Combined vaccine for inhibiting and / or preventing type A streptococcal infection
A type A streptococcus and streptococcus technology, applied in antibacterial drugs, bacterial antigen components, etc., can solve problems such as hindering vaccine development, lack of serotype crossover, and difficulty in developing vaccines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
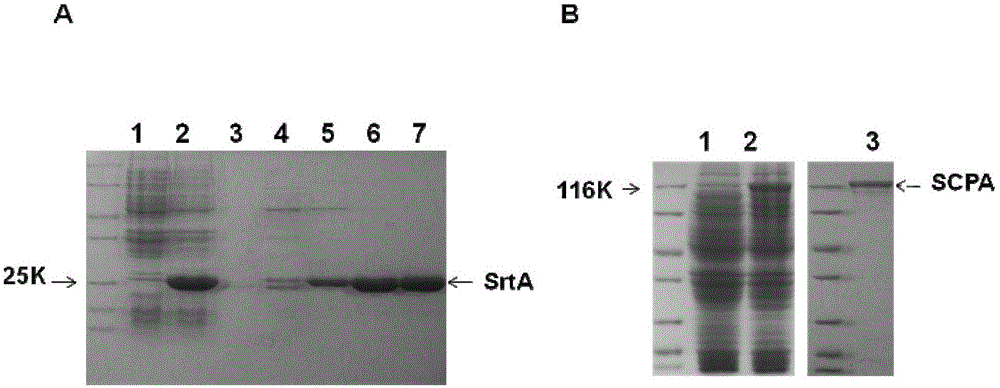
[0061] Embodiment 1, the preparation of combined vaccine component sortase A and C5a protease
[0062] 1. Preparation of sortase A
[0063] 1. Preparation of recombinant expression vector
[0064] 1) Using the genomic DNA of type A Streptococcus M1 as a template, perform PCR amplification with a primer pair composed of F1 and R1 to obtain a 516bp PCR amplification product, which is the sortase A gene SrtA.
[0065] F1: 5'-CTTA CATATG GTCTTGCAAGCACAAATGG-3';
[0066] R1: 5'-ATGTT CTCGAG CTAGGTAGATACTTGGTTATAAGA-3'.
[0067] 2), using restriction endonucleases NdeI and XhoI to double digest the PCR amplified product of step 1, and reclaim the digested product.
[0068] 3) The vector pET28a(+) was double digested with restriction endonucleases NdeI and XhoI, and the vector backbone of about 6000 bp was recovered.
[0069] 4) Ligate the digested product of step 2 with the vector backbone of step 3 to obtain the recombinant plasmid pET28a-SrtA.
[0070] After sequencing, t...
Embodiment 2
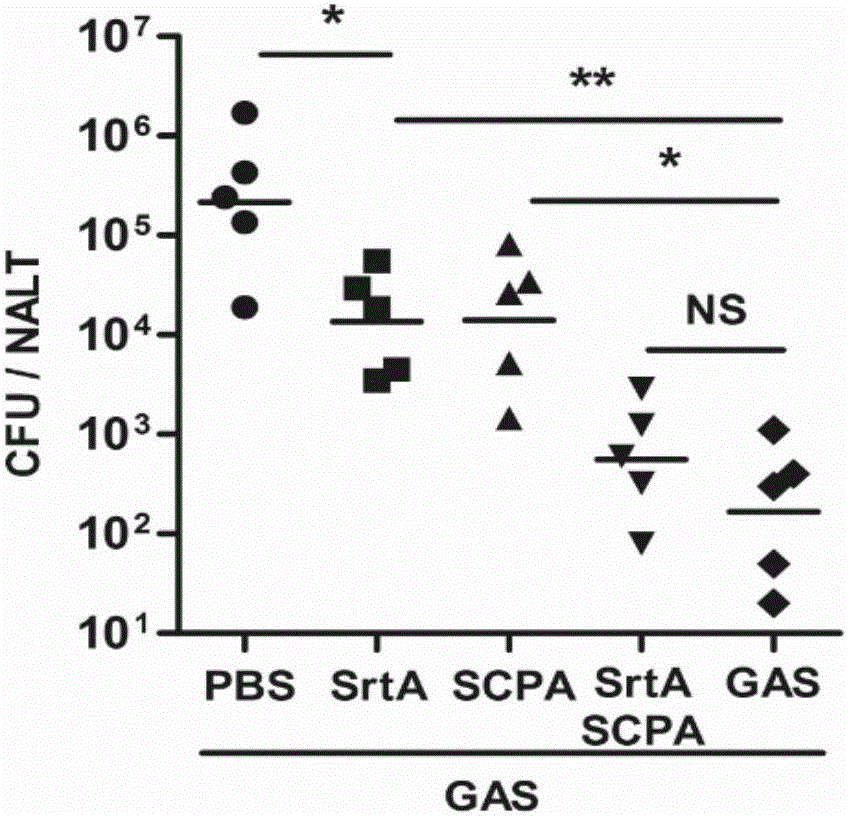
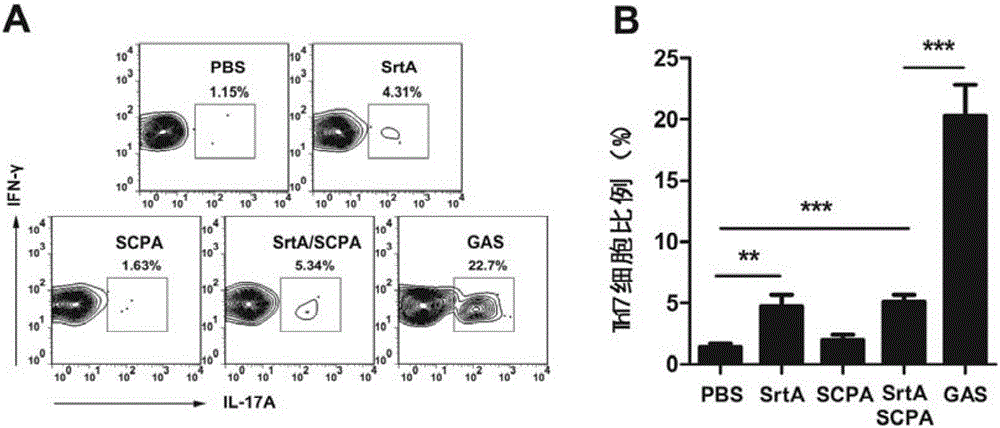
[0109] Embodiment 2, the functional research of combined vaccine
[0110] 1. The immune protection of the combined vaccine against Streptococcus A
[0111] The immunogens are grouped as follows:
[0112] Sortase A plus CTB group (SrtA): the sortase A prepared in Example 1 and the adjuvant CTB were mixed according to the mass ratio of 10:1, and immunized, and the immunization dose was 10 μg sortase A / time / piece;
[0113] C5a protein plus CTB group (SCPA): the C5a protease prepared in Example 1 and the adjuvant CTB were mixed according to the mass ratio of 20:1, and immunized, and the immunization dose was 20 μg C5a protein / time;
[0114] Combined vaccine plus CTB group (SrtA / SCPA): Combined vaccine immunization prepared in Example 1, immunization dose is 20 μg C5a protein / time;
[0115] Streptococcus A full bacteria group (GAS): Type A Streptococcus M1 type immunity, the immune dose is 5×10 7 cfu / time;
[0116] PBS group: use PBS (10mMNa 2 HPO 4 , 1.8mMKH 2 PO 4 , 140mM...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com