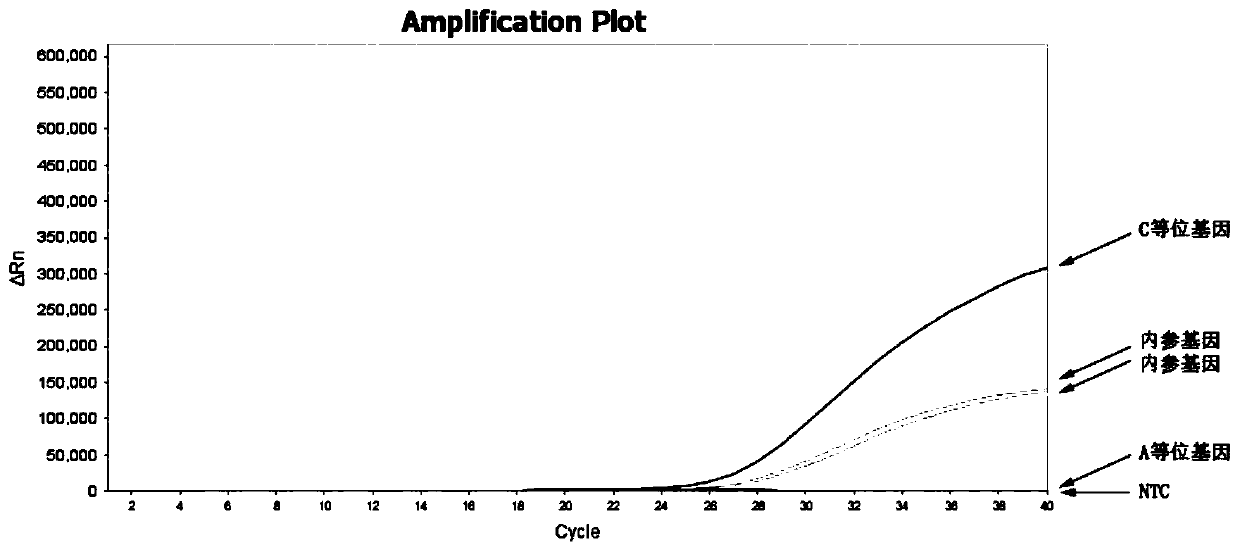
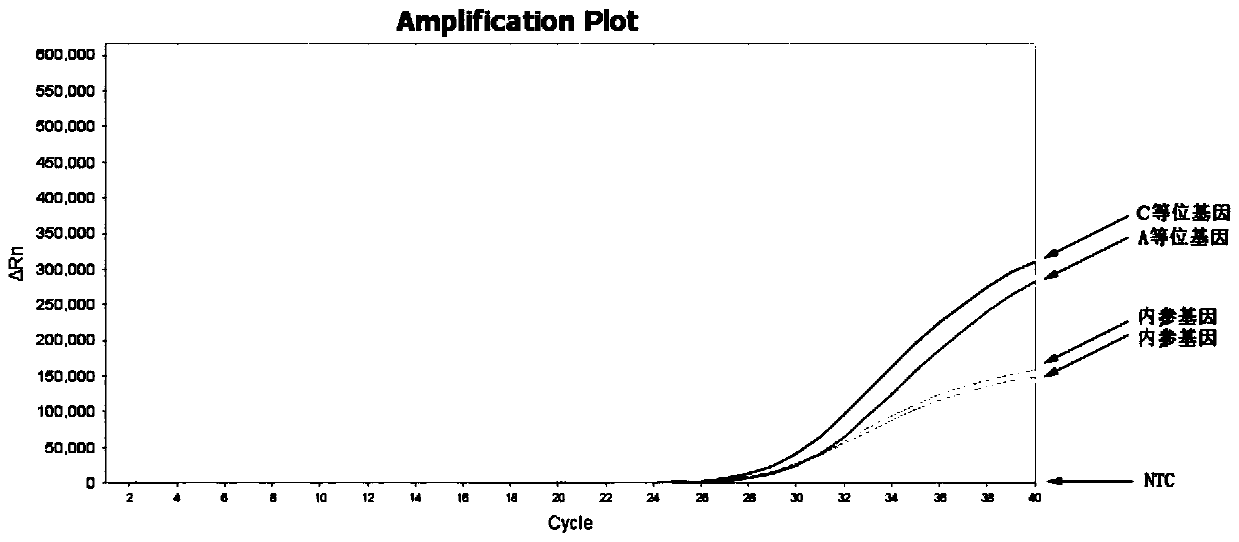
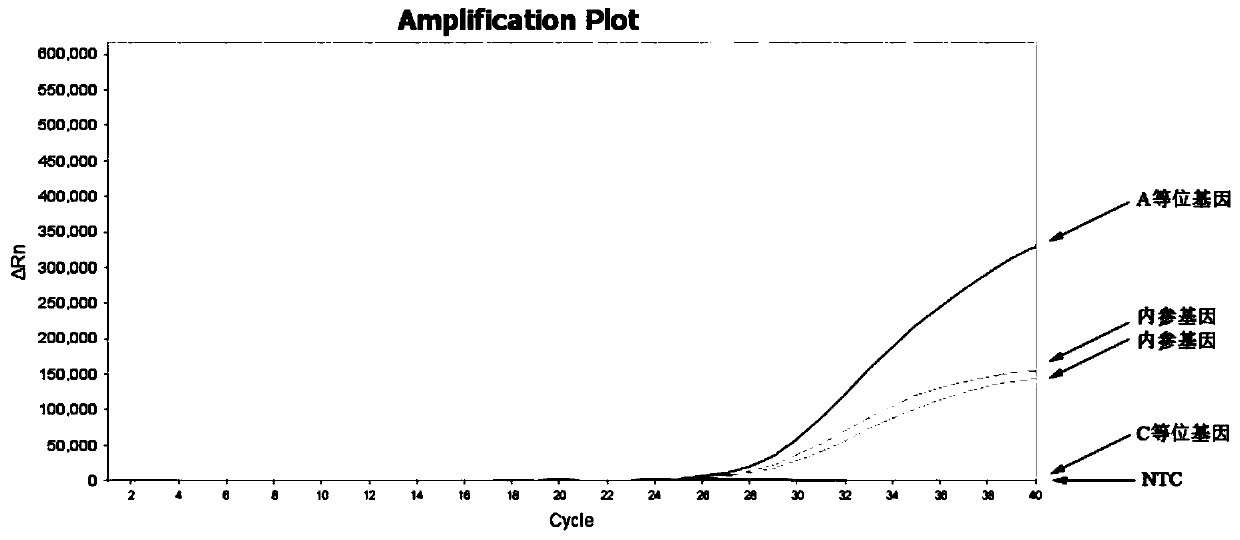
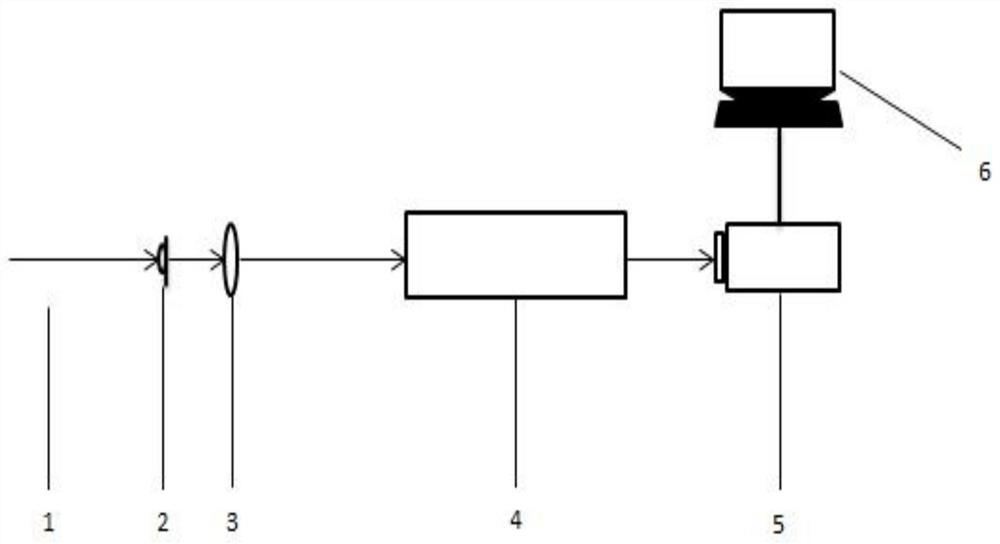
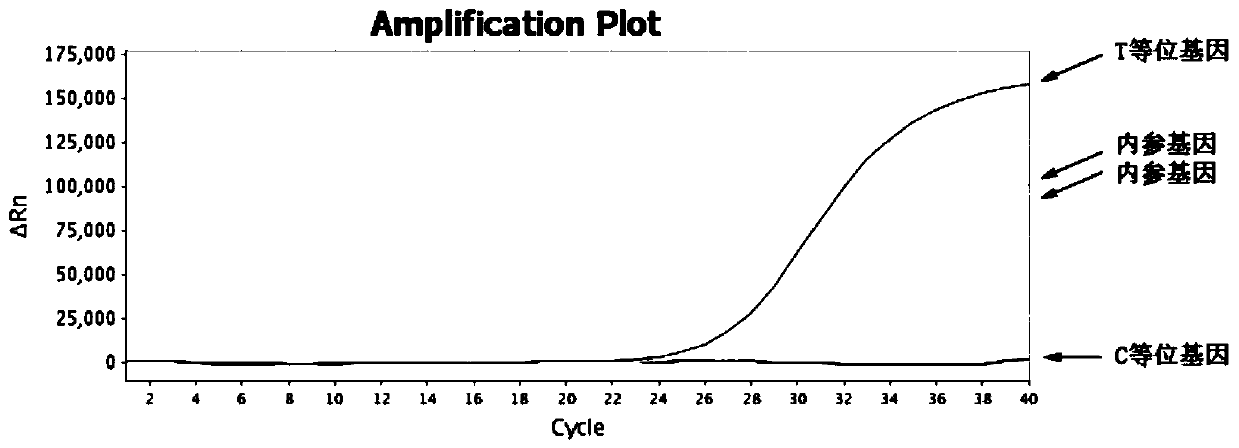
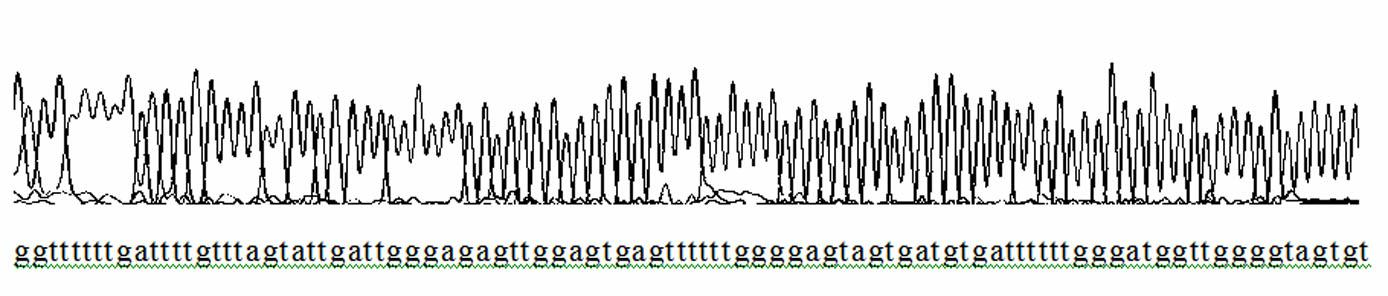
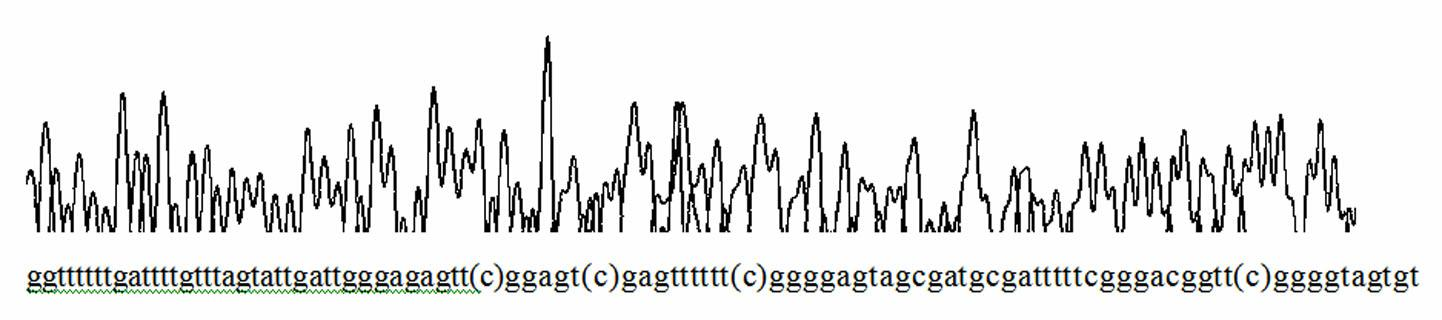
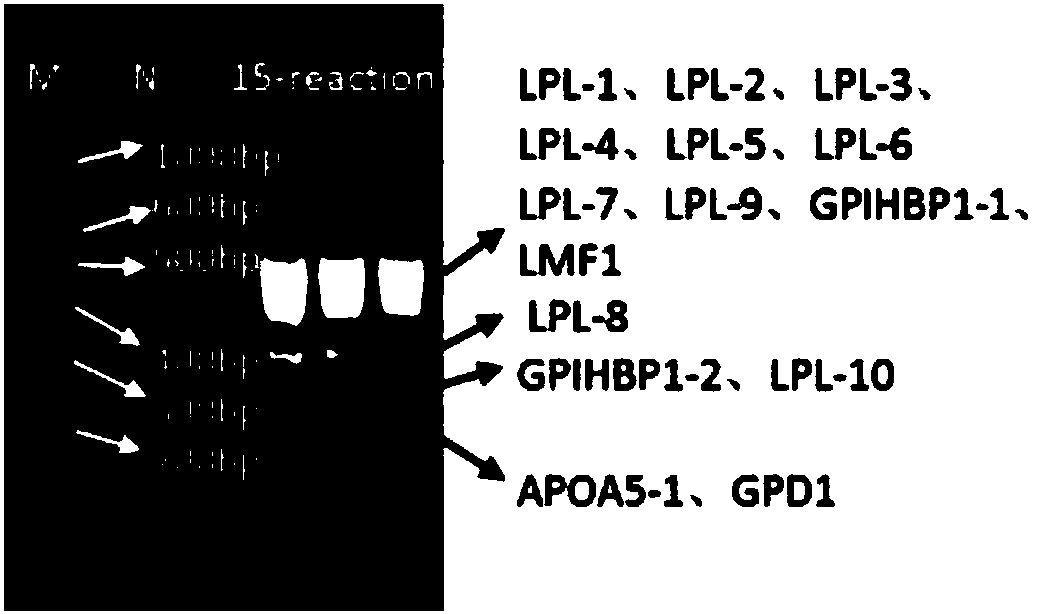
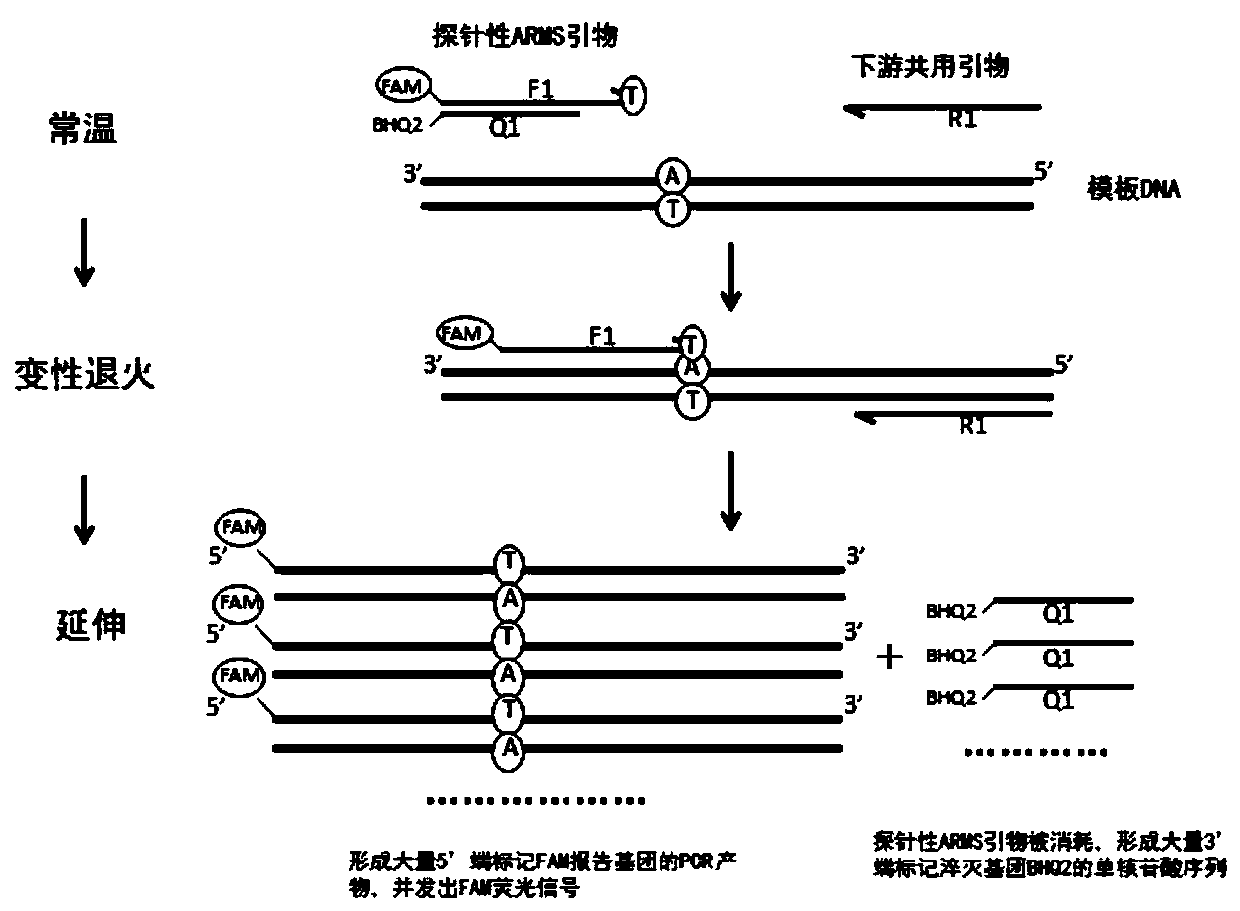
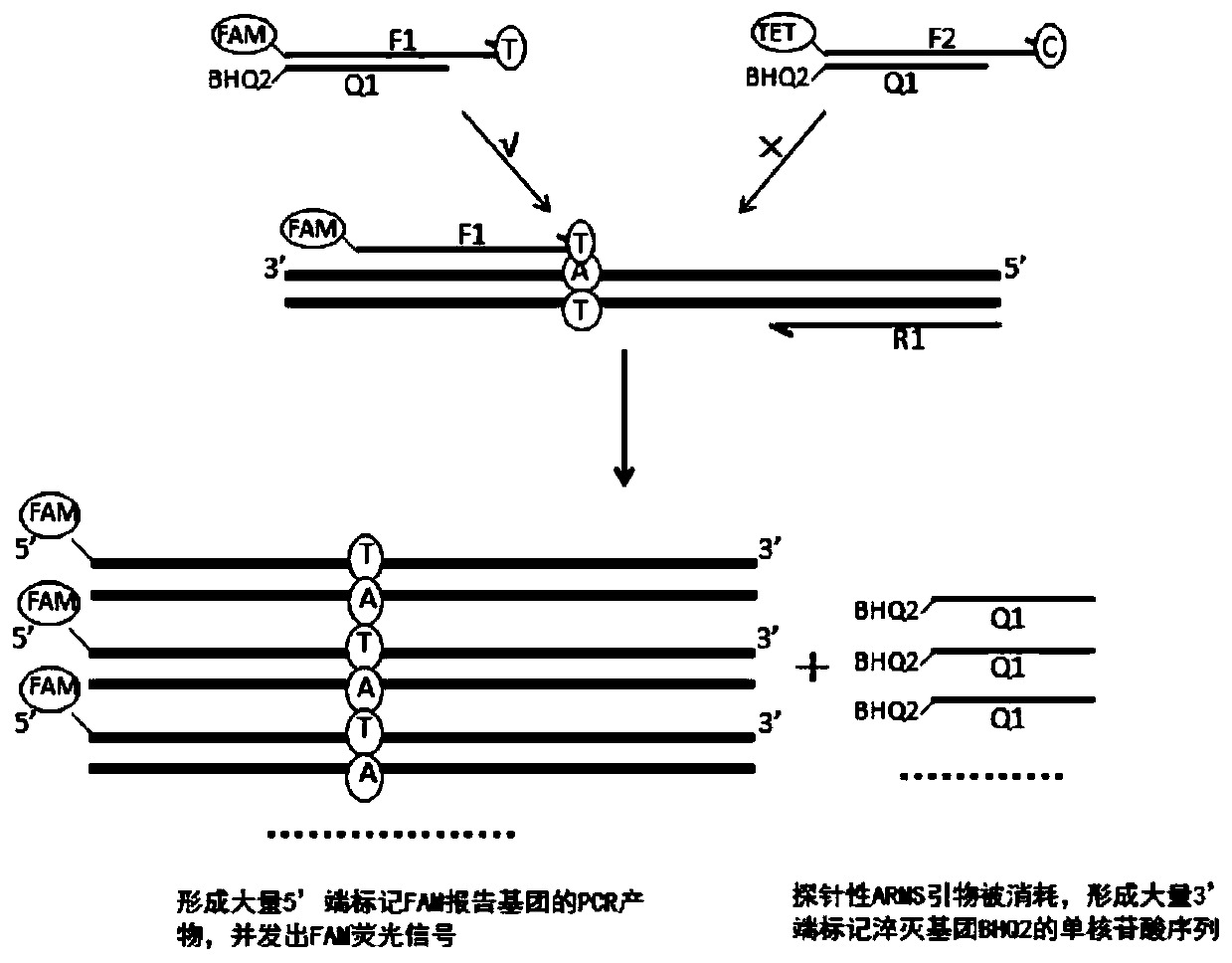
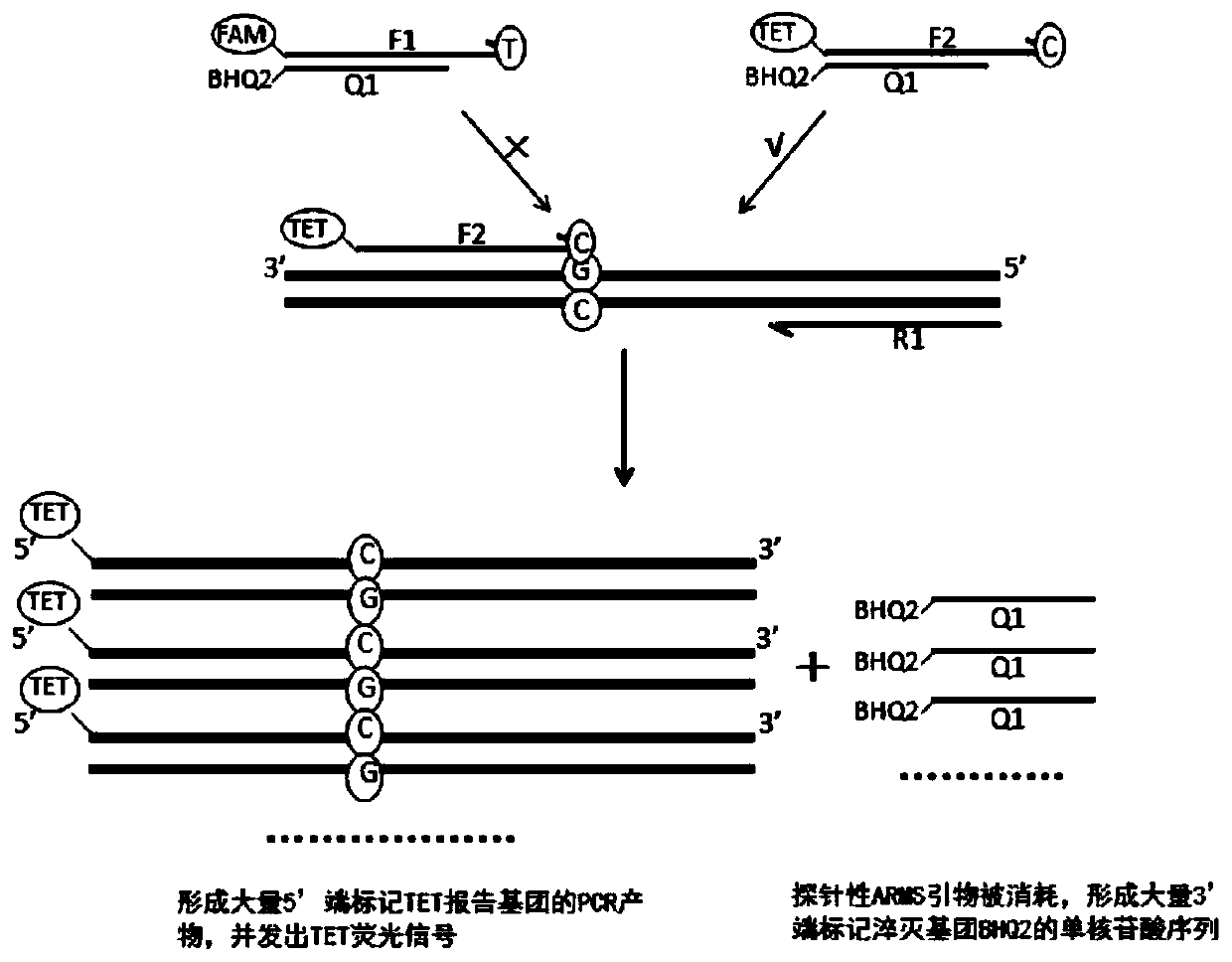
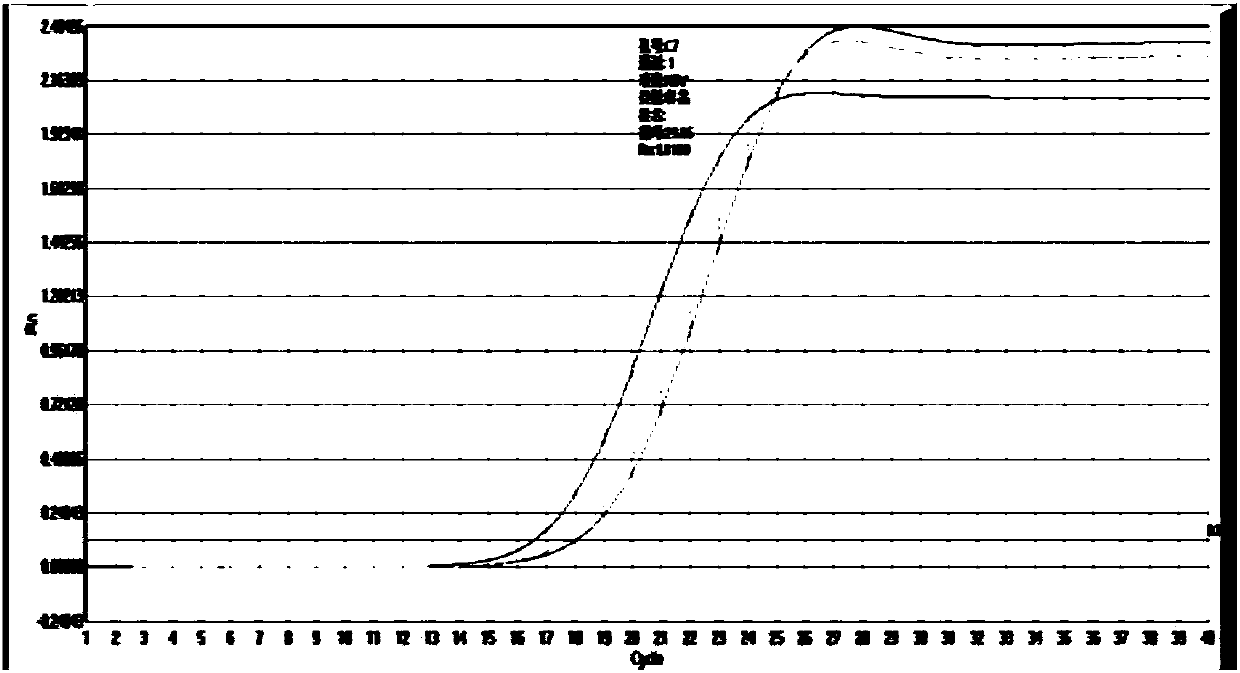
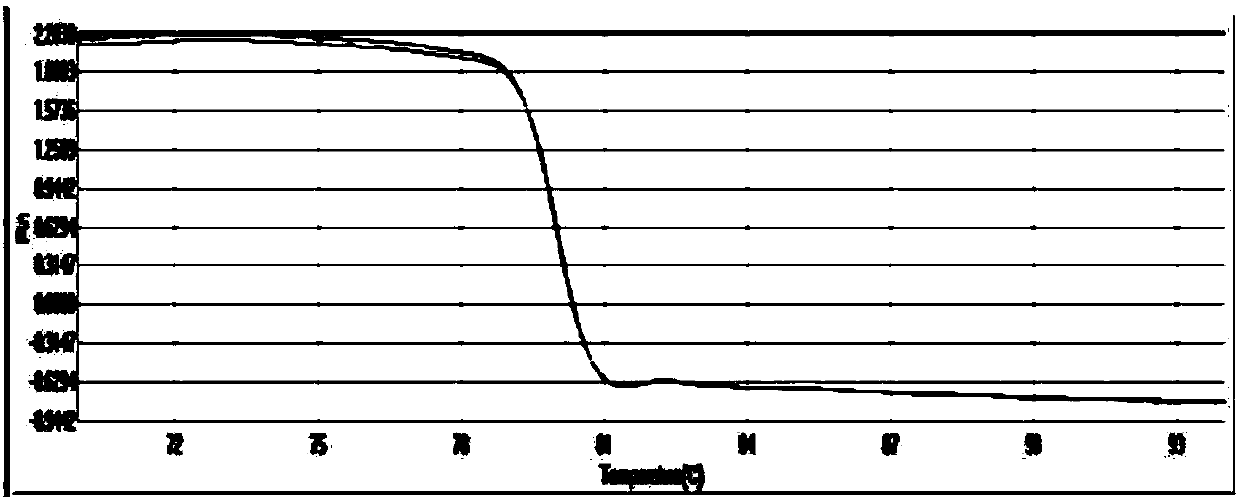
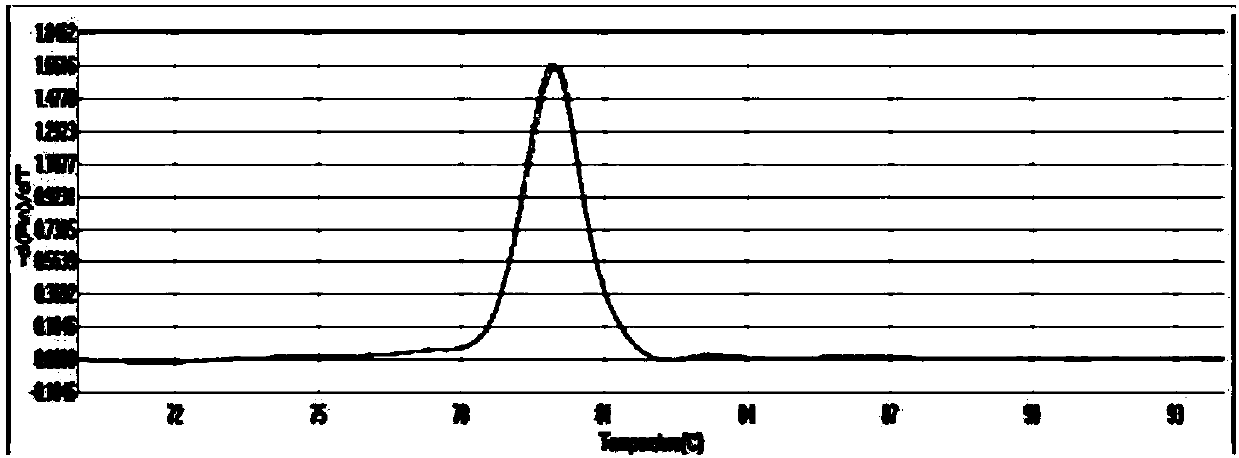
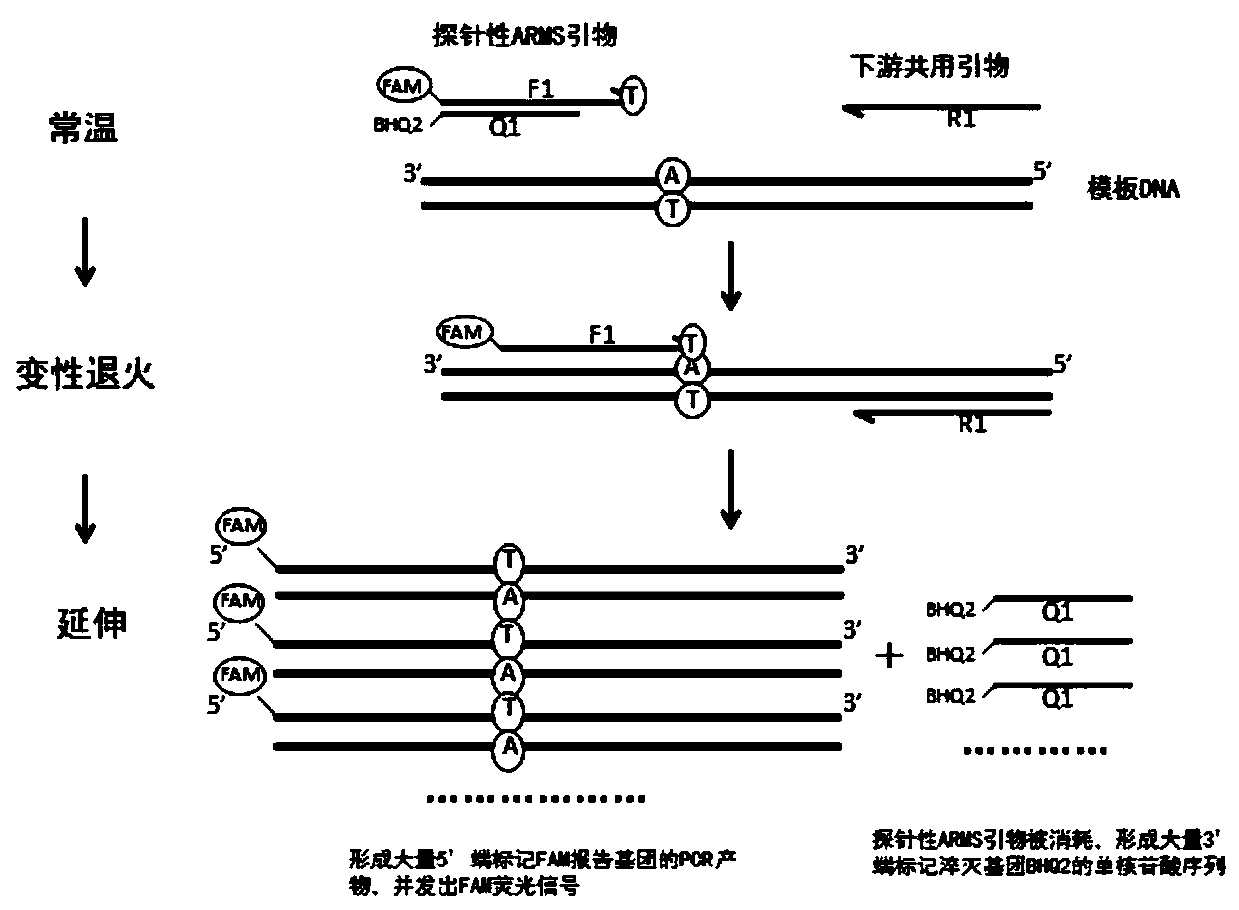
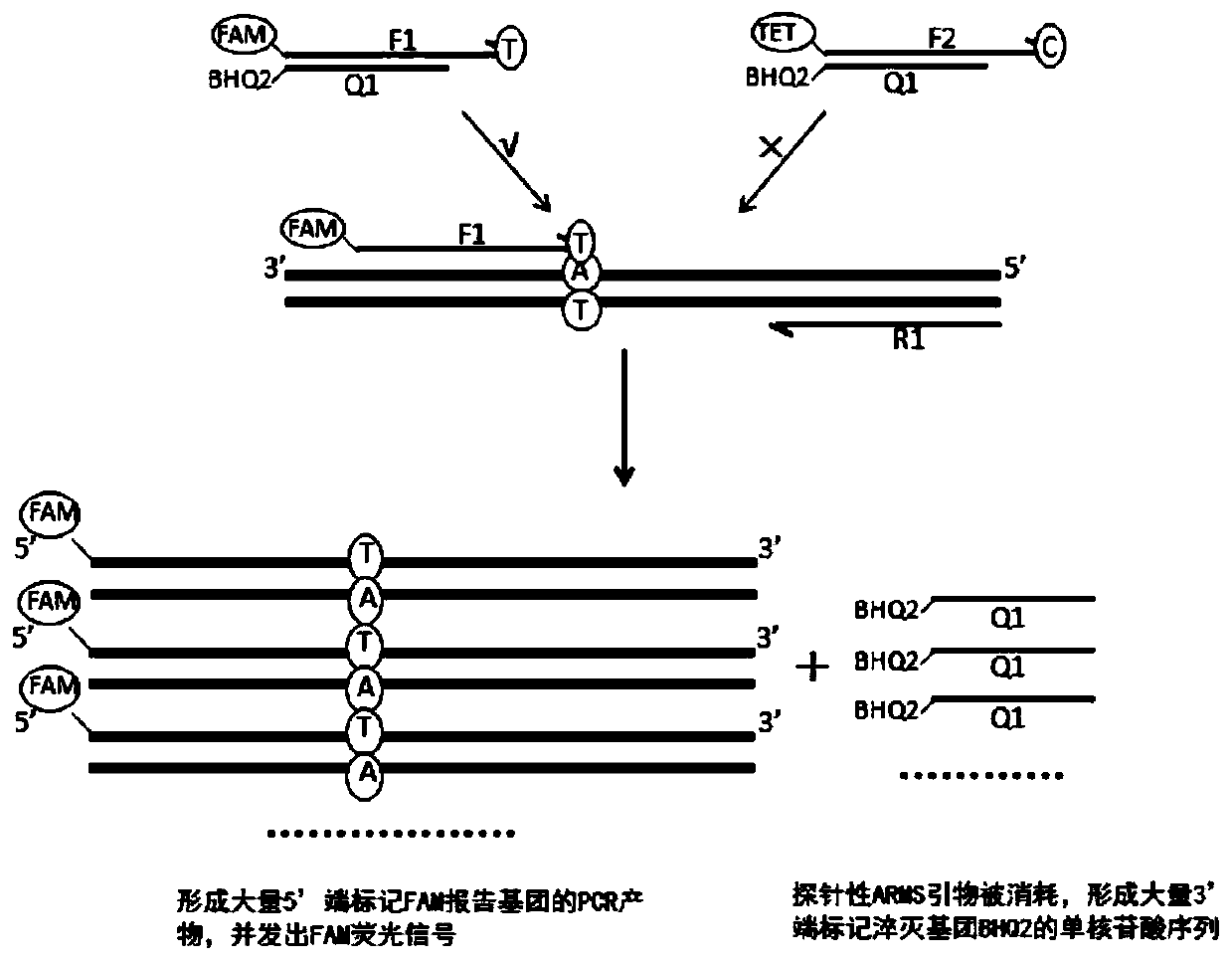
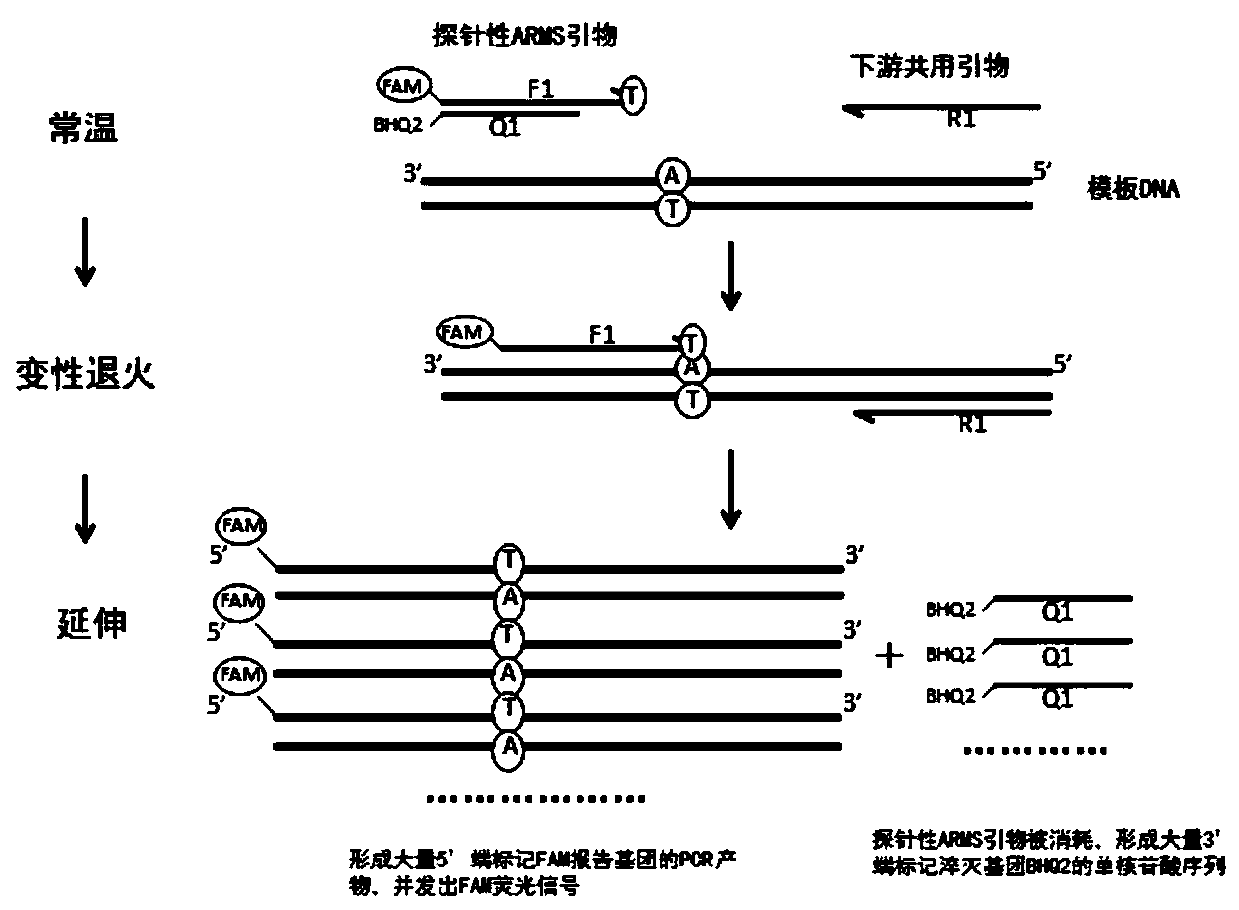
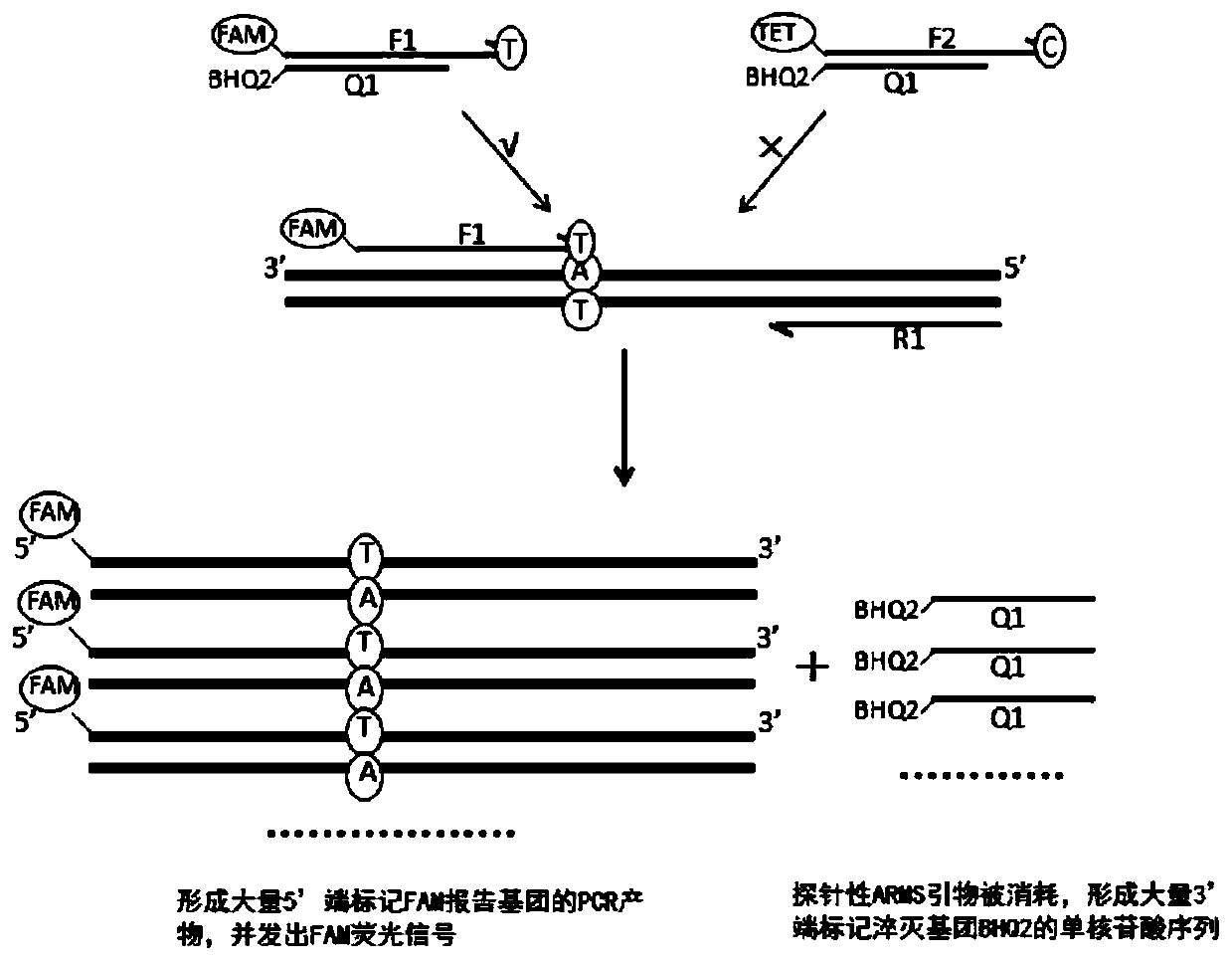
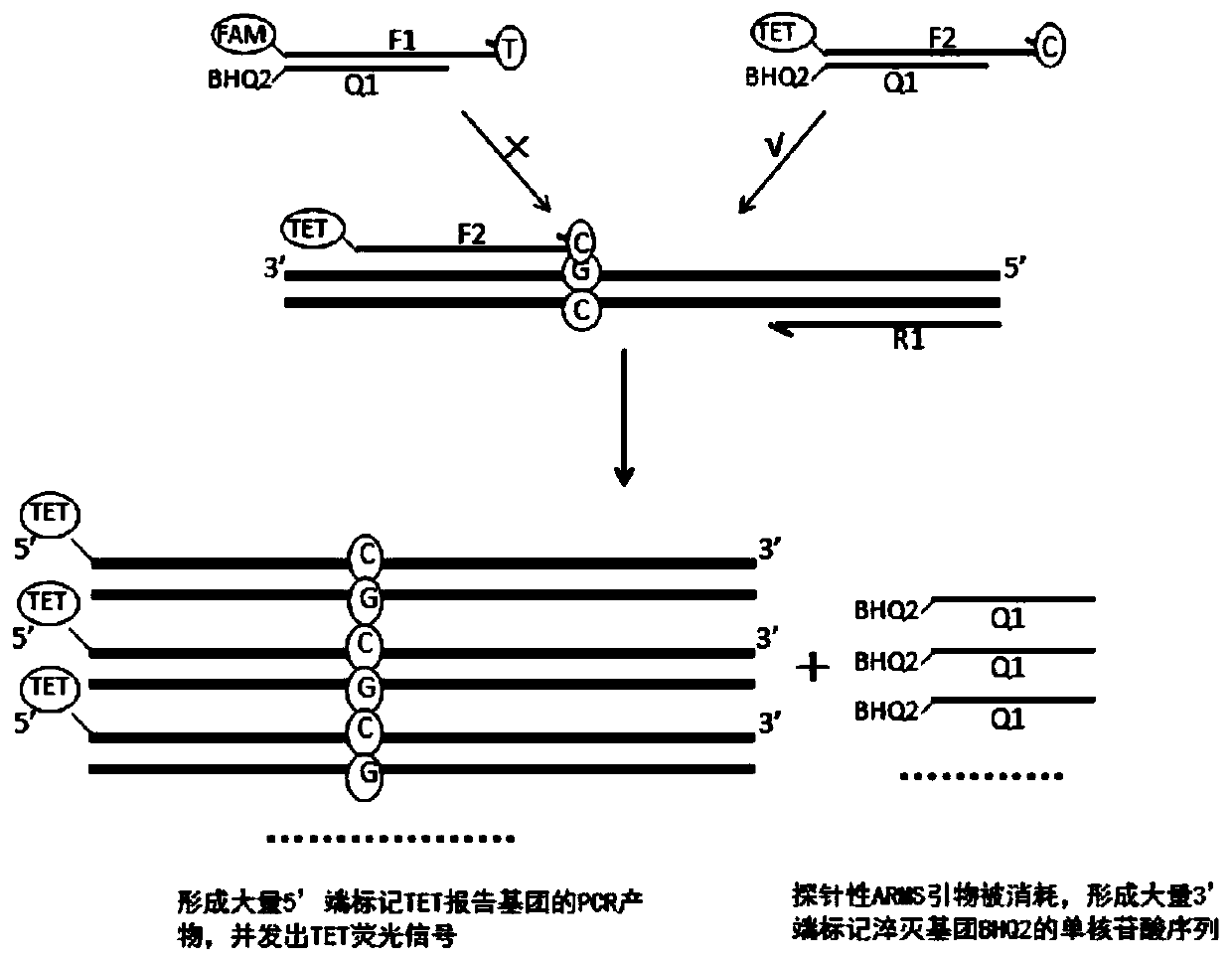
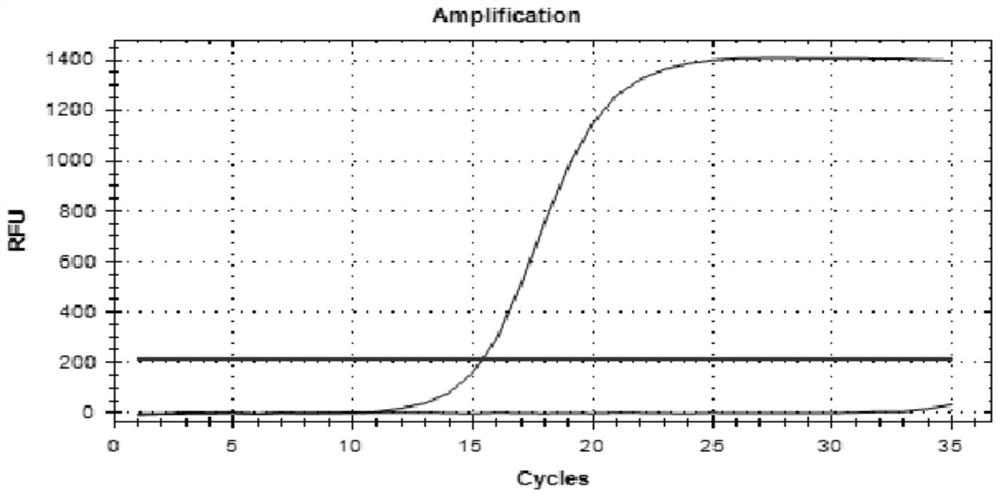
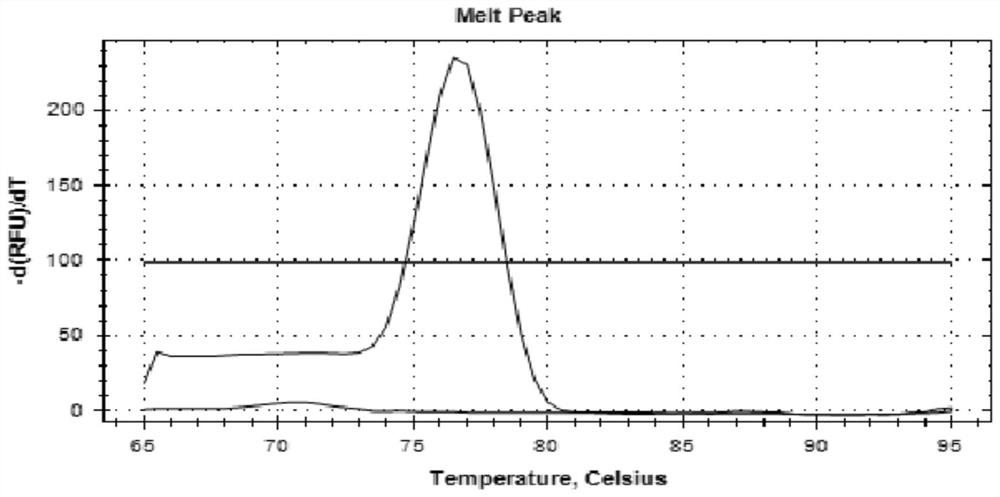
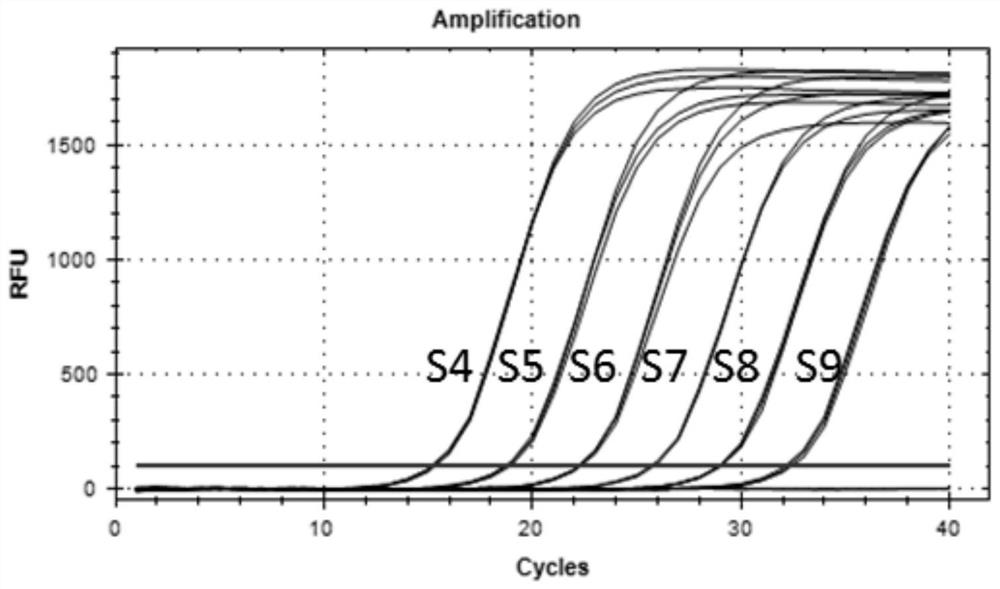
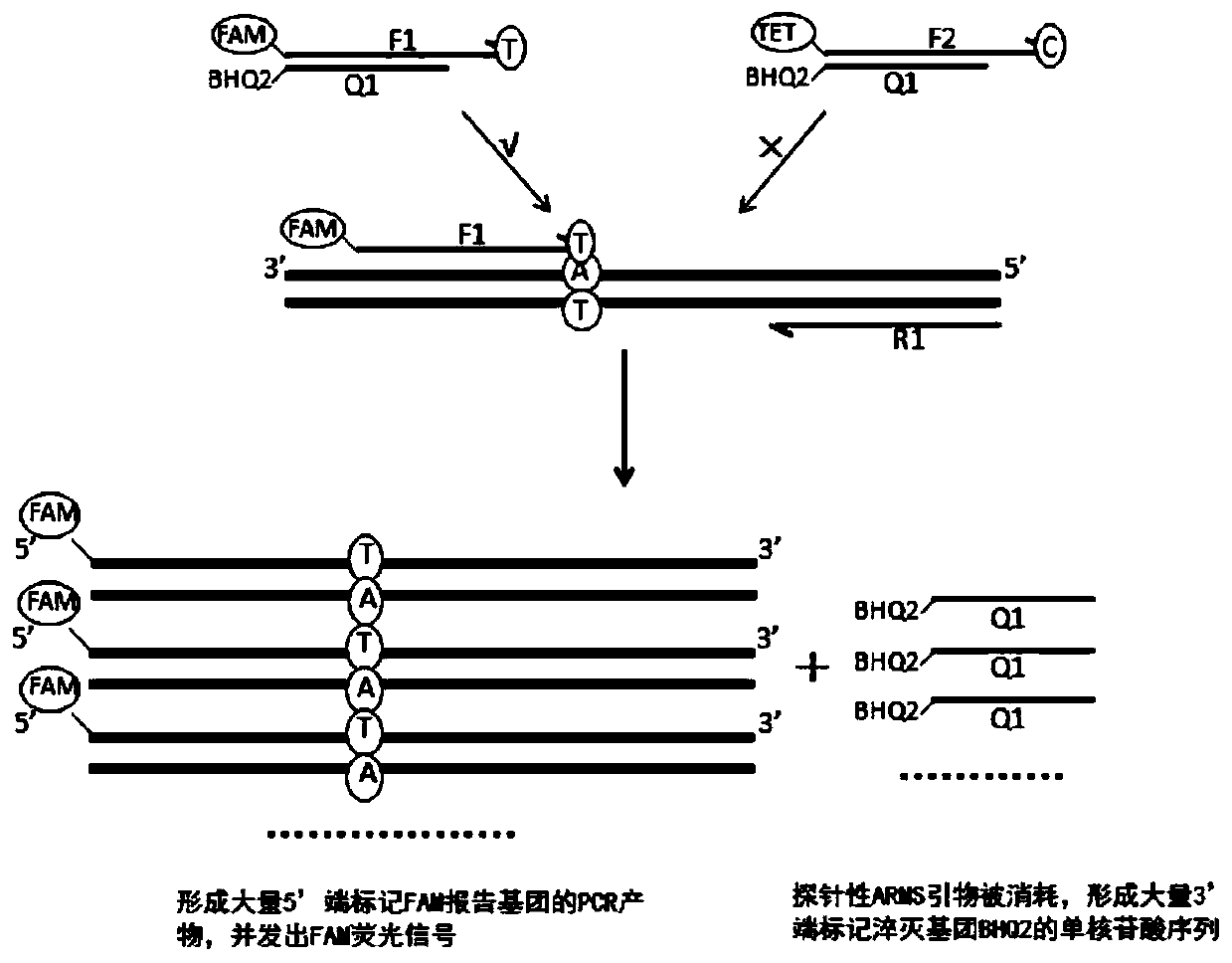
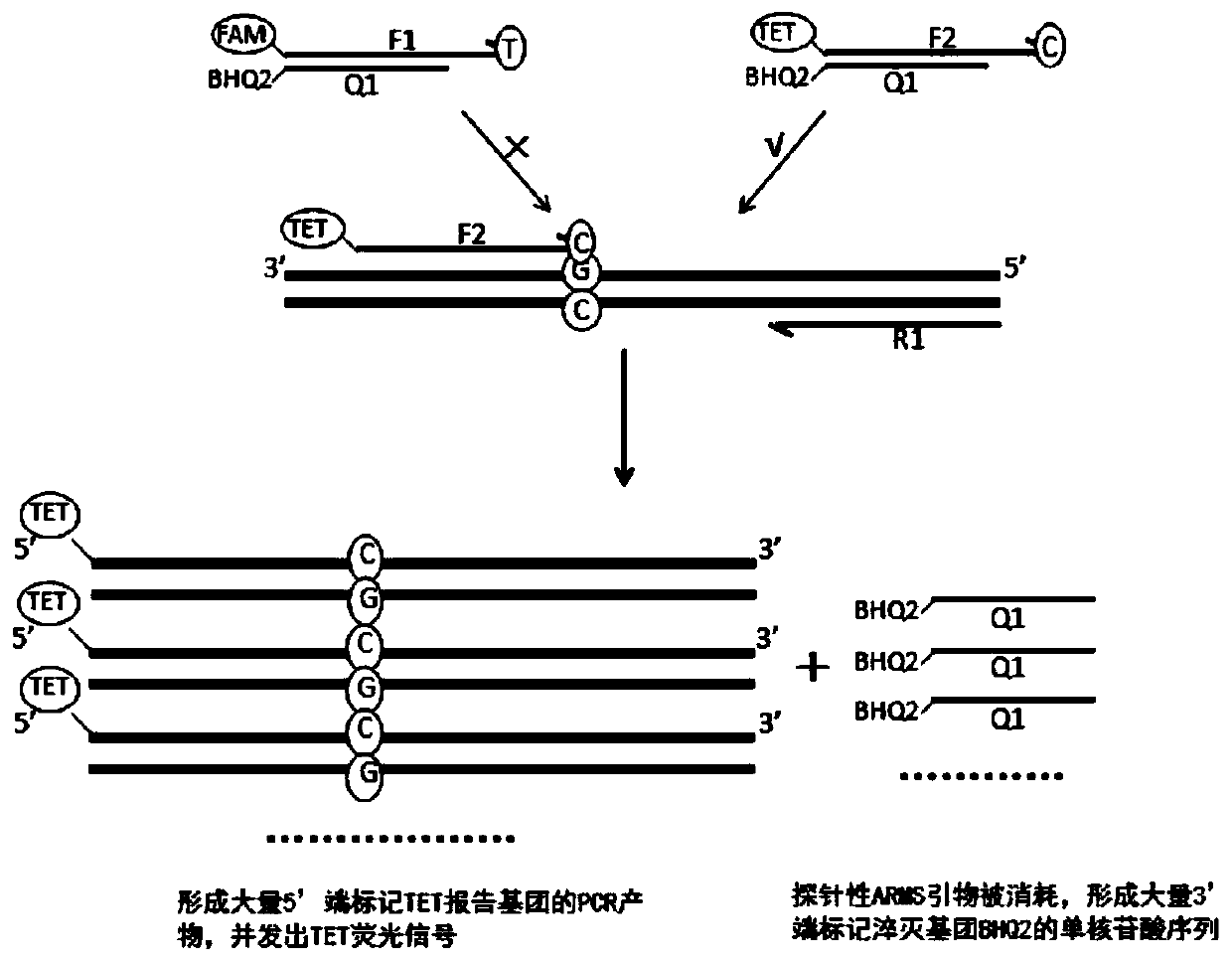
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
42results about How to "Analysis of results is simple" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
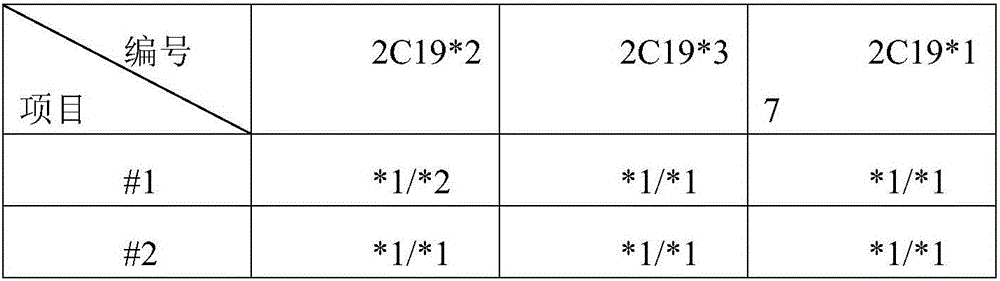
Detection probe for SNP (Single Nucleotide Polymorphism) of human CYP2C19 gene and application of detection probe
InactiveCN106702019AIncreased sensitivityHigh Primer Match RateMicrobiological testing/measurementDNA/RNA fragmentationFluoProbesMedicine
The invention provides a detection probe for an SNP (Single Nucleotide Polymorphism) of a human CYP2C19 gene. A detection primer consists of a specific upstream and downstream primer pair of the gene and a specific Taqman double fluorescent probe which are respectively used for correspondingly detecting SNPs at CYP2C19*2, CYP2C19*3 and CYP2C19*17 sites. The detection probe has the characteristics of quick detection and high specificity; furthermore, the detection method is simple; positive contrast and negative contrast which are necessary to conventional fluorescent quantitation PCR are eliminated, so that the operation steps and the experimental cost are reduced; the subsequent clinical treatment strategy can be guided more quickly and better.
Owner:北京一立科技发展有限公司
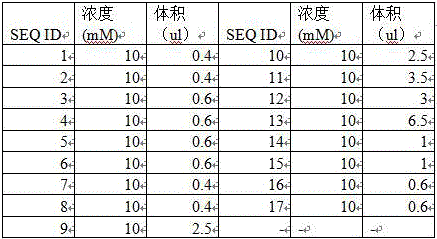
Method and kit for detecting alpha and beta thalassemia point mutation based on next generation sequencing technology
ActiveCN106755329AWide detection rangeHigh sensitivityMicrobiological testing/measurementLibrary creationBeta globinBeta thalassemia
The invention relates to a method and kit for detecting alpha and beta thalassemia point mutation based on a next generation sequencing technology .In the detection method, PCR (polymerase chain reaction) amplification primer sequences SEQ ID NO1-17 in exon, regulation and transcription regions of HBA1, HBA2 and HBB genes, PCR marker primer sequences SEQ ID NO18-115 at the 5'-terminal and PCR marker primer sequences SEQ ID NO116-213 at the 3'-terminal involved with alpha and beta thalassemia are included. The detection method comprises the following detection steps: (1) constructing a next generation sequencing library; (2) purifying the next generation sequencing library; (3) sequencing through a next generation sequencer; and (4) performing bioinformatic analysis to obtain a result. The kit for detection comprises the above-mentioned primer sequences and the next generation sequencer solexa. The detection method provided by the invention has the characteristics of simple operation steps, high detection specificity, wide detection range, low cost and the like.
Owner:MATERNAL & CHILD HEALTH HOSPITAL OF GUANGXI ZHUANG AUTONOMOUS REGION GUANGXI ZHUANG AUTONOMOUS REGION
Kit for detecting CYP3A4 and CYP3A5 polymorphic sites and method thereof
ActiveCN108410961AIncrease the Tm valueRaise the annealing temperatureMicrobiological testing/measurementDNA/RNA fragmentationFluorescenceRepeatability
The invention discloses a kit for detecting CYP3A4 and CYP3A5 polymorphic sites and a method thereof. The kit comprises specificity upstream and downstream primer sequences of CYP3A4*1G shown in sequences 1-4 in a sequence table, specificity TaqMan-MGB bi-fluorescence probe sequences of CYP3A4*1G shown in sequences 5-8, specificity upstream and downstream primer sequences of CYP3A5*3 shown in sequences 9-12 and specificity TaqMan-MGB bi-fluorescence probe sequences of CYP3A5*3 shown in sequences 13-16. The kit has the advantages that the specificity is high, pollution is prevented, the sensitivity is high, the detection speed is high, result analysis is simple, and the accuracy is high; the kit can judge the metabolism capacity of to-be-detected objects to fentanyl medicine by detecting polymorphic sites of CYP3A4 and CYP3A5 genes, the using amount of medicine such as fentanyl can be more accurately and effectively guided accordingly, and the kit has the actual clinical application value. The kit is applied to clinical diagnosis, the fit degree between the kit and the prior art is higher, the cost is low, and repeatability is high.
Owner:浙江鼎创医疗科技有限公司
Primer sequence and test kit for gene detection of safe medication for children
PendingCN111304320AHigh sensitivityEasily damagedMicrobiological testing/measurementDNA/RNA fragmentationMedicineGenotype
The invention discloses a primer sequence and a test kit for gene detection of safe medication for children, belonging to the technical field of molecular diagnosis. The test kit comprises 22 pairs ofamplification primers and can specifically amplify 22 common locus regions of the safe medication gene for children; the 22 single-base extension primers are used for detecting genotypes of 22 loci of the safe medication gene for children; in addition, the test kit comprises a special reagent for pretreatment and detection. The test kit provided by the invention can realize one-hole detection ofdifferent genotypes of 22 common loci related to clinical safe medication genes for the children, and has the advantages of high sensitivity, strong specificity, high accuracy, simple and convenient operation, low cost, high throughput, rapid detection, automatic result interpretation and easy clinical popularization and application. The test kit provided by the invention can be applied to gene detection of safe medication for the children, provides a reliable detection system and test kit for safe medication for the children, and has important clinical application value and good market application prospect.
Owner:ZHEJIANG DIGENA DIAGNOSTIC TECH CO LTD
Primer combination sequence and kit for detecting child safe medication-related gene mutation sites
PendingCN111304321AHigh sensitivityGood curative effectMicrobiological testing/measurementDNA/RNA fragmentationMedication riskFull Term Neonate
The invention discloses a primer combination sequence and kit for detecting child safe medication-related gene mutation sites. The kit includes 23 pairs of amplification primers and 23 single-base extension primers; the 23 pairs of amplification primers can specifically amplify the 23 common mutation site regions of child safe medication-related genes, and the 23 single-base extension primers areused to detect the 23 mutation site genotypes of the child safe medication-related genes; and the kit further includes special reagents for pretreatment and detection. The kit provided by the invention can realize one-hole detection of the 23 mutations related to the clinical child safe medication-related genes, has high sensitivity, strong specificity and high accuracy, and is simple to operate,low in cost and high in throughput, the detection is fast, automatic interpretation of results is realized, and the clinical promotion and application are easy to realize; and the kit can be applied to the detection of the genes related to safe medication of newborns or children, guide clinical correct medication, avoid medication risks, reduce medication injury, and truly achieve precise medication.
Owner:ZHEJIANG DIGENA DIAGNOSTIC TECH CO LTD
Kit for detecting human PEAR1 gene polymorphism and application thereof
InactiveCN105671142AImprove detection accuracyAnalysis of results is simpleMicrobiological testing/measurementPEAR1 geneFluorescence
The invention relates to a kit for detecting human PEAR1 gene polymorphism and an application thereof. The kit includes the following substances: a specific primer and a specific fluorescent probe for detecting an rs2768759 locus on PEAR1 gene, a specific primer and a specific fluorescent probe for detecting an rs12041331 locus on the PEAR1 gene, a Taq DNA polymerase, dNTP mixed liquid, a MgCl2 solution, a fluorescent quantitative PCR reaction buffer liquid and deionized water. The kit overcomes the defects in various gene mutation detection methods, is high in detection accuracy, is simple and convenient, is short in detection time and is simple in result analysis. The kit is suitable for clinical laboratories and can perform qualitative detection to the two polymorphic sites, the rs2768759 and the rs12041331, on the PEAR1 gene, wherein a PCR fluorescent amplification reaction is carried out according to the SNP locus. A result can be determined just on the basis of whether two different fluorescent curves are positive or not without manual error, so that the kit is improved in efficiency and reduced in detection cost. The kit is free of DNA extraction, wherein a suspension liquid after cell pyrolysis is added to the reaction system to complete amplification.
Owner:南京仁天生物科技有限公司
Primer sequence and kit for detecting glucose-6-phosphate dehydrogenase (G6PD) gene mutation
ActiveCN111118141AHigh sensitivityStrong specificityMicrobiological testing/measurementAgainst vector-borne diseasesPrenatal diagnosisGenotype
The invention discloses a primer sequence and kit for detecting pathogenic mutation related to glucose-6-phosphate dehydrogenase (G6PD) deficiency. The kit comprises five pairs of amplification primers, 16 single-base extension primers and a special reagent for pretreatment and detection, wherein the amplification primers can specifically amplify 16 common mutation site regions of G6PD genes related to G6PD deficiency; and the 16 single-base extension primers are used for detecting genotypes of 16 mutation sites of the G6PD genes. By the kit, one-hole detection of 16 common mutations related to clinical G6PD deficiency pathopoiesis can be realized; and the kit is high in sensitivity, specificity and accuracy, simple and convenient to operate, low in cost, high in throughput, quick in detection, automatic in result interpretation and easy for clinical popularization and application. The primer sequence and kit can be applied to prenatal diagnosis or neonatal hereditary disease screeningdetection, and a reliable detection reagent is provided for quick detection of G6PD deficiency carriers or patients.
Owner:ZHEJIANG DIGENA DIAGNOSTIC TECH CO LTD
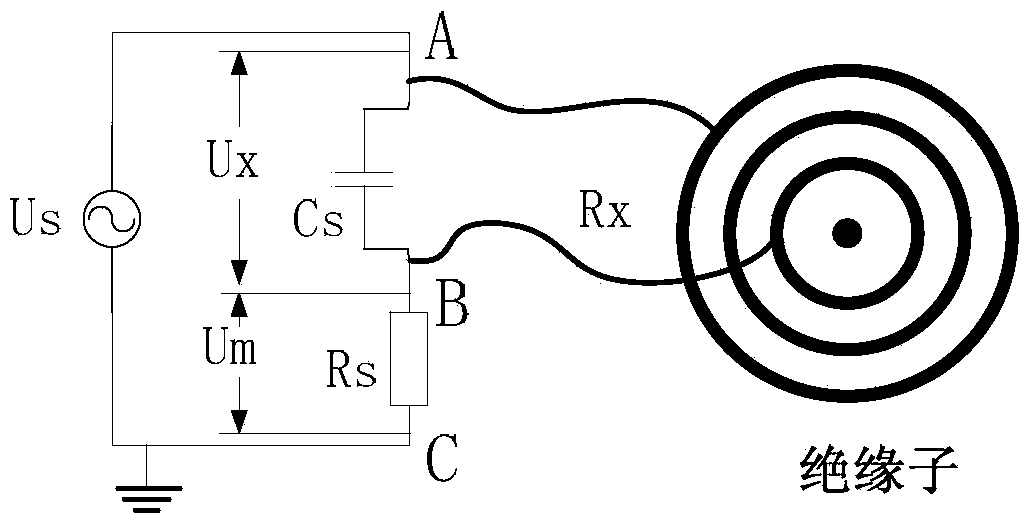
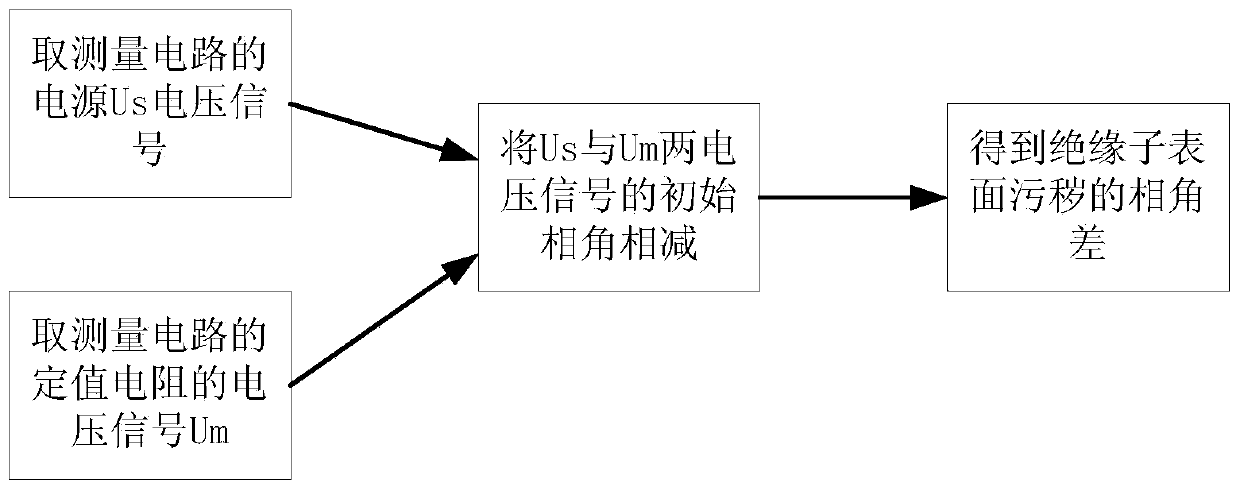
Method for monitoring foul and damp degree on surface of insulator
ActiveCN103728344AGrasp the situation of pollution and dampReduce measurement errorMaterial resistanceElectrical resistance and conductanceObservational error
The invention discloses a method for monitoring foul and damp degree on the surface of an insulator, which comprises the following steps: S1, in a measuring circuit, collecting the voltage signals of a fixed resistor and a power supply; S2, subtracting the initial phase angles of the two voltage signals, and taking the absolute value of the difference value to obtain phase angle difference theta; S3, obtaining the relational expression between the phase angle difference theta and the equivalent resistance Rx of foulness on the surface of the insulator; S4, according to the change of the phase angle difference, obtaining the foul and damp degree on the surface of the insulator. According to the invention, the measurement error is less, the equipment is cheap , and the analysis of the monitoring result is simple.
Owner:SOUTH CHINA UNIV OF TECH
Kit for detecting polymorphism of human ABCB1 gene and application thereof
InactiveCN105695453AImprove detection accuracyAnalysis of results is simpleMicrobiological testing/measurementDNA/RNA fragmentationFluorescenceBiology
The invention relates to a kit for detecting polymorphism of a human ABCB1 gene and an application thereof. Substances in the kit comprise a specific primer and a specific fluorescent probe for detecting an rs1045642 SNP locus on the ABCB1 gene, Taq DNA polymerase, a dNTP mixed liquid, an MgCl2 solution, a fluorescence quantitative PCR reaction buffer solution and deionized water. The defects of various genetic mutation detection methods are overcome. The kit has the advantages of high detection accuracy, simple and convenient detection, short detection time, and simple result analysis, is suitable for use in clinical laboratories, can perform qualitative detection on a CYP2C19 gene polymorphism locus, performs PCR fluorescent amplification reaction according to the SNP locus, can judge a result just according to a condition whether two different fluorescence curves are started or no, cannot produce artificial errors, has low false positive and false negative rates, reduces detection costs, has no need for DNA extraction, can complete amplification only with cracking of complete blood cells, and improves the detection accuracy.
Owner:南京仁天生物科技有限公司
Dual polymerase chain reaction-denaturing high performance liquid chromatography (PCR-DHPLC) detection method for staphylococcus aureus in aquatic products
ActiveCN102399897AImprove throughputHighly automated detectionComponent separationMicrobiological testing/measurementDuplex pcrStaphylococcus cohnii
The invention relates to a dual polymerase chain reaction-denaturing high performance liquid chromatography (PCR-DHPLC) detection method for staphylococcus aureus in aquatic products, which is characterized by comprising the following steps of: 1, staphylococcus aureus culture: aquatic product samples to be tested are mixed with enrichment liquid for culture; 2, staphylococcus aureus genome deoxyribonucleic acid (DNA) extraction; 3, primer design; 4, dual PCR amplification by using the staphylococcus aureus genome DNA as templates; and 5, DHPLC peak type map obtaining through PCR product DHPLC detection for analysis and identification. The integral detection method has the advantages that the reaction condition is simplified, the detection sensitivity is improved, in addition, the operation is simpler and more convenient, and the goal of high flux is reached.
Owner:中华人民共和国舟山出入境检验检疫局
Method for rapidly detecting LOX (lipoxygenase) transgenic wheat and kit using method
ActiveCN106520981AShort detection cycleReduce testing costsMicrobiological testing/measurementSkyGenetically modified wheat
The invention relates to the field of molecular genetic breeding and particularly provides a method for rapidly detecting LOX (lipoxygenase) transgenic wheat and a kit using the method. Specific and sensitive outer primers including F3 and B3 and inner primers including FIP and BIP are taken as amplification target sequences. The principle of the detection method is as follows: if the color of a reaction liquid turns from purplish blue to sky blue through DNA (deoxyribonucleic acid) extraction and LAMP (loop-mediated isothermal amplification) detection, detected wheat belongs to a transgenic wheat strain. The method has low requirement for the quality of a DNA template, the LAMP detection cycle is short, the detection cost is low, the operation process is simple and convenient, and a result is easy to analyze.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
Real-time fluorescence PCR method of detecting rs762551 site of CYP1A2 gene, and primer-and-probe combination of real-time fluorescence PCR method
PendingCN110923312AStrong specificityGuaranteed specificityMicrobiological testing/measurementDNA/RNA fragmentationForward primerPcr method
The invention belongs to the field of gene diagnosis, and discloses a real-time fluorescence PCR method of detecting a rs762551 site of a CYP1A2 gene, and a primer-and-probe combination of the real-time fluorescence PCR method. A sequence of one forward primer FpG is 5'-GGTGAGCTCTGTGGGGC-3', a sequence of the other forward primer FpG is 5'-GGTGAGCTCTGTGGGGA-3', a sequence of a reverse primer Rp is5'-GCTGAGGGTTGAGATGGAGAC-3', and a sequence of a probe Probe is 5'-Cy5-ACGCATGGTAGATGGAGCTTAG-BHQ2-3'. After a PCR is completed, results can be analyzed through whether or not an amplification curveexists and in combination with a Ct value. The real-time fluorescence PCR method of detecting the rs762551 site of the CYP1A2 gene is applied to polymorphism detection of the rs762551 site, and has the advantages of being easy to operate, high in resolution, free of pollution, and high in throughput.
Owner:SHANXI LIFEGEN
Decidua and villus pairing cDNA chip
ActiveCN107190328ARealize functional presentationImprove throughputMicrobiological testing/measurementLibrary creationClinical informationQuantitative accuracy
The invention relates to a decidua and villus pairing cDNA chip. The cDNA chip is prepared through the following method that the decidua and villus tissue of a woman with early pregnancy abortion are taken, the RNA is extracted, cDNA is formed by reverse transcription and preset in a PCR reaction plate, and cDNA chip is made. A preparation method and application of cDNA chip are further provided. The method and application have the advantages that the decidua and villus tissue of every abortion woman are respectively taken, pairing is formed by cDNA, the cDNA pairs are concentrated on the same chip, and the function presentation of parent-fetal interface of the physiological structure is achieved on the chip outside the body. The decidua and villus pairing cDNA chip has the advantages of being high in throughput, strong in specificity, high in sensitivity, simple and fast in operation, high in quantitative accuracy, strong in result reliability, simple in result analysis, complete in clinical information for samples and the like, and the cDNA chip can be used for quantitative detection of the expression statuses of a target gene in different patients and for conducting in-depth correlation analyses of clinical information.
Owner:夷希微医学科技(上海)有限公司
Device for detecting topological charge of optical vortex beam based on radial grating spoke number
The invention provides a device for detecting the topological charge of an optical vortex beam based on a radial grating spoke number. A core element of the device is a radial grating lifting apparatus with a spoke number ranging from 2 to 20, the radial gratings with different spoke numbers can fall onto a light path platform in sequence, and a to-be-detected optical vortex beam can just pass through. A to-be-detected optical vortex beam enters the radial grating lifting apparatus and passes through the falling radial grating, and a charge-coupled device therebehind receives a diffraction pattern and displays the diffraction pattern in a computer. The topological charge of the optical vortex beam is detected by observing whether a bright spot exists in an optical axis part of the diffraction pattern or not. Different from a traditional topological charge detection device, the device does not need to introduce an additional reference light beam, the diffraction pattern can be observedvery visually, and the judgment of the topological charge number is faster and more accurate. The device provided by the invention simple and easy to operate.
Owner:CHINA JILIANG UNIV
Fluorescent polarization based homogeneous phase detection method of single nucleotide polymorphism of codon118 of ERCC1 (excision repair cross-complementing 1) gene
InactiveCN102676661AThe method steps are simpleEasy to operateMicrobiological testing/measurementDNA/RNA fragmentationERCC1Nucleotide
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Kit for detecting human COX-2 gene polymorphism and application thereof
InactiveCN105671036AImprove detection accuracyAnalysis of results is simpleMicrobiological testing/measurementDNA/RNA fragmentationFluorescenceDNA extraction
The invention relates to a kit for detecting human COX-2 gene polymorphism and an application thereof. The kit includes the following substances: a specific primer and a specific fluorescent probe for detecting an rs20417 SNP locus on COX-2 gene, a Taq DNA polymerase, a dNTP mixed liquid, a MgCl2 solution, a fluorescent quantitative PCR reaction buffer liquid and deionized water. The kit overcomes the defects in various gene mutation detection methods, is high in detection accuracy, is simple and convenient, is short in detection time and is simple in result analysis. The kit is suitable for clinical laboratories and can perform qualitative detection to COX-2 gene polymorphic sites, wherein a PCR fluorescent amplification reaction is carried out according to the SNP locus. A result can be determined just on the basis of whether two different fluorescent curves are positive or not, so that the kit is free of manual error, is low in false positive and false negative rate, and is improved in efficiency and reduced in detection cost.
Owner:南京仁天生物科技有限公司
TaqMan probe real-time fluorescence PCR method for detecting rs6313 locus of HTR2A gene and primer probe combination
PendingCN110643689AStrong specificityGuaranteed specific amplificationMicrobiological testing/measurementDNA/RNA fragmentationPcr methodFluorescent pcr
The invention belongs to the field of gene diagnosis, and discloses a TaqMan probe real-time fluorescent PCR method for detecting the rs6313 locus (HTR2A, 102C>T) of a HTR2A gene, and a primer probe combination. A upstream primer FpC-T is 5'-GCTCTACAGTAATGACTTTAACTTC-3', a upstream primer FpT-T is 5'-GCTCTACAGTAATGACTTTAACTTT-3', a downstream primer Rp is 5'-GATGAAGTAAGGAGAGACACGAC-3', and a probeProbe is 5'-FAM-TAACACTTCTGATGCATTTAACTGGAC-BHQ2-3'. After PCR reaction is completed, the presence or absence of an amplification curve can be used for analyzing the result. The TaqMan probe real-time fluorescent PCR method has the advantages of simple operation, high resolution, no pollution, high throughput and the like.
Owner:SHANXI LIFEGEN
Homogeneous phase detection method for methylation state of epidermal growth factor receptor (EGFR) gene promoter based on fluorescence polarization
InactiveCN102618645AThe method steps are simpleEasy to operateMicrobiological testing/measurementFluorescence/phosphorescenceFluorescent polarizationPromoter
The invention relates to a homogeneous phase detection method for a methylation state of an epidermal growth factor receptor (EGFR) gene promoter based on fluorescence polarization. The method solves the problems that the cost is high, the operation steps are fussy, pollution is easily produced and the accuracy of the detection result is affected in the prior art; the method is simple and easy to operate; and the method is finished by one step in a closed tube, and does not produce pollution. The method is low in cost, and does not require any special reagent and fluorescence quenching or micro groove bonding agent. Methylation of a target region is detected by using change of a fluorescence polarization value due to a single end-marked methylated specific probe, so that the detection result is objective and accurate. The method is simple in result analysis; and only digital comparison is required during result judgment, so the method easily realizes standardization and automation, has a wide application range and can be used for detecting clinical blood or tissue specimens.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Detection method of hypertriglyceridemia mutant site and detection kit
InactiveCN108103181ATime-consuming and labor-intensive solutionLow costMicrobiological testing/measurementHypertriglyceridemiaProblem of time
The invention discloses a detection method of a hypertriglyceridemia mutant site. Through the design of a specific primer, a target fragment is amplified by single-tube multiplex PCR (polymerase chainreaction); a sequencing primer is designed; a non-glue-cutting purified Sanger sequencing method is used for performing sequencing on the obtained target fragment. The invention also discloses a detection kit of the hypertriglyceridemia mutant site. The multiplex amplification LPL (lipoprotein lipase) isogene and the non-glue-cutting purification sequencing can be realized; only the target gene is amplified; the advantages of low cost and exact target goal are realized. Dozens of fragments are amplified at the same time through a single tube; the detection flux is high; the problem of time consumption and labor waste of the single PCR are solved; the operation is simple and fast; the operation can be performed by ordinary experiment personnel according to the description; during the sequencing, the glue cutting purification is not needed; the result shows that the operation is convenient; the PCR multiple number is sufficient; the identical or similar band strip size does not need tobe worried. The popularization is easy; the market prospects are wide.
Owner:GWP BIOTECHNOLOGIES INC
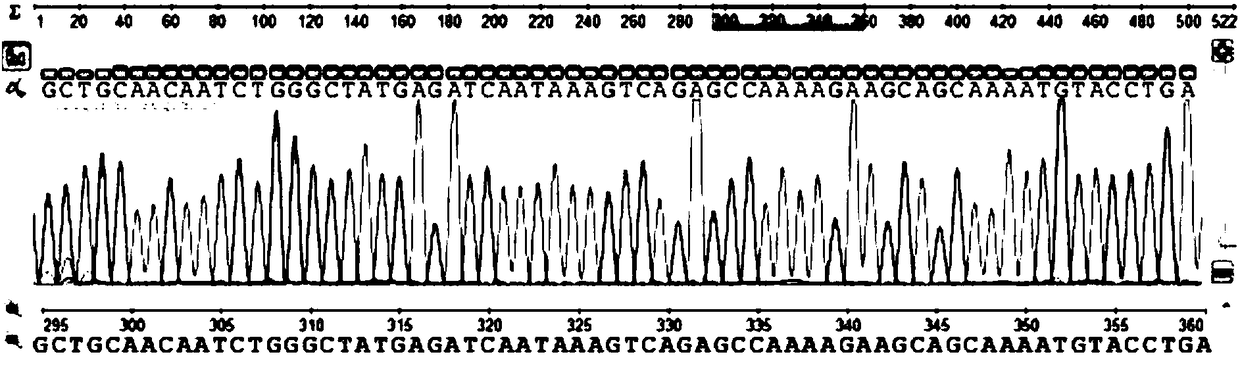
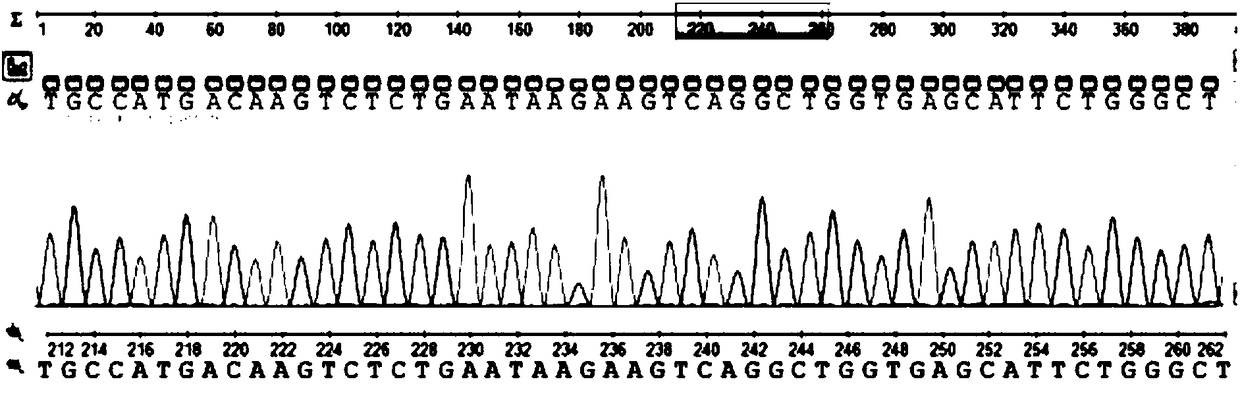
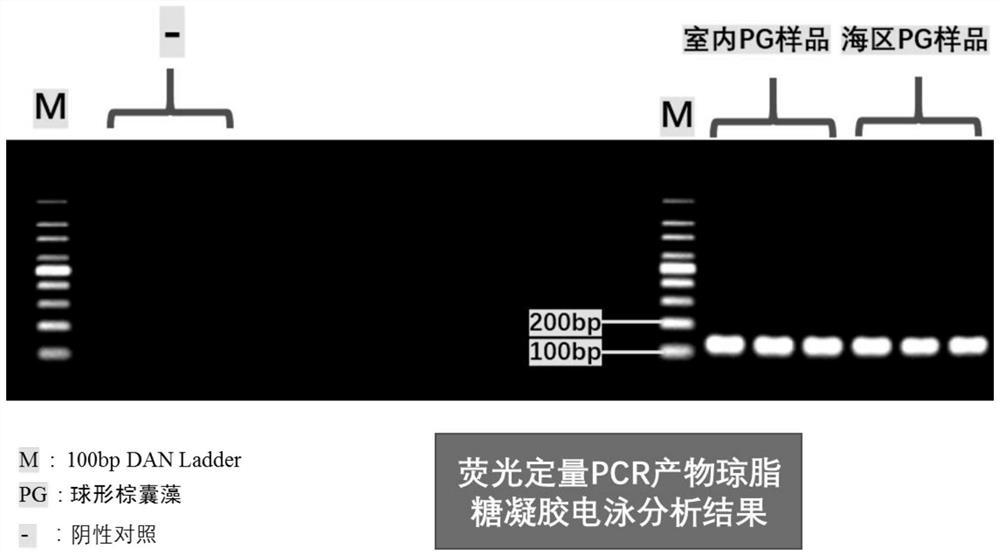
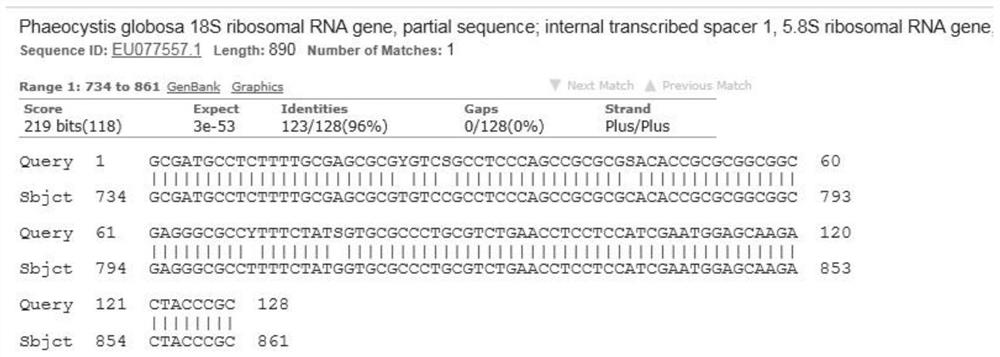
Marine phaeocystis globosa single-cell detection method based on a TaqMan probe technology
PendingCN111996240AEasy to operateHigh detection throughputMicrobiological testing/measurementSequence analysisGenomic DNA
The invention belongs to the technical field of detection of environmental microorganisms by a biological method, and discloses a marine phaeocystis globosa single-cell detection method based on a TaqMan probe technology. The method comprises the following steps: S1, designing and synthesizing a primer and a probe; S2, preparing a positive control sample of the phaeocystis globosa; S3, performingnegative control quality control; S4, extracting genomic DNA of a marine single-cell phaeocystis globosa sample and a positive control sample; S5, performing fluorescent quantitative PCR detection byadopting the primer and the probe designed in the S1; S6, judging a real-time fluorescent quantitative PCR amplification signal; S7, carrying out agarose gel electrophoresis analysis on a fluorescentquantitative PCR product; S8, carrying out sequencing analysis on a fluorescent quantitative PCR product; and S9, detecting and analyzing marine single-cell phaeocystis globosa. According to the method, low-density harmful red tide algae in the early stage of harmful red tide outbreak can be detected, so that an effective early warning tool is provided for early warning and early prevention and control of red tide outbreak, and the economic loss of mass culture and tourism nuclear power industries is avoided.
Owner:GUANGXI ACAD OF SCI +1
Primer set, method and kit for detecting slco1b1 and apoe gene polymorphisms based on shared primer probes
ActiveCN110863039BIncrease the Tm valueSimple designMicrobiological testing/measurementDNA/RNA fragmentationSLCO1B1Medicine
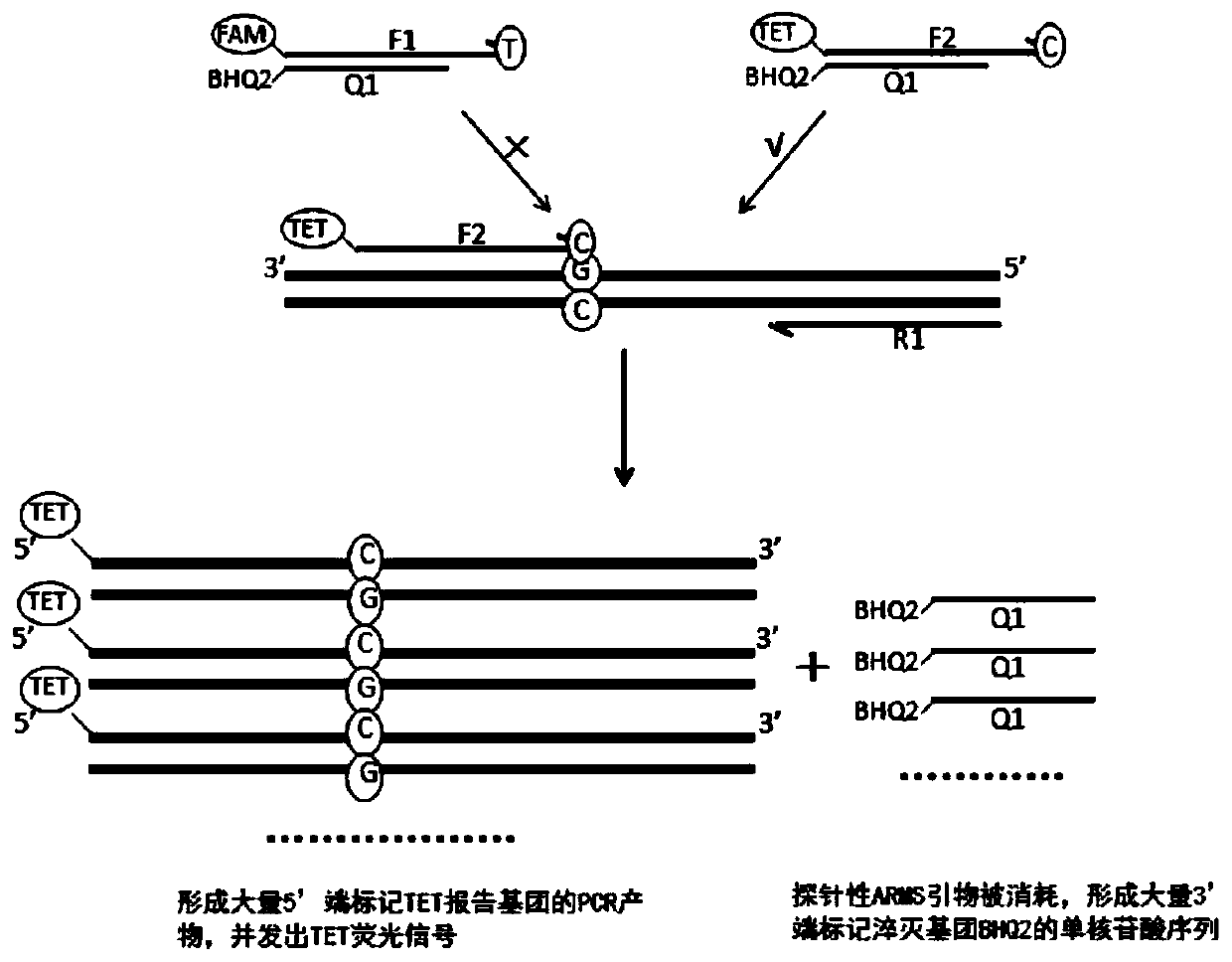
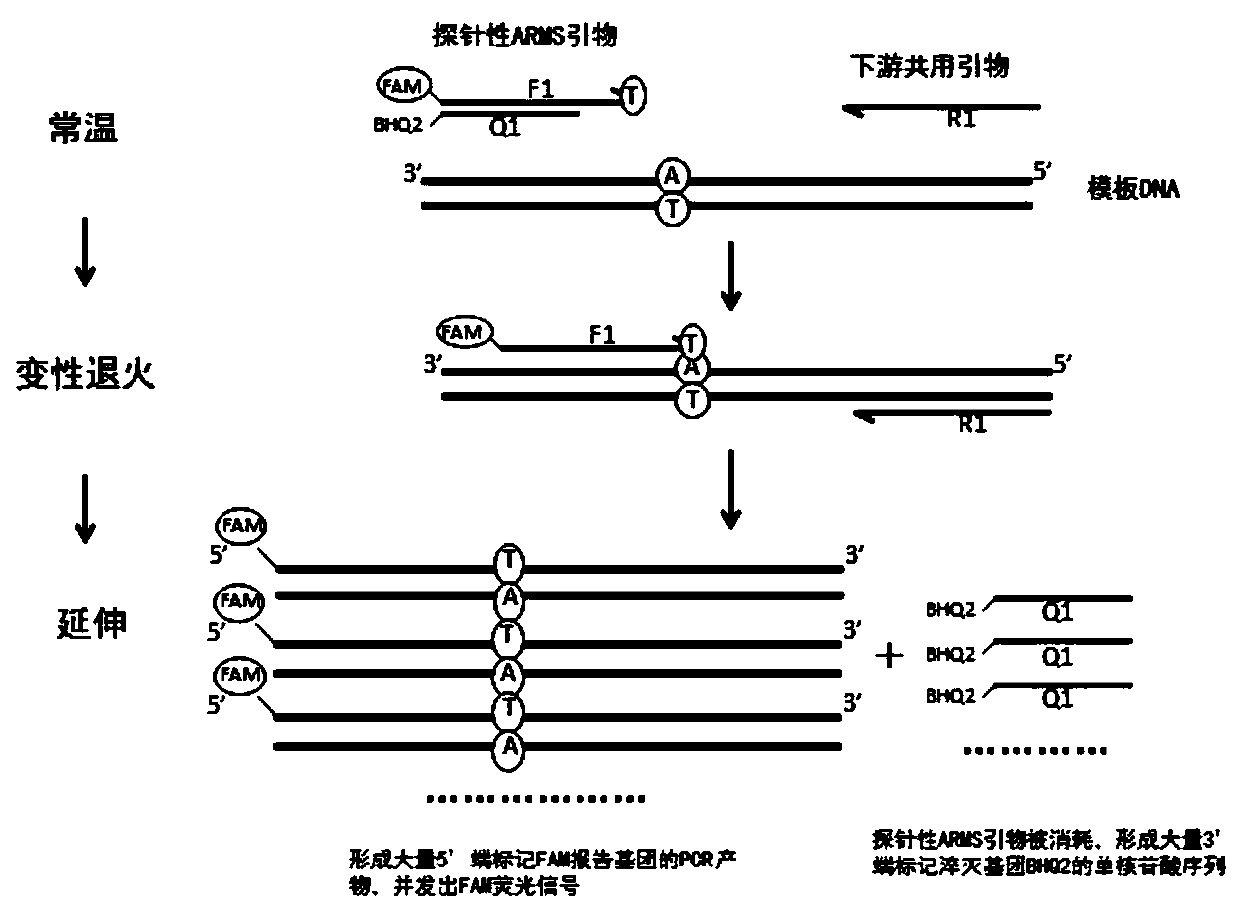
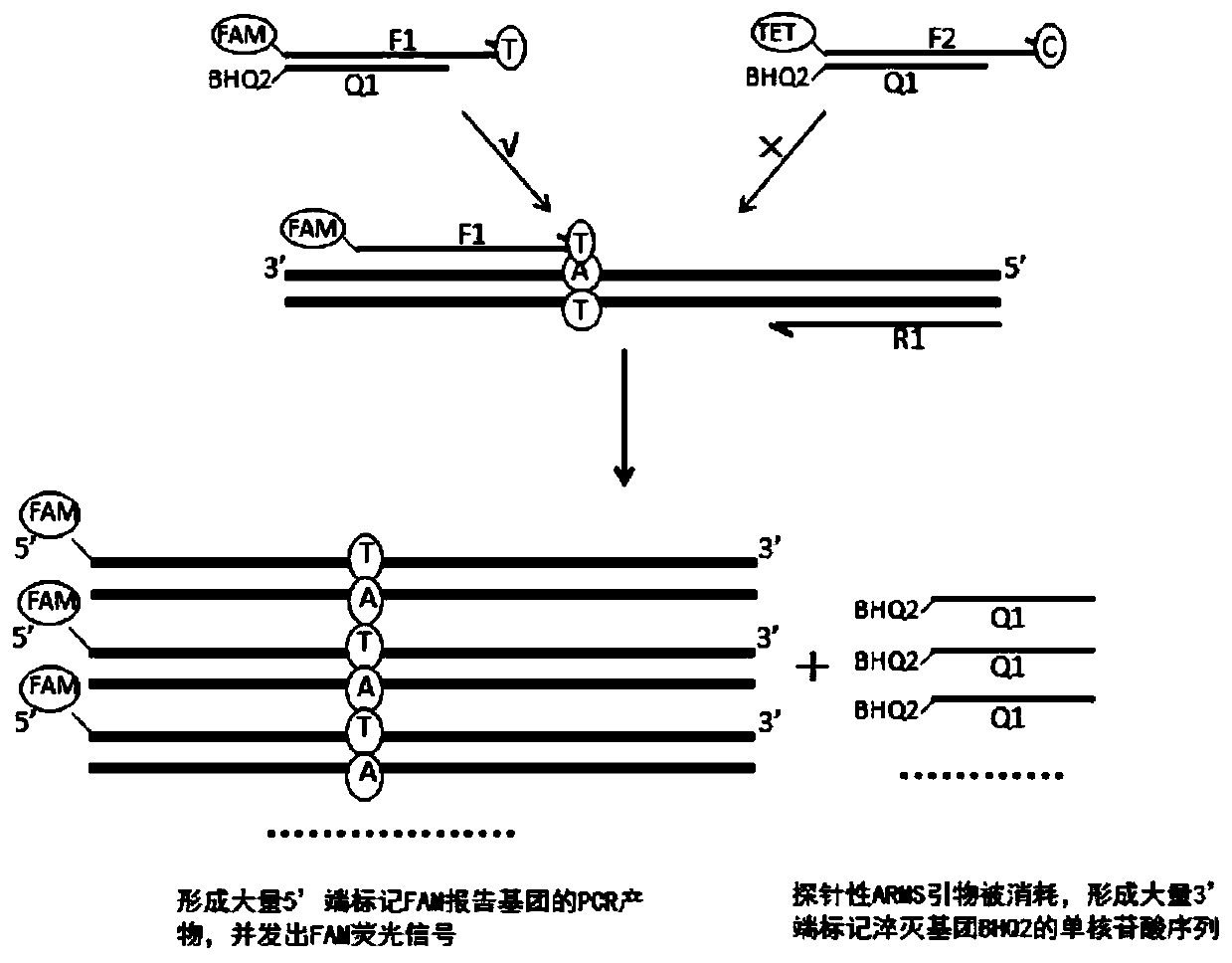
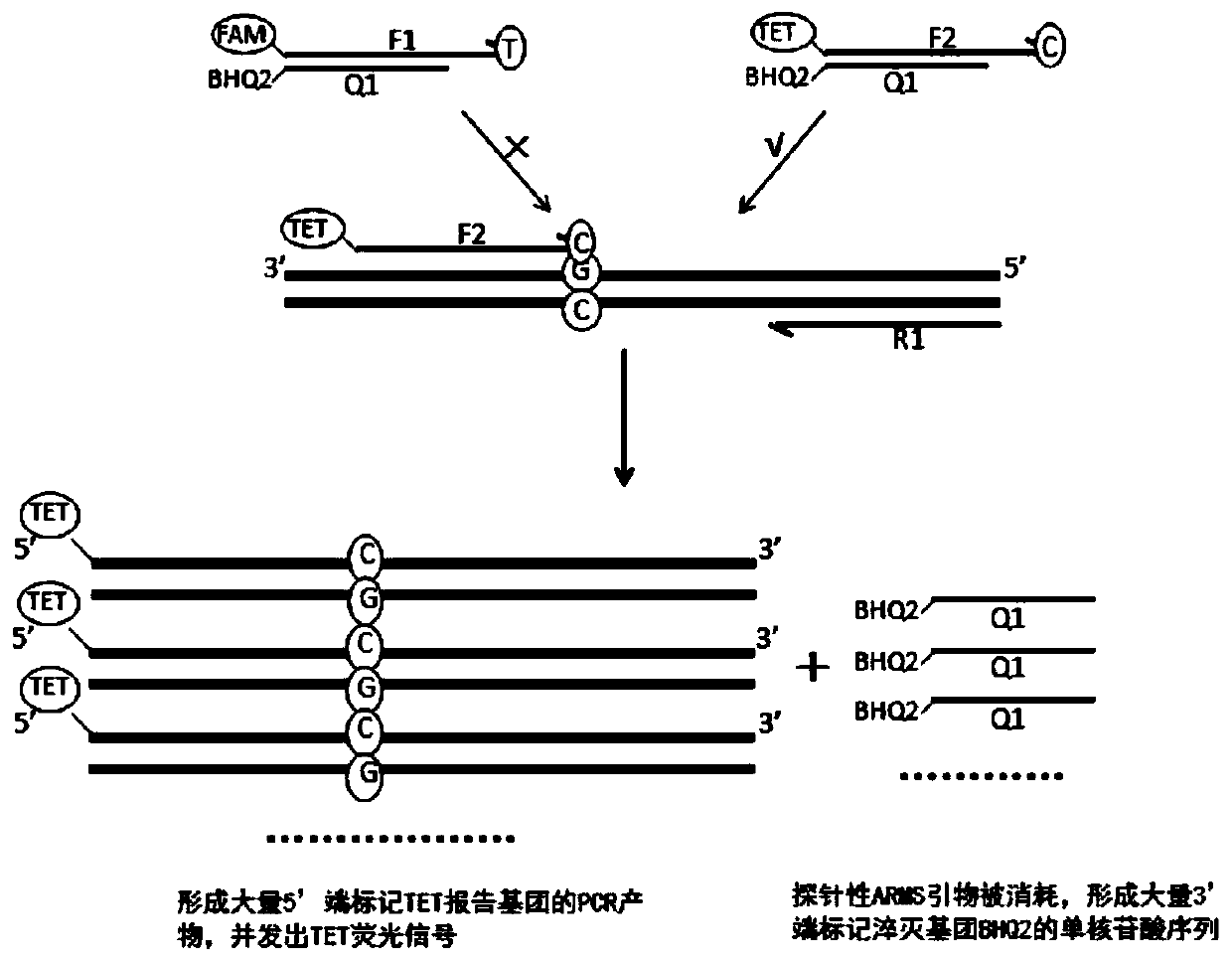
The invention discloses a primer group, a method and a kit for detecting SLCO1B1 and APOE gene polymorphism based on a shared primer probe. According to the method, reporter groups are marked at 5'ends of wild ARMS primers and mutant ARMS primers which are designed in the same direction to prepare ARMS primers with probes, and the ARMS primers are used for polymorphism detection of genes after being screened. The method is higher in detection sensitivity, stronger in specificity, more accurate and objective in result, simple in design and low in cost.
Owner:ANNGEEN BIOTECHNOLOGY CO LTD
Primer probe for diagnosing corneal dystrophy caused by human TGF beta I gene 555 locus mutation and detection method
InactiveCN107805661ALess impuritiesImprove accuracyMicrobiological testing/measurementDNA/RNA fragmentationGene specific primerFluorescence
The present invention provides a primer probe for diagnosing corneal dystrophy caused by variation of human TGFβI gene 555 site, including specific primer designed for TGFβI gene 555 site, specific wild-type Taqman for wild-type site R555 Fluorescent probes, and at least one of the two specific mutant Taqman fluorescent probes for mutant sites W555 and Q555, each probe is connected to a different fluorescent reporter group, so as to separate monitoring, and does not require the addition of internal references. The present invention also provides a detection method for human TGFβI gene 555 site variation based on the above-mentioned primer probe, which has the advantages of high sensitivity, strong specificity, non-pollution, good accuracy, convenience and quickness, and is suitable for application in clinical case analysis and testing at work.
Owner:奥斯汀生命科学技术公司
Kit, method and application for detecting gene polymorphism based on shared primer probe
ActiveCN110628883BIncrease the Tm valueSimple designMicrobiological testing/measurementDNA/RNA fragmentationMedicineGenetics
The present application discloses a method, kit and application for detecting gene polymorphism based on shared primer probes. In the method, the reporter group is labeled on the 5' ends of the wild-type ARMS primers and mutant ARMS primers designed in the same direction to prepare the probe-specific ARMS primers, which are used for gene polymorphism detection after screening, especially for Human CYP2C19 polymorphism detection. The method has simple design, low cost, higher detection sensitivity, stronger specificity, and more accurate and objective results.
Owner:ANNGEEN BIOTECHNOLOGY CO LTD
Kit and method for detecting gene polymorphism based on shared primer probe, and application
ActiveCN110628883AIncrease the Tm valueSimple designMicrobiological testing/measurementDNA/RNA fragmentationWild typeGene
The invention discloses a method and kit for detecting gene polymorphism based on a shared primer probe, and application. According to the method, reporter groups are marked at 5'ends of a wild-type ARMS primer and a mutant-type ARMS primer which are designed isotropically to prepare ARMS primers with the probe property, and the ARMS primers are screened and then used for polymorphism detection ofgenes, especially for polymorphism detection of human CYP2C19. The method is simple in design, low in cost, higher in detection sensitivity, higher in specificity and more accurate and more objectivein result.
Owner:ANNGEEN BIOTECHNOLOGY CO LTD
Kit and method for detecting gene polymorphism based on shared primer probes and application of method or kit
ActiveCN111363792AIncrease the Tm valueSimple designMicrobiological testing/measurementDNA/RNA fragmentationWild typePolymorphism Detection
The present application discloses a method and kit for detecting gene polymorphism based on shared primer probes and an application of the method or the kit. According to the method, reporter groups are labeled at the 5' ends of a wild-type ARMS primer and a mutant ARMS primer codirectionally designed, and ARMS primers with probe performance are prepared and used for gene polymorphism detection after being screened, especially for polymorphism detection of human CYP2C19. The method is simple in design, low in cost, and has higher detection sensitivity, stronger specificity, and more accurate and objective results.
Owner:ANNGEEN BIOTECHNOLOGY CO LTD
PCR primers, PCR methods and kits for detecting Staphylococcus cocci
ActiveCN110373486BStrong specificityHigh sensitivityMicrobiological testing/measurementMicroorganism based processesPathogenic microorganismStaphylococcus cohnii
Owner:SUZHOU XISHAN BIOLOGICAL TECH
Primer group, method and kit for detecting SLCO1B1 and APOE gene polymorphism based on shared primer probe
ActiveCN110863039AIncrease the Tm valueSimple designMicrobiological testing/measurementDNA/RNA fragmentationSLCO1B1Wild type
The invention discloses a primer group, a method and a kit for detecting SLCO1B1 and APOE gene polymorphism based on a shared primer probe. According to the method, reporter groups are marked at 5'ends of wild ARMS primers and mutant ARMS primers which are designed in the same direction to prepare ARMS primers with probes, and the ARMS primers are used for polymorphism detection of genes after being screened. The method is higher in detection sensitivity, stronger in specificity, more accurate and objective in result, simple in design and low in cost.
Owner:ANNGEEN BIOTECHNOLOGY CO LTD
Malignant hyperpyrexia multiplex PCR-LDR molecular diagnosis kit and application thereof
PendingCN111961720AImprove throughputDetection speedMicrobiological testing/measurementDNA/RNA fragmentationMultiplexNucleotide
The invention discloses a malignant hyperpyrexia multiplex PCR-LDR molecular diagnosis kit. The malignant hyperpyrexia multiplex PCR-LDR molecular diagnosis kit comprises 18 pairs of specific primers(the nucleotide sequences are shown as SEQ ID NO. 1-36), one group of universal fluorescent probes and 49 groups of detection probes (the nucleotide sequences are shown as SEQ ID NO. 37-179). The kitalso comprises a PCR amplification reagent, an LDR ligation reagent, a genotyping reagent and quality control products, wherein the quality control products comprise a negative quality control product, a wild type quality control product and a positive quality control product. The kit can simultaneously detect 49 gene polymorphism sites, and can be used for genotyping, DNA polymorphism detection,malignant hyperpyrexia (MH) molecular diagnosis and the like. By the kit, the problems of false positive and false negative of results are solved due to directness of sequencing results. The kit hasthe advantages of high flux, high sensitivity, simplicity in operation, short flow, simpler result analysis, cost saving, low cost and the like.
Owner:诚谨医学检验所(山东)有限公司
Paired cDNA arrays for decidua and villi
ActiveCN107190328BRealize functional presentationImprove throughputMicrobiological testing/measurementLibrary creationClinical informationMedicine
The invention relates to a decidua and villus pairing cDNA chip. The cDNA chip is prepared through the following method that the decidua and villus tissue of a woman with early pregnancy abortion are taken, the RNA is extracted, cDNA is formed by reverse transcription and preset in a PCR reaction plate, and cDNA chip is made. A preparation method and application of cDNA chip are further provided. The method and application have the advantages that the decidua and villus tissue of every abortion woman are respectively taken, pairing is formed by cDNA, the cDNA pairs are concentrated on the same chip, and the function presentation of parent-fetal interface of the physiological structure is achieved on the chip outside the body. The decidua and villus pairing cDNA chip has the advantages of being high in throughput, strong in specificity, high in sensitivity, simple and fast in operation, high in quantitative accuracy, strong in result reliability, simple in result analysis, complete in clinical information for samples and the like, and the cDNA chip can be used for quantitative detection of the expression statuses of a target gene in different patients and for conducting in-depth correlation analyses of clinical information.
Owner:夷希微医学科技(上海)有限公司
Kit for detecting human ABCC4 gene polymorphism and application thereof
InactiveCN105695565AImprove detection accuracyAnalysis of results is simpleMicrobiological testing/measurementFluorescenceBiology
The invention relates to a kit for detecting polymorphism of a human ABCC4 gene and an application thereof. Substances in the kit comprise a specific primer and a specific fluorescent probe for detecting an rs868853 SNP locus on the ABCC4 gene, Taq DNA polymerase, a dNTP mixed liquid, an MgCl2 solution, a fluorescence quantitative PCR reaction buffer solution and deionized water. The defects of various conventional genetic mutation detection methods are overcome. The kit has the advantages of high detection accuracy, simple and convenient detection, short detection time, and simple result analysis, is suitable for use in clinical laboratories, can perform qualitative detection on a CYP2C19 gene polymorphism locus, performs PCR fluorescent amplification reaction according to the SNP locus, can judge a result just according to a condition whether two different fluorescence curves are started or no, cannot produce artificial errors, has low false positive and false negative rates, improves the detection accuracy, and reduces detection costs.
Owner:南京仁天生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com