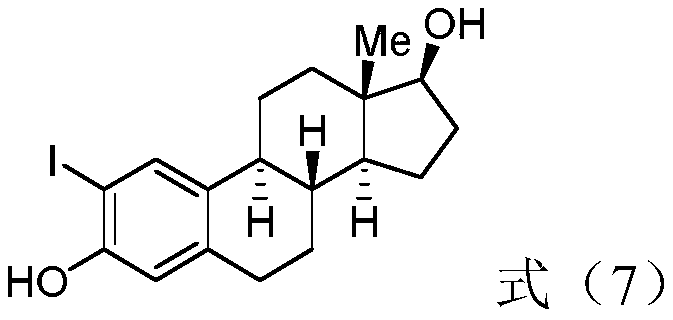
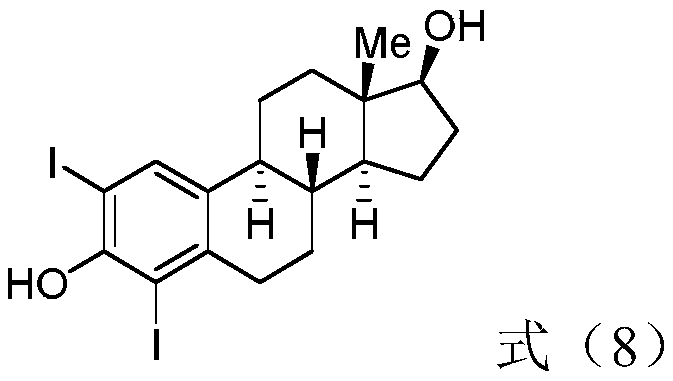
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
40 results about "Therapeutic Estradiol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
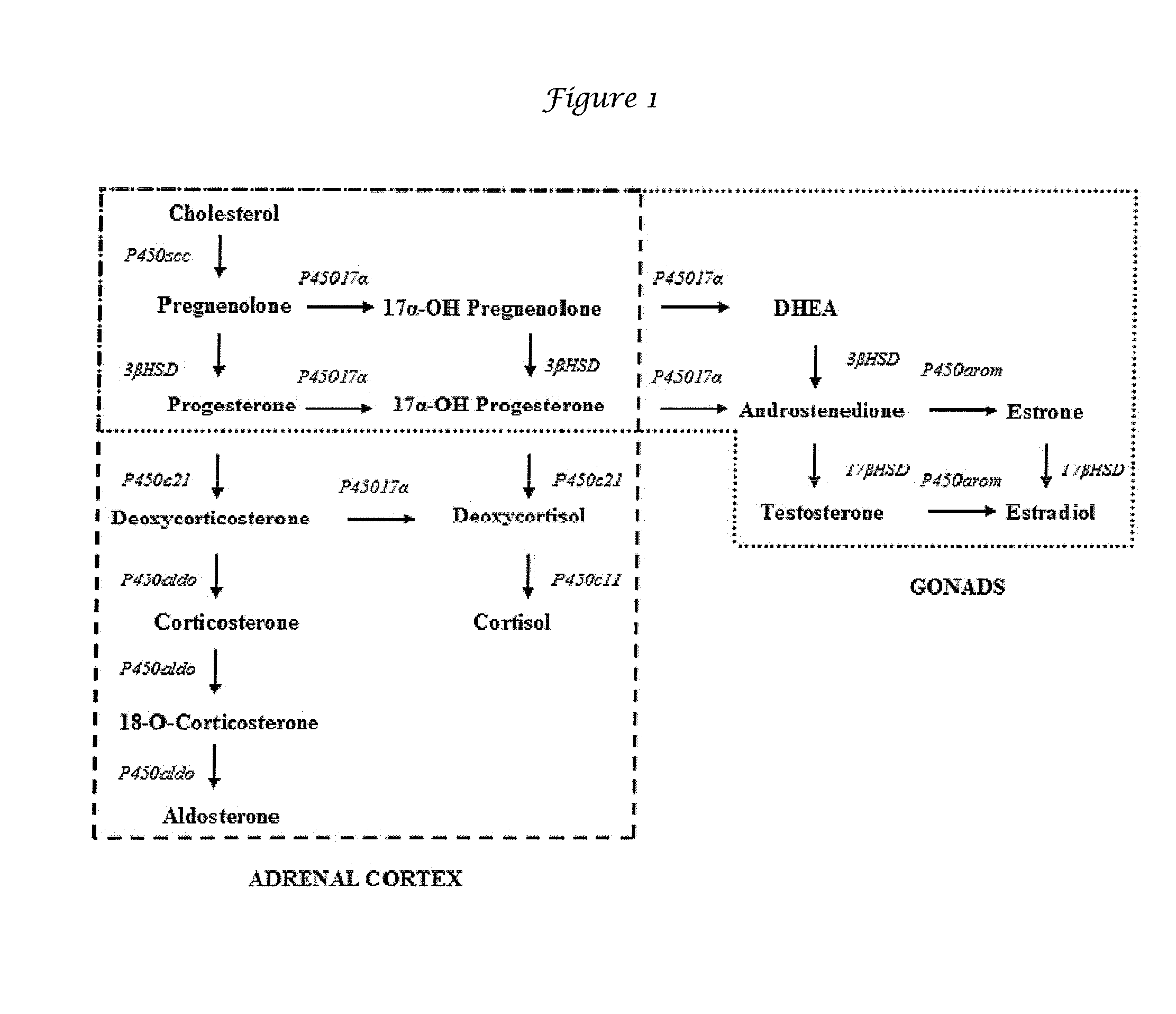
A steroid sex hormone vital to the maintenance of fertility and secondary sexual characteristics in females. Typically esterified, estradiol derivatives are formulated for oral or parenteral administration. As the primary, most potent estrogen hormone produced by the ovaries, estradiol binds to and activates specific nuclear receptors. Estradiol exhibits mild anabolic and metabolic properties, and increases blood coagulability.
Hormone replacement formulation
The formulation comprises a combination of three estrogens and selected amount of other elements. The three estrogens include 2-hydroxyestrone, 17-beta estradiol, and estriol. The amount of 17-beta estradiol is substantially less than the amounts of 2-hydroxyestrone and estriol, both which are approximately equal in amount. The amounts of pyridoxine, folic acid, selenium and cobalt are therapeutically effective amounts.
Owner:WRIGHT JONATHAN V
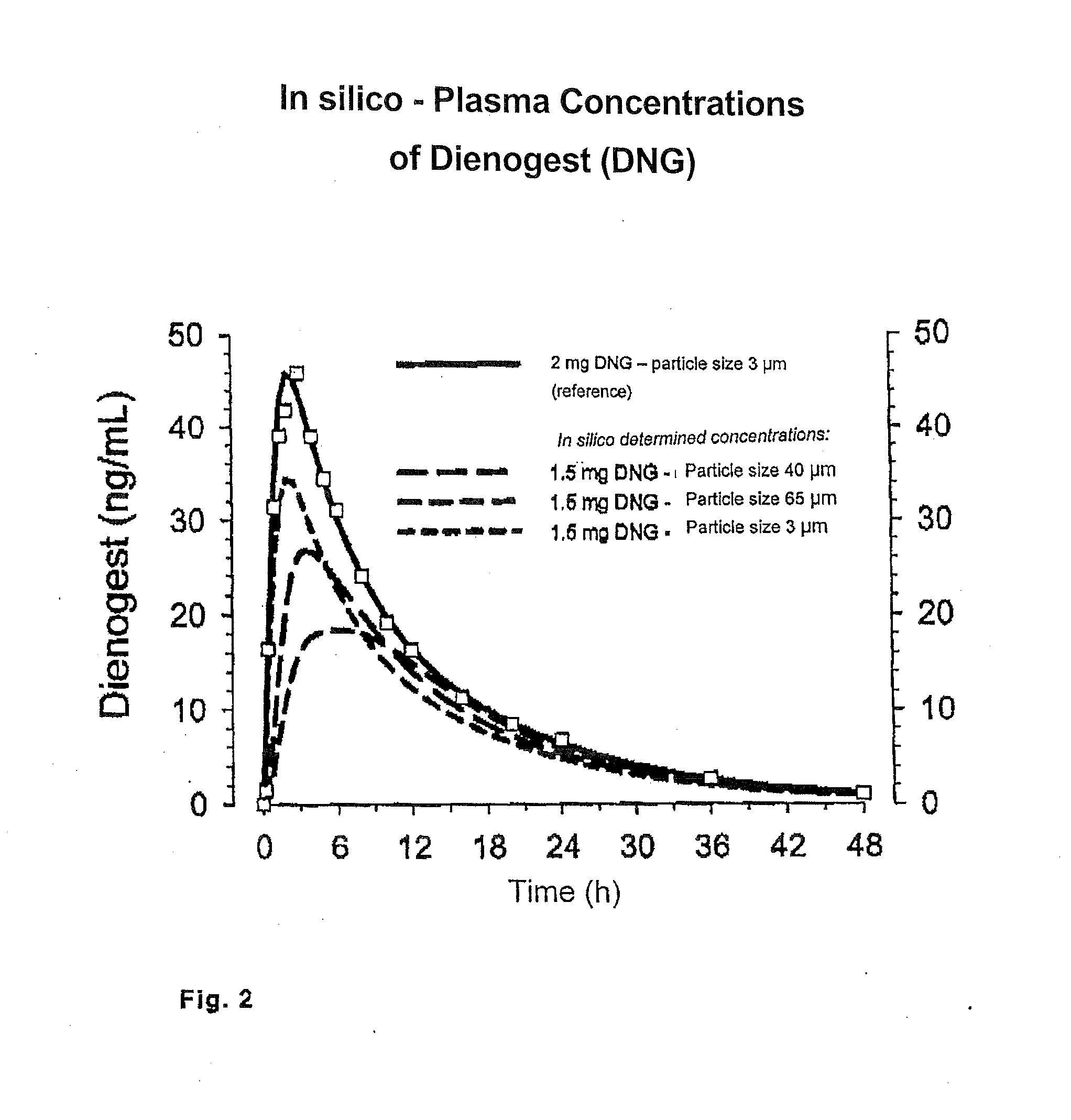
Low-dosage peroral medication for contraception containing crystalline dienogest and ethinyl estradiol
InactiveUS20080038350A1Reduce contentReduce loadBiocideOrganic active ingredientsEthinylestradiolBULK ACTIVE INGREDIENT
The peroral medication for prevention of conception contains as one active ingredient crystalline 17α-cyanomethyl-17β-hydroxyestra-4,9-dien-3-one (dienogest) at a daily dosage equal to or less than 2.0 mg and as another active ingredient 17α-ethinyl estradiol at a daily dosage of less than 0.030 mg, together with one or more pharmaceutically acceptable carriers. The active ingredient dienogest is contained in the medication in crystalline form with an average particle size of preferably 25 to 70 μm. The other active ingredient ethinyl estradiol is incorporated during granulation in micronized form or by spraying an ethanolic solution containing it.
Owner:BAYER SCHERING PHARMA AG
Natural combination hormone replacement formulations and therapies
Owner:THERAPEUTICSMD
Methods of extended use oral contraception
A method of female contraception involves administering a combination of estrogen and progestin for 60-110 consecutive days in which the daily amounts of estrogen and progestin are equivalent to about 5-35 mcg of ethinyl estradiol and about 0.025 to 10 mg of norethindrone acetate, respectively. The advantages include less menstrual bleeding, less patient anemia, less total exposure to medication, higher compliance rates and more lifestyle convenience for patients.
Owner:TEVA WOMENS HEALTH
Low dose estrogen interrupted hormone replacement therapy
A hormone replacement therapy, comprising a plurality of daily doses of a pharmaceutical preparation, the doses being administered continuously and consecutively in alternating phases of three daily doses, a relatively dominant estrogenic activity phase comprising three daily doses of a substance exhibiting estrogenic activity equivalent to about 1 mg per day of 17beta-estradiol per day, and a relatively dominant progestagenic activity phase of a combination of a substance exhibiting estrogenic activity equivalent to about 1 mg per day of 17beta-estradiol and a substance exhibiting progestogenic activity equivalent to about 90 mug per day of norgestimate.
Owner:DURAMED PHARMA +1
Oral contraceptive regimen
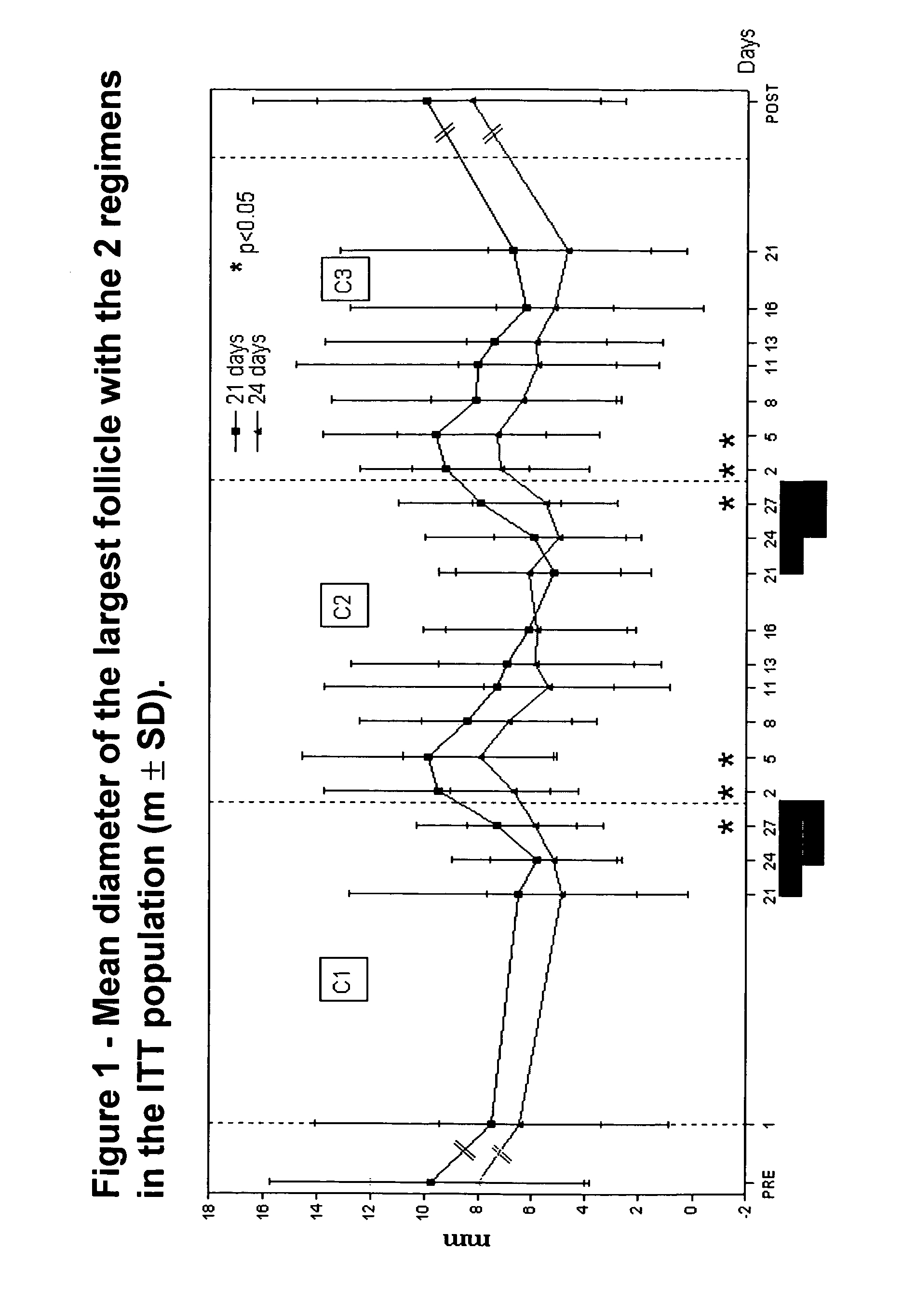
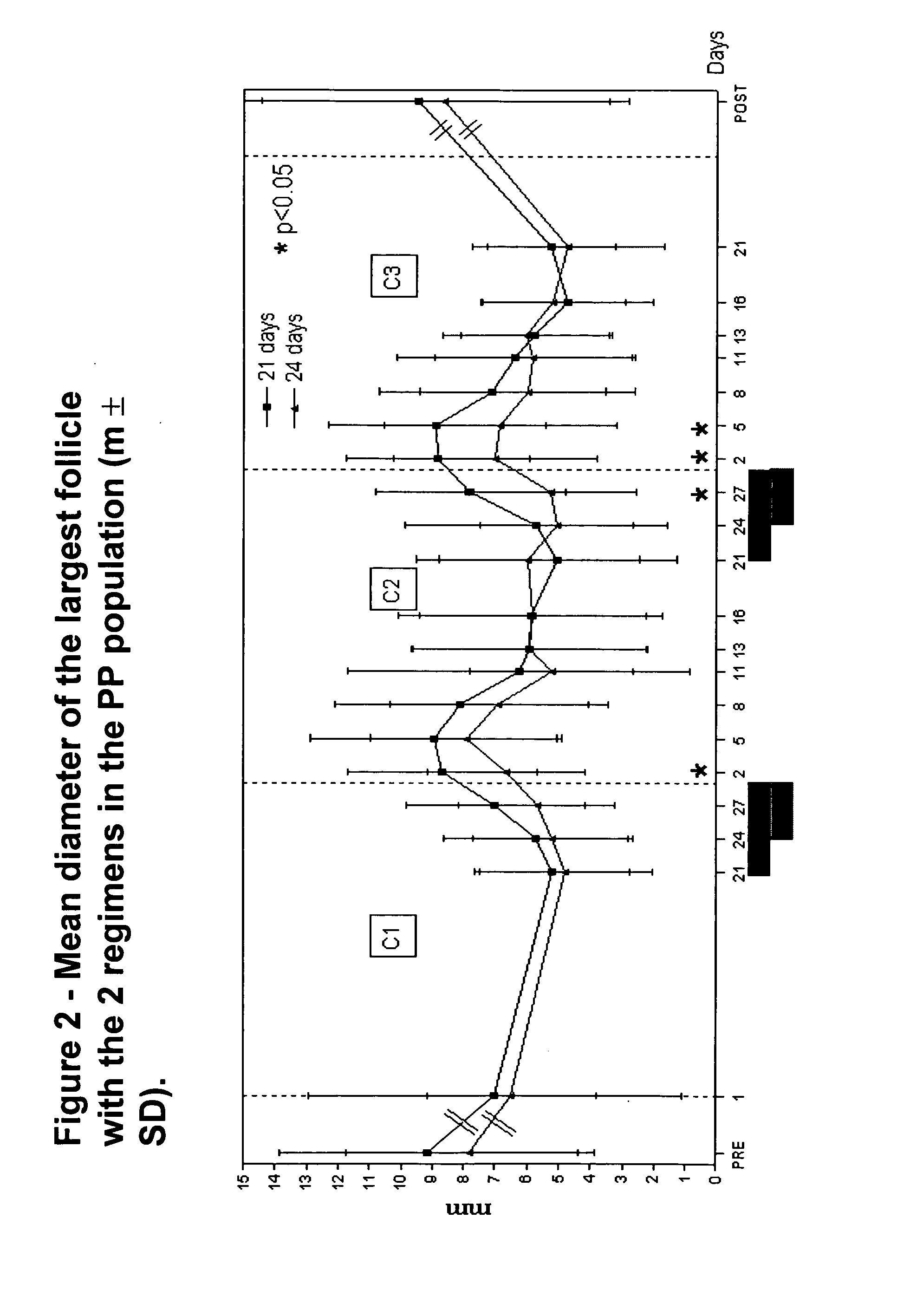
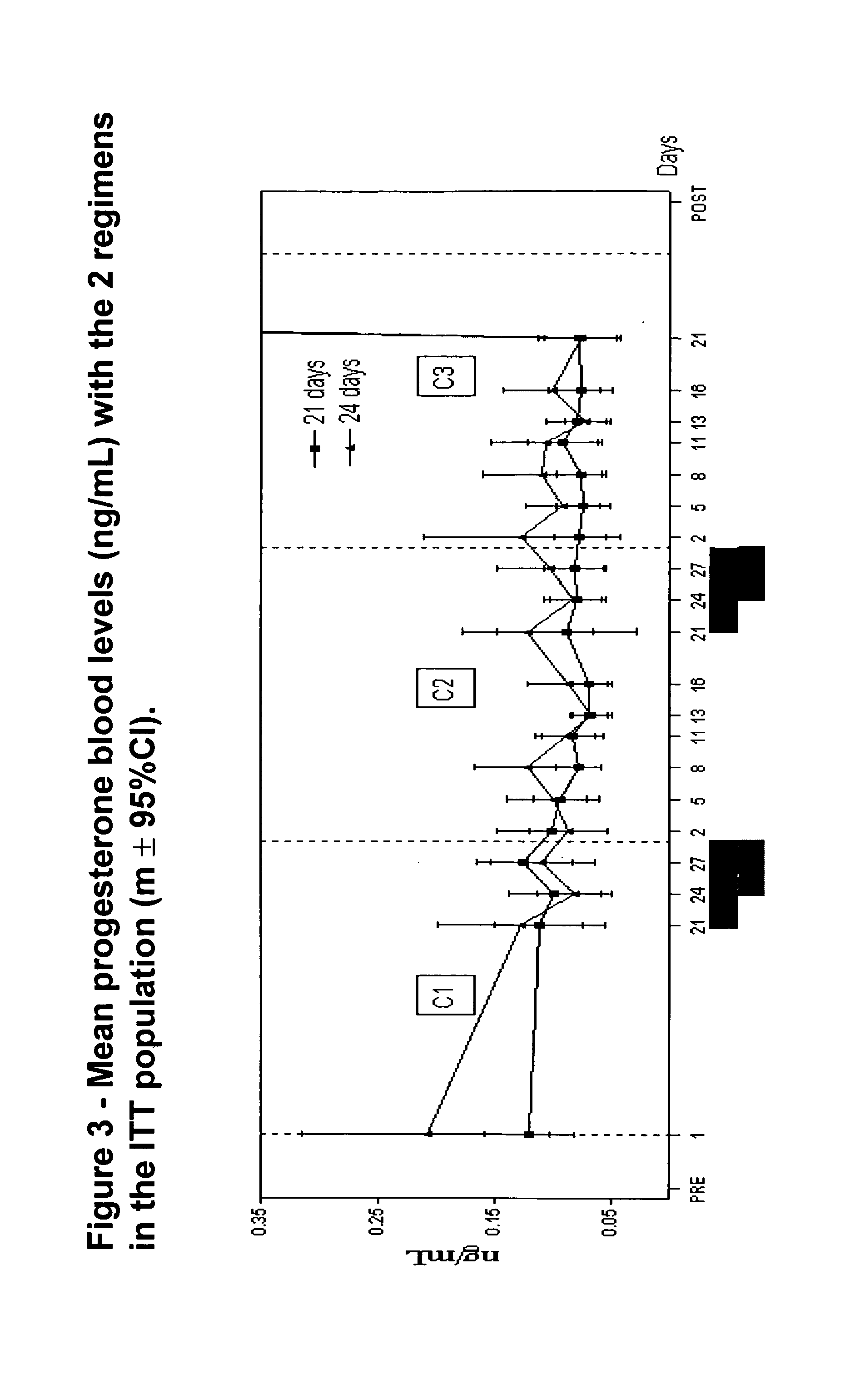
A monophasic method of achieving contraception in a human female comprising orally administering to the human female a composition comprising 1.5 mg of 17-beta-estradiol and 2.5 mg of nomegestrol acetate for 24 days followed by a hormone-free period of 4 days.
Owner:LAB THERAMEX SA
Estrogenic components for use in the treatment of neurological disorders
ActiveUS9238035B2Reduce brain damageGood treatment effectOrganic active ingredientsNervous disorderDiseaseNervous system
The invention relates to the prophylactic and therapeutic applications of certain estrogenic components, such as estetrol, in neurological disorders, such as neonatal hypoxic-ischemic encephalopathy (HIE).
Owner:NEURALIS SA
Cyclodextrin-drospirenone inclusion complexes
InactiveUS6958326B2Improve stabilityShelf-life of an estrogen-containing product is improvedBiocideSugar derivativesDrospirenoneEthinylestradiol
Pharmaceutical compositions comprising low doses of sensitive complexes between an estrogen and a cyclodextrin are provided with improved stability. In specific embodiments the composition comprises a complex between ethinyl estradiol and β-cyclodextrin in a granulate preparation and in yet another embodiment the composition comprises a limited amount of polyvinylpyrrolidone since this excipient was found to degrade ethinyl estradiol. Furthermore, a method for improving the stability of an estrogen in a composition and for manufacturing such a stable composition is provided. Essentially, the granulate preparation are manufactured under careful control of the relative humidity.
Owner:BAYER INTELLECTUAL PROPERTY GMBH
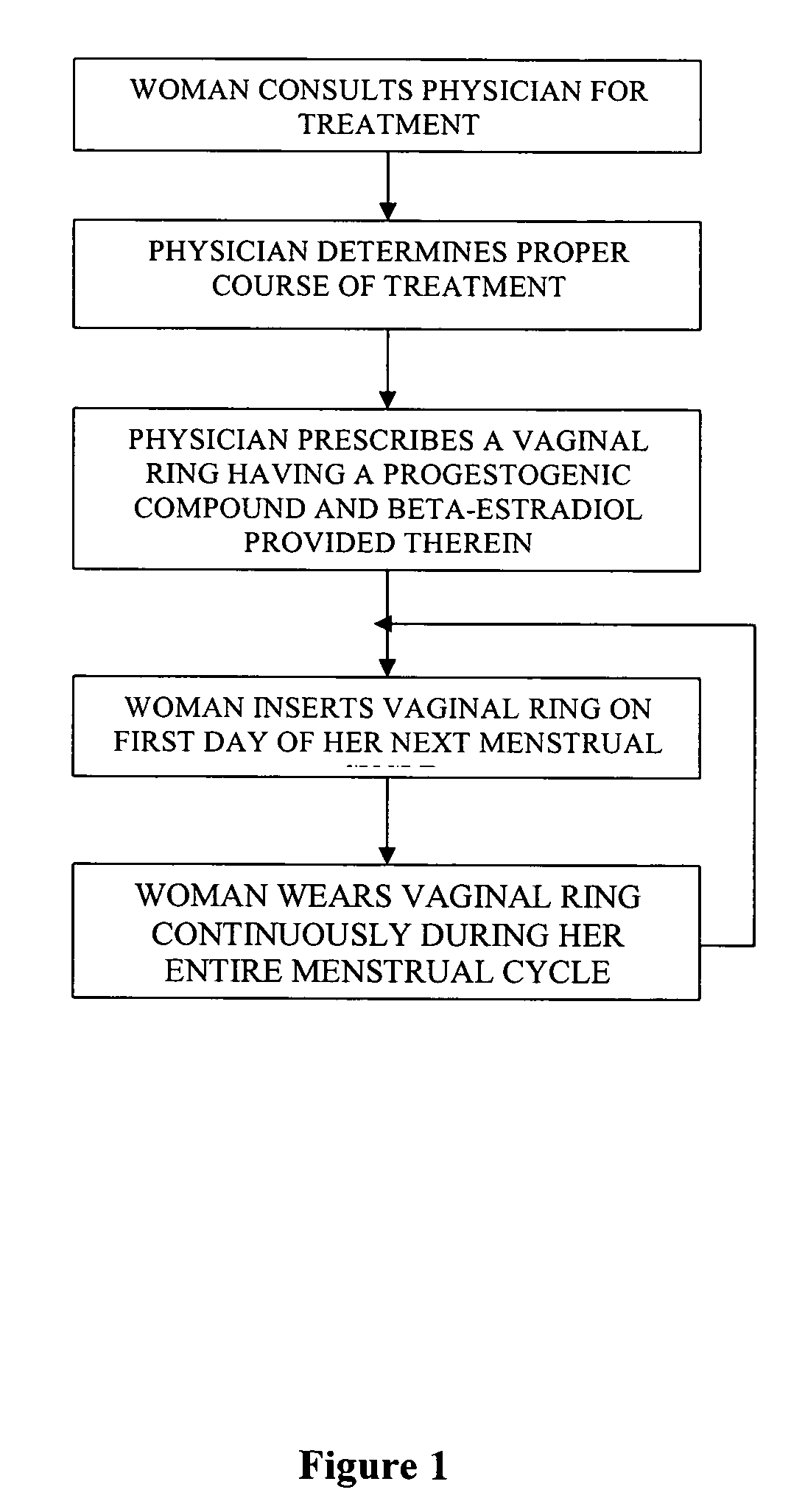
Method of birth control and hormone regulation
InactiveUS20070077269A1Avoid problemsReduce doseOrganic active ingredientsBiocideObstetricsSexual hormones
A method of birth control and hormone regulation comprises utilizing a vaginal ring worn continuously during each menstrual cycle. A vaginal ring delivers predetermined doses of progesterone and beta-estradiol. Beta-estradiol does not cause an increase in sex hormone binding globulin and thereby does not decrease a woman's levels of free testosterone and libido.
Owner:LES MEDECINS
Application of human mature follicle fluid in external mature technology of immature ovum
InactiveCN1385523AConvenient sourceSignificant clinical effectMedical devicesGerm cellsGestationCulture fluid
The present invention belongs to the field of auxiliary reproduction technology. Said invention described culture solution containing human mature follicular fluid is used for external culture technique of human immature ovum, and its composition is formed from Ham'e F-10 or TCM199, human serum substitute SSS, rFSH, HCG, 17 beta-estradiol and human mature follicular fluid. The in-vitro mature (IVM) technique of immature ovum can be used to take out the immature ovum of sterility woman with ovum maturation disturbance, and the culture solution prepared by said invention is used to culture it, and make it mature and make in-vitro fertilization and embryo transplantation to help gestation. It can obtain 65% of ovum mature rate and 33.3% of gestatino rate.
Owner:JIANGSU PROVINCE HOSPITAL
Chemiluminescence quantitative detection kit for estradiol
InactiveCN102095880AQuick checkEasy to detectChemiluminescene/bioluminescenceBiological testingDiseaseHypoplasia
The invention discloses a chemiluminescence quantitative detection kit for estradiol. The kit comprises an estradiol detection reaction plate, an enzyme conjugate, a luminescent substrate, a calibrator, a quality control material and washing concentrate, wherein the reaction plate is coated with an estradiol antibody. The kit can specifically and quantitatively detect the content of the estradiol in patient serum, and is used for the auxiliary diagnosis of diseases such as ovarian hypoplasia, primary ovarian failure, pituitary amenorrhea or infertility, Cushing's syndrome, Addison's disease, malignant tumors, focal lesion of brain and hypophysis, and the like. Compared with the conventional enzyme linked immunosorbent assay (ELISA) technology, the chemiluminescence immunoassay keeps high specificity of the ELISA technology, stability and reliability of a detection result, and convenience of operation, and can improve detection sensitivity simultaneously.
Owner:上海裕隆生物科技有限公司
Detection of infertility risk and premature ovarian aging
ActiveUS20140206756A1Microbiological testing/measurementGenetic material ingredientsRegimenHuman Females
Method of early detection of risk of infertility and ovarian aging in and treatment of a human female who has not experienced infertility and is not otherwise indicated to have premature ovarian aging. A number of CGG repeats on each allele of the isolated FMR1 gene is measured by using an assay, and a testing regimen is performed only when the determined number of CGG repeats on one of the FMR1 gene alleles is less than 26. The testing regimen includes periodically measuring serum level of a hormone related to fertility, such as Anti-Müllerian Hormone, Follicle Stimulating Hormone and / or estradiol over a period of about three to eight years and, after each measurement, determining if the measured serum level is less than a set confidence interval for a human female of the same age of the female. If so, the human female is treated for premature ovarian aging.
Owner:THE FOUND FOR REPRODUCTIVE MEDICINE INC
Low dose estrogen interrupted hormone replacement therapy
A hormone replacement therapy, comprising a plurality of daily doses of a pharmaceutical preparation, the doses being administered continuously and consecutively in alternating phases of three daily doses, a relatively dominant estrogenic activity phase comprising three daily doses of a substance exhibiting estrogenic activity equivalent to about 1 mg per day of 17beta-estradiol per day, and a relatively dominant progestagenic activity phase of a combination of a substance exhibiting estrogenic activity equivalent to about 1 mg per day of 17beta-estradiol and a substance exhibiting progestogenic activity equivalent to about 90 mug per day of norgestimate.
Owner:DURAMED PHARMA +1
Extended estrogen dosing contraceptive regimen
A method of contraception that provides for sequentially administering to a female of child bearing age: (a) a first composition containing a progestin in an amount equivalent to about 0.3 to about 1.5 mg norethindrone acetate and an estrogen in an amount equivalent to about 5 to about 20 mcg of ethinyl estradiol for about 22 to about 26 days; (b) a second composition containing an estrogen in an amount equivalent to about 5 to about 20 mcg of ethinyl estradiol for about 2 to about 3 days and an optional third composition that is a placebo provided that (i) if estrogen administration is continuous then the first composition is administered for 25 to 26 days, the second composition is administered for 2 to 3 days and no third composition is administered and (ii) if estrogen administration is not continuous then the first composition is administered for 22 to 24 days, the second composition is administered for 2 to 3 days and the third composition is administered for 1 to 4 days. The total cycle length is 28 days, with the first composition administered on day 1 of the menstrual cycle, defined as the first day of menstrual bleeding, or on the first Sunday after the first day of the menstrual cycle.
Owner:APTALIS PHARMA
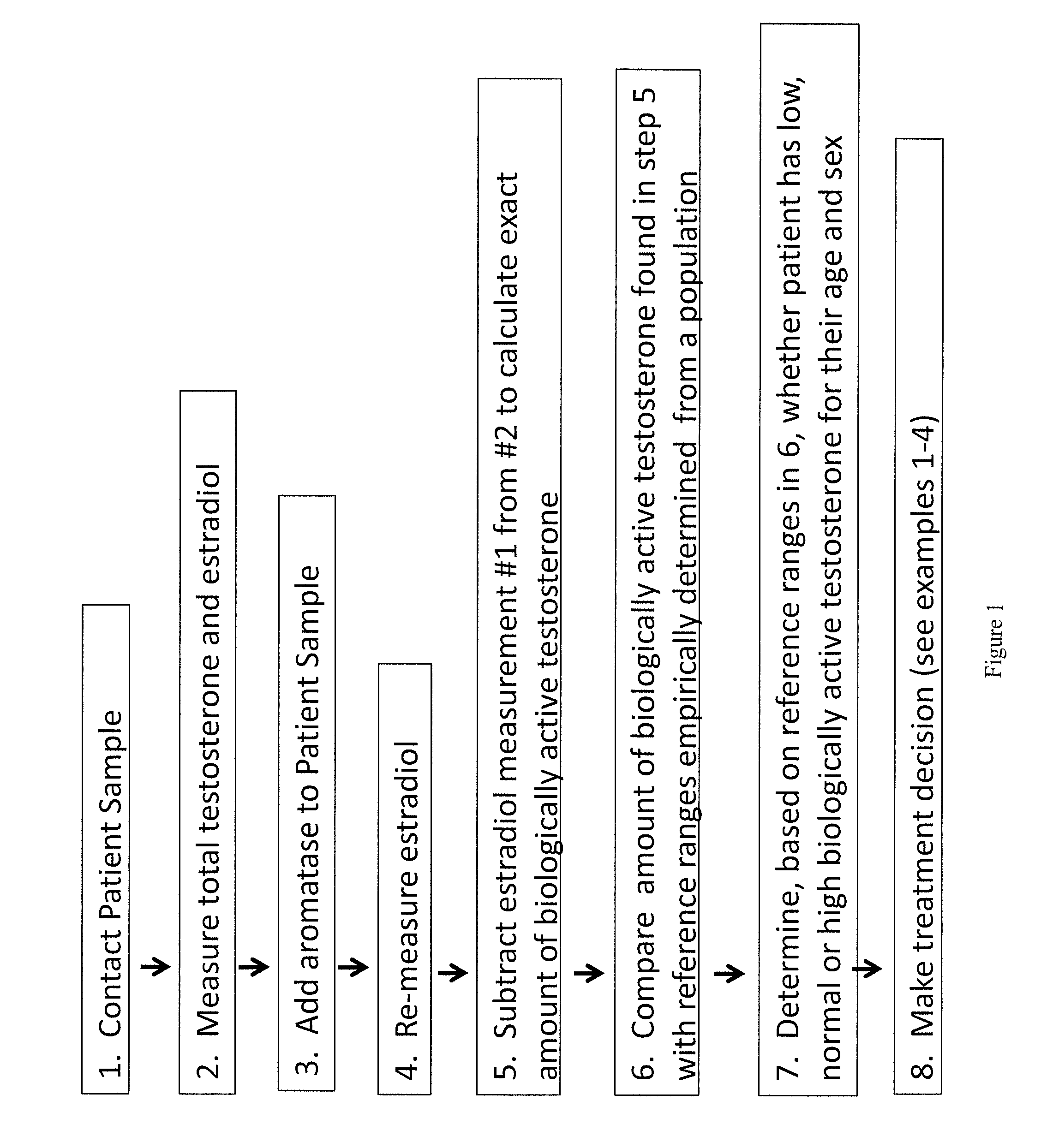
Method for measurement of bioavailable testosterone
Provided are methods for determining level of bioactive testosterone in a biological sample. In one aspect, aromatase enzyme is utilized to convert free, bio-available testosterone into estradiol and the amount of estradiol is measured before and after the addition of enzyme. The difference in measurements provides the amount of bioactive testosterone in the sample. In another aspect, a competitor of testosterone binding to SHBG is utilized to displace testosterone bound to SHBG. Measurements of total testosterone in the sample before addition of competitor and afterwards are taken, such that the delta reflects the amount of testosterone that was bound on SHBG.
Owner:NAVIGA LLC
Cationic 17 α-substituted-estradiol derivatives useful as anti-cancer agent
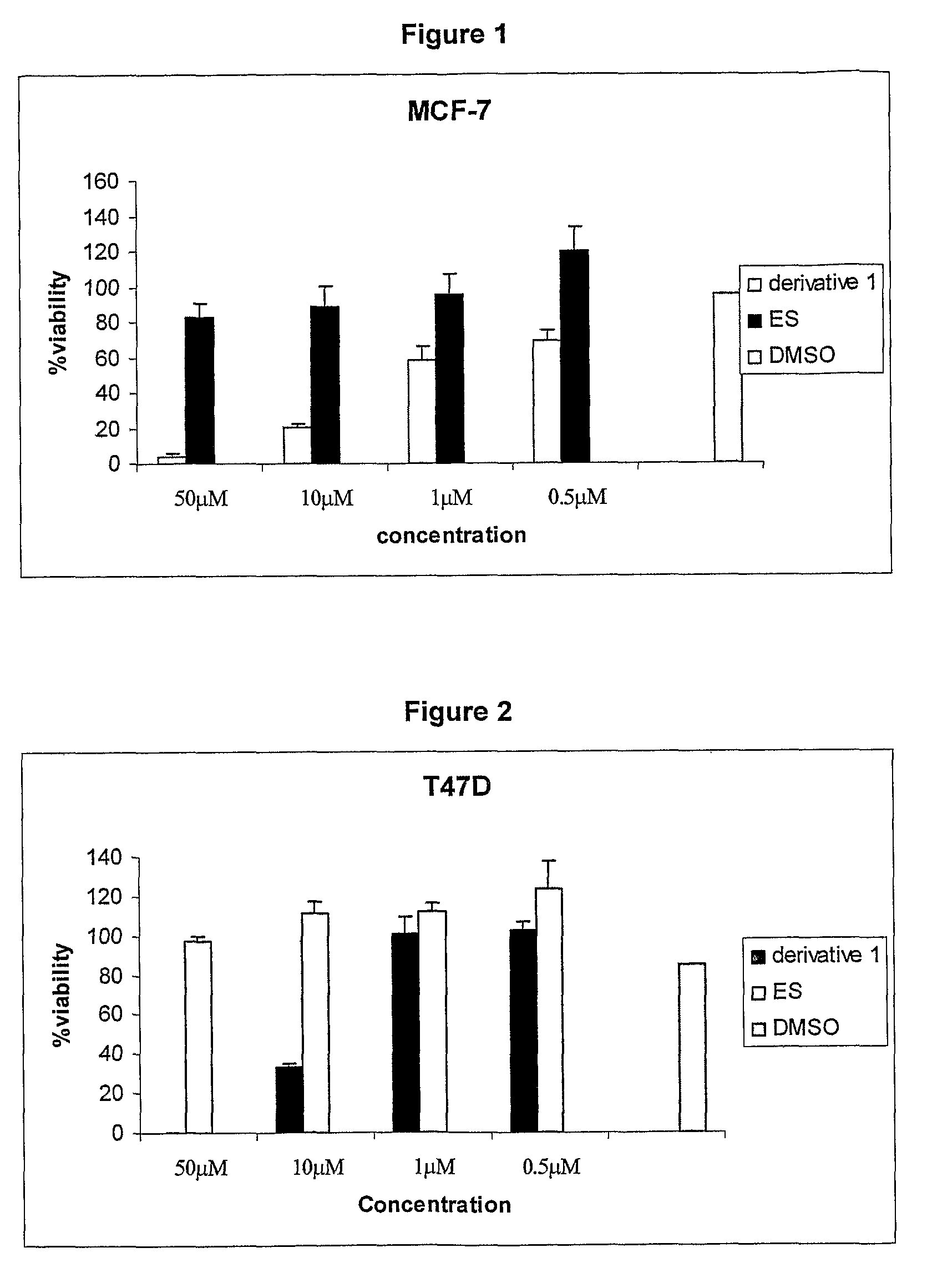
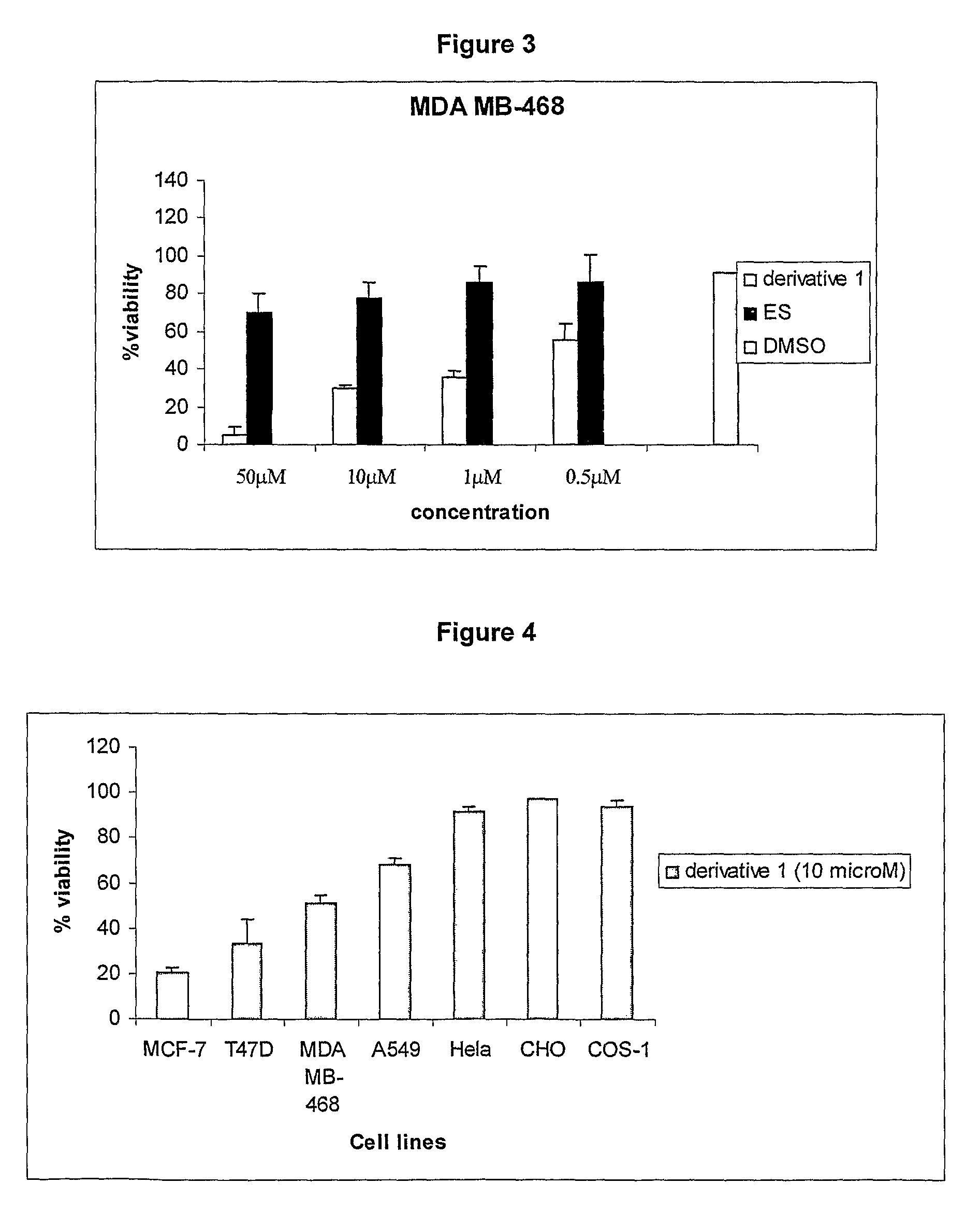
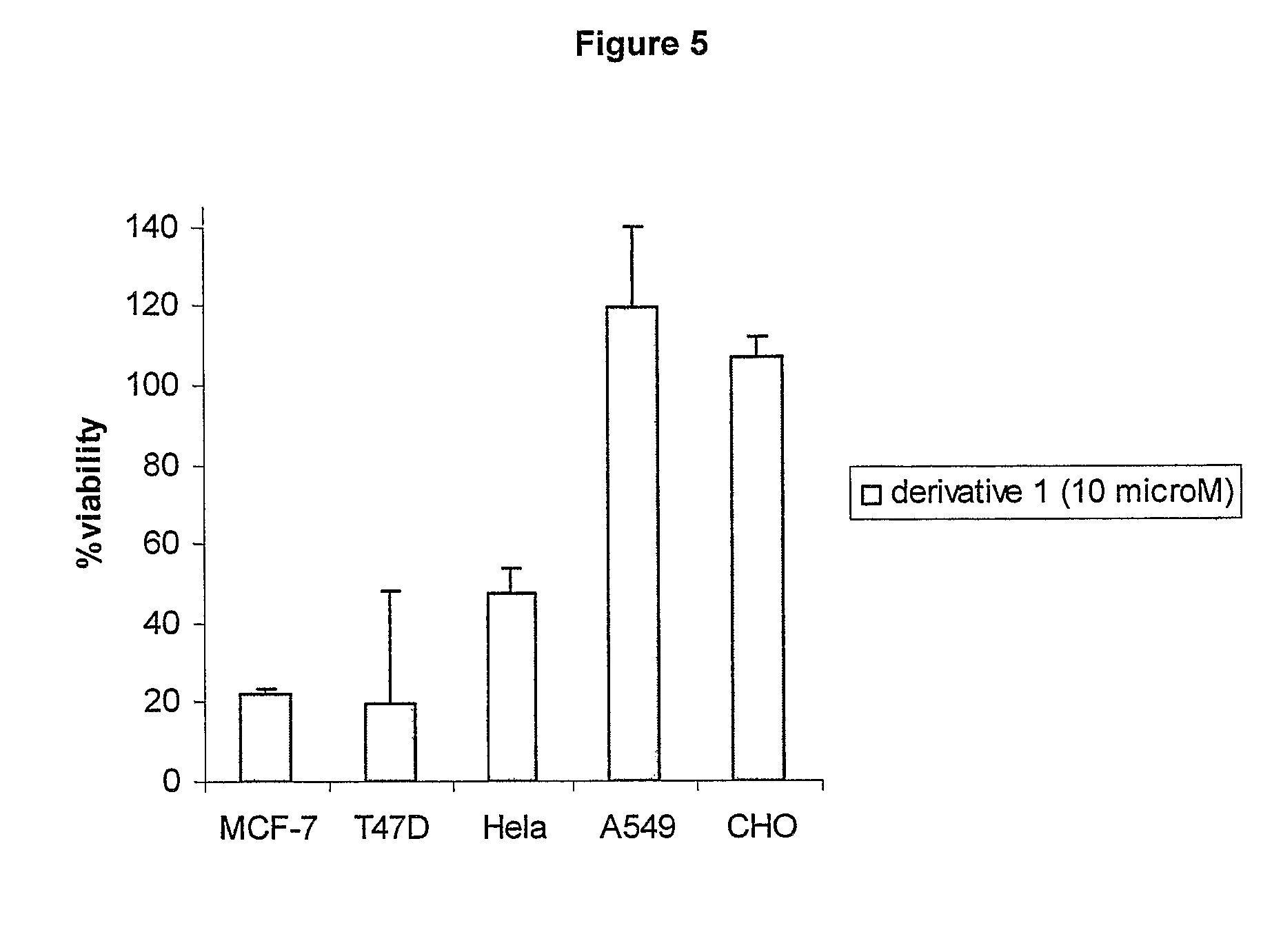
The present invention provides a novel series of cationic, lipid-based, 17α-substituted-estradiol derivatives. The present invention further provides a process for the preparation of a novel series of 17α-substituted-estradiol derivatives. The invention also provides information about highly selective anticancer activities of these molecules in estrogen responsive cell lines. The compound elicits high level of toxicity to gynecological cancer cell lines such as MCF-7, T47D (estrogen receptor positive cell lines), MDA-MB-468 (estrogen receptor knock-out cell line), HeIa (cervical cancer). The present class of cationic lipid-based, estradiol derivatives is likely to find specific use in developing target specifically deliverable anticancer drugs for the treatment of gynecological cancers that are most prevalent in women population irrespective of ethnicity.
Owner:COUNCIL OF SCI & IND RES
Composition and method for affecting male and female hormone levels
Owner:NOKOMIS RES
Aromatase inhibition to enhance assisted reproduction
The use of at least one aromatase inhibitor in the production of a medicament for improving the implantation and pregnancy rates for a female undergoing assisted reproduction treatment, which comprises one or more daily doses of an aromatase inhibitor (AI) for administration during assisted reproduction cycles or ovarian stimulation cycles, wherein the doses of AI are selected from amounts effective to reduce serum estradiol levels. Also disclosed are related pharmaceutical preparations and packages.
Owner:ARES TRADING SA
Pharmaceutical product and analysis model for hormone replacement therapy for women and prevention of some cancers and uterine myomas
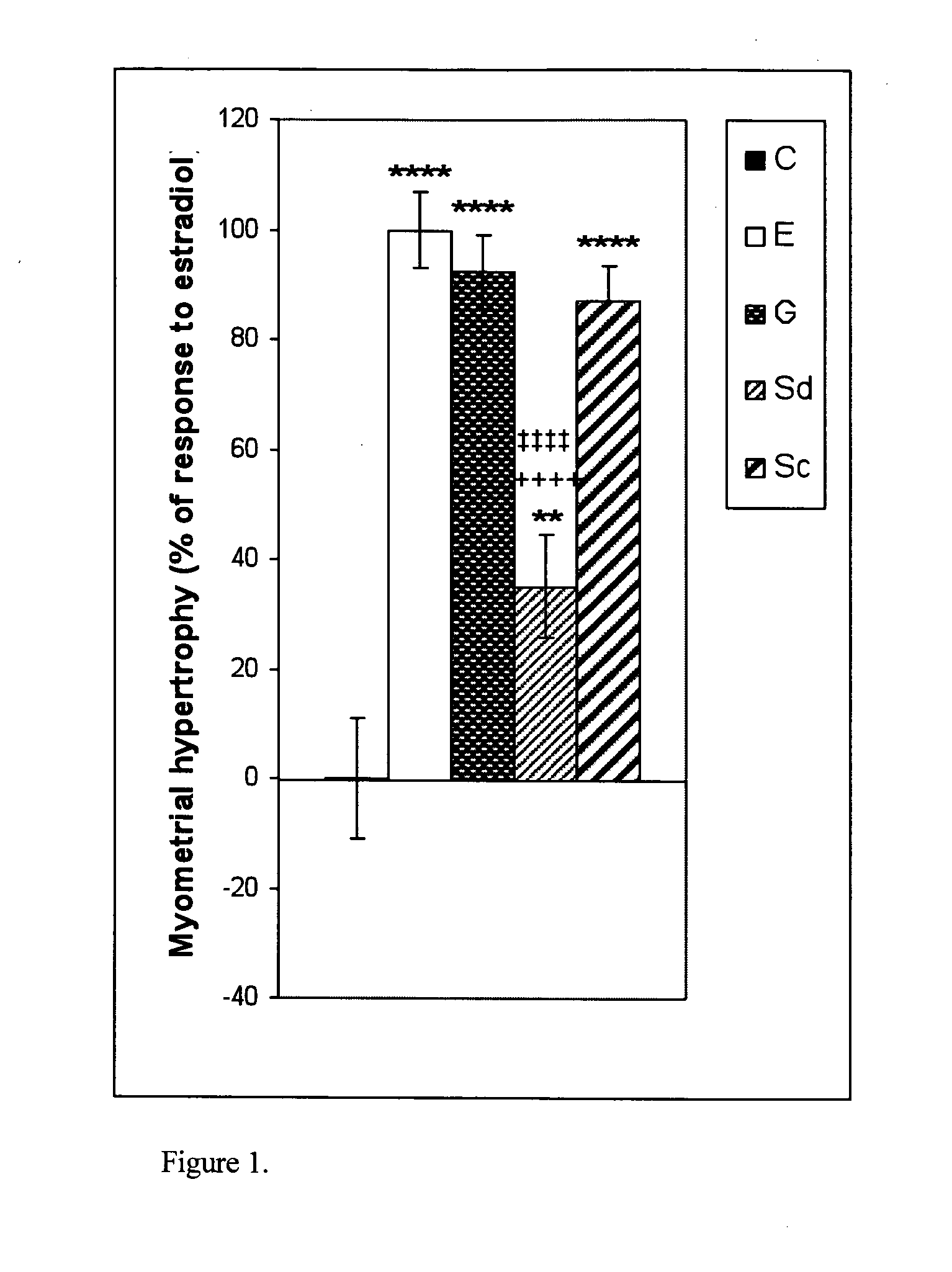
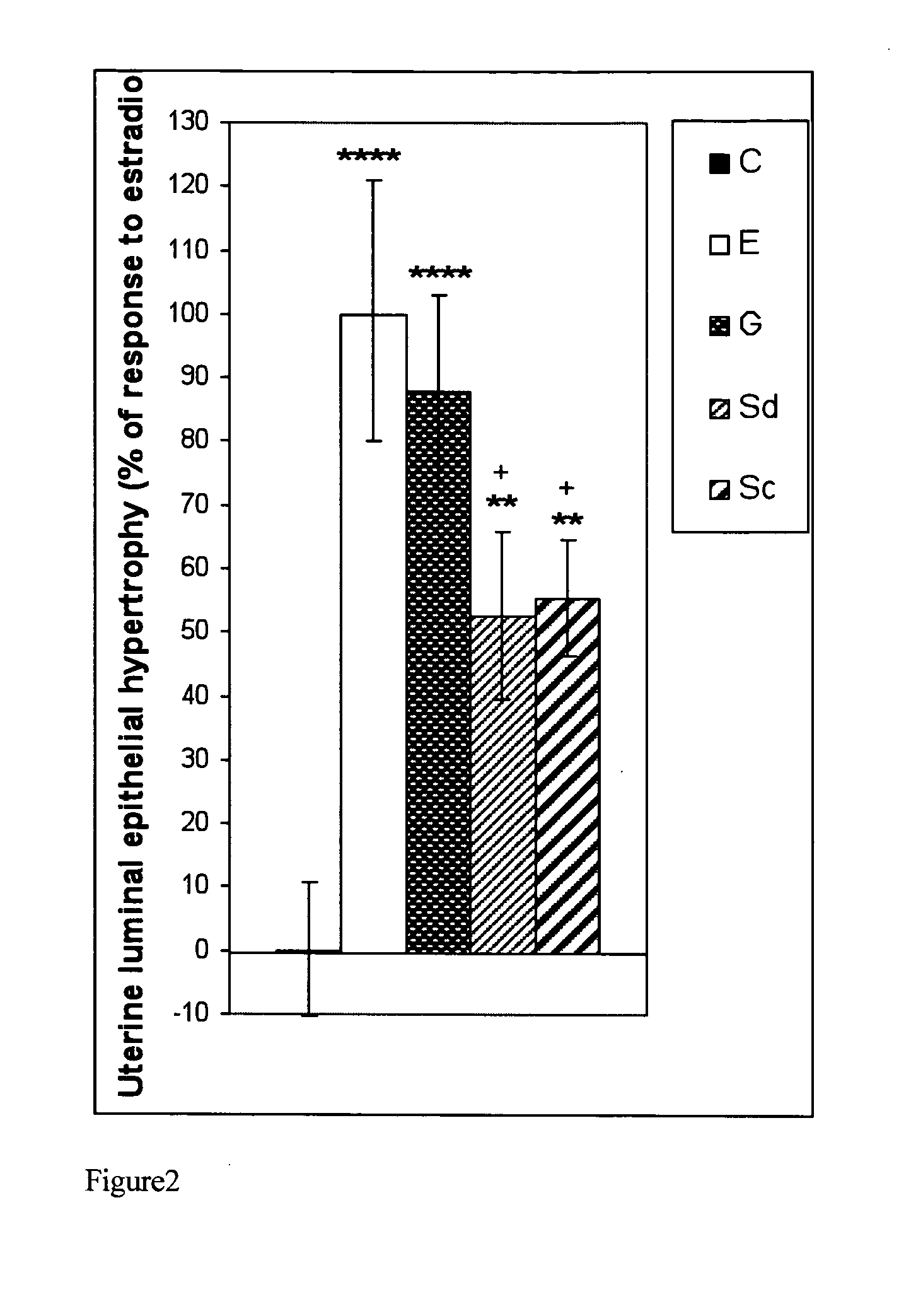
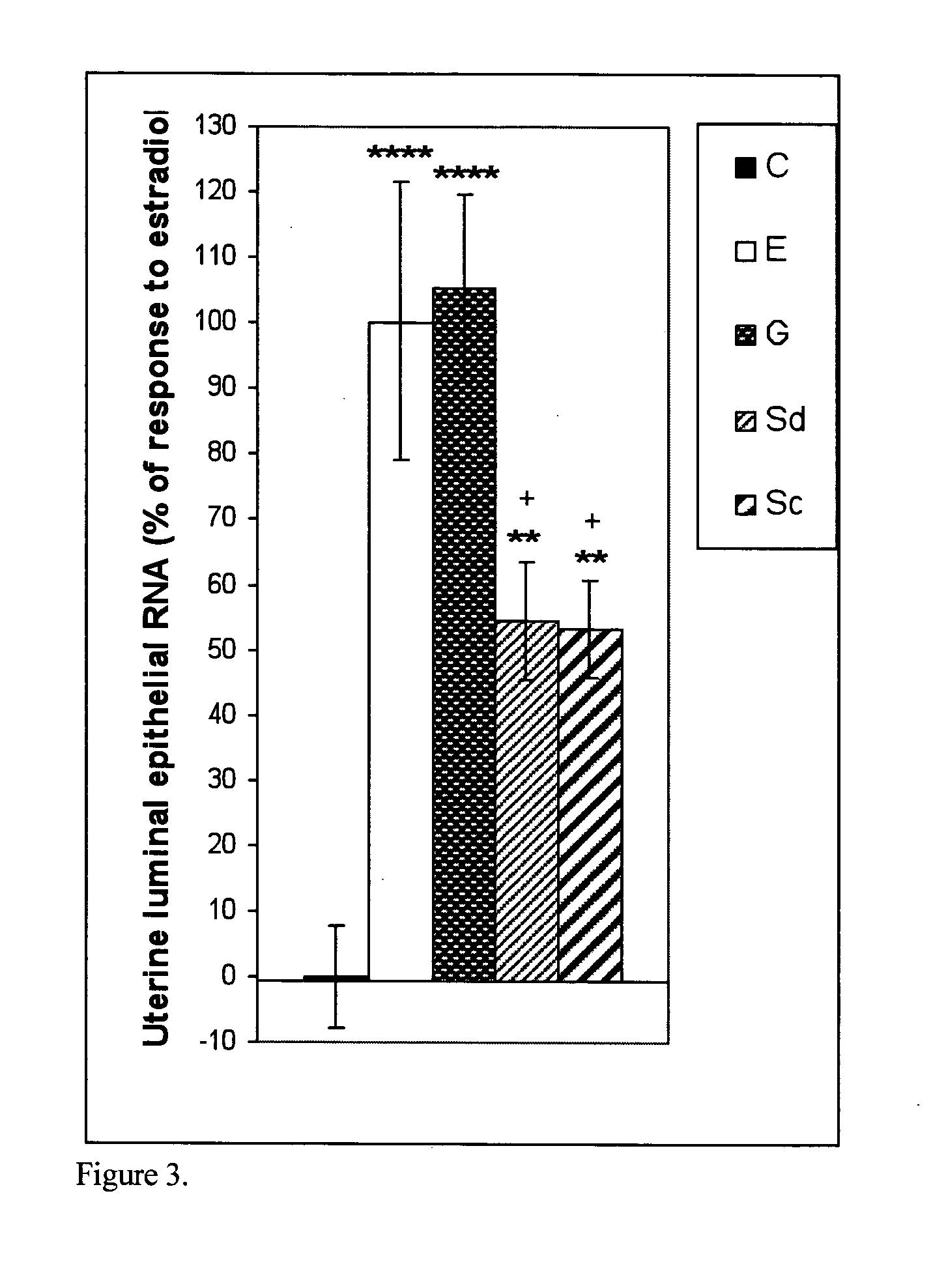
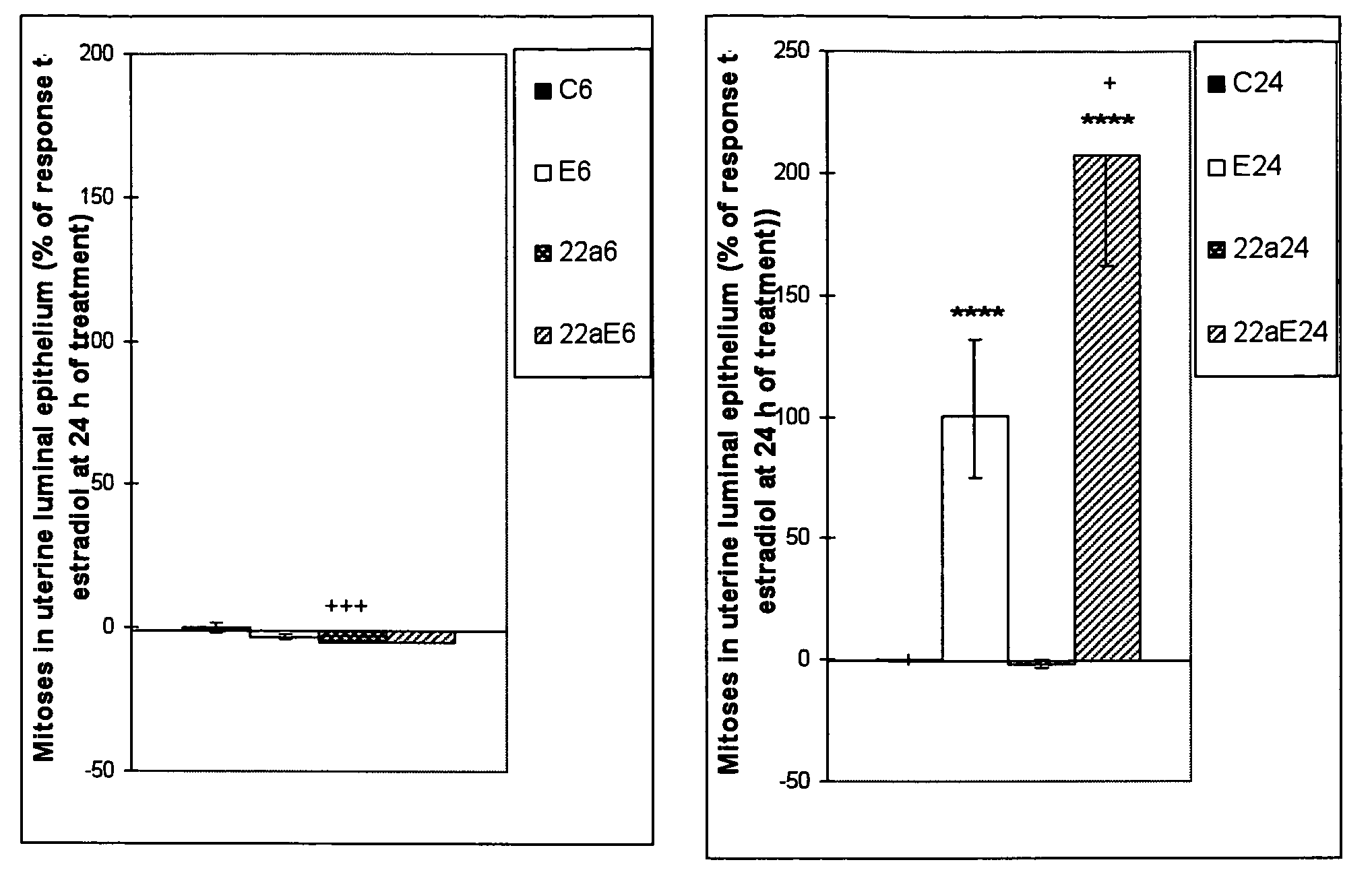
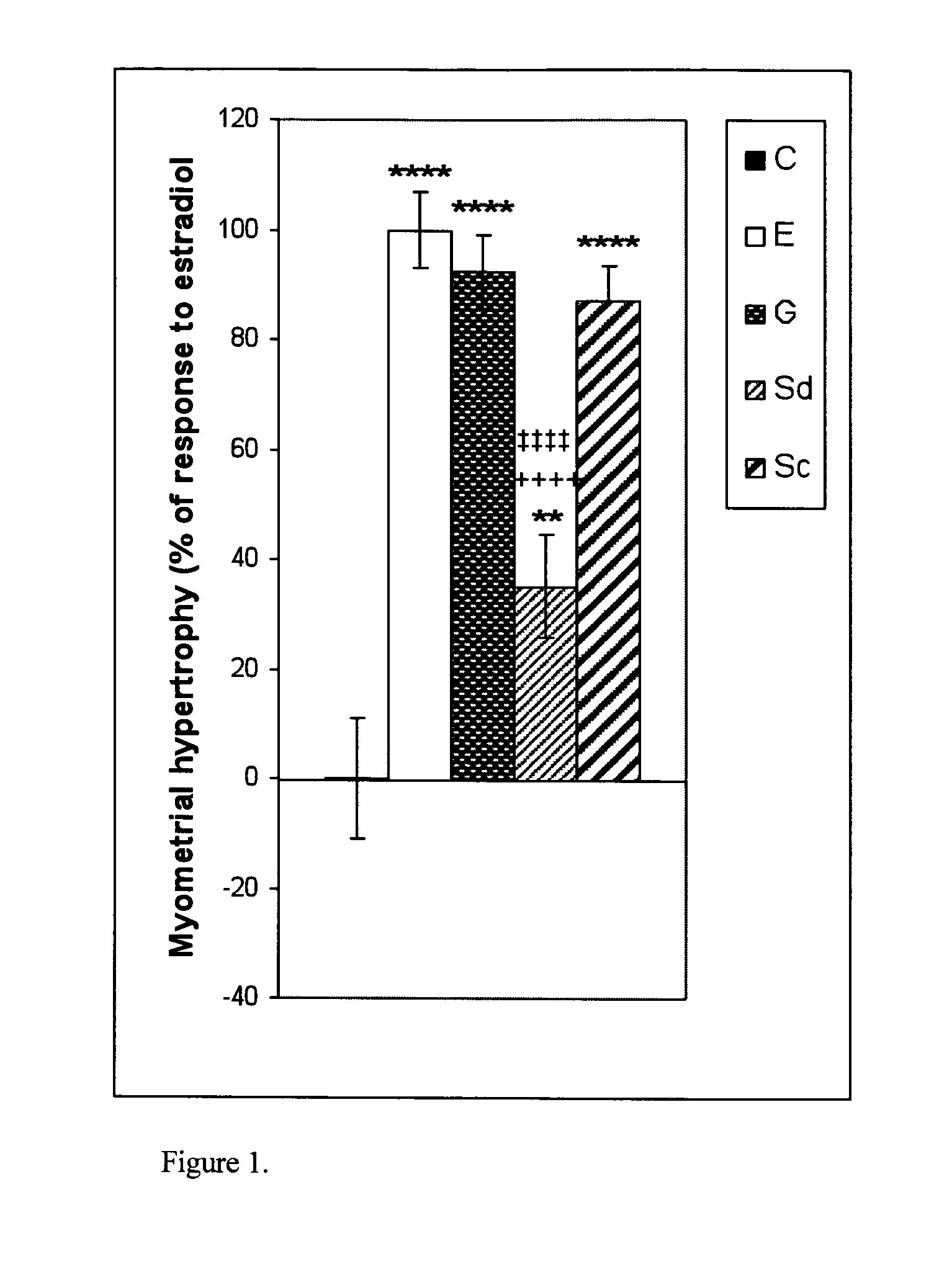
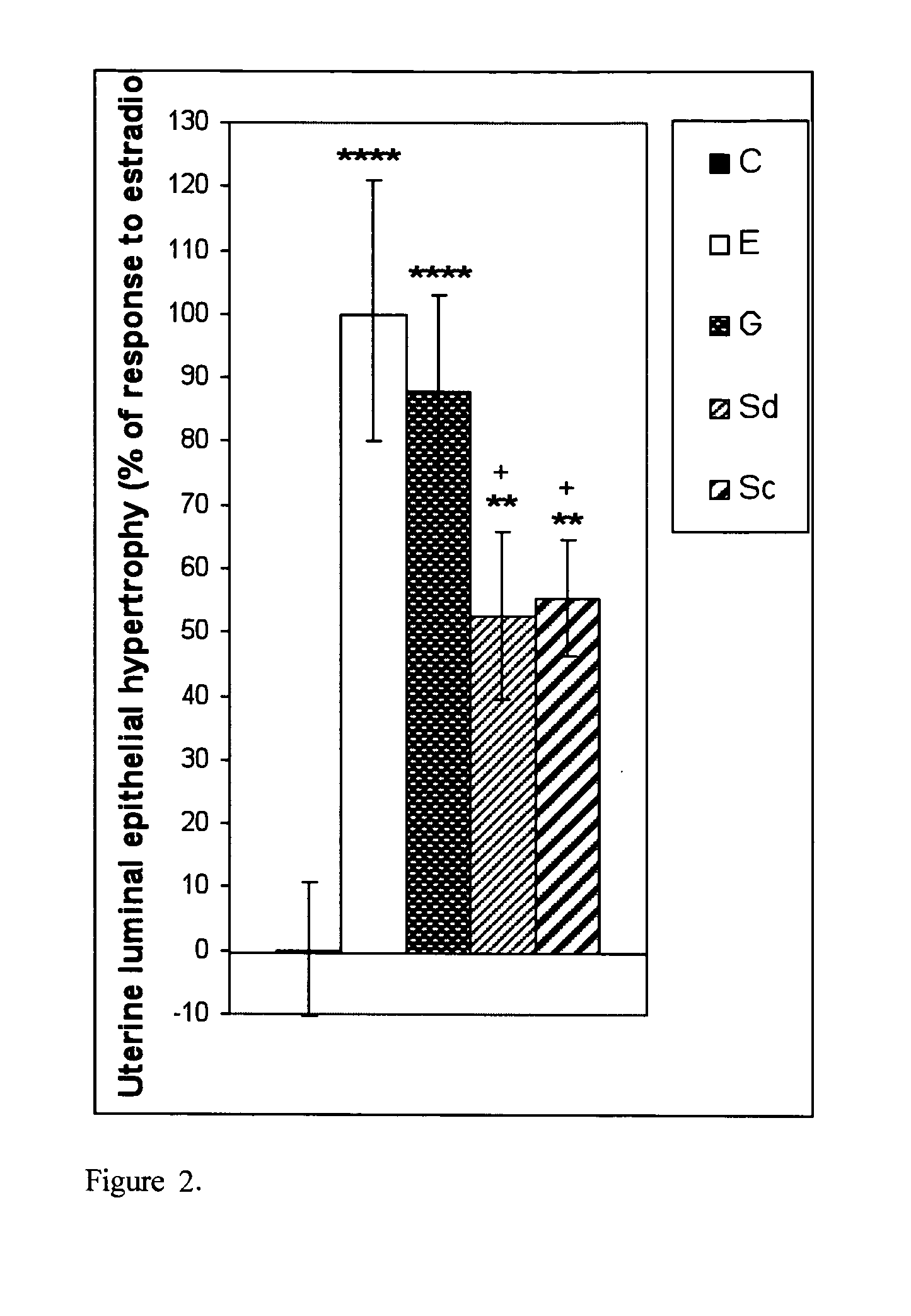
The present disclosure describes a study of estrogenic activity present in various plant species, selectively inducing some but not all estrogenic responses in the uterus. Prepubertal female rats were treated sequentially with various extracts or decoctions of different plant species or its vehicle, followed 1 h later by treatment with estradiol-17β (E) or its solvent. Uteri were excised under anesthesia and histologically processed for eosinophil quantification and morphometric evaluation of various uterine responses to estrogen, at 6 or 24 h after hormone or vehicle treatment. Besides extracts or decoctions, pure phytoestrogens were also used. Additionally, human mammary cancer cells MCF-7 or MDAMB-231 were cultured in presence of the extract (or decoction), E, both or solvent and cell proliferation was evaluated. Various extracts or decoction displayed selective estrogenic and / or antiestrogenic action for some but not all parameters of estrogen stimulation in the uterus and inhibited growth of human mammary cells in culture or antagonized the estrogen-induced increase in their growth. Present results reveal, for the first time, a dissociation of responses to estrogen by phytoestrogens, suggesting its possible therapeutic application as estrogenic compounds not inducing cell proliferation and reveal the anticancerous effect of some of the extracts with possible therapeutic relevance. The dissociation of responses to estrogen additionally suggest therapeutic applications in estrogen-related diseases (for instance, premenstrual syndrome, endometriosis, etc.); the inhibition of eosinophil degranulation suggest an application in diseases related to eosinophils (hypereosinophilic syndrome, allergic and hypersensitivity diseases).
Owner:CHERNIKALIFE +1
Extended cycle multiphasic oral contraceptive method
Owner:APTALIS PHARMA
Pharmaceutical product and analysis model for hormone replacement therapy for women and prevention of some cancers and uterine myomas
The present disclosure describes a study of estrogenic activity present in various plant species, selectively inducing some but not all estrogenic responses in the uterus. Prepubertal female rats were treated sequentially with various extracts or decoctions of different plant species or its vehicle, followed 1 h later by treatment with estradiol-17β (E) or its solvent. Uteri were excised under anesthesia and histologically processed for eosinophil quantification and morphometric evaluation of various uterine responses to estrogen, at 6 or 24 h after hormone or vehicle treatment. Besides extracts or decoctions, pure phytoestrogens were also used. Additionally, human mammary cancer cells MCF-7 or MDAMB-231 were cultured in presence of the extract (or decoction), E, both or solvent and cell proliferation was evaluated. Various extracts or decoction displayed selective estrogenic and / or antiestrogenic action for some but not all parameters of estrogen stimulation in the uterus and inhibited growth of human mammary cells in culture or antagonized the estrogen-induced increase in their growth. Present results reveal, for the first time, a dissociation of responses to estrogen by phytoestrogens, suggesting its possible therapeutic application as estrogenic compounds not inducing cell proliferation and reveal the anticancerous effect of some of the extracts with possible therapeutic relevance. The dissociation of responses to estrogen additionally suggest therapeutic applications in estrogen-related diseases (for instance, premenstrual syndrome, endometriosis, etc.); the inhibition of eosinophil degranulation suggest an application in diseases related to eosinophils (hypereosinophilic syndrome, allergic and hypersensitivity diseases).
Owner:CHERNIKALIFE +1
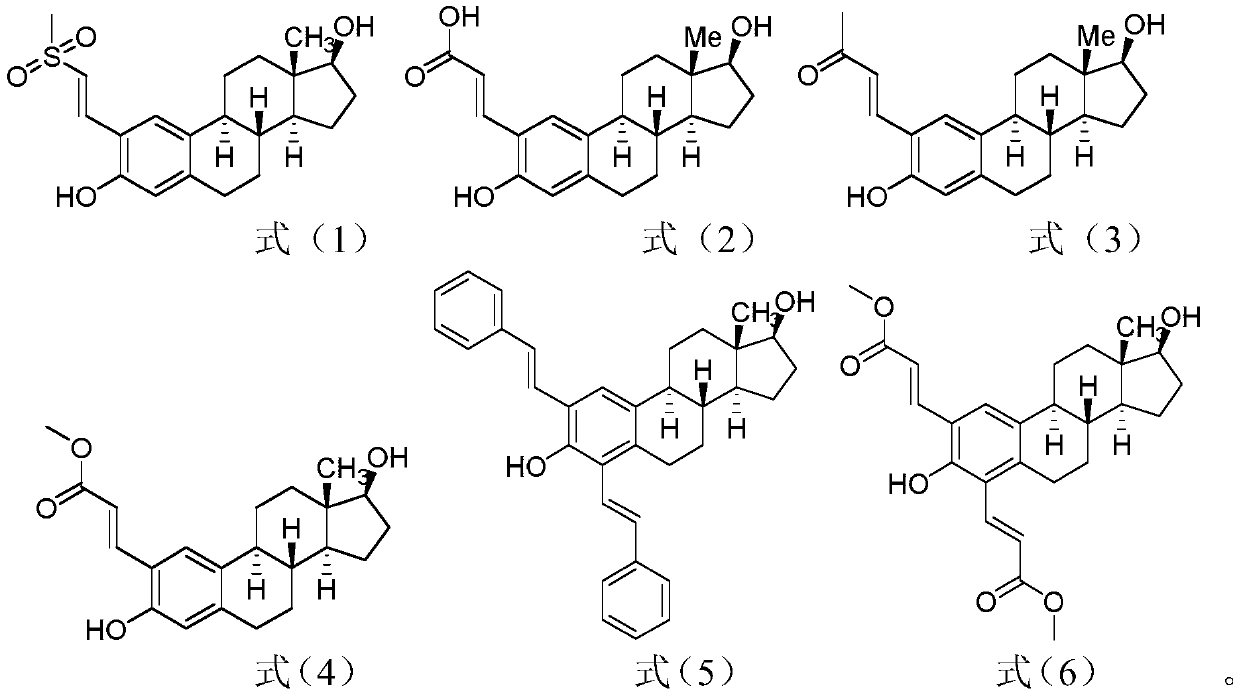
Olefinated estradiol compounds and preparation and application thereof
ActiveCN109988209AGood antitumor activityImprove anti-tumor activitySteroidsAntineoplastic agentsProstate cancer cellWilms' tumor
The invention relates to olefinated estradiol compounds, a preparation thereof and application thereof, and optimal olefination modification sites for estradiol drugs are provided through screening. The structures of estradiol drugs modified at different sites are as shown in formulas (1) to (6). The estradiol drugs have excellent inhibitory activity on prostate cancer cell line PC-3 and breast cancer cell MCF-7, and the anti-tumor activity can be increased by 2-3 times. The present invention provides a basis for modification of estradiol drugs by providing the optimal olefination modificationsites for estradiol drugs (position No. 2 is an optimal modification site) through screening.
Owner:ZHEJIANG UNIV OF TECH
Compositions and methods for mucosal delivery
The present invention relates to a dosage unit comprising a water-soluble hydrocolloid and a mucosal surface-coat-forming film, such film including an effective dose of active agent. In the dosage unit slidenafil citrate, nicotine, hydromorphone, oxybutynine or estradiol are used as active agents.
Owner:LAVIPHARM LABORATORIES INC
Extended cycle multiphasic oral contraceptive method
InactiveCN101394845AReduce the number of timesAvoid irregular bleedingOrganic active ingredientsSexual disorderPhysiologyPlacebo
A multiphasic method of contraception comprising the steps of sequentially administering to a female of child bearing age a Phase I composition containing a progestogen in an amount equivalent to about 0.3 to about 1.5 mg norethiiidrpne acetate and an estrogen in an amount equivalent to about 5 to abo[mu]t 15 mcg of ethinyl estradiol for about 7 to about 14 days; a Phase II composition containing a progestogen in an amount equivalent to about 0.3 to about 1.5 mg of norethindrone acetate and an estrogen in an amount equivalent to about 10 to about 25 meg of ethinyl estradiol for about 14 to about 22 days; a Phase m composition containing a progestogen in an amount equivalent to about 0.3 to about 1.5 mg of norethindrone acetate and an estrogen in an amount equivalent to about 15 to about 35 meg of ethinyl estradiol for about 20 to about 31 days; and an optional Phase IV composition containing (i) an estrogen in an amount equivalent to about 5 to about 20 meg of ethinyl estradiol, or (ii) a placebo or a non-steroidal component, or (iii) a combination of (i) and (ii), for about 2 to about 8 days. The ethinyl estradiol equivalent amount of estrogen in each of the successive Phases II and III is at least 5 meg greater than the ethinyl estradiol equivalent amount of estrogen in the immediately-preceding phase.
Owner:沃纳奇尔科特公司
Transdermal Pharmaceutical Compositions Including Testosterone and a C-SERM
InactiveUS20160051498A1Improve the level ofRelieve symptomsOrganic active ingredientsBiocideRegimenLotion
Formulations for transdermal pharmaceutical compositions including a synergistic combination of low doses of testosterone with a selective estrogen receptor modulator (C-SERM) that are combined with transdermal permeation enhancers are disclosed. Transdermal pharmaceutical compositions can be designed with various release rates, and can be administered to increase bloodstream testosterone levels and thereby reduce symptoms of testosterone deficiency. Transdermal pharmaceutical compositions include liquid dosage forms, such as, for example solutions, liquid sprays, lotions, and the like. Additionally, transdermal pharmaceutical compositions include semi-solid dosage forms, such as, for example emulsions, creams, gels, pastes, ointments, and the like. Transdermal pharmaceutical compositions will deliver testosterone and C-SERM through the skin and directly into the patient's bloodstream, thereby providing high bioavailability of testosterone and C-SERM. The dosage regimen of the transdermal pharmaceutical compositions can be easily tailored for individual patients according to the baseline blood levels of testosterone and estradiol.
Owner:PROFESSIONAL COMPOUNDING CENTS OF AMERICA PCCA
Therapeutically active steroidal derivatives
ActiveUS20180354984A1Little and no inhibitory effectImprove the level ofOrganic active ingredientsSteroidsSteroid prophylaxisBULK ACTIVE INGREDIENT
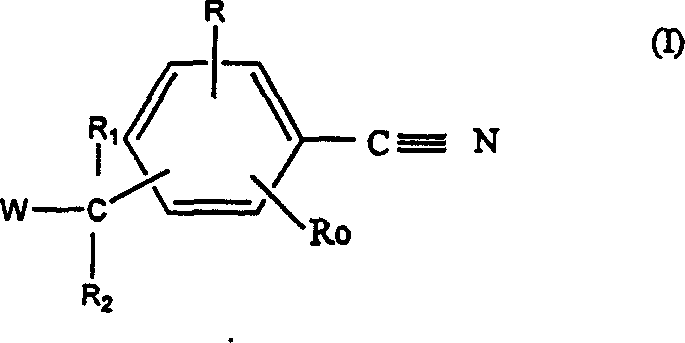
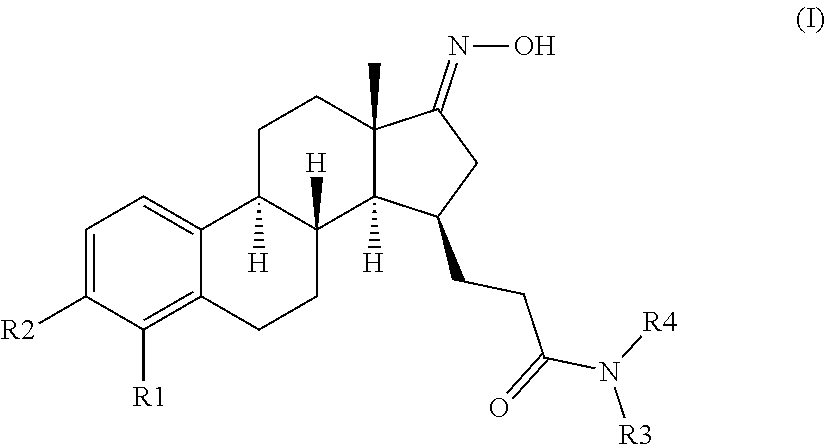
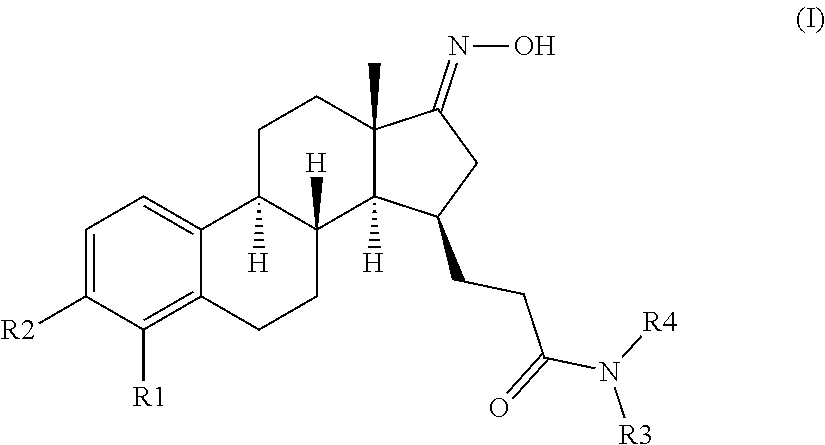
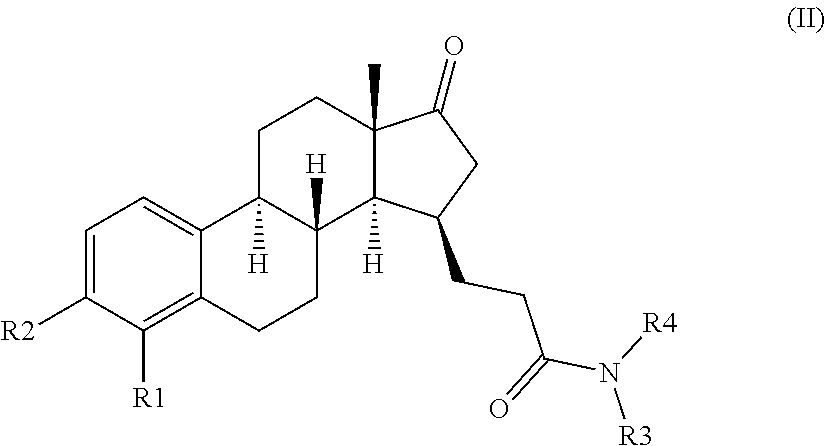
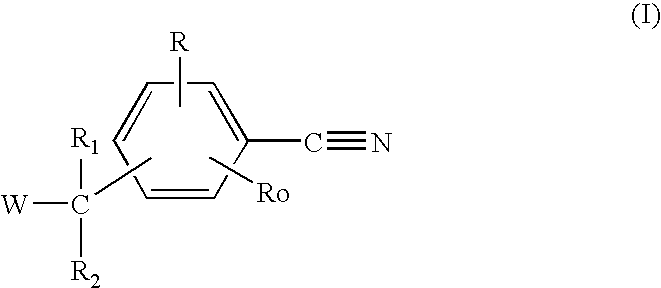
The invention relates to compounds of formula (I) and pharmaceutically acceptable salts thereofwherein R1 to R4 are as defined in the claims. The invention further relates to their use as inhibitors of 17β-HSD1 and in treatment or prevention of steroid hormone dependent diseases or disorders, such as steroid hormone dependent diseases or disorders requiring the inhibition of the 17β-HSD1 enzyme and / or requiring the lowering of the endogenous estradiol concentration. The present invention also relates to the preparation of the aforementioned compounds and to pharmaceutical compositions comprising as an active ingredient(s) one or more of the aforementioned compounds or pharmaceutically acceptable salts thereof.
Owner:FORENDO PHARMA LTD
Methods and compositions for enhancing visual function
InactiveUS20140378426A1Improve eyesightOrganic active ingredientsPharmaceutical delivery mechanismVisual functionDegenerative Disorder
The invention provides compositions of and methods for using estradiol and related compounds to improve visual function and to treat ocular degenerative disorders.
Owner:THE SCRIPPS RES INST
Aromatase inhibition to enhance assisted reproduction
InactiveUS7846957B2Low amountReduces supraphysiologic level of estrogenBiocidePeptide/protein ingredientsSerum igeGynecology
The use of at least one aromatase inhibitor in the production of a medicament for improving the implantation and pregnancy rates for a female undergoing assisted reproduction treatment, which comprises one or more daily doses of an aromatase inhibitor (AI) for administration during assisted reproduction cycles or ovarian stimulation cycles, wherein the doses of AI are selected from amounts effective to reduce serum estradiol levels. Also disclosed are related pharmaceutical preparations and packages.
Owner:ARES TRADING SA
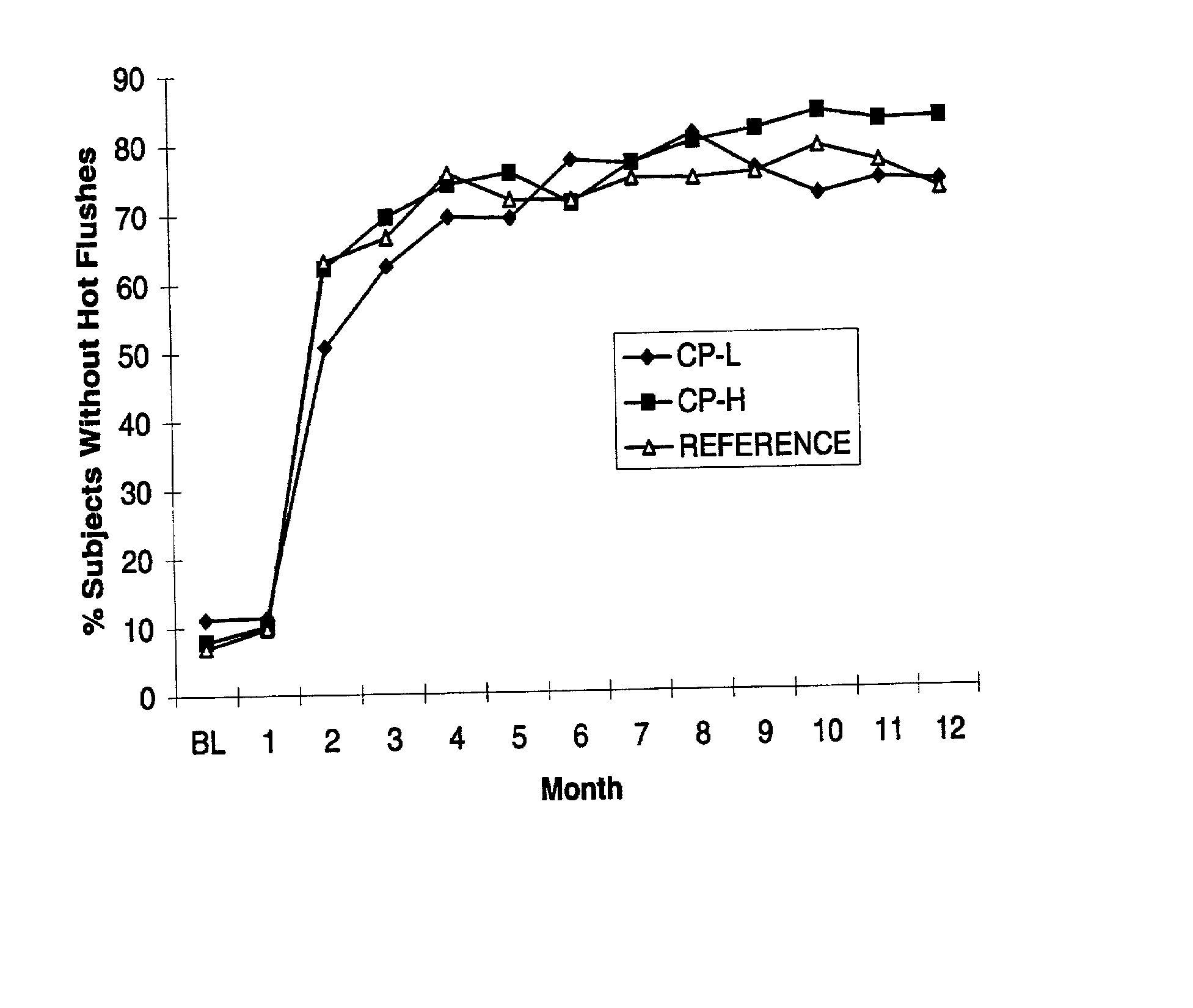
Low-dosed solid oral dosage forms for hrt
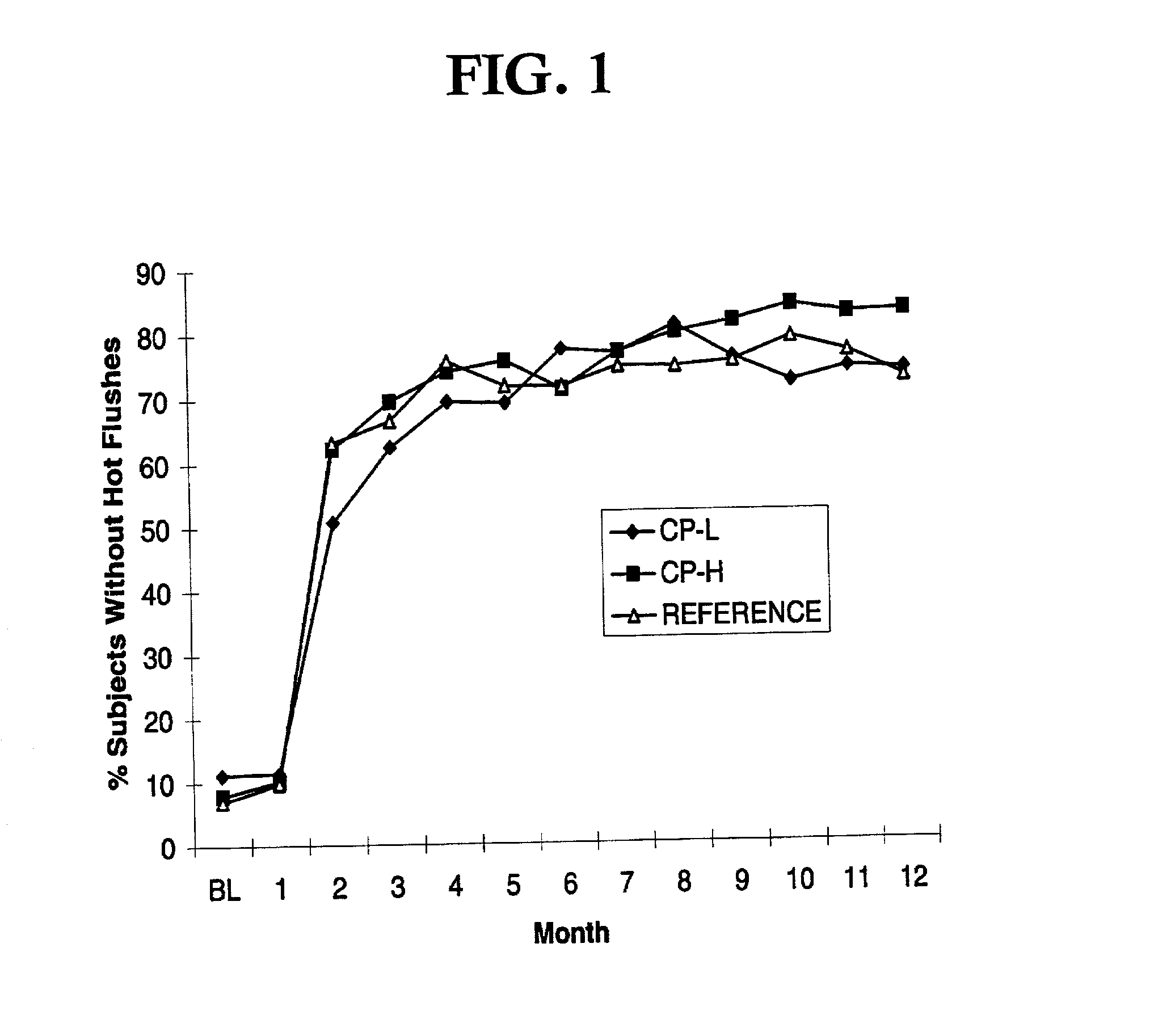
InactiveUS20130137664A1Rapid and adequate relief of moderate to severe vasomotor symptomsReduce frequencyOrganic active ingredientsBiocideDrospirenoneHormone replacement
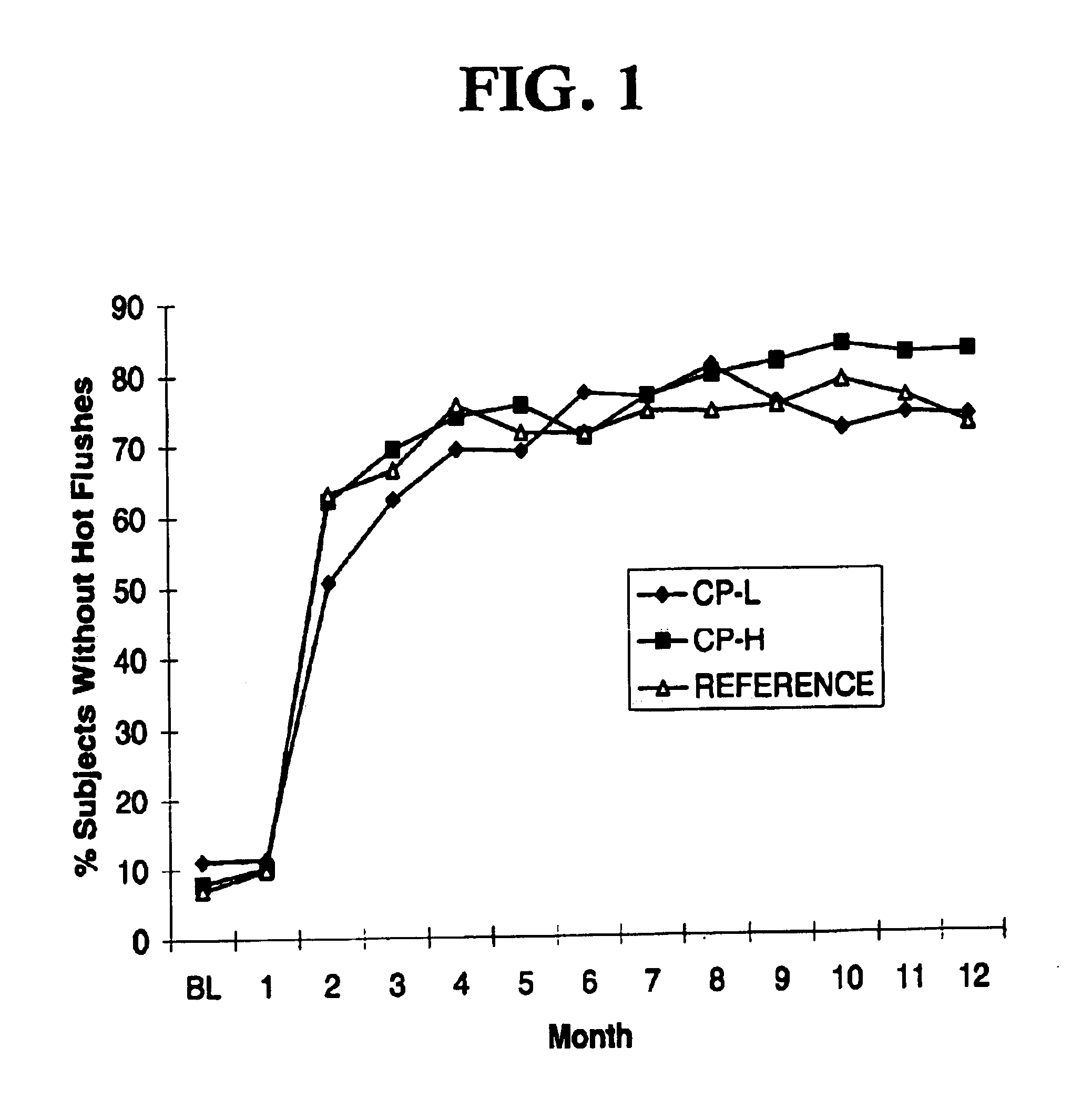
The present invention relates to a very low-dosed dosage form for hormone replacement therapy (HRT). More particularly, the present invention concerns a solid oral dosage form comprising about 0.5 mg estradiol and about 0.25 mg drospirenone, and at least one pharmaceutically acceptable excipient. Despite the very low E2 and DRSP doses it has surprisingly been found that a high proportion of the women suffering from moderate to severe hot flushes actually respond to this treatment. Accordingly, the dosage form of the invention may be used as maintenance HRT or may be used already when HRT is initiated.
Owner:BAYER INTELLECTUAL PROPERTY GMBH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com