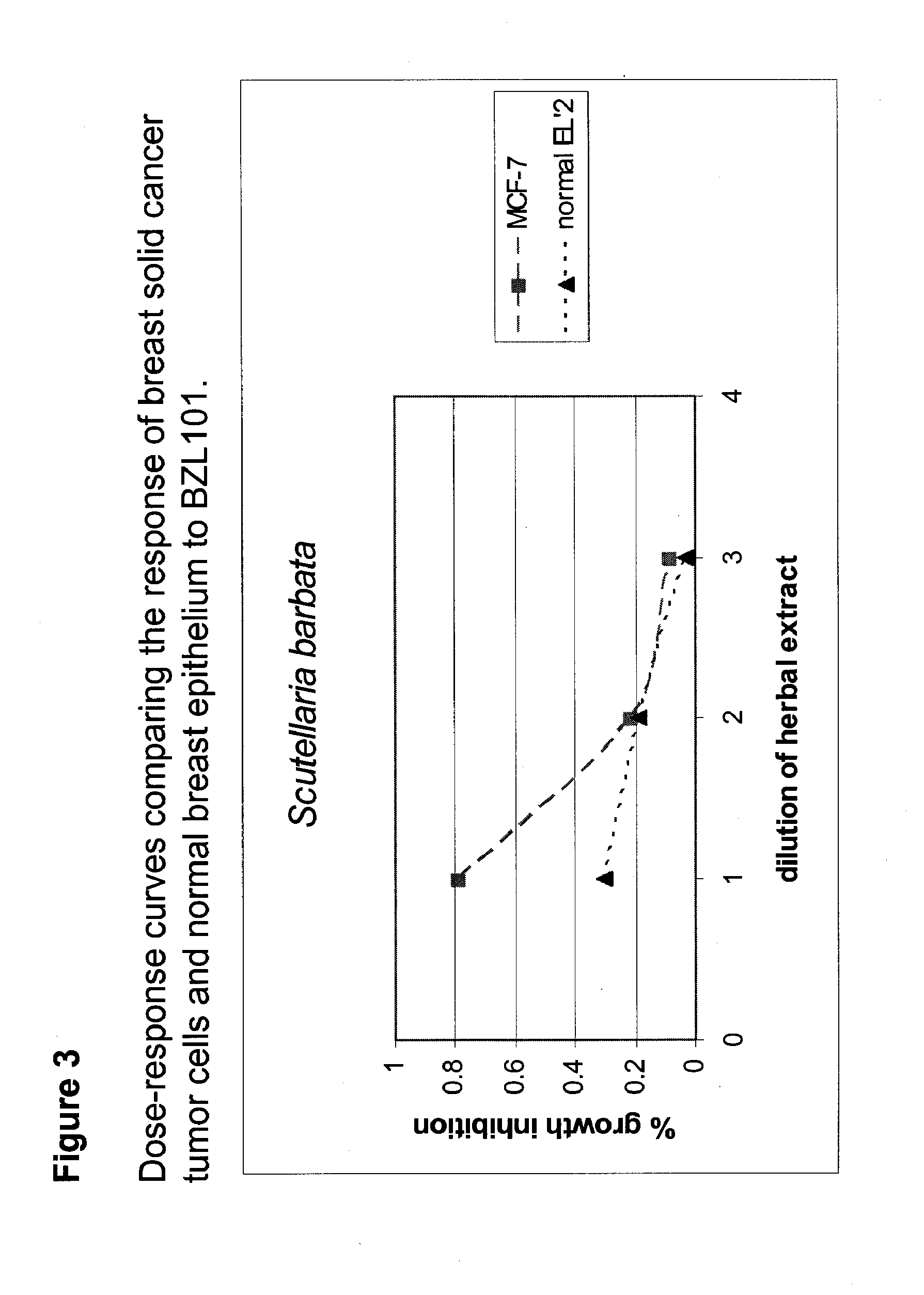
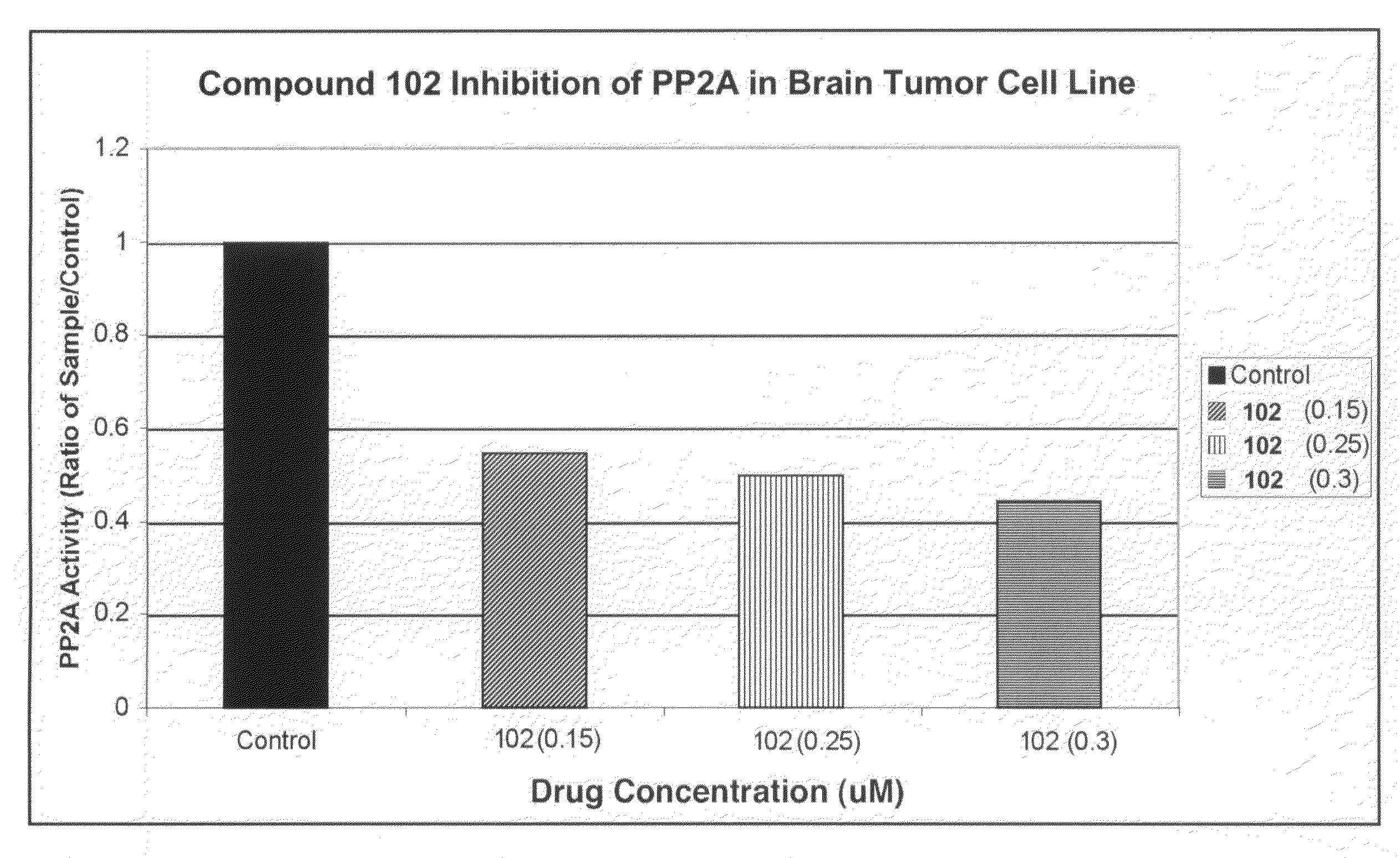
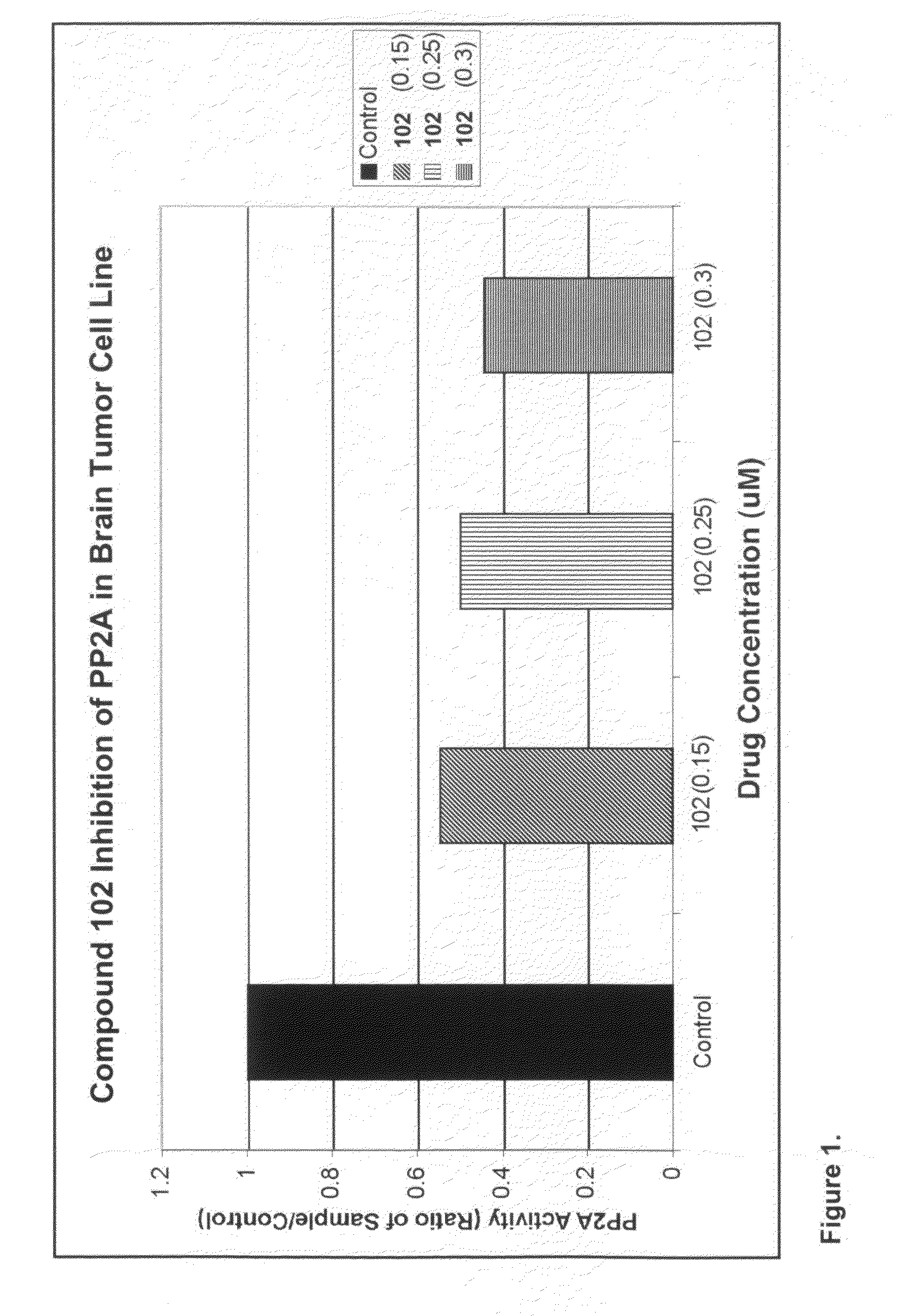
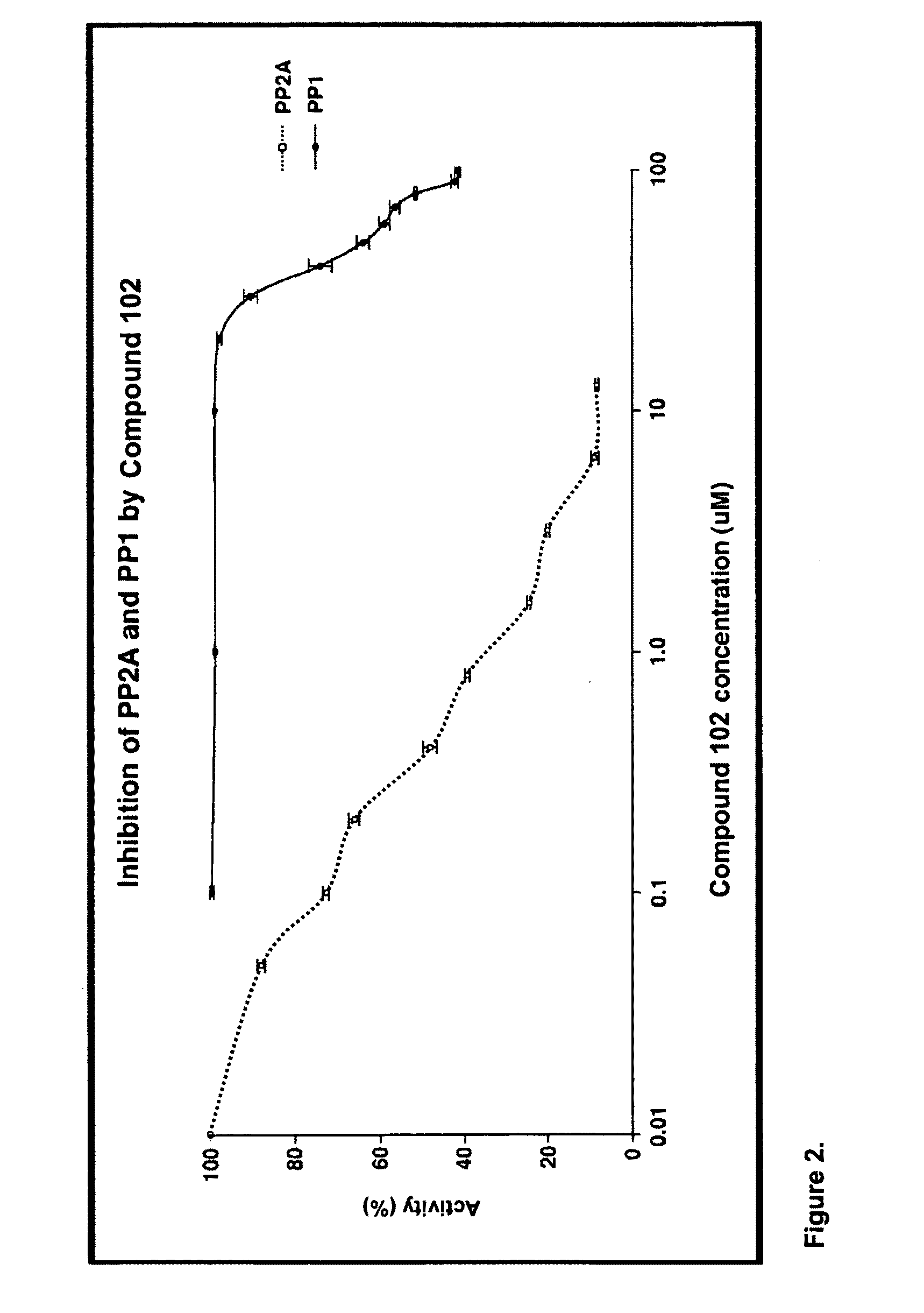
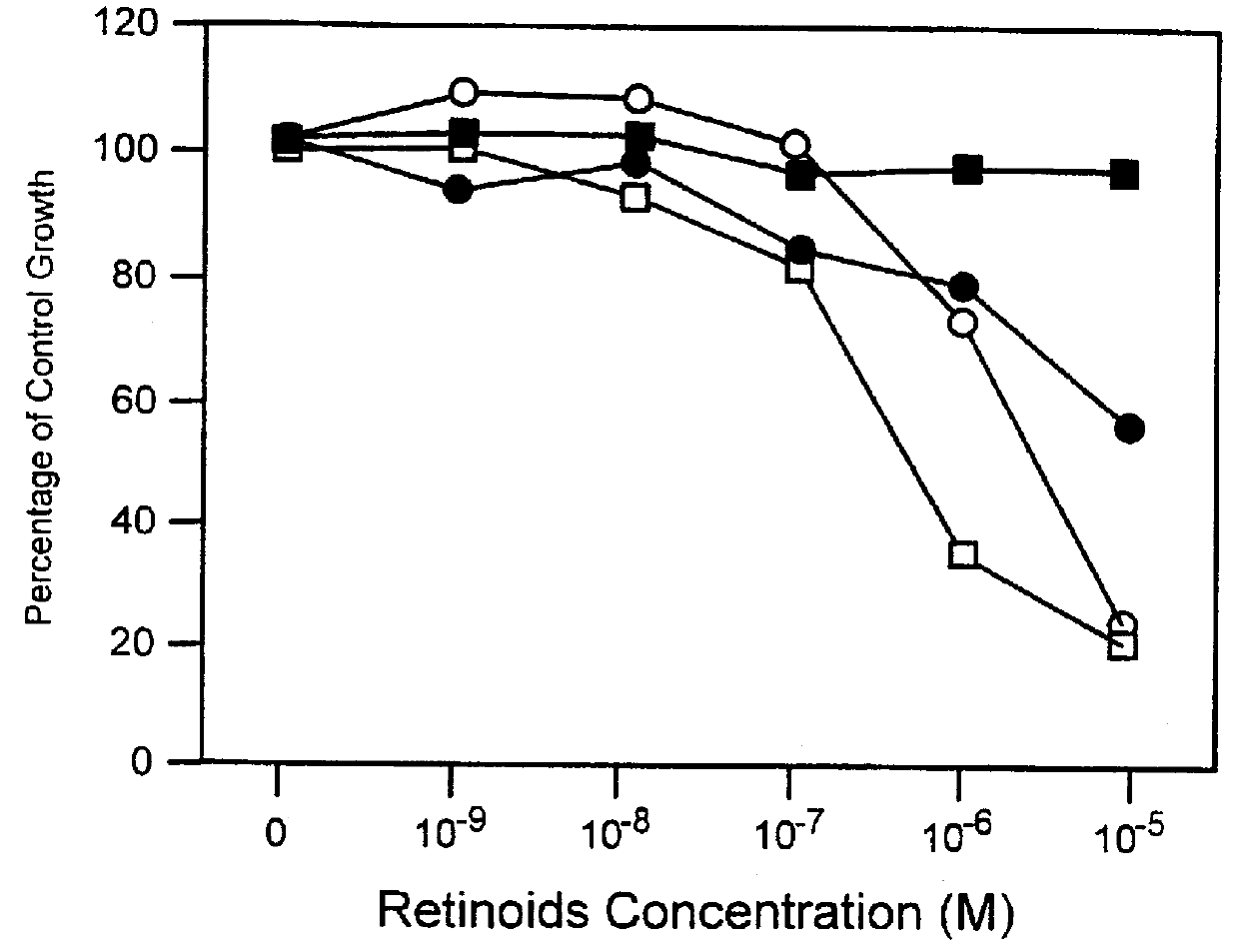
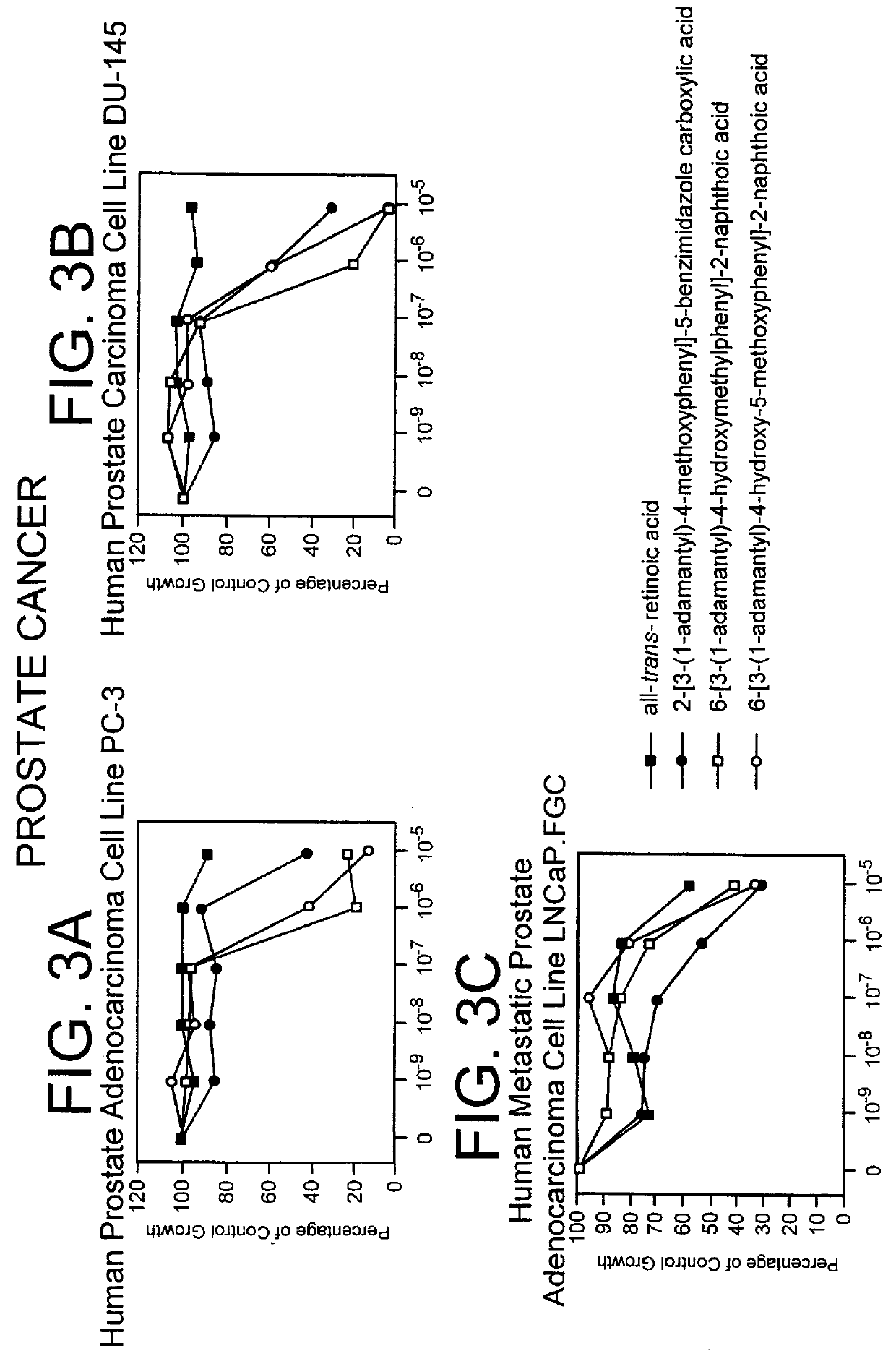
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
350 results about "Cancer cell apoptosis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Apoptosis inducers cause cancer cells to undergo a process of controlled cell death called apoptosis. Apoptosis is one method the body uses to get rid of unneeded or abnormal cells, but cancer cells have strategies to avoid apoptosis. Apoptosis inducers can get around these strategies to cause the death of cancer cells.
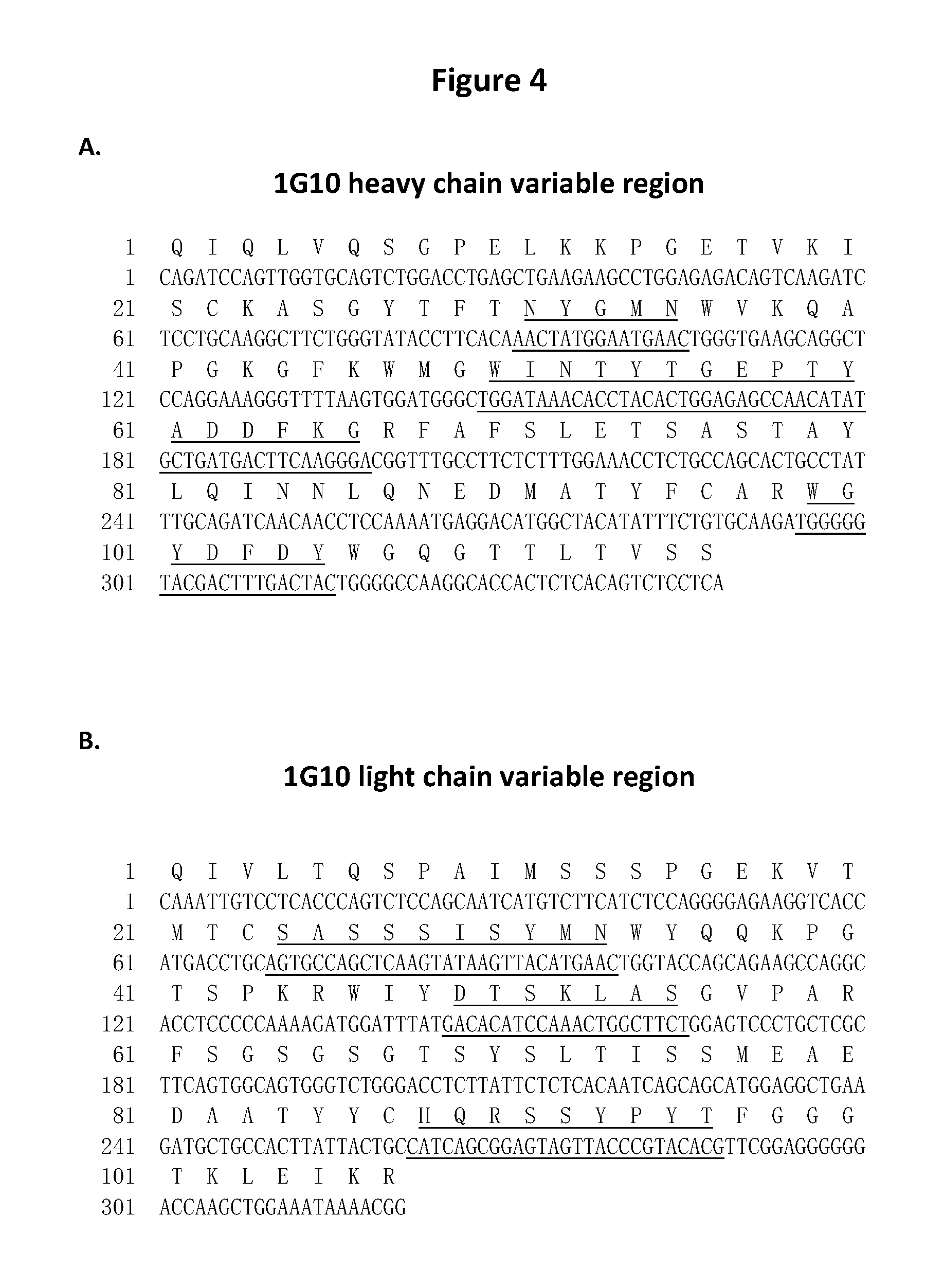
Dock-and-lock (DNL) vaccines for cancer therapy
Owner:IBC PHARMACEUTICALS INC
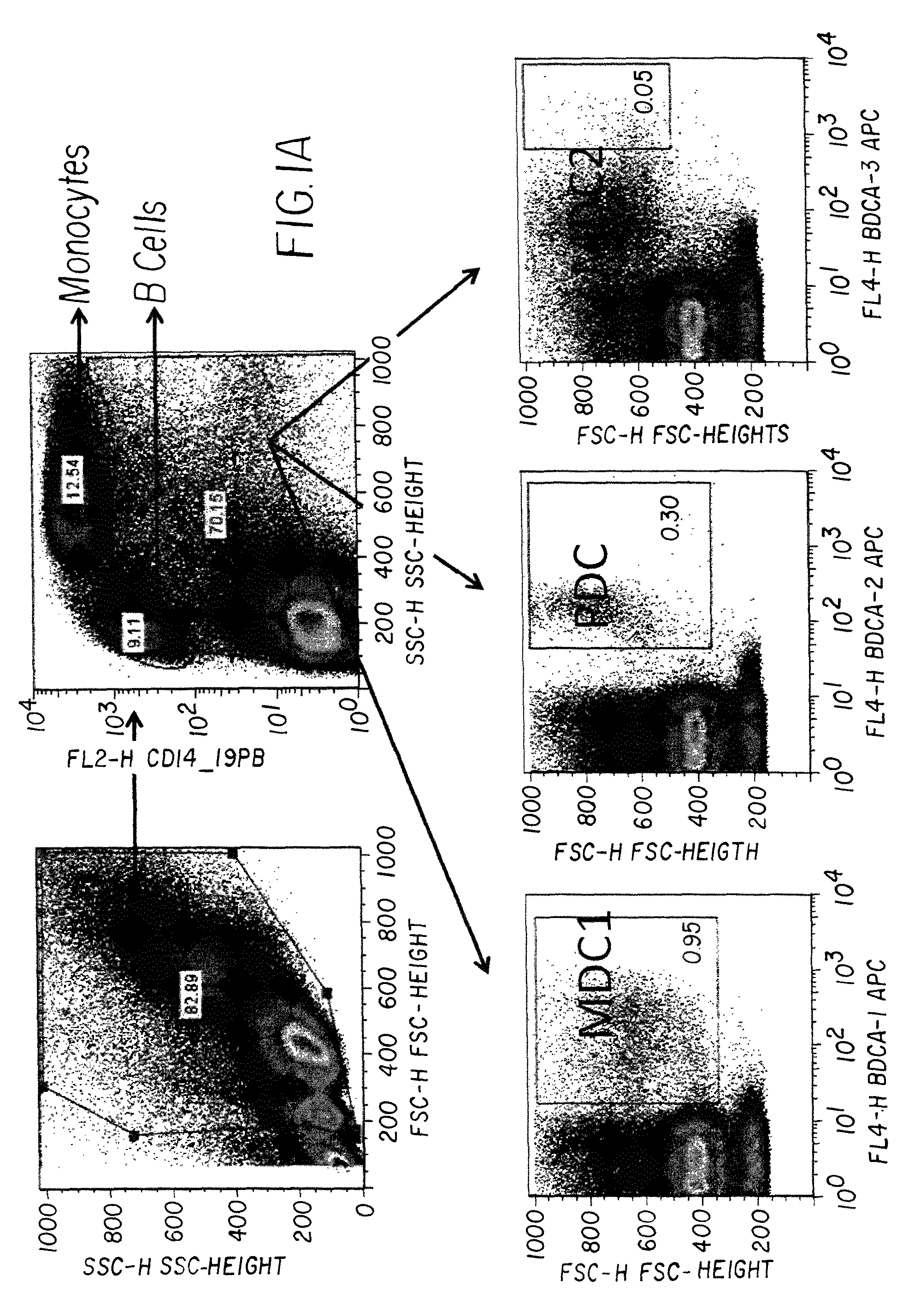
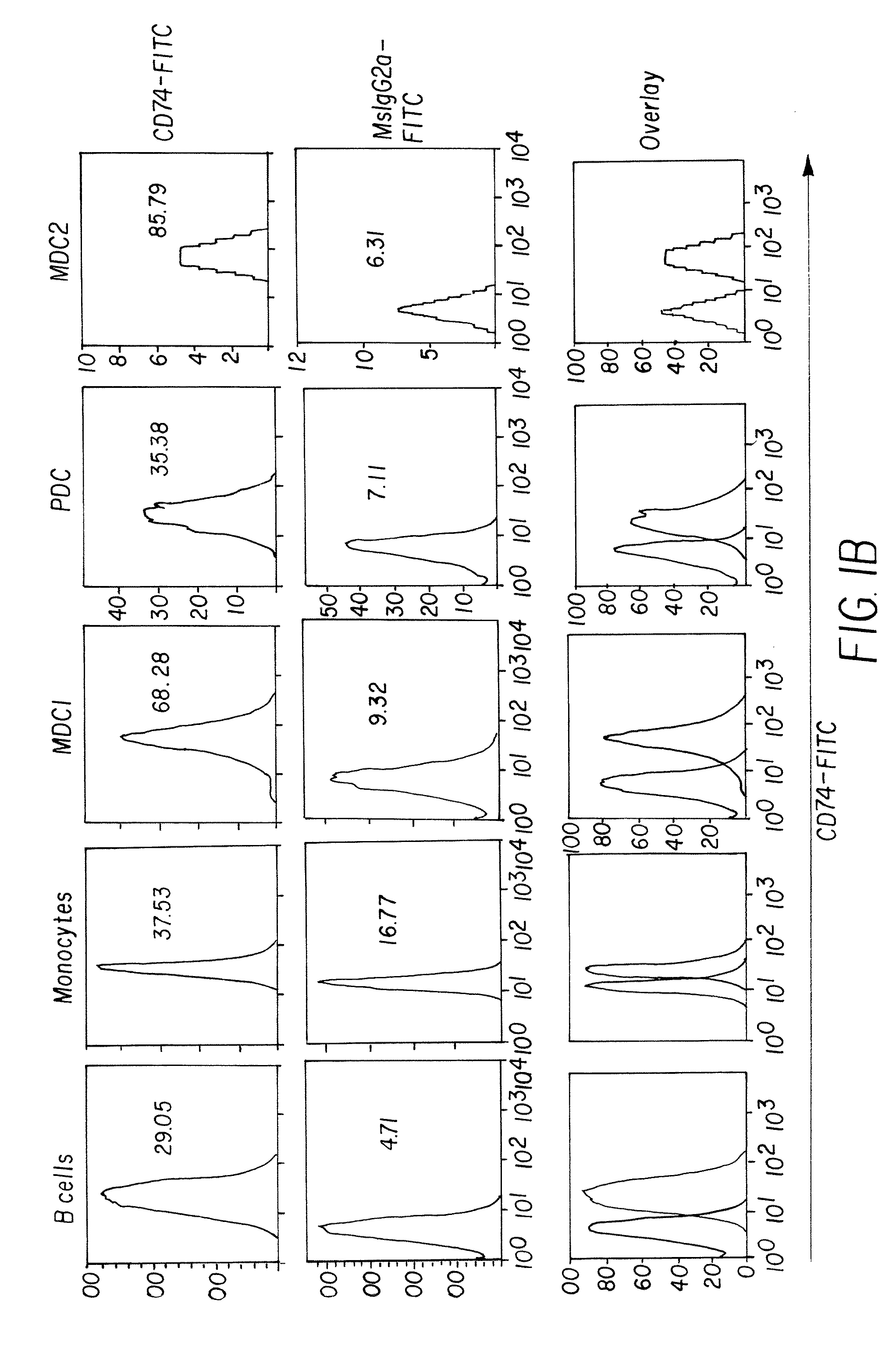
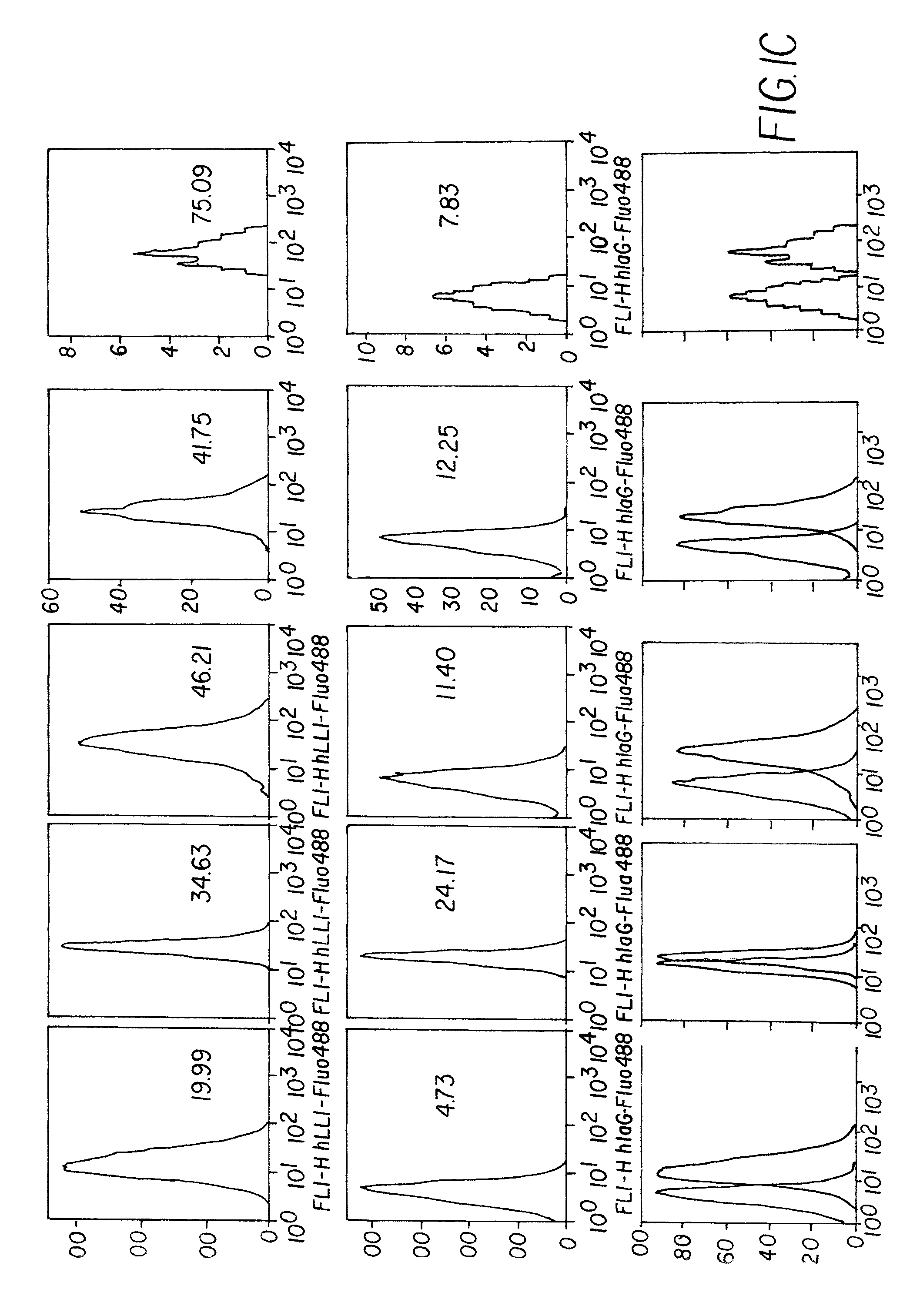
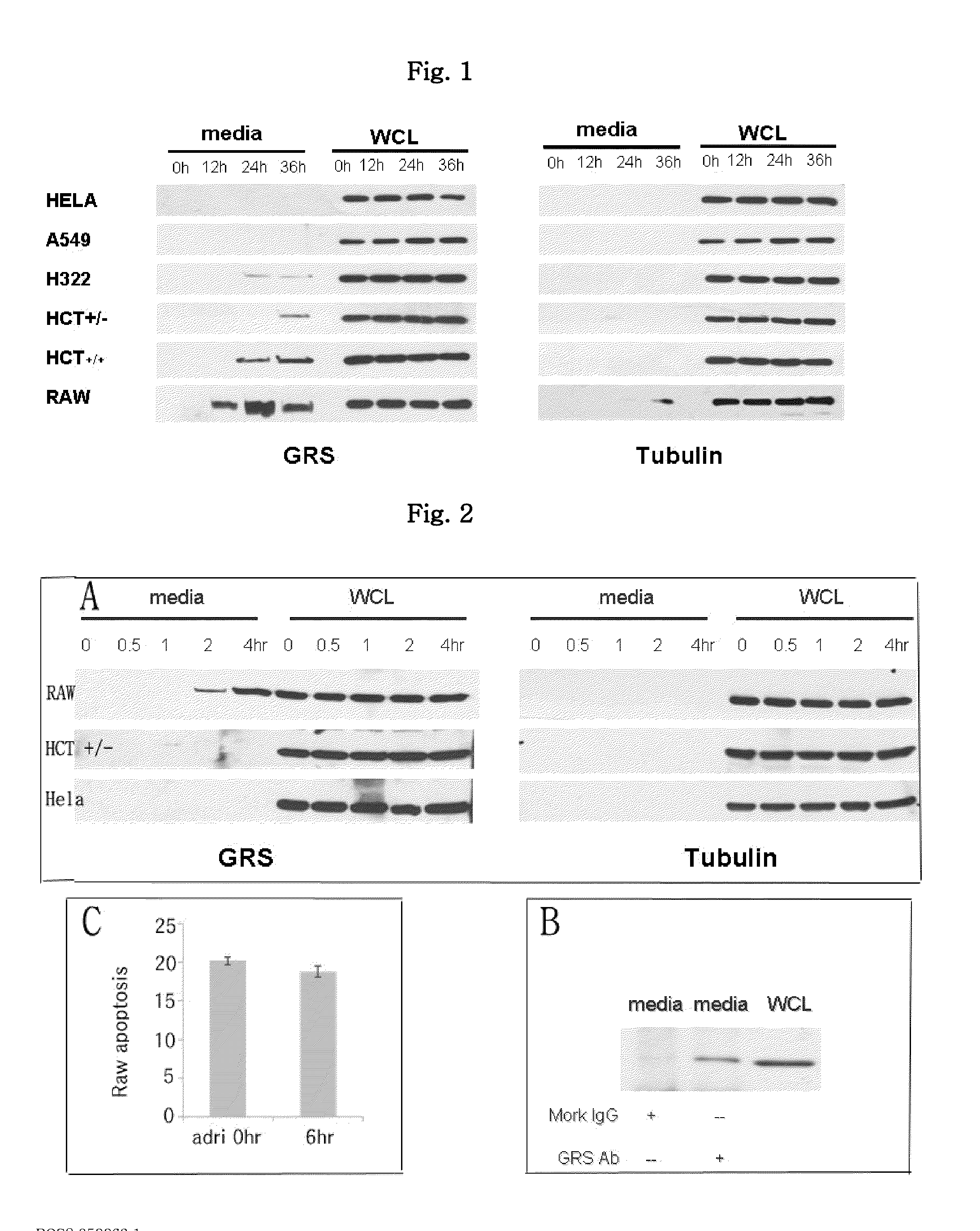
Novel uses of grs proteins or fragments thereof
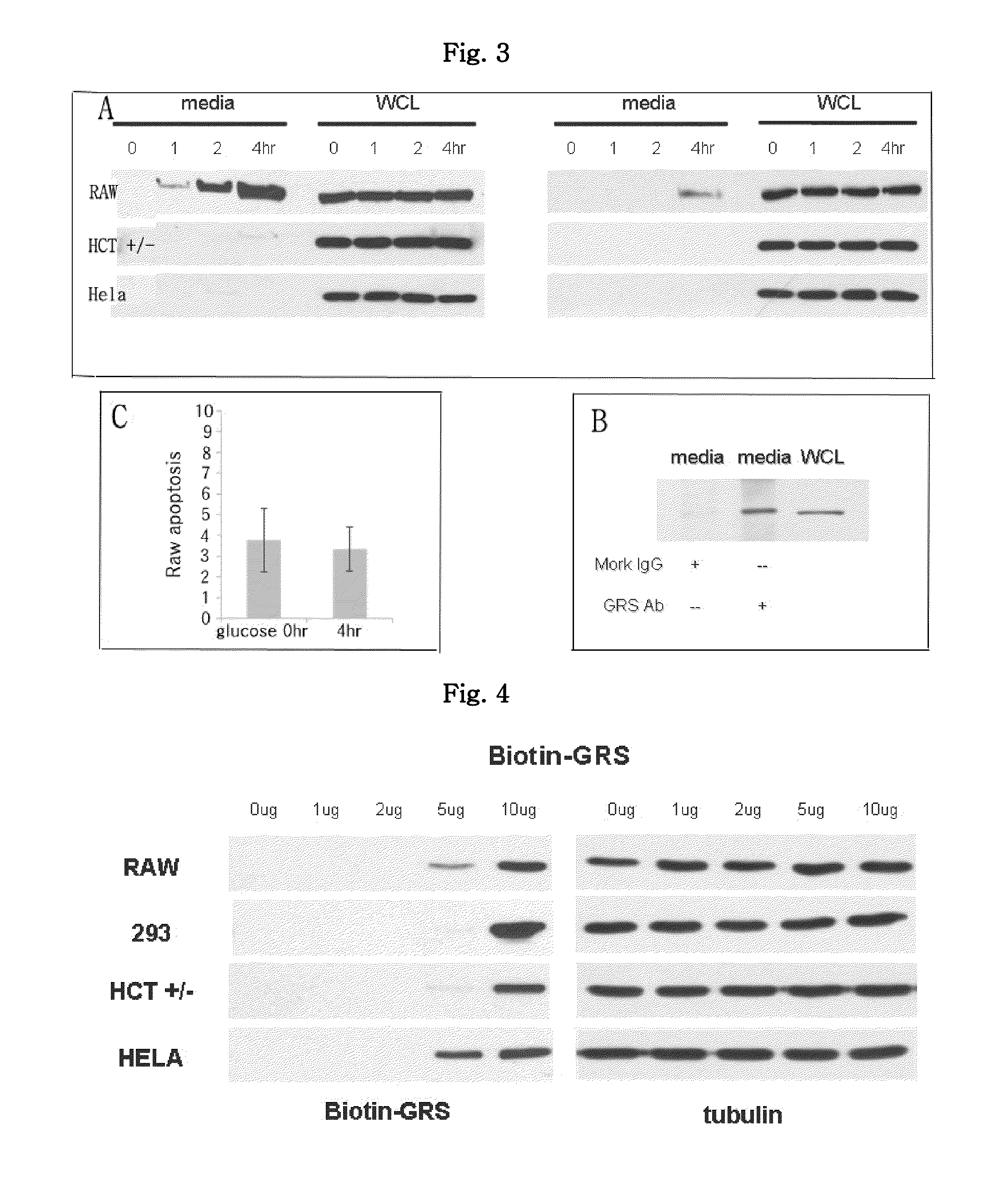
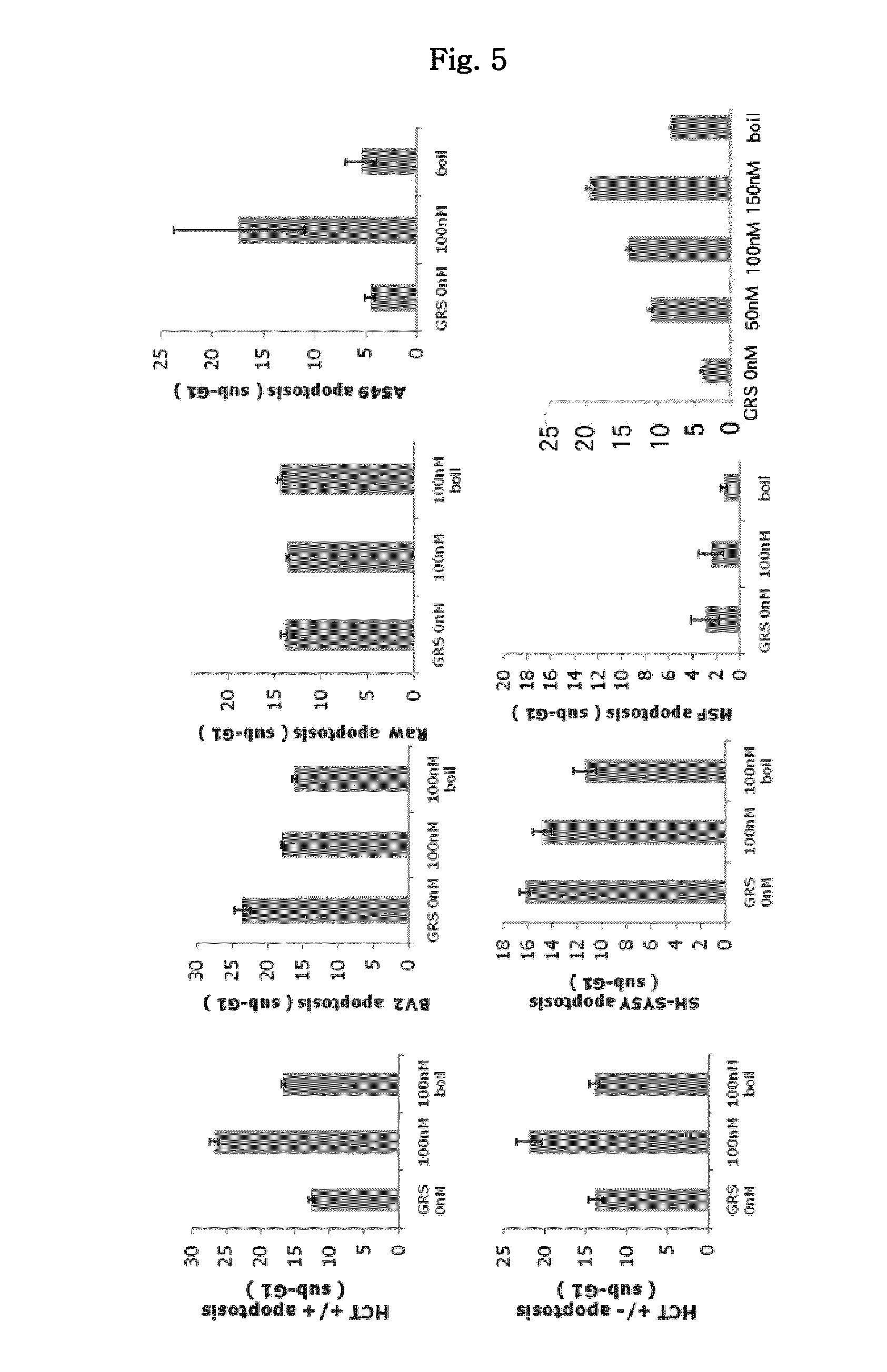
The present invention is related to a anticancer composition comprising full length GRS protein or a fragment thereof, a nucleic acid encoding the GRS protein or a fragment thereof. Since the GRS proteins or fragments thereof have activity to induce apoptosis of cancer cell specifically, a composition comprising the GRS proteins or fragments thereof or a nucleic acid encoding thereof may be useful to treatment of cancer.
Owner:SEOUL NAT UNIV R&DB FOUND
Haloacetamide and azide substituted compounds and methods of use thereof
InactiveUS20050137172A1Effective treatmentPrevent cancerBiocideOrganic chemistryCancer preventionMetabolite
The present invention relates to a novel class of anti-cancer compounds, which contain a haloacetamide or azide moiety and are, in one embodiment, alkylating agents. These agents, either alone or in a composition, are useful for treating cancer, preventing cancer, delaying the progression of cancer, treating and / or preventing the recurrence of cancer, suppressing, inhibiting or reducing the incidence of cancer, or inducing apoptosis in a cancer cell. Accordingly, the present invention provides a) methods of treating cancer in a subject; b) methods of preventing cancer in a subject; c) methods of delaying the progression of cancer in a subject; d) methods of treating the recurrence of cancer in a subject; e) methods of preventing the recurrence of cancer in a subject; f) methods of suppressing, inhibiting or reducing the incidence of cancer in a subject; and g) methods of inducing apoptosis in a cancer cell; by administering to the subject an anti-cancer compound of the present invention or an analog or metabolite thereof, its N-oxide, ester, pharmaceutically acceptable salt, hydrate, or any combination thereof as described herein.
Owner:DALTON JAMES +3
Antibodies against cancer
InactiveUS7318924B2Improve permeabilityPeptide/protein ingredientsGenetic material ingredientsAnticarcinogenCripto
An isolated binding partner of a Cripto-1 protein, Pim-1 protein or an antigen present in a colon cancer cell lysate is described. The binding partner inhibits growth of one or more cancer cell types and may be used in an anti-cancer agent for treating cancer in a subject. The binding partner may also be used in a method of inducing apoptosis in a cancer cell, as well as in a method of sensitizing a cancer cell to a cytotoxic compound. In addition, a cancer vaccine is described wherein the vaccine comprises a Cripto-1 protein (or an antigenic fragment thereof), Pim-1 protein (or an antigenic fragment thereof) or an antigen present in a colon cancer cell lysate or, alternatively, comprises an expressible DNA molecule encoding a Cripto-1 protein (or an antigenic fragment thereof), Pim-1 protein (or an antigenic fragment thereof) or an antigen present in a colon cancer cell lysate.
Owner:THE MACFARLANE BURNET INST FOR MEDICAL RES & PUBLIC HEALTH LTD
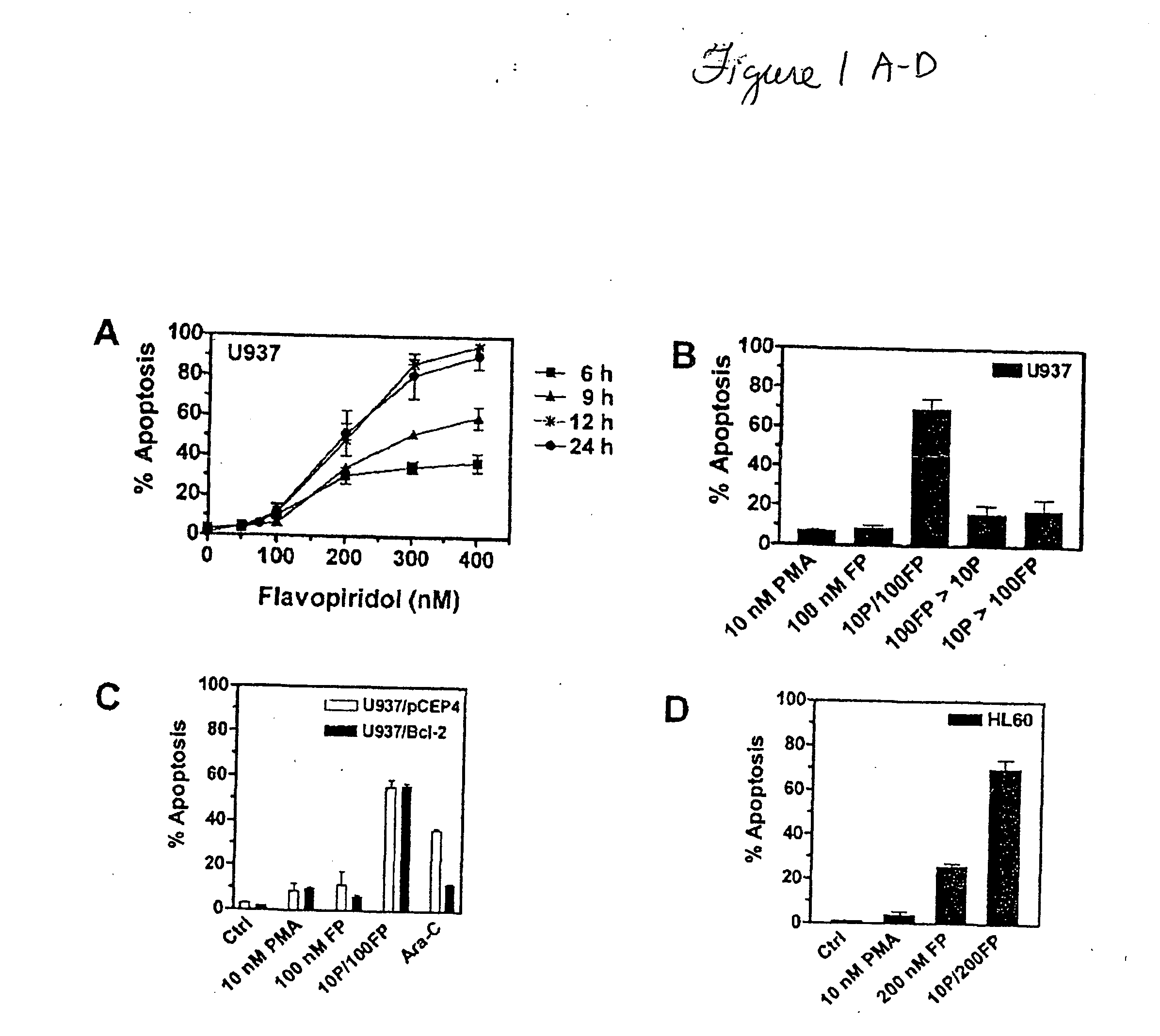
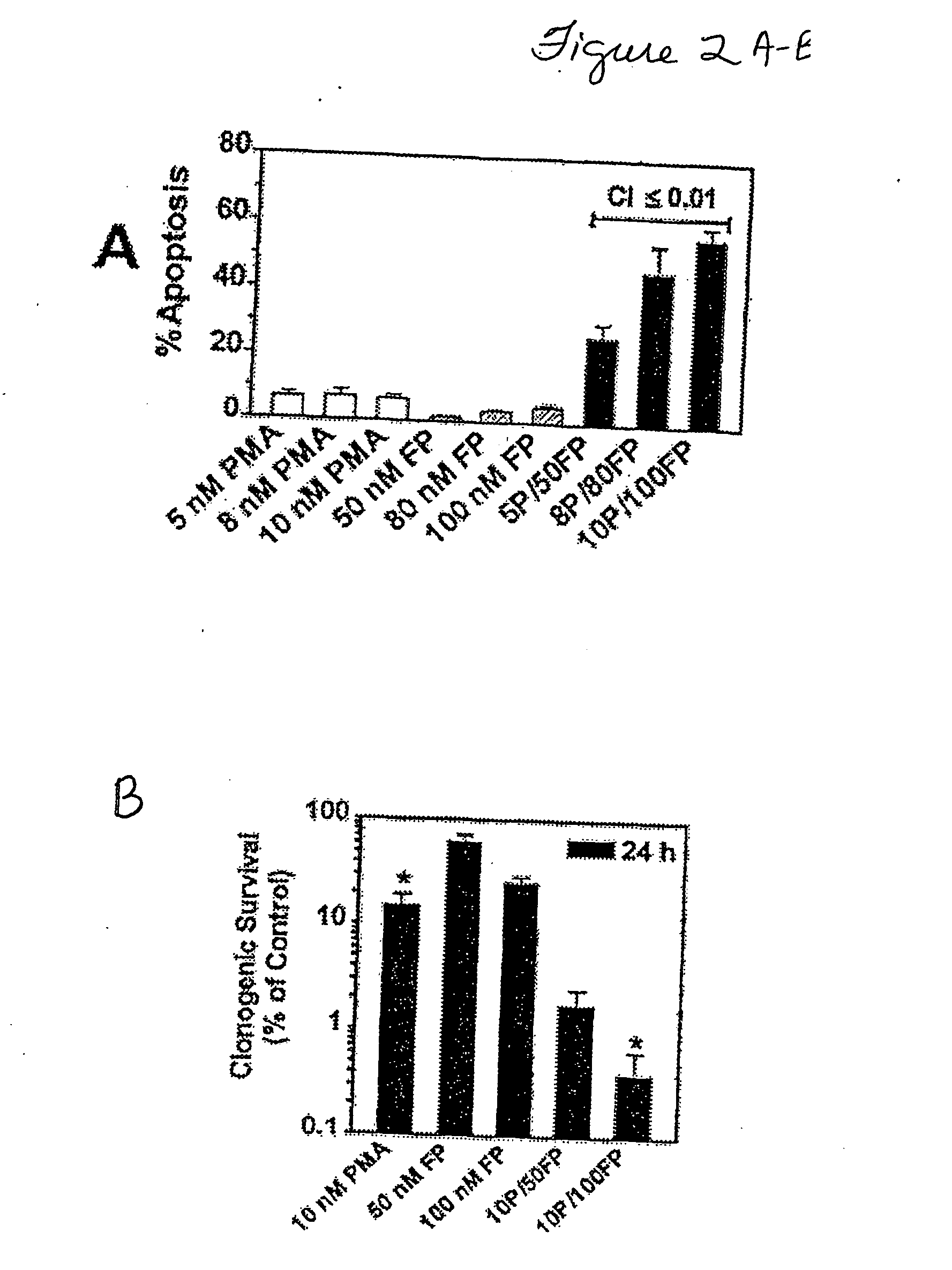
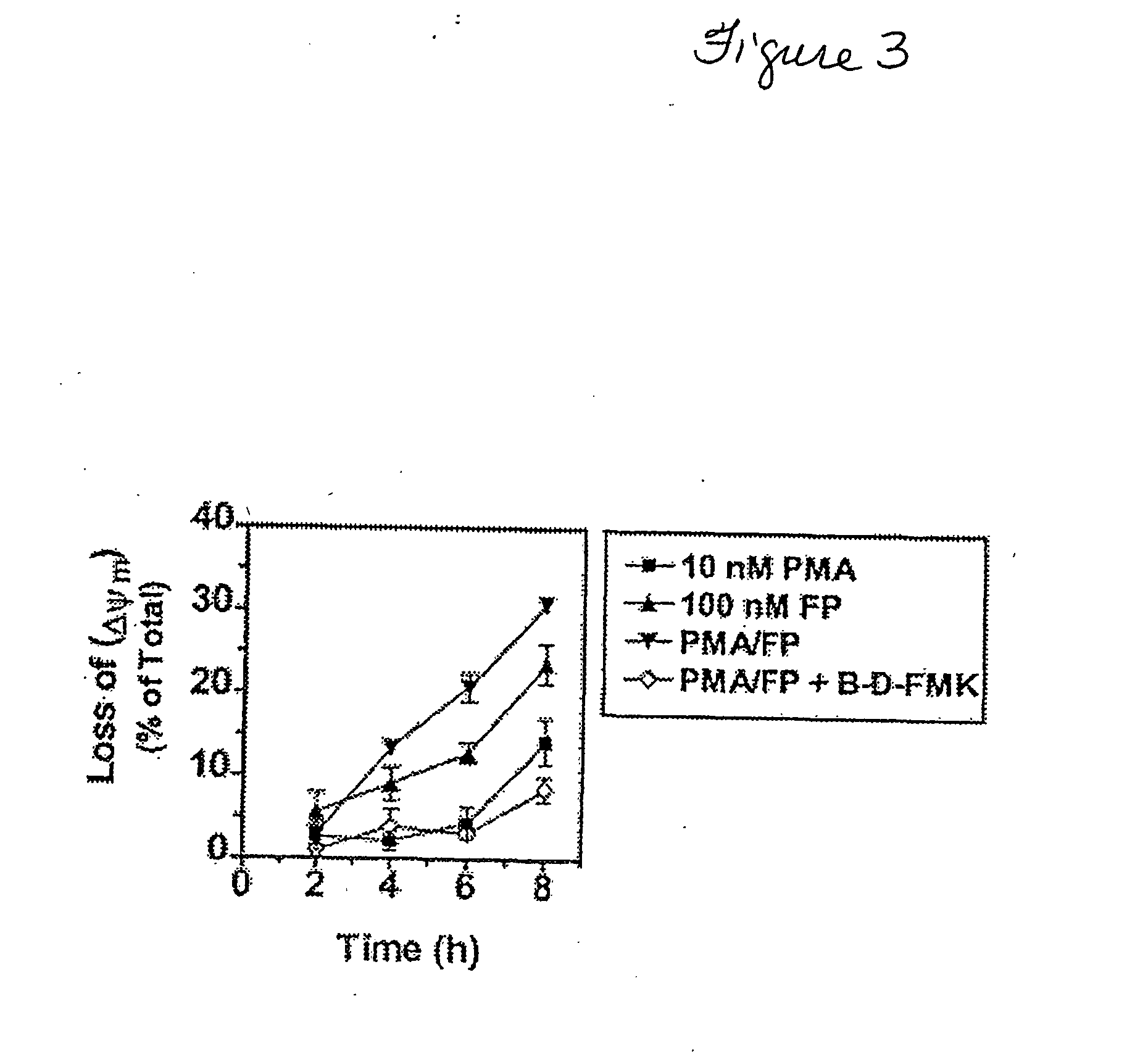
Promotion of adoptosis in cancer cells by co-administration of cyclin dependent kinase inhibitiors and cellular differentiation agents
The invention provides compositions and methods for promoting apoptosis of cancer cells, and methods for treating cancer. The compositions comprise cyclin dependent kinase inhibitor and an agent that induces cellular differentiation. The methods of promoting apoptosis of cancer cells involve the co-administration to the cancer cells of a cyclin dependent kinase inhibitor and an agent that induces cell differentiation. The method for treating cancer involves the co-administration of a cyclin dependent kinase inhibitor and an agent that induces cellular differentiation to a patient. Examples of cyclin dependent kinase inhibitors include histone deacetylase inhibitors, protein kinase C activators, retinoids, and Vitamin D3.
Owner:VIRGINIA COMMONWEALTH UNIV
Methods and compounds useful to induce apoptosis in cancer cells
InactiveUS20050027000A1Reduced viabilityCompound screeningHalogenated hydrocarbon active ingredientsApoptosisCompound (substance)
Owner:SANFORD BURNHAM PREBYS MEDICAL DISCOVERY INST
Strategies for improved cancer vaccines
The present invention concerns methods and compositions for forming anti-cancer vaccine complexes. In preferred embodiments, the anti-cancer vaccine complex comprises an antibody moiety that binds to dendritic cells, such as an anti-CD74 antibody or antigen-binding fragment thereof, attached to an AD (anchoring domain) moiety and a xenoantigen, such as CD20, attached to a DDD (dimerization and docking domain) moiety, wherein two copies of the DDD moiety form a dimer that binds to the AD moiety, resulting in the formation of the vaccine complex. The anti-cancer vaccine complex is capable of inducing an immune response against xenoantigen expressing cancer cells, such as CD138negCD20+ MM stem cells, and inducing apoptosis of and inhibiting the growth of or eliminating the cancer cells.
Owner:IBC PHARMACEUTICALS INC
Scutellaria barbata extract for the treatment of cancer
An extract of Scutellaria barbata D. Don is effective in the arrest of cancer cell growth in the G1 phase, the induction of apoptosis in cancer cells and the shrinking of solid cancers. The extract may be prepared as a pharmaceutical composition for administration to mammals for the treatment of solid cancers, such as epithelial cancers. Such epithelial cancers include breast cancer and ovarian cancers. The extract is obtained from Scutellaria barbata D. Don by contacting aerial portions of a plant from the species Scutellaria barbata D. Don with an aqueous or alcoholic solvent.
Owner:BIONOVO
Methods for regulating cell mitosis by inhibiting serine/threonine phosphateses
InactiveUS20100029683A1Reduce the amount requiredBiocideMicrobiological testing/measurementApoptosisThreonine
Disclosed herein are methods of inhibiting proliferation of a cancer cell or inducing apoptosis of a cancer cell, which does not overexpress N—CoR. Also disclosed herein are methods of inhibiting proliferation or inducing apoptosis of a cancer cell that overexpresses TCTP and methods for determining whether a compound is effective in inducing cell death.
Owner:LIXTE BIOTECH +1
Apoptosis inducing adamantyl derivatives and their usage as anti-cancer agents
InactiveUS6127415APreventing and controlling photoinducedPreventing and controlling and chronologic agingBiocideCosmetic preparationsDiseaseAnticarcinogen
PCT No. PCT / US97 / 11564 Sec. 371 Date Apr. 14, 1999 Sec. 102(e) Date Apr. 14, 1999 PCT Filed Jul. 8, 1997 PCT Pub. No. WO98 / 01132 PCT Pub. Date Jan. 15, 1998The present invention relates to specific adamantyl or adamantyl group derivative containing retinoid compounds induce apoptosis of cancer cells. These adamantyl retinoid derivatives are useful for the treatment of many cancers and solid tumors, especially androgen-independent prostate cancer, skin cancer, pancreatic carcinomas, colon cancer, melanoma, ovarian cancer, liver cancer, small cell lung carcinoma, non-small cell lung carcinoma, cervical carcinoma, brain cancer, bladder cancer, breast cancer, neuroblastoma / glioblastoma, and leukemia. Also, the invention relates to novel adamantyl or adamantyl group derivative compounds which are useful as active agents for the treatment or prevention of keratinization disorders and other dermatological conditions, and other diseases.
Owner:GALDERMA RES & DEV SNC
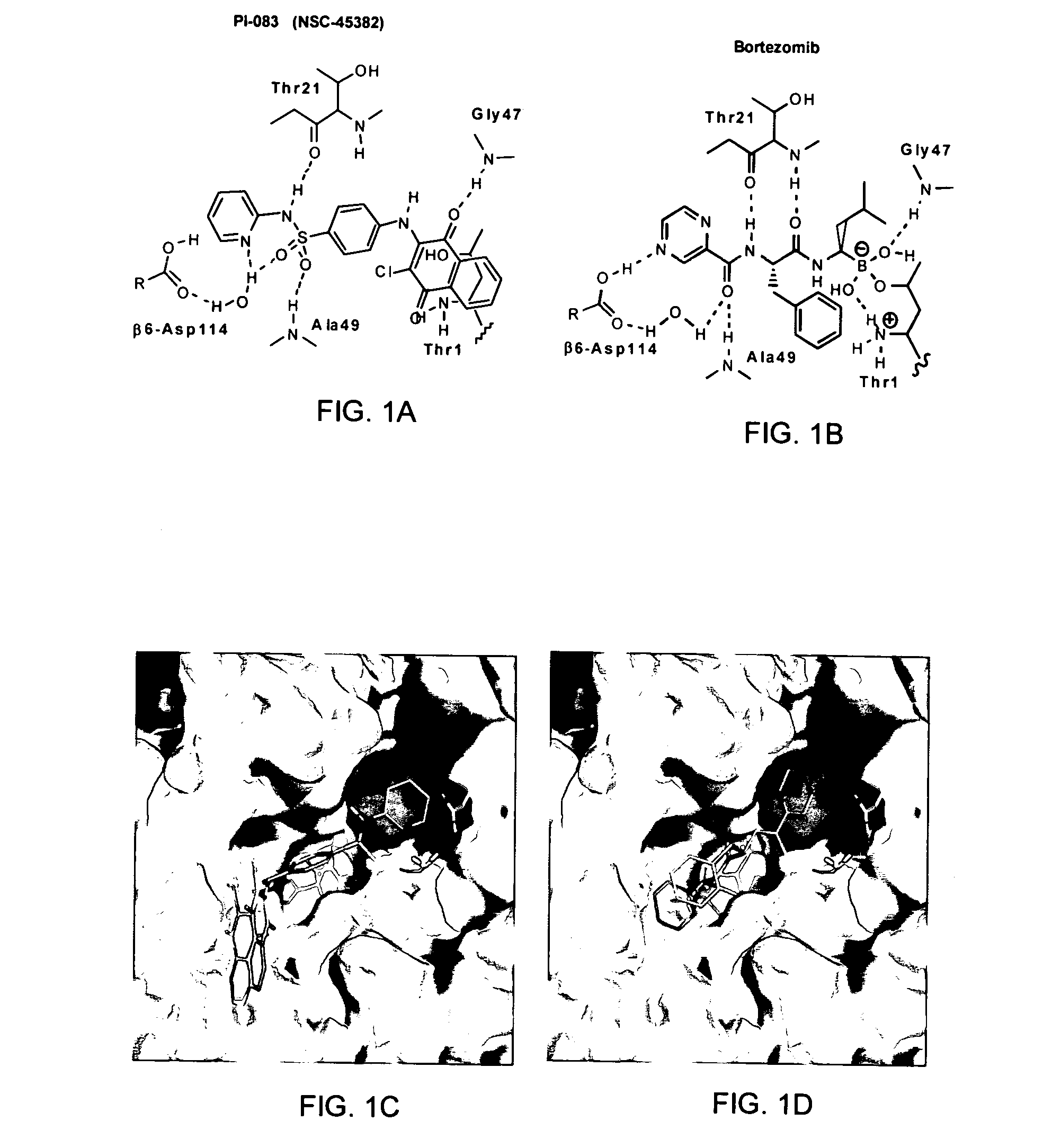
Proteasome inhibitors for selectively inducing apoptosis in cancer cells
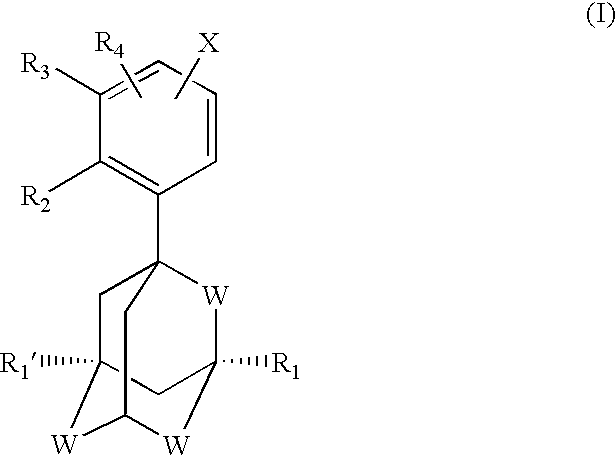
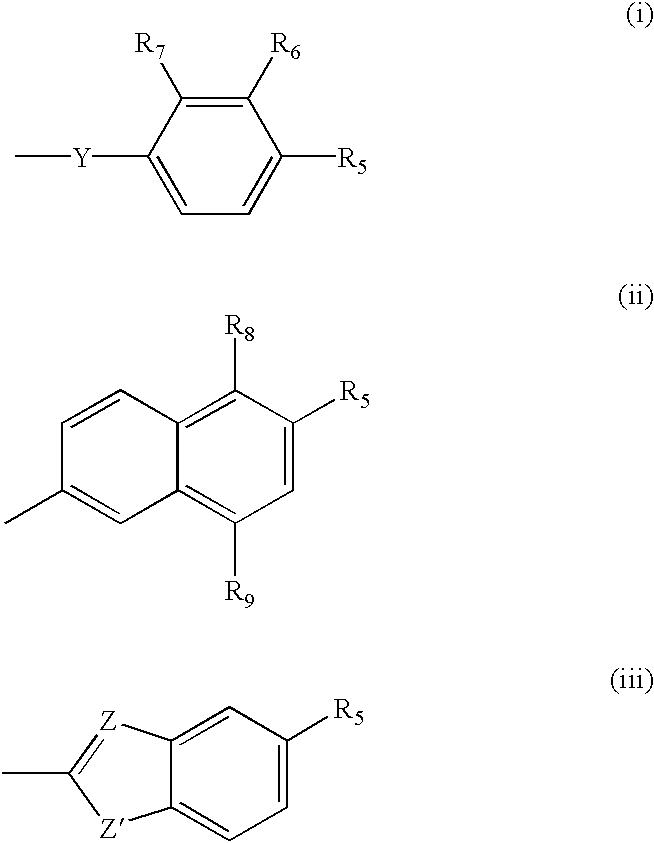
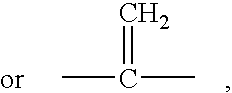
The subject invention concerns compounds having activity as inhibitors of proteasomes and methods of using the subject compounds. In one embodiment, a compound of the invention has the chemical structure shown in formula I:whereinR1 is an organic cyclic ring structure bonded to a sulfonamide structure;R2 is H, halogen, alkyl, —NR6R7, or heteroalkyl;R3 is H, halogen, —OH, —O-alkyl, alkyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, —NO2, —NH2 or substituted amines;R4 is H, alkyl, heteroalkyl, aryl, or heteroaryl, any of which can be optionally substituted with one or more of —NO2, alkyl, heteroalkyl, aryl, or heteroaryl, or halogen;R5 is H, —OH, halogen, alkyl, aryl, heteroaryl, cycloalkyl, heterocycloalkyl, —O-alkyl, —O-aryl, heteroalkyl, —NO2, —NH2, or substituted amine; andR6 and R7 are independently H, O, alkyl, aryl, heterocycloalkyl, or heteroaryl, or together can form a heterocycloalkyl or a heteroaryl, any of which can be optionally substituted with one or more of —NO2, alkyl, heteroalkyl, aryl, or halogen;or a pharmaceutically acceptable salt or hydrate thereof.In another embodiment, a compound of the invention has the chemical structure shown in formula II:whereinQ, W, X, Y, Z are each independently carbon, oxygen, or nitrogen;R1 is H, or X1R8;R2 is heteroalkyl, which can be optionally substituted with one or more of —OH, halogen, —C(O)OR4, alkyl, heteroalkyl, heterocycloalkyl, or heteroaryl;R3 is heterocycloalkyl, aryl, heteroaryl, any of which can be optionally substituted with one or more of a halogen or —OH; andR4 is H or alkyl;R5 is halogen, alkyl or nitro;R6 is nitro, X2R9 or a halogen;R7 is H or alkyl;R8 is H, alkyl, aryl, CH2-alkyl-aryl, -alkyl-C(O)OH, or alkyl-tetrazole (aromatic and aliphatic heterocyclic groups);R9 is H or alkyl;X1 is oxygen, nitrogen, or sulfur;X2 is oxygen, nitrogen, or sulfur;or a pharmaceutically acceptable salt or hydrate thereof.
Owner:UNIV OF SOUTH FLORIDA +1
Methods and compositions for treating cancer
ActiveUS20100221246A1Reduce the overall heightPromote growthBiocideAntibody ingredientsApoptosisCell growth
The present invention provides methods of treating cancer using 2-amino-6-trifluoromethoxybenzothiazole (riluzole). In one aspect, the present invention provides methods of reducing cancer cell growth. In another aspect, the present invention provides a method of inducing apoptosis in a cancer cell. In another aspect, the present invention provides a method of reducing the growth of a glutamate-releasing tumor.
Owner:RUTGERS THE STATE UNIV
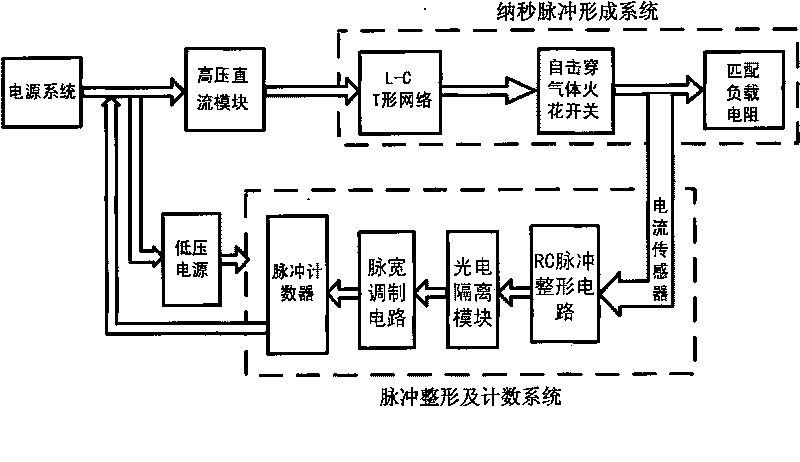
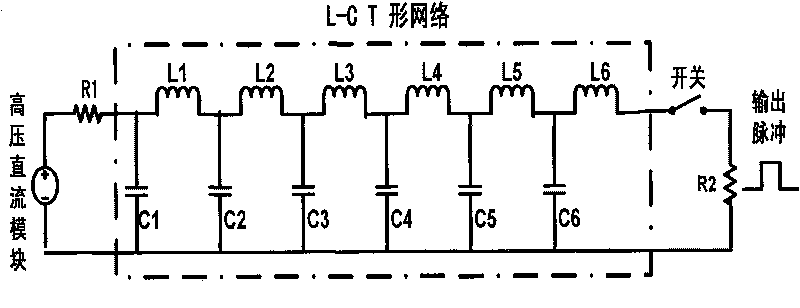
Portable high-voltage nanosecond squarer
ActiveCN101745178AImprove breakdown voltageReduce weightElectrotherapyStress based microorganism growth stimulationLow voltageHigh-voltage direct current
The invention relates to a portable high-voltage nanosecond squarer which belongs to a medicinal device for inducing cancer cell apoptosis by using a high-voltage nanosecond square wave pulse electric field. The portable high-voltage nanosecond squarer mainly comprises a power supply system, a high-voltage direct current module, a low-voltage power supply, a pulse forming system and a pulse shaping and counting system. The portable high-voltage nanosecond squarer has output pulses with highest amplitude of 15kV, pulse width adjustment range of 50ns-1us, rising edge gradient of reaching 10ns and controllable recurrence frequency of 0.2-15Hz; and the quantity of the output pulses of the portable high-voltage nanosecond squarer can be set. The portable high-voltage nanosecond squarer can be adjusted in multiple-parameters (pulse amplitude, pulse width, recurrence frequency, pulse quantity and the like), large-range and flexible and independent way, and is beneficial to researching the optimal window parameter of the cancer cell apoptosis and promoting the cancer cell apoptosis as well as improving the cancer treatment effect. Meanwhile, the portable high-voltage nanosecond squarer has compact and light structure, reliable work, simple operation and convenient popularization and application, and can be widely applied to the treatment of various cancers.
Owner:HANGZHOU WKNIFE MEDICAL TECH CO LTD
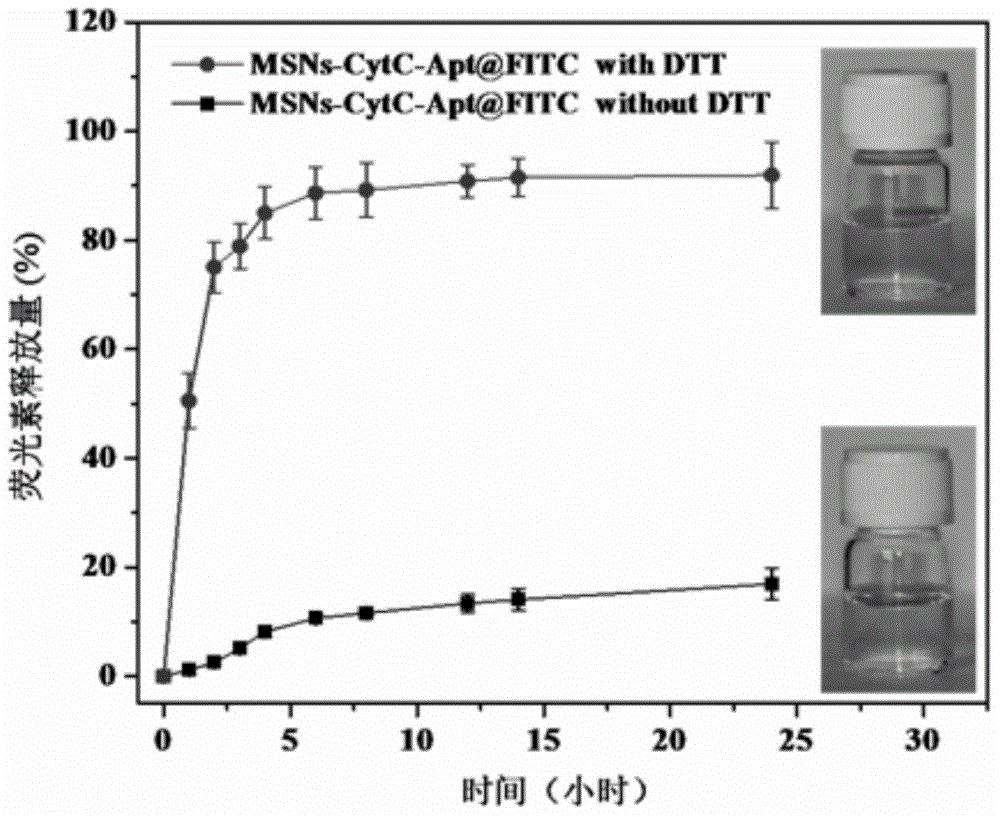
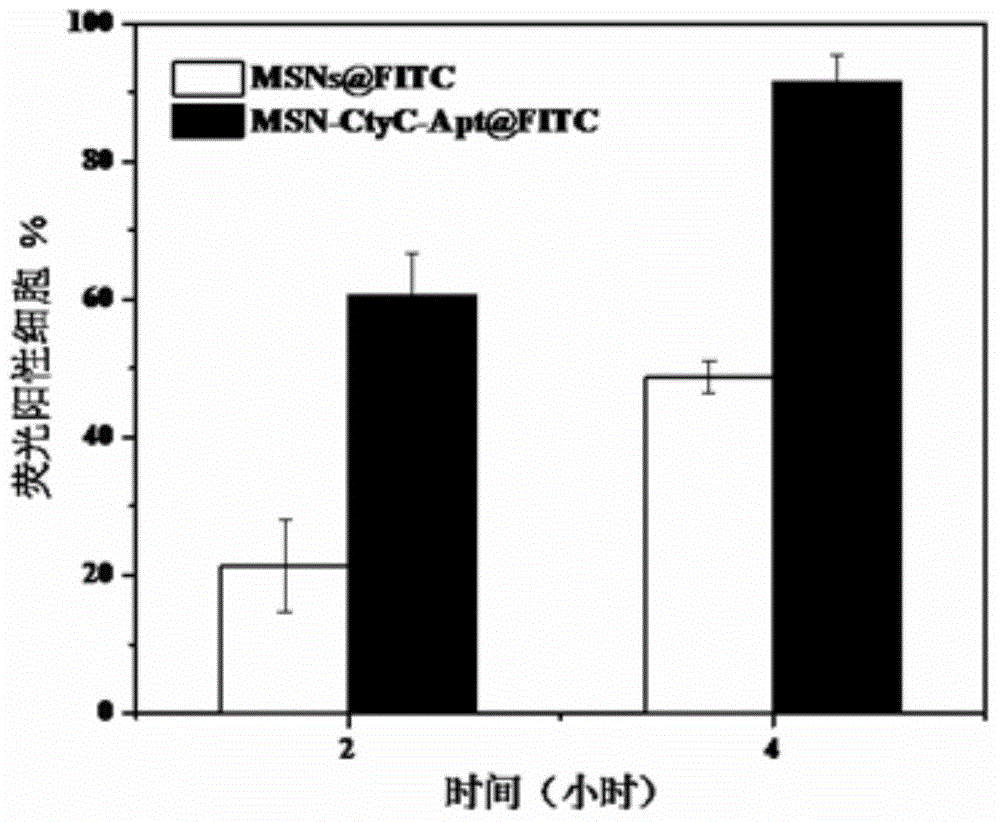
Method for preparing meso-porous silicon nano medicine carrier with cell specificity target, reduction responsiveness and triple anticancer treatment effects
InactiveCN104013965AOvercome toleranceEasy to operatePowder deliveryMacromolecular non-active ingredientsCell membraneCarrier system
The invention discloses a method for preparing a meso-porous silicon nano medicine carrier with cell specificity target, reduction responsiveness and triple anticancer treatment effects. The method comprises the following steps: firstly, synthesizing meso-porous silicon nano particles by using a gel dissolution method, subsequently, introducing a disulfide bond onto the surface of a meso-porous silicon nano reservoir by using a chemical modification method, innovatively fixing cytochrome C with an apoptosis-inducing function onto the surface of the meso-porous silicon nano reservoir, blocking meso-porous channels with medicines, finally modifying DNA (Deoxyribose Nucleic Acid) aptamer single chain molecules (AS1411, with a cancer cell apoptosis-inducing function) onto the surface of a meso-porous silicon / cytochrome C nano composite system, and taking the system as specificity ligand of a receptor (nucleolin protein) which is overexpressed on the surface of liver cancer cytomembrane, thereby establishing a multifunctional composite type nano medicine carrier system for achieving triple anticancer treatment under combined action of medicines, blocking substances and target molecules inside meso-pores.
Owner:CHONGQING UNIV
Antibodies against cancer
InactiveUS20040176576A1Reduce the number of cellsEnhanced inhibitory effectPeptide/protein ingredientsGenetic material ingredientsAnticarcinogenCripto
An isolated binding partner of a Cripto-1 protein, Pim-1 protein or an antigen present in a colon cancer cell lysate is described. The binding partner inhibits growth of one or more cancer cell types and may be used in an anti-cancer agent for treating cancer in a subject. The binding partner may also be used in a method of inducing apoptosis in a cancer cell, as well as in a method of sensitising a cancer cell to a cytotoxic compound. In addition, a cancer Vaccine is described wherein the vaccine comprises a Cripto-1 protein (or an antigenic fragment thereof), Pim-1 protein (or an antigenic fragment thereof) or an antigen present in a colon cancer cell lysate or, alternatively, comprises an expressible DNA molecule encoding a Cripto-1 protein (or an antigenic fragment thereof), Pim-1 protein (or an antigenic fragment thereof) or an antigen present in a colon cancer cell lysate.
Owner:THE MACFARLANE BURNET INSTITUTE FOR MEDICAL RESEARCH AND PUBLIC HEALTH LTD
Apoptosis inducing adamantyl derivatives and their usage as anti-cancer agents, especially for cervical cancers and dysplasias
InactiveUS6462064B1Easy to convertCombating the greasy appearance of the skin orBiocideHydroxy compound active ingredientsCancer preventionRetinoid
The invention relates to the discovery that specific adamantyl or adamantyl group derivatives containing retinoid-related compounds induce apoptosis of cancer cells and therefore may be used for the treatment of cancer, including advanced cancer. Also, the present invention relates to novel adamantyl or adamantyl group derivatives containing retinoid compounds and their usage for treatment and / or prevention of cancer, keratinization disorders, dermatological conditions, and other therapies More specifically, it has been shown that such adamantyl compounds, e.g., 6-[3-(1-adamantyl)-4-methoxyphenyl]-2-naphthoic acid, 2-[3-(1-adamantyl)-4-methoxyphenyl]-5-benzimidazole carboxylic acid, and 6-[3-(1-adamantyl)-4,5-methylenedioxyphenyl]-2-naphthoic acid, can be used to treat or prevent cervical cancers and precancers such as cervical dysplasias, including high grade and low grade dysplasias.
Owner:GALDERMA RES & DEV SNC +1
Anti-epcam antibodies that induce apoptosis of cancer cells and methods using same
InactiveUS20110165161A1High apoptosis-inducing activityAvoid developmentSugar derivativesMicroorganismsApoptosisTherapeutic intent
The present invention provides antibodies (such as chimeric and humanized antibodies) specifically bind to epithelial cell adhesion / activating molecule EpCAM expressed on cancer cells and induce cancer cell apoptosis. In addition, the present invention also provides use of the antibodies described herein for diagnostic and therapeutic purposes.
Owner:BIOALLIANCE CV
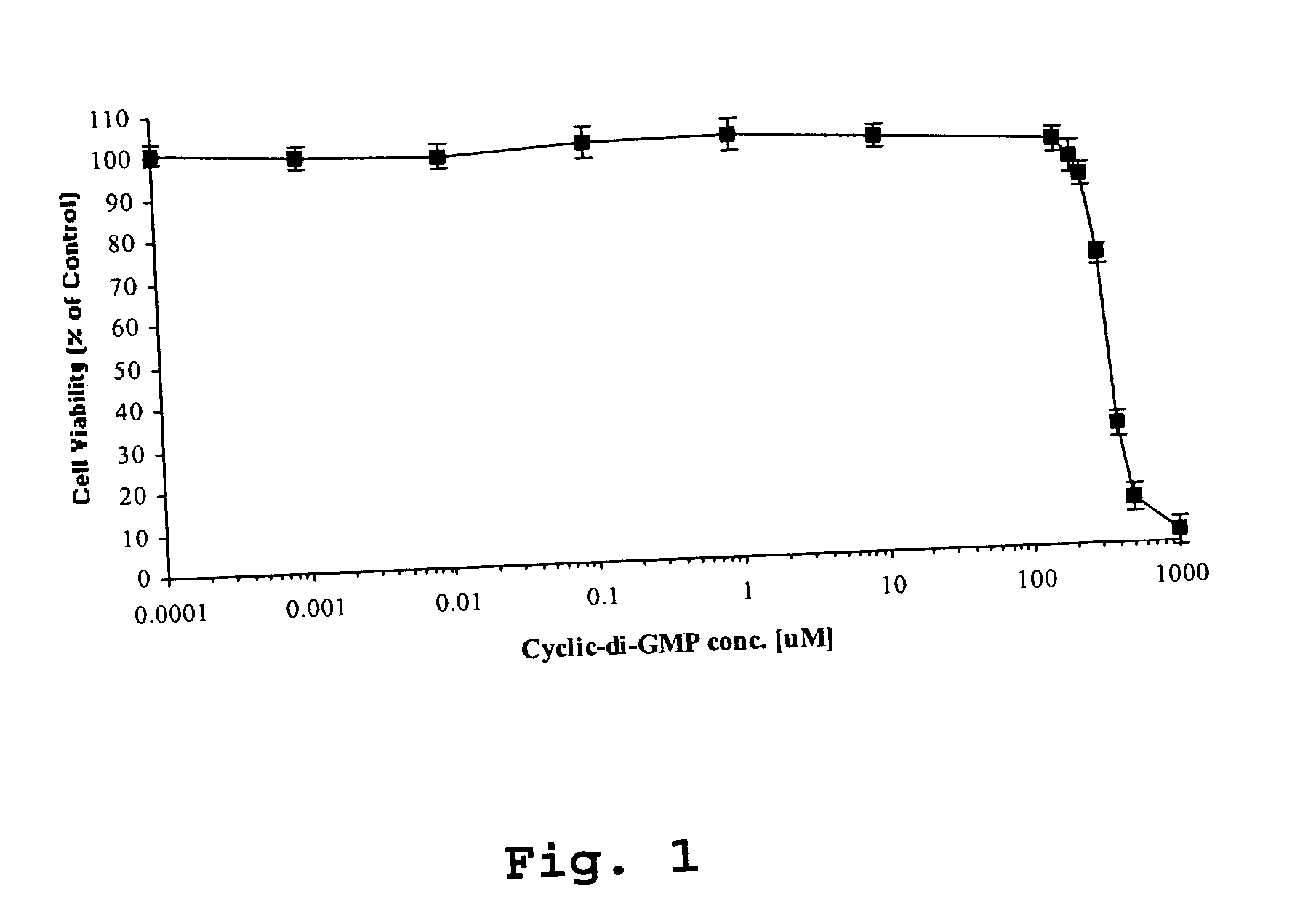
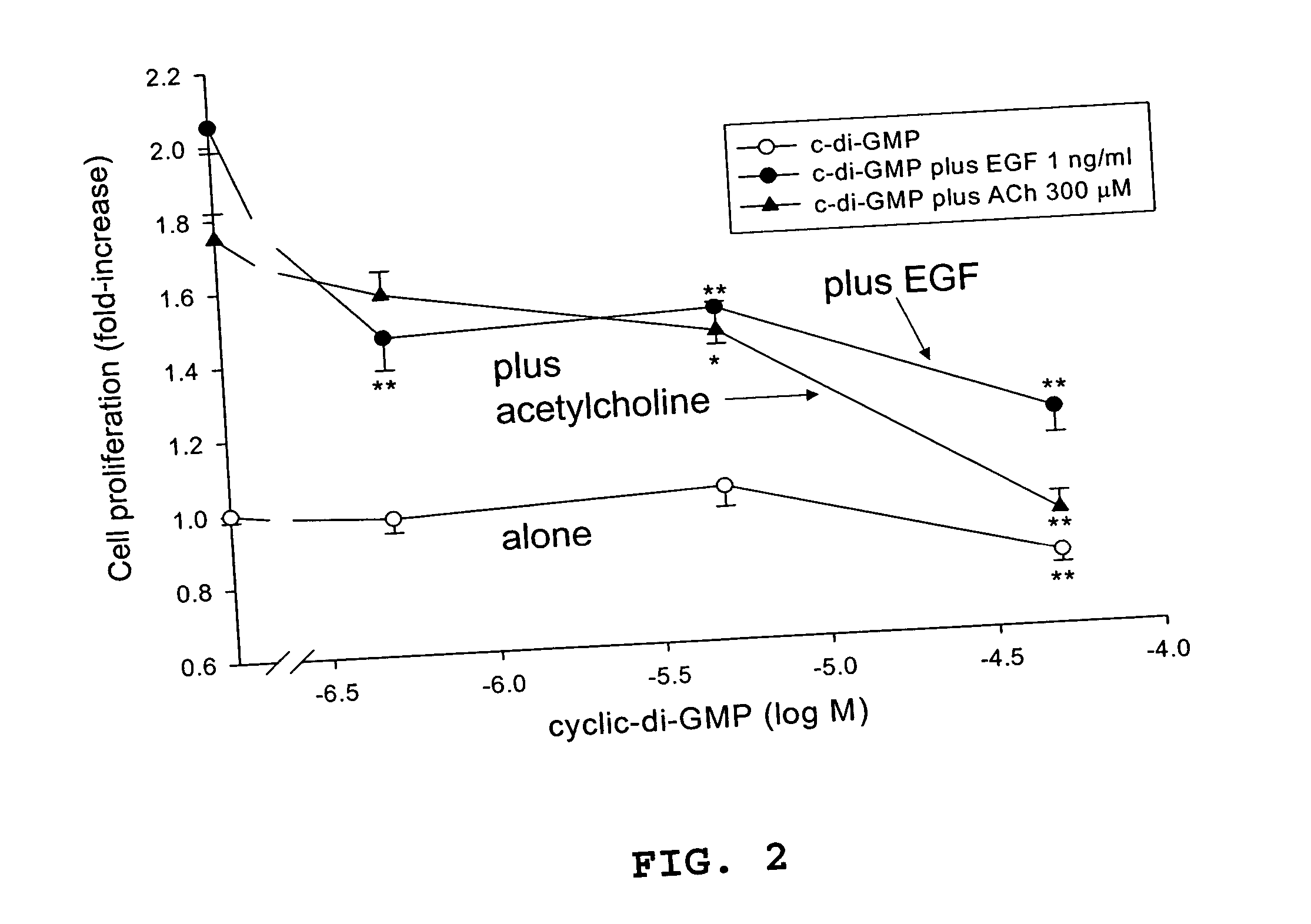
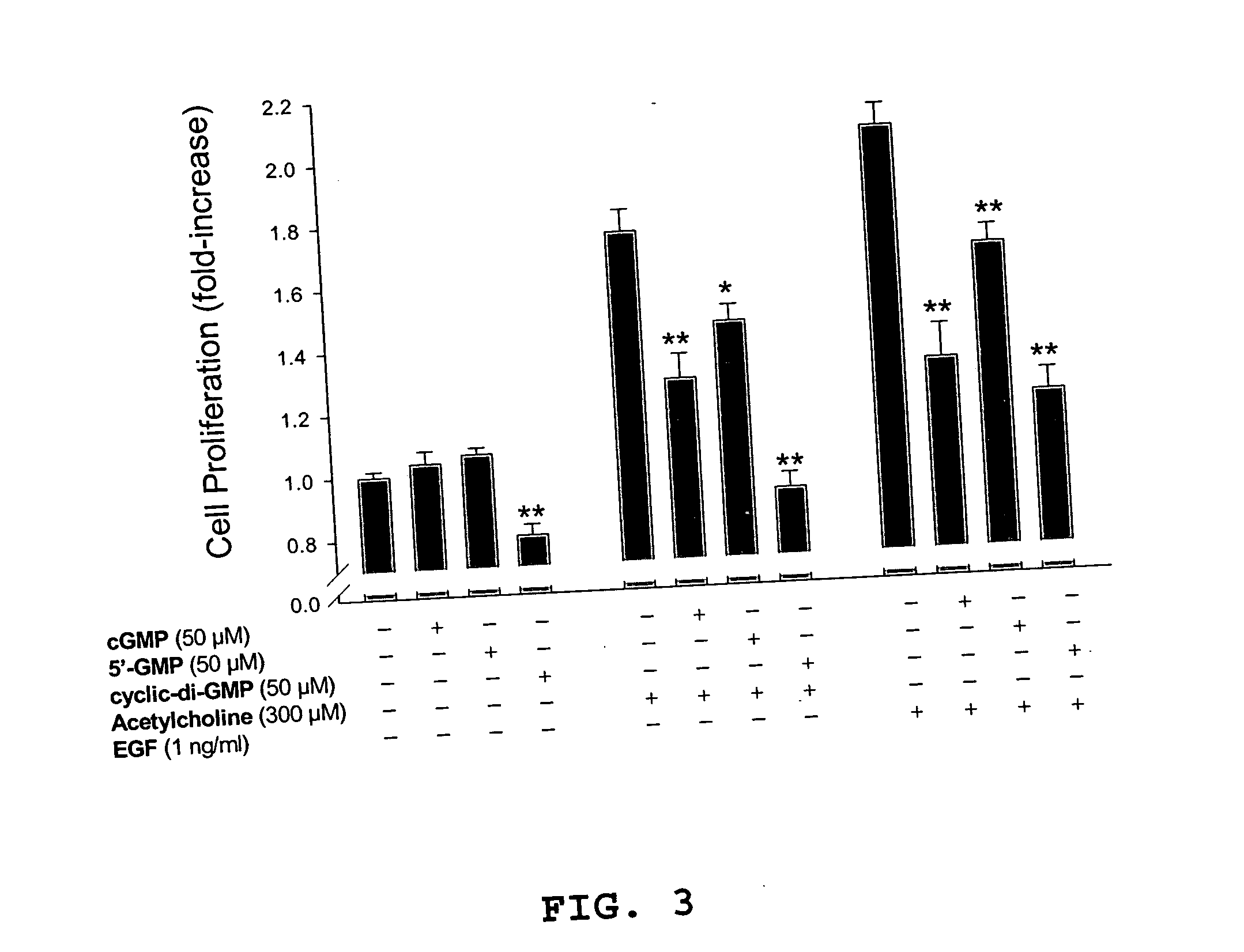
Method for inhibiting cancer cell proliferation or increasing cancer cell apoptosis
ActiveUS20050203051A1Inhibit cancer cell proliferationIncreasing cancer cell apoptosisBiocideSenses disorderCyclic di-GMPIn vivo
Owner:KARAGEN PHARMA INC
Recombination of human soluble TRAIL protein, the preparing method and the application in preparing antineoplastic medicine
InactiveCN1500808AHigh purityInduce apoptosisPeptide/protein ingredientsFermentationCancer cellTRAIL Protein
The present invention discloses recombinant soluble human tumore necrosis factor related death inducing ligand and its preparation process and application in preparing antitumor medicine. The present invention adopts human tonsil tissue mRNA as template to amplify TRAIL coding sequence, constitute engineering colibacillus for expressing rhsTRAIL and engineering saccharomycete, establish the rhsTRAIL engineering colibacillus fermentation, occlusion body washing and destination protein purification process and the engineering saccharomycete and destination protein purification process separately, and obtain pure rhsTRAIL product, which has molecular weight of 19.6-24 KD and exists in both monomer and dimmer. The rhsTRAIL can induce death of several kinds of cancer cells outside body, inhibit amplification of liver cancer cell in tumor loading mouse obviously and kill tumor cell selectively, so that it may be used in preparing effective antitumor medicine.
Owner:李宏
Reversible crosslinked biodegradable polymer vesicle having positive charges on inner membrane, preparation method thereof and application in preparation of antineoplastic drugs
ActiveCN106137968AEfficient specific bindingEfficient combinationPharmaceutical non-active ingredientsAntineoplastic agentsTumor targetingApoptosis
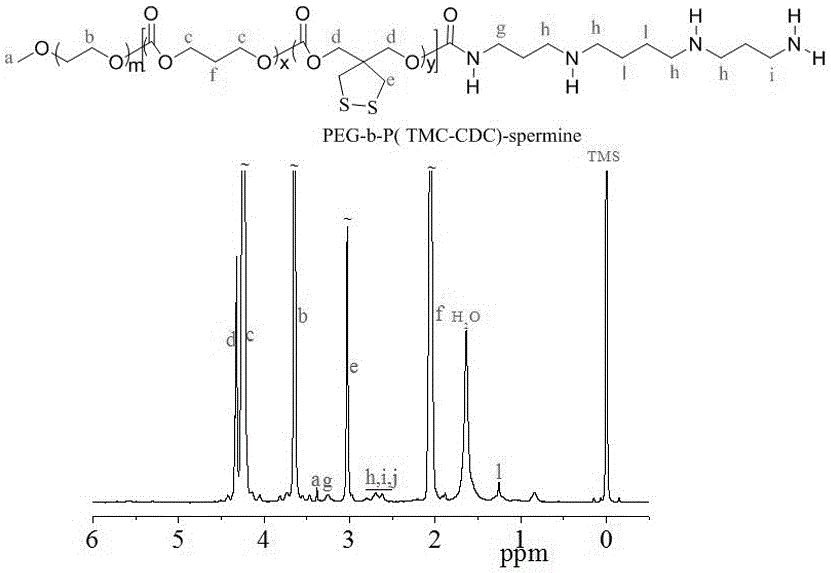
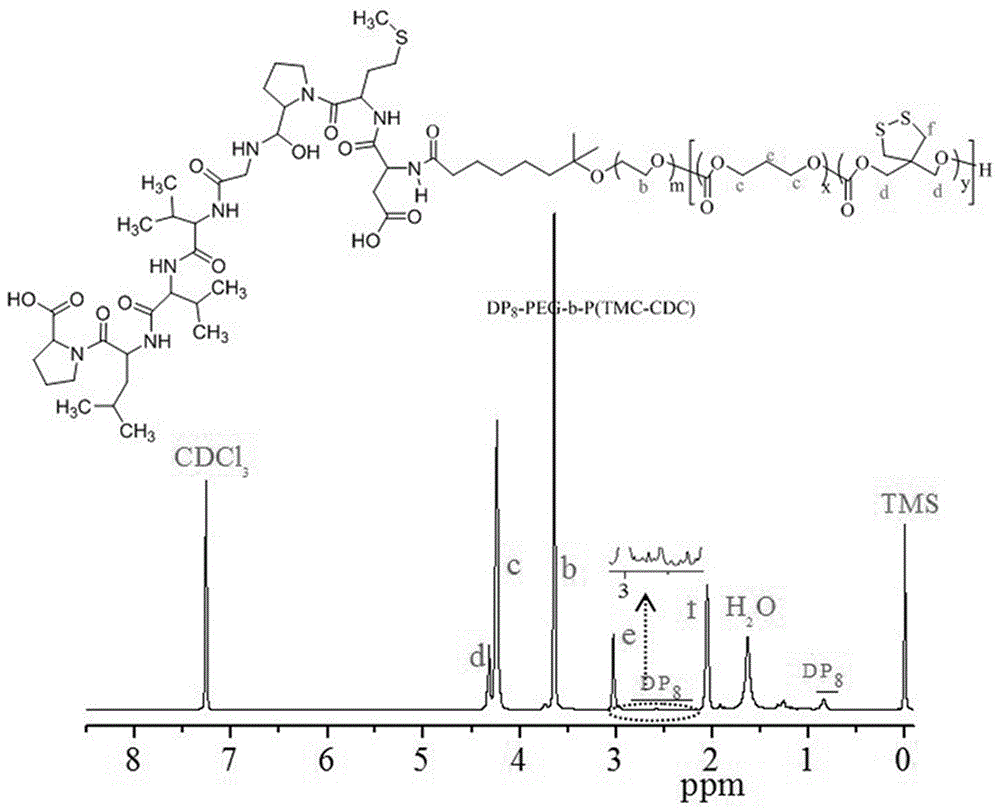
The invention discloses a reversible crosslinked biodegradable polymer vesicle having positive charges on an inner membrane, a preparation method thereof and application in preparation of antineoplastic drugs. The biodegradable polymer vesicle based on tumor targeting of a block polymer PEG-P (TMC-DTC)-SP or PEG-P (LA-DTC)-SP, having the positively charged membrane, reversibly crosslinked in reduction sensitivity and intracellularly solvable and crosslinked can effectively support and protect biological macromolecules such as proteins, DNA and siRNA and micromolecular drugs with negative charges in a physical environment and can be delivered to intravital tumor cells to induce apoptosis. The system has lots of unique advantages including simple and easy controllability of preparation, excellent biocompatibility, excellent controlled drug release property, super-strong in-vivo circulation stability, superior cancerous cell targeting and remarkable cancer cell apoptosis capability and the like. Therefore, the reversible crosslinked biodegradable polymer vesicle is expected to become a simple, stable, multifunctional nano system platform integrating multiple advantages and is used for efficient, active and targeted delivery from nucleic acids to in-situ tumors.
Owner:SUZHOU UNIV
Compositions for Inhibiting Cell Growth and Inducing Apoptosis in Cancer Cells and Methods of Use Thereof
InactiveUS20080220416A1Sufficient inhibitionPeptide/protein ingredientsMicrobiological testing/measurementChemotherapeutic drugsApoptosis
Owner:LOYOLA UNIV OF CHICAGO +1
Compounds and methods for inducing apoptosis in proliferating cells
InactiveUS7026346B2Treating and inhibiting and delaying onsetBiocideOrganic active ingredientsMelanomaApoptosis
Compounds useful for inducing apoptosis in proliferative cells, particularly cancer cells, including but not limited to prostate cancer, leukemia, non-smalll cell lung cancer, colon cancer, CNS cancer, melanoma, ovarian cancer, renal cancer, bladder cancer, lymphoma, and breast cancer. These compounds are particularly useful in the treatment of androgen-independent cancers, including hormone-refractory prostate cancer. Further provided are methods of treating cancer in a subject in need of such treatment using the compounds of the present invention. Further provided are methods for using the compounds of the present invention to treat, inhibit, or delay the onset of cancer in a subject. Further provided are methods of inducing apoptosis in rapidly proliferating cells, particularly, though not necessarily cancer cells, using the compounds of the present invention.
Owner:THE OHIO STATE UNIV RES FOUND
Lactic acid bacteria and their use for treating and preventing cancer
InactiveUS20050208033A1Inhibiting tumor progressionReinforcing immune systemOrganic active ingredientsBiocideBacteroidesCancer prevention
The invention concerns the isolation of novel properties of lactic acid bacteria stains. Said novel properties are advantageously useful for preventing and treating cancer. More particularly, the invention concerns the use of lactic acid bacteria to facilitate induction of cell apoptosis of a cancer. The invention also concerns the use of lactic acid bacterial strains, such as Lactobacillus acidophilus and Lactobacillus casei in methods and compositions for preventing and treating cancer, in particular colon cancer.
Owner:BIO K PLUS INT
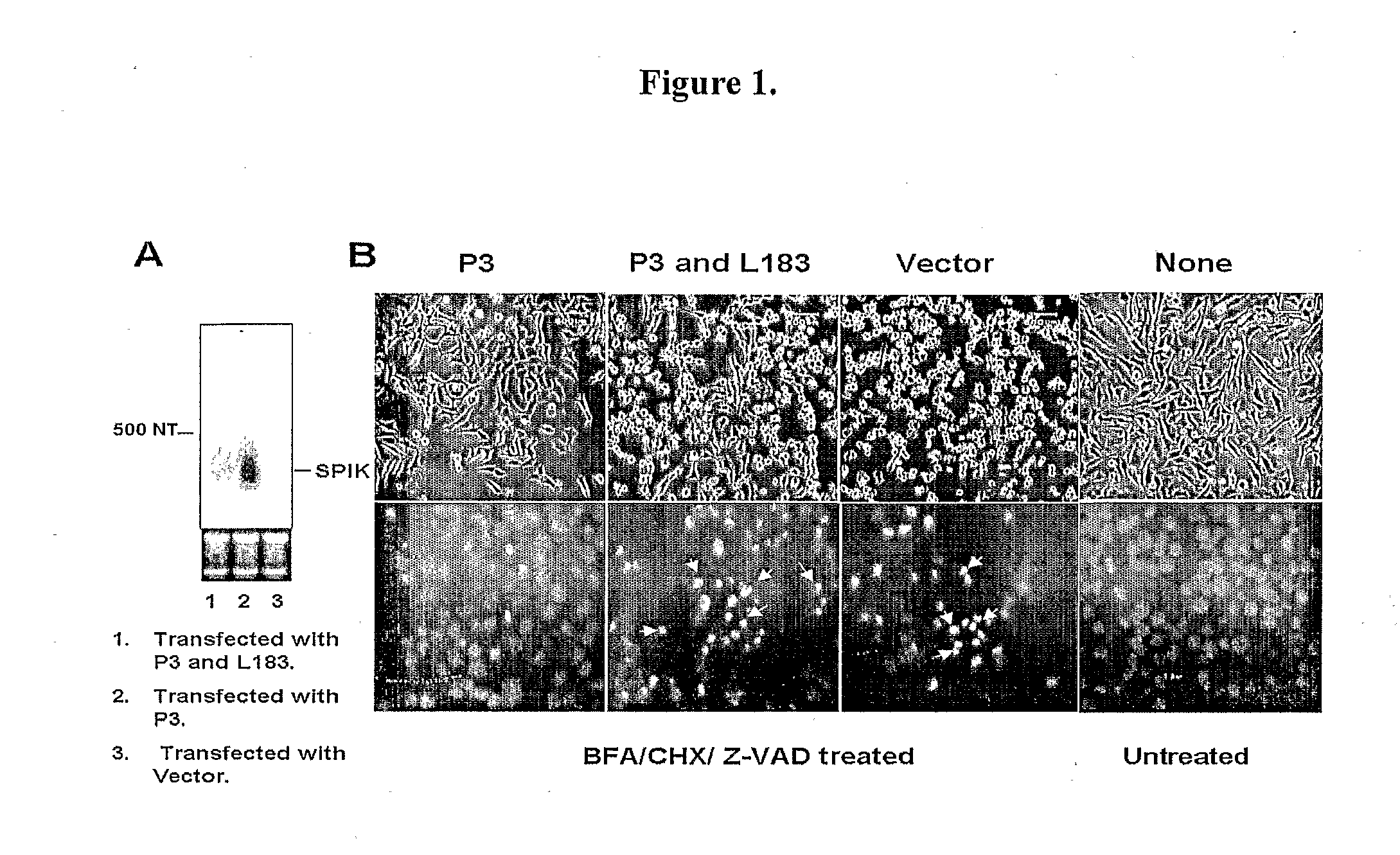
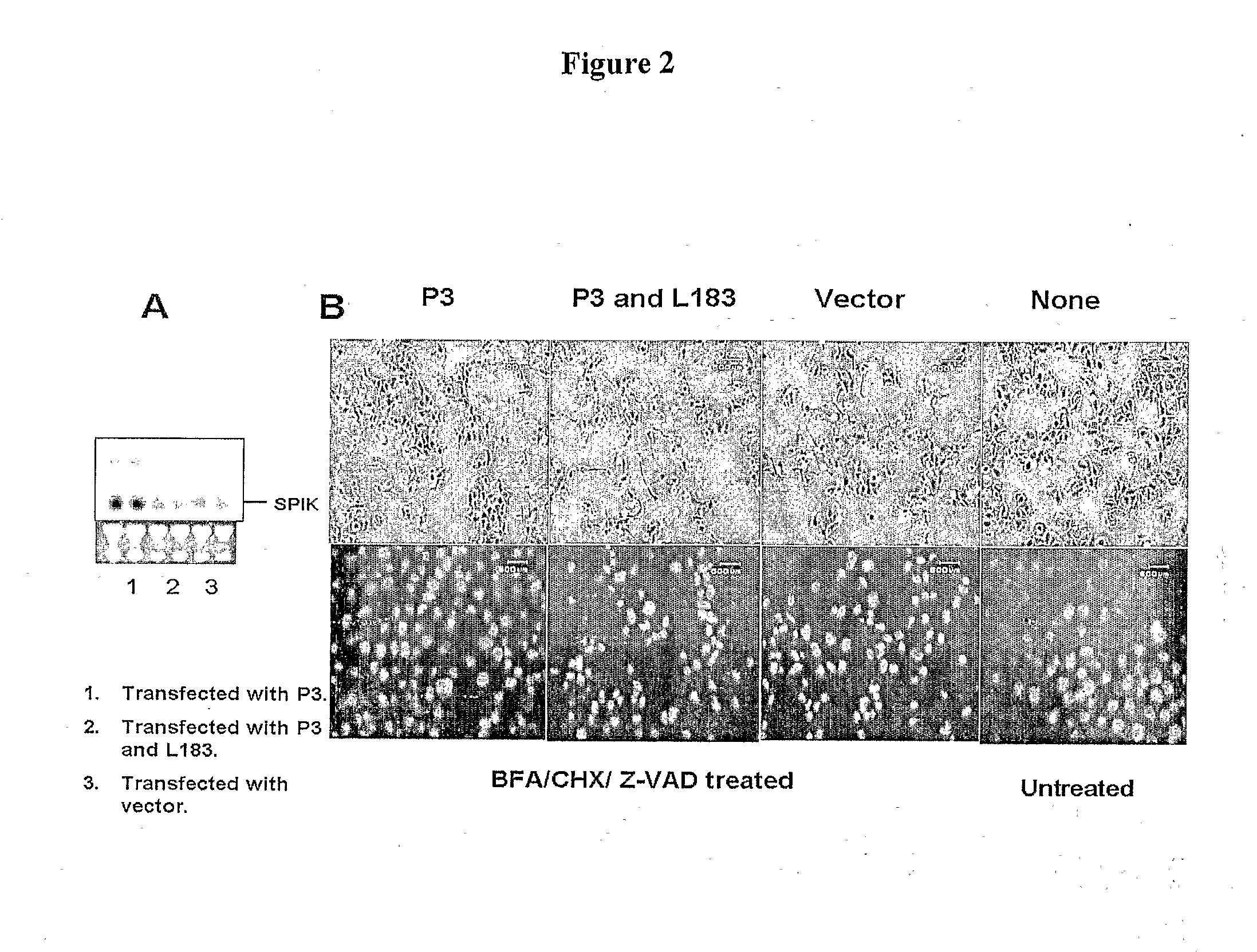
Identification of modulators of serine protease inhibitor kazal and their use as Anti-cancer and Anti-viral agents
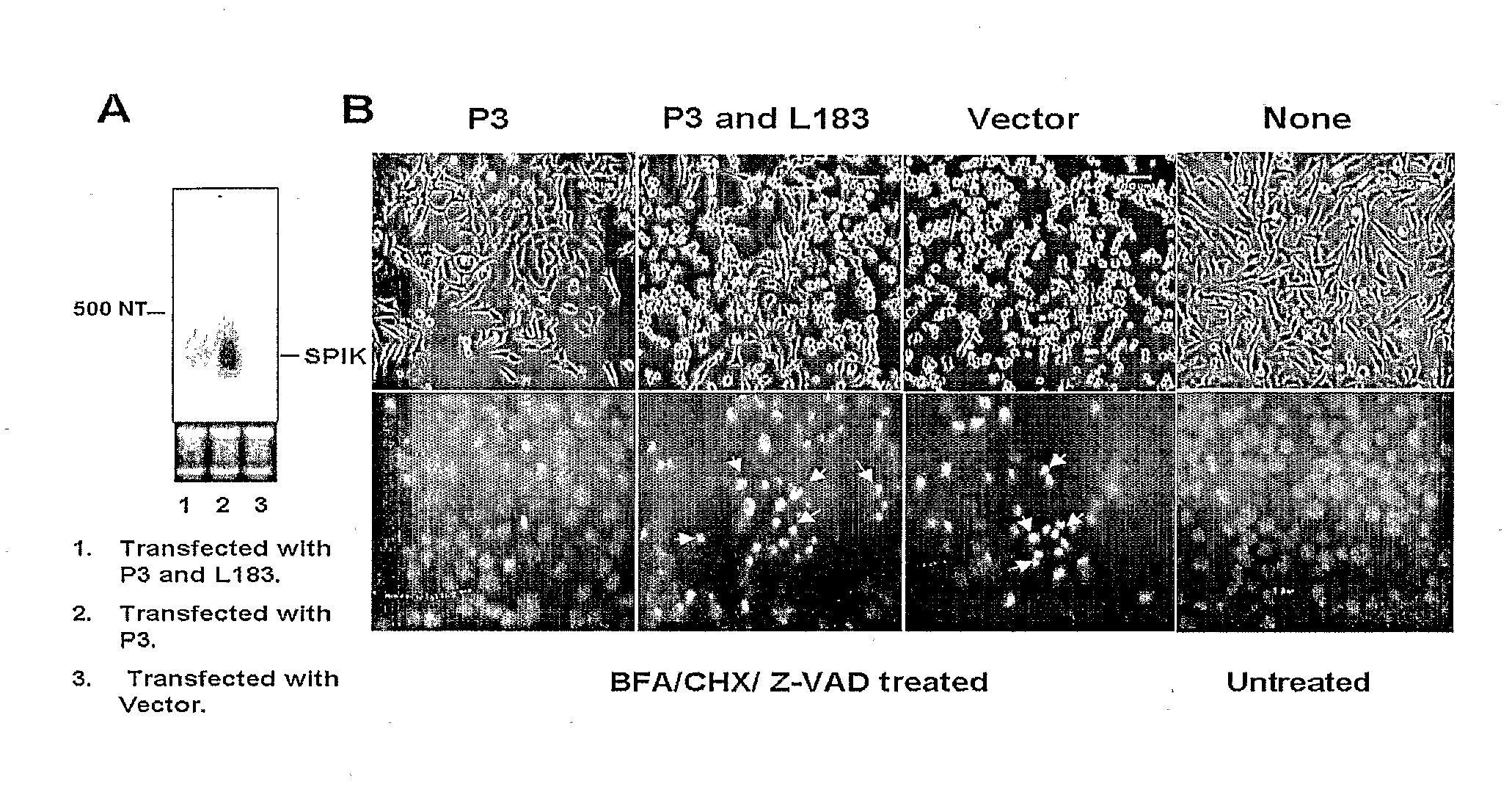
InactiveUS20090017457A1Suppressed SPIK expressionRestore sensitivityCompound screeningApoptosis detectionEtiologyAnticarcinogen
This invention describes a relevant etiology of cancer and a novel anti-cancer therapeutic strategy, based on the discovery that a protein named serine protease inhibitor (SPIK / SPINK / PSTI) was up-regulated by hepatitis B and C virus infections consequently suppressing the cell apoptosis. Accordingly, this invention provides an inhibitor of SPIK and / or a technology of suppression of over-expression of SPIK in cells. The inhibitors include: 1) chemical compounds, which can inhibit SPIK transcripts, protein activity, and gene expression, 2) SPIK siRNA (RNAi gene silence or dsRNA of SPIK, 3) DNA anti-sense and anti-SPIK antibody. Further, this invention provides a method of using the inhibitor as an anti-cancer agent to re-instate cancer cell apoptosis (e.g., serine protease dependent cell apoptosis).
Owner:PHILADELPHIA HEALTH & EDUCATION CORP
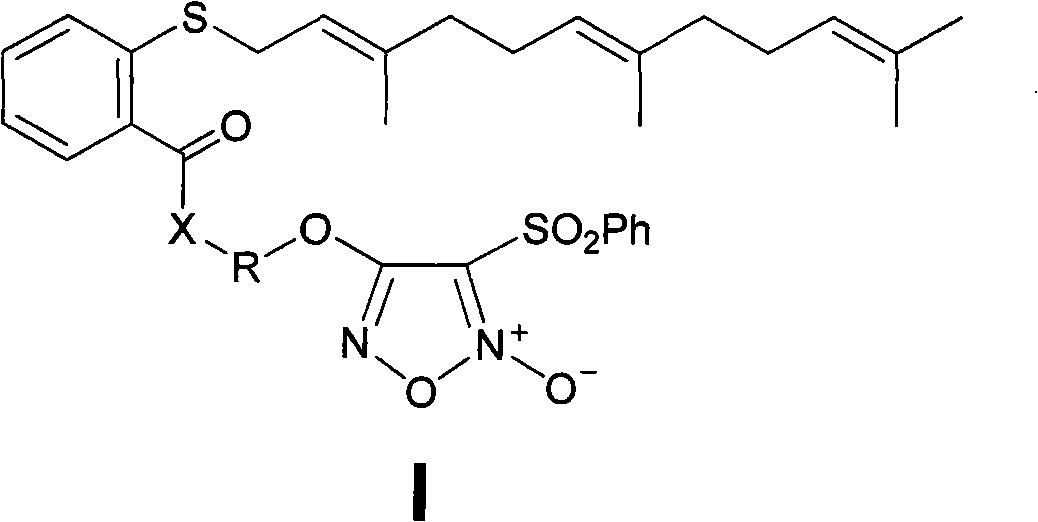
Nitric oxide donor-type farnesyl thiosalicylic acid derivative, and preparation method and medical application thereof
The invention discloses a nitric oxide (NO) donor-type farnesyl thiosalicylic acid (FTA) derivative, and pharmaceutically acceptable salt, a preparation method and medical application thereof. The FTA derivative is a compound obtained by carrying out heterozygosis on a NO donor furazan nitrogen oxide and Ras protein inhibitor FTA by an ester bond or an amido bond. Pharmacological test results show that the FTA derivative can reserve the Ras protein inhibiting activity of FTA and simultaneously releases high-concertration NO to induce cancer cell apoptosis and enhance the inhibiting action on cancer cell proliferation; compared with the FTA, the FTA derivative has more excellent anti-tumor activity, and therefore, the compound can be suitable for treating various clinical malignant tumours.
Owner:CHINA PHARM UNIV
Tumour-dissolving adenovirus mutant possessing multiple specific anti-tumour mechanism
InactiveCN1884556AGood curative effectPlay a therapeutic roleFermentationGenetic engineeringHuman tumorReverse transcriptase
This invention involved oncolytic adenovirus mutant with the multiple antitumoral Mec. It belongs to the BME field. The adenovirus mutant Elb-55kDa and Elb-19kDa has missing gene, inserts chimeric promoter composed by the human telomerase reverse transcriptase core sequence and human tumor epidermal growth factor receptor enhancer before the replication required gene Ela codons mEla289R and mEla243R. So the virus can specific proliferate in cancer cell. Oncolytic viruses and mEla protein can exert thire influence. It can also increase the sensitivity of cancer cell to chemoradiation and have no influence to normal cell. It inserts the human h-endostatin gene expression cassette in adenovirus mutant gene group; along the replication of virus in cancer cell to amplificate h-endostatin gene and effective expression in tumor so to restrain neovascularization of tumor so to realize the result of restrain the increase and transformation of cancer cell and apoptotic cell. This new oncolytic adenovirus mutant has good clinical prospect in gene curing so to used in curing many kinds of human tumors.
Owner:JIANGSU SHUNTANG BIOENG
Nano hydroxyapatite-gene-medicament complex as well as preparation method and application thereof
ActiveCN104288784AGood biocompatibilityAcid pH sensitiveOrganic active ingredientsGenetic material ingredientsPhosphateApoptosis
The invention discloses a nano hydroxyapatite-gene-medicament complex as well as a preparation method and application thereof, belonging to the field of biomedicine. The complex disclosed by the invention comprises nano hydroxyapatite, anti-tumor genes and an anti-tumor medicament, wherein the size of nano hydroxyapatite is 20-300nm, the genes are anti-tumor genes and the medicament is an anti-tumor medicament. The preparation method comprises the following steps: mixing a calcium salt with a phosphate solution to prepare nano hydroxyapatite, adsorbing the anti-tumor genes to form a nano hydroxyapatite-gene precursor complex and further adsorbing the anti-tumor medicament by the precursor complex to form the complex, wherein the complex can efficiently pass through cancer cell membranes and promote the apoptosis of cancer cells. Hydroxyapatite with good biocompatibility is used as a co-transfer carrier, thereby having high safety, high loading rate of the genes and the medicament, high transfection efficiency and great effect of killing the cancer cells. Taking nano-hydroxyapatite as the co-transfer carrier of the genes and the medicament has great application prospects in the field of treatment of cancers.
Owner:ZHEJIANG SCI-TECH UNIV
Multifunctional nanomicelle for early diagnosis and phototherapy of tumors and application of multifunctional nanomicelle
ActiveCN105012970AProlong circulation time in the bodyExtend cycle timeEnergy modified materialsIn-vivo testing preparationsThermal energySide effect
The invention relates to a multifunctional nanomicelle for early diagnosis and phototherapy of tumors. The multifunctional nanomicelle comprises a monolayer functionalized lipid membrane shell, photosensitive molecules and optothermal absorbents, wherein the photosensitive molecules and the optothermal absorbents are embedded into the monolayer functionalized lipid membrane shell. The multifunctional nanomicelle has the advantages that the nanomicelle can be used for multi-modal imaging diagnosis such as early magnetic resonance imaging, photoacoustic imaging or near-infrared fluorescence imaging of cancer; multiple kinds of diagnosis effects can be achieved as long as a patient suffers from one contrast medium administration; since sensitivity is improved, contrast medium injecting dosage is further decreased, and toxic and side effects on the patient can be alleviated; when the tumors are under single-wavelength near-infrared laser radiation, chlorine 6 and Pdots inside the nanomicelle are capable of converting light energy into chemical energy and thermal energy effectively to produce a great number of ROS (reactive oxygen species) oxygen free radicals and overhigh heat at 50-60 DEG C, and the ROS oxygen free radicals and the overhigh heat are used for inducing apoptosis and necrosis of cancer cells, so that a photodynamic and photothermal collaborative therapy function is achieved; the multifunctional nanomicelle not only can be used as a contrast medium for cancer diagnosis, but also can be used as a therapeutic agent for collaborative therapy of the cancer.
Owner:榕知科技(武汉)有限公司
Application of scutellaria root extract in preparation of anti esophageal cancer medicine
InactiveCN1442134AInhibition of divisionSuppress generationOrganic active ingredientsAntineoplastic agentsBaicaleinPharmaceutical drug
An application of the scutellaria extract in preparing the medicine for esophagus cancer is disclosed, and it features that scutellarin, scutellaroside, or their mixture can be used to prepare the pharmacologically receptable sale for suppressing the splitting of cancer cell, inducing the withering of cancer cell and inhibiting the generation of the blood vessel inside esophagus cancer.
Owner:HANGZHOU HUADONG MEDICINE GRP PHARMA RES INST
Traditional Chinese medicine compound preparation for treating ovarian cancer and preparation method of traditional Chinese medicine compound preparation
InactiveCN102670944AImprove clinical symptomsImprove the quality of lifeUnknown materialsAntineoplastic agentsTolerabilityWhite blood cell
The invention belongs to the field of traditional Chinese medicine and relates to a traditional Chinese medicine compound preparation for treating ovarian cancer and a preparation method of the traditional Chinese medicine compound preparation. According to the traditional Chinese medicine compound preparation, extracts of bulk medicine such as astragalus mongholicus, codonopsis pilosula, rehmannia root, sculellaria barbata, asparagus cochinchinensis, medlar, cornu cervi, fiveleaf akebia fruit, elecampane and radix paeoniae alba are prepared into solid and liquid preparations. Animal experiment results show that the weight loss and the white cell reduction can be relieved, the T lymphocyte function is improved, the bax expression is increased, the cancer cell apoptosis is promoted, the anti-tumor angiogenesis effect is realized, the oxygen deficiency microenvironment of tumor cells is improved, and the sensitivity of ovarian cancer cells on chemotherapeutics is enhanced. Clinical test results show that the clinical symptoms of later-stage ovarian cancer patients can be relieved, and the survival quality of patients is improved; the chemotherapy toxic and side effect is lightened, and the chemotherapy tolerance and the compliance of the patients are improved; and the uncontrolled and recurrence rate of later-stage ovarian cancer can be reduced, and the five-year survival rate is improved; and no obvious adverse reaction exists, and good safety is realized. The traditional Chinese medicine compound preparation is applicable to the treatment on ovarian cancer and ovarian cancer postoperative chemotherapy patients.
Owner:SHUGUANG HOSPITAL AFFILIATED WITH SHANGHAI UNIV OF T C M
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com