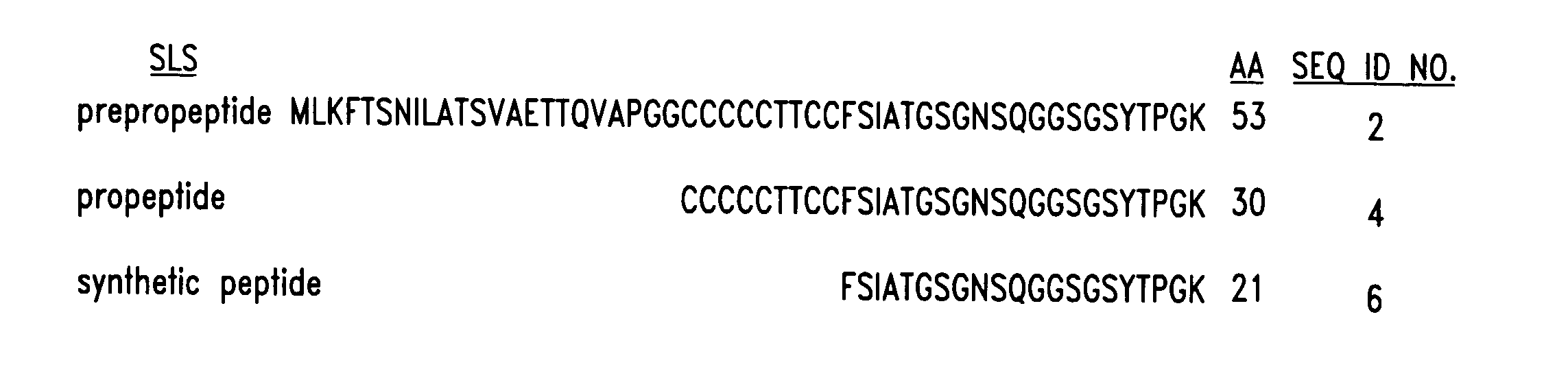Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
70 results about "STREPTOCOCCAL INFECTIONS" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Collagen-binding proteins from Streptococcus pyogenes
InactiveUS7169902B2Avoid infectionInhibit bindingBacterial antigen ingredientsBacteriaStreptococcus pyogenesOrganism
Isolated proteins, designated Cpa1 and Cpa49, and their corresponding amino acid and nucleic acid sequences are provided which are useful in the prevention and treatment of infection caused by group A streptococcal bacteria such as Streptococcus pyogenes. These proteins have been observed to bind to collagen, and thus methods are provided, such as by administration of the proteins or antibodies generated thereto, whereby streptococcal binding of collagen can be inhibited, and streptococcal infection can be greatly reduced. In addition, medical instruments can be treated using the collagen-binding proteins of the invention in order to reduce or eliminate the possibility of their becoming infected or further spreading the infection. In particular, the proteins are advantageous because they may be used as vaccine components or antibodies thereof, and they may be administered to wounds or used to coat biomaterials in order to act as collagen blocking agents and reduce or prevent severe infection by group A streptococcal bacteria.
Owner:PODBIELSKI ANDREAS
Streptococcal heat shock proteins of the Hsp60 family
Methods and compositions comprising isolated nucleic acid molecules specific to Streptococcus pneumoniae and Streptococcus pyogenes, as well as vector constructs and isolated polypeptides specific to Streptococcus pneumoniae and Streptococcus pyogenes are provided. Such compositions and methods are useful for the diagnosis of Streptococcal infection and for generating an immune response to Streptococcal bacteria.
Owner:NVENTA BIOPHARMACEUTICALS CORP
Collagen-binding proteins from Streptococcus pyogenes
InactiveUS20050019345A1Avoid infectionInhibit bindingBacterial antigen ingredientsSugar derivativesStreptococcus pyogenesNucleic acid sequencing
Isolated proteins, designated Cpa1 and Cpa49, and their corresponding amino acid and nucleic acid sequences are provided which are useful in the prevention and treatment of infection caused by group A streptococcal bacteria such as Streptococcus pyogenes. These proteins have been observed to bind to collagen, and thus methods are provided, such as by administration of the proteins or antibodies generated thereto, whereby streptococcal binding of collagen can be inhibited, and streptococcal infection can be greatly reduced. In addition, medical instruments can be treated using the collagen-binding proteins of the invention in order to reduce or eliminate the possibility of their becoming infected or further spreading the infection. In particular, the proteins are advantageous because they may be used as vaccine components or antibodies thereof, and they may be administered to wounds or used to coat biomaterials in order to act as collagen blocking agents and reduce or prevent severe infection by group A streptococcal bacteria.
Owner:PODBIELSKI ANDREAS
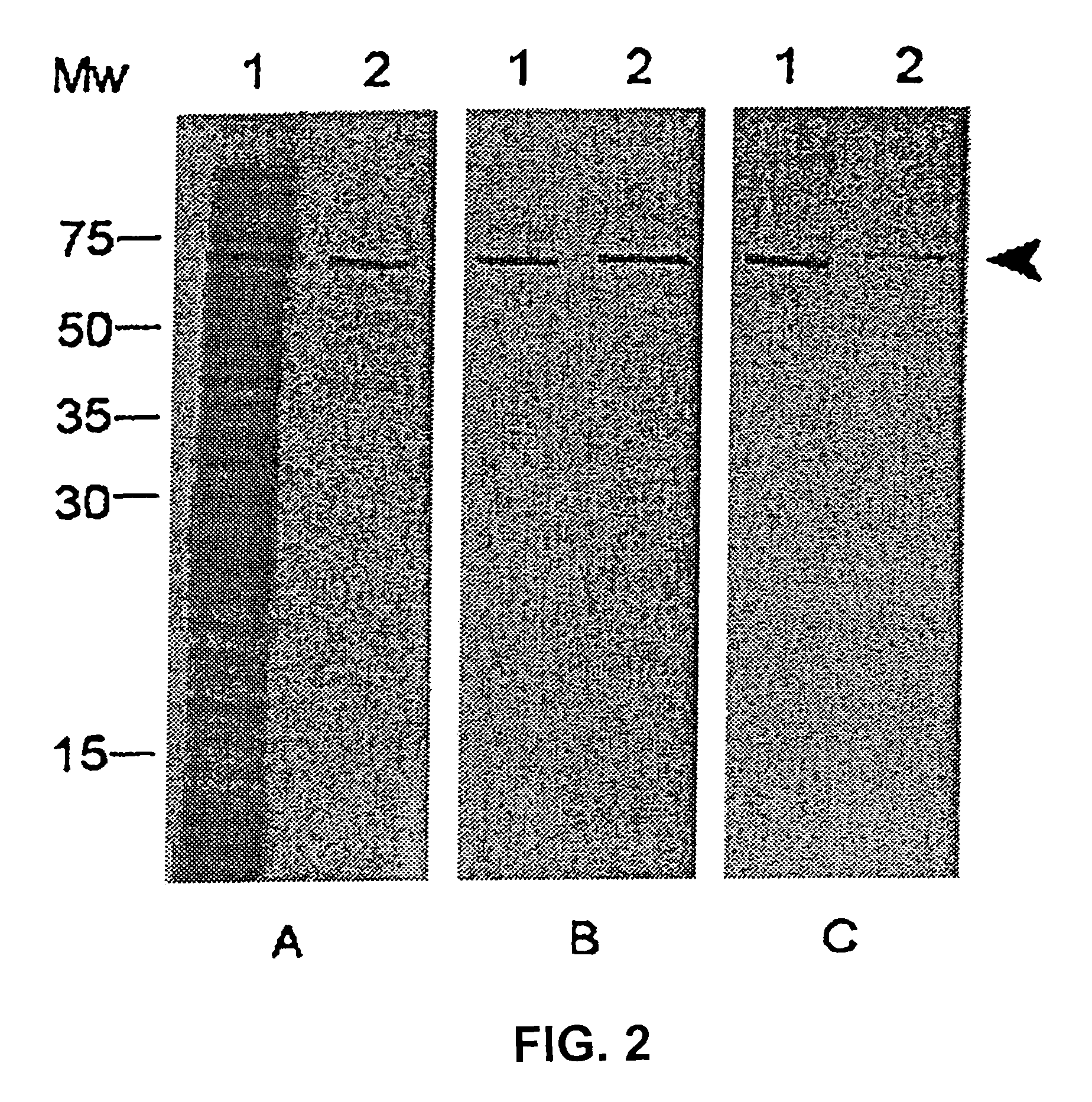
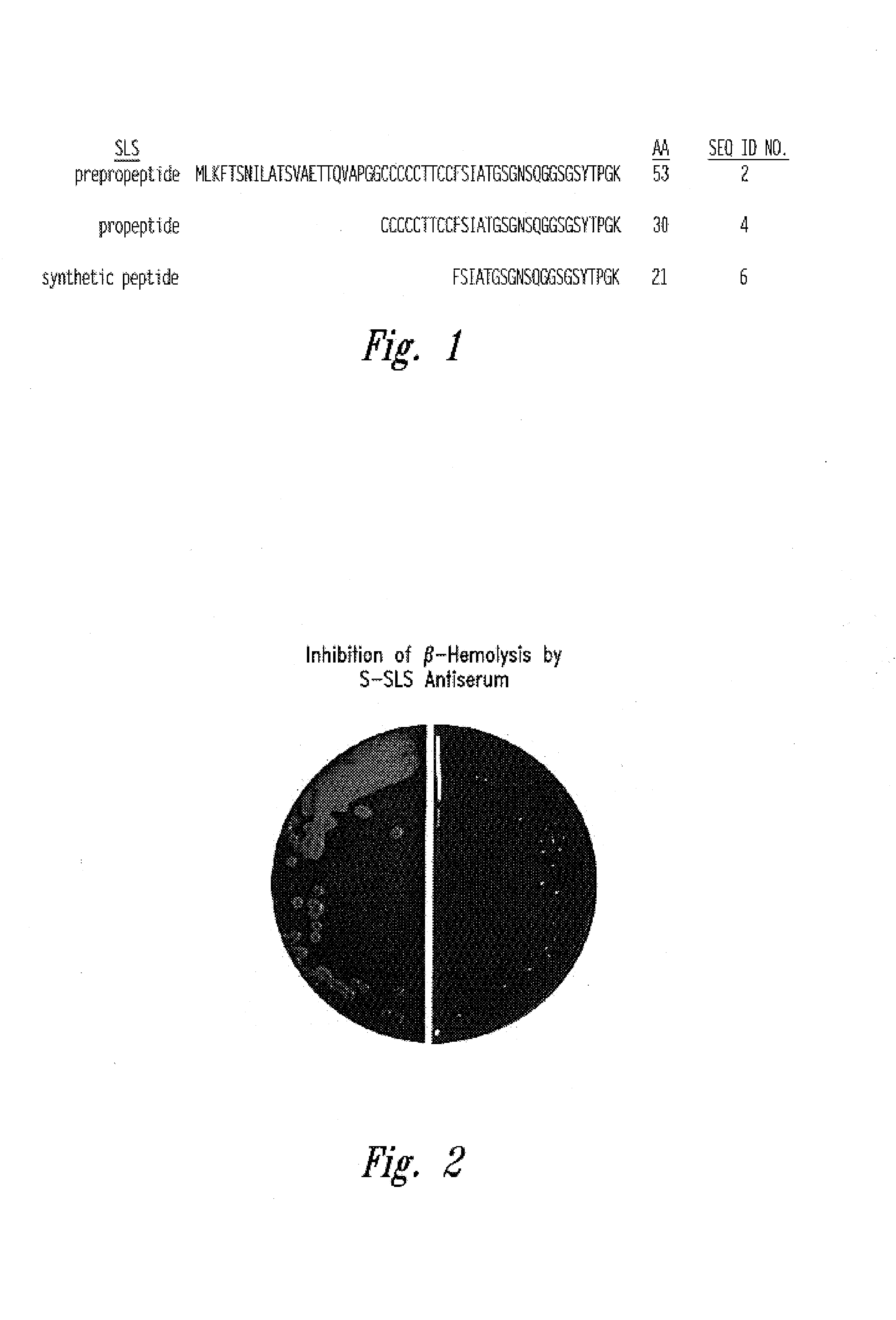
Streptococcal streptolysin S vaccines
InactiveUS20070098737A1Bacterial antigen ingredientsImmunoglobulins against bacteriaStreptolysinToxin
Provided are streptolysin S (SLS) polypeptides, peptides, and variants thereof, antibodies directed thereto, and isolated nucleic acids encoding such proteins. In one embodiment, a method is provided wherein a synthetic peptide of SLS is used to elicit an immune response specific for SLS in a subject to treat or prevent a streptococcal infection. In other embodiments, antibodies that neutralize the hemolytic activity of the SLS toxin may be used as a vaccinating agent.
Owner:UNIV OF TENNESSEE RES FOUND
Vaccine
InactiveUS20040081662A1Avoid toxicityEnhance immune responseAntibacterial agentsBacterial antigen ingredientsStreptococcus pneumoniaeMedicine
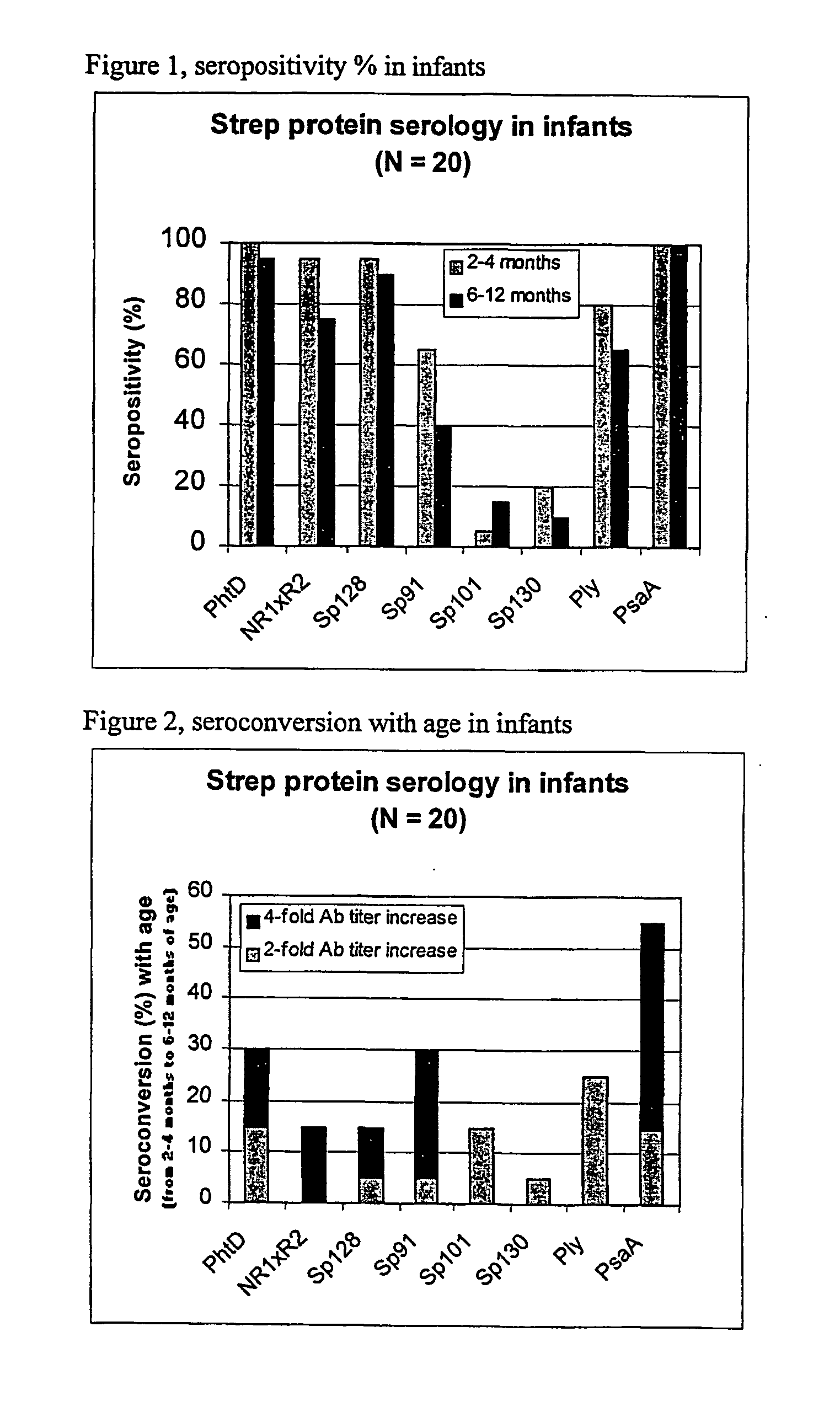
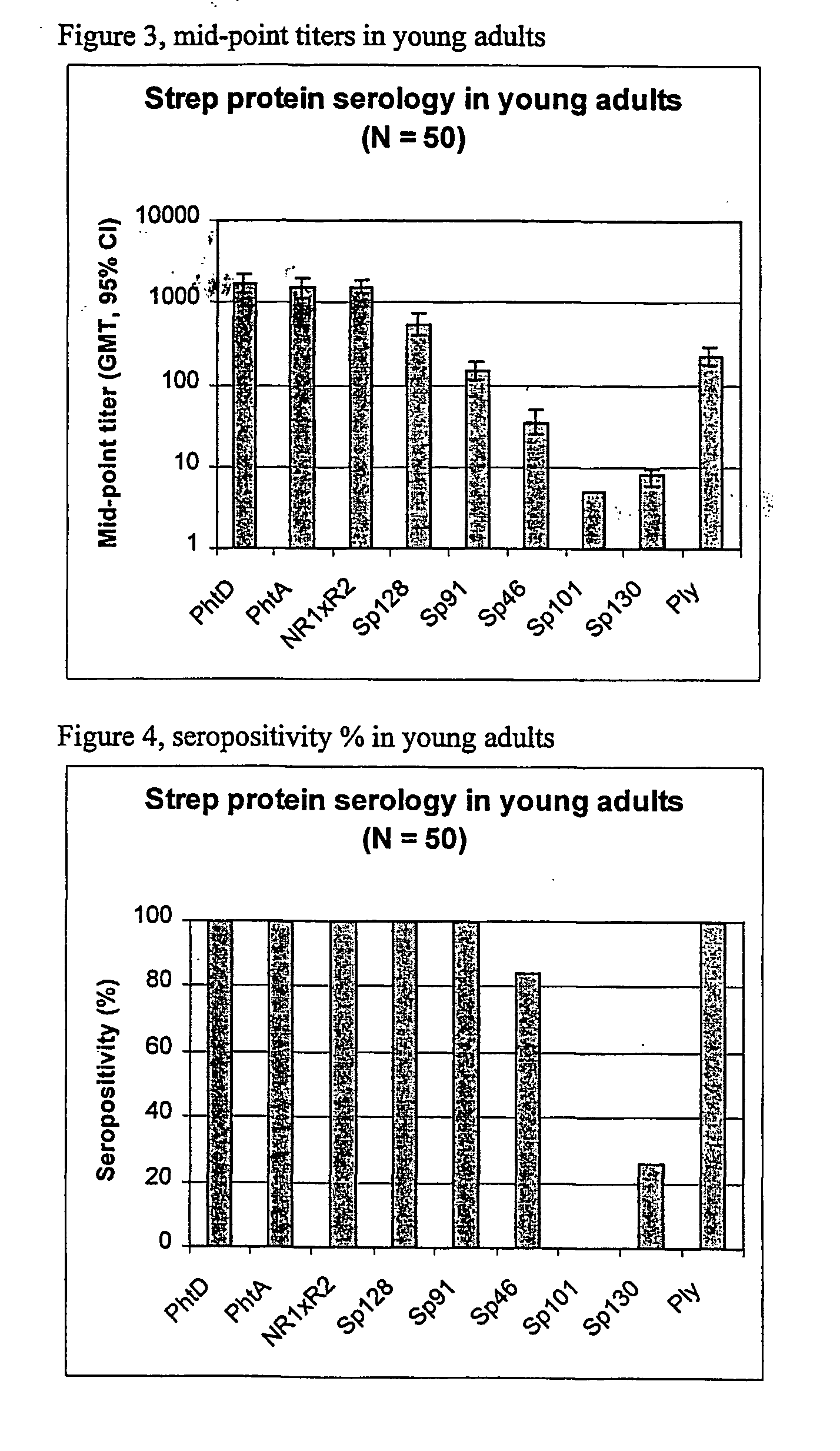
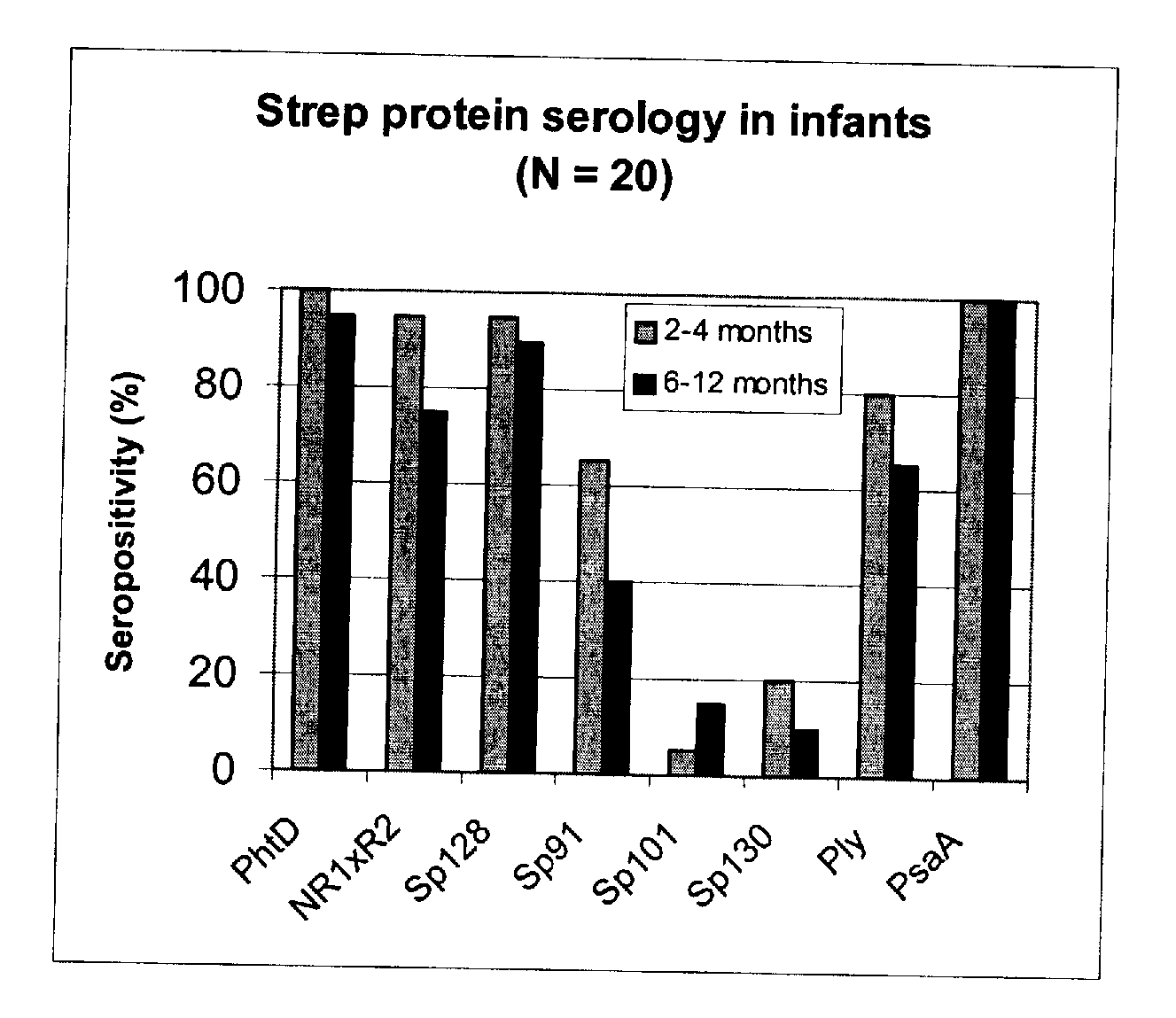
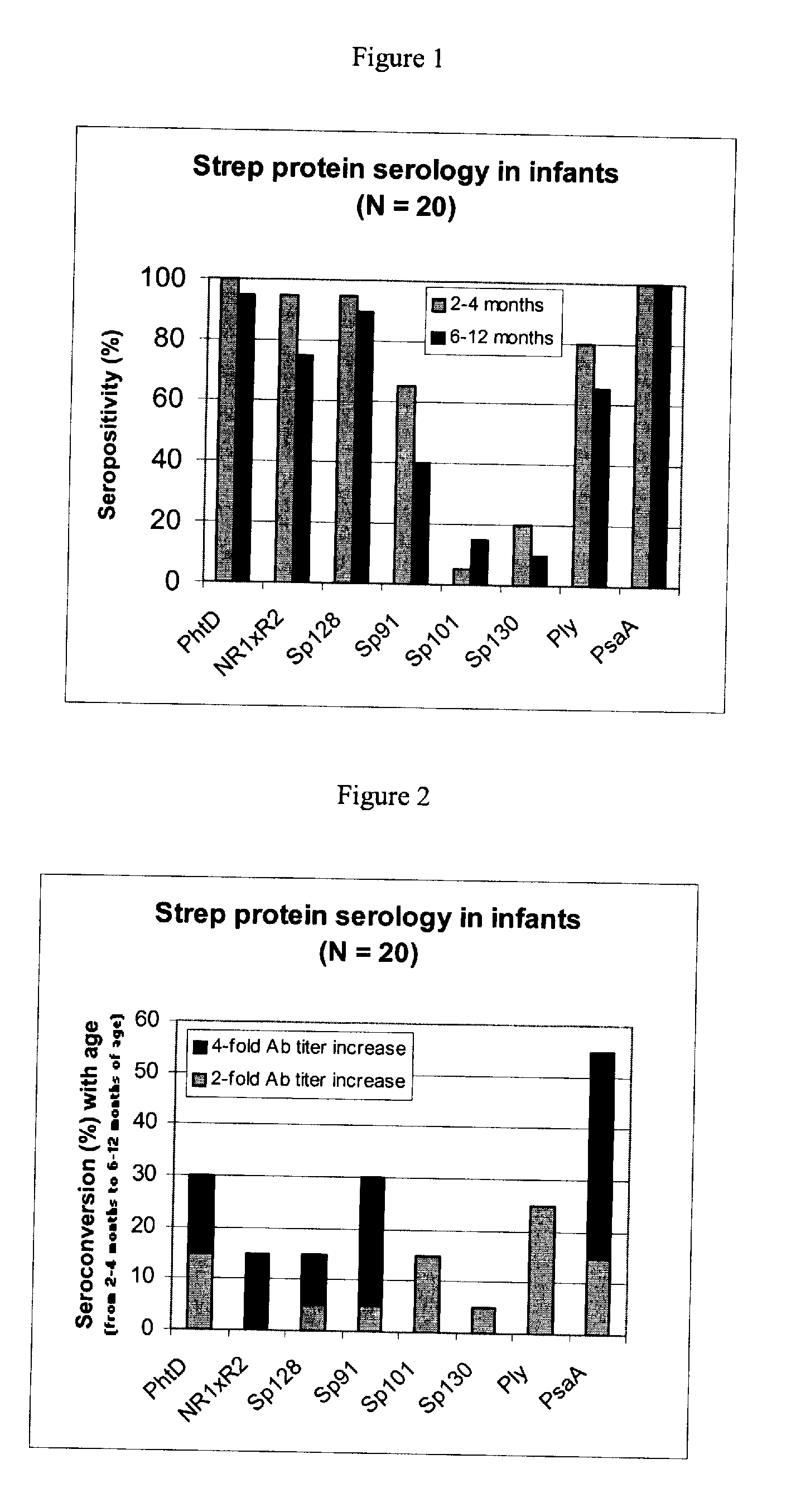
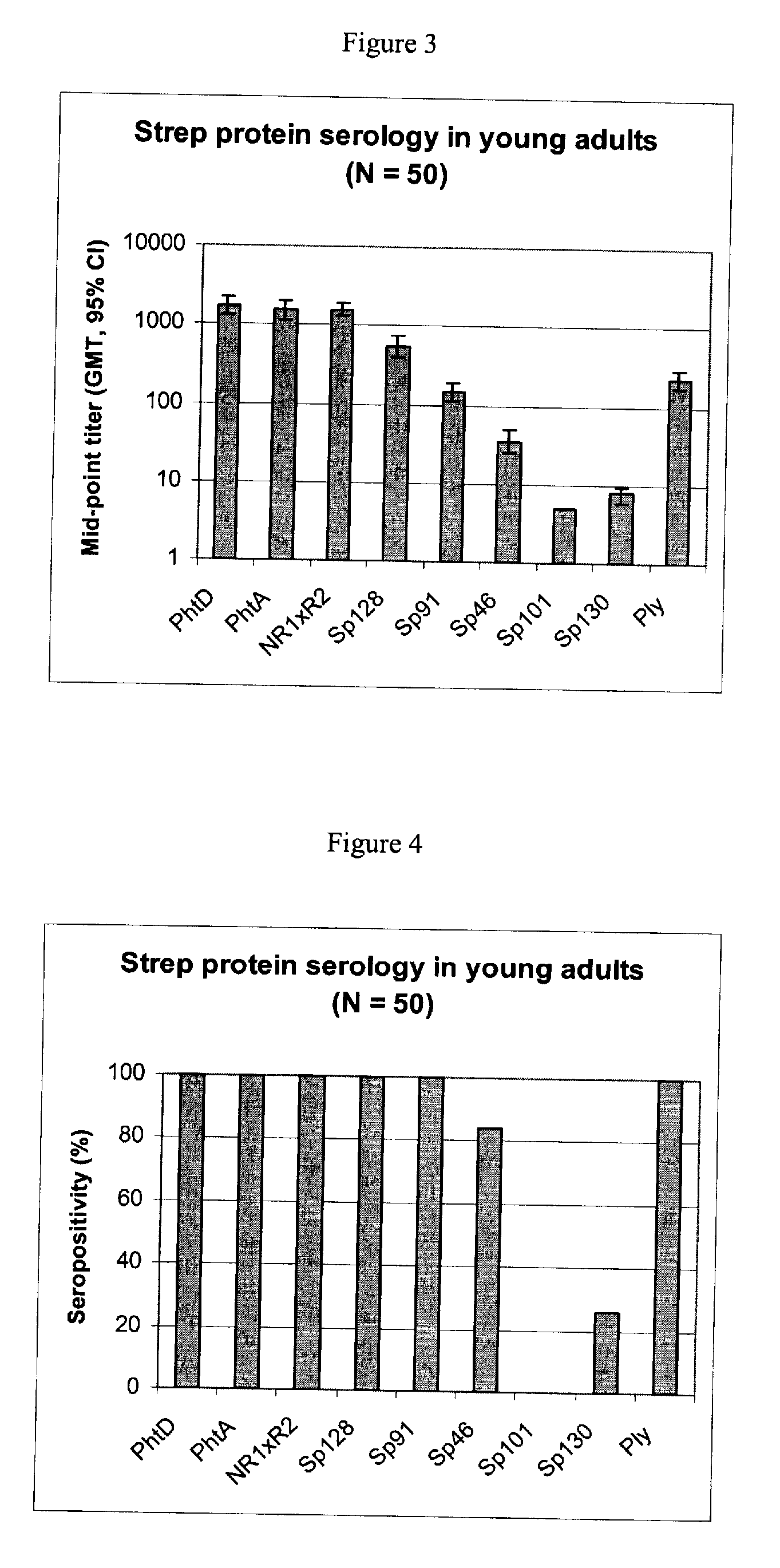
The present invention relates to a combination of 2 or more S pneumoniae proteins, their manufacture and use in medicine as a vaccine. Such combinations are particularly useful for the protection of infants and elderly against streptococcal infection.
Owner:SMITHKLINE BECKMAN CORP +1
Virulence of streptococci
InactiveUS7670835B2Increase virulenceMinimal effectAntibacterial agentsBacterial antigen ingredientsGenomic SegmentVaccination
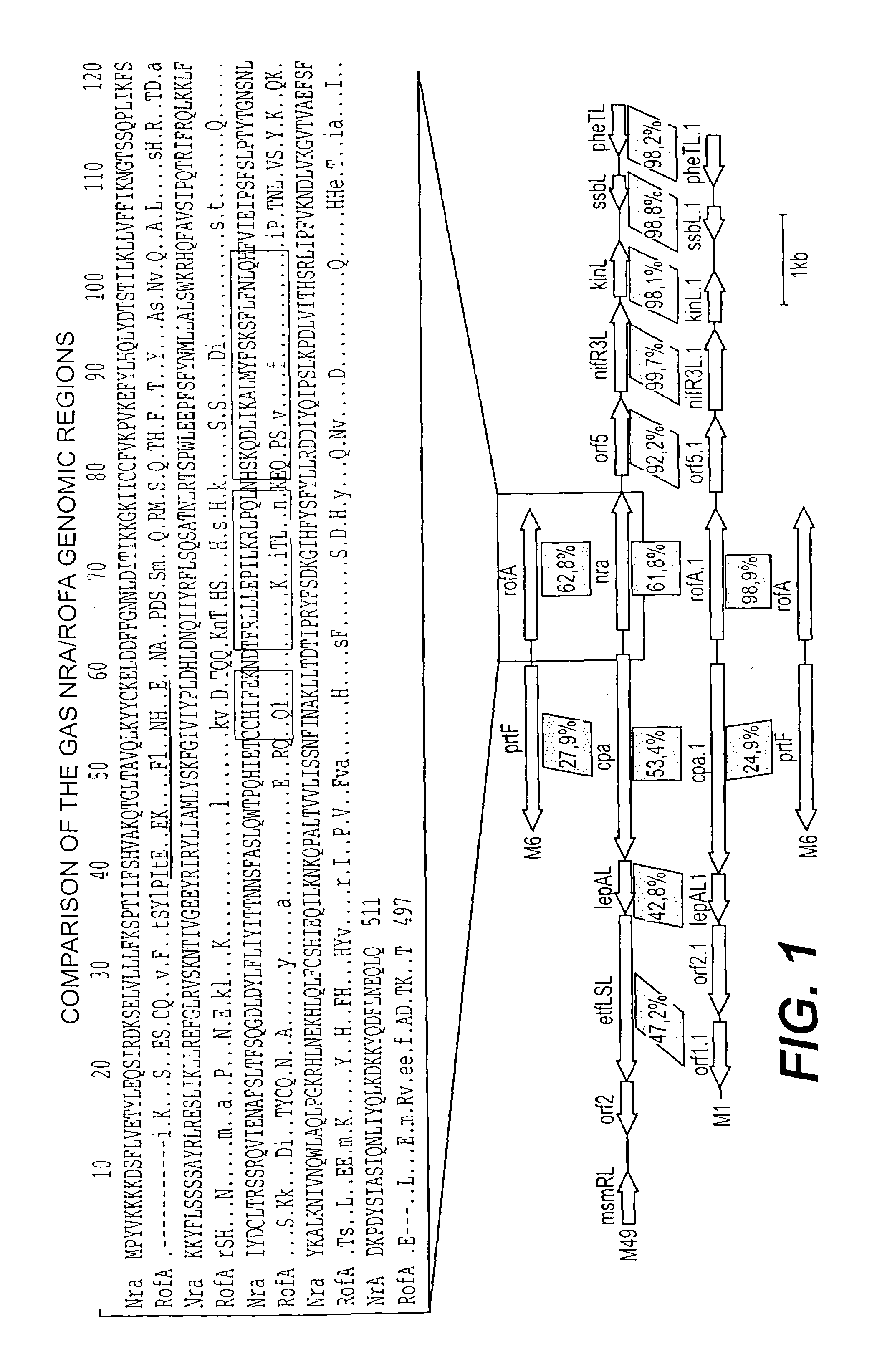
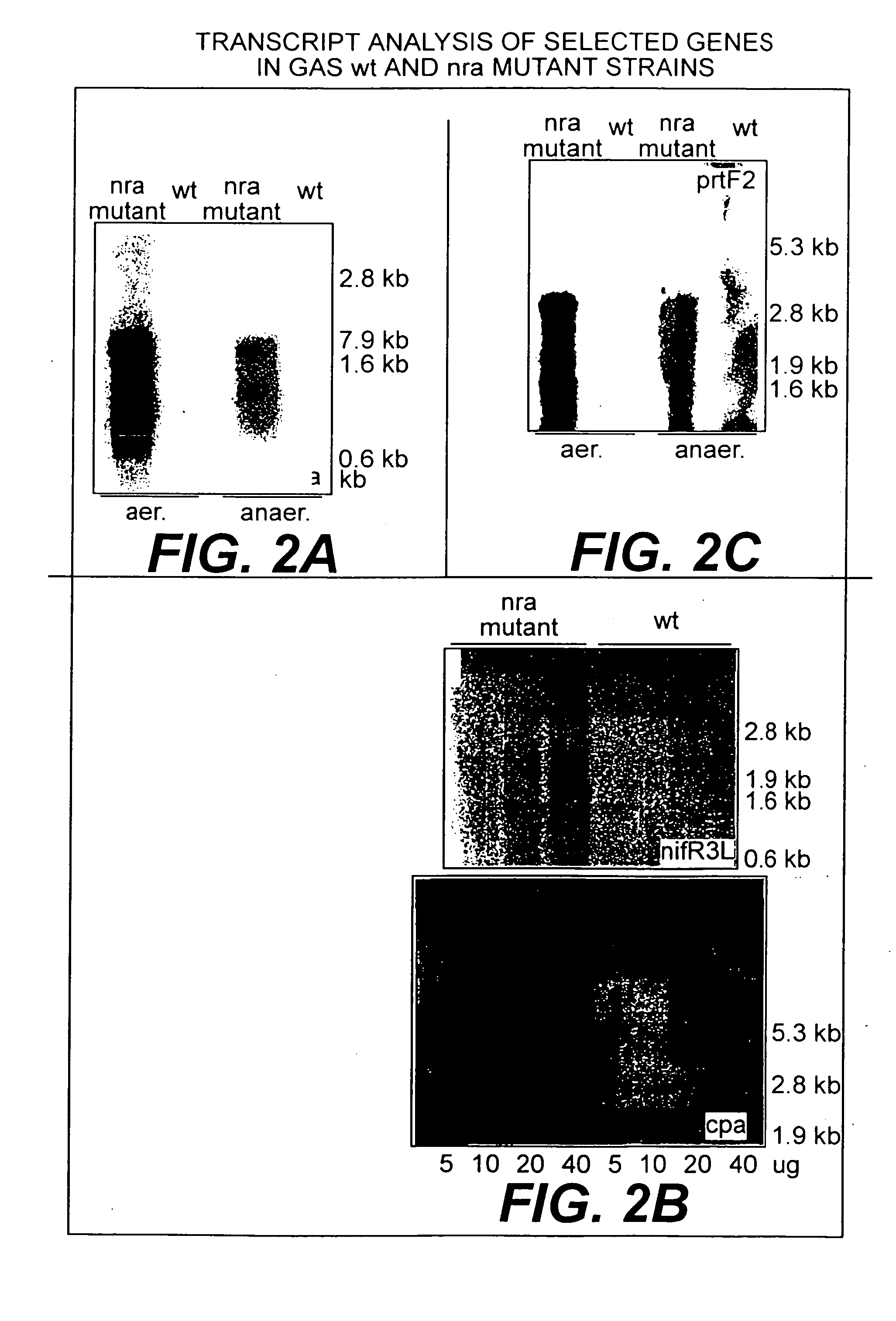
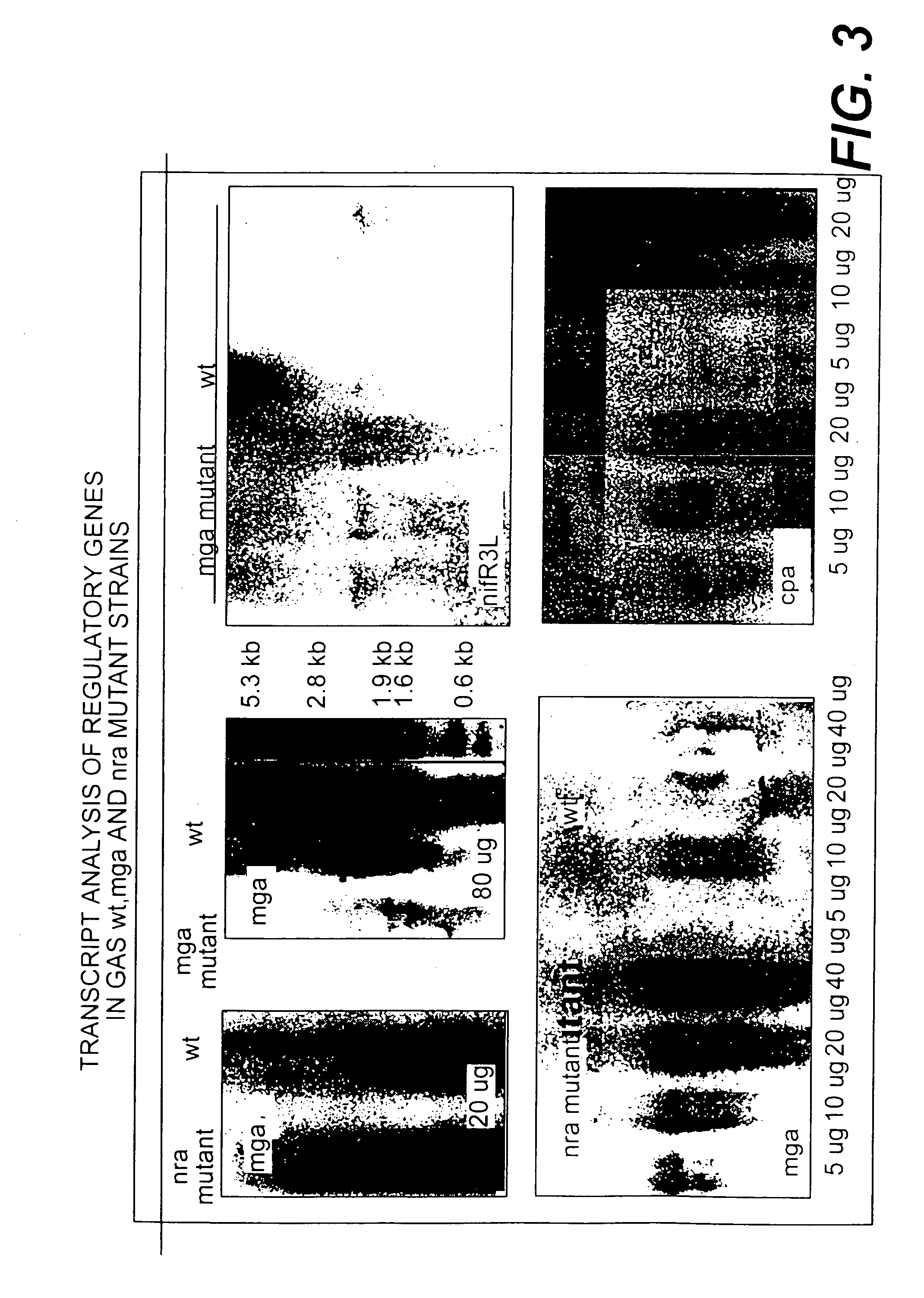
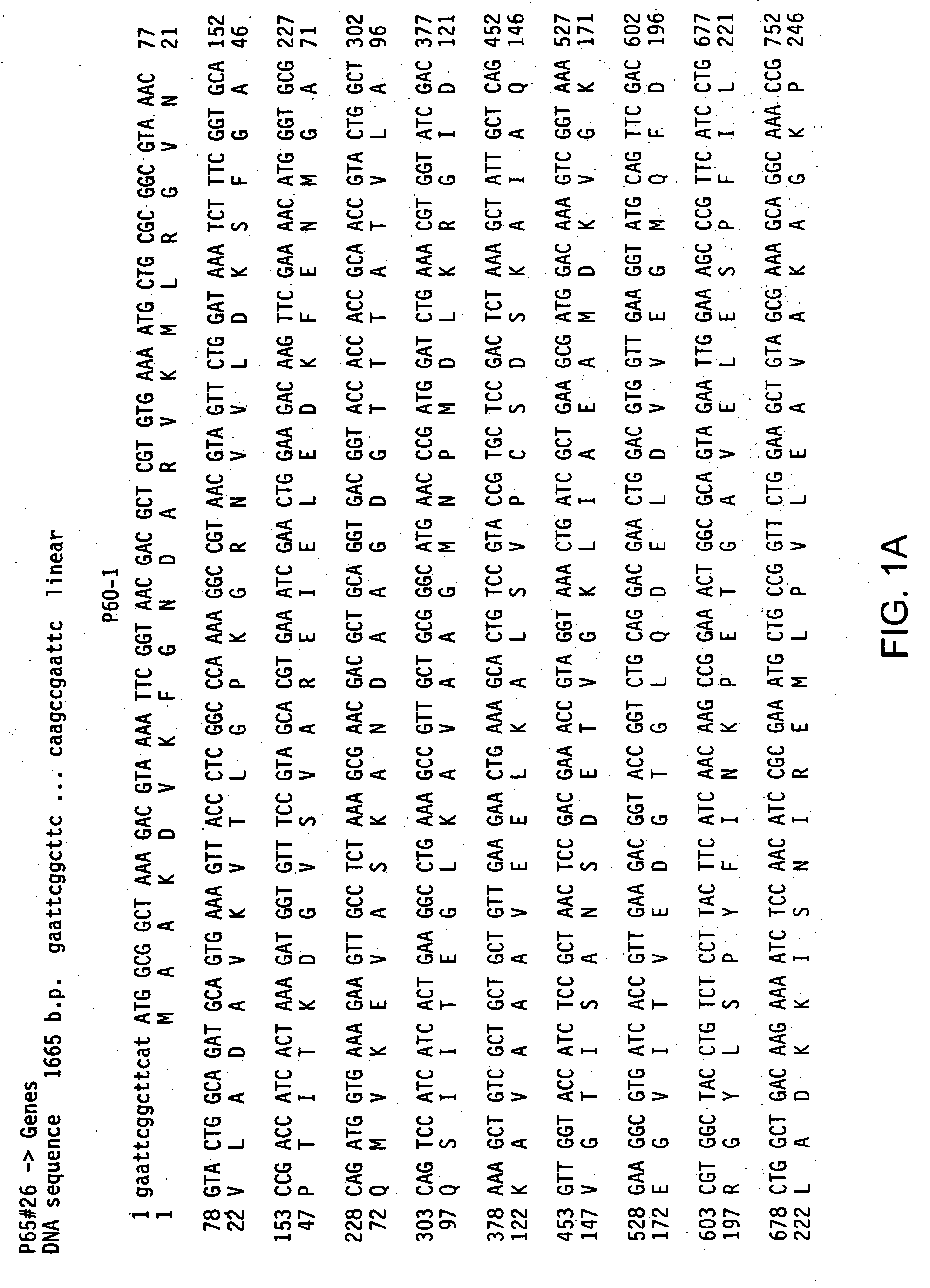
The invention relates to the field of diagnosis of and vaccination against Streptococcal infections and to the detection of virulence markers of Streptococci. The invention discloses a method for modulating virulence of a Streptococcus, the method comprising modifying a genomic fragment of Streptococcus wherein the genomic fragment comprises at least a functional part of a fragment identifiable by hybridization in Streptococcus suis to a nucleic acid or fragment thereof as shown in FIG. 5.
Owner:STICHTING WAGENINGEN RES
Surface-located streptococcus pneumoniae polypeptides
InactiveCN101163499AAntibacterial agentsBacterial antigen ingredientsStreptococcus pneumoniaeStreptococcus mitis
The present invention relates to cell-surface-located polypeptides of Streptococcus pneumoniae and their use in immunisation against Streptococcal infection, in diagnosis of Streptococcus and in identification of compounds with anti-Streptococcus activity. In a further aspect, the invention relates to antibodies capable of recognising cell surface-located polypeptides of Streptococcus pneumoniae and uses thereof.
Owner:ACE BIOSCIENCES AS
Use of bacterial phage associated lysing enzymes for treating streptococcal infections of the upper respiratory tract-
A composition for treatment of bacterial infections of the eye is disclosed which comprises a lytic enzyme composition specific for the infecting bacteria, and a carrier for delivering said lytic enzyme.. The carrier for delivering at least one lytic enzyme to the eye may be but is not limited to the use of an isotonic solution..
Owner:THE ROCKEFELLER UNIV
Surface-Located Streptococcus Pneumoniae Polypeptides
InactiveUS20090202528A1High sensitivityReduce impactAntibacterial agentsOrganic active ingredientsStreptococcus pneumoniaeStreptococcus mitis
The present invention relates to cell-surface-located polypeptides of Streptococcus pneumoniae and their use in immunisation against Streptococcal infection, in diagnosis of Streptococcus and in identification of compounds with anti-Streptococcus activity. In a further aspect, the invention relates to antibodies capable of recognising cell surface-located polypeptides of Streptococcus pneumoniae and uses thereof.
Owner:ACE BIOSCIENCES AS
Virulence of Streptococci
InactiveUS7109006B2Increase virulenceMinimal effectAntibacterial agentsBacteriaGenomic SegmentVirulent characteristics
The invention relates to the field of diagnosis of and vaccination against Streptococcal infections and to the detection of virulence markers of Streptococci. The invention discloses a method for modulating virulence of a Streptococcus comprising modifying a genomic fragment of Streptococcus wherein the genomic fragment comprises at least a functional part of a fragment identifiable by hybridization in Streptococcus suis to a nucleic acid or fragment thereof as shown in FIG. 5.
Owner:STICHTING WAGENINGEN RES
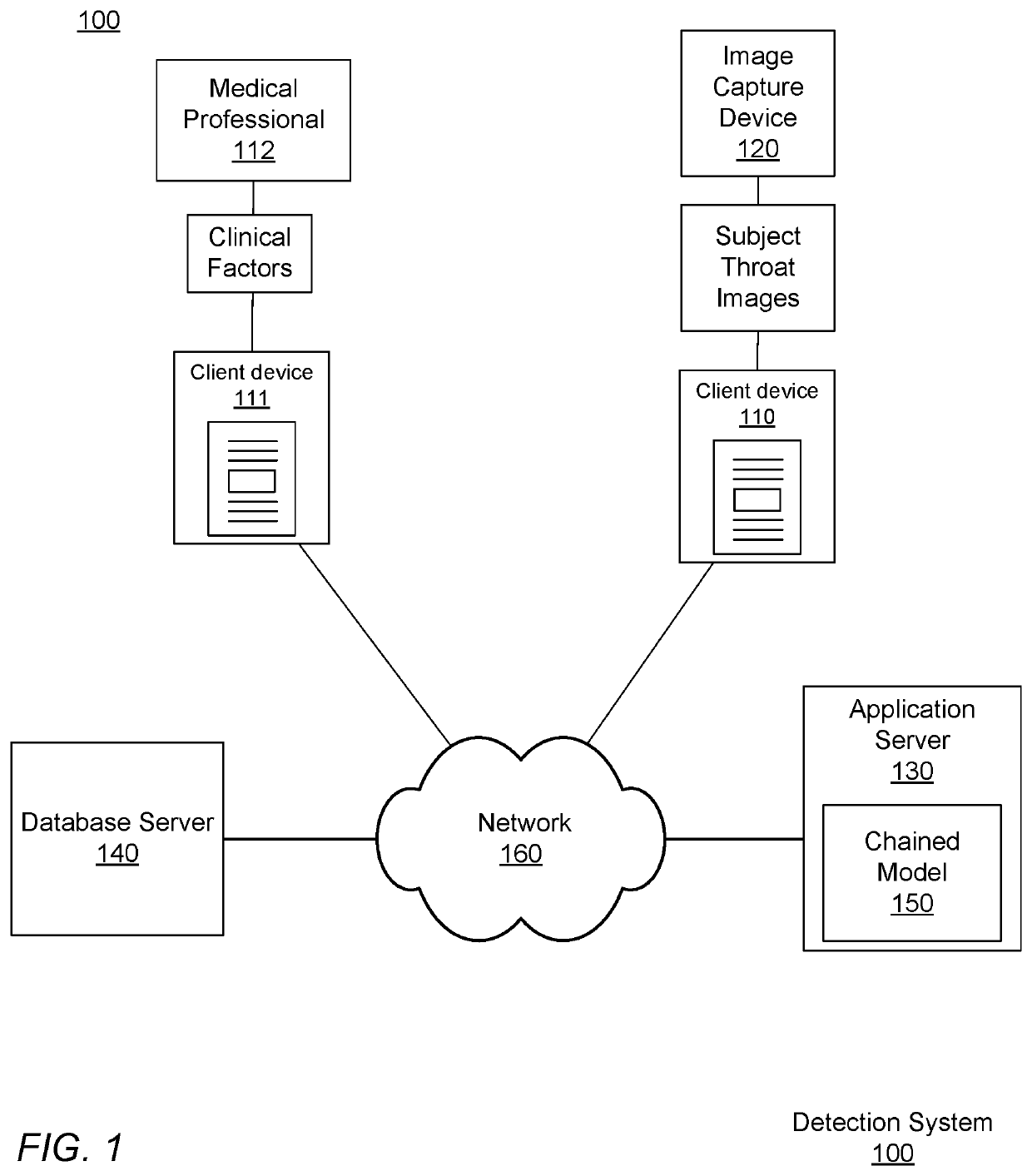
Image Processing of Streptococcal Infection in Pharyngitis Subjects
A method for determining a disease state prediction, relating to a potential disease or medical condition of a subject, includes accessing a set of subject images, the subject images capturing a part of a subject's body, and accessing a set of clinical factors from the subject. The clinical factors are collected by a device or a medical practitioner substantially contemporaneously with the capture of the subject images. The subject images are inputted into an image model to generate disease metrics for disease prediction for the subject. The disease metrics generated by the image model and the clinical factors are inputted into a classifier to determine the disease state prediction, and the disease state prediction is returned.
Owner:LIGHT AI INC
Vaccine against streptococci
The invention relates to subunit immunogenic or vaccine compositions which may comprise at least one polypeptide of Streptococcus equi and methods for preparing and / or formulating such compositions. The invention also relates to the use of such subunit compositions, such as a method for eliciting an immunogenic response or a protective immune response, which may comprise administering the composition to a mammal susceptible to streptococcal infection.
Owner:MERIAL INC
Vaccine
Effective stimulation of immune responses is achieved through the use of a group A streptococcal antigen combined with proteosome adjuvant. The compositions are provided in particular for intranasal administration. The vaccine compositions are provided for use in inducing an immune response in an individual for the treatment or prophylaxis of group A streptococcal infection in an individual, preferably via prevention or reduction of colonisation of the throat following intranasal administration.
Owner:COUNCIL OF THE QUEENSLAND INST OF MEDICAL RES +1
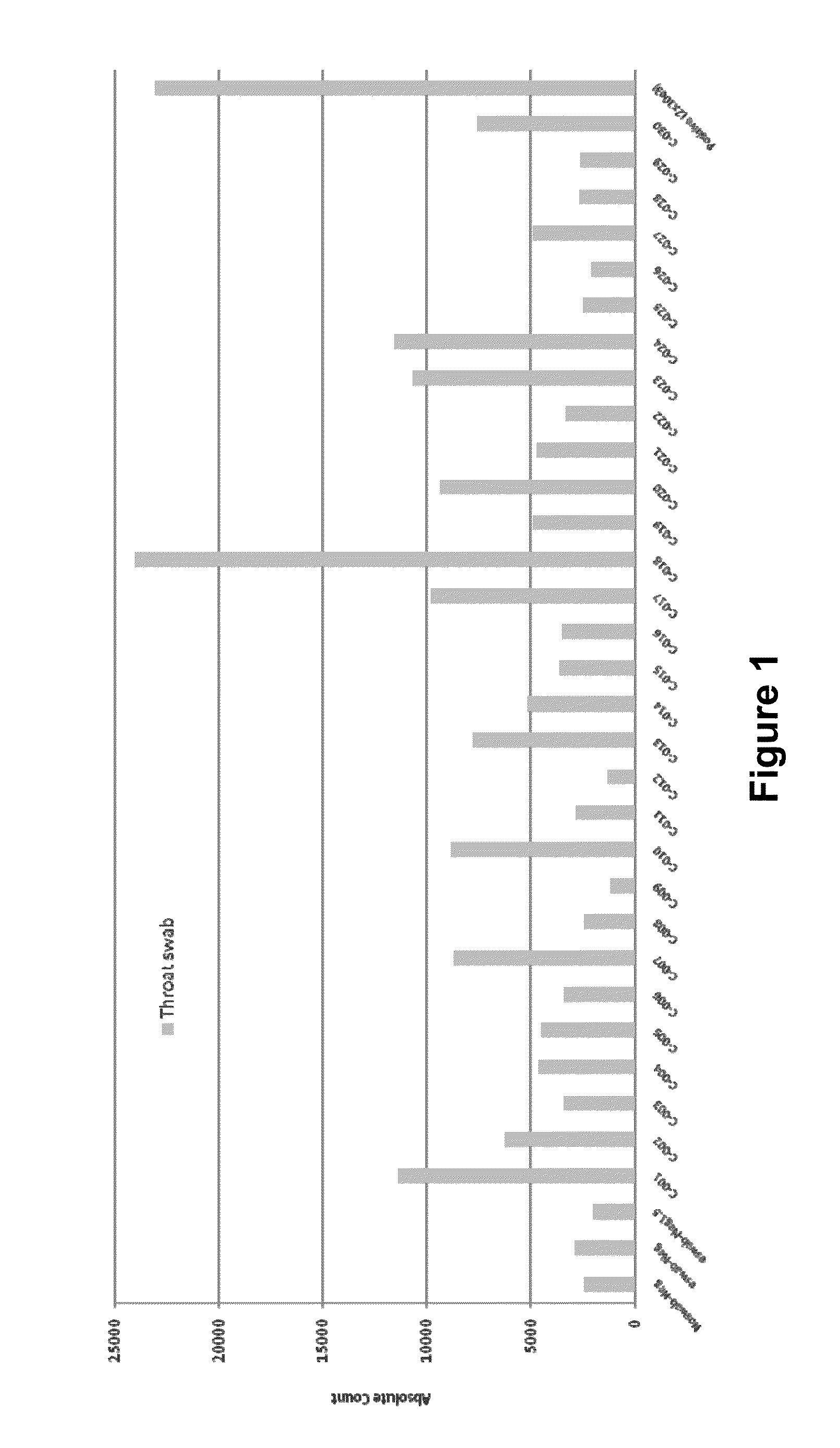
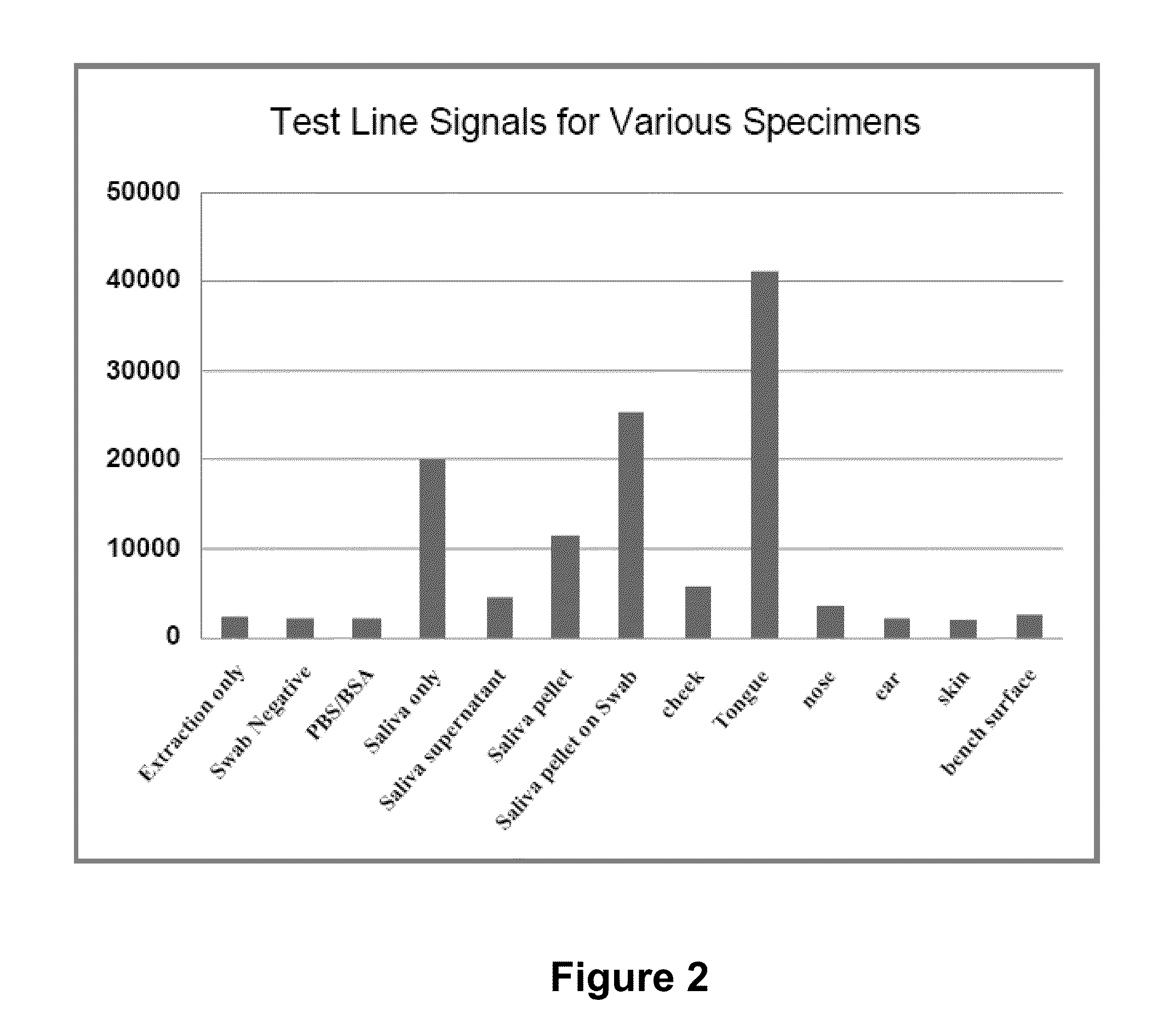
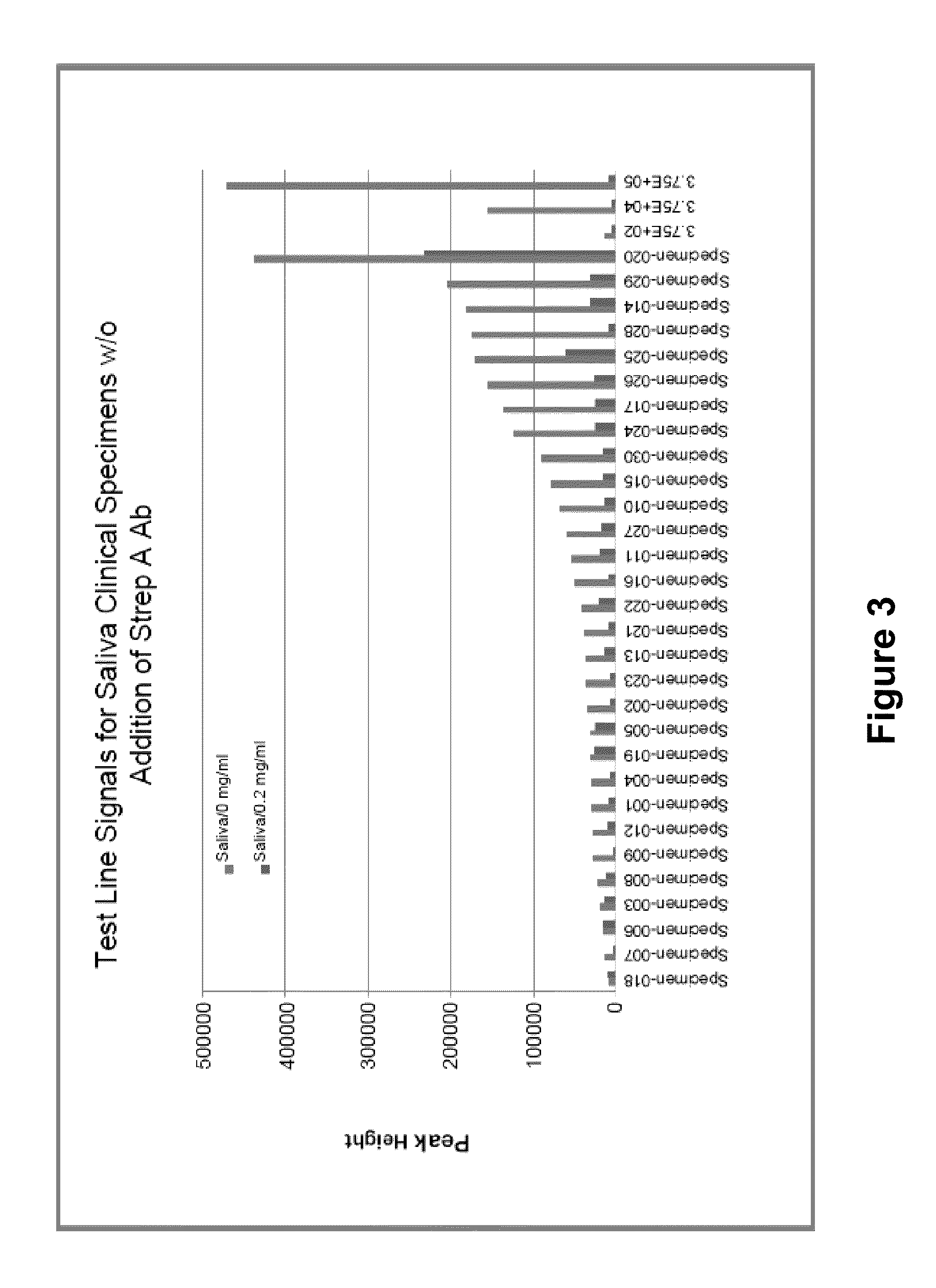
N-acetyl-D-glucosamine for enhanced specificity of Strep A immunoassay
ActiveUS10168329B2Reduce probabilityReduce false alarm rateMaterial analysisN Acetyl D GlucosamineStreptococcus mitis
Methods, compositions and kits for detecting Group A streptococcus in a biological sample are described. More particularly, the present disclosure provides an immunoassay in which the specificity of detection of Group A streptococcus is enhanced by addition of N-acetyl-D-glucosamine. These methods, compositions and kits are useful in convenient, reliable and early diagnosis of streptococcal infection in a human subject.
Owner:QUIDEL
Group b streptococcus antigens
The present invention relates to polypeptides, epitopes and antibodies directed to these epitopes, more particularly to the Sip polypeptide of Group B streptococcus (GBS), also called Streptococcus Agalactiae which may be used to prevent, diagnose and / or treat streptococcal infection.
Owner:ID BIOMEDICAL
Assays and methods for the diagnosis of post-streptococcal disorders
Provided are methods for diagnosing a disease in a subject with a previous streptococcal infection by determining the presence or absence of one or more autoantibodies in a biological sample from the subject, wherein the one or more autoantibodies recognize an antigen from a protein selected from the group consisting of ELAVL2, ELAVL3, ELAVL4, Nova-1, Nova-2, Cdr1, Cdr2; and Cdr3. The presence of such autoantibodies is indicative of a positive diagnosis for a post-streptococcal disease such as PANDAS, post-GABHS glomerulonephritis, rheumatic fever, autism and Syndenham's chorea.
Owner:QUEST DIAGNOSTICS INVESTMENTS INC
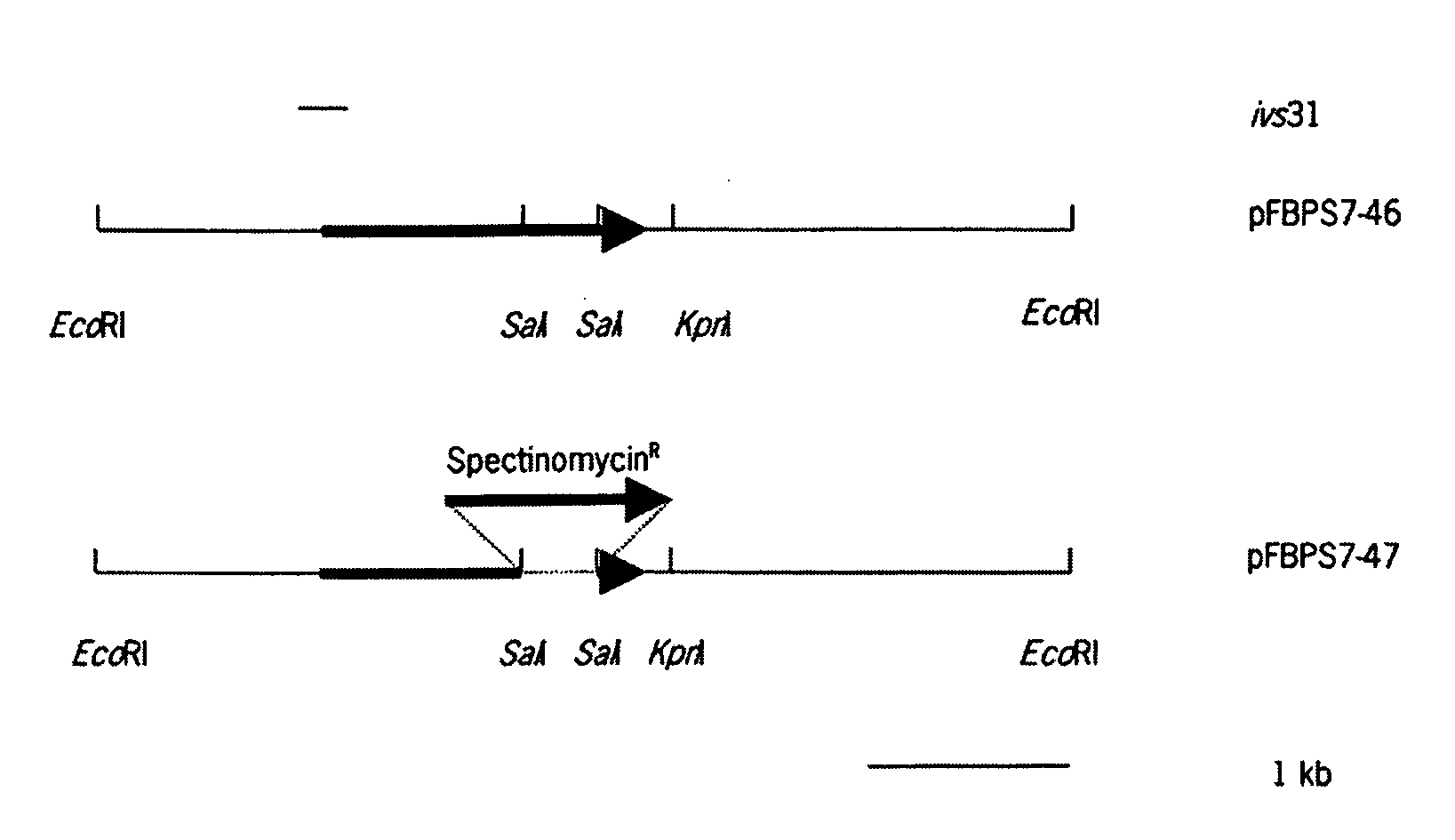
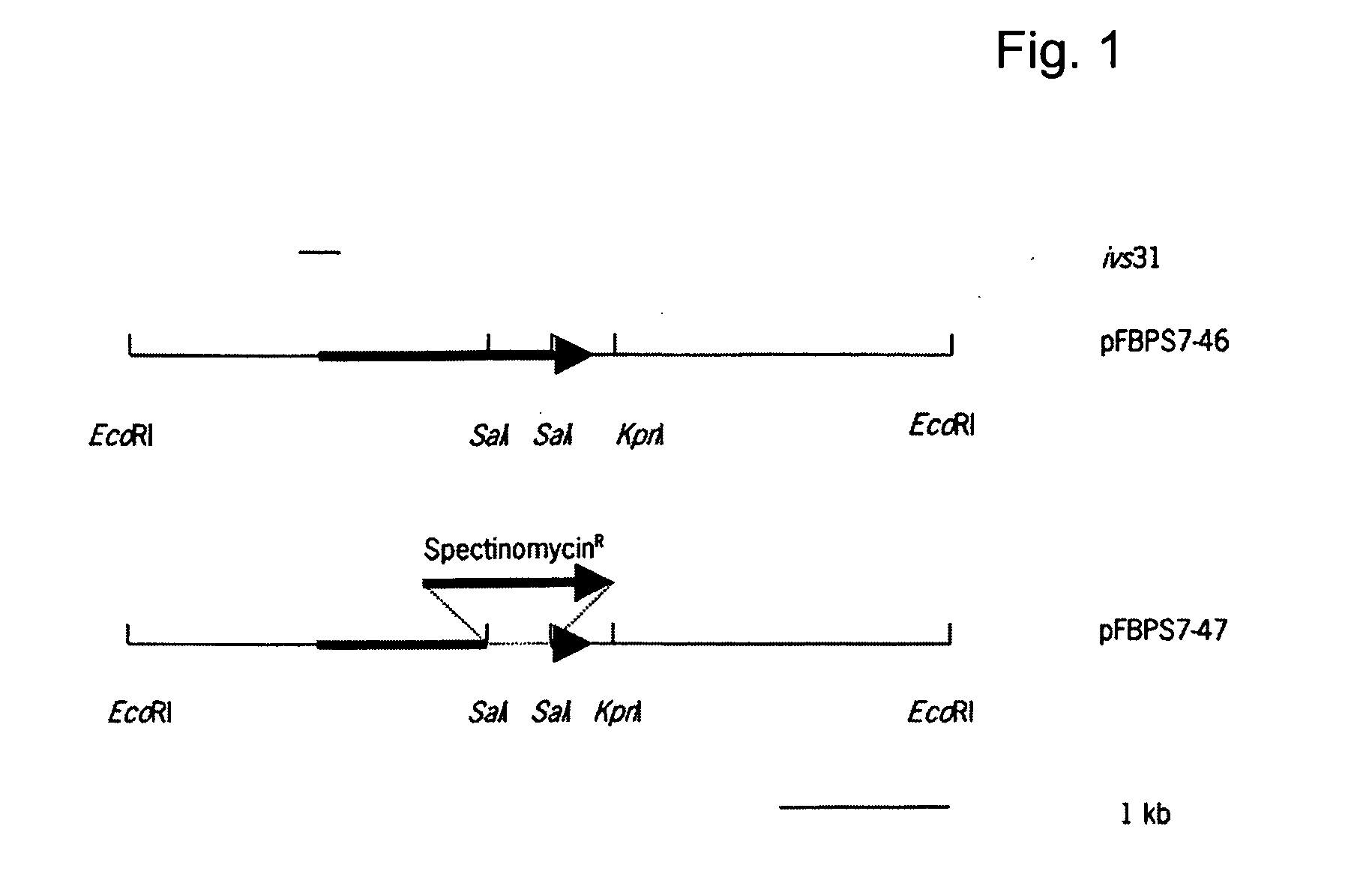
Environmentally regulated genes of Streptococcus suis
The invention relates to the field of the diagnosis of and vaccination against Streptococcal infections, and to the detection of virulence markers of Streptococci. The invention discloses a method for modulating virulence of a Streptococcus comprising modifying a genomic fragment of the Streptococcus, wherein the genomic fragment comprises at least a functional part of a fragment identifiable by hybridization in Streptococcus suis to a nucleic acid or fragment thereof.
Owner:STICHTING DIENST LANBOUWKUNDIG ONDERZOEK
Streptococcal streptolysin S vaccines
Provided are streptolysin S (SLS) polypeptides, peptides, and variants thereof, antibodies directed thereto, and isolated nucleic acids encoding such proteins. In one embodiment, a method is provided wherein a synthetic peptide of SLS is used to elicit an immune response specific for SLS in a subject to treat or prevent a streptococcal infection. In other embodiments, antibodies that neutralize the hemolytic activity of the SLS toxin may be used as a vaccinating agent.
Owner:UNIV OF TENNESSEE RES FOUND
Environmentally regulated genes of Streptococcus suis
The invention relates to the field of the diagnosis of and vaccination against Streptococcal infections, and to the detection of virulence markers of Streptococci. The invention discloses a method for modulating virulence of a Streptococcus comprising modifying a genomic fragment of the Streptococcus, wherein the genomic fragment comprises at least a functional part of a fragment identifiable by hybridization in Streptococcus suis to a nucleic acid or fragment thereof.
Owner:STICHTING DIENST LANBOUWKUNDIG ONDERZOEK
Broad-spectrum multi-subunit vaccine for preventing A type streptococcal infection
ActiveCN107737334ANo tissue damageNo local side effectsAntibacterial agentsBacterial antigen ingredientsSide effectMucosal adjuvant
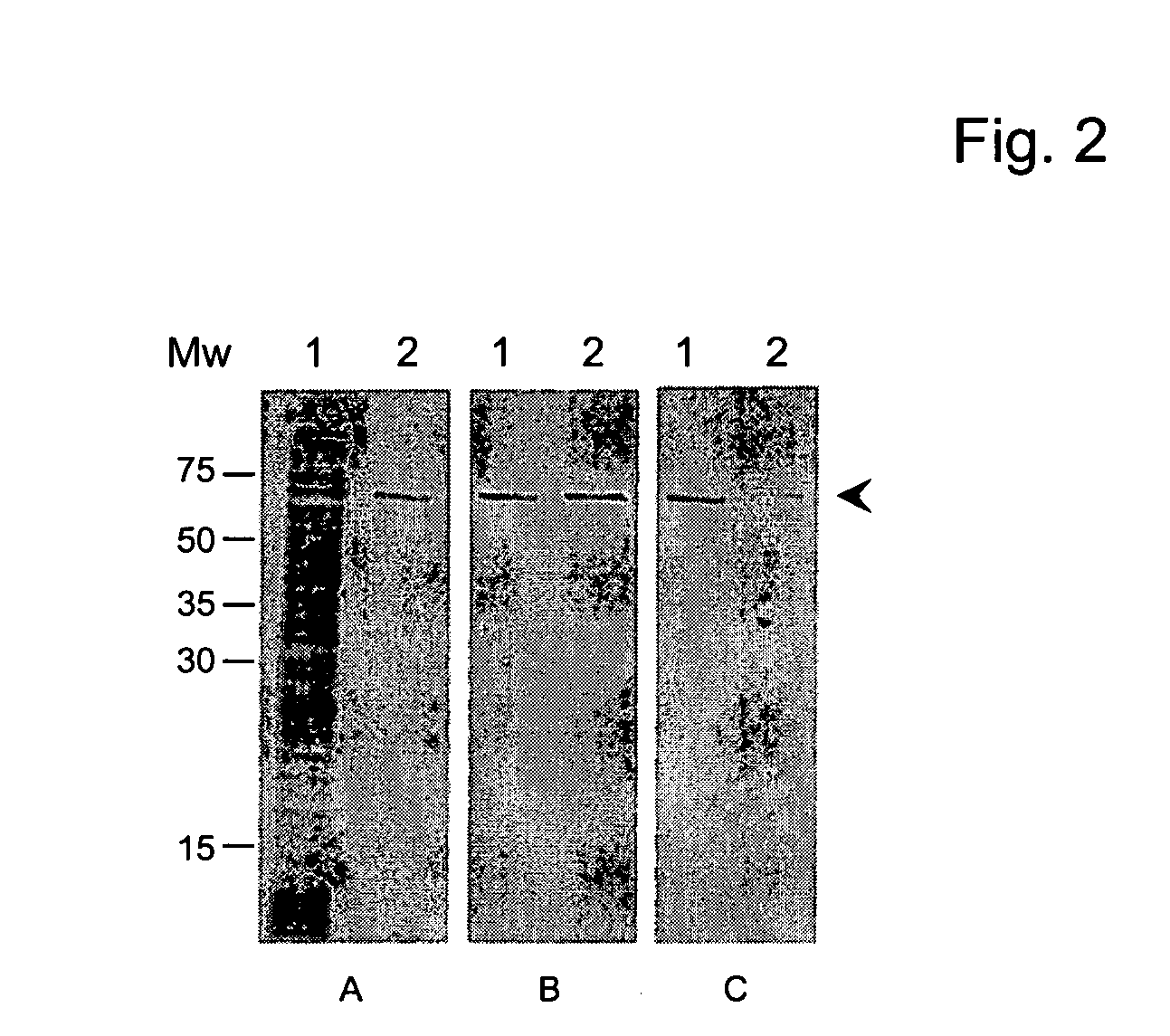
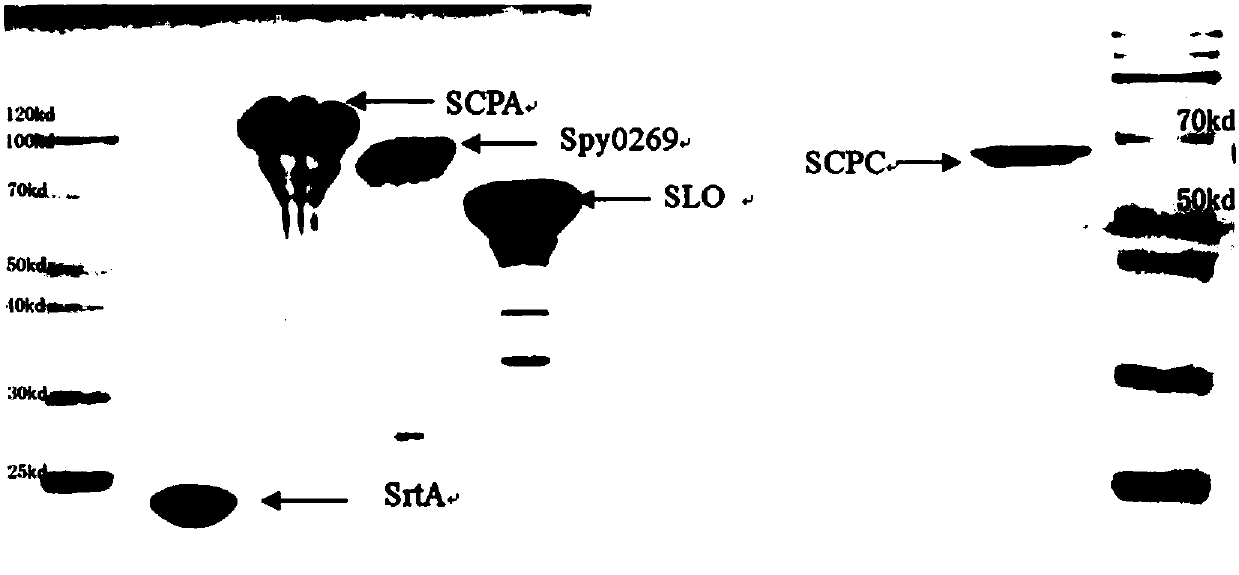
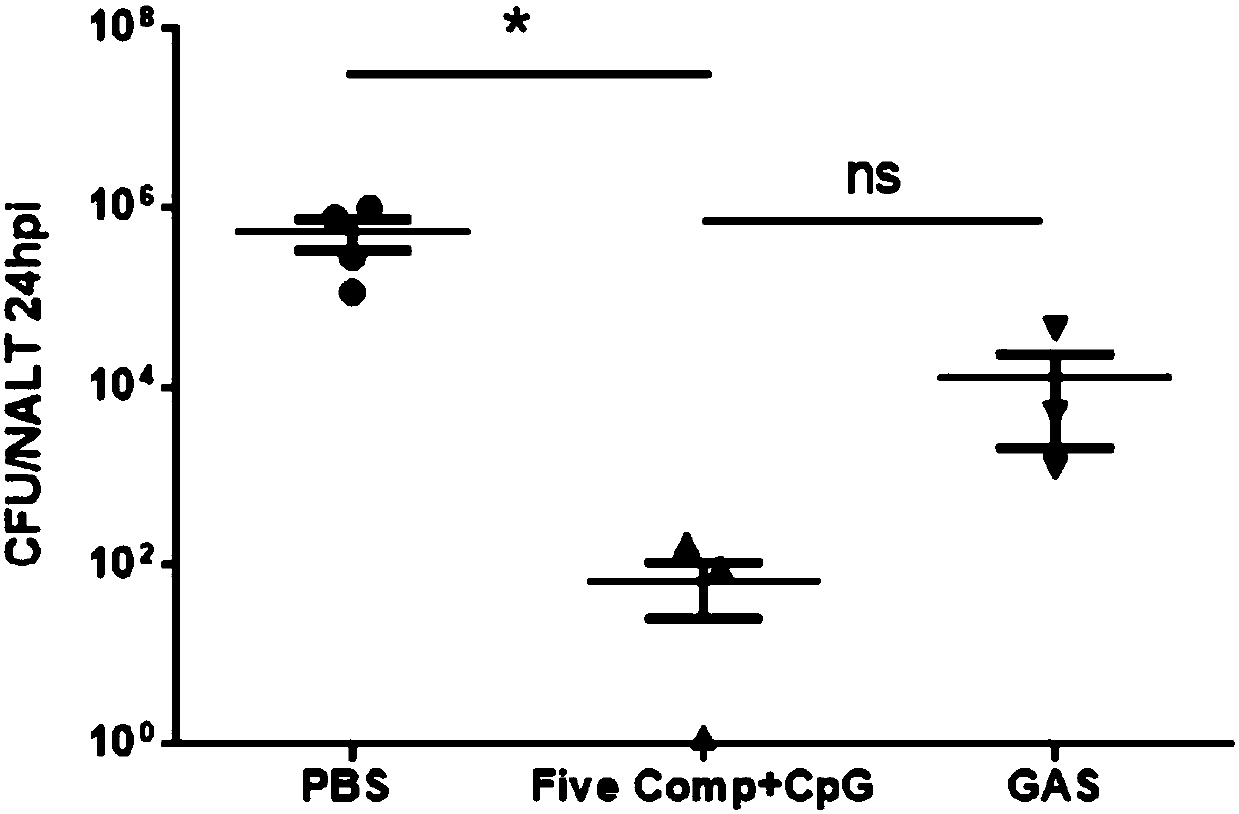
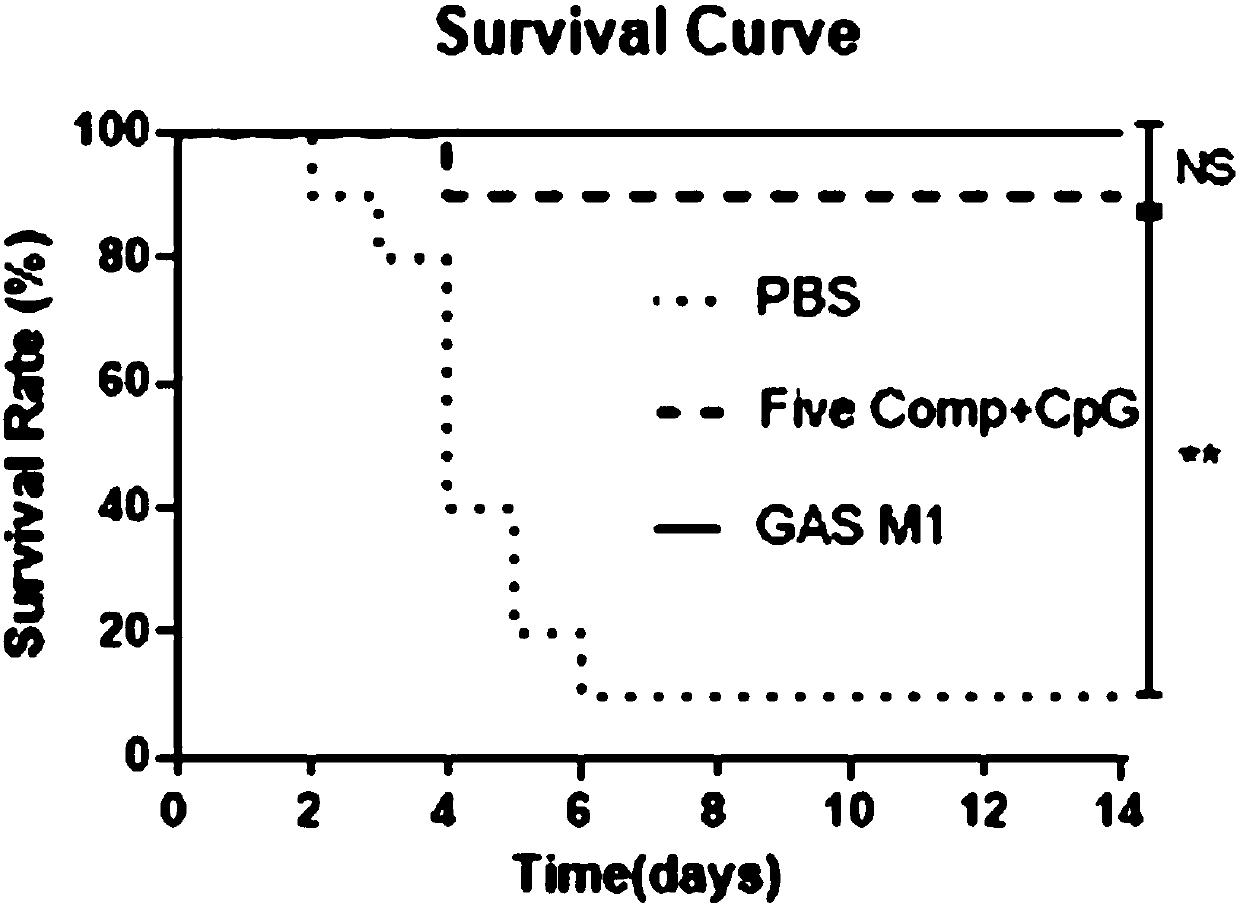
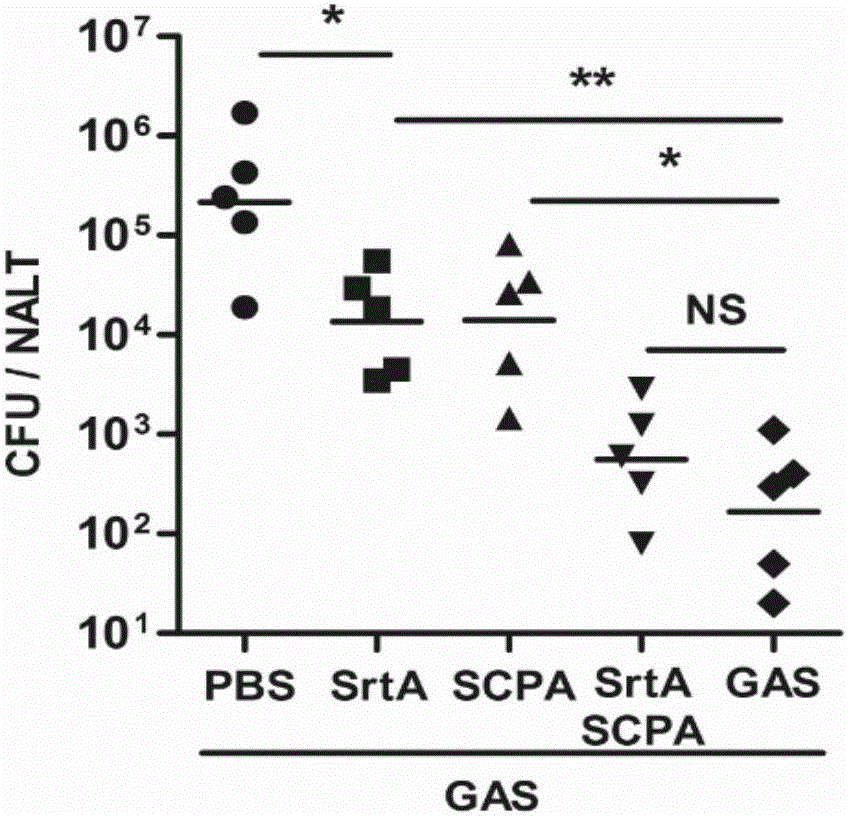
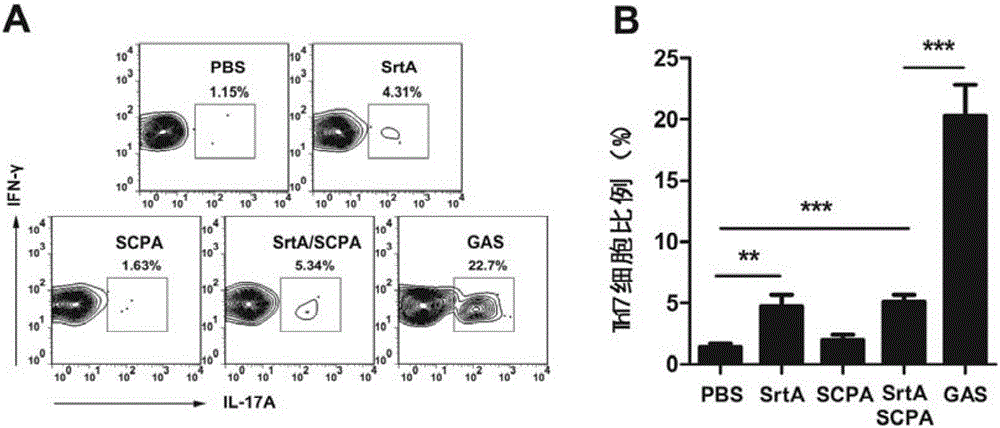
The invention discloses a vaccine for preventing A type streptococcal infection. The vaccine provided by the invention has the active ingredients such as ingredient A, ingredient B, ingredient C, ingredient D, ingredient E and ingredient F; the ingredient A is sortase or fusion protein with sortase; the ingredient B is SCPA or fusion protein with SCPA; the ingredient C is Spy0269 or fusion proteinwith Spy0269; the ingredient D is SCPC or fusion protein with SCPC; the ingredient E is SLO or fusion protein with SLO; and the ingredient F is adjuvant CpG or other mucosal adjuvants. The vaccine provided by the invention has the advantages of high efficiency, broad spectrum and low cost. Meanwhile, the vaccine provided by the invention adopts the way of mucosal immunity, has the characteristicsof no tissue damage, no local side effect and simple and convenient use, and is easy to popularize and use.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
N-acetyl-d-glucosamine for enhanced specificity of strep a immunoassay
ActiveUS20130196337A1Reduce probabilityReduce false alarm rateBioreactor/fermenter combinationsBiological substance pretreatmentsN Acetyl D GlucosamineStreptococcus mitis
Methods, compositions and kits for detecting Group A streptococcus in a biological sample are described. More particularly, the present disclosure provides an immunoassay in which the specificity of detection of Group A streptococcus is enhanced by addition of N-acetyl-D-glucosamine. These methods, compositions and kits are useful in convenient, reliable and early diagnosis of streptococcal infection in a human subject.
Owner:QUIDEL
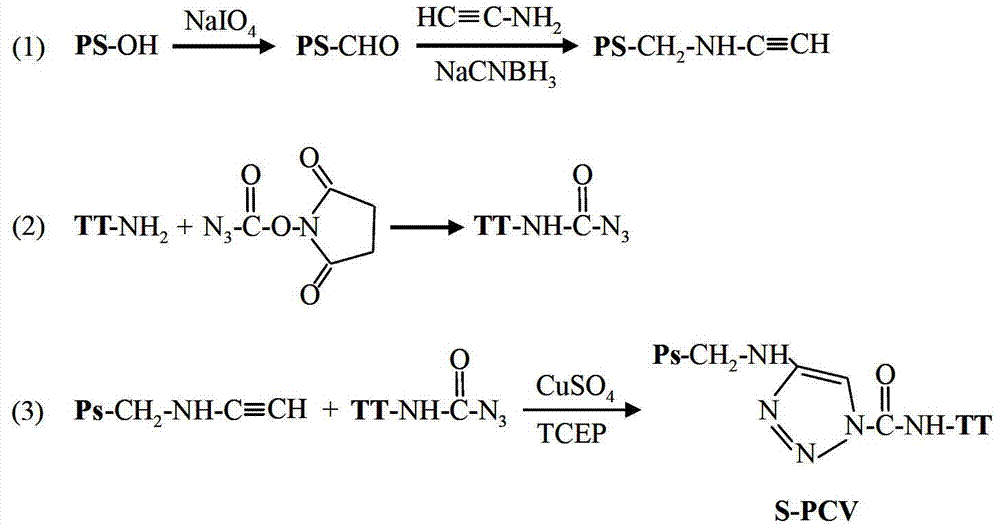
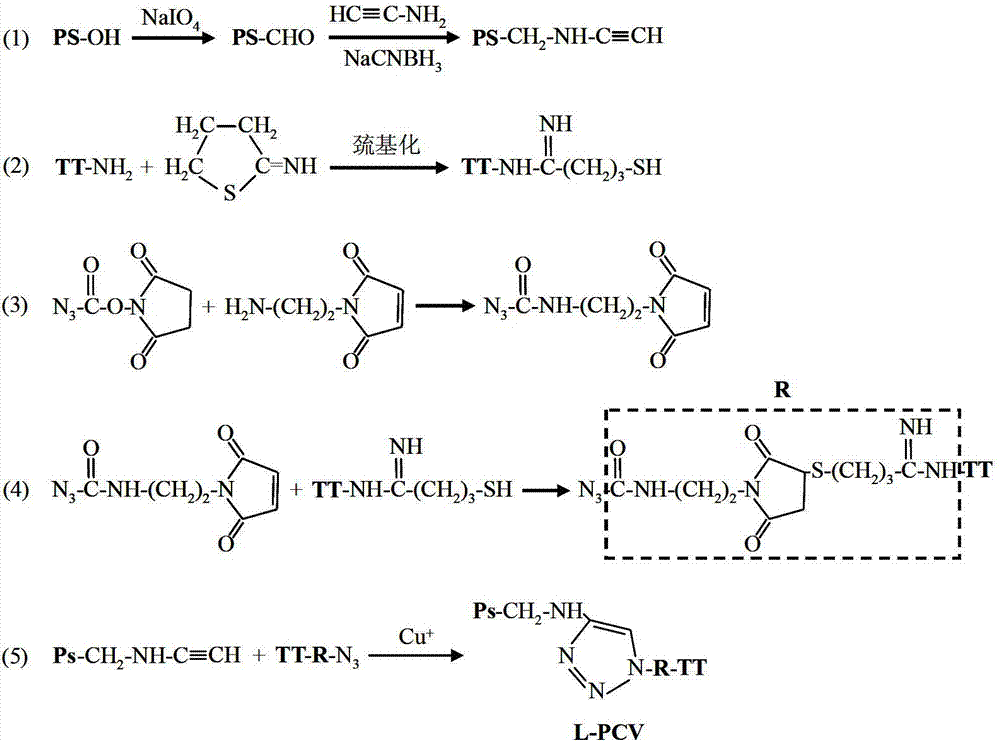
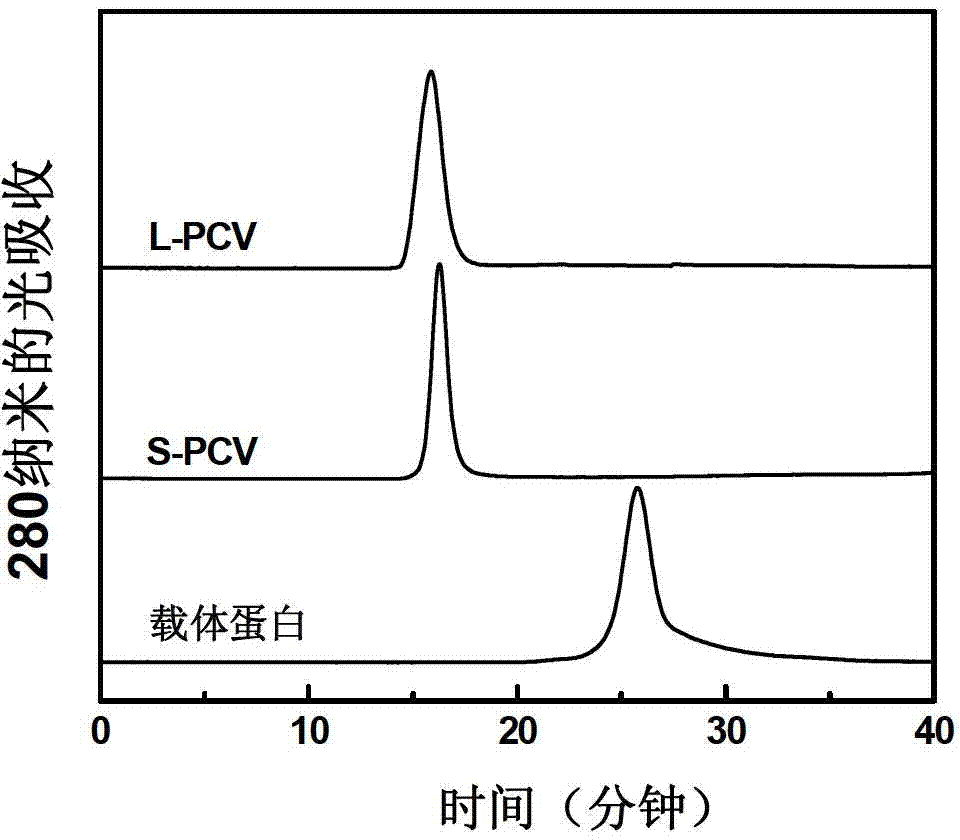
Click-chemistry-based pneumococcal polysaccharide conjugate vaccine and preparation method thereof
ActiveCN103110940AIncrease space distanceReduce space shielding effectAntibacterial agentsBacterial antigen ingredientsConjugate vaccineMedicine
The invention relates to a polysaccharide conjugate vaccine for preventing pneumonia streptococcal infection, which is prepared by connecting pneumococcal capsular polysaccharide to tetanus toxoid by using a 'click chemistry' method. The immunogenicity of the polysaccharide conjugate vaccine is improved by prolonging the length of a connecting bridge between the pneumococcal capsular polysaccharide and the tetanus toxoid.
Owner:华兰生物疫苗股份有限公司 +1
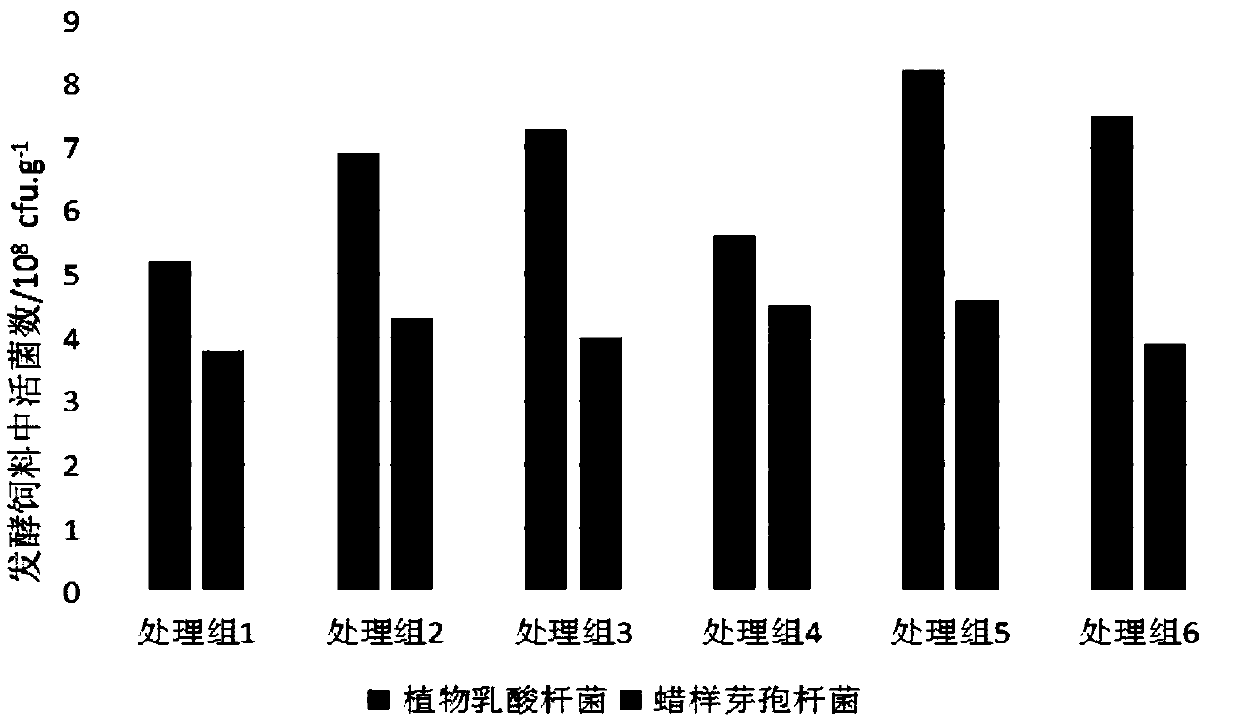
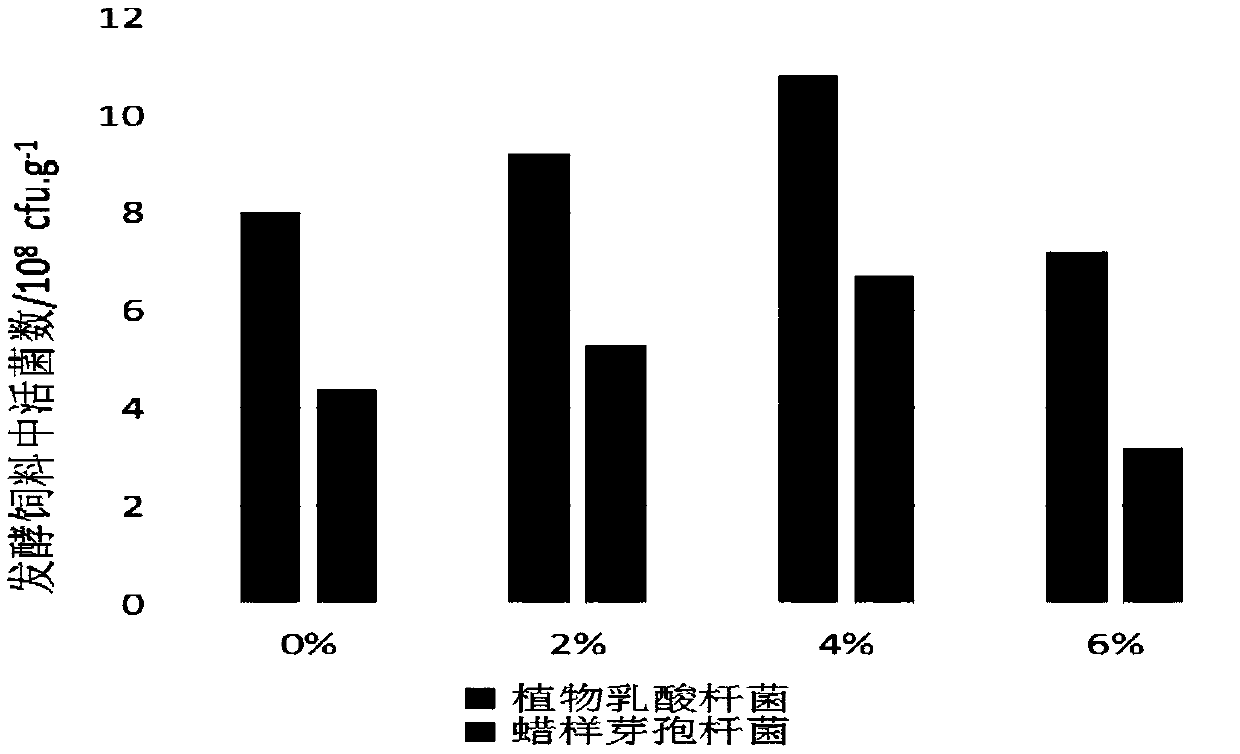
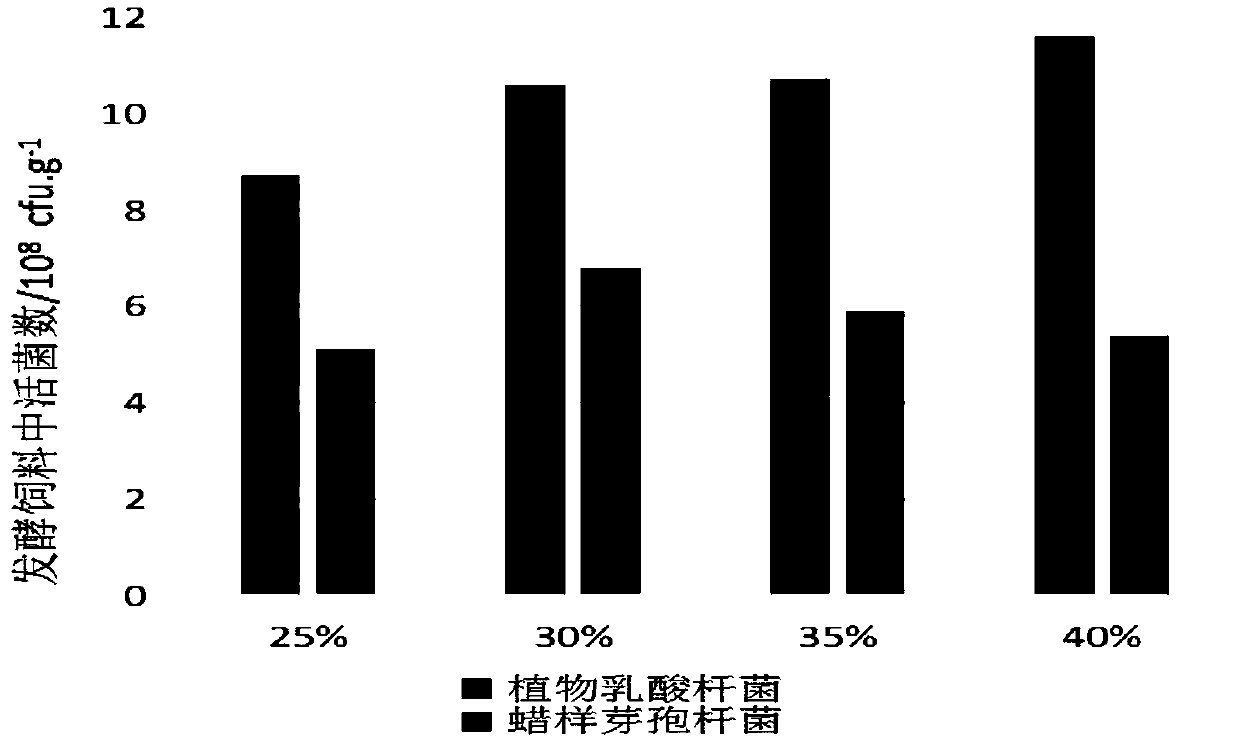
Aquatic feed pre-digestion method and product and application thereof
PendingCN109619324AImprove securityDisease resistantFood processingClimate change adaptationWater contentBacillus cereus
The invention provides an aquatic feed pre-digestion method, and belongs to the technical field of feed processing. The pre-digestion method firstly mixes an aquatic feed, lactobacillus plantarum, bacillus cereus and molasses according to a mass ratio of 100:2-6:0.2-0.4:2-6,adjusts the water content of a mixture to 25-40% by adding water, and finally places a material to be fermented in a sealed package for fermenting for 18-36 hours to obtain a pre-digestion aquatic feed. The pre-digestion method can maximize the functions of detoxifying and antagonizing pathogenic bacteria of a functional microbial feed;the fermentation of the method is basically not limited by an environmental temperature, the equipment investment is small, the operation is simple, and the process is stable. The application of the methodto tilapia culture can effectively improve the growth performance of the tilapia and the ability to resist streptococcal infection, increase the survival rate after infection of thebacteria, and be a pre-digestion treatment technology for the aquatic feed before feeding which is worthy of popularization and application.
Owner:GUANGXI ACADEMY OF FISHERY SCI
Vaccine against streptococcus pneumoniae
Owner:HERMAND PHILIPPE +3
Tilapia feed additive capable of reducing proinflammatory response and enhancing immune response and preparation method and application thereof
ActiveCN110432404AReduce expressionPromote healthy farmingAntibacterial agentsAntipyreticFeed additiveBiology
The invention provides a tilapia feed additive for reducing proinflammatory response and enhancing immune response and a preparation method and an application thereof, and belongs to the technical field of aquaculture. The tilapia feed additive for reducing proinflammatory response and enhancing immune response comprises the following components in parts by weight: 25-35 parts of manyprickle acanthopanax stems, 15-25 parts of loquat leaves, 15-25 parts of tripterygium wilfordii, 10-20 parts of hawthorn fruits and 10-20 parts of mulberry leaves. The invention also provides a tilapia feed containing the tilapia feed additive, and an application of the tilapia feed additive or the tilapia feed in tilapia breeding. The application can effectively reduce the proinflammatory response of the headkidney tissue and the spleen tissue of the tilapia, and enhance the immune response capability of the tilapia, thereby improving the capability of the tilapia against streptococcal infection.
Owner:FRESHWATER FISHERIES RES CENT OF CHINESE ACAD OF FISHERY SCI
Group A streptococcal polysaccharide immunogenic compositions and methods
This invention provides a novel immunogenic composition and vaccine, processes for producing them and methods for immunization against infections and disease caused by group A Streptococci. The compositions include group A streptococcal polysaccharide covalently linked to protein or liposomes to form immunogenic conjugates. The method of immunization for this invention comprises administering to an individual an immunogenic amount of group A polysaccharide. The group A polysaccharide may be administered as a vaccine either on its own, conjugated to proteins or conjugated to liposomes. Additionally, the group A polysaccharides may be associated with an adjuvant. This invention is particularly useful for providing both active and passive immunogenic protection for those populations most at risk of contracting group A Streptococcal infections and disease namely adults, pregnant women and in particular infants and children.
Owner:THE ROCKEFELLER UNIV +1
Diagnostic Method for Pediatric Acute-Onset Neuropsychiatric Syndrome (PANS) and Pediatric Autoimmune Neuropsychiatric Disorder Associated with Streptococci Infection (PANDAS)
InactiveUS20140271678A1Convenient and accurate diagnosticReliable diagnosisAntibody ingredientsDisease diagnosisPresent methodCalmodulin-dependent protein kinase activity
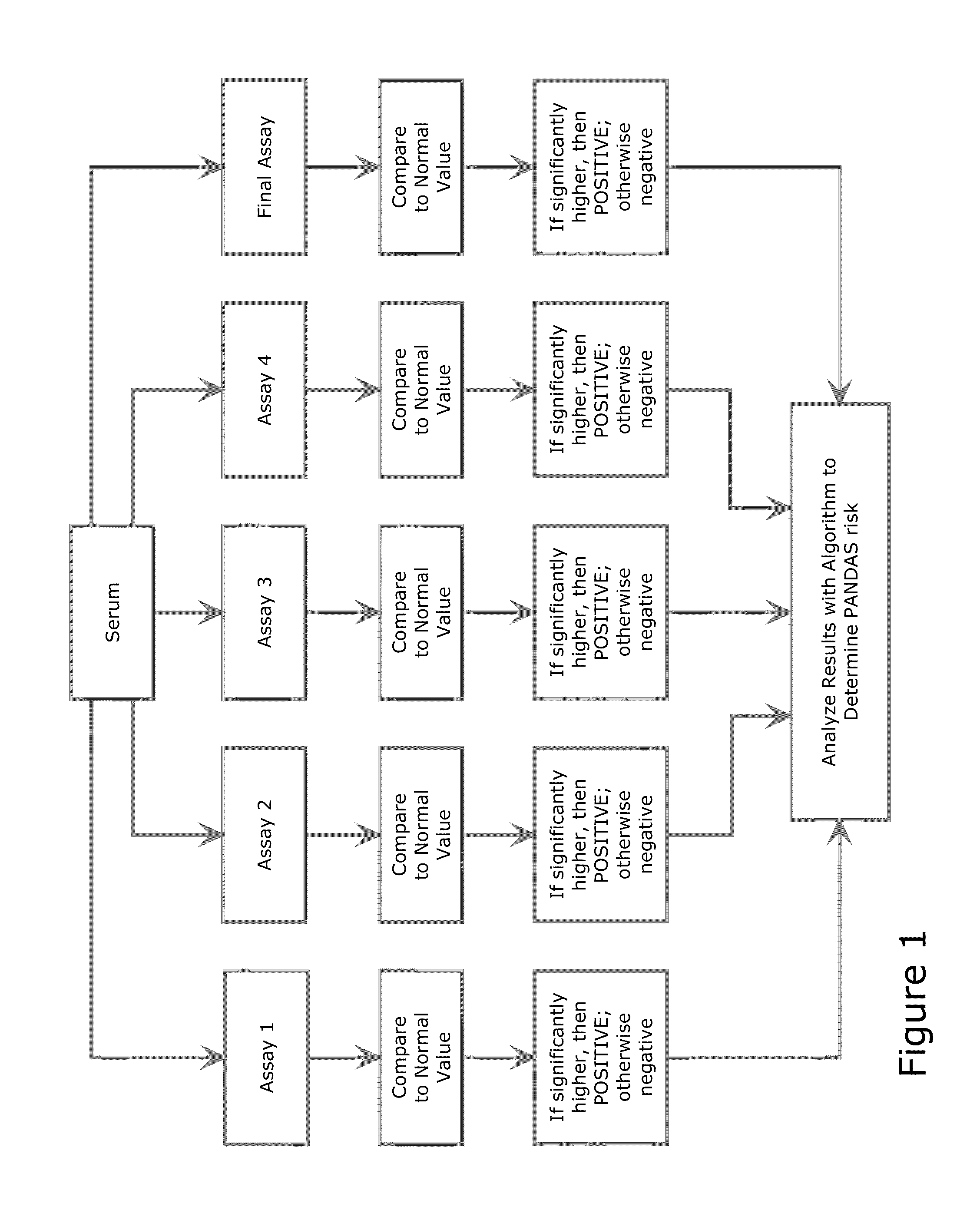
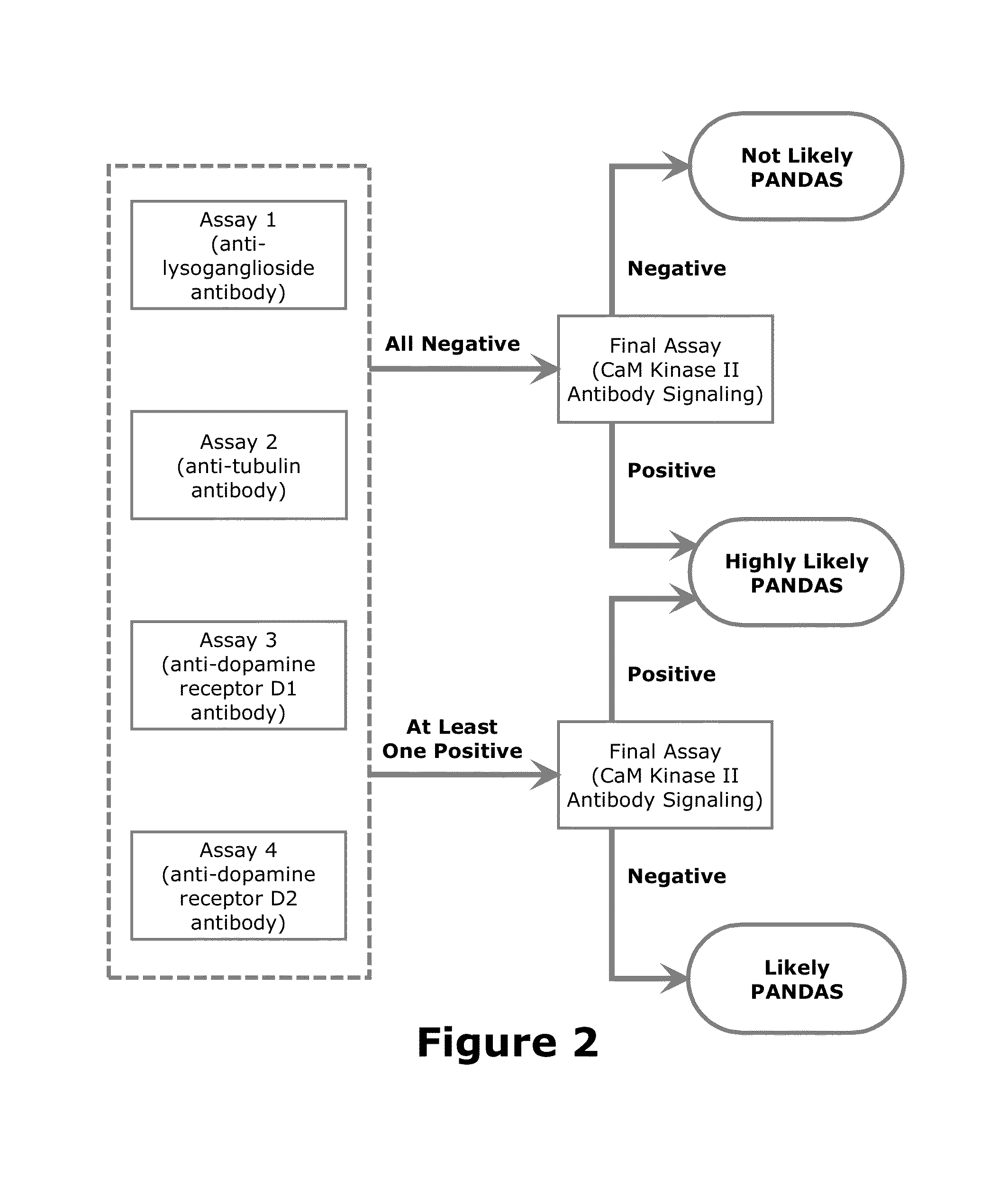
The present invention provides a panel of at least five clinical analyses or tests (using serum samples) to determine the risk of pediatric acute-onset neuropsychiatric syndrome (PANS) and / or pediatric autoimmune neuropsychiatric disorder associated with streptococcal infection (PANDAS) in an individual. These include enzyme linked immunosorbent assays (ELISAs) to measure antibody titers against neuronal antigens present in the brain; the neuronal antigens include lysoganglioside, tubulin, dopamine receptor D1, dopamine receptor D2, serotonin receptor 5HT2A, and serotonin receptor 5HT2C. Antibody titers against at least four of these neuronal antigens are required in the present methods; preferably antibody tiers against all of these neuronal antigens are measured. A final assay is used to quantify calcium / calmodulin-dependent protein kinase activity using a neuronal cell line. The results of these analyses or tests are then combined using an algorithm to determine whether a PANS or PANDAS diagnosis is appropriate for the individual. Depending on the diagnosis, an appropriate treatment can be determined.
Owner:THE BOARD OF RGT UNIV OF OKLAHOMA +2
Combined vaccine for inhibiting and / or preventing type A streptococcal infection
ActiveCN105056223AEffective protectionImproving immunogenicityAntibacterial agentsBacterial antigen ingredientsSortase ASTREPTOCOCCAL INFECTIONS
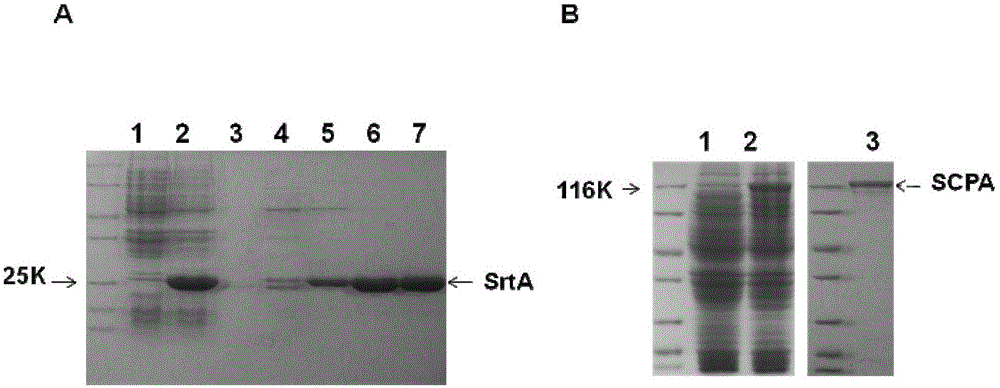
The present invention discloses a combined vaccine for inhibiting and / or preventing type A streptococcal infection. The present invention provides a composition for inhibiting streptococcus and / or preventing streptococcal infection, and the composition comprise an immunogen. The immunogen is one of the following 1)-4): 1) the immunogen comprises a sortase A and a C5a protease; 2) the immunogen comprises a sortase A solution and a C5a protease solution; 3) the immunogen comprise a fusion protein containing sortase A and a fusion protein containing C5a protease; and 4) the immunogen comprises a junctional complex of sortase A and polysaccharide and a junctional complex of C5a protease and polysaccharide. The specific amino acid sequence of the sortase A is the N-terminal amino acid residues from site No.22 to No.189 of a sequence 2; and the amino acid sequence of the C5a protease is a sequence 4 in the sequence table. The combined vaccine can be applied to prevention and treatment of mucosal system infections caused a variety of Gram-positive bacteria.
Owner:HAIKOU PHARMA FACTORY
Prophylactic and/or therapeutic agent for pneumococcal infection
ActiveCN111542330APrevention and/or treatment of infectionsAntibacterial agentsUnknown materialsGenus EnterococcusTherapeutic food
Provided is a novel prophylactic and / or therapeutic agent for a pneumococcal infection. It is found that bacteria belonging to the genus enterococcus can prevent and / or treat a pneumococcal infection.Provided is a prophylactic and / or therapeutic agent for a pneumococcal infection, the agent containing bacteria belonging to the genus enterococcus. Provided is a prophylactic and / or therapeutic medicine for a pneumococcal infection, the medicine containing bacteria belonging to the genus enterococcus. Provided is a prophylactic and / or therapeutic food for a pneumococcal infection, the food containing bacteria belonging to the genus enterococcus.
Owner:NUTRI CO LTD
Methods and Compositions for Diagnosing and Preventing a Group B Streptococcal Infection
The present invention provides a group B streptococcal (GBS) surface antigen, designated epsilon antigen, that is co-expressed with the delta antigen on a subset of serotype III GBS. Epsilon is expressed on more pathogenic Restriction Digest Pattern (RDP) III-3 GBS, but not on RDP types 1, 2, or 4. Accordingly, the present invention provides compositions and methods for detecting a group B streptococcus serotype III, RDP III-3 strain. Vaccines and methods of identifying agents which inhibit adhesion of a group B streptococcal cell to a host cell are also provided.
Owner:ST JUDE CHILDRENS RES HOSPITAL INC +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com